Abstract
Impaired tissue oxygen delivery is a major cause of organ damage and failure in critically ill patients, which can occur even when systemic parameters, including cardiac output and arterial hemoglobin saturation, are close to normal. This review addresses oxygen transport mechanisms at the microcirculatory scale, and how hypoxia may occur in spite of adequate convective oxygen supply. The structure of the microcirculation is intrinsically heterogeneous, with wide variations in vessel diameters and flow pathway lengths, and consequently also in blood flow rates and oxygen levels. The dynamic processes of structural adaptation and flow regulation continually adjust microvessel diameters to compensate for heterogeneity, redistributing flow according to metabolic needs to ensure adequate tissue oxygenation. A key role in flow regulation is played by conducted responses, which are generated and propagated by endothelial cells and signal upstream arterioles to dilate in response to local hypoxia. Several pathophysiological conditions can impair local flow regulation, causing hypoxia and tissue damage leading to organ failure. Therapeutic measures targeted to systemic parameters may not address or may even worsen tissue oxygenation at the microvascular level. Restoration of tissue oxygenation in critically ill patients may depend on restoration of endothelial cell function, including conducted responses.
Keywords: conducted responses, critical illness, heterogeneity, microvascular networks, oxygen transport
1 |. INTRODUCTION
In caring for the critically ill, clinicians may face the conundrum of a patient with normal ventilation, cardiac output, and arterial oxygen saturation, but with evidence of renal1 or hepatic2 failure. Conventional clinical decision-making in these situations is often based on macroscopic parameters such as heart rate and blood pressure that do not provide insight into conditions at the microvascular level. As a result, therapeutic decisions may not address the underlying pathophysiological derangements leading to organ failure.
Normal tissue function depends on adequate oxygen supply. Although cellular hypoxia can result from defects anywhere along the oxygen transport pathway (pulmonary uptake, blood flow, uptake by mitochondria), deficits not attributable to impairment of overall ventilation or blood flow can result from heterogeneous oxygen transport at the microvascular level. Tissue oxygen levels vary widely over short length scales (tens of microns). Microvascular networks are heterogeneous in structure (diameter, length of flow pathways) and function (flow velocity, oxygen content). This heterogeneity can result in impaired oxygen extraction,3 and regions of hypoxia or anoxia can occur even in tissue that receives an adequate overall oxygen supply.4,5 This review addresses the role of local regulation of blood flow in overcoming this heterogeneity and matching perfusion to metabolic demand under normal and pathological conditions.
2 |. MICROVASCULAR HETEROGENEITY
2.1 |. Causes of heterogeneity
Oxygen is transported throughout the body by convection in flowing blood and by diffusion from blood into surrounding tissue. The continuous need for oxygen together with its short diffusion distance4 necessitates a convective delivery system that places erythrocytes close to every living cell. The network of microvessels that fulfills this requirement is heterogeneous in structure (Figure 1). This heterogeneity, coupled with spatial and temporal variations in flow and demand, requires local regulation of blood flow to prevent regions of hypoxia. Similarly, local regulation of blood flow in the lung is needed to avoid high blood flow to poorly ventilated regions, which would result in impaired blood oxygenation. The importance for tissue oxygenation of ventilation-perfusion matching in the lungs and metabolism-perfusion matching in the systemic circulation is illustrated schematically in Figure 2A and B.
FIGURE 1.
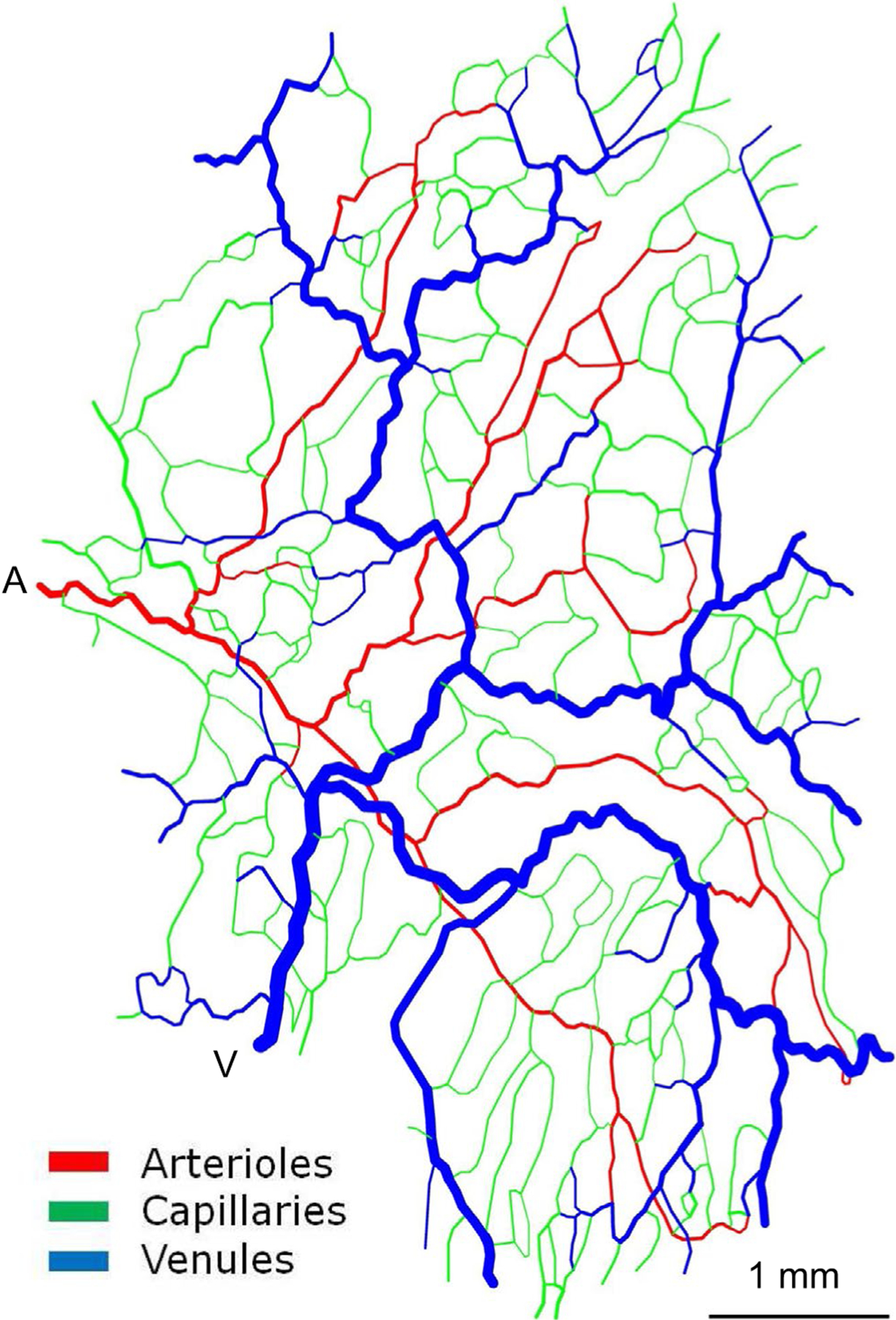
Representative microvascular network derived from observations of rat mesentery illustrating structural heterogeneity. A, Main arteriolar inflow. V: Main venular outflow (reprinted from Roy et al.56)
FIGURE 2.
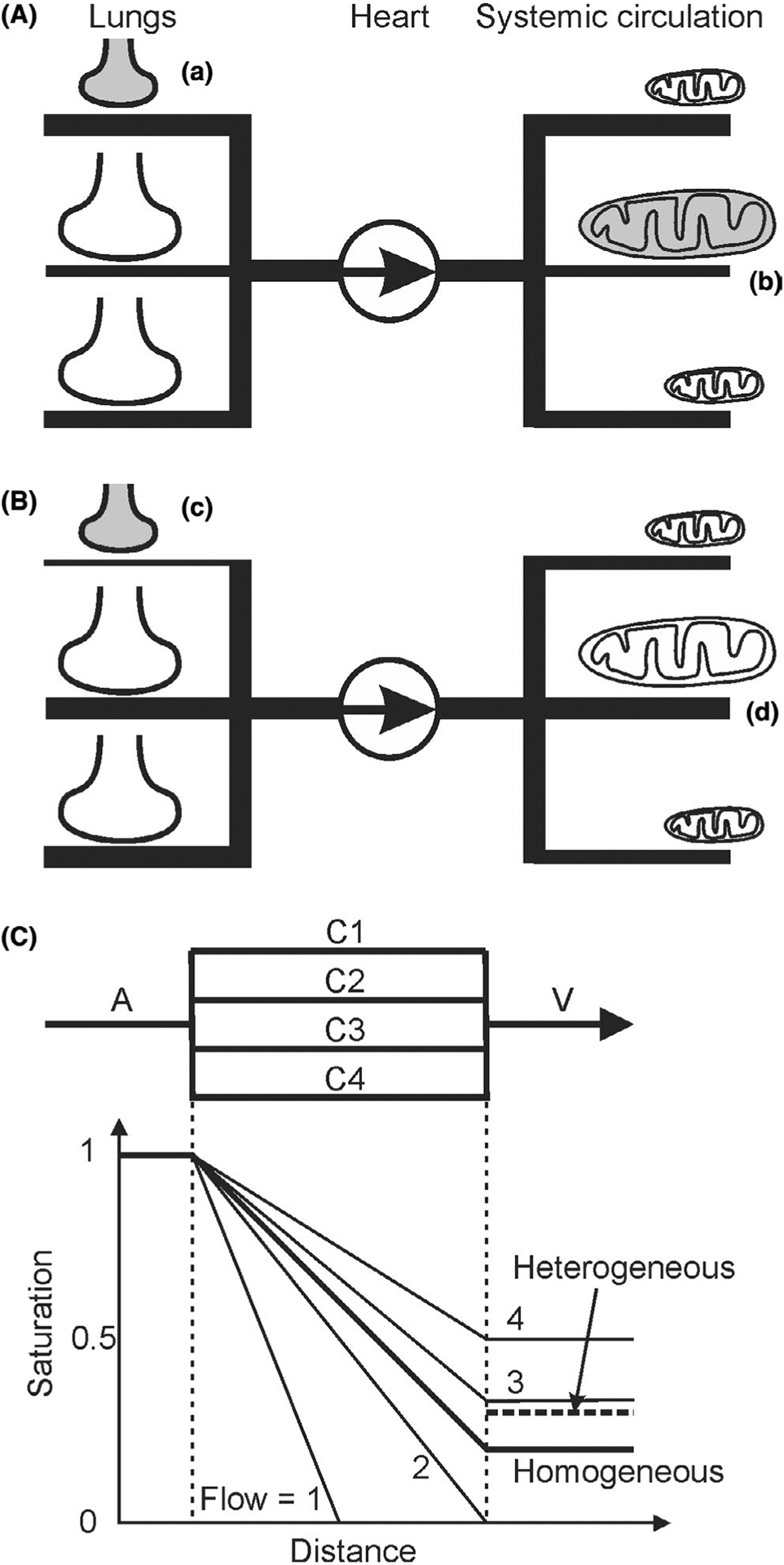
Schematic diagrams indicating how lack of ventilation-perfusion matching or metabolism-perfusion matching can cause hypoxia. A, (a) In the lungs, if poorly ventilated alveoli (indicated by small shaded shape) receive high perfusion, then blood may be poorly oxygenated. Thicknesses of lines represent relative distribution of blood flow. (b) In the systemic circulation, if tissue regions with high metabolic demand by mitochondria (represented by large shaded shape) receive low perfusion, then oxygen supply may be inadequate. B, (c) Redistribution of flow in the lungs, for example, by hypoxic vasoconstriction, reduces flow to poorly ventilated regions, improving overall blood oxygenation. (d) Redistribution of flow in peripheral circulation, for example, by local metabolic regulation of blood flow, increases flow to regions of high metabolic demand, improving tissue oxygenation (represented by large unshaded shape). C, Effects of heterogeneous capillary flow rates on oxygen delivery. Oxygen saturation in arteriole A is set to 1. In the homogeneous case, capillaries C1-C4 all have flow of 2.5 (arbitrary units). Saturation in venule V is 0.2, that is, 80% extraction. In the heterogeneous case, the same flow is distributed (1, 2, 3, 4) to capillaries C1-C4. Mixed saturation in V is 0.3 (dashed line), that is, 70% extraction. C1 is anoxic along its downstream half, implying tissue hypoxia. For simplicity, oxygen delivery per unit length is held constant if saturation is above zero
2.1.1 |. Structural heterogeneity
Microvascular network structures are not genetically predetermined, but arise from angiogenesis,6,7 a stochastic process involving vessel growth in response to metabolic signals and pruning of redundant vessels. This leads to variations in vessel length and diameter.8–12 An additional source of heterogeneity is the “dimensional problem”.13 A three-dimensional delivery system can provide spatially uniform convective transport to a two-dimensional region by means of symmetrically branching networks, all flow pathways being equivalent in geometry and flow (Figure 3A). If, however, a three-dimensional region must be supplied, then the branching network is embedded within the region itself, which results in non-equivalent flow pathways. This problem is exacerbated when feeding and draining vessels run adjacent to each other, as is often the case (Figure 3B). A high degree of heterogeneity among flow pathways is inevitable.
FIGURE 3.
