ABSTRACT
Borrelia recurrentis is the causative agent of louse-borne relapsing fever and the only Borrelia species transmitted by an insect rather than a tick vector. While bed bugs (Cimex lectularius L.) are not established vectors of any human pathogens, a recent study reported that they may be competent vectors of B. recurrentis. However, many aspects of infection and transmission remain unclear in this possible secondary vector. Here, we carried out several quantitative laboratory studies to gain a better understanding of the host suitability of bed bugs relative to the established body louse vector as well as the factors that may affect the ability of bed bugs to transmit the pathogen. We fed bed bugs B. recurrentis and estimated the level and duration of infection in the hemolymph using live imaging. We performed quantitative PCR (qPCR) to examine whole-body spirochete levels and the occurrence of vertical transmission to progeny. We also developed an assay to compare the amounts of force required to release infectious hemolymph from recently engorged bed bugs and body lice. Finally, we analyzed humoral antibacterial activity in the hemolymph, hemolymph pH, and hemocyte activity in both insect species. Our results confirm that within 24 h of ingestion, B. recurrentis can penetrate the midgut epithelium of bed bugs and enter the hemolymph, overcoming a major host barrier, as in body lice. Once in the hemolymph, spirochetes remain visible for at least 4 days. Moreover, we show that bed bugs are more physically susceptible to crushing than body lice, suggesting that crushing is a feasible route for the natural dissemination of B. recurrentis from the hemolymph of bed bugs, as for body lice. Nonetheless, our data also indicate that bed bugs are suboptimal hosts for B. recurrentis, as the bacterium does not appear to proliferate to high levels or stably colonize the hemolymph and exhibits pleomorphism in this environment. In particular, our data suggest that hemolymph pH and unique cellular immune responses, rather than humoral effectors, may be involved in limiting spirochete survival in bed bugs. Notably, we document the formation of extracellular DNA traps by bed bug hemocytes for the first time. For these reasons, while bed bugs may be capable of limited transmission given their ecology, vector competence is probably minimal relative to body lice. Additional mechanistic studies of human pathogen infection of bed bugs may provide much-needed insight into the biological factors that restrict their ability to act as vectors and may reveal novel mechanisms of immunity.
KEYWORDS: Cimex, bed bug, lice, Pediculus, Borrelia recurrentis, hemolymph, host, vector, transmission, competence, Borrelia, arthropod vectors, vector-borne diseases
INTRODUCTION
Borrelia recurrentis is the causative agent of louse-borne relapsing fever, a historically significant disease that remains endemic in the Horn of Africa today (1, 2). Imported infections are also emerging in Europe (3). B. recurrentis is the only species of Borrelia known to be transmitted by an insect rather than a tick. As it has no known nonhuman reservoir, the transmission cycle begins when bacteria are ingested by body lice (Pediculus humanus humanus) from an infected human harboring spirochetes in the blood (4). The spirochete load can exceed 106 spirochetes/mL in the symptomatic stages of infection (5, 6). Within days, spirochetes penetrate the gut barrier and reach the hemolymph of the lice, where they replicate to a high concentration (7–9). Lice remain infected for life, without experiencing significant adverse effects (9), and go on to transmit the bacteria when they are transferred to and crushed on a new human host, releasing infectious hemolymph onto the skin or mucous membranes. Crushing of the body louse vector is thought to be the primary transmission mechanism due to multiple seminal reports indicating that the bacteria are not shed in the feces and do not colonize the salivary glands (7, 10–12). However, some more recent results suggest that shedding in the feces of the vector could be an additional mechanism for transmission (9). There is conflicting evidence as to whether vertical (transovarial) transmission takes place in infected lice (9, 12).
Bed bugs, including the common bed bug Cimex lectularius L., are blood-feeding urban pests that are widespread throughout the globe. The potential for bed bugs to transmit human infectious disease agents has been of interest for decades (13, 14). However, despite being able to transmit some bacterial and parasitic agents (e.g., Bartonella quintana and Trypanosoma cruzi) under laboratory conditions (15, 16), the insects have not been directly implicated in the transmission of any human pathogens in the field to date. This lack of evidence has led to the hypothesis that bed bugs may possess special antipathogen defenses that prevent them from acting as efficient vectors, although little work has been done to investigate this hypothesis.
There is ecological overlap between body lice and bed bugs under conditions of deteriorated hygiene, including in locations where louse-borne relapsing fever epidemics occur (17–19). This relationship coupled with the unique ability of B. recurrentis to colonize an insect has led to much speculation about the potential for bed bugs to serve as vectors. Initial attempts to test the vector competence of bed bugs for B. recurrentis produced unclear results. A study from 1923 found that unspecified relapsing fever spirochetes fed to bed bugs invaded the hemolymph, surviving and apparently replicating there for up to 15 days while remaining infectious (20). Another set of experiments from 1938 indicated that B. recurrentis ingested by bed bugs mostly died in the gut within 24 h, was present in the hemolymph only transiently, and could not be transmitted to progeny or squirrels during feeding (21). A paper published in 1953 reported that Borrelia duttonii survived in bed bugs for up to 150 days, but the results regarding B. recurrentis were considered inconclusive (22). All these studies were limited by the availability of quantitative experimental techniques at the time. More recently, a 2019 study using culture and staining techniques not available during previous investigations documented the presence of viable, infectious spirochetes in the feces of bed bugs for up to 10 days after ingesting a blood meal containing the bacteria (23). As in body lice, B. recurrentis did not appear to affect the survival of bed bugs and was not detected in the salivary glands. While El Hamzaoui et al. established the ability of the spirochetes to survive in the gut and feces, the techniques used did not clearly address whether colonization of hemolymph and bacterial replication took place, as in lice, or whether vertical transmission to progeny occurred. These critical processes could be limited by immunological or biochemical differences between bed bugs and body lice, significantly affecting transmission potential.
It also remains unclear whether the behavior and physiology of bed bugs are conducive to the transmission of B. recurrentis through the same mechanisms as those for lice. Bed bugs defecate soon after feeding, which could facilitate transmission via the feces (15), but little work has been done to study bed bug defecation in the context of pathogen transmission. On the other hand, spotting and streaking of blood on mattresses and surfaces are considered classical signs of bed bug infestation (24), which indicates that bed bugs are frequently crushed on or near human hosts. Although the phenomenon is not common, bed bugs may also linger or nest directly on the human host during severe infestations, in a manner similar to that of permanent ectoparasites such as body lice (18, 25–28). Thus, if the susceptibility of bed bugs to crushing is similar to that of body lice and bed bugs are able to maintain significant levels of B. recurrentis in the hemolymph, then bed bugs may be capable of contributing to natural transmission.
