Abstract
Vibrio cholerae, a noninvasive mucosal pathogen, is endemic in more than 50 countries. Oral cholera vaccines, based on killed whole-cell strains of Vibrio cholerae, can provide significant protection in adults and children for 2–5 years. However, they have relatively limited direct protection in young children. To overcome current challenges, in this study, a potential conjugate vaccine was developed by linking O-specific polysaccharide (OSP) antigen purified from V. cholerae O1 El Tor Inaba strain PIC018 with Qβ virus-like particles efficiently via squarate chemistry. The Qβ-OSP conjugate was characterized with mass photometry (MP) on the whole particle level. Pertinent immunologic display of OSP was confirmed by immunoreactivity of the conjugate with convalescent phase samples from humans with cholera. Mouse immunization with the Qβ-OSP conjugate showed that the construct generated prominent and long-lasting IgG antibody responses against OSP, and the resulting antibodies could recognize the native lipopolysaccharide from Vibrio cholerae O1 Inaba. This was the first time that Qβ was conjugated with a bacterial polysaccharide for vaccine development, broadening the scope of this powerful carrier.
Keywords: bacteriophage Qβ, mass photometry, O-specific polysaccharide, vaccine, Vibrio cholera
Graphical Abstract
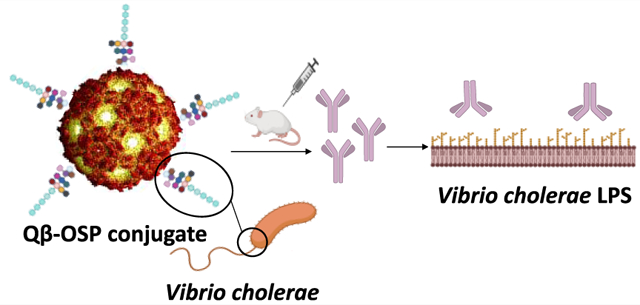
Cholera is an acute, secretory diarrheal disease caused by the highly transmissible bacterium Vibrio cholerae (V. cholerae). V. cholerae are Gram-negative and highly motile bacteria with a single polar flagellum. There are more than 200 serogroups of V. cholerae based on the O-antigen of surface lipopolysaccharide (LPS) structures, but only serogroups O1 and O139 are capable of causing epidemic cholera. V. cholerae O1 has two serotypes, i.e., Ogawa and Inaba, based on the presence or absence of a methyl group on the nonreducing terminal perosamine moiety of the surface O-specific polysaccharide (OSP, O-antigen).1 There are 2–3 million cases of cholera each year, resulting in tens of thousands of deaths annually.2 Current cholera vaccines include oral killed whole cell vaccine with or without cholera toxin B subunit (CtxB), and attenuated oral cholera vaccine.3 Inclusion of the cholera vaccine into global cholera control strategies has been transformative, but current oral vaccines have the lowest level and duration of protection in young children,4–8 who bear a large share of global cholera burden, especially in cholera-endemic countries.2,3,9–11 As such, there is a need to develop new cholera vaccines that can provide high-level and long-term immunity.
Immunity protection against cholera infection targets the OSP of V. cholerae.12,13 However, as O-antigens are T cell independent B cells antigens, direct administration of the O-antigens often only leads to low titers of low-affinity IgM antibodies with limited duration of antibody responses and a lack of induction of immunological memory, rendering O-antigen-based vaccination suboptimal.14–16 Covalent linkage of carbohydrate to a carrier protein provides a T cell-dependent immune response by activating CD4+ T cells and enables memory B cell proliferation for long lasting antibody protection. Recently, we have demonstrated that self-assembled virus-like particles (VLPs) such as bacteriophage Qβ could be used to conjugate with carbohydrate antigens such as the Thomsen–Nouveau (Tn) antigen, ganglioside GM2 and GD2 as potential vaccines.17–19 The resulting glycoconjugates were able to induce strong glycan specific IgG antibody responses. However, to date, only low molecular weight (MW generally below 2000 Da) glycans have been investigated for Qβ based anticarbohydrate vaccine studies. It is not known whether bacterial polysaccharide antigens could be conjugated with Qβ and whether such conjugates could induce strong IgG antibody responses to polysaccharides.
Herein, we report that the native O-specific polysaccharide (OSP) 1 of Vibrio cholerae O1, Inaba serotype was successfully conjugated with Qβ through squarate chemistry, which is the first time that a bacterial polysaccharide antigen is covalently linked with Qβ as a potential vaccine. This approach provides direct conjugation without prior introduction of a linker to the protein carrier. High levels of antipolysaccharide IgG antibodies were induced by the conjugate in mice, and the antibodies were effective in killing the bacteria.
RESULTS
Conjugation of the OSP Core Antigen to Qβ.
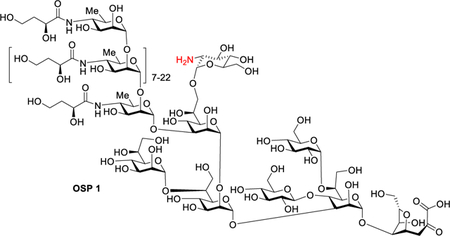
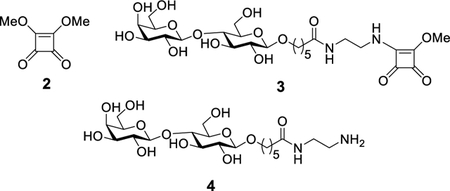
To efficiently link the OSP antigen to Qβ, we built upon previous work using squarate based conjugation of polysaccharide.20–22 3,4-Dimethoxy-3-cyclobutene-1,2-dione (dimethyl squarate) 2 is a unique bifunctional linker, both of whose methoxy groups are reactive with amines but under different pH conditions. The first methoxy group can be substituted by a primary amine in neutral pH and the second one is active toward amines in a basic solution. The squaramide moiety itself is stable to hydrolysis under the aqueous reaction conditions.23 To optimize the conjugation reaction with the squarate ester chemistry, Qβ was conjugated with a squarate functionalized lactose 324 as a model reaction. Lactoside 3 was prepared by derivatizing lactose 424 with dimethyl squarate 2 in 76% yield (Scheme S1). In parallel, the coat protein of Qβ triple mutant A38K/A40C/D102C was expressed in E. coli,25 which self-assembled into nanoparticles with average diameters of 28 nm consisting of 180 copies of the monomer. The Qβ triple mutant A38K/A40C/D102C was selected as the carrier as it has been shown to induce lower levels of antibodies against the carrier itself as compared to the wild type Qβ, thus leading to stronger IgG antibody responses against the target antigen.25 The lactoside 3 (14 equiv per monomer) was then incubated with Qβ at 22 °C for 20 h leading to the Qβ-lactose conjugate 5 (Scheme S1). In order to quantify the degree of modification, surface-enhanced laser desorption/ionization time-of-flight (SELDI-TOF) mass spectrometry analysis was performed (Figure S1). On the basis of the intensities of the mass spectrometry (MS) peaks for Qβ coat protein monomer conjugated with lactosides, it was estimated there was an average loading of 4.5 haptens per Qβ monomer, corresponding to 810 copies per Qβ capsid. Increasing the amount of 3 to 28 equiv led to an average of 8 lactosides conjugated per Qβ monomer unit (1440 copies per Qβ capsid). As each monomer of the Qβ triple mutant A38K/A40C/D102C has 9 total free amines (8 lysines plus the free N-terminus), the ability to nearly fully functionalize Qβ suggests the squarate chemistry is highly efficient in promoting glycan conjugation with Qβ. The 1440 copies per capsid are one of the highest ligand loading levels on Qβ reported to date.18,19,26,27 MS sequencing of Qβ conjugated with lactoside 3 indicated high functionalization efficiency (80–100%) of lysines (K14, K17, K61, K64, and K68) on the capsid surface and moderate efficiency (46%) of the lysine K3 that lays between monomers in the capsid compared to almost no functionalization (~1%) of K47, the least accessible lysine residue based on the crystal structure of Qβ (Table S1).
To further characterize the conjugate, gel electrophoresis analysis was carried out (Figure S2). In the presence of reducing agents, the Qβ capsid disassembled to its subunits showing bands at 14 and 28 kDa corresponding to the monomer and dimer of the coat protein respectively (Figure S2, lane 11). After conjugation, the monomer band of the Qβ coat protein shifted to about 19 kDa, correlating well with the addition of ~8 lactose units per Qβ monomer on average (lane 10).
