Abstract
Objective:
Even though antibiotic resistance is one of the most serious threats to global public health, it is becoming more common due to inappropriate antibiotic prescribing patterns. Thus, the purpose of this study is to assess antibiotic prescribing patterns among inpatients at an Ethiopian comprehensive specialized hospital.
Methods:
An institutional-based cross-sectional study was used. During the study period, data were collected from the charts of admitted patients in selected wards of Debre Tabor comprehensive specialized hospital. The World Health Organization’s developed questionnaire and conventional antibiotic prescribing indicators were used to assess rational drug usage, with an emphasis on antibiotic prescribing trends. The data were analyzed using SPSS 25.0 statistical software.
Results:
For 861 patients admitted to medical and pediatric wards, a total of 1444 antibiotics were prescribed. Overall, 60.6% of inpatients were prescribed at least one antibiotic, with an average (mean ± SD) number of antibiotics prescribed per patient of 1.7 ± 1.6. During their hospital stay, patients were given antibiotics for an average (mean ± SD) of 6.4 ± 2.7 days. Furthermore, 83.3% of antibiotics were prescribed for therapeutic purposes, whereas 100% were provided for empiric purposes. Ceftriaxone was the most commonly administered antibiotic in the study settings (49.2%). During the study period, Debre Tabor comprehensive specialized hospital had access to 67.5% of key antibiotics.
Conclusion:
The antibiotic prescribing pattern in our study diverged from the World Health Organization-recommended guidelines. Furthermore, all antibiotics were given without a culture or sensitivity test in every case. Setting up an antibiotic stewardship program, introducing antibiotic use based on culture and sensitivity tests, and adopting institutional guidelines could all help to address this issue.
Keywords: WHO, antibiotics, prescribing pattern, prescribing indicators, resistance
Introduction
Antibiotics are medications that have saved countless lives by treating bacterial infections. These medications work by selectively killing or blocking the formation of disease-causing microorganisms, thereby destroying or inhibiting their growth. They are currently the most widely prescribed medications in hospitals around the world.1,2 Antibiotics are crucial in the fight against infectious disease and preserving good health, particularly in underdeveloped countries where infectious diseases are still a major problem. In contrast, inappropriate antibiotic use contributes to bacterial resistance development by hastening the establishment and spread of resistant microbes and influencing treatment outcomes.1,3,4
Antibiotic resistance develops when potentially dangerous bacteria adapt in a way that limits or eliminates the antibiotic’s effectiveness. Even though antibiotic resistance is a prevalent problem, it is becoming more common as a result of improper antibiotic use and prescriptions. It has been found all over the world and is currently one of the most serious threats to global public health. During the past few decades, antibiotic resistance has become a global concern due to its rapid onset and spread.1,2,4,5
Antibiotic resistance is thought to be controlled if antibiotics are used wisely, and practical antibiotic use is associated with rational antibiotic use. Patients are said to be using medicines rationally if they obtain pharmaceuticals that are appropriate for their clinical needs, in doses that fit their specific needs, for an adequate period of time, and at the lowest feasible cost.6,7
In 1985, the World Health Organization (WHO) held an international conference on rational drug use in Nairobi, Kenya, to develop recommendations for rational drug use. 6 The most common reasons for irrational drug use are self-medication, polypharmacy, inappropriate antibiotic use, abuse of intractable pharmaceuticals, and prescription medicines not prescribed according to applicable clinical practice guidelines.1,7–9 In many African countries, including Ethiopia, polypharmacy is a term used to describe the practice of prescribing many medications on a single prescription paper. 7
Antibiotic resistance is a global health concern that affects both poor and developed countries and is one of the most serious challenges facing public health to date. Inappropriate prescribing practices result in poor and unsafe therapy, disease deterioration and perpetuation, and expense increases owing to disease complications and the need for additional treatment. Inappropriate prescribing patterns also lower the standard of medical care and waste resources.10,11
Supervision, audit, and feedback are the key strategies that support reasonable medication usage. Prescription audit and feedback entails examining prescriptions for suitability before providing feedback.6,11 An essential step in encouraging rational drug use is evaluating drug use trends using WHO drug use indicators. To encourage rational medication use, researchers must first identify and characterize the different ways in which drug usage is illogical, such as polypharmacy, antibiotic misuse, and injectable medicines.6,7
Much of the research carried out in Ethiopia has revealed that the majority of bacteria that cause illnesses in humans have evolved significant resistance to frequently used first-line antibiotics. 5 The WHO core indicators help to improve prescribing patterns and, as a result, promote the practical use of pharmaceuticals in healthcare settings. The ability of clinicians to prescribe rationally must be assessed, and this can be done by conducting frequent prescription audits.2,7 As a result, this study aimed to use WHO prescribing indicators such as antibiotics prescribed per prescription and antibiotics prescribed by generic or brand name to evaluate antibiotic prescribing patterns at an Ethiopian comprehensive specialized hospital.
Methodology
Study setting
This study was carried out at Debre Tabor Comprehensive Specialized Hospital (DTCSH) in Debre Tabor, Ethiopia. This hospital is one of the largest multispecialty hospitals in Ethiopia’s Amhara region. It serves as a teaching hospital for Debre Tabor University’s undergraduate health science students. It also serves as a training facility for health professionals who work in the region’s South Gondar zone’s various health sectors. Emergency, surgical, medical, pediatrics, psychiatric, gynecological, and obstetrics are some of the wards that make up the hospital. The medical and pediatrics wards were among those where we conducted our study. Specialists, general practitioners, clinical pharmacists, nurses, and other healthcare professionals work on the wards of the hospital.