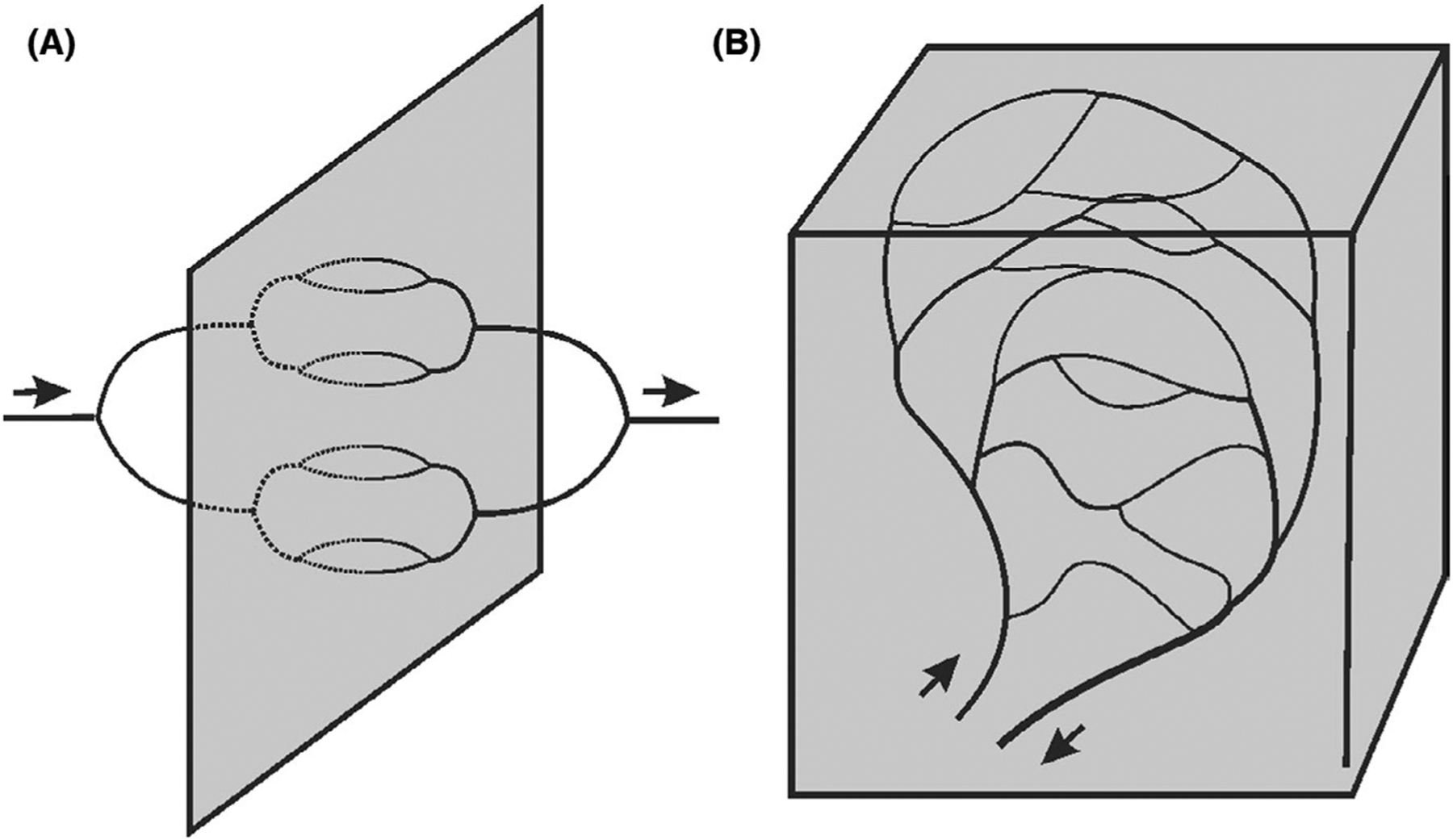
Schematic illustrating the “dimensional problem.” A, Network supplying a two-dimensional region. B. Network supplying a three-dimensional region
2.1.2 |. Flow heterogeneity
According to Poiseuille’s law, flow resistance of a blood vessel is proportional to segment length and inversely proportional to the fourth power of diameter.11 The structural heterogeneity of microvascular networks thus results in wide variations in flow rates.14–18 A further source of heterogeneity is the particulate nature of blood.19 At diverging bifurcations, erythrocytes preferentially enter the branch with higher flow (phase separation), leading to wide variations in microvessel hematocrit.11,20,21 With hemodilution,22 anemia, or other pathophysiological conditions, some microvessels may receive few or no red blood cells, leading to hypoxia.23 Conditions resulting in lowered hematocrit such as hemorrhagic shock can lead to increased temporal and spatial heterogeneity, particularly following fluid resuscitation.24,25 Spontaneous oscillations in vessel diameter, termed vasomotion, can redistribute blood flow and may affect oxygen transport.26,27 As measured by Poole and colleagues in skeletal muscle using phosphorescence quenching, a substantial oxygen gradient exists between the microvasculature and the interstitium,28,29 highlighting the importance of a homogeneous red blood cell distribution in maintaining adequate supply to tissues with high demand.
The effect of heterogeneity on hemodynamic parameters can be quantified in terms of capillary transit time heterogeneity (CTH), defined as the standard deviation of the transit time distribution,30 which increases in pathophysiological conditions. Alternatively, capillary outflow saturation heterogeneity has been utilized as an index of functional heterogeneity.31,32 Figure 2C illustrates how heterogeneity in capillary flow rates results in heterogeneous capillary outflow saturation, tissue hypoxia, and reduced oxygen extraction. Capillary flow in vivo is typically much more heterogeneous than shown in this example.8 In this schematic representation, capillaries are shown as having equal lengths for simplicity. In reality, flow pathways are heterogeneous in length, and heterogeneous outflow saturation can be caused by poor matching of perfusion to metabolic demand. This representation does not include the effect of oxygen diffusion from arterioles to tissue, which affects oxygen delivery by capillaries and can, for example, result in reverse oxygen diffusion from tissue into capillaries.33,34
2.1.3 |. Demand heterogeneity
In many organs, spatial and temporal changes in demand necessitate concomitant changes in blood flow to maintain oxygen availability. One example is differential activation of muscle fiber types during exercise.35,36 A recent computational model accounting for spatial heterogeneity in types and sizes of muscle fibers demonstrated that fiber size heterogeneity was a primary cause of local hypoxia,37 but that non-equilibrium states and high demand conditions such as heavy exercise could exacerbate local discrepancies between supply and demand. A biphasic pattern of oxygen delivery relative to consumption has been demonstrated in skeletal muscle, with increased heterogeneity at low-to-moderate exercise intensities but a decrease at high exercise intensity corresponding to more uniform capillary perfusion.38,39 Another example is increased regional cerebral metabolic activity, which can occur due to localized neuronal activation. Increased oxygen demand is matched by increased blood flow via neurovascular coupling, a process that relies on multiple cell types and signaling pathways. Dysfunction in neurovascular coupling resulting in hypoxia is suspected in the pathophysiology of various diseases including vascular dementia.40
2.2 |. Role of flow regulation in metabolism-perfusion matching
Active vascular responses are needed to counteract the inherent heterogeneity of microvascular flow and ensure adequate tissue oxygenation. These responses can be classified according to the time scales over which they act. Over time scales of hours to weeks, all blood vessels, from capillaries to arteries, are subject to structural adaptation. This occurs during growth and development and in response to changing functional needs6 and mitigates the intrinsic heterogeneity of microvascular perfusion.13 Over time scales of seconds to minutes, flow regulation is responsible for modulation of local perfusion.41 Contraction and relaxation of vascular smooth muscle in small arteries and arterioles cause diameter changes that redirect flow via changes in flow resistance.
Vascular smooth muscle tone is modulated by local concentrations of vasoactive metabolites and mediators, autonomic influences, and hemodynamic factors, and conducted responses from downstream vessels.42 Hemodynamic factors affecting vessel diameter include responses to wall shear stress and circumferential wall stress generated by transmural pressure. Increases in wall shear stress are sensed by endothelial cells and typically result in vasodilation (decreased vascular tone) due to the release of mediators including NO, prostaglandins, and EDHF (endothelium-derived hyperpolarizing factor). Increases in transmural pressure activate mechanosensitive ion channels in vascular smooth muscle leading to vasoconstriction (increased vascular tone), termed the myogenic response.43,44 Sympathetic stimulation causes vasoconstriction and serves to direct flow to skeletal muscle during exercise.45–47
2.2.1 |. Metabolic signals
The mechanisms by which metabolic needs are signaled to the vasculature are not well understood. Multiple signaling mechanisms have been proposed, and more than one mechanism may be active in any given situation. Sensing of oxygen levels is typically involved. In the brain, however, metabolic flow regulation is considered to be independent of oxygen levels.48 Metabolic signals may originate in erythrocytes, in vessel walls, or in surrounding tissue.41 One mechanism of oxygen sensing involves the release of ATP by erythrocytes at a rate that depends on saturation, and ATP binding to receptors on endothelial cells.49–51 Erythrocyte-dependent NO vasodilator activity has also been implicated as a potential mechanism.52 Endothelial cells release metabolites under hypoxic conditions, including NO, prostaglandins, EDHF, and adenosine. Possible metabolites arising from tissue include carbon dioxide (resulting in decreased pH), and breakdown products of ATP including adenosine.53 Neural activity causes increases in extracellular potassium levels. This may be an important metabolic signal in neurovascular coupling and may also play a role in initiating vasodilation when skeletal muscle is stimulated. Another possible mechanism involves a direct effect of hypoxia on smooth muscle cell function causing vasodilation.54 The differing functional ranges of oxygen tension at which these mechanisms operate may imply that their effect varies depending on local conditions. The relative roles of signals derived from erythrocytes, vessel walls, and parenchyma are not known.55 Theoretical arguments imply that erythrocyte-derived signals alone cannot provide adequate flow regulation in heterogeneous networks.56 Experiments have demonstrated the role of non–erythrocyte-dependent mechanisms.57 A similar conclusion applies with regard to the role of erythrocyte-derived signals in structural adaptation.41,58
2.2.2 |. Role of conducted responses
The blood flow rate in any vessel depends on the resistance not only of that segment but also of the flow pathway feeding and draining it. The largest component of overall flow resistance resides in the arterioles. Modulation of capillary flow therefore requires control of the diameters of upstream arterioles.59 This is achieved largely by conducted responses propagated along vessel walls.60,61 Conducted responses involve changes in cell membrane potential and/or intra-cellular ion concentrations, which are transmitted via gap junctions along the endothelial cell layer over distances on the scale of mm62 and between endothelial and vascular smooth muscle cells.63 Gap junction connexins involved in conducted responses include Cx37, Cx40, and Cx43 in endothelium and Cx37, Cx40, Cx43, and Cx45 in vascular smooth muscle.64 Although other mechanisms exist for co-ordinating vascular constriction and dilation along flow pathways,65 upstream conducted responses play a critical role in both flow regulation63,66 and structural adaptation.6 These findings suggest that impairment of conducted responses may play a role in the poor tissue oxygenation observed in pathophysiological conditions, even when perfusion is adequate.67
3 |. IMPAIRED METABOLISM-PERFUSION MATCHING: IMPLICATIONS FOR OXYGEN DELIVERY AND UPTAKE
3.1 |. Mechanisms and consequences of impaired flow regulation
Failure of local flow regulation can lead to local areas of inadequate oxygen delivery and impaired oxygen extraction.3 Acute and chronic pathophysiological conditions such as sepsis, peripheral vascular disease, and myocardial ischemia can impair regulatory mechanisms68,69 and result in local heterogeneity leading to hypoxia and potential organ failure. Potential modes of dysregulation include endothelial cell dysfunction70–72 and loss of endothelial cell coupling causing attenuation of conducted signals.73 Examples of clinical conditions resulting in increased microvascular perfusion heterogeneity and organ dysfunction are discussed below, followed by organ-specific considerations.
3.2 |. Clinical implications of impaired flow regulation
3.2.1 |. Sepsis
Sepsis results from a dysregulated systemic inflammatory response to an infectious pathogen.74 Global vasodilation resulting from inflammation results in maldistribution of oxygen delivery and impaired oxygen extraction.75 The syndrome has been defined based on a set of clinical criteria.76 If not treated aggressively within an appropriate time, it can lead to hypoperfusion, organ failure, and death.77 Failure of one or more organ systems (most commonly respiratory, cardiovascular, and renal) correlates with mortality, and survivors often require continued care.78
Features of sepsis include a decrease in systemic vascular resistance (distributive shock) and impaired oxygen extraction despite increased cardiac output and increased systemic oxygen delivery.77 Although cardiac output is often preserved or even increased following fluid resuscitation, sepsis can also cause reversible depression in left ventricular ejection fraction and diastolic dysfunction,79 leading to superimposed cardiogenic shock. Various classes of sepsis biomarkers have been proposed and used for prognostication,80 reflecting the variable presentation and course of this condition.
At the microcirculatory level, derangements in microvascular flow regulation, erythrocyte deformability, leukocyte and platelet activation, and microvascular permeability result in increased heterogeneity of microvascular flow and oxygen delivery, which in combination with increased oxygen demand and mitochondrial dysfunction can result in regional hypoxia and organ failure.81–83 Furthermore, inflammation and increased capillary permeability can cause hypovolemic shock and tissue edema as fluid leaks out of the microcirculation84; this can be exacerbated by damage to the glycocalyx.85 In resistance vessels, altered vascular reactivity86 due to increased release of vasoactive substances such as adenosine and nitric oxide87,88 along with impairment of autonomic tone89 and conducted responses result in altered patterns of blood flow including shunting.90
The term shunting refers to partial diversion of oxygen delivery away from target tissues, which can result in inadequate oxygen utilization.91 It is characterized by values of venous PO2 higher than microcirculatory PO2, and this difference (termed the PO2 gap) has been observed in hemorrhagic shock.92 Anatomical shunting via collaterals has been observed in several organs.93–95 Functional shunting can result from (i) diffusive transfer of oxygen from arterioles to venules33,96,97; (ii) failure of flow regulation and increased flow heterogeneity (the focus of this review); or (iii) the theoretical inability of Hb to unload rapidly enough as it passes through the capillaries due to rapid transit times.98,99 The poor matching of perfusion and oxygen delivery to metabolic needs is a hallmark of sepsis and a likely cause of tissue damage and organ failure.91 A classic study investigating blood flow under conditions of sepsis and endotoxemia using microspheres in dogs showed little evidence of systemic shunting but demonstrated the presence of splanchnic and renal shunting.100 As illustrated in Figure 2, heterogeneity in capillary flow can lead to an oxygen extraction deficit, suggesting the use of vasodilators to open up these “weak microcirculatory units”.101 Alternatively, the use of oxygen carriers including perfluorocarbons102 and hemoglobin-based oxygen carriers to improve transport from these vessels has been investigated.92,103
The role of mitochondrial dysfunction in sepsis also remains unclear. The presence of impaired organ function in sepsis in the face of adequate oxygen delivery naturally leads to impaired cellular respiration as a possible explanation. Singer and colleagues assessed muscle biopsies for levels of ATP along with markers of mitochondrial activity, NO production, and oxidative stress in critically ill patients relative to controls104 and found that shock severity (as measured by pressor requirement) correlated with mitochondrial dysfunction. Impaired oxygen extraction as a result of impaired cellular respiration has been termed cytopathic hypoxia.105
Several other mechanisms have also been implicated in the impaired oxygen extraction seen in sepsis. Heterogeneity of perfusion75,90 can lead to impaired metabolism-perfusion matching.106 Aggregation and plugging of microvessels due to altered erythrocyte and leukocyte rheology77,90,107 can occur along with vascular microthrombosis.108 Decreased sensitivity of adrenergic receptors to norepinephrine with acidosis and hypoxia can lead to impairment of the autonomic regulation that contributes to metabolism-perfusion matching. Also, in spite of increased sympathetic activity,109 widespread vasodilation may occur due to various mechanisms, including adenosine88 and increased NO production due to upregulation of iNOS,110 causing decreased whole body and regional extraction. These characteristics are consistent with the paradigm of sepsis as a disease of the microcirculation.81,111–113 Since the severity of microcirculatory dysfunction is associated with poor outcome in critical illness,114,115 several interventions including fluid resuscitation,116–118 vasoactive agents,115,119,120 and NOS inhibition110 have been proposed and utilized to optimize oxygen delivery at the microcirculatory level.84,121
Mathematical models of the microcirculation in sepsis have simulated heterogeneity in tissue perfusion by assuming populations of capillaries with stopped flow, and have demonstrated its detrimental effect on oxygen transport.122,123 As models increase in sophistication, the ability to quantitatively characterize the mechanisms of decreased extraction and response to interventions may allow optimization of pharmacologic management (ie, choice of vasopressors) to reverse maldistribution of flow in vasodilatory states.