Due to the controversial nature of pathogen transmission by bed bugs, confirming and expanding upon previous work in this field are essential to achieving progress and reaching a consensus. Here, we sought to clarify several aspects of the relationship between bed bugs and B. recurrentis using quantitative methods. Specifically, we aimed to determine whether B. recurrentis could escape the gut and colonize the hemolymph, whether infection could be vertically transmitted from infected adults to progeny, and whether crushing of bed bugs could be a plausible route for the dissemination of infectious hemolymph similarly to body lice. More critically, we sought to compare several immunological and biochemical aspects of the hemolymph of bed bugs and body lice to identify factors that could contribute to differential vector competence, addressing an important question in vector biology.
RESULTS
Dynamics of Borrelia recurrentis infection in the hemolymph of bed bugs.
Within 24 h of ingesting B. recurrentis in a blood meal, individual motile spirochetes with typical morphology were observed in the hemolymph of some but not all bed bugs by phase-contrast microscopy, indicating successful penetration of the gut barrier (Fig. 1). Based on an estimated blood meal volume of 5 μL, bed bugs in our experiments were expected to have consumed ∼250 to 500 spirochetes each. Therefore, those detected in the hemolymph on day 1 postfeeding (mean = 112.5 spirochetes/individual) represented a nontrivial proportion of those ingested. Individual motile spirochetes were still seen in the hemolymph of some bugs 4 days after feeding. The spirochete load declined slightly (mean = 68.75 spirochetes/individual), but this difference was not statistically different from the spirochete load on day 1 postfeeding (P = 0.543 by analysis of variance [ANOVA]). On days 7 and 11 postfeeding, no spirochetes were observed in the hemolymph of any of the insects examined, which was a statistically significant reduction in the spirochete load relative to day 1 postfeeding (P = 0.009 by ANOVA). Quantitative PCR (qPCR) analysis supported our microscopy results (Fig. 2). B. recurrentis was detected in all six adult insects tested 1 day after feeding (mean cycle threshold [CT] = 36.1). However, it was not detected in any insects at the other time points examined, nor was it detected in nymphs derived from eggs produced by infected adults.
FIG 1.

Detection of Borrelia recurrentis in the hemolymph of bed bugs. Adult bed bugs fed a blood meal containing B. recurrentis were collected at 1, 4, 7, and 11 days postfeeding. Individual, motile B. recurrentis bacteria with characteristic spirochete morphology (arrow) were observed in the hemolymph of bed bugs by phase-contrast microscopy on day 1 and day 4 postinfection but were not seen at later time points. Estimated numbers of motile B. recurrentis spirochetes present in the hemolymph were calculated for individual insects by counting on a hemocytometer. Shown are a representative image and the mean numbers of Borrelia spirochetes per insect with standard errors (SE) (n = 4 biological replicates for 2 adult insects per time point [8 total insects per time point]). Data were analyzed by one-way ANOVA with Tukey’s post hoc testing. ND, not detected; NS, not significant.
FIG 2.

Detection of Borrelia recurrentis in whole bed bugs by qPCR. Adult bed bugs fed a blood meal containing B. recurrentis were collected at 1, 4, 7, and 11 days postfeeding. In addition, offspring (nymphs) of bed bugs fed B. recurrentis were also collected at the end of each trial (day 11). DNA was extracted from individual adults or pools of nymphs for the detection of B. recurrentis by qPCR using specific primers (for analyses of adults, n = 3 biological replicates for 2 insects per time point [6 total insects per time point]; for analyses of nymphs, n = 3 biological replicates for 5 to 10 pooled insects per replicate). Mean and individual cycle threshold (CT) values are plotted at each time point. ND, not detected.
Interestingly, B. recurrentis displayed pleomorphism in the hemolymph of bed bugs, which was not previously reported. Borrelia spirochetes with aberrant morphology and membrane “blebs” (29) (Fig. 3A) as well as slightly motile aggregates of spirochetes (30, 31) (Fig. 3B) were sporadically noted in the hemolymph on day 1 and day 4 postfeeding. Both of these forms are also present in in vitro cultures of strain PAbn.
FIG 3.
Pleomorphic and aggregated forms of Borrelia recurrentis in the hemolymph of bed bugs. (A) In addition to individual spirochetes, “blebbed” forms of Borrelia with aberrant morphology were randomly observed by phase-contrast microscopy on both day 1 and day 4 postinfection. (B) Motile aggregates of B. recurrentis were also observed on both days. Shown are representative images.
Susceptibility of bed bugs and body lice to crushing.
Mean force values at which mechanical crushing occurs for engorged bed bugs and body lice were determined on a custom-fabricated compressive-force device (Fig. 4A to C). Significantly lower force was required to rupture the exoskeleton of recently engorged bed bugs and release hemolymph (2.8 N) than the force required to crush recently engorged body lice (6.1 N) (P < 0.001 by a t test) (Fig. 4D).
FIG 4.
Susceptibility of bed bugs and body lice to crushing. (A) Schematic of the custom-fabricated compression device. (B) Compression force function from a typical trial. Initial increases in compression forces can be seen up until the point at which the specimen exoskeleton fails. (C) In the corresponding rate of change in compression force function, the point of exoskeleton failure is easily identified as the peak rate of change in the compression force. (D) Individual insects were placed into a custom-fabricated compression device 1 h after engorging on a blood meal. The force required to reach exoskeleton failure (crushing) and release hemolymph was determined in newtons. Mean values with SE are plotted. The results were analyzed using a t test (n = 6 to 8 insects per group).
Activity of acellular hemolymph from bed bugs and body lice against Borrelia.
Intrigued by our observations of the dynamics of B. recurrentis in bed bug hemolymph, we sought to further investigate the factors that may limit B. recurrentis survival in this environment relative to body lice hemolymph. To do so, we first compared the activity of humoral factors present in the hemolymph of both species against a more tractable surrogate spirochete, B. duttonii. In these in vitro experiments, treatment of spirochete cultures with a positive control of human plasma significantly inhibited growth (Fig. 5) (P = 0.035 by a one-sample t test with Bonferroni correction), as expected given the presence of humoral immune factors. However, inhibition was only partial, possibly due to the complement evasion activity of relapsing fever spirochetes (32). Meanwhile, treatment with equivalent volumes of acellular hemolymph from bed bugs or body lice had no significant impact relative to treatment with the vehicle (Barbour-Stoenner-Kelly-H [BSK-H] medium), indicating that neither has strong borreliacidal/borreliastatic properties.
FIG 5.
