Abstract
PFAS are persistent and toxic chemicals used in many commercial and industrial applications that are often added to consumer products, including those used by children and adolescents, to impart water and stain resistance. Since product labels rarely list chemical additives, including PFAS, we evaluated whether other information on product labels can be used by consumers to select products without PFAS. We selected 93 items marketed to or often used by children and adolescents across three product types (furnishings, apparel, bedding) and five labeling groups representing different combinations of water and/or stain resistance and “green” (including “nontoxic”) assurances. We screened all products for total fluorine (F) and analyzed solvent extracts from a subset (n = 61) for 36 targeted PFAS and from a smaller subset (n = 30) for perfluoroalkyl acids (PFAAs) generated by precursor oxidation using the total oxidizable precursor (TOP) assay. Products advertised as water- and/or stain-resistant had more frequent detections and higher concentrations of total F than those without such claims, and targeted PFAS were detected only in products labeled as water- and/or stain-resistant. Concentrations of PFAAs generated by precursor oxidation using the TOP assay often exceeded pre-oxidation concentrations, suggesting that PFAA precursors contribute to solvent-extractable PFAS from products. Among products advertised as water- and/or stain-resistant, detection frequencies and concentrations of targeted PFAS were similar regardless of green assurances. This study illustrates many nonessential uses of PFAS in products used by children and adolescents and suggests that while water- and stain-resistant assurances can identify products likely to contain PFAS, current green assurances do not consistently indicate the absence of PFAS.
Keywords: textiles, stain resistance, fluorine, product labels, precursors
Short abstract
PFAS are commonly found in stain- and water-resistant products used by children and adolescents, regardless of green or nontoxic assurances on product labels.
Introduction
Per- and polyfluoroalkyl substances (PFAS) are a class of over 9000 compounds,1 many of which are used globally in industrial and commercial applications, including firefighting foams, paints, food packaging, cookware, textiles, medical devices, and electronic devices.2 PFAS have been detected in drinking water supplies serving millions of Americans3,4 and in blood samples from over 99% of the U.S. population,5 consistent with the widespread use, persistence, mobility, and long half-lives in the human body of some PFAS. While biomonitoring studies have shown that blood levels of some long-chain PFAS are declining in countries that have phased out their production,6,7 recent evidence of increasing concentrations of short-chain PFAS in breast milk suggests that exposures to short-chain PFAS are increasing.8
Widespread exposures to PFAS raise public health concerns because some PFAS have been linked to numerous adverse health outcomes, including cancer, thyroid disease, elevated cholesterol, decreased birth weight, developmental toxicity, ulcerative colitis, preeclampsia, and immunotoxicity.9,10 Moreover, a 2017 review concluded that children may be especially vulnerable to harmful effects of some PFAS, with the strongest evidence for dyslipidemia, reduced vaccine response, changes in renal function, asthma, and delayed age at menarche.11
The presence of PFAS in everyday consumer products is likely an important source of exposure among the general population, although the extent of exposure and contribution relative to drinking water and diet are not well understood and may vary by individual. PFAS added to consumer products can contribute to exposure via multiple pathways, including inhalation and ingestion of dust, inhalation of volatile PFAS, hand-to-mouth transfer, and potentially, dermal absorption.12 PFAS are frequently found in dust,13 and consumer products can be a source of PFAS into dust via fibers that are shed from PFAS-treated products and PFAS that volatilize from products and then partition from air into dust.14 A case study found elevated levels of perfluorohexane sulfonic acid (PFHxS) in the blood of members of a household that was traced to frequent application of stain-resistant carpet treatment,15 and the presence of stain-resistant furniture or carpets in homes has been associated with serum PFAS in cohorts in North America and Europe.16−20 Children may have relatively elevated exposures due to frequent hand-to-mouth contact and more direct contact with PFAS in carpets and dust.21
PFAS are most commonly applied to textiles as fluorinated polymers to impart stain and water resistance. However, nonpolymer PFAS can also be present in textiles, either as a result of impurities, processing aids from fluorinated polymer production, or degradation products of side-chain fluorinated polymers.22−25 The types of PFAS that have typically been measured in household items such as carpets, furniture, and clothing include perfluoroalkane sulfonic acids (PFSAs) and perfluoroalkyl carboxylic acids (PFCAs), which are both types of perfluoroalkyl acids (PFAAs), as well as fluorotelomer alcohols (FTOHs) and perfluoroalkane sulfonamides (FASAs), which are PFAA precursors.26,27 PFAA precursors released from products can be transformed within the human body or in the environment into highly stable terminal PFCAs and PFSAs that are frequently detected in blood or tissues.28,29 Several studies of consumer products have found greater concentrations of PFAAs in sample extracts after oxidation with the total oxidizable precursor (TOP) assay compared to pre-oxidation concentrations, attributable to generation from oxidizable precursors.30,31
The presence of PFAS ingredients in consumer products, including those used by children and adolescents, is not typically disclosed to consumers on product labels. The primary goal of this study was to investigate the extent to which other product information available to consumers, such as labeling for stain or water resistance and “green” (including “nontoxic”) assurances and certifications, can be used by consumers to identify products likely to contain PFAS. To accomplish this, we purchased a range of household items (bedding, furnishings, apparel) that children and adolescents may frequently come into contact with, using product label information to select and categorize products. We used measurements of total fluorine as a screening tool to select products for analysis of targeted PFAS analytes and oxidizable PFAA precursors using the TOP assay. To our knowledge, this is the first study to evaluate whether marketing language on product labels is indicative of the presence of PFAS in consumer products. The results from this study can inform exposure assessment efforts, particularly for children and adolescents, and support implementation of the essential use concept,32 which aims to reduce the use of these toxic and persistent chemicals by helping manufacturers, retailers, and regulators identify and avoid noncritical uses of PFAS.
Methods
Product Selection
We selected products that children and adolescents are likely to have frequent contact with on a regular basis. When selecting products, we reviewed online product descriptions for specific keywords to classify products within five product label categories (Table 1): (1) trademarked water and/or stain resistance (e.g., Teflon, Scotchgard) (WS-T); (2) water and/or stain resistance with no trademark (WS); (3) water and/or stain resistance and green assurances, including nontoxic terms or green certification (e.g., Greenguard Gold, Oeko-Tex 100) (WS-G); (4) green assurances with no water- or stain-resistance claims (G); and (5) no claims of water or stain resistance or green assurances (N-WS-G). Several products were classified as WS based on online descriptions but were reclassified as WS-G based on green language on product packaging.
Table 1. Descriptions and Keywords Associated with Five Label Groups for Study Products.
| label group | description | trademark (e.g., Teflon, Scotchgard, Protekt, Stainmaster, Carpet Guard) | water- and/or stain-resistant (e.g., waterproof, water-resistant, stainproof, stain-resistant, leak-proof) | green (e.g., nontoxic, toxics free, PFC-free, eco-friendly, chemical-free, Oeko-Tex 100, organic cotton, Green Label Plus, GOTS, Bluesign, Greenguard Gold) |
|---|---|---|---|---|
| WS-T | trademarked water and/or stain resistance | √ | √ | |
| WS | water- and/or stain-resistance claims with no trademark | √ | ||
| WS-G | water- and/or stain-resistance claims plus green language/certification | √ | √ | |
| G | green language/certification with no water- or stain-resistance claims | √ | ||
| N-WS-G | no language regarding water or stain resistance or green assurances |
We conducted our searches using Google and major retailer websites and purchased products online from major retailers and retailers specializing in products for children and adolescents in March–September 2020. We selected a total of 93 products from eight product types among three primary product categories (apparel, bedding, furnishings). Apparel included clothing (school uniform shirts and pants, casual wear, infant wear) (n = 14), face masks (n = 6), and menstrual underwear (n = 6). Bedding included sheets (twin, crib-sized) (n = 12), mattress protectors (twin, crib-sized) (n = 15), and pillow protectors (n = 10). Furnishings included area rugs (n = 16) and upholstered chairs (n = 14). When available, rug and upholstery swatches were purchased to reduce material waste and expense. Most products were marketed specifically for children, while some bedding and menstrual underwear were selected because of presumed use by children or adolescents, even if they were not explicitly marketed for their use.