We next explored the conjugation of Vibrio cholerae O1 Inaba OSP from PIC01822 with Qβ using the squarate chemistry. V. cholerae O1 Inaba OSP 1 was treated with dimethyl squarate 2 first, which was then incubated with Qβ. To preserve the valuable material, the amount of OSP-squarate was reduced to 8 equiv per Qβ monomer (Scheme 1). After 120 h, the conjugation reaction was analyzed by SELDI-TOF, which only showed the peak for Qβ monomer at 14.1 kDa with very weak signals from the potential OSP adduct (Figure S3). The SDS-PAGE of the Qβ-OSP conjugate (Figure S2, lane 9) showed a very faint band close to the Qβ monomer MW under the reducing condition with the majority of the protein sample appearing smeared at the high MW region of the gel. The incomplete disassembly of Qβ-OSP under the reducing condition as compared to Qβ-lactose may be due to the relatively large size of the OSP (~6 kDa). The loading of multiple OSP molecules on Qβ surface may sterically impede the access of reducing agents to the capsid, hindering reduction of the disulfides resulting in multiple monomers remaining bound together. As there was little Qβ monomer observed on the gel, the low signal from the OSP conjugate observed in SELDI-TOF (Figure S3) was most likely due to the difficulty in ionizing the Qβ-OSP conjugate by MS. Thus, we needed to employ additional techniques beyond SELDI-TOF and SDS-PAGE to provide more quantitative information on the degree of OSP functionalization on Qβ.
Scheme 1.
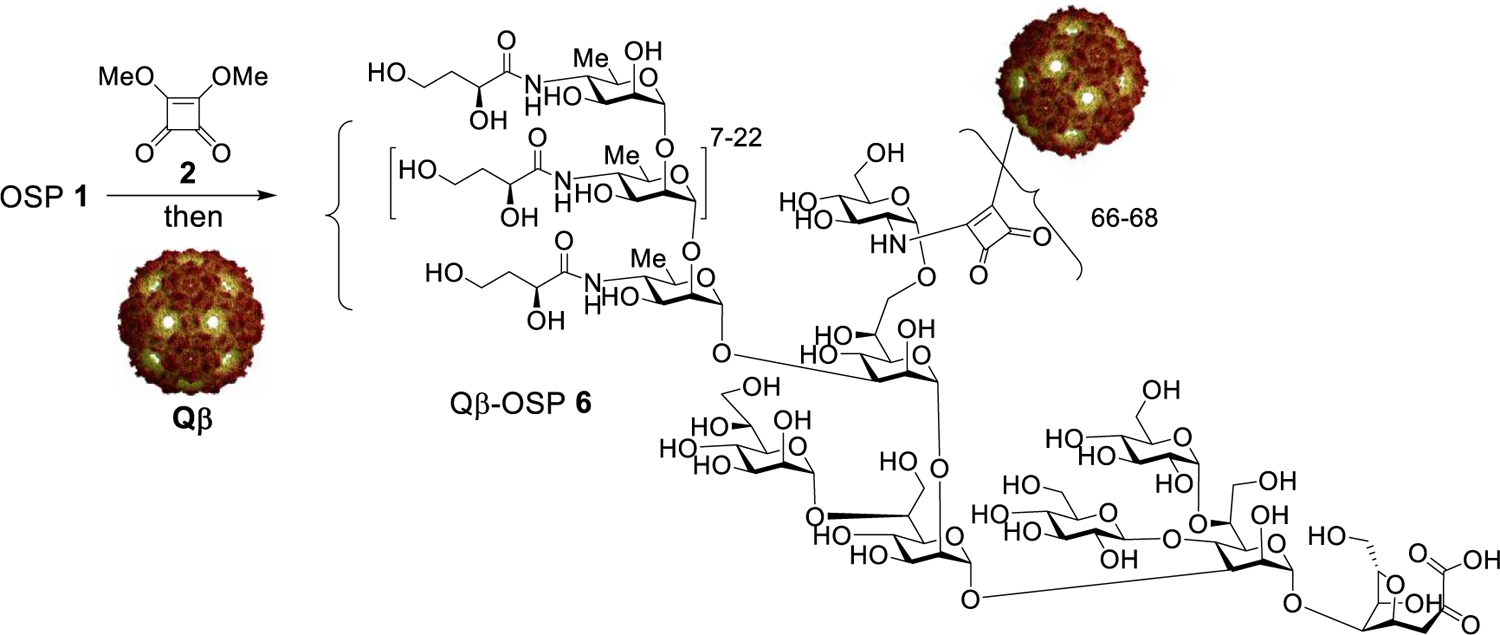
Conjugation of OSP 1 to Qβ VLPa
aOSP 1 was activated with dimethyl squarate 2, and subsequently added to a Qβ solution in 0.5 M borate buffer, pH = 9.0. After 120 h, the reaction was worked up by ultrafiltration against pH 7.2 (1×) PBS buffer.
We next tested Mass Photometry (MP)28 to quantify OSP functionalization of Qβ. While the aforementioned SELDI-TOF and SDS-PAGE methods for VLP analysis rely on disassembly of the particles and assessing the conjugation at the individual monomeric coat protein levels, MP measures light scattering from the intact particle, which is proportional to the mass of the scattering particles. By analyzing hundreds to thousands of particles, the mass distribution of the sample is generated. To the best of our knowledge, MP has only been applied twice to study VLPs to date.29,30 When we measured the Qβ sample, there were two populations and the MW was reducing over time (Figure S4). This could be resulting from the instability and degradation of RNA encapsulated inside the Qβ. To test this possibility, we prepared a Qβ sample without RNA by cleaving the RNA chemically with lead acetate31 and measured the empty particles with MP. Although we still observed two populations in the sample, the MW remained stable in two different measurements of the same sample over a 3-month interval (Figure S5). This supported that the result is reproducible and that MW reduction over time observed with full Qβ was likely due to RNA degradation. The population of Qβ particles with smaller molecular weight observed in the MP spectrum may be the result of partially disassembled VLPs.
In order to calibrate the mass shifts in MP, we conjugated the Qβ capsid without RNA with lactoside 3 as a control sample. On the basis of the MW of this conjugate obtained from MP, the lactose loading was about 7 per monomer (Figure S6), which was close to the average loading of 6 obtained from the same sample on QTOF-ESI (Figure S8) and SELDI-TOF (Figure S9). With this result, we confirmed that the mass shift in Qβ sample after conjugation is due to the loading of the carbohydrate and hence the difference in mass can be used to calculate the loading.
We analyzed the Qβ-OSP conjugate with MP next. Upon OSP conjugation, the mean mass shifted about 400 kDa based on the MW of intact particles (Figure 1). With the average MW of OSP at ~6000 Da, it was calculated that on average there were 68 OSP molecules per Qβ capsid. The OSP conjugation and MP protocols were reproducible, giving 66–68 OSP units per Qβ on two independent batches of Qβ-OSP conjugates.
Figure 1.
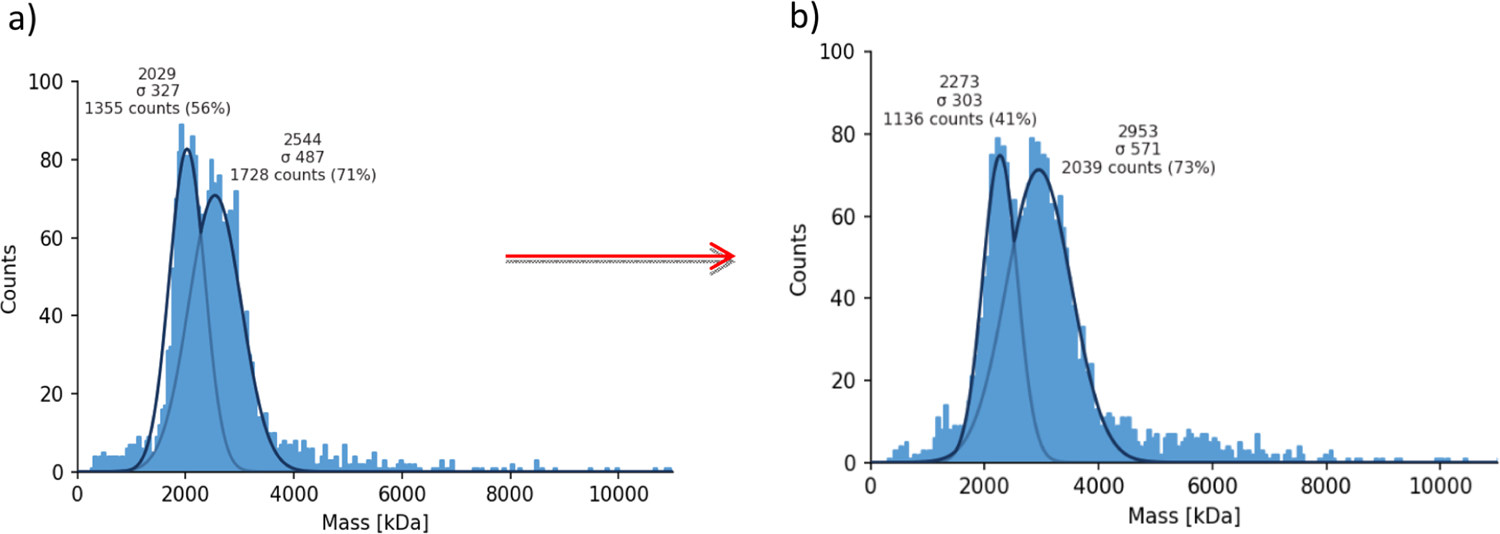
MP results of (a) Qβ and (b) Qβ-OSP conjugate. The right peak shifted from 2544 kDa to 2953 kDa, which suggests the conjugation of an average of 68 OSP per full Qβ capsid calculated based on the mass of the intact particle.
