Abstract
While pregnancy presents a strong motivation to seek and comply with treatment for opioid use disorder (OUD), many women relapse within the first year of childbirth. Addressing relapse risk, we examined the perinatal experiences of mothers with OUD through 6 months postpartum. We recruited mothers (N = 42) with a history of OUD into the Newborn Attachment and Wellness study, all of whom met with a child welfare worker immediately after giving birth. In qualitative interviews, mothers described their social, physical, emotional, and psychological perinatal experiences. Seven themes categorically informed relapse risk (i.e., related to childhood bond, mother-infant attachment, birth support, child protective services, breastfeeding, mental health, and recovery planning). In conclusion, we noted a critical window in which clinical social workers and other health/behavioral health providers have the opportunity to capitalize on mothers' desire not to “ever want to touch it again." We outline specific avenues for directed support in the perinatal and postpartum period associated with reduced risk for relapse, and we make recommendations to enhance risk assessment practices.
Keywords: Postpartum, Mothers, Opioid use disorder, Substance use disorder, Addiction, Relapse
From 2010 to 2017, the rates of opioid-related diagnoses such as opioid use disorder (OUD) among new mothers at delivery have increased 131% (Hirai et al., 2021). Consequently, the rate of babies born with neonatal abstinence syndrome (i.e., neurobehavioral signs of withdrawal in a newborn) has increased to 82% of those exposed in utero and affects all demographic groups and geographic locations (Hirai et al., 2021; Substance Abuse and Mental Health Services Administration [SAMHSA], 2018). Despite a critical need for interventions, guidelines, and clinical support during the postpartum period, multiple barriers prevent women with OUD from receiving the care they need (SAMHSA, 2018). Stigma, shame, and misinformation exacerbate the growing national problem as many healthcare professionals are reluctant to provide essential care and support to women after giving birth (SAMHSA, 2018).
The postpartum period, typically 6 to 8 weeks after giving birth, is an important time for both parent1 and baby, where forming an attachment is critical for the newborn's developmental needs while the parent's body is physically recovering. This period of time can be challenging for new mothers, especially for those with OUD (Ellis et al., 2019). Women with OUD may experience a host of mental health challenges, including anxiety, depression, and post-traumatic stress disorder (Agius et al., 2016; Benningfield et al., 2012; Davie-Gray et al., 2013; Hazelgrove et al., 2021; Holbrook & Kaltenbach, 2012; Söderquist et al., 2009). Ultimately, the postpartum period among women with OUD is a volatile time during which parent/child attachments may be strengthened or put at-risk as a function of relapse. Clinical social workers and other behavioral health care providers allied with obstetrical care are particularly well-position to strengthen potential for continued recovery and help mitigate the risk of relapse through comprehensive early assessment.
Risk of Relapse
The first year after giving birth, rates of opioid relapse, overdose, and death increase substantially as compared to the year before delivery (Schiff et al., 2018). Relapse is "a return to substance use after a significant period of abstinence" (SAMHSA, 2020, p. 4). One study indicated that 83% of women are likely to abstain from alcohol and drug use during pregnancy, though the study excluded women who identified opioids as a primary drug of choice (Forray et al., 2015). Less is known about the rates of pregnant people who use opioids prenatally. However, pregnancy may be the only time a parent presents for medical care, creating an opportunity for OUD treatment (Reddy et al., 2017). Under the supervision of a health care provider during pregnancy, parents may decrease opioid use, or they may be more likely to accept treatment utilizing medication for OUD (e.g., buprenorphine or methadone). After birth, however, the frequency of visits to health care providers may decrease, creating an environment that increases the risk of relapse (Schiff et al., 2018).
Ensuring adequate support during the postpartum period means that health and behavioral health providers can identify the risk of relapse and offer access to effective preventative measures and interventions. When there is limited access to interprofessional care and interpersonal support, women with OUD experience significantly increased rates of postpartum relapse (Chapman & Wu, 2013; SAMHSA, 2018). Given these high rates of relapse, it is clear that close postpartum monitoring and accompanying treatment systems will help decrease rates of overdose and death (Irvine et al., 2019). What is less clear, however, are the specific areas that need to be addressed to achieve these desired outcomes. Determining salient risk and protective factors such that interventions may be targeted for the needs of postpartum parents is an area in need of further inquiry.
Factors Associated with Relapse
Several risk factors are associated with postpartum relapse, including the new demands of parenting, parenting a child who exhibits neonatal opioid withdrawal syndrome (NOWS; Wachman et al., 2018), and lack of preparedness (Krans et al., 2018). Other factors include sleep deprivation (Committee on Obstetric Practice, 2017), the threat of loss of child custody (Proulx & Fantasia, 2021), postpartum depression and anxiety (Corr et al., 2020), lack of interpersonal social support (Bollampally, 2019; Young & Martin, 2012), and postpartum pain (Committee on Obstetric Practice, 2018). Additionally, fewer days in formal prenatal treatment, entering treatment late in pregnancy, opioid use during pregnancy, having a cesarean section, and receiving opioid medication at discharge also contribute to the increased risk of relapse (Ellis et al., 2019). Furthermore, women who are on medication for opioid use disorder (MOUD), such as with methadone, are far more likely (than women who did not use prenatally) to have contact with the child welfare system, which is stressful and anxiety-provoking (Lean et al., 2013). As the rate of pregnant women with OUD has increased by 131% between 2010 and 2017 (Hirai et al., 2021), the above challenges have grown exponentially. Addressing said challenges will require a comprehensive understanding of risk and protective factors associated with relapse.
Mitigating and Protective Factors
In addition to the protective function of support systems, it is increasingly evident that for mothers with a history of SUD, bonding and attachment may act as protective factors due to the oxytocin released after delivery and with breastfeeding (Patterson et al., 2021). Oxytocin is a hormone that helps develop attachment between newborns and mothers through early interaction (Scatliffe et al., 2019). Research demonstrates that elevated oxytocin levels in mothers are related to more affectionate contact behaviors, and parents with higher oxytocin levels were found to have more synchrony and responsiveness in their interactions with their infants (Scatliffe et al., 2019). Breastfeeding is recommended for women who are stable on MOUD (SAMHSA, 2018). Other mitigating factors include comprehensive and careful postpartum pharmacological care for pain management. Researchers have stressed the need for physicians to have adequate education in pain management, especially for mothers with MOUD (Ellis et al., 2019). Furthermore, related to mitigation and protective factors, empirical studies have concluded that increased recovery supports (e.g., formal treatment setting) are critical in the month after delivery (Ellis et al., 2019). This evidence suggests that women with OUD may benefit from increased and early postpartum visits that include discussions related to psychiatric care and long-term pharmacotherapy (Rizk et al., 2019). Guidelines for clinical care are increasingly available, mainly in risk assessment and pharmacotherapies. However, there is room to enhance the practice of risk assessment with a more profound understanding of patients' experiences. Thus, part of our purpose herein is to offer suggestions for the enhancement of risk assessment practices among social workers and other behavioral health care providers.
