Abstract
‘You can't roll the clock back and reverse the effects of experiences' Bruce McEwen used to say when explaining how allostasis labels the adaptive process. Here we will for once roll the clock back to the times that the science of the glucocorticoid hormone was honored with a Nobel prize and highlight the discovery of their receptors in the hippocampus as inroad to its current status as master regulator in control of stress coping and adaptation. Glucocorticoids operate in concert with numerous neurotransmitters, neuropeptides, and other hormones with the aim to facilitate processing of information in the neurocircuitry of stress, from anticipation and perception of a novel experience to behavioral adaptation and memory storage. This action, exerted by the glucocorticoids, is guided by two complementary receptor systems, mineralocorticoid receptors (MR) and glucocorticoid receptors (GR), that need to be balanced for a healthy stress response pattern. Here we discuss the cellular, neuroendocrine, and behavioral studies underlying the MR:GR balance concept, highlight the relevance of hypothalamic-pituitary-adrenal (HPA) -axis patterns and note the limited understanding yet of sexual dimorphism in glucocorticoid actions. We conclude with the prospect that (i) genetically and epigenetically regulated receptor variants dictate cell-type-specific transcriptome signatures of stress-related neuropsychiatric symptoms and (ii) selective receptor modulators are becoming available for more targeted treatment. These two new developments may help to ‘restart the clock’ with the prospect to support resilience.
Keywords: Mineralocorticoid receptor, Glucocorticoid receptor, Hypothalamic-pituitary-adrenal-axis, Allostatic load, Stress response, Stress coping and adaptation, Resilience, Vulnerability, Brain, Hippocampus, Cortisol, Glucocorticoid, Regulation, Early life programming, Cell type
Abbreviations: MR, Mineralocorticoid receptor; GR, Glucocorticoid receptor; HPA-axis, Hypothalamic-pituitary-adrenal-axis; ACTH, Adrenocorticotropic hormone; CRH, Corticotrophin-releasinghormone; GREs, Glucocorticoid response elements
1. Introduction
It was at the wonderful Frontiers in Stress and Cognition conference, September 23–26, 2012 in Ascona organized by Carmen Sandi, that Bruce McEwen gave a presentation entitled the “Brain on Stress: how the social environment gets under the skin.” The story evolved along the allostasis/allostatic load concept until Bruce stated rhetorically that the magic bullet to treat the health risk due to maladaptive stress-coping has not been discovered yet. A voice with a Dutch accent arose from the audience stating: “but Bruce the magic bullet is staring right in your face: that bullet is related to cortisol, your life's work!”
2. Stress and glucocorticoids
And why not? If cortisol is a master regulator of the myriad of neural, endocrine, immune and metabolic stress mediators for better or worse, it also should harbor somehow a key towards prevention and treatment of stress-related health problems. This notion, however, has been entertained before when the science of the adrenal evolved. Remember, the 1950 Nobel Prize in Physiology or Medicine was awarded jointly to Edward Calvin Kendall, Tadeus Reichstein and Philip Showalter Hench “for their discoveries relating to the hormones of the adrenal cortex, their structure and biological effects.” (see www.nobelprize.org/prizes/medicine/1950/hench/lecture/).
To explain the miraculous action of cortisone in relief from rheumatoid arthritis and other inflammatory diseases one of the Nobel laureates stated, by using a figure of speech: “these hormones appear not to extinguish the fire, not to act like a carpenter to repair the damage of fire. Instead, they appear to dampen the fire or to provide, as it were, an asbestos suit behind which the patient, like some biblical Shadrach, Meshach, or Abednego, protects his tissues from the fire” (Philip Hench, Nobel Lecture, December 11, 1950). In retrospect, that view was entirely wrong. The correct view is that the endogenous glucocorticoid action prevents the initial stress (e.g., inflammatory, and immune) reactions to overshoot and become damaging (Munck et al., 1984). Or as Marius Tausk (1952), Director of NV Organon in The Netherlands, metaphorically stated in the language of the Nobel laureates: “glucocorticoids are required to limit the water damage caused by the fire brigade”.
This all happened at the time Selye had formulated the ‘stress concept’ more than 10 years before (Selye, 1936). To that effect Selye distinguished specific actions (e.g., tissue damage) from the apparent ‘non-specific stereotypical reaction of stress’, that activates the HPA-axis and increases the level of its end-product the ‘adaptive hormone’ cortisol. The regulation of the inflammatory or tissue reactivity (in Selye's terms) by cortisol determines whether “the body succumbs or resists by means of adaptation”, distinguished as distress vs eustress, respectively. All effects of cortisol depend on external and internal (hereditary, constitution, previous exposure) “conditioning factors”. Of note, the ‘non-specific stereotypical action of stress’ can -in retrospect-be disputed; there are numerous highly specific pathways and neuronal circuits that mediate the activation of the HPA-axis and that show plasticity over time. Moreover, cortisol has in every cell- and tissue-type distinct and conditional actions. Such pleiotropic action allows the hormone to coordinate and integrate cell and tissue function over time at the organismal level.
According to Selye “the adaptation syndrome in itself is not pathogenic, but an indispensable physiological defense reaction to damage as such”. The imperfection (e.g., an absolute or relative excess or deficiency of one or more adaptive hormones) plays an important role in the pathogenesis of many diseases. “It is not stress that kills us, it is our reaction to it”. Such maladies, in which inadequacies of cortisol actions, are even more important than the specific actions of the pathogen itself, are considered “diseases of adaptation”. This line of reasoning led to the ‘pendulum hypothesis’ by which the action of antagonistic adaptive hormones (pro-inflammatory mineralocorticoids and anti-inflammatory glucocorticoids) can set disease susceptibility at different levels (Selye, 1950).
The process of restoring balance towards a new homeostatic setpoint, is at the root of the allostasis concept (McEwen and Wingfield, 2010). Thus, information, perceived and/or anticipated as a psychological stressor, is processed in the brain, and triggers the release of multiple stress mediators that operate in complex self-regulating feedforward and feedback regulations with the goal to establish homeostasis via physiological and behavioral adaptations (Levine, 2005). This adaptive process in anticipation of, or in response to, is called allostasis and can be described in terms of metastable energetic states of the fitness landscape, according to Brian Fertig in a two volume book on Metabolism and Medicine, advised by Bruce (Fertig, 2022). The energy expenditure is ‘the cost’ or allostatic load and subsequent ‘wear and tear’ is a sign of allostatic overload. Attempts are under way to provide a quantification-the allostatic load index based on levels of circulating stress mediators of the autonomic, immune and HPA-axis systems, blood lipids and glucose (Guidi et al., 2021; Juster et al., 2010; Wiley et al., 2016).
Bruce was capable to explain the ‘good and bad’ glucocorticoid action in succinct and comprehensible language. For instance, in a commentary in Chronic Stress (McEwen, 2017) he addressed in just 1000 words the question: “What is the confusion of Cortisol?”. The narrative highlighted the composite of ultradian, circadian and stress response patterns of cortisol secretion as critical for balanced neuroendocrine, immune and metabolic adaptations. During chronic stress, cortisol promotes adaptive changes in structure and function of the brain: the shrinkage of hippocampus and medial prefrontal cortex vs. growth of amygdala and orbital frontal cortex, are manifestations of adaptation and need to be interpreted as sign of plasticity rather than damage. Cortisol's and corticosterone's actions vary over the life time, from programming of stress-induced ‘early’ amygdala awakening (see Box 2) and potentiation of stress-related functions in sex-dependent fashion during puberty (Brydges et al., 2014; Papilloud et al., 2019) to facilitation of age-related adaptations (Landfield, 1987; Lupien et al., 2009; Sapolsky et al., 1986).
Box 1.
An overactive renin-angiotensin-aldosterone system (RAAS) aggravates the course of COVID-19; the RAAS system may become overactive because SARS-CoV-2 targets ACE2 (Rysz et al., 2021; Young et al., 2020). Such RAAS imbalance can be restored with angiotensin II inhibitors, and in vitro more recently mineralocorticoid antagonists appeared active in reducing pro-inflammatory mediators including galectin-3 (Jover et al., 2021). While this preliminary observation supports a pro-inflammatory mineralocorticoid action in the course of COVID-19, in the later stage when the cytokine storm arises, the potent anti-inflammatory synthetic glucocorticoid dexamethasone appeared a life-saver, which is in line with the role of glucocorticoids in limiting overshoot of immune defense (Horby et al., 2020). Collectively, these findings illustrate the pendulum hypothesis, if further validated for mineralocorticoids (Akin et al., 2022).
Alt-text: Box 1
Box 2.
In rodents, there is a stress hyporesponsive period (SHRP) in which responses to mild and moderate stressors do not increase glucocorticoid secretion (Levine et al., 1991), and pups learn to remain with the dam irrespective the aversiveness of the environment (Moriceau and Sullivan, 2006). The SHRP provides the context for the brain to develop, while being protected from excessive glucocorticoid-mediated actions. However, the SHRP can be interrupted by challenges. For instance, in response to maternal absence the offspring's HPA-axis is activated. Interestingly, baseline ACTH and corticosterone secretion can be reinstated in the deprived animals by mimicking different aspects of maternal care: increased CRH and ACTH is normalized by tactile stimulation and corticosterone release by feeding (van Oers et al., 1998). Another interesting phenomenon is the premature expression of amygdala-based aversion learning upon corticosterone exposure during early life (Moriceau et al., 2006, 2009; Moriceau and Sullivan, 2006). The programming of an amygdala-based fearful phenotype was supported in a model of early stressful experience during repeated daily separations. The premature amygdala awakening resulted during later life in reduced social interaction, increased emotional reactivity and memory, increased stereotypy, and impaired sensorimotor gating (Daskalakis et al., 2011, 2012, 2014b). In clinical realm, a role of maternal depression in programming of childhood anxiety disorder involving placenta 11HSD-2, DNA methylation of NR3C2 and infant cortisol reactivity anxiety was suggested recently in a series of publications arising from the Mercy Pregnancy and Emotional Wellbeing Study (Galbally et al., 2019, 2020, 2021). See also section 6.
Alt-text: Box 2
The key question posed by Bruce was: “when conditions change, and the stressor is gone, can the brain adapt to the new situation?” If not, information processing gets “stuck” and resilience becomes compromised, leading to the equally important query “how can this be treated?” The commentary is clarified with a cartoon illustrating the inverted U-shape effects of increasing glucocorticoid and excitatory amino acid exposure on synaptic function and plasticity culminating into risk for excitotoxicity. In the next sections the avenue towards understanding this overarching action of the glucocorticoid hormone is discussed in terms of cooperation between its two complementary receptor systems (McEwen, 2017).
3. Discovery of MR and GR function
It was in the pioneering phase of the neurosciences in the late 1960's, heralding the emergence of Psychoneuroendocrinology, that Bruce identified the receptors for the rat's glucocorticoid corticosterone in the purified cell nuclear fraction of the rat hippocampus, thus, surprisingly, not in the hypothalamic PVN, which releases corticotrophin releasing hormone (CRH). As Bruce pleasantly recalled, he initially thought having catastrophically changed the labels of the samples to be counted in the liquid scintillation counter. But the replication inexorably confirmed that corticosterone targets the hippocampus, a finding that was further supported by high resolution autoradiography visualizing the hour-long retention of radioactive labelled corticosterone in all pyramidal and dentate gyrus neurons and abundantly also in other limbic areas (lateral septum and amygdala), but not in the PVN (Gerlach and McEwen, 1972; McEwen et al., 1968).
Attempts to reproduce the in vivo identification of the hippocampal corticosterone receptors with the potent synthetic glucocorticoid dexamethasone initially turned out to be a total failure. It was found that dexamethasone targets the pituitary corticotrophs, and not the hippocampus [(de Kloet et al., 1974; De Kloet et al., 1975); for a detailed account see the Box ‘The dexamethasone story’ in (de Kloet et al., 2018)]. By acting at the pituitary, dexamethasone potently suppressed stress-induced HPA-axis activity leading to a condition of minimal amounts of circulating corticosterone, which was called ‘chemical adrenalectomy’. Twenty years later it was discovered that the synthetic glucocorticoid poorly penetrates the blood brain barrier, because it is a substrate for multidrug resistance P-glycoprotein (Meijer et al., 1998; Schinkel et al., 1995).
In the early 1980's it was already current wisdom that in vitro in hippocampus cytosol two receptor populations were present that could be distinguished by inclusion of the pure glucocorticoid RU28362 (Krozowski and Funder, 1983; Moguilewsky and Raynaud, 1980; Veldhuis et al., 1982) in the medium: one soluble receptor did bind with high affinity not only the naturally occurring glucocorticoids corticosterone and cortisol, but also aldosterone, deoxycorticosterone and progesterone. It was also known that in vivo aldosterone and corticosterone did compete for this apparent promiscuous mineralocorticoid receptor (MR) retained in hippocampal cell nuclei, while progesterone and deoxycorticosterone although having appreciable affinity to MR, did not interfere with in vivo cell nuclear retention of corticosterone (De Kloet et al., 1983; McEwen et al., 1976). The other soluble receptor population, the classical glucocorticoid receptor (GR), did bind with a ten-fold lower affinity corticosterone, but with high affinity dexamethasone and the ‘pure’ glucocorticoid RU28362 (Veldhuis et al., 1982).
We realized that in vivo the low affinity GR escaped detection by the tracer amount of corticosterone (see Box 3). Indeed, in vivo dose-response studies with corticosterone revealed a differential occupancy of the high and low affinity corticosterone receptors; the neuro-anatomical distribution of the dual corticosterone receptor system was mapped with micropunches of brain nuclei and by using in vitro autoradiography (Reul and de Kloet, 1985, 1986; Reul et al., 1987). It was an interesting time, full of confusion. For instance, the glucocorticoid cascade theory (Sapolsky et al., 1986), and the developmental programming actions of corticosterone were initially still based on high-affinity 3H-corticosterone binding in hippocampus called GR, while these high affinity sites are in fact the MR.
Box 4.
Writing this MR:GR overview is a reminder to a friendship of more than half a century ago that started even before the time of ERdK's postdoctoral fellowship in the McEwen lab [see Box 1 in (de Kloet et al., 2018)]. That postdoc project was inspired by the failure of ERdK to demonstrate -as a PhD student in The Netherlands-dexamethasone binding to hippocampal corticosterone receptors. And indeed, in the Bruce lab we established that dexamethasone and corticosterone could not be lumped together in binding to, what later appeared to be, the mineralocorticoid receptor (De Kloet et al., 1975). At that time the McEwen lab, located at the Gasser Hall, was already densely populated and night shifts were needed to accommodate all students of stress in the brain. Bruce's office was so small that the lab had to be evacuated to provide sufficient space if he decided to roll back his chair for consultation of a literature archive. In the subsequent 50 years ERdK has visited numerous times the 13th floor of the Weiss building, sometimes unexpectedly, by pretending he called from Holland, but, in reality, was just sitting next door to Bruce's office in New York. Bruce returned briefly to his Dutch roots, the Lenters family in Hardenberg in the east of The Netherlands, when he was awarded in 2006 in Leiden the Marius Tausk Guest Professorship. Such events reinforced our ever-ongoing friendship and resulted in several more recent common publications of the Leiden-New York labs along Bruce's Leitmotiv that ‘you can't do things on your own’ (Datson et al., 2013; Hunter et al., 2012, 2016; Polman et al., 2012).
Alt-text: Box 4
Box 3.
In 1984 at a French Riviera conference organized by Roussel Uclaf, Jan-Åke Gustafsson presented the very first brain immunocytochemistry (ICC) with antibodies raised against the liver glucocorticoid receptor (GR). However, the rat hippocampal CA2 and CA3 areas, surprisingly, appeared poorly labelled with immunoreactive GR, while these pyramidal neurons were shown by high resolution to abundantly retain the 3H-corticosterone tracer (De Kloet et al., 1983; Fuxe et al., 1985). A voice with a Dutch accent arose from the audience stating rather bluntly the error of the mismatch between GR ICC and 3H-corticosterone autoradiography. The chairman then interfered and expressed his dismay by proposing this remark ought to be withdrawn from the proceedings since it discredited the craftmanship of the Swede, which was of course not the intention of the discussant. However, in a flash, the mystery was resolved: the 3H-corticosterone tracer apparently did not provide enough ligand to visualize in vivo the lower affinity GR, while 3H-dexamethasone did not reach the brain in sufficient quantity!
Alt-text: Box 3
In the spring of 1987 Jeff Arriza and Ron Evans cloned the NR3C2 (MR gene), after having revealed the genetic structure of NR3C1 (GR gene) two years earlier (Arriza et al., 1987; Hollenberg et al., 1985). By referring to the receptor pharmacology they suggested the two receptor types may operate as a “binary signaling system” (Arriza et al., 1988; Evans and Arriza, 1989). Then, in 1988 in Edinburgh, Chris Edwards discovered the critical role of 11β-hydroxysteroid dehydrogenase type 2 (11HSD-2) in conferring aldosterone specificity to MR in kidney epithelial cells. Accordingly, we showed in a collaborative study with in vivo autoradiography that after a licorice block of 11HSD-2 activity the aldosterone-selective receptor in kidney was labelled with the 3H-corticosterone tracer (Edwards et al., 1988). A similar conclusion on the role of 11HSD-2 in conferring aldosterone specificity was around the same time reached by John Funder (Funder et al., 1988). Meanwhile, the concentration of immunoreactive (ir) aldosterone present in purified rat hippocampal nuclei was in vivo only 1–5% of the amount of ir-corticosterone suggesting that the MR is exposed to predominantly the naturally occurring glucocorticoid (Yongue and Roy, 1987). Actually, in hippocampus, the abundance of glucocorticoids over aldosterone is probably even much larger in many brain cells given their co-localization with 11-HSD1 reductase that regenerates locally bio-active glucocorticoids (Chapman et al., 2013).
During fetal rat life, 11HSD-2 is abundantly expressed in brain, but soon after birth the oxidase is found mainly restricted to the subcommissural organs, locus coeruleus, ventromedial hypothalamus and amygdala and n tractus solitarii (NTS) in non-aminergic cells near the periventricular area postrema (Geerling et al., 2006; Robson et al., 1998). These aldosterone-selective MR are involved in salt appetite and contribute to central cardiovascular regulation (Geerling and Loewy, 2009; van den Berg et al., 1990). The aldosterone-selective MR expressing neurons project from the NTS to discrete midbrain and forebrain regions, notably the parabrachial nucleus near the locus coeruleus and the ventrolateral bed nucleus of the stria terminalis (vlBNST) (Gasparini et al., 2019). From these nodes extensive crosstalk is possible with limbic forebrain and hypothalamic agouti related peptide (AgRP) networks in regulation of emotional, motivational and cognitive functions associated with salt appetite and energy metabolism (Gasparini et al., 2019). This crosstalk likely underlies the anxiety, dysphoric and anhedonic symptoms experienced by patients suffering from hyperaldosteronism (Hlavacova and Jezova, 2008; Murck et al., 2020).
These initial findings on MR and GR diversity were shared with Bruce in his influential 1986 Physiological Review (McEwen et al., 1986). The various versions + corrections of the manuscript were communicated by snail mail, for all of us the trigger to start writing with word processors. In a subsequent 1987 position statement with Hans Reul the GR-mediated feedback action was distinguished from tonic influences exerted via the corticosterone-preferring limbic MR in regulation of the stress response (De Kloet and Reul, 1987). This reasoning was based on the extensive occupancy of the brain MR even under basal conditions, while GR activation only occurred with rising corticosterone concentrations during the circadian peak and after stress (but see (Mifsud et al., 2021, Polman et al., 2013)). Moreover, rat brain MR expression was highest in the morning when corticosterone levels are low. MRs decreased during the aging process, while neurotrophic peptides and surprisingly, ginsenoside RG1, could restore this age-dependent decrease, a finding that was recently confirmed in the zebrafish (De Kloet and Reul, 1987; He et al., 2020; Reul et al., 1988). Accordingly, we concluded that for brain MR the receptor activity rather than the ligand concentration is rate-limiting. The notion that the brain MR was predicted to be involved in regulation of the setpoint of the stress response system rather than it mediated the feedback action exerted via GR (De Kloet and Reul, 1987) is until today a source of inspiration.
4. MR and GR: neuroendocrine, cellular and behavioral aspects
4.1. Neuroendocrine regulation
The notion that brain MR could be linked to the setpoint of the HPA-axis was readily demonstrated by Mary Dallman's group. They showed that the increased level of adrenocorticotropic hormone (ACTH) after adrenalectomy (ADX) was normalized with amounts of corticosterone that were sufficient for MR occupancy, and only little GR activation (Dallman et al., 1989). At the same time, it was demonstrated that, in adrenally-intact animals, the MR antagonist RU28318 administered systemically, icv or in hippocampus, increased basal am and pm circulating ACTH and corticosterone levels and enhanced stress-induced HPA-axis activity (Oitz, 1997; Oitzl et al., 1995; Ratka et al., 1989; van Haarst et al., 1997). That blockade of the brain MR increased HPA-axis activity was confirmed in humans (Deuschle et al., 1998; Young et al., 1998). Accordingly, the MR is important for the threshold or sensitivity of the stress response system. This role of the MR in the onset of the stress response is also demonstrated in genetically modified as well as genetically selected mice: high hippocampal MR expression corresponds with low basal and attenuated stress-induced HPA-axis activity (Harris et al., 2013; Veenema et al., 2003).
The GR antagonist mifepristone only caused increased glucocorticoid secretion during the circadian peak when there is sufficient GR occupancy in pituitary corticotrophs and brain. If the GR antagonist was given under the stressful conditions of a systemic injection a large and prolonged HPA-axis activation occurred that lasted almost 24 h; the same is observed with a 100 000 fold lower dose locally in the PVN (Dalm et al., 2019; De Kloet et al., 1988; Ratka et al., 1989). Surprisingly, the stress-induced HPA-axis activation was strongly suppressed, upon subsequent daily administrations of the GR antagonist (Dalm et al., 2019). How that is possible is not precisely known. One explanation may be the MR, which gets a more prominent role if colocalized GR is blocked. Indeed, when the antagonist was infused locally in the dorsal hippocampus in amounts of 10 ng per rat, a blunting of stress-induced levels of ACTH and corticosterone was observed (van Haarst et al., 1997). Such opposing MR- and GR-mediated effects on the HPA-axis were supported in a transgenic animal model where MR and GR were expressed in different expression ratios (Harris et al., 2013).
