Abstract
In spite of choline’s importance in fungal metabolism, its sources in cytoplasm have not been fully established. 13C nuclear magnetic resonance analysis of mycelial extracts from day-5 Penicillium fellutanum cultures showed that, as well as choline-O-sulfate, intracellular glycine betaine is another reserve form of choline, depending on the availability of sulfate in the culture medium. These observations are discussed relative to the multiple roles of choline and its precursors in P. fellutanum.
Choline is a major component of membranes and a structural component of some microbial cell wall polymers (12, 16, 20). In some bacteria and higher plants, choline is a precursor of the osmolyte glycine betaine (GB) (2, 3, 15). In Penicillium fellutanum cultured in a medium containing 3 M NaCl, 46 and 70 mM concentrations of the osmolytes choline-O-sulfate (COS) and GB, respectively, accumulated (14). Choline stimulates hyphal extension, inhibits initiation of branching (19, 22), and is an essential nutrient for growth of choline auxotrophs of Neurospora crassa (6) and Aspergillus nidulans (9, 10).
COS is a known sulfate storage molecule in fungi (4, 5, 7), and it is also a known endogenous reserve source of choline that stimulates growth in choline-requiring auxotrophs of A. nidulans cultured in insufficient choline (11). However, Markham et al. (11) suggested that A. nidulans carrying mutations blocked in sulfate metabolism did not synthesize COS and that residual growth must have resulted from an unknown endogenous storage precursor of choline.
We previously reported that, as phosphate in the nutrient medium becomes limiting, choline phosphodiesters of P. fellutanum extracellular glycopeptide (peptidophosphogalactomannan) provide phosphate and choline, and excess choline accumulates as cytoplasmic GB and COS (13, 14). This finding was exploited to determine the relationship between COS, GB, and choline in P. fellutanum under sulfate-limiting conditions. We assume that COS is a storage form of both sulfate and choline in filamentous fungi (11). This study was focused on determining if an alternative intracellular soluble precursor of choline or COS accumulates in P. fellutanum cultured in limiting sulfate or if the concentration of choline increases in the cytoplasm.
Influence of phosphate concentration in the nutrient medium on accumulation of COS and GB.
The sources of P. fellutanum, nutrients, and l-[methyl-13C]methionine, preparation of mycelial extracts, and 13C nuclear magnetic resonance (NMR) spectroscopy analysis have been described recently (14). The 13C-methyl signals of COS (56.77 ppm) and GB (56.23 ppm) in extracts of mycelium from 200 ml of 8-day cultures in low-phosphate standard growth (LPSG) (2 mM Pi) or standard growth (SG) (20 mM Pi) medium containing l-[methyl-13C]methionine were integrated, and their magnitudes were compared with that of the 0.22% TSP [(trimethylsilane)-1-propanesulfonate] (0.00 ppm) signal, all as described previously (13, 14). COS in extracts of day-5 mycelium from LPSG and SG medium was 9.3 and 6.3 mg (dry weight) per g, respectively (14). No significant GB was found in extracts of SG mycelium. The increases in COS and GB in mycelium from LPSG medium may result from decreases in the requirements for choline and ethanolamine. No detectable soluble choline occurred in mycelium from LPSG medium. This suggests that COS and GB are the primary choline precursors or storage products.
Age-dependent accumulation of COS and GB.
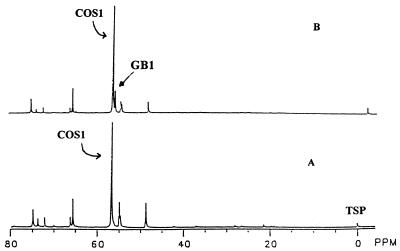
Mycelium from day-5 LPSG medium enriched with l-[methyl-13C]methionine accumulated [methyl-13C]COS with a major signal (COS1, 56.77 ppm) and two unidentified signals at 54.94 and 48.75 ppm (Fig. 1A). These signals were confirmed as 13C-methyl signals by comparison with those in an extract of mycelium of a culture supplemented with l-methionine not enriched with 13C (data not shown).
FIG. 1.