Immunogenicity of the Qβ-OSP Conjugate.
With the Qβ-OSP conjugate in hand, to analyze whether the Qβ-OSP was displaying OSP in an immunologically relevant manner, we assessed the ability of convalescent plasma from humans recovering from cholera to recognize Qβ-OSP. Plasma was collected and analyzed as previously described.22 As shown in Figure 2, Qβ-OSP was recognized by convalescent phase plasma of humans recovering from cholera (day 7 sample), but not by acute phase plasma (day 2 sample). In comparison to Qβ-OSP, there was little binding of convalescent sera to Qβ by itself. Furthermore, there was no increased immune-recognition of Qβ-OSP by plasma from Salmonella Typhi infected patients (typhoid fever) suggesting the binding of Qβ-OSP was a result from cholera infection.
Figure 2.
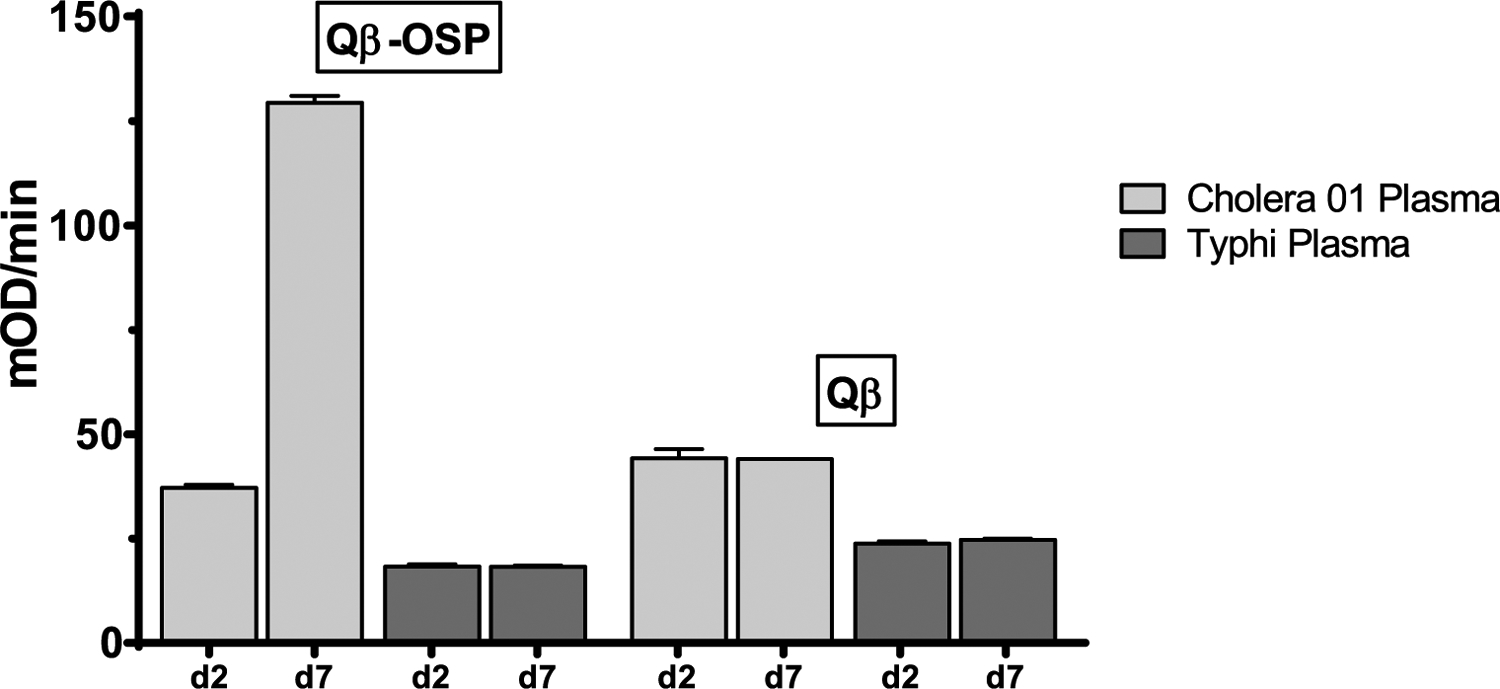
Immunoreactivities of human plasma toward Qβ and Qβ-OSP were measured by acute phase plasma (day 2 sample) versus convalescent phase plasma (day 7 sample) of patients with cholera versus typhoid fever in Dhaka, Bangladesh.
Next, we evaluated the ability of Qβ-OSP to generate anti-OSP antibodies in animals. A group of five female Swiss-Webster (3–5 week old) mice was injected intramuscularly on days 0, 21, 42, and 265 with the Qβ-OSP construct (10 μg OSP per mouse) in the absence of any exogenous adjuvants (Figure 3a). Blood was collected from the immunized mice on days 0, 7, 28, 49, 56 and during the study as shown in Figure 3a. A control group of Swiss-Webster mice received Qβ only following the same protocol. To analyze the levels of anti-OSP antibodies in the sera by enzyme-linked immunosorbent assay (ELISA), a bovine serum albumin (BSA) conjugate of OSP was prepared to avoid the interference of anti-Qβ antibodies. The anti-OSP IgG titer, the highest dilution above background that gives optical density (OD) = 0.1, was determined in pooled sera at different time points by ELISA. While there were weak IgG responses 1 week after the first immunization, after the second immunization, significantly higher levels of IgG were observed on days 28, 49, and 56 (Figures 3b and 3c). The average IgG titers reached the maximum value of 226 504 on day 56 (Figure 3d). The IgG titer from the Qβ-OSP group remained at high levels over time with IgG titers still detectable at day 265. In contrast, there was no detectable anti-OSP IgG responses in the control group at any time point suggesting Qβ-OSP potently induced antibody responses against OSP. No anti-OSP IgM antibodies were detected.
Figure 3.
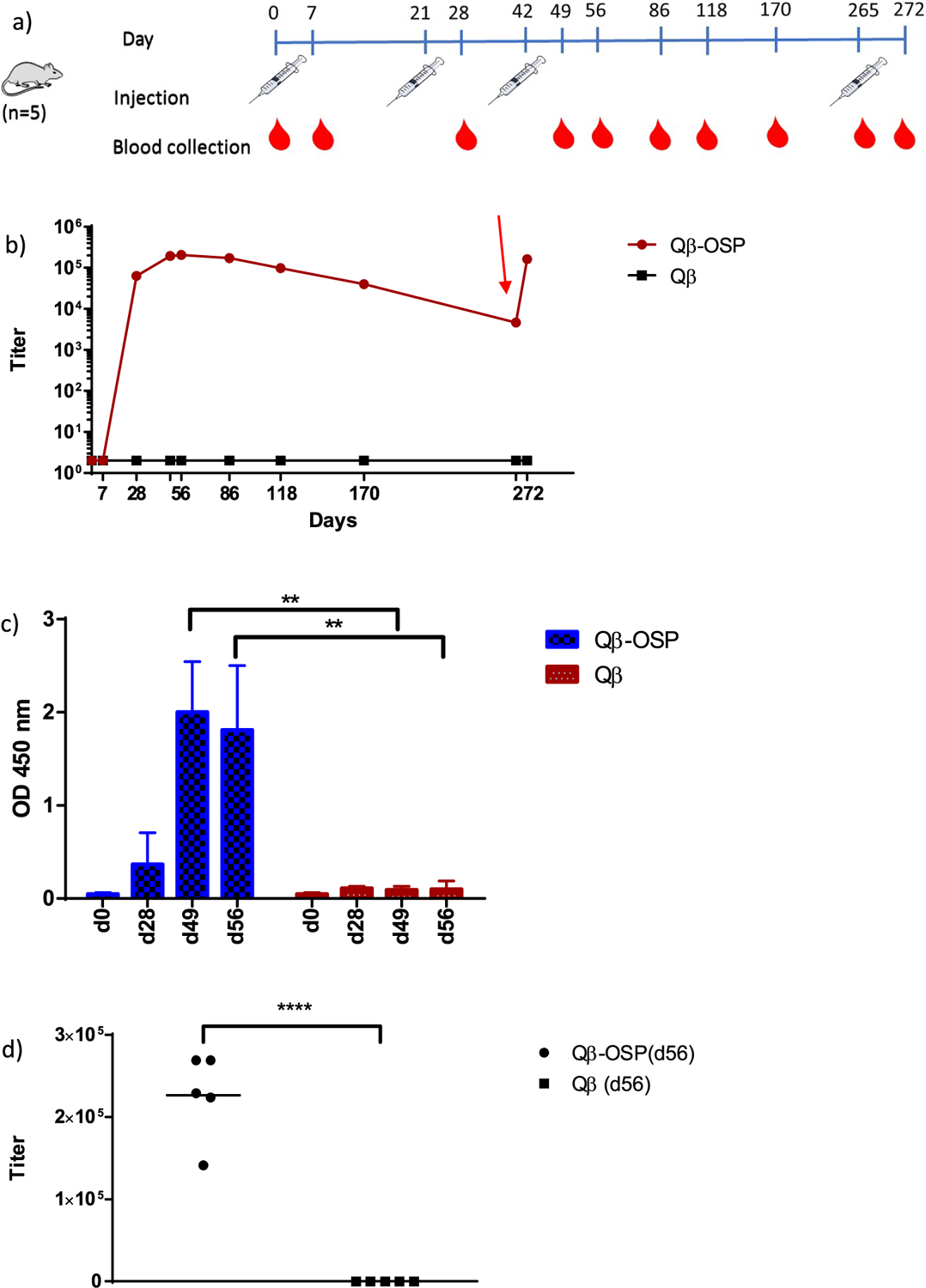
Evaluation of Qβ-OSP immunogenicity. (a) Immunization and blood collection schedule. Each group received 3 immunizations 3 weeks apart with blood collected at day 0 and on days 7, 28, 49, 56, 86, 118, 170, 265, and 272 respectively. (b) OSP-specific IgG titer of pooled sera from Qβ and Qβ-OSP groups up to day 272 postimmunization. The red arrow indicates a booster injection at day 265. (c) ELISA analysis showed significant IgG binding to BSA–OSP by postimmune sera at d49 and d56 (p = 0.0014 and 0.0065, respectively), compared to the control sera from mice immunized with Qβ only. Each bar represents data for 5 mice at 20 000 fold of serum dilution. (d) Individual mouse serum OSP-specific IgG titer of Qβ and Qβ-OSP groups at day 56. The statistical significance was determined through a two-tailed t test using GraphPad Prism. **p < 0.0001.
