Abstract
The role of surface proteins in Vibrio cholerae attachment to chitin particles in vitro was studied. Treatment of V. cholerae O1 ATCC 14034 and ATCC 14035 with pronase E reduced the attachment of bacteria to chitin particles by 57 to 77%. A statistically significant reduction was also observed when the attachment to chitin was evaluated in the presence of homologous Sarkosyl-insoluble membrane proteins (MPs) (67 to 84%), N-acetylglucosamine (GlcNAc) (62%), the sugar that makes up chitin, and wheat germ agglutinin (40 to 56%), a lectin that binds GlcNAc. The soluble oligomers N,N′-diacetylchitobiose or N,N′,N"-triacetylchitotriose caused an inhibition of 14 to 23%. Sarkosyl-insoluble MPs able to bind chitin particles were isolated and visualized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis; two of these peptides (molecular sizes, 36 and 53 kDa) specifically bind GlcNAc.
Vibrio cholerae is the etiological agent of a severe diarrheal disease (cholera) which continues to devastate many developing countries (12, 16). Given the observation that it can live and multiply in seawater, great attention has been given to the identification and characterization of its environmental reservoirs. It has been shown that the persistence of V. cholerae in the aquatic environment is facilitated by its ability to colonize various substrates, including zooplankton surfaces (i.e., copepods) and detrital chitin (4, 5, 8–10, 24). The attachment, survival, and proliferation of vibrios on the surfaces of small crustacea are well documented; adhering bacteria have also been shown to assume special survival forms, including a viable but nonculturable state (1, 5, 14, 20, 22). As a result of these studies, it has been suggested that altered forms of V. cholerae in specific association with plankton organisms are the most plausible reservoir from which epidemic, fully virulent strains could spring (5).
V. cholerae, as other Vibrio species, produces a chitinase(s) responsible for the degradation of chitin to soluble oligosaccharides (2, 6). Without such bacterial activity that returns the insoluble polysaccharide to the ecosystem in a biologically useful form, ocean waters would be depleted of carbon and nitrogen in a relatively short time. Therefore, the study of the interactions occurring between vibrios and chitin-containing surfaces is important for both its impact on human health and its ecological significance.
Bacterial binding to various surfaces involves several forces, including hydrophobic and ionic bonds and also lectin-like interactions between bacterial ligands and complementary receptors on the substrate. Few examples of specific interactions between bacteria and chitin-containing surfaces are known. Lectins with specificity for N-acetylglucosamine (GlcNAc), the sugar that makes up chitin, have been demonstrated in Vibrio harveyi, Vibrio damsela, and Vibrio furnissii (17, 18, 25, 26); the existence of chitin-binding proteins (CBPs) in Vibrio alginolyticus (3, 21) has recently been shown. These results have prompted us to verify whether in V. cholerae, the ability to colonize chitin-containing surfaces is dependent on the presence of a similar chitin recognition system.
V. cholerae O1 classical strains ATCC 14034 (Inaba serotype) and ATCC 14035 (Okawa serotype) (15) were used throughout this study. Marine broth 2216 (Difco Laboratories, Detroit, Mich.) and thiosulfate-citrate-bile salts sucrose agar (Difco) were used; plates were poured with Bacto Agar at a final concentration of 15 g liter−1. To radiolabel bacteria, strains were grown in marine broth 2216 containing 10 μCi of [methyl-3H]thymidine (25 Ci/mmol) ml−1. After overnight growth, cells were harvested by centrifugation (3,000 × g for 15 min at 4°C), washed three times with phosphate-buffered 3% (wt/vol) NaCl solution (pH 8), and resuspended in the same buffer to an A650 of 0.1. The efficiencies of cell labelling varied from 1,100 to 3,500 cells per count per min. Bacterial attachment to chitin particles was evaluated as described previously (17, 21). Briefly, 1 volume of