Study design and period
An institutional-based cross-sectional study was undertaken from May to August 2021.
Study population
Our study population consisted of all patients who were hospitalized in the pediatric and medical wards of the hospital during the study period and met the eligibility criteria.
Eligibility criteria
All patients hospitalized in the hospital’s designated wards were included in the study. Patients using long-term antibiotics on a schedule were not included in the study.
Sample size and sampling technique
The data were collected from the medical and pediatric wards of the hospital. The sample size was estimated using a single proportion formula and the assumption that no previous studies in the study setting had been done, and we chose a proportion (P) of 50%. The formula is as follows:
where:
n = sample size
p = the proportion of antibiotics use
Z = the 95% confidence level (1.96);
d = the marginal error (3.5% = 0.001225). We utilized 3.5% to enhance the sample size, as advised in the literature (2.5−5%). 4
The sample size was 785 based on this single proportion formula. Using a 10% contingency rate for nonresponse, the study’s final sample size was 864 patients from the hospital’s wards. Using a quota sampling technique, patients were recruited systematically. During the study period, 1420 patients were admitted to the wards. We enrolled 861 patients who were taking at least one antibiotic in our study (with a 99.7% response rate).
Study variables
The antibiotic prescribing pattern is the study’s dependent variable (WHO indicators). Sociodemographics (gender, age, and residence) and antibiotic information (indication, frequency, and regimen/combination) are the independent variables.
Data collection tools and procedure
To collect data, we used a questionnaire adapted from previous studies, as well as a WHO-developed structured questionnaire and standard antibiotic prescribing indicators.12,13 The four types of indicators used were prescribing indicators, patient care indicators, hospital indicators, and complimentary indicators. Supplemental material summarizes the WHO antibiotic prescribing indicators as well as the standard data abstraction format.
On the study days, data were collected from hospitalized patients who came in for treatment. Data were collected in a systematic manner using a standard data abstraction format. Patients admitted during this time were closely monitored. Patients’ sociodemographic characteristics; frequency, indication, and duration of prescribed antibiotics; dosage form and route of administration; and type and number of prescribed antibiotics were all collected from patients’ medical charts. Throughout the study period, patients’ medical charts were reviewed daily, and any changes in the drug chart or laboratory details were noted. Antibiotic prescription patterns were assessed using the Ethiopian Essential Medicines List (EML). Four pharmacists were hired as data collectors.
Statistical analysis
Using EpiData Manager and EpiData Entry Client Version 4.0.2.00, variables were coded, the database was set, and data were entered. For analysis, the data were exported to SPSS version 25.0. To describe sociodemographic and antibiotic prescribing patterns, descriptive statistics such as mean and standard deviation for continuous variables and frequency and percentage for categorical variables were used. Antibiotic prescribing trends were calculated, assessed, and compared with WHO prescribing indicator standard values. 12 The supplementary data summarize the details of the calculation formulae.
Data quality control
A week before the actual data collection began, 5% of the sample was pretested, and changes were made as a result. The data collectors had been trained, and they were closely supervised. The data collectors were trained, and close supervision was carried out daily. The completeness of the filled information was checked at the end of each data collection day to ensure the quality of the recorded data. If any errors were found, they would be corrected right away.
Operational definition
Antibiotics
Antibiotics are drugs that are used to treat various infections caused by different types of bacteria. Antibiotics are antibiotics that are used to prevent and treat bacterial infections. 14
Results
Sociodemographic characteristics
Eight hundred sixty-one patients out of 1420 were found to be on at least one antibiotic and were enrolled in this study. The bulk of the study participants (53.7% and 67.9%, respectively) were male and lived in rural areas (Table 1).
Table 1.
Sociodemographic characteristics of patients admitted to DTCSH, Debre Tabor, Northwest Ethiopia, 2021.
| Variables | Category | Selected wards | Frequency (%) | |
|---|---|---|---|---|
| Pediatrics | Medical | |||
| Sex | Male | 283 (62.3) | 179 (44) | 462 (53.7) |
| Female | 171 (37.7) | 228 (56) | 399 (46.3) | |
| Total | 454 (52.7) | 407 (47.3) | 861 (100) | |
| Residence | Rural | 303 (66.7) | 282 (69.3) | 585 (67.9) |
| Urban | 151 (33.3) | 125 (30.7) | 276 (32.1) | |
WHO indicators
Prescribing indicators
A total of 1444 antibiotics were prescribed for 861 (60.6%) of the hospital’s 1420 inpatients during the study period. The average (mean ± SD) number of antibiotics prescribed per patient was 1.7 ± 1.6, with the highest value (five antibiotics) occurring on the medical ward for a mean ± SD period of 6.4 ± 2.7 days, and the longest time (14 days) occurring on the selected wards.
Furthermore, 98.5% of antibiotics were prescribed using their generic names, and 100% of antibiotics were prescribed from Ethiopia’s EML. Table 2 summarizes the details of prescribing indicators. Because culture and sensitivity testing of antibiotics was not done at all at the hospital, all antibiotics were prescribed empirically in all cases. Antibiotics were prescribed for therapeutic purposes in the cases (83.3%) patients for the indication of pneumonia, sepsis, and urinary tract infection and for empiric purposes in 100% of the cases. The most widely used route of antibiotic administration was an injection, which was utilized in 78.7% of patients, mostly in pediatric wards (89.9%).
