Abstract
The biotransformation of the polycyclic aromatic hydrocarbons (PAHs) naphthalene and phenanthrene was investigated by using two dioxygenase-expressing bacteria, Pseudomonas sp. strain 9816/11 and Sphingomonas yanoikuyae B8/36, under conditions which facilitate mass-transfer limited substrate oxidation. Both of these strains are mutants that accumulate cis-dihydrodiol metabolites under the reaction conditions used. The effects of the nonpolar solvent 2,2,4,4,6,8,8-heptamethylnonane (HMN) and the nonionic surfactant Triton X-100 on the rate of accumulation of these metabolites were determined. HMN increased the rate of accumulation of metabolites for both microorganisms, with both substrates. The enhancement effect was most noticeable with phenanthrene, which has a lower aqueous solubility than naphthalene. Triton X-100 increased the rate of oxidation of the PAHs with strain 9816/11 with the effect being most noticeable when phenanthrene was used as a substrate. However, the surfactant inhibited the biotransformation of both naphthalene and phenanthrene with strain B8/36 under the same conditions. The observation that a nonionic surfactant could have such contrasting effects on PAH oxidation by different bacteria, which are known to be important for the degradation of these compounds in the environment, may explain why previous research on the application of the surfactants to PAH bioremediation has yielded inconclusive results. The surfactant inhibited growth of the wild-type strain S. yanoikuyae B1 on aromatic compounds but did not inhibit B8/36 dioxygenase enzyme activity in vitro.
Polycyclic aromatic hydrocarbons (PAHs) are a major cause of concern as anthropogenic pollutants in the environment. They arise from diverse sources, including petrochemical products and the combustion of fossil fuels (3). Concern arises for two reasons, first because many are recalcitrant, and second because of the health hazards associated with these compounds. Many, such as benzo[a]pyrene, chrysene, and benz[a]anthracene, are also carcinogens in animals.
One approach that has been considered for enhancing PAH bioremediation in contaminated soils is the application of nonionic surfactants (26). The theoretical justification for this solution is based upon two hypotheses, first that surfactant micelles may sequester PAHs which are sorbed to the soil matrix, and second that the surfactant micelles may increase the concentration of PAHs in the aqueous phase because the PAHs are more soluble in the micelles. Where the rate of PAH degradation is limited by mass transfer from the solid phase to the aqueous phase, the PAH oxidation rates by microorganisms may then be enhanced (11, 23). Furthermore, there is also some evidence to suggest that certain microorganisms may absorb PAHs directly from surfactant micelles (27).
A number of studies to test these hypotheses have been carried out, but the results have been inconclusive. While some reports suggest that surfactants may increase PAH biodegradation rates (20, 26, 28, 29), others have shown that the effect of their application may be negligible or even detrimental (19, 26, 28, 29).
To date, the typical approach to evaluating surfactants in laboratory studies with monocultures has involved the analysis of their effect on microbial growth characteristics, such as specific growth rate and cellular yields, with PAHs as growth substrates (11). However, the conclusions may not be wholly applicable because this type of study excludes those microorganisms present in the environment which can oxidize the PAHs by cometabolism (i.e., concomitant metabolism of growth and nongrowth substrates). For many PAHs, especially those with four or more aromatic rings, cometabolism may serve as the main route for their degradation (10, 15, 22, 23). Furthermore, growth of monocultures in these experiments (where specific growth rates are relatively high) may not be limited by the same factors in situ, where specific growth rates of bacteria are likely to be much lower. If the results from experiments using monocultures are to be used to optimize surfactant use in bioremediation, then the effect of the surfactants on the rate of PAH oxidation by the di- and mono-oxygenase enzymes involved in the initial degradation of these molecules might prove to be more useful.