For each of the eight product types, we aimed to purchase at least three products within each of the five label groups. In some cases, we could not find products for each label group because of the nature of the product; for example, all menstrual underwear had leak-proof (coded as water-resistant) claims. We noted the country of origin for each product, indicated in online product descriptions or product labels, although this information was not considered when selecting products. Product and label descriptions are provided in Table S1.
Sample Processing
All products remained in their original packaging until processing, typically within 2 weeks of receipt. All products were handled using nitrile gloves, and gloves were either changed or wiped with isopropyl alcohol between products. Swatches of approximately uniform areas were cut using stainless steel dissecting scissors from upholstery, bedding, and clothing items, and dimensions of individual swatches were recorded. Scissors were cleaned after each use with isopropyl alcohol wipes. Rug fibers were collected by cutting fibers from rug backings, which were not included in the analyses. Forty-nine of the 93 products arrived folded or rolled in their packaging, which allowed us to cut samples from areas of these products that had not been in contact with packaging.
For total fluorine (F) analysis, approximately 200 mg samples (an individual swatch or equivalent weight of carpet fibers) were cut from products and placed in 5 mL polypropylene screw-top centrifuge tubes. For analyses of targeted PFAS analytes and oxidizable precursors, approximately 4.0–4.5 g samples, comprising individual swatches each weighing 0.5–1.0 g or equivalent weight of carpet fibers, were cut from each product and placed in 50 mL polypropylene screw-top centrifuge tubes. Samples were stored at room temperature before shipping to laboratories, and sample analyses commenced within 4 months.
Total Fluorine Analyses
A total of 122 samples from 93 products were analyzed for total F (Galbraith Laboratories, Knoxville, TN). For products with multiple layers (face masks, menstrual underwear, mattress protectors), each layer was analyzed for total F. Total F was analyzed using an ion-selective electrode (Orion) coupled to a digital meter (Fisher Accumet AR25) following combustion in an oxygen flask containing a known volume of buffer solution as the absorbing medium. Standard solutions made with sodium fluoride (NaF) were used to calibrate the electrode, and a potassium fluoride (KF) standard solution was run at the beginning, end, and after every tenth sample of each batch (generally 25–35 samples per batch) to monitor calibration drift (99.0–100.6% consistency observed). A method blank (reagent grade sucrose) was run with each batch. Buffer solution concentrations were converted to mg F/kg product using the weight of the material combusted. The LOD was 10 ppm for almost all products. Additional information is provided in the Supporting Information (SI). This method produces a low bias on heavily fluorinated organic materials, such as PFAS, because the oxygen flask combustion preparation may not separate all tightly bound carbon–fluorine bonds. A diagram outlining our criteria for additional analyses is shown in Figure S1.
Targeted PFAS Analysis
All products with total F ≥ 10 ppm (n = 54) were analyzed for 36 targeted PFAS via methanol extraction followed by liquid chromatography/tandem mass spectrometry (LC/MS/MS) with isotope dilution (Alpha Analytical, Mansfield, MA) (Table S2). If multiple layers of a product had detectable total F, only one layer (typically the layer in contact with skin) was selected for PFAS analysis. Targeted PFAS analysis was also performed on seven products with no detectable total F, including at least two products from each of the three primary product categories and one product from each of the five label groups.
Full details of the analysis are provided in the Supporting Information. Approximately 1–4 g of the sample (depending on the density of the sample material) was removed from the initial storage tube, weighed, and placed into another 50 mL polypropylene centrifuge tube. Ottawa sand was used as a solid matrix substitute for the procedural blanks because it had been documented by the laboratory as being free of any target analyte interference. Each sample and associated quality control sample were spiked with a suite of extracted internal standard (EIS) primary dilution standards (Table S5). Tubes were capped, inverted for mixing, and vortexed for 25 s at 2500 RPM after adding 10 mL of methanol with 2% ammonium hydroxide. Additional methanol was required for some fabric samples. Samples were sonicated for 30 min, allowed to sit overnight, and then centrifuged at 3500 RPM for 10 min. Half of the supernatant was processed and analyzed for targeted PFAS, and the other half was stored for possible analysis with the TOP assay.
Clean-up procedures for sample extracts are described in the Supporting Information, and LC/MS/MS operating conditions are summarized in Tables S3–S5. In short, sample clean-up was performed using solid-phase extraction (SPE) and weak anion exchange (WAX) reverse-phase stacked on a carbon black cartridge. For targeted PFAS analysis, a 3 μL extract was injected into the LC equipped with the C18-column and interfaced to the MS/MS. Thirty-six PFAS (Table S2) were quantified by separating and identifying analytes by comparing the acquired mass spectra and retention times to reference spectra and retention times for calibration standards acquired under identical LC/MS/MS conditions (Table S5). Targeted analytes were quantified using the isotope dilution technique. Extracted Internal Standards (EISs) were used to monitor the extraction efficiency of method analytes. All native and isotopically labeled standards for LC/MS/MS analyses were purchased from Wellington Laboratories (Guelph, ON, Canada) (Table S5). Derivation of the limit of detection (LOD) and limit of quantitation (LOQ) is provided in the SI. Estimated values, or values between the LOD and LOQ, are provided in Table S1 but are not presented in the main results.
Oxidizable Precursor Analysis
Aliquots of methanol extracts from 30 products, all of which had at least one detection of a targeted PFAS analyte in the LC/MS/MS analysis or total F ≥ 100 ppm (Figure S1), were analyzed using a commercialized method for the total oxidizable precursor (TOP) assay. This method measures PFAAs derived from precursors that undergo oxidation by hydroxyl radicals.33 In this oxidation procedure modified from Houtz and Sedlak,33 aliquots of the original extracts containing EIS were treated by adding potassium persulfate until reaching a concentration of 60 mM (approx. 4 g). Sodium hydroxide was added to achieve 125 mM (approx. 1.25 g), and sample pH was monitored to ensure it remained >12. Samples were placed in a water bath at 85 °C for 6 h, removed, and pH was adjusted to 6–8 with hydrochloric acid to stop the oxidation reaction. Samples were then cleaned up, processed, and analyzed in the same manner as unoxidized samples, targeting 18 terminal PFCA and PFSA analytes (Table S2). Net generation of PFAA precursors (ΔPFAA) was calculated as the difference between corresponding pairs of PFAA concentrations after oxidation and before oxidation. ΔPFAA values were only calculated in instances where post-oxidation concentrations were above the corresponding LOQ. Five of 73 (7%) post-oxidation concentrations (≥LOQ) were less than their respective pre-oxidation concentrations, shown as “not calculated” (rather than a negative value) in Table S1.
Quality Control
Total F analyses were conducted in eight batches and included 4–5 replicates for six products, two products from each of the three primary product categories and 1–2 products from each of the five label groups. Six of eight batches included at least one replicate. Among two of six replicate samples with ≥10 ppm total F, the relative standard deviation (RSD) was <8.7% for one product and 26% for the other (Table S6).