Table 2.
WHO prescribing indicators of antibiotics among patients admitted to DTCSH, Northwest Ethiopia, 2021.
| Prescribing indicator | Selected wards, n (%) [N = 1420] | Total, n = 861 n (%), mean ± SD | |
|---|---|---|---|
| Pediatrics | Medical | ||
| Percentage of patients admitted with at least one antibiotic | 454 (52.7) | 407 (47.3) | 861 (60.6) |
| The average number of antibiotics prescribed per patient | 661 (1.5) | 783 (1.9) | 1.7 ± 1.6 |
| Percentage of antibiotics prescribed from national FL/EML | 661 (100) | 783 (100) | 1444 (100) |
| Percentage of antibiotics prescribed by generic name | 659 (99.7) | 763 (97.4) | 1422 (98.5) |
| Percentage of antibiotics prescribed in injection form | 594 (89.9) | 542 (69.2) | 1136 (78.7) |
| Percentage of antibiotics prescribed in oral form | 67 (10.1) | 241 (30.8) | 308 (21.3) |
| The average duration of days antibiotic prescribed in the hospital stay | 6.7 | 6.0 | 6.4 ± 2.7 |
| Percentage of patients who received antibiotics for therapeutic purposes | 420 (82.5) | 594 (83.9) | 1014 (83.3) |
| Percentage of patients who received antibiotics for prophylaxis | 81 (15.9) | 78 (11.0) | 159 (13.1) |
| Percentage of patients who received antibiotics for an unknown purpose | 8 (1.6) | 36 (5.1) | 44 (3.6) |
| Percentage of patients who received antibiotics for empiric therapy | 152 (100) | 75 (100) | 227 (100) |
SD: standard deviation; FL/EML: formulary list/essential medicine list.
Hospital and patient care indicators
A copy of the national standard treatment guidelines (STGs) for hospitals, as well as a Drug and Therapeutic Committee (DTC), were available at the hospital. In the hospital, there was no national formulary list or hospital formulary list of drugs, especially for infectious disorders. 67.5% of the antibiotics were found to be in stock during the study period. Furthermore, the average number of days those essential antibiotics were out of stock per month was found to be 5.1 days (Table 3).
Table 3.
Hospital and patient indicators for prescribing antibiotics for patients admitted to DTCSH, Debre Tabor, Northwest Ethiopia, 2021.
| Hospital indicator | Results | Recommended |
|---|---|---|
| Existence of DTC * | Yes | Yes |
| Existence of a copy of the national STGs # for hospitals | Yes | Yes |
| Existence of institutional STGs/clinical guidelines for infectious diseases | No | Yes |
| Existence of a copy of the national FL/EML ** | No | Yes |
| Existence of institutional FL/EML | No | Yes |
| The availability of a set of key antibiotics in the hospital during the study period | 67.5% | 100% |
| The average number of days that a set of key antibiotics is out of stock per month | 5.1 | 0 |
| Number of sensitivity tests performed for the prescribed antibiotics | No | Yes |
DTC: Drug and Therapeutic Committee; EML: essential medicines list.
Drug and Therapeutics Committee; #Standard Treatment Guideline; **Formulary List/Essential Medicine List.
Distribution of prescribed antibiotics
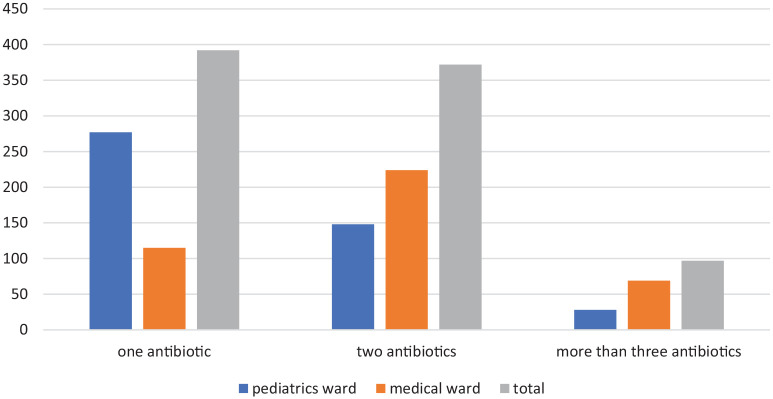
As shown in Table 4, antibiotics were provided to nearly half of the patients (54.2%) admitted to the medical ward. Ceftriaxone was the most commonly prescribed antibiotic on the wards, accounting for 49.2% of all prescriptions, followed by azithromycin (12.5%) and metronidazole (9.3%). Ceftriaxone with azithromycin was the most commonly prescribed combination (33.6%), followed by ceftriaxone with metronidazole (17.2%). In terms of the number of antibiotics prescribed per patient, more than half of the patients (54.5%) were given a combination of antibiotics (Figure 1).
Table 4.
