ABSTRACT
Humans are considered “superorganisms,” harboring a diverse microbial collective that outnumbers human cells 10 to 1. Complex and gravely understudied host- and microbe-microbe interactions—the product of millions of years of host-microbe coevolution—govern the superorganism in almost every aspect of life functions and overall well-being. Abruptly disrupting these interactions via extrinsic factors has undesirable consequences for the host. On the other hand, supplementing commensal or beneficial microbes may mitigate perturbed interactions or enhance the interactive relationships that ultimately benefit all parties. Hence, immense efforts have focused on dissecting the innumerable host- and microbe-microbe relationships to characterize if a “positive” or “negative” interaction is at play and to exploit such behavior for broader implications. For example, microbiome research has worked to identify and isolate naturally antipathogenic microbes that may offer therapeutic potential either in a direct, one-on-one application or by leveraging its unique metabolic properties. However, the discovery and isolation of such desired therapeutic microbes from complex microbiota have proven challenging. Currently, there is no conventional technique to universally and functionally screen for these microbes. With this said, we first describe in this review the historical (probiotics) and current (fecal microbiota or defined consortia) perspectives on therapeutic microbes, present the discoveries of therapeutic microbes through exploiting microbe-microbe and host-microbe interactions, and detail our team’s efforts in discovering therapeutic microbes via our novel microbiome screening platform. We conclude this minireview by briefly discussing challenges and possible solutions with therapeutic microbes’ applications and paths ahead for discovery.
KEYWORDS: probiotics, fecal microbiota transplant, defined microbial consortia, therapeutic microbes, microbe-microbe interactions, host-microbe interactions, next-generation sequencing, unculturables
INTRODUCTION
Every animal (and plant) on Earth is a result of multitudinous host-microbe interactions, wherein at least one-member (microbe and/or host) benefits. Microbes assist their animal hosts in various physiological processes: metabolizing and digesting food nutrients, stimulating mucosal immune responses, producing essential vitamins, energizing physioanatomical development, and providing resistance to colonization by intruding pathogens (1–3). Without microbial interactions, the host becomes predisposed to various disease conditions. This intellection can be appreciated, as germfree animals exhibit anatomical and functional differences in the gut, liver, lungs, enteric nervous system, and suboptimal immunity and endocrine functions (4, 5). However, the mechanistic aspects of microbe-microbe interactions (MMIs) and host-microbe interactions (HMIs) have been a major challenge to dissect. Many external factors (e.g., antimicrobials, diet, and environment) and internal factors (e.g., genotype) influence MMIs and HMIs (6). Studies have correlated perturbed HMIs with acute (7, 8) and chronic (9) conditions; furthermore, the duration of HMI abruption and host age covary with the severity of outcome (10). For example, vaginal delivery is an essential process for seeding selective species of microbes on the skin surface and internal gut of newborn babies. In contrast, caesarean births preclude mother-child microbe transfer, and such microbes are replaced with environmentally acquired microbes; thereupon, newborns are prone to develop chronic diseases in adulthood such as diabetes, obesity, asthma, and neurological disorders (11, 12). During adulthood, broad-spectrum-antibiotic usage drastically affects HMIs, leading to acute discomfort (i.e., antibiotic-associated diarrhea [13]). Hence, where HMI imbalance is inevitable (e.g., caesarean birth and septicemia), limiting the duration of disruption by supplying essential and beneficial microbes can potentially ameliorate the deleterious health outcomes associated with perturbed HMIs (14).
The emergence of modern medical practices, germ theory, and the hygiene hypothesis have erroneously imprinted on humanity that all microbes are pathogens. Such ideologies have contemporized an increase in hygiene practices and overuse of antimicrobial products in attempts to eradicate the microbial collective. Indeed, this notion has prevented millions of morbidities from infectious diseases across the world. However, a paradigm shift is occurring with newly acquired knowledge of microbial communities (i.e., the microbiome and microbiota) and their role in health. Emerging evidence regarding microbes is revealing that not all microorganisms are necessarily pathogens. In fact, only a limited fraction of known microbes are responsible for the development of disease (15). Any given microbial species in a complex community will likely have various kinds of interactions with its neighboring native organisms. Some of these interactions may provide advantages and/or disadvantages to a given cohabiting neighbor. Therefore, harnessing such microbial species by dissecting MMIs and HMIs is an opportune path for discovery of next-generation therapeutics (16).
It is important to mention that the term “therapeutic microbes” can also refer to genetically engineered microbes that sense and respond to their environmental cues by secreting therapeutic molecules, and such organisms are not covered in this review (17, 18). Unlike conventional antimicrobials, therapeutic microbes have many favorable traits, such as consisting of live cells, having self-renewal abilities, being unlikely to induce resistance, employing broad mechanisms to inhibit pathogens, being pathogen specific, and inducing minimal side effects (19). In this minireview, we present the historical integration of therapeutic microbes (probiotics), the current perception of their use in therapy (i.e., microbiome based or rationally selected defined consortia), discovery through exploiting MMI and HMI, and our team’s efforts in banking therapeutic microbes by use of a novel microbiome-screening platform. We conclude by briefly discussing challenges and possible solutions with therapeutic microbes’ applications and present anticipated future directions of discovery.
EARLY HISTORY OF THERAPEUTIC MICROBES
Bipedalism, tool design, domestication of fire, transition to an agriculturist society, and the development of written language are the decorated hallmarks of human evolution. Subsequently, the human adaptations have coincided with climate variation, thereby biasing distinct physiological and socioeconomic characteristics that have promoted our health (20–23). An often-underappreciated feat in human evolution is the resourcefulness and implementation of microbiology practices (24). The first serendipitous applications originated during early civilization with the realization of the beneficial health effects of fermented foods. Although humans were unaware of the existence of microscopic organisms, breakthroughs in fermentation increased food preservation and enhanced recovery from some diseases (25, 26). Detailed archeological methodologies (analogical, ethnographical, archaeobotanical, etc.) and chemical analyses (mass spectrometry [MS], nuclear magnetic resonance [NMR], high-pressure liquid chromatography [HPLC], etc.) of vessel sherds have dated the earliest uses of fermented dairy, vegetables, bread, and beverages to 8000 BCE (Fig. 1) (27–29). Furthermore, collections from bronze vessels over the succeeding 5,000 years reveal optimizations of the fermentation process, resulting in improved wines, breads, and medicine. As material trade expanded, numerous civilizations (Mesopotamian, Egyptian, Greek, and Roman) traded knowledge of the dynamic use of fermentation (30). As such, fermentation expanded across continental distances, and in the 21st century, these practices have been adapted with human culture.
FIG 1.
Milestones in the discovery of potential therapeutic microbes in human evolution (80, 81, 91, 99, 135–138).
With the definitive falsification of spontaneous generation in 1863 by Louis Pasteur’s germ theory, microbes have been recognized in society as being influential. At the beginning of the 19th century, marketing tools promoting health introduced microbes to the public. For example, groups advertised and differentiated their milk from competitors by advertising increased health benefits through their use of beneficial microbes (now identified as Streptococcus thermophilus and Lactobacillus delbrueckii) (31). In Spain in 1919, Isaac Carasso made a similar push with marketing tactics to increase yogurt sales. At the same time, viruses—particularly bacteriophages, which specifically infect and kill bacterial species—were used as treatments for Shigella dysenteries in humans (32). Interestingly, modern history attributes the first eukaryotic therapeutic microbe to the accidental discovery of the mold Penicillium notatum, which inhibited the growth of pathogenic Staphylococcus aureus. However, earlier records suggest the Greeks and Indians used molds to treat infections prior to this. Ironically, the rise of interest in conducive therapeutic microbes (bacteria, viruses, and fungi) rapidly halted with the emergence of antibiotics. Carrying over to the next century (1930 to the present), antibiotics became ubiquitous and were viewed as a panacea. However, the overuse of antibiotics has escalated the prevalence of antibiotic resistance in bacterial pathogens.
As a response to the alarming rise of antibiotic resistance in bacteria, the 1980s marked a global shift for discovering novel therapeutic microbes and public sponsorship as a route for disease mitigation (33). At the same time, probiotics were touted as beneficial microbes with a plethora of supported health claims. In 1991, the Office of Alternative Medicine was established to evaluate and provide scientific data on the use of probiotics in an effort to prevent customer exploitation by ungoverned marketing strategies. Intense microbiome research over the last decade has indicated that unexplored Archaea species that make up ∼1.5% of the human gut microbiota also possess therapeutic potential in remediating atherosclerosis and kidney disease by metabolizing trimethylamine N-oxide (TMAO) levels in blood (19, 34). In summary, the interdependency between humans and microbe interactions has given rise to a long record of accomplishment in the use of therapeutic microbes to maximize health.
CURRENT METHODS OF PROBIOTIC DISCOVERY
A myriad of in vitro assays exist to evaluate a microbe’s inherent antagonist activities toward known microbial pathogens. Such assays focus on determining a microbe’s potential antagonism via direct interaction or through collected extracts (e.g., exoproteins). In vitro methods have been routinely used to discover organisms that display antimicrobial characteristics by using semisolid medium platforms (Table 1) (35). Live-cell antimicrobial activity is assessed by coculturing two species but does not distinguish whether antagonism is a product of diffusible inhibitors or direct contact (36, 37). Alternatively, layered-agar-based methods can proliferate probiotics followed by harvested cell-free supernatant to minimize confounding variables, thereby attributing antagonistic activity to secreted diffusible proteins. In this method, inoculated agar plates containing the pathogen are seeded with a layer of the microbe’s extract, where both pathogen and extract are titrated (35, 38). Gelatin- and agar-based methods crudely express antimicrobial activity as either inhibition zones (in square millimeters) or arbitrary units (AU) per milliliter. Notably, semisolid-substrate techniques demand large amounts of materials and present reproducibility issues, whereas novel high-throughput microdilution techniques provide elevated reproducibility and rapid microbe screening. Microdilution protocols employ microwell plates, where each well contains an inoculum of a predefined concentration of a pathogenic strain. Thereafter, dilutions of the probiotic extracts are introduced to evaluate dose-dependent inhibition of microbial growth (35, 39). Furthermore, the microdilution method provides a quantitative estimate of a suspected therapeutic microbe’s lowest concentration at which it inhibits complete growth of the pathogen to determine the minimum inhibitory concentration, which is the universally accepted metric to quantify the lowest concentration of an antimicrobial compound that inhibits bacterial growth. Different versions of these two forms of assays have been utilized since the discovery of bacteria, but these methods remain time intensive, have relatively low throughput, possess limited application in evaluating MMI, and presume underlying characteristics of cultured strains (e.g., high penetrance).
TABLE 1.
Summarized attributes of therapeutic microbesa
| Attribute | Probiotics | Fecal microbiota transplantb | Defined consortiumc | Therapeutic microbesc |
|---|---|---|---|---|
| Definition | “Live microorganisms which when administered in adequate amounts confer a health benefit on the host” (41). Contains mainly bacteria and yeast | Screened fecal transplant from healthy donor to recipient (90). Contains bacteria, virus, fungi and/or metabolites | Collection of characterized microbes for therapeutic use (111). May contain a pure or mixed culture of bacteria and/or fungi | Adequately characterized, or engineered, single and/or collections of microbes with targeted therapeutic applications May contain single or a cocktail of bacteria, virus, and/or fungi species |
| Discovery and/or rationale | Beneficial relationship through the fermentation of food that promoted health | Administration of fecal suspensions performed by Ge Hong from healthy individual to diarrheic patient (82, 83) | Depleted commensal microbe collections and/or cocktails of probiotic species with beneficial effect (100–102) | First instance of antagonistic MMI: lytic phages against Shigella species and Penicillium notatum against S. aureus |
| Methods |
Diffusiona (41, 147–155) Agar disk Etest Agar well Agar plug Cross streak TLCa (156–158) Agar diffusion Direct bioautography Agar overlay bioassay |
Screening: identify donors satisfying prerequisite criteria Preparation: samples suspended in saline Administration: colonoscopy, nasogastric, oral capsules (139, 143, 144, 159, 160) |
NGS and other omics approaches followed by bioinformatics analysis to shortlist essential microbes (100, 101, 103). | Engineering strain via synthetic biology approach (17, 18, 120) Development of microbiome screening platform [HiSCI] (115) |
| FDA categorization (Table 2) | Dietary supplement | Biological product | Biological product | Drug |
| Applications | Allergy (161–164) Carcinogenicity (165) Carcinogenicity and mutagenicity (166, 167) Cholesterol reduction (168) Gastrointestinal (169, 170) Endotoxemia (171) Hypertension (135) Immunomodulation (136) Kidney stone (oxaluria) (137) Lactose tolerance (140) Urinary tract infections (172) |
Selected noninfectious diseases Psychological (144) Obesity (97, 141) Drug-induced dysbiosis (142) Gastrointestinal (143) Selected infectious diseases Rotavirus (88) Bacterial infections (87) Fungal infections (58) Parasitic prevention (173, 174) |
Recurrent C. difficile infections | Engineered strain: familial adenomatous polyposis, urea cycle disorder, cirrhosis, phenylketonuria, type 1 diabetes mellitus, oral mucositis (120) Therapeutic microbes: C. scindens and C. amalonaticus (103, 119) |
| Mechanism/microbes involved |
Direct Cholesterol clearance Nutrient competition Toxin suppression AMP production Inhibitory peptide production Competitive exclusion Indirect Immunomodulation Gut barrier reinforcement Enterocyte inflammatory stimulation |
Direct Establishing eubiosis Novel antibacterial species Pathogen-specific lytic bacteriophages Beneficial fungi Unidentified microbial metabolites Indirect Stimulation of host immunity and physiology (see probiotics mechanisms) |
Direct and indirect May employ probiotic or FMT mechanisms |
Direct Engineered strain: sense and respond to external stimuli (i.e., pathogen presence) (17, 18) Therapeutic microbes: may employ multiple mechanisms to inhibit or suppress pathogen of interest: nutrient competition, toxin suppression, AMP secretion, inhibitory peptide production, competitive exclusion Indirect Stimulate host immunity and physiology |
| Advantages | Derived from human and animals Consistent history of overall safe use Considered GRASE by FDA |
Minimal side effects in contrast to antibiotic treatment (77) Multifaceted clinical applications (77) >70% eradication rate against multidrug-resistant organisms (77) |
Absence of known pathogens Minimal vertical or horizontal antibiotic resistant gene transfer Known mechanism of action Reduced patient anxiety compared to FMT Reflects patient-driven health care (175) |
Target specific and minimal side effect (19) High success rate in pathogen eradication Well-characterized and defined mechanism of action towards target and host No risk of transferable antibiotic resistance genes (17, 18) |
| Disadvantages/limitations | Limited data of mechanisms of action Exhaustive list of prerequisite criteria (Table 2) Various beneficial effect dependent on species and strain Batch-to-batch variation in large-scale manufacturing May harbor and transfer antibiotic resistant genes Vaguely regulated by FDA |
Relatively high failure rates, non-C. difficile conditions (10–20%) (91) Difficulty in finding compatible donor(s) (92) Limited longitudinal studies for safety, recipient compatibility, and its benefits (93) Inadequate screening for pathogens (101) Transplantation of uncharacterized microorganisms with unknown interactions (102) Enforced discretion by FDA for temporary use against recurrent C. difficile and non-C. difficile infection (107) Laborious process and elevated patient anxiety |
Difficult to discover and isolate essential microbes from microbiota Currently less efficient than FMT in disease remediation Lack of inclusion of other essential microbes |
Technology development in early stages Requires personalized medicine approach Standard treatment guidelines are not yet established (121) Biocontainment, strain stability, and quality control assurances have not been proposed or attempted (121) |
Not an exhaustive list of current methodologies. MMI, microbe-microbe interaction; TLC, thin-layer chromatography; GRASE, generally recognized as safe and effective; FDA, Federal Drug Administration. Underlines indicate appropriate subgroupings that the topic within the matrix's element can be divided into.
