Abstract
The coronavirus disease 2019 (COVID-19) pandemic has caused a tremendous global impact both socially and economically. The mechanisms behind the disparity in the severity, vaccine coverage, and variant replacement patterns across European countries are unclear. In this work, we aim to reveal the possible reasons via data visualization and model fitting. We developed a model with a vaccination component to simulate the mortality waves in these countries. Deaths averted by the vaccination campaign were estimated. Finally, we discuss the potential reasons behind the differences in vaccine coverage across European countries. Contemporary transportation and global trade bring significant convenience to our daily life but also facilitate the spread of the novel virus COVID-19 to anywhere globally within a short time. The observations and results in this work highlight the importance of the global campaign to mitigate the COVID-19 pandemic and future pandemics under the One Health approach.
Keywords: COVID-19, Southeast Asia, South Asia, Mathematical modelling, Delta variants
Highlights
-
•
We reveal disparity in COVID-19 vaccine coverage across European counties.
-
•
We reveal different patterns of COVID-19 variants across European countries.
-
•
Using a mathematical model, we calculate deaths averted by the vaccine in Europe.
-
•
We discuss the reasons behind the disparity in vaccine coverage in Europe.
1. Introduction
Since its emergence in 2019, the Coronavirus Disease 2019 (COVID-19) pandemic has caused a worldwide health emergency across five continents. COVID-19 is caused by severe acute respiratory syndrome–coronavirus 2 (SARS-CoV-2), with an incubation period of 2–14 days. The SARS-CoV-2 is a zoonotic virus that can spread between humans and animals and be transmitted through contaminated environments [1]. The majority of SARS-CoV-2 infected individuals experience a mild to moderate respiratory illness and recover without requiring special treatment. However, a certain proportion of infected individuals develop serious symptoms and need medical treatment. As of January 6, 2022, 296 million confirmed cases of COVID-19 worldwide with approximately 5.46 million deaths were reported.
On November 4, 2021, the World Health Organization declared that “Europe is again at the epicenter of the coronavirus pandemic” [2]. Starting from November 1, 2021, Europe accounted for more than half of globally reported weekly deaths over five consecutive weeks [3]. For example, 27,000 deaths were reported across Europe during the single week beginning on November 1, 2021. However, the severity of the pandemic in different European countries during this resurgence differed markedly. Despite the variation in severity across nations, the resurgence of the COVID-19 pandemic has reinforced the importance of the One Health approach in the global control of current and future diseases, in particular zoonotic diseases [4].
2. Materials and methods
Based on our previous susceptible-exposed-infected-hospitalized-death-recovered models [[41], [42]], we propose the following model with a vaccinated class:
Here SV represents the vaccinated class. We denote V(t) as the cumulative proportion of the population fully vaccinated (second dose) by day t. We assumed that a proportion (η) of vaccinated individuals enter class R and gain relatively long-term immunity, and a proportion (1 − η) of vaccinated individuals enter class SV and regain susceptibility to breakthrough infection. Parameter ψ accounts for the reduced susceptibility of vaccinated individuals. Parameter θ represents the death risk of hospitalized individuals and we assume the death rate θ declines as the vaccination coverage, V(t) increases, θ = [1 − εV(t)] θ0. The vaccination rate per unvaccinated (including susceptible) at day t can be represented as [7,8]. The parameter π denotes the hospitalization risk of infected individuals. The infection fatality rate (IFR) is the product of π and θ. Based on preliminary tests, we found that it is convenient to assume θ0 = π to reduce one parameter without severely impacting the fitting performance [9]. We define the transmission rate β(t), as an exponential cubic spline function with a fixed number of nodes (nβ = 12) spanning the study period. We fixed the mean latent period, mean infectious period and mean duration from loss infectiousness to death, at σ−1 = 2 days, γ−1 = 3 days and κ−1 = 8 days, following previous studies [7,8,10].
We fit the above model to the adjusted COVID-19 death data with the reported V(t). Using maximum log-likelihood estimates of β(t) and π, we re-ran our model while setting V(t) = 0 to obtain the simulated deaths under the counterfactual scenario in the absence of a vaccine [7,8]. We compared the simulated deaths under the baseline and counterfactual scenarios to obtain the deaths averted by the vaccination campaign. We considered three different parameter settings: ε = 0, ε = 0.25, and ε = 0.5. We fixed the other parameters η = 0.85 to reflect the high efficacy of the vaccine in preventing infection and death. We noted that the vaccine provided differing efficacy in preventing infection and death. However, for the sake of simplicity, we did not explicitly separate natural infection from breakthrough infection. Thus, we argued that our parameters reflect effects against both infection and death. We considered the effect of the vaccine in reducing the IFR by incorporating the parameter ε. Thus, our focus in this research was to explore ε in terms of averted deaths in various European countries.