radiolabelled bacterial suspension was added to 1 volume of phosphate-buffered 3% (wt/vol) NaCl solution (pH 8) containing UV-sterilized chitin purified from crab shell (2.5 mg ml−1; Sigma Chemical Co., St. Louis, Mo.), and the mixture was incubated at 20°C with shaking; a control sample without chitin was also prepared. At timed intervals, three replicates of each treatment were filtered onto 8-μm-pore-size filters (25-mm-diameter polycarbonate membranes; Bio-Rad Laboratories Srl, Milan, Italy), which were then rinsed with marine broth 2216 (10 ml) and radioassayed with a Beckman model L5 1801 scintillation counter. The total number of cells attached to chitin particles was calculated according to the efficiency of cell labelling. To evaluate background counts due to the attachment of bacteria to filtration membranes, duplicate samples for each treatment were incubated without chitin and filtered to correct for unattached cells left on the filter. The radioactivity of these control filters was subtracted from the sample values to measure the radioactivity of cells that had attached to the chitin particles. Other experiments were performed by incubating chitin with bacterial membrane proteins (MPs) isolated as described below (from 5 to 15 μg per mg of chitin), wheat germ agglutinin (WGA), or concanavalin A (ConA) (100 μg per mg of chitin). Other tests were performed in the presence of either N,N′-diacetylchitobiose (chitobiose), N,N′,N"-triacetylchitotriose (chitotriose), GlcNAc, d-glucose, d-fructose, or d-fucose (final concentration, 10 mg ml−1). Treatment of bacteria with either pronase E or sodium m-periodate (Sigma) was performed as described previously (21). To isolate N-dodecanoylsarcosinate (Sarkosyl)-insoluble MPs, bacteria grown overnight were centrifuged (10,000 × g for 20 min at 4°C) three times and resuspended in 125 mM Tris-HCl (pH 6.7). Concentrated cells were ultrasonicated (Ultrasonic liquid processor model XL 2020 with heat system) at 20% power for 30 s on ice. This sonication step was repeated five times, with a 60-s cooling period between each sonication. The samples were centrifuged at 10,000 × g (for 20 min at 4°C) to pellet unbroken cells and then at 100,000 × g (for 40 min at 4°C) to pellet cell membranes. The sediment was resuspended in Tris, treated for 30 min at 20°C with 0.5% (wt/vol) Sarkosyl (Sigma), and then centrifuged at 100,000 × g (for 40 min at 20°C). This step was repeated three times, and the last pellet, containing Sarkosyl-insoluble MPs, was washed with Tris and resuspended in the same buffer. Proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (13) with a 3.85% (wt/vol) acrylamide stacking gel and a 12.5% (wt/vol) separating gel. The determination of protein concentrations was performed with a protein assay (Bio-Rad Laboratories Srl). To isolate CBPs, aliquots of the Sarkosyl-insoluble MP fraction were incubated (for 30 min at 25°C) with 5-mg portions of chitin particles. The mixture was then washed with Tris-buffered saline (25 mM Tris buffer [pH 7.5] and 150 mM NaCl) and centrifuged (10,000 × g) to remove proteins not binding to chitin; this step was repeated five times. Forty-microliter aliquots of the pellet, resuspended in Tris-buffered saline, were then added to 40 μl of loading buffer (100 mM Tris-HCl [pH 6.8], 100 mM dithiothreitol, 2% [wt/vol] SDS, 0.2% [wt/vol] bromophenol blue, 20% [vol/vol] glycerol), heated in boiling water (for 10 min) to remove CBPs from the chitin particles, and centrifuged (10,000 × g for 10 min at 25°C). The supernatant was assayed for total proteins and analyzed by SDS-PAGE.
To study whether V. cholerae interactions with chitin are mediated by surface proteins as previously shown for V. alginolyticus (21), the attachment of strains 14034 and 14035 to chitin particles was evaluated after bacteria had been treated with pronase E; as a control, bacteria were treated with sodium m-periodate, which oxidizes polysaccharides. As shown in Table 1, pronase E reduced attachment by 69 to 70%, depending on the strain, while sodium m-periodate had no effect.
TABLE 1.