The terms “fecal microbiota transplant” (FMT) and “fecal transplant” (FT) are used interchangeably in literature but refer to the same concept. Sometimes “bacteriotherapy” is also used in place of “FMT” or “FT,” but this is not accurate, as “bacteriotherapy” refers to the use of bacterial species for therapy, and FMT/FT contains other microbial organisms, like viruses, archaea, and fungi.
Defined consortium and therapeutic microbes may appear to be the same but differ in selection, strain generation, and discovery approach; therefore, we put therapeutic microbes in a separate therapeutic class.
Historical probiotic discoveries, during the inception of germ theory and early agar-based experiments, are limited to accidental identification. Refinements of methods, specifically ones that illustrate direct microbe-microbe inhibition, marked an increase in the prospects for detection of beneficial bacteria. Until recently, testing probiotics has been restricted by generic methods and biased selection of known bacterial species (e.g., Lactobacillus, Bifidobacterium, etc.). The combination of interdisciplinary efforts, specifically through computational analysis and culture independent sequencing, fostered the rapid discovery of the next generation of beneficial microbes by predicting the existence of exploitable microbes found in the most unusual places: sewage, soil, and the gut (40).
DEFINING PROBIOTICS: FDA
The Food and Agriculture Organization of the United Nations (FAO), the U.S. Food and Drug Administration (FDA), and the World Health Organization (WHO) define a probiotic as “a live microorganism(s) which when administered in adequate amounts confer a health benefit on the host” (41). The FDA monitors marketed use, premarketing requirements, and endpoint analysis; with a level of constraint based on intended use of the product. To this end, there is still great confusion as to how the FDA views probiotics. With convoluted definitions and liberal use of health claim benefits, manufacturers have been bypassing regulatory categories—many of which promote a level of safety, function, and usability. Manufacturers often include disclaimers such as “These statements have not been evaluated by the FDA” printed in nearly illegible font size to bypass additional testing requirements. Adding a disclosure statement on the product permits manufactures to apply conjectural assertions in advertisement. For example, businesses may cite relief of nonspecific symptoms, thereby potentially putting consumers at great risk by delaying professional medical attention. From Table 2, we can see that as a company transitions from advertising “management” to “prevention” of a disease condition in their product’s health claim(s), supervision and board approval from the FDA concomitantly increase. Greater efforts are required when a probiotic ventures into increasing health claims. The advertisement of a product’s therapeutic quality requires years of data accumulation, investigative new drug applications, and review board approval, among other requirements, to be recognized as generally safe and effective for human use. Thus, manufacturers are finding loopholes in current FDA policies for profit, thereby prompting a need to refine existing guidelines in order to protect the consumer.
TABLE 2.
Tentative FDA categorization of foods and drugs based on intended use and their premarketing prerequisites, according to the 2021 Amended Federal Food, Drug, and Cosmetic Acta
| Category [U.S. Code] | Intended use | Premarketing requirements |
|---|---|---|
| Medical food [Title 21, chapter 9, section 360ee; 2011] | Used under the supervision of a physician and is administered “internally” with the intended application for the “management of a disease or condition” Dietary management |
If GRASE, no clinical studies required, but prior research is needed; otherwise, IRB approval and clinical studies required. |
| Food [Title 21, chapter 9, section 321(f); 2011] | Articles used for food, drink, and its components Permits “health claims” to appear on foods and supplements Includes natural foodborne microorganisms Limited claim to “help maintain (micro)flora” |
Typically, GRASE If not GRASE, requires FDA disclosure |
| Dietary supplement [Title 21, chapter 9, section 321(ff); 2011] | A product, excluding tobacco, used to “supplement the diet,” intended for ingestion, and “not present for use as a conventional food” Application of “new dietary ingredient is required” if product not marketed before 15 October 1994 |
Not required for ingredient marketed before 15 October 1994 May be placed on the market with limited claims Manufacturer must disclose to FDA intended claims; however, the FDA may request substantial evidence If manufacturer makes claims regarding ingredients’ use in health remediation, then premarketing clearance is required Cannot be marketed as “drug-like” or a disease preventative |
| Drug [Title 21, chapter 9, section 321(g)(1); 2011] | Claims to “diagnose, mitigate, treat, cure, or prevent a specific disease or class of diseases” Product not GRASE Therapeutic microbes (single strain or consortium) with the above claims fall within the definition of a “new drug” |
IRB approval before preclinical and clinical trials Must complete NDA and IND trials before marketing Possession of biologics license Formal communication with FDA prior to clinical testing Submission of test protocols and endpoint measurements 30 days prior Transcript of investigational plan Requires IRB review and approval |
| Biological product [Title 42, chapter 6A, section 262; 2010] | “Application for prevention, treatment and/or prevention of a disease condition” Article contains no living cells but can include any of the following: “virus, therapeutic serum, toxin, antitoxin, vaccine, blood, blood component or derivative, or allergenic” |
Submission and approval of biologics license application IND studies |
IND, investigative new drug; IRB, institutional review board; GRASE, generally recognized as safe and effective; NDA, new drug application.
APPLICATIONS OF PROBIOTICS
Numerous studies on probiotic research have been published; however, only a fraction have shown the product to produce efficacious outcomes in disease remission that are supported by mechanistic evidence (Table 1) (42, 43). Applicable probiotics commonly demonstrate at least one of the following characteristics; (i) antagonism to pathogen proliferation, (ii) competitive inhibition or outcompetition for nutrients; (iii) beneficial immunomodulatory effects on the host; and (iv) inhibition of endotoxin production (44–46). Altogether, these mechanisms present a probiotic’s advantage directly via association with the infectious agent or indirectly by enhancement of a host’s immunity.
Investigation into direct effects of a probiotic on other microbes convey two primary mechanisms of action. Lactobacilli, abundantly available in dairy products, are known to induce their antimicrobial effects by the production of bacteriocins (47, 48). Release of these low-molecular-weight lipids (LMWL) induces its antimicrobial influence through depolarization of its target organism, consequently introducing pores into the cytoplasmic membrane and thus compromising cell viability. Structure-specific toxicity of LMWL results from the influx of fatty acids coinciding with a pathogen’s inability to rapidly metabolize LMWL (49). Second, probiotics (e.g., Lactobacillus and Bifidobacterium) exhibit antimicrobial properties by secreting conjugated bile acids in concert with LMWL (50, 51). Detergent properties of bile acids result in dissolution of bacterial membranes by membrane fragmentation and cytosolic organelle dissolution (52–54). In light of these properties, bacteriocin-producing probiotics present a major advantage in disease mitigation and treatment by interacting directly with the target pathogen.
Alternatively, probiotics may present benefits by conditioning the host immune system. Immunomodulatory effects of the intestinal microbiome facilitate development of tolerance to environmental antigens and govern immunological responses against pathogens, in effect diminishing autoaggressive or allergic reactions. For example, although not in direct contact with mucosa-resident bacteria, resident M cells are able to take up and undergo transcytosis of bacterial fragments from the luminal surface to the subepithelial surface (55, 56). Nearby dendritic cells collect these fragments and present them to naive CD4+ cells. In an ex vivo study, coculturing dendritic cells with Lactobacillus rhamnosus on exposure to T cells led to a significant decrease in production of T-cell-specific cytokines (interleukin 2 [IL-2], IL-4, and IL-10). This suggests that potential immunomodulatory interactions could occur between the resident microbiota and the host (57). In similar studies, the probiotic Escherichia coli Nissle 1917 was able to negate deleterious effects of chemically induced colitis by strengthening the mucosal gut barrier (58). Mouse models and supporting in vitro experiments reported positive correlations of zonula occludens proteins 1 and 2 with resistance to enteropathogenic E. coli (59, 60). In summary, probiotics’ benefits have either direct action on a pathogen or indirect host immunomodulation effects; hence, possession of any of these features flags a microbe as a potential therapeutic.
The aforementioned examples demonstrate why probiotics have taken the limelight and how they are currently being discussed as the next generation of medicine (61). A recent clinical trial illustrated the effective use of probiotics to substantially diminish the overall presence of antimicrobial resistance genes and the bacteria that harbor them (62). Systematic searches for underlying mechanisms support probiotics’ potential to mitigate infection, combat cancer, and stabilize/enhance a host’s microbiome. However, the issue of identifying a particular probiotic strain with the desired specificity remains, as the beneficial effect may not be conserved across probiotic strains (63).
LIMITATIONS OF PROBIOTICS AND REQUIREMENTS
The reemergence of probiotics research over the last 45 years varies jointly with elevated interest among researchers and consumers. This billion-dollar industry is based on four predominant market factors: (i) an increased demand for alternative therapeutics to combat the accelerating rise of antibiotic resistance in bacteria, (ii) shifts toward patient-driven health care, (iii) elevated interest in natural products to minimize side effects associated with conventional therapies, and (iv) increasing evidence of strain-specific beneficial effects (64–66). Hence, agencies like the FDA, European Medicines Agency (EMA), and Codex Alimentarius (a joint FAO/WHO Food Standards Program) are extending their influence in the realm of probiotics development to provide assurance to users. As a result, these agencies are requiring demonstrations of safety, functionality, and manufacturing usability before marketing (Table 3) (67).
TABLE 3.
Prerequisite traits for probiotic selectiona
| Measure | Criterion |
|---|---|
| Safety | GRASE No evidence of competition or parasitism with its host Open-accessible data Produced by GMP Commensal or mutualistic to native microflora Minimal threat of vertical and horizontal transfer of antibiotic resistant genes Phenotypic and genotypic identification of strain Resistance to bacteriophage |
| Functionality | Characterized mechanism of action Resistant to low pH and bile acids produced by host |
| Usability | Manufacturing capabilities Long-term storage QA for manufacturing and distribution Genetic stability over high biomass production |
GRASE, generally recognized as safe and effective; GMP, good manufacturing practices; QA, quality assurance.
Most probiotics are generally recognized as safe and effective (GRASE) but do not necessarily have similar beneficial effects. Safety assessments focus on identifying antibiotic-resistant populations, in addition to the likelihood of the traits being transferred to cohabiting species (68–70). With this in mind, vertical gene transfer, measured by shifts in antibiotic median effective concentration (EC50), is extensively recorded over succeeding generations (71). Species and strains that rapidly develop antibiotic resistance are disregarded as probiotic candidates. Defining functional mechanisms regarding a probiotic’s endpoint aims to provide empirical data regarding its ability to mitigate disease and requires substantial efforts. Before application, probiotic strains’ antagonism toward the pathogen and the mechanistic pathway of action are required to be validated (69). Finally, new standards for manufacturing practices may disqualify or delay some identified therapeutic candidates. For example, the probiotic must have a stable shelf life and be genetically stable over repeated generations of culturing. Probiotics being investigated are undergoing elevated surveillance and, depending on the declared application, are subject to various degrees of certification (Table 2), all of which are geared toward products safety, function, and usability, thereby delaying the translation to specific clinical application.
CURRENT NOTION OF THERAPEUTIC MICROBES
FMT.