Time-dependent accumulation of choline derivatives in mycelium. Mycelial extracts were obtained from P. fellutanum cultured in 50 mg of l-[methyl-13C]methionine in 200 ml of LPSG medium. NMR spectra from day-5 (A) and day-8 (B) cultures are shown. Peak symbol abbreviations are defined in the text.
Unlike day-5 cultures, mycelium from day-8 cultures contained [methyl-13C]GB (Fig. 1B) (GB1, 56.23 ppm) as well as COS at 56.77 ppm in LPSG cultures. This suggests that accumulation of COS precedes that of GB. Accumulation of GB may depend on the depletion of a certain nutrient(s) in the culture medium. Choline did not accumulate in either set of mycelia. This conclusion is based on the absence of 13C-hydroxymethyl and 13C-N+-methylene signals for choline at 58.59 and 70.22 ppm, respectively, in the NMR spectra (13).
Effects of sulfate limitation in the culture medium on accumulation of COS and GB.
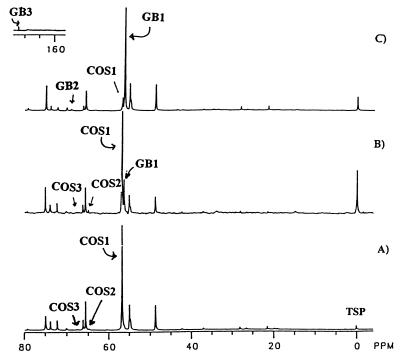
Because COS is a sulfate and choline storage molecule in filamentous fungi (11), it was reasoned that a significant level of choline might accumulate in mycelium cultured in limiting sulfate and low phosphate concentrations. Mycelium was prelabeled for 5 days with 13C by addition of 50 mg of l-[methyl-13C]methionine to LPSG cultures. The 13C spectrum of an extract from the enriched mycelium is shown in Fig. 2A. The remainder of the culture was harvested aseptically from separate flasks and transferred either to fresh LPSG medium without added l-[methyl-13C]methionine, as a control, or to fresh LPSG low-sulfate unenriched medium containing 1.53 μM Cr2(SO4)3 · 12H2O and 6.41 μM CuSO4 · 5H2O with FeCl2 · 2H2O substituted for FeSO4 · 7H2O. These 13C-labeled P. fellutanum cells were cultured for an additional 8 days; the mycelial extract from each culture was then subjected to 13C NMR analysis with 0.51% TSP as a reference. Mycelium which was transferred to fresh LPSG medium (control) (Fig. 2B) shows a slightly decreased level of cytosolic COS (carbons are designated COS1, COS2, and COS3) and the appearance of a [methyl-13C]GB signal at 56.23 ppm. In contrast, the extract of mycelium transferred to and cultured in LPSG low-sulfate medium (Fig. 2C) shows a significant decrease in COS and an increase in the GB level to that of COS shown in Fig. 2A. No choline signals are present in these spectra. These results clearly indicate that COS is a sulfate storage molecule in P. fellutanum as well as other filamentous fungi. The precipitous decrease in [methyl-13C]COS, and the large increase in [methyl-13C]GB, in the extracts of mycelium cultured in LPSG low-sulfate medium (Fig. 2C) suggests that (i) COS was converted to GB or (ii) any excess [methyl-13C]choline synthesized was converted to GB.
FIG. 2.
Influence of the concentration of sulfate in the culture medium on accumulation of intracellular COS and GB. The cytoplasmic solutes of mycelium cultured for 5 days in 200 ml of LPSG medium that was enriched with 50 mg of l-[methyl-13C]methionine are shown (A). On day 5, 13C-labeled mycelium was transferred to fresh LPSG medium and cultured for an additional 8 days (B) or transferred to fresh LPSG low-sulfate medium and cultured for 8 days (C).
Effects of adding sulfate to cultures in LPSG low-sulfate medium.