Risk Assessment
A well-established first step in determining the risk of relapse among anyone with substance use disorder is early assessment. Many screening methods exist to help social workers and other health/behavioral health providers determine the biopsychosocial implications of OUD among postpartum parents, such as The World Health Organization’s (WHO) Guidelines for the Identification and Management of Substance Use and Substance Use Disorders in Pregnancy (WHO, 2014). Multiple instruments can help clinicians determine the next steps for patient care with substance use (e.g., the Substance Use Risk Profile-Pregnancy [SURP-P]). Furthermore, growing information is available to healthcare providers who are interested in reducing adverse outcomes by utilizing pharmacotherapy with new parents who have an OUD diagnosis (e.g., American Society of Addiction Medicine [ASAM] National Practice Guidelines for the Use of Medications in the Treatment of Addiction Involving Opioid Use [American Society of Addiction Medicine, 2020]; the Clinician Consultation Center at the University of California [National Clinician Consultation Center, n.d.]). Clinical assessment and medication guidance add to the practitioner toolkit; however, not all mitigating factors have been unearthed. A standardized method of detecting illicit drug use among pregnant people has not been well-established (US Preventive Services Task Force, 2020). Furthermore, a standard of practice for clinical social workers related to assessment for people who use opioids has yet to fully materialize (Vakharia & Little, 2017). What is clear however, is that the best prepared clinician is the one who knows both what and how to assess; meaning, s/he/they must have a full understanding of risk and protective factors while starting where the client is (Vakharia & Little, 2017). Ultimately, more inquiry related to the risk of relapse among postpartum parents will add rigor to the assessment. While momentum in risk assessment and intervention grows, we aim to add to the knowledge base related to risk and mitigation factors such that clinicians are armed with increasingly specific areas for assessment and inquiry.
With our study, we examined the lived experience of having a baby among mothers with SUDs and identified potential factors that may influence maternal and newborn attachment, which may then influence their desires to use substances postpartum. With these qualitative methods, we add richness and depth to what is known in the empirical foundation. The purpose of the present study was to examine the qualitative differences in experience among mothers who had urges to use substances postpartum compared to mothers who did not have urges to use substances. We informed our efforts by considering empirical studies that examine both risk and protective factors associated with relapse. This study will contribute to our understanding of risk factors associated with opioid use relapse among pregnant and postpartum parents, enabling providers to be better equipped to work with new parents and instruments that are better tailored to assess both risk and protective factors.
Methods
The Newborn Attachment and Wellness (NAW) Study assesses perinatal factors potentially associated with attachment for mothers with a history of substance use, primarily from opioids, and their newborns with NAS. The institutional review boards for human subjects research at the [University] and [Hospital] assumed study oversight. The study was approved on 02/13/2018.
Participants
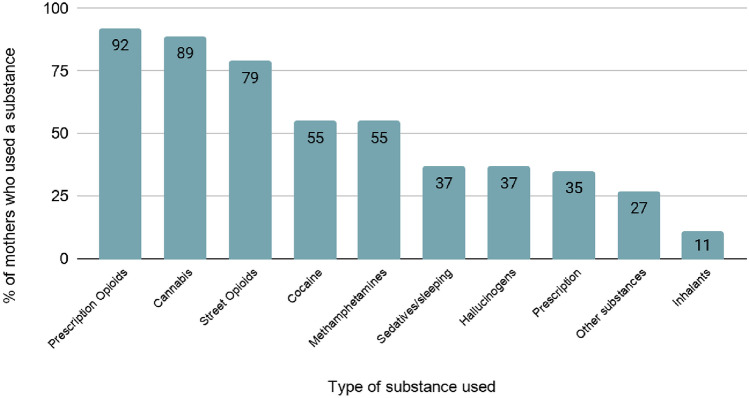
Participants were biological mothers (N = 42) with a self-reported history of substance use (92% prescription opioids, 89% cannabis, 79% street opioids, 55% cocaine, 55% methamphetamines, 37% sedatives/sleeping pills, 37% hallucinogens, 35% prescription stimulants, 27% other substances, and 11% inhalants) (see Fig. 1). In cases where mothers denied opioid use, the mother and/or the infant had a positive toxicology screen that indicated opioid exposure during pregnancy and were therefore included in the study. Mothers were interviewed when they gave birth (wave 1), at 3 months (wave 2), and at 6 months postpartum (wave 3). The final sample (aged 19 to 41 years, Mage = 28.56; SD 5.19) represents the diversity of the southwest United States region (i.e., 50.0% White, 19.0% Hispanic, 4.8% Native American, 7.1% African American).
Fig. 1.
Substances used by mothers before pregnancy (N = 42)
Measures
Participants were interviewed by social work graduate students and social work interns for approximately 1 hour in-person (February 2018–March 2020) or by Zoom (March 2020–April 2021) at each wave (birth, 3-months, and 6-months). They were asked a series of open-ended questions about their lived experiences leading up to, surrounding, and following their birth across social, physical, emotional, psychological, and environmental factors. The questions inquired about a range of topics including, "What was your birth experience like?", "What was your first reaction when you found out you were pregnant?", "What has your bonding experience with your baby been like?", and "Describe your relationship with your biological mother when you were a child." Participants were probed with follow-up questions to expand when further clarification was needed (e.g., “Can you tell me more about what that was like?” “Could you give me an example?”).
Additionally, participants responded to a quantitative measure at each wave via a self-report survey on how often they had a strong desire or urge to use the following substances in the past 3 months: cannabis, cocaine, prescription stimulants, methamphetamine, inhalants, sedatives, hallucinogens, and street or prescription opioids. Data were summed across substances (1 = any urges, 0 = no urges) to create a total urge score and was then dummy coded at each wave as any urges to use substances, no initial urges who later developed urges, and no urges to use substances. Questions regarding current substance use were not asked at the 3- and 6-month interviews. This information was obtained at baseline either by maternal admission of use or positive toxicology screen of mother and/or infant.