Effects of treatment of bacteria with pronase E, sodium m-periodate, and sugars on V. cholerae attachment to chitin
| Strain | Mean no. of bacteria (106) per 2.5 mg of chitin ± SD (% inhibition)a treated with:
|
||||||
|---|---|---|---|---|---|---|---|
| Nothing (control) | Pronase E | Na m-periodate | GlcNAc | Chitobiose | Chitotriose | d-Glucoseb | |
| 14034 | 37 ± 0.8 | 11 ± 0.5 (70) | 37 ± 1.4 (NI) | 14 ± 0.8 (62) | 32 ± 1.2 (14) | 29 ± 0.5 (22) | 37 ± 0.5 (NI) |
| 14035 | 39 ± 2.1 | 12 ± 0.8 (69) | 39 ± 1.6 (NI) | 15 ± 0.8 (62) | 33 ± 1.6 (15) | 30 ± 1.7 (23) | 39 ± 1.7 (NI) |
Results are averages of three independent experiments performed with three cultures on different days. Percentages of attachment inhibition were calculated by comparing the treated samples with untreated controls. NI, no inhibition. Differences between samples treated with GlcNAc, chitobiose, chitotriose, and pronase E and untreated controls were statistically significant (P ≤ 0.05).
Results similar to those obtained with d-glucose were obtained with d-fucose and d-fructose.
To further examine the role of cell envelope peptides in V. cholerae interactions with chitin, Sarkosyl-insoluble MPs were isolated from strains 14034 and 14035, and their capabilities to inhibit attachment to chitin particles of homologous strains were evaluated. As a control, MPs extracted from V. alginolyticus T3, which attaches to chitin particles through CBPs (21), or from Escherichia coli DH5α (7), which does not attach to this substrate, were used. The level of attachment was lower in the presence of homologous (67 to 84% reduction) and V. alginolyticus (40 to 42% reduction) MPs (Table 2) and was unchanged with MPs isolated from E. coli DH5α.
TABLE 2.
Effects of treatment of chitin with MPs and lectins on V. cholerae attachment to chitin
| Strain | Mean no. of bacteria (106) per 2.5 mg of chitin ± SD (% inhibition)a treated with:
|
|||||
|---|---|---|---|---|---|---|
| Nothing (control) | MPsb from:
|
WGA | ConA | |||
| V. cholerae | V. alginolyticus | E. coli | ||||
| 14034 | 45 ± 3.9 | 7 ± 0.4 (84) | 27 ± 1.1 (40) | 46 ± 2.1 (NI) | 20 ± 2.7 (56) | 45 ± 3.0 (NI) |
| 14035 | 48 ± 0.9 | 16 ± 0.5 (67) | 28 ± 1.2 (42) | 49 ± 2.1 (NI) | 19 ± 2.5 (40) | 51 ± 2.7 (NI) |
Results are averages of three independent experiments performed with three cultures on different days. Percentages of attachment inhibition were calculated by comparing the treated samples with untreated controls. NI, no inhibition. Only differences between controls and samples treated with either WGA or MPs isolated from the homologous strains and V. alginolyticus were statistically significant (P ≤ 0.01).
Sarkosyl-insoluble MPs isolated from the homologous V. cholerae strain, V. alginolyticus T3, and E. coli DH5α were used; 5 μg of MPs per mg of chitin was used in each case.
To analyze the sugar specificities of the proteins involved in binding to chitin, the level of bacterial attachment was evaluated in the presence of either GlcNAc, the sugar that makes up chitin, or the soluble oligomers chitobiose and chitotriose. Moreover, chitin was treated with WGA, a lectin that binds this sugar. As shown in Table 1, GlcNAc inhibited V. cholerae attachment by 62%, while chitobiose and chitotriose caused inhibitions ranging from 14 to 23%. d-Glucose, d-fructose, and d-fucose, used as controls, had no effects. Chitin treatment with WGA (Table 2) reduced bacterial attachment by 40 to 56%, while ConA, which binds polymers containing d-mannose and d-glucose, had no effect.
