Abstract
Solid-state fermentation of eucalypt wood with several fungal strains was investigated as a possible biological pretreatment for decreasing the content of compounds responsible for pitch deposition during Cl2-free manufacture of paper pulp. First, different pitch deposits were characterized by gas chromatography (GC) and GC-mass spectrometry (MS). The chemical species identified arose from lipophilic wood extractives that survived the pulping and bleaching processes. Second, a detailed GC-MS analysis of the lipophilic fraction after fungal treatment of wood was carried out, and different degradation patterns were observed. The results showed that some basidiomycetes that decreased the lipophilic fraction also released significant amounts of polar extractives, which were identified by thermochemolysis as originating from lignin depolymerization. Therefore, the abilities of fungi to control pitch should be evaluated after analysis of compounds involved in deposit formation and not simply by estimating the decrease in the total extractive content. In this way, Phlebia radiata, Funalia trogii, Bjerkandera adusta, and Poria subvermispora strains were identified as the most promising organisms for pitch biocontrol, since they degraded 75 to 100% of both free and esterified sterols, as well as other lipophilic components of the eucalypt wood extractives. Ophiostoma piliferum, a fungus used commercially for pitch control, hydrolyzed the sterol esters and triglycerides, but it did not appear to be suitable for eucalypt wood treatment because it increased the content of free sitosterol, a major compound in pitch deposits.
Biotechnology has been introduced into pulp and paper manufacturing, the first nonfood industrial use of plant biomass (7). Xylanase-aided elementary chlorine-free (ECF) bleaching of paper pulp is the best example of the applications developed in recent years (22). However, other aspects of paper pulp manufacturing also offer promising avenues for using microorganisms and enzymes; one of these is biological control of the so-called pitch deposits. Accumulation of wood extractives in pulp and paper mills (pitch deposits) results in low-quality pulp and blockages that cause shutdowns of operations and important economic losses (15). The increasing need for recirculating water in pulp mills and the need to reduce effluents in order to meet environmental protection requirements are leading to increases in the concentrations of pitch compounds in the production process. This situation and the use of totally chlorine-free (TCF) and ECF bleaching processes based on the replacement of Cl2 by milder chemical oxidants or enzymes result in greater pitch deposition problems in mills. Traditionally, pitch deposits during pulping processes have been reduced by debarking and seasoning logs and wood chips and by adding pitch control agents (1, 5). However, often the results are far from satisfactory. Alternatively, biological control of pitch deposits by treatment of pulp with enzymes (9–11) and treatment of wood with different microorganisms (2, 6, 8, 12), have been suggested in recent years. These biotechnological approaches have involved mainly treatment of pine wood with Ophiostoma piliferum and related species, as well as some basidiomycetes. The studies of pitch removal performed with basidiomycetes, such as Phanerochaete chrysosporium, Poria subvermispora (synonym, Ceriporiopsis subvermispora), and Phlebiopsis gigantea, are in the preliminary stages and are often associated with studies of the use of these fungi for so-called wood biopulping (i.e., biological removal of lignin for paper pulp manufacturing). A white strain of O. piliferum (Cartapip from Clariant) has been used commercially to depitch some types of wood prior to pulping. However, successful use of Cartapip to control pitch deposition in Kraft pulp obtained from eucalypt wood (which is extensively used as a raw material for paper pulp manufacturing in Spain, Portugal, Brazil, and other countries) has not been reported. Moreover, no information about biological depitching of this type of wood with other fungal species is available.
Designing effective biotechnological solutions for wood extractive removal requires thorough characterization of the compounds responsible for pitch deposition. In this context, the first aim of this work was to identify the specific constituents of pitch deposits during Kraft pulping of Eucalyptus globulus wood compared with wood extractives obtained from this eucalypt species. Then, the main aim was to evaluate the viability of biotechnological solutions for eliminating these problematic pitch compounds by analyzing in detail the patterns of removal of the main lipophilic compounds present in eucalypt wood by a selection of fungal strains.
MATERIALS AND METHODS
Samples.