3.2.2 |. Metabolic syndrome
A recurring theme in microvascular dysfunction is the role of oxidative stress. Metabolic syndrome represents a constellation of clinical conditions including obesity, hypertension, dyslipidemia and atherosclerosis, and impaired glycemic control that is associated with chronic inflammatory and prothrombotic states.124 The underlying pathophysiology of metabolic syndrome is thought to originate with impaired vascular reactivity, decreased perfusion, and microcirculatory dysfunction, causing peripheral vascular disease (PVD) and affecting the brain, heart, and skeletal muscle. Although many pathways are involved in the generation of reactive oxygen species (ROS) in affected individuals, a major mechanism is the uncoupling of nitric oxide production by eNOS so that superoxide is formed instead. This occurs when necessary cofactors are unavailable and/or peroxynitrite (generated by NO scavenging) is present.125 The presence of superoxide along with other ROS (such as hydrogen peroxide) and reactive nitrogen species interferes with the regulation of vascular tone. Overproduction of NO can also lead to endothelial dysfunction by causing peroxynitrite formation. The resultant oxidative stress leads to a blunting of the vasodilatory response in hypoxia due to increased endogenous vasoconstrictor production via the cyclooxygenase pathway.126 These changes manifest as structural and functional alterations in the vascular wall in affected patients, leading to limitations in skeletal muscle and cerebral blood flow. In an animal model of metabolic syndrome (the obese Zucker rat), cerebral arteries were found to be stiffer and narrower with impaired vasodilatory capacity and decreased nitric oxide availability relative to controls.124 Similarly (in a range of rat models with increasing risk), vascular reactivity and NO bioavailability were found to be progressively more impaired with increasing PVD risk. Additionally, the perfusion distribution coefficient (a measure of temporal perfusion heterogeneity at arteriolar bifurcations) was found to be higher in higher-risk rats and trended toward affecting larger vessels as risk increased.127 Finally, in a study using the obese Zucker rat, Frisbee and colleagues investigated the nature of vascular dysfunction by using adrenergic stimulation in addition to pressor response and endothelium-dependent vasodilation; blocking the adrenergic response to simply improve blood flow did not improve oxygen uptake and muscle performance as much as also restoring endothelial function,128 suggesting distinct mechanisms of dysfunction affecting different calibers of vessels.129 Modeling results for skeletal muscle confirm that perfusion heterogeneity adversely affects tissue oxygenation in the metabolic syndrome.130
3.2.3 |. Diabetes mellitus
Diabetes is characterized by high glucose levels either due to inadequate insulin production (T1D) or due to insulin resistance in peripheral tissues (T2D) as seen in metabolic syndrome.131 Many of its common complications (retinopathy, nephropathy, neuropathy,132 myocardial dysfunction) are vascular in origin and result from oxidative stress and inflammation. Abnormal angiogenesis results from increased VEGF (vascular endothelial growth factor) levels in the retina and kidneys, and may also destabilize vascular walls.133 Advanced glycation end products (AGEs) and microRNAs have been implicated in the development of vascular disease in T2D.134 Walls of large vessels show structural changes secondary to inflammation (cell proliferation, hypertrophy), with decreased compliance. Both small and large vessels show impaired endothelium-dependent vasodilation, increased endothelial permeability, and oxidative stress. In an animal model of T2D, the observed decreased vascular reactivity and blunted active hyperemia were attributed to increased thromboxane production and oxidative stress131; muscle perfusion was partially restored with antioxidant and anti-thromboxane therapy. The significant role that oxidative stress and inflammation play in diabetic microangiopathy suggests that targeting these mediators and controlling comorbid conditions such as hypertension may be as important as normalizing glucose levels.134 This is corroborated by differences in functional abnormalities of the microvasculature in T1D vs T2D.135 Finally, diabetes may influence muscle glucose uptake via a direct effect on muscle perfusion,136 although this remains controversial.137
3.3 |. Organ-specific considerations
3.3.1 |. Kidney
The kidney receives a disproportionate amount of blood flow for its size and has one of the highest oxygen consumption rates in the body.138 Its high metabolic demand required to facilitate sodium transport, and its complex vascular architecture makes it particularly vulnerable to hypoxic injury. The renal cortex receives the majority of renal blood flow (RBF) relative to the medulla and renal PO2 decreases from the cortex to the medulla as the osmotic gradient increases.139 Acute kidney injury (AKI) in the critically ill can result from ischemic or nephrotoxic insults, but is also common in sepsis even among patients who have not experienced hypoxemia or hypotension resulting in decreased RBF and/or oxygen delivery to the kidney.140 The severity of AKI is characterized by the degree of oliguria, an increase in serum creatine, and the need for renal replacement therapy (KDIGO–Kidney Disease: Improving Global Outcomes Criteria),141 but these are often late signs and highlight the need for early diagnosis and treatment. Since histological studies of AKI particularly in sepsis may not show overt evidence of structural injury such as tubular necrosis,142,143 focus has shifted to possible proinflammatory and prooxidant effects causing microcirculatory dysfunction and alterations in vascular reactivity. This is corroborated by studies showing that simply targeting higher perfusion pressures does not lead to improvements in oxygenation.144
The inflammatory response that characterizes sepsis is thought to initiate a cascade culminating in the release of cytokines, chemokines, and other mediators that cause endothelial cell activation and microvascular dysfunction.145 Consequences include impaired flow regulation due to regional deficits in NO-mediated vasodilation causing heterogeneity in perfusion and oxygen levels, enhanced release of ROS, platelet aggregation, and damage to the glycocalyx and endothelial junctions146 leading to increased vascular permeability and edema. Regional variations in renal perfusion and oxygen levels were recently observed in renal injury using pimonidazole staining,147 suggesting that shunting of oxygen might explain the impaired oxygen utilization and extraction seen in AKI.91 This is supported by a study showing that arteriovenous shunting was an important contributor in maintaining renal PO2 in the face of changing RBF.148 Since renal oxygen delivery normally exceeds demand, the possibility that preglomerular shunting could serve to spare the renal parenchyma from exposure to high oxygen levels and ROS was proposed.149,150 Arteriovenous oxygen transport may be facilitated by the anatomical arrangement of the renal vasculature.151,152 This concept is supported by mathematical modeling94,153,154 although recent models153,155 predict a relatively small shunt fraction under normal conditions. With regard to the role of mitochondria in AKI, a recent in vitro study showed that mitochondrial function was impaired in renal tissue exposed to endotoxin140 and that mitochondrial damage may occur even in the absence of tubular injury.156
3.3.2 |. Skeletal muscle
In skeletal muscle, autonomic regulation modulates flow distribution to recruited motor units.35 The baseline diameter of skeletal muscle arterioles is determined in part by resting sympathetic nerve activity (SNA).157 Increased SNA (eg, during exercise) leads to increased cardiac contractility and arteriolar vasoconstriction, which maintains systemic blood pressure as the skeletal muscle vasculature dilates. The local metabolic vasodilation (termed functional sympatholysis) serves to direct blood to metabolically active areas.158–160 This interaction is mediated by the differential response of larger vs. smaller arterioles to vasodilatory metabolites46,161 due to the distribution of α adrenergic receptor subtypes. The sympathetic vasoconstriction of smaller vessels with higher concentrations of α2 receptors appears to be more susceptible to inhibition by vasodilatory metabolites as compared to larger vessels with higher concentrations of α1 receptors,162,163 providing a mechanism for increasing perfusion to metabolically active areas. Attempts to increase oxygen delivery by pharmacologic vasodilation have resulted in either no improvement in maximal aerobic capacity164 or decreased oxygen extraction.165 Despite an increase in blood flow, vasodilation via adenosine results in impaired metabolism-perfusion matching (ie, decreased extraction), likely due to shunting of blood to non-exercising tissue.164,166 Similarly, experiments performed at altitude with adenosine vasodilation show a paradoxical worsening of oxygen uptake with reduced extraction due to shunting.165 These results indicate that adrenergic tone is important for directing flow to metabolically active areas.167,168 Implications for impaired vascular reactivity (as seen in metabolic syndrome) include the development of peripheral vascular disease and mitigation of this risk by exercise training.169
3.3.3 |. Brain
Under normal conditions, increases in regional cerebral metabolic demand either increase extraction170 or increase perfusion via neurovascular coupling.171,172 This involves conducted responses that vasodilate upstream arterioles and small arteries.171 Several disorders have been linked to cerebrovascular dysfunction and impaired neurovascular regulation including vascular dementia,40 stroke,171 and Alzheimer’s disease.171,173–176 Cerebral autoregulation maintains blood flow under a wide range of perfusion pressures.40 The cerebral blood supply arises from the Circle of Willis, and the vascular arrangement in the deep white matter is such that the watershed regions between the anterior and middle cerebral artery distributions have poor collateral flow. This area is vulnerable to disruptions in perfusion and can be affected by decreases in global cerebral perfusion and neuropathological small vessel lesions affecting arterioles.177 Vascular dementia is often functional but is sometimes associated with pathological features including leukoaraiosis, lacunar infarcts, microbleeds, microinfarcts, and cerebral amyloid angiopathy.40 In many cases, these lesions are accompanied by attributes of neurodegenerative diseases such as the neurofibrillary tangles and amyloid plaques seen in Alzheimer’s disease. These processes may be synergistic in that vascular insufficiency promotes amyloid deposition and impaired clearance, and amyloid reduces cerebral blood flow and impairs functional hyperemia.40 Both vascular dementia and Alzheimer’s disease exhibit vascular dysfunction including impaired reactivity, altered cerebrovascular autoregulation, and disruption of the blood-brain barrier.174,178 The demyelinated neurons and damaged glia are unable to support the endothelium, leading to capillary rarefaction. The underlying cause of vascular dysfunction is thought to be oxidative stress and inflammation leading to endothelial damage and disruption of neurovascular coupling.40,176 Since no effective treatment has been identified, efforts have focused on preventive measures such as control of hypertension and other risk factors.40 Ischemic strokes result in similar impairment of reactivity and autoregulation177 and can even result in decreased blood flow and metabolism in intact areas.171 Eventual consequences of impaired cerebral blood flow (as seen in metabolic syndrome for example) can include cognitive decline and stroke.124
3.3.4 |. Heart
The myocardium is characterized by regional flow heterogeneity179,180 and regional variations in oxygen demand.10 These variations lead to heterogeneity in oxygen levels at the microvascular level.10 Recent evidence suggests that the fractal nature of myocardial blood flow correlates more with metabolic activity than vascular structure,181 suggesting the strong influence of metabolic flow regulation in the heart. Failure of flow regulation is seen in a condition called microvascular angina (previously referred to as cardiac syndrome X)182,183 in which patients (women more often than men) experience angina in response to microvascular dysregulation without arterial occlusion184; these symptoms may or may not be accompanied by ECG changes and/or regional wall motion abnormalities. The pathophysiology involves endothelial and autonomic dysfunction resulting in vasoconstriction and/or inadequate vasodilation in response to metabolic demand.183,185 The condition is difficult to diagnose since patients typically have normal coronary angiograms. Noninvasive methods such as vasodilator stress cardiac MRI185 and invasive methods such as coronary flow reserve (CFR) and the index of microcirculatory resistance (IMR) have been used.183,186,187 Patients with symptoms of microvascular angina are typically treated with drugs used for ischemia such as beta-adrenergic blocking agents, calcium channel blockers, and nitrates185 as well as preventative measures, since microvascular angina can coexist with atherosclerotic heart disease. Microvascular dysfunction is often associated with atherosclerotic heart disease183,188 and can persist even after revascularization of large coronary vessels189; capillaries experience oxidative stress and in some cases exhibit loss of pericytes and rarefaction, limiting blood flow. The systemic implications of the atherogenic, inflammatory, and prothrombotic state seen in the metabolic syndrome include impaired vascular reactivity and peripheral vascular disease, culminating in angina or heart failure from hypertension and increased afterload.127
3.3.5 |. Lung
Efficient uptake of oxygen through the alveolar-capillary membrane requires a normal diffusing capacity190 and intact flow regulation to match perfusion to ventilation.191 ARDS (acute respiratory distress syndrome) is characterized by severe hypoxemia resulting in high mortality among critically ill patients. Its pathogenesis includes pulmonary endothelial injury resulting in increased vascular permeability and pulmonary edema as well as alveolar epithelial injury and increased interstitial fluid.192–194 Inflammation and accumulation of protein-rich edema fluid impair gas exchange and cause alveolar flooding.195 The increased diffusion distance across the alveolar-capillary membrane coupled with impaired flow regulation and local or regional heterogeneity of ventilation and perfusion contributes to decreased arterial oxygenation.196
Lung injury in patients with ARDS is associated with changes in ventilation at the regional and local levels, leading to heterogeneity at multiple scales. Increased heterogeneity is associated with increased dead space fraction.197,198 Ventilation changes in distal aspects of the tracheobronchial tree can occur due to mucous plugging, particularly in cases of infection. Pulmonary edema due to alveolar epithelial injury can alter alveolar mechanics (preventing alveoli from opening during tidal ventilation); alveolar flooding (an extreme case of increased alveolar-capillary membrane thickness) can prevent ventilation altogether.
In the absence of well-distributed ventilation, adequate matching of perfusion to ventilation through flow regulation is crucial. Hypoxic vasoconstriction (HPV) is a key mechanism for matching perfusion to ventilation.199–202 Capillary endothelial cells act as the sensors of local oxygen tension203 along with pulmonary artery smooth muscle cells (PASMC).204 Upstream conduction of this signal via gap junctions in endothelial cells has been proposed as the mechanism for the vasoconstriction of upstream arterioles via contraction of PASMC.205 Endothelial injury and/or decreased Cx40 expression may explain the impairment of HPV and failure of flow regulation in ARDS206 and illnesses such as COVID-19.207 In addition, the cystic fibrosis transmembrane conductance regulator (CFTR) has been shown to be necessary for an intact HPV response, and recent evidence demonstrates that some lung infections block HPV by inhibiting CFTR.208 The extent to which HPV is impaired in ARDS and is attenuated by various agents used in the treatment of critically ill patients remains unclear.196
Inhaled NO (iNO) and inhaled prostacyclin (PGI2) have been used to improve ventilation/perfusion matching in mechanically ventilated patients209,210; although these agents can improve oxygen saturation, they have not been shown to decrease mortality.210,211 Regional changes in blood flow with iNO have been observed in hypoxia and normoxia on imaging.212 Other agents that have been used to redistribute blood flow include almitrine, a selective pulmonary vasoconstrictor of nonventilated lung areas,213 and phenylephrine, an α-receptor agonist causing vasoconstriction.214,215 Further investigation is needed to determine optimal pharmacologic combinations to improve arterial oxygen saturation.
When supplemental oxygen is not sufficient to raise arterial oxygen saturation, patients are placed on positive pressure ventilation (PPV). Mechanical ventilation itself can cause lung injury216 and was shown to worsen ventilation-perfusion matching and oxygenation in an animal model of lung injury by decreasing blood flow to poorly ventilated areas during inspiration.217 Flow-controlled ventilation has been proposed to attenuate lung injury and improves homogeneity of aeration in dependent portions of the lung.218
In summary, local regulation of pulmonary blood flow is crucial for maintaining oxygen saturation in critically ill patients. In ARDS, the combination of impaired flow regulation and impaired diffusing capacity results in severe hypoxemia and requires judicious application of pharmacologic interventions and appropriate ventilator strategies to redistribute blood flow.