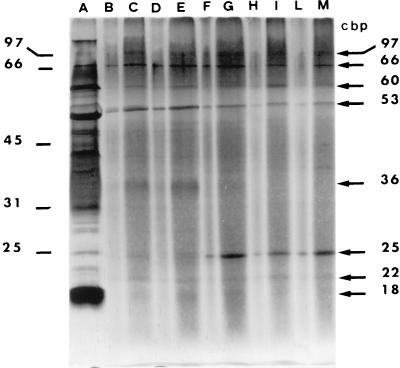
Finally, to identify the V. cholerae protein(s) able to bind chitin (CBPs), Sarkosyl-insoluble MPs isolated from the tested strains were incubated with chitin, and the fraction bound to the particles was separated by SDS-PAGE. As shown in Fig. 1, which reports the results obtained with strain 14034, at least eight CBPs (molecular sizes of 97, 66, 60, 53, 36, 25, 22, and 18 kDa) were visualized (lanes B and C). To define the binding properties of these peptides, the same experiment was performed by adding increasing amounts of GlcNAc (from 2 to 100 mg ml−1) to chitin-MP complexes (Fig. 1, lanes D to M). At concentrations starting from 10 mg ml−1 (Fig. 1, lanes F to M) GlcNAc strongly inhibits the binding of the 36-kDa peptide to chitin particles. In the presence of the sugar, a reduction of binding of the 53-kDa peptide was also observed (Fig. 1, lanes F to M), while the amount of the 25-kDa peptide bound to chitin particles increased. Chitobiose and chitotriose, at the concentration used (10 mg ml−1), did not have any effect (data not shown).
FIG. 1.
SDS-PAGE of V. cholerae 14034 Sarkosyl-insoluble MPs that bind chitin particles in the presence or absence of GlcNAc. Electrophoresed proteins were visualized by silver staining. Molecular sizes (in kilodaltons) of markers and CBPs are indicated on the left and right, respectively. Results are shown for Sarkosyl-insoluble MPs with 100 μg of proteins loaded on the gel (A), Sarkosyl-insoluble MPs bound to chitin particles with 5 (B) or 10 (C) μg of proteins loaded on the gel, and Sarkosyl-insoluble MPs bound to chitin particles in the presence of GlcNAc (2 mg ml−1) with 5 (D) or 10 (E) μg of proteins loaded on the gel, in the presence of GlcNAc (10 mg ml−1) with 5 (F) or 10 (G) μg of proteins loaded on the gel, in the presence of GlcNAc (50 mg ml−1) with 5 (H) or 10 (I) μg of proteins loaded on the gel, and in the presence of GlcNAc (100 mg ml−1) with 5 (L) or 10 (M) μg of proteins loaded on the gel.
The results presented in this paper indicate that surface proteins are involved in in vitro interactions between V. cholerae O1 classical strains and chitin particles. Although eight peptides were visualized by SDS-PAGE, only two seemed to specifically recognize chitin: the 36-kDa protein, whose binding to chitin is completely inhibited by GlcNAc, and the 53-kDa protein, whose binding is partly reduced by the sugar. Since Sarkosyl-insoluble MPs are present as membrane-detergent-protein aggregates, the other proteins visualized by SDS-PAGE (insensitive to GlcNAc inhibition) could only interact indirectly with the chitin particles as part of a complex. Apparently, the 36- and 53-kDa proteins have affinity for the GlcNAc monosaccharide and not for di- and trisaccharides, as suggested by the fact that chitobiose and chitotriose, at the same concentration (10 mg ml−1) at which binding reduction by GlcNAc was already evident (Fig. 1, lanes F and G), did not have any effect. The slight inhibition of bacterial binding to chitin (Table 1) may be due to chitobiose and chitotriose interaction with other surface components.
The increase in the amount of the 25-kDa protein bound to chitin in the presence of GlcNAc (Fig. 1, lanes F to M) may be due, at least in part, to the fact that the same protein amount was loaded on all gel lanes. Therefore, diminishing the 36- and 53-kDa bands leads to an increase in some of the other peptides. Alternatively, two types of CBP with different affinities toward chitin may exist: the high-affinity CBPs rapidly and efficiently interact with chitin, while the low-affinity ones can interact with this substrate only when the former are inhibited.