In order to assess whether memory responses were generated, on day 265 post initial immunization, mice received an additional vaccination. One week (day 272) after the booster, the average anti-OSP IgG antibody levels of the mice increased over 35 times compared to those on day 265 and reached the similar level of IgG titer as day 56. These results suggest that Qβ-OSP vaccination induced memory B cell responses and the anti-OSP humoral immunity could be boosted (Figure 3b).
In order to determine whether immunization with Qβ-OSP would elicit antibodies recognizing the native LPS containing OSP from V. cholerae, ELISA analysis was also performed using V. cholerae O1 Inaba LPS PIC018 as the coating antigen. Sera from mice immunized with Qβ-OSP had significantly higher levels of anti-LPS IgG antibodies as compared to those from mice receiving Qβ alone (Figure 4). Furthermore, serum binding to Inaba LPS was significantly higher compared to binding to LPS from E. coli, suggesting the antibodies induced by Qβ-OSP were selective toward Inaba (Figure 4).
Figure 4.
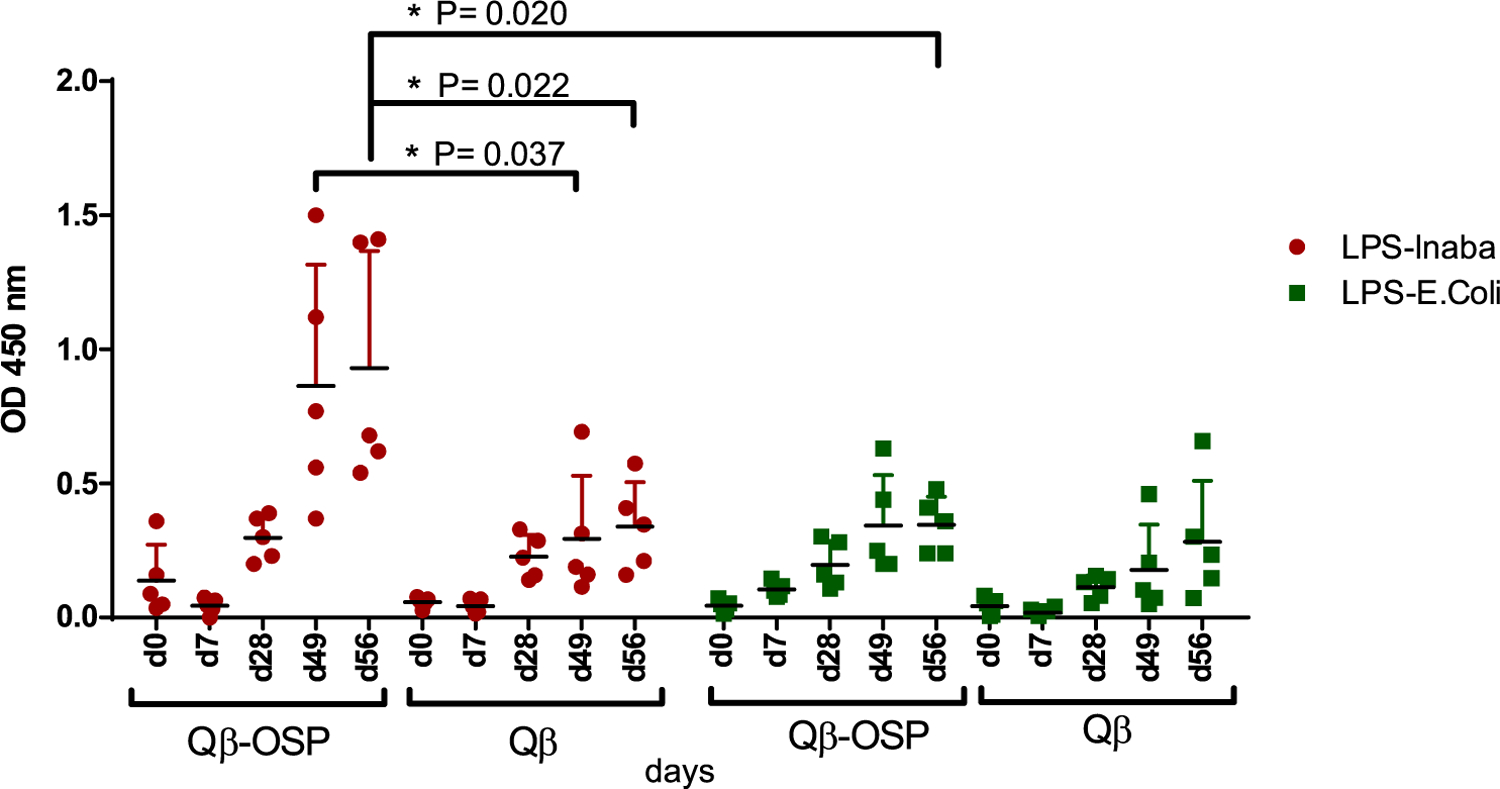
Binding of mouse serum immunized with Qβ-OSP and Qβ to LPS from Inaba vs E. coli. Serum binding against Inaba LPS was observed in the Qβ-OSP immunized group while sera from the Qβ group had lower binding. The binding to E. coli LPS was lower by sera from both Qβ-OSP and Qβ immunized mice. The statistical significance was determined through an unpaired two-tailed t test using GraphPad Prism. *p < 0.05.
With the ability to selectively bind native V. cholerae O1 Inaba LPS and OSP by the Qβ-OSP induced antibodies established, we next measured the vibriocidal activities of the postimmune sera.22 In the presence of an exogenous source of complement, V. cholerae cells are incubated with serial serum dilutions. Anti-V. cholerae antibodies present in the serum sample(s) in combination with complement can lyse the live bacteria. While none of the mice from the Qβ immunized group showed any vibriocidal activities, sera from 2 of the 5 Qβ-OSP immunized mice were able to kill the bacteria at dilutions higher than those in mice immunized with Qβ alone (Figure 5).
Figure 5.