3.3.6 |. Tumors
As a tumor develops, angiogenesis is stimulated by its need for oxygen and nutrients, and enables tumors to grow to sizes larger than the diffusion distances for those substances.219 Angiogenic activators such as VEGF are instrumental in this process; the expression of VEGF and its receptor is induced by the effects of hypoxia on HIF-1α (hypoxia-inducible factor 1, α subunit).220 Tumor microvessel networks have an aberrant structure and exhibit increased vascular permeability, resulting in heterogeneous blood flow and oxygenation.4,221,222 The abnormal conditions in tumors cause hypoxia and affect the transport of anticancer drugs, often limiting their effectiveness.223 Strategies to mitigate these effects include limiting stromal development to minimize microvascular compression and improve flow, normalizing the vascular network by using VEGF inhibitors, and using supplemental oxygen or pharmacologic therapies to reduce oxygen consumption to minimize hypoxia.224
3.4 |. Implications for therapeutic interventions
Optimizing tissue oxygenation and perfusion at the microvascular level in addition to restoring systemic physiological parameters (‘hemodynamic coherence’225) is the objective of ‘microvascular resuscitation’.115,226–228 Several strategies have been proposed and employed to address the spatial and temporal heterogeneities in flow and oxygenation (‘microcirculatory shock’229) seen at the microvascular level.230–233 Methods for monitoring the microvascular response to these interventions234–236 are also discussed.
3.4.1 |. Vasoactive agents
Pharmacologic agents commonly used in critically ill patients include vasopressors (to increase systemic perfusion pressure),119,120,237 vasodilators (to increase microvascular flow and decrease shunting),119,238 and inotropes (to increase cardiac output).119,120 Clinically, these therapies are typically used following intravascular volume resuscitation, but can be used concurrently with fluid239–241 or in combination with each other.242,243 Despite multiple clinical trials involving pharmacologic agents, no definitive correlation has been found between achieving systemic hemodynamic endpoints and improved microcirculatory metabolism-perfusion matching.119,244
Vasoactive drugs differentially affect vessels of different caliber due to differing distributions of adrenergic receptors.120,245–247 Many vasopressors (and vasodilators) also have systemic effects.101,119,248 For example, norepinephrine has significant α-adrenergic properties and causes peripheral vasoconstriction, and also increased cardiac output due to its β-adrenergic effects.119,120 Phenylephrine (a selective α1-adrenergic agonist) causes peripheral vasoconstriction and an increase in mean arterial pressure (MAP), but may decrease cardiac output via increased afterload.120 Epinephrine increases MAP via α- and β-adrenergic effects on the peripheral circulation as well as cardiac output; β-effects predominate at lower doses.119,120 Vasopressin causes contraction of vascular smooth muscle and increases sensitivity to catecholamines.120
Although some of these drugs preserve or improve systemic perfusion pressure,249–251 their effect on microvascular perfusion remains unclear,119,248,252–254 especially in conjunction with fluid resuscitation. In fact, the use of vasodilators has also been proposed to increase overall microvascular perfusion and reduce stopped-flow capillaries and/or plasma channels.255,256 Another proposed unconventional therapy is inhaled nitric oxide to stimulate nitrite and S-nitrosothiol production, causing release of NO by erythrocytes and potentially improving flow regulation in sepsis.257 Potential exists for personalized therapy based on patient phenotype (adrenergic receptor subtypes, responses to pharmacologic agents). Similar techniques could be used in other conditions that involve inflammatory responses and heterogeneity of flow. Hemorrhagic shock and free flap perfusion are conditions in which elucidating the microcirculatory response to fluid resuscitation and vasoactive agents would be of interest.
3.4.2 |. Fluid therapy
Intravenous crystalloid administration has long been the mainstay of therapy in patients with sepsis or distributive shock, to restore intravascular volume, maintain stroke volume and cardiac output, and preserve blood pressure in the context of vasodilation and capillary leak. However, the optimal amount and type of fluid remain a subject of debate.258,259 Judicious fluid resuscitation is the initial component of protocol-based management (“early goal-directed therapy”)260 as formalized in the commonly utilized “Surviving Sepsis” guidelines,74,261 although these are controversial.262 Various systemic hemodynamic endpoints for fluid resuscitation have been proposed,263 including central venous pressure, mean arterial pressure, cardiac index, oxygen delivery, oxygen consumption, and mixed venous oxygen saturation. Clinical trials utilizing such endpoints have not reliably demonstrated improved outcomes, however264,265 implying that restoring systemic hemodynamics may be insufficient to restore oxygen delivery to tissue.266 One possible cause may be endothelial damage due to the effect of crystalloids on the integrity of the glycocalyx, a luminal barrier layer that plays a role in regulation of vascular permeability, immune function, cellular adhesion, and flow regulation.267–269 Additionally, alterations in plasma viscosity and erythrocyte deformability induced by administered crystalloids and colloids can alter the flow and distribution of blood and cause heterogeneity in oxygen transport.270–272 In summary, potential detrimental effects from fluid resuscitation include hemodilution (decreased hematocrit), damage to the glycocalyx resulting in increased permeability leading to tissue and pulmonary edema (and therefore increased diffusion distances),116 altered hemorheology, shunting/malperfusion (resulting in plasma channels and poor tissue oxygenation), and venous congestion.85,116,273,274
Risks of under-resuscitation include hypotension, hypoperfusion, and inadequate oxygen delivery leading to organ failure, but over-resuscitation, even if indicated by systemic hemodynamic parameters, may be detrimental.116,275 Studies of microvascular oxygenation using phosphorescence techniques in rat intestinal microcirculation have demonstrated the preservation of tissue oxygen levels with crystalloid resuscitation.276 Increased flow may improve perfusion and oxygen delivery at the microcirculatory level only up to a point, at which the effect of hemodilution would supervene and result in hypoxia due to redistribution of erythrocytes, the development of plasma channels,277 and decreased oxygen delivery.22,278 This is consistent with evidence of shunting seen in experimental models.279,280 Due to the lack of correlation between systemic hemodynamics and microcirculatory flow, the optimal fluid resuscitation endpoint remains unclear274 and individualized therapy may be indicated.266,281 Observations of microvascular flow have therefore been used to identify candidates for fluid resuscitation282 based on measured microvascular flow parameters.116
3.4.3 |. Blood transfusion
Blood transfusion improves sublingual microcirculatory density (as measured by video microscopy) and oxygenation (as measured by spectrophotometry).283,284 Administering whole blood or packed red blood cells prevents hemodilution and may increase vascular tone via the myogenic mechanism, while increasing oxygen delivery. Increased delivery has been shown to improve oxygen utilization in patients with poor baseline extraction285 but may be counteracted to some extent by the increase in flow resistance resulting from hemoconcentration.286,287 Furthermore, less tissue edema would likely result since more administered volume would be retained within the vasculature. Transfusion of blood with altered oxygen affinity may also serve to improve oxygen unloading in peripheral tissues.288–290
3.4.4 |. Anesthetic agents
Agents used for sedation and anesthesia comprise another class of commonly used drugs in critically ill patients and are known to affect the vasculature.291 However, the effects of anesthetic agents on the microcirculation are variable292 and poorly understood. Effects of inhalational and intravenous agents on erythrocyte deformability,293,294 integrity of the glycocalyx,295 vascular permeability,296 and vascular reactivity to vasoactive agents297,298 have all been variously reported.299 As an example, results of a recent study evaluating the effect of general anesthesia on attenuation of renal perfusion and oxygen delivery in an ovine model suggest that the use of IV anesthetics may be preferable to volatile agents if subjects are at risk of renal injury.300 Much work remains to be done to define the most appropriate sedative or anesthetic agents for a particular patient.
3.4.5 |. Monitoring
Conventional methods of monitoring the progress of septic patients include so-called “upstream” variables reflecting oxygen delivery (cardiac output, mean arterial pressure) and “downstream” variables reflecting oxygen utilization (mixed venous oxygen saturation,301 lactate levels, urine output). Because these measures may not reflect microcirculatory derangement, methods of clinically evaluating the microcirculation have been developed302–306 (eg, sublingual capnometry, sidestream dark-field video microscopy, orthogonal polar spectral imaging307–313). While imaging methods are not in widespread clinical use, algorithms to analyze such images314,315 can provide serial measurements of local flow velocities, the percentage of perfused vessels, and effective vessel density.316,317 Derived quantities used to monitor therapy include microvascular flow index (MFI),282,318 perfused vessel density (PVD),59 proportion of perfused vessels (PPV), and a heterogeneity index as well as functional measurements of local tissue oxygen tension as obtained by near-infrared spectroscopy (NIRS).318 These measurements are often site-specific117 but have been found to reflect conditions at other sites.319 The significance and relevant therapeutic endpoints for these measures are still under investigation.144,320–323
4 |. DISCUSSION
When considered at the whole-organ level, the occurrence of tissue hypoxia in a well-perfused tissue may seem paradoxical. However, this paradox is resolved when microvascular-scale behaviors are considered. The microcirculation is inherently heterogeneous in structure, and active vascular responses on short and long time scales are essential to overcome this heterogeneity and provide adequate oxygenation throughout tissues. Any disturbance of flow regulation can cause local hypoxia. In patients with sepsis or other critical illness, flow regulation may be disturbed due to effects of pathophysiological conditions on endothelial cell function. Conducted responses, which play a vital role in flow regulation, require not only the normal function of individual endothelial cells but also maintenance of their connectivity via intact gap junctions. This aspect of endothelial function is likely to be particularly vulnerable to disruption in abnormal states such as inflammation. Therapeutic measures targeted to systemic parameters may not address or may even worsen such dysfunction. The goal of therapy should therefore be restoration of endothelial cell function, including conducted responses. Further investigation of these aspects of microvascular function with theoretical, experimental, and clinical studies could lead to improved therapies for improving oxygen transport and preventing organ failure in critically ill patients.
5 |. PERSPECTIVES
Critically ill patients depend on adequate oxygen availability at the microvascular level to prevent tissue and organ damage, and regions of hypoxia or anoxia can occur even in a tissue that receives an adequate overall convective oxygen supply. Intact local regulation of blood flow is required to counteract the structural and functional heterogeneity of microcirculatory networks and prevent hypoxia. Given that endothelial injury in critical illness can impair the upstream conducted responses that mediate arteriolar vasodilation and that correcting systemic parameters alone may not improve microvascular oxygen delivery, restoring endothelial function and flow regulation may be the key to preventing organ failure in these “diseases of the microcirculation.”
ACKNOWLEDGMENTS
This study was supported by the Grant NIH U01 HL133362.