In this study, a comparison was made between two groups of microorganisms which play an important role in the degradation of aromatic hydrocarbons in the environment. Sphingomonads have been shown to metabolize a number of PAHs, including the larger molecules, such as benzo[a]pyrene (10) and chrysene (2). Pseudomonads are known to be particularly important in the biodegradation of monocyclic aromatic hydrocarbons, such as toluene and benzene (7, 8, 30), and of the smaller PAHs, such as naphthalene and phenanthrene (5, 12, 17).
In this paper, we propose that the inconclusive observations which have resulted from the earlier surfactant-PAH bioremediation studies can be explained if the surfactants were inhibitory to PAH degradation by some bacteria while enhancing the rate of PAH degradation in others. If this were the case, then the composition of the microbial population in a PAH-contaminated site would determine the effectiveness of such nonionic surfactants in PAH bioremediation applications.
We would then expect that when different PAH-degrading microorganisms are used to biotransform PAHs, they will show varying responses to the addition of nonionic surfactants. This is because if a surfactant is not toxic to a microorganism under conditions where PAH oxidation is limited by substrate mass transfer, then an increase in the rate of PAH oxidation is expected. If the PAH is toxic, then inhibition of PAH oxidation should occur.
The aim of the work presented here was to investigate the effect of a common surfactant on PAH oxidation by arene dioxygenase enzymes in two different microorganisms, which can convert the PAHs to their corresponding cis-dihydrodiol metabolites. The enzymes are expressed in mutant strains of bacteria which do not have cis-dihydrodiol dehydrogenase enzyme activity and therefore accumulate the cis-dihydrodiols. This work therefore provided an opportunity to test the explanation proposed above. One advantage of this approach is that any conclusions drawn may be applicable to degradation processes that involve cometabolism.
The bacteria used were the wild-type Sphingomonas yanoikuyae B1 (9, 16) and a mutant (B8/36) derived from this strain. The mutant accumulates cis-dihydrodiol metabolites when grown under experimental conditions, facilitating the expression of a biphenyl dioxygenase (BPO) enzyme (25). Also used were Pseudomonas sp. strain NCIMB 9816 (5) and a mutant strain, 9816/11, which also accumulates cis-dihydrodiol metabolites under conditions that facilitate expression of a naphthalene dioxygenase enzyme (NDO) (25). Pseudomonas sp. strain NCIMB 9816 was obtained from the National Collection of Industrial and Marine Bacteria Ltd., Aberdeen, United Kingdom.
Biotransformation of PAHs in a two-phase HMN-water system.
Surfactant solutions at concentrations above the critical micelle concentration (CMC) can be considered “two-phase” systems, where the secondary phase is made up of the surfactant micelle pseudophase (11).
We investigated the biotransformation of the PAHs naphthalene and phenanthrene by the two mutant strains in a two-phase system to establish conditions under which PAH degradation was mass transfer limited. We would then expect to see an increased yield of PAH cis-dihydrodiol metabolites in this system, where the substrates are dissolved in a suitable nonpolar solvent. The solvent 2,2,4,4,6,8,8-heptamethylnonane (HMN) was chosen (24).
In biotransformation experiments, the concentration of the products, cis-1R,2S-dihydroxy-1,2-dihydronaphthalene (naphthalene cis-1,2-dihydrodiol) and cis-3S,4R-dihydroxy-3,4-dihydrophenanthrene (phenanthrene cis-3,4-dihydrodiol), was determined. Naphthalene is converted to naphthalene cis-1,2-dihydrodiol by both of the two strains (12), and phenanthrene is converted to a mixture of phenanthrene cis-3,4-diol (95% relative yield) and cis-1R,2S-dihydroxy-1,2-dihydrophenanthrene (phenanthrene cis-1,2-dihydrodiol; 5% relative yield) (13, 18). By analysis of biotransformation crude extracts using 1H-nuclear magnetic resonance (500 MHz, CDCl3), we have found the same relative yields of the 3,4- and 1,2-dihydrodiols produced in phenanthrene biotransformations with both S. yanoikuyae B8/36 and Pseudomonas sp. strain 9816/11. Both of these compounds coeluted when they were detected in time course biotransformations by reverse-phase high-pressure liquid chromatography (HPLC). Purified phenanthrene cis-3,4-dihydrodiol was therefore used as a standard. For quantification by HPLC, the cis-dihydrodiol standards were prepared by using published methods (1, 13).
