Abstract
Although conventional cigarette smoking is declining, emerging tobacco related products (ETRPs) are currently gaining ground, especially among the youth. These products include electronic cigarettes, waterpipes/hookah, cigars/cigarillo, smokeless tobacco, and heat-not-burn cigarettes. The observed increase in the use of ETRPs is multifactorial and complex but appears to be mainly driven by efforts from the major tobacco companies to reinvent themselves, and present more appealing and allegedly safe(r) tobacco products. However, it is becoming apparent that these products produce substantial amounts of toxic chemicals, many of which have been shown to exert negative health effects, including in the context of the cardiovascular system. Thus, there has been research efforts, albeit limited in general, to characterize the health impact of these products on occlusive/thrombotic cardiovascular diseases (CVD). In this review, we will discuss the potential impact of ETRPs on thrombosis-based CVD. Specifically, we will review how these products and the major chemicals they produce and/or emit can trigger key players in the process of thrombosis, namely inflammation, oxidative stress, platelets, coagulation, and the vascular endothelium, and the relationship between these effects.
Introduction
There is overwhelming evidence that conventional cigarette smoking (CS) is a major risk of cardiovascular disease (CVD), not only to active users, but also individuals within close vicinity [1, 2]. For instance, CS in both men and women increases the incidence of myocardial infarction (MI) and fatal coronary artery disease (CAD) [3]. Similarly, studies also demonstrated that even low-tar cigarettes increase the risk of acute cardiovascular events in comparison to nonsmokers [4]. Along the same lines, passive smoking or environmental tobacco exposure- with a smoke exposure about that is only 0.01% that of active CS- was found to be associated with approximately a 30% increase in risk of CAD, compared with an 80% increase in active smokers [1]. It is worth mentioning that the risk of CVD due to CS is both dose and duration dependent [3, 5, 6]. In short, the available set of facts- that took decades to accumulate- document a clear link of CS to major negative health consequences, particularly in the case of CVD. This body of evidence, along with efforts from private and public entities mounted “push back” campaigns against CS use, which is considered a public health problem. Consequently, these efforts have resulted in noticeable decline in CS use in the United States (US) and Europe [7]. Nonetheless, although CS has experienced a declining trend on a global scale [8], emerging tobacco related products (ETRP) are gaining ground [9], especially among the youth [10, 11], and women of childbearing age [12, 13]. The observed increase in the use of ETRPs is multifactorial and complex but appears to be mainly driven by efforts from the major tobacco companies to reinvent themselves, and present more appealing and allegedly safe(r) tobacco products [14–16]. Fortunately, nonetheless, these ETRP are now the focus of rigorous research efforts-including from our team- to understand their safety profile and their effects on health outcomes, including in the context of thrombotic CVD. It is of utmost importance to note that thrombosis is a major mechanism of occlusive CVDs. To this end, thrombosis is defined as the pathological condition in which a blood clot is formed within blood vessels, lead to obstruction of blood flow, and that can manifest as a MI or stroke. Major players in thrombosis include hyperactive platelets, activated coagulation system, and vascular endothelium injury. Therefore, in this review we will discuss the potential impact of ETRPs on thrombotic based CVD. Furthermore, we will review how these products and the major chemicals they produce/emit can trigger inflammation, and oxidative stress, as well as how they impact platelets, coagulation, and the vascular endothelium- all of which are key players in the process of thrombosis- and the relationship between these effects.
Emerging Tobacco Related Products
ETRPs are group of products that include electronic cigarettes, waterpipes/hookah, cigars/cigarillo, smokeless tobacco, and heat-not-burn (HNB) cigarettes (see Table 1). These products emerged on the premise of their safe profile – especially in current smokers trying to quit - in comparison to traditional cigarettes, and this assumption made them very appealing to many including the youth (e.g., high and middle school students; Figure 1A). In the next section, we will describe the different ETRPs and their health impact especially in the context of thrombotic CVDs.
Table 1:
different type of emerging tobacco products and the impact on different aspect of hemostasis.
| Product | Common brand | Common Flavors | Common Thrombosis-Dependent Mechanisms | Toxicant / Chemical |
|---|---|---|---|---|
| Electronic cigarettes (e-cigarettes, vapes) Open and Close systems 
|
NJOY, JUUL, Vuse, Mart Ten, Blu, Logic |
|
||
| Waterpipes (Hookah, E-Hookah) 
|
Starbuzz, Tangiers, Al-Fakher tobacco brands* |
|
||
Cigars and cigarillo 
|
|
|
No sufficient data available | |
| Heat-not-burn tobacco product (Heated tobacco products) |
iQos, Plom | Tobacco, menthol, bubble gum and lime[209] | ||
Smokeless tobacco products
|
Pouches: ZYN, DRYFT, On! Snus: Swedish Snus |
Figure 1:
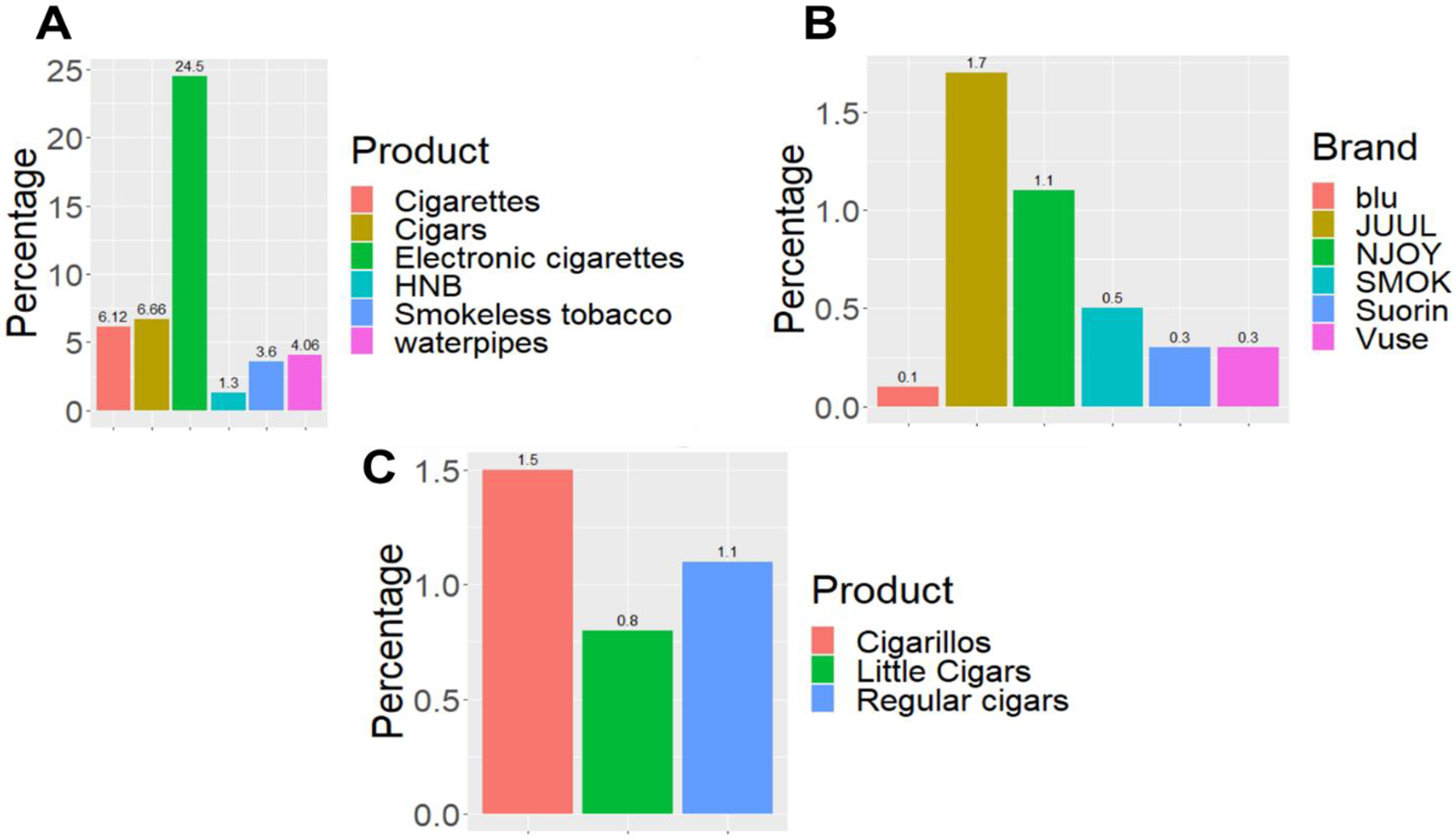
A) Current Use Estimates for Selected Tobacco Products for Middle and High School Students-United states (2020). Data for the national youth tobacco survey, total population (N=14495). B) Percentage of E-cigarette Brands Used Among Middle and High School Students-United states (2020). Data obtained from the national youth tobacco survey, and based on the question (During the past 30 days, what brand of e-cigarettes did you usually use? Choose only one answer). C) Percentage of Cigar Products Used Among Middle and High School Students-United states (2020). Data obtained from the national youth tobacco survey. and based on the question (During the past 30 days, which of the following types of cigars, cigarillo, or little cigars have you smoked?)
E-cigarettes
Electronic cigarettes, also referred to as “e-cigs”, “vapes”, and “electronic nicotine delivery systems” (ENDS), are devices that include a power source, a heating element, and a prefilled or e-liquid refillable tank or cartridge (pods). These battery-operated devices exist in different sizes, colors, and shapes, some of which are like cigarettes and pipes. Newer generations on the other hand, are similar to USB flash drives or slim pens [17]. Notably, e-cigarettes contain different percentages of nicotine and flavors [18] that are dissolved in glycerol and propylene glycol. These are the main constituents of the e-liquid, which is heated to generate aerosols [19]. An analysis of brands (Figure 1B) websites revealed that there are more than 15,000 flavors and the most common are tobacco, menthol, alcohol/drink, fruit and dessert/candy [20] Since 2015, the popularity of the USB-like device JUUL has grown significantly, becoming the most popular device sold in the US [21]. To this end, and likely due to their success, other companies have also manufactured separate e-cigarette devices containing prefilled e-liquid in pods like JUUL [22]. It is noteworthy that although e-cigarettes do not emit large smoke clouds in comparison with traditional cigarettes, the pods may in fact contain as much nicotine as a pack of 20 regular cigarettes [22]. Indeed, e-cigarettes have the capacity to deliver levels of nicotine similar to traditional cigarettes. Additionally, a vast number of e-cigarette users are dual users, meaning that they use both types of cigarettes (traditional and electronic), and hence are consuming toxic substances from both products [23]. Importantly, e-cigarettes were found to be associated with MI even after adjusting for conventional cigarette use [24]. Furthermore, it was observed that there is an association between some days e-cigarette use and MI (odd ratio: 2.11, 95% CI: 1.14–3.88, p = 0.017), as well as between coronary heart disease and daily e-cigarette use (odd ratio: 1.89, 95% CI: 1.01–3.53, p = 0.047; [25]). Also, there is evidence that e-cigarette use is associated with stroke, when used with conventional cigarette smoke (dual use), even when compared with current sole conventional cigarette use in young adults [26]. Finally, the use of e-cigarettes is shown to be associated with increase in platelet activity[27], a cell that is known to be a main player in thrombotic CVDs.