Analytical Approach and Data Coding
The interviews were transcribed verbatim (including field notes) and entered into QSR NVivo, a qualitative data analysis software tool (Gibbs, 2002). In the coding process we utilized reflexivity, including documenting each step by establishing an audit trail and triangulating the data through the involvement of multiple researchers. These steps strengthen the trustworthiness of the study (Creswell & Miller, 2000; Padgett, 2008). Using a triple-blind design, the first coder did not conduct any interviews and analyzed the data blind to the participants' condition. The coding process gave weight to the transcribed interviews, including responses to the interview questions and any unprompted comments. The text was examined using inductive content analysis. The resulting codes yielded patterns of recurring or outstanding responses grouped into emerging themes based on content frequency, specificity, emotion, and extensiveness (Krueger & Casey, 2000). The themes were then charted and analyzed to determine theme weightiness in all three categories of mothers. The resulting themes and subthemes were refined in collaboration with a second coder. Three waves of data were included in the coding process, depending on the availability of transcriptions from each participant. Supplemental waves (i.e., 4 or 5) were included among participants who did not have data on file for waves 2 and 3. Participants were placed into three categories: MNUs—mothers with no urges (no urges to use illicit substances across the study period, n = 10; 24%), MNIUs—mothers with no initial urges (wave 1 = 0, wave 2 or 3 = 1 or more, n = 6; 14%), and MUs—mothers with urges (wave 1 = 1 or more, n = 26; 62%).
Results
Based on frequency, richness, specificity, and emotionality of the responses, seven main themes related to mother's urges to use illicit substances were identified. We present the themes in an approximate chronological order: Maternal Childhood Bond, Pregnancy and Postpartum Attachment, Birth Experience Support, Department of Child Safety (DCS) Involvement, Breastfeeding, Mental Health, and Recovery Plan (see Fig. 2). Aliases are used in reporting the results to protect the privacy of participants.
Fig. 2.
Relating urges to relapse risk
Maternal Childhood Bond: “It was Like How, How I Want, How I Want to Be with my Kids”
Mothers across categories described their childhood bonding experience with their mother as a motivator for creating a positive relationship with their newborn, however, there were differences in the perceived impact of their childhood attachment. For MNUs, many described that they did not have a positive bond with their mothers as children, but this motivated their desire to create a better bond between themselves and their infants. For example, Jane, aged 32, MNU, explained,
The fact that my mom left me when I was little, I think that made me want to be with my kids and be a better mom. Because I could never imagine leaving my kids. Every kid deserves to bond with their mom and be with their mom and be loved by their mom.
Samantha, 22, MNU, also explained,
I don't have that kind of bonding or that kind of relationship with my mom… She wasn't caring or nurturing or anything like that, while growing up. So, I try to do better and be the best that I can for my kid. I learned not to be like my mom. I learned patience is a key for parenting. Time and attention is what matters the most when having a child. And being a positive role model is what they need. But [my mom], she definitely strengthened those traits about me.
On the other hand, some MNIUs and MUs described a close childhood bond with their mothers and that this motivated them to want to create the same type of bond with their babies. Lara, age 30, MU, explained her maternal childhood relationship, stating, "It was close… My mom was on drugs my whole life… but she never failed to take care of me… I never had to work for anything." She went on to explain that she wanted the same with her baby, saying, "I can say from dealing with my own mom, [I hope my baby and I] establish and get that good grounds for communication from the beginning." Priscilla, age 26, MNIU, also explained, "I'm so grateful that my mom raised me because she showed me like how to be a mom and how like to take care of my kids." Similarly, Ana, 35, MU, explained, "It was, it was, it was really good. As far as I can remember you know, um, it was like how, how I want, how I want to be with my kids you know."
Pregnancy and Postpartum Attachment: "I didn't Talk to my Tummy a Lot…that was During the Time When I was Still Kind of Upset"
In general, mothers in all three categories described the news of their pregnancy as being unexpected or shocking and described feeling like they did not know what they would do. For example, Kristen, age 32, MNU, explained, "[I was] shocked cause I already had three and didn't plan—I didn't plan on anymore." Kiana, 34, MNU, described, "I got pregnant. I was like, this wasn't part of the plan… I was shocked to say the least. I was surprised. I wasn't expecting it." MNIUs described similar expressions of shock. However, in addition to feeling shocked and unprepared for the pregnancy, MNIUs were more likely to report feeling upset and scared upon learning about the pregnancy. Alicia, 27, MNIU, stated, "I couldn't believe it… It made me nervous. Scared. I didn't know what to do or what to expect really, you know?" Mayra, 21, MNIU, explained, "honestly, I was really sad and depressed, I didn't expect it—having another child." Sarah, age 32, MNIU, also stated, "I was very surprised and scared… I was not planning on having a baby yet." While MUs also described feeling shocked and scared, they were more likely to find out about their baby late into their pregnancy. Lara, 30, MU, explained her reaction to finding out about her pregnancy late, stating, "I cried because, uh, I didn't find out till I was 6 or 7 months… There wasn't even time to develop all of those emotions and place all of those emotions." Ava, 27, MU, stated, "I didn't find out until pretty late, like believe it or not… I didn't know. I was like 20 weeks."
In addition to feeling unprepared, mothers' connection to their baby during pregnancy was also prominent. Among MNUs and MNIUs, there was no mention of having difficulty developing an attachment with their baby during pregnancy. Mia, 22, MNU, described the bond she felt during her pregnancy, saying, "It's gotten stronger, um like when I was pregnant, I would talk to my stomach, I'd be so happy you know." Similarly, Kiana, 34, MNU, felt an attachment with her newborn during pregnancy. "I think he always has, cause like even when I was pregnant, when I would cry, or I'd be sad, he'd move. He'd comfort me." However, several MUs explained that they did not have a strong attachment with their newborn during pregnancy. Lara, age 30, MU, described, "I-it was rough in the beginning because I felt like we weren't connecting. And that was when she was in my stomach… I didn't talk to my tummy a lot you know and that was during the time when I was still kind of upset." Aaliyah, age 32, MU, also described, "I think that at first, when before I had the baby… I was really, really scared to get attached because… I didn't know what was going to happen with DCS if I was going to like keep my children."
Mothers more often talked about connecting with their babies once they were born, particularly through skin-to-skin contact. Michelle, 41, MNU, described that "Especially with the skin-to-skin, [she felt baby truly started to bond with her] like instantly." Priscilla, 26, MNIU, described, "I'll lay him on my chest, you know, skin-to-skin because they recommended that and I feel maybe a little stronger bond… because when I lay him on my chest skin-to-skin he'll always lay his face a certain way, like right on my heart." Rachel, age 26, MNU, described, "The skin-to-skin, and with her going through like the withdrawals and stuff, she was really dependent on me I guess for comfort and everything. So that's when it, when the bond really started."