4.2. Cellular studies
Meanwhile, pioneering in vitro cellular studies by Marian Joëls et al. with the dorsal hippocampal CA1 neurons had clearly demonstrated an U-shaped dependency of opposing MR- and GR-mediated actions (Joels, 2006; Joels and de Kloet, 1989, 1990). The depolarization-induced influx of Ca2+ via L-type voltage-dependent Ca2+ channels is high after ADX, suppressed via predominant MR activation, but increased again with high concentrations of corticosterone in a process requiring GR homodimerization (Chameau et al., 2007; Karst et al., 1994, 2000). A similar U-shape dose response was observed for other processes that depend on Ca2+ influx such as cell firing accommodation and slow afterhyperpolarization (Joels and de Kloet, 1989, 1990). Neurotransmitter responses in the dorsal hippocampal CA1 neurons obey the U-shaped dose-response relationship such as the inwardly rectifying K+-hyperpolarization linked to serotonin 1A (5HT1A) receptor activation (Joels and De Kloet, 1992; Joels et al., 1991) which is suppressed by MR and increased after ADX as well as high dose GR activation.
An U-shaped action of glucocorticoids on neuronal excitability by a complementary MR- and GR-mediated action is also observed in e.g., the ventral hippocampus (Maggio and Segal, 2009) and the basolateral amygdala (BLA) (Karst et al., 2010). Neurons that express predominantly GR as in hypothalamus and the ascending aminergic neurons, lack this U-shaped curve upon corticosterone stimulation. However, in the hippocampal granular dentate neurons that express both MR and GR, the ADX-induced increase in Ca2+ current and the 5HT1A linked K+ hyperpolarization (and 5HT1A expression) is suppressed by MR occupancy, while GR activation does not reverse this effect (Joels, 2006; Meijer et al., 1997). There is no straightforward explanation for this lack of GR-mediated effect in dentate gyrus neurons. It could be, however, related to the observation that GR activation following stress occurs in hippocampus only in sparsely distributed neurons, that are linked to the memory engram (Bonapersona et al., 2022; Lesuis et al., 2021; Reul et al., 2015), but this obvious needs further investigation. In the dentate gyrus, cellular differentiation depends on MR, while proliferation and migration is GR-dependent. When both MR and GR are deleted the granular dentate gyrus neurons do not survive and apoptosis is observed (Fitzsimons et al., 2013; Oakley et al., 2021; Sloviter et al., 1989; Woolley et al., 1991).
Altogether, the role of MR in maintaining a stable and high excitatory tone is important for viability, while GR activation suppresses transiently raised excitability [for overview see (Joels et al., 2008, 2012, 2018)]. Neuronal function under basal conditions with predominant MR occupation (the trough in the U-shape) is characterized by a high and stable excitatory tone. The high excitatory hippocampal outflow activates the inhibitory hypothalamic GABA-ergic network suppressing CRH neuronal activity as can be measured with Ca fiber photometry (Kim et al., 2019; Ulrich-Lai and Herman, 2009). Accordingly, this mechanism would explain why hippocampal inhibition of HPA-axis activity is an MR-rather than GR-dependent phenomenon.
While the above cellular mechanisms are genomic, a fast non-genomic GR-mediated suppression of PVN neuronal excitability was discovered that depends on the trans-synaptic action of endocannabinoid (Di et al., 2003, 2016). Next to this GR-mediated endocannabinoid-driven mechanism, a non-genomic MR-mediated action was identified in hippocampal CA1 neurons that rapidly increases miniature excitatory postsynaptic potential (mEPSP) as measure of pre-synaptic glutamate release (Karst et al., 2005; Olijslagers et al., 2008). The actual visualization of the putative membrane localization of MR and GR has met so far only limited success (Groeneweg et al., 2011), but see imaging of the nuclear receptor localization (Groeneweg et al., 2014).
Subsequent studies focused on ‘metaplasticity’ of the amygdala neurons, i.e., the phenomenon that various signals cooperate to ensure responsivity. Metaplasticity was studied by exposure of the amygdala neurons to different concentrations of the β-adrenergic agonist isoproterenol and corticosterone. By combined exposure to concentrations of isoproterenol mimicking moderate stress initially excitability increased, before it was suppressed by the steroid. However, by combining high concentrations of noradrenaline agonist and corticosterone, mimicking severe stress, the later excitability suppression ‘flips’ to excitation (Karst et al., 2010; Karst and Joels, 2016). Such a mechanism would make sense in behavioral realm when an extended period of increased excitability would reflect the excessive strong encoding of a severely stressful experience (Henckens et al., 2012, 2015). The findings on the cellular level are therefore important for translation to behavioral studies that showed a similar synergy of glucocorticoid and noradrenergic signaling in consolidation of fear-motivated behavior (see next section) (Roozendaal et al., 2009a).
4.3. Behavior
On the behavioral level, the pioneering studies by Melly Oitzl et al. revealed the outcome of MR and GR complementarity over the different domains of information processing. Using the Morris water maze as example, it was shown that GR activation in hippocampus promoted memory consolidation of the escape platform location, which could be blocked by the GR antagonist mifepristone icv (Oitzl and de Kloet, 1992; Oitzl et al., 2001). In contrast, 24 h after memory storage, MR appeared involved in memory retrieval to locate this escape platform. Interestingly, the MR antagonist icv not only impaired retrieval, but also facilitated a switch in search strategy when the original escape platform was removed. Intact animals continued to swim to the original platform location, but with MR blockade the animal seemed to have forgotten the location and started to search elsewhere for an escape (Oitzl and de Kloet, 1992). Also, in fear-motivated behavior, MR blockade was found to interfere with memory retrieval, to affect risk assessment and response selection. The latter selection of a behavioral response was very nicely demonstrated in the radial maze where animals either used a stimulus response or a spatial learning strategy to locate an exit. Without stress, all animals (males) used spatial and contextual (hippocampal) information, but upon stress exposure the animals readily relied on a habitual stimulus-response (striatal) strategy (Arp et al., 2014; Dias-Ferreira et al., 2009; Schwabe et al., 2010b, 2013; Souza et al., 2014, Ter Horst et al., 2013a).
GR activation promotes memory storage in fear conditioning paradigms which is further enhanced by a noradrenergic – cAMP mechanism, possibly involving endocannabinoid interaction (Roozendaal et al., 2009b). Accordingly, GR activation promotes consolidation and memory storage of a great variety of paradigms based on active or passive fear-motivated behaviors, social competence, problem solving behavior, drug and alcohol dependence (De Kloet et al., 1988; Roozendaal and McGaugh, 2011; Sandi and Haller, 2015; Vendruscolo et al., 2015).
To test the effect of MR:GR imbalance on behavior, male mice were generated (with littermates as controls to avoid different maternal influences) with limbic brain overexpression of MR (MRhi) and global GR underexpression (GRlo) (Harris et al., 2013). The mutants were not affected in basal secretion of corticosterone but showed an HPA-axis response pattern that is consistent with knowledge on MR and GR function gained from pharmacological manipulations and genetically selected lines (Veenema et al., 2005). Thus, MRlo/GRlo animals show an enhanced and prolonged HPA-axis response but this overshoot in peak secretion was strongly suppressed in MRhi mutants. Behaviorally, the MRhi animals maintained readily their acquired coping strategy suggesting superior retrieval or a more readily switch to habitual behavior, which is typically facilitated in case of excess limbic MR functionality (Harris et al., 2013). The switch from spatial/declarative (thinking) to habitual (doing) behavior is a characteristic feature of human behavior under stress, particularly in individuals carrying a gain of function MR polymorphism that are protected against depression and display dispositional optimism (Klok et al., 2011b; Wirz et al., 2017). Gain of function MR supports healthy aging, as observed in Brown Norway rats (Marissal-Arvy et al., 2004; Oitzl et al., 2000).
Coordination of MR- and GR-mediated actions in learning and memory processes was also demonstrated during the circadian cycle. Using live imaging with transcranial 2-photon microscopy, Conor Liston reported that a non-transcriptional GR-mediated action promotes during the circadian corticosterone peak the formation of dendritic spines in layer 5 of the motor cortex, while the animals are learning the rotarod task (Liston and Gan, 2011). This GR-mediated non-genomic mechanism readily triggered the LIM1-kinase cofilin pathway, which underlies modulation of the actin cytoskeleton. The response to high glucocorticoid concentrations is superimposed on a transcriptional MR-mediated action, which operates during the circadian trough as a prerequisite for retention of the motor skill. This MR-mediated action is involved in pruning the apparent superfluous dendritic spines (Heshmati and Russo, 2013; Liston et al., 2013). Accordingly, over the sleep-wake cycle stabilization of dendritic spine formation is under control of the genomic MR (Hall et al., 2015; Ikeda et al., 2015).
Interestingly, also dexamethasone interferes with learning and spine fate in the mouse cerebral cortex, but formation, stabilization and pruning can be reinstated however with a corticosterone regime mimicking the circadian rhythm (Liston et al., 2013). Previous research had demonstrated that dexamethasone cannot replace corticosterone in the brain because the potent synthetic GR ligand shows only little affinity for the MR and poorly penetrates the blood brain barrier (Karssen et al., 2005). In humans, dexamethasone can have severe side effects (Judd et al., 2014). Dexamethasone reduces slow wave sleep and causes dysphoric effects, which can be turned into euphoria by co-administration of cortisol activating MR (Born et al., 1991; Groch et al., 2013; Plihal et al., 1996). The utility of cortisol add-on was shown with dexamethasone treatment of children with acute lymphoblastic leukemia. In about 30% of the children's severe adverse neuropsychological effects and sleep disturbances occurred, which were ameliorated by cortisol add-on (Warris et al., 2016).
4.4. MR:GR balance concept
The cellular studies demonstrate coordinate MR- and GR-mediated actions of corticosterone in metaplasticity: MR activation increases excitability, and the transient increase in excitability is suppressed via GR. The neuroendocrine data show that central MR activation regulates the onset and peak HPA-axis activity, and GR its duration. The behavioral data show MR-mediated actions are engaged in risk assessment, response selection and behavioral flexibility. MR activation enhances learning, encoding and retrieval of the experience. The subsequent GR-mediated actions promote contextualization and rationalization of the experience, while supporting memory consolidation, behavioral adaptation and recovery. These actions mediated by MR and GR were disturbed with selective antagonists and genetic deletion of either receptor and reinstated by restoring the balance in MR- and GR-mediated actions. Accordingly, the neuroendocrine, cellular, and behavioral data illustrate the concept that “upon imbalance of the MR- and GR-mediated actions, the initiation and/or management of the stress response becomes compromised. At a certain threshold this may lead to a condition of neuroendocrine dysregulation and impaired behavioral adaptation, which potentially can aggravate stress-related deterioration and promote vulnerability” (de Kloet et al., 2005, 2018; De Kloet et al., 1998; Holsboer, 2000).
5. HPA-axis patterns matter
Imaging of the human brain has provided insight in the circuits that are activated sequentially during behavioral adaptation (Fig. 1). Initially, a salience circuit is recruited that processes perception of sensory information towards increased attention, vigilance, and emotional reactivity, along the fight-flight-fright scenario (Berretz et al., 2021; Hermans et al., 2014). This alarm reaction is coordinated by CRH, vasopressin and other stress neuropeptides, central aminergic and sympathetic nervous system and the HPA-axis.
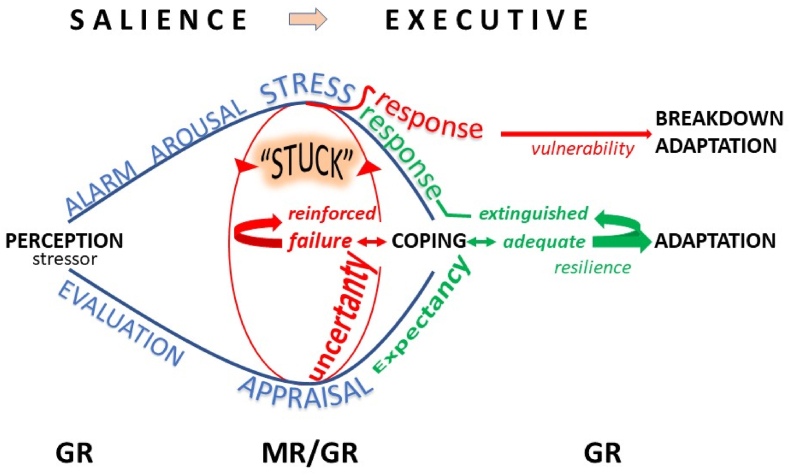
Fig. 1.
The Brain Gets “Stuck”. The anticipation and/or perception of a stressor may trigger an alarm reaction causing arousal while a behavioral (vigilance, attention), autonomic (sympathetic nervous system) and neuroendocrine stress response develops. At the same the stressor is appraised for its controllability. While appraisal and stress response networks interact (Cabib et al., 2020; Douma and de Kloet, 2020), resources are shifted from the salience to the executive network underlying rationalization, selection of an appropriate coping style and contextualization to label the experience for memory storage (Cabib et al., 2020; Henckens et al., 2012, 2015; Hermans et al., 2014). If coping fails because of uncertainty about outcome, the stress response is reinforced and prolonged (in red). Upon repeated failure to cope with the stressor, the neurons that underlie emotional reactivity grow (amygdala, orbital frontal cortex), while hippocampus and medial PFC shrink and compromises their role in cognitive control (Wellman et al., 2020), a condition described by Bruce as ‘the brain gets stuck’ (McEwen, 2017; McEwen and Akil, 2020; McEwen et al., 2016). Under these ‘chronic stress' conditions a novel stimulus cannot be processed appropriately, which may lead to breakdown of adaptation, a condition that can be read from altered patterns of glucocorticoid secretion upon challenge by an acute stressor (McEwen, 1998, 2007; Papilloud et al., 2019; Tzanoulinou et al., 2020). If coping is adequate, because expectancy is rewarded and control is regained, the activated stress response system is extinguished, and adaptation promoted (Douma and de Kloet, 2020). Using fMRI, optogenetics and DREADD technology much progress has been made in recent years to understand how medial PFC neuronal ensembles gain control over stress- and emotional reactions, and how this control is translated top down into an altered pattern of neuroendocrine and behavioral responses; glucocorticoids integrate and coordinate in bottom up fashion the brain-body dialogue in stress-coping and adaptation (de Kloet et al., 2019; Herman et al., 2020; Lingg et al., 2020; Ulrich-Lai and Herman, 2009). GR seems involved in regulation of detection thresholds that are relevant for perception of sensory signals (Henkin and Daly, 1968; Obleser et al., 2021) and MR for the threshold or sensitivity of the stress response system; the balance in MR:GR-mediated actions is crucial for proper processing of information in the salience and executive networks (de Kloet et al., 1999, 2018; Joels et al., 2018; Wirz et al., 2017). MR activation facilitates retrieval processes, risk assessment and response selection; GR activation promotes rationalization and contextualization facilitating memory storage and behavioral adaptation (Oitzl and de Kloet, 1992; Roozendaal and McGaugh, 2011). Cartoon inspiration from discussions with Pieter Smelik, Nuno Sousa and Bruce McEwen. Green denotes that adequate coping extinguishes the stress response and promotes resilience, while red color denotes that failure to cope reinforces the stress response and leads to a crash of information processing. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Meanwhile resources are gradually made available to the executive circuitry to mobilize higher cognitive functions directed by neuronal ensembles in the medial prefrontal cortex (PFC). The goal is then to suppress emotional reactivity and to support coping and adaptation, and thus recovery from the stressor. Underlying the shift towards executive control are appraisal processes such as risk assessment as prelude to decision-making in selection of behavioral solutions to cope and to adapt, which coincides with hippocampus-based contextualization and memory storage. The executive function is more than just a homeostatic reset to prevent the initial stress reaction from overshoot, which is the essential of glucocorticoid physiology; it is allostasis-at work underlying adaptation to change and its cost weighted as allostatic load (Henckens et al., 2012).
Studies have demonstrated that the salient phase tapers off over 30–60 min, until shortly after the cortisol peak, while meanwhile executive control is gradually reinforced when genomic actions of the hormone take shape (Henckens et al., 2015). In animal studies, using pharmacological, optogenetic, DREADD and CRISPR-cas technology, the prefrontal-limbic-periaquaductal circuitry that underlies executive cognitive control over salient emotional reactivity is being identified (Cabib et al., 2020; Herman et al., 2020; Lingg et al., 2020; McKlveen et al., 2019; Nawreen et al., 2020; Radley and Johnson, 2018).
It is not unreasonable to postulate that the balance in activity of salient and executive networks can be read from patterns of HPA-axis activity and glucocorticoid secretion that drives the complementary MR- and GR-mediated actions. This is demonstrated in animal studies on the effects of chronic stress showing profound adaptations in structure and function of the salient and executive networks. Thus, the amygdala and orbital cortex hypertrophy, while the dendritic organization in medial PFC and hippocampus atrophies, including suppression of neurogenesis (McEwen et al., 2016). While under these conditions the individual may still manage with a shift towards habitual behavior, adaptation breaks down upon exposure to a heterotypic stressor, since the executive network is not equipped anymore at that time to deal with the novel challenge. This can be read in hippocampus from gene expression patterns. Expression patterns do not differ very much under steady-state conditions in chronically stressed individuals from controls. However, an acute challenge with corticosterone or with a heterotypic stressor shows a profoundly different response between the two conditions. In chronically stressed animals the challenge reveals prominent expression of e.g., inflammatory, epigenetic, chromatin reorganization pathways, while pathways supporting neurogenesis and synaptic plasticity are suppressed (Datson et al., 2013; Gray et al., 2014; Polman et al., 2012).
In the influential 1998 NEJM article (McEwen, 1998), Bruce proposed that under these conditions of chronic stress three potential maladaptive cortisol secretion patterns can be distinguished. Firstly, upon exposure to a repeated homologous stressor a vulnerable condition would develop if the corresponding glucocorticoid response does not adapt or habituate and continues to show a response of the same or perhaps even larger amplitude. Secondly, if a ‘chronic stress’ condition is challenged with an acute heterologous stressor a too low or too high cortisol peak would signal a problem with containment of stress- and emotional reactions, functions ascribed to the MR. Thirdly, a prolonged cortisol response would indicate lack of control, inadequate coping and thus poor adaptation, which is typical GR business. An aberrant initiation or termination of the stress reaction thus can be read from HPA-axis activity and cortisol and implies risk for compromised resilience and increased vulnerability.
For understanding the significance of HPA-axis activity and cortisol secretion, elaborate tests are needed that record patterns rather than a single cortisol value, preferably based on certified challenges such as theTrier Social Stress Test (TSST) (Rothe et al., 2020) or dexamethasone suppression test (Carroll et al., 1976; Heuser et al., 1994; Shorter and Fink, 2010). Alternatively, such patterns have been exploited in animal experiments for the identification of susceptible phenotypes. In a series of studies in the laboratory of Carmen Sandi animals were selected by a protocol of repeated peripubertal stress exposure. While in part of the animals the corticosterone secretion pattern adapts, there are also animals that show resistance to adaptation of the peak corticosterone secretion, while others maintain a prolonged secretion. Then, during adulthood the peak-resistant animals showed deficits in cognitive functions, as shown in learning a maze task, while those displaying resistance to shut-off are characterized by impaired social competence (Papilloud et al., 2019, 2020; Tzanoulinou et al., 2020). Interestingly, these altered corticosterone patterns and impaired behaviors can be reset with mifepristone (Papilloud et al., 2019). Thus, the glucocorticoid hormone is a master regulator and its function in stress coping and adaptation can be read from its secretory pattern. MR-mediated actions are prominent during the onset and peak of the stress reaction, while GR is involved in its duration.
6. Sex differences
In a systematic review and meta-analysis (Zorn et al., 2017) revealed sex differences in saliva free cortisol secretion patterns of psychiatric patients in response to the TSST or a similar psychosocial stressor characterized by socio-evaluative threat and lack of control. Data collected from 14 studies showed a blunted cortisol response in women diagnosed with major depressive disorder, whereas cortisol secretion of depressed men was exaggerated, both in comparison with controls. In remitted male patients, the enhanced cortisol reactivity disappeared, while in females the difference with controls became less. The TSST-induced cortisol profiles from 9 studies with patients suffering from anxiety disorders (including posttraumatic stress disorder (PTSD) and social anxiety disorder) showed also decreased cortisol reactivity in females vs., increased reactivity in males. In healthy individuals the HPA-axis response shows a similar sex difference in the TSST: adult males show higher ACTH, saliva free and blood total cortisol responses than females in the luteal phase; generally HPA-axis is increased during the follicular phase and contraceptive use (Kudielka and Kirschbaum, 2005; Stephens et al., 2016).
In contrast, the rodents’ HPA-axis activity in response to a variety of stressors is, however, generally more enhanced in females over males. Highest ACTH and corticosterone levels are in the pro-estrus phase when estrogen and progesterone levels peak (Bangasser and Valentino, 2014; Heck and Handa, 2019). Interestingly, estrogens decrease hippocampal MR mRNA and protein expression rather than GR, while progesterone is a competitive inhibitor of the hippocampal MR (Carey et al., 1995). Accordingly, when the levels of both sex steroids are high, as is the case in the pro-estrus, the consequent reduced MR functionality predicts elevated HPA-axis activity. Indeed, in ovariectomized animals the stress-induced ACTH and corticosterone levels are highest when substituted with both sex steroids (Carey et al., 1995). However, under conditions of chronic mild stress or chronic variable stress, male rather than female animals have the highest HPA-axis response, if challenged with an acute novel stressor (Dalla et al., 2005; Kokras et al., 2021). For understanding the implication of the sex difference in circulating glucocorticoids various other factors play a role beyond the balance of MR and GR, such as e.g., the sex difference in bio-available amount of free vs. total corticosterone because of increased corticosteroid binding globulin in females, liver metabolism and clearance rate and last possibly sexual dimorphism in glucocorticoid action (see below).