Targeted PFAS analyses were conducted in four batches, and matrix spike recoveries and surrogate recoveries were determined for each analyte in each batch. Of the 36 target analytes, 27 had acceptable (50–150%) lab control sample (i.e., matrix spike) recoveries and extracted internal standard recoveries (Table S7). Of the nine target analytes outside that range, N-ethyl perfluorooctane sulfonamidoethanol (NEtFOSE) and N-methyl perfluorooctane sulfonamidoethanol (NMeFOSE) were the only two detected in at least one sample; measurements for these two analytes were not included in the presented results, but all measurements, including estimated concentrations, are reported in Table S1. No product samples exhibited consistently low or high recoveries among the suite of targeted PFAS, suggesting that product-specific matrix effects did not affect analyte recoveries. All results for an empty centrifuge tube extracted and analyzed in the same manner as samples were <LOD, and their corresponding surrogate recoveries were consistent with surrogate recoveries for product samples.
Duplicate samples were analyzed for five products, and each duplicate was analyzed in a different batch. For each chemical detected among each duplicate pair, the relative percent difference (RPD) was below 20%, except for perfluorotridecanoic acid (PFTrA), perfluoropentanesulfonic acid (PFPeS), and NMeFOSE (Table S7).
Results
Total Fluorine
Total F was detected above 10 ppm in 54 of 93 products (58%; Table 2). Total F ranged from 10 to 3660 ppm, with the highest concentration in a WS school uniform shirt (product ID C4). Twenty-eight of these 54 products had total F concentrations >100 ppm and 13 products had concentrations >1000 ppm (Figure 1).
Table 2. Number of Products with ≥10 ppm Total Fluorine (Number with Detectable F/Number Tested) and with Concentrations above the LOQ for At Least One Targeted PFAS Analyte or Net Generation of PFAAs via TOP (Number with Detectable PFAS/Number Tested).
|
WS-Ta |
WS |
WS-G |
G |
N-WS-G |
All |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| product category | TFb | PFASc | TOPd | TF | PFAS | TOP | TF | PFAS | TOP | TF | PFAS | TOP | TF | PFAS | TOP | TF | PFAS | TOP |
| clothing | 1/2 | 1/1 | 1/1 | 5/5 | 4/5 | 4/5 | 4/4 | 2/4 | 1/3 | 1/3 | 0/2 | 11/14 | 7/12 | 7/9 | ||||
| face mask | 1/3 | 0/1 | 0/1 | 3/3 | 0/3 | 0/1 | 4/6 | 0/4 | 0/2 | |||||||||
| menstrual underwear | 2/3 | 0/3 | 2/3 | 0/2 | 2/2 | 4/6 | 1/5 | 2/2 | ||||||||||
| mattress protector | 3/3 | 2/3 | 2/2 | 2/5 | 0/3 | 5/7 | 1/5 | 1/1 | 10/15 | 3/11 | 3/3 | |||||||
| sheets | 1/3 | 0/2 | 2/5 | 0/2 | 0/1 | 0/1 | 0/1 | 0/2 | 3/12 | 0/5 | ||||||||
| pillow protector | 3/3 | 3/3 | 2/3 | 1/3 | 0/1 | 3/4 | 1/3 | 1/1 | 7/10 | 4/7 | 3/4 | |||||||
| rugs | 2/2 | 1/2 | 2/2 | 2/4 | 0/2 | 0/1 | 0/2 | 0/1 | 0/3 | 0/5 | 0/1 | 4/16 | 1/6 | 2/3 | ||||
| upholstered chairs | 4/4 | 3/4 | 2/4 | 4/6 | 0/4 | 1/3 | 3/3 | 0/3 | 0/1 | 11/14 | 3/11 | 4/7 | ||||||
| all categories | 10/13 | 7/11 | 7/8 | 19/32 | 7/21 | 6/11 | 18/27 | 4/19 | 6/10 | 3/8 | 0/4 | 4/14 | 0/6 | 54/93 | 18/61 | 19/30 | ||
WS-T, trademark for water- and/or stain-resistant treatment; WS, water- and/or stain-resistant; WS-G, water- and/or stain-resistant plus green assurance; G, green assurance; N-WS-G, not water- or stain-resistant, no green assurances.
TF = Total fluorine.
PFAS = targeted PFAS in samples prior to oxidation using the total oxidizable precursor assay (TOP).
TOP = net generation of PFAAs from oxidizable precursors via the TOP assay (ΔPFAA).
Figure 1.
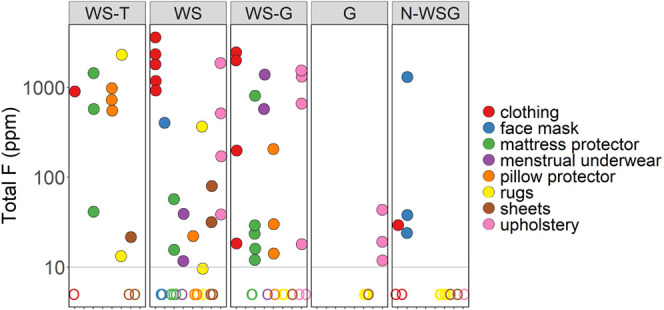
Total fluorine for 93 products arranged by label groups. The detection limit is 10 ppm. Label group abbreviations are defined in Table 1. Filled circles indicate samples at or above the detection limit and open circles indicate samples below the detection limit.
Total F concentrations and detection frequencies were similar for WS-T, WS, and WS-G products. Detection frequencies for total F were somewhat higher among WS-T products (10/13; 77%) compared to WS (19/32; 59%) and WS-G (18/27; 67%) products (Table 2), but the proportion of samples with total F > 1000 ppm was similar among these three groups (WS-T, 15%; WS, 16%; WS-G, 19%). The detection frequency for total F was substantially lower (7/22; 32%) for products with no water- or stain-resistance language (G or N-WS-G) (Table 2). When detected, total F concentrations in G and N-WS-G products were relatively low, with six of seven total F detections <50 ppm; the one exception was the outer layer of an N-WS-G face mask (F5-O) with 1250 ppm total F (Figure 1).
Among the product types tested, clothing and upholstery had the highest detection frequencies, both overall (both 79%) and among WS items (both 100%). Rugs and sheets had the lowest detection frequencies overall (both 25%), while among WS items, masks and pillow protectors had the lowest detection frequency (both 33%). It is important to note, however, that comparisons among product types should be interpreted with caution given the small sample sizes. Further, comparisons among product types are complicated by the fact that different product types had different distributions among product labels. For instance, some product types were only available with water and/or stain resistance, and other product types were not available with trademark treatments. Only the WS label group is represented in all eight product types, with 3–5 items per product type.
Targeted PFAS Analytes
Eighteen of 61 products (30%) analyzed for targeted PFAS had measured concentrations above the LOQ for at least one of 27 target analytes with acceptable surrogate recoveries (Table 2). PFAS detection frequencies were 2–3 times higher for WS-T products (7/11; 64%) than for WS (7/21; 33%) and WS-G products (4/19; 21%). None of the G (n = 4) or N-WS-G (n = 6) products tested for targeted PFAS had any detections. Clothing and pillow protectors had the highest detection frequencies for at least one PFAS (7/12; 58% and 4/7; 57%, respectively), while face masks and sheets had no target PFAS detections (Table 2). Among products with at least one target PFAS analyte detected, the average number of detections was three, and one product (a WS-G pillow protector, P7) contained seven target analytes (Table S1).