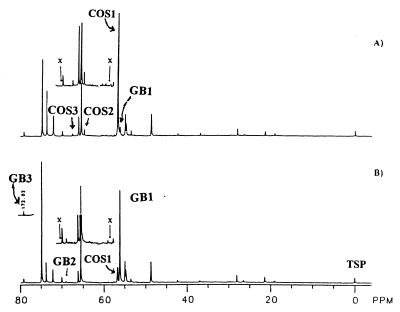
13C-methyl-labeled mycelium from LPSG low-sulfate (8 μM SO4) medium (Fig. 2C) was transferred to an LPSG–5 mM sulfate medium (Fig. 3A) or to fresh LPSG low-sulfate medium as a control (Fig. 3B) and cultured for 5 days. This experiment was performed to determine whether the levels of GB and COS in the mycelium are influenced by addition of sulfate to the culture medium. The [methyl-13C]COS signal increased significantly with a concomitant decrease of [methyl-13C]GB in mycelium cultured in LPSG high-sulfate medium (Fig. 3A). The high ratio of GB1 to COS1 signal intensities remained relatively unchanged in control mycelium (Fig. 3B). No detectable quantity of choline was observed in extracts of mycelium obtained from either set of nutritional conditions. These results suggest that COS and GB are metabolically closely related and that they are interconvertible depending upon the concentrations of sulfate and phosphate in the culture medium. However, the intensities of signals from the primary and secondary hydroxyl groups of erythritol and glycerol at 65.5 and 75.1 ppm, respectively, as well as other minor signals, were severalfold larger than those noted in Fig. 1 and 2. This effect may have resulted from the synthesis of polyhydroxy alcohols after the mycelium was transferred to fresh media.
FIG. 3.
Influence of sulfate on the accumulation of intracellular COS and GB. 13C-methyl-labeled mycelium cultured for 5 days in LPSG low-sulfate medium (shown in Fig. 2C) was transferred to fresh LPSG medium containing 5 mM Na2SO4 (A) or to fresh LPSG low-sulfate medium (B) as a control. The region of 72.50 to 56.80 ppm is shown with twofold enlargement in signal height in the insets. “x” placed at 58.59 and 70.22 ppm indicates the absence of signals representing the choline hydroxymethyl and N-methylene carbons, respectively.
Similar experiments, with [2-13C]glycine as the source of 13C, resulted in enriching of all carbons in GB (GB1, 56.23 ppm; GB2, 69.04 ppm; GB3, 172.04 ppm) and COS (COS1, 56.77 ppm; COS2, 64.90 ppm; COS3, 67.68 ppm) with 13C (data not shown). No signals indicative of 13C-enriched choline were noted. These data suggest that all carbon atoms of GB are converted to COS.
The results suggest that choline accumulates primarily as COS when the medium contains adequate sulfate but that choline is stored in the cytoplasm as GB if the medium is deficient in sulfate. The organism has the capability of converting GB to COS upon transfer of the culture to LPSG–5 mM sulfate nutrient medium.
GB as a potential intracellular alternative reserve of choline.
COS accumulation is common among filamentous fungi (8, 17, 18), and Markham et al. (11) concluded that the role of COS in fungal physiology is as a storage source of sulfur, based on the observation (9) that choline-requiring auxotrophs of A. nidulans continued growth in the absence of added choline. However, Arst (1) argued against COS being the endogenous source of choline because, under choline-deficient conditions, A. nidulans double mutants, carrying the choA1 mutation and a mutation that makes the organism unable to either synthesize or utilize COS, showed growth equivalent to that of the choA1 mutant. It was suggested that such residual growth is due to choline supplied from sources other than membrane phospholipids or endogenously stored choline (11). We reported previously that a phosphocholine-containing P. fellutanum peptidophosphogalactomannan is a precursor of intracellular COS and GB (13) when the organism is cultured in LPSG medium. Based on the observation that a detectable level of soluble choline does not accumulate in the mycelium in a sulfate-deficient medium and that a loss of cytoplasmic COS and an increase of GB results, we now conclude that GB is another endogenous storage precursor of choline in P. fellutanum and is likely the unknown precursor of choline that Markham et al. predicted (11). This conclusion was strengthened by the demonstration that adjusting the culture’s concentration of sulfate to 5 mM resulted in the near depletion of GB and the return of cytoplasmic COS as the major storage form of choline.
