Abstract
Mutacin II is a ribosomally synthesized peptide lantibiotic produced by group II Streptococcus mutans. DNA sequencing has revealed that the mutacin II biosynthetic gene cluster consists of seven specific open reading frames: a regulator (mutR), the prepromutacin structural gene (mutA), a modifying protein (mutM), an ABC transporter (mutT), and an immunity cluster (mutFEG). Transformations of a non-mutacin-producing strain, S. mutans UA159, and a mutacin I-producing strain, S. mutans UA140, with chromosomal DNA from S. mutans T8 with an aphIII marker inserted upstream of the mutacin II structural gene yielded transformants producing mutacin II and mutacins I and II, respectively.
Many bacteria produce antimicrobial peptides, termed bacteriocins. One group of bacteriocins is the lanthionine-containing lantibiotic group. The lantibiotics are ribosomally synthesized as prepeptides that undergo several posttranslational modification events (20, 21). The posttranslational modifications involve dehydration of the hydroxyl amino acids serine and threonine and addition of sulfur atoms of cysteines across the formed double bonds, leading to formation of the thioether amino acids lanthionine and methyllanthionine, respectively. Cleavage of the leader peptide liberates the active final product.
Mutacin II (4, 17) has been purified from a human isolate, Streptococcus mutans T8, and characterized (7, 15, 16). Mutacin II, a small thermostable peptide, has a molecular mass of 3,245 Da and exhibits bactericidal activity over a wide range of pHs against several gram-positive bacteria by inhibiting energy metabolism of sensitive cells (7). Mature mutacin II contains two lanthionine residues, one methyllanthionine, and one didehydro amino acid residue (16). Accordingly, mutacin II is a member of the lantibiotic family of antibiotics (21).
Two genes, mutA and mutM, encoding the prepromutacin and modification enzyme, respectively, have been cloned, sequenced, and characterized (29). The deduced primary sequence of mutacin II showed the presence of 27 amino acids in mature mutacin II, including three cysteine residues and four hydroxy amino acids (29). In this study, we provide evidence that all of the specific genes for mutacin II production are clustered and can be transferred as a unit. In addition, we identified the rest of the mutacin II biosynthetic genes in the cluster. Moreover, we constructed a strain capable of producing both mutacin I and mutacin II.
Transformation of non-mutacin-producing S. mutans UA159.
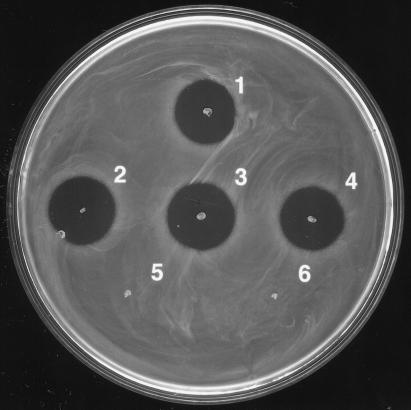
In order to have a selection marker for the transformation process, we inserted the kanamycin resistance gene (aphIII) from Streptococcus faecalis (27) into the chromosomal DNA of S. mutans T8 at the HpaI site upstream of the mutacin II structural gene (mutA) by using a plasmid, pCBM6 (5). Plasmid pCBM6 was constructed in previous structure-activity study (5). The insert in pCBM6 was linearized by restriction enzyme XbaI digestion and then transformed into competent S. mutans T8 by a previously published method (22). The kanamycin resistance gene (aphIII) was integrated into the chromosome via the mechanism of double-crossover recombination. The resultant transformants were mutacin positive and kanamycin resistant and were designated S. mutans T8kan. Chromosomal DNA from strain T8kan was isolated with the QIAGEN genomic-tip DNA isolation kit (QIAGEN, Santa Clarita, Calif.) and used to transform S. mutans UA159, which is naturally competent and non-mutacin producing (22). Randomly selected kanamycin-resistant transformants were tested for mutacin production by using a deferred-antagonism assay (4, 17). All transformants tested produced mutacin. As shown in Fig. 1, three randomly picked UA159 transformants, unlike the parental strain UA159, produced mutacin and inhibited the growth of the indicator strain, Streptococcus sobrinus OMZ176.