That there are sex differences in the stress induced HPA-axis response is perhaps not so surprising since males and females may have different coping strategies (Bale and Epperson, 2017; Bangasser and Valentino, 2014; Moisan, 2021). While the preferred initial response to a threat in males is ‘fight or flight’, that of females seems rather pro-social ‘tend-and-befriend’ strategy (Taylor et al., 2000). In rodent studies, sex differences can be demonstrated in coping with an inescapable forced swim stressor, in fear conditioning paradigms, during spatial learning and in social discrimination tests (Kokras et al., 2021; ter Horst et al., 2012). For instance, acute and chronic stress impairs performance of males in a variety of spatial memory tasks, but not of females, who showed actually an improved spatial performance (Luine et al., 2017). Such impaired performance of males in spatial learning tasks during stress seems to be compensated by a switch towards stimulus-response (habitual) behavior. For this purpose a circular ‘hole board’ is used where the mice had to locate an exit hole using extra- (spatial) and intra-maze (stimulus) cues (Schwabe et al., 2010a) (see section 4.3.). In this test, the switch of behavior in females is opposite to that of males: the females show under stress rather improved spatial skills -a sign of cognitive resilience-particularly in the estrous phase, rather than the habitual behavior displayed by the males (ter Horst et al., 2013b). However, the switch in coping strategy of both sexes under stress is lost following MR deletion from the forebrain (Ter Horst et al., 2013a). In an intriguing series of experiments, yet another sexual dimorphic aspect was uncovered using BDNFVal66Met mutants in a spatial learning: the object placement (or recognition) test. It was found that, in this mutant, estrogens impair spatial learning, while males are not deficient. This deficiency in females could be predicted by a particular transcriptional signature in the hippocampal CA3 region, where MR dominates GR expression (Marrocco et al., 2017).
The studies reported in the previous paragraphs suggested that hippocampal MR is involved in sex-specific risk assessment and response selection. This is further demonstrated in a test that measures social approach and discrimination between familiar and non-familiar conspecifics. After forebrain MR deletion, it appeared that the male rather than the female mutants had lost their ability to discriminate between a familiar and un-familiar mouse. Administration of a MR antagonist, and not a GR antagonist, to male mice also impaired their ability of social discrimination (Ter Horst et al., 2014). Recently, a MR hotspot, with relatively little GR expression, was identified in the hippocampal CA2 network that displayed a molecular identity distinct from the other pyramidal cell groups (McCann et al., 2021; Oakley et al., 2021). Deletion of the MR from the CA2 hub impaired social behavior. Interestingly, the CA2 neurons are also densely innervated by vasopressin acting via V1B receptors which are known to regulate social behavior (Pagani et al., 2015; Young et al., 2006).
The different learning strategies could be due to ’activational’ effects of sex steroids on structure and function of limbic forebrain circuitry, a field of science that was explored by the McEwen lab for half a century (Marrocco and McEwen, 2016; McEwen, 2020a). In addition, the MR seems involved because it binds progesterone and responds to estrogen. Besides these direct effects, the sex steroids have long-term ‘organizational’ actions that can enable the sexual differentiation because of “interaction between genetic influences of the X and Y chromosome and mitochondrial DNA inherited primarily via the mother” (McEwen, 2020a). In the ‘classic’ view this regards the function of the Y chromosome Sry causing testis formation and subsequent masculinization of the fetal brain by testosterone (McEwen, 2020a). In the ‘revisionist’ view, transcriptomics of the sex chromosome complement revealed that the developing male brain does express much more immune and inflammatory genes. A mechanism was proposed involving the increased immune function of microglia's that seem to bias sexual differentiation in the brain towards a male signature (McCarthy et al., 2017). Glucocorticoids also have sexual dimorphic effects firstly because of mutual interaction with sex steroids via their respective receptors (Kroon et al., 2020). Secondly, because of the much larger number of glucocorticoid responsive immune genes in males than females (Duma et al., 2010). These two aspects raise the question why glucocorticoids, either via MR and/or GR, would not be an additional signal in sexual differentiation of the brain (Bale, 2016; Marrocco et al., 2017; McCarthy, 2020; Quinn et al., 2014), a notion that received recently further support by the sex-dependent role of GR-responsive miRNA's in neuronal architecture (Tejos-Bravo et al., 2021). The interaction between glucocorticoid, sex steroids and potentially immune factors is important for understanding the perinatal basis of sex differences in the brain mechanism underlying stress resilience (Maccari et al., 2017).
In the human, several studies have shown associations between increased maternal cortisol, or maternal depression characterized by a history of early life trauma, with increased volume c.q. connectivity of the ‘right’ amygdala, in female offspring only (Buss et al., 2012; Graham et al., 2019; Soe et al., 2018). Remarkable is that the female prevalence of the maternal programming effect is associated with the 12 months infant cortisol reactivity and with internalization and emotional problems at an age of 4 years (Galbally et al., 2022b). In a subsequent study, the association of maternal depression appeared to be female-specific for infant cortisol reactivity and later emotional disorder of the 4 year old. These associations were found mediated by reduced expression of placental 11HSD-2 mRNA. A diminished 11HSD-2 activity would result in excess cortisol that passes through the placenta, and which then could become engaged in female-specific programming of an emotional ‘amygdala’ (Galbally et al., 2021, 2022a, 2022b; Jahnke et al., 2021). In animal studies the GR-mediated glucocorticoid amygdala programming of an emotional phenotype was uncovered before (Daskalakis et al., 2014b; Moriceau et al., 2006), but if this is also sex-specific and involves lateralization of the amygdala awaits further research.
7. What next?
A signature of chronic stress can be revealed as aberrant glucocorticoid secretion pattern and signaling in response to an acute stressor, taking also into account the sex difference (Bale, 2016; Kokras et al., 2021; Kroon et al., 2020; Quinn et al., 2014). Furthermore, it has been demonstrated that changes in cortisol secretion pattern may signal not only an upcoming precipitation of depression and other affective disorders, but also its remission (Holsboer, 2000). This link between the pattern of cortisol secretion and disease vulnerability or resilience raises the question how it can be exploited as biomarker for a successful treatment strategy. In other words, would it be possible with pharmacological and/or psychological approaches to restore a healthy glucocorticoid pattern as point of departure ‘to restart the clock’. Such an approach would better match Bruce's positivity rather than figuring out ‘why one cannot roll the clock back’ question to cure stress-related disorders.
A fruitful approach ‘to restart the clock’ could be to teach glucocorticoid patterns with interventions such as e.g., psychotherapy, mindfulness, exercise, and other non-pharmacological approaches that have been advocated by Bruce repeatedly himself (McEwen, 2020b). Another approach to restart would be pharmacological, but unfortunately, the application of a GR antagonist, CRH antagonists or V1B antagonists has made little progress in this respect (Kokras et al., 2019). One problem with using glucocorticoid-based pharmacotherapy, however, is that the stress hormone is pleiotropic with different actions in every cell and tissue, also over time. This is because -as noted before-glucocorticoids coordinate and integrate the function of cells and tissues over the ultradian pulses and circadian cycle, and during stress coping and adaptation. That's why cortisol substitution therapy is so cumbersome; one needs to mimic the ultradian/circadian and stress-induced patterns to meet the need) (Kalafatakis et al., 2016; Violaris et al., 2021).
For real progress, therefore, it is absolutely required to precisely identify the defect in glucocorticoid signaling down to the level of individual cells. This would allow a precise repair of the defect with a targeted treatment, i.e., personalized medicine ‘avant la lettre’. In the next sections progress in two novel developments is highlighted. Firstly, the identification of cell type-specific MR and GR genomics allowing to detect molecular changes responsible for defects in glucocorticoid action. Secondly, the development of an entirely novel generation of highly specific modulators of MR and/or GR function based on bringing selectivity to the interactions of the multimeric receptor complex with DNA/chromatin.
7.1. Genomics to predict stress resilience or vulnerability
Predisposition for altered glucocorticoid signaling has been studied in a variety of genomic studies as biomarker of trait and state, and predictor of longitudinal outcomes such disease progression and treatment response. Genetic studies focused on common NR3C1 single nucleotide polymorphisms (SNPs) linked to enhanced transactivation [primarily Bcll (rs41423247) and N363S (rs6195/; alias rs56149945)] and revealed association with altered HPA-axis response to stress and to the dexamethasone suppression test (DST) (Huizenga et al., 1998; Jewell et al., 2016; van West et al., 2010; Wust et al., 2004). Genetic variation in NR3C2 in interaction with childhood maltreatment is associated with DST cortisol outcome, hippocampal and amygdala volume and depression status (top SNP, rs17581262) (Gerritsen et al., 2017; Vinkers et al., 2015). A NR3C2 haplotype consisting of the 2G/C (rs2070951) and I180V (rs5522) SNPs in the promoter region that affects gene transcription, translation, and transactivation was associated with increased subjective stress- and cortisol stress response (van Leeuwen et al., 2011), changes in amygdala activation (Bogdan et al., 2012), dispositional optimism and protection against depression (Gerritsen et al., 2017; Klok et al., 2011a; Vinkers et al., 2015).
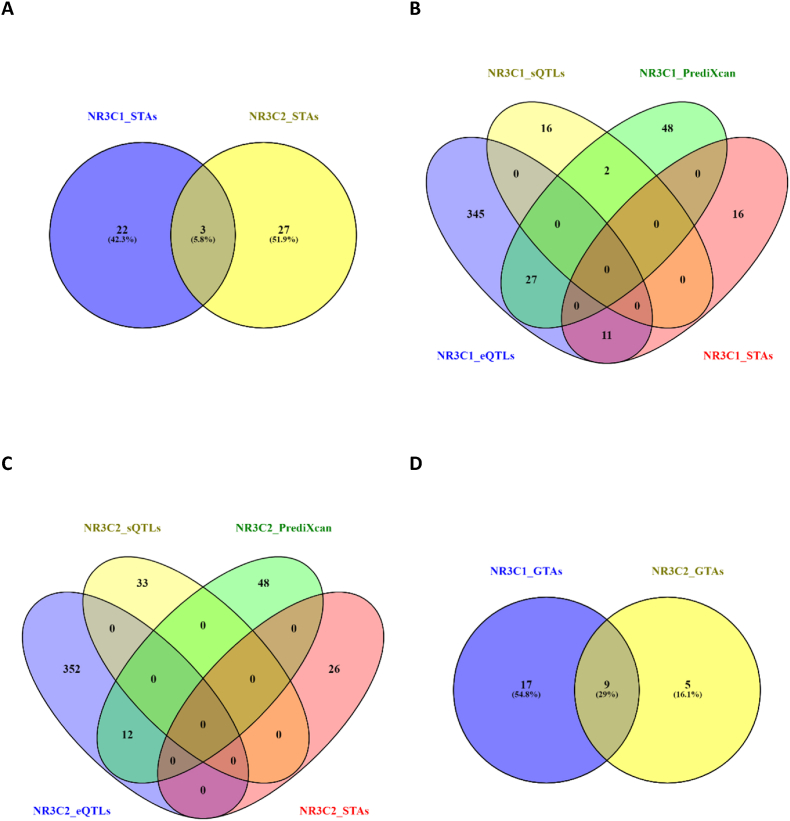
The latest GWAS of basal plasma cortisol did not identify any genome wide significant associations for SNPs in NR3C1 and NR3C2 genes but rather in the SERPINA6 gene, which encodes corticosteroid binding globulin (Crawford et al., 2021). The GWAS Catalog however includes multiple SNP-trait associations (STAs) primarily with non-brain phenotypes (NR3C1: atrial fibrillation, height, lung function; NR3C2: blood pressure, electrocardiogram morphology, lymphocyte counts; Supplementary Tables 1–2), but also brain phenotypes (NR3C1: migraine, response to ketamine, sleep; NR3C2: Alzheimer's disease, hippocampal volume, neurofibrillary tangles; Supplementary Tables 1–2). Finally, rare variants in NR3C2 are associated with autism spectrum disorder (De Rubeis et al., 2014; Ruzzo et al., 2019) and schizophrenia (Singh et al., 2022). The two genes did not share many trait-associations (∼6%, Fig. 2A) with the shared traits being generic/not disease-specific: lymphocyte percentage, neutrophil percentage, and liver protein quantitative trait loci.
Fig. 2.
NR3C1 and NR3C2 trait associations. (A) Overlap of NR3C1 and NR3C2 SNP trait associations (STAs) based on GWAS (NHGRI-EBI GWAS catalog database: https://www.ebi.ac.uk/gwas/). Overlap of NR3C1 (B) and NR3C2 (C) STAs with their expression and splicing quantitative trait loci (GTEx project: https://gtexportal.org/home/) and predictive models of expression based on cis-regulation (Barbeira et al., 2021). Overlap of NR3C1 and NR3C2 gene-trait-associations (GTAs; D) based on TWAS included in webTWAS database (http://www.webtwas.net/#/).
Approximately a third of the disease-associated NR3C1 variants are involved in the cis-regulation of NR3C1 gene expression (Fig. 2B, Table S1) either as expression or splicing quantitative trait loci (eQTLs or sQTLS) or as members of polygenic prediction of gene expression [e.g., PrediXcan models (Gamazon et al., 2015)]. Interestingly, the disease-associated NR3C2 variants had none of those properties (Fig. 2C Table S2). Using expression predictive models to conduct transcriptome-wide association studies (TWAS) of tissue- and cell-type-specific genetically regulated mRNA expressions and pathways with traits (Chatzinakos et al., 2020, 2021a). Using the webTWAS resource (Cao et al., 2022), the identified gene-trait associations (GTAs) for NR3C1 and NR3C2 were more overlapping (∼30%, Fig. 2D) compared to the STAs. The shared traits were centered around heart and thyroid diseases. For stress-related mental disorders, TWAS-based GTAs have been identified for glucocorticoid regulated genes and pathways (Dalvie et al., 2021; Gelernter et al., 2019; Huckins et al., 2020; Stein et al., 2021).
NR3C1 and NR3C1 exon expression patterns are tissue-specific (Fig. 3). Seminal studies by the Meaney group focused on epigenetic regulation (increased methylation) of the hippocampus Nr3c1 primary exon 1F promoter by early life stress (i.e., low maternal care) that was found linked to reduced GR mRNA and protein expression, hyperresponsivity to stress and impaired cognitive performance (Liu et al., 1997; Weaver et al., 2004). Further investigation revealed that these methylation changes were not unique to Nr3c1 (Suderman et al., 2012) and that also MR levels were reduced (Champagne et al., 2008). A study of analogous genomic loci in the human GR gene (i.e., NR3C1) was conducted in brain (McGowan et al., 2009) and peripheral tissue as a function of stress exposure during different phases of life (intergenerational, fetal, child and adult) and/or in relation to stress-related psychopathology (Daskalakis and Yehuda, 2014). Trauma exposure during military deployment was associated with an increase in all methylation measures, but development of mental health problems after deployment was only significantly associated with an increased functional methylation (i.e., methylation sites affecting gene expression) (Schur et al., 2017). DNA methylation patterns in NR3C2 are less studied, with the first study showing an association with aggressive behavior in males (Qing et al., 2021). Finally, a pilot study revealed that genetic variation and DNA methylation in NR3C1 were predictors of treatment response to psychotherapy in PTSD subjects (Yehuda et al., 2013). This was an intriguing study that was followed up by genome wide investigations of DNA methylation changes associated with psychotherapy (Vinkers et al., 2021; Yang et al., 2021).
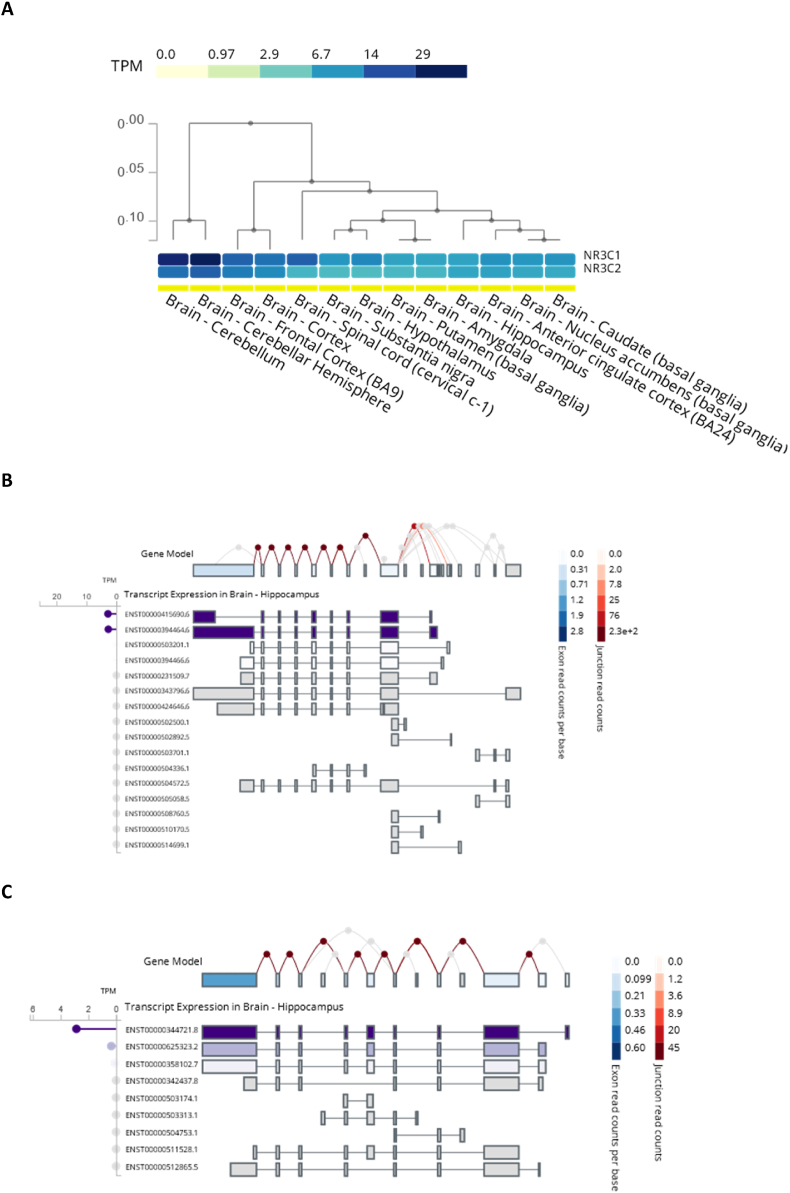
Fig. 3.
NR3C1 and NR3C2 expression in the brain. A: Clustering of NR3C1 and NR3C2 expression in the brain loci. B/C: Exon-specific transcription of NR3C1 (B) and NR3C2 (C) genes in hippocampus. Plots generated at GTEx project portal: https://gtexportal.org/home/.
Gene expression studies have investigated components of the GR signaling complex as biomarkers and predictors of stress-related psychopathology. The largest studies and meta-analyses revealed the involvement of GR as a master regulator of the expression signatures, but not as an explicitly altered GR gene expression which of course would not be necessary when glucocorticoid concentration patterns are altered (Breen et al., 2015, 2018; Daskalakis et al., 2016; Glatt et al., 2013; Guardado et al., 2016; Kuan et al., 2017; Logue et al., 2015; Mehta et al., 2013; Neylan et al., 2011; Segman et al., 2005; Su et al., 2008; Tylee et al., 2015; Yehuda et al., 2009, 2013; Zhang et al., 2015; Zieker et al., 2007). Animal models confirmed this notion by revealing, in the brain, glucocorticoids and the glucocorticoid-activated GR as a primary regulator of transcription in association with individual differences in behavioral responses to stress (Caradonna et al., 2022; Daskalakis et al., 2014a). In the first five studies evaluating gene expression in postmortem brain tissue of PTSD patients, changes were found in immune, neuronal, and stress regulatory pathways (Holmes et al., 2017; Licznerski et al., 2015; Morrison et al., 2019; Su et al., 2008; Young et al., 2015). Next, the first three adequately sized postmortem RNA-seq PTSD studies of cortical and subcortical tissue indicated changes in cell type specific functions like GABA-ergic interneuron pathways and immune/cytokine pathways (Girgenti et al., 2021; Jaffe et al., 2021; Logue et al., 2021).
Nowadays, tissue-specific gene expression has advanced to the single cell level. Thus, using single cell transcriptomics of dorsolateral PFC, we have recently identified in post-mortem brains of PTSD and major depressive disorder (MDD) patients differentially expressed genes within excitatory and inhibitory neurons (not the other cell types), suggesting that cell-type-specific dysregulations are associated with both disorders (Chatzinakos et al., 2021b; Chatzinakos et al., Under Review). Expression signatures of PTSD and MDD in excitatory- and inhibitory-neurons were differentially enriched for GR-dependent cortisol-induced gene expression alterations, especially in Ex-neurons (GR activation in PTSD, GR deactivation in MDD). These findings need to be expanded to other brain regions and need to incorporate MR as well, but they already confirm the hypothesis proposing that PTSD and MDD differ in excitatory- and inhibitory-neuronal GC signaling (Daskalakis et al., 2013b; Yehuda, 2002).
Taken together, stress-related psychopathology arises from differences at various levels of gene regulation in diverse brain cell types that converge on specific pathways (e.g., GC signaling) potentially harboring clinical significance (Dalvie et al., 2021). Adopting the three-hit concept of stress susceptibility (Daskalakis et al., 2013a), the interaction of genetic factors (hit-1) with early-life environmental factors (hit-2) precipitates in altered cell-type-specific glucocorticoid signaling that is mediated by epigenetic modifications, which in turn program brain plasticity with consequences for susceptibility (resilience or vulnerability) to later-life environment (hit-3). This hypothesis is being tested in animal stress studies [e.g., (Bouet et al., 2021)], with the goal to model human findings on stress susceptibility [e.g., (Daskalakis et al., 2021; Vinkers et al., 2014)].