Waterpipes
The use of waterpipes, also known as hookah or shisha, has been rising around the world, mainly in young adults [28]. Many factors have been attributed to their popularity, such as the use of flavored tobacco, marketing, and the misperceptions about their adverse health effects [29]. The tobacco used in waterpipes is also called mouassal “honeyed tobacco”, which usually contains 30% tobacco and around 70% honey/sugarcane, as well as glycerol and flavors [30]. Most recently, e-hookah was introduced as a healthier alternative to the regular charcoal operated waterpipes, and it was “picked up” quickly by users [31]. Two types of e-hookahs are available in the market, e-hookah pens (they are mostly disposable) and e-hookah bowls (used in similar manner to the regular hookah or waterpipe). Interestingly, a recent study has shown that e-hookahs have a distinct user profile (common among females and higher prevalence of substance use) when compared to e-cigs [32]. In terms of their negative health effects, indeed, data has shown that smoking waterpipes is associated with MI [33, 34], and we have recently shown that they can directly induce occlusive thrombotic disorders [35]. Nevertheless, data that link waterpipes to negative clinical outcomes are generally limited, and hence more research is needed in this area.
Cigars/Cigarillos
Cigars represent perhaps the simplest form of tobacco products, in which tobacco is wrapped in a “tobacco” leaf, which is different from a cigarette in which tobacco roll is wrapped in paper. Cigarillo is a common and distinct cigar product [36], and is shown to be increasingly used among youth [37] (Figure 1C). In addition, cigarillo users are most likely to also be users of multiple tobacco products[38], and tend to be low income, and represent racial/ethnic minority youth living in urban centers[39, 40]. Concerningly, studies on the use of cigars, toxicant exposure, and health effects are limited [41]. Nonetheless, cigar smoking has been associated with increased risk of CAD, and aortic aneurysms [42]. This should be reconciled with the rather interesting fact that the use of cigars is becoming more common among youth; primarily because of curiosity, peer influence, and low cost [43].
Heat-Not-Burn tobacco products
The heated tobacco products, also known as heat-not-burn (HNB) tobacco products, represent the most recent “invention” from the tobacco industry, and are claimed to be less harmful. These battery-operated devices heat tobacco to a maximum of 350°C (500°F) to generate an inhalable aerosol [44]. They also contain nicotine, propylene glycol, flavors and other tobacco particles [45, 46]. HNB products contain as much nicotine as traditional cigarettes, although the levels of nicotine in the aerosols may be lower than those in cigarettes [47]. Of note, the heated tobacco products are the least well-characterized products among the ETRP. Nevertheless, it has been indicated that due to the lack of combustion in HNB, less toxicants are formed in the aerosol of these products than in cigarette smoke [48]. However, the aerosol of heated tobacco contains the same acrolein, formaldehyde, benzaldehyde, acenaphthylene, nicotine, carbon monoxide, and particulates that are the harmful constituents of conventional cigarette smoke [45]. Nonetheless, there is limited data on the effects of HNB on general health and cardiovascular endpoints; a knowledge gap that warrants attention.
Smokeless Tobacco
Smokeless tobacco products contain fire-cured tobacco that is powdered for nasal or oral snuff use, and is also grated for chewing use[49]. The effect of smokeless tobacco on the cardiovascular system is controversial. On one hand, a systematic review indicated that there is insufficient data to link smokeless tobacco to CVD, including thrombotic events[50]. On the other hand, data have shown that smokeless tobacco products are not harmless, and that for instance, their long-term use may be associated with an elevated risk of MI and stroke [51]. One main assumption driving the use of smokeless tobacco is that they help in smoking cessation. This assumption was disputed by a randomized trial, which concluded that success of smokeless tobacco products as means of smoking cessation is low, and that they are indeed associated with risks for long-term adverse events [52]. A large case-control study found that smokeless/chewed tobacco increased the risk of MI and the highest increase in the risk of acute MI was in smokers who also chewed tobacco [53].
In summary, although the available data are in general still preliminary and there is an urgent need for more longitudinal studies, they indicate that ETRPs are instigators of thrombotic events. Thus; in the next section we will focus on what is known regarding the major mechanisms that play a role in ETRF-mediated thrombosis
Emerging Tobacco Related Products and Thrombosis
Thrombosis, which is the formation of blood clots inside blood vessels, can lead to a reduction in the blood supply to organs, and consequently hypoxia and/or tissue damage. Hemostasis on the other hand, is the physiological balanced response to vascular/circulatory injury that results in the formation of a platelet-fibrin clot, which prevents further bleeding. The hemostatic machinery integrates many players, including the endothelium, the clotting factors (e.g., fibrinogen) and importantly platelets, working along the fibrinolytic system [54]. These elements are there to ensure the formation of a stable clot that can seal the damaged blood vessel. Exaggerated responses to endothelial injury can lead to thrombosis [55], a critical event that is associated with MI and stroke [56]. Thrombosis can also lead to venous thromboembolic events, which account for considerable morbidity and mortality [57]. It has been shown that for thrombosis to take place there must be 1) an abnormal pro-thrombotic activity (e.g., hyperactive platelets[58], increase plasma fibrinogen[59]), 2) abnormal antithrombotic activity (e.g., defect in the inhibitory prostaglandin/PGI2’s antiplatelet function[60]), 3) an increase in thrombin generation[61], 4) vascular cell damage[62], 6) and fibrinolysis system failure or inhibition[63]. These processes are shown to be impacted by a number of ETRPs (Table1). These data provide evidence that ETRPs indeed present a risk in developing thrombotic CVDs, through modulating different aspects of hemostasis. Nonetheless, these studies are limited by the sample size used and the observational nature of their design, thereby opening the opportunity for more longitudinal studies, which would be expected to contribute more meaningful results in linking these products to thrombotic CVDs.
In this connection, and much like CS[64, 65], ETRPs are found/shown to produce proinflammatory toxic chemicals and reactive oxygen species [66–70], both of which are instigators/promoters of the thrombotic process, which is essential for development of occlusive CVDs. Surprisingly, there has not yet been any clear model to link these processes together in a manner that would aid not only in understanding the complete picture of the disease conditions, but also how these processes work in an intertwined manner to form a continuous cycle of harm to the users of these products.
Emerging Tobacco Related Products, Inflammation, Oxidative Stress, and Thrombosis
It is inferred from the evidence in the literature that inflammation, oxidative stress, and thrombosis are interconnected processes that can be very detrimental to our cardiovascular system health. Therefore, it is imperative to discuss this process in one framework to appreciate their role in the disease state. To this end, animal and human studies (including data from Population Assessment of Tobacco and Health Study (PATH) indicate that the majority if not all of the tobacco products can induce inflammation and oxidative stress [66–71]. For instance, e-cigarette usage was found-some of which by our group- to be associated with increase in proinflammatory proteins IL6, TNFα, and CXCL8[67], ROS[72] and a heightened risk of thrombosis [27, 73]. These effects are possibly due to the proinflammatory toxic chemicals produced by heating the e-liquid. Similarly, waterpipes are known to produce many proinflammatory toxic chemicals [66]. In fact, it is now known that waterpipes increase proinflammatory cytokines IL-6, IL-8, IL1β and TNFα, as well as markers of oxidative stress such as 8-isoprostane, myeloperoxidase, and matrix metalloproteinase-9[74], all of which are preconditions for development of CVD [66, 75, 76]. Smokeless tobacco has also been shown to cause inflammation [77] and to produce oxidative stress [78]. Most recently, HNB products were found to induce oxidative stress and inflammation [79–81] in a manner similar to conventional cigarettes. It should be noted that inflammation and oxidative stress are strong instigators of thrombosis, the main pathology of acute occlusive cardiovascular events.
With regard to the interconnectedness of these processes, it has been observed that inflammation can shift the hemostasis response toward a prothrombotic state [82]. In fact, data have shown that inflammation may also cause a tendency to develop arterial and venous thrombosis, leading to a wide range of clinical presentations ranging from mild disease to life-threatening situations [82]. It is noteworthy that inflammation is considered one of the main mechanisms by which a host defends itself against external foreign entities, including toxic chemicals[83]. Physiological inflammation is self-limited and beneficial to the host. However, if inappropriately stimulated, excessive inflammation may fail to resolve, thereby creating an environment for “pathogenesis” [84]. One reason that the inflammatory process may fail to resolve is because of continuous exposure to toxic chemicals, including those emitted by tobacco products [85]. The activation of immune cells (e.g., neutrophils, and monocytes)- driven by proinflammatory stimuli- leads to the production of reactive oxygen species (ROS; e.g. hydrogen peroxide, and hydroxyl radical), reactive nitrogen species (e.g., peroxynitrite), and chlorine species (hypochlorous acid), which are the main players of the oxidative stress state [84]. Exaggerated production of ROS predisposes the host to thrombotic state by modulating endothelial function [86], and activating platelets[87] - potentially through leukocyte release of superoxide. Furthermore, ROS impacts polymorphonuclear leukocytes, leading to the production of tissue factor, which is a major trigger of the coagulation system [88, 89]. In fact, a previous study suggested that NAD(P)H oxidase activation and ROS generation are involved in tissue factor upregulation in activated platelets[90]. In addition, superoxide generated in hyperactive platelets might be another mechanism that contributes to the thrombotic state [90]. Oxidative stress also upregulates plasminogen activator inhibitor-1 (PAI-1) and alters the bioavailability of nitric oxide in endothelial cells, which consequently promotes thrombus formation [91]. Interestingly, studies have shown that platelets and coagulation, which are part of the thrombotic state, can augment the inflammatory process by means of modulating proinflammatory cytokines and growth factors[82]. The mechanism by which coagulation modulates inflammation is primarily through binding of thrombin and some other coagulation factors to the protease-activated receptors (PARs)[92]; which are located on the endothelium, platelets, fibroblasts, and smooth muscle cells. While PAR-1, PAR-3, and PAR-4 are mainly interacting with thrombin, PAR-2 is thought to potentially serve as a receptor for both the “TF-factor VIIa” complex and factor Xa. Moreover, thrombin is known to interact with these receptors, which induces the production of growth factors and cytokines (e.g., IL-6 and IL-8), thereby leading to up-regulation of inflammatory responses[93]. Moreover, studies have shown that platelet activation leads to production of ROS, which becomes a potential source of further oxidative stress[94]. Taken together, the use of ETRPs-in a manner similar to conventional cigarette- can induce inflammation and oxidative stress (through the action of toxic chemicals). Consequently, these activities modulate hemostasis and lead to thrombosis, which can directly potentiate the inflammatory and oxidative stress processes, thereby creating a potentially dangerous, yet vicious cycle (Figure 2)[82, 95, 96].