FIG. 1.
Deferred-antagonism assay of mutacin phenotypes in selected S. mutans strains. 1, T8 wild type; 2 to 4, UA159 transformants; 5, UA159 wild type; 6, indicator strain S. sobrinus OMZ176. Mutacin production was indicated by the inhibition zones around the producing strains (strain T8 and UA159 transformants) in the lawn of S. sobrinus OMZ176. The absence of such an inhibition zone indicated a lack of mutacin production (parental strain UA159 and the negative control, S. sobrinus OMZ176).
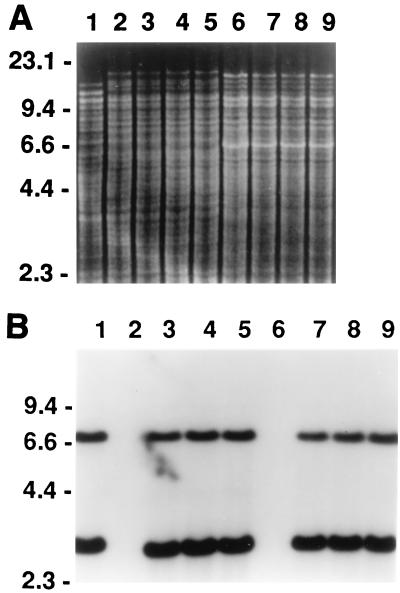
To further confirm the transfer of the mutacin II biosynthetic gene(s) into these transformants, Southern blot analysis was carried out with mutA and mutM as the probe. Chromosomal DNA isolated from the three randomly picked transformants was digested with the restriction enzyme HindIII. Restriction fragments were resolved on 0.65% agarose gels, transferred to a nylon membrane after denaturation and neutralization, and hybridized to the probe. The results (Fig. 2B) confirmed transfer of the biosynthetic genes, mutA and mutM, and a contiguous downstream region by competence transformation. Comparison of chromosomal DNA restriction patterns of the transformants and the parental strain UA159 (Fig. 2A) showed that all transformants were derivatives of the parental strain.
FIG. 2.
Restriction and Southern blot analysis of S. mutans mutacin-producing and non-mutacin-producing strains and corresponding transformants. Chromosomal DNA was digested with HindIII, and the resultant fragments were resolved on a 0.65% agarose gel in Tris-borate-EDTA buffer and photographed under UV light (A). The gel was transferred to a nylon membrane and probed with part of the mutacin II biosynthetic operon (mutAM) (B). Lanes: 1, T8 wild type; 2, UA159 wild type; 3 to 5, UA159 transformants; 6, UA140 wild type, 7 to 9, UA140 transformants. DNA marker sizes are indicated on the left side of each panel, in kilobases.
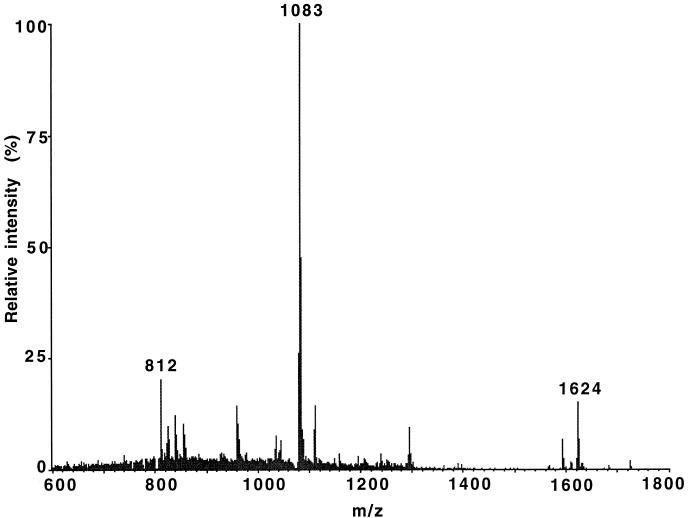
Biosynthesis of lantibiotics involves complex posttranslational modifications (12, 20). While Southern blot analysis indicated the transfer of the mutacin II biosynthetic genes mutA and mutM and the downstream region into these transformants, it was not obvious whether the active mutacins from these transformants were fully modified, as was the wild type from T8. To verify the correct processing of the mutA gene product in these transformants, we purified the mutacin from these transformants, as described previously (15), and analyzed it by electrospray ionization mass spectroscopy on a PE Sciex API III biomolecular mass analyzer (15). The results (Fig. 3) showed that the mutacins from the S. mutans UA159 transformants and S. mutans T8 (15) were identical, indicating that the transferred genetic determinants were sufficient to catalyze the synthesis of fully modified lantibiotic molecules.