Many mothers described using babywearing to continue their bonding relationship with their newborn. For example, Aaliyah, age 32, MU, explained, "It kind of imitates skin-to-skin even though it's not skin-to-skin. I think it creates that bonding, especially for a dad too. It gives him that experience." Similarly, Olivia, 36, MU, said, "…it [babywearing] felt really secure, you know… I felt the closest probably when she was… I was wearing her… like putting her in the carrier was a moment when I felt like my baby truly started to bond with me." Sarah, 32, MNIU, described how babywearing substituted for her struggles with breastfeeding by giving her a way to stay close to her baby, saying,
I think most people know like [breastfeeding is] such a special connection with your child… [so] I was so sad that I couldn't [breastfeed] but we're still wearing the carrier… I would just wear her most of the time, just get her to bond to like just to have that contact… Yeah, it really helped substitute for the lack of nursing.
There were no differences across mothers in babywearing or skin-to-skin contact and bonding with their infant once the baby was born.
Birth Experience Support: “I Had to Experience Everything by Myself”
In general, MNUs described how having support figures, such as a partner or family members, present during their birth experience made their overall birth experience better. For example, Kristen, age 32, MNU, described, "we had a lot of support and it was very peaceful and everything completely just worked out." On the other hand, MUs explained that they missed having any social support. Olivia, age 36, MU, explained, "I would have wanted it to be different, you know, if I had my choice… I would have liked to have my partner there." Similarly, Celia, age 36, MU described missing out on the support of her partner at the birth because of the quick onset of labor, "I went in an ambulance by myself because the kids were there at the house…it was the worst experience of my life." MNIUs did not talk about support figures as part of their birth experience.
In addition to partner support, participants described the hospital staff's support, or lack of support, as impactful to their overall birth experience. MNUs generally described feeling accepted while at the hospital. For example, Lucille, age 32, MNU stated,
Being on methadone, you can like come across people who don't understand or don't get it and [the hospital staff] were all very respectful and patient and... not judgmental at all. They were very helpful, so it was a really, really good experience.
Conversely, MUs talked about the stigma of recovering from a substance use disorder. Olivia, age 36, MU, shared, "There's a definite treatment that was like, probably you know, because of my history. So I feel like I was treated overall like a dumbass. I just thought they were gonna like take my kid and pretend like everything was gonna be okay." This perception was echoed in some participants' experiences that their birth pain was not perceived as valid. For example, Celia, age 36 said, "I just wanted medicine because I was in so much pain and they wouldn't give it to me and I was screaming and yelling and they're like, telling me they're like, calm down because I was scaring people but they didn't understand. They didn't know what was going on inside my body like my uterus tore. When [the doctor] opened me up she goes, 'oh, that's why she was in so much pain'."
Department of Child Safety (DCS) Involvement: “They were Involved When She was Born Because I was on the Methadone”
All the mothers in the study faced potential DCS involvement. However, most MNUs did not have any DCS involvement after the initial interview. Michelle, 41, MNU, explained how the potential involvement of DCS impacted her emotional well-being in her response to the interviewer’s question,
I felt nothing to look forward to…I would say sometimes and a lot of this in the last week is more situational with the DCS meeting. So now that that’s past and you have a resolution in a sense, I feel a lot less stressed out and [less] anxiety. I feel like I can enjoy him now…Everything has been revolving around this DCS and not knowing until yesterday…and the threat of losing him.
In Michelle’s case, her mother was identified as the safety monitor for the next 30 days, until the baby had no levels of THC: “Once that’s at zero, then my mom is able to go. But we’ll be taking intense in-home services. So, I’ll have a person from two different programs come out.”
For some MNIUs, DCS became involved within the initial weeks following birth. For example, Mayra, 21, MNIU, explained that her baby was placed into DCS care at 9 days old, and "I see her three times a week, like 2 (h) each visit." However, almost all DCS involvements initiating at birth were found with MUs. For example, Olivia, 36, MU, discussed how her feelings of attachment have changed due to her involvement with DCS. "I felt like because I haven't had my kids for a while, they haven't lived with me for a while. And it's like the longer they're away from you, the less you feel like a mom."
Breastfeeding: "Breastfeeding isn't All What People- Like- Make It"
Mothers with No Urges (MNUs) described a positive breastfeeding experience with their infants. For example, Jane, age 32, explained, "yes, [I breastfed] for 2 to 3 months, I wanna say," and had few difficulties with breastfeeding. Furthermore, even among MNUs that did not initially plan to breastfeed, some were still breastfeeding successfully at 6 months. In contrast, a common pattern for MNIUs was that many breastfed successfully for only a short period of time and often had difficulties in their breastfeeding experience. Sarah, 32, explains, "she only breastfed for 2 months, and it was a fight the whole time… So, I hardly breastfed her, which was sad because I loved how much bonding it was and how much me and my first daughter bonded while breastfeeding." Similarly, Alma, 28, MNIU, explained, "no, I don't breastfeed. I did in the beginning, but I didn't make enough." Most MUs did not breastfeed or described significant struggles with breastfeeding their newborn. Joanna, age 29, describes the difficulties she faced trying to breastfeed, explaining, "I can't breastfeed, which sucks… He wouldn't latch, so I pumped but it wasn't enough to fulfill him… eventually, I just couldn't make enough at all. It was so sad."
Mothers in all categories described feeling sadness and frustration about their experiences when difficulties with breastfeeding were present. However, disinterest in breastfeeding was only displayed in MUs and was not seen in MNUs and MNIUs. For example, Judith, age 31, MU, described, "even though they did recommend it [breastfeeding], I just personally, I just chose not to." Despite these patterns, mothers in all three categories made statements about breastfeeding as a significant source of bonding between them and their newborns.
Mental Health: “Sometimes Hormones and Pregnancy Like Mess People Up”
Differences in participants’ perceptions of their own mental health emerged as well. Specifically, the experiences of MUs were noted by explicit statements of challenges with mental health. For example, at age 33, MU, Natalie stated,
Often I have felt downhearted and blue… I'm just disgusted. I'm just to the point where I lost so much weight, where I don't even care like it doesn't bother me. I look sick. I don't really care anymore. I know what's going on with me and I'm just sad you know. I'm just sad [baby crying].
Lara, age 30, MU, also explained, "My postpartum [depression] when I got home was a little, a little bit hard at first… [the baby’s] so far away." Similarly, Joanna, age 29, MU, stated, "The postpartum [depression] has really taken a toll on me." Mary, 21, MU felt similarly and stated how critical it was that she received help:
Mary: I wasn’t expecting it [to be hard] at all, like I’d want to kill this dude sometimes…that’s not a lie, especially if I didn’t take my medication. I had no idea about postpartum, I had no idea.