2.1. Data
We obtained daily new cases, weekly excess deaths, and daily vaccination coverage from [[11], [12], [13], [14], [15], [16]]. We obtained biweekly proportion of SARS-COV-2 variants among samples process from [[17], [18], [19], [20]].
3. Results
3.1. Adjusted deaths and vaccine coverage
Countries in Western Europe, including the United Kingdom, France, Germany, Switzerland, the Netherlands, and Belgium, have a relatively high vaccine coverage, with first-dose coverage of 70–80% and second-dose coverage of 65–75% by the end of 2021. This high coverage could be due to better medical access rates and high awareness and acceptance due to a large number of deaths in the severe disease waves in 2020. Also, a large proportion of the population was infected and gained a certain level of natural immunity in 2020. A high level of immunity due to natural infection and vaccination helped reduce the transmission of SARS-CoV-2 in 2021.
In contrast to Western Europe, countries in Central and Eastern Europe have relatively low vaccine coverage. These countries in Central Europe, including Austria, Czechia, Serbia, Poland, and Hungary, have a vaccine coverage of 40–70%. This coverage is lower than the average coverage in Western Europe. For countries in Eastern Europe, including Romania, Bulgaria, Ukraine, and the Russian Federation, the vaccine coverage is 20–40%, which is much lower than in neighboring Central and Western European countries. From the death rate data, we can see that the new wave of disease beginning in September 2021 in Eastern Europe was much worse than in Central or Western Europe. The differences in death rate in this recent resurgence could be due to differences in vaccine coverage. In Bulgaria, only 23% of people are fully vaccinated, and it is among the countries with the highest COVID-19 mortality rate. In Romania, only 40% of people are fully vaccinated. Eastern Europe accounts for 7 of the 10 countries with the lowest vaccine confidence among a survey of 67 countries [21] conducted in 2016, and Bulgaria has the least-confident parents, with only 23% of respondents indicating no hesitation with vaccine services, which is consistent with the current vaccine coverage of 23% in Bulgaria. Hesitation related to perceptions regarding effectiveness, safety, and potential side effect has been the main driver of limited vaccine acceptance. Lack of knowledge regarding the severity of infection with the virus and distance from the epicenter of prior global pandemics might also play a role in the low uptake rate of the COVID vaccine.
The strategy of each country in combating COVID-19 has varied in terms of testing, tracing, and reporting rates; thus, the quality and accuracy of data (disease-induced death) regarding COVID-19 also vary. Some countries, such as the United Kingdom and France, have relatively strict policies governing disease control and monitoring, and their reported cases and deaths are close to the actual scope of the pandemic. Data regarding reported COVID-19 deaths in some other countries, such as Ukraine, Russian, and Germany, may not accurately reflect the actual pandemic impact. To accurately estimate the actual scope of the pandemic, excess deaths (calculated as all-cause mortality in the pandemic years minus the average number of deaths inferred from all-cause mortality in the previous 5 years) are frequently used to estimate the actual pandemic severity [14,15].