7.2. From pleiotropic action of glucocorticoids to design of novel specific modulators of resilience
Although MR- and GR-mediated processes are complementary and sometimes opposite, at first sight molecular signaling is very similar. The long-term effects of MR and GR activation depend on their activity as transcription factors. Without ligand, both receptor types are predominantly complexed to chaperone proteins in the cytoplasm, and they translocate to the cell nucleus upon binding of agonists and most antagonists. Once nuclear, they can bind to the chromatin/DNA to affect gene expression. The best understood transcriptional mechanism involves binding of receptor dimers to two palindromic hexanucleotide sequences: the glucocorticoid response elements (GREs). Also, the progesterone and the androgen receptors can bind the GRE. Accordingly, PR, AR, MR and GR share a number of target genes that are induced in many different cell types. However, interactions with transcription factors that bind in the vicinity of GREs can result in exclusive DNA binding of a specific receptor, such as NeuroD factors that confer specific binding for MR in the hippocampus (van Weert et al., 2017).
A strongly induced shared target gene codes for FKBP5. The FK506 binding protein 5 (FKBP5) protein is a co-chaperone in the cytoplasmic steroid receptor complex, and negatively affects ligand binding of the GR (and presumably the other receptors). Thus, the transcriptional regulation of FKBP5 constitutes an intracellular negative feedback system, and the fact that MR is also able to induce its expression is one of the ways by which MR activation can limit GR functionality in cells that express both receptor types (Hartmann et al., 2021). Interestingly, the GRE sequence in the FKBP5 gene can be methylated in a haplotype dependent manner, and this has been suggested to be a mechanism for long term regulation of GR sensitivity, e.g., by early life stress in association with PTSD (Klengel et al., 2013), and FKBP5 inhibitors are being considered for clinical development. Of note, the consequences of FKBP5 regulation have been almost exclusively interpreted in relation to GR function, and effects on the MR functionality are understudied. Genes like FKBP5 that are regulated via both MR and GR should respond to corticosterone over a broader concentration range given the affinity differences. However, GR and MR may also differ in their strength of transactivation of the FKBP5 gene. Understanding the details and relevance of ‘joint targetness’ await further experimentation.
Classically, GR-mediated actions have been distinguished as to depend on GRE binding, binding in a ‘tethering’ mode to other transcription factors, and binding to negative GREs (nGREs) to exclusively repress (transiently induced) transcription. CRH (encoding CRH) and POMC (encoding proopiomelanocortin, the precursor of ACTH) are two examples of genes that likely are transrepressed through nGREs, as part of slow negative feedback. The nGRE mechanism is unique for GR, based on its biophysical properties (Hudson et al., 2013). Also, transrepressive interactions with other transcription factors (classically called ‘transrepression’) seem to be stronger for GR than for MR. However, a number of recent papers in cell models suggest that some form of direct (monomeric or dimeric) GR binding to GREs always involved in its transcriptional activity in a chromatin setting (Escoter-Torres et al., 2020; Johnson et al., 2021). The obligatory involvement of some form of GR-DNA interaction is somewhat of a paradigm shift away from what became a classical ‘transactivation-transrepression’ dogma (Reichardt et al., 1998; Saatcioglu et al., 1994). Indeed, genome wide occupancy studies in the hippocampus suggest that the vast majority of GR binding occurs at the GRE (Buurstede et al., 2021; Mifsud and Reul, 2016; Polman et al., 2013; Pooley et al., 2017). We also found this for MR binding (van Weert et al., 2017), but a recent study by Hans Reul and coworkers suggests that MR also may bind other elements (Mifsud et al., 2021). Of note, any call on the presence of GREs depends on statistical algorithms and is complicated by binding of receptors to half sites (Johnson et al., 2021). Thus, the GRE for now seems to be the most important way by which the hippocampal genome responds to changes in glucocorticoids.
MR and GR bind as dimers to the GRE, either as homodimers or MR:GR heterodimers, or in fact as tetrameric complexes, stabilized by one DNA-docking dimer (Presman and Hager, 2017). The existence of MR:GR heterodimers was elegantly shown by two complementary mutations in the DNA binding domain, that forced MR:GR heterodimers (Liu et al., 1995). Later co-occupancy of MR and GR at the same chromatin fragments (re-ChIP) strongly suggested the existence of heterodimers in the rat hippocampus (Mifsud and Reul, 2016). The presence of MR and GR in the same molecular complexes was also elegantly shown by proximity ligation assay (Oakley et al., 2021). With the emergence of higher order complexes, MR and GR may occur in different stoichiometries in tetramers (Rivers et al., 2019). Almost 30 years after their discovery, the jury is still out with respect to the consequences that MR and GR can form heterodimers for transcription.
Heterodimers would not necessarily be needed to explain differential effects like those on CA1 pyramidal cell excitability. For such phenomena, one would expect that the distinct DNA binding of each receptor type would lead to receptor-specific target genes. From whole hippocampus analysis, the transcription factor Jdp2 may be such a MR specific target gene (van Weert et al., 2019). The work by Mifsud suggests that the formation or maintenance of the cellular cilia may be a function process uniquely regulated via MR (Mifsud et al., 2021). However, the exact molecular underpinning of the differential MR and GR-mediated effects on cellular excitability remains to be determined – although the relevant target genes are known. In fact, the genomic effects on cellular responsiveness may well involve sets of cooperating target genes. Likely, revealing these will rely on single cell sequencing approaches, as RNA sequencing on bulk hippocampus, or even laser-dissected micropunches (Datson et al., 2013), reflects effects in many different cell types, often diluting each other (Buurstede et al., 2021).
The statement that steroid receptors are transcription factors is seemingly straightforward, but in fact involves an extensive ‘signal transduction’ from the GRE-bound receptor in the form of steroid receptor coregulators. First identified by Bert O'Malley, coactivator proteins link the DNA-bound receptors (often many thousands of base pairs away from the gene) to the RNA polymerase at the promoter of target genes. Some of these proteins can also remodel the local chromatin structure to allow other transcription factors to also access the DNA (Lonard and O'Malley, 2012). Conversely, corepressor proteins bind to steroid receptors mediate inhibition of transcriptional responses, e.g., at nGREs (Hudson et al., 2013). Of note, the coregulator recruitment differs between genomic loci, and therefore genes (Zwart et al., 2011). GR (and MR) mechanisms that involve chromatin remodeling may enable the GR to reprogram neurons and circuits during (early life) traumatic stressors.
Coregulators should be of considerable interest to stress researchers. First, they are shared to varying degrees between members of the nuclear receptor superfamily. This implies that they may become limiting for receptor function and provide a mechanism for cross talk between these types of receptors (Lonard and O'Malley, 2012). Second, coregulators show substantial differences in neuroanatomical distribution (Mahfouz et al., 2016; Viho et al., 2022). This may explain why in the PVN glucocorticoids can suppress the expression of the Crh gene, while in other brain regions such as the amygdala GR potently stimulates its expression (Zalachoras et al., 2016). Thirdly, coregulator recruitment is strongly ligand dependent, and this may allow targeting a subset of MR and/or GR dependent transcriptional targets (Zalachoras et al., 2013b).
MR and GR both have two large protein domains that can bind coregulators. The AF-1 function is localized in the intrinsically unstructured N-terminal part of the receptor, and shows little apparent overlap between MR and GR. The AF-2 function is localized in the LBD, and -at least in vitro-there are only modest differences in the coregulator recruitment between MR and GR via this domain (Broekema et al., 2018; Meijer et al., 2005). For some coregulators MR specificity has been shown, but this knowledge has not yet been integrated with MR function in the brain (Fuller et al., 2017). Thus, which coregulators are recruited by a particular receptor at a particular locus, the subsequent consequences for gene expression remain unknown for now. Given cell type specific (co-)expression of MR, GR and the many coregulators, also here progress in knowledge awaits single cell level -omics approaches in specific brain structures and behavioral contexts (Viho et al., 2022); similarly to the analysis of changes in gene expression in the brains of different patient population (see section 7.1).
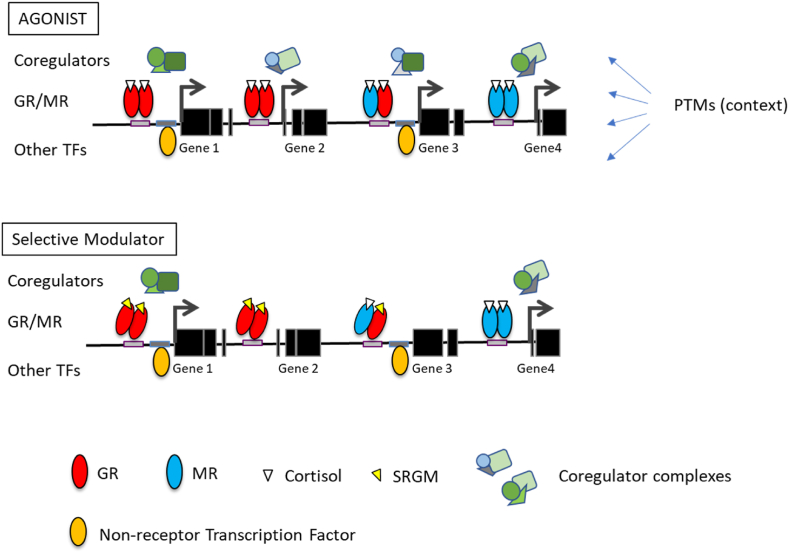
The coregulator recruitment is of specific interest because it may be targetable by GR and MR ligands. By definition, a full MR or GR agonist like corticosterone should allow recruitment of a broad range of coregulators, and an antagonist should not. However, new receptor ligands have shown coregulator recruitment profiles that are intermediates of these extremes [(Atucha et al., 2015; Zalachoras et al., 2013a), Fig. 4]. It is likely that GR-negative feedback regulation uses different coregulators compared to other brain cells (Meijer, 2002). Also, the coregulator repertoire of brain microglia is distinct from that other brain cells with respect to the important p160 Steroid Receptor Coactivator family (Viho et al., 2022). These are just two examples that could allow specific targeting of e.g., negative feedback mechanisms distinct from brain inflammatory processes with ‘selective receptor modulators’ (Viho et al., 2019).
Fig. 4.
DNA context and ligand dependent mineralocorticoid receptor (MR)/glucocorticoid receptors (GR) signaling. A. At high concentrations of agonist, MR and GR may bind as homo/or heterodimers to GREs, in conjunction with other, non-receptor, transcription factors (TFs). Depending on the DNA locus, coregulator complexes are recruited to mediate effects on transcription (arrows) and chromatin remodeling. Different ensembles of geometric shapes reflect different coregulator complexes. Other extracellular signals such as synaptic input can impinge at all levels via post-translational modifications (PTMs). B. When selective receptor modulators bind (here: for GR: a selective GR modulator or SGRM), the conformation of the receptor changes in such a way that only part of the interactions with coregulators may be formed. This allows for agonistic activity at some genes, but no or partial activity (antagonism) at others. Selective receptor modulation may allow to separate wanted from unwanted effects of MR/GR activation, although it is yet hard to predict which effects will separate.
In fact, the new GR antagonist, Relacorilant, differs substantially from the classical mifepristone in its interference with GR negative feedback (Hunt et al., 2018). One of the explanations for this phenomenon may be differential recruitment of coregulators by the antagonist-bound GR. Such selective modulators may be used in research to link coregulators to specific biological processes, and they may turn out to be of clinical interest, as exemplified by the activity of CORT118335 in treating fatty liver in mice (Koorneef et al., 2018). Also for MR, selective receptor modulation has been explored (Young and Clyne, 2021), and this new pharmacology may offer readily applicable ways to study the mechanisms of MR and GR effects in the brain. An important caveat is that at present it is impossible to predict coregulator interactions of newly designed ligands. Yet, with the available ligands it should be possible to link specific GR-dependent processes to particular (sets of) coregulators.
In sum, the transcriptional effects of glucocorticoids depend not only on cell type specific receptor expression, but also on chromatin accessibility, the presence of transcription factors that confer DNA binding specificity to specific loci, and on the cell type and locus specific recruitment of steroid receptor coregulators to the transcription complex. These ensembles mediate acute transcriptional effects, but also are responsible for the layer of epigenetic modification in adaptive processes. An exciting prospect is that ‘straightforward’ pharmacological tools are becoming available to target these processes with a certain degree of specificity.
8. Epilogue
In the past half a century Bruce McEwen has been an indisputable leader in the neuroendocrinology of stress and adaptation. His serendipitous discovery of corticosterone receptors in the hippocampus opened an entirely new field of research on the question how glucocorticoids can coordinate body and mind in dealing with environmental challenges. This action exerted by the glucocorticoid was further dissected in fast non-genomic and slower genomic components that allow integration over time from seconds and minutes to hours and even years when the programming effects on the developing brain are taken into account. The distinction between MR- and GR-mediated actions reflected the feedforward and feedback cascades in distinct neuronal networks. Such cascades can be translated in secretory patterns of stress mediators that critically regulate the MR/GR-mediated balance in emotional reactivity and cognitive control, pro- and anti-inflammatory/immune regulations and metabolic performance.
The secretory patterns themselves may serve as indicator of resilience i.e., the ability to bounce back. The depth of this analysis is embodied by the understanding that altered glucocorticoid exposure due to the repeated failure to cope with a stressor, can alter structure and function of the networks underlying emotional reactivity and cognitive control. An altered balance in these networks can progress until a point of ‘no return’, so the brain gets ‘stuck’, according to Bruce. Such emotion/cognition imbalance can be probed by an acute heterotypic stressor to reveal the altered secretory patterns of glucocorticoids. In collaborative studies between the New York and Leiden labs such a heterotypic challenge was also found instrumental in demonstrating the entirely different genomic responsivity in the brains of chronically stressed rodents (Datson et al., 2013; Gray et al., 2014). It also revealed striking sex differences in the brain, perhaps illustrating the potential mechanism underlying the different strategies males and females may use to cope with stress (McEwen, 2020a).
This recognition of a dynamic brain was the impetus for advancing the homeostasis 2.0, or allostasis concept. While homeostasis describes the negative feedback processes necessary to guard the constancy of the ‘milieu intérieure’, allostasis is a feedforward process towards a new equilibrium; you ‘can't roll the clock’ back, Bruce used to say, ‘and truly reverse the effects of experiences’ while he explained allostasis as the process of adaptation to (anticipated) change with its price tag of allostatic load. Entirely in line with Bruce's take on life, he used the allostasis concept as basis to launch ideas how to revitalize mechanisms of resilience by ‘restarting the clock again’. The future of the concepts of allostasis and allostatic load will depend, however, on how these can be translated into clinically accessible measures. The current measure based on blood levels of glucose, lipids and stress mediators is only the beginning of the ambition to calibrate fitness (Fertig, 2022; Guidi et al., 2021; Picard et al., 2017).
Based on the pioneering research of Bruce the contours of three new developments are becoming visible. Firstly, the combined genetic/bioinformatics approach where genetic variants are translated into cell-type specific transcriptome signatures of resilience to stress-related neuropsychiatric symptoms (see 7.1.). The idea is to use this computation strategy in combination with imaging and digital biomarkers as a novel means to identify individuals at risk with the option to follow the effect of preventive or treatment interventions. Secondly, the pharmacological approach to activate cell-type- and tissue-type-specific functions within the pleiotropic spectrum of glucocorticoid actions. Although CNS cell type-specific drug delivery is challenging, recent advances in bispecific antibody drug design have made feasible this important/promising treatment paradigm (Sedykh et al., 2018). Indeed, glucocorticoid analogs are currently being developed that can activate a specific cocktail of receptor coregulators to allow curative modulation of, for instance, only inflammatory processes without devastating neuroendocrine and metabolic side effects (see 7.2.).
The third approach refers to the new discipline of molecular sociology that precipitated in a hallmark paper by Bruce and brother Craig (McEwen and McEwen, 2017). Central in this approach is the open question whether lifestyle patterns, exercise, social support, socio-economic conditions and interventions of all kind actually maybe helpful in ‘taking back control’ and “teach” secretory and action patterns of stress mediators to create conditions that may facilitate recovery (Chen et al., 2017; Collins et al., 2009; Guidi and Fava, 2021; Lee et al., 2021). Or, as stated in the McEwen and McEwen (2017) paper, to exploit a model that “captures ways in which social structures interact with biological characteristics and systems to shape life trajectories”. While uncovering the biology underlying social patterns Bruce would say: “What I have always liked about science is discovering the unexpected”, a notion that is underscored by his seminal discovery of hippocampal corticosterone receptors as inroad to a potential magic bullet for boosting resilience.
Declaration of competing interest
In the past 3 years, NP Daskalakis has been a consultant for Sunovion Pharmaceuticals and is on the scientific advisory board for Sentio Solutions and Circular Genomics for unrelated work. OC Meijer collaborates with and receives research funding from Corcept Therapeutics. ER de Kloet owns stock of Corcept Therapeutics.
Acknowledgement
The support by the Stichting Universitas and Dr. RH de Rijk, the Bontius Foundation, National Institute of Mental Health (R21MH121909 to NPD), and the Royal Netherlands Academy of Arts and Sciences (to ERdK) is gratefully acknowledged.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2022.100455.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Akin S., Schriek P., van Nieuwkoop C., Neuman R.I., Meynaar I., van Helden E.J., El Bouazzaoui H., Baak R., Veuger M., Mairuhu R.A., van den Berg L. A low aldosterone/renin ratio and high soluble ACE2 associate with COVID-19 severity. J. Hypertens. 2022;40(3):606. doi: 10.1097/HJH.0000000000003054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arp J.M., ter Horst J.P., Kanatsou S., Fernandez G., Joels M., Krugers H.J., Oitzl M.S. Mineralocorticoid receptors guide spatial and stimulus-response learning in mice. PLoS One. 2014;9 doi: 10.1371/journal.pone.0086236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriza J.L., Simerly R.B., Swanson L.W., Evans R.M. The neuronal mineralocorticoid eeceptor as a mediator of glucocorticoid response. Neuron. 1988;1:887–900. doi: 10.1016/0896-6273(88)90136-5. [DOI] [PubMed] [Google Scholar]
- Arriza J.L., Weinberger C., Cerelli G., Glaser T.M., Handelin B.L., Housman D.E., Evans R.M. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237:268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- Atucha E., Zalachoras I., van den Heuvel J.K., van Weert L.T., Melchers D., Mol I.M., Belanoff J.K., Houtman R., Hunt H., Roozendaal B., Meijer O.C. A mixed glucocorticoid/mineralocorticoid selective modulator with dominant antagonism in the male rat brain. Endocrinology. 2015;156:4105–4114. doi: 10.1210/en.2015-1390. [DOI] [PubMed] [Google Scholar]
- Bale T.L. The placenta and neurodevelopment: sex differences in prenatal vulnerability. Dialogues Clin. Neurosci. 2016;18:459–464. doi: 10.31887/DCNS.2016.18.4/tbale. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale T.L., Epperson C.N. Sex as a biological variable: who, what, when, why, and how. Neuropsychopharmacology. 2017;42:386–396. doi: 10.1038/npp.2016.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Valentino R.J. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 2014;35:303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeira A.N., Bonazzola R., Gamazon E.R., Liang Y., Park Y., Kim-Hellmuth S., Wang G., Jiang Z., Zhou D., Hormozdiari F., Liu B., Rao A., Hamel A.R., Pividori M.D., Aguet F., Group G.T.G.W., Bastarache L., Jordan D.M., Verbanck M., Do R., Consortium G.T., Stephens M., Ardlie K., McCarthy M., Montgomery S.B., Segre A.V., Brown C.D., Lappalainen T., Wen X., Im H.K. Exploiting the GTEx resources to decipher the mechanisms at GWAS loci. Genome Biol. 2021;22:49. doi: 10.1186/s13059-020-02252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretz G., Packheiser J., Kumsta R., Wolf O.T., Ocklenburg S. The brain under stress-A systematic review and activation likelihood estimation meta-analysis of changes in BOLD signal associated with acute stress exposure. Neurosci. Biobehav. Rev. 2021;124:89–99. doi: 10.1016/j.neubiorev.2021.01.001. [DOI] [PubMed] [Google Scholar]
- Bogdan R., Williamson D.E., Hariri A.R. Mineralocorticoid receptor Iso/Val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. Am. J. Psychiatr. 2012;169:515–522. doi: 10.1176/appi.ajp.2011.11060855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonapersona V., Schuler H., Damsteegt R., Adolfs Y., Pasterkamp R.J., van den Heuvel M.P., Joels M., Sarabdjitsingh R.A. The mouse brain after foot shock in four dimensions: temporal dynamics at a single-cell resolution. Proc. Natl. Acad. Sci. U. S. A. 2022;119 doi: 10.1073/pnas.2114002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J., DeKloet E.R., Wenz H., Kern W., Fehm H.L. Gluco- and antimineralocorticoid effects on human sleep: a role of central corticosteroid receptors. Am. J. Physiol. 1991;260:E183–E188. doi: 10.1152/ajpendo.1991.260.2.E183. [DOI] [PubMed] [Google Scholar]
- Bouet V., Percelay S., Leroux E., Diarra B., Leger M., Delcroix N., Andrieux A., Dollfus S., Freret T., Boulouard M. A new 3-hit mouse model of schizophrenia built on genetic, early and late factors. Schizophr. Res. 2021;228:519–528. doi: 10.1016/j.schres.2020.11.043. [DOI] [PubMed] [Google Scholar]
- Breen M.S., Maihofer A.X., Glatt S.J., Tylee D.S., Chandler S.D., Tsuang M.T., Risbrough V.B., Baker D.G., O'Connor D.T., Nievergelt C.M., Woelk C.H. Gene networks specific for innate immunity define post-traumatic stress disorder. Mol. Psychiatr. 2015;20:1538–1545. doi: 10.1038/mp.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen M.S., Tylee D.S., Maihofer A.X., Neylan T.C., Mehta D., Binder E.B., Chandler S.D., Hess J.L., Kremen W.S., Risbrough V.B., Woelk C.H., Baker D.G., Nievergelt C.M., Tsuang M.T., Buxbaum J.D., Glatt S.J. PTSD blood transcriptome mega-analysis: shared inflammatory pathways across biological sex and modes of trauma. Neuropsychopharmacology. 2018;43:469–481. doi: 10.1038/npp.2017.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekema M.F., Hollman D.A.A., Koppen A., van den Ham H.J., Melchers D., Pijnenburg D., Ruijtenbeek R., van Mil S.W.C., Houtman R., Kalkhoven E. Profiling of 3696 nuclear receptor-coregulator interactions: a resource for biological and clinical discovery. Endocrinology. 2018;159:2397–2407. doi: 10.1210/en.2018-00149. [DOI] [PubMed] [Google Scholar]
- Brydges N.M., Jin R., Seckl J., Holmes M.C., Drake A.J., Hall J. Juvenile stress enhances anxiety and alters corticosteroid receptor expression in adulthood. Brain Behav. 2014;4:4–13. doi: 10.1002/brb3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C., Davis E.P., Shahbaba B., Pruessner J.C., Head K., Sandman C.A. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E1312–E1319. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buurstede J.C., van Weert L., Colucci P., Gentenaar M., Viho E.M.G., Koorneef L.L., Schoonderwoerd R.A., Lanooij S.D., Moustakas I., Balog J., Mei H., Kielbasa S.M., Campolongo P., Roozendaal B., Meijer O.C. Hippocampal glucocorticoid target genes associated with enhancement of memory consolidation. Eur. J. Neurosci. 2021 doi: 10.1111/ejn.15226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib S., Campus P., Conversi D., Orsini C., Puglisi-Allegra S. Functional and dysfunctional neuroplasticity in learning to cope with stress. Brain Sci. 2020;10:127. doi: 10.3390/brainsci10020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C., Wang J., Kwok D., Cui F., Zhang Z., Zhao D., Li M.J., Zou Q. webTWAS: a resource for disease candidate susceptibility genes identified by transcriptome-wide association study. Nucleic Acids Res. 2022;50:D1123–D1130. doi: 10.1093/nar/gkab957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caradonna S.G., Zhang T.Y., O'Toole N., Shen M.J., Khalil H., Einhorn N.R., Wen X., Parent C., Lee F.S., Akil H., Meaney M.J., McEwen B.S., Marrocco J. Genomic modules and intramodular network concordance in susceptible and resilient male mice across models of stress. Neuropsychopharmacology. 2022;47:987–999. doi: 10.1038/s41386-021-01219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey M.P., Deterd C.H., de Koning J., Helmerhorst F., de Kloet E.R. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J. Endocrinol. 1995;144:311–321. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- Carroll B.J., Curtis G.C., Mendels J. Neuroendocrine regulation in depression. II. Discrimination of depressed from nondepressed patients. Arch. Gen. Psychiatr. 1976;33:1051–1058. doi: 10.1001/archpsyc.1976.01770090041003. [DOI] [PubMed] [Google Scholar]
- Chameau P., Qin Y., Spijker S., Smit A.B., Joels M. Glucocorticoids specifically enhance L-type calcium current amplitude and affect calcium channel subunit expression in the mouse hippocampus. J. Neurophysiol. 2007;97:5–14. doi: 10.1152/jn.00821.2006. [DOI] [PubMed] [Google Scholar]
- Champagne D.L., Bagot R.C., van Hasselt F., Ramakers G., Meaney M.J., De Kloet E.R., Joëls M., Krugers H. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J. Neurosci. 2008;28(23):6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K., Holmes M., Seckl J. 11beta-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol. Rev. 2013;93:1139–1206. doi: 10.1152/physrev.00020.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzinakos C., Georgiadis F., Daskalakis N.P. GWAS meets transcriptomics: from genetic letters to transcriptomic words of neuropsychiatric risk. Neuropsychopharmacology. 2021;46:255–256. doi: 10.1038/s41386-020-00835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzinakos C., Georgiadis F., Lee D., Cai N., Vladimirov V.I., Docherty A., Webb B.T., Riley B.P., Flint J., Kendler K.S., Daskalakis N.P., Bacanu S.A. TWAS pathway method greatly enhances the number of leads for uncovering the molecular underpinnings of psychiatric disorders. Am. J. Med. Genet B Neuropsychiatr Genet. 2020;183:454–463. doi: 10.1002/ajmg.b.32823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzinakos C., Morrison F., McCullough K., Pernia C., Logue M., Wolf E., Carlezon W., Kellis M., Ressler K., Miller M., Huber B., Daskalakis N. Single-nucleus transcriptomic dissection of PTSD and MDD in human post-mortem DLPFC reveals genetic and environmental regulation. Biol. Psychiatr. 2021;89:S71. [Google Scholar]
- Chatzinakos, C., Pernia, C.D., Morrison, F.G., Schuler, H., McCullough, K.M., Snijders, C., Logue, M.W., Bajaj, T., Gassen, N.C., Bharadwaj, R.A., Kleinman, J.E., Anastasopoulos, C., Bowlby, B.C., DiPietro, C.P., Hartmann, J., Maihofer, A., Nievergelt, C.M., Ressler, N.M., Wolf, E.J., Traumatic Stress Brain Research, G., PsychEncode Consortium PTSD, W.g., Psychiatric Genomics Consortium PTSD, W.g, Carlezon, W.A., Jr, Krystal, J.H., Girgenti, M.J., Huber, B.R., Kellis, M., Miller, M.W., Ressler, K.J., Daskalakis, N.P., Under Review. Transcriptomic profiling of 573,408 PFC single nuclei 1 reveals roles for neuronal gene expression underlying stress response and white matter integrity in PTSD.