Interviewer: So you’re experiencing postpartum depression?
Mary: Yeah
Interviewer: And maybe postpartum anxiety?
Mary: Yes.
Interviewer: And then it was hard on your relationship?
Mary: It was…there were times when I wouldn’t take my medication because I was getting mad, like why do I need this to function, I shouldn’t have to take this. It took me a while to get that understanding of what was going on, I would get really manic without it, wouldn’t eat, wouldn’t sleep, so at first it was very difficult to deal with and I was feeling extremely alone with it. But now, it’s like a complete 180 you know. I make sure I take my medication every day.
In contrast to MUs, explicit statements regarding mental health were rare among MNUs and MNIUs. Georgia, 26, MNU, explained how she copes with her mental health:
I struggled with PTSD, anxiety, depression, and I've learned so much grounding techniques and self-soothing and honestly just living differently…you think you have all those problems, but a lot of it is drug or detox induced so after you’ve been through all that and you have some really good amount of time—like it’s been almost three years for me now, so it’s nice—a lot of those things don’t exist anymore...
Alma, age 28, MNIU, also stated, "I have plenty of purpose."
Recovery Plan: “Relapse Could Be Around the Corner, any Day”
Finally, mothers' treatment and perceptions of their recovery appeared to be a meaningful factor that varied across each category of mothers. Most mothers across categories indicated they were prescribed methadone as pharmacotherapy for their substance use recovery. Suboxone was more commonly prescribed to MNUs (N = 10) or MNIUs (N = 6) compared to MUs (N = 26). See Table 1.
Table 1.
Medication prescribed for OUD for each respective group
| Methadone | Suboxone/subutex | Vivitrol | No meds | |
|---|---|---|---|---|
| MNU | 70% | 30% | 0 | 0 |
| MNIU | 50% | 33.3% | 0 | 16.7% |
| MU | 69.2% | 11.5% | 3.9% | 15.4% |
Most mothers across categories described their baby as a motivator for working towards recovery. However, there were some notable differences in how mothers in each category articulated their steps to recovery and the role their baby played in recovering. MNUs made slight adjustments in their lives to stay present for their baby and prioritize recovery. For example, Kiana, age 34, MNUs, described, "I look back and I think of—all the time that I missed… Just little things that I missed. That I didn't get to see for the first time, so, I try not to miss anything now… I want to see everything." Georgia, age 26, MNU, also advised, "keep working on your recovery and love your baby and you know, if you're feeling bad reach out and just keep going, you know?".
In contrast, MNIUs and MUs described their approach to recovery as a change in their thought process. For example, Priscilla, age 26, MNIU, explained that having her baby decreased her urges to use substances. It was hard, you know but- especially after having him, it’s, it’s pushed me even further away from it… like when you were asking me about the urges…it's more of the urge not to ever want to touch it again." Aaliyah, 32, MU, talked about what she has learned after having her baby, stating that "I've learned that the mistakes I've made, they're not worth losing my family over. Like referring to my use… [I learned] not to take your baby for granted because it's a privilege to be a parent."
Discussion
Pregnant and postpartum mothers with OUD face a myriad of challenges psychologically, interpersonally, and across the service continuum, which could contribute to relapse (SAMHSA, 2018). Specific to the risk of relapse, the purpose of this study was to assess the qualitative differences in experience among mothers who had urges to use substances postpartum and mothers who did not have urges. Further, our objective was to offer clarity around risk and protective factors associated with opioid relapse so that clinical social workers and other behavioral health providers may be better prepared and risk assessments may be tailored to address specific areas of parent concern or strength. Seven themes for consideration regarding risk and protection are associated with perceptions of childhood bonding, mother-infant attachment, birth support, DCS involvement, breastfeeding, mental health, and mother’s recovery plan. These themes offer a road map for providers, such as clinical social workers, in direct practice and researchers developing new and enhanced means of relapse risk assessment tailored for pregnant and postpartum parents.
Adding to rigorous relapse-related risk assessment, our findings will offer clear areas for provider insight. However, our study is not without its limitations. Specifically, data collection methods were altered as a function of the COVID-19 pandemic. Thus, all interviews were switched into a remote Zoom format, which may have impacted participant responses, opportunities for follow-up interviews, and the ability to observe body language, which may have contributed to the results of the research. Nonetheless, we are confident that these findings and our recommendations will lead to enhanced clinical practice related to risk assessment.
Critically, enhanced clinical practitioners must convey the importance of postpartum bonding; attachment must be secured after birth (see Abdel-Latif et al., 2006; Ballard, 2002; McQueen et al., 2011; O’Connor et al., 2013; Welle-Strand et al., 2013). In our study, mothers in all three categories explained that they felt closer to their infant during moments of skin-to-skin contact, in which they felt they developed a stronger bond in this moment. This finding holds implications for increasing opportunities for skin-to-skin contact and babywearing in hospitals and clinics, especially after birth. Providing new parents—especially those with a history of substance use—with the opportunity to hold and carry their baby in close contact may potentially contribute to a closer attachment and decrease their risk of relapse. The process by which attachment occurs via the release of the hormone oxytocin is related to more affectionate behavior and responsiveness on the mothers' part (Scatliffe et al., 2019). While it is increasingly evident that bonding is a protective factor for parents with OUD and their babies, more evidence is needed to better understand the role of oxytocin in attachment between opioid-affected dyads.
Recommendations for both health and behavioral health care providers seeking to mitigate relapse are related to a concerted focus on helping to enhance and solidify attachment early during pregnancy and after birth via breastfeeding, perhaps through increased education and support for breastfeeding parents. Moreover, it is essential to address provider stigma associated with breastfeeding and educate providers that breastfeeding is safe and recommended when postpartum parents are receiving MOUD. Notably, the majority of women in the study, despite their urges or lack of urges to use substances, described that breastfeeding allowed them to feel closer to their infant. Thus, having a positive breastfeeding experience is important (particularly for parents with urges) in creating an attachment and potentially reducing their desire to use substances. Our findings related to breastfeeding highlight the need for SAMHSA's (2018) recommendation for providers to articulate the benefits of breastfeeding in delivery plans. Clinical social workers and other behavioral health providers can play an integral role in ensuring new parents have the tools to inform and prepare themselves for bonding.