Our results and those obtained by others (17, 18, 21) indicate that CBPs are present in at least three species: V. harveyi, V. alginolyticus, and V. cholerae. Since preliminary data from our laboratory suggest that the same system is present in other Vibrio species, CBPs may give these bacteria an important advantage in competing with other microorganisms for this particular substrate. Interestingly, V. alginolyticus MPs reduced the attachment of V. cholerae to chitin particles, albeit less efficiently than homologous proteins (40 to 42% versus 67 to 84%), confirming the similarities between the chitin-binding systems present in the two species. It was previously shown that in both V. harveyi and V. alginolyticus a 53-kDa MP mediates attachment to chitin (17, 21). Since we show in this paper that a CBP of the same molecular mass is expressed by V. cholerae, this protein seems to be a conserved ligand and may be a good candidate for the preparation of Vibrio-specific probes and primers.
Both animate (e.g., copepod surface) and inanimate (detrital chitin) substrates are targets for these bacteria that, after binding, may initiate the solubilization of the polysaccharide through the activity of their chitin-hydrolyzing enzymes. The mechanisms underlying bacterial attachment to chitin are complex and include several types of physical-chemical reactions; CBPs may be involved in fostering bacterial attachment to this substrate. It was previously advanced that the association of V. cholerae with zooplankton is a key factor in deciphering the global nature of cholera epidemics (5). Therefore, the ability of vibrios to bind chitin seems to be crucial for their survival in the environment and for their transmission to humans. It was also shown that the attachment of vibrios to copepods is less efficient than their attachment to chitin particles (11). This could be due to the presence on the copepod surface of a wax epicuticle which prevents close contact until bacterial enzymatic activities (e.g., lipase) have digested the epicuticle. To define the role of the detected CBPs in bacterial attachment to copepods we are studying the ability of V. cholerae mutants lacking MPs to adhere to these plankton organisms (data not shown).
A direct relationship between the attachment of V. cholerae O1 bacteria to chitin surfaces and human diseases was hypothesized by Nalin et al. (19), who showed that chitin protects V. cholerae O1 from the lethal effects of a low pH. Recently, Singh et al. (23) found that a V. cholerae 53-kDa protein is involved in intestinal colonization. In a forthcoming paper we intend to show that the mutant that does not express the peptide (23) attaches to chitin particles less efficiently than the parental strain (data not shown). This suggests that the same bacterial surface ligand, binding a widespread compound (GlcNAc), may have a dual function in the V. cholerae life cycle, mediating the attachment to inanimate substrates (e.g., detrital chitin and copepod exoskeleton) in the environment and to cell membranes in the intestinal tract. The capability to utilize the same structure to interact with different substrates could be a common feature of pathogenic bacteria that have environmental reservoirs and could constitute the discriminating feature between harmless and potentially pathogenic environmental species.
Acknowledgments
This work was supported by CNR grant 97.04239.CT04 and CNR Target Project on Biotechnology 97.01187.PF49.
REFERENCES
- 1.Barcina I, Lebaron P, Vives-Rego J. Survival of allochthonous bacteria in aquatic systems: a biological approach. FEMS Microbiol Ecol. 1997;23:1–9. [Google Scholar]
- 2.Baumann P, Schubert R H W. Family II. Vibrionaceae. In: Krieg N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore, Md: The Williams & Wilkins Co.; 1984. p. 516. [Google Scholar]
- 3.Carli A, Pane L, Casareto L, Bertone S, Pruzzo C. Occurrence of Vibrio alginolyticus in Ligurian coast rock pools (Tyrrhenian Sea, Italy) and its association with the copepod Tigriopus fulvus (Fisher 1860) Appl Environ Microbiol. 1993;59:1960–1962. doi: 10.1128/aem.59.6.1960-1962.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colwell R R. Microbial ecology and biofouling. In: Colwell R R, Pariser E R, Siskey A J, editors. Biotechnology in the marine sciences. New York, N.Y: Wiley Intersciences; 1981. p. 221. [Google Scholar]