Funding information
National Institutes of Health
REFERENCES
- 1.Ince C The central role of renal microcirculatory dysfunction in the pathogenesis of acute kidney injury. Nephron Clin Pract. 2014;127:124–128. [DOI] [PubMed] [Google Scholar]
- 2.Ring A, Stremmel W. The hepatic microvascular responses to sepsis. Semin Thromb Hemost. 2000;26:589–594. [DOI] [PubMed] [Google Scholar]
- 3.Walley KR. Heterogeneity of oxygen delivery impairs oxygen extraction by peripheral tissues: Theory. J Appl Physiol. 1996;81:885–894. [DOI] [PubMed] [Google Scholar]
- 4.Secomb TW, Hsu R, Dewhirst MW, Klitzman B, Gross JF. Analysis of oxygen transport to tumor tissue by microvascular networks. Int J Radiat Oncol Biol Phys. 1993;25:481–489. [DOI] [PubMed] [Google Scholar]
- 5.Secomb TW, Hsu R, Beamer NB, Coull BM. Theoretical simulation of oxygen transport to brain by networks of microvessels: Effects of oxygen supply and demand on tissue hypoxia. Microcirculation. 2000;7:237–247. [PubMed] [Google Scholar]
- 6.Pries AR, Secomb TW. Making microvascular networks work: Angiogenesis, remodeling, and pruning. Physiology (Bethesda). 2014;29:446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Secomb TW, Alberding JP, Hsu R, Dewhirst MW, Pries AR. Angiogenesis: An adaptive dynamic biological patterning problem. PLoS Comput Biol. 2013;9:e1002983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pries AR, Secomb TW, Gaehtgens P. Structure and hemodynamics of microvascular networks: heterogeneity and correlations. Am J Physiol. 1995;269:H1713–1722. [DOI] [PubMed] [Google Scholar]
- 9.Sarelius IH. An analysis of microcirculatory flow heterogeneity using measurements of transit time. Microvasc Res. 1990;40:88–98. [DOI] [PubMed] [Google Scholar]
- 10.Zuurbier CJ, van Iterson M, Ince C. Functional heterogeneity of oxygen supply-consumption ratio in the heart. Cardiovasc Res. 1999;44:488–497. [DOI] [PubMed] [Google Scholar]
- 11.Pries A, Secomb T. Blood Flow in Microvascular Networks. In: Tuma R, Duran W, Ley K, eds. Handbook of Physiology: Microcirculation, 2nd edn. Amsterdam: Elsevier; 2008:3–36. [Google Scholar]
- 12.Frisbee JC, Wu F, Goodwill AG, Butcher JT, Beard DA. Spatial heterogeneity in skeletal muscle microvascular blood flow distribution is increased in the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol. 2011;301:R975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pries AR, Secomb TW. Origins of heterogeneity in tissue perfusion and metabolism. Cardiovasc Res. 2009;81:328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pries AR, Secomb TW, Gaehtgens P. Relationship between structural and hemodynamic heterogeneity in microvascular networks. Am J Physiol. 1996;270:H545–553. [DOI] [PubMed] [Google Scholar]
- 15.Duling BR, Damon DH. An examination of the measurement of flow heterogeneity in striated muscle. Circ Res. 1987;60:1–13. [DOI] [PubMed] [Google Scholar]
- 16.Klitzman B, Johnson PC. Capillary network geometry and red cell distribution in hamster cremaster muscle. Am J Physiol. 1982;242:H211–H219. [DOI] [PubMed] [Google Scholar]
- 17.Duling BR. Is red cell flow heterogeneity a critical variable in the regulation and limitation of oxygen transport to tissue? Adv Exp Med Biol. 1994;361:237–247. [DOI] [PubMed] [Google Scholar]
- 18.Ellsworth ML, Popel AS, Pittman RN. Assessment and impact of heterogeneities of convective oxygen transport parameters in capillaries of striated muscle: experimental and theoretical. Microvasc Res. 1988;35:341–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Secomb TW. Red blood cell mechanics and capillary blood rheology. Cell Biophys. 1991;18:231–251. [DOI] [PubMed] [Google Scholar]
- 20.Pries AR, Secomb TW, Gaehtgens P, Gross JF. Blood flow in microvascular networks. Experiments and simulation. Circ Res. 1990;67:826–834. [DOI] [PubMed] [Google Scholar]
- 21.Pries AR, Ley K, Gaehtgens P. Generalization of the Fahraeus principle for microvessel networks. Am J Physiol. 1986;251:H1324–1332. [DOI] [PubMed] [Google Scholar]
- 22.Pries AR, Fritzsche A, Ley K, Gaehtgens P. Redistribution of red blood cell flow in microcirculatory networks by hemodilution. Circ Res. 1992;70:1113–1121. [DOI] [PubMed] [Google Scholar]
- 23.Morisaki H, Sibbald W, Martin C, Doig G, Inman K. Hyperdynamic sepsis depresses circulatory compensation to normovolemic anemia in conscious rats. J Appl Physiol. 1996;80:656–664. [DOI] [PubMed] [Google Scholar]
- 24.Torres Filho IP, Barraza D, Hildreth K, Williams C, Dubick MA. Cremaster muscle perfusion, oxygenation, and heterogeneity revealed by a new automated acquisition system in a rodent model of prolonged hemorrhagic shock. J Appl Physiol. 1985;127(1548–1561):2019. [DOI] [PubMed] [Google Scholar]
- 25.Vajda K, Szabó A, Boros M. Heterogeneous microcirculation in the rat small intestine during hemorrhagic shock: quantification of the effects of hypertonic-hyperoncotic resuscitation. Eur Surg Res. 2004;36:338–344. [DOI] [PubMed] [Google Scholar]
- 26.Aalkjaer C, Boedtkjer D, Matchkov V. Vasomotion - what is currently thought? Acta Physiol (Oxf). 2011;202:253–269. [DOI] [PubMed] [Google Scholar]
- 27.Arciero JC, Secomb TW. Spontaneous oscillations in a model for active control of microvessel diameters. Math Med Biol. 2012;29:163–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirai DM, Craig JC, Colburn TD, et al. Skeletal muscle microvascular and interstitial PO2 from rest to contractions. J Physiol. 2018;596:869–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirai DM, Colburn TD, Craig JC, et al. Skeletal muscle interstitial O(2) pressures: bridging the gap between the capillary and myocyte. Microcirculation. 2019;26:e12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jespersen SN, Ostergaard L. The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab. 2012;32:264–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucker A, Secomb TW, Weber B, Jenny P. The relation between capillary transit times and hemoglobin saturation heterogeneity. Part 1: theoretical models. Front Physiol. 2018;9:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucker A, Secomb TW, Barrett MJP, Weber B, Jenny P. The relation between capillary transit times and hemoglobin saturation heterogeneity. Part 2: Capillary Networks. Front Physiol. 2018;9:1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellsworth ML, Ellis CG, Popel AS, Pittman RN. Role of microvessels in oxygen supply to tissue. News Physiol Sci. 1994;9:119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Secomb TW, Hsu R. Simulation of O2 transport in skeletal muscle: diffusive exchange between arterioles and capillaries. Am J Physiol. 1994;267:H1214–1221. [DOI] [PubMed] [Google Scholar]
- 35.Lo A, Fuglevand AJ, Secomb TW. Oxygen delivery to skeletal muscle fibers: Effects of microvascular unit structure and control mechanisms. Am J Physiol Heart Circ Physiol. 2003;285:H955–963. [DOI] [PubMed] [Google Scholar]
- 36.Henneman E, Olson CB. Relations between structure and function in the design of skeletal muscles. J Neurophysiol. 1965;28:581–598. [DOI] [PubMed] [Google Scholar]
- 37.Liu G, Mac Gabhann F, Popel AS. Effects of fiber type and size on the heterogeneity of oxygen distribution in exercising skeletal muscle. PLoS One. 2012;7:e44375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heinonen I, Koga S, Kalliokoski KK, Musch TI, Poole DC. Heterogeneity of muscle blood flow and metabolism: Influence of exercise, aging, and disease states. Exerc Sport Sci Rev. 2015;43:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koga S, Poole DC, Fukuoka Y, et al. Methodological validation of the dynamic heterogeneity of muscle deoxygenation within the quadriceps during cycle exercise. Am J Physiol Regul Integr Comp Physiol. 2011;301:R534–541. [DOI] [PubMed] [Google Scholar]
- 40.Iadecola C The pathobiology of vascular dementia. Neuron. 2013;80:844–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis MJ, Hill MA, Kuo L. Local regulation of microvascular perfusion. In: Terjung R, ed. Comprehensive Physiology. 2011. 10.1002/cphy.cp020406 [DOI] [Google Scholar]
- 42.Arciero JC, Carlson BE, Secomb TW. Theoretical model of metabolic blood flow regulation: Roles of ATP release by red blood cells and conducted responses. Am J Physiol Heart Circ Physiol. 2008;295:H1562–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schubert R, Mulvany MJ. The myogenic response: Established facts and attractive hypotheses. Clin Sci (Lond). 1999;96:313–326. [PubMed] [Google Scholar]
- 44.Carlson BE, Secomb TW. A theoretical model for the myogenic response based on the length-tension characteristics of vascular smooth muscle. Microcirculation. 2005;12:327–338. [DOI] [PubMed] [Google Scholar]
- 45.Calbet JA, Joyner MJ. Disparity in regional and systemic circulatory capacities: Do they affect the regulation of the circulation? Acta Physiol (Oxf). 2010;199:393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joyner MJ, Thomas GD. Having it both ways? Vasoconstriction in contracting muscles. J Physiol. 2003;550:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roy TK, Secomb TW. Functional sympatholysis and sympathetic escape in a theoretical model for blood flow regulation. Front Physiol. 2014;5:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leithner C, Royl G. The oxygen paradox of neurovascular coupling. J Cereb Blood Flow Metab. 2014;34:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ellsworth ML. The red blood cell as an oxygen sensor: What is the evidence? Acta Physiol Scand. 2000;168:551–559. [DOI] [PubMed] [Google Scholar]
- 50.Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: Oxygen sensors and modulators of vascular tone. Physiology (Bethesda). 2009;24:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ellsworth ML, Ellis CG, Sprague RS. Role of erythrocyte-released ATP in the regulation of microvascular oxygen supply in skeletal muscle. Acta Physiol (Oxf). 2016;216:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dufour SP, Patel RP, Brandon A, et al. Erythrocyte-dependent regulation of human skeletal muscle blood flow: Role of varied oxyhemoglobin and exercise on nitrite, S-nitrosohemoglobin, and ATP. Am J Physiol Heart Circ Physiol. 2010;299:H1936–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berne RM. Cardiac nucleotides in hypoxia: Possible role in regulation of coronary blood flow. Am J Physiol. 1963;204:317–322. [DOI] [PubMed] [Google Scholar]
- 54.Taggart MJ, Wray S. Hypoxia and smooth muscle function: Key regulatory events during metabolic stress. J Physiol. 1998;509:315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buerk DG, Hirai DM, Roseguini BT, et al. Commentaries on viewpoint: A paradigm shift for local blood flow regulation. J Appl Physiol. 1985;116(706–707):2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roy TK, Pries AR, Secomb TW. Theoretical comparison of wall-derived and erythrocyte-derived mechanisms for metabolic flow regulation in heterogeneous microvascular networks. Am J Physiol Heart Circ Physiol. 2012;302:H1945–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ngo AT, Jensen LJ, Riemann M, Holstein-Rathlou NH, Torp-Pedersen C. Oxygen sensing and conducted vasomotor responses in mouse cremaster arterioles in situ. Pflugers Arch. 2010;460:41–53. [DOI] [PubMed] [Google Scholar]
- 58.Reglin B, Secomb TW, Pries AR. Structural adaptation of microvessel diameters in response to metabolic stimuli: where are the oxygen sensors? Am J Physiol Heart Circ Physiol. 2009;297:H2206–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fry BC, Roy TK, Secomb TW. Capillary recruitment in a theoretical model for blood flow regulation in heterogeneous microvessel networks. Physiol Rep. 2013;1:e00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Segal SS. Integration of blood flow control to skeletal muscle: Key role of feed arteries. Acta Physiol Scand. 2000;168:511–518. [DOI] [PubMed] [Google Scholar]
- 61.Diep HK, Vigmond EJ, Segal SS, Welsh DG. Defining electrical communication in skeletal muscle resistance arteries: A computational approach. J Physiol. 2005;568:267–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bearden SE, Payne GW, Chisty A, Segal SS. Arteriolar network architecture and vasomotor function with ageing in mouse gluteus maximus muscle. J Physiol. 2004;561:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gustafsson F, Holstein-Rathlou N. Conducted vasomotor responses in arterioles: characteristics, mechanisms and physiological significance. Acta Physiol Scand. 1999;167:11–21. [DOI] [PubMed] [Google Scholar]
- 64.McKinnon RL, Bolon ML, Wang HX, et al. Reduction of electrical coupling between microvascular endothelial cells by NO depends on connexin 37. Am J Physiol Heart Circ Physiol. 2009;297:H93–H101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Secomb TW, Pries AR. Information transfer in microvascular networks. Microcirculation. 2002;9:377–387. [DOI] [PubMed] [Google Scholar]
- 66.Bagher P, Segal SS. Regulation of blood flow in the microcirculation: Role of conducted vasodilation. Acta Physiol (Oxf). 2011;202:271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pries AR, Hopfner M, le Noble F, Dewhirst MW, Secomb TW. The shunt problem: Control of functional shunting in normal and tumour vasculature. Nat Rev Cancer. 2010;10:587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Houben A, Martens RJH, Stehouwer CDA. Assessing microvascular function in humans from a chronic disease perspective. JASN. 2017;28:3461–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reynolds T, Vivian-Smith A, Jhanji S, Pearse RM. Observational study of the effects of age, diabetes mellitus, cirrhosis and chronic kidney disease on sublingual microvascular flow. Perioperative medicine (London. 2013;England) 2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ince C, Mayeux PR, Nguyen T, et al. The endothelium in sepsis. Shock (Augusta, Ga). 2016;45:259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leligdowicz A, Richard-Greenblatt M, Wright J, Crowley VM, Kain KC. Endothelial activation: The Ang/Tie axis in sepsis. Front Immunol. 2018;9:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cross D, Drury R, Hill J, Pollard AJ. Epigenetics in sepsis: Understanding its role in endothelial dysfunction, immunosuppression, and potential therapeutics. Front Immunol. 2019;10:1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bearden SE. Effect of aging on the structure and function of skeletal muscle microvascular networks. Microcirculation. 2006;13:279–288. [DOI] [PubMed] [Google Scholar]
- 74.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. [DOI] [PubMed] [Google Scholar]
- 75.Ellis CG, Bateman RM, Sharpe MD, Sibbald WJ, Gill R. Effect of a maldistribution of microvascular blood flow on capillary O(2) extraction in sepsis. Am J Physiol Heart Circ Physiol. 2002;282:H156–164. [DOI] [PubMed] [Google Scholar]
- 76.Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: For the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bateman RM, Sharpe MD, Ellis CG. Bench-to-bedside review: Microvascular dysfunction in sepsis–hemodynamics, oxygen transport, and nitric oxide. Crit Care. 2003;7:359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. [DOI] [PubMed] [Google Scholar]
- 79.Parker MM, Shelhamer JH, Bacharach SL, et al. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100:483–490. [DOI] [PubMed] [Google Scholar]
- 80.Kim MH, Choi JH. An update on sepsis biomarkers. Infect Chemother. 2020;52:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ince C The microcirculation is the motor of sepsis. Crit Care. 2005;9(Suppl 4):S13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ince C, Mik EG. Microcirculatory and mitochondrial hypoxia in sepsis, shock, and resuscitation. J Appl Physiol. 1985;120(226–235):2016. [DOI] [PubMed] [Google Scholar]
- 83.Pool R, Gomez H, Kellum JA. Mechanisms of organ dysfunction in sepsis. Crit Care Clin. 2018;34:63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hernandez G, Bruhn A, Ince C. Microcirculation in sepsis: New perspectives. Curr Vasc Pharmacol. 2013;11:161–169. [PubMed] [Google Scholar]