Figure 2: The vicious cycle of thrombotic disease.
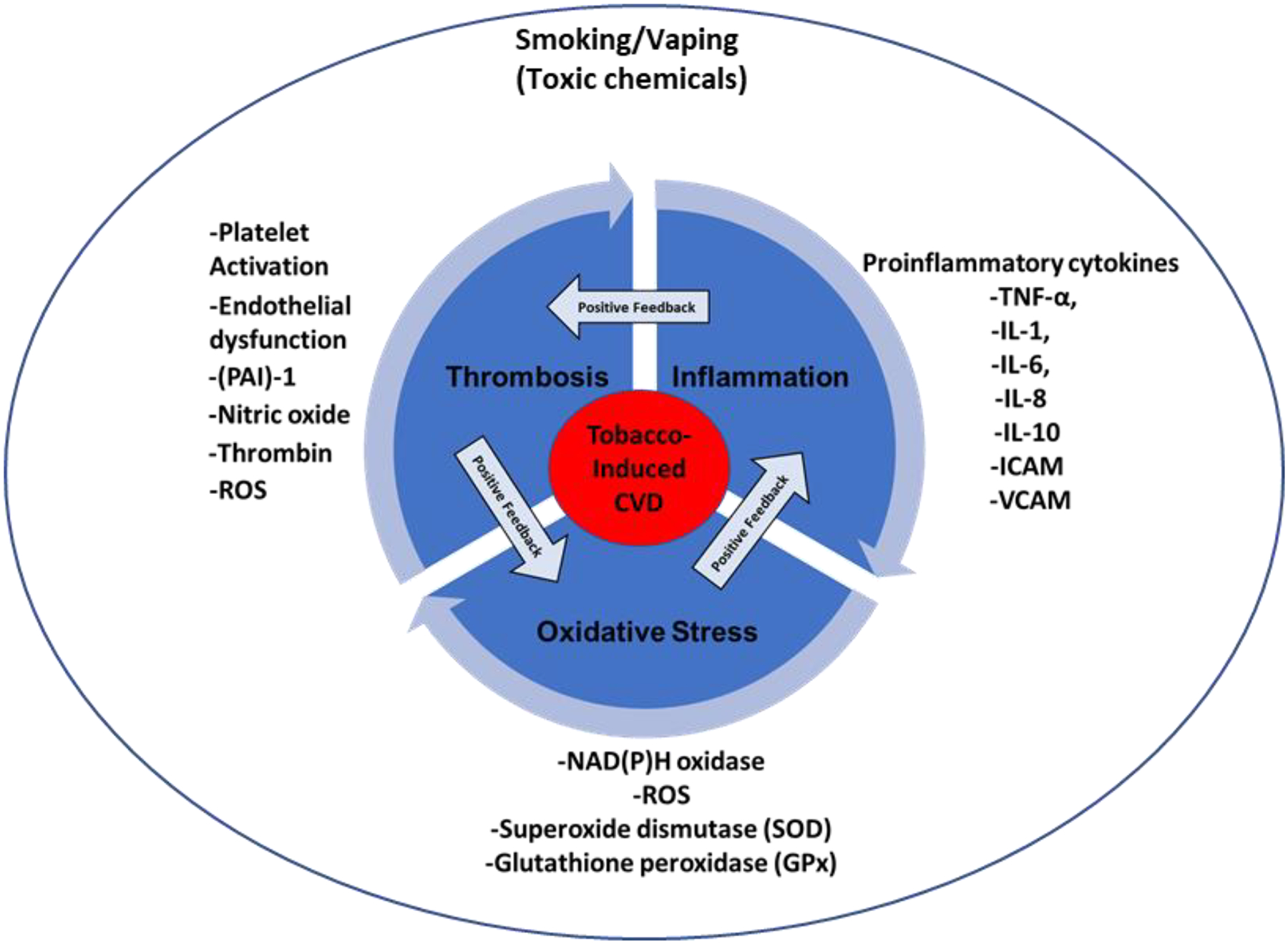
Inflammation instigates oxidative stress through the release of ROS from excessively activated immune cells and the consequent production of proinflammatory cytokines. Oxidative stress impacts different aspects of hemostatic process, which consequently leads to thrombosis. Thrombosis participates in increasing the inflammatory process through different mechanisms including thrombin-induced proinflammatory production.
Toxic Profile of Emerging Tobacco Related Products.
Although the levels of toxic chemicals emitted by ETRPs vary, it is important to note that these tobacco products are not emission free, and the toxic chemicals they emit are hazardous (Table 1), and have the capacity to lead to serious health issues (including CVD), even at low levels [97]. According to accumulating data, tobacco’s toxic chemicals that received the greatest scrutiny as possible contributors to CVD, include nicotine[98], particulate matter[99], polyaromatic hydrocarbons (PAHs)[100], and to a lesser extent tobacco-specific nitrosamine (TSNAs). Indeed, some of these chemicals have been proven to profoundly contribute to the cardiovascular hemodynamic instability and thrombotic effects associated with tobacco use[1]. Furthermore, they also participate in the development of a proinflammatory state that is mechanistically involved in “smokers”-associated thrombogenesis [82]. In the next section, we will focus on the specific role of the major toxic components of ETRPs, and the data so far linking them to thrombotic conditions.
Role of various chemical components of emerging tobacco related products in thrombogenesis
Nicotine
Like conventional cigarettes, ETRPs contain considerable amounts of nicotine. For example, e-liquids, which are used in most ENDS such as e-cigs typically contain nicotine at concentrations ranging between 0–87.2 mg/ml[101], albeit some e-liquids are “nicotine free”. Furthermore, JUUL, which is one of the popular ENDS, contains e-liquid with nicotine concentrations that could reach as high as 59 mg/mL [102]. With regard to waterpipe, the average nicotine content ranges between 67 – 713 mg per head [103]. Smokeless tobacco also contains considerable amounts of nicotine, which is absorbed more slowly than that of cigarettes. It is noteworthy that the nicotine concentration delivered/inhaled/absorbed by the user depends on a multitude of factors, including frequency of use and puffing patterns (puff number and duration) [104, 105].
Rather interestingly, and perhaps contrary to common beliefs, the majority of published data attached little importance to the role of nicotine as a contributor to tobacco-induced thrombotic diseases[1]. In fact, a significant body of knowledge concluded that nicotine’s direct effect on the development of tobacco induced thrombosis is negligible, and its role centers more on hemodynamic instability[106]. Furthermore, these studies suggested that nicotine contributes indirectly to thrombosis, by inducing atherosclerotic plaque development [1, 106–108]. In contrast, other data showed that exposure to nicotine may increase PAI-1, which is a major regulator of fibrinolysis [109]. Also, it was found that nicotine is a potential modulator of nitric oxide, which normally inhibits platelet activation [110]. It is important to note that some studies found nicotine to be involved in modulating platelet reactivity [111–115], some of which by employing urinary Thromboxane A2 (TXA2) metabolite excretion. Of note, while we employed the well-known nicotine metabolite cotinine, we showed that it potentiates platelet aggregation in response to thrombin[35]. This is significant given that platelets are a critical player in the process of thrombosis.
Particulate Matter (PM)
Studies have shown that conventional cigarettes expose humans to nearly 40,000 μg of particulate matter (PM) [116]. These particles have a mean diameter of <1 μm, which allows a high degree of deposition inside the human body [117], and thus makes them very hazardous. In fact, studies have shown that PM is associated with increased hospitalization and mortality due to CVD [118]. In a striking similarity, ETRPs emit very comparable levels of PM to conventional cigarettes. Thus, studies have shown that e-cigs and waterpipes produce TPM in the range of 0.87–5.8 mg/puff, and 1.8–9.3 mg/puff, respectively, in comparison to 0.1–1.7 mg/puff from conventional cigarettes [119]. Consequently, in light of the substantial difference in the puffing topography between cigarettes (have an average of 18 puff/cigarette)[120–122] and other products, such as e-cigs (average >150 puff/day) [123] and waterpipe (<171 puff/session)[66, 124], these products are even more dangerous with regard to PM emission. Likewise, there is data showing that HNB tobacco emits an average of 44 mg/cig compared to 36 mg/cig with conventional cigarettes, indicating that heating tobacco might be as “bad” as combustion in generating PM [10, 125]. To this end, accumulating evidence shows that exposure to PM does induce a prothrombotic state, which might involve arterial and venous thrombotic events [126, 127]. Consistent with this notion, exposure to PM triggers the production of fibrinogen, Von Willebrand factor, sP-selectin, and sCD40L, all of which are important players in the processes of hemostasis and thrombosis [128–131]. Furthermore, data have also shown that PM exposure causes a defect in the fibrinolysis mechanisms that normally would help in limiting clot expansion, which is consistent with the prothrombotic state. Specifically, exposure to PM led to inhibition of tissue the plasminogen activator (t-PA) [132]. Additionally, this effect was not only limited to t-PA, as PM exposure was also found to upregulate PAI-1, which is considered a risk factor for thrombosis [133, 134]. Taken together, these reports are in complete accord with previous studies that linked PM exposure with increases in plasma viscosity, platelet activation, and modulation of coagulation [135–138]. Interestingly, evidence appears to suggest that a far greater impact on hemostasis is associated with ultrafine particles (PM0.1) rather than coarse particles (PM10) and fine dust (PM2.5). Thus, in patients with metabolic syndrome, short-term exposure (2 h) to PM0.1 caused a decrease in blood plasminogen and thrombomodulin and increased C-reactive protein (CRP; a biomarker for CVD) [139]. In addition, separate cohort studies (humans) have showed that ultrafine particles are associated with modulation of the hemostatic process and increase in the inflammatory state in exposed in comparison to control [140, 141]. Another study with a large sample size found that mortality from ischemic heart disease was more strongly associated with PM0.1 relative to other PM [142]. Finally, it has been documented that cardiovascular mortality is correlated with particle size, and the correlation tends to be more profound as the particle size decreases [143, 144]. Collectively, there is ample evidence indicating that PM is indeed a common harmful toxic element across variety of emergent tobacco products, even/including those that claimed to be less harmful than the conventional cigarette. Nevertheless, more studies are needed to investigate the mechanism and pharmacodynamics of all sizes and types of tobacco-derived PM on the various elements of hemostasis and thrombotic mechanisms.