- Chen C., Nakagawa S., An Y., Ito K., Kitaichi Y., Kusumi I. The exercise-glucocorticoid paradox: how exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels. Front. Neuroendocrinol. 2017;44:83–102. doi: 10.1016/j.yfrne.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Collins A., Hill L.E., Chandramohan Y., Whitcomb D., Droste S.K., Reul J.M. Exercise improves cognitive responses to psychological stress through enhancement of epigenetic mechanisms and gene expression in the dentate gyrus. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A.A., Bankier S., Altmaier E., Barnes C.L.K., Clark D.W., Ermel R., Friedrich N., van der Harst P., Joshi P.K., Karhunen V., Lahti J., Mahajan A., Mangino M., Nethander M., Neumann A., Pietzner M., Sukhavasi K., Wang C.A., Bakker S.J.L., Bjorkegren J.L.M., Campbell H., Eriksson J., Gieger C., Hayward C., Jarvelin M.R., McLachlan S., Morris A.P., Ohlsson C., Pennell C.E., Price J., Rudan I., Ruusalepp A., Spector T., Tiemeier H., Volzke H., Wilson J.F., Michoel T., Timpson N.J., Smith G.D., Walker B.R., consortium C.O.N. Variation in the SERPINA6/SERPINA1 locus alters morning plasma cortisol, hepatic corticosteroid binding globulin expression, gene expression in peripheral tissues, and risk of cardiovascular disease. J. Hum. Genet. 2021;66:625–636. doi: 10.1038/s10038-020-00895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C., Antoniou K., Drossopoulou G., Xagoraris M., Kokras N., Sfikakis A., Papadopoulou-Daifoti Z. Chronic mild stress impact: are females more vulnerable? Neuroscience. 2005;135:703–714. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Dallman M.F., Levin N., Cascio C.S., Akana S.F., Jacobson L., Kuhn R.W. Pharmacological evidence that the inhibition of diurnal adrenocorticotropin secretion by corticosteroids is mediated via type I corticosterone-preferring receptors. Endocrinology. 1989;124:2844–2850. doi: 10.1210/endo-124-6-2844. [DOI] [PubMed] [Google Scholar]
- Dalm S., Karssen A.M., Meijer O.C., Belanoff J.K., de Kloet E.R. Resetting the stress system with a mifepristone challenge. Cell. Mol. Neurobiol. 2019;39:503–522. doi: 10.1007/s10571-018-0614-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalvie S., Chatzinakos C., Al Zoubi O., Georgiadis F., workgroup P.-P.S.B., Lancashire L., Daskalakis N.P. From genetics to systems biology of stress-related mental disorders. Neurobiol Stress. 2021;15:100393. doi: 10.1016/j.ynstr.2021.100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis N.P., Bagot R.C., Parker K.J., Vinkers C.H., de Kloet E.R. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology. 2013;38:1858–1873. doi: 10.1016/j.psyneuen.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis N.P., Claessens S.E., Laboyrie J.J., Enthoven L., Oitzl M.S., Champagne D.L., de Kloet E.R. The newborn rat's stress system readily habituates to repeated and prolonged maternal separation, while continuing to respond to stressors in context dependent fashion. Horm. Behav. 2011;60:165–176. doi: 10.1016/j.yhbeh.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Daskalakis N.P., Cohen H., Cai G., Buxbaum J.D., Yehuda R. Expression profiling associates blood and brain glucocorticoid receptor signaling with trauma-related individual differences in both sexes. Proc. Natl. Acad. Sci. U. S. A. 2014;111:13529–13534. doi: 10.1073/pnas.1401660111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis N.P., Cohen H., Nievergelt C.M., Baker D.G., Buxbaum J.D., Russo S.J., Yehuda R. New translational perspectives for blood-based biomarkers of PTSD: from glucocorticoid to immune mediators of stress susceptibility. Exp. Neurol. 2016;284:133–140. doi: 10.1016/j.expneurol.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis N.P., Diamantopoulou A., Claessens S.E.F., Remmers E., Tjalve M., Oitzl M.S., Champagne D.L., de Kloet E.R. Early experience of a novel-environment in isolation primes a fearful phenotype characterized by persistent amygdala activation. Psychoneuroendocrinology. 2014;39:39–57. doi: 10.1016/j.psyneuen.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Daskalakis N.P., Lehrner A., Yehuda R. Endocrine aspects of post-traumatic stress disorder and implications for diagnosis and treatment. Endocrinol Metab. Clin. N. Am. 2013;42:503–513. doi: 10.1016/j.ecl.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Daskalakis N.P., Oitzl M.S., Schachinger H., Champagne D.L., de Kloet E.R. Testing the cumulative stress and mismatch hypotheses of psychopathology in a rat model of early-life adversity. Physiol. Behav. 2012;106:707–721. doi: 10.1016/j.physbeh.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Daskalakis N.P., Schultz L.M., Visoki E., Moore T.M., Argabright S.T., Harnett N.G., DiDomenico G.E., Warrier V., Almasy L., Barzilay R. Contributions of PTSD polygenic risk and environmental stress to suicidality in preadolescents. Neurobiol Stress. 2021;15:100411. doi: 10.1016/j.ynstr.2021.100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis N.P., Yehuda R. Site-specific methylation changes in the glucocorticoid receptor exon 1F promoter in relation to life adversity: systematic review of contributing factors. Front. Neurosci. 2014;8:369. doi: 10.3389/fnins.2014.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datson N.A., van den Oever J.M., Korobko O.B., Magarinos A.M., de Kloet E.R., McEwen B.S. Previous history of chronic stress changes the transcriptional response to glucocorticoid challenge in the dentate gyrus region of the male rat hippocampus. Endocrinology. 2013;154:3261–3272. doi: 10.1210/en.2012-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet E.R., de Kloet S.F., de Kloet C.S., de Kloet A.D. Top-down and bottom-up control of stress-coping. J. Neuroendocrinol. 2019;31 doi: 10.1111/jne.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet E.R., De Kock S., Schild V., Veldhuis H.D. Antiglucocorticoid RU 38486 attenuates retention of a behaviour and disinhibits the hypothalamic-pituitary adrenal axis at different brain sites. Neuroendocrinology. 1988;47:109–115. doi: 10.1159/000124900. [DOI] [PubMed] [Google Scholar]
- de Kloet E.R., Joels M., Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- de Kloet E.R., Meijer O.C., de Nicola A.F., de Rijk R.H., Joels M. Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Front. Neuroendocrinol. 2018;49:124–145. doi: 10.1016/j.yfrne.2018.02.003. [DOI] [PubMed] [Google Scholar]
- de Kloet E.R., Oitzl M.S., Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- De Kloet E.R., Reul J.M. Feedback action and tonic influence of corticosteroids on brain function: a concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology. 1987;12:83–105. doi: 10.1016/0306-4530(87)90040-0. [DOI] [PubMed] [Google Scholar]
- de Kloet E.R., van der Vies J., de Wied D. The site of the suppressive action of dexamethasone on pituitary-adrenal activity. Endocrinology. 1974;94:61–73. doi: 10.1210/endo-94-1-61. [DOI] [PubMed] [Google Scholar]
- De Kloet E.R., Versteeg D.H., Kovacs G.L. Aldosterone blocks the response to corticosterone in the raphe-hippocampal serotonin system. Brain Res. 1983;264:323–327. doi: 10.1016/0006-8993(83)90834-x. [DOI] [PubMed] [Google Scholar]
- De Kloet E.R., Vreugdenhil E., Oitzl M.S., Joels M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- De Kloet R., Wallach G., McEwen B.S. Differences in corticosterone and dexamethasone binding to rat brain and pituitary. Endocrinology. 1975;96:598–609. doi: 10.1210/endo-96-3-598. [DOI] [PubMed] [Google Scholar]
- De Rubeis S., He X., Goldberg A.P., Poultney C.S., Samocha K., Cicek A.E., Kou Y., Liu L., Fromer M., Walker S., Singh T., Klei L., Kosmicki J., Shih-Chen F., Aleksic B., Biscaldi M., Bolton P.F., Brownfeld J.M., Cai J., Campbell N.G., Carracedo A., Chahrour M.H., Chiocchetti A.G., Coon H., Crawford E.L., Curran S.R., Dawson G., Duketis E., Fernandez B.A., Gallagher L., Geller E., Guter S.J., Hill R.S., Ionita-Laza J., Jimenz Gonzalez P., Kilpinen H., Klauck S.M., Kolevzon A., Lee I., Lei I., Lei J., Lehtimaki T., Lin C.F., Ma'ayan A., Marshall C.R., McInnes A.L., Neale B., Owen M.J., Ozaki N., Parellada M., Parr J.R., Purcell S., Puura K., Rajagopalan D., Rehnstrom K., Reichenberg A., Sabo A., Sachse M., Sanders S.J., Schafer C., Schulte-Ruther M., Skuse D., Stevens C., Szatmari P., Tammimies K., Valladares O., Voran A., Li-San W., Weiss L.A., Willsey A.J., Yu T.W., Yuen R.K., Study D.D.D., Homozygosity Mapping Collaborative for A., Consortium U.K., Cook E.H., Freitag C.M., Gill M., Hultman C.M., Lehner T., Palotie A., Schellenberg G.D., Sklar P., State M.W., Sutcliffe J.S., Walsh C.A., Scherer S.W., Zwick M.E., Barett J.C., Cutler D.J., Roeder K., Devlin B., Daly M.J., Buxbaum J.D. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–215. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschle M., Weber B., Colla M., Muller M., Kniest A., Heuser I. Mineralocorticoid receptor also modulates basal activity of hypothalamus-pituitary-adrenocortical system in humans. Neuroendocrinology. 1998;68:355–360. doi: 10.1159/000054384. [DOI] [PubMed] [Google Scholar]
- Di S., Itoga C.A., Fisher M.O., Solomonow J., Roltsch E.A., Gilpin N.W., Tasker J.G. Acute stress suppresses synaptic inhibition and increases anxiety via endocannabinoid release in the basolateral amygdala. J. Neurosci. 2016;36:8461–8470. doi: 10.1523/JNEUROSCI.2279-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S., Malcher-Lopes R., Halmos K.C., Tasker J.G. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J. Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Ferreira E., Sousa J.C., Melo I., Morgado P., Mesquita A.R., Cerqueira J.J., Costa R.M., Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Douma E.H., de Kloet E.R. Stress-induced plasticity and functioning of ventral tegmental dopamine neurons. Neurosci. Biobehav. Rev. 2020;108:48–77. doi: 10.1016/j.neubiorev.2019.10.015. [DOI] [PubMed] [Google Scholar]
- Duma D., Collins J.B., Chou J.W., Cidlowski J.A. Sexually dimorphic actions of glucocorticoids provide a link to inflammatory diseases with gender differences in prevalence. Sci. Signal. 2010;3:ra74. doi: 10.1126/scisignal.2001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C.R.W., Burt D., McIntyre M.A., De Kloet E.R., Stewart P.M., Brett L., Sutanto W.S., Monder C. Localisation of 11β-hydroxysteroid dehydrogenase—tissue specific protector of the mineralocorticoid receptor. Lancet. 1988;332:986–989. doi: 10.1016/s0140-6736(88)90742-8. [DOI] [PubMed] [Google Scholar]
- Escoter-Torres L., Greulich F., Quagliarini F., Wierer M., Uhlenhaut N.H. Anti-inflammatory functions of the glucocorticoid receptor require DNA binding. Nucleic Acids Res. 2020;48:8393–8407. doi: 10.1093/nar/gkaa565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R.M., Arriza J.L. A molecular framework for the actions of glucocorticoid hormones in the nervous system. Neuron. 1989;2:1105–1112. doi: 10.1016/0896-6273(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Fertig B.J. CRC Press; 2022. Metabolism and Medicine: the Metabolic Landscape of Health and Disease. [Google Scholar]
- Fitzsimons C.P., van Hooijdonk L.W., Schouten M., Zalachoras I., Brinks V., Zheng T., Schouten T.G., Saaltink D.J., Dijkmans T., Steindler D.A., Verhaagen J., Verbeek F.J., Lucassen P.J., de Kloet E.R., Meijer O.C., Karst H., Joels M., Oitzl M.S., Vreugdenhil E. Knockdown of the glucocorticoid receptor alters functional integration of newborn neurons in the adult hippocampus and impairs fear-motivated behavior. Mol. Psychiatr. 2013;18:993–1005. doi: 10.1038/mp.2012.123. [DOI] [PubMed] [Google Scholar]
- Fuller P.J., Yang J., Young M.J. 30 years OF the mineralocorticoid receptor: coregulators as mediators of mineralocorticoid receptor signalling diversity. J. Endocrinol. 2017;234:T23–T34. doi: 10.1530/JOE-17-0060. [DOI] [PubMed] [Google Scholar]
- Funder J.W., Pearce P.T., Smith R., Smith A.I. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988;242:583–585. doi: 10.1126/science.2845584. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Wikstrom A.C., Okret S., Agnati L.F., Harfstrand A., Yu Z.Y., Granholm L., Zoli M., Vale W., Gustafsson J.A. Mapping of glucocorticoid receptor immunoreactive neurons in the rat tel- and diencephalon using a monoclonal antibody against rat liver glucocorticoid receptor. Endocrinology. 1985;117:1803–1812. doi: 10.1210/endo-117-5-1803. [DOI] [PubMed] [Google Scholar]
- Galbally M., van Rossum E.F.C., Watson S.J., de Kloet E.R., Lewis A.J. Trans-generational stress regulation: mother-infant cortisol and maternal mental health across the perinatal period. Psychoneuroendocrinology. 2019;109:104374. doi: 10.1016/j.psyneuen.2019.104374. [DOI] [PubMed] [Google Scholar]
- Galbally M., Watson S.J., Lappas M., de Kloet E.R., van Rossum E., Wyrwoll C., Mark P., Lewis A.J. Fetal programming pathway from maternal mental health to infant cortisol functioning: the role of placental 11beta-HSD2 mRNA expression. Psychoneuroendocrinology. 2021;127:105197. doi: 10.1016/j.psyneuen.2021.105197. [DOI] [PubMed] [Google Scholar]
- Galbally M., Watson S.J., Lappas M., de Kloet E.R., Wyrwoll C.S., Mark P.J., Lewis A.J. Exploring sex differences in fetal programming for childhood emotional disorders. Psychoneuroendocrinology. 2022:105764. doi: 10.1016/j.psyneuen.2022.105764. [DOI] [PubMed] [Google Scholar]
- Galbally M., Watson S.J., van IJzendoorn M., Saffery R., Ryan J., de Kloet E.R., Oberlander T.F., Lappas M., Lewis A.J. The role of glucocorticoid and mineralocorticoid receptor DNA methylation in antenatal depression and infant stress regulation. Psychoneuroendocrinology. 2020;115:104611. doi: 10.1016/j.psyneuen.2020.104611. [DOI] [PubMed] [Google Scholar]
- Galbally M., Watson S.J., van Rossum E.F.C., Chen W., de Kloet E.R., Lewis A.J. The perinatal origins of childhood anxiety disorders and the role of early-life maternal predictors. Psychol. Med. 2022;52:506–514. doi: 10.1017/S0033291720002147. [DOI] [PubMed] [Google Scholar]
- Gamazon E.R., Wheeler H.E., Shah K.P., Mozaffari S.V., Aquino-Michaels K., Carroll R.J., Eyler A.E., Denny J.C., Consortium G.T., Nicolae D.L., Cox N.J., Im H.K. A gene-based association method for mapping traits using reference transcriptome data. Nat. Genet. 2015;47:1091–1098. doi: 10.1038/ng.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini S., Resch J.M., Narayan S.V., Peltekian L., Iverson G.N., Karthik S., Geerling J.C. Aldosterone-sensitive HSD2 neurons in mice. Brain Struct. Funct. 2019;224:387–417. doi: 10.1007/s00429-018-1778-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling J.C., Kawata M., Loewy A.D. Aldosterone-sensitive neurons in the rat central nervous system. J. Comp. Neurol. 2006;494:515–527. doi: 10.1002/cne.20808. [DOI] [PubMed] [Google Scholar]
- Geerling J.C., Loewy A.D. Aldosterone in the brain. Am. J. Physiol. Ren. Physiol. 2009;297:F559–F576. doi: 10.1152/ajprenal.90399.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J., Sun N., Polimanti R., Pietrzak R., Levey D.F., Bryois J., Lu Q., Hu Y., Li B., Radhakrishnan K., Aslan M., Cheung K.H., Li Y., Rajeevan N., Sayward F., Harrington K., Chen Q., Cho K., Pyarajan S., Sullivan P.F., Quaden R., Shi Y., Hunter-Zinck H., Gaziano J.M., Concato J., Zhao H., Stein M.B., Department of Veterans Affairs Cooperative Studies, P. Million Veteran P. Genome-wide association study of post-traumatic stress disorder reexperiencing symptoms in >165,000 US veterans. Nat. Neurosci. 2019;22:1394–1401. doi: 10.1038/s41593-019-0447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach J.L., McEwen B.S. Rat brain binds adrenal steroid hormone: radioautography of hippocampus with corticosterone. Science. 1972;175:1133–1136. doi: 10.1126/science.175.4026.1133. [DOI] [PubMed] [Google Scholar]
- Gerritsen L., Milaneschi Y., Vinkers C.H., van Hemert A.M., van Velzen L., Schmaal L., Penninx B.W. HPA Axis genes, and their interaction with childhood maltreatment, are related to cortisol levels and stress-related phenotypes. Neuropsychopharmacology. 2017;42:2446–2455. doi: 10.1038/npp.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgenti M.J., Wang J., Ji D., Cruz D.A., Traumatic Stress Brain Research, G. Stein M.B., Gelernter J., Young K.A., Huber B.R., Williamson D.E., Friedman M.J., Krystal J.H., Zhao H., Duman R.S. Transcriptomic organization of the human brain in post-traumatic stress disorder. Nat. Neurosci. 2021;24:24–33. doi: 10.1038/s41593-020-00748-7. [DOI] [PubMed] [Google Scholar]
- Glatt S.J., Tylee D.S., Chandler S.D., Pazol J., Nievergelt C.M., Woelk C.H., Baker D.G., Lohr J.B., Kremen W.S., Litz B.T., Tsuang M.T., Marine Resiliency Study I. Blood-based gene-expression predictors of PTSD risk and resilience among deployed marines: a pilot study. Am. J. Med. Genet B Neuropsychiatr Genet. 2013;162B:313–326. doi: 10.1002/ajmg.b.32167. [DOI] [PubMed] [Google Scholar]
- Graham A.M., Rasmussen J.M., Entringer S., Ben Ward E., Rudolph M.D., Gilmore J.H., Styner M., Wadhwa P.D., Fair D.A., Buss C. Maternal cortisol concentrations during pregnancy and sex-specific associations with neonatal amygdala connectivity and emerging internalizing behaviors. Biol. Psychiatr. 2019;85:172–181. doi: 10.1016/j.biopsych.2018.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J.D., Rubin T.G., Hunter R.G., McEwen B.S. Hippocampal gene expression changes underlying stress sensitization and recovery. Mol. Psychiatr. 2014;19:1171–1178. doi: 10.1038/mp.2013.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groch S., Wilhelm I., Lange T., Born J. Differential contribution of mineralocorticoid and glucocorticoid receptors to memory formation during sleep. Psychoneuroendocrinology. 2013;38:2962–2972. doi: 10.1016/j.psyneuen.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Groeneweg F.L., Karst H., de Kloet E.R., Joels M. Rapid non-genomic effects of corticosteroids and their role in the central stress response. J. Endocrinol. 2011;209:153–167. doi: 10.1530/JOE-10-0472. [DOI] [PubMed] [Google Scholar]
- Groeneweg F.L., van Royen M.E., Fenz S., Keizer V.I., Geverts B., Prins J., de Kloet E.R., Houtsmuller A.B., Schmidt T.S., Schaaf M.J. Quantitation of glucocorticoid receptor DNA-binding dynamics by single-molecule microscopy and FRAP. PLoS One. 2014;9 doi: 10.1371/journal.pone.0090532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardado P., Olivera A., Rusch H.L., Roy M., Martin C., Lejbman N., Lee H., Gill J.M. Altered gene expression of the innate immune, neuroendocrine, and nuclear factor-kappa B (NF-kappaB) systems is associated with posttraumatic stress disorder in military personnel. J. Anxiety Disord. 2016;38:9–20. doi: 10.1016/j.janxdis.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Guidi J., Fava G.A. Sequential combination of pharmacotherapy and psychotherapy in major depressive disorder: a systematic review and meta-analysis. JAMA Psychiatr. 2021;78:261–269. doi: 10.1001/jamapsychiatry.2020.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi J., Lucente M., Sonino N., Fava G.A. Allostatic load and its impact on health: a systematic review. Psychother. Psychosom. 2021;90:11–27. doi: 10.1159/000510696. [DOI] [PubMed] [Google Scholar]