In humans, the lack of a heathy gut microbiota due to an imbalance of the normal gut fauna (also called dysbiosis) is associated with various acute and chronic conditions (72–75). This idea is not novel, as the Greek physician Hippocrates postulated that “all disease begins in the gut” nearly 2,500 years ago (76). Recently, physicians and scientists have been exploring ways to reestablish baseline biodiversity in a dysbiotic gut microbiome through the use of fecal microbiota transplantation (FMT) to correct the underlying condition (77). The most common practice is to derive fecal samples from healthy individuals who meet the stringent donor criteria (78). These samples are then used to inoculate alimentary tracts of patients exhibiting dysbiosis (79, 80). As mentioned, fecal transplant practices are not novel, and variations have been used for centuries, with the earliest known implementation having been dated to 300 CE. Ge Hong in China performed the first iterations of FMT to treat gut diseases, wherein he orally administered fecal material mixed with water (“yellow soup”) to patients suffering from diarrhea or food poisoning (81, 82). For unknown reasons, over the next 1,200 years, no records of the practice are found, and references to FMT resurfaced in the 16th century during the Ming Dynasty (81, 83). Discussion of fecal microbiota transplants continued in veterinary medicine into the 17th century (84). Interestingly, the use of FMT overlapped the era of microbe discovery, thus providing a primitive explanation for its beneficial outcomes (85). The compounding evidence of historical FMT usage and modern experimentation in treating recurrent Clostridioides difficile (previously known as Clostridium difficile) infections, in addition to other gut conditions, has further cemented the concept of therapeutic microbes.
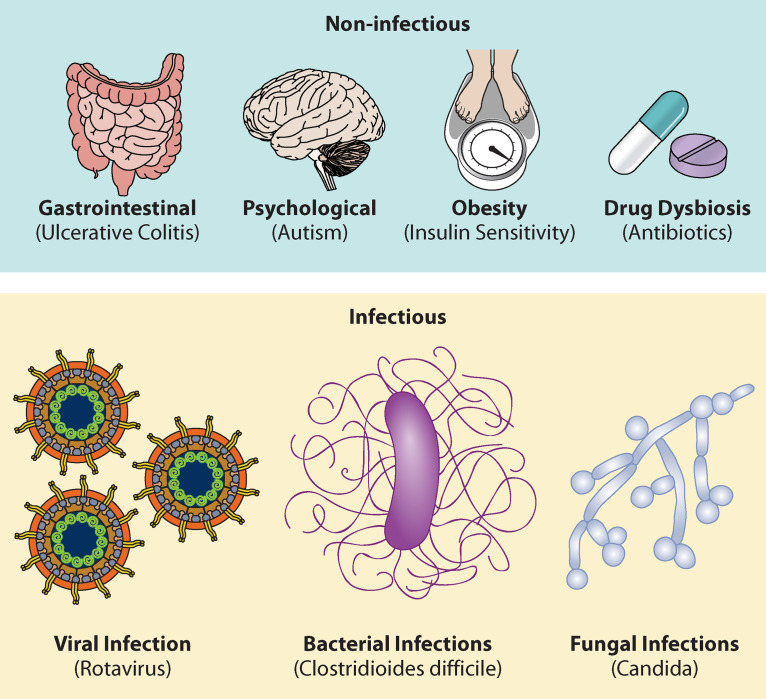
Investigating microbial communities has elucidated covariation between dysbiosis and gut infections. Many patients treated with favorable biodiverse microfloras, via FMTs, have experienced remediation of infectious and noninfectious conditions. Figure 2 presents a graphical summary of recorded FMT applications for review. Under infectious-disease conditions, FMT improved fungal (Candida), viral (rotavirus), and bacterial (MRSA) infectious outcomes (58, 77, 86–88). Notably, C. difficile infection (CDI)—among the most commonly diagnosed and well-studied gastrointestinal diseases—is currently the main target for FMT. A 2019 Centers for Disease Control and Prevention (CDC) report lists CDI as one of the top threats in the United States, with an estimated annual 224,000 cases and 13,000 deaths (89). Physicians have turned to FMT treatment to combat recurrent CDI and its associated secondary infections. Preliminary evidence from FMT-treated patients reported substantial relief from acute CDI symptoms (22). Further randomized trials of FMT against CDI resulted in 81% remission of symptoms, in contrast to an average 27% in groups receiving antibiotics (90). These initial successes of FMT heavily influenced the FDA to authorize it as a recommended treatment against recurrent CDI and waived prerequisites for investigational new drug (IND) (91). Despite the expeditious route of FMT to clinical application, the agency categorizes FMT as an investigational therapeutic drug based on limited empirical data.
FIG 2.
FMT applications in selected noninfectious conditions and infectious diseases (58, 89, 91, 133, 139–142). These include gastrointestinal illness (143), obesity (141), autism (144), dysbiosis resulting from antibiotics (142), and infection with rotavirus (88), Candida (58), and C. difficile (90, 145).
Limitations and risks associated with FMT.
Fecal microbiota transplants remain experimental in composition, efficacy, and safety with respect to non-CDI conditions. For example, FMT failure rates are reportedly on average 18.6% in non-CDI bacterial infections, such as those caused by Enterobacteriaceae, enterococci, and MRSA (77, 92). Another concern involves the potential failure to screen for pathogens (such as Shiga toxin-producing Escherichia coli) and/or transferable antibiotic resistance genes (such as those for ampicillin, ciprofloxacin, and nitrofurantoin resistance) during donor selection. To emphasize the importance of screening, recent clinical trials reported eight patients becoming severely ill, with one death, due to the presence of multidrug-resistant pathogens (93, 94). Similarly, noninfectious diseases (i.e., neurological dysregulation and obesity), in the absence of diligent screening, have been reported to result in transfer of pathogens from donor to recipient (95–98). Unfortunately, the symbiosis between donor and patient, their resident microbiotas, and the target pathogen remains uncharacterized for FMT. The above-mentioned examples of FMT highlight issues that have come to light from the limited research and screening practices. While FMT effectively demonstrates the therapeutic potential of certain gut-derived microbes to benefit human health, considerable work remains warranted, not only to understand the mechanisms leading to its therapeutic outcome but also for the refinement of standard practices of FMT. Furthermore, recipients’ genetics, immunity, microbiota, and lifestyle also shown to influence the clinical efficacy of FMT in infectious and noninfectious conditions. Therefore, a list of strategies must be taken into consideration to improve FMT treatment: (i) optimization of donor-recipient matching in terms of genetics, immunity, and microbiota compatibility; (ii) preparation of the gut environment using antibiotics and bowel cleaning, (iii) targeting the recipient’s immune system with immunosuppressive therapy, and (iv) controlling the recipient’s lifestyle through dietary intervention (99).
Defined microbial consortia.
One noteworthy alternative to FMT is the use of a defined microbial consortium (DMC), which integrates a rationally selected collection of known microbes at specific abundances for treatment of disease conditions. Thus far, authors have used a genome-guided approach to design a DMC, which on administration to germfree mice provided resistance against colonization by pathogenic Salmonella enterica serovar Typhimurium (100). The isolates acquired by genomic sequencing and antibiotic susceptibility testing can be combined to generate DMC against specific pathogens. In another study, researchers prepared a DMC by characterizing a healthy donor fecal sample through culturing of favorable bacteria and then administered it to individuals with recurrent CDI. Patients given the characterized DMC exhibited remission of CDI symptoms over a 24-week span (101). In a similar fashion, researchers provided 55 individuals with recurrent CDI a collection of defined probiotics (Lactobacillus, Bacteroides, Clostridium, etc.) and reported 80% remission in acute cases, with no recurrence for 30 days (102). Recent phase II trials investigated the efficacy and safety of nonpathogenic C. difficile spore administration for the inhibition of pathogenic C. difficile strains and observed 89% remission in patients receiving DMC treatment (103, 104). In a novel pursuit for CDI treatment, data from antibiotic therapy and 16S rRNA profiling revealed that Clostridium scindens to exert an inhibitory influence on C. difficile colonization via the production of secondary bile acids (105). To understand the mechanism behind resistance to colonization by pathogenic C. difficile strains, authors characterized the unique presence of baiCD genes, encoding bile acid metabolism in C. scindens (105, 106). Collectively, these studies indicate that specific microbes with specific genes present in DMC have antagonistic activity toward target pathogens. Hence, DMC has a number of advantages over FMT, such as (i) minimum safety concerns, (ii) lack of pathogenic organisms, and (iii) rationally tailored therapeutic microbes.
Limitations associated with defined consortia.
Defined consortia are not without limitations and concerns. The removal of potential pathogens or antibiotic-resistant organisms is largely beneficial; other aspects of DMC (preparation and efficacy) are questionable. The selection of microbes to include in consortia can be resource intensive and warrant metagenomics analyses, safety testing, and postengraftment evaluation to determine the efficacy of treatment (101). Second, rationally generated DMC may be inferior to FMT due to missed key microbes within the consortia. A recent randomized trial reported 52% efficacy with DMC compared to 76% with FMT engraftments (107). These data suggest the possibility of excluding therapeutic microbes when creating DMC, resulting in a subefficacious outcome. Alternatively, advanced cultivation and identification methods like culturomics—a high-throughput bacterial culturing method that comprehensively identifies strains/species from a complex microbiota and acts as a complement to metagenomics—will be beneficial in discovering and isolating novel therapeutic microbes (108–110). Attesting to the utility of this method, a recent study discovered 174 novel human gut commensals, including rare, previously unidentified bacteria like Deinococcus-Thermus and Synergistetes (108). Finally, consortia rarely include beneficial fungi or viruses, which may also be of therapeutic value. In an interesting study, sterile-filtered stool, which retained virus-like particles, effectively cured six recurrent CDI patients (111). In essence, symbiotic interactions between therapeutic microbes, host, commensals, and pathogens are difficult to characterize due to their complexity. We cannot ignore the limitations of DMC and must consider them if these therapies are utilized in medicine. Preparing DMC with the inclusion of other beneficial microbial organisms (i.e., fungi or viruses), validation of their safety and efficacy, and understanding the mechanisms of action are the pathways for bringing DMC into real clinical practice.
EXPLOITING MMIs TO DISCOVER THERAPEUTIC MICROBES
Microbe-microbe interactions are ubiquitous in nature, occupy all ecosystems, can be extremely complex, and usually involve exchange of chemical signaling, nutrients, gene transfer, and other relevant mechanisms (112). Microbial interactions are broadly classified as positive (mutualism, syntrophism, protocooperation, and commensalism) and negative (amensalism, parasitism, predation, competition) (Table 4) (15). Exploiting inter- and intraspecies MMIs that promote or suppress targeted organisms can provide opportunities to revolutionize medicine and improve patient quality of life with minimal side effects. Conveniently, diversity-rich multispecies ecosystems grant an opening to study microbial communication in an ecosystem and detect therapeutic candidates.
TABLE 4.
Interactions among species grouped broadly into two categories depended if interaction is positive or negativea
| Type | Interaction | Definition/description | Effect on species 1 | Effect on species 2 |
|---|---|---|---|---|
| Positive | Mutualismb | Each species benefits from one another Species 1 is irreplaceable to species 2 and vice versa One species not dependent on other species metabolic product |
+ | + |
| Proto-cooperationc | Both species benefit, but an individual can survive in the absence of the other | + | + | |
| Syntrophismb | Species 1 is dependent on the metabolic product of species 2 | + | + | |
| Commensalism (commensal and host) | + | ∅ | ||
| Negative | Amensalism | ∅ | − | |
| Parasitism (parasite/pathogen and host)b,d |
An individual species derives nutrients from the host species In most cases the parasite does not kill the host |
+ | − | |
| Predationd | One organism kills and consumes the other | + | − | |
| Competition | Intraspecific competition (species strain 1a ≠ species strain 1b) Interspecific competition (species 1 ≠ species 2) |
− | − | |
∅, neutral effect on species.
Obligatory interaction (mutualist and host are metabolically dependent).
Nonobligatory interaction (independent).
Antagonistic interaction.
The classical approach to investigate MMIs, derived from Robert Koch’s postulates, involves obtaining an isolated microbial species that is then one-to-one probed in vitro to appreciate positive and negative interactions. Even today, such is the gold standard to investigate MMIs and etiologies of disease. Given that less than 2% of microbes are culturable using conventional methods (113, 114), only a few candidate organisms can be evaluated with assays; hence, investigating culture-recalcitrant microbial species appears impossible. Additionally, microbial communities may differ conspicuously in species richness. For this reason, we face rapidly increasing dimensions of MMIs that are quickly becoming laborious to untangle (115). Nonetheless, culture-independent, next-generation sequencing (NGS), and bioinformatics analyses followed by predictive modeling have to some extent addressed these challenges. Bioinformatics have simplified MMIs’ principal components and identified candidates for exerting positive or negative influences on microbes of interest that theoretically exist (115). However, the problem of how to isolate lead microbes that have therapeutic potential from their complex microbial community remains.
As just mentioned, postponement of in-depth research of MMIs is due to the current limitations in cultivation technologies and lack of screening methods, ultimately delaying clinical application of therapeutic microbes. Of the cultivable 2% of microbes, therapeutic microbes were haphazardly identified, easily isolated, and fortuitously cultivated using archaic methods. For example, in 1917, Alfred Nissle sampled fecal content from a dysentery-resistant individual and correlated resistance with an abundance of an E. coli biovar. Afterward, he successfully isolated and cultivated the particular E. coli strain exhibiting antagonistic activity against tested enteropathogens. This biovar, named E.coli Nissle 1917, became widely tested as a probiotic of choice for various enteric conditions (116).