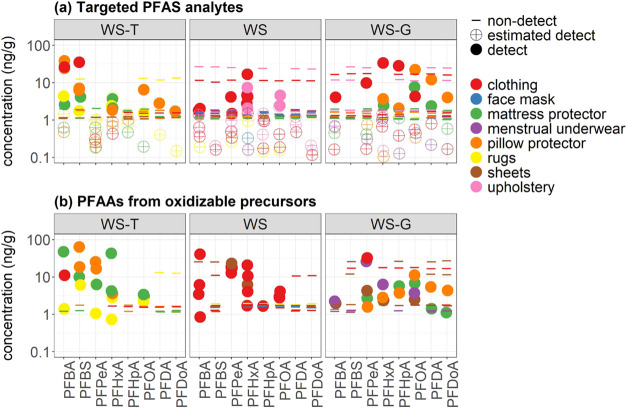
Eleven PFAS target analytes with acceptable surrogate recoveries were detected at least once among the analyzed products (Table 3). Among these 11, nine were PFCAs (C4-C10, C12-C13) and two were PFSAs (C4, C8). The most frequently detected were perfluorohexanoic acid (PFHxA) (14/18 products; 78%), perfluorobutanoic acid (PFBA) (8/18; 44%), perfluorooctanoic acid (PFOA) (7/18; 39%), perfluorobutane sulfonic acid (PFBS) (5/18; 28%), and perfluorodecanoic acid (PFDA) and perfluoropentanoic acid (PFPeA) (each 3/18; 17%) (Table 3). Maximum concentrations measured for these analytes ranged from 10.2 to 36.6 ng/g (0.108 to 0.872 ng/cm2) (Table 3). Perfluoroheptanoic acid (PFHpA), perfluorooctane sulfonic acid (PFOS), perfluorononanoic acid (PFNA), perfluorododecanoic acid (PFDoA), and perfluorotetradecanoic acid (PFTA) were each detected once or twice. PFOA and PFOS were detected despite the phase-out of their production in the U.S., with seven products containing PFOA and one containing PFOS. The maximum detected PFOA concentration (22.5 ng/g) was found in a WS-G pillow protector (P7) and 2.1 ng/g PFOS was measured in a WS cover fabric of a children’s upholstered chair (U2). The maximum PFOA concentration was close to the highest compound-specific maximum concentrations, which ranged from 28.9 to 36.6 ng/g for four short-chain compounds (PFBA, PFHxA, PFHpA, PFBS) (Figure 2, Tables 3 and S1). Three of the five highest PFAS concentrations were found in WS-G products even though only 30% of samples were WS-G.
Table 3. Detection Frequency (Number of Samples in Parentheses) and Maximum Concentrations of PFAS Measured in 61 Products and PFAAs Generated from Oxidizable Precursors in 30 Products Using the TOP Assay.
| number
of detections |
maximum
concentration (ng/g) |
maximum
concentration (ng/cm2) |
||||
|---|---|---|---|---|---|---|
| chemical | PFASa | ΔPFAAb | PFAS | ΔPFAAc | PFAS | ΔPFAAc |
| PFBA | 13% (8/61) | 30% (9/30) | 36.6 | 48.2 | 0.664 | 0.689 |
| PFBS | 8.2% (5/61) | 17% (5/30) | 34.4 | 227 | 0.872 | 5.75 |
| PFPeA | 4.9% (3/61) | 57% (17/30) | 10.2 | 271 | 0.210 | 4.67 |
| PFHxA | 23% (14/61) | 47% (14/30) | 35.7 | 42.1 | 0.736 | 0.442 |
| PFHpA | 3.3% (2/61) | 10% (3/30) | 28.9 | 5.53 | 0.596 | 0.100 |
| PFOA | 11% (7/61) | 37% (11/30) | 22.5 | 11.2 | 0.207 | 0.123 |
| PFOS | 1.6% (1/61) | 3.3% (1/30) | 2.15 | 0.780 | 0.051 | 0.033 |
| PFNA | 1.6% (1/61) | 6.7% (2/30) | 1.62 | 1.91 | 0.015 | 0.020 |
| PFDA | 4.9% (3/61) | 6.7% (2/30) | 11.8 | 5.20 | 0.108 | 0.048 |
| PFUnA | n.d.d | 3.3% (1/30) | n.d. | 0.636 | n.d. | 0.006 |
| PFDoA | 3.3% (2/61) | 6.7% (2/30) | 3.99 | 4.47 | 0.037 | 0.041 |
| PFTA | 1.6% (1/61) | 3.3% (1/30) | 2.59 | 0.690 | 0.024 | 0.006 |
Number of detections among 61 samples. LOQ range: 1.11–26.6 ng/g.
Number of detections among 30 samples. Δ = post-oxidation minus pre-oxidation PFAA concentration. LOQ range: 1.11–18.7 ng/g.
After subtracting pre-oxidation PFAA concentration. Only net positive PFAA concentrations are included.
n.d., not detected.
Figure 2.
(a) Targeted PFAS analyte concentrations in 61 products prior to oxidation with TOP. (b) ΔPFAA concentrations in 30 samples generated from oxidizable precursors during TOP analysis. PFOS, PFNA, and PFTA concentrations are not shown because they were detected in only one sample. Nondetects are plotted at each sample’s LOQ. Label group abbreviations are provided in Table 1.
Precursor-Derived PFAAs
Concentrations of PFAAs in extracts from 19 of 30 products oxidized according to the TOP assay were greater than pre-oxidation concentrations, indicating the presence of oxidizable PFAA precursors in those samples (Table 2). Net generation of individual PFAAs (ΔPFAA) was observed most frequently in WS-T products (7 of 8; 88%), which also had the highest pre-oxidation detection frequencies. Frequencies of positive ΔPFAA values were lower, but similar, for WS and WS-G products (55%; 6/11 and 60%; 6/10, respectively). No PFAAs were detected after oxidation in the one N-WS-G product (a face mask, F5-O) included in the TOP analysis, despite having 1250 ppm total F. The number of PFAAs generated from oxidizable precursors per product ranged from 0 to 9 (Table S1), with a median number of detections per product of 1 and an average of 2.3. WS-T, WS, and WS-G products all had similar average numbers of PFAAs generated from oxidizable precursors (2–3 per product), although two WS-G products (a pillow protector, P7, and a crib mattress protector, M12-O) had the highest number of PFAAs generated from oxidizable precursors (9 and 7, respectively) (Table S1).
Individual PFAA concentrations were often substantially higher in extracts following oxidation using the TOP assay compared to pre-oxidation PFAA concentrations. In products with targeted PFAS detection before oxidation and positive ΔPFAA values for those same analytes after oxidation (n = 14), individual ΔPFAA concentrations were 39 times greater on average (range: 0.27–558) compared to pre-oxidation concentrations (Table S1). The largest multiple was measured in a WS-T mattress protector (M1) for which ΔPFPeA was 108 ng/g and the pre-oxidation PFPeA concentration was 0.19 ng/g (Table S1). The highest ΔPFAA concentration, 271 ng/g ΔPFPeA, was measured in a WS school uniform shirt (C2). Note that in some cases, ΔPFAA values were within the variability of pre-oxidation measurements (median RPD; Table S7) and may not always indicate the presence of precursors.
Net generation was observed at least once for 12 of the 18 PFAAs targeted in the TOP analysis, with ΔPFPeA detected most frequently (17/30 products), followed by ΔPFHxA (14 products) and ΔPFOA (11 products) (Table 3). The proportion of products with ΔPFOA was similar among the WS-T (3/8), WS (4/11), and WS-G (4/10) label groups, although the two highest ΔPFOA concentrations (11.2 and 6.78 ng/g) were found in the same two WS-G products (a pillow protector, P7, and crib mattress protector, M12-O, respectively) with the two highest pre-oxidation PFOA concentrations (Figure 2; Table S1). These two WS-G products were also the only products subjected to the TOP assay for which net generation of PFAAs with a carbon chain length ≥9 was observed. The identity of the specific precursors present in the samples is not known since the TOP assay does not provide information about the chemical structures of PFAA precursors and each precursor can be oxidized to form a mixture of PFAA products with varying chain lengths.