- Hall B.S., Moda R.N., Liston C. Glucocorticoid mechanisms of functional connectivity changes in stress-related neuropsychiatric disorders. Neurobiol Stress. 2015;1:174–183. doi: 10.1016/j.ynstr.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A.P., Holmes M.C., de Kloet E.R., Chapman K.E., Seckl J.R. Mineralocorticoid and glucocorticoid receptor balance in control of HPA axis and behaviour. Psychoneuroendocrinology. 2013;38:648–658. doi: 10.1016/j.psyneuen.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Hartmann J., Bajaj T., Klengel C., Chatzinakos C., Ebert T., Dedic N., McCullough K.M., Lardenoije R., Joels M., Meijer O.C., McCann K.E., Dudek S.M., Sarabdjitsingh R.A., Daskalakis N.P., Klengel T., Gassen N.C., Schmidt M.V., Ressler K.J. Mineralocorticoid receptors dampen glucocorticoid receptor sensitivity to stress via regulation of FKBP5. Cell Rep. 2021;35:109185. doi: 10.1016/j.celrep.2021.109185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., Halima M., Xie Y., Schaaf M.J.M., Meijer A.H., Wang M. Ginsenoside Rg1 acts as a selective glucocorticoid receptor agonist with anti-inflammatory action without affecting tissue regeneration in zebrafish larvae. Cells. 2020;9:1107. doi: 10.3390/cells9051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck A.L., Handa R.J. Sex differences in the hypothalamic-pituitary-adrenal axis' response to stress: an important role for gonadal hormones. Neuropsychopharmacology. 2019;44:45–58. doi: 10.1038/s41386-018-0167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens M.J., Pu Z., Hermans E.J., van Wingen G.A., Joels M., Fernandez G. Dynamically changing effects of corticosteroids on human hippocampal and prefrontal processing. Hum. Brain Mapp. 2012;33:2885–2897. doi: 10.1002/hbm.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens M.J., van der Marel K., van der Toorn A., Pillai A.G., Fernandez G., Dijkhuizen R.M., Joels M. Stress-induced alterations in large-scale functional networks of the rodent brain. Neuroimage. 2015;105:312–322. doi: 10.1016/j.neuroimage.2014.10.037. [DOI] [PubMed] [Google Scholar]
- Henkin R.I., Daly R.L. Auditory detection and perception in normal man and in patients with adrenal cortical insufficiency: effect of adrenal cortical steroids. J. Clin. Invest. 1968;47:1269–1280. doi: 10.1172/JCI105819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.P., Nawreen N., Smail M.A., Cotella E.M. Brain mechanisms of HPA axis regulation: neurocircuitry and feedback in context Richard Kvetnansky lecture. Stress. 2020;23:617–632. doi: 10.1080/10253890.2020.1859475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans E.J., Henckens M.J., Joels M., Fernandez G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014;37:304–314. doi: 10.1016/j.tins.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Heshmati M., Russo S.J. Learning to deal with life's ups and downs. Nat. Neurosci. 2013;16:658–659. doi: 10.1038/nn.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser I., Yassouridis A., Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J. Psychiatr. Res. 1994;28:341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Hlavacova N., Jezova D. Chronic treatment with the mineralocorticoid hormone aldosterone results in increased anxiety-like behavior. Horm. Behav. 2008;54:90–97. doi: 10.1016/j.yhbeh.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Hollenberg S.M., Weinberger C., Ong E.S., Cerelli G., Oro A., Lebo R., Thompson E.B., Rosenfeld M.G., Evans R.M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes S.E., Girgenti M.J., Davis M.T., Pietrzak R.H., DellaGioia N., Nabulsi N., Matuskey D., Southwick S., Duman R.S., Carson R.E., Krystal J.H., Esterlis I., Traumatic Stress Brain Study, G Altered metabotropic glutamate receptor 5 markers in PTSD: in vivo and postmortem evidence. Proc. Natl. Acad. Sci. U. S. A. 2017;114:8390–8395. doi: 10.1073/pnas.1701749114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Cold Spring Harbor Laboratory; 2020. Effect of Dexamethasone in Hospitalized Patients with COVID-19 – Preliminary Report. [Google Scholar]
- Huckins L.M., Chatzinakos C., Breen M.S., Hartmann J., Klengel T., da Silva Almeida A.C., Dobbyn A., Girdhar K., Hoffman G.E., Klengel C., Logue M.W., Lori A., Maihofer A.X., Morrison F.G., Nguyen H.T., Park Y., Ruderfer D., Sloofman L.G., van Rooij S.J.H., Consortium, P.W.G.o.P.G. Baker D.G., Chen C.Y., Cox N., Duncan L.E., Geyer M.A., Glatt S.J., Im H.K., Risbrough V.B., Smoller J.W., Stein D.J., Yehuda R., Liberzon I., Koenen K.C., Jovanovic T., Kellis M., Miller M.W., Bacanu S.A., Nievergelt C.M., Buxbaum J.D., Sklar P., Ressler K.J., Stahl E.A., Daskalakis N.P. Analysis of genetically regulated gene expression identifies a prefrontal PTSD gene, SNRNP35, specific to military cohorts. Cell Rep. 2020;31:107716. doi: 10.1016/j.celrep.2020.107716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson W.H., Youn C., Ortlund E.A. The structural basis of direct glucocorticoid-mediated transrepression. Nat. Struct. Mol. Biol. 2013;20:53–58. doi: 10.1038/nsmb.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizenga N.A., Koper J.W., De Lange P., Pols H.A., Stolk R.P., Burger H., Grobbee D.E., Brinkmann A.O., De Jong F.H., Lamberts S.W. A polymorphism in the glucocorticoid receptor gene may be associated with and increased sensitivity to glucocorticoids in vivo. J. Clin. Endocrinol. Metab. 1998;83:144–151. doi: 10.1210/jcem.83.1.4490. [DOI] [PubMed] [Google Scholar]
- Hunt H., Donaldson K., Strem M., Zann V., Leung P., Sweet S., Connor A., Combs D., Belanoff J. Assessment of safety, tolerability, pharmacokinetics, and pharmacological effect of orally administered CORT125134: an adaptive, double-blind, randomized, placebo-controlled phase 1 clinical study. Clin Pharmacol Drug Dev. 2018;7:408–421. doi: 10.1002/cpdd.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter R.G., Murakami G., Dewell S., Seligsohn M., Baker M.E., Datson N.A., McEwen B.S., Pfaff D.W. Acute stress and hippocampal histone H3 lysine 9 trimethylation, a retrotransposon silencing response. Proc. Natl. Acad. Sci. U. S. A. 2012;109:17657–17662. doi: 10.1073/pnas.1215810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter R.G., Seligsohn M., Rubin T.G., Griffiths B.B., Ozdemir Y., Pfaff D.W., Datson N.A., McEwen B.S. Stress and corticosteroids regulate rat hippocampal mitochondrial DNA gene expression via the glucocorticoid receptor. Proc. Natl. Acad. Sci. U. S. A. 2016;113:9099–9104. doi: 10.1073/pnas.1602185113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M., Hojo Y., Komatsuzaki Y., Okamoto M., Kato A., Takeda T., Kawato S. Hippocampal spine changes across the sleep-wake cycle: corticosterone and kinases. J. Endocrinol. 2015;226:M13–M27. doi: 10.1530/JOE-15-0078. [DOI] [PubMed] [Google Scholar]
- Jaffe A.E., Tao R., Tran M.N., Page S.C., Maynard K.R., Pattie E.A., Nguyen C.V., Deep-Soboslay A., Bharadwaj R., Young K.A., Friedman M.J., Williamson D.E., Shin J.H., Hyde T.M., Martinowich K., Kleinman J.E. Decoding shared versus divergent transcriptomic signatures across cortico-amygdala circuitry in PTSD and depressive disorders. bioRxiv. 2021;2001(2012):426438. doi: 10.1176/appi.ajp.21020162. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnke J.R., Teran E., Murgueitio F., Cabrera H., Thompson A.L. Maternal stress, placental 11beta-hydroxysteroid dehydrogenase type 2, and infant HPA axis development in humans: psychosocial and physiological pathways. Placenta. 2021;104:179–187. doi: 10.1016/j.placenta.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell C.M., Katen K.S., Barber L.M., Cannon C., Garantziotis S., Cidlowski J.A. Healthy glucocorticoid receptor N363S carriers dysregulate gene expression associated with metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2016;311:E741–E748. doi: 10.1152/ajpendo.00105.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol. Sci. 2006;27:244–250. doi: 10.1016/j.tips.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Joels M., de Kloet E.R. Effects of glucocorticoids and norepinephrine on the excitability in the hippocampus. Science. 1989;245:1502–1505. doi: 10.1126/science.2781292. [DOI] [PubMed] [Google Scholar]
- Joels M., de Kloet E.R. Mineralocorticoid receptor-mediated changes in membrane properties of rat CA1 pyramidal neurons in vitro. Proc. Natl. Acad. Sci. U. S. A. 1990;87:4495–4498. doi: 10.1073/pnas.87.12.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M., De Kloet E.R. Coordinative mineralocorticoid and glucocorticoid receptor-mediated control of responses to serotonin in rat hippocampus. Neuroendocrinology. 1992;55:344–350. doi: 10.1159/000126135. [DOI] [PubMed] [Google Scholar]
- Joels M., Hesen W., de Kloet E.R. Mineralocorticoid hormones suppress serotonin-induced hyperpolarization of rat hippocampal CA1 neurons. J. Neurosci. 1991;11:2288–2294. doi: 10.1523/JNEUROSCI.11-08-02288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M., Karst H., DeRijk R., de Kloet E.R. The coming out of the brain mineralocorticoid receptor. Trends Neurosci. 2008;31:1–7. doi: 10.1016/j.tins.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Joels M., Karst H., Sarabdjitsingh R.A. The stressed brain of humans and rodents. Acta Physiol. 2018;223 doi: 10.1111/apha.13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M., Sarabdjitsingh R.A., Karst H. Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacol. Rev. 2012;64:901–938. doi: 10.1124/pr.112.005892. [DOI] [PubMed] [Google Scholar]
- Johnson T.A., Paakinaho V., Kim S., Hager G.L., Presman D.M. Genome-wide binding potential and regulatory activity of the glucocorticoid receptor's monomeric and dimeric forms. Nat. Commun. 2021;12:1987. doi: 10.1038/s41467-021-22234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jover E., Matilla L., Garaikoetxea M., Fernandez-Celis A., Muntendam P., Jaisser F., Rossignol P., Lopez-Andres N. Beneficial effects of mineralocorticoid receptor pathway blockade against endothelial inflammation induced by SARS-CoV-2 spike protein. Biomedicines. 2021;9:639. doi: 10.3390/biomedicines9060639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd L.L., Schettler P.J., Brown E.S., Wolkowitz O.M., Sternberg E.M., Bender B.G., Bulloch K., Cidlowski J.A., de Kloet E.R., Fardet L., Joels M., Leung D.Y., McEwen B.S., Roozendaal B., Van Rossum E.F., Ahn J., Brown D.W., Plitt A., Singh G. Adverse consequences of glucocorticoid medication: psychological, cognitive, and behavioral effects. Am. J. Psychiatr. 2014;171:1045–1051. doi: 10.1176/appi.ajp.2014.13091264. [DOI] [PubMed] [Google Scholar]
- Juster R.P., McEwen B.S., Lupien S.J. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kalafatakis K., Russell G.M., Harmer C.J., Munafo M.R., Marchant N., Wilson A., Brooks J.C., Thai N.J., Ferguson S.G., Stevenson K., Durant C., Schmidt K., Lightman S.L. Effects of the pattern of glucocorticoid replacement on neural processing, emotional reactivity and well-being in healthy male individuals: study protocol for a randomised controlled trial. Trials. 2016;17:44. doi: 10.1186/s13063-016-1159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karssen A.M., Meijer O.C., Berry A., Sanjuan Pinol R., de Kloet E.R. Low doses of dexamethasone can produce a hypocorticosteroid state in the brain. Endocrinology. 2005;146:5587–5595. doi: 10.1210/en.2005-0501. [DOI] [PubMed] [Google Scholar]
- Karst H., Berger S., Erdmann G., Schutz G., Joels M. Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc. Natl. Acad. Sci. U. S. A. 2010;107:14449–14454. doi: 10.1073/pnas.0914381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H., Berger S., Turiault M., Tronche F., Schutz G., Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc. Natl. Acad. Sci. U. S. A. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H., Joels M. Severe stress hormone conditions cause an extended window of excitability in the mouse basolateral amygdala. Neuropharmacology. 2016;110:175–180. doi: 10.1016/j.neuropharm.2016.07.027. [DOI] [PubMed] [Google Scholar]
- Karst H., Karten Y.J., Reichardt H.M., de Kloet E.R., Schutz G., Joels M. Corticosteroid actions in hippocampus require DNA binding of glucocorticoid receptor homodimers. Nat. Neurosci. 2000;3:977–978. doi: 10.1038/79910. [DOI] [PubMed] [Google Scholar]
- Karst H., Wadman W.J., Joels M. Corticosteroid receptor-dependent modulation of calcium currents in rat hippocampal CA1 neurons. Brain Res. 1994;649:234–242. doi: 10.1016/0006-8993(94)91069-3. [DOI] [PubMed] [Google Scholar]
- Kim J.S., Han S.Y., Iremonger K.J. Stress experience and hormone feedback tune distinct components of hypothalamic CRH neuron activity. Nat. Commun. 2019;10:5696. doi: 10.1038/s41467-019-13639-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T., Mehta D., Anacker C., Rex-Haffner M., Pruessner J.C., Pariante C.M., Pace T.W., Mercer K.B., Mayberg H.S., Bradley B., Nemeroff C.B., Holsboer F., Heim C.M., Ressler K.J., Rein T., Binder E.B. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok M.D., Giltay E.J., Van der Does A.J., Geleijnse J.M., Antypa N., Penninx B.W., de Geus E.J., Willemsen G., Boomsma D.I., van Leeuwen N., Zitman F.G., de Kloet E.R., DeRijk R.H. A common and functional mineralocorticoid receptor haplotype enhances optimism and protects against depression in females. Transl. Psychiatry. 2011;1:e62. doi: 10.1038/tp.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok M.D., Vreeburg S.A., Penninx B.W., Zitman F.G., de Kloet E.R., DeRijk R.H. Common functional mineralocorticoid receptor polymorphisms modulate the cortisol awakening response: interaction with SSRIs. Psychoneuroendocrinology. 2011;36:484–494. doi: 10.1016/j.psyneuen.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Kokras N., Hodes G.E., Bangasser D.A., Dalla C. Sex differences in the hypothalamic-pituitary-adrenal axis: an obstacle to antidepressant drug development? Br. J. Pharmacol. 2019;176:4090–4106. doi: 10.1111/bph.14710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokras N., Krokida S., Varoudaki T.Z., Dalla C. Do corticosterone levels predict female depressive-like behavior in rodents? J. Neurosci. Res. 2021;99:324–331. doi: 10.1002/jnr.24686. [DOI] [PubMed] [Google Scholar]
- Koorneef L.L., van den Heuvel J.K., Kroon J., Boon M.R., t Hoen P.A.C., Hettne K.M., van de Velde N.M., Kolenbrander K.B., Streefland T.C.M., Mol I.M., Sips H.C.M., Kielbasa S.M., Mei H., Belanoff J.K., Pereira A.M., Oosterveer M.H., Hunt H., Rensen P.C.N., Meijer O.C. Selective glucocorticoid receptor modulation prevents and reverses nonalcoholic fatty liver disease in male mice. Endocrinology. 2018;159:3925–3936. doi: 10.1210/en.2018-00671. [DOI] [PubMed] [Google Scholar]
- Kroon J., Pereira A.M., Meijer O.C. Glucocorticoid sexual dimorphism in metabolism: dissecting the role of sex hormones. Trends Endocrinol. Metabol. 2020;31:357–367. doi: 10.1016/j.tem.2020.01.010. [DOI] [PubMed] [Google Scholar]
- Krozowski Z.S., Funder J.W. Renal mineralocorticoid receptors and hippocampal corticosterone-binding species have identical intrinsic steroid specificity. Proc. Natl. Acad. Sci. U. S. A. 1983;80:6056–6060. doi: 10.1073/pnas.80.19.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan P.F., Waszczuk M.A., Kotov R., Clouston S., Yang X., Singh P.K., Glenn S.T., Cortes Gomez E., Wang J., Bromet E., Luft B.J. Gene expression associated with PTSD in World Trade Center responders: an RNA sequencing study. Transl. Psychiatry. 2017;7:1297. doi: 10.1038/s41398-017-0050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka B.M., Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol. Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Landfield P.W. Prog Brain Res. Elsevier; 1987. Chapter 25 Modulation of Brain Aging Correlates by Long-Term Alterations of Adrenal Steroids and Neurally-Active Peptides; pp. 279–300. [DOI] [PubMed] [Google Scholar]
- Lee E.H., Park J.Y., Kwon H.J., Han P.L. Repeated exposure with short-term behavioral stress resolves pre-existing stress-induced depressive-like behavior in mice. Nat. Commun. 2021;12:6682. doi: 10.1038/s41467-021-26968-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesuis S.L., Brosens N., Immerzeel N., van der Loo R.J., Mitric M., Bielefeld P., Fitzsimons C.P., Lucassen P.J., Kushner S.A., van den Oever M.C., Krugers H.J. Glucocorticoids promote fear generalization by increasing the size of a dentate gyrus engram cell population. Biol. Psychiatr. 2021;90:494–504. doi: 10.1016/j.biopsych.2021.04.010. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Levine S., Huchton D.M., Wiener S.G., Rosenfeld P. Time course of the effect of maternal deprivation on the hypothalamic-pituitary-adrenal axis in the infant rat. Dev. Psychobiol. 1991;24:547–558. doi: 10.1002/dev.420240803. [DOI] [PubMed] [Google Scholar]
- Licznerski P., Duric V., Banasr M., Alavian K.N., Ota K.T., Kang H.J., Jonas E.A., Ursano R., Krystal J.H., Duman R.S., Traumatic Stress Brain Study, G Decreased SGK1 expression and function contributes to behavioral deficits induced by traumatic stress. PLoS Biol. 2015;13 doi: 10.1371/journal.pbio.1002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingg R.T., Johnson S.B., Emmons E.B., Anderson R.M., Romig-Martin S.A., Narayanan N.S., McGaugh J.L., LaLumiere R.T., Radley J.J. Bed nuclei of the stria terminalis modulate memory consolidation via glucocorticoid-dependent and -independent circuits. Proc. Natl. Acad. Sci. U. S. A. 2020;117:8104–8114. doi: 10.1073/pnas.1915501117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C., Cichon J.M., Jeanneteau F., Jia Z., Chao M.V., Gan W.B. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat. Neurosci. 2013;16:698–705. doi: 10.1038/nn.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C., Gan W.B. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16074–16079. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Diorio J., Tannenbaum B., Caldji C., Francis D., Freedman A., Sharma S., Pearson D., Plotsky P.M., Meaney M.J. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Liu W., Wang J., Sauter N.K., Pearce D. Steroid receptor heterodimerization demonstrated in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 1995;92:12480–12484. doi: 10.1073/pnas.92.26.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue M.W., Smith A.K., Baldwin C., Wolf E.J., Guffanti G., Ratanatharathorn A., Stone A., Schichman S.A., Humphries D., Binder E.B., Arloth J., Menke A., Uddin M., Wildman D., Galea S., Aiello A.E., Koenen K.C., Miller M.W. An analysis of gene expression in PTSD implicates genes involved in the glucocorticoid receptor pathway and neural responses to stress. Psychoneuroendocrinology. 2015;57:1–13. doi: 10.1016/j.psyneuen.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue M.W., Zhou Z., Morrison F.G., Wolf E.J., Daskalakis N.P., Chatzinakos C., Georgiadis F., Labadorf A.T., Girgenti M.J., Young K.A., Williamson D.E., Zhao X., Grenier J.G., Traumatic Stress Brain Research, G. Huber B.R., Miller M.W. Gene expression in the dorsolateral and ventromedial prefrontal cortices implicates immune-related gene networks in PTSD. Neurobiol Stress. 2021;15:100398. doi: 10.1016/j.ynstr.2021.100398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonard D.M., O'Malley B.W. Nuclear receptor coregulators: modulators of pathology and therapeutic targets. Nat. Rev. Endocrinol. 2012;8:598–604. doi: 10.1038/nrendo.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V., Gomez J., Beck K., Bowman R. Sex differences in chronic stress effects on cognition in rodents. Pharmacol. Biochem. Behav. 2017;152:13–19. doi: 10.1016/j.pbb.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maccari S., Polese D., Reynaert M.L., Amici T., Morley-Fletcher S., Fagioli F. Early-life experiences and the development of adult diseases with a focus on mental illness: the Human Birth Theory. Neuroscience. 2017;342:232–251. doi: 10.1016/j.neuroscience.2016.05.042. [DOI] [PubMed] [Google Scholar]