In an effort to overcome the “growth barrier,” our team is investigating MMIs in a relatively high-throughput fashion to identify and isolate therapeutic microbes by screening different microbiotas. For a phenotype-based microbiome screening approach, we have developed a high-throughput screening of cell-to-cell interactions (HiSCI) platform by integrating microfluidics, cocultivation methods, flow cytometry, and sequencing techniques. The HiSCI platform has a number of advantages: (i) it creates millions of microdroplets (MDs) comprising defined growth medium and effectively representing an independent petri dish at a picoliter scale, (ii) it preserves microbe-microbe interactions confined in each MD as observed in a complex community, (iii) it facilitates high-throughput screening of phenotypes postcultivation via fluorescence-activated cell sorting (FACS), and (iv) it helps in recovery of microbes exhibiting a desired phenotype(s). We have successfully applied this platform to identify and isolate a bacterial species that promotes (positive interaction) the growth and biomass of Chlorella sorokiniana algae (117). In this study, we isolated bacteria from freshwater ponds and randomly cocaptured a few bacterial cells (≤8 cells) with a single algal cell suspended in MDs containing growth medium. With the help of FACS, enrichment of tens of millions of MDs followed for samples containing algal chlorophyll fluorescence. Down-selected algal-bacterial MDs were cocultivated in a single chip under defined dark-light cycles. Subsequently, via FACS, we rapidly identified and recovered the MDs having increased algal growth with greater fluorescent signals than single captured algal MD controls. We then isolated and propagated the bacterial MDs and subjected them to subsequent rounds of algal cocapture and screening, until the same bacterial species were repeatedly identified. Ultimately, this set of bacterial pond isolates promoted faster and improved algal biomass via conventional growth assays. This first successful demonstration of the HiSCI platform led us to tailor its application for investigating human microbiota interactions. Recently, similar ultrahigh-throughput methods have been developed and applied to identify oral microbiota species with antagonistic characteristics toward pathogenic S. aureus (118, 119).
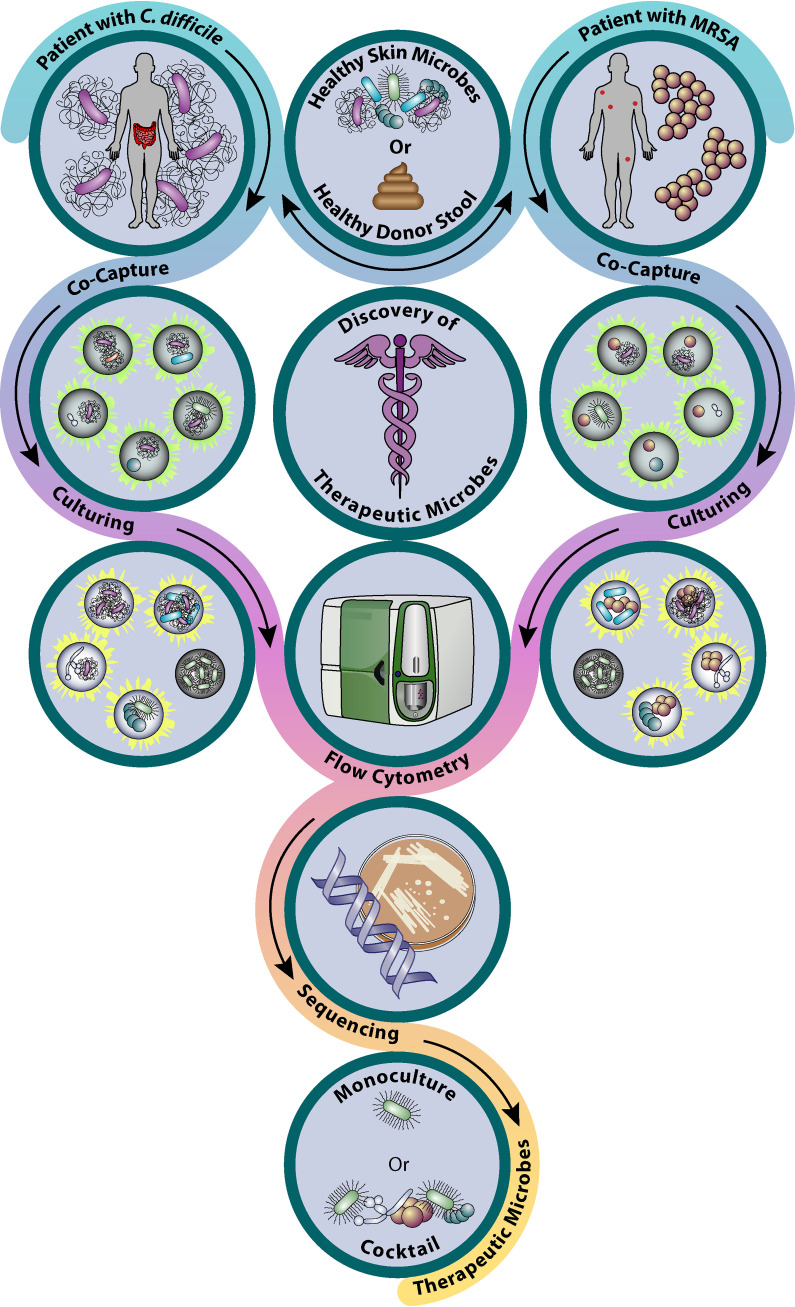
Currently, we are using the modified version of the HiSCI platform to address the public health antibiotic resistance crisis. Specifically, we have adapted our current HiSCI protocols to identify therapeutic microbes that naturally inhibit multidrug-resistant pathogens like C. difficile and methicillin-resistant S. aureus (MRSA) (Fig. 3). In the former project, we are cocapturing microbes harvested from healthy human fecal samples and suspending them with a single C. difficile cell in millions of MDs. In the latter project, a similar method is used to harvest human skin microbes to be cocaptured with a single MRSA cell. Subsequently, cocaptured MDs are cultured under optimal growth conditions—anaerobic (gut) or aerobic (skin)—that mimic natural community conditions. Using flow cytometry with differential staining techniques (antibody staining or fluorescent pathogen strain generation), we sort only MDs where significant growth inhibition of the pathogen strain occurred, as indicated by a low fluorescent signal. At present, we are in the process of identifying associated bacterial species by sequencing efforts (e.g., bacterial 16S rRNA phylotyping), which will, in turn, aid in identifying and isolating the desired therapeutic bacterial species after iterative rounds of selection. Finally, we are in the process of developing an analogous platform wherein we propose to screen for bacterial-bacteriophage interactions in order to rapidly and efficiently identify lytic bacteriophages against MRSA.
FIG 3.
Schematic representation of a microbiome screening platform to identify therapeutic microbes against the antibiotic-resistant pathogens C. difficile and MRSA (146).
Limitations of the HiSCI platform.
Our developed platforms have many advantages in identifying lead microbes; however, we also observed a small number of notable challenges. (i) The current process of capturing microbial cells in MDs is highly inefficient, random, and nonuniform. (ii) Due to randomness, at best 10% of the microbial cell input is captured with the desired cell number inside the MDs, thereby losing most of the microbial consortium. (iii) Microdroplets with diameters greater than 70 μm are discarded, as they are too large to sort via flow cytometry, which in turn contributes to additional loss of the captured cells (117). (iv) Microspheres are made of biocompatible materials, such as agarose or collagen, that limit the capture of certain microbial species that naturally degrade MDs through their enzymatic activities (e.g., agarases). (v) Because of MD volume, only early MMIs are able to be investigated, screened, and phenotyped (117). (vi) HiSCI is biased in favor of rapidly multiplying microbial species, thus ignoring slow growers. (vii) Bacterial species that resist broth culturing cannot be analyzed, as the HiSCI platform is dependent on liquid culturing.
EXPLOITING HMIs TO DISCOVER THERAPEUTIC MICROBES
Like MMIs, HMIs are equally multifaceted and crucial for complex eukaryotic life (e.g., animals, humans, etc.). In fact, higher-order eukaryotic species are believed to contain more microorganisms than eukaryotic cells; therefore, the term “holobiont” or “superorganism” refers to a host plus all the associated microbes in a complex eukaryotic species (120, 121). The concepts of positive and negative interactions applied to MMIs remain consistent for HMIs, with subtle difference in terminology (Table 4) (122). Unlike the pathways of MMIs, where therapeutic microbes indirectly benefit the host by suppressing pathogens or benefitting indigenous microflora, investigating HMI leads to the discovery of microbes that directly benefit the host. This is similar to the case with oncolytic microbes (oncolytic virotherapy and bacterial therapy), wherein therapeutic microbes selectively infect and kill cancerous cells while stimulating a more robust anticancer immune response (123). In 1867, the German physician Wilhelm Busch was the first to observe the phenomenon of cancer remission in patients infected with the agent of erysipelas (now known as Streptococcus pyogenes) (124). Subsequently, William Coley championed the same practice of using killed S. pyrogens with another killed bacterial species (now identified as Serratia marcescens) (123) to successfully treat a number of cancer patients (125). Currently, various genera of modified bacteria and viruses are being tested in preclinical and clinical studies as oncolytic microbes (123).
Although Edward Jenner was the first to immunize against the smallpox virus using material from the pustules of cowpox-infected patients, modern history remembers Louis Pasteur and Robert Koch as the pioneers of investigating HMIs. Pasteur’s and Koch’s work led to revolutions in microbial control and the understanding of disease, such as “pasteurization” and Koch’s postulates (126). Pasteur’s research further contributed to medicine through the development of inoculation strategies using live and dead microbial cells to prevent anthrax in sheep, cattle, and chicken; cholera in fowl; and rabies in humans and dogs (126). The outcome from generations of refinements of the technique have led to a myriad of models, including in vitro models such as cell culture, organoids, bioreactors, and organ-on-a-chip (127); ex vivo models (e.g., harvested living tissue); and in vivo models (e.g., invertebrate and vertebrate models). Notably, the digital revolution and the search for unculturable microbials have made some conventional methods obsolete. With the inception of NGS, machine learning, time-series predictive modeling, and HiSCI, we are able to identify millions of potential therapeutic microbes and extrapolate outcomes in complex life forms. However, the conventional methods still have a degree of applicability in the digital age, specifically validating computational models with observable biological outcomes. For example, authors have isolated and identified a C. scindens strain and demonstrated with metagenomic and time-series analysis modeling in a mouse that this strain inhibited the growth of clinically relevant C. difficile (105). Furthermore, in a recent study using mouse models, researchers identified and demonstrated the potential therapeutic effect of Citrobacter amalonaticus on inhibiting the growth of Citrobacter rodentium, an opportunistic pathogen of humans (128).
Orchestrating computational and laborious in vivo HMI models has made it possible to identify, isolate, and determine the potential of therapeutic microbes. Furthermore, these studies suggest that commensal bacterial species in the presence of pathogens can act as therapeutic microbes by conferring resistance to colonization. Overall, exploiting either HMIs or MMIs to investigate microbes antagonistic toward disease-causing pathogens serves as a novel area for future medicine.
CHALLENGES AND POSSIBLE SOLUTIONS TO THERAPEUTIC MICROBES’ APPLICATIONS
Technologies to discover therapeutic microbes are still being pioneered and, as such, have elucidated many challenges to overcome. The first concerns live cells and growth rate. Viable cells with self-renewal properties complicate calculation of pharmacokinetics and pharmacodynamics during clinical testing (129). For example, the growth rate of these microbes is dependent on person-specific factors like food intake, native microbiota composition, and disease status. To address person-to-person variability, we can screen individuals and group them based on microbiome composition, diet, and physical activities, etc. To achieve this the current population-based medicine approach (i.e., one size fits all) needs to be replaced with personalized medicine. The second challenge involves mixed strains, complex interactions, and downstream effects. Multistrain therapeutic microbes have another layer of complexity—multiple off-target interactions (129). Cultivation of mixed strains and strain integrity will challenge the manufacturing process, wherein genotypes can be highly variable from batch to batch. Evolving or developing a species with target specificity, gene stability, an inherited self-destructive kill switch, and a built-in strain characterization can address these challenges and ensure safety. The third challenge is that of drug delivery and dosing. The standard pharmacological processes—absorption, distribution, metabolism, and excretion—have limited application in the use of therapeutic microbe products. Instead, gastrointestinal distribution, attachment (colonization), multiplication, and shedding (clearance) need consideration. (129). The fourth challenge concerns manufacturing and quality assurance. Therapeutic microbes need special consideration regarding temperature, pH, salt concentration, etc., to maintain the product’s quality. The use of enteric-coated gelatin capsules filled with buffers and low-temperature storage are possibilities for mitigating these concerns. Last, there is the question of FDA regulation. The FDA is currently working with leading startup companies in this area and developing regulation for such products (e.g., SER-109; Seres Therapeutics). Given that information, placement in the FDA’s categorization regarding IND application status is still in debate (129, 130).