Carbon Monoxide
Carbon monoxide (CO) is a product of partial combustion or oxidation of carbon in tobacco products. It is established that a conventional cigarette produces CO, which is known to be associated with negative health effects[145]. Once generated, CO finds its way to the pulmonary system and gets absorbed into the circulation. With the exception of e-cigs, almost all ETRPs have been shown to produce CO, albeit at varying concentrations [119, 146]. For instance, conventional cigarettes can emit 1–2.3 mg/puff in comparison to 1.15–1.67 mg/puff and 0.531mg/12 puff in case of waterpipe and HNB tobacco, respectively. As for its effect on the hemostasis system, excessive exposure to CO was found to increase the risk of deep venous thrombosis and pulmonary embolism; disease states known to be associated with high mortality [147]. Moreover, CO can enhance the coagulation cascade, inhibit fibrinolysis, and expediate clot growth, all of which favor a prothrombotic state [148]. It was also shown that water-soluble CO-releasing molecules (CORMs) are able to increase the speed by which clot formation happens, as well as inhibit t-PA-based fibrinolysis [149]. Interestingly, CO seems to have an opposite effect on platelets in comparison to coagulation, as it was found to suppress their activation. Thus, it was observed that CORM-3 reduces both collagen- and thrombin-induced platelet aggregation. This effect was mediated by the guanylyl cyclase pathway [150, 151]; albeit other pathways might be also involved [152, 153]. Given the apparent conflicting effects CO exerts with regards to platelets, thrombus formation (and coagulation), further studies are needed to clarify these effects. Taken together, tobacco product users are clearly exposed to a multitude of toxic chemicals at once, and so far, data have shown that the “collective” effect of tobacco on hemostasis is shifted towards thrombosis, rather than the other way around.
Polycyclic Aromatic Hydrocarbons (PAHs)
The PAHs- compounds that are composed of a minimum of two fused benzenoid rings- are known to be among the numerous toxic chemicals produced by incomplete combustion of tobacco. In addition, one study that analyzed 70 brands in the US market, confirmed the presence of PAHs in smokeless tobacco. It is noteworthy that PAHs do not occur naturally in plants, therefore the presence of PAHs in smokeless tobacco might be due to fire-curing, which is a process employed to transform green wet tobacco into a dry “ready to use” product [154]. PAHs are known to possess mutagenic properties [155], and experimental studies have shown that exposure to them might be associated with negative cardiovascular consequences, including thrombotic events such as MI [156]. Also, PAHs are linked to accelerated development of endothelial atherosclerotic plaques [157]. In addition, it has been suggested that PAHs induce apoptosis of endothelial cells by a mechanism that involves activation of phospholipase A2 [158]. Separate lines of evidence suggested that exposure to PAHs increases mean platelet volume (MPV) [159], which is linked to hyperactive platelets. Increases in MPV is also associated with cardiovascular inflammation [160], another risk element for thrombogenesis [161]. Likewise, there is convincing experimental data that appears to positively link PAHs exposure with oxidative stress and inflammation [162, 163], which are strong modulators of hemostasis. Indeed, reports indicate that almost all ETRPs with perhaps the exception of e-cigs (not enough data to support such conclusion) can produce PAHs, but at varying levels. This is of significance from a health standpoint, as these chemicals are not safe and have the potential to participate in the genesis of a host of diseases overtime, even at low concentrations.
Heavy Metals
ETRPs carry a considerable profile of heavy metals such as aluminum, antimony, arsenic, cadmium, cobalt, chromium, copper, iron, lead, manganese, mercury, and zinc[164–167]. These heavy metals are of major concern due to their toxic impact on different body systems, including their potential to cause cardiovascular disease such CAD[168, 169]. Furthermore, animal studies have shown that heavy metals such as cadmium can in fact modulate the coagulation system and increase platelet activation, thereby increasing the risk of thrombosis[170]. Heavy metals are also known to increase proinflammatory cytokines, such as IL-1β, IL- 6, and TNF α as well as ROS in both humans and animals[171]. Nonetheless, more studies are needed to mechanistically address how heavy metals contribute to the thrombotic process, which can consequently lead to cardiovascular events.
Tobacco-Specific Nitrosamines (TSNAs)
TSNAs are one of the carcinogenic chemicals produced by tobacco smoke, but are also among the contents of smokeless tobacco [172]. In fact, like conventional tobacco smoke, almost all ETRPs produce TSNAs, albeit in differing amounts. To this end, there is evidence that tobacco smoke produces significantly higher amounts of TSNAs, in comparison to e-cigs and waterpipe [119]. On the other hand, HNB tobacco shows comparable levels of TSNAs relative to conventional cigarettes [146, 173]; which perhaps is inconsistent with the fact that it is being promoted as a safer option to conventional cigarettes [174]. Notably, studies linking TSNAs to CVD are limited, which makes it difficult to appreciate their harmful effects in this regard. Nonetheless, in term of TSNAs impact on the cardiovascular health, studies have shown that they increase CVD biomarkers such as creatine kinase-MB and lactate dehydrogenase. The same study [175] also concluded that TSNAs increased the levels of free radicals and decreased the activity of antioxidant enzymes; all of which are known to favor a state of thrombotic CVDs. Regarding their direct effect on thrombosis, it does not seem to have been explored yet, but clearly warrants investigation.
In summary, it is evident that ETRPs-emitted toxic substances are an important cause of thrombosis. These toxic substances impact different aspects of hemostatic processes such as platelet activation, coagulation, and endothelial function which predisposes to thrombosis and eventual cardiac events such as MI and stroke. And although it is challenging to characterize the effect of different toxic chemicals on the different aspects of thrombosis, there is a huge need for more rigors studies to address this issue mechanistically.
Conclusion
ETRPs were introduced into the market as safe/safer options, mainly for individuals who would like to transition and quit cigarette smoking. However, the safety of these products is questionable. In fact, in many instances, some of these products appear to exert greater negative health effects than cigarette smoking. This- at least in part- derives from the fact that ETRPs emit considerable and often comparable levels of toxic chemicals, in comparison with conventional cigarettes. A host of these toxic chemicals are known to promote inflammation and oxidative stress, both of which are important instigators of thrombosis-based disease states. Indeed, the link between inflammation, oxidative stress, and thrombosis is well established in the literature. However, this relationship is not merely in one direction, as it involves positive feedback and is more of augmentative in nature. In this mini, yet comprehensive review, we attempted to put these relationships in a model that we hope will better help the readers in not only understanding the big picture, but also appreciating smoke-induced thrombotic diseases. Furthermore, the literature appears to indicate that emerging tobacco products, similar to conventional cigarettes, impact all elements of hemostasis, such as the endothelium, platelets, and the coagulation system, albeit with some variability. These effects are associated with a host of negative health consequences, such as myocardial infraction and stroke.. Finally, this review also revealed that more research is warranted to investigate the mechanistic effects of ETRPs in the context of thrombotic cardiovascular disease, and the pathways involved in such disease processes.
Sources of Funding
Research reported in this publication was supported by the National Institute of Environmental Health Sciences and the National Heart, Lung, And Blood Institute of the National Institutes of Health under Awards Number R21ES029345, R03ES030486 and R01HL145053. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- CS
Cigarette Smoking
- CVD
Cardiovascular Disease
- CAD
Coronary Artery Disease
- E-cig
Electronic Cigarettes
- ETRPs
Emerging Tobacco Related Products
- ENDS
Electronic Nicotine Delivery System
- PAHs
Polyaromatic Hydrocarbons
- ROS
Reactive Oxygen Species
- TSNAs
Tobacco-Specific Nitrosamines
- TXA2
Thromboxane A2
Footnotes
Conflict of Interest statement:
The authors declare that there are no conflicts of interest.
References
- 1.Ambrose JA and Barua RS, The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol, 2004. 43(10): p. 1731–7. [DOI] [PubMed] [Google Scholar]
- 2.Pan B, et al. , The relationship between smoking and stroke: A meta-analysis. Medicine (Baltimore), 2019. 98(12): p. e14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns DM, Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Dis, 2003. 46(1): p. 11–29. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease, C., et al. , Publications and Reports of the Surgeon General, in How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. 2010, Centers for Disease Control and Prevention (US): Atlanta (GA). [PubMed] [Google Scholar]
- 5.Honjo K, et al. , The effects of smoking and smoking cessation on mortality from cardiovascular disease among Japanese: pooled analysis of three large-scale cohort studies in Japan. Tob Control, 2010. 19(1): p. 50–7. [DOI] [PubMed] [Google Scholar]
- 6.Lakier JB, Smoking and cardiovascular disease. The American Journal of Medicine, 1992. 93(1, Supplement 1): p. S8–S12. [DOI] [PubMed] [Google Scholar]
- 7.Miech RA, et al. , Monitoring the future national survey results on drug use, 1975–2017: volume I, secondary school students. 2018.
- 8.Cigarette smoking among adults and trends in smoking cessation - United States, 2008. MMWR Morb Mortal Wkly Rep, 2009. 58(44): p. 1227–32. [PubMed] [Google Scholar]
- 9.Bhatnagar A, et al. , New and Emerging Tobacco Products and the Nicotine Endgame: The Role of Robust Regulation and Comprehensive Tobacco Control and Prevention: A Presidential Advisory From the American Heart Association. Circulation, 2019. 139(19): p. e937–e958. [DOI] [PubMed] [Google Scholar]
- 10.Bekki K, et al. , Comparison of Chemicals in Mainstream Smoke in Heat-not-burn Tobacco and Combustion Cigarettes. J uoeh, 2017. 39(3): p. 201–207. [DOI] [PubMed] [Google Scholar]
- 11.Cornelius ME, et al. , Tobacco Product Use Among Adults - United States, 2019. MMWR Morb Mortal Wkly Rep, 2020. 69(46): p. 1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braillon A, Electronic Cigarette Use Among Populations of Women During Reproductive Years. JAMA Pediatr, 2019. 173(12): p. 1213–1214. [DOI] [PubMed] [Google Scholar]
- 13.Azab M, et al. , Exposure of pregnant women to waterpipe and cigarette smoke. Nicotine Tob Res, 2013. 15(1): p. 231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staal YC, et al. , New Tobacco and Tobacco-Related Products: Early Detection of Product Development, Marketing Strategies, and Consumer Interest. JMIR Public Health Surveill, 2018. 4(2): p. e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parascandola M, et al. , Consumer awareness and attitudes related to new potential reduced-exposure tobacco product brands. Nicotine Tob Res, 2009. 11(7): p. 886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bialous SA and Glantz SA, Heated tobacco products: another tobacco industry global strategy to slow progress in tobacco control. Tob Control, 2018. 27(Suppl 1): p. s111–s117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Electronic Cigarettes. 2020; Available from: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/index.htm.