- 5.Colwell R R. Global climate and infectious disease: the cholera paradigm. Science. 1996;274:2025–2031. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- 6.Farmer J J, III, Hickman-Brenner F W, Kelly M T. Vibrio. In: Lennette E H, Balows A, Hausler W J, Shadomy H J, editors. Manual of clinical microbiology. 4th ed. Washington, D.C: American Society for Microbiology; 1991. p. 281. [Google Scholar]
- 7.Hanhan D. Studies on transformation of Escherichia coli with plasmid. J Mol Biol. 1983;166:557–579. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 8.Hood M A, Winter P A. Attachment of Vibrio cholerae under various environmental conditions and to selected substrates. FEMS Microbiol Ecol. 1997;22:215–223. [Google Scholar]
- 9.Huq A, West P A, Small E B, Huq M I, Colwell R R. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl Environ Microbiol. 1983;45:275–283. doi: 10.1128/aem.45.1.275-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huq A, West P A, Small E B, Huq M I, Colwell R R. Influence of water temperature, salinity, and pH on survival and growth of toxigenic Vibrio cholerae serovar O1 associated with live copepods in laboratory microcosms. Appl Environ Microbiol. 1984;48:420–424. doi: 10.1128/aem.48.2.420-424.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaneko T, Colwell R R. Adsorption of Vibrio parahaemolyticus onto chitin and copepods. Appl Microbiol. 1975;29:269–274. doi: 10.1128/am.29.2.269-274.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaper J B, Morris J G, Jr, Levine M M. Cholera. Clin Microbiol Rev. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.McDougald D, Rice S A, Weichart D, Kjelleberg S. Nonculturability: adaptation or debilitation? FEMS Microbiol Ecol. 1998;25:1–9. [Google Scholar]
- 15.Mekalanos J J. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983;35:253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- 16.Mekelanos J J, Rubin E J, Waldor M K. Cholera; a molecular basis for emergence and pathogenesis. FEMS Immunol Med Microbiol. 1997;18:241–248. doi: 10.1111/j.1574-695X.1997.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery M T, Kirchman D L. Role of chitin-binding proteins in the specific attachment of the marine bacterium Vibrio harveyi to chitin. Appl Environ Microbiol. 1993;59:373–379. doi: 10.1128/aem.59.2.373-379.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montgomery M T, Kirchman D L. Induction of chitin-binding proteins during the specific attachment of the marine bacterium Vibrio harveyi to chitin. Appl Environ Microbiol. 1994;60:4284–4288. doi: 10.1128/aem.60.12.4284-4288.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nalin D R, Daya V, Reid A, Levine M M, Wu H C. Adsorption and growth of Vibrio cholerae on chitin. Infect Immun. 1979;25:768–770. doi: 10.1128/iai.25.2.768-770.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliver J D. Formation of viable but nonculturable cells. In: Kjelleberg S K, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. p. 239. [Google Scholar]
- 21.Pruzzo C, Crippa A, Bertone S, Pane L, Carli A. Attachment of Vibrio alginolyticus to chitin mediated by chitin binding proteins. Microbiology. 1996;142:2181–2186. doi: 10.1099/13500872-142-8-2181. [DOI] [PubMed] [Google Scholar]
- 22.Roszak D B, Colwell R R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987;51:365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh S N, Srivastava R, Sinha V B, Srivastava B S. A 53 kDa protein of Vibrio cholerae classical strain 00395 involved in intestinal colonization. Microb Pathog. 1994;17:69–78. doi: 10.1006/mpat.1994.1053. [DOI] [PubMed] [Google Scholar]
- 24.Tamplin M L, Gauzens A L, Huq A, Sack D A, Colwell R R. Attachment of Vibrio cholerae serogroup O1 to zooplankton and phytoplankton of Bangladesh waters. Appl Environ Microbiol. 1990;56:1977–1980. doi: 10.1128/aem.56.6.1977-1980.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu C, Lee A M, Roseman S. The sugar-specific adhesion/deadhesion apparatus of the marine bacterium Vibrio furnissii is a sensorium that continously monitors nutrient levels in the environment. Biochem Biophys Res Commun. 1987;149:86–92. doi: 10.1016/0006-291x(87)91608-1. [DOI] [PubMed] [Google Scholar]
- 26.Yu C, Lee A M, Bassler B L, Roseman S. Chitin utilization by marine bacteria. A physiological function for bacterial adhesion to immobilized carbohydrates. J Biol Chem. 1991;25:24260–24267. [PubMed] [Google Scholar]