Antibiotics prescribed for patients admitted to selected wards of DTCSH in 2021, Debre Tabor, Northwest Ethiopia.
| Antibiotics | Involved wards, n (%) | Total, n (%) | |
|---|---|---|---|
| Pediatrics | Medical | ||
| Ceftriaxone | 342 (51.7) | 368 (47.0) | 710 (49.2) |
| Azithromycin | 10 (1.5) | 171 (21.8) | 181 (12.5) |
| Metronidazole | 61 (9.2) | 74 (9.5) | 135 (9.3) |
| Vancomycin | 35 (5.3) | 72 (9.2) | 107 (7.4) |
| Gentamycin | 46 (7.0) | 3 (0.4) | 49 (3.4) |
| Ampicillin | 43 (6.5) | 0 (0.0) | 43 (3.0) |
| Doxycycline | 5 (0.8) | 36 (4.6) | 41 (2.8) |
| Crystalline penicillin | 37 (5.6) | 0 (0.0) | 37 (2.6) |
| Ciprofloxacin | 17 (2.6) | 18 (2.3) | 35 (2.4) |
| Amoxicillin | 27 (4.1) | 6 (0.8) | 33 (2.3) |
| Cloxacillin | 24 (3.6) | 6 (0.8) | 30 (2.1) |
| Ceftazidime | 4 (0.6) | 16 (2.0) | 20 (1.4) |
| Cotrimoxazole | 10 (1.5) | 0 (0.0) | 10 (0.7) |
| Clindamycin | 0 (0.0) | 4 (0.5) | 4 (0.3) |
| Norfloxacin | 0 (0.0) | 4 (0.5) | 4 (0.3) |
| Clarithromycin | 0 (0.0) | 3 (0.4) | 3 (0.2) |
| Amoxicillin–clavulanic acid | 0 (0.0) | 2 (0.3) | 2 (0.14) |
| Total | 661 (45.8) | 783 (54.2) | 1444 (100) |
| Combination of antibiotics | |||
| Ceftriaxone + azithromycin | 11 (7.1) | 133 (48.7) | 144 (33.6) |
| Ceftriaxone + metronidazole | 40 (25.6) | 34 (12.5) | 74 (17.2) |
| Ampicillin + gentamycin | 38 (24.4) | 0 (0.0) | 38 (8.9) |
| Ceftriaxone + doxycycline | 5 (3.2) | 32 (11.7) | 37 (8.6) |
| Ceftriaxone + vancomycin | 17 (10.9) | 18 (6.6) | 35 (8.2) |
| Ceftriaxone + metronidazole + gentamycin | 7 (4.5) | 14 (5.1) | 21 (4.9) |
| Ceftriaxone + cloxacillin | 19 (12.2) | 0 (0.0) | 19 (4.4) |
| Ceftriaxone + vancomycin + azithromycin | 0 (0.0) | 18 (6.6) | 18 (4.2) |
| Ceftriaxone + vancomycin + ciprofloxacin | 5 (3.2) | 8 (2.9) | 13 (3.0) |
| Vancomycin + ceftazidime | 4 (2.6) | 6 (2.2) | 10 (2.3) |
| Amoxicillin + metronidazole | 5 (3.2) | 3 (1.1) | 8 (1.9) |
| Metronidazole + clarithromycin | 5 (3.2) | 3 (1.1) | 8 (1.9) |
| Ceftriaxone + doxycycline | 0 (0.0) | 4 (1.5) | 4 (0.9) |
Figure 1.
Numbers of antibiotics prescribed per prescription for patients admitted to selected wards of DTCSH, Debre Tabor, Northwest Ethiopia, 2021.
Complementary indicators
During their hospital stay, patients received antibiotics for an average (mean ± SD) of 6.4 ± 2.7 days (Table 2). Antibiotics were prescribed for 7 days or more in half of the patients (50%). Antibiotics were used by nearly one-third of the patients (32%) for 5 days, and 18% for 3 days or less (Figure 2).
Figure 2.
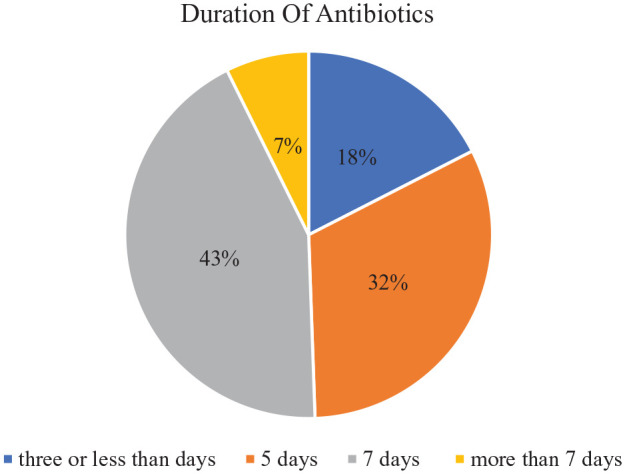
Number of days antibiotics prescribed for patients admitted to selected wards of DTCSH, Debre Tabor, northwest Ethiopia, 2021.