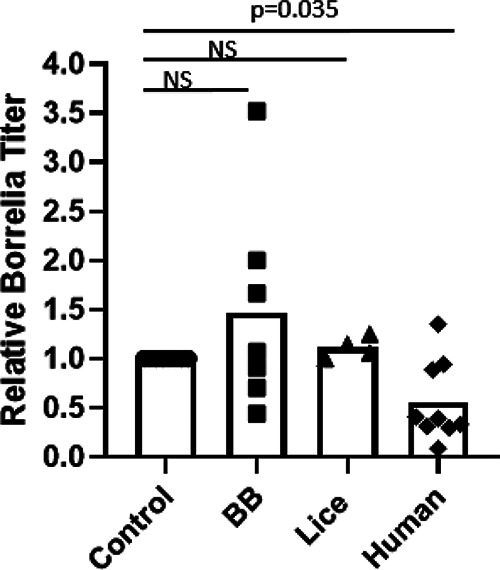
Activity of acellular hemolymph from bed bugs and body lice against Borrelia. Hemolymph was extracted from bed bugs (BB) or body lice and filtered to remove cellular components. Equal volumes of hemolymph were added to in vitro cultures of Borrelia duttonii, and borreliacidal/borreliastatic activity was measured by quantitation of the titer (motile spirochetes per milliliter) 24 h later. Treated groups were compared to control cultures treated with sterile BSK-H medium. Human plasma was included as a positive control, and the results were analyzed using one-sample t testing with Bonferroni correction.
Hemolymph pH.
The pH of the environment can be an important factor influencing the survival and growth of Borrelia and other bacteria. Thus, to determine whether this property may be involved in affecting B. recurrentis survival in bed bug hemolymph, we compared the pHs of the hemolymph of both species. A slight difference in the hemolymph pH was evident, with that of bed bugs being slightly more acidic. The pHs of hemolymph from the insects that were examined individually by the semiquantitative method were estimated to fall between ∼6.4 and 7.0 for bed bugs and between ∼7.0 and 7.6 for body lice. Consistent with these results, quantitative measurements of pooled hemolymph samples gave average pH values of 6.70 for bed bug hemolymph and 7.41 for body louse hemolymph (Fig. 6) (P = 0.002 by a t test).
FIG 6.

pH of hemolymph from bed bugs and body lice. Hemolymph samples from bed bugs or body lice were pooled, and the pH was measured using a benchtop pH meter equipped with a microelectrode. Each data point represents a pool of 3 insects, and bars represent the means. The results were analyzed using a t test. Neutral pH (7) is represented by the dashed line.
Activity of hemocytes from bed bugs and body lice stimulated with Borrelia.
In vitro fluorescence microscopy of hemocytes exposed to B. recurrentis revealed obvious morphological differences. Most notably, we observed that bed bug hemocytes frequently formed extracellular DNA traps (Fig. 7A), which are a known, multifunctional innate immune defense that is conserved across diverse animal taxa (33). Extracellular DNA traps formed as either diffuse cloud-like structures or individual strands that were often many times longer than the cell diameter. Extracellular DNA staining was observed in 12.1% of stimulated bed bug hemocytes randomly examined across multiple fields of view from two biological replicates. The vast majority of louse hemocytes contained DNA only in very small condensed nuclei, indicating a lack of extracellular DNA trap formation (Fig. 7B). In fact, only a single louse hemocyte of those examined displayed vague extracellular DNA staining in response to Borrelia, which was a significantly lower proportion than that of bed bug hemocytes (Fig. 7B and C) (P = 0.015 by Fisher’s exact test). DNA staining in unstimulated bed bug hemocyte controls was similar in appearance to that seen in stimulated louse hemocytes (Fig. 7D).
FIG 7.
Bed bug hemocytes but not body louse hemocytes form extracellular DNA traps. Hemocytes were extracted from bed bugs (BB) or body lice (L) and maintained in vitro. The hemocytes were incubated with B. recurrentis for 1 h and stained for fluorescence microscopy analysis. (A) DAPI-stained bed bug hemocytes with representative examples of extracellular DNA traps formed after stimulation (arrows). Magnification = ×200. (B) DAPI-stained body louse hemocytes displaying small condensed nuclei without extracellular DNA. Magnification = ×200. (C) Proportion of hemocytes from each species that displayed extracellular DNA staining after stimulation. The results were analyzed using Fisher’s exact test. (D) DAPI-stained unstimulated bed bug hemocyte controls.
Direct immunofluorescence staining using fluorescein isothiocyanate (FITC)-conjugated polyclonal anti-Borrelia antibody revealed instances of spirochetes colocalized with extracellular DNA strands from bed bug hemocytes, indicating that the structures can ensnare Borrelia (Fig. 8). The Borrelia spirochetes associated with hemocyte extracellular DNA did not exhibit normal morphology but rather appeared diffusely stained or fragmented.
FIG 8.
Extracellular DNA traps from bed bug hemocytes interact with Borrelia. Hemocytes extracted from bed bugs were incubated in vitro with B. recurrentis for 1 h and fixed and stained for fluorescence microscopy analysis. B. recurrentis was stained using cross-reactive, FITC-conjugated polyclonal antibody, while hemocyte DNA was stained with DAPI. Numerous instances of stained Borrelia spirochetes colocalized with hemocyte extracellular DNA strands were observed (arrows).
DISCUSSION
With the present study, we provide new information regarding the vector competence of bed bugs and the factors that may influence this, addressing a topic of long-standing controversy. In the established body louse vector, a key feature of B. recurrentis infection is the proliferation of spirochetes to achieve a high concentration in the hemolymph after escaping the midgut barrier (7, 8). A recent study of the vector competence of bed bugs for B. recurrentis documented the presence of spirochetes in the legs and hemolymph for up to 20 days after ingestion using immunofluorescence but appropriately acknowledged that the observed localization could be confounded by the fixing and staining methods that were used and did not discern if these spirochetes were viable (23). Our work confirms that B. recurrentis can in fact penetrate the midgut of bed bugs, overcoming a major defensive host barrier to reach the hemolymph. Furthermore, using live-imaging methods, we extend this finding by showing that the spirochetes remain viable in the hemolymph of bed bugs for at least 4 days. However, both microscopy and qPCR indicated that despite reaching the hemolymph, spirochetes subsequently declined in numbers rather than proliferating to a high concentration in this environment as they do in lice, consistent with data from a previous study (21).
Interestingly, our results obtained using microscopy and qPCR varied slightly. There were also some intriguing differences between our results and those reported in a similar recent study of bed bugs (23). While we microscopically visualized B. recurrentis in the hemolymph for up to 4 days after ingestion, all bed bugs tested by qPCR beyond the first day after ingestion were negative. This may be explained by a difference in the limits of detection of the assays. The large amount of host DNA present in extractions may prevent the detection of small numbers of spirochetes by molecular methods. On the other hand, by collecting and imaging most of the hemolymph volume of individual insects, it is feasible to detect just a few spirochetes. For similar reasons, our negative results beyond day 4 postfeeding are not necessarily at odds with the results of El-Hamzaoui et al., who did not specify the concentration of spirochetes administered to bed bugs. It is entirely possible that viable spirochetes were present in the gut and feces during later time points in our study, as documented by El-Hamzaoui et al. via culture and immunofluorescence, but at concentrations below the limit of detection of our qPCR assay. Moreover, El-Hamzaoui et al. reported the presence of B. recurrentis in the legs of bed bugs at later time points by immunofluorescence, but these may have been dead or tissue-associated spirochetes that would not have been detected by our hemolymph microscopy or an artifact of the method used. Finally, it is possible that El-Hamzaoui et al. administered higher doses of spirochetes, which could prolong their presence, or that there are differences in colonization abilities among strains of B. recurrentis.