FIG. 3.
Electrospray ionization mass spectroscopic analysis of purified mutacin isolated from S. mutans UA159 transformants by a previously described method (15). The molecular mass indicated by multiple charged ions was calculated as 3,245.4 ± 0.6 Da, which is identical to that of mutacin II from S. mutans T8 (15).
Transformation of mutacin I-producing S. mutans UA140.
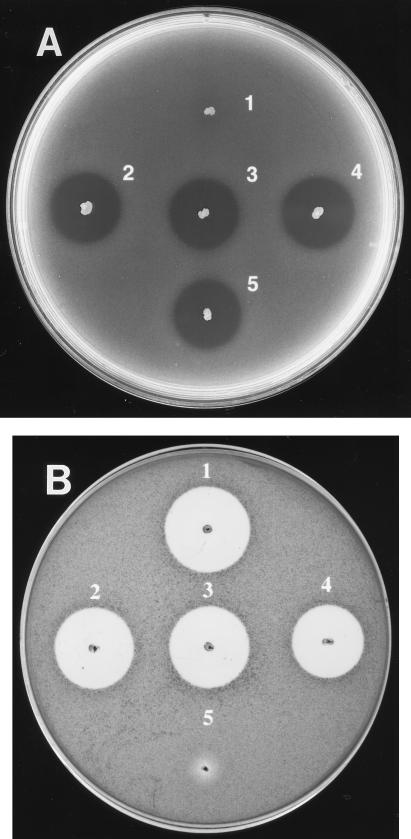
The above results suggested that the entire mutacin biosynthetic locus was transferred into a non-mutacin-producing strain as a unit via competence transformation. It was not known, however, whether this locus could be also transferred into strains producing other mutacins. A group I strain, S. mutans UA140, which produces mutacin I (4), was transformed with the chromosomal DNA from strain T8kan. The resultant kanamycin-resistant transformants inhibited the growth of strain T8, as shown in Fig. 4A, suggesting that these transformants continued to produce mutacin I. Mutacin II production by these transformants was indicated by their ability to inhibit the growth of Staphylococcus aureus MSSA4, a strain susceptible only to mutacin II, in a deferred-antagonism assay (Fig. 4B). The Southern blot (Fig. 2B, lanes 7 to 9) indicated the successful transfer of the mutacin II biosynthetic operon, which was absent in the UA140 wild type (lane 6). The restriction patterns of the corresponding chromosomal DNA preparations (Fig. 2A, lanes 6 to 9) confirmed that these transformants were truly derivatives of UA140.
FIG. 4.
Deferred-antagonism assay of the mutacin I and II phenotypes in selected S. mutans strains. (A) When S. mutans T8 was used as the indicator strain, the inhibition zones around S. mutans UA140 wild type (5) and its transformants (2 to 4) indicated mutacin I production in these strains, while the absence of such an inhibition zone around T8 wild type (1) indicated that mutacin I was not produced by this strain. (B) Mutacin II production by T8 wild type (1) and UA140 transformants (2 to 4) was indicated by their ability to inhibit growth of S. aureus MSSA4.