Tools more specific to recovery planning are related to medication for parents with OUD. In our study, methadone and suboxone were the two most common medications mothers take during pregnancy or in the postpartum period for treatment with MOUD. In particular, suboxone was more commonly prescribed among mothers with no urges and no initial urges. This may hold implications for an increase in providers licensed to prescribe suboxone, which may potentially decrease the urges to use substances in parents who have a history of substance use and later prevent their likelihood of relapse. Notably, all of the mothers with no urges were participating in MOUD. Supporting other research in the area (see Ellis et al., 2019) on the importance of MOUD, our study calls for clinical social workers and other health/behavioral health providers to have education in MOUD services. MOUD services provide additional layers of support, and ultimately, are associated with a reduced risk for relapse.
Reduced risk for relapse occurs when new parents see recovery and the influence of a new baby as inextricably linked; there is an opportunity to achieve sobriety now that they have the responsibility of a child and a reason not to use. This link may be strengthened with support and encouragement from healthcare providers (Ellis et al., 2019). Stigma-free support from providers, friends, and family is an obvious and critical variable associated with more successful outcomes (i.e., those with no urges). Having support present during their birth experience held significance for mothers in all categories. Mothers with no urges explained that having a partner or family members present during the birth contributed to a better experience overall. Related, the difference in mothers with no urges and mothers with urges/no initial urges suggested that a lack of a positive maternal relationship in childhood was a stronger motivator than the role of a present positive relationship. MNUs may be more cognizant of the impact of maternal substance use on the mother-infant relationship and subsequently, be more motivated to disrupt a generational pattern of parenting. This differed for mothers with urges, some of which noted that having a support figure present would have created a better birth experience for them. There are implications for creating an atmosphere that provides personal support to postpartum parents, especially with urges to use substances, that will help support and facilitate bonding.
Areas for improvement and increased support are also evident within the child welfare system. Involvement with the child welfare system and the threat of losing custody may have profound implications for the emotional and mental health of the postpartum parent. Because DCS involvement plays a role in how often they see their baby, this factor may prevent postpartum parents from forming an attachment with their baby, and thus may potentially contribute to a higher risk of relapse. In our study, mothers with no urges did not have further DCS involvement after hospital discharge. Conversely, DCS involvement some time after giving birth was experienced by mothers who had no initial urges, and DCS involvement from birth was reported by only mothers with urges. Regardless of the DCS custody decision, the fear of child welfare involvement or the loss of custody "guides all aspects" of a new mothers' decision-making process (Proulx & Fantasia, 2021, p. 212). Though mothers with no urges were less likely to face removal, there was a clear link to additional stress and anxiety, at a time when stress and anxiety is already heightened. When aligned with other findings in the empirical knowledge base, implications related to child welfare practice are compounded. Ultimately, greater access to resources for parents afforded by DCS, particularly to support postpartum mental health, will allow more opportunities to solidify bonding. Clinical social workers are particularly well-positioned to assist with connecting service systems such that new parents are linked across systems of care mitigating potential for relapse (see West et al., 2022).
Clinical social workers may also play a fundamental and profound role in addressing co-occurring concerns of new parents such as anxiety and depression (Nwabuzor Ogbonnaya et al., 2019). Stressors associated with relapse also include generalized anxiety and depression. Our study highlights a critical need for emotional and mental health resources for postpartum parents with OUD. Experiencing symptoms related to anxiety and depression may contribute to their urges to use substances, making them likelier to relapse, especially when combined with the experience of having a new baby. There was an apparent link between co-occurring disorders and the experience of urges, and this may be exacerbated in the postpartum period. Furthermore, our findings point to the potential role of trauma-related symptomatology. For women in our study, early childhood experiences were linked to urges, as were links to DCS involvement. In sum, pre-existing mental health concerns and co-occurring disorders are notable complications for new parents. However, early assessment conducted by clinical social workers or other behavioral health practitioners may minimize the impact of mental health risks given targeted and person-centered approaches that start where the client is (Adynski et al., 2019; Vakharia & Little, 2017).
In addition to specific practice recommendations for clinical social workers and other behavioral health providers included herein, we stress that the most important clinical tools are those that are strengths-based and consider the individual person within their environment (e.g., Harden et al., 2021). Said another way, it will always be important to assume that every individual has within themselves strength and resiliency to do what is necessary for their health and the health of their child. Furthermore, each individual will draw on those strengths differently as a function of their environments and resources to which they have access. Lastly, drawing from social work roots in ecological systems theory (see Bronfenbrenner, 2005), we stress that prevention, intervention, and harm reduction efforts must necessarily occur within and across all systems. The focus for work is not only on the patient/client, it should be a multi-pronged approach involving policy (macro), agencies (meso), and the provider–client relationship (micro) (e.g., McCormick & Steiker, 2021).
Conclusion
Our study adds to the growing literature related to the risk assessment for relapse in postpartum parents. For new parents with OUD, the risk of relapse is greater the year after giving birth (as compared to the year before giving birth), due to factors such as sleep deprivation, caring for a high-needs infant, decreased coping mechanisms, postpartum depression and anxiety, lack of social support, and postpartum pain. Identifying risk and protective factors is the first step in offering needed support to pregnant and postpartum parents. While myriad assessments exist to examine and address risk factors, there is a lack of consensus about which factors are critical in addressing relapse and where we can bolster strengths. As clinical social workers and other health/behavioral health providers look to tried assessment methods such as those developed by ASAM (2020) and the WHO (2014), they can target assessment to include inquiry related to bonding and attachment, support during birth, child welfare involvement, breastfeeding, mental health concerns, and recovery planning. Ensuring that these factors are fully explored—in consideration of multiple systems—guarantees that new parents are afforded the necessary, equitable, and consistent support needed as they transition to parenthood and maintain sobriety.
Biographies
Lela Rankin
is a professor in the School of Social Work at Arizona State University. Dr. Rankin’s scholarship focuses on preventive interventions for children and families. She has established strong community partnerships that have led to the development, implementation, and management of several federally funded intervention studies.
Natasha Mendoza
is an associate professor in the School of Social Work at Arizona State University. Dr. Mendoza’s scholarship is focused on substance use, co-occurring disorders, and connecting service systems (i.e., treatment, crisis response, health care, criminal justice, and child welfare).
Lisa Grisham
is a Neonatal Nurse Practitioner at Banner University Medical Center Tucson. Lisa was one of the founders of the Family Centered Neonatal Abstinence Syndrome Care Program, which has become a Center of Excellence providing care for infants withdrawing from opiates they were exposed to in utero.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
When possible, we use “parent” to refer to the person giving birth to ensure the inclusivity of gender-expansive individuals. However, when the cited literature uses "mother" or "maternal," we remain consistent with the cited author. Participants in the current study all identified as "mothers," thus, results also refer to "mothers.".
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lela Rankin, Email: lrw@asu.edu.