PROSPECTS OF THERAPEUTIC-MICROBE DISCOVERY
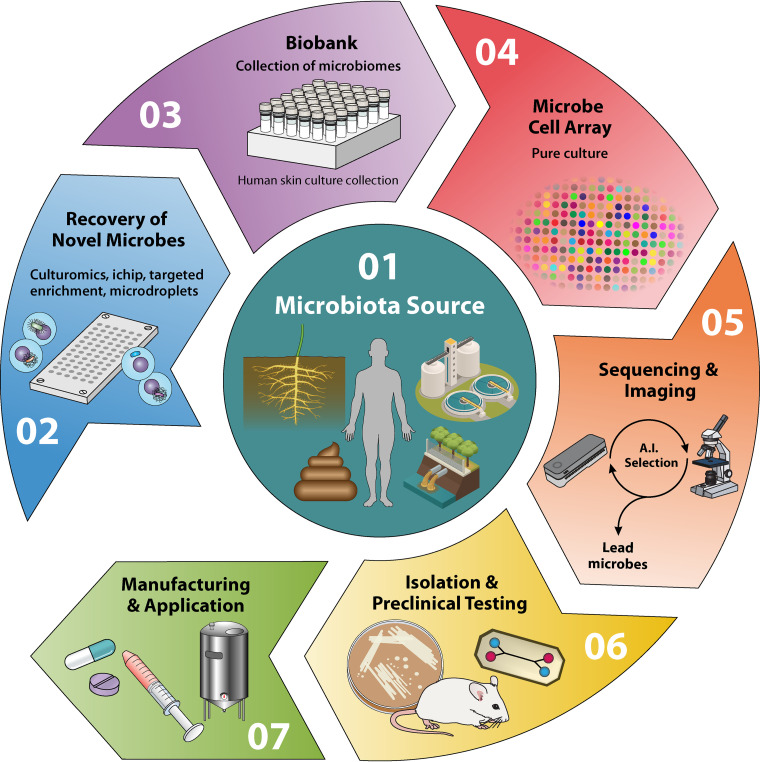
We are just beginning to appreciate the value of microbial ecosystems in dictating human health and to be able to gauge the usefulness of microbes for therapeutic and diagnostic applications. In order to truly understand and exploit microbiotas for therapeutic applications, we need extensive interdisciplinary efforts (Fig. 4). Advances in any of the following avenues of research, alone or in combination, will be a step forward in discovering therapeutic microbes: (i) next-generation culturing methods to grow unculturable organisms (e.g., in situ culturing via an isolation chip [ichip] [131], targeted phenotypic culturing [132], etc.), (ii) extensive collection of a microbe biobank for species-specific and tissue-specific microbiotas (e.g., skin microbiome, gut microbiome, etc.), (iii) advanced microfluidics (pico- or nanovolume ichips) culturing (117, 133), (iv) highly sensitive and specific rapid screening assays, (v) high-density microbial cell arrays (134) that can readily be used to screen against any pathogens, (vi) real-time sequencing and data analysis, and finally, (vii) artificial intelligence (AI)-integrated advanced and rapid imaging techniques.
FIG 4.
Circular flow diagram highlighting the critical steps needed for therapeutic-microbe discovery. (1) Therapeutic-microbe source: human, animal, or environment. (2) Next-generation culturing methodologies (ichip, large-cohort cultivation, etc.) to collect unculturable microbes. (3) Isolating, cataloguing, and preservation of cultured novel microbial species to generate a large microbial biobank. (4) Generating and sharing high-density microbial cell arrays for microbe-pathogen screening. (5) Concomitant analysis of real-time genome sequencing and high-resolution microscopy to identify potential therapeutic microbes. (6) Preclinical testing of isolated therapeutic microbes. (7) Initiation of clinical trials to appreciate efficacy of therapeutic microbes and subsequent mass production, per FDA guidelines. A.I., artificial intelligence.
CONCLUSION
Due to limited technologies, exploiting a microbial community’s structure and function for the discovery of favorable therapeutic microbes remains a daunting task. Recent bursts of microbiome research have ignited this research field, but it needs significant interdisciplinary collaborative efforts to address discovery and application challenges. We expect living therapeutics and diagnostics to be the next medical revolution, similar to the discovery of antibiotics in the early 19th century.
ACKNOWLEDGMENTS
Anand Kumar’s research work is supported by a Los Alamos National Laboratory (LANL) internal grant [20210612ECR: A high-throughput (RapidPhage) platform for the discovery of lytic bacteriophages against multidrug resistance pathogens]. The majority of Anand Kumar’s previous research was also funded by two internal grants, Using therapeutic bacteria to treat human diseases (grant 20160340ER) and Developing a unique technology to discover a novel gut microbial cocktail to treat antibiotic resistance pathogens (grant 20170671PRD2), to develop and establish a microbiome screening platform.
Conceptualization and project administration, A.K.; minireview design, A.K., N.C., G.A.A.; writing—original draft preparation, N.C., A.K., G.A.; writing—review and editing, N.C., A.K., G.A.A., A.E.K.D.; supervision, A.K., visualization, G.A.A., N.C. All authors have read and agreed to the published version of the manuscript.
We declare no conflict of interest.
Biographies

Nathan Cruz is a post-master’s student at Los Alamos National Laboratory (LANL). He holds a B.S. in psychological sciences and a M.S. in biology from Northern Arizona University (NAU). During his undergraduate studies, he assisted Dr. Christopher Woodruff in recording neuron activity using electroencephalography in humans to understand the neuroscience of empathy. In his senior year, he joined Dr. Robert Kellar’s regenerative medicine laboratory to gain experience in mammalian cell culture techniques. By securing the NAU Research Initiative for Scientific Enhancement scholarship, he enrolled in a M.S. program and continued research under Dr. Kellar. With cell culture experience, in his M.S. studies he used an in vitro model to investigate the nocuous effects of uranyl nitrate on wound healing. While analyzing his research data, Mr. Cruz realized how important statistical tools are in hypothesis-driven biological research. This led him to simultaneously graduate with a certification in applied statistics. At LANL, he works with Dr. Anand Kumar in basic and applied aspects of STEM research. Concurrently, he is supporting the development of a microfluidic-based bacteriophage discovery platform. In the next few years, his ambition is to pursue a Ph.D. in Applied Mathematics to continue his passion in data-driven medical research.

George A. Abernathy is a post-master’s student working with Dr. Anand Kumar at LANL. He holds a B.S. in genetics and biotechnology from New Mexico State University and an M.S. in integrative genomics from Black Hills State University. While an undergraduate student, he received a research scholarship from the Howard Hughes Medical Institute to acquire basic microbiological skills. After obtaining his B.S., he worked with veterinary physicians as a full-time assistant to support surgeries and routine care for small and exotic animals. During his five years of veterinary assistantship, he developed a passion for research with the ultimate goal of promoting animal health. He enrolled in a graduate program and conducted research under the guidance of Dr. Cynthia Anderson. His research focused on the effect of a natural antifungal agent against fungal species pathogenic to humans and animals. With his M.S. in integrative genomics, he joined Dr. Kumar’s team at LANL to develop an innovative and rapid phage technology to discover an effective lytic phage cocktail against antibiotic resistant pathogens. Mr. Abernathy plans to pursue an integrated D.V.M. research program to conduct translational research that benefits human and animal health.

Armand E. K. Dichosa, Ph.D., M.S., is a staff scientist in Bioscience Division at LANL. He received both his M.S. and Ph.D. from the University of New Mexico in biology, investigating extremophile archaeal and bacterial communities and their potential roles in their respective environments. His core research interests focus on the genomic and viable recovery of novel bacterial species from various environments and determining their potential beneficial or adverse impacts on human health, national security, and the environment. Dr. Dichosa pioneered the integration of gel microdroplets with single-cell genomics to dissect complex microbiomes for various research efforts involving biodegradation, probiotic therapies, and novel bacterial characterization. He collaborates on various projects with Dr. Anand Kumar and his team to discover therapeutic microbes. Dr. Dichosa is involved in promoting global cooperatives for microbial biosurveillance through genomics and bioinformatics outreach efforts, as well as leading microbiology-genomics STEM efforts for our nation’s high school students.

Anand Kumar, D.V.M., Ph.D., is a staff scientist in the Bioscience Division at LANL. He received his D.V.M. in India and M.S. and Ph.D. degrees from the Ohio State University. His Ph.D. research focused on understanding host responses to probiotics as well as investigating the impact of diet and rotavirus infection on a humanized pig model. During his M.S. research, he investigated Campylobacter pathogenesis and developed prophylactic (nanoparticle vaccine) and therapeutic (small-molecule discovery) measures against it. During his postdoctoral tenure at LANL, he led several efforts to establish and develop microbiome-screening platforms. Dr. Kumar’s research is currently geared toward microbiome engineering by (i) discovering and developing novel microbiome-based therapeutics to tackle the threat of emerging bacterial pathogens, (ii) engineering probiotic strains to create a living diagnostic-therapeutic system, and (iii) evolving a bioreactor model of the human gut to investigate host-microbe interactions.
Contributor Information
Anand Kumar, Email: akumar@lanl.gov.
Karen M. Ottemann, University of California, Santa Cruz
REFERENCES
- 1.Stilling RM, Bordenstein SR, Dinan TG, Cryan JF. 2014. Friends with social benefits: host-microbe interactions as a driver of brain evolution and development? Front Cell Infect Microbiol 4:147. 10.3389/fcimb.2014.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y-J, Li S, Gan R-Y, Zhou T, Xu D-P, Li H-B. 2015. Impacts of gut bacteria on human health and diseases. Int J Mol Sci 16:7493–7519. 10.3390/ijms16047493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaiko GE, Stappenbeck TS. 2014. Host–microbe interactions shaping the gastrointestinal environment. Trends Immunol 35:538–548. 10.1016/j.it.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker A, Lawson MAE, Vaux L, Pin C. 2018. Host-microbe interaction in the gastrointestinal tract. Environ Microbiol 20:2337–2353. 10.1111/1462-2920.13926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Asmakh M, Zadjali F. 2015. Use of germ-free animal models in microbiota-related research. J Microbiol Biotechnol 25:1583–1588. 10.4014/jmb.1501.01039. [DOI] [PubMed] [Google Scholar]
- 6.Benson AK, Kelly SA, Legge R, Ma F, Low SJ, Kim J, Zhang M, Oh PL, Nehrenberg D, Hua K, Kachman SD, Moriyama EN, Walter J, Peterson DA, Pomp D. 2010. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci USA 107:18933–18938. 10.1073/pnas.1007028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sencio V, Machado MG, Trottein F. 2021. The lung–gut axis during viral respiratory infections: the impact of gut dysbiosis on secondary disease outcomes. Mucosal Immunol 14:296–304. 10.1038/s41385-020-00361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rea MC, O'Sullivan O, Shanahan F, O'Toole PW, Stanton C, Ross RP, Hill C. 2012. Clostridium difficile carriage in elderly subjects and associated changes in the intestinal microbiota. J Clin Microbiol 50:867–875. 10.1128/JCM.05176-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkins LJ, Monga M, Miller AW. 2019. Defining dysbiosis for a cluster of chronic diseases. Sci Rep 9:12918. 10.1038/s41598-019-49452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuman H, Forsythe P, Uzan A, Avni O, Koren O. 2018. Antibiotics in early life: dysbiosis and the damage done. FEMS Microbiol Rev 42:489–499. 10.1093/femsre/fuy018. [DOI] [PubMed] [Google Scholar]
- 11.Blustein J, Liu J. 2015. Time to consider the risks of caesarean delivery for long term child health. BMJ 350:h2410–h2410. 10.1136/bmj.h2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magne F, Puchi Silva A, Carvajal B, Gotteland M. 2017. The elevated rate of Cesarean section and its contribution to non-communicable chronic diseases in Latin America: the growing involvement of the microbiota. Front Pediatr 5:192. 10.3389/fped.2017.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou H, Xu Q, Liu Y, Guo L-T. 2020. Risk factors, incidence, and morbidity associated with antibiotic-associated diarrhea in intensive care unit patients receiving antibiotic monotherapy. World J Clin Cases 8:1908–1915. 10.12998/wjcc.v8.i10.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson BC, Butler ÉM, Grigg CP, Derraik JGB, Chiavaroli V, Walker N, Thampi S, Creagh C, Reynolds AJ, Vatanen T, O'Sullivan JM, Cutfield WS. 2021. Oral administration of maternal vaginal microbes at birth to restore gut microbiome development in infants born by caesarean section: a pilot randomised placebo-controlled trial. EBioMedicine 69:103443. 10.1016/j.ebiom.2021.103443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg G, Rybakova D, Fischer D, Cernava T, Vergès M-CC, Charles T, Chen X, Cocolin L, Eversole K, Corral GH, Kazou M, Kinkel L, Lange L, Lima N, Loy A, Macklin JA, Maguin E, Mauchline T, McClure R, Mitter B, Ryan M, Sarand I, Smidt H, Schelkle B, Roume H, Kiran GS, Selvin J, Souza RSC, de van Overbeek L, Singh BK, Wagner M, Walsh A, Sessitsch A, Schloter M. 2020. Microbiome definition re-visited: old concepts and new challenges. Microbiome 8:103. 10.1186/s40168-020-00875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shanahan F. 2010. Gut microbes: from bugs to drugs. Am J Gastroenterol 105:275–279. 10.1038/ajg.2009.729. [DOI] [PubMed] [Google Scholar]
- 17.Kang M, Choe D, Kim K, Cho B-K, Cho S. 2020. Synthetic biology approaches in the development of engineered therapeutic microbes. Int J Mol Sci 21:8744. 10.3390/ijms21228744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charbonneau MR, Isabella VM, Li N, Kurtz CB. 2020. Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nat Commun 11:1738. 10.1038/s41467-020-15508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hania WB, Ballet N, Vandeckerkove P, Ollivier B, O’Toole PW, Brugère J-F. 2017. Archaebiotics: Archaea as pharmabiotics for treating chronic disease in humans? In Sghaier H, Najjari A, Ghedira K (ed), Archaea—new biocatalysts, novel pharmaceuticals and various biotechnological applications. IntechOpen, London, United Kingdom. [Google Scholar]
- 20.deMenocal PB. 1995. Plio-Pleistocene African climate. Science 270:53–59. 10.1126/science.270.5233.53. [DOI] [PubMed] [Google Scholar]
- 21.Potts R. 1996. Evolution and climate variability. Science 273:922–923. 10.1126/science.273.5277.922. [DOI] [Google Scholar]
- 22.Schwartz GT. 2012. Growth, development, and life history throughout the evolution of Homo. Curr Anthropol 53:S395–S408. 10.1086/667591. [DOI] [Google Scholar]
- 23.Winder IC, Devès MH, King GCP, Bailey GN, Inglis RH, Meredith-Williams M. 2015. Evolution and dispersal of the genus Homo: a landscape approach. J Hum Evol 87:48–65. 10.1016/j.jhevol.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Crittenden AN, Schnorr SL. 2017. Current views on hunter-gatherer nutrition and the evolution of the human diet. Am J Phys Anthropol 162:84–109. 10.1002/ajpa.23148. [DOI] [PubMed] [Google Scholar]
- 25.Nakazawa Y, Hosono A, Howells BW. 1992. Functions of fermented milk challenges for the health sciences. Elsevier Applied Science, London, United Kingdom. [Google Scholar]
- 26.Ozen M, Dinleyici EC. 2015. The history of probiotics: the untold story. Benef Microbes 6:159–165. 10.3920/BM2014.0103. [DOI] [PubMed] [Google Scholar]
- 27.McGovern PE, Zhang J, Tang J, Zhang Z, Hall GR, Moreau RA, Nunez A, Butrym ED, Richards MP, Wang C.-s, Cheng G, Zhao Z, Wang C. 2004. Fermented beverages of pre- and proto-historic China. Proc Natl Acad Sci USA 101:17593–17598. 10.1073/pnas.0407921102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anonymous . 2008. Origin, history and manufacturing process of Egyptian dairy products: an overview. Alex J Food Sci Technol 5:51–62. [Google Scholar]