Identification and characterization of the mutacin II biosynthetic gene cluster.
The above-mentioned results indicated that mutacin II-specific genes are likely to be clustered. To confirm this, up- and downstream fragments of the mutacin II structural gene (mutA) from S. mutans T8 were cloned by single-specific-primer PCR chromosomal walking (23, 24). The cloned fragments were sequenced by automated sequencing (Applied Biosystems 373A DNA sequencer), and the resultant sequence data were analyzed by using several programs in the GCG package (Genetics Computer Group, Madison, Wis.). The entire mutacin II biosynthetic gene cluster was determined.
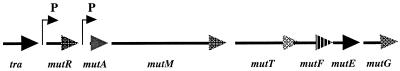
This cluster consists of seven genes (Fig. 5) in the order mutRAMTFEG, based on BLAST similarity search. Southern blot analyses with an internal portion of each gene as a probe indicated that these seven genes exist only in mutacin II-producing strains (data not shown). In addition to the previously identified MutA and MutM (29), the products of five additional open reading frames (MutR, MutT, MutF, MutE, and MutG) were identified. The gene upstream of mutA was designated mutR because it encodes a protein with similarity (25% identity and 49% positives) to a positive transcriptional regulator, Rgg (25, 26), which regulates expression of glucosyltransferase G and influences the Spp phenotype of Streptococcus gordonii Challis. MutR is therefore proposed to be the regulator for the mutacin biosynthetic operon. Internal disruption of mutR resulted in mutants that did not produce any mutacin, due to abolished transcription of mutA (data not shown). This result further supported the proposed function of MutR as a regulator. Approximately 130 bp upstream of mutR resides a putative structural gene with a deduced amino acid sequence having similarity to transposases (tra) from lactococcal insertion elements (9, 28, 30) but lacking a translation start codon and a ribosomal binding site. PCR analyses indicated that this transposase does not exist in S. mutans UA140 (data not shown). A 1.2-kb intergenic region and CTP synthetase (UTP-ammonium ligase) gene follows the tra gene.
FIG. 5.
Genetic organization of the mutacin II biosynthetic gene cluster. The cluster consists of seven specific genes in the following order: regulator gene (mutR), structural gene (mutA), modifying enzyme gene (mutM), transporter and leader peptidase gene (mutT), and accessory self-protection genes (mutFEG). Upstream of mutR is a silent transposase gene (tra).
The amino acid sequence deduced downstream of the mutM gene, MutT, exhibits a high degree of similarity with sequences of ATP-dependent transport proteins, especially LanT (38% identity and 59% positives) of lacticin 481 from Lactococcus lactis (18). Based on what is known about LanT, it is reasonable to propose that MutT has the dual function of cleaving the leader peptide and excreting mature mutacin. Insertion inactivation of mutT with a terminatorless antibiotic cassette completely abolished mutacin production (data not shown).
The products of the open reading frames after MutT were MutFEG. MutF showed its strongest sequence identities with LacF (44% identity and 65% positives) (19) and several other immunity proteins and ATP-binding membrane proteins. Thus, it is likely that MutF is involved in mutacin immunity. MutF is an ATP-binding protein without a transmembrane domain, and MutE and MutG are membrane proteins (data not shown) with homology to Cdd4, Cdd3, and Cdd2 from Clostridium difficile (2). As cdd4, cdd3, and cdd2 do in C. difficile, mutF, mutE, and mutG code for an ABC transporter system, which is likely to be involved in mutacin immunity. Downstream of mutG was a 0.7-kb intergenic region followed by a fructose biphosphate adolase (fba) gene.