Natasha S. Mendoza, Email: Tadoza@asu.edu
Lisa Grisham, Email: lgrisham@arizona.edu.
References
- Abdel-Latif ME, Pinner J, Clews S, Cooke F, Lui K, Oei J. Effects of breast milk on the severity and outcome of neonatal abstinence syndrome among infants of drug dependent mothers. Pediatrics. 2006 doi: 10.1542/peds.2005-1561. [DOI] [PubMed] [Google Scholar]
- Adynski H, Zimmer C, Thorp J, Jr, Santos HP., Jr Predictors of psychological distress in low-income mothers over the first postpartum year. Research in Nursing & Health. 2019;42(3):205–216. doi: 10.1002/nur.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agius A, Xuereb RB, Carrick-Sen D, Sultana R, Rankin J. The co-existence of depression, anxiety and post-traumatic stress symptoms in the perinatal period: A systematic review. Midwifery. 2016;36:70–79. doi: 10.1016/j.midw.2016.02.013. [DOI] [PubMed] [Google Scholar]
- American Society of Addiction Medicine [ASAM]. (2020). The ASAM National Practice Guideline For the Treatment of Opioid Use Disorder: 2020 Focused Upate. Rockville, MD. Retrieved November 5, 2021, from https://www.asam.org/quality-care/clinical-guidelines/national-practice-guideline
- Ballard JL. Treatment of neonatal abstinence syndrome with breast milk containing methadone. Journal of Perinatal and Neonatal Nursing. 2002;15(4):76–85. doi: 10.1097/00005237-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Benningfield MM, Dietrich MS, Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, O'Grady KE, Fischer G, Martin PR. Opioid dependence during pregnancy: Relationships of anxiety and depression symptoms to treatment outcomes. Addiction (abingdon, England) 2012;107(Suppl 1 (0 1)):74–82. doi: 10.1111/j.1360-0443.2012.04041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollampally, P. R. (2019). Violence and postpartum drug abstinence among substance-using mothers. Public Health Thesis 1817. (Open Access). Yale University. Retrieved from https://elischolar.library.yale.edu/ysphtdl/1817.
- Bronfenbrenner U. Ecological systems theory (1992) In: Bronfenbrenner U, editor. Making human beings human: Bioecological perspectives on human development. Sage Publications Ltd.; 2005. pp. 106–173. [Google Scholar]
- Chapman SLC, Wu L-T. Postpartum substance use and depressive symptoms: A review. Women & Health. 2013;53(5):479–503. doi: 10.1080/03630242.2013.804025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Obstetric Practice Committee opinion no. 711: Opioid use and opioid use disorder in pregnancy. Obstetrics and Gynecology. 2017;130(2):e81–e94. doi: 10.1097/AOG.0000000000002235. [DOI] [PubMed] [Google Scholar]
- Committee on Obstetric Practice American College of Obstetricians and Gynecologists, Committee Opinion No 742: Postpartum pain management. Obstetrics and Gynecology. 2018;132(1):e35–43. doi: 10.1097/AOG.0000000000002683. [DOI] [PubMed] [Google Scholar]
- Corr TE, Schaefer EW, Hollenbeak CS, Leslie DL. One-year postpartum mental health outcomes of mothers of infants with neonatal abstinence syndrome. Maternal and Child Health Journal. 2020;24(3):283–290. doi: 10.1007/s10995-019-02839-9. [DOI] [PubMed] [Google Scholar]
- Creswell JW, Miller DL. Determining validity in qualitative inquiry. Theory into Practice. 2000;39:124–130. doi: 10.1207/s15430421tip3903_2. [DOI] [Google Scholar]
- Davie-Gray A, Moor S, Spencer C, et al. Psychosocial characteristics and poly-drug use of pregnant women enrolled in methadone maintenance treatment. Neurotoxicology and Teratology. 2013;38:46–52. doi: 10.1016/j.ntt.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Ellis JD, Cairncross M, Struble CA, Carr MM, Ledgerwood DM, Lundahl LH. Correlates of treatment retention and opioid misuse among postpartum women in methadone treatment. Journal of Addiction Medicine. 2019;13(2):153–158. doi: 10.1097/ADM.0000000000000467. [DOI] [PubMed] [Google Scholar]
- Forray A, Merry B, Lin H, Ruger JP, Yonkers KA. Perinatal substance use: A prospective evaluation of abstinence and relapse. Drug and Alcohol Dependence. 2015;150:147–155. doi: 10.1016/j.drugalcdep.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs GR. Qualitative data analysis: Explorations with NVivo. Open University Press; 2002. [Google Scholar]
- Harden V, Romas I, Romines J, Lewis M. Interlocking theories and practice in treatment and recovery for women with opioid use disorders. Journal of Social Work Practice in the Addictions. 2021 doi: 10.1080/01634372.2021.1954398. [DOI] [Google Scholar]
- Hazelgrove K, Biaggi A, Waites F, Fuste M, Osborne S, Conroy S, Howard LM, Mehta MA, Miele M, Nikkheslat N, Seneviratne G, Zunszain PA, Pawlby S, Pariante CM, Dazzan P. Risk factors for postpartum relapse in women at risk of postpartum psychosis: The role of psychosocial stress and the biological stress system. Psychoneuroendocrinology. 2021;128:105218. doi: 10.1016/j.psyneuen.2021.105218. [DOI] [PubMed] [Google Scholar]
- Hirai AH, Ko JY, Owens PL, Stocks C, Patrick SW. Neonatal abstinence syndrome and maternal opioid-related diagnoses in the US, 2010–2017. JAMA. 2021;325(2):146–155. doi: 10.1001/jama.2020.24991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook A, Kaltenbach K. Co-occurring psychiatric symptoms in opioid-dependent women. Journal of Substance Abuse Treatment. 2012;32(1):19–25. [Google Scholar]
- Irvine MA, Kuo M, Buxton JA, Balshaw R, Otterstatter M, Macdougall L, Milloy M-J, Bharmal A, Henry B, Tyndall M, Coombs D, Gilbert M. Modeling the combined impact of interventions in averting deaths during a synthetic-opioid overdose epidemic. Addiction. 2019;114:1602–1613. doi: 10.1111/add.14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RA, Casey MA. Focus groups: A practical guide for applied research. Sage Publications; 2000. [Google Scholar]
- Krans EE, Tong ST, Terplan M. Postpartum care for women with substance use disorders. Opioid-use disorders in pregnancy. In: Wright TE, editor. Opioid-use disorders in pregnancy: Management guidelines for improving outcomes. Cambridge University Press; 2018. [Google Scholar]