- 29.Wang J, Liu L, Ball T, Yu L, Li Y, Xing F. 2016. Revealing a 5,000-y-old beer recipe in China. Proc Natl Acad Sci USA 113:6444–6448. 10.1073/pnas.1601465113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breasted JH. 1906. Ancient records of Egypt: historical documents from the earliest times to the Persian conquest. University of Chicago Press, Chicago, IL. http://jhir.library.jhu.edu/handle/1774.2/35053. Retrieved 28 October 2021. [Google Scholar]
- 31.Perles C. 1977. Le strategie alimentari nella preistoria, p 12–25. In Flandrin JL, Montanari M (ed) Storia dell’alimentazione. Editori Laterza, Rome, Italy. [Google Scholar]
- 32.Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM. 2011. Phage treatment of human infections. Bacteriophage 1:66–85. 10.4161/bact.1.2.15845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witzany C, Bonhoeffer S, Rolff J. 2020. Is antimicrobial resistance evolution accelerating? PLoS Pathog 16:e1008905. 10.1371/journal.ppat.1008905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramezani A, Nolin TD, Barrows IR, Serrano MG, Buck GA, Regunathan-Shenk R, West RE, Latham PS, Amdur R, Raj DS. 2018. Gut colonization with methanogenic Archaea lowers plasma trimethylamine N-oxide concentrations in apolipoprotein e−/− mice. Sci Rep 8:14752. 10.1038/s41598-018-33018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balouiri M, Sadiki M, Ibnsouda SK. 2016. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6:71–79. 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi EA, Chang HC. 2015. Cholesterol-lowering effects of a putative probiotic strain Lactobacillus plantarum EM isolated from kimchi. LWT - Food Sci Technol 62:210–217. 10.1016/j.lwt.2015.01.019. [DOI] [Google Scholar]
- 37.Macaluso G, Fiorenza G, Gaglio R, Mancuso I, Scatassa ML. 2016. In vitro evaluation of bacteriocin-like inhibitory substances produced by lactic acid bacteria isolated during traditional Sicilian cheese making. Ital J Food Saf 5:5503. 10.4081/ijfs.2016.5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoelzer K, Cummings KJ, Warnick LD, Schukken YH, Siler JD, Gröhn YT, Davis MA, Besser TE, Wiedmann M. 2011. Agar disk diffusion and automated microbroth dilution produce similar antimicrobial susceptibility testing results for Salmonella serotypes Newport, Typhimurium, and 4,5,12:i. Foodborne Pathog Dis 8:8:1281–1288. 10.1089/fpd.2011.0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfaller MA, Sheehan DJ, Rex JH. 2004. Determination of fungicidal activities against yeasts and molds: lessons learned from bactericidal testing and the need for standardization. Clin Microbiol Rev 17:268–280. 10.1128/CMR.17.2.268-280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cani PD, de Vos WM. 2017. Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front Microbiol 8:1765. 10.3389/fmicb.2017.01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Food and Agriculture Organization. 2006. Probiotics in food: health and nutritional properties and guidelines for evaluation. Food and Agriculture Organization, Rome, Italy. [Google Scholar]
- 42.Caetano-Silva ME, Capitani CD, Antunes AEC, Vougt E, da Silva VSN, Pacheco MTB. 2015. Whey protein-carboxymethylcellulose obtained by complex coacervation as an ingredient in probiotic fermented milk. Food Nutr Sci 06:571–580. 10.4236/fns.2015.66060. [DOI] [Google Scholar]
- 43.Sanders ME. 2003. Probiotics: considerations for human health. Nutr Rev 61:91–99. 10.1301/nr.2003.marr.91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brandão RL, Castro IM, Bambirra EA, Amaral SC, Fietto LG, Tropia MJM, Neves MJ, Dos Santos RG, Gomes NCM, Nicoli JR. 1998. Intracellular signal triggered by cholera toxin in Saccharomyces boulardii and Saccharomyces cerevisiae. Appl Environ Microbiol 64:564–568. 10.1128/AEM.64.2.564-568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chenoll E, Casinos B, Bataller E, Astals P, Echevarría J, Iglesias JR, Balbarie P, Ramón D, Genovés S. 2011. Novel probiotic Bifidobacterium bifidum CECT 7366 strain active against the pathogenic bacterium Helicobacter pylori. Appl Environ Microbiol 77:1335–1343. 10.1128/AEM.01820-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schachtsiek M, Hammes WP, Hertel C. 2004. Characterization of Lactobacillus coryniformis DSM 20001T surface protein Cpf mediating coaggregation with and aggregation among pathogens. Appl Environ Microbiol 70:7078–7085. 10.1128/AEM.70.12.7078-7085.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma C, Singh BP, Thakur N, Gulati S, Gupta S, Mishra SK, Panwar H. 2017. Antibacterial effects of Lactobacillus isolates of curd and human milk origin against food-borne and human pathogens. 3 Biotech 7:31. 10.1007/s13205-016-0591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. 2019. Mechanisms of Action of Probiotics. Adv Nutr 10:S49–S66. 10.1093/advances/nmy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parsons JB, Yao J, Frank MW, Jackson P, Rock CO. 2012. Membrane disruption by antimicrobial fatty acids releases low-molecular-weight proteins from Staphylococcus aureus. J Bacteriol 194:5294–5304. 10.1128/JB.00743-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Begley M, Hill C, Gahan CGM. 2006. Bile salt hydrolase activity in probiotics. Appl Environ Microbiol 72:1729–1738. 10.1128/AEM.72.3.1729-1738.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McAuliffe O, Cano RJ, Klaenhammer TR. 2005. Genetic analysis of two bile salt hydrolase activities in Lactobacillus acidophilus NCFM. Appl Environ Microbiol 71:4925–4929. 10.1128/AEM.71.8.4925-4929.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Valdez GF, Martos G, Taranto MP, Lorca GL, Oliver G, de Ruiz Holgado AP. 1997. Influence of bile on β-galactosidase activity and cell viability of Lactobacillus reuteri when subjected to freeze-drying. J Dairy Sci 80:1955–1958. 10.3168/jds.S0022-0302(97)76137-X. [DOI] [PubMed] [Google Scholar]
- 53.Grill J, Schneider F, Crociani J, Ballongue J. 1995. Purification and characterization of conjugated bile salt hydrolase from Bifidobacterium longum BB536. Appl Environ Microbiol 61:2577–2582. 10.1128/aem.61.7.2577-2582.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taranto MP, Fernandez Murga ML, Lorca G, Valdez GF. 2003. Bile salts and cholesterol induce changes in the lipid cell membrane of Lactobacillus reuteri. J Appl Microbiol 95:86–91. 10.1046/j.1365-2672.2003.01962.x. [DOI] [PubMed] [Google Scholar]
- 55.Gómez-Llorente C, Muñoz S, Gil A. 2010. Role of Toll-like receptors in the development of immunotolerance mediated by probiotics. Proc Nutr Soc 69:381–389. 10.1017/S0029665110001527. [DOI] [PubMed] [Google Scholar]
- 56.Lebeer S, Vanderleyden J, De Keersmaecker SCJ. 2010. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol 8:171–184. 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- 57.Braat H, van den Brande J, van Tol E, Hommes D, Peppelenbosch M, van Deventer S. 2004. Lactobacillus rhamnosus induces peripheral hyporesponsiveness in stimulated CD4+ T cells via modulation of dendritic cell function. Am J Clin Nutr 80:1618–1625. 10.1093/ajcn/80.6.1618. [DOI] [PubMed] [Google Scholar]
- 58.Matsuo K, Haku A, Bi B, Takahashi H, Kamada N, Yaguchi T, Saijo S, Yoneyama M, Goto Y. 2019. Fecal microbiota transplantation prevents Candida albicans from colonizing the gastrointestinal tract. Microbiol Immunol 63:155–163. 10.1111/1348-0421.12680. [DOI] [PubMed] [Google Scholar]
- 59.Ukena SN, Singh A, Dringenberg U, Engelhardt R, Seidler U, Hansen W, Bleich A, Bruder D, Franzke A, Rogler G, Suerbaum S, Buer J, Gunzer F, Westendorf AM. 2007. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One 2:e1308. 10.1371/journal.pone.0001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. 2007. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCζ redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol 9:804–816. 10.1111/j.1462-5822.2006.00836.x. [DOI] [PubMed] [Google Scholar]
- 61.O’Toole PW, Marchesi JR, Hill C. 2017. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol 2:17057. 10.1038/nmicrobiol.2017.57. [DOI] [PubMed] [Google Scholar]
- 62.Ouwehand AC, Forssten S, Hibberd AA, Lyra A, Stahl B. 2016. Probiotic approach to prevent antibiotic resistance. Ann Med 48:246–255. 10.3109/07853890.2016.1161232. [DOI] [PubMed] [Google Scholar]
- 63.Vital M, Chai B, Østman B, Cole J, Konstantinidis KT, Tiedje JM. 2015. Gene expression analysis of E. coli strains provides insights into the role of gene regulation in diversification. ISME J 9:1130–1140. 10.1038/ismej.2014.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Puebla-Barragan S, Reid G. 2019. Thirty-year evolution of probiotic therapy. Microb Cell 6:184–196. 10.15698/mic2019.04.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kandasamy S, Vlasova AN, Fischer DD, Chattha KS, Shao L, Kumar A, Langel SN, Rauf A, Huang H-C, Rajashekara G, Saif LJ. 2017. Unraveling the differences between Gram-positive and Gram-negative probiotics in modulating protective immunity to enteric infections. Front Immunol 8:334. 10.3389/fimmu.2017.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar A, Vlasova AN, Liu Z, Chattha KS, Kandasamy S, Esseili M, Zhang X, Rajashekara G, Saif LJ. 2014. In vivo gut transcriptome responses to Lactobacillus rhamnosus GG and Lactobacillus acidophilus in neonatal gnotobiotic piglets. Gut Microbes 5:152–164. 10.4161/gmic.27877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Markowiak P, Śliżewska K. 2017. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9:1021. 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanders ME, Lenoir-Wijnkoop I, Salminen S, Merenstein DJ, Gibson GR, Petschow BW, Nieuwdorp M, Tancredi DJ, Cifelli CJ, Jacques P, Pot B. 2014. Probiotics and prebiotics: prospects for public health and nutritional recommendations: probiotics and prebiotics. Ann N Y Acad Sci 1309:19–29. 10.1111/nyas.12377. [DOI] [PubMed] [Google Scholar]
- 69.Ganguly NK, Bhattacharya SK, Sesikeran B, Nair GB, Ramakrishna BS, Sachdev HPS, Batish VK, Kanagasabapathy AS, Muthuswamy V, Kathuria SC, Katoch VM, Satyanarayana K, Toteja GS, Rahi M, Rao S, Bhan MK, Kapur R, Hemalatha R. 2011. ICMR-DBT guidelines for evaluation of probiotics in food. Indian J Med Res 134:22–25. [PMC free article] [PubMed] [Google Scholar]
- 70.European Food Safety Authority. 2005. Opinion of the Scientific Committee on a request from EFSA related to a generic approach to the safety assessment by EFSA of microorganisms used in food/feed and the production of food/feed additives. EFSA J 3(6):226. 10.2903/j.efsa.2005.226. [DOI] [Google Scholar]
- 71.Temmerman R, Pot B, Huys G, Swings J. 2003. Identification and antibiotic susceptibility of bacterial isolates from probiotic products. Int J Food Microbiol 81:1–10. 10.1016/S0168-1605(02)00162-9. [DOI] [PubMed] [Google Scholar]
- 72.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. 2007. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 104:13780–13785. 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.The Human Microbiome Project Consortium. 2012. Structure, function and diversity of the healthy human microbiome. Nature 486:207–214. 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parracho HM, Bingham MO, Gibson GR, McCartney AL. 2005. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol 54:987–991. 10.1099/jmm.0.46101-0. [DOI] [PubMed] [Google Scholar]
- 75.Proal AD, Lindseth IA, Marshall TG. 2017. Microbe-microbe and host-microbe interactions drive microbiome dysbiosis and inflammatory processes. Discov Med 23:51–60. [PubMed] [Google Scholar]
- 76.Lyon L. 2018. ‘All disease begins in the gut’: was Hippocrates right? Brain 141:e20. 10.1093/brain/awy017. [DOI] [PubMed] [Google Scholar]
- 77.Yoon YK, Suh JW, Kang E-J, Kim JY. 2019. Efficacy and safety of fecal microbiota transplantation for decolonization of intestinal multidrug-resistant microorganism carriage: beyond Clostridioides difficile infection. Ann Med 51:379–389. 10.1080/07853890.2019.1662477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bibbò S, Settanni CR, Porcari S, Bocchino E, Ianiro G, Cammarota G, Gasbarrini A. 2020. Fecal microbiota transplantation: screening and selection to choose the optimal donor. J Clin Microbiol 9:1757. 10.3390/jcm9061757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ray A, Jones C. 2016. Does the donor matter? Donor vs patient effects in the outcome of a next-generation microbiota-based drug trial for recurrent Clostridium difficile infection. Future Microbiol 11:611–616. 10.2217/fmb.16.10. [DOI] [PubMed] [Google Scholar]
- 80.Wang J-W, Kuo C-H, Kuo F-C, Wang Y-K, Hsu W-H, Yu F-J, Hu H-M, Hsu P-I, Wang J-Y, Wu D-C. 2019. Fecal microbiota transplantation: review and update. J Formos Med Assoc 118:S23–S31. 10.1016/j.jfma.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 81.Brandt LJ. 2012. Response to Zhang et al. Am J Gastroenterol 107:1755–1756. 10.1038/ajg.2012.253.23160295 [DOI] [Google Scholar]
- 82.Hong G. 2000. Zhou hou bei ji fang. Tianjin Science & Technology Press, Tianjin, China. [Google Scholar]
- 83.Shizhen L. 2011. Ben cao gang mu. Huaxia Press, Beijing, China. [Google Scholar]
- 84.Niederwerder MC. 2018. Fecal microbiota transplantation as a tool to treat and reduce susceptibility to disease in animals. Vet Immunol Immunopathol 206:65–72. 10.1016/j.vetimm.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Groot PF, Frissen MN, de Clercq NC, Nieuwdorp M. 2017. Fecal microbiota transplantation in metabolic syndrome: history, present and future. Gut Microbes 8:253–267. 10.1080/19490976.2017.1293224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jacobson A, Lam L, Rajendram M, Tamburini F, Honeycutt J, Pham T, Van Treuren W, Pruss K, Stabler SR, Lugo K, Bouley DM, Vilches-Moure JG, Smith M, Sonnenburg JL, Bhatt AS, Huang KC, Monack D. 2018. A gut commensal-produced metabolite mediates colonization resistance to Salmonella infection. Cell Host Microbe 24:296–307.E7. 10.1016/j.chom.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wei Y, Gong J, Zhu W, Guo D, Gu L, Li N, Li J. 2015. Fecal microbiota transplantation restores dysbiosis in patients with methicillin resistant Staphylococcus aureus enterocolitis. BMC Infect Dis 15:265. 10.1186/s12879-015-0973-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi Z, Zou J, Zhang Z, Zhao X, Noriega J, Zhang B, Zhao C, Ingle H, Bittinger K, Mattei LM, Pruijssers AJ, Plemper RK, Nice TJ, Baldridge MT, Dermody TS, Chassaing B, Gewirtz AT. 2019. Segmented filamentous bacteria prevent and cure rotavirus infection. Cell 179:644–658.E13. 10.1016/j.cell.2019.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Centers for Disease Control and Prevention. 2019. Antibiotic resistance threats in the United States, 2019. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 90.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JFWM, Tijssen JGP, Speelman P, Dijkgraaf MGW, Keller JJ. 2013. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 368:407–415. 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 91.Food and Drug Administration. 2013. Enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies; availability (docket no. FDA-2013-D-0811).