Discussion
In this study, the prescribing pattern of antibiotics in two DTCSH wards was analyzed using WHO prescribing indicators. During the study period, 60.6% of patients were prescribed antibiotics. This percentage was higher than the WHO’s 15 recommended range of 20–25.4%. This indicates that DTCSH has a high rate of antibiotic prescriptions. This percentage matched that of prior research, which found 58.1% in southern Ethiopia, 6 58.5% in Gondar, 2 and 64.7% in southwest Ethiopia. 16 On the other hand, our antibiotic prescribing rate was low when compared with the findings of Harar (66.9%), 17 India (66%), 18 Congo (68%), 19 Eritrea (79.05%), 13 and Pakistan (82.3%). 12
The percentage of patients using at least one antibiotic in this study was greater than the figures of 24.37% reported in Wello town, Ethiopia, 20 29.4% in Gondar hospital, Ethiopia, 21 48.5% in Gondar town, Ethiopia, 22 and 32.0% in Ayder hospital, Northern Ethiopia. 10 Similarly, it was also higher than in studies reported from Cameroon (36.71%), 23 Pakistan (51.5%), 24 China (54.6%), 25 and Ghana (55.2%). 26
This disparity could be explained by the fact that these studies were conducted on outpatients who only received oral antibiotics in the community, whereas our study was conducted on hospitalized patients who received both oral and parenteral antibiotics. Furthermore, compared with our 11 month study (nearly 1 year), these studies looked at antibiotic prescribing patterns for a shorter period of time (no more than 6 months). In addition, in Ayder hospital, Ethiopia, 10 all drugs were included, whereas other studies were conducted on outpatients, with a rate of 58% in Hawassa, Ethiopia, 6 and in primary health care in Saudi Arabia. 27
This study found that the average number of antibiotics per patient was 1.7, which is within the range recommended by WHO, 15 with a limit of 1.6–1.8. The antibiotic prescription pattern in the study area was similar to the 1.6 antibiotics per patient reported in another Gondar study. 2 Our finding, on the other hand, was higher than those reported from Eritrea and Congo, where 1.29 and 1.4 antibiotics were prescribed per patient, respectively.13,19 This finding was lower than the optimal values reported from eastern Ethiopia, Aksum, Addis Ababa, and Gujarat (2.0–2.2 antibiotics per patient).4,5,16,28 The lower the number of drugs prescribed per patient, the more appropriate the prescription practice. It decreases polypharmacy, which reduces disease complications caused by drug–drug interactions and adverse drug reactions. This is in line with a study undertaken in Aksum, 4 in which the national EML was used to prescribe 100% of antibiotics.
All of the antibiotics prescribed in this current study (100%) were from Ethiopia’s EML. 29 This is in line with a study undertaken in Aksum, 4 in which 100% of antibiotics were prescribed from the national EML. This could be explained by the fact that Ethiopian public hospitals procure the majority of their drugs from a government supplier, which mostly distributes pharmaceuticals based on the national EML. As a result, prescribers in DTCSH are encouraged to follow the EML, resulting in 100% compliance. Similarly, all antibiotics were prescribed from the national EML in Eritrea. 13 Many studies have revealed deviations from the target endorsed by their national EML, such as 98.0% in India, 30 98.8% in Pakistan, 24 84.8% in Gujarat, 5 and 79% in Lesotho. 31 In this study, 100% of antibiotics were prescribed from the EML, which corresponded to the WHO’s 32 recommended value.
Another significant finding from our study was that injectable antibiotics were frequently prescribed (78.7%). This compares with 81.4% in Asmara, Eritrea 13 and 82.4% in Addis Ababa, Ethiopia, 28 but is lower than the 84.8% in Aksum 4 and higher than the 68.2% in Congo. 19 Other studies in Ethiopia found lower rates of injection prescribing at 4%, 2 11.2%, 33 26.5%, 17 and 38%. 6 Those studies on outpatients, in particular, may account for the lower percentage of injections being prescribed. Injectable antibiotics were most likely readily available in our study because they are often reserved for hospitalized patients, who would be managed onward in the setting of this investigation, whereas outpatients would not. Injectable antibiotics are overprescribed, which is considered inappropriate antibiotic use.24,34 As a result, DTCSH prescribers should emphasize the use of oral antibiotics over injectable antibiotics to prevent injection-related infections, shorten hospital stays and lower healthcare costs. 35 This could indicate that prescribers are aware of the fact that they should not be administering as many injections as they now do to patients.
During the study period, 67.5% of 54 key antibiotics assessed from Ethiopia’s national EML 29 had stock availability in DTCSH. A comparable result (65.2% and 65.7%) has been reported in Aksum and southern Ethiopia, respectively.4,36 Although our findings were lower than those reported in other studies, with stock availability of key antibiotics of 72.4% in Pakistan, 24 81.3% in Sudan, 37 83.3% in Zambia, 38 and 87.5% in Eritrea, 13 these values are much lower than the ideal value, as key antibiotics should always be available. 32
This finding could point to a scarcity of key antibiotics, leading prescribers to prescribe antibiotics with inferior efficacy, higher prices, and higher resistance. Key antibiotics were out of stock in the study area for 5.1 days per month, which is significantly higher than the 30 days per year reported in another study in Ethiopia 9 and 3.8 days per month in Aksum, 4 but lower than in Eritrea’s 6.52 days per month (78.18 days per year). 13 The finding that key antibiotics were unavailable for 5.1 days per month should be considered by policymakers, who may need to enact legislation to ensure better access to these critical drugs.
Despite the goal of prescribing antibiotics by their generic names 100% of the time, we found that only 98.5% of medicines were prescribed by their generic names. According to the WHO 15 indicators, prescription antibiotics by their generic names are one of the clearest markers of the adoption of low-cost antibiotics. In this study, antibiotics were prescribed by their generic names 98.5% of the time. This was slightly less than the WHO-recommended criterion of prescribing medications by their generic names (100%). 34 This result, however, was higher than the rates of 49.3% and 56.1% found in Sudan 37 and Zambia 38 studies, respectively. Furthermore, our findings are similar to those of Gondar, Ethiopia (96%), 2 Aksum, Ethiopia (97.6%), 4 Sidama, Ethiopia (95.8%), 39 Cameroon (98.36%), 23 India (98.0%), 30 and Eritrea (97%). 13
In truth, there is no difference in pharmacological efficacy between prescribing antibiotics by their brand and generic names. 40 This may be justified because prescribing pharmaceuticals by their generic names minimizes the danger of drug replication, as patients may be unaware that the same drug is being used by two doctors if one prescribes a brand name and the other a generic name, or if both prescribe different brands. However, there may be discrepancies in perceptions and understandings of the differences between generic and brand names. 41 As a result, in our setting, prescribing antibiotics by generic names is a beneficial practice that should be encouraged.