Genes for lantibiotic biosynthesis (8, 11, 14) can be found on plasmids (epidermin, Pep5, cytolysin, lacticin 481, lactocin S, and streptococcin A-FF22), on a conjugative transposon (nisin), or on the chromosome (subtilin, epilancin K7, salivaricin A, and variacin). Buchman et al. (3) suggested that many, if not all, lantibiotics probably evolved from a common ancestor, and the ability to make lantibiotics has become dispersed among gram-positive bacteria by transfer of mobile genetic elements such as plasmids or transposons that encode lantibiotics. Although mutacin II biosynthesis genes are located on the chromosome, the presence of a silent transposase gene (tra) in the upstream region of the operon suggested that the ability to produce mutacin II in S. mutans T8 might be obtained from an ancestral transposon. It may be that, after entering a new host (for example, S. mutans T8), this ancestral transposon began to evolve to suit the needs of the new host by the usual means of spontaneous mutation and natural selection. Once stably situated within the host chromosome, the tra function, we hypothesize, may have become nonfunctional.
One way to identify the genes required for lantibiotic biosynthesis is to transfer the capacity for lantibiotic production from a producer strain to a nonproducer strain. Conversion of a non-bacteriocin-producing strain into a bacteriocin producer by transformation was reported for several lantibiotics, including subtilin (13), epidermin (1), nisin (10), and lacticin 481 (18). For subtilin, conversion was achieved by competence transformation from a natural producer (Bacillus subtilis ATCC 6633) into a nonproducing strain (B. subtilis 168), and a 40-kb region of chromosomal DNA was associated with conversion (13). For epidermin production, the transfer of an 8-kb DNA fragment containing six complete open reading frames into heterologous strains (Staphylococcus carnosus and Staphylococcus xylosus) was sufficient for antibacterial activity (1). The genes for nisin production are within a 68- to 70-kb transposon that can be transferred among strains of L. lactis by conjugative transposition (10). In the case of lacticin 481, two genes, lcnA and lcnM, cloned on a plasmid vector were sufficient to convert a nonproducing strain into a lacticin-producing strain (18). Although mutA and mutM have high homology with lcnA and lcnM, this is not the case in the conversion of S. mutans UA159. Transforming only mutA and mutM is not sufficient to convert the wild-type strain UA159 into a mutacin-producing strain (unpublished results). The conversion of strain UA159 into a mutacin producer by integration of a fragment of T8 DNA does not conclusively prove that all of the genes required for mutacin production reside on the integrated DNA. The integrated DNA may merely provide specific genes required for mutacin II production. Another required regulator gene(s), such as the diacylglycerol kinase gene dgk (6), is already present in strain UA159.
In summary, we have identified the specific mutacin II biosynthetic gene cluster and successfully converted S. mutans UA159 into a mutacin II producer by competence transformation. In addition, we have successfully transformed a strain (UA140) producing another mutacin into a mutant strain with the ability to produce the resident mutacin I as well as the introduced mutacin II.
Nucleotide sequence accession numbers.
The nucleotide sequences of mutR, mutT, and mutFEG have been deposited in the GenBank database under accession no. AF007761, AF026468, and AF082183, respectively.
Acknowledgments
This work was supported by NIH grant DE09082. The mass spectrometer was purchased by funds from an NIH Instrumentation Grant (SI0RR06487) and from the University of Alabama at Birmingham (UAB). Operation of the Mass Spectrometer Shared Facility was supported in part by an NCI Core Research Support Grant to the UAB Comprehensive Cancer Center (P30 CA13148).