- 18.U.S. Department of Health and Human Services. The health consequences of smoking: nicotine addiction: a report of the surgeon general. 1988; Available from: http://profiles.nlm.nih.gov/ps/access/NNBBZD.pdf.
- 19.Hutzler C, et al. , Chemical hazards present in liquids and vapors of electronic cigarettes. Arch Toxicol, 2014. 88(7): p. 1295–308. [DOI] [PubMed] [Google Scholar]
- 20.Hsu G, Sun JY, and Zhu SH, Evolution of Electronic Cigarette Brands From 2013–2014 to 2016–2017: Analysis of Brand Websites. J Med Internet Res, 2018. 20(3): p. e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King BA, et al. , Electronic Cigarette Sales in the United States, 2013–2017. JAMA, 2018. 320(13): p. 1379–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. E-cigarettes shaped like USB flash drives: information for parents, educators and health care providers. 2018; Available from: https://www.cdc.gov/tobacco/infographics/youth/pdfs/e-cigarettes-usb-flash-508.pdf.
- 23.Darville A and Hahn EJ, E-cigarettes and Atherosclerotic Cardiovascular Disease: What Clinicians and Researchers Need to Know. Curr Atheroscler Rep, 2019. 21(5): p. 15. [DOI] [PubMed] [Google Scholar]
- 24.Alzahrani T, et al. , Association Between Electronic Cigarette Use and Myocardial Infarction. Am J Prev Med, 2018. 55(4): p. 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farsalinos KE, et al. , Is e-cigarette use associated with coronary heart disease and myocardial infarction? Insights from the 2016 and 2017 National Health Interview Surveys. Ther Adv Chronic Dis, 2019. 10: p. 2040622319877741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parekh T, Pemmasani S, and Desai R, Risk of Stroke With E-Cigarette and Combustible Cigarette Use in Young Adults. Am J Prev Med, 2020. 58(3): p. 446–452. [DOI] [PubMed] [Google Scholar]
- 27.Qasim H, et al. , Short-Term E-Cigarette Exposure Increases the Risk of Thrombogenesis and Enhances Platelet Function in Mice. J Am Heart Assoc, 2018. 7(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maziak W, et al. , The global epidemiology of waterpipe smoking. Tob Control, 2015. 24 Suppl 1: p. i3–i12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salloum RG, Asfar T, and Maziak W, Toward a Regulatory Framework for the Waterpipe. Am J Public Health, 2016. 106(10): p. 1773–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khater AE, et al. , Radiological hazards of Narghile (hookah, shisha, goza) smoking: activity concentrations and dose assessment. J Environ Radioact, 2008. 99(12): p. 1808–14. [DOI] [PubMed] [Google Scholar]
- 31.Dube SR, et al. , Electronic Cigarette and Electronic Hookah: A Pilot Study Comparing Two Vaping Products. Prev Med Rep, 2015. 2: p. 953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rezk-Hanna M, et al. , E-Hookah Versus E-Cigarettes: Findings From Wave 2 of the PATH Study (2014–2015). Am J Prev Med, 2019. 57(5): p. e163–e173. [DOI] [PubMed] [Google Scholar]
- 33.Al-Amri A, et al. , Waterpipe smoking and the risk of myocardial infarction: A hospital-based case-control study. Tob Induc Dis, 2019. 17: p. 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Platt DE, et al. , Association of waterpipe smoking with myocardial infarction and determinants of metabolic syndrome among catheterized patients. Inhal Toxicol, 2017. 29(10): p. 429–434. [DOI] [PubMed] [Google Scholar]
- 35.Alarabi AB, et al. , Short-Term Exposure to Waterpipe/Hookah Smoke Triggers a Hyperactive Platelet Activation State and Increases the Risk of Thrombogenesis. Arterioscler Thromb Vasc Biol, 2020. 40(2): p. 335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corey CG, et al. , Little filtered cigar, cigarillo, and premium cigar smoking among adults--United States, 2012–2013. MMWR Morb Mortal Wkly Rep, 2014. 63(30): p. 650–4. [PMC free article] [PubMed] [Google Scholar]
- 37.Leung WH, et al. , Cor triatriatum masked by coexisting COPD in an adult. Chest, 1989. 96(3): p. 676–8. [DOI] [PubMed] [Google Scholar]
- 38.Messer K, et al. , Trends in use of little cigars or cigarillos and cigarettes among U.S. smokers, 2002–2011. Nicotine Tob Res, 2015. 17(5): p. 515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cullen J, et al. , Seven-year patterns in US cigar use epidemiology among young adults aged 18–25 years: a focus on race/ethnicity and brand. Am J Public Health, 2011. 101(10): p. 1955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts ME, et al. , Rural Versus Urban Use of Traditional and Emerging Tobacco Products in the United States, 2013–2014. Am J Public Health, 2017. 107(10): p. 1554–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pickworth WB and Thanner MH, Cigar Use Symposium: Epidemiology, Toxicant Exposure, Health and Policy Implications. Tob Regul Sci, 2017. 3(Suppl 1): p. S3–s7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iribarren C, et al. , Effect of cigar smoking on the risk of cardiovascular disease, chronic obstructive pulmonary disease, and cancer in men. N Engl J Med, 1999. 340(23): p. 1773–80. [DOI] [PubMed] [Google Scholar]
- 43.Kong G, et al. , Reasons for Cigarillo Initiation and Cigarillo Manipulation Methods among Adolescents. Tob Regul Sci, 2017. 3(2 Suppl 1): p. S48–s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kondo T, et al. , Effects of Tobacco Smoking on Cardiovascular Disease. Circ J, 2019. 83(10): p. 1980–1985. [DOI] [PubMed] [Google Scholar]
- 45.Auer R, et al. , Heat-Not-Burn Tobacco Cigarettes: Smoke by Any Other Name. JAMA Intern Med, 2017. 177(7): p. 1050–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caputi TL, et al. , They’re heating up: Internet search query trends reveal significant public interest in heat-not-burn tobacco products. PLoS One, 2017. 12(10): p. e0185735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farsalinos KE, et al. , Nicotine Delivery to the Aerosol of a Heat-Not-Burn Tobacco Product: Comparison With a Tobacco Cigarette and E-Cigarettes. Nicotine Tob Res, 2018. 20(8): p. 1004–1009. [DOI] [PubMed] [Google Scholar]
- 48.Mallock N, et al. , Heated Tobacco Products: A Review of Current Knowledge and Initial Assessments. Front Public Health, 2019. 7: p. 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Savitz DA, et al. , Public health implications of smokeless tobacco use as a harm reduction strategy. Am J Public Health, 2006. 96(11): p. 1934–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta R, et al. , A systematic review on association between smokeless tobacco & cardiovascular diseases. Indian J Med Res, 2018. 148(1): p. 77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piano MR, et al. , Impact of smokeless tobacco products on cardiovascular disease: implications for policy, prevention, and treatment: a policy statement from the American Heart Association. Circulation, 2010. 122(15): p. 1520–44. [DOI] [PubMed] [Google Scholar]
- 52.Nelson PR, et al. , Randomized Trial to Compare Smoking Cessation Rates of Snus, With and Without Smokeless Tobacco Health-Related Information, and a Nicotine Lozenge. Nicotine Tob Res, 2019. 21(1): p. 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teo KK, et al. , Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet, 2006. 368(9536): p. 647–58. [DOI] [PubMed] [Google Scholar]
- 54.Versteeg HH, et al. , New fundamentals in hemostasis. Physiol Rev, 2013. 93(1): p. 327–58. [DOI] [PubMed] [Google Scholar]
- 55.Hvas AM, Platelet Function in Thrombosis and Hemostasis. Semin Thromb Hemost, 2016. 42(3): p. 183–4. [DOI] [PubMed] [Google Scholar]
- 56.Wendelboe AM and Raskob GE, Global Burden of Thrombosis: Epidemiologic Aspects. Circ Res, 2016. 118(9): p. 1340–7. [DOI] [PubMed] [Google Scholar]
- 57.Giordano NJ, et al. , Epidemiology, Pathophysiology, Stratification, and Natural History of Pulmonary Embolism. Tech Vasc Interv Radiol, 2017. 20(3): p. 135–140. [DOI] [PubMed] [Google Scholar]
- 58.Tomaiuolo M, Brass LF, and Stalker TJ, Regulation of Platelet Activation and Coagulation and Its Role in Vascular Injury and Arterial Thrombosis. Interv Cardiol Clin, 2017. 6(1): p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Hylckama Vlieg A and Rosendaal FR, High levels of fibrinogen are associated with the risk of deep venous thrombosis mainly in the elderly. J Thromb Haemost, 2003. 1(12): p. 2677–8. [DOI] [PubMed] [Google Scholar]
- 60.Moncada S and Vane JR, The role of prostacyclin in vascular tissue. Fed Proc, 1979. 38(1): p. 66–71. [PubMed] [Google Scholar]
- 61.Carcaillon L, et al. , Increased thrombin generation is associated with acute ischemic stroke but not with coronary heart disease in the elderly: the Three-City cohort study. Arterioscler Thromb Vasc Biol, 2011. 31(6): p. 1445–51. [DOI] [PubMed] [Google Scholar]
- 62.Pearson JD, Endothelial cell function and thrombosis. Baillieres Clin Haematol, 1994. 7(3): p. 441–52. [DOI] [PubMed] [Google Scholar]
- 63.Booth NA and Bennett B, Fibrinolysis and thrombosis. Baillieres Clin Haematol, 1994. 7(3): p. 559–72. [DOI] [PubMed] [Google Scholar]
- 64.Kamceva G, et al. , Cigarette Smoking and Oxidative Stress in Patients with Coronary Artery Disease. Open Access Maced J Med Sci, 2016. 4(4): p. 636–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van der Vaart H, et al. , Acute effects of cigarette smoke on inflammation and oxidative stress: a review. Thorax, 2004. 59(8): p. 713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qasim H, et al. , The effects of hookah/waterpipe smoking on general health and the cardiovascular system. Environ Health Prev Med, 2019. 24(1): p. 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scott A, et al. , Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax, 2018. 73(12): p. 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shields PG, et al. , A Review of Pulmonary Toxicity of Electronic Cigarettes in the Context of Smoking: A Focus on Inflammation. Cancer Epidemiol Biomarkers Prev, 2017. 26(8): p. 1175–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leigh NJ, et al. , Cytotoxic effects of heated tobacco products (HTP) on human bronchial epithelial cells. Tob Control, 2018. 27(Suppl 1): p. s26–s29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rezk-Hanna M, et al. , P941Acute effects of electronic hookah smoking on endothelial function, inflammation and oxidative stress. European Heart Journal, 2019. 40(Supplement_1). [Google Scholar]