Discussion
In this study, we found that many water- and/or stain-resistant products that may be frequently used by children and adolescents contained total F, as well as methanol-extractable long- and short-chain targeted PFAS and oxidizable PFAA precursors. Products labeled as water- and/or stain-resistant had more frequent detections and higher concentrations of methanol-extractable targeted PFAS than products not labeled that way; in fact, of the products analyzed for 36 target PFAS, methanol-extractable targeted PFAS were detected only in those labeled as water- and/or stain-resistant. Total F and targeted PFAS were also frequently detected in water- and/or stain-resistant products with green assurances or certifications (WS-G), with concentrations similar to those in water- and/or stain-resistant products without these assurances (WS-T, WS). We found that targeted long-chain PFAS, including PFOA, were detected across multiple product categories, even in green products. Notably, three of the seven products with detectable PFOA concentrations were WS-G items, and two of those products, a pillow protector (P7) and crib mattress protector (M12), had the highest concentrations of PFOA and PFOA generated from oxidizable PFAA precursors.
The range of total F and PFAS concentrations detected in the consumer products analyzed in this study overlaps with values observed in other studies of similar consumer products26,30 and is consistent with what would be expected from the treatment of products with fluorinated polymers.34 Products without detectable total fluorine (<10 ppm) did not contain PFAS detections above the LOQ (n = 7), although of the 54 products with detectable total F (≥10 ppm), only 18 (33%) had detections above the LOQ for any of the 36 target PFAS. Consistent with prior studies, the total amount of fluorine attributable to methanol-extractable target PFAS analytes and to PFAAs generated from oxidizable precursors in our samples never exceeded 0.1% of the total F measured in these products, and total F concentrations were not correlated with the sum of methanol-extractable PFAS.30 For example, in five of the water- and/or stain-resistant products we tested with total F > 100 ppm (including two products with >1000 ppm total F), we measured no extractable targeted PFAS either before oxidation or after the TOP procedure. These results may be due to several factors. First, our targeted PFAS and TOP assay analyses did not capture fluorinated polymers that may have been present in products. Furthermore, our analyses included only a limited number of targeted PFAS, and our methods were not optimized for volatile PFAS, which have been shown to represent a substantial pool of extractable PFAS in some children’s fabrics and other consumer products.30 It may also be possible that there are PFAS present in these recently purchased products that are not readily extractable at present but may become more readily mobilized as products age with exposure to sunlight or repeated washing.22,35 Finally, we cannot rule out the presence of inorganic fluorine contributing to total F measurements.
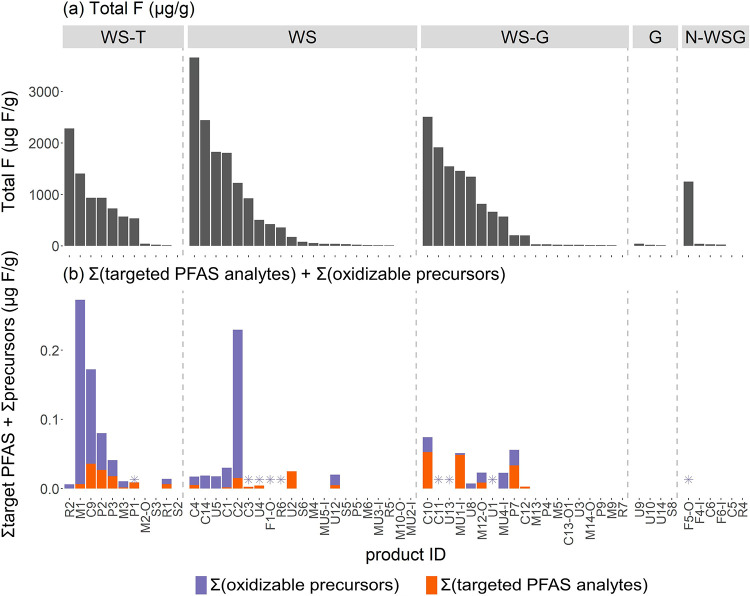
Nevertheless, the frequency of targeted PFAS detections was greater for products with total F concentrations greater than 100 ppm compared to products with lower total F concentrations. Targeted PFAS were detected in 16 of 28 products with total F > 100 ppm, with WS-T products more likely to have extractable PFAS (6 of 7) compared to WS (6 of 10) and WS-G (4 of 10) (Figure 3). By contrast, targeted PFAS were detected in only 2 of 26 products (R1, U12) with total F in the range of 10–100 ppm (Figure 3; Table S1). These results suggest that while total F concentrations may not be linearly correlated with the amount of extractable PFAS we targeted for analysis in these products, products with total F concentrations >100 ppm, especially WS-T products, were more likely to contain extractable targeted PFAS. Although the TOP assay was only performed on a subset of products, we note that consideration of ΔPFAA results increased the overall PFAS detection frequency among the 28 products with total F > 100 ppm, from 16 to 22 products.
Figure 3.
(a) Total F (μg/g) and (b) ∑(targeted PFAS analytes) and ∑(oxidizable precursors) (μg/g as F) for 61 products that underwent targeted analysis for 36 PFAS. Only 30 of the 61 products were analyzed for oxidizable precursors. Products with no target PFAS detected are blank, and products with no net precursor formation are noted with an asterisk. Only concentrations ≥LOQ are plotted. Product IDs correspond to Table S1. The inner and outer layers of a sample are indicated by “-I” and “-O” in the product ID.
Only a few other studies have tested for PFAS in everyday apparel items, such as shorts, shirts, school uniforms, and other clothes regularly worn by children, and most prior analyses have focused on outerwear, which is less likely to contribute to year-round everyday exposures.30,31,35−39 To our knowledge, only one other study has included face masks in analyses for PFAS.37 Similar to the results of Tokronov et al.,37 the four mask samples analyzed for targeted PFAS analytes in the current study had no PFAS detections above the LOQ. Our school uniform results were most similar to those of Liu et al., who also frequently detected PFHxA at similar concentrations in school uniforms purchased in 2011.36 However, in addition to PFHxA, Liu et al. also detected PFOA frequently in their 2011 school uniforms, whereas we did not detect PFOA in any of the school uniform items we analyzed for targeted PFAS (n = 5), and we detected PFOA derived from oxidization of precursors in only two of those five school uniform items. In those two school uniform items subjected to the TOP assay, the resulting ΔPFOA concentrations (4.24 and 2.81 ng/g; C1 and C4, respectively) were within the range of PFOA concentrations measured by Liu et al.36 Considering the 2011 purchase date for the Liu et al.’s samples, their results may already reflect global efforts to reduce PFOA manufacturing and intentional use.40
We observed substantial variability in PFAS detections among the different types of bedding items analyzed. Four out of the seven pillowcases and pillow protectors we analyzed had at least one detection of a targeted PFAS, and one WS-G pillow protector (P7) had seven individual PFAS detected, higher than any other product in this study. Robel et al. also detected a similar range of targeted PFAS, including PFOA, in one pillowcase included in their study (collected in 2015), at concentrations similar to those we found in the P7 pillow protector.30 By contrast, none of the bedsheets analyzed in our study (n = 5) had any targeted PFAS detections above our reporting limits, regardless of the label category. Vestergren et al. detected PFHxA and PFOA in the one bedsheet included in their study (collected in 2012/2013), at lower concentrations than many other textiles in their study39 and within the range of estimated PFHxA detections observed in two of our bedsheets. These results suggest that particular bedding items, such as pillow protectors, are more likely to contain PFAS than other types of bedding, such as bedsheets.