- 92.Fischer M, Kao D, Mehta SR, Martin T, Dimitry J, Keshteli AH, Cook GK, Phelps E, Sipe BW, Xu H, Kelly CR. 2016. Predictors of early failure after fecal microbiota transplantation for the therapy of Clostridium difficile infection: a multicenter study. Am J Gastroenterol 111:1024–1031. 10.1038/ajg.2016.180. [DOI] [PubMed] [Google Scholar]
- 93.DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, Turbett S, Chung RT, Chen Y-B, Hohmann EL. 2019. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med 381:2043–2050. 10.1056/NEJMoa1910437. [DOI] [PubMed] [Google Scholar]
- 94.Zellmer C, Sater MRA, Huntley MH, Osman M, Olesen SW, Ramakrishna B. 2021. Shiga toxin-producing Escherichia coli transmission via fecal microbiota transplant. Clin Infect Dis 72:e876–e880. 10.1093/cid/ciaa1486. [DOI] [PubMed] [Google Scholar]
- 95.Alang N, Kelly CR. 2015. Weight gain after fecal microbiota transplantation. Open Forum Infect Dis 2ofv004. 10.1093/ofid/ofv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ma Y, Liu J, Rhodes C, Nie Y, Zhang F. 2017. Ethical issues in fecal microbiota transplantation in practice. Am J Bioeth 17:34–45. 10.1080/15265161.2017.1299240. [DOI] [PubMed] [Google Scholar]
- 97.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI. 2013. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341:1241214. 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, Zhang X, Yang D, Yang Y, Meng H, Li W, Melgiri ND, Licinio J, Wei H, Xie P. 2016. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 21:786–796. 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- 99.Danne C, Rolhion N, Sokol H. 2021. Recipient factors in faecal microbiota transplantation: one stool does not fit all. Nat Rev Gastroenterol Hepatol 18:503–513. 10.1038/s41575-021-00441-5. [DOI] [PubMed] [Google Scholar]
- 100.Brugiroux S, Beutler M, Pfann C, Garzetti D, Ruscheweyh H-J, Ring D, Diehl M, Herp S, Lötscher Y, Hussain S, Bunk B, Pukall R, Huson DH, Münch PC, McHardy AC, McCoy KD, Macpherson AJ, Loy A, Clavel T, Berry D, Stecher B. 2017. Genome-guided design of a defined mouse microbiota that confers colonization resistance against Salmonella enterica serovar Typhimurium. Nat Microbiol 2:16215. 10.1038/nmicrobiol.2016.215. [DOI] [PubMed] [Google Scholar]
- 101.Petrof EO, Gloor GB, Vanner SJ, Weese SJ, Carter D, Daigneault MC, Brown EM, Schroeter K, Allen-Vercoe E. 2013. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘rePOOPulating’ the gut. Microbiome 1:3. 10.1186/2049-2618-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tvede M, Tinggaard M, Helms M. 2015. Rectal bacteriotherapy for recurrent Clostridium difficile-associated diarrhoea: results from a case series of 55 patients in Denmark 2000–2012. Clin Microbiol Infect 21:48–53. 10.1016/j.cmi.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 103.Gerding DN, Meyer T, Lee C, Cohen SH, Murthy UK, Poirier A, Van Schooneveld TC, Pardi DS, Ramos A, Barron MA, Chen H, Villano S. 2015. Administration of spores of nontoxigenic Clostridium difficile strain M3 for prevention of recurrent C difficile infection: a randomized clinical trial. JAMA 313:1719. 10.1001/jama.2015.3725. [DOI] [PubMed] [Google Scholar]
- 104.Wilson KH, Sheagren JN. 1983. Antagonism of toxigenic Clostridium difficile by nontoxigenic C. difficile. J Infect Dis 147:733–736. 10.1093/infdis/147.4.733. [DOI] [PubMed] [Google Scholar]
- 105.Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A, Littmann E, van den Brink MRM, Jenq RR, Taur Y, Sander C, Cross JR, Toussaint NC, Xavier JB, Pamer EG. 2015. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 517:205–208. 10.1038/nature13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Forster SC, Lawley TD. 2015. Systematic discovery of probiotics. Nat Biotechnol 33:47–48. 10.1038/nbt.3111. [DOI] [PubMed] [Google Scholar]
- 107.Rode AA, Chehri M, Krogsgaard LR, Heno KK, Svendsen AT, Ribberholt I, Helms M, Engberg J, Schønning K, Tvede M, Andersen CØ, Jensen US, Petersen AM, Bytzer P. 2021. Randomised clinical trial: a 12-strain bacterial mixture versus faecal microbiota transplantation versus vancomycin for recurrent Clostridioides difficile infections. Aliment Pharmacol Ther 53:999–1009. [DOI] [PubMed] [Google Scholar]
- 108.Lagier J-C, Armougom F, Million M, Hugon P, Pagnier I, Robert C, Bittar F, Fournous G, Gimenez G, Maraninchi M, Trape J-F, Koonin EV, La Scola B, Raoult D. 2012. Microbial culturomics: paradigm shift in the human gut microbiome study. Clin Microbiol Infect 18:1185–1193. 10.1111/1469-0691.12023. [DOI] [PubMed] [Google Scholar]
- 109.Greub G. 2012. Culturomics: a new approach to study the human microbiome. Clin Microbiol Infect 18:1157–1159. 10.1111/1469-0691.12032. [DOI] [PubMed] [Google Scholar]
- 110.Lagier J-C, Khelaifia S, Alou MT, Ndongo S, Dione N, Hugon P, Caputo A, Cadoret F, Traore SI, Seck EH, Dubourg G, Durand G, Mourembou G, Guilhot E, Togo A, Bellali S, Bachar D, Cassir N, Bittar F, Delerce J, Mailhe M, Ricaboni D, Bilen M, Dangui Nieko NPM, Dia Badiane NM, Valles C, Mouelhi D, Diop K, Million M, Musso D, Abrahão J, Azhar EI, Bibi F, Yasir M, Diallo A, Sokhna C, Djossou F, Vitton V, Robert C, Rolain JM, La Scola B, Fournier P-E, Levasseur A, Raoult D. 2016. Culture of previously uncultured members of the human gut microbiota by culturomics. Nat Microbiol 1:16203. 10.1038/nmicrobiol.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 111.Ott SJ, Waetzig GH, Rehman A, Moltzau-Anderson J, Bharti R, Grasis JA, Cassidy L, Tholey A, Fickenscher H, Seegert D, Rosenstiel P, Schreiber S. 2017. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology 152:799–811.E7. 10.1053/j.gastro.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 112.Scherlach K, Hertweck C. 2018. Mediators of mutualistic microbe–microbe interactions. Nat Prod Rep 35:303–308. 10.1039/C7NP00035A. [DOI] [PubMed] [Google Scholar]
- 113.Wade W. 2002. Unculturable bacteria–the uncharacterized organisms that cause oral infections. J R Soc Med 95:81–83. 10.1258/jrsm.95.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tang L. 2019. Microbial interactions. Nat Methods 16:19–19. 10.1038/s41592-018-0272-z. [DOI] [PubMed] [Google Scholar]
- 115.Faust K, Raes J. 2012. Microbial interactions: from networks to models. Nat Rev Microbiol 10:538–550. 10.1038/nrmicro2832. [DOI] [PubMed] [Google Scholar]
- 116.Sonnenborn U. 2016. Escherichia coli strain Nissle 1917—from bench to bedside and back: history of a special Escherichia coli strain with probiotic properties. FEMS Microbiol Lett 363:fnw212. 10.1093/femsle/fnw212. [DOI] [PubMed] [Google Scholar]
- 117.Ohan J, Pelle B, Nath P, Huang J-H, Hovde B, Vuyisich M, Dichosa AE, Starkenburg SR. 2019. High-throughput phenotyping of cell-to-cell interactions in gel microdroplet pico-cultures. Biotechniques 66:218–224. 10.2144/btn-2018-0124. [DOI] [PubMed] [Google Scholar]
- 118.Terekhov SS, Smirnov IV, Malakhova MV, Samoilov AE, Manolov AI, Nazarov AS, Danilov DV, Dubiley SA, Osterman IA, Rubtsova MP, Kostryukova ES, Ziganshin RH, Kornienko MA, Vanyushkina AA, Bukato ON, Ilina EN, Vlasov VV, Severinov KV, Gabibov AG, Altman S. 2018. Ultrahigh-throughput functional profiling of microbiota communities. Proc Natl Acad Sci USA 115:9551–9556. 10.1073/pnas.1811250115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Terekhov SS, Smirnov IV, Stepanova AV, Bobik TV, Mokrushina YA, Ponomarenko NA, Belogurov AA, Rubtsova MP, Kartseva OV, Gomzikova MO, Moskovtsev AA, Bukatin AS, Dubina MV, Kostryukova ES, Babenko VV, Vakhitova MT, Manolov AI, Malakhova MV, Kornienko MA, Tyakht AV, Vanyushkina AA, Ilina EN, Masson P, Gabibov AG, Altman S. 2017. Microfluidic droplet platform for ultrahigh-throughput single-cell screening of biodiversity. Proc Natl Acad Sci USA 114:2550–2555. 10.1073/pnas.1621226114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Youle M, Knowlton N, Rohwer F, Gordon J, Relman DA. 2013. Superorganisms and holobionts: looking for a term for the functional entity formed by a macrobe and its associated symbiotic microbes and viruses? The term is “holobiont.” Microbe Mag 8:152–153. [Google Scholar]
- 121.Bordenstein SR, Theis KR. 2015. Host biology in light of the microbiome: ten principles of holobionts and hologenomes. PLoS Biol 13:e1002226. 10.1371/journal.pbio.1002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Casadevall A, Pirofski L. 2000. Host-pathogen interactions: basic concepts of microbial commensalism, colonization, infection, and disease. Infect Immun 68:6511–6518. 10.1128/IAI.68.12.6511-6518.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Forbes NS, Coffin RS, Deng L, Evgin L, Fiering S, Giacalone M, Gravekamp C, Gulley JL, Gunn H, Hoffman RM, Kaur B, Liu K, Lyerly HK, Marciscano AE, Moradian E, Ruppel S, Saltzman DA, Tattersall PJ, Thorne S, Vile RG, Zhang HH, Zhou S, McFadden G. 2018. White paper on microbial anti-cancer therapy and prevention. J Immunother Cancer 6:78. 10.1186/s40425-018-0381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hoffman RM (ed). 2016. Bacterial therapy of cancer: methods and protocols. Humana Press, New York, NY. [Google Scholar]
- 125.Richardson MA, Ramirez T, Russell NC, Moye LA. 1999. Coley toxins immunotherapy: a retrospective review. Altern Ther Health Med 5:42–47. [PubMed] [Google Scholar]
- 126.Blevins SM, Bronze MS. 2010. Robert Koch and the ‘golden age’ of bacteriology. Int J Infect Dis 14:e744–e751. 10.1016/j.ijid.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 127.Bermudez-Brito M, Plaza-Díaz J, Fontana L, Muñoz-Quezada S, Gil A. 2013. In vitro cell and tissue models for studying host–microbe interactions: a review. Br J Nutr 109:S27–S34. 10.1017/S0007114512004023. [DOI] [PubMed] [Google Scholar]