- 71.Stokes AC, et al. , Association of Cigarette and Electronic Cigarette Use Patterns With Levels of Inflammatory and Oxidative Stress Biomarkers Among US Adults: Population Assessment of Tobacco and Health Study. Circulation, 2021. 143(8): p. 869–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chatterjee S, et al. , Acute exposure to e-cigarettes causes inflammation and pulmonary endothelial oxidative stress in nonsmoking, healthy young subjects. Am J Physiol Lung Cell Mol Physiol, 2019. 317(2): p. L155–l166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramirez JEM, et al. , The JUUL E-Cigarette Elevates the Risk of Thrombosis and Potentiates Platelet Activation. J Cardiovasc Pharmacol Ther, 2020. 25(6): p. 578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khan NA, et al. , Systemic biomarkers of inflammation, oxidative stress and tissue injury and repair among waterpipe, cigarette and dual tobacco smokers. Tob Control, 2020. 29(Suppl 2): p. s102–s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Badran M and Laher I, Waterpipe (shisha, hookah) smoking, oxidative stress and hidden disease potential. Redox Biol, 2020. 34: p. 101455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jebai R, et al. , Markers of oxidative stress and toxicant exposure among young waterpipe smokers in the USA. Environ Sci Pollut Res Int, 2021. 28(21): p. 26677–26683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Furie MB, et al. , Extracts of smokeless tobacco induce pro-inflammatory changes in cultured human vascular endothelial cells. Immunopharmacology, 2000. 47(1): p. 13–23. [DOI] [PubMed] [Google Scholar]
- 78.Bagchi M, et al. , Smokeless tobacco, oxidative stress, apoptosis, and antioxidants in human oral keratinocytes. Free Radic Biol Med, 1999. 26(7–8): p. 992–1000. [DOI] [PubMed] [Google Scholar]
- 79.Ito Y, et al. , Heat-Not-Burn cigarette induces oxidative stress response in primary rat alveolar epithelial cells. PLoS One, 2020. 15(11): p. e0242789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kopa PN and Pawliczak R, IQOS - a heat-not-burn (HnB) tobacco product - chemical composition and possible impact on oxidative stress and inflammatory response. A systematic review. Toxicol Mech Methods, 2020. 30(2): p. 81–87. [DOI] [PubMed] [Google Scholar]
- 81.Fried ND and Gardner JD, Heat-not-burn tobacco products: an emerging threat to cardiovascular health. Am J Physiol Heart Circ Physiol, 2020. 319(6): p. H1234–h1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aksu K, Donmez A, and Keser G, Inflammation-induced thrombosis: mechanisms, disease associations and management. Curr Pharm Des, 2012. 18(11): p. 1478–93. [DOI] [PubMed] [Google Scholar]
- 83.Medzhitov R, Inflammation 2010: new adventures of an old flame. Cell, 2010. 140(6): p. 771–6. [DOI] [PubMed] [Google Scholar]
- 84.Biswas SK, Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid Med Cell Longev, 2016. 2016: p. 5698931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parke DV and Parke AL, Chemical-induced inflammation and inflammatory diseases. Int J Occup Med Environ Health, 1996. 9(3): p. 211–7. [PubMed] [Google Scholar]
- 86.Loscalzo J, Oxidative stress in endothelial cell dysfunction and thrombosis. Pathophysiol Haemost Thromb, 2002. 32(5–6): p. 359–60. [DOI] [PubMed] [Google Scholar]
- 87.Violi F and Pignatelli P, Platelet oxidative stress and thrombosis. Thromb Res, 2012. 129(3): p. 378–81. [DOI] [PubMed] [Google Scholar]
- 88.McGee MP and Li LC, Functional difference between intrinsic and extrinsic coagulation pathways. Kinetics of factor X activation on human monocytes and alveolar macrophages. J Biol Chem, 1991. 266(13): p. 8079–85. [PubMed] [Google Scholar]
- 89.Stary HC Changes in the Cells of Atherosclerotic Lesions as Advanced Lesions Evolve in Coronary Arteries of Children and Young Adults. 1990. New York, NY: Springer New York. [Google Scholar]
- 90.Ferguson DJ, Dural puncture and epidural catheters. Anaesthesia, 1992. 47(3): p. 272. [DOI] [PubMed] [Google Scholar]
- 91.Lubos E, Handy DE, and Loscalzo J, Role of oxidative stress and nitric oxide in atherothrombosis. Front Biosci, 2008. 13: p. 5323–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coughlin SR, Thrombin signalling and protease-activated receptors. Nature, 2000. 407(6801): p. 258–64. [DOI] [PubMed] [Google Scholar]
- 93.Matta F, et al. , Risk of venous thromboembolism with rheumatoid arthritis. Thromb Haemost, 2009. 101(1): p. 134–8. [PubMed] [Google Scholar]
- 94.Wachowicz B, et al. , Generation of reactive oxygen species in blood platelets. Platelets, 2002. 13(3): p. 175–82. [DOI] [PubMed] [Google Scholar]
- 95.Esmon CT, Crosstalk between inflammation and thrombosis. Maturitas, 2008. 61(1–2): p. 122–31. [DOI] [PubMed] [Google Scholar]
- 96.Kim YW, West XZ, and Byzova TV, Inflammation and oxidative stress in angiogenesis and vascular disease. J Mol Med (Berl), 2013. 91(3): p. 323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Talhout R, et al. , Hazardous compounds in tobacco smoke. Int J Environ Res Public Health, 2011. 8(2): p. 613–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Price LR and Martinez J, Cardiovascular, carcinogenic and reproductive effects of nicotine exposure: A narrative review of the scientific literature. F1000Res, 2019. 8: p. 1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pope CA 3rd, et al. , Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation, 2009. 120(11): p. 941–8. [DOI] [PubMed] [Google Scholar]
- 100.Holme JA, et al. , Potential role of polycyclic aromatic hydrocarbons as mediators of cardiovascular effects from combustion particles. Environ Health, 2019. 18(1): p. 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.National Academies of Sciences, E., et al. , in Public Health Consequences of E-Cigarettes, Eaton DL, Kwan LY, and Stratton K, Editors. 2018, National Academies Press (US)Copyright 2018 by the National Academy of Sciences. All rights reserved.: Washington (DC). [PubMed] [Google Scholar]
- 102.Yingst JM, et al. , Nicotine Absorption Profile Among Regular Users of a Pod-Based Electronic Nicotine Delivery System. JAMA Netw Open, 2019. 2(11): p. e1915494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hadidi KA and Mohammed FI, Nicotine content in tobacco used in hubble-bubble smoking. Saudi Med J, 2004. 25(7): p. 912–7. [PubMed] [Google Scholar]
- 104.Farsalinos K, Poulas K, and Voudris V, Changes in Puffing Topography and Nicotine Consumption Depending on the Power Setting of Electronic Cigarettes. Nicotine Tob Res, 2018. 20(8): p. 993–997. [DOI] [PubMed] [Google Scholar]
- 105.Sutton SR, et al. , Relationship between cigarette yields, puffing patterns, and smoke intake: evidence for tar compensation? Br Med J (Clin Res Ed), 1982. 285(6342): p. 600–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith CJ and Fischer TH, Particulate and vapor phase constituents of cigarette mainstream smoke and risk of myocardial infarction. Atherosclerosis, 2001. 158(2): p. 257–67. [DOI] [PubMed] [Google Scholar]
- 107.Benowitz NL, The role of nicotine in smoking-related cardiovascular disease. Prev Med, 1997. 26(4): p. 412–7. [DOI] [PubMed] [Google Scholar]
- 108.Schedel A, et al. , Megakaryocytes and platelets express nicotinic acetylcholine receptors but nicotine does not affect megakaryopoiesis or platelet function. Platelets, 2016. 27(1): p. 43–50. [DOI] [PubMed] [Google Scholar]
- 109.Prasad DS, et al. , Smoking and cardiovascular health: a review of the epidemiology, pathogenesis, prevention and control of tobacco. Indian J Med Sci, 2009. 63(11): p. 520–33. [PubMed] [Google Scholar]
- 110.Ichiki K, et al. , Long-term smoking impairs platelet-derived nitric oxide release. Circulation, 1996. 94(12): p. 3109–14. [DOI] [PubMed] [Google Scholar]
- 111.Renaud S, et al. , Platelet function after cigarette smoking in relation to nicotine and carbon monoxide. Clin Pharmacol Ther, 1984. 36(3): p. 389–95. [DOI] [PubMed] [Google Scholar]
- 112.Fahim MA, et al. , Thromboembolic injury and systemic toxicity induced by nicotine in mice. Gen Physiol Biophys, 2014. 33(3): p. 345–55. [DOI] [PubMed] [Google Scholar]
- 113.Nowak J, et al. , Effect of nicotine infusion in humans on platelet aggregation and urinary excretion of a major thromboxane metabolite. Acta Physiol Scand, 1996. 157(1): p. 101–7. [DOI] [PubMed] [Google Scholar]
- 114.Rausch JL, et al. , Effect of nicotine on human blood platelet serotonin uptake and efflux. Prog Neuropsychopharmacol Biol Psychiatry, 1989. 13(6): p. 907–16. [DOI] [PubMed] [Google Scholar]
- 115.Renaud S, Cigarette smoking and platelet function: relation to nicotine, carbon monoxide and saturated fat. Adv Exp Med Biol, 1990. 273: p. 161–71. [DOI] [PubMed] [Google Scholar]
- 116.Traboulsi H, et al. , Inhalation Toxicology of Vaping Products and Implications for Pulmonary Health. Int J Mol Sci, 2020. 21(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Valente P, et al. , Exposure to fine and ultrafine particles from secondhand smoke in public places before and after the smoking ban, Italy 2005. Tob Control, 2007. 16(5): p. 312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brook RD, et al. , Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation, 2010. 121(21): p. 2331–78. [DOI] [PubMed] [Google Scholar]
- 119.Münzel T, et al. , Effects of tobacco cigarettes, e-cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur Heart J, 2020. 41(41): p. 4057–4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.St Helen G, et al. , Nicotine Delivery and Vaping Behavior During ad Libitum E-cigarette Access. Tob Regul Sci, 2016. 2(4): p. 363–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim S and Yu S, Smoking Topography among Korean Smokers: Intensive Smoking Behavior with Larger Puff Volume and Shorter Interpuff Interval. Int J Environ Res Public Health, 2018. 15(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bover Manderski MT, Delnevo CD, and Warner KE, Toward a More Comprehensive Index of Youth Cigarette Smoking: Average Number of Cigarettes Smoked per Day among Students in the United States over Two Decades. Int J Environ Res Public Health, 2021. 18(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Qasim H, et al. , Impact of Electronic Cigarettes on the Cardiovascular System. J Am Heart Assoc, 2017. 6(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Neergaard J, et al. , Waterpipe smoking and nicotine exposure: a review of the current evidence. Nicotine Tob Res, 2007. 9(10): p. 987–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Haziza C, et al. , Assessment of the reduction in levels of exposure to harmful and potentially harmful constituents in Japanese subjects using a novel tobacco heating system compared with conventional cigarettes and smoking abstinence: A randomized controlled study in confinement. Regul Toxicol Pharmacol, 2016. 81: p. 489–499. [DOI] [PubMed] [Google Scholar]