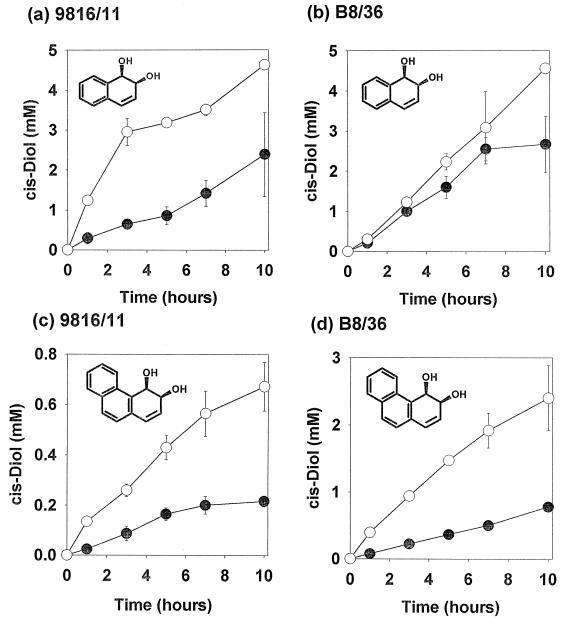
The results of these time course studies are shown in Fig. 1. At the end of the experiments, the concentration of cis-dihydrodiol in the HMN phase was determined, and the partition coefficient of phenanthrene cis-dihydrodiol concentration in the organic phase versus the concentration in the aqueous phase was found to be approximately 0.1 for both cis-dihydrodiols. This indicated that most of the cis-dihydrodiol metabolites produced were present in the aqueous phase.
FIG. 1.
Effect of HMN on the biotransformation of naphthalene by Pseudomonas sp. strain 9816/11 (a) and S. yanoikuyae B8/36 (b) and of phenanthrene by Pseudomonas sp. strain 9816/11 (c) and S. yanoikuyae B8/36 (d). HMN was added at a ratio of 5 ml per 100 ml of cell suspension (○). Control flasks (●) had no HMN added. Strains were grown using published methods (induction being required for expression of dioxygenase enzymes for both biotransformation and enzyme assay experiments) (25). For all biotransformation experiments, cells were resuspended in 0.1 M potassium phosphate buffer (pH 7.5; A600 = 1.1). Sodium succinate (31 mM) was used as a cosubstrate, and (unless otherwise stated) PAH substrates were added at a concentration of 0.9 g liter−1. All biotransformations were conducted in triplicate; data points show the mean concentrations of cis-dihydrodiol metabolites in the aqueous phase. HPLC analysis using an octyldecyl silane 15-cm reverse-phase column (50 to 70% methanol-water gradient over 30 min, 0.5 ml min−1 flow rate) of phenanthrene metabolites was performed at 260 nm, and analysis of naphthalene metabolites was performed at 265 nm.
These data clearly show that in all cases HMN increased the yield of cis-dihydrodiols accumulating over the period of the experiment. The effect was most noticeable with the phenanthrene biotransformations, but this might be expected, as phenanthrene is considerably less soluble in water (1.3 mg liter−1) than naphthalene (31.7 mg liter−1) at ambient temperature (21). Therefore, where biotransformation rates are limited by mass transfer of the PAH from the solid phase via the liquid phase to the biocatalyst, the large increase in interfacial area which results from dissolving the PAHs in HMN would be expected to increase the rate of microbial oxidation.
It is interesting to note that there were some differences between biotransformations with the two microorganisms. Similar concentrations of the cis-dihydrodiol were observed for the naphthalene biotransformations with both microorganisms when HMN was added. However, S. yanoikuyae B8/36 accumulated the cis-dihydrodiol of phenanthrene at approximately four times the amount of that achieved with the 9816/11 strain.