- 85.Marik PE. Iatrogenic salt water drowning and the hazards of a high central venous pressure. Annals of intensive care. 2014;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Doerschug KC, Delsing AS, Schmidt GA, Haynes WG. Impairments in microvascular reactivity are related to organ failure in human sepsis. Am J Physiol Heart Circ Physiol. 2007;293:H1065–1071. [DOI] [PubMed] [Google Scholar]
- 87.De Backer D, Ortiz JA, Salgado D. Coupling microcirculation to systemic hemodynamics. Curr Opin Crit Care. 2010;16:250–254. [DOI] [PubMed] [Google Scholar]
- 88.Sam AD 2nd, Sharma AC, Rice AN, Ferguson JL, Law WR. Adenosine and nitric oxide regulate regional vascular resistance via interdependent and independent mechanisms during sepsis. Crit Care Med. 2000;28:1931–1939. [DOI] [PubMed] [Google Scholar]
- 89.Annane D, Trabold F, Sharshar T, et al. Inappropriate sympathetic activation at onset of septic shock: a spectral analysis approach. Am J Respir Crit Care Med. 1999;160:458–465. [DOI] [PubMed] [Google Scholar]
- 90.Hinshaw LB. Sepsis/septic shock: participation of the microcirculation: an abbreviated review. Crit Care Med. 1996;24:1072–1078. [DOI] [PubMed] [Google Scholar]
- 91.Ince C, Sinaasappel M. Microcirculatory oxygenation and shunting in sepsis and shock. Crit Care Med. 1999;27:1369–1377. [DOI] [PubMed] [Google Scholar]
- 92.van Iterson M, Sinaasappel M, Burhop K, Trouwborst A, Ince C. Low-volume resuscitation with a hemoglobin-based oxygen carrier after hemorrhage improves gut microvascular oxygenation in swine. The Journal of laboratory and clinical medicine. 1998;132:421–431. [DOI] [PubMed] [Google Scholar]
- 93.Cronenwett JL, Shellito JL, Luce JL, Stanley JC, Lindenauer SM. Interaction of hindlimb blood flow, A-V shunting and A-V oxygen difference. The Journal of Surgical Research. 1984;36:223–229. [DOI] [PubMed] [Google Scholar]
- 94.Gardiner BS, Thompson SL, Ngo JP, et al. Diffusive oxygen shunting between vessels in the preglomerular renal vasculature: anatomic observations and computational modeling. Am J Physiol Renal Physiol. 2012;303:F605–618. [DOI] [PubMed] [Google Scholar]
- 95.Stickland MK, Welsh RC, Haykowsky MJ, et al. Intra-pulmonary shunt and pulmonary gas exchange during exercise in humans. J Physiol. 2004;561:321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sharan M, Popel AS. A mathematical model of countercurrent exchange of oxygen between paired arterioles and venules. Math Biosci. 1988;91:17–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hsu R, Secomb TW. Analysis of oxygen exchange between arterioles and surrounding capillary-perfused tissue. J Biomech Eng. 1992;114:227–231. [DOI] [PubMed] [Google Scholar]
- 98.Gutierrez G The rate of oxygen release and its effect on capillary O2 tension: a mathematical analysis. Respir Physiol. 1986;63:79–96. [DOI] [PubMed] [Google Scholar]
- 99.Sharan M, Selvakumar S. A note on Gutierrez’s kinetics model for oxygen delivery to tissue. Bio Systems. 1992;26:171–176. [DOI] [PubMed] [Google Scholar]
- 100.Archie JP Jr. Anatomic arterial-venous shunting in endotoxic and septic shock in dogs. Ann Surg. 1977;186:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Buwalda M, Ince C. Opening the microcirculation: Can vasodilators be useful in sepsis? Intensive Care Med. 2002;28:1208–1217. [DOI] [PubMed] [Google Scholar]
- 102.Eggleton CD, Roy TK, Popel AS. Predictions of capillary oxygen transport in the presence of fluorocarbon additives. Am J Physiol. 1998;275:H2250–2257. [DOI] [PubMed] [Google Scholar]
- 103.van Iterson M, Siegemund M, Burhop K, Ince C. Hemoglobin-based oxygen carrier provides heterogeneous microvascular oxygenation in heart and gut after hemorrhage in pigs. J. Trauma 2003;55:1111–1124. [DOI] [PubMed] [Google Scholar]
- 104.Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet (London, England). 2002;360:219–223. [DOI] [PubMed] [Google Scholar]
- 105.Fink MP, Cytopathic hypoxia. Is oxygen use impaired in sepsis as a result of an acquired intrinsic derangement in cellular respiration? Crit Care Clin. 2002;18:165–175. [DOI] [PubMed] [Google Scholar]
- 106.Refsum HE, Opdahl H, Leraand S. Effect of extreme metabolic acidosis on oxygen delivery capacity of the blood–an in vitro investigation of changes in the oxyhemoglobin dissociation curve in blood with pH values of approximately 6.30. Crit Care Med. 1997;25:1497–1501. [DOI] [PubMed] [Google Scholar]
- 107.Edul VK, Ferrara G, Dubin A. Microcirculatory dysfunction in sepsis. Endocr Metab Immune Disord Drug Targets. 2010;10:235–246. [DOI] [PubMed] [Google Scholar]
- 108.Chang JC. Sepsis and septic shock: Endothelial molecular pathogenesis associated with vascular microthrombotic disease. Thrombosis J. 2019;17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ramchandra R, Wan L, Hood SG, Frithiof R, Bellomo R, May CN. Septic shock induces distinct changes in sympathetic nerve activity to the heart and kidney in conscious sheep. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1247–1253. [DOI] [PubMed] [Google Scholar]
- 110.Bateman RM, Sharpe MD, Goldman D, Lidington D, Ellis CG. Inhibiting nitric oxide overproduction during hypotensive sepsis increases local oxygen consumption in rat skeletal muscle. Crit Care Med. 2008;36:225–231. [DOI] [PubMed] [Google Scholar]
- 111.De Blasi RA, Palmisani S, Alampi D, et al. Microvascular dysfunction and skeletal muscle oxygenation assessed by phase-modulation near-infrared spectroscopy in patients with septic shock. Intensive Care Med. 2005;31:1661–1668. [DOI] [PubMed] [Google Scholar]
- 112.Lehr HA, Bittinger F, Kirkpatrick CJ. Microcirculatory dysfunction in sepsis: a pathogenetic basis for therapy? J Pathol. 2000;190:373–386. [DOI] [PubMed] [Google Scholar]
- 113.Spronk PE, Zandstra DF, Ince C. Bench-to-bedside review: sepsis is a disease of the microcirculation. Crit Care. 2004;8:462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Verdant C, De Backer D. How monitoring of the microcirculation may help us at the bedside. Curr Opin Crit Care. 2005;11:240–244. [DOI] [PubMed] [Google Scholar]
- 115.Bateman RM, Walley KR. Microvascular resuscitation as a therapeutic goal in severe sepsis. Crit Care. 2005;9(Suppl 4):S27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ince C The rationale for microcirculatory guided fluid therapy. Curr Opin Crit Care. 2014;20:301–308. [DOI] [PubMed] [Google Scholar]
- 117.Ospina-Tascon G, Neves AP, Occhipinti G, et al. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med. 2010;36:949–955. [DOI] [PubMed] [Google Scholar]
- 118.Boldt J, Ince C. The impact of fluid therapy on microcirculation and tissue oxygenation in hypovolemic patients: A review. Intensive Care Med. 2010;36:1299–1308. [DOI] [PubMed] [Google Scholar]
- 119.Boerma EC, Ince C. The role of vasoactive agents in the resuscitation of microvascular perfusion and tissue oxygenation in critically ill patients. Intensive Care Med. 2010;36:2004–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hollenberg SM. Inotrope and vasopressor therapy of septic shock. Crit Care Clin 2009;25:781–802, ix. [DOI] [PubMed] [Google Scholar]
- 121.Marik PE. Early management of severe sepsis: Concepts and controversies. Chest. 2014;145:1407–1418. [DOI] [PubMed] [Google Scholar]
- 122.Goldman D, Bateman RM, Ellis CG. Effect of sepsis on skeletal muscle oxygen consumption and tissue oxygenation: Interpreting capillary oxygen transport data using a mathematical model. Am J Physiol Heart Circ Physiol. 2004;287:H2535–2544. [DOI] [PubMed] [Google Scholar]
- 123.Goldman D, Bateman RM, Ellis CG. Effect of decreased O2 supply on skeletal muscle oxygenation and O2 consumption during sepsis: role of heterogeneous capillary spacing and blood flow. Am J Physiol Heart Circ Physiol. 2006;290:H2277–2285. [DOI] [PubMed] [Google Scholar]
- 124.Brooks SD, DeVallance E, d’Audiffret AC, et al. Metabolic syndrome impairs reactivity and wall mechanics of cerebral resistance arteries in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2015;309:H1846–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Goodwill AG, Frisbee JC. Oxidant stress and skeletal muscle microvasculopathy in the metabolic syndrome. Vascul Pharmacol. 2012;57:150–159. [DOI] [PubMed] [Google Scholar]
- 126.Goodwill AG, James ME, Frisbee JC. Increased vascular thromboxane generation impairs dilation of skeletal muscle arterioles of obese Zucker rats with reduced oxygen tension. Am J Physiol Heart Circ Physiol. 2008;295:H1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Frisbee JC, Butcher JT, Frisbee SJ, et al. Increased peripheral vascular disease risk progressively constrains perfusion adaptability in the skeletal muscle microcirculation. Am J Physiol Heart Circ Physiol. 2016;310:H488–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Frisbee JC, Goodwill AG, Butcher JT, Olfert IM. Divergence between arterial perfusion and fatigue resistance in skeletal muscle in the metabolic syndrome. Exp Physiol. 2011;96:369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Frisbee JC, Lewis MT, Wiseman RW. Skeletal muscle performance in metabolic disease: Microvascular or mitochondrial limitation or both? Microcirculation. 2019;26:e12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mason McClatchey P, Wu F, Olfert IM, et al. Impaired tissue oxygenation in metabolic syndrome requires increased microvascular perfusion heterogeneity. Cardiovasc Transl Res. 2017;10:69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Frisbee JC, Lewis MT, Kasper JD, Chantler PD, Wiseman RW. Type 2 diabetes mellitus in the Goto-Kakizaki rat impairs microvascular function and contributes to premature skeletal muscle fatigue. J Appl Physiol. 1985;126(626–637):2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ostergaard L, Finnerup NB, Terkelsen AJ, et al. The effects of capillary dysfunction on oxygen and glucose extraction in diabetic neuropathy. Diabetologia. 2015;58:666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shi Y, Vanhoutte PM. Macro- and microvascular endothelial dysfunction in diabetes. Journal of diabetes. 2017;9:434–449. [DOI] [PubMed] [Google Scholar]
- 134.Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: Clinical insights and vascular mechanisms. Can J Cardiol. 2018;34:575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tooke JE. Microvascular function in human diabetes. A physiological perspective. Diabetes. 1995;44:721–726. [DOI] [PubMed] [Google Scholar]
- 136.Keske MA, Dwyer RM, Russell RD, et al. Regulation of microvascular flow and metabolism: An overview. Clin Exp Pharmacol Physiol. 2017;44:143–149. [DOI] [PubMed] [Google Scholar]
- 137.Clark MG. Impaired microvascular perfusion: a consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am J Physiol Endocrinol Metab. 2008;295:E732–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nourbakhsh N, Singh P. Role of renal oxygenation and mitochondrial function in the pathophysiology of acute kidney injury. Nephron Clin Pract. 2014;127:149–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Verma SK, Molitoris BA. Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin Nephrol. 2015;35:96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Arulkumaran N, Pollen S, Greco E, et al. Renal tubular cell mitochondrial dysfunction occurs despite preserved renal oxygen delivery in experimental septic acute kidney injury. Crit Care Med. 2018;46:e318–e325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bellomo R, Kellum JA, Ronco C, et al. Acute kidney injury in sepsis. Intensive Care Med. 2017;43:816–828. [DOI] [PubMed] [Google Scholar]
- 142.Aslan A, van den Heuvel MC, Stegeman CA, et al. Kidney histopathology in lethal human sepsis. Crit Care. 2018;22:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maiden MJ, Otto S, Brealey JK, et al. Structure and function of the kidney in septic shock. A prospective controlled experimental study. Am J Respir Crit Care Med. 2016;194:692–700. [DOI] [PubMed] [Google Scholar]
- 144.Legrand M, Mik EG, Balestra GM, et al. Fluid resuscitation does not improve renal oxygenation during hemorrhagic shock in rats. Anesthesiology. 2010;112:119–127. [DOI] [PubMed] [Google Scholar]
- 145.Abdulmahdi W, Patel D, Rabadi MM, et al. HMGB1 redox during sepsis. Redox Biol. 2017;13:600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yu WK, McNeil JB, Wickersham NE, Shaver CM, Bastarache JA, Ware LB. Vascular endothelial cadherin shedding is more severe in sepsis patients with severe acute kidney injury. Crit Care. 2019;23:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Abdelkader A, Ho J, Ow CP, et al. Renal oxygenation in acute renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2014;306:F1026–1038. [DOI] [PubMed] [Google Scholar]
- 148.Leong CL, Anderson WP, O’Connor PM, Evans RG. Evidence that renal arterial-venous oxygen shunting contributes to dynamic regulation of renal oxygenation. Am J Physiol Renal Physiol. 2007;292:F1726–1733. [DOI] [PubMed] [Google Scholar]
- 149.O’Connor PM, Anderson WP, Kett MM, Evans RG. Renal preglomerular arterial-venous O2 shunting is a structural anti-oxidant defence mechanism of the renal cortex. Clin Exp Pharmacol Physiol. 2006;33:637–641. [DOI] [PubMed] [Google Scholar]
- 150.O’Connor PM, Evans RG. Structural antioxidant defense mechanisms in the mammalian and nonmammalian kidney: different solutions to the same problem? Am J Physiol Regul Integr Comp Physiol. 2010;299:R723–727. [DOI] [PubMed] [Google Scholar]
- 151.Ngo JP, Kar S, Kett MM, et al. Vascular geometry and oxygen diffusion in the vicinity of artery-vein pairs in the kidney. Am J Physiol Renal Physiol. 2014;307:F1111–1122. [DOI] [PubMed] [Google Scholar]
- 152.Ngo JP, Ow CP, Gardiner BS, et al. Diffusive shunting of gases and other molecules in the renal vasculature: physiological and evolutionary significance. Am J Physiol Regul Integr Comp Physiol. 2016;311:R797–r810. [DOI] [PubMed] [Google Scholar]
- 153.Lee CJ, Ngo JP, Kar S, Gardiner BS, Evans RG, Smith DW. A pseudo-three-dimensional model for quantification of oxygen diffusion from preglomerular arteries to renal tissue and renal venous blood. Am J Physiol Renal Physiol. 2017;313:F237–f253. [DOI] [PubMed] [Google Scholar]
- 154.Gardiner BS, Smith DW, O’Connor PM, Evans RG. A mathematical model of diffusional shunting of oxygen from arteries to veins in the kidney. Am J Physiol Renal Physiol. 2011;300:F1339–1352. [DOI] [PubMed] [Google Scholar]
- 155.Olgac U, Kurtcuoglu V. Renal oxygenation: preglomerular vasculature is an unlikely contributor to renal oxygen shunting. Am J Physiol Renal Physiol. 2015;308:F671–688. [DOI] [PubMed] [Google Scholar]
- 156.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Investig. 2009;119:1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Charkoudian N, Joyner MJ, Sokolnicki LA, et al. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J Physiol. 2006;572:821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Delp MD, Laughlin MH. Regulation of skeletal muscle perfusion during exercise. Acta Physiol Scand. 1998;162:411–419. [DOI] [PubMed] [Google Scholar]
- 159.Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand. 1998;162:421–436. [DOI] [PubMed] [Google Scholar]
- 160.Sarelius I, Pohl U. Control of muscle blood flow during exercise: local factors and integrative mechanisms. Acta Physiol (Oxf). 2010;199:349–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Thomas GD, Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol. 2004;97:731–738. [DOI] [PubMed] [Google Scholar]
- 162.Anderson KM, Faber JE. Differential sensitivity of arteriolar alpha 1- and alpha 2-adrenoceptor constriction to metabolic inhibition during rat skeletal muscle contraction. Circ Res. 1991;69:174–184. [DOI] [PubMed] [Google Scholar]
- 163.VanTeeffelen JW, Segal SS. Interaction between sympathetic nerve activation and muscle fibre contraction in resistance vessels of hamster retractor muscle. J Physiol. 2003;550:563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Barden J, Lawrenson L, Poole JG, et al. Limitations to vasodilatory capacity and VO2max in trained human skeletal muscle. Am J Physiol Heart Circ Physiol. 2007;292:H2491–2497. [DOI] [PubMed] [Google Scholar]