Pitch deposits after TCF and ECF bleaching were obtained from eucalypt Kraft pulp mills in Huelva and Pontevedra, Spain. The TCF sequence used included two oxygen delignification stages, a chelation stage, an H2O2 stage under pressure (with oxygen), and a final H2O2 stage. The ECF sequence used included an oxygen delignification stage, followed by two ClO2 stages with an intermediate alkaline extraction stage. E. globulus wood chips were ground to sawdust. The different pitch deposits and sawdust were Soxhlet extracted with acetone for 6 h (21). The acetone extracts were evaporated to dryness and redissolved in chloroform before they were analyzed by gas chromatography (GC) and GC-mass spectrometry (MS).
Fungal strains and wood treatment conditions.
The first screening of fungi for removal of eucalypt extractives under solid-state fermentation conditions was carried out with 73 strains (18). Many of these strains were isolated from fruiting bodies growing on eucalypt wood in forests or in log piles and identified. Additional strains were obtained from the Centraalbureau voor Schimmelcultures (Baarn, The Netherlands), Wageningen Agricultural University (Wageningen, The Netherlands), and the fungal culture collection of the Centro de Investigaciones Biológicas (Madrid, Spain).
Treatment of wood with all of the fungi (in duplicate) was carried out in flasks containing 2 g (dry weight) of small wood chips (1 to 2 by 10 to 20 mm) and 5 ml of water (sterilized at 120°C) that were inoculated with two portions of mycelium from cultures on 2% malt extract agar. After 40 days of incubation at 28°C and a constant humidity, the wood was dried with air at 60°C, milled, and extracted with acetone as described above. Then, the Klason lignin content was estimated after hot-water extraction (21). Wood treatment with the 14 most promising strains was repeated in quadruplicate to confirm the results obtained in the first screening.
GC and GC-MS.
The GC analysis were performed with a Hewlett-Packard model HP 5890 GC equipped with a flame ionization detector by using a high- temperature polyimide-coated fused-silica capillary column (5 m by 0.25 mm; type DB5-HT; film thickness, 0.1 μm; J & W Scientific). The injector and detector temperatures were 300 and 350°C, respectively. The oven temperature was programmed to increase from 100°C (1 min) to 350°C (3 min) at a rate of 15°C/min. Samples were injected in the splitless mode. Helium was used as the carrier gas. A mixture of standard compounds (palmitic acid, sitosterol, cholesteryl oleate, and triheptadecanoin) was used to construct a calibration curve for quantitation of wood extractives at concentrations ranging from 0.1 to 1 mg/ml. The correlation coefficient was greater than 0.99 in all cases. Peaks were quantified by determining areas.
GC-MS analyses were performed with a Varian model Star 3400 GC equipped with an ion trap detector (Varian model Saturn 2000) by using a type DB-5HT capillary column (15 m by 0.25 mm; film thickness, 0.1 μm; J & W Scientific). Helium was used as the carrier gas. Samples were injected directly into the column with an autoinjector (Varian model 8200) by using a septum-equipped programmable injector system. The temperature of the injector during injection was 120°C, and 0.1 min after injection the temperature was programmed to increase to 380°C (10 min) at a rate of 200°C/min. The oven temperature was programmed to increase from 120°C (1 min) to 380°C (5 min) at a rate of 10°C/min. The temperatures of the ion trap detector and the transfer line were set at 200 and 300°C, respectively. Compounds were identified by comparing their mass spectra with mass spectra in the Wiley and Nist libraries, by performing mass fragmentography, and, when possible, by using standards.
Thermochemolysis.
A thermochemolysis analysis was performed with a Varian model Saturn 2000 GC-MS coupled to a Curie point pyrolyser (Horizon Instruments Ltd.) by using a type DB-5 column (30 m by 0.25 mm; film thickness, 0.25 μm). A finely divided sample was deposited onto ferromagnetic wire, mixed with approximately 0.5 μl of tetramethylammonium hydroxide (25% [wt/wt] aqueous solution) (14), inserted into the glass liner, and then immediately located in the pyrolyser, and pyrolysis was carried out at 610°C. The temperature of the chromatograph was programmed to increase from 40°C (1 min) to 300°C (20 min) at a rate of 6°C/min. The temperature of the injector, which was equipped with a liquid carbon dioxide cryogenic unit, was programmed to increase from 30°C (1 min) to 300°C at a rate of 200°C/min, while the GC-MS interface was kept at 300°C.
RESULTS AND DISCUSSION
Lipophilic compounds in pitch deposits and E. globulus wood.