Physiological functions, such as regulation of the rate of hyphal extension and the frequency of branching of hypha, were shown in Fusarium graminearum (strain A3.5) to be sensitive to the concentration of choline in a range of 1 to 5 μM (22). A biochemical mechanism in P. fellutanum for simultaneously maintaining a low cytoplasmic choline concentration and storing excess absorbed choline as COS and/or GB under widely variable nutritional and environmental conditions must occur. This ability to store excess choline as GB and COS in concentrations ranging up to 70 mM (14) provides evidence of the importance of conservation of choline by this Penicillium sp. and some insight into the interrelationships between the apparently unrelated physiological functions of hyphal extension, medium osmolarity, and sulfate storage.
P. fellutanum cultures in SG medium with 20 mM phosphate and added l-[methyl-13C]methionine apparently store a large portion of excess 13C-methyl residues in phosphocholine phosphodiester residues of peptidophosphogalactomannan (13); methyl carbons of GB and COS are not significantly enriched with 13C under these conditions, nor are signals at the chemical shifts of naturally abundant [13C]choline carbons detected (data not shown).
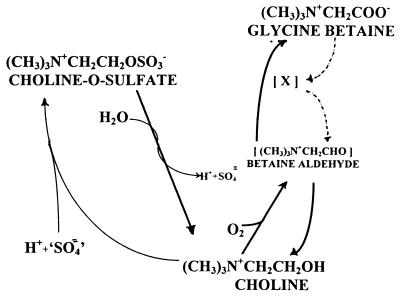
The pathway of reaction intermediates through which GB is converted to choline is unknown. However, there exists in the l-threonine biosynthetic pathway an ATP-dependent conversion of aspartate to aspartyl-β-phosphate, followed by reductive formation of aspartyl-β-semialdehyde and phosphate (21). A similar reaction type, shown in Fig. 4, in which the carboxylate group of GB is activated (phosphorylated) and followed by reductive release of the acid group, resulting in the formation of betaine aldehyde and its reduction to choline, has biochemical precedent.
FIG. 4.
Metabolic relationships of choline and three of its derivatives in biological systems. The known reactions (solid lines) and the proposed conversion of GB to betaine aldehyde (broken lines) are shown. The latter two reactions depict the activation of the GB carboxyl group to form [X], followed by the reductive cleavage of [X] to betaine aldehyde and an unknown activating acidic agent (not shown). ‘SO4,’ activated SO4.
Acknowledgments
This work was supported by the Florida Agricultural Experiment Station (journal series no. R06449), Gainesville, Fla.
We thank Sandra J. Bonetti, Department of Chemistry, University of Southern Colorado, Pueblo, and Clifford J. Unkefer, Los Alamos National Laboratory, Los Alamos, N.M., for reviewing the manuscript and for useful comments.
REFERENCES
- 1.Arst H N. Genetic analysis of the first steps of sulphate metabolism in Aspergillus nidulans. Nature. 1968;219:268–270. doi: 10.1038/219268a0. [DOI] [PubMed] [Google Scholar]
- 2.Canovas D, Vargas C, Csonka L N, Ventosa A, Nieto J J. Synthesis of glycine betaine from exogenous choline in the moderately halophilic bacterium Halomonas elongata. Appl Environ Microbiol. 1998;64:4095–4097. doi: 10.1128/aem.64.10.4095-4097.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Csonka L N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989;53:121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gravel R A. Choline-O-sulphate utilization in Aspergillus nidulans. Genet Res. 1976;28:261–276. doi: 10.1017/s0016672300016955. [DOI] [PubMed] [Google Scholar]
- 5.Harada T, Spencer B. Choline sulphate in fungi. J Gen Microbiol. 1960;22:520–527. doi: 10.1099/00221287-22-2-520. [DOI] [PubMed] [Google Scholar]
- 6.Horowitz N H, Bonner D, Houlahan M B. The utilization of choline analogues by cholineless mutants of Neurospora. J Biol Chem. 1945;15:145–151. [Google Scholar]