REFERENCES
- 1.Augustin J, Rosenstein R, Wieland B, Schneider U, Norbert S, Engelke G, Entain K D, Gotz F. Genetic analysis of epidermin biosynthetic genes and epidermin-negative mutants of Staphylococcus epidermidis. Eur J Biochem. 1992;204:1149–1154. doi: 10.1111/j.1432-1033.1992.tb16740.x. [DOI] [PubMed] [Google Scholar]
- 2.Braun V, Hundsberger T, Leukel P, Sauerborn M, von Eichel-Streiber C. Definition of the single integration site of the pathogenicity locus in Clostridium difficile. Gene. 1996;181:29–38. doi: 10.1016/s0378-1119(96)00398-8. [DOI] [PubMed] [Google Scholar]
- 3.Buchman G W, Banerje S, Hansen J N. Structure, expression, and evolution of a gene encoding the precursor of nisin, a small protein antibiotic. J Biol Chem. 1988;263:16260–16266. [PubMed] [Google Scholar]
- 4.Caufield P W, Childers N K, Allen D N, Hansen J B. Distinct bacteriocin groups correlate with different groups of Streptococcus mutans plasmids. Infect Immun. 1985;48:51–56. doi: 10.1128/iai.48.1.51-56.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen P, Novak J, Kirk M, Barnes S, Qi F, Caufield P W. Structure-activity study of the lantibiotic mutacin II from Streptococcus mutans T8 by a gene replacement strategy. Appl Environ Microbiol. 1998;64:2335–2340. doi: 10.1128/aem.64.7.2335-2340.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen P, Novak J, Qi F, Caufield P W. Diacylglycerol kinase is involved in regulation of expression of the lantibiotic mutacin II of Streptococcus mutans. J Bacteriol. 1998;180:167–170. doi: 10.1128/jb.180.1.167-170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chikindas M L, Novak J, Driessen A J M, Konings W N, Schilling K M, Caufield P W. Mutacin II, a bactericidal lantibiotic from Streptococcus mutans. Antimicrob Agents Chemother. 1995;39:2656–2660. doi: 10.1128/aac.39.12.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vos W M, Kuipers O P, van der Meer J R, Siezen R J. Maturation pathway of nisin and other lantibiotics: post-translationally modified antimicrobial peptides exported by Gram-positive bacteria. Mol Microbiol. 1995;17:427–437. doi: 10.1111/j.1365-2958.1995.mmi_17030427.x. [DOI] [PubMed] [Google Scholar]
- 9.Dodd H M, Horn N, Gasson M J. Characterization of IS905, a new multicopy insertion sequence identified in lactococci. J Bacteriol. 1994;176:3393–3396. doi: 10.1128/jb.176.11.3393-3396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gireesh T, Davidson B E, Hillier A J. Conjugal transfer in Lactococcus lactis of a 68-kilobase-pair chromosomal fragment containing the structural gene for the peptide bacteriocin nisin. Appl Environ Microbiol. 1992;58:1670–1676. doi: 10.1128/aem.58.5.1670-1676.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jack R, Bierbaum G, Heidrich C, Sahl H-G. The genetics of lantibiotic biosynthesis. Bioessays. 1995;17:793–802. doi: 10.1002/bies.950170909. [DOI] [PubMed] [Google Scholar]
- 12.Kupke T, Gotz F. Post-translational modifications of lantibiotics. Antonie Leeuwenhoek. 1996;69:139–150. doi: 10.1007/BF00399419. [DOI] [PubMed] [Google Scholar]
- 13.Liu W, Hansen J N. Conversion of Bacillus subtilis 168 to a subtilin producer by competence transformation. J Bacteriol. 1991;173:7387–7390. doi: 10.1128/jb.173.22.7387-7390.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nes I F, Tagg J R. Novel lantibiotics and their pre-peptides. Antonie Leeuwenhoek. 1996;69:89–97. doi: 10.1007/BF00399414. [DOI] [PubMed] [Google Scholar]
- 15.Novak J, Caufield P W, Miller E J. Isolation and biochemical characterization of a novel lantibiotic mutacin from Streptococcus mutans. J Bacteriol. 1994;176:4316–4320. doi: 10.1128/jb.176.14.4316-4320.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novak J, Kirk M, Caufield P W, Barnes S, Morrison K, Baker J. Detection of modified amino acids in lantibiotic peptide mutacin II by chemical derivatization followed by electrospray ionization mass spectroscopic analysis. Anal Biochem. 1996;236:358–360. doi: 10.1006/abio.1996.0181. [DOI] [PubMed] [Google Scholar]