- Maggio N., Segal M. Differential modulation of long-term depression by acute stress in the rat dorsal and ventral hippocampus. J. Neurosci. 2009;29(27):8633–8638. doi: 10.1523/JNEUROSCI.1901-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfouz A., Lelieveldt B.P., Grefhorst A., van Weert L.T., Mol I.M., Sips H.C., van den Heuvel J.K., Datson N.A., Visser J.A., Reinders M.J., Meijer O.C. Genome-wide coexpression of steroid receptors in the mouse brain: identifying signaling pathways and functionally coordinated regions. Proc. Natl. Acad. Sci. U. S. A. 2016;113:2738–2743. doi: 10.1073/pnas.1520376113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marissal-Arvy N., Lombes M., Petterson J., Moisan M.P., Mormede P. Gain of function mutation in the mineralocorticoid receptor of the Brown Norway rat. J. Biol. Chem. 2004;279:39232–39239. doi: 10.1074/jbc.M407436200. [DOI] [PubMed] [Google Scholar]
- Marrocco J., McEwen B.S. Sex in the brain: hormones and sex differences. Dialogues Clin. Neurosci. 2016;18:373–383. doi: 10.31887/DCNS.2016.18.4/jmarrocco. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrocco J., Petty G.H., Rios M.B., Gray J.D., Kogan J.F., Waters E.M., Schmidt E.F., Lee F.S., McEwen B.S. A sexually dimorphic pre-stressed translational signature in CA3 pyramidal neurons of BDNF Val66Met mice. Nat. Commun. 2017;8:808. doi: 10.1038/s41467-017-01014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann K.E., Lustberg D.J., Shaughnessy E.K., Carstens K.E., Farris S., Alexander G.M., Radzicki D., Zhao M., Dudek S.M. Novel role for mineralocorticoid receptors in control of a neuronal phenotype. Mol. Psychiatr. 2021;26:350–364. doi: 10.1038/s41380-019-0598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M.M. A new view of sexual differentiation of mammalian brain. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020;206:369–378. doi: 10.1007/s00359-019-01376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M.M., Nugent B.M., Lenz K.M. Neuroimmunology and neuroepigenetics in the establishment of sex differences in the brain. Nat. Rev. Neurosci. 2017;18:471–484. doi: 10.1038/nrn.2017.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Neurobiological and systemic effects of chronic stress. Chronic Stress. 2017;1 doi: 10.1177/2470547017692328. Thousand Oaks) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Hormones and behavior and the integration of brain-body science. Horm. Behav. 2020;119:104619. doi: 10.1016/j.yhbeh.2019.104619. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. The untapped power of allostasis promoted by healthy lifestyles. World Psychiatr. 2020;19:57–58. doi: 10.1002/wps.20720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Akil H. Revisiting the stress concept: implications for affective disorders. J. Neurosci. 2020;40:12–21. doi: 10.1523/JNEUROSCI.0733-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., De Kloet E.R., Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol. Rev. 1986;66:1121–1188. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., de Kloet R., Wallach G. Interactions in vivo and in vitro of corticoids and progesterone with cell nuclei and soluble macromolecules from rat brain regions and pituitary. Brain Res. 1976;105:129–136. doi: 10.1016/0006-8993(76)90928-8. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Nasca C., Gray J.D. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Weiss J.M., Schwartz L.S. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220:911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Wingfield J.C. What is in a name? Integrating homeostasis, allostasis and stress. Horm. Behav. 2010;57:105–111. doi: 10.1016/j.yhbeh.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen C.A., McEwen B.S. Social structure, adversity, toxic stress, and intergenerational poverty: an early childhood model. Annu. Rev. Sociol. 2017;43:445–472. [Google Scholar]
- McGowan P.O., Sasaki A., D’alessio A.C., Dymov S., Labonté B., Szyf M., Turecki G., Meaney M.J. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKlveen J.M., Moloney R.D., Scheimann J.R., Myers B., Herman J.P. Braking" the prefrontal cortex: the role of glucocorticoids and interneurons in stress adaptation and pathology. Biol. Psychiatr. 2019;86:669–681. doi: 10.1016/j.biopsych.2019.04.032. [DOI] [PubMed] [Google Scholar]
- Mehta D., Klengel T., Conneely K.N., Smith A.K., Altmann A., Pace T.W., Rex-Haffner M., Loeschner A., Gonik M., Mercer K.B., Bradley B., Muller-Myhsok B., Ressler K.J., Binder E.B. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proc. Natl. Acad. Sci. U. S. A. 2013;110:8302–8307. doi: 10.1073/pnas.1217750110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer O.C. Coregulator proteins and corticosteroid action in the brain. J. Neuroendocrinol. 2002;14:499–505. doi: 10.1046/j.1365-2826.2002.00795.x. [DOI] [PubMed] [Google Scholar]
- Meijer O.C., Cole T.J., Schmid W., Schutz G., Joels M., De Kloet E.R. Regulation of hippocampal 5-HT1A receptor mRNA and binding in transgenic mice with a targeted disruption of the glucocorticoid receptor. Brain Res Mol Brain Res. 1997;46:290–296. doi: 10.1016/s0169-328x(97)00002-8. [DOI] [PubMed] [Google Scholar]
- Meijer O.C., de Lange E.C., Breimer D.D., de Boer A.G., Workel J.O., de Kloet E.R. Penetration of dexamethasone into brain glucocorticoid targets is enhanced in mdr1A P-glycoprotein knockout mice. Endocrinology. 1998;139:1789–1793. doi: 10.1210/endo.139.4.5917. [DOI] [PubMed] [Google Scholar]
- Meijer O.C., Kalkhoven E., van der Laan S., Steenbergen P.J., Houtman S.H., Dijkmans T.F., Pearce D., de Kloet E.R. Steroid receptor coactivator-1 splice variants differentially affect corticosteroid receptor signaling. Endocrinology. 2005;146:1438–1448. doi: 10.1210/en.2004-0411. [DOI] [PubMed] [Google Scholar]
- Mifsud K.R., Kennedy C.L.M., Salatino S., Sharma E., Price E.M., Haque S.N., Gialeli A., Goss H.M., Panchenko P.E., Broxholme J., Engledow S., Lockstone H., Cordero Llana O., Reul J. Distinct regulation of hippocampal neuroplasticity and ciliary genes by corticosteroid receptors. Nat. Commun. 2021;12:4737. doi: 10.1038/s41467-021-24967-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifsud K.R., Reul J.M. Acute stress enhances heterodimerization and binding of corticosteroid receptors at glucocorticoid target genes in the hippocampus. Proc. Natl. Acad. Sci. U. S. A. 2016;113:11336–11341. doi: 10.1073/pnas.1605246113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moguilewsky M., Raynaud J.P. Evidence for a specific mineralocorticoid receptor in rat pituitary and brain. J. Steroid Biochem. 1980;12:309–314. doi: 10.1016/0022-4731(80)90285-x. [DOI] [PubMed] [Google Scholar]
- Moisan M.-P. Sexual dimorphism in glucocorticoid stress response. Int. J. Mol. Sci. 2021;22:3139. doi: 10.3390/ijms22063139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Shionoya K., Jakubs K., Sullivan R.M. Early-life stress disrupts attachment learning: the role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. J. Neurosci. 2009;29:15745–15755. doi: 10.1523/JNEUROSCI.4106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Sullivan R.M. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat. Neurosci. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Wilson D.A., Levine S., Sullivan R.M. Dual circuitry for odor-shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. J. Neurosci. 2006;26:6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison F.G., Miller M.W., Wolf E.J., Logue M.W., Maniates H., Kwasnik D., Cherry J.D., Svirsky S., Restaino A., Hildebrandt A., Aytan N., Stein T.D., Alvarez V.E., McKee A.C., Traumatic Stress Brain Study, G. Huber B.R. Reduced interleukin 1A gene expression in the dorsolateral prefrontal cortex of individuals with PTSD and depression. Neurosci. Lett. 2019;692:204–209. doi: 10.1016/j.neulet.2018.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munck A., Guyre P.M., Holbrook N.J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr. Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Murck H., Schlageter L., Schneider A., Adolf C., Heinrich D., Quinkler M., Beuschlein F., Reincke M., Kunzel H. The potential pathophysiological role of aldosterone and the mineralocorticoid receptor in anxiety and depression - lessons from primary aldosteronism. J. Psychiatr. Res. 2020;130:82–88. doi: 10.1016/j.jpsychires.2020.07.006. [DOI] [PubMed] [Google Scholar]
- Nawreen N., Cotella E.M., Morano R., Mahbod P., Dalal K.S., Fitzgerald M., Martelle S., Packard B.A., Franco-Villanueva A., Moloney R.D., Herman J.P. Chemogenetic inhibition of infralimbic prefrontal cortex GABAergic parvalbumin interneurons attenuates the impact of chronic stress in male mice. eNeuro. 2020;7 doi: 10.1523/ENEURO.0423-19.2020. ENEURO.0423-0419.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylan T.C., Sun B., Rempel H., Ross J., Lenoci M., O'Donovan A., Pulliam L. Suppressed monocyte gene expression profile in men versus women with PTSD. Brain Behav. Immun. 2011;25:524–531. doi: 10.1016/j.bbi.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley R.H., Whirledge S.D., Petrillo M.G., Riddick N.V., Xu X., Moy S.S., Cidlowski J.A. Combinatorial actions of glucocorticoid and mineralocorticoid stress hormone receptors are required for preventing neurodegeneration of the mouse hippocampus. Neurobiol Stress. 2021;15:100369. doi: 10.1016/j.ynstr.2021.100369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obleser J., Kreitewolf J., Vielhauer R., Lindner F., David C., Oster H., Tune S. Circadian fluctuations in glucocorticoid level predict perceptual discrimination sensitivity. iScience. 2021;24:102345. doi: 10.1016/j.isci.2021.102345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitz M. Behavioral and neuroendocrine responses controlled by the concerted action of central mineralocorticoid (MRS) and glucocorticoid receptors (GRS) Psychoneuroendocrinology. 1997;22:S87–S93. doi: 10.1016/s0306-4530(97)00020-6. [DOI] [PubMed] [Google Scholar]
- Oitzl M.S., de Kloet E.R. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behav. Neurosci. 1992;106:62–71. doi: 10.1037//0735-7044.106.1.62. [DOI] [PubMed] [Google Scholar]
- Oitzl M.S., Reichardt H.M., Joels M., de Kloet E.R. Point mutation in the mouse glucocorticoid receptor preventing DNA binding impairs spatial memory. Proc. Natl. Acad. Sci. U. S. A. 2001;98:12790–12795. doi: 10.1073/pnas.231313998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl M.S., van Haarst A.D., Sutanto W., Ron de Kloet E. Corticosterone, brain mineralocorticoid receptors (MRS) and the activity of the hypothalamic-pituitary-adrenal (hpa) axis: the Lewis rat as an example of increased central MR capacity and a hyporesponsive HPA axis. Psychoneuroendocrinology. 1995;20:655–675. doi: 10.1016/0306-4530(95)00003-7. [DOI] [PubMed] [Google Scholar]
- Oitzl M.S., Workel J.O., Fluttert M., Frosch F., De Kloet E.R. Maternal deprivation affects behaviour from youth to senescence: amplification of individual differences in spatial learning and memory in senescent Brown Norway rats. Eur. J. Neurosci. 2000;12:3771–3780. doi: 10.1046/j.1460-9568.2000.00231.x. [DOI] [PubMed] [Google Scholar]
- Olijslagers J.E., de Kloet E.R., Elgersma Y., van Woerden G.M., Joels M., Karst H. Rapid changes in hippocampal CA1 pyramidal cell function via pre- as well as postsynaptic membrane mineralocorticoid receptors. Eur. J. Neurosci. 2008;27:2542–2550. doi: 10.1111/j.1460-9568.2008.06220.x. [DOI] [PubMed] [Google Scholar]
- Pagani J.H., Zhao M., Cui Z., Avram S.K., Caruana D.A., Dudek S.M., Young W.S. Role of the vasopressin 1b receptor in rodent aggressive behavior and synaptic plasticity in hippocampal area CA2. Mol. Psychiatr. 2015;20:490–499. doi: 10.1038/mp.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papilloud A., Veenit V., Tzanoulinou S., Riccio O., Zanoletti O., Guillot de Suduiraut I., Grosse J., Sandi C. Peripubertal stress-induced heightened aggression: modulation of the glucocorticoid receptor in the central amygdala and normalization by mifepristone treatment. Neuropsychopharmacology. 2019;44:674–682. doi: 10.1038/s41386-018-0110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papilloud A., Weger M., Bacq A., Zalachoras I., Hollis F., Larrieu T., Battivelli D., Grosse J., Zanoletti O., Parnaudeau S., Tronche F., Sandi C. The glucocorticoid receptor in the nucleus accumbens plays a crucial role in social rank attainment in rodents. Psychoneuroendocrinology. 2020;112:104538. doi: 10.1016/j.psyneuen.2019.104538. [DOI] [PubMed] [Google Scholar]
- Picard M., Juster R.P., Sloan R.P., McEwen B.S. Mitochondrial nexus to allostatic load biomarkers. Psychosom. Med. 2017;79:114–117. doi: 10.1097/PSY.0000000000000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plihal W., Krug R., Pietrowsky R., Fehm H.L., Born J. Corticosteroid receptor mediated effects on mood in humans. Psychoneuroendocrinology. 1996;21:515–523. doi: 10.1016/s0306-4530(96)00011-x. [DOI] [PubMed] [Google Scholar]
- Polman J.A., de Kloet E.R., Datson N.A. Two populations of glucocorticoid receptor-binding sites in the male rat hippocampal genome. Endocrinology. 2013;154:1832–1844. doi: 10.1210/en.2012-2187. [DOI] [PubMed] [Google Scholar]
- Polman J.A., Hunter R.G., Speksnijder N., van den Oever J.M., Korobko O.B., McEwen B.S., de Kloet E.R., Datson N.A. Glucocorticoids modulate the mTOR pathway in the hippocampus: differential effects depending on stress history. Endocrinology. 2012;153:4317–4327. doi: 10.1210/en.2012-1255. [DOI] [PubMed] [Google Scholar]
- Pooley J.R., Flynn B.P., Grontved L., Baek S., Guertin M.J., Kershaw Y.M., Birnie M.T., Pellatt A., Rivers C.A., Schiltz R.L., Hager G.L., Lightman S.L., Conway-Campbell B.L. Genome-wide identification of basic helix-loop-helix and NF-1 motifs underlying GR binding sites in male rat Hippocampus. Endocrinology. 2017;158:1486–1501. doi: 10.1210/en.2016-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presman D.M., Hager G.L. More than meets the dimer: what is the quaternary structure of the glucocorticoid receptor? Transcription. 2017;8:32–39. doi: 10.1080/21541264.2016.1249045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing L., Gao C., Ji A., Lu X., Zhou L., Nie S. Association of mineralocorticoid receptor gene (NR3C2) hypermethylation in adult males with aggressive behavior. Behav. Brain Res. 2021;398:112980. doi: 10.1016/j.bbr.2020.112980. [DOI] [PubMed] [Google Scholar]
- Quinn M., Ramamoorthy S., Cidlowski J.A. Sexually dimorphic actions of glucocorticoids: beyond chromosomes and sex hormones. Ann. N. Y. Acad. Sci. 2014;1317:1–6. doi: 10.1111/nyas.12425. [DOI] [PubMed] [Google Scholar]
- Radley J.J., Johnson S.B. Anteroventral bed nuclei of the stria terminalis neurocircuitry: towards an integration of HPA axis modulation with coping behaviors - curt Richter Award Paper 2017. Psychoneuroendocrinology. 2018;89:239–249. doi: 10.1016/j.psyneuen.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratka A., Sutanto W., Bloemers M., de Kloet E.R. On the role of brain mineralocorticoid (type I) and glucocorticoid (type II) receptors in neuroendocrine regulation. Neuroendocrinology. 1989;50:117–123. doi: 10.1159/000125210. [DOI] [PubMed] [Google Scholar]
- Reichardt H.M., Kaestner K.H., Tuckermann J., Kretz O., Wessely O., Bock R., Gass P., Schmid W., Herrlich P., Angel P., Schütz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- Reul J.M., Collins A., Saliba R.S., Mifsud K.R., Carter S.D., Gutierrez-Mecinas M., Qian X., Linthorst A.C. Glucocorticoids, epigenetic control and stress resilience. Neurobiol Stress. 2015;1:44–59. doi: 10.1016/j.ynstr.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul J.M., de Kloet E.R. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Reul J.M., de Kloet E.R. Anatomical resolution of two types of corticosterone receptor sites in rat brain with in vitro autoradiography and computerized image analysis. J. Steroid Biochem. 1986;24:269–272. doi: 10.1016/0022-4731(86)90063-4. [DOI] [PubMed] [Google Scholar]
- Reul J.M., Tonnaer J.A., De Kloet E.R. Neurotrophic ACTH analogue promotes plasticity of type I corticosteroid receptor in brain of senescent male rats. Neurobiol. Aging. 1988;9:253–260. doi: 10.1016/s0197-4580(88)80062-9. [DOI] [PubMed] [Google Scholar]
- Reul J.M., van den Bosch F.R., de Kloet E.R. Relative occupation of type-I and type-II corticosteroid receptors in rat brain following stress and dexamethasone treatment: functional implications. J. Endocrinol. 1987;115:459–467. doi: 10.1677/joe.0.1150459. [DOI] [PubMed] [Google Scholar]
- Rivers C.A., Rogers M.F., Stubbs F.E., Conway-Campbell B.L., Lightman S.L., Pooley J.R. Glucocorticoid receptor-tethered mineralocorticoid receptors increase glucocorticoid-induced transcriptional responses. Endocrinology. 2019;160:1044–1056. doi: 10.1210/en.2018-00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson A.C., Leckie C.M., Seckl J.R., Holmes M.C. 11β-Hydroxysteroid dehydrogenase type 2 in the postnatal and adult rat brain. Mol. Brain Res. 1998;61:1–10. doi: 10.1016/s0169-328x(98)00161-2. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., McEwen B.S., Chattarji S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., McGaugh J.L. Memory modulation. Behav. Neurosci. 2011;125:797–824. doi: 10.1037/a0026187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B., McReynolds J.R., Van der Zee E.A., Lee S., McGaugh J.L., McIntyre C.K. Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. J. Neurosci. 2009;29:14299–14308. doi: 10.1523/JNEUROSCI.3626-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe N., Steffen J., Penz M., Kirschbaum C., Walther A. Examination of peripheral basal and reactive cortisol levels in major depressive disorder and the burnout syndrome: a systematic review. Neurosci. Biobehav. Rev. 2020;114:232–270. doi: 10.1016/j.neubiorev.2020.02.024. [DOI] [PubMed] [Google Scholar]
- Ruzzo E.K., Perez-Cano L., Jung J.Y., Wang L.K., Kashef-Haghighi D., Hartl C., Singh C., Xu J., Hoekstra J.N., Leventhal O., Leppa V.M., Gandal M.J., Paskov K., Stockham N., Polioudakis D., Lowe J.K., Prober D.A., Geschwind D.H., Wall D.P. Inherited and de novo genetic risk for autism impacts shared networks. Cell. 2019;178:850–866 e826. doi: 10.1016/j.cell.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rysz S., Al-Saadi J., Sjostrom A., Farm M., Campoccia Jalde F., Platten M., Eriksson H., Klein M., Vargas-Paris R., Nyren S., Abdula G., Ouellette R., Granberg T., Jonsson Fagerlund M., Lundberg J. COVID-19 pathophysiology may be driven by an imbalance in the renin-angiotensin-aldosterone system. Nat. Commun. 2021;12:2417. doi: 10.1038/s41467-021-22713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatcioglu F., Claret F.X., Karin M. Negative transcriptional regulation by nuclear receptors. Semin. Cancer Biol. 1994;5:347–359. [PubMed] [Google Scholar]