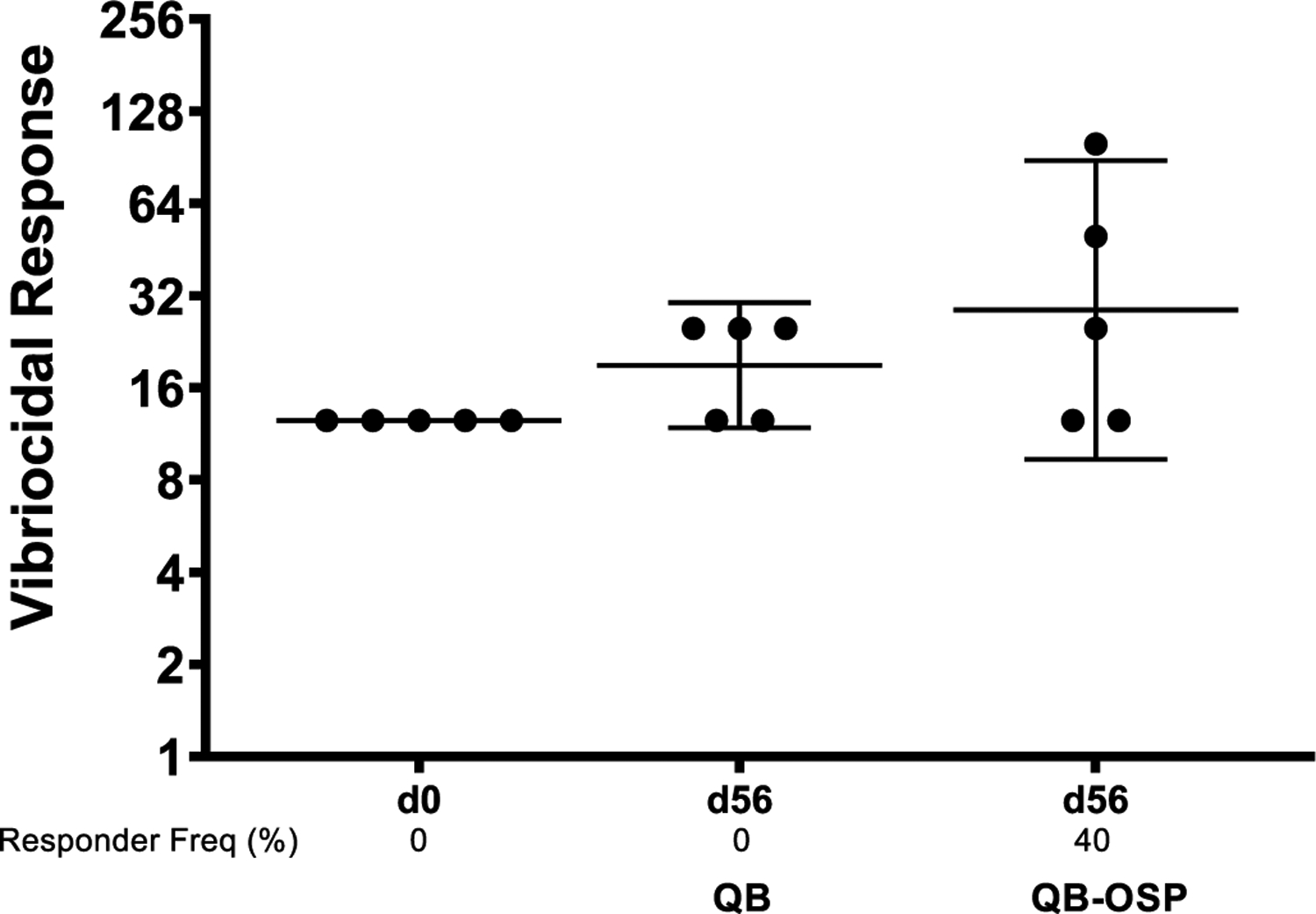
Vibriocidal responses in vaccine cohorts. We defined responders as having an increase in vibriocidal titer by 4-fold at day 56 than day 0.
DISCUSSION
OSP of V. cholerae has been used as an antigen in conjugation with BSA and recombinant heavy chain of tetanus toxin, and synthetic hexasaccharide and synthetic hexasaccharide cluster conjugates have also been evaluated as vaccine antigens against V. cholerae.22,32–34 A virus-like particle (VLP) based approach has a number of attractive features for vaccine applications since the highly ordered organization of the protein(s) in the VLPs is well recognized via pathogen-associated molecular patterns (PAMPs).35 VLPs can present antigens in an organized and polyvalent manner to cross-link B-cell receptors to induce intense cellular signaling for strong immune activation. Bacteriophage Qβ VLP is a promising platform for organized display to induce antibody responses against a target antigen.
For polysaccharide based conjugate vaccines, there are several coupling methods such as periodate activation for reductive amination, cyanylation, and carbodiimide-mediated coupling.36 These approaches can suffer from incompatibility with some proteins and substrates and low selectivity. Squaric acid esters are favorable linker molecules in glyco-conjugate formation between amino-saccharides and proteins due to the amine-selectivity, high reactivity at room temperature, possibility of stoichiometric modification, and recovery of high-value unreacted (oligo)saccharide.37 In the current study, we have prepared the squaric acid monoester derivative of V. cholerae O1 OSP core antigen utilizing the core amine group of the OSP and conjugated the monoester with VLP (carrier protein). While bacteriophage Qβ has been conjugated with glycan antigens,18,19,26,27 this is the first example of using a squarate linker for glycan conjugation with Qβ VLP. The squarate chemistry was highly efficient, leading to close to full derivatization of all free amines of Qβ coat proteins with a small glycan such as lactose 4.
With squarate chemistry, the polysaccharide of V. cholerae O1 OSP was conjugated to the Qβ carrier protein via single point attachment due to the presence of single amino group per OSP molecule. The final construct may mimic native bacteria by presenting multiple OSP polysaccharide on the surface. OSP display on Qβ was in an immunologically relevant manner, which was recognized by convalescent sera of cholera infected humans but not of typhoid fever patients. The Qβ-OSP vaccine was immunogenic in mice, inducing persistent IgG responses against OSP. Such long-term IgG production may assist with the long-term protective goal of anticholera vaccination.38
The vaccine was administrated in the absence of an exogenous adjuvant. This in part may be due to inherent adjuvant properties of Qβ VLP, which can encapsulate E. coli RNA molecules, thus stimulating internal cellular signals via TLR7/8 in the antigen presenting cells.39 The induced IgG antibodies recognized native V. cholerae LPS and had vibriocidal activities. Induction of vibriocidal antibodies correlate with protection against cholera,40 and these responses largely target V. cholerae OSP.40,41 The mechanism of protection against V. cholerae in the intestinal lumen is currently unclear, but may involve inhibition of V. cholerae motility through the binding of OSP-specific antibodies.42,43 The induction of low levels E. coli LPS-specific IgG antibody responses (Figure 4) may be due to the presence of trace amounts of residual E. coli LPS in Qβ VLP preps. Studies are ongoing to express Qβ VLPs in LPS deficient E. coli strains.44
Our study has several limitations. We focused on developing suitable coupling chemistry and analytical tools to synthesize and characterize the Qβ-OSP conjugate. We evaluated the immunogenicity of the conjugate, and future assessments could evaluate protective efficacy in a wild type V. cholerae challenge assay. We did not investigate the effect of adjuvant administration on anti-OSP IgG production, or the mechanism by which induced antibodies can provide protection.
CONCLUSIONS
In this work, we have conjugated the V. cholerae O1 OSP polysaccharide to Qβ carrier for the first time. The MP technique yielded critical information on the level of OSP loading on Qβ. The Qβ-OSP conjugate was recognized by sera from humans with cholera and was able to induce long lasting antibody production in the absence of adjuvant in a mice immunization study. The resulting antibodies exhibited vibriocidal activities. VLP-based display of bacterial OSP may warrant additional evaluation as a next generation anticholera vaccine.
METHODS
All chemicals were reagent grade and were used as received from the manufacturer, unless otherwise noted. Protein concentration was measured using the Coomassie Plus Protein Reagent (Bradford Assay, Pierce) with BSA as the standard. OSP and BSA-OSP was produced as previously described.22 Qβ capsid was expressed recombinantly following the reported procedure.25,45
Qβ Conjugation to Lactoside 3 and Purification.
A stock solution of 4.8 mg/mL Qβ in 0.1 M (pH 7.0) KPB buffer (208 μL) was placed in a Millipore Amicon Ultra-0.5 (10k Da cutoff) ultrafiltration device and the content was ultrafiltered against 0.5 M pH 9.0 borate buffer for three times (at 10 °C, 7500 rcf, 10 min/run) to exchange the buffer to 0.5 M pH 9.0 borate buffer. The filtrates were discarded and the final retentate was transferred into a 0.5 mL V-shaped reaction vessel and the same buffer was added to adjust the overall volume to 200 μ. Lactose squarate 3 (0.6 mg, 0.986 mmol) was carefully added into the reaction mixture and the content of the vessel was stirred at r.t. for 20 h. SELDI-TOF-MS analysis showed that an average loading (lactose/Qβ monomer ratio) of ~4.5 was achieved (Figure S1b). Another 0.6 mg (0.986 mmol) of compound 3 was added and the reaction mixture was further stirred for 72 h. SELDI-TOF-MS showed that the average loading reached ~8.0 per monomer. The reaction was worked up by ultrafiltering the reaction mixture in a Millipore Amicon Ultra-0.5 (30k Da cutoff) tube against pH 7.2 PBS (1×) buffer for 6 times to remove the unconjugated lactose derivatives. The final retentate was transferred into a conical tube for storage.
SELDI-TOF-MS Analysis of Samples.
The above Qβ-lactose reaction mixture (1 mL) was diluted with 0.5 M pH 7.0 phosphate buffer (10 mL) and then mixed with dithiothreitol (DTT) solution (0.1 M in water, 11 mL). The mixture was incubated at 37 °C for 30 min. The above solution (1 mL) was withdrawn from the mixture for SELDI analysis20 using sinapinic acid (SPA) as the matrix.
Preparation of SPA Matrix.
To SPA (5 mg) in a 1 mL Eppendorf tube was added acetonitrile (100 μL) followed by 1% TFA aqueous solution (100 μL). The mixture was vigorously vortexed for 20 s and then centrifuged for 10 min at 1000 rcf and the supernatant was used as the matrix solution.
MS Sequencing of Qβ Lactoside 3 Conjugates.
Qβ conjugated with lactoside 3 (10 μg) was run on SDS-PAGE gel under reducing conditions. The gel was fixed and stained with Coomassie and the band corresponding to the Qβ monomers was excised. In gel trypsin digestion was performed and the resulting peptides were analyzed by mass spectrometry. The resulting data was analyzed using Scaffold v5.1.0.