Effect of Triton X-100 on PAH biotransformations.
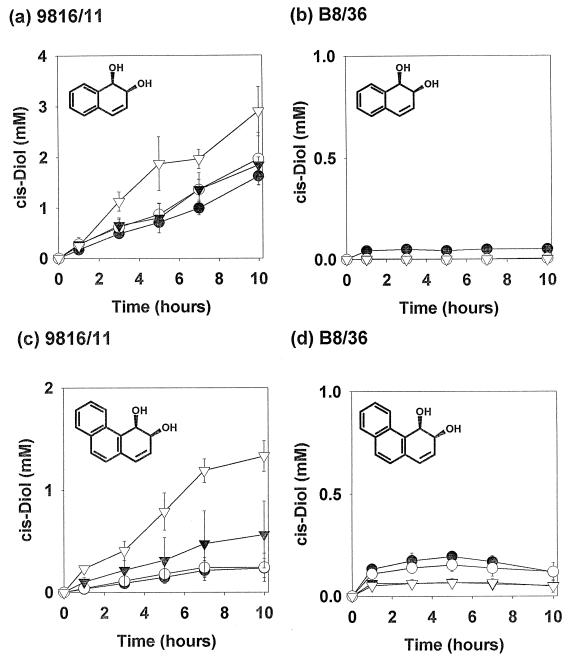
Having established that two-phase systems can be used to enhance the yields of PAH metabolites under mass-transfer limited biotransformation conditions with the two strains, the effect of a surfactant was then determined. We chose to use Triton X-100 in these experiments because a number of reports suggest that it is effective in PAH bioremediation and also relatively nontoxic to microorganisms (19, 20, 29). The surfactant was tested by using the same biotransformation conditions as in the earlier experiments and at a number of concentrations above the cited CMC of 0.17 mM (20). The results from these experiments are shown in Fig. 2.
FIG. 2.
Effect of Triton X-100 on biotransformation of naphthalene by Pseudomonas sp. strain 9816/11 (a) and S. yanoikuyae B8/36 (b) and of phenanthrene by Pseudomonas sp. strain 9816/11 (c) and S. yanoikuyae B8/36 (d). Triton X-100 (variable concentrations as described, prepared in a filter-sterilized 100 mM stock solution) was added to final concentrations of 0.2 mM (●), 0.5 mM (○), 1.0 mM (▾), and 2.0 mM (▿). All biotransformations were conducted in triplicate; data points show the total mean concentrations of cis-dihydrodiol metabolites present.
If the effects of the surfactant on Pseudomonas sp. strain 9816/11 are considered, it is clear that there is a significant enhancement of cis-dihydrodiol accumulation with both PAHs in the presence of increasing concentrations of the surfactant. Furthermore, it should be noted that the effect with phenanthrene was again greater than that with naphthalene. The concentration of phenanthrene cis-dihydrodiol which accumulated in biotransformations with this strain at the maximum surfactant concentration studied was greater than that which was observed with the same microorganism and HMN, and with S. yanoikuyae B8/36 in the absence of any secondary phase. These results point to the conclusion that where surfactants are not toxic, significant enhancement of the PAH oxidation rate may be facilitated where the rate is otherwise limited by mass transfer.
Although other studies have shown that surfactants can increase the rate of growth of microorganisms on PAHs (11, 28), here we show conclusively that they also increase the rate of oxidation of PAHs by dioxygenase enzymes in vivo. The scale of this enhancement is likely to be influenced by factors such as the solubility of the PAHs in both the aqueous phase and the surfactant pseudophase and also by the concentration of surfactant present. Cox and Williams (4) investigated the effect of surfactants on the oxidation of naphthalene to naphthalene cis-1,2-dihydrodiol by an NDO-expressing P. putida mutant. In that study, a mixture of the surfactants Tergitol NP-10 and Neodol 25-3A was used, and the rates of substrate oxidation by the bacteria in the presence of the surfactant mixture were approximately three times that of the control. However it should be noted that the surfactant concentrations used in those experiments (1% [vol/vol]) were significantly higher than those employed here.