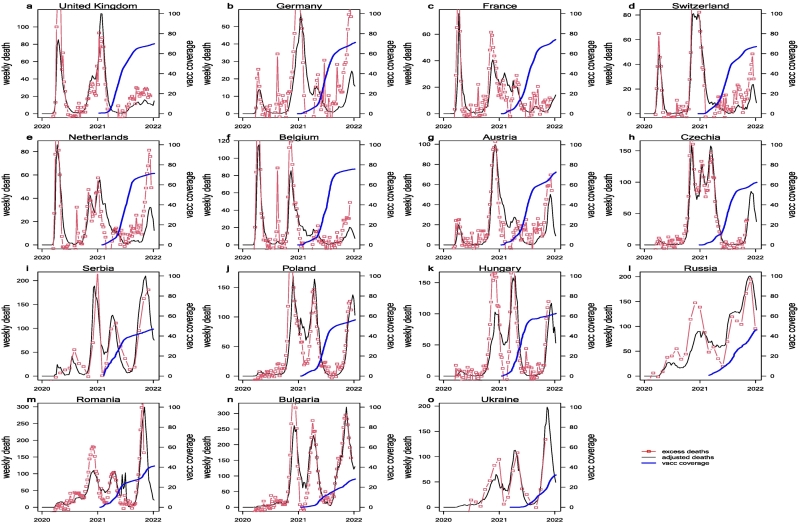
Excess deaths equal the all-cause mortality in the pandemic years minus the expected all-cause mortality in the absence of a pandemic, inferred from all-cause mortality in the previous 5 years [11]. Excess deaths in 2020–2021 should equal the COVID-19–related deaths minus the deaths expected to have been prevented by pandemic control measures. If we assume that deaths due to other causes (e.g. traffic) prevented by control measures are relatively low, then excess deaths data should be a good proxy for true COVID-19 deaths. On the one hand, excess deaths may yield negative values for some periods. On the other hand, COVID-19 deaths were likely under-reported. In this work, we assumed that the total number of excess deaths from January 2020 to December 2021 equaled the true number of COVID-19 deaths, Thus, we obtained the under-reporting ratio of COVID-19 as the ratio of the total number of reported COVID-19 deaths to the total number of excess deaths. We note that the under-reporting ratio should vary over time; that is, we would expect severe under-reporting in the early phase of the pandemic due to low awareness and lack of testing kits. For simplicity, we assumed that under-reporting was constant. We then divided the number of weekly reported deaths by the under-reporting ratio to generate the adjusted number of COVID-19 deaths, which matched the number of excess deaths. In Fig. 1, we show the adjusted number of weekly COVID-19 deaths (black curves) and the number of weekly excess deaths (if the excess deaths were monthly, we divided the value by four, the number of weeks in a month) (red curves). The two curves matched reasonably well, demonstrating that the excess deaths were mainly associated with COVID-19. The blue curves in Fig. 1 show the number of fully vaccinated individuals per 100 population in each country.
Fig. 1.
Weekly adjusted COVID-19 death (black curve), excess deaths (red curve), and daily fully vaccinated coverage (second dose) in 15 Europe countries. It was generally believed the excess deaths (the extra all-cause deaths in pandemic years compared above the average all-cause deaths in pre-pandemic five years) is a better proxy of true COVID-related deaths. Here we multiplied the reported COVID-19 deaths by a factor (>1) such that the total excess deaths equal the total of adjusted-reported COVID-19 deaths. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Fitting the model to adjusted deaths
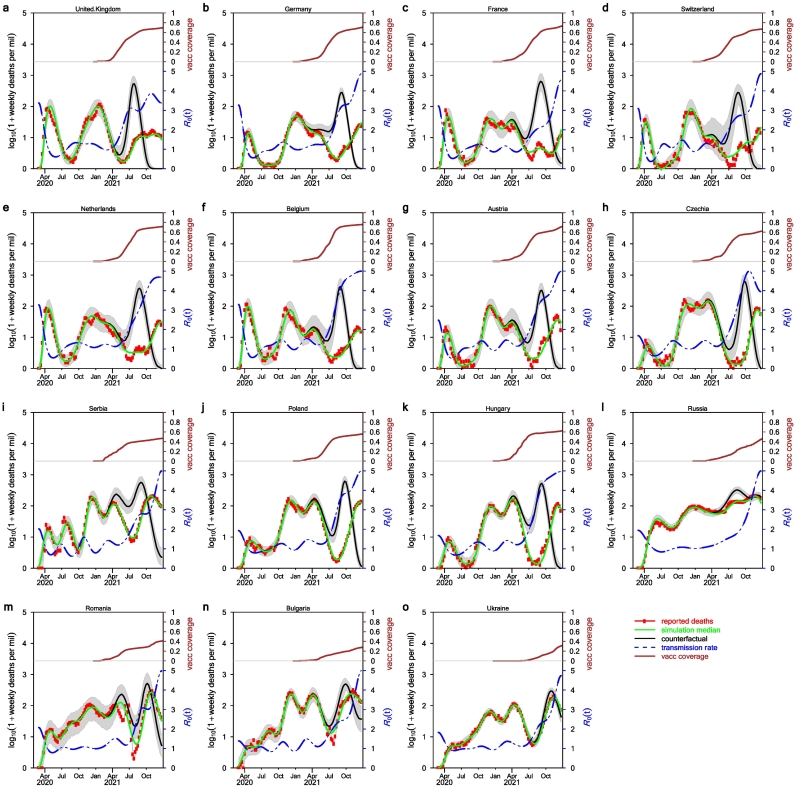
Fig. 2 shows our model simulation with ε = 0.5. We did not separate breakthrough infections and other infections among unvaccinated individuals in our model. The IFR is for all infections. When vaccination coverage increases, the proportion of breakthrough infections among all infections will increase. Thus, the IFR should probably decrease, but the magnitude of the decrease in IFR is unclear. The effect of vaccination in our model was reflected by the absence of infection and mortality among the majority of the vaccinated individuals. We simulated weekly deaths under two scenarios: a real scenario with vaccination which matched the adjusted deaths well, and a counterfactual scenario without vaccination but with other parameter estimates kept the same as in the real scenario. The difference in death toll under these two scenarios is deaths averted. Deaths averted data are shown in Fig. 3 (raw numbers) and Supplementary Fig. S1 (population scaled).