- 165.Lundby C, Boushel R, Robach P, Moller K, Saltin B, Calbet JA. During hypoxic exercise some vasoconstriction is needed to match O2 delivery with O2 demand at the microcirculatory level. J Physiol. 2008;586:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Calbet JA, Lundby C, Sander M, Robach P, Saltin B, Boushel R. Effects of ATP-induced leg vasodilation on VO2 peak and leg O2 extraction during maximal exercise in humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R447–453. [DOI] [PubMed] [Google Scholar]
- 167.Moore AW, Jackson WF, Segal SS. Regional heterogeneity of alpha-adrenoreceptor subtypes in arteriolar networks of mouse skeletal muscle. J Physiol. 2010;588:4261–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Thomas GD. Ready, set, flow! But how does the flow know where to go? J Physiol. 2010;588:3633–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Branyan KW, Devallance ER, Lemaster KA, et al. Role of chronic stress and exercise on microvascular function in metabolic syndrome. Med Sci Sports Exerc. 2018;50:957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Angleys H, Ostergaard L, Jespersen SN. The effects of capillary transit time heterogeneity (CTH) on brain oxygenation. J Cereb Blood Flow Metab. 2015;35:806–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 1985;100(328–335):2006. [DOI] [PubMed] [Google Scholar]
- 172.Steinback CD, Poulin MJ. Influence of hypoxia on cerebral blood flow regulation in humans. Adv Exp Med Biol. 2016;903:131–144. [DOI] [PubMed] [Google Scholar]
- 173.Iadecola C Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. [DOI] [PubMed] [Google Scholar]
- 174.Iadecola C Vascular and metabolic factors in Alzheimer’s disease and related dementias: Introduction. Cell Mol Neurobiol. 2016;36:151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Iadecola C, Gottesman RF. Cerebrovascular alterations in alzheimer disease. Circ Res. 2018;123:406–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18:419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jackman K, Iadecola C. Neurovascular regulation in the ischemic brain. Antioxid Redox Signal. 2015;22:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Di Marco LY, Farkas E, Martin C, Venneri A, Frangi AF. Is vasomotion in cerebral arteries impaired in Alzheimer’s disease? JAD. 2015;46:35–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bassingthwaighte JB, King RB, Roger SA. Fractal nature of regional myocardial blood flow heterogeneity. Circ Res. 1989;65:578–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ostergaard L, Kristiansen SB, Angleys H, et al. The role of capillary transit time heterogeneity in myocardial oxygenation and ischemic heart disease. Basic Res Cardiol. 2014;109:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yipintsoi T, Kroll K, Bassingthwaighte JB. Fractal regional myocardial blood flows pattern according to metabolism, not vascular anatomy. Am J Physiol Heart Circ Physiol. 2016;310:H351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cannon RO III, Epstein SE. “Microvascular angina” as a cause of chest pain with angiographically normal coronary arteries. Am J Cardiol. 1988;61:1338–1343. [DOI] [PubMed] [Google Scholar]
- 183.Lanza GA, Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation. 2010;121:2317–2325. [DOI] [PubMed] [Google Scholar]
- 184.Cannon RO 3rd. Microvascular angina and the continuing dilemma of chest pain with normal coronary angiograms. J Am Coll Cardiol. 2009;54:877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cannon RO 3rd. MY APPROACH to chest pain with normal coronary arteries. Trends Cardiovasc Med. 2016;26:391–392. [DOI] [PubMed] [Google Scholar]
- 186.Fearon WF, De Bruyne B. The shifting sands of coronary microvascular dysfunction. Circulation. 2019;140:1817–1819. [DOI] [PubMed] [Google Scholar]
- 187.Rahman H, Ryan M, Lumley M, et al. Coronary microvascular dysfunction is associated with myocardial ischemia and abnormal coronary perfusion during exercise. Circulation. 2019;140:1805–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Drexler H, Hornig B. Endothelial dysfunction in human disease. J Mol Cell Cardiol. 1999;31:51–60. [DOI] [PubMed] [Google Scholar]
- 189.Ziegler T, Abdel Rahman F, Jurisch V, Kupatt C. Atherosclerosis and the capillary network; Pathophysiology and potential therapeutic strategies. Cells 9, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Roy TK, Secomb TW. Theoretical analysis of the determinants of lung oxygen diffusing capacity. J Theor Biol. 2014;351:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Roy TK, Secomb TW. Effects of pulmonary flow heterogeneity on oxygen transport parameters in exercise. Respir Physiol Neurobiol. 2019;261:75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ryan D, Frohlich S, McLoughlin P. Pulmonary vascular dysfunction in ARDS. Ann Inten Care. 2014;4:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Uhlig S, Yang Y, Waade J, Wittenberg C, Babendreyer A, Kuebler WM. Differential regulation of lung endothelial permeability in vitro and in situ. Cell Physiol Biochem. 2014;34:1–19. [DOI] [PubMed] [Google Scholar]
- 195.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Investig. 2012;122:2731–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jolin A, Bjertnaes L. Hypoxic pulmonary vasoconstriction in the adult respiratory distress syndrome. Acta Anaesthesiol Scand Suppl 95:40–52; discussion 53–44, 1991. [DOI] [PubMed] [Google Scholar]
- 197.Cressoni M, Cadringher P, Chiurazzi C, et al. Lung inhomogeneity in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2014;189:149–158. [DOI] [PubMed] [Google Scholar]
- 198.Nuckton TJ, Alonso JA, Kallet RH, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346:1281–1286. [DOI] [PubMed] [Google Scholar]
- 199.Lumb AB, Slinger P. Hypoxic pulmonary vasoconstriction: Physiology and anesthetic implications. Anesthesiology. 2015;122:932–946. [DOI] [PubMed] [Google Scholar]
- 200.Naeije R, Brimioulle S. Physiology in medicine: Importance of hypoxic pulmonary vasoconstriction in maintaining arterial oxygenation during acute respiratory failure. Crit Care. 2001;5:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sylvester JT. Hypoxic pulmonary vasoconstriction: a radical view. Circ Res. 2001;88:1228–1230. [DOI] [PubMed] [Google Scholar]
- 202.Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev. 2012;92:367–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Grimmer B, Kuebler WM. The endothelium in hypoxic pulmonary vasoconstriction. J Appl Physiol. 1985;123(1635–1646):2017. [DOI] [PubMed] [Google Scholar]
- 204.Dasgupta A, Wu D, Tian L, et al. Mitochondria in the pulmonary vasculature in health and disease: Oxygen-sensing, metabolism, and dynamics. Compr Physiol. 2020;10:713–765. [DOI] [PubMed] [Google Scholar]
- 205.Wang L, Yin J, Nickles HT, et al. Hypoxic pulmonary vasoconstriction requires connexin 40-mediated endothelial signal conduction. J Clin Investig. 2012;122:4218–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bhattacharya J Lung capillaries raise the hypoxia alarm. J Clin Investig. 2012;122:3845–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020. [DOI] [PubMed] [Google Scholar]
- 208.Tabeling C, Yu H, Wang L, et al. CFTR and sphingolipids mediate hypoxic pulmonary vasoconstriction. Proc Natl Acad Sci U S A. 2015;112:E1614–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ryter SW, Choi AM. Gaseous therapeutics in acute lung injury. Compr Physiol. 2011;1:105–121. [DOI] [PubMed] [Google Scholar]
- 210.Spieth PM, Zhang H. Pharmacological therapies for acute respiratory distress syndrome. Curr Opin Crit Care. 2014;20:113–121. [DOI] [PubMed] [Google Scholar]
- 211.Frohlich S, Murphy N, Ryan D, Boylan JF. Acute respiratory distress syndrome: current concepts and future directions. Anaesth Intensive Care. 2013;41:463–472. [DOI] [PubMed] [Google Scholar]
- 212.Asadi AK, Sa RC, Kim NH, et al. Inhaled nitric oxide alters the distribution of blood flow in the healthy human lung, suggesting active hypoxic pulmonary vasoconstriction in normoxia. J Appl Physiol. 1985;118(331–343):2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gallart L, Lu Q, Puybasset L, Umamaheswara Rao GS, Coriat P, Rouby JJ. Intravenous almitrine combined with inhaled nitric oxide for acute respiratory distress syndrome. The NO Almitrine Study Group. Am J Respir Crit Care Med. 1998;158:1770–1777. [DOI] [PubMed] [Google Scholar]
- 214.Doering EB, Hanson CW 3rd, Reily DJ, Marshall C, Marshall BE. Improvement in oxygenation by phenylephrine and nitric oxide in patients with adult respiratory distress syndrome. Anesthesiology. 1997;87:18–25. [DOI] [PubMed] [Google Scholar]
- 215.Schloss B, Martin D, Beebe A, Klamar J, Tobias JD. Phenylephrine to treat hypoxemia during one-lung ventilation in a pediatric patient. Thorac Cardiovasc Surg Rep. 2013;2:16–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Marini JJ. Firmer footing for ventilating and monitoring the injured lung. J Thorac Dis. 2018;10:S4047–s4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cronin JN, Crockett DC, Farmery AD, et al. Mechanical ventilation redistributes blood to poorly ventilated areas in experimental lung injury. Crit Care Med. 2020;48:e200–e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Schmidt J, Wenzel C, Spassov S, et al. Flow-controlled ventilation attenuates lung injury in a porcine model of acute respiratory distress syndrome: a preclinical randomized controlled study. Crit Care Med. 2020;48:e241–e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pries AR, Cornelissen AJ, Sloot AA, et al. Structural adaptation and heterogeneity of normal and tumor microvascular networks. PLoS Comput Biol. 2009;5:e1000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag. 2006;2:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Secomb TW, Hsu R, Braun RD, Ross JR, Gross JF, Dewhirst MW. Theoretical simulation of oxygen transport to tumors by three-dimensional networks of microvessels. Adv Exp Med Biol. 1998;454:629–634. [DOI] [PubMed] [Google Scholar]
- 222.Secomb TW, Hsu R, Ong ET, Gross JF, Dewhirst MW. Analysis of the effects of oxygen supply and demand on hypoxic fraction in tumors. Acta oncologica (Stockholm, Sweden). 1995;34:313–316. [DOI] [PubMed] [Google Scholar]
- 223.Dewhirst MW, Secomb TW. Transport of drugs from blood vessels to tumour tissue. Nat Rev Cancer. 2017;17:738–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Dewhirst MW, Mowery YM, Mitchell JB, Cherukuri MK, Secomb TW. Rationale for hypoxia assessment and amelioration for precision therapy and immunotherapy studies. J Clin Investig. 2019;129:489–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ince C Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care 19 Suppl. 2015;3:S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Harrois A, Dupic L, Duranteau J. Targeting the microcirculation in resuscitation of acutely unwell patients. Curr Opin Crit Care. 2011;17:303–307. [DOI] [PubMed] [Google Scholar]
- 227.Legrand M, Ait-Oufella H, Ince C. Could resuscitation be based on microcirculation data? Yes. Intensive Care Med. 2018;44:944–946. [DOI] [PubMed] [Google Scholar]
- 228.Shapiro NI, Angus DC. A review of therapeutic attempts to recruit the microcirculation in patients with sepsis. Minerva Anestesiol. 2014;80:225–235. [PubMed] [Google Scholar]
- 229.Kanoore Edul VS, Ince C, Dubin A. What is microcirculatory shock? Curr Opin Crit Care. 2015;21:245–252. [DOI] [PubMed] [Google Scholar]
- 230.Chierego M, Verdant C, De Backer D. Microcirculatory alterations in critically ill patients. Minerva Anestesiol. 2006;72:199–205. [PubMed] [Google Scholar]
- 231.Domizi R, Damiani E, Scorcella C, et al. Association between sublingual microcirculation, tissue perfusion and organ failure in major trauma: A subgroup analysis of a prospective observational study. PLoS One. 2019;14:e0213085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Krupickova P, Mlcek M, Huptych M, et al. Microcirculatory blood flow during cardiac arrest and cardiopulmonary resuscitation does not correlate with global hemodynamics: an experimental study. J Transl Med. 2016;14:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lam C, Tyml K, Martin C, Sibbald W. Microvascular perfusion is impaired in a rat model of normotensive sepsis. J Clin Investig. 1994;94:2077–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bezemer R, Bartels SA, Bakker J, Ince C. Clinical review: Clinical imaging of the sublingual microcirculation in the critically ill–where do we stand? Crit Care. 2012;16:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Donati A, Domizi R, Damiani E, Adrario E, Pelaia P, Ince C. From macrohemodynamic to the microcirculation. Critical care research and practice. 2013;2013:892710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Donati A, Tibboel D, Ince C. Towards integrative physiological monitoring of the critically ill: from cardiovascular to microcirculatory and cellular function monitoring at the bedside. Crit Care 17 Suppl. 2013;1:S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Vincent JL, Leone M. Optimum treatment of vasopressor-dependent distributive shock. Expert Rev Anti Infect Ther. 2017;15:5–10. [DOI] [PubMed] [Google Scholar]
- 238.Spronk PE, Ince C, Gardien MJ, Mathura KR, Oudemans-van Straaten HM, Zandstra DF. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet (London, England). 2002;360:1395–1396. [DOI] [PubMed] [Google Scholar]
- 239.Ospina-Tascon GA, Hernandez G, Alvarez I, et al. Effects of very early start of norepinephrine in patients with septic shock: A propensity score-based analysis. Crit Care. 2020;24:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hamzaoui O, Shi R. Early norepinephrine use in septic shock. J Thorac Dis. 2020;12:S72–s77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lesur O, Delile E, Asfar P, Radermacher P. Hemodynamic support in the early phase of septic shock: A review of challenges and unanswered questions. Ann Inten Care. 2018;8:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Morelli A, Donati A, Ertmer C, et al. Effects of vasopressinergic receptor agonists on sublingual microcirculation in norepinephrine-dependent septic shock. Crit Care. 2011;15:R217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Nascente APM, Freitas FGR, Bakker J, et al. Microcirculation improvement after short-term infusion of vasopressin in septic shock is dependent on noradrenaline. Clinics (Sao Paulo, Brazil). 2017;72:750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.den Uil CA, Lagrand WK, van der Ent M, et al. Conventional hemodynamic resuscitation may fail to optimize tissue perfusion: an observational study on the effects of dobutamine, enoximone, and norepinephrine in patients with acute myocardial infarction complicated by cardiogenic shock. PLoS One. 2014;9:e103978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis*. Crit Care Med. 2012;40:725–730. [DOI] [PubMed] [Google Scholar]
- 246.De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779–789. [DOI] [PubMed] [Google Scholar]