Infected body lice likely do not transmit B. recurrentis vertically to their progeny (9), and most infected lice likely die during transmission due to crushing such that a single infected louse can usually infect only one naive host. Therefore, outbreaks of B. recurrentis rely on a high density of lice. Likewise, we found no evidence of vertical transmission in bed bugs. Yet the persistence of spirochetes in the hemolymph and gut (23) of bed bugs is well within the typical interval between blood meals. Under natural conditions, bed bugs seek a blood meal every 2.5 days on average (34). These data indicate that bed bugs that ingest B. recurrentis by feeding on an infected human could harbor viable spirochetes upon seeking a second blood meal, rendering them biologically capable of transmission not only through shedding in the feces, as previously shown, but also via the hemolymph if crushed on a naive host.
From a behavioral perspective, it is also plausible for bed bugs to contribute to the transmission of B. recurrentis shed in the feces or circulating in their hemolymph albeit probably rarely. Bed bugs defecate within ∼5 min of a blood meal (15). Furthermore, our data demonstrate quantitatively that body lice are much more difficult to crush than bed bugs. Yet they are able to disseminate pathogens through this route. Body lice feed more frequently, take longer to engorge, and linger on the body for longer, which may provide more frequent and consistent opportunities for crushing. Nonetheless, bed bugs linger on the body for at least several minutes during feeding and in severe infestations can be persistently found on the host (25–28). They are also more adept at active dispersal and feeding on multiple hosts than body lice. Coupled with the low force required to crush them, it is entirely possible that bed bugs are crushed on sleeping hosts, especially in severe infestations. Indeed, spotting and streaking of blood on mattresses and surfaces are common in bed bug infestations (24). Importantly, our results suggest the potential for bed bugs to transmit B. recurrentis through crushing, but they do not provide direct evidence of transmission, which has never been documented in nature. B. recurrentis is largely restricted to East Africa and circulates in environments of poor hygiene where both body lice and bed bugs can be prevalent, and the individual roles of each in transmission may be confounded, especially if bed bugs play only a secondary role. Thus, further epidemiological studies of B. recurrentis transmission that consider the involvement of bed bugs should be encouraged (17).
On a different front, our comparative studies of bed bug and body louse hemolymph samples provide some new preliminary insight into the factors that may limit the vector competence of bed bugs, which remain poorly understood. B. recurrentis is the only insect-adapted species of Borrelia, as most are transmitted by ticks (35). Our data indicate that this adaptation enables the bacteria to also complete part of their life cycle in bed bugs, supporting recent work. However, while B. recurrentis survives in the gut of bed bugs and breaches the epithelial barrier, it does not appear to colonize the hemolymph. The implication of this observation is that the vector competence of lice depends primarily on the permissiveness of their hemolymph for B. recurrentis persistence and proliferation. Based on the results presented in Fig. 5, circulating humoral effectors present in the hemolymph of bed bugs do not have particularly strong borreliacidal or borreliastatic activity, arguing against the hypothesis that clearance of B. recurrentis from the hemolymph of bed bugs is driven by special humoral activity. The activity of hemolymph humoral effectors against Borrelia burgdorferi has previously been shown to contribute to differential vector competence among ixodid ticks (36). On the other hand, consistent with our findings, the vector competence of argasid ticks for specific North American relapsing fever spirochetes appears to be mediated by the ability of the bacteria to persist in the salivary glands rather than by immune responses in the hemolymph (37).
Resistance to B. recurrentis in bed bugs may be driven in part by suboptimal environmental conditions, such as pH, that result in the passive clearance of spirochetes from the hemolymph. The pH of body louse hemolymph is slightly basic, like that of BSK-H medium, while that of bed bug hemolymph is slightly acidic. The optimal pH for B. recurrentis is not known, but for B. burgdorferi, it is 7.6 (38). Although in the tick vector Ixodes scapularis, B. burgdorferi can encounter pH levels as low as ∼6.8 (39), acid stress has been shown to induce morphological and gene expression changes in B. burgdorferi (39–42). Our observation of pleomorphic forms of B. recurrentis in bed bug hemolymph is indicative of a stress response. While aggregation of B. recurrentis has been observed in lice during the late stages of infection (9), membrane blebbing is typically associated with unfavorable growth conditions (29). Additional in vitro studies to determine the effects of pH on B. recurrentis are necessary to establish causality but are complicated by the fastidious nature of culture of this species.
Alternatively, cell-mediated immunity in the hemolymph of bed bugs may play a role in limiting vector competence. The novel finding that bed bug hemocytes form extracellular DNA traps is of particular importance. These multifunctional structures that are typically associated with granulocytes are recognized as important components of cellular innate immunity in a wide range of vertebrate animals (33) but have been documented in only a limited number of insect species (43–45). Other investigators have previously exposed body louse hemocytes to bacterial pathogens besides B. recurrentis without any evidence of extracellular DNA trap formation (46). In line with our observation that bed bug hemocyte extracellular DNA strands sometimes interacted with Borrelia, studies of mammalian neutrophils demonstrate that extracellular DNA traps are involved in ensnaring and killing Borrelia (47). It is plausible that DNA extracellular traps could directly inhibit the colonization of bed bug hemolymph by B. recurrentis and perhaps other pathogens through the same mechanisms, although our experiments were not designed to test the activity of said structures. In addition, extracellular nucleic acids may indirectly contribute to pathogen clearance from bed bugs by enhancing other innate responses such as coagulation or antimicrobial peptide expression, as shown during bacterial infection in the wax moth (43). The scope of the present study was to provide a broad assessment of the factors that could contribute to the differential vector competence of bed bugs and body lice. Nonetheless, future work should investigate bed bug hemocyte biology in the context of infection with B. recurrentis as well as other vector-borne disease agents in greater mechanistic detail, as the cells may drive additional immune responses that contribute to their pathogen resistance (48). Understanding these responses could ultimately inform the development of transmission-blocking strategies against diverse vector-borne pathogens.
MATERIALS AND METHODS
Bed bug and body louse colonies.