- 126.Park JM, et al. , Differential Effects between Cigarette Total Particulate Matter and Cigarette Smoke Extract on Blood and Blood Vessel. Toxicol Res, 2016. 32(4): p. 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Khandoga A, et al. , Ultrafine particles exert prothrombotic but not inflammatory effects on the hepatic microcirculation in healthy mice in vivo. Circulation, 2004. 109(10): p. 1320–5. [DOI] [PubMed] [Google Scholar]
- 128.Riediker M, et al. , Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care Med, 2004. 169(8): p. 934–40. [DOI] [PubMed] [Google Scholar]
- 129.Riediker M, et al. , Cardiovascular effects in patrol officers are associated with fine particulate matter from brake wear and engine emissions. Part Fibre Toxicol, 2004. 1(1): p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rich DQ, et al. , Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. Jama, 2012. 307(19): p. 2068–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu C, et al. , The Acute Effects of Fine Particulate Matter Constituents on Blood Inflammation and Coagulation. Environ Sci Technol, 2017. 51(14): p. 8128–8137. [DOI] [PubMed] [Google Scholar]
- 132.Mills NL, et al. , Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation, 2005. 112(25): p. 3930–6. [DOI] [PubMed] [Google Scholar]
- 133.Vaughan DE, PAI-1 and atherothrombosis. J Thromb Haemost, 2005. 3(8): p. 1879–83. [DOI] [PubMed] [Google Scholar]
- 134.Chuang KJ, et al. , The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med, 2007. 176(4): p. 370–6. [DOI] [PubMed] [Google Scholar]
- 135.Lucking AJ, et al. , Particle traps prevent adverse vascular and prothrombotic effects of diesel engine exhaust inhalation in men. Circulation, 2011. 123(16): p. 1721–8. [DOI] [PubMed] [Google Scholar]
- 136.Lucking AJ, et al. , Diesel exhaust inhalation increases thrombus formation in man. Eur Heart J, 2008. 29(24): p. 3043–51. [DOI] [PubMed] [Google Scholar]
- 137.Jacobs L, et al. , Air pollution related prothrombotic changes in persons with diabetes. Environ Health Perspect, 2010. 118(2): p. 191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cascio WE, et al. , Cardiac and vascular changes in mice after exposure to ultrafine particulate matter. Inhal Toxicol, 2007. 19 Suppl 1: p. 67–73. [DOI] [PubMed] [Google Scholar]
- 139.Devlin RB, et al. , Controlled exposure of humans with metabolic syndrome to concentrated ultrafine ambient particulate matter causes cardiovascular effects. Toxicol Sci, 2014. 140(1): p. 61–72. [DOI] [PubMed] [Google Scholar]
- 140.Delfino RJ, Sioutas C, and Malik S, Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environ Health Perspect, 2005. 113(8): p. 934–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Samet JM, et al. , Concentrated ambient ultrafine particle exposure induces cardiac changes in young healthy volunteers. Am J Respir Crit Care Med, 2009. 179(11): p. 1034–42. [DOI] [PubMed] [Google Scholar]
- 142.Ostro B, et al. , Associations of mortality with long-term exposures to fine and ultrafine particles, species and sources: results from the California Teachers Study Cohort. Environ Health Perspect, 2015. 123(6): p. 549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stölzel M, et al. , Daily mortality and particulate matter in different size classes in Erfurt, Germany. J Expo Sci Environ Epidemiol, 2007. 17(5): p. 458–67. [DOI] [PubMed] [Google Scholar]
- 144.Meng X, et al. , Size-fractionated particle number concentrations and daily mortality in a Chinese city. Environ Health Perspect, 2013. 121(10): p. 1174–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dorey A, et al. , Acute and Chronic Carbon Monoxide Toxicity from Tobacco Smoking. Mil Med, 2020. 185(1–2): p. e61–e67. [DOI] [PubMed] [Google Scholar]
- 146.Schaller JP, et al. , Evaluation of the Tobacco Heating System 2.2. Part 2: Chemical composition, genotoxicity, cytotoxicity, and physical properties of the aerosol. Regul Toxicol Pharmacol, 2016. 81 Suppl 2: p. S27–s47. [DOI] [PubMed] [Google Scholar]
- 147.Chung WS, Lin CL, and Kao CH, Carbon monoxide poisoning and risk of deep vein thrombosis and pulmonary embolism: a nationwide retrospective cohort study. J Epidemiol Community Health, 2015. 69(6): p. 557–62. [DOI] [PubMed] [Google Scholar]
- 148.Nielsen VG and Pretorius E, Carbon monoxide: Anticoagulant or procoagulant? Thromb Res, 2014. 133(3): p. 315–21. [DOI] [PubMed] [Google Scholar]
- 149.Nielsen VG and Garza JI, Comparison of the effects of CORM-2, CORM-3 and CORM-A1 on coagulation in human plasma. Blood Coagul Fibrinolysis, 2014. 25(8): p. 801–5. [DOI] [PubMed] [Google Scholar]
- 150.Chlopicki S, et al. , Carbon monoxide released by CORM-3 inhibits human platelets by a mechanism independent of soluble guanylate cyclase. Cardiovasc Res, 2006. 71(2): p. 393–401. [DOI] [PubMed] [Google Scholar]
- 151.Brüne B and Ullrich V, Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol Pharmacol, 1987. 32(4): p. 497–504. [PubMed] [Google Scholar]
- 152.Vostal JG and Fratantoni JC, Econazole inhibits thapsigargin-induced platelet calcium influx by mechanisms other than cytochrome P-450 inhibition. Biochem J, 1993. 295 (Pt 2)(Pt 2): p. 525–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Truss NJ and Warner TD, Gasotransmitters and platelets. Pharmacol Ther, 2011. 132(2): p. 196–203. [DOI] [PubMed] [Google Scholar]
- 154.McAdam KG, et al. , Polycyclic aromatic hydrocarbons in US and Swedish smokeless tobacco products. Chem Cent J, 2013. 7(1): p. 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vu AT, et al. , Polycyclic Aromatic Hydrocarbons in the Mainstream Smoke of Popular U.S. Cigarettes. Chem Res Toxicol, 2015. 28(8): p. 1616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Alhamdow A, et al. , Early markers of cardiovascular disease are associated with occupational exposure to polycyclic aromatic hydrocarbons. Sci Rep, 2017. 7(1): p. 9426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Penn A and Snyder C, Arteriosclerotic plaque development is ‘promoted’ by polynuclear aromatic hydrocarbons. Carcinogenesis, 1988. 9(12): p. 2185–9. [DOI] [PubMed] [Google Scholar]
- 158.Tithof PK, et al. , Polycyclic aromatic hydrocarbons present in cigarette smoke cause endothelial cell apoptosis by a phospholipase A2-dependent mechanism. Faseb j, 2002. 16(11): p. 1463–4. [DOI] [PubMed] [Google Scholar]
- 159.Hu C, et al. , Association of polycyclic aromatic hydrocarbons exposure with atherosclerotic cardiovascular disease risk: A role of mean platelet volume or club cell secretory protein. Environ Pollut, 2018. 233: p. 45–53. [DOI] [PubMed] [Google Scholar]
- 160.Sansanayudh N, et al. , Mean platelet volume and coronary artery disease: a systematic review and meta-analysis. Int J Cardiol, 2014. 175(3): p. 433–40. [DOI] [PubMed] [Google Scholar]
- 161.Gasparyan AY, et al. , Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des, 2011. 17(1): p. 47–58. [DOI] [PubMed] [Google Scholar]
- 162.Curfs DM, et al. , Polycyclic aromatic hydrocarbons induce an inflammatory atherosclerotic plaque phenotype irrespective of their DNA binding properties. Faseb j, 2005. 19(10): p. 1290–2. [DOI] [PubMed] [Google Scholar]
- 163.Jeng HA, et al. , Polycyclic aromatic hydrocarbon-induced oxidative stress and lipid peroxidation in relation to immunological alteration. Occup Environ Med, 2011. 68(9): p. 653–8. [DOI] [PubMed] [Google Scholar]
- 164.Zhao D, et al. , Metal/Metalloid Levels in Electronic Cigarette Liquids, Aerosols, and Human Biosamples: A Systematic Review. Environ Health Perspect, 2020. 128(3): p. 36001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Al-Kazwini AT, Said AJ, and Sdepanian S, Compartmental analysis of metals in waterpipe smoking technique. BMC Public Health, 2015. 15: p. 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ruprecht AA, et al. , Environmental pollution and emission factors of electronic cigarettes, heat-not-burn tobacco products, and conventional cigarettes. Aerosol Science and Technology, 2017. 51(6): p. 674–684. [Google Scholar]
- 167.Pappas RS, et al. , Determination of Toxic Metals in Little Cigar Tobacco with ‘Triple Quad’ ICP-MS. J Anal Toxicol, 2015. 39(5): p. 347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chowdhury R, et al. , Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. Bmj, 2018. 362: p. k3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Moon K, Guallar E, and Navas-Acien A, Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep, 2012. 14(6): p. 542–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Arbi S, et al. , Effects of chronic exposure to mercury and cadmium alone and in combination on the coagulation system of Sprague-Dawley rats. Ultrastruct Pathol, 2017. 41(4): p. 275–283. [DOI] [PubMed] [Google Scholar]
- 171.Milnerowicz H, Ściskalska M, and Dul M, Pro-inflammatory effects of metals in persons and animals exposed to tobacco smoke. J Trace Elem Med Biol, 2015. 29: p. 1–10. [DOI] [PubMed] [Google Scholar]
- 172.Oldham MJ, et al. , Variability of TSNA in U.S. Tobacco and Moist Smokeless Tobacco Products. Toxicol Rep, 2020. 7: p. 752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jeong WT, et al. , Comparison of the content of tobacco alkaloids and tobacco-specific nitrosamines in ‘heat-not-burn’ tobacco products before and after aerosol generation. Inhal Toxicol, 2018. 30(13–14): p. 527–533. [DOI] [PubMed] [Google Scholar]