Fig. 2.
Fitting model to data and the effects of vaccination. Here, we used ε = 0.5 namely in the limit of vaccine coverage reaching 100%, the overall infection fatality rate will reduce by half. Red circles show the adjusted deaths, the green curve is the weekly median of 1000 model simulations under the real scenario. The black curve is the weekly median under the counterfactual scenario when vaccination is absent. The blue dash curve is the estimated transmission rate in the form β(t)/γ. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
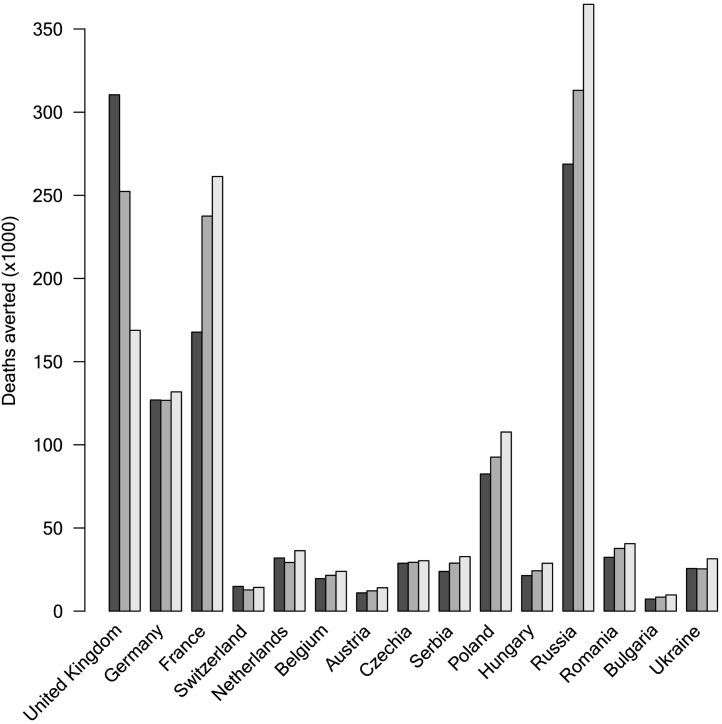
Fig. 3.
Deaths averted by vaccination campaign in 15 European countries by January 7, 2022. For each country, we compared three cases ε = 0, 0.25, and 0.5. For most countries, the effects of introducing ε lead to the effect of increasing the number of deaths averted, except for the UK, where the effect is the opposite.
Fig. 3 shows the number of deaths averted by the vaccination campaign in 15 European countries. From the raw numbers, we can see that the United Kingdom, Germany, France, Russia, and Poland had the most lives saved by the vaccination campaign. The population-scaled deaths averted are shown in Supplementary Fig. S1.