In contrast, it was apparent that the surfactant inhibited the biotransformation of both substrates with S. yanoikuyae B8/36, with the maximum concentrations of metabolites occurring at levels less than those observed without a second phase (Fig. 1). Therefore, we can conclude that Triton X-100 was toxic to this microorganism under these experimental conditions. The data presented here show conclusively that the effect of a nonionic surfactant on PAH transformation by dioxygenase-expressing microorganisms is greatly affected by the type of bacteria involved. It therefore strongly supports the earlier proposal that the inconclusive results of bioremediation studies may be explained by differences in the population of PAH-degrading bacteria present. This observation is especially important if we consider that Sphingomonas spp. have been implicated in the biodegradation of a number of the larger recalcitrant PAHs (2, 10, 22), and that therefore they may play an important role in the biodegradation of these compounds in nature.
Effect of Triton X-100 on growth of bacteria with aromatic substrates.
The observation that a surfactant should have different effects on the S. yanoikuyae and Pseudomonas sp. oxidations was considered novel in the context of biodegradation and was investigated further. Table 1 shows the effect of the surfactant on the specific growth rate of the wild-type S. yanoikuyae B1 when it was grown on a variety of carbon sources under otherwise identical conditions. The data corroborate the conclusion that the surfactant inhibits oxidation of phenanthrene in S. yanoikuyae B8/36. Furthermore, the data show that Triton X-100 also inhibited growth of this organism on the aromatic substrates biphenyl, phenanthrene, and sodium benzoate. However, there was no growth inhibition when sodium pyruvate was used as a growth substrate. These data might indicate that while the inhibitory effect was not specific for a particular aromatic pathway, neither was it a general toxic effect. In the control experiments, the effect of the surfactant was determined on the growth of the wild-type Pseudomonas sp. strain NCIMB 9816 with naphthalene, sodium benzoate, and sodium succinate provided as carbon sources. In this case, no detrimental effect of the surfactant on growth was found.
TABLE 1.
Effect of Triton X-100 on maximum specific growth rate of strains B1 and NCIMB 9816
| Strain | Growth substrate | Specific growth rate (h−1) at Triton X-100 concentration ofa:
|
||||
|---|---|---|---|---|---|---|
| 0 mM | 0.2 mM | 0.5 mM | 1.0 mM | 2.0 mM | ||
| B1 | Succinate | 0.73 ± 0.03 | 0.68 ± 0.03 | 0.62 ± 0.06 | 0.68 ± 0.06 | 0.66 ± 0.04 |
| Biphenyl | 0.31 ± 0.04 | 0.20 ± 0.04 | 0.15 ± 0.01 | 0.06 ± 0.04 | 0.03 ± 0.02 | |
| Phenanthrene | 0.30 ± 0.06 | <0.02 | <0.02 | <0.02 | <0.02 | |
| Benzoate | 0.32 ± 0.03 | 0.23 ± 0.07 | <0.02 | <0.02 | <0.02 | |
| NCIMB 9816 | Succinate | 0.53 ± 0.04 | 0.58 ± 0.02 | 0.57 ± 0.03 | 0.54 ± 0.03 | 0.62 ± 0.01 |
| Naphthalene | 0.40 ± 0.08 | 0.50 ± 0.01 | 0.36 ± 0.02 | 0.32 ± 0.08 | 0.36 ± 0.11 | |
| Benzoate | 0.49 ± 0.02 | 0.49 ± 0.01 | 0.50 ± 0.01 | 0.51 ± 0.03 | 0.49 ± 0.03 | |
All measurements shown are the means of triplicate shake-flask experiments, with standard deviations as shown. Pseudomonas sp. strain NCIMB 9816 and S. yanoikuyae B1 were grown at 30°C. Carbon sources were used at the following concentrations: biphenyl (5 g liter−1), phenanthrene (5 g liter−1), naphthalene (5 g liter−1), sodium benzoate (filter sterilized; 1 mM), and sodium succinate (12.3 mM).