Qβ Conjugation to OSP and Purification.
V. cholerae O1 Inaba OSP 1 (5.4 mg, 0.0009 mmol) was converted to its squarate derivative by reacting with 3,4-dimethoxy-3-cyclobutene-1,2-dione (2, 2.56 mg, 0.018 mmol) in 0.5 M pH 7.0 phosphate buffer as described previously.21 A white fluffy solid (5.4 mg, 0.00088 mmol, 98%) was obtained after workup. A stock solution of 6.7 mg/mL Qβ in 0.1 M (pH 7.0) KPB buffer (240 μL) was placed in a Millipore Amicon Ultra-0.5 (10 kDa cutoff) ultrafiltration device and the content was ultrafiltered against 0.5 M pH 9.0 borate buffer for three times (at 10 °C, for speed/rpm, time and volume of the concentrate, manufacturer’s suggestions were followed) to exchange the buffer to 0.5 M pH 9.0 borate buffer. The filtrates were discarded and the final retentate was transferred into a 1 mL V-shaped reaction vessel and the same buffer was added to adjust the overall volume to 225 μL. The squarate derivative obtained above (5.4 mg, 0.00088 mmol) was added into the vessel and the clear solution formed was stirred at r.t. After 120 h, the reaction was worked up by ultrafiltering the reaction mixture in a Millipore Amicon Ultra-4 (30 kDa cutoff) tube against pH 7.2 PBS (1×) buffer for 6 times to remove the unconjugated antigen. The final retentate was transferred into a conical tube for storage.
MP Procedure.
The 24 × 50 mm microscope coverslips (Fisher Scientific, Waltham, MA) and precut 2 × 2 silicon gasket wells (GBL103250, Sigma, MO) were cleaned and assembled as described in the literature.46 Measurements were performed on OneMP instrument (Refeyn, Oxford, UK) at room temperature. PBS (1×, pH 7.2) buffer was filtered through 0.22 μM filters before use. Ten microliters of the buffer were loaded in gasket well to focus the objective on the coverslip surface. Qβ conjugates stock were diluted 100 times in PBS (1×, pH 7.2) buffer and added to buffer in the well. The MP video was immediately recorded after the sample loading using the AcquireMP software (Refeyn, Oxford, UK). A 1 min video was recorded for each sample, and each sample was repeated twice. Data were processed using the DiscoverMP software (Refeyn, Oxford, UK) with the threshold filter values of 5. The mass distribution was plotted as histograms with bin width of 50 kDa and fit with Gaussian peaks to obtain the average mass of different species. The contrast-to-mass calibration was performed using an unstained protein ladder (LC0725, Thermo Fisher, Wattham, MA) and empty AAV5 sample (Virovek, Hayward, CA).
Immunization.
All animal experiments were approved by and performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Michigan State University. The animal usage protocol number is PROTO201900423. Female Swiss-Webster (3–5 week old) mice were used for studies (n = 5 for each group). Mice were injected intramuscularly on days 0, 21, and 42 with 0.06 mL Qβ-OSP construct (10 μg OSP, 65 μg Qβ per mouse) without an exogenous adjuvant. The control group was injected with the same amount of Qβ carrier (65 μg Qβ per mouse) as the experimental group. The final boost was given at day 265. Serum samples were collected on days 0 (before immunization), 7, 28, 49, 56, 86, 118, 170, 265, and 272.
Evaluation of Antibody Titers by ELISA.
The Nunc MaxiSorp flat-bottom 96-well microtiter plates were coated with 1 μg mL−1 of the BSA-OSP conjugate (100 μL/well)1 in NaHCO3/Na2CO3 buffer (0.05 M, pH 9.6) containing 0.02% NaN3 by incubation at 4 °C overnight. For ELISA study against LPS, plates were coated with 10 μg mL−1 of LPS, Inaba or E. coli, in PBS buffer overnight at room temperature. The coated plates were washed with PBS/0.5% Tween-20 (PBST) (4 × 200 μL) and blocked with 1% BSA in PBS (200 μL/well) at rt for 1 h. The plates were washed again with PBST (4 × 200 μL) and incubated with serial dilutions of mouse sera in 0.1% BSA/PBS (100 μL/well, 2 wells for each dilution). The plates were incubated for 2 h at 37 °C and then washed with PBST (4 × 200 μL). A 1:2000 dilution of HRP-conjugated goat antimouse IgG or IgM (Jackson ImmunoResearch Laboratory, 115-035-003) in 0.1% BSA/PBS (100 μL) was added to the wells respectively to determine the titers of antibodies generated. The plates were incubated for 1 h at 37 °C and then washed with PBST (4 × 200 μL). A solution of the enzymatic substrate 3,3′,5,5′- tetramethylbenzidine (TMB, 200 μL) was added to the plates (for one plate: 5 mg of TMB was dissolved in 2 mL of DMSO plus 18 mL of citric acid buffer containing 20 μL of H2O2). Color was allowed to develop for 15 min and then quenched by adding 50 μL of 0.5 M H2SO4. The readout was measured at 450 nm using a microplate reader. The titer was determined by regression analysis with log10 dilution plotted with optical density and reported as the highest fold of dilution giving the optical absorbance value of 0.1 over those of the preimmune control sera. 1:5000 serum dilution was used for LPS binding study as shown in Figure 4.
Evaluation of Qβ-OSP Conjugates Using Human Serum.
To assess immunoreactivity of the OSP display on the Qβ conjugates, antigen-specific ELISA using sera collected from humans with cholera in Bangladesh was performed. The responses to human sera with cholera were compared to the response detected in humans with typhoid fever in Bangladesh. All samples were collected following informed consent, and human subjects work was approved by the Institutional Review Boards of the Massachusetts General Hospital and the International Centre for Diarrheal Disease Research, Bangladesh (icddr,b). Plates coated with 100 ng of Qβ or Qβ-OSP (based on protein mass) per well. After blocking and washing of plates, acute and convalescent phase sera from humans with cholera or typhoid (diluted 1:250 in 0.1% BSA in phosphate buffered saline-Tween) were added and incubated for 90 min at 37 °C. HRP-conjugate antihuman IgG antibody at 1:5000 dilution in 0.1% BSA in phosphate buffered saline-Tween was used to detect antigen-specific antibodies. After 90 min incubation at 37 °C, 0.55 mg/mL solution of 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS; Sigma) with 0.03% H2O2 (Sigma) were added to the plates and optical density was read at 405 nm for 5 min at 30 s intervals. The maximum slope for an optical density change of 0.2 U was reported as millioptical density units per minute (mOD/min).
Serum Vibriocidal Responses.
The vibriocidal antibody titers against V. cholerae O1 El Tor Inaba strain PIC018 were assessed in a microassay as previously reported.47 The endogenous complement activity of mouse serum was inactivated by heating it for 30 min at 56 °C. 50 μL aliquots of serial dilution of heat-inactivated sera in 0.15 M saline were added to wells of sterile 96-well tissue culture plates containing 25 μL/well of V. cholerae O1 El Tor Inaba strain PIC018 (OD 0.3) in 0.15 M saline and 22% guinea pig complement. After 1 h incubation at 37 °C, 150 μL of brain heart infusion broth was added to each well, and plates were incubated for an additional 2 h at 37 °C. The optical density of plates was then measured at 595 nm. A responder was defined as having a 4-fold increase of vibriocidal titer at day 56 compared with baseline day 0 titer.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported in part by grants from the National Institutes of Health: (R01AI146210 (XH), R01AI106878 (ETR)), the Fogarty International Center, Training Grant in Vaccine Development and Public Health (TW005572 (MK, TRB)), and Emerging Global Fellowship Award TW010362 (TRB), the Intramural Research Program of the NIH and NIDDK (PX), and Michigan State University. We would like to thank the Michigan State University RTSF Proteomics Core for their help with the sequencing of Qβ-lactose conjugates.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsinfecdis.1c00585.
Scheme S1: Qβ conjugation to lactose squarate 3; Figure S1: SELDI-TOF MS result of the conjugation of Qβ and lactose; Figure S2: The SDS-PAGE of different samples; Figure S3: SELDI-TOF MS result of conjugation of Qβ to OSP, Figure S4: The MP result of Qβ triple mutant A38K/A40C/D102C; Figure S5: The MP result of Qβ WT mutant without RNA; Figure S6: The MP result of Qβ WT mutant without RNA (a) before and (b) after Qβ conjugation to lactoside 3; Figure S7: Mass spectrum of wild-type Qβ without RNA; Figure S8: Mass spectrum of wild-type Qβ without RNA after conjugation to lactoside; Figure S9: SELDI-TOF MS result of wild type Qβ without RNA before and after conjugation to lactoside; Figure S10: Sequence coverage of wild type Qβ conjugated with lactoside 3; Table S1: TIC from sequencing of wild type Qβ conjugated with lactoside 3 (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acsinfecdis.1c00585
The authors declare the following competing financial interest(s): Xuefei Huang is the founder of Iaso Therapeutics Inc., which is dedicated to the development of next generation of vaccines using the bacteriophage Qbeta platform. All authors declare no other competing interests.