- Sandi C., Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat. Rev. Neurosci. 2015;16:290–304. doi: 10.1038/nrn3918. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M., Krey L.C., McEwen B.S. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr. Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Schinkel A.H., Wagenaar E., van Deemter L., Mol C.A., Borst P. Absence of the mdr1a P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J. Clin. Invest. 1995;96:1698–1705. doi: 10.1172/JCI118214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schur R.R., Boks M.P., Rutten B.P.F., Daskalakis N.P., de Nijs L., van Zuiden M., Kavelaars A., Heijnen C.J., Joels M., Kahn R.S., Geuze E., Vermetten E., Vinkers C.H. Longitudinal changes in glucocorticoid receptor exon 1F methylation and psychopathology after military deployment. Transl. Psychiatry. 2017;7:e1181. doi: 10.1038/tp.2017.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L., Schachinger H., de Kloet E.R., Oitzl M.S. Corticosteroids operate as a switch between memory systems. J. Cognit. Neurosci. 2010;22:1362–1372. doi: 10.1162/jocn.2009.21278. [DOI] [PubMed] [Google Scholar]
- Schwabe L., Schachinger H., de Kloet E.R., Oitzl M.S. Stress impairs spatial but not early stimulus-response learning. Behav. Brain Res. 2010;213:50–55. doi: 10.1016/j.bbr.2010.04.029. [DOI] [PubMed] [Google Scholar]
- Schwabe L., Tegenthoff M., Hoffken O., Wolf O.T. Mineralocorticoid receptor blockade prevents stress-induced modulation of multiple memory systems in the human brain. Biol. Psychiatr. 2013;74:801–808. doi: 10.1016/j.biopsych.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Sedykh S.E., Prinz V.V., Buneva V.N., Nevinsky G.A. Bispecific antibodies: design, therapy, perspectives. Drug Des. Dev. Ther. 2018;12:195–208. doi: 10.2147/DDDT.S151282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segman R.H., Shefi N., Goltser-Dubner T., Friedman N., Kaminski N., Shalev A.Y. Peripheral blood mononuclear cell gene expression profiles identify emergent post-traumatic stress disorder among trauma survivors. Mol. Psychiatr. 2005;10:500–513. doi: 10.1038/sj.mp.4001636. 425. [DOI] [PubMed] [Google Scholar]
- Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138 doi: 10.1176/jnp.10.2.230a. 32-32. [DOI] [PubMed] [Google Scholar]
- Selye H. Stress and the general adaptation syndrome. Br. Med. J. 1950;1:1383–1392. doi: 10.1136/bmj.1.4667.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter E., Fink M. Oxford University Press; 2010. Endocrine Psychiatry: Solving the Riddle of Melancholia. [Google Scholar]
- Singh T., Poterba T., Curtis D., Akil H., Al Eissa M., Barchas J.D., Bass N., Bigdeli T.B., Breen G., Bromet E.J., Buckley P.F. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature. 2022 doi: 10.1038/s41586-022-04556-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter R.S., Valiquette G., Abrams G.M., Ronk E.C., Sollas A.L., Paul L.A., Neubort S. Selective loss of hippocampal granule cells in the mature rat brain after adrenalectomy. Science. 1989;243:535–538. doi: 10.1126/science.2911756. [DOI] [PubMed] [Google Scholar]
- Soe N.N., Wen D.J., Poh J.S., Chong Y.S., Broekman B.F., Chen H., Shek L.P., Tan K.H., Gluckman P.D., Fortier M.V., Meaney M.J., Qiu A. Perinatal maternal depressive symptoms alter amygdala functional connectivity in girls. Hum. Brain Mapp. 2018;39:680–690. doi: 10.1002/hbm.23873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza R.R., Dal Bo S., de Kloet E.R., Oitzl M.S., Carobrez A.P. Paradoxical mineralocorticoid receptor-mediated effect in fear memory encoding and expression of rats submitted to an olfactory fear conditioning task. Neuropharmacology. 2014;79:201–211. doi: 10.1016/j.neuropharm.2013.11.017. [DOI] [PubMed] [Google Scholar]
- Stein M.B., Levey D.F., Cheng Z., Wendt F.R., Harrington K., Pathak G.A., Cho K., Quaden R., Radhakrishnan K., Girgenti M.J., Ho Y.A., Posner D., Aslan M., Duman R.S., Zhao H., Department of Veterans Affairs Cooperative Studies, P. Program V.A.M.V., Polimanti R., Concato J., Gelernter J. Genome-wide association analyses of post-traumatic stress disorder and its symptom subdomains in the Million Veteran Program. Nat. Genet. 2021;53:174–184. doi: 10.1038/s41588-020-00767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M.A., Mahon P.B., McCaul M.E., Wand G.S. Hypothalamic-pituitary-adrenal axis response to acute psychosocial stress: effects of biological sex and circulating sex hormones. Psychoneuroendocrinology. 2016;66:47–55. doi: 10.1016/j.psyneuen.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y.A., Wu J., Zhang L., Zhang Q., Su D.M., He P., Wang B.D., Li H., Webster M.J., Traumatic Stress Brain Study, G. Rennert O.M., Ursano R.J. Dysregulated mitochondrial genes and networks with drug targets in postmortem brain of patients with posttraumatic stress disorder (PTSD) revealed by human mitochondria-focused cDNA microarrays. Int. J. Biol. Sci. 2008;4:223–235. doi: 10.7150/ijbs.4.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suderman M., McGowan P.O., Sasaki A., Huang T.C., Hallett M.T., Meaney M.J., Turecki G., Szyf M. Conserved epigenetic sensitivity to early life experience in the rat and human hippocampus. Proc. Natl. Acad. Sci. U. S. A. 2012;109(Suppl. 2):17266–17272. doi: 10.1073/pnas.1121260109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tausk M. 1952. Das Hormon: Hat die Nebennierrinde tatsächlich eine Verteidigungsfunktion? Organon International BV. Oss The Netherlands. [Google Scholar]
- Taylor S.E., Klein L.C., Lewis B.P., Gruenewald T.L., Gurung R.A., Updegraff J.A. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol. Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Tejos-Bravo M., Oakley R.H., Whirledge S.D., Corrales W.A., Silva J.P., Garcia-Rojo G., Toledo J., Sanchez W., Roman-Albasini L., Aliaga E., Aguayo F., Olave F., Maracaja-Coutinho V., Cidlowski J.A., Fiedler J.L. Deletion of hippocampal Glucocorticoid receptors unveils sex-biased microRNA expression and neuronal morphology alterations in mice. Neurobiol Stress. 2021;14:100306. doi: 10.1016/j.ynstr.2021.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Horst J.P., de Kloet E.R., Schachinger H., Oitzl M.S. Relevance of stress and female sex hormones for emotion and cognition. Cell. Mol. Neurobiol. 2012;32:725–735. doi: 10.1007/s10571-011-9774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Horst J.P., Kentrop J., Arp M., Hubens C.J., de Kloet E.R., Oitzl M.S. Spatial learning of female mice: a role of the mineralocorticoid receptor during stress and the estrous cycle. Front. Behav. Neurosci. 2013;7:56. doi: 10.3389/fnbeh.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Horst J.P., Kentrop J., de Kloet E.R., Oitzl M.S. Stress and estrous cycle affect strategy but not performance of female C57BL/6J mice. Behav. Brain Res. 2013;241:92–95. doi: 10.1016/j.bbr.2012.11.040. [DOI] [PubMed] [Google Scholar]
- Ter Horst J.P., van der Mark M., Kentrop J., Arp M., van der Veen R., de Kloet E.R., Oitzl M.S. Deletion of the forebrain mineralocorticoid receptor impairs social discrimination and decision-making in male, but not in female mice. Front. Behav. Neurosci. 2014;8:26. doi: 10.3389/fnbeh.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylee D.S., Chandler S.D., Nievergelt C.M., Liu X., Pazol J., Woelk C.H., Lohr J.B., Kremen W.S., Baker D.G., Glatt S.J., Tsuang M.T., Marine Resiliency Study I. Blood-based gene-expression biomarkers of post-traumatic stress disorder among deployed marines: a pilot study. Psychoneuroendocrinology. 2015;51:472–494. doi: 10.1016/j.psyneuen.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanoulinou S., Gantelet E., Sandi C., Marquez C. Programming effects of peripubertal stress on spatial learning. Neurobiol Stress. 2020;13:100282. doi: 10.1016/j.ynstr.2020.100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai Y.M., Herman J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg D.T.W.M., de Kloet E.R., de Jong W. Role of central corticosteroid receptors in cardiovascular control. Eur. J. Pharmacol. 1990;183:843. [Google Scholar]
- van Haarst A.D., Oitzl M.S., de Kloet E.R. Facilitation of feedback inhibition through blockade of glucocorticoid receptors in the hippocampus. Neurochem. Res. 1997;22:1323–1328. doi: 10.1023/a:1022010904600. [DOI] [PubMed] [Google Scholar]
- van Leeuwen N., Bellingrath S., de Kloet E.R., Zitman F.G., DeRijk R.H., Kudielka B.M., Wust S. Human mineralocorticoid receptor (MR) gene haplotypes modulate MR expression and transactivation: implication for the stress response. Psychoneuroendocrinology. 2011;36:699–709. doi: 10.1016/j.psyneuen.2010.10.003. [DOI] [PubMed] [Google Scholar]
- van Oers H.J., de Kloet E.R., Whelan T., Levine S. Maternal deprivation effect on the infant's neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. J. Neurosci. 1998;18:10171–10179. doi: 10.1523/JNEUROSCI.18-23-10171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weert L., Buurstede J.C., Mahfouz A., Braakhuis P.S.M., Polman J.A.E., Sips H.C.M., Roozendaal B., Balog J., de Kloet E.R., Datson N.A., Meijer O.C. NeuroD factors discriminate mineralocorticoid from glucocorticoid receptor DNA binding in the male rat brain. Endocrinology. 2017;158:1511–1522. doi: 10.1210/en.2016-1422. [DOI] [PubMed] [Google Scholar]
- van Weert L., Buurstede J.C., Sips H.C.M., Vettorazzi S., Mol I.M., Hartmann J., Prekovic S., Zwart W., Schmidt M.V., Roozendaal B., Tuckermann J.P., Sarabdjitsingh R.A., Meijer O.C. Identification of mineralocorticoid receptor target genes in the mouse hippocampus. J. Neuroendocrinol. 2019;31 doi: 10.1111/jne.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van West D., Del-Favero J., Deboutte D., Van Broeckhoven C., Claes S. Associations between common arginine vasopressin 1b receptor and glucocorticoid receptor gene variants and HPA axis responses to psychosocial stress in a child psychiatric population. Psychiatr. Res. 2010;179:64–68. doi: 10.1016/j.psychres.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Veenema A.H., Meijer O.C., de Kloet E.R., Koolhaas J.M., Bohus B.G. Differences in basal and stress-induced HPA regulation of wild house mice selected for high and low aggression. Horm. Behav. 2003;43:197–204. doi: 10.1016/s0018-506x(02)00013-2. [DOI] [PubMed] [Google Scholar]
- Veenema A.H., Sijtsma B., Koolhaas J.M., de Kloet E.R. The stress response to sensory contact in mice: genotype effect of the stimulus animal. Psychoneuroendocrinology. 2005;30:550–557. doi: 10.1016/j.psyneuen.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Veldhuis H.D., Van Koppen C., Van Ittersum M., De Kloet E.R. Specificity of the adrenal steroid receptor system in rat hippocampus. Endocrinology. 1982;110:2044–2051. doi: 10.1210/endo-110-6-2044. [DOI] [PubMed] [Google Scholar]
- Vendruscolo L.F., Estey D., Goodell V., Macshane L.G., Logrip M.L., Schlosburg J.E., McGinn M.A., Zamora-Martinez E.R., Belanoff J.K., Hunt H.J., Sanna P.P., George O., Koob G.F., Edwards S., Mason B.J. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J. Clin. Invest. 2015;125:3193–3197. doi: 10.1172/JCI79828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viho E.M.G., Buurstede J.C., Berkhout J.B., Mahfouz A., Meijer O.C. Cell type specificity of glucocorticoid signaling in the adult mouse hippocampus. J. Neuroendocrinol. 2022;34 doi: 10.1111/jne.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viho E.M.G., Buurstede J.C., Mahfouz A., Koorneef L.L., van Weert L., Houtman R., Hunt H.J., Kroon J., Meijer O.C. Corticosteroid action in the brain: the potential of selective receptor modulation. Neuroendocrinology. 2019;109:266–276. doi: 10.1159/000499659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkers C.H., Geuze E., van Rooij S.J.H., Kennis M., Schur R.R., Nispeling D.M., Smith A.K., Nievergelt C.M., Uddin M., Rutten B.P.F., Vermetten E., Boks M.P. Successful treatment of post-traumatic stress disorder reverses DNA methylation marks. Mol. Psychiatr. 2021;26:1264–1271. doi: 10.1038/s41380-019-0549-3. [DOI] [PubMed] [Google Scholar]
- Vinkers C.H., Joels M., Milaneschi Y., Gerritsen L., Kahn R.S., Penninx B.W., Boks M.P. Mineralocorticoid receptor haplotypes sex-dependently moderate depression susceptibility following childhood maltreatment. Psychoneuroendocrinology. 2015;54:90–102. doi: 10.1016/j.psyneuen.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Vinkers C.H., Joels M., Milaneschi Y., Kahn R.S., Penninx B.W., Boks M.P. Stress exposure across the life span cumulatively increases depression risk and is moderated by neuroticism. Depress. Anxiety. 2014;31:737–745. doi: 10.1002/da.22262. [DOI] [PubMed] [Google Scholar]
- Violaris I.G., Kalafatakis K., Zavala E., Tsoulos I.G., Lampros T., Lightman S.L., Tsipouras M.G., Giannakeas N., Tzallas A., Russell G.M. Modelling hydrocortisone pharmacokinetics on a subcutaneous pulsatile infusion replacement strategy in patients with adrenocortical insufficiency. Pharmaceutics. 2021;13 doi: 10.3390/pharmaceutics13060769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warris L.T., van den Heuvel-Eibrink M.M., Aarsen F.K., Pluijm S.M., Bierings M.B., van den Bos C., Zwaan C.M., Thygesen H.H., Tissing W.J., Veening M.A., Pieters R., van den Akker E.L. Hydrocortisone as an intervention for dexamethasone-induced adverse effects in pediatric patients with acute lymphoblastic leukemia: results of a double-blind, randomized controlled trial. J. Clin. Oncol. 2016;34:2287–2293. doi: 10.1200/JCO.2015.66.0761. [DOI] [PubMed] [Google Scholar]
- Weaver I.C., Cervoni N., Champagne F.A., D’Alessio A.C., Sharma S., Seckl J.R., Dymov S., Szyf M., Meaney M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wellman C.L., Bollinger J.L., Moench K.M. Effects of stress on the structure and function of the medial prefrontal cortex: insights from animal models. Int. Rev. Neurobiol. 2020;150:129–153. doi: 10.1016/bs.irn.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley J.F., Gruenewald T.L., Karlamangla A.S., Seeman T.E. Modeling multisystem physiological dysregulation. Psychosom. Med. 2016;78:290–301. doi: 10.1097/PSY.0000000000000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz L., Reuter M., Wacker J., Felten A., Schwabe L. A haplotype Associated with enhanced mineralocorticoid receptor expression facilitates the stress-induced shift from. Cognitive" to "Habit" Learning. eNeuro. 2017;4 doi: 10.1523/ENEURO.0359-17.2017. ENEURO.0359-0317.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley C.S., Gould E., Sakai R.R., Spencer R.L., McEwen B.S. Effects of aldosterone or RU28362 treatment on adrenalectomy-induced cell death in the dentate gyrus of the adult rat. Brain Res. 1991;554:312–315. doi: 10.1016/0006-8993(91)90207-c. [DOI] [PubMed] [Google Scholar]
- Wust S., Van Rossum E.F., Federenko I.S., Koper J.W., Kumsta R., Hellhammer D.H. Common polymorphisms in the glucocorticoid receptor gene are associated with adrenocortical responses to psychosocial stress. J. Clin. Endocrinol. Metab. 2004;89:565–573. doi: 10.1210/jc.2003-031148. [DOI] [PubMed] [Google Scholar]
- Yang R., Xu C., Bierer L.M., Flory J.D., Gautam A., Bader H.N., Lehrner A., Makotkine I., Desarnaud F., Miller S.A., Jett M., Hammamieh R., Yehuda R. Longitudinal genome-wide methylation study of PTSD treatment using prolonged exposure and hydrocortisone. Transl. Psychiatry. 2021;11:398. doi: 10.1038/s41398-021-01513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Post-traumatic stress disorder. N. Engl. J. Med. 2002;346:108–114. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Cai G., Golier J.A., Sarapas C., Galea S., Ising M., Rein T., Schmeidler J., Muller-Myhsok B., Holsboer F., Buxbaum J.D. Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biol. Psychiatr. 2009;66:708–711. doi: 10.1016/j.biopsych.2009.02.034. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Daskalakis N.P., Desarnaud F., Makotkine I., Lehrner A.L., Koch E., Flory J.D., Buxbaum J.D., Meaney M.J., Bierer L.M. Epigenetic biomarkers as predictors and correlates of symptom improvement following psychotherapy in combat veterans with PTSD. Front. Psychiatr. 2013;4:118. doi: 10.3389/fpsyt.2013.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yongue B.G., Roy E.J. Endogenous aldosterone and corticosterone in brain cell nuclei of adrenal-intact rats: regional distribution and effects of physiological variations in serum steroids. Brain Res. 1987;436:49–61. doi: 10.1016/0006-8993(87)91555-1. [DOI] [PubMed] [Google Scholar]
- Young E.A., Lopez J.F., Murphy-Weinberg V., Watson S.J., Akil H. The role of mineralocorticoid receptors in hypothalamic-pituitary-adrenal axis regulation in humans. J. Clin. Endocrinol. Metab. 1998;83:3339–3345. doi: 10.1210/jcem.83.9.5077. [DOI] [PubMed] [Google Scholar]
- Young K.A., Thompson P.M., Cruz D.A., Williamson D.E., Selemon L.D. BA11 FKBP5 expression levels correlate with dendritic spine density in postmortem PTSD and controls. Neurobiol Stress. 2015;2:67–72. doi: 10.1016/j.ynstr.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M.J., Clyne C.D. Mineralocorticoid receptor actions in cardiovascular development and disease. Essays Biochem. 2021;65:901–911. doi: 10.1042/EBC20210006. [DOI] [PubMed] [Google Scholar]
- Young M.J., Clyne C.D., Chapman K.E. Endocrine aspects of ACE2 regulation: RAAS, steroid hormones and SARS-CoV-2. J. Endocrinol. 2020;247:R45–R62. doi: 10.1530/JOE-20-0260. [DOI] [PubMed] [Google Scholar]
- Young W.S., Li J., Wersinger S.R., Palkovits M. The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience. 2006;143:1031–1039. doi: 10.1016/j.neuroscience.2006.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalachoras I., Houtman R., Atucha E., Devos R., Tijssen A.M., Hu P., Lockey P.M., Datson N.A., Belanoff J.K., Lucassen P.J., Joels M., de Kloet E.R., Roozendaal B., Hunt H., Meijer O.C. Differential targeting of brain stress circuits with a selective glucocorticoid receptor modulator. Proc. Natl. Acad. Sci. U. S. A. 2013;110:7910–7915. doi: 10.1073/pnas.1219411110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalachoras I., Houtman R., Meijer O.C. Understanding stress-effects in the brain via transcriptional signal transduction pathways. Neuroscience. 2013;242:97–109. doi: 10.1016/j.neuroscience.2013.03.038. [DOI] [PubMed] [Google Scholar]
- Zalachoras I., Verhoeve S.L., Toonen L.J., van Weert L.T., van Vlodrop A.M., Mol I.M., Meelis W., de Kloet E.R., Meijer O.C. Isoform switching of steroid receptor co-activator-1 attenuates glucocorticoid-induced anxiogenic amygdala CRH expression. Mol. Psychiatr. 2016;21:1733–1739. doi: 10.1038/mp.2016.16. [DOI] [PubMed] [Google Scholar]
- Zhang L., Li H., Hu X., Benedek D.M., Fullerton C.S., Forsten R.D., Naifeh J.A., Li X., Wu H., Benevides K.N., Le T., Smerin S., Russell D.W., Ursano R.J. Mitochondria-focused gene expression profile reveals common pathways and CPT1B dysregulation in both rodent stress model and human subjects with PTSD. Transl. Psychiatry. 2015;5 doi: 10.1038/tp.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieker J., Zieker D., Jatzko A., Dietzsch J., Nieselt K., Schmitt A., Bertsch T., Fassbender K., Spanagel R., Northoff H., Gebicke-Haerter P.J. Differential gene expression in peripheral blood of patients suffering from post-traumatic stress disorder. Mol. Psychiatr. 2007;12:116–118. doi: 10.1038/sj.mp.4001905. [DOI] [PubMed] [Google Scholar]
- Zorn J.V., Schur R.R., Boks M.P., Kahn R.S., Joels M., Vinkers C.H. Cortisol stress reactivity across psychiatric disorders: a systematic review and meta-analysis. Psychoneuroendocrinology. 2017;77:25–36. doi: 10.1016/j.psyneuen.2016.11.036. [DOI] [PubMed] [Google Scholar]
- Zwart W., Theodorou V., Kok M., Canisius S., Linn S., Carroll J.S. Oestrogen receptor-co-factor-chromatin specificity in the transcriptional regulation of breast cancer. EMBO J. 2011;30:4764–4776. doi: 10.1038/emboj.2011.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.