Effect of Triton X-100 on dioxygenase enzyme activity.
One explanation for the observed effects of the surfactant on strain B1 may be that the surfactant inhibits arene dioxygenase enzymes in this organism. To test this hypothesis, cell extracts of both S. yanoikuyae B8/36 and Pseudomonas sp. strain 9816/11 were assayed for their ability to oxidize 14C-labelled naphthalene by using an assay for NDO activity (6), in both the presence and absence of the surfactant. The specific activity of the dioxygenase in strain B8/36 towards naphthalene was 0.17 nmol min−1 mg−1; in the presence of 1 or 2 mM Triton X-100, there was no noticeable effect on this activity (specific activity estimates were 0.19 and 0.18 nmol min−1 mg−1, respectively; in all assays, these activities are the means of duplicate measurements). The specific activity of the dioxygenase in strain 9816/11 towards naphthalene was 4.84 nmol min−1 mg−1. In the presence of Triton X-100, the activity of this enzyme actually increased, to 7.05 (1 mM) and 7.69 (2 mM) nmol min−1 mg−1. These data show that the surfactant had no inhibitory effect on dioxygenase activity in either strain. This would indicate that the site of inhibition in the Sphingomonas sp. is not at the dioxygenase but elsewhere. A supply of electrons (via NADH or NADPH) is critical for dioxygenase activity in vivo, and therefore any disruptive effects on the cell membrane of the Sphingomonas cell would cause loss of dioxygenase enzyme activity. The structure of the cell wall in this microorganism is quite different from that in pseudomonads (14), and this should be considered a possible site of inhibition.
An unexpected observation from these experiments was that enzyme activity in Pseudomonas sp. strain 9816/11 was increased in the presence of low levels of the surfactant. Negative control experiments, in which the assay was repeated in the absence of any cell extract and in the absence of NADH, clearly indicated that this effect was enzyme mediated and also NADH dependent. The reason for this increase in activity is at present uncertain and warrants further investigation.
In summary, the results in this report have shown that the surfactant Triton X-100 can have quite opposite effects on the biotransformation of PAHs by resting cells of PAH-degrading bacteria. While inhibition of biotransformation occurred with the S. yanoikuyae B8/36 mutant, the surfactant was also found to prevent growth of the wild-type S. yanoikuyae B1 strain with aromatic substrates. Furthermore, our results suggest that the site of this inhibition is not the PAH-dioxygenase enzyme.
Acknowledgments
This work was supported in part by grants from The Queen’s University of Belfast Environmental Science and Technology Research (QUESTOR) Centre (to C.C.R.A.) and the Biotechnology and Biological Sciences Research Council (BBSRC) (to N.D.S.).
We thank David T. Gibson for supplying S. yanoikuyae B1 and B8/36 and Pseudomonas sp. strain 9816/11.