- 247.Bangash MN, Kong ML, Pearse RM. Use of inotropes and vasopressor agents in critically ill patients. Br J Pharmacol. 2012;165:2015–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Dubin A, Pozo MO, Casabella CA, et al. Increasing arterial blood pressure with norepinephrine does not improve microcirculatory blood flow: a prospective study. Crit Care. 2009;13:R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Silva S, Teboul JL. Defining the adequate arterial pressure target during septic shock: not a ‘micro’ issue but the microcirculation can help. Crit Care. 2011;15:1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Thooft A, Favory R, Salgado DR, et al. Effects of changes in arterial pressure on organ perfusion during septic shock. Crit Care. 2011;15:R222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Xu JY, Ma SQ, Pan C, et al. A high mean arterial pressure target is associated with improved microcirculation in septic shock patients with previous hypertension: a prospective open label study. Crit Care. 2015;19:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Asfar P, De Backer D, Meier-Hellmann A, Radermacher P, Sakka SG. Clinical review: influence of vasoactive and other therapies on intestinal and hepatic circulations in patients with septic shock. Crit Care. 2004;8:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Annane D, Ouanes-Besbes L, de Backer D, et al. A global perspective on vasoactive agents in shock. Intensive Care Med. 2018;44:833–846. [DOI] [PubMed] [Google Scholar]
- 254.Jhanji S, Stirling S, Patel N, Hinds CJ, Pearse RM. The effect of increasing doses of norepinephrine on tissue oxygenation and microvascular flow in patients with septic shock. Crit Care Med. 2009;37:1961–1966. [DOI] [PubMed] [Google Scholar]
- 255.Walley KR. Use of central venous oxygen saturation to guide therapy. Am J Respir Crit Care Med. 2011;184:514–520. [DOI] [PubMed] [Google Scholar]
- 256.Almac E, Siegemund M, Demirci C, Ince C. Microcirculatory recruitment maneuvers correct tissue CO2 abnormalities in sepsis. Minerva Anestesiol. 2006;72:507–519. [PubMed] [Google Scholar]
- 257.Trzeciak S, Cinel I, Phillip Dellinger R, et al. Resuscitating the microcirculation in sepsis: the central role of nitric oxide, emerging concepts for novel therapies, and challenges for clinical trials. Acad Emerg Med. 2008;15:399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Bennett VA, Vidouris A, Cecconi M. Effects of fluids on the macro-and microcirculations. Crit Care. 2018;22:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Siegemund M, Hollinger A, Gebhard EC, Scheuzger JD, Bolliger D. The value of volume substitution in patients with septic and haemorrhagic shock with respect to the microcirculation. Swiss Med Wkly. 2019;149:w20007. [DOI] [PubMed] [Google Scholar]
- 260.Rivers EP, Kruse JA, Jacobsen G, et al. The influence of early hemodynamic optimization on biomarker patterns of severe sepsis and septic shock. Crit Care Med. 2007;35:2016–2024. [DOI] [PubMed] [Google Scholar]
- 261.Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med. 2018;44:925–928. [DOI] [PubMed] [Google Scholar]
- 262.Spiegel R, Farkas JD, Rola P, et al. The 2018 surviving sepsis campaign’s treatment bundle: When guidelines outpace the evidence supporting their use. Ann Emerg Med. 2019;73:356–358. [DOI] [PubMed] [Google Scholar]
- 263.Jhanji S, Vivian-Smith A, Lucena-Amaro S, Watson D, Hinds CJ, Pearse RM. Haemodynamic optimisation improves tissue microvascular flow and oxygenation after major surgery: A randomised controlled trial. Crit Care. 2010;14:R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Fischer MO, Lemoine S, Tavernier B, et al. Individualized fluid management using the pleth variability index: A randomized clinical trial. Anesthesiology. 2020;133:31–40. [DOI] [PubMed] [Google Scholar]
- 265.Maheshwari K, Sessler DI. Goal-directed therapy: Why benefit remains uncertain. Anesthesiology. 2020;133(1):5–7. [DOI] [PubMed] [Google Scholar]
- 266.Marik PE, Byrne L, van Haren F. Fluid resuscitation in sepsis: The great 30 mL per kg hoax. J Thorac Dis. 2020;12:S37–s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Dostalova V, Astapenko D, Dostalova V Jr, et al. The effect of fluid loading and hypertonic saline solution on cortical cerebral microcirculation and glycocalyx integrity. J Neurosurg Anesthesiol. 2019;31:434–443. [DOI] [PubMed] [Google Scholar]
- 268.Naumann DN, Beaven A, Dretzke J, Hutchings S, Midwinter MJ. Searching for the optimal fluid to restore microcirculatory flow dynamics after haemorrhagic shock: A systematic review of preclinical studies. Shock (Augusta, Ga). 2016;46:609–622. [DOI] [PubMed] [Google Scholar]
- 269.Torres Filho IP, Torres LN, Salgado C, Dubick MA. Plasma syndecan-1 and heparan sulfate correlate with microvascular glycocalyx degradation in hemorrhaged rats after different resuscitation fluids. Am J Physiol Heart Circ Physiol. 2016;310:H1468–1478. [DOI] [PubMed] [Google Scholar]
- 270.Guerci P, Tran N, Menu P, Losser MR, Meistelman C, Longrois D. Impact of fluid resuscitation with hypertonic-hydroxyethyl starch versus lactated ringer on hemorheology and microcirculation in hemorrhagic shock. Clin Hemorheol Microcirc. 2014;56:301–317. [DOI] [PubMed] [Google Scholar]
- 271.Villela NR, Salazar Vázquez BY, Intaglietta M. Microcirculatory effects of intravenous fluids in critical illness: Plasma expansion beyond crystalloids and colloids. Curr Opinion Anaesthesiol. 2009;22:163–167. [DOI] [PubMed] [Google Scholar]
- 272.Zhao L, Wang B, You G, Wang Z, Zhou H. Effects of different resuscitation fluids on the rheologic behavior of red blood cells, blood viscosity and plasma viscosity in experimental hemorrhagic shock. Resuscitation. 2009;80:253–258. [DOI] [PubMed] [Google Scholar]
- 273.Correa TD, Vuda M, Takala J, Djafarzadeh S, Silva E, Jakob SM. Increasing mean arterial blood pressure in sepsis: Effects on fluid balance, vasopressor load and renal function. Crit Care. 2013;17:R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Vellinga NA, Ince C, Boerma EC. Elevated central venous pressure is associated with impairment of microcirculatory blood flow in sepsis: a hypothesis generating post hoc analysis. BMC Anesthesiol. 2013;13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Montomoli J, Donati A, Ince C. Acute kidney injury and fluid resuscitation in septic patients: Are we protecting the kidney? Nephron. 2019;143:170–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.van Bommel J, Siegemund M, Henny CP, van den Heuvel DA, Trouwborst A, Ince C. Preservation of intestinal microvascular Po2 during normovolemic hemodilution in a rat model. J Lab Clin Med. 2000;135:476–483. [DOI] [PubMed] [Google Scholar]
- 277.Pries AR, Ley K, Claassen M, Gaehtgens P. Red cell distribution at microvascular bifurcations. Microvasc Res. 1989;38:81–101. [DOI] [PubMed] [Google Scholar]
- 278.Kuo L, Pittman RN. Effect of hemodilution on oxygen transport in arteriolar networks of hamster striated muscle. Am J Physiol. 1988;254:H331–339. [DOI] [PubMed] [Google Scholar]
- 279.Johannes T, Mik EG, Nohe B, Unertl KE, Ince C. Acute decrease in renal microvascular PO2 during acute normovolemic hemodilution. Am J Physiol Renal Physiol. 2007;292:F796–803. [DOI] [PubMed] [Google Scholar]
- 280.Schwarte LA, Fournell A, van Bommel J, Ince C. Redistribution of intestinal microcirculatory oxygenation during acute hemodilution in pigs. J Appl Physiol. 2005;98:1070–1075. [DOI] [PubMed] [Google Scholar]
- 281.Marik PE. Surviving sepsis: Going beyond the guidelines. Annals of intensive care. 2011;1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Pranskunas A, Koopmans M, Koetsier PM, Pilvinis V, Boerma EC. Microcirculatory blood flow as a tool to select ICU patients eligible for fluid therapy. Intensive Care Med. 2013;39:612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Yuruk K, Almac E, Bezemer R, Goedhart P, de Mol B, Ince C. Blood transfusions recruit the microcirculation during cardiac surgery. Transfusion. 2011;51:961–967. [DOI] [PubMed] [Google Scholar]
- 284.Atasever B, van der Kuil M, Boer C, et al. Red blood cell transfusion compared with gelatin solution and no infusion after cardiac surgery: effect on microvascular perfusion, vascular density, hemoglobin, and oxygen saturation. Transfusion. 2012;52:2452–2458. [DOI] [PubMed] [Google Scholar]
- 285.Conrad SA, Dietrich KA, Hebert CA, Romero MD. Effect of red cell transfusion on oxygen consumption following fluid resuscitation in septic shock. Circulatory shock. 1990;31:419–429. [PubMed] [Google Scholar]
- 286.Kuo L, Pittman RN. Influence of hemoconcentration on arteriolar oxygen transport in hamster striated muscle. Am J Physiol. 1990;259:H1694–1702. [DOI] [PubMed] [Google Scholar]
- 287.Vicaut E, Stucker O, Teisseire B, Duvelleroy M. Effects of changes in systemic hematocrit on the microcirculation in rat cremaster muscle. Int J Microcirc Clin Exp. 1987;6:225–235. [PubMed] [Google Scholar]
- 288.Dominelli PB, Baker SE, Wiggins CC, et al. Dissociating the effects of oxygen pressure and content on the control of breathing and acute hypoxic response. J Appl Physiol. 1985;127(1622–1631):2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Dominelli PB, Wiggins CC, Baker SE, et al. Influence of high affinity haemoglobin on the response to normoxic and hypoxic exercise. J Physiol. 2020;598:1475–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Shepherd JRA, Dominelli PB, Roy TK, et al. Modelling the relationships between haemoglobin oxygen affinity and the oxygen cascade in humans. J Physiol. 2019;597:4193–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Nyvad J, Mazur A, Postnov DD, et al. Intravital investigation of rat mesenteric small artery tone and blood flow. J Physiol. 2017;595:5037–5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.De Blasi RA, Palmisani S, Boezi M, et al. Effects of remifentanil-based general anaesthesia with propofol or sevoflurane on muscle microcirculation as assessed by near-infrared spectroscopy. Br J Anaesth. 2008;101:171–177. [DOI] [PubMed] [Google Scholar]
- 293.Yang XM, Liu J, Ji J, Xie J. Effects of dexmedetomidine on the deformability of erythrocytes in vitro and in anesthesia. Experimental and therapeutic medicine. 2014;7:1631–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Yerer MB, Aydogan S, Comu FM, et al. The red blood cell deformability alterations under desfluran anesthesia in rats. Clin Hemorheol Microcirc. 2006;35:213–216. [PubMed] [Google Scholar]
- 295.Astapenko D, Benes J, Pouska J, Lehmann C, Islam S, Cerny V. Endothelial glycocalyx in acute care surgery - what anaesthesiologists need to know for clinical practice. BMC Anesthesiol. 2019;19:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Bruegger D, Bauer A, Finsterer U, Bernasconi P, Kreimeier U, Christ F. Microvascular changes during anesthesia: Sevoflurane compared with propofol. Acta Anaesthesiol Scand. 2002;46:481–487. [DOI] [PubMed] [Google Scholar]
- 297.Chiarandini P, Pompei L, Costa MG, et al. Effects of catecholamines on microcirculation during general inhalation anesthesia. J Cardiothorac Vasc Anesth. 2013;27:1239–1245. [DOI] [PubMed] [Google Scholar]
- 298.Loeb AL, Godeny I, Longnecker DE. Anesthetics alter relative contributions of NO and EDHF in rat cremaster muscle microcirculation. Am J Physiol. 1997;273:H618–627. [DOI] [PubMed] [Google Scholar]
- 299.Turek Z, Sykora R, Matejovic M, Cerny V. Anesthesia and the microcirculation. Seminars in Cardiothoracic and Vascular Anesthesia. 2009;13:249–258. [DOI] [PubMed] [Google Scholar]
- 300.Iguchi N, Kosaka J, Booth LC, et al. Renal perfusion, oxygenation, and sympathetic nerve activity during volatile or intravenous general anaesthesia in sheep. Br J Anaesth. 2019;122:342–349. [DOI] [PubMed] [Google Scholar]
- 301.Jones AE, Puskarich MA. Sepsis-induced tissue hypoperfusion. Crit Care Clin. 2009;25(4):769–779. [DOI] [PubMed] [Google Scholar]
- 302.Erdem O, Ince C, Tibboel D, Kuiper JW. Assessing the microcirculation with handheld vital microscopy in critically Ill neonates and children: Evolution of the technique and its potential for critical care. Frontiers in pediatrics. 2019;7:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Hariri G, Joffre J, Leblanc G, et al. Narrative review: clinical assessment of peripheral tissue perfusion in septic shock. Annals Intens Care. 2019;9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Huber W, Zanner R, Schneider G, Schmid R, Lahmer T. Assessment of regional perfusion and organ function: Less and non-invasive techniques. Front Med. 2019;6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Tafner P, Chen FK, Rabello RF, Correa TD, Chaves RCF, Serpa AN. Recent advances in bedside microcirculation assessment in critically ill patients. Revista Brasileira de terapia intensiva. 2017;29:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Vos JJ, Ellermann SF, Scheeren TWL. Journal of clinical monitoring and computing 2017/2018 end of year summary: Monitoring-and provocation-of the microcirculation and tissue oxygenation. J Clin Monit Comput. 2019;33:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.De Backer D, Dubois MJ. Assessment of the microcirculatory flow in patients in the intensive care unit. Curr Opin Crit Care. 2001;7:200–203. [DOI] [PubMed] [Google Scholar]
- 308.De Backer D, Ospina-Tascon G, Salgado D, Favory R, Creteur J, Vincent JL. Monitoring the microcirculation in the critically ill patient: Current methods and future approaches. Intensive Care Med. 2010;36:1813–1825. [DOI] [PubMed] [Google Scholar]
- 309.Cerny V, Turek Z, Parizkova R. Orthogonal polarization spectral imaging. Physiol Res. 2007;56:141–147. [DOI] [PubMed] [Google Scholar]
- 310.Barcelos A, Lamas C, Tibirica E. Evaluation of microvascular endothelial function in patients with infective endocarditis using laser speckle contrast imaging and skin video-capillaroscopy: Research proposal of a case control prospective study. BMC Res Notes. 2017;10:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Hessler M, Nelis P, Ertmer C, et al. Optical coherence tomography angiography as a novel approach to contactless evaluation of sublingual microcirculation: A proof of principle study. Sci Rep. 2020;10:5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Scheeren TW. Monitoring the microcirculation in the critically ill patient: reflectance spectroscopy. Intensive Care Med. 2011;37:1045–1046. author reply 1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ince C, Boerma EC, Cecconi M, et al. Second consensus on the assessment of sublingual microcirculation in critically ill patients: results from a task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2018;44:281–299. [DOI] [PubMed] [Google Scholar]
- 314.Guven G, Hilty MP, Ince C. Microcirculation: Physiology, pathophysiology, and clinical application. Blood Purif. 2020;49:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Hilty MP, Guerci P, Ince Y, Toraman F, Ince C. MicroTools enables automated quantification of capillary density and red blood cell velocity in handheld vital microscopy. Communications biology. 2019;2:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Lundy DJ, Trzeciak S. Microcirculatory dysfunction in sepsis. Crit Care Clin 2009;25:721–731, viii. [DOI] [PubMed] [Google Scholar]
- 317.Sand CA, Starr A, Wilder CD, et al. Quantification of microcirculatory blood flow: a sensitive and clinically relevant prognostic marker in murine models of sepsis. J Appl Physiol. 1985;118(344–354):2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.De Backer D, Hollenberg S, Boerma C, et al. How to evaluate the microcirculation: report of a round table conference. Crit Care. 2007;11:R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Verdant CL, De Backer D, Bruhn A, et al. Evaluation of sublingual and gut mucosal microcirculation in sepsis: A quantitative analysis. Crit Care Med. 2009;37:2875–2881. [DOI] [PubMed] [Google Scholar]
- 320.Monnet X, Saugel B. Could resuscitation be based on microcirculation data? We are not sure. Intensive Care Med. 2018;44:950–953. [DOI] [PubMed] [Google Scholar]
- 321.Naumann DN, Lima A. Could resuscitation be based on microcirculation data? No. Intensive Care Med. 2018;44:947–949. [DOI] [PubMed] [Google Scholar]
- 322.Lopez A, Grignola JC, Angulo M, et al. Effects of early hemodynamic resuscitation on left ventricular performance and microcirculatory function during endotoxic shock. Intensive care medicine experimental. 2015;3:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.van Beest PA, Vos JJ, Poterman M, Kalmar AF, Scheeren TW. Tissue oxygenation as a target for goal-directed therapy in high-risk surgery: a pilot study. BMC Anesthesiol. 2014;14:122. [DOI] [PMC free article] [PubMed] [Google Scholar]