Contributor Information
Zahra Rashidijahanabad, Department of Chemistry and Institute for Quantitative Health Science and Engineering, Michigan State University, East Lansing, Michigan 48824, United States.
Meagan Kelly, Division of Infectious Diseases, Massachusetts General Hospital, Boston, Massachusetts 02114, United States.
Mohammad Kamruzzaman, International Centre for Diarrheal Disease Research Bangladesh (icddr,b), Dhaka 1212, Bangladesh.
Firdausi Qadri, International Centre for Diarrheal Disease Research Bangladesh (icddr,b), Dhaka 1212, Bangladesh.
Taufiqur R. Bhuiyan, International Centre for Diarrheal Disease Research Bangladesh (icddr,b), Dhaka 1212, Bangladesh
Hunter McFall-Boegeman, Department of Chemistry and Institute for Quantitative Health Science and Engineering, Michigan State University, East Lansing, Michigan 48824, United States.
Di Wu, Biophysics Core Facility, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland 20892, United States.
Grzegorz Piszczek, Biophysics Core Facility, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland 20892, United States.
Peng Xu, Laboratory of Bioorganic Chemistry, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland 20892, United States.
Edward T. Ryan, Division of Infectious Diseases, Massachusetts General Hospital, Boston, Massachusetts 02114, United States Department of Medicine, Harvard Medical School, Boston, Massachusetts 02115, United States; Department of Immunology and Infectious Diseases, Harvard T.H. Chan School of Public Health, Boston, Massachusetts 02115, United States.
Xuefei Huang, Department of Chemistry, Institute for Quantitative Health Science and Engineering, and Department of Biomedical Engineering, Michigan State University, East Lansing, Michigan 48824, United States.
REFERENCES
- (1).Du JJ; Wang CW; Xu WB; Zhang L; Tang YK; Zhou SH; Gao XF; Yang GF; Guo J Multifunctional Protein Conjugates with Built-in Adjuvant (Adjuvant-Protein-Antigen) as Cancer Vaccines Boost Potent Immune Responses. iScience 2020, 23, 100935–100935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Pezzoli L Global oral cholera vaccine use, 2013–2018. Vaccine 2020, 38, A132–A140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Harris JB Cholera: Immunity and prospects in vaccine development. J. Infect. Dis 2018, 218, S141–S146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Kanungo S; Paisley A; Lopez AL; Bhattacharya M; Manna B; Kim DR; Han SH; Attridge S; Carbis R; Rao R; Holmgren J; Clemens JD; Sur D Immune responses following one and two doses of the reformulated, bivalent, killed, whole-cell, oral cholera vaccine among adults and children in Kolkata, India: A randomized, placebo-controlled trial. Vaccine 2009, 27, 6887–6893. [DOI] [PubMed] [Google Scholar]
- (5).Saha A; Chowdhury MI; Khanam F; Bhuiyan MS; Chowdhury F; Khan AI; Khan IA; Clemens J; Ali M; Cravioto A; Qadri F Safety and immunogenicity study of a killed bivalent (O1 and O139) whole-cell oral cholera vaccine Shanchol, in Bangladeshi adults and children as young as 1 year of age. Vaccine 2011, 29, 8285–8292. [DOI] [PubMed] [Google Scholar]
- (6).Leung DT; Rahman MA; Mohasin M; Patel SM; Aktar A; Khanam F; Uddin T; Riyadh MA; Saha A; Alam MM; Chowdhury F; Khan AI; Charles R; LaRocque R; Harris JB; Calderwood SB; Qadri F; Ryan ET Memory B cell and other immune responses in children receiving two doses of an oral killed cholera vaccine compared to responses following natural cholera infection in Bangladesh. Clin. Vaccine Immunol 2012, 19, 690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Sinclair D; Abba K; Zaman K; Qadri F; Graves PM Oral vaccines for preventing cholera. Cochrane Database Syst. Rev 2011, DOI: 10.1002/14651858.CD008603.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Sur D; Kanungo S; Sah B; Manna B; Ali M; Paisley AM; Niyogi SK; Park JK; Sarkar B; Puri MK; Kim DR; Deen JL; Holmgren J; Carbis R; Rao R; van Thu N; Han SH; Attridge S; Donner A; Ganguly NK; Bhattacharya SK; Nair GB; Clemens JD; Lopez AL Efficacy of a Low-Cost, inactivated Whole-Cell oral cholera vaccine: Results from 3 years of Follow-Up of a randomized, controlled trial. PLoS Neglected Trop. Dis 2011, 5, e1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Shaikh H; Lynch J; Kim J; Excler JL Current and future cholera vaccines. Vaccine 2020, 38, A118–A126. [DOI] [PubMed] [Google Scholar]
- (10).Gabutti G; Rossanese A; Tomasi A; Giuffrida S; Nicosia V; Barriga J; Florescu C; Sandri F; Stefanati A Cholera, the current status of cholera vaccines and recommendations for travellers. Vaccines 2020, 8, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Ali M; Lopez AL; You YA; Kim YE; Sah B; Maskery B; Clemens J The global burden of cholera. Bull. World Health Org 2012, 90, 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Islam K; Hossain M; Kelly M; Mayo Smith LM; Charles RC; Bhuiyan TR; Kováč P; Xu P; LaRocque RC; Calderwood SB; Simon JK; Chen WH; Haney D; Lock M; Lyon CE; Kirkpatrick BD; Cohen M; Levine MM; Gurwith M; Harris JB; Qadri F; Ryan ET Anti-O-specific polysaccharide (OSP) immune responses following vaccination with oral cholera vaccine CVD 103-HgR correlate with protection against cholera after infection with wild-type Vibrio cholerae O1 El Tor Inaba in North American volunteers. PLoS Neglected Trop. Dis 2018, 12, e0006376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Aktar A; Rahman MA; Afrin S; Akter A; Uddin T; Yasmin T; Sami MIN; Dash P; Jahan SR; Chowdhury F; Khan AI; LaRocque RC; Charles RC; Bhuiyan TR; Mandlik A; Kelly M; Kováč P; Xu P; Calderwood SB; Harris JB; Qadri F; Ryan ET Plasma and memory B cell responses targeting O-specific polysaccharide (OSP) are associated with protection against Vibrio cholerae O1 infection among household contacts of cholera patients in Bangladesh. PLoS Neglected Trop. Dis 2018, 12, No. e0006399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Sun X; Stefanetti G; Berti F; Kasper DL Polysaccharide structure dictates mechanism of adaptive immune response to glycoconjugate vaccines. Proc. Natl. Acad. Sci. U. S. A 2019, 116, 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Micoli F; Bjarnarson SP; Arcuri M; Pind AAA; Magnusdottir GJ; Necchi F; Di Benedetto R; Carducci M; Schiavo F; Giannelli C; Pisoni I; Martin LB; Del Giudice G; MacLennan CA; Rappuoli R; Jonsdottir I; Saul A Short Vipolysaccharide abrogates T-independent immune response and hyporesponsiveness elicited by long Vi-CRM197 conjugate vaccine. Proc. Natl. Acad. Sci. U. S. A 2020, 117, 24443–24449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Enotarpi J; Tontini M; Balocchi C; van der Es D; Auberger L; Balducci E; Carboni F; Proietti D; Casini D; Filippov DV; Overkleeft HS; van der Marel GA; Colombo C; Romano MR; Berti F; Costantino P; Codeé JDC; Lay L; Adamo R A stabilized glycomimetic conjugate vaccine inducing protective antibodies against Neisseria meningitidis serogroup A. Nat. Commun 2020, DOI: 10.1038/s41467-020-18279-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Yin Z; Dulaney S; McKay CS; Baniel C; Kaczanowska K; Ramadan S; Finn MG; Huang X Chemical Synthesis of GM2 Glycans, Bioconjugation with Bacteriophage Qβ, and the Induction of Anticancer Antibodies. ChemBioChem. 2016, 17, 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Yin Z; Chowdhury S; McKay C; Baniel C; Wright WS; Bentley P; Kaczanowska K; Gildersleeve JC; Finn MG; BenMohamed L; Huang X Significant Impact of Immunogen Design on the Diversity of Antibodies Generated by Carbohydrate-Based Anticancer Vaccine. ACS Chem. Biol 2015, 10, 2364–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Wu X; Ye J; DeLaitsch AT; Rashidijahanabad Z; Lang S; Kakeshpour T; Zhao Y; Ramadan S; Saavedra PV; Yuzbasiyan-Gurkan V; Kavunja H; Cao H; Gildersleeve JC; Huang X Chemoenzymatic Synthesis of 9NHAc-GD2 Antigen to Overcome the Hydrolytic Instability of O-Acetylated-GD2 for Anticancer Conjugate Vaccine Development. Angew. Chem., Int. Ed 2021, 60, 24179–24188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Xu P; Kováč P Direct conjugation of bacterial polysaccharides to proteins by squaric acid chemistry. In Methods Mol. Biol; Humana Press: New York, NY, 2019; Vol. 1954, pp 89–98. [DOI] [PubMed] [Google Scholar]