- Lean RE, Pritchard VE, Woodward LJ. Child protection and out-of-home placement experiences of preschool children born to mothers enrolled in methadone maintenance treatment during pregnancy. Children and Youth Services Review. 2013;35(11):1878–1885. doi: 10.1016/j.childyouth.2013.09.003. [DOI] [Google Scholar]
- McCormick KA, Steiker LKH. A social work perspective on the opioid solution: A community-based expansion of the hub-and-spoke ecosystem. Journal of Social Work Practice in the Addictions. 2021;21(3):308–315. doi: 10.1080/1533256X.2021.1923894. [DOI] [Google Scholar]
- McQueen KA, Murphy-Oikonen J, Gerlach K, Montelpare W. The impact of infant feeding method on neonatal abstinence scores of methadone-exposed infants. Advances in Neonatal Care. 2011;11(4):282–290. doi: 10.1097/ANC.0b013e318225a30c. [DOI] [PubMed] [Google Scholar]
- National Clinician Consultation Center. (n.d.). Substance Use Management. Retrieved November 5, 2021, from https://nccc.ucsf.edu/clinician-consultation/substance-use-management/
- Nwabuzor Ogbonnaya I, Keeney AJ, Villodas MT. The role of co-occurring intimate partner violence, alcohol use, drug use, and depressive symptoms on disciplinary practices of mothers involved with child welfare. Child Abuse & Neglect. 2019;90:76–87. doi: 10.1016/j.chiabu.2019.02.002. [DOI] [PubMed] [Google Scholar]
- O’Connor AB, Collett A, Alto WA, O’Brien LM. Breastfeeding rates and the relationship between breastfeeding and neonatal abstinence syndrome in women maintained on buprenorphine during pregnancy. Journal of Midwifery and Women’s Health. 2013;58(4):383–388. doi: 10.1111/jmwh.12009. [DOI] [PubMed] [Google Scholar]
- Padgett DK. Qualitative methods in social work research. Sage Publications; 2008. [Google Scholar]
- Patterson DK, Pollock D, Carter CS, Chambers JE. Treating opioid use disorder in peripartum mothers: A look at the psychodynamics, neurobiology, and potential role of oxytocin. Psychodynamic Psychiatry. 2021;49(1):48–72. doi: 10.1521/pdps.2021.49.1.48. [DOI] [PubMed] [Google Scholar]
- Proulx D, Fantasia HC. The Lived experience of postpartum women attending outpatient substance treatment for opioid or heroin use. Journal of Midwifery & Women’s Health. 2021;66(2):211–217. doi: 10.1111/jmwh.13165. [DOI] [PubMed] [Google Scholar]
- Reddy UM, Davis JM, Ren Z, Greene MF. Opioid use in pregnancy, neonatal abstinence syndrome, and childhood outcomes: Executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, American Congress of Obstetricians and Gynecologists, American Academy of Pediatrics, Society for Maternal-Fetal Medicine, Centers for Disease Control and Prevention, and the March of Dimes Foundation. Obstetrics and Gynecology. 2017;130(1):10–28. doi: 10.1097/AOG.0000000000002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk AH, Simonsen SE, Roberts L, Taylor-Swanson L, Lemoine JB, Smid M. Maternity care for pregnant women with opioid use disorder: A review. Journal of Midwifery & Women's Health. 2019;64(5):532–544. doi: 10.1111/jmwh.13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2018). Clinical guidance for treating pregnant and parenting women with opioid use disorder and their infants. HHS Publication No. (SMA) 18-5054. Substance Abuse and Mental Health Services Administration.
- Substance Abuse and Mental Health Services Administration. (2020). Substance use disorder treatment for people with co-occurring disorders. Treatment improvement protocol (TIP) Series, No. 42. SAMHSA Publication No. PEP20-02-01-004. Substance Abuse and Mental Health Services Administration.
- Scatliffe N, Casavant S, Vittner D, Cong X. Oxytocin and early parent-infant interactions: A systematic review. International Journal of Nursing Sciences. 2019;6(4):445–453. doi: 10.1016/j.ijnss.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff DM, Nielsen T, Terplan M, Hood M, Bernson D, Diop H, Bharel M, Wilens TE, LaRochelle M, Walley AY, Land T. Fatal and nonfatal overdose among pregnant and postpartum women in Massachusetts. Obstetrics and Gynecology. 2018;132(2):466. doi: 10.1097/AOG.0000000000002734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderquist J, Wijma B, Thorbert G, Wijma K. Risk factors in pregnancy for post-traumatic stress and depression after childbirth. BJOG: An International Journal of Obstetrics & Gynecology. 2009;116:672–680. doi: 10.1111/j.1471-0528.2008.02083.x. [DOI] [PubMed] [Google Scholar]
- US Preventive Services Task Force Screening for unhealthy drug use: US preventive services task force recommendation statement. JAMA. 2020;323(22):2301–2309. doi: 10.1001/jama.2020.8020. [DOI] [PubMed] [Google Scholar]
- Vakharia SP, Little J. Starting where the client is: Harm reduction guidelines for clinical social work practice. Clinical Social Work Journal. 2017;45(1):65–76. doi: 10.1007/s10615-016-0584-3. [DOI] [Google Scholar]
- Wachman EM, Schiff DM, Silverstein M. Neonatal abstinence syndrome: Advances in diagnosis and treatment. JAMA. 2018;319(13):1362–1374. doi: 10.1001/jama.2018.2640. [DOI] [PubMed] [Google Scholar]
- Welle-Strand GK, Skurtveit S, Jansson LM, Bakstad B, Bjarkø L, Ravndal E. Breastfeeding reduces the need for withdrawal treatment in opioid exposed infants. Acta Paediatrica. 2013;102(11):1060–1066. doi: 10.1111/apa.12378. [DOI] [PubMed] [Google Scholar]
- West A, Schultz D, Schacht RL, Barnet B, DiClemente C, Leonardi LaCasse M. Evaluation of interprofessional training to strengthen communication and coordination among providers working with expectant mothers and infants affected by substance use. Children and Youth Services Review. 2022;132:106331. doi: 10.1016/j.childyouth.2021.106331. [DOI] [Google Scholar]
- World Health Organization [WHO]. (2014). Guidelines for the Identfication and Management of Substance Use and Substance Use Disorders in Pregnancy. World Health Organization. Retreived November 5, 2021, from https://www.who.int/publications/i/item/9789241548731 [PubMed]
- Young JL, Martin PR. Treatment of opioid dependence in the setting of pregnancy. Psychiatric Clinics of North America. 2012;35(2):441–460. doi: 10.1016/j.psc.2012.03.008. [DOI] [PubMed] [Google Scholar]