REFERENCES
- 1.Boyd D R, McMordie R A S, Sharma N D, Dalton H, Williams P, Jenkins R O. Stereospecific benzylic hydroxylation of bicyclic alkenes by Pseudomonas putida: isolation of (+)-R-1-hydroxy-1,2-dihydronaphthalene, an arene hydrate of naphthalene from metabolism of 1,2-dihydronaphthalene. J Chem Soc Chem Commun. 1989;1989:339–340. [Google Scholar]
- 2.Boyd D R, Sharma N D, Agarwal R, Resnick S M, Shocken M J, Gibson D T, Sayer J M, Yagi H, Jerina D M. Bacterial dioxygenase-catalysed dihydrozylation and chemical resolution routes to enantiopure cis-dihydrodiols of chrysene. J Chem Soc Perkin Trans I. 1998;1998:1715–1723. [Google Scholar]
- 3.Cerniglia C E. Biodegradation of polycylic aromatic hydrocarbons. Biodegradation. 1992;3:351–368. [Google Scholar]
- 4.Cox D P, Williams A L. Biological process for converting naphthalene to cis-1,2-dihydroxyl-1,2-dihydronaphthalene. Appl Environ Microbiol. 1980;39:320–326. doi: 10.1128/aem.39.2.320-326.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies J I, Evans W C. Oxidative metabolism of naphthalene nucleus by soil pseudomonads—ring fission mechanism. Biochem J. 1964;91:251–261. doi: 10.1042/bj0910251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ensley B D, Gibson D T, Laborde A L. Oxidation of naphthalene by a multicomponent enzyme system from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1982;149:948–954. doi: 10.1128/jb.149.3.948-954.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson D T, Hensley M, Yoshioka H, Mabry T J. Formation of (+)-cis-1,2-dihydroxy-3-methylcyclohexa-3,5-diene from toluene by Pseudomonas putida. Biochemistry. 1970;9:1626–1630. doi: 10.1021/bi00809a023. [DOI] [PubMed] [Google Scholar]
- 8.Gibson D T, Cardini G E, Maseles F C, Kallio R E. Incorporation of oxygen-18 into benzene by Pseudomonas putida. Biochemistry. 1970;9:1631–1635. doi: 10.1021/bi00809a024. [DOI] [PubMed] [Google Scholar]
- 9.Gibson D T, Roberts R L, Wells M C, Kobal V M. Oxidation of biphenyl by a Beijerinckia species. Biochem Biophys Res Commun. 1973;50:211–219. doi: 10.1016/0006-291x(73)90828-0. [DOI] [PubMed] [Google Scholar]
- 10.Gibson D T, Mahadevan V, Jerina D M, Jagi H, Yeh H J C. Oxidation of the carcinogens benzo[a]pyrene and benzo[a]anthracene to dihydrodiols by a bacterium. Science. 1975;189:295–297. doi: 10.1126/science.1145203. [DOI] [PubMed] [Google Scholar]
- 11.Grimberg S J, Stringfellow W T, Aitken M D. Quantifying the biodegradation of phenanthrene by Pseudomonas stutzeri P16 in the presence of a nonionic surfactant. Appl Environ Microbiol. 1996;62:2387–2392. doi: 10.1128/aem.62.7.2387-2392.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeffrey A M, Yeh H J C, Jerina D M, Patel T R, Davey J F, Gibson D T. Initial reactions in the oxidation of naphthalene by Pseudomonas putida. Biochemistry. 1975;14:575–584. doi: 10.1021/bi00674a018. [DOI] [PubMed] [Google Scholar]
- 13.Jerina D M, Selander H, Yagi H, Wells M C, Davey J F, Mahadevan V, Gibson D T. Dihydrodiols from anthracene and phenanthrene. J Am Chem Soc. 1976;98:5988–5996. doi: 10.1021/ja00435a035. [DOI] [PubMed] [Google Scholar]
- 14.Kawasaki S, Moriguchi R, Sekiya K, Nakai T, Ono E, Kume K, Kawahara K. The cell envelope structure of the lipopolysaccharide-lacking gram-negative bacterium Sphingomonas paucimobilis. J Bacteriol. 1994;176:284–290. doi: 10.1128/jb.176.2.284-290.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keck J, Sims R C, Coover M, Park K, Symons B. Evidence for cooxidation of polynuclear aromatic hydrocarbons in soil. Water Res. 1989;23:1467–1476. [Google Scholar]