- (21).Xu P; Kelly M; Vann WF; Qadri F; Ryan ET; and Kováč P Conjugate Vaccines from Bacterial Antigens by Squaric Acid Chemistry: A Closer Look. ChemBioChem 2017, 18, 799–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Sayeed MA; Bufano MK; Xu P; Eckhoff G; Charles RC; Alam MM; Sultana T; Rashu MR; Berger A; Escobedo GG; Mandlik A; Bhuiyan TR; Leung DT; LaRocque RC; Harris JB; Calderwood SB; Qadri F; Vann WF; Kováč P; Ryan ET A cholera conjugate vaccine containing ospecific polysaccharide (OSP) of V. cholera o1 inaba and recombinant fragment of tetanus toxin heavy chain (OSP:rTTHC) induces serum, memory and lamina proprial responses against OSP and is protective in mice. PLoS Neglected Trop. Dis 2015, 9, e0003881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Storer RI; Aciro C; Jones LH Squaramides: Physical properties, synthesis and applications. Chem. Soc. Rev 2011, 40, 2330–2346. [DOI] [PubMed] [Google Scholar]
- (24).Hou S-J; Saksena R; and Kovác P Preparation of glycoconjugates by dialkyl squarate chemistry revisited. Carbohydr. Res 2008, 343, 196–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Sungsuwan S; Wu X; Shaw V; Kavunja H; McFall-Boegeman H; Rashidijahanabad Z; Tan Z; Lang S; Tahmasebi Nick SYZ; Ramadan S; Jin X; Huang X Structure Guided Design of Bacteriophage Qβ Mutants as Next Generation Carriers for Conjugate Vaccines. ACS Chem. Biol 2022, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Dhara D; Baliban SM; Huo CX; Rashidijahanabad Z; Sears KT; Nick ST; Misra AK; Tennant SM; Huang X Syntheses of Salmonella Paratyphi A Associated Oligosaccharide Antigens and Development towards Anti-Paratyphoid Fever Vaccines. Chem. Eur. J 2020, 26, 15953–15968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Wu X; Yin Z; McKay C; Pett C; Yu J; Schorlemer M; Gohl T; Sungsuwan S; Ramadan S; Baniel C; Allmon A; Das R; Westerlind U; Finn MG; Huang X Protective Epitope Discovery and Design of MUC1-based Vaccine for Effective Tumor Protections in Immunotolerant Mice. J. Am. Chem. Soc 2018, 140, 16596–16609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Young G; Hundt N; Cole D; Fineberg A; Andrecka J; Tyler A; Olerinyova A; Ansari A; Marklund EG; Collier MP; Chandler SA; Tkachenko O; Allen J; Crispin M; Billington N; Takagi Y; Sellers JR; Eichmann C; Selenko P; Frey L; Riek R; Galpin MR; Struwe WB; Benesch JLP; Kukura P Quantitative mass imaging of single biological macromolecules. Science 2018, 360, 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Garmann RF; Goldfain AM; Manoharan VN Measurements of the self-assembly kinetics of individual viral capsids around their RNA genome. Proc. Natl. Acad. Sci. U. S. A 2019, 116, 22485–22490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Wu D; Hwang P; Li T; Piszczek G Rapid Characterization of AAV gene therapy vectors by Mass Photometry. bioRxiv, February 19, 2021. DOI: 10.1101/2021.02.18.431916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Hovlid ML; Lau JL; Breitenkamp K; Higginson CJ; Laufer B; Manchester M; Finn MG Encapsidated atom-transfer radical polymerization in Qβ virus-like nanoparticles. ACS Nano 2014, 8, 8003–8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Xu P; Alam MM; Kalsy A; Charles RC; Calderwood SB; Qadri F; Ryan ET; Kováč P Simple, direct conjugation of bacterial O-SP-core antigens to proteins: Development of cholera conjugate vaccines. Bioconjugate Chem. 2011, 22, 2179–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Rollenhagen JE; Kalsy A; Saksena R; Sheikh A; Alam MM; Qadri F; Calderwood SB; Kovác P; Ryan ET Transcutaneous immunization with a synthetic hexasaccharide-protein conjugate induces anti-Vibrio cholerae lipopolysaccharide responses in mice. Vaccine 2009, 27, 4917–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Pfister HB; Kelly M; Qadri F; Ryan ET; Kováč P Synthesis of glycocluster-containing conjugates for a vaccine against cholera. Org. Biomol. Chem 2019, 17, 4049–060. [DOI] [PubMed] [Google Scholar]
- (35).Bachmann MF; Zinkernagel RM Neutralizing antiviral B cell responses. Annu. Rev. Immunol 1997, 15, 235–270. [DOI] [PubMed] [Google Scholar]
- (36).Micoli F; Adamo R; Costantino P Protein carriers for glycoconjugate vaccines: History, selection criteria, characterization and new trends. Molecules 2018, 23, 1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Wurm FR; Klok HA Be squared: Expanding the horizon of squaric acid-mediated conjugations. Chem. Soc. Rev 2013, 42, 8220–8236. [DOI] [PubMed] [Google Scholar]
- (38).Patel SM; Rahman MA; Mohasin M; Riyadh MA; Leung DT; Alam MM; Chowdhury F; Khan AI; Weil AA; Aktar A; Nazim M; LaRocque RC; Ryan ET; Calderwood SB; Qadri F; Harris JB Memory B cell responses to Vibrio cholerae O1 lipopolysaccharide are associated with protection against infection from household contacts of patients with cholera in Bangladesh. Clin. Vaccine Immunol 2012, 19, 842–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Akache B; Weeratna RD; Deora A; Thorn JM; Champion B; Merson JR; Davis HL; McCluskie MJ Anti-IgE Qβ-VLP conjugate vaccine self-adjuvants through activation of TLR7. Vaccines 2016, 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Johnson RA; Uddin T; Aktar A; Mohasin M; Alam MM; Chowdhury F; Harris JB; LaRocque RC; Bufano MK; Yu Y; Wu-Freeman Y; Leung DT; Sarracino D; Krastins B; Charles RC; Xu P; Kováč P; Calderwood SB; Qadri F; Ryan ET Comparison of immune responses to the O-specific polysaccharide and lipopolysaccharide of Vibrio cholerae O1 in Bangladeshi adult patients with cholera. Clin. Vaccine Immunol 2012, 19, 1712–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Saha D; LaRocque RC; Khan AI; Harris JB; Begum YA; Akramuzzaman SM; Faruque ASG; Ryan ET; Qadri F; Calderwood SB Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J. Infect. Dis 2004, 189, 2318–2322. [DOI] [PubMed] [Google Scholar]
- (42).Kauffman RC; Adekunle O; Yu H; Cho A; Nyhoff LE; Kelly M; Harris JB; Bhuiyan MS; Qadri F; Calderwood SB; Charles RC; Ryan ET; Kong J; Wrammert J Impact of Immunoglobulin Isotype and Epitope on the Functional Properties of Vibrio cholerae O-Specific Polysaccharide-Specific Monoclonal Antibodies. mBio 2021, 12, No. e03679–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Charles RC; Kelly M; Tam JM; Akter A; Hossain M; Islam K; Biswas R; Kamruzzaman M; Chowdhury F; Khan AI; Leung DT; Weil A; Larocque RC; Bhuiyan TR; Rahman A; Mayo-Smith LM; Becker RL; Vyas JM; Faherty CS; Nickerson KP; Giffen S; Ritter AS; Waldor MK; Xu P; Kováč P; Calderwood SB; Kauffman RC; Wrammert J; Qadri F; Harris JB; Ryan ET Humans surviving cholera develop antibodies against vibrio cholerae o-specific polysaccharide that inhibit pathogen motility. mBio 2020, 11, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Mamat U; Wilke K; Bramhill D; Schromm AB; Lindner B; Kohl TA; Corchero JL; Villaverde A; Schaffer L; Head SR; Souvignier C; Meredith TC; Woodard RW Detoxifying Escherichia coli for endotoxin-free production of recombinant proteins. Microb. Cell Fact 2015, DOI: 10.1186/s12934-015-0241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Fiedler JD; Brown SD; Lau JL; Finn MG RNA-directed packaging of enzymes within virus-like particles. Angew. Chem., Int. Ed 2010, 49, 9648–9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Wu D; Piszczek G Standard protocol for mass photometry experiments. Eur. Biophys. J 2021, 50, 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Sayeed MA; Bufano MK; Xu P; Eckhoff G; Charles RC; Alam MM; Sultana T; Rashu MR; Berger A; Escobedo GG; Mandlik A; Bhuiyan TR; Leung DT; LaRocque RC; Harris JB; Calderwood SB; Qadri F; Vann WF; Kováč P; Ryan ET A cholera conjugate vaccine containing ospecific polysaccharide (OSP) of V. cholera o1 inaba and recombinant fragment of tetanus toxin heavy chain (OSP:rTTHC) induces serum, memory and lamina proprial responses against OSP and is protective in mice. PLoS Neglected Trop. Dis 2015, 9, e0003881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