Another finding in this study showed that patients were given antibiotics for an average (mean ± SD) of 6.4 ± 2.7 days during their hospital stay, which is consistent with the typical antibiotic use length. Antibiotics were prescribed to half of the patients (50%) for 7 days or longer, while nearly one-third (32%) were prescribed for 5 days, and 18% were prescribed for 3 days or less. This statistic corresponds to research conducted in Eritrea, 13 in which antibiotics were prescribed for 6.36 days. This result is higher than that of a similar study conducted in Aksum, 4 in which antibiotics were prescribed for 4.2 ± 2.3 days. Use of antibiotics for shorter or longer durations in the hospital necessitates special attention, which could be addressed by adopting an institutional guideline.
In this study, 17 different antibiotics were administered as single agents or in combination. Since DTCSH does not do culture and local sensitivity testing of microorganisms, all antibiotics are prescribed empirically. As evidenced by similar studies,4,24,25,28 prescribing broad-spectrum antibiotics is common practice. There may be no need for cultures to isolate microorganisms when prescribing broad-spectrum antibiotics. In contrast to a research by Demoz et al., 4 we found that more than 80% of antibiotics were recommended for therapeutic purposes. This difference could be attributed to the study’s inclusion of surgical and gynecology/obstetrics wards. Ceftriaxone was the most commonly prescribed antibiotic (49.2%), followed by azithromycin (12.5%) and metronidazole (9.3%). This finding was consistent with a similar study conducted in Aksum and Saudi Arabia,4,42 in which ceftriaxone was the most commonly prescribed antibiotic. Among hospitalized patients, surgical site infection (ceftriaxone) and pneumonia (azithromycin) were suggested as conceivable explanations for antibiotic utilization. Such antibiotic prescriptions have also been documented in Ethiopia’s tertiary and primary healthcare settings.4,9,16,28,36
In studies conducted in southern and eastern Ethiopia,17,43 Zambia, 38 and Cameroon, 23 amoxicillin was the most commonly prescribed antibiotic, whereas, in Congo and Eritrea,13,19 ampicillin (injection) was the most commonly prescribed antibiotic. This disparity may be explained by the fact that our study was conducted in a tertiary hospital where all prescribers are at least general practitioners and are capable of prescribing antibiotics with lower resistance data to treat complicated medical problems in patients referred from various primary healthcare settings.
Limitations of the study
This study has certain limitations. Because this antibiotic prescribing pattern was explored at a single hospital, the findings of the study cannot be applied to all Ethiopian hospitals, and because the study was conducted on inpatients, the results may not represent outpatients. The data were also gathered solely through the evaluation of prescription papers and registry books, with no interviews with prescribers and/or patients, and thus did not assess factors contributing to the current practice. Furthermore, because there is a scarcity of data on key antibiotic prescribing indicators among inpatients, we compared our findings to data based on outpatient results.
Conclusion
In this study, the antibiotic prescribing pattern deviated from the WHO-recommended standard. This necessitates the implementation of ongoing interventional techniques as well as periodic audits at all levels of health care to avoid the harmful repercussions of incorrect antibiotic prescribing. More than half of the patients in this study were taking at least one antibiotic, and all antibiotics were obtained from the EML of Ethiopia. However, in all cases, antibiotics were given without a culture or sensitivity test. This finding indicates that the hospital’s antibiotic prescribing practice deviates from and is in violation of the WHO-endorsed standard. Establishing an antibiotic stewardship program, introducing antibiotic use based on culture and sensitivity tests, and developing institutional guidelines could all help to alleviate this problem. As a result, this study provides evidence for the importance of establishing an antibiotic stewardship program at the hospital as well as a path ahead for doing so.
Supplemental Material
Supplemental material, sj-docx-1-smo-10.1177_20503121221096608 for Evaluation of antibiotic prescribing patterns among inpatients using World Health Organization indicators: A cross-sectional study by Tesfaye Yimer Tadesse, Mulugeta Molla, Yohannis Shumet Yimer, Benyas Shishigie Tarekegn and Belayneh Kefale in SAGE Open Medicine
Acknowledgments
The authors acknowledge the DTCSH Inpatient pharmacy department for their invaluable assistance in providing information and other materials during the research process.
Footnotes
Author contributions: All authors made a significant contribution to the work reported in the conception, study design, execution, acquisition of data, analysis, and interpretation; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Availability of data and materials: The datasets used during the current study are available in the main document.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: The study was approved by Debre Tabor University’s Ethical Review Board (approval number: DTU/RE/1/212/13), and subsequent permission was obtained from DTCSH’s Medical Record Department.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Due to the nature of the study, Debre Tabor University’s Ethical Review Board and DTCSH waived informed written consent from study participants. Patients’ personal information and medication information were recorded while maintaining patient confidentiality and omitting their names and addresses.