- 16.Khan A A, Wang R-F, Cao W-W, Franklin W, Cerniglia C E. Reclassification of a polycylic aromatic-hydrocarbon metabolizing bacterium, Beijerinckia sp. strain B1, as Sphingomonas yanoikuyae by fatty acid analysis, protein pattern analysis, DNA-DNA hybridization, and 16S ribosomal DNA sequencing. Int J Syst Bacteriol. 1996;46:466–469. doi: 10.1099/00207713-46-2-466. [DOI] [PubMed] [Google Scholar]
- 17.Kiyohara H, Torigoe S, Kaida N, Asaki T, Iida T, Hayashi H, Takizawa N. Cloning and characterization of a chromosomal gene cluster that encodes the upper pathway for phenanthrene and naphthalene utilization by Pseudomonas putida OUS82. J Bacteriol. 1994;176:2439–2443. doi: 10.1128/jb.176.8.2439-2443.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koreeda M, Akhtar M N, Boyd D R, Neil J P, Gibson D T, Jerina D M. Absolute stereochemistry of cis-1,2-, trans-1,2- and cis-3,4-dihydrodiol metabolites of phenanthrene. J Org Chem. 1978;43:1023–1027. [Google Scholar]
- 19.Laha S, Luthy R G. Inhibition of phenanthrene mineralisation by nonionic surfactants in soil-water systems. Environ Sci Technol. 1991;25:1920–1930. [Google Scholar]
- 20.Liu Z, Jacobson A M, Luthy R G. Biodegradation of naphthalene in aqueous nonionic surfactant systems. Appl Environ Microbiol. 1995;61:145–151. doi: 10.1128/aem.61.1.145-151.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackay D, Shiu W Y. Aqueous solubility of polynuclear aromatic hydrocarbons. J Chem Eng Data. 1977;22:399–402. [Google Scholar]
- 22.Mahaffey W R, Gibson D T, Cerniglia C E. Bacterial oxidation of chemical carcinogens: formation of polycyclic aromatic acids from benzo[a]anthracene. Appl Environ Microbiol. 1988;54:2415–2423. doi: 10.1128/aem.54.10.2415-2423.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mueller J G, Chapman P J, Pritchard P H. Action of a fluoranthene-utilizing bacterial community on polycyclic aromatic hydrocarbon components of creosote. Appl Environ Microbiol. 1989;55:3085–3090. doi: 10.1128/aem.55.12.3085-3090.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortega-Calvo J-J, Alexander M. Roles of bacterial attachment and spontaneous partitioning in the biodegradation of naphthalene initially present in nonaqueous-phase liquids. Appl Environ Microbiol. 1994;60:2643–2646. doi: 10.1128/aem.60.7.2643-2646.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Resnick S M, Gibson D T. Oxidation of 6,7-dihydro-5H-benzocycloheptene by bacterial strains expressing naphthalene dioxygenase, biphenyl dioxygenase, and toluene dioxygenase yields homochiral monol or cis-diol enantiomers as major products. Appl Environ Microbiol. 1996;62:1364–1368. doi: 10.1128/aem.62.4.1364-1368.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rouse J D, Sabatini D A, Suflita J M, Harwell J H. Influence of surfactants on microbial degradation of organic compounds. Crit Rev Environ Sci Technol. 1994;24:325–370. [Google Scholar]
- 27.Stringfellow W T, Aitken M D. Comparative physiology of phenanthrene degradation by two dissimilar pseudomonads isolated from creosote-contaminated soil. Can J Microbiol. 1994;40:432–438. doi: 10.1139/m94-071. [DOI] [PubMed] [Google Scholar]
- 28.Tiehm A. Degradation of polycyclic aromatic hydrocarbons in the presence of synthetic surfactants. Appl Environ Microbiol. 1994;60:258–263. doi: 10.1128/aem.60.1.258-263.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsomides H J, Hughes J B, Thomas J M, Ward C H. Effect of surfactant addition on phenanthrene biodegradation in sediments. Environ Toxicol Chem. 1995;14:953–959. [Google Scholar]
- 30.Worsley M J, Williams P A. Metabolism of toluene and xylene by Pseudomonas putida (arvilla) mt-2. Evidence for a new function of the TOL plasmid. J Bacteriol. 1975;124:7–13. doi: 10.1128/jb.124.1.7-13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]