3.3. Differences in dominant variants and the resulting impact
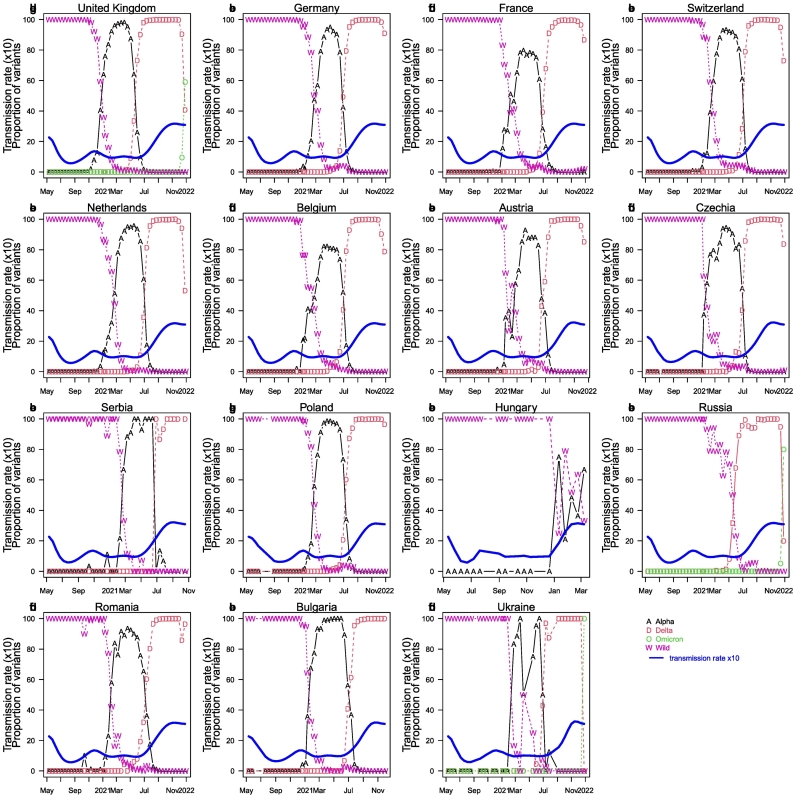
Fig. 4 shows the composition of dominant strains or variants over time in 15 countries. Most countries went through a pattern of “wild-type → Alpha → Delta” variants (namely, wild-type strain dominance followed by alpha variant, then followed by delta variant), but Hungary exhibited a “wild-type → Alpha+wild-type” variants pattern (wild-type and Alpha variant cocirculation), and Russia exhibited a “wild-type → Delta” variant pattern. In these strain/variant replacements, the latter variant possessed a transmission advantage compared with the earlier variant, such that the latter variant can replace the earlier variant in a population. Namely, the delta variant transmitted faster than the alpha variant, whereas the alpha variant transmitted faster than the wild-type strain. All countries shared virtually the same pattern of variant prevalence, except for Hungry and Russia, where alpha arrived very late or did not dominate. The delta variant was not identified in Hungry for some unknown reason. In Fig. 4, we also include the estimated overall transmission rate (solid blue curves) in our one-strain model. In the one-strain model, the transmission rate reflects the transmissibility of the dominant strain/variant in its dominant time interval. The delta strain exhibited the strongest transmissibility and became the dominant strain in most European countries before the omicron variant. The proportion of the omicron variant in the United Kingdom, Germany, France, Switzerland, Netherlands, Belgium, Austria, Russia, and Romania is increasing and may overtake the delta strain and become the next dominant strain in these countries as time passes, which might sweep other countries as well. The impact of omicron on the pandemic remains unclear.
Fig. 4.
The proportion of dominant strains or variants (a variant of concern VOC) out of all samples was sequenced biweekly in 15 countries. The blue curve shows the shows transmission rate (see later method and results). We downloaded aggregated variant proportion data from “The our world in data” which obtained their data originally from GISAID. [[17], [18], [19],22]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Conclusion
Tremendous hope was placed on the COVID-19 vaccine program in the earlier phase of the pandemic. However, a vaccine campaign is not solely determined by the vaccine efficacy and safety. Vaccine distribution and vaccine hesitancy became major obstacles in combating COVID-19 [23], along with the rapid mutation rate leading to the different variants. The vaccine acceptance rate among the public (laymen and healthcare workers) appears to have played a decisive role in controlling the pandemic [24]. The primary barriers to vaccine acceptance were fears regarding the quality and safety of the vaccines, as well as mistrust of the government [23].
The low vaccine coverage in Eastern Europe is probably not due solely to the availability of vaccines. It could also be due to public vaccine hesitancy rooted in distrust in the efficacy or safety of the vaccines, which is enhanced by misinformation on social media that spreads as fast as the virus [25]. Due to mistrust of the vaccine, the uptake rates of vaccines have been relatively low, even though countries such as Romania and Bulgaria have ample supply of vaccines [26]. Only 34.5% of Romania's inhabitants have received two injections, and 23.04% of Bulgaria's inhabitants had received two injections by November 2021. A Eurobarometer survey conducted earlier this year found a high degree of distrust of government and medical staff, with only 22% of Bulgarians, 26% of Latvians, and 31% of Romanians expressing trust in their government. In addition, 34% of Bulgarians and 40% of Romanians said they did not trust medical staff [27]. Trust in the vaccine, health system, and the government is a key to reducing anti-vaccine attitudes so that COVID-19 [21] can be successfully combatted globally.