ORCID iDs: Tesfaye Yimer Tadesse  https://orcid.org/0000-0002-5747-7120
https://orcid.org/0000-0002-5747-7120
Mulugeta Molla  https://orcid.org/0000-0001-7904-5823
https://orcid.org/0000-0001-7904-5823
Yohannis Shumet Yimer  https://orcid.org/0000-0001-5871-3589
https://orcid.org/0000-0001-5871-3589
Belayneh Kefale  https://orcid.org/0000-0003-4841-0861
https://orcid.org/0000-0003-4841-0861
Supplemental material: Supplemental material for this article is available online.
References
- 1. Nia SS, Hiremath SRR, Prasad S. Assessment of antimicrobial use pattern using World Health Organization prescribing indicators at a tertiary hospital: a prospective, observational study. J Appl Pharm Sci 2018; 8(6): 132–138. [Google Scholar]
- 2. Yimenu DK, Emam A, Elemineh E, et al. Assessment of antibiotic prescribing patterns at outpatient pharmacy using World Health Organization prescribing indicators. J Prim Care Community Health. Epub ahead of print 6 November 2019. DOI: 10.1177/2150132719886942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. White A, Hughes JM. Critical importance of a one health approach to antimicrobial resistance. Ecohealth 2019; 16(3): 404–409. [DOI] [PubMed] [Google Scholar]
- 4. Demoz GT, Kasahun GG, Hagazy K, et al. Prescribing pattern of antibiotics using WHO prescribing indicators among inpatients in Ethiopia: a need for antibiotic stewardship program. Infect Drug Resist 2020; 13: 2783–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel J, Deshpande S. Assessment of antibiotic prescribing pattern using World Health Organization prescribing indicators in a Tertiary Care Hospital, Gujarat. Int J Pharm Sci Invent 2020; 9: 17–22. [Google Scholar]
- 6. Desalegn AA. Assessment of drug use pattern using WHO prescribing indicators at Hawassa University Teaching and Referral Hospital, south Ethiopia: a cross-sectional study. BMC Health Serv Res 2013; 13(1): 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yilma Z, Liben M. Assessment of drug prescription pattern in Mekelle general hospital, Mekelle, Ethiopia, using World Health Organization prescribing indicators. Biomed Res Int 2020; 2020: 3809157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tassew SG, Abraha HN, Gidey K, et al. Assessment of drug use pattern using WHO core drug use indicators in selected general hospitals: a cross-sectional study in Tigray region, Ethiopia. BMJ Open 2021; 11(10): e045805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sisay M, Mengistu G, Molla B, et al. Evaluation of rational drug use based on World Health Organization core drug use indicators in selected public hospitals of eastern Ethiopia: a cross sectional study. BMC Health Serv Res 2017; 17(1): 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Demeke B, Molla F, Assen A, et al. Evaluation of drugs utilization pattern using WHO prescribing indicators in Ayder Referral Hospital, Northern Ethiopia. Int J Pharm Sci Res 2015; 6(2): 343–347. [Google Scholar]
- 11. Ayenew W, Asmamaw G, Getaneh A. Prescribing pattern of medications prescribed to outpatients based on WHO prescribing indicators in Ethiopia: a systematic review and meta-analysis of observational studies. Afr J Pharm Pharmaco 2020; 14(7): 240–249. [Google Scholar]
- 12. Atif M, Azeem M, Saqib A, et al. Investigation of antimicrobial use at a tertiary care hospital in Southern Punjab, Pakistan using WHO methodology. Antimicrob Resist Infect Control 2017; 6(1): 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amaha ND, Berhe YH, Kaushik A. Assessment of inpatient antibiotic use in Halibet National Referral Hospital using WHO indicators: a retrospective study. BMC Res Notes 2018; 11(1): 904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hart J, Phillips P. What out-of-hours antibiotic prescribing practices are contributing to antibiotic resistance: a literature review. Br Paramed J 2020; 4(4): 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization (WHO). How to investigate antimicrobial use in hospitals: selected indicators. Geneva: WHO, 2012. [Google Scholar]
- 16. Gube A, Gonfa R, Tadesse T. Evaluation of antibiotic use in medical Ward of Fitche District Hospital, north Showa zone, Oromia region, Ethiopia. Adv Pharmacoepidemiol Drug Saf 2017; 6: 217. [Google Scholar]
- 17. Gashaw T, Sisay M, Mengistu G, et al. Investigation of prescribing behavior at outpatient settings of governmental hospitals in eastern Ethiopia: an overall evaluation beyond World Health Organization core prescribing indicators. J Pharm Policy Pract 2018; 11(1): 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Landstedt K, Sharma A, Johansson F, et al. Antibiotic prescriptions for inpatients having non-bacterial diagnosis at medicine departments of two private sector hospitals in Madhya Pradesh, India: a cross-sectional study. BMJ Open 2017; 7(4): e012974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iyamba J-ML, Mathe DM, Kavuo SK. Point prevalence study of antibiotic use in hospitals in Butembo. Int J Med Med Sci 2016; 8(12): 133–139. [Google Scholar]
- 20. Getachew E, Aragaw S, Adissie W, et al. Antibiotic prescribing pattern in a referral hospital in Ethiopia. Afr J Pharm Pharmaco 2013; 7(38): 2657–2661. [Google Scholar]
- 21. Admassie E, Begashaw B, Hailu W. Assessment of drug use practices and completeness of prescriptions in Gondar University Teaching Referral Hospital. Int J Pharm Sci Res 2013; 4(1): 265–275. [Google Scholar]