- 7.Hussey C, Oris B A, Scott J, Spencer B. Mechanisms of choline sulphate utilization in fungi. Nature. 1965;207:632–633. doi: 10.1038/207632b0. [DOI] [PubMed] [Google Scholar]
- 8.Itahashi M. Comparative biochemistry of choline sulfate metabolism. J Biochem. 1961;50:52–61. doi: 10.1093/oxfordjournals.jbchem.a127408. [DOI] [PubMed] [Google Scholar]
- 9.Markham P, Bainbridge B W. Effect of choline deprivation on the growth, morphology and ultra-structure of a choline-requiring mutant of Aspergillus nidulans. FEMS Microbiol Lett. 1992;90:217–222. doi: 10.1016/0378-1097(92)90746-b. [DOI] [PubMed] [Google Scholar]
- 10.Markham P, Bainbridge B W. Characterization of a new choline locus in Aspergillus nidulans and its significance for choline metabolism. Genet Res. 1979;32:303–310. doi: 10.1017/s0016672300018802. [DOI] [PubMed] [Google Scholar]
- 11.Markham P, Robson G D, Bainbridge B W, Trinci A P J. Choline: its role in the growth of filamentous fungi and the regulation of mycelial morphology. FEMS Microbiol Rev. 1993;104:287–300. doi: 10.1111/j.1574-6968.1993.tb05872.x. [DOI] [PubMed] [Google Scholar]
- 12.Mosser J L, Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970;245:287–298. [PubMed] [Google Scholar]
- 13.Park Y-I, Buszko M L, Gander J E. Recycling of phosphocholine from extracellular complex polysaccharide into cytoplasmic choline derivatives in Penicillium fellutanum. J Bacteriol. 1997;179:1186–1192. doi: 10.1128/jb.179.4.1186-1192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park Y-I, Gander J E. Choline derivatives involved in osmotolerance of Penicillium fellutanum. Appl Environ Microbiol. 1998;64:273–278. doi: 10.1128/aem.64.1.273-278.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhodes D, Hanson A D. Quaternary ammonium and ternary sulfonium compounds in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:357–384. [Google Scholar]
- 16.Rolin D B, Pfeffer P E, Osman S F, Szwergold B S, Kappler F, Benesi A J. Structural studies of a phosphocholine substituted β-(1,3)-β-(1,6) macrocyclic glucan from Bradyrhizobium japonicum USDA 110. Biochim Biophys Acta. 1992;1116:215–225. doi: 10.1016/0304-4165(92)90014-l. [DOI] [PubMed] [Google Scholar]
- 17.Spencer B, Hussey E C, Orsi B A, Scott J M. Mechanism of choline-O-sulfate utilization in fungi. Biochem J. 1968;106:461–469. doi: 10.1042/bj1060461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spencer B, Harada T. The role of choline sulfate in the sulphur metabolism of fungi. Biochem J. 1960;77:305–315. doi: 10.1042/bj0770305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trinci A P J. Regulation of hyphal branching and hyphal orientation. In: Jennings D H, Rayner A D M, editors. Ecology and physiology of the fungal mycelium. Cambridge, United Kingdom: Cambridge University Press; 1984. pp. 23–52. [Google Scholar]
- 20.Unkefer C J, Jackson C L, Gander J E. The 5-O-β-d-galactofuranosyl-containing exocellular glycopeptide from Penicillium charlesii. Identification of phosphocholine attached to C6 mannopyranosyl residues of the mannan region. J Biol Chem. 1982;257:2491–2497. [PubMed] [Google Scholar]
- 21.Vernon M, Falcoz-Kelly F, Cohen G N. The threonine-sensitive homoserine dehydrogenase and aspartokinase activities of Escherichia coli K12. The two catalytic activities are carried by two independent regions of the polypeptide chain. Eur J Biochem. 1972;28:520–527. doi: 10.1111/j.1432-1033.1972.tb01939.x. [DOI] [PubMed] [Google Scholar]
- 22.Wiebe M G, Robson G D, Trinci A P J. Effect of choline on the morphology, growth and phospholipid composition of Fusarium graminearum. J Gen Microbiol. 1989;135:2155–2162. doi: 10.1099/00221287-135-8-2155. [DOI] [PubMed] [Google Scholar]