A lack of confidence in vaccines among health workers might be another factor driving vaccine resistance in Eastern Europe. For example, approximately half of Ukrainian medical workers remain hesitant to get vaccinated [28]. Surveys of healthcare workers (doctors and nurses) found vaccine acceptance rates ranging from 27.7% to 78.1%, with the highest in Israel [24]. As pointed out by Obregon et al. [21], the skills and knowledge of health professionals or insufficient information they provide regarding the vaccine can cause people to rely on the internet or social media, where there can be misinformation and fake stories and a lack of trusted resources. The low acceptance rate of the vaccine could a major issue in combating COVID-19 globally in the short term.
One study showed that the vaccine can effectively reduce the hospitalization rate and in particular the death rate associated with a variant of concern (VOC), including the alpha, beta, gamma, and delta variants [29]. Approximately 92% of hospitalized patients are unvaccinated [28]. In Bulgaria, approximately 94% of deaths were of unvaccinated individuals [28]. People are highly encouraged to take two doses or even a third booster dose since the reduced severity of cases can help reduce the burden on public health systems so that patients in need can get appropriate care without crashing the system. Although the vaccines have demonstrated certain protection against the delta strain, fully vaccinated individuals are still urged to exercise caution, as the Delta strain is twice as contagious as previous variants [[31], [40]].
To overcome vaccine hesitancy or resistance, some studies have attempted to define the challenges in vaccine administration or campaigns and propose solutions. These studies have targeted Romania [32] and eight other countries in Europe [23]. Suggested actions include improving distribution chains and delivery of the vaccines and combating the spread of misinformation. Information campaigns aimed at the public, such as conveying the reduced risk with vaccination, non-medical benefits of a vaccination certificate, and personal benefits, have also been shown to effectively increase vaccine uptake in the United Kingdom and Germany [23]. It is suggested that health authorities or governments should tailor their vaccination campaigns to target special populations in particular economically disadvantaged groups that are less likely to have access to vaccines due to a lack of information, knowledge, or poverty.
Another study [33] also expressed concerns regarding the supply of vaccines worldwide and suggested that ensuring equitable vaccine access should be a global priority. Disparities in the distribution of vaccine doses exist worldwide. A report in Nature [34] indicated that more than 80% of doses have gone to people in high-income and upper-middle-income countries, but only 1% of people in low-income countries have been given at least one dose. However, we also see that help can extend beyond borders. For example, Albania vaccinated people who crossed the border before Kosovo started its vaccination campaign. Serbia and Romania have donated vaccines to North Macedonia or Moldova [35]. Global vaccine equity and collaboration are necessary for the global control of the pandemic. As pointed out by Padma [34], it is a long journey from the design of the vaccines to achieving global herd immunity, which cannot be achieved without collaborative and global responses.
As pointed out by Iftekhar et al. [36], three critical factors that affect the COVID-19 pandemic are population immunity/vaccination, VOCs, and public responses to pandemic policy. A study [37] covering 22 European countries found that COVID-19 vaccination public opinion/acceptance rates have more impact on the vaccination rate than factors related to government vaccine administration. As pointed out in [38], collaboration across nations under the ‘One Health approach is crucial in controlling and preventing future pandemics. It is important to speed up vaccine development and vaccine delivery and enhance vaccine coverage across countries to save lives and close the opportunity of emergence of new variants. In 2022, Omicron VOC replaced previous VOCs and dominated in most countries due to its strong immune evasion ability. However, the vaccine efficacy against death caused by Omicron VOC is still very high. For example, the case fatality rate of Omicron VOC among unvaccinated is 16-fold of that among vaccinated in the fifth wave in Hong Kong, China [39]. It is foremost important to deliver vaccine to those high-risk groups or countries. All together, we can finally get out of the pandemic.
Availability of data and materials
All data used in this work were publicly available.
Funding
The work described in this paper was partially supported by a grant from the Research Grants Council of the Hong Kong Special Administrative Region, China (HKU C7123-20G), the National Natural Science Foundation of China (12171291), the Fundamental Research Program of Shanxi Province (20210302124018) and the Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province (20200001).