Detection frequencies for PFAS and the diversity of individual PFAS detected among the rugs included in our study are substantially lower than observed in other recent studies,21,37,41 although the ages of the rugs analyzed in those studies are mostly unknown. Of the six rugs we analyzed for PFAS, only one had any targeted PFAS detections (R1), while PFAAs generated from oxidizable precursors were measured in two rugs (R1 and R2). The four PFAS (PFBA, PFBS, PFHxA, PFOA) detected in rug item R1 prior to oxidation were, however, each within the ranges previously reported for childcare nap mats21 and childcare carpets.41 The less frequent detections and lower concentrations of PFAS in rugs in our study may reflect recent retailer actions and policy and regulatory changes in the U.S., such as phase-out campaigns for PFAS in residential carpeting42,43 and the focus of California’s Safer Consumer Products program on PFAS in carpeting.44
The presence of PFAS in green-certified products marketed with water- and/or stain-resistance claims (WS-G products) was not unexpected, since many green certifications either do not consider PFAS or limit concentrations of individual PFAS at levels higher than we found in this study. For example, Oeko-Tex 100 has individual limits of 25–50 ng/g for a number of PFAS,45 while Greenguard Gold certification pertains to volatile organic compound emissions from products, and no PFAS were included in its chemicals of concern list as of June 2021.46 Of the 16 WS-G products with certifications that we analyzed initially for targeted PFAS, only two (C10, an Oeko-Tex 100 product, and M12, a Greenguard Gold product) had detections above the LOQ, at levels either below the certification criteria (for Oeko-Tex 100) or for targeted PFAS not specifically prohibited for certification (Greenguard Gold). However, of the subset of seven WS-G products that were then subjected to the TOP assay, five had positive ΔPFAA values, a much higher detection frequency that illustrates that consideration of PFAA precursors may be important when setting criteria.
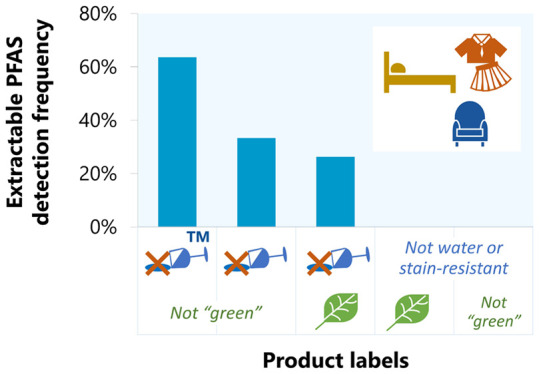
To explore whether water- versus stain-resistant products were more or less likely to contain our targeted PFAS, we coded “water-resistant,” “stain-resistant,” and green labels separately (with some products included in multiple groups). Products labeled as stain-resistant had a higher total F detection frequency (76.3%) compared to products labeled as water-resistant (63.2%) or green (60%), and the detection frequency was much lower (32%) for products that lacked any stain- or water-resistant language (Figure S2). Products labeled as stain-resistant also had a higher detection frequency for target PFAS analytes (52%) compared to products labeled as water-resistant (35%) or green (17%) (Figure S3). These differences may be due to the fact that manufacturers can more readily achieve water resistance without using PFAS than stain resistance. Nine of the water-resistant products in our study with product information indicating the use of a physical barrier, such as a polyurethane (PU) or vinyl layer, were analyzed for PFAS, and only two (a bib, C12, and a pair of menstrual underwear, MU1-I) had targeted PFAS detections. These were also the only two products of the nine with total F >100 ppm (201 and 1456 ppm, respectively). While these results might be anticipated for the bib, which was also advertised as both stainproof and waterproof with a PU layer, the menstrual underwear product information mentioned only waterproofing with a PU layer, without any stain-resistance language. These results suggest that products labeled as waterproof may still contain PFAS even if a physical barrier is used for water resistance.
Consistent with other studies,30,31 PFAA precursors—PFAS that can be transformed to highly stable PFAAs in the body and in the environment—are present in many children’s products we tested, as indicated by results from the TOP assay. In sample extracts subjected to TOP, individual PFAA concentrations often exceeded their concentrations in pre-oxidation samples. For example, we observed ΔPFOA in 6 products for which PFOA was not detected before oxidation: baby jeggings (C14), two upholstery items (U8, U5), two mattress protectors (M1, M3), and menstrual underwear (MU1). Similarly, Zhu and Kannan et al. observed that detection frequencies for PFOA were three times higher after subjecting 160 apparel and apparel textile samples, including infant items, to the TOP assay.31 However, the mean ΔPFOA concentration observed by Zhu and Kannan was over 20 times higher than in our products (1.48 ng/g),31 and unlike our study, they detected PFCAs of chain lengths up to 12, both before and after oxidation, which may reflect differences in product formulations. Interestingly, net production of PFBS was observed in five samples, one of which also showed formation of PFOS, even though PFSA formation is not expected to occur from oxidation of precursors by the hydroxyl radical.33 Zhu and Kannan31 and Robel et al.30 both previously observed PFBS and/or PFOS formation in consumer products according to the TOP assay. These results could be attributable to analytical error or to formation from unidentified precursors present in consumer products.
When we evaluated the country of origin for products with extractable PFAS, we found that products containing long-chain PFAS disproportionately came from China (Figure S4). This is not surprising, since long-chain PFAS are still being manufactured in China.47 Approximately half of the products we purchased in the study came from China, while 75% of the products with long-chain detections (n = 8) came from China. By contrast, products containing short-chain PFAS came from a wider range of countries, including China and the U.S., and more closely reflected the countries of origin among the products in our study overall.
The ability of PFAS to migrate out of consumer products used by children or adolescents raises concerns about exposures to these toxic chemicals during sensitive developmental periods. Few studies have addressed the breadth of PFAS exposures among children and adolescents from consumer products, and future exposure assessments should consider a broad range of PFAS in consumer products, including volatile PFAS and other PFAA precursors. Because many PFAS have been shown to activate similar biological pathways, they may impart toxicity in an additive manner.48−50 Further, PFAA precursors may be more toxic than the corresponding PFAA degradation products, as illustrated by a recent study that showed that 6:2 FTOH was more toxic than PFHxA.51 The toxicity of many PFAS and the potential for long-term exposures to children and adolescents from intimate contact with these products support removing noncritical uses of PFAS from products used by children or adolescents as a way to protect their health.
Acknowledgments
This work was supported by the Commonwealth of Massachusetts, the National Institute of Environmental Health Sciences of the National Institutes of Health (Award Numbers P42ES027706, R01ES028311), and charitable donations to Silent Spring Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.1c05175.
Additional information about standards and reagents, liquid chromatography and mass spectrometry (LC/MS/MS) analytical procedures, LC and MS settings, quality assurance/quality control, total fluorine and PFAS relative abundances by marketing language, and the country of origin (PDF)
Concentrations of total fluorine, target PFAS analytes, and TOP precursors in all samples (XLSX)
The authors declare no competing financial interest.
Supplementary Material
References
- US EPA (U.S. Environmental Protection Agency). PFAS Master List of PFAS Substances (Version 2), 2020, https://comptox.epa.gov/dashboard/chemical_lists/pfasmaster (Accessed Jan 8, 2021).