The bed bugs used in this study were taken from a colony of the Cincinnati field strain of Cimex lectularius (49). This strain originated from a population collected in 2007 in Cincinnati, OH, by Sierra Research Laboratories, Inc. (Modesto, CA). Bed bug colonies were maintained at the University of South Dakota in plastic jars with corrugated cardboard harborages at 28°C ± 1°C at 60 to 70% relative humidity with a 12-h-light/12-h-dark photoperiod. Colonies were fed mechanically defibrinated rabbit blood (Hemostat Laboratories, Dixon, CA) once per week using a Hemotek membrane feeding system (Hemotek Ltd., Blackburn, United Kingdom). The body lice (Pediculus humanus humanus) used in this study were also from a colony maintained at the University of South Dakota. This body louse strain is derived from a long-standing colony at the Hebrew University of Jerusalem and originated from a colony at the London School of Tropical Medicine and Hygiene established in 1984. The lice were reared using a previously described in vitro system (50) on small pieces of cotton fabric in ventilated plastic jars at 30°C ± 1°C at 70 to 80% relative humidity with a 12-h-light/12-h-dark photoperiod.
Borrelia recurrentis culture and bed bug infections.
The PAbn strain of Borrelia recurrentis, isolated from a human subject in 2015 (51), was grown in BSK-H medium (Sigma-Aldrich, St. Louis, MO) supplemented with 6% rabbit serum at 34°C in a 5% CO2 atmosphere as recommended by the supplier (BEI Resources, Manassas, VA). Log-phase cultures were harvested after 7 to 21 days of growth and diluted directly into aseptic, defibrinated rabbit blood (Hemostat Laboratories) for feeding to bed bugs via a Hemotek membrane feeding system (Hemotek Ltd.). The concentrations of spirochetes fed in each replicate were determined by counting viable spirochetes on a hemocytometer under a phase-contrast microscope and ranged between 5.6 × 104 spirochetes/mL and 1.1 × 105 spirochetes/mL. Importantly, these are physiologically relevant values that do not exceed typical spirochetemias observed in infected patients (5, 6). Groups of 15 to 20 adult bed bugs were fed a single infected blood meal and subsequently maintained under the conditions described above for the colonies. Four independent infection sets were carried out.
Detection of Borrelia recurrentis in bed bug hemolymph by live microscopy.
Adult bed bugs were collected 1, 4, 7, and 11 days after feeding on a blood meal containing B. recurrentis. At each time point, hemolymph was extracted from individual insects for live imaging. This approach was chosen to avoid the confounding effects of fixing, sectioning, and staining procedures on spirochete localization (23). In brief, insects were immobilized by cooling on ice. Subsequently, a pulled glass needle was inserted into the ventral thorax to extract circulating hemolymph by capillary action. Each insect was drained of hemolymph. The volume was not specifically measured but is typically <1 μL/insect, with some expected variation. The hemolymph collected from each insect was immediately diluted in 10 μL of phosphate-buffered saline (PBS) and placed into a hemocytometer for visualization by phase-contrast microscopy at a ×400 magnification using a Zeiss (Oberkochen, Germany) Primo Star instrument equipped with a Zeiss AxioCam 208 color camera. For each of four infection sets, two adult insects were examined at each time point (8 insects total per time point). The number of individual, motile spirochetes present in the 10-μL diluted hemolymph sample extracted from each insect was estimated by counting on a hemocytometer under a phase-contrast microscope and using the equation total spirochetes = average spirochetes observed in 1 mm2 × 10,000/100 to account for the volume of the diluted sample. Differences in spirochete loads over time were assessed by one-way ANOVA with Tukey’s post hoc testing.
Detection of Borrelia recurrentis in whole bed bugs by qPCR.
Separate groups of adult bed bugs were also collected 1, 4, 7, and 11 days after feeding on a blood meal containing B. recurrentis for qPCR analysis. DNA was extracted from individual bed bug bodies using the DNeasy blood and tissue kit (Qiagen, Germantown, MD) according to the manufacturer’s instructions. DNA concentrations were then determined on a Qubit fluorometer (Thermo Fisher, Waltham, MA), and samples were stored at −20°C until further use. Borrelia-positive samples were identified by performing a 40-cycle qPCR assay using 30 ng of the above-mentioned DNA and primers specific for the internal transcribed spacer (ITS) of Borrelia on an Applied Biosystems (Foster City, CA) StepOne Plus instrument as previously described (52). DNA extracted from a B. recurrentis culture served as a positive control, and reactions with no template were run as negative controls. Three independent replicates of these experiments were conducted. Each replicate consisted of one male and one female per time point as well as one pool of 5 to 10 nymphs derived from eggs laid after infection (collected on day 11) to test for vertical transmission. Therefore, six individual insects were tested per time point in total. Each sample was tested in triplicate, and average cycle threshold (CT) values were taken for semiquantitative comparison of spirochete loads over time.
Susceptibility of bed bugs and body lice to crushing.
To determine the amount of force required to release infectious hemolymph from lice and bed bugs through crushing, a custom-fabricated compressive-force device was constructed. The device consisted of a base and a sliding ceiling instrumented with a force/torque sensor (Nano 17 F/T; ATI Industrial Automation, Garner, NC, USA). The sensor was capped with a transparent glass slide, allowing the precise measurement of the tangential force applied to the specimen up until the point of mechanical crushing of the specimen. Analog data were collected using an NI USB-6218 DAQ device and recorded using custom-developed LabVIEW software (NI, Austin, TX, USA).
Six individual adult bed bugs and eight individual adult body lice were collected 1 h after engorging on aseptic blood via a membrane feeding system (Hemotek Ltd.). In each trial, a single specimen was immobilized and placed on the base of the compressive-force device. Following insect placement, the top plate was lowered to contact the insect. Once placed, the mechanical compressive force was slowly increased to the point that exoskeleton failure (crushing) was observed, evidenced by a sudden reduction in downward applied pressure when displayed in real time and a corresponding “popping” sound. Between each trial, the base and ceiling of the device were cleaned using an alcohol wipe.
All analyses were conducted using custom-developed LabVIEW software. Vertical force from the sensor was sampled at 500 Hz. Raw force signals were low-pass filtered using a 4th-order, zero-phase-lag Butterworth filter with a cutoff frequency of 14 Hz. The signal representing the vertical force applied to the insect was plotted as a function of time. The processed signal was then differentiated with respect to time using a 1st-order central-difference equation to obtain the rate of change in compression force, or the compression force rate. For each trial, we determined the compression force at the time of the first substantial reduction in the load force rate, representing the time at which exoskeleton failure (crushing) occurred. The data were analyzed using a t test.
Activity of acellular hemolymph against Borrelia.