- 174.McAlinden KD, Sohal SS, and Sharma P, There can be smoke without fire: warranted caution in promoting electronic cigarettes and heat not burn devices as a safer alternative to cigarette smoking. ERJ Open Res, 2019. 5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sheweita SA, El-Bendery HA, and Mostafa MH, Novel study on N-nitrosamines as risk factors of cardiovascular diseases. Biomed Res Int, 2014. 2014: p. 817019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Krüsemann EJZ, et al. , An E-Liquid Flavor Wheel: A Shared Vocabulary Based on Systematically Reviewing E-Liquid Flavor Classifications in Literature. Nicotine Tob Res, 2019. 21(10): p. 1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kuntic M, et al. , Short-term e-cigarette vapour exposure causes vascular oxidative stress and dysfunction: evidence for a close connection to brain damage and a key role of the phagocytic NADPH oxidase (NOX-2). Eur Heart J, 2020. 41(26): p. 2472–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Carnevale R, et al. , Acute Impact of Tobacco vs Electronic Cigarette Smoking on Oxidative Stress and Vascular Function. Chest, 2016. 150(3): p. 606–12. [DOI] [PubMed] [Google Scholar]
- 179.Kaisar MA, et al. , Offsetting the impact of smoking and e-cigarette vaping on the cerebrovascular system and stroke injury: Is Metformin a viable countermeasure? Redox Biol, 2017. 13: p. 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Elkhalifa AM, Effects of cigarette smoking on coagulation screening tests and platelet counts in a Sudanese male adults population. Saudi Med J, 2018. 39(9): p. 897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Harada HA, et al. , A 20-Year-Old Man with e-Cigarette or Vaping Product Use-Associated Lung Injury (EVALI) and Thrombotic Coagulopathy. Am J Case Rep, 2021. 22: p. e929915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kalantary A, et al. , Coagulopathy and Acute Respiratory Distress Syndrome: Dual Complications of E-Cigarette-Associated Lung Injury. Cureus, 2021. 13(2): p. e13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chaumont M, et al. , Differential Effects of E-Cigarette on Microvascular Endothelial Function, Arterial Stiffness and Oxidative Stress: A Randomized Crossover Trial. Sci Rep, 2018. 8(1): p. 10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hiler M, et al. , Electronic cigarette user plasma nicotine concentration, puff topography, heart rate, and subjective effects: Influence of liquid nicotine concentration and user experience. Exp Clin Psychopharmacol, 2017. 25(5): p. 380–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wagener TL, et al. , Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob Control, 2017. 26(e1): p. e23–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jensen RP, Strongin RM, and Peyton DH, Solvent Chemistry in the Electronic Cigarette Reaction Vessel. Sci Rep, 2017. 7: p. 42549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Salamanca JC, et al. , E-cigarettes can emit formaldehyde at high levels under conditions that have been reported to be non-averse to users. Sci Rep, 2018. 8(1): p. 7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Samburova V, et al. , Aldehydes in Exhaled Breath during E-Cigarette Vaping: Pilot Study Results. Toxics, 2018. 6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Williams M, et al. , Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One, 2013. 8(3): p. e57987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jain RB, Concentrations of cadmium, lead, and mercury in blood among US cigarettes, cigars, electronic cigarettes, and dual cigarette-e-cigarette users. Environ Pollut, 2019. 251: p. 970–974. [DOI] [PubMed] [Google Scholar]
- 191.Canistro D, et al. , E-cigarettes induce toxicological effects that can raise the cancer risk. Sci Rep, 2017. 7(1): p. 2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kim HJ and Shin HS, Determination of tobacco-specific nitrosamines in replacement liquids of electronic cigarettes by liquid chromatography-tandem mass spectrometry. J Chromatogr A, 2013. 1291: p. 48–55. [DOI] [PubMed] [Google Scholar]
- 193.Lerner CA, et al. , Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One, 2015. 10(2): p. e0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Soussy S, et al. , Detection of 5-hydroxymethylfurfural and furfural in the aerosol of electronic cigarettes. Tob Control, 2016. 25(Suppl 2): p. ii88–ii93. [DOI] [PubMed] [Google Scholar]
- 195.Oh JA and Shin HS, Identification and Quantification of Several Contaminated Compounds in Replacement Liquids of Electronic Cigarettes by Gas Chromatography-Mass Spectrometry. J Chromatogr Sci, 2015. 53(6): p. 841–8. [DOI] [PubMed] [Google Scholar]
- 196.Alomari MA, et al. , Central and peripheral cardiovascular changes immediately after waterpipe smoking. Inhal Toxicol, 2014. 26(10): p. 579–87. [DOI] [PubMed] [Google Scholar]
- 197.Alomari MA, et al. , Acute vascular effects of waterpipe smoking: Importance of physical activity and fitness status. Atherosclerosis, 2015. 240(2): p. 472–6. [DOI] [PubMed] [Google Scholar]
- 198.Bentur L, et al. , Laboratory and clinical acute effects of active and passive indoor group water-pipe (narghile) smoking. Chest, 2014. 145(4): p. 803–809. [DOI] [PubMed] [Google Scholar]
- 199.Nemmar A, et al. , Waterpipe Tobacco Smoke Inhalation Triggers Thrombogenicity, Cardiac Inflammation and Oxidative Stress in Mice: Effects of Flavouring. Int J Mol Sci, 2020. 21(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shihadeh A, et al. , Toxicant content, physical properties and biological activity of waterpipe tobacco smoke and its tobacco-free alternatives. Tob Control, 2015. 24 Suppl 1(Suppl 1): p. i22–i30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Schubert J, et al. , Mainstream smoke of the waterpipe: does this environmental matrix reveal as significant source of toxic compounds? Toxicol Lett, 2011. 205(3): p. 279–84. [DOI] [PubMed] [Google Scholar]
- 202.Aljadani RH, et al. , Waterpipe Tobacco Chemical Content, Microbial Contamination, and Genotoxic Effects: A Systematic Review. Int J Toxicol, 2020. 39(3): p. 256–262. [DOI] [PubMed] [Google Scholar]
- 203.Shihadeh A, Investigation of mainstream smoke aerosol of the argileh water pipe. Food Chem Toxicol, 2003. 41(1): p. 143–52. [DOI] [PubMed] [Google Scholar]
- 204.Monn C, et al. , Ultrafine particle emissions from waterpipes. Tob Control, 2007. 16(6): p. 390–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gurung G, Bradley J, and Delgado-Saborit JM, Effects of shisha smoking on carbon monoxide and PM2.5 concentrations in the indoor and outdoor microenvironment of shisha premises. Sci Total Environ, 2016. 548–549: p. 340–346. [DOI] [PubMed] [Google Scholar]
- 206.Gammon DG, et al. , National and state patterns of concept-flavoured cigar sales, USA, 2012–2016. Tob Control, 2019. 28(4): p. 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Koszowski B, et al. , Nicotine Content and Physical Properties of Large Cigars and Cigarillos in the United States. Nicotine Tob Res, 2018. 20(3): p. 393–398. [DOI] [PubMed] [Google Scholar]
- 208.Brunnemann KD and Hoffmann D, Assessment of the carcinogenic N-nitrosodiethanolamine in tobacco products and tobacco smoke. Carcinogenesis, 1981. 2(11): p. 1123–7. [DOI] [PubMed] [Google Scholar]
- 209.Cho YJ and Thrasher JF, Flavour capsule heat-sticks for heated tobacco products. Tob Control, 2019. 28(e2): p. e158–e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nabavizadeh P, et al. , Vascular endothelial function is impaired by aerosol from a single IQOS HeatStick to the same extent as by cigarette smoke. Tob Control, 2018. 27(Suppl 1): p. s13–s19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Biondi-Zoccai G, et al. , Acute Effects of Heat-Not-Burn, Electronic Vaping, and Traditional Tobacco Combustion Cigarettes: The Sapienza University of Rome-Vascular Assessment of Proatherosclerotic Effects of Smoking (SUR - VAPES) 2 Randomized Trial. J Am Heart Assoc, 2019. 8(6): p. e010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lüdicke F, et al. , Effects of Switching to the Menthol Tobacco Heating System 2.2, Smoking Abstinence, or Continued Cigarette Smoking on Clinically Relevant Risk Markers: A Randomized, Controlled, Open-Label, Multicenter Study in Sequential Confinement and Ambulatory Settings (Part 2). Nicotine Tob Res, 2018. 20(2): p. 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Leigh NJ, et al. , Tobacco-specific nitrosamines (TSNA) in heated tobacco product IQOS. Tob Control, 2018. 27(Suppl 1): p. s37–s38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pacitto A, et al. , Characterization of airborne particles emitted by an electrically heated tobacco smoking system. Environ Pollut, 2018. 240: p. 248–254. [DOI] [PubMed] [Google Scholar]
- 215.Oliver AJ, et al. , Flavored and nonflavored smokeless tobacco products: rate, pattern of use, and effects. Nicotine Tob Res, 2013. 15(1): p. 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wennmalm A, et al. , Relation between tobacco use and urinary excretion of thromboxane A2 and prostacyclin metabolites in young men. Circulation, 1991. 83(5): p. 1698–704. [DOI] [PubMed] [Google Scholar]
- 217.Malovichko MV, et al. , Systemic Toxicity of Smokeless Tobacco Products in Mice. Nicotine Tob Res, 2019. 21(1): p. 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Laytragoon-Lewin N, et al. , Direct effects of pure nicotine, cigarette smoke extract, Swedish-type smokeless tobacco (Snus) extract and ethanol on human normal endothelial cells and fibroblasts. Anticancer Res, 2011. 31(5): p. 1527–34. [PubMed] [Google Scholar]
- 219.Eliasson M, et al. , Relationship of cigarette smoking and snuff dipping to plasma fibrinogen, fibrinolytic variables and serum insulin. The Northern Sweden MONICA Study. Atherosclerosis, 1995. 113(1): p. 41–53. [DOI] [PubMed] [Google Scholar]
- 220.Pappas RS, et al. , Analysis of toxic metals in commercial moist snuff and Alaskan iqmik. J Anal Toxicol, 2008. 32(4): p. 281–91. [DOI] [PubMed] [Google Scholar]
- 221.Fant RV, et al. , Pharmacokinetics and pharmacodynamics of moist snuff in humans. Tob Control, 1999. 8(4): p. 387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Stepanov I, et al. , New and traditional smokeless tobacco: comparison of toxicant and carcinogen levels. Nicotine Tob Res, 2008. 10(12): p. 1773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]