- 128.Mullineaux-Sanders C, Carson D, Hopkins EGD, Glegola-Madejska I, Escobar-Zepeda A, Browne HP, Lawley TD, Frankel G. 2021. Citrobacter amalonaticus inhibits the growth of Citrobacter rodentium in the gut lumen. mBio 12:e02410-21. 10.1128/mBio.02410-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jimenez M, Langer R, Traverso G. 2019. Microbial therapeutics: new opportunities for drug delivery. J Exp Med 216:1005–1009. 10.1084/jem.20190609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McGovern BH, Ford CB, Henn MR, Pardi DS, Khanna S, Hohmann EL, O’Brien EJ, Desjardins CA, Bernardo P, Wortman JR, Lombardo M-J, Litcofsky KD, Winkler JA, McChalicher CWJ, Li SS, Tomlinson AD, Nandakumar M, Cook DN, Pomerantz RJ, Auninš JG, Trucksis M. 2021. SER-109, an investigational microbiome drug to reduce recurrence after Clostridioides difficile infection: lessons learned from a phase 2 trial. Clin Infect Dis 72:2132–2140. 10.1093/cid/ciaa387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nichols D, Cahoon N, Trakhtenberg EM, Pham L, Mehta A, Belanger A, Kanigan T, Lewis K, Epstein SS. 2010. Use of Ichip for high-throughput in situ cultivation of “uncultivable” microbial species. Appl Environ Microbiol 76:2445–2450. 10.1128/AEM.01754-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Browne HP, Forster SC, Anonye BO, Kumar N, Neville BA, Stares MD, Goulding D, Lawley TD. 2016. Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature 533:543–546. 10.1038/nature17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chakraborty S, Gourain V, Benz M, Scheiger JM, Levkin PA, Popova AA. 2021. Droplet microarrays for cell culture: effect of surface properties and nanoliter culture volume on global transcriptomic landscape. Mater Today Bio 11:100112. 10.1016/j.mtbio.2021.100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Melamed S, Elad T, Belkin S. 2012. Microbial sensor cell arrays. Curr Opin Biotechnol 23:2–8. 10.1016/j.copbio.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 135.Hata Y, Yamamoto M, Ohni M, Nakajima K, Nakamura Y, Takano T. 1996. A placebo-controlled study of the effect of sour milk on blood pressure in hypertensive subjects. Am J Clin Nutr 64:767–771. 10.1093/ajcn/64.5.767. [DOI] [PubMed] [Google Scholar]
- 136.Isolauri E, Sütas Y, Kankaanpää P, Arvilommi H, Salminen S. 2001. Probiotics: effects on immunity. Am J Clin Nutr 73:444s–450s. 10.1093/ajcn/73.2.444s. [DOI] [PubMed] [Google Scholar]
- 137.Campieri C, Campieri M, Bertuzzi V, Swennen E, Matteuzzi D, Stefoni S, Pirovano F, Centi C, Ulisse S, Famularo G, De Simone C. 2001. Reduction of oxaluria after an oral course of lactic acid bacteria at high concentration. Kidney Int 60:1097–1105. 10.1046/j.1523-1755.2001.0600031097.x. [DOI] [PubMed] [Google Scholar]
- 138.Twort FW. 1915. An investigation on the nature of ultra-microscopic viruses. Lancet 186:1241–1243. 10.1016/S0140-6736(01)20383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, Sokol H, Arkkila P, Pintus C, Hart A, Segal J, Aloi M, Masucci L, Molinaro A, Scaldaferri F, Gasbarrini G, Lopez-Sanroman A, Link A, de Groot P, de Vos WM, Högenauer C, Malfertheiner P, Mattila E, Milosavljević T, Nieuwdorp M, Sanguinetti M, Simren M, Gasbarrini A, European FMT Working Group. 2017. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 66:569–580. 10.1136/gutjnl-2016-313017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.de Vrese M, Stegelmann A, Richter B, Fenselau S, Laue C, Schrezenmeir J. 2001. Probiotics—compensation for lactase insufficiency. Am J Clin Nutr 73:421s–429s. 10.1093/ajcn/73.2.421s. [DOI] [PubMed] [Google Scholar]
- 141.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, Dallinga–Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JET, Bloks VW, Groen AK, Heilig HGHJ, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JBL, Nieuwdorp M. 2012. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143:913–916.E7. 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 142.Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, Zur M, Regev-Lehavi D, Ben-Zeev Brik R, Federici S, Horn M, Cohen Y, Moor AE, Zeevi D, Korem T, Kotler E, Harmelin A, Itzkovitz S, Maharshak N, Shibolet O, Pevsner-Fischer M, Shapiro H, Sharon I, Halpern Z, Segal E, Elinav E. 2018. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 174:1406–1423.E16. 10.1016/j.cell.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 143.Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, Armstrong D, Marshall JK, Kassam Z, Reinisch W, Lee CH. 2015. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 149:102–109.E6. 10.1053/j.gastro.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 144.Kang D-W, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, Geis E, Maldonado J, McDonough-Means S, Pollard EL, Roux S, Sadowsky MJ, Lipson KS, Sullivan MB, Caporaso JG, Krajmalnik-Brown R. 2017. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 5:10. 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Eiseman B, Silen W, Bascom GS, Kauvar AJ. 1958. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 44:854–859. [PubMed] [Google Scholar]
- 146.Lilly DM, Stillwell RH. 1965. Probiotics: growth-promoting factors produced by microorganisms. Science 147:747–748. 10.1126/science.147.3659.747. [DOI] [PubMed] [Google Scholar]
- 147.Heatley NG. 1944. A method for the assay of penicillin. Biochem J 38:61–65. 10.1042/bj0380061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hausdorfer J, Sompek E, Allerberger F, Dierich MP, Rüsch-Gerdes S. 1998. E-test for susceptibility testing of Mycobacterium tuberculosis. Int J Tuber Lung Dis 2:751–755. [PubMed] [Google Scholar]
- 149.Magaldi S, Mata-Essayag S, Hartung de Capriles C, Perez C, Colella MT, Olaizola C, Ontiveros Y. 2004. Well diffusion for antifungal susceptibility testing. Int J Infect Dis 8:39–45. 10.1016/j.ijid.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 150.Valgas C, de Souza SM, Smânia EF, Smânia A, Jr.. 2007. Screening methods to determine antibacterial activity of natural products. Braz J Microbiol 38:369–380. 10.1590/S1517-83822007000200034. [DOI] [Google Scholar]
- 151.Jiménez-Esquilín AE, Roane TM. 2005. Antifungal activities of actinomycete strains associated with high-altitude sagebrush rhizosphere. J Ind Microbiol Biotechnol 32:378–381. 10.1007/s10295-005-0007-x. [DOI] [PubMed] [Google Scholar]
- 152.Elleuch L, Shaaban M, Smaoui S, Mellouli L, Karray-Rebai I, Fourati-Ben Fguira L, Shaaban KA, Laatsch H. 2010. Bioactive secondary metabolites from a new terrestrial Streptomyces sp. Appl Biochem Biotechnol 162:579–593. 10.1007/s12010-009-8808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lertcanawanichakul M, Sawangnop S. 2008. A comparison of two methods used for measuring the antagonistic activity of Bacillus species. Walailak J Sci Technol 5:161–171. [Google Scholar]
- 154.Ali-Shtayeh MS, Abu Ghdeib SI. 1999. Antifungal activity of plant extracts against dermatophytes. Mycoses 42:665–672. 10.1046/j.1439-0507.1999.00499.x. [DOI] [PubMed] [Google Scholar]
- 155.Mukherjee PK, Raghu K. 1997. Effect of temperature on antagonistic and biocontrol potential of shape Trichoderma sp. on Sclerotium rolfsii. Mycopathologia 139:151–155. 10.1023/A:1006868009184. [DOI] [PubMed] [Google Scholar]
- 156.Kumar SN, Nambisan B, Sundaresan A, Mohandas C, Anto RJ. 2014. Isolation and identification of antimicrobial secondary metabolites from Bacillus cereus associated with a rhabditid entomopathogenic nematode. Ann Microbiol 64:209–218. 10.1007/s13213-013-0653-6. [DOI] [Google Scholar]
- 157.Marston A. 2011. Thin-layer chromatography with biological detection in phytochemistry. J Chromatogr A 1218:2676–2683. 10.1016/j.chroma.2010.12.068. [DOI] [PubMed] [Google Scholar]
- 158.Dewanjee S, Gangopadhyay M, Bhattacharya N, Khanra R, Dua TK. 2015. Bioautography and its scope in the field of natural product chemistry. J Pharm Anal 5:75–84. 10.1016/j.jpha.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Aas J, Gessert CE, Bakken JS. 2003. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis 36:580–585. 10.1086/367657. [DOI] [PubMed] [Google Scholar]
- 160.Hirsch BE, Saraiya N, Poeth K, Schwartz RM, Epstein ME, Honig G. 2015. Effectiveness of fecal-derived microbiota transfer using orally administered capsules for recurrent Clostridium difficile infection. BMC Infect Dis 15:191. 10.1186/s12879-015-0930-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Isolauri E, Rautava S, Kalliomäki M, Kirjavainen P, Salminen S. 2002. Role of probiotics in food hypersensitivity. Curr Opin Allergy Clin Immunol 2:263–271. 10.1097/00130832-200206000-00018. [DOI] [PubMed] [Google Scholar]
- 162.Malin M, Verronen P, Mykkänen H, Salminen S, Isolauri E. 1996. Increased bacterial urease activity in faeces in juvenile chronic arthritis: evidence of altered intestinal microflora? Rheumatology 35:689–694. 10.1093/rheumatology/35.7.689. [DOI] [PubMed] [Google Scholar]
- 163.Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. 2001. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 357:1076–1079. 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 164.Matricardi PM. 2002. Probiotics against allergy: data, doubts, and perspectives Allergy 57:185–187. 10.1034/j.1398-9995.2002.1a3299.x. [DOI] [PubMed] [Google Scholar]
- 165.Näse L, Hatakka K, Savilahti E, Saxelin M, Pönkä A, Poussa T, Korpela R, Meurman JH. 2001. Effect of long–term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children. Caries Res 35:412–420. 10.1159/000047484. [DOI] [PubMed] [Google Scholar]
- 166.Wollowski I, Rechkemmer G, Pool-Zobel BL. 2001. Protective role of probiotics and prebiotics in colon cancer. Am J Clin Nutr 73:451s–455s. 10.1093/ajcn/73.2.451s. [DOI] [PubMed] [Google Scholar]
- 167.Rafter J. 2002. Lactic acid bacteria and cancer: mechanistic perspective. Br J Nutr 88:S89–S94. 10.1079/BJN2002633. [DOI] [PubMed] [Google Scholar]
- 168.de Roos NM, Katan MB. 2000. Effects of probiotic bacteria on diarrhea, lipid metabolism, and carcinogenesis: a review of papers published between 1988 and 1998. Am J Clin Nutr 71:405–411. 10.1093/ajcn/71.2.405. [DOI] [PubMed] [Google Scholar]
- 169.Marteau P, Seksik P, Jian R. 2002. Probiotics and intestinal health effects: a clinical perspective. Br J Nutr 88:s51–s57. 10.1079/BJN2002629. [DOI] [PubMed] [Google Scholar]
- 170.Madden JAJ, Hunter JO. 2002. A review of the role of the gut microflora in irritable bowel syndrome and the effects of probiotics. Br J Nutr 88:s67–s72. 10.1079/BJN2002631. [DOI] [PubMed] [Google Scholar]
- 171.Nanji AA, Khettry U, Sadrzadeh SMH. 1994. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). Exp Biol Med 205:243–247. 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- 172.Reid G. 2002. Probiotics for urogenital health. Nutr Clin Care 5:3–8. 10.1046/j.1523-5408.2002.00512.x. [DOI] [PubMed] [Google Scholar]
- 173.Singer SM, Nash TE. 2000. The role of normal flora in Giardia lamblia infections in mice. J Infect Dis 181:1510–1512. 10.1086/315409. [DOI] [PubMed] [Google Scholar]
- 174.Faivre B, Bellenger J, Rieu A, Guivier E, Galan M, Ollivier A, Poloni L, Sorci G. 2019. Disentangling the effect of host genetics and gut microbiota on resistance to an intestinal parasite. Int J Parasitol 49:873–883. 10.1016/j.ijpara.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 175.Swan M. 2009. Emerging patient-driven health care models: an examination of health social networks, consumer personalized medicine and quantified self-tracking. Int J Environ Res Public Health 6:492–525. 10.3390/ijerph6020492. [DOI] [PMC free article] [PubMed] [Google Scholar]