The effects of the acellular fractions of hemolymph (plasma) from bed bugs and body lice against Borrelia duttonii (strain CR2A; NIH Rocky Mountain Laboratories, Hamilton, MT) in culture were compared to assess humoral borreliacidal/borreliastatic activity. B. duttonii was used in this assay in place of B. recurrentis because it consistently grows to higher titers in vitro, thereby increasing the sensitivity and precision of the assay while reducing the volume of hemolymph used, which is a limited resource. B. duttonii is genetically extremely similar to B. recurrentis (53) and similarly invades the hemolymph of body lice in experimental infections (54, 55), indicating that it is a suitable surrogate for this assay.
Log-phase cultures of B. duttonii grown under the same conditions as those used for B. recurrentis were treated with acellular fractions of hemolymph using methods similar to those described previously by Johns et al. and Urbanová et al. (36, 56). In brief, hemolymph from 30 bed bugs or lice was passed through a 0.22-μm filter column to remove cellular components. Subsequently, 50-μL aliquots of the Borrelia culture were seeded into individual wells of a 96-well tissue culture plate. Aliquots of 3 μL of hemolymph were diluted to a volume of 10 μL using BSK-H medium and then added to individual wells of the Borrelia culture, bringing the final volume to 60 μL/well. Equivalent volumes of human plasma were used as positive controls, while equivalent volumes of BSK-H medium were used as negative controls (56, 57). The cultures were incubated at 34°C in a 5% CO2 atmosphere for 24 h, and Borrelia titers were then determined by counting on a hemocytometer using phase-contrast microscopy as described above. Titers in treated wells were normalized to those of the untreated controls, and the data were analyzed using one-sample t testing with Bonferroni correction to compare the values for treated groups to the control value (set at 1).
Hemolymph pH.
The pH of the environment can be an important factor influencing the survival and growth of Borrelia and other bacteria. To estimate the pH of small volumes of hemolymph from individual body lice and bed bugs, we first used a simple, semiquantitative assay involving colorimetric pH paper with a pH range of 5.5 to 8.0 (Hydrion; Micro Essential Laboratory, Inc., Brooklyn, NY). Hemolymph was extracted from six individual insects of each species using a pulled glass capillary needle and immediately spotted onto the pH paper. The color change was then evaluated by comparison to the reference provided by the manufacturer to estimate the pH. To obtain more precise pH values, hemolymph samples from 3 individuals of each species were pooled to reach a larger volume, and quantitative measurements were taken using an Orion Star benchtop pH meter equipped with a microelectrode (Thermo Fisher). The data were analyzed using a t test.
Fluorescence microscopy of hemocytes stimulated with Borrelia recurrentis.
To explore whether hemocytes exhibited any interesting responses to B. recurrentis, cells from each species were exposed to spirochetes in vitro and examined by fluorescence microscopy. First, hemocytes from 20 adult body lice or bed bugs were extracted into Schneider’s insect medium (Sigma-Aldrich) using a pulled glass capillary needle as described above for our other assays and allowed to adhere to chamber slides for 1 h at 28°C. Borrelia recurrentis was concentrated from a log-phase culture by centrifugation at 1,000 relative centrifugal force (rcf) for 10 min, resuspended in Schneider’s medium, and then added directly to the hemocytes in the chamber slides. The hemocytes and Borrelia were incubated together at 28°C for an additional hour. Subsequently, the slides were washed once with PBS to remove free spirochetes. The cytoplasm of hemocytes was stained with Vybrant CM-DiI for 20 min (Thermo Fisher) (1:500 in medium) (58), and three additional washes with PBS were performed. The slides were then mounted with 4′,6-diamidino-2-phenylindole (DAPI) plus Vectashield (Vector Laboratories, Burlingame, CA) to stain hemocyte DNA. In separate experiments, the slides were fixed in cold acetone after stimulation and washing, and Borrelia was stained with cross-reactive FITC-conjugated polyclonal antibody (catalog no. PA1-73005; Thermo Fisher) diluted 1:50 in PBS as previously described (59). Slides were visualized by epifluorescence microscopy using a Zeiss AxioSkop2 instrument equipped with a Zeiss AxioCam 208 color camera. For quantitative analysis, two independent replicates were conducted.
ACKNOWLEDGMENTS
This work was supported in part by the University of South Dakota Sanford School of Medicine and by the Department of the Army, U.S. Army Contracting Command, Aberdeen Proving Ground, Natick Contracting Division, Ft. Detrick, MD, under Deployed Warfighter Protection (DWFP) program grant W911QY-19-1-0013 to J.E.P.
Borrelia recurrentis strain PAbn, NR-51673, was obtained through BEI Resources, NIAID, NIH. We thank Frank Gherardini of Rocky Mountain Laboratories, NIAID, NIH, for providing Borrelia duttonii strain CR2A.
Contributor Information
Jose E. Pietri, Email: Jose.Pietri@usd.edu.
De’Broski R. Herbert, University of Pennsylvania
REFERENCES
- 1.Raoult D, Roux V. 1999. The body louse as a vector of reemerging human diseases. Clin Infect Dis 29:888–911. 10.1086/520454. [DOI] [PubMed] [Google Scholar]
- 2.Kahlig P, Paris DH, Neumayr A. 2021. Louse-borne relapsing fever—a systematic review and analysis of the literature: part 1. Epidemiology and diagnostic aspects. PLoS Negl Trop Dis 15:e0008564. 10.1371/journal.pntd.0008564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antinori S, Mediannikov O, Corbellino M, Grande R, Parravicini C, Bestetti G, Longhi E, Ricaboni D, Ehounoud CB, Fenollar F, Raoult D, Rimoldi SG. 2016. Louse-borne relapsing fever (Borrelia recurrentis) in a Somali refugee arriving in Italy: a re-emerging infection in Europe? PLoS Negl Trop Dis 10:e0004522. 10.1371/journal.pntd.0004522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackie FP. 1907. The part played by Pediculus corporis in the transmission of relapsing fever. Br Med J ii:1706–1709. 10.1136/bmj.2.2450.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler T, Hazen P, Wallace CK, Awoke S, Habte-Michael A. 1979. Infection with Borrelia recurrentis: pathogenesis of fever and petechiae. J Infect Dis 140:665–675. 10.1093/infdis/140.5.665. [DOI] [PubMed] [Google Scholar]
- 6.Reller ME, Clemens EG, Schachterle SE, Mtove GA, Sullivan DJ, Dumler JS. 2011. Multiplex 5′ nuclease-quantitative PCR for diagnosis of relapsing fever in a large Tanzanian cohort. J Clin Microbiol 49:3245–3249. 10.1128/JCM.00940-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung HL, Feng LC. 1936. Studies on the development of Spirochaeta recurrentis in body louse. A preliminary report. Chin Med J 50:1181–1184. [Google Scholar]
- 8.Heisch RB, Sparrow H, Harvey AEC. 1960. The behaviour of Spirochaeta recurrentis Lebert in lice. Bull Soc Pathol Exot Filiales 53:140–143. [PubMed] [Google Scholar]
- 9.Houhamdi L, Raoult D. 2005. Excretion of living Borrelia recurrentis in feces of infected human body lice. J Infect Dis 191:1898–1906. 10.1086/429920. [DOI] [PubMed] [Google Scholar]
- 10.Chung H-L, Wei Y-L. 1938. Studies on the transmission of relapsing fever in North China. II. Observations on the mechanism of transmission of relapsing fever in man. Am J Trop Med Hyg s1-18:661–674. 10.4269/ajtmh.1938.s1-18.661. [DOI] [Google Scholar]
- 11.Nicolle C, Blaizot L, Conseil E. 1912. Conditions de transmission de la fièvre récurrente par le pou. C R Hebd Seances Acad Sci 155:481–484. [Google Scholar]
- 12.Nicolle C, Blaizot L, Conseil E. 1913. Etiologie de la fièvre récurrente: son mode de transmission par les poux. Ann Inst Pasteur (Paris) 27:204–225. [Google Scholar]
- 13.Pietri JE. 2020. Case not closed: arguments for new studies of the interactions between bed bugs and human pathogens. Am J Trop Med Hyg 103:619–624. 10.4269/ajtmh.20-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delaunay P, Blan V, Del Giudice P, Levy-Bencheton A, Chosidow O, Marty P, Brouqui P. 2011. Bedbugs and infectious diseases. Clin Infect Dis 52:200–210. 10.1093/cid/ciq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salazar R, Castillo-Neyra R, Tustin AW, Borrini-Mayorí K, Náquira C, Levy MZ. 2015. Bed bugs (Cimex lectularius) as vectors of Trypanosoma cruzi. Am J Trop Med Hyg 92:331–335. 10.4269/ajtmh.14-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leulmi H, Bitam I, Berenger JM, Lepidi H, Rolain JM, Almeras L, Raoult D, Parola P. 2015. Competence of Cimex lectularius bed bugs for the transmission of Bartonella quintana, the agent of trench fever. PLoS Negl Trop Dis 9:e0003789. 10.1371/journal.pntd.0003789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung HL. 1936. Studies on the transmission of relapsing fever in North China. I. Preliminary observations. Chin Med J 50:1723–1734. [Google Scholar]
- 18.Pietri JE, Yax JA, Agany DDM, Gnimpieba EZ, Sheele JM. 2020. Body lice and bed bug co-infestation in an emergency department patient, Ohio, USA. IDCases 19:e00696. 10.1016/j.idcr.2020.e00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brouqui P. 2011. Arthropod-borne diseases associated with political and social disorder. Annu Rev Entomol 56:357–374. 10.1146/annurev-ento-120709-144739. [DOI] [PubMed] [Google Scholar]
- 20.Dunn LH. 1923. The tropical bed bug in relation to the transmission of relapsing fever in Panama. Am J Trop Med Hyg s1-3:345–350. 10.4269/ajtmh.1923.s1-3.345. [DOI] [Google Scholar]
- 21.Chung HL, Feng LC. 1938. Studies on the development of the Chinese strain of Spirochaeta recurrentis in Cimex lectularius. Chin J Med Suppl 2:563–577. [Google Scholar]
- 22.Blanc G, Bruneau J, Chabaud A. 1953. The behavior of certain spirochaetes in Cimex lectularius. Arch Inst Pasteur Maroc 4:411–428. [Google Scholar]
- 23.El Hamzaoui B, Laroche M, Bechah Y, Bérenger JM, Parola P. 2019. Testing the competence of Cimex lectularius bed bugs for the transmission of Borrelia recurrentis, the agent of relapsing fever. Am J Trop Med Hyg 100:1407–1412. 10.4269/ajtmh.18-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C, Singh N, Zha C, Cooper R. 2016. Bed bugs: prevalence in low-income communities, resident’s reactions, and implementation of a low-cost inspection protocol. J Med Entomol 53:639–646. 10.1093/jme/tjw018. [DOI] [PubMed] [Google Scholar]
- 25.Pietri JE. 2021. Bed bugs (Cimex spp.) as permanent ectoparasites: a rare but potentially significant phenomenon. J Med Entomol 58:2038–2039. 10.1093/jme/tjab136. [DOI] [PubMed] [Google Scholar]
- 26.Sabou M, Imperiale DG, Andrès E, Abou-Bacar A, Foeglé J, Lavigne T, Kaltenbach G, Candolfi E. 2013. Bed bugs reproductive life cycle in the clothes of a patient suffering from Alzheimer’s disease results in iron deficiency anemia. Parasite 20:16. 10.1051/parasite/2013018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Izri A, Marteau A, Ferreira T, Bruel C, Benainous R, Dhote R, Akhoundi M. 2020. Severe anemia due to bed bugs hyperinfestation. Microb Pathog 149:104564. 10.1016/j.micpath.2020.104564. [DOI] [PubMed] [Google Scholar]
- 28.Debarbieux S, Delaunay P, Raymond C, Dupont D, Persat F. 2020. Unusual location for bedbugs. Clin Microbiol Infect 26:895–896. 10.1016/j.cmi.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Meriläinen L, Herranen A, Schwarzbach A, Gilbert L. 2015. Morphological and biochemical features of Borrelia burgdorferi pleomorphic forms. Microbiology (Reading) 161:516–527. 10.1099/mic.0.000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aberer E, Duray PH. 1991. Morphology of Borrelia burgdorferi: structural patterns of cultured borreliae in relation to staining methods. J Clin Microbiol 29:764–772. 10.1128/jcm.29.4.764-772.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srivastava SY, de Silva AM. 2009. Characterization of Borrelia burgdorferi aggregates. Vector Borne Zoonotic Dis 9:323–329. 10.1089/vbz.2008.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meri T, Cutler SJ, Blom AM, Meri S, Jokiranta TS. 2006. Relapsing fever spirochetes Borrelia recurrentis and B. duttonii acquire complement regulators C4b-binding protein and factor H. Infect Immun 74:4157–4163. 10.1128/IAI.00007-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniel C, Leppkes M, Munoz LE, Schley G, Schett G, Herrmann M. 2019. Extracellular DNA traps in inflammation, injury and healing. Nat Rev Nephrol 15:559–575. 10.1038/s41581-019-0163-2. [DOI] [PubMed] [Google Scholar]
- 34.Reinhardt K, Isaac D, Naylor R. 2010. Estimating the feeding rate of the bedbug Cimex lectularius in an infested room: an inexpensive method and a case study. Med Vet Entomol 24:46–54. 10.1111/j.1365-2915.2009.00847.x. [DOI] [PubMed] [Google Scholar]
- 35.Talagrand-Reboul E, Boyer PH, Bergström S, Vial L, Boulanger N. 2018. Relapsing fevers: neglected tick-borne diseases. Front Cell Infect Microbiol 8:98. 10.3389/fcimb.2018.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johns R, Ohnishi J, Broadwater A, Sonenshine DE, De Silva AM, Hynes WL. 2001. Contrasts in tick innate immune responses to Borrelia burgdorferi challenge: immunotolerance in Ixodes scapularis versus immunocompetence in Dermacentor variabilis (Acari: Ixodidae). J Med Entomol 38:99–107. 10.1603/0022-2585-38.1.99. [DOI] [PubMed] [Google Scholar]
- 37.Schwan T. 2021. Vector specificity of the relapsing fever spirochete Borrelia hermsii (Spirochaetales: Borreliaceae) for the tick Ornithodoros hermsi (Acari: Argasidae) involves persistent infection of the salivary glands. J Med Entomol 58:1926–1930. 10.1093/jme/tjab060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi W, Yang Z, Geng Y, Wolinsky LE, Lovett MA. 1998. Chemotaxis in Borrelia burgdorferi. J Bacteriol 180:231–235. 10.1128/JB.180.2.231-235.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X, Goldberg MS, Popova TG, Schoeler GB, Wikel SK, Hagman KE, Norgard MV. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol Microbiol 37:1470–1479. 10.1046/j.1365-2958.2000.02104.x. [DOI] [PubMed] [Google Scholar]
- 40.Carroll JA, Garon CF, Schwan TG. 1999. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect Immun 67:3181–3187. 10.1128/IAI.67.7.3181-3187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dulebohn DP, Richards CL, Su H, Lawrence KA, Gherardini FC. 2017. Weak organic acids decrease Borrelia burgdorferi cytoplasmic pH, eliciting an acid stress response and impacting RpoN- and RpoS-dependent gene expression. Front Microbiol 8:1734. 10.3389/fmicb.2017.01734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murgia R, Cinco M. 2004. Induction of cystic forms by different stress conditions in Borrelia burgdorferi. APMIS 112:57–62. 10.1111/j.1600-0463.2004.apm1120110.x. [DOI] [PubMed] [Google Scholar]
- 43.Altincicek B, Stotzel S, Wygrecka M, Preissner KT, Vilcinskas A. 2008. Host-derived extracellular nucleic acids enhance innate immune responses, induce coagulation, and prolong survival upon infection in insects. J Immunol 181:2705–2712. 10.4049/jimmunol.181.4.2705. [DOI] [PubMed] [Google Scholar]
- 44.Chen RY, Keddie BA. 2021. Galleria mellonella (Lipidoptera: Pyralidae) hemocytes release extracellular traps that confer protection against bacterial infection in the hemocoel. J Insect Sci 21:7. 10.1093/jisesa/ieab092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nascimento MTC, Silva KP, Garcia MCF, Medeiros MN, Machado EA, Nascimento SB, Saraiva EM. 2018. DNA extracellular traps are part of the immune repertoire of Periplaneta americana. Dev Comp Immunol 84:62–70. 10.1016/j.dci.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 46.Coulaud PJ, Lepolard C, Bechah Y, Berenger JM, Raoult D, Ghigo E. 2014. Hemocytes from Pediculus humanus humanus are hosts for human bacterial pathogens. Front Cell Infect Microbiol 4:183. 10.3389/fcimb.2014.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menten-Dedoyart C, Faccinetto C, Golovchenko M, Dupiereux I, Van Lerberghe PB, Dubois S, Desmet C, Elmoualij B, Baron F, Rudenko N, Oury C, Heinen E, Couvreur B. 2012. Neutrophil extracellular traps entrap and kill Borrelia burgdorferi sensu stricto spirochetes and are not affected by Ixodes ricinus tick saliva. J Immunol 189:5393–5401. 10.4049/jimmunol.1103771. [DOI] [PubMed] [Google Scholar]
- 48.Potts R, King JG, Pietri JE. 2020. Ex vivo characterization of the circulating hemocytes of bed bugs and their responses to bacterial exposure. J Invertebr Pathol 174:107422. 10.1016/j.jip.2020.107422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pietri JE, Liang D. 2020. Insecticidal activity of doxycycline against the common bedbug. Antimicrob Agents Chemother 64:e00005-20. 10.1128/AAC.00005-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pietri JE, Ray R. 2020. A simplified protocol for in vitro rearing of human body lice. Parasite 27:8. 10.1051/parasite/2020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marosevic D, Margos G, Wallich T, Wieser A, Sing A, Fingerle V. 2017. First insights in the variability of Borrelia recurrentis genomes. PLoS Negl Trop Dis 11:e0005865. 10.1371/journal.pntd.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Socolovschi C, Reynaud P, Kernif T, Raoult D, Parola P. 2012. Rickettsiae of spotted fever group, Borrelia valaisiana, and Coxiella burnetii in ticks on passerine birds and mammals from the Camargue in the south of France. Ticks Tick Borne Dis 3:355–360. 10.1016/j.ttbdis.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 53.Lescot M, Audic S, Robert C, Nguyen TT, Blanc G, Cutler SJ, Wincker P, Couloux A, Claverie JM, Raoult D, Drancourt M. 2008. The genome of Borrelia recurrentis, the agent of deadly louse-borne relapsing fever, is a degraded subset of tick-borne Borrelia duttonii. PLoS Genet 4:e1000185. 10.1371/journal.pgen.1000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mooser H, Weyer F. 1954. Artificial infection of lice with Borrelia duttonii. Z Tropenmed Parasitol 5:28–45. [PubMed] [Google Scholar]
- 55.Heisch RB, Harvey AEC. 1962. The development of Spirochaeta duttoni and S. recurrentis in Pediculus humanus. Parasitology 52:77–88. 10.1017/S003118200002401X. [DOI] [Google Scholar]
- 56.Urbanová V, Hajdušek O, Mondeková HH, Šíma R, Kopáček P. 2017. Tick thioester-containing proteins and phagocytosis do not affect transmission of Borrelia afzelii from the competent vector Ixodes ricinus. Front Cell Infect Microbiol 7:73. 10.3389/fcimb.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Shaughnessy CM, Cunningham AF, MacLennan CA. 2012. The stability of complement-mediated bactericidal activity in human serum against Salmonella. PLoS One 7:e49147. 10.1371/journal.pone.0049147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.King JG, Hillyer JF. 2012. Infection-induced interaction between the mosquito circulatory and immune systems. PLoS Pathog 8:e1003058. 10.1371/journal.ppat.1003058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sapi E, Pabbat N, Datar A, Davies EM, Rattelle A, Kuo BA. 2013. Improved culture conditions for the growth and detection of Borrelia from human serum. Int J Med Sci 10:362–376. 10.7150/ijms.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]