- 22. Erku DA, Aberra SY. Non-prescribed sale of antibiotics for acute childhood diarrhea and upper respiratory tract infection in community pharmacies: a 2 phase mixed-methods study. Antimicrob Resist Infect Control 2018; 7(1): 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chem ED, Anong DN, Akoachere J-FK. Prescribing patterns and associated factors of antibiotic prescription in primary health care facilities of Kumbo East and Kumbo West Health Districts, North West Cameroon. PLoS One 2018; 13(3): e0193353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Atif M, Sarwar MR, Azeem M, et al. Assessment of WHO/INRUD core drug use indicators in two tertiary care hospitals of Bahawalpur, Punjab, Pakistan. J Pharm Policy Pract 2016; 9(1): 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yan K, Xue M, Ye D, et al. Antibiotic prescribing practices in secondary and tertiary hospitals in Shaanxi province, western China, 2013-2015. PLoS One 2018; 13(12): e0207229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prah J, Kizzie-Hayford J, Walker E, et al. Antibiotic prescription pattern in a Ghanaian primary health care facility. Pan Afr Med J 2017; 28(1): 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. El Mahalli A, Akl OAM, Al-Dawood SF, et al. WHO/INRUD patient care and facility-specific drug use indicators at primary health care centres in Eastern province, Saudi Arabia. East Mediterr Health J 2012; 18(11): 1086–1090. [DOI] [PubMed] [Google Scholar]
- 28. Gutema G, Håkonsen H, Engidawork E, et al. Multiple challenges of antibiotic use in a large hospital in Ethiopia —a ward-specific study showing high rates of hospital-acquired infections and ineffective prophylaxis. BMC Health Serv Res 2018; 18(1): 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takemoto H, Nishimura J, Komori T, et al. Combination antiemetic therapy with aprepitant/fosaprepitant in patients with colorectal cancer receiving oxaliplatin-based chemotherapy in the SENRI trial: analysis of risk factors for vomiting and nausea. Int J Clin Oncol 2017; 22(1): 88–95. [DOI] [PubMed] [Google Scholar]
- 30. Mugada V, Mahato V, Andhavaram D, et al. Evaluation of prescribing patterns of antibiotics using selected indicators for Antimicrobial Use in Hospitals and the Access, Watch, Reserve (AWaRe) classification by the World Health Organization. Turk J Pharm Sci 2021; 18(3): 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ntšekhe M, Hoohlo-Khotle N, Tlali M, et al. Antibiotic prescribing patterns at six hospitals in Lesotho Published for the US Agency for International Development by the Strengthening Pharmaceutical Systems Program. Arlington, VA: Management Sciences for Health, 2011. [Google Scholar]
- 32. World Health Organization (WHO). How to investigate drug use in health facilities: selected drug use indicators (EDM research series no. 007). Geneva: WHO, 1993. [Google Scholar]
- 33. Bilal AI, Osman ED, Mulugeta A. Assessment of medicines use pattern using World Health Organization’s prescribing, patient care and health facility indicators in selected health facilities in eastern Ethiopia. BMC Health Serv Res 2016; 16(1): 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kallen MC, Prins JM. A systematic review of quality indicators for appropriate antibiotic use in hospitalized adult patients. Infect Dis Rep 2017; 9(1): 6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tanaka A, Yano A, Watanabe S, et al. Impact of switching from intravenous to oral linezolid therapy in Japanese patients: a retrospective cohort study. J Pharm Policy Pract 2016; 9(1): 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gidebo KD, Summoro TS, Kanche ZZ, et al. Assessment of drug use patterns in terms of the WHO patient-care and facility indicators at four hospitals in Southern Ethiopia: a cross-sectional study. BMC Health Serv Res 2016; 16(1): 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ahmed AM, Awad AI. Drug use practices at pediatric hospitals of Khartoum State, Sudan. Ann Pharmacother 2010; 44(12): 1986–1993. [DOI] [PubMed] [Google Scholar]
- 38. Mudenda W, Chikatula E, Chambula E, et al. Prescribing patterns and medicine use at the University Teaching Hospital, Lusaka, Zambia. Med J Zambia 2016; 43(2): 94–102. [Google Scholar]
- 39. Xu K, Huang L, Xu Z, et al. Design, synthesis, and antifungal activities of novel triazole derivatives containing the benzyl group. Drug Des Devel Ther 2015; 9: 1459–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Veronin MA. Should we have concerns with generic versus brand antimicrobial drugs? A review of issues. J Pharm Health Serv Res 2011; 2(3): 135–150. [Google Scholar]
- 41. Fadare JO, Adeoti AO, Desalu OO, et al. The prescribing of generic medicines in Nigeria: knowledge, perceptions and attitudes of physicians. Expert Rev Pharmacoecon Outcomes Res 2016; 16(5): 639–650. [DOI] [PubMed] [Google Scholar]
- 42. Al Shimemeri A, Al Ghadeer H, Memish Z. Antibiotic utilization pattern in a general medical ward of a tertiary medical center in Saudi Arabia. Avicenna J Med 2011; 1(1): 8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Summoro TS, Gidebo KD, Kanche ZZ, et al. Evaluation of trends of drug-prescribing patterns based on WHO prescribing indicators at outpatient departments of four hospitals in southern Ethiopia. Drug Des Devel Ther 2015; 9: 4551–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-smo-10.1177_20503121221096608 for Evaluation of antibiotic prescribing patterns among inpatients using World Health Organization indicators: A cross-sectional study by Tesfaye Yimer Tadesse, Mulugeta Molla, Yohannis Shumet Yimer, Benyas Shishigie Tarekegn and Belayneh Kefale in SAGE Open Medicine