- Glüge J.; Scheringer M.; Cousins I. T.; DeWitt J. C.; Goldenman G.; Herzke D.; Lohmann R.; Ng C. A.; Trier X.; Wang Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Proc. Imp. 2020, 22, 2345–2373. 10.1039/D0EM00291G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X. C.; Andrews D.; Lindstrom A. B.; Bruton T. A.; Schaider L. A.; Grandjean P.; Lohmann R.; Carignan C. C.; Blum A.; Balan S. A.; Higgins C. P.; Sunderland E. M. Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas and wastewater treatment plants. Environ. Sci. Technol. Lett. 2016, 3, 344–350. 10.1021/acs.estlett.6b00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews D. Q.; Naidenko O. V. Population-wide exposure to per- and polyfluoroalkyl substances from drinking water in the United States. Environ. Sci. Technol. Lett. 2020, 7, 931–936. 10.1021/acs.estlett.0c00713. [DOI] [Google Scholar]
- CDC (U.S. Centers for Disease Control and Prevention). Fourth National Report on Human Exposure to Environmental Chemicals. Updated Tables, January 2019. U.S. Centers for Disease Control and Prevention: Atlanta, GA, 2019. [Google Scholar]
- Kato K.; Wong L. Y.; Jia L. T.; Kuklenyik Z.; Calafat A. M. Trends in exposure to polyfluoroalkyl chemicals in the U.S. population: 1999-2008. Environ. Sci. Technol. 2011, 45, 8037–8045. 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- Toms L. M. L.; Thompson J.; Rotander A.; Hobson P.; Calafat A. M.; Kato K.; Ye X.; Broomhall S.; Harden F.; Mueller J. F. Decline in perfluorooctane sulfonate and perfluorooctanoate serum concentrations in an Australian population from 2002 to 2011. Environ. Int. 2014, 71, 74–80. 10.1016/j.envint.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G.; Schreder E.; Dempsey J. C.; Uding N.; Chu V.; Andres G.; Sathyanarayana S.; Salamova A. Per- and polyfluoroalkyl substances (PFAS) in breast milk: Concerning trends for current-use PFAS. Environ. Sci. Technol. 2021, 55, 7510–7520. 10.1021/acs.est.0c06978. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) . Toxicological Profile for Perfluoroalkyls; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, 2021. [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 110: Perfluorooctanoic acid; International Agency for Research on Cancer: Lyon, France, December 2016, pp 37–110. https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Some-Chemicals-Used-As-Solvents-And-In-Polymer-Manufacture-2016. [Google Scholar]
- Rappazzo K. M.; Coffman E.; Hines E. P. Exposure to perfluorinated alkyl substances and health outcomes in children: A systematic review of the epidemiologic literature. Int. J. Environ. Res. Public Health 2017, 14, 691 10.3390/ijerph14070691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva A. O.; Armitage J. M.; Bruton T. A.; Dassuncao C.; Heiger-Bernays W.; Hu X. C.; Karrman A.; Kelly B.; Ng C.; Robuck A.; Sun M.; Webster T. F.; Sunderland E. M. PFAS exposure pathways for humans and wildlife: A synthesis of current knowledge and key gaps in understanding. Environ. Toxicol. Chem. 2021, 40, 631–657. 10.1002/etc.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitro S. D.; Dodson R. E.; Singla V.; Adamkiewicz G.; Elmi A. F.; Tilly M. K.; Zota A. R. Consumer product chemicals in indoor dust: A quantitative meta-analysis of U.S. studies. Environ. Sci. Technol. 2016, 50, 10661–10672. 10.1021/acs.est.6b02023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines S. R.; Adams R. I.; Boor B. E.; Bruton T. A.; Downey J.; Ferro A. R.; Gall E.; Green B. J.; Hegarty B.; Horner E.; Jacobs D. E.; Lemieux P.; Misztal P. K.; Morrison G.; Perzanowski M.; Reponen T.; Rush R. E.; Virgo T.; Alkhayri C.; Bope A.; Cochran S.; Cox J.; Donohue A.; May A. A.; Nastasi N.; Nishioka M.; Renninger N.; Tian Y.; Uebel-Niemeier C.; Wilkinson D.; Wu T.; Zambrana J.; Dannemiller K. C. Ten questions concerning the implications of carpet on indoor chemistry and microbiology. Build. Environ. 2020, 170, 106589 10.1016/j.buildenv.2019.106589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesoon S.; Genuis S. J.; Benskin J. P.; Martin J. W. Exceptionally high serum concentrations of perfluorohexanesulfonate in a Canadian family are linked to home carpet treatment applications. Environ. Sci. Technol. 2012, 46, 12960–12967. 10.1021/es3034654. [DOI] [PubMed] [Google Scholar]
- Boronow K. E.; Brody J. G.; Schaider L. A.; Peaslee G. F.; Havas L.; Cohn B. A. Serum concentrations of PFASs and exposure-related behaviors in African American and non-Hispanic white women. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 206–217. 10.1038/s41370-018-0109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X. C.; Dassuncao C.; Zhang X.; Grandjean P.; Weihe P.; Webster G. M.; Nielsen F.; Sunderland E. M. Can profiles of poly-and perfluoroalkyl substances (PFASs) in human serum provide information on major exposure sources?. Environ. Health 2018, 17, 11. 10.1186/s12940-018-0355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley S.; Goldberg D.; Wang M. M.; Park J. S.; Petreas M.; Bernstein L.; Anton-Culver H.; Nelson D. O.; Reynolds P. Time trends in per- and polyfluoroalkyl substances (PFASs) in California women: Declining serum levels, 2011–2015. Environ. Sci. Technol. 2018, 52, 277–287. 10.1021/acs.est.7b04650. [DOI] [PubMed] [Google Scholar]
- Karásková P.; Venier M.; Melymuk L.; Bečanová J.; Vojta Š.; Prokeš R.; Diamond M. L.; Klánová J. Perfluorinated alkyl substances (PFASs) in household dust in Central Europe and North America. Environ. Int. 2016, 94, 315–324. 10.1016/j.envint.2016.05.031. [DOI] [PubMed] [Google Scholar]
- Harris M. H.; Rifas-Shiman S. L.; Calafat A. M.; Ye X.; Mora A. M.; Webster T. F.; Oken E.; Sagiv S. K. Predictors of per- and polyfluoroalkyl substance (PFAS) plasma concentrations in 6-10 year old American children. Environ. Sci. Technol. 2017, 51, 5193–5204. 10.1021/acs.est.6b05811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Romanak K.; Bruton T.; Blum A.; Venier M. Per- and polyfluoroalkyl substances in paired dust and carpets from childcare centers. Chemosphere 2020, 251, 126771 10.1016/j.chemosphere.2020.126771. [DOI] [PubMed] [Google Scholar]
- Schellenberger S.; Jonsson C.; Mellin P.; Levenstam O. A.; Liagkouridis I.; Ribbenstedt A.; Hanning A.-C.; Schultes L.; Plassmann M. M.; Persson C.; Cousins I. T.; Benskin J. P. Release of side-chain fluorinated polymer-containing microplastic fibers from functional textiles during washing and first estimates of perfluoroalkyl acid emissions. Environ. Sci. Technol. 2019, 53, 14329–14338. 10.1021/acs.est.9b04165. [DOI] [PubMed] [Google Scholar]
- California Environmental Protection Agency. Product – Chemical Profile for Carpets and Rugs Containing Perfluoroalkyl or Polyfluoroalkyl Substances; Department of Toxic Substances Control: Sacramento, CA, 2019. https://dtsc.ca.gov/wp-content/uploads/sites/31/2020/02/Final_Product-Chemical_Profile_Carpets_Rugs_PFASs_a.pdf. [Google Scholar]
- Lohmann R.; Cousins I. T.; DeWitt J. C.; Gluege J.; Goldenman G.; Herzke D.; Lindstrom A. B.; Miller M. F.; Ng C. A.; Patton S.; Scheringer M.; Trier X.; Wang Z. Are fluoropolymers really of low concern for human and environmental health and separate from other PFAS?. Environ. Sci. Technol. 2020, 54, 12820–12828. 10.1021/acs.est.0c03244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S. G.; Letcher R. J. Side-chain fluorinated polymer surfactants in aquatic sediment and biosolid-augmented agricultural soil from the Great Lakes basin of North America. Sci. Total Environ. 2017, 607–608, 262–270. 10.1016/j.scitotenv.2017.06.252. [DOI] [PubMed] [Google Scholar]