- 17.Parrot M, Caufield P W, Lavoie M C. Preliminary characterization of four bacteriocins from Streptococcus mutans. Can J Microbiol. 1990;36:123–130. doi: 10.1139/m90-022. [DOI] [PubMed] [Google Scholar]
- 18.Rincé A, Dufour A, Le Pogam S, Thuault D, Bourgeois C M, Le Pennec J P. Cloning, expression, and nucleotide sequence of genes involved in production of lactococcin DR, a bacteriocin from Lactococcus lactis subsp. lactis. Appl Environ Microbiol. 1994;60:1652–1657. doi: 10.1128/aem.60.5.1652-1657.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rincé A, Dufour A, Uguen P, Le Pennec J-P, Haras D. Characterization of the lacticin 481 operon: the Lactococcus lactis genes lctF, lctE, and lctG encode a putative ABC transporter involved in bacteriocin immunity. Appl Environ Microbiol. 1997;63:4252–4260. doi: 10.1128/aem.63.11.4252-4260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahl H-G, Jack R W, Bierbaum G. Lantibiotics: biosynthesis and biological activities of peptides with unique post-translational modifications. Eur J Biochem. 1995;230:827–853. doi: 10.1111/j.1432-1033.1995.tb20627.x. [DOI] [PubMed] [Google Scholar]
- 21.Schnell N, Entian K-D, Schneider U, Götz F, Zähner H, Kellner R, Jung G. Prepeptide sequence of epidermin, a ribosomally synthesized antibiotic with four sulfide-rings. Nature. 1988;333:276–278. doi: 10.1038/333276a0. [DOI] [PubMed] [Google Scholar]
- 22.Shah G R, Caufield P W. Enhanced transformation of Streptococcus mutans by modifications in culture conditions. Anal Biochem. 1993;214:343–346. doi: 10.1006/abio.1993.1503. [DOI] [PubMed] [Google Scholar]
- 23.Shyamala V, Ames G F-L. Genome walking by single-specific-primer polymerase chain reaction. Methods Enzymol. 1993;217:436–446. doi: 10.1016/0076-6879(93)17082-g. [DOI] [PubMed] [Google Scholar]
- 24.Shyamala V, Ames G F-L. Genome walking by single-specific-primer polymerase chain reaction: SSP-PCR. Gene. 1989;84:1–8. doi: 10.1016/0378-1119(89)90132-7. [DOI] [PubMed] [Google Scholar]
- 25.Sulavik M C, Clewell D B. Rgg is a positive transcriptional regulator of the Streptococcus gordonii gtfG gene. J Bacteriol. 1996;178:5826–5830. doi: 10.1128/jb.178.19.5826-5830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sulavik M C, Tardif G, Clewell D B. Identification of a gene, rgg, which regulates expression of glucosyltransferase and influences the Spp phenotype of Streptococcus gordonii Challis. J Bacteriol. 1992;174:3577–3586. doi: 10.1128/jb.174.11.3577-3586.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trieu-Cuot P, Courvalin P. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3′5"-amino glycoside phosphotransferase type III. Gene. 1983;23:331–341. doi: 10.1016/0378-1119(83)90022-7. [DOI] [PubMed] [Google Scholar]
- 28.Van Kranenburg R, Marugg J D, Van Swam I I, Willem N J, De Vos W M. Molecular characterization of the plasmid-encoded eps gene cluster essential for exopolysaccharide biosynthesis in Lactococcus lactis. Mol Microbiol. 1997;24:387–397. doi: 10.1046/j.1365-2958.1997.3521720.x. [DOI] [PubMed] [Google Scholar]
- 29.Woodruff W A, Novak J, Caufield P W. Sequence analysis of mutA and mutM genes involved in the biosynthesis of the lantibiotic mutacin II in Streptococcus mutans. Gene. 1998;206:37–43. doi: 10.1016/s0378-1119(97)00578-7. [DOI] [PubMed] [Google Scholar]
- 30.Yu W, Mierau I, Mars A, Johnson E, Dunny G, McKay L L. Novel insertion sequence-like element IS982 in lactococci. Plasmid. 1995;33:218–225. doi: 10.1006/plas.1995.1023. [DOI] [PubMed] [Google Scholar]