Contributions
DH, GF, HS, SY, and TZ conceived the study, carried out the analyses, and wrote the manuscript draft.
DH, GF, HS, SY, and TZ discussed the results, revised the manuscript critically, and approved it for publishing.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2022.100402.
Appendix A. Supplementary data
Supplementary Fig. S1. Deaths (per million inhabitants) averted by vaccination campaign in 15 European countries by January 7, 2022.
References
- 1.Importance of One Health for COVID-19 and Future Pandemics Centers for Disease Control and Prevention. https://www.cdc.gov/media/releases/2021/s1103-one-health.html Available from.
- 2.WHO says Europe is back at the epicenter of Covid pandemic, despite vaccines NBC news. 2021 Nov 4. [Google Scholar]
- 3.European Centre for Disease Prevention and Control . 2021 [December 9]. COVID-19 situation update worldwide, as of week 48, updated 9 December 2021.https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases Available from: [Google Scholar]
- 4.Ruckert A., Zinszer K., Zarowsky C., Labonté R., Carabin H. What role for One Health in the COVID-19 pandemic? Can. J. Public Health. 2020;111(5):641–644. doi: 10.17269/s41997-020-00409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin L., Zhao Y., Chen B., He D. Multiple COVID-19 waves and vaccination effectiveness in the United States. Int. J. Environ. Res. Public Health. 2022;19(4):2282. doi: 10.3390/ijerph19042282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin L., Chen B., Zhao Y., Wang W., He D. Two waves of COVID-19 in Brazilian cities and vaccination impact. Math. Biosci. Eng. 2021;19(5):4657–4671. doi: 10.3934/mbe.2022216. [DOI] [PubMed] [Google Scholar]
- 9.Musa S.S., Tariq A., Yuan L., Haozhen W., He D. Infection fatality rate and infection attack rate of COVID-19 in south American countries. Infect. Dis. Pov. 2022;11(1):1–11. doi: 10.1186/s40249-022-00961-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang X., Musa S.S., Zhao S., Mei S., He D. Using proper mean generation intervals in modeling of COVID-19. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.691262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannah Ritchie E.M., Rodés-Guirao Lucas, Appel Cameron, Giattino Charlie, Ortiz-Ospina Esteban, Hasell Joe, Macdonald Bobbie, Beltekian Diana. 2020. Max Roser. Coronavirus Pandemic (COVID-19)https://ourworldindata.org/coronavirus [cited 2022 Feb 28]. Available from: [Google Scholar]
- 12.Johns Hopkins University CSSE Available from. 2022. https://github.com/CSSEGISandData/COVID-19
- 13.Vladimir Shkolnikov M.B. John Wilmoth. Human Mortality Database. 2022 doi: 10.1093/ije/dyv105. https://www.mortality.org Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The pandemic’s true death toll Our daily estimate of excess deaths around the world: The Economist. 2022. https://www.economist.com/graphic-detail/coronavirus-excess-deaths-estimates Available from: [Google Scholar]
- 15.Karlinsky A., Kobak D. Tracking excess mortality across countries during the COVID-19 pandemic with the World Mortality Dataset. Elife. 2021;10 doi: 10.7554/eLife.69336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathieu E., Ritchie H., Ortiz-Ospina E., Roser M., Hasell J., Appel C., et al. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 2021;5(7):947–953. doi: 10.1038/s41562-021-01122-8. [DOI] [PubMed] [Google Scholar]
- 17.Shu Y., McCauley J. GISAID: global initiative on sharing all influenza data–from vision to reality. Eurosurveillance. 2017;22(13):30494. doi: 10.2807/1560-7917.ES.2017.22.13.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khare S., Gurry C., Freitas L., Schultz M.B., Bach G., Diallo A., et al. GISAID’s role in pandemic response. China CDC Weekly. 2021;3(49):1049. doi: 10.46234/ccdcw2021.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elbe S., Buckland-Merrett G. Data, disease and diplomacy: GISAID’s innovative contribution to global health. Global Chall. 2017;1(1):33–46. doi: 10.1002/gch2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodcroft E.B. CoVariants: SARS-CoV-2 Mutations and Variants of Interest. 2021. https://covariants.org Available from:
- 21.Obregon R., Mosquera M., Tomsa S., Chitnis K. Vaccine hesitancy and demand for immunization in Eastern Europe and Central Asia: implications for the region and beyond. J. Health Commun. 2020;25(10):808–815. doi: 10.1080/10810730.2021.1879366. [DOI] [PubMed] [Google Scholar]
- 22.Hodcroft E.B. CoVariants: SARS-CoV-2 Mutations and Variants of Interest. 2021. https://covariants.org/ Available from:
- 23.Steinert J., Sternberg H., Prince H., Fasolo B., Galizzi M., Büthe T., et al. COVID-19 vaccine hesitancy in eight European countries: prevalence, determinants and heterogeneity. Sci. Advances. 2022;8(17) doi: 10.1126/sciadv.abm9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallam M. COVID-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines. 2021;9(2):160. doi: 10.3390/vaccines9020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pietsch B. Eastern Europe, facing coronavirus pandemic surge, also battles vaccine hesitancy. Wash. Post. 2021 Nov;11 [Google Scholar]
- 26.Oltermann J.H.P. Why is Europe returning to the dark days of Covid? Guardian News. 2021 Sept 13. [Google Scholar]
- 27.The Ecomomist (Europe) 2021 Nov 13. The arc of susceptibility: Eastern European countries are being hit by a wave of covid deaths: But only where vaccination rates are poor. [Google Scholar]
- 28.Karmanau Y. 2021 Oct 28. Vaccine Reluctance in Eastern Europe Brings High COVID Cost AP News. [Google Scholar]
- 29.WHO COVID-19 Weekly Epidemiological Update 2021 [September 7] https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---7-september-2021 Available from.
- 31.Heathline: Even if you are vaccinated, the Delta variant can still impact you 2021. Available from: https://www.healthline.com/health-news/even-if-youre-vaccinated-the-delta-variant-can-still-impact-you.
- 32.Dascalu S., Geambasu O., Covaciu O., Chereches R.M., Diaconu G., Dumitra G.G., et al. Prospects of COVID-19 vaccination in Romania: challenges and potential solutions. Front. Public Health. 2021;9:90. doi: 10.3389/fpubh.2021.644538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forman R., Shah S., Jeurissen P., Jit M., Mossialos E. COVID-19 vaccine challenges: what have we learned so far and what remains to be done? Health Policy. 2021;125(5):553–567. doi: 10.1016/j.healthpol.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Padma T. COVID vaccines to reach poorest countries in 2023—despite recent pledges. Nature. 2021;595(7867):342–343. doi: 10.1038/d41586-021-01762-w. [DOI] [PubMed] [Google Scholar]
- 35.Kluge H., McKee M. COVID-19 vaccines for the European region: an unprecedented challenge. Lancet. 2021;397(10286):1689–1691. doi: 10.1016/S0140-6736(21)00709-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iftekhar E.N., Priesemann V., Balling R., Bauer S., Beutels P., Valdez A.C., et al. A look into the future of the COVID-19 pandemic in Europe: an expert consultation. The Lancet Regional Health-Europe. 2021;8 doi: 10.1016/j.lanepe.2021.100185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kozlovskyi S., Bilenko D., Kuzheliev M., Ivanyuta N., Butenko V., Lavrov R. Comparison and assessment of factors affecting the COVID-19 vaccination in European countries. Problemy Ekorozwoju. 2021;16(2) [Google Scholar]
- 38.Strengthen ‘One Health approach’ to prevent future pandemics – WHO chief. United Nation News. Available from: https://news.un.org/en/story/2021/02/1084982.
- 39.Latest situation of COVID-19 (as of 4 April 2022): Centre for Health Protection (CHP) of the Department of Health (DH) Hong Kong. 2022. https://www.chp.gov.hk/files/pdf/local_situation_covid19_en.pdf [cited 2022 April 4]. Available from:
- 40.2022. K. Katella. YaleMedicine: 5-things-to-know-delta-variant-covid
- 41.Song H., Fan H., Liu Y., Wang X., He D. The Second Wave of COVID-19 in South and Southeast Asia and the Effects of Vaccination. Front. Med. 2021;8 doi: 10.3389/fmed.2021.773110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song H., Fan G., Zhao S., Li H., Huang Q., He D. Forecast of the COVID-19 trend in India: A simple modelling approach. Mathemat. Biosci. Eng. 2021;18(6):9775–9786. doi: 10.3934/mbe.2021479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S1. Deaths (per million inhabitants) averted by vaccination campaign in 15 European countries by January 7, 2022.
Data Availability Statement
All data used in this work were publicly available.