- Lassen C.; Kjølhol J.; Mikkelsen S. H.; Warming M.; Jensen A. A.; Boss R.; Nielsen I. B.. Polyfluoroalkyl Substances (PFASs) in Textiles for Children; Danish Environmental Protection Agency, Ministry of Environment and Food, 2015; https://www2.mst.dk/Udgiv/publications/2015/04/978-87-93352-12-4.pdf. [Google Scholar]
- Kotthoff M.; Müller J.; Jürling H.; Schlummer M.; Fiedler D. Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Env. Sci. Pollut. Res. Int. 2015, 22, 14546–14559. 10.1007/s11356-015-4202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Eon J. C.; Mabury S. A. Exploring indirect sources of human exposure to perfluoroalkyl carboxylates (PFCAs): Evaluating uptake, elimination, and biotransformation of polyfluoroalkyl phosphate esters (PAPs) in the rat. Environ. Health Perspect. 2011, 119, 344–350. 10.1289/ehp.1002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Eon J. C.; Mabury S. A. Production of perfluorinated carboxylic acids (PFCAs) from the biotransformation of polyfluoroalkyl phosphate surfactants (PAPS): exploring routes of human contamination. Environ. Sci. Technol. 2007, 41, 4799–4805. 10.1021/es070126x. [DOI] [PubMed] [Google Scholar]
- Robel A. E.; Marshall K.; Dickinson M.; Lunderberg D.; Butt C.; Peaslee G.; Stapleton H. M.; Field J. A. Closing the mass balance on fluorine on papers and textiles. Environ. Sci. Technol. 2017, 51, 9022–9032. 10.1021/acs.est.7b02080. [DOI] [PubMed] [Google Scholar]
- Zhu H.; Kannan K. Total oxidizable precursor assay in the determination of perfluoroalkyl acids in textiles collected from the United States. Environ. Pollut. 2020, 265, 114940 10.1016/j.envpol.2020.114940. [DOI] [PubMed] [Google Scholar]
- Cousins I. T.; Goldenman G.; Herzke D.; Lohmann R.; Miller M.; Ng C. A.; Patton S.; Scheringer M.; Trier X.; Vierke L.; Wang Z.; DeWitt J. C. The concept of essential use for determining when uses of PFASs can be phased out. Environ. Sci. Proc. Imp. 2019, 21, 1803–1815. 10.1039/C9EM00163H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtz E. F.; Sedlak D. L. Oxidative conversion as a means of detecting precursors to perfluoroalkyl acids in urban runoff. Environ. Sci. Technol. 2012, 46, 9342–9349. 10.1021/es302274g. [DOI] [PubMed] [Google Scholar]
- Knepper T.; Fromel T.; Gremmel C.; Driezum I. v.; Weil H.; Vestergren R.; Cousins I.. Understanding the Exposure Pathways of Per- and Polyfluoralkyl Substances (PFASs) via Use of PFASs-Containing Products—Risk Estimation For Man And Environment. In Environmental Research of the Federal Ministry of the Environment Nature Conservation and Nuclear Safety; German Federal Environment Agency: Germany, 2014. [Google Scholar]
- van der Veen I.; Hanning A.-C.; Stare A.; Leonards P. E. G.; de Boer J.; Weiss J. M. The effect of weathering on per- and polyfluoroalkyl substances (PFASs) from durable water repellent (DWR) clothing. Chemosphere 2020, 249, 126100 10.1016/j.chemosphere.2020.126100. [DOI] [PubMed] [Google Scholar]
- Liu X.; Guo Z.; Krebs K. A.; Pope R. H.; Roache N. F. Concentrations and trends of perfluorinated chemicals in potential indoor sources from 2007 through 2011 in the US. Chemosphere 2014, 98, 51–57. 10.1016/j.chemosphere.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Tokranov A. K.; Nishizawa N.; Amadei C. A.; Zenobio J. E.; Pickard H. M.; Allen J. G.; Vecitis C. D.; Sunderland E. M. How do we measure poly- and perfluoroalkyl substances (PFASs) at the surface of consumer products?. Environ. Sci. Technol. Lett. 2019, 6, 38–43. 10.1021/acs.estlett.8b00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danish Ministry of the Environment. Short-chain Polyfluoroalkyl Substances (PFAS): A Literature Review of Information on Human Health Effects And Environmental Fate and Effect Aspects of Short-chain PFAS; Danish Environmental Protection Agency: Copenhagen, Denmark, 2015. [Google Scholar]
- Vestergren R.; Herzke D.; Wang T.; Cousins I. T. Are imported consumer products an important diffuse source of PFASs to the Norwegian environment?. Environ. Pollut. 2015, 198, 223–30. 10.1016/j.envpol.2014.12.034. [DOI] [PubMed] [Google Scholar]
- US EPA (U.S. Environmental Protection Agency), Fact Sheet: 2010/2015 PFOA Stewardship Program, 2015, https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/fact-sheet-20102015-pfoa-stewardship-program.
- Zheng G.; Boor B. E.; Schreder E.; Salamova A. Indoor exposure to per- and polyfluoroalkyl substances (PFAS) in the childcare environment. Environ. Pollut. 2020, 258, 113714 10.1016/j.envpol.2019.113714. [DOI] [PubMed] [Google Scholar]
- Home Depot. The Home Depot Phasing Out Products Containing PFAS. https://corporate.homedepot.com/newsroom/phasing-out-products-containing-pfas (accessed June 16, 2021).
- Lowe’s. Lowe’s Safer Chemicals Policy, https://corporate.lowes.com/our-responsibilities/corporate-responsibility-reports-policies/lowes-safer-chemicals-policy (accessed June 16, 2021).
- California DTSC (Department of Toxic Substances Control). Safer Consumer Products Regulations – Listing Carpets and Rugs Containing Perfluoroalkyl or Polyfluoroalkyl Substances as a Priority Product. In Department of Toxic Substances Control Reference Number: R-2019-02, Office of Administrative Law Notice Reference Number: Z-2020-0218-04; California Department of Public Health: Sacramento, CA, 2021. [Google Scholar]
- OEKO-TEX. Standard 100 by OEKO-TEX, https://www.oeko-tex.com/en/downloads (Accessed June 16, 2021).
- Underwriters Laboratories. GREENGUARD Certification Criteria for Building Products and Interior Finishes, https://www.ul.com/sites/g/files/qbfpbp306/files/2019-05/GG_VOC_tables.pdf (Accessed June 16, 2021).
- Wang Z. Y.; Boucher J. M.; Scheringer M.; Cousins I. T.; Hungerbühler K. Toward a comprehensive global emission inventory of C4–C10 perfluoroalkanesulfonic acids (PFSAs) and related precursors: Focus on the life cycle of C8-based products and ongoing industrial transition. Environ. Sci. Technol. 2017, 51, 4482–93. 10.1021/acs.est.6b06191. [DOI] [PubMed] [Google Scholar]
- Lopez-Espinosa M.-J.; Mondal D.; Armstrong B.; Bloom M. S.; Fletcher T. Thyroid function and perfluoroalkyl acids in children living near a chemical plant. Environ. Health Perspect. 2012, 120, 1036–1041. 10.1289/ehp.1104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar S.; Sepulveda M. S.; Roy K.; Leszczynski J. Endocrine-disrupting activity of per- and polyfluoroalkyl substances: Exploring combined approaches of ligand and structure based modeling. Chemosphere 2017, 184, 514–523. 10.1016/j.chemosphere.2017.06.024. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski C. F.; Andrews D. Q.; Birnbaum L. S.; Bruton T. A.; DeWitt J. C.; Knappe D. R. U.; Maffini M. V.; Miller M. F.; Pelch K. E.; Reade A.; Soehl A.; Trier X.; Venier M.; Wagner C. C.; Wang Z.; Blum A. Scientific basis for managing PFAS as a chemical class. Environ. Sci. Technol. Lett. 2020, 7, 532–543. 10.1021/acs.estlett.0c00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A.; Aungst J.; Cooper J.; Bandele O.; Kabadi S. V. Comparative analysis of the toxicological databases for 6:2 fluorotelomer alcohol (6:2 FTOH) and perfluorohexanoic acid (PFHxA). Food Chem. Toxicol. 2020, 138, 111210 10.1016/j.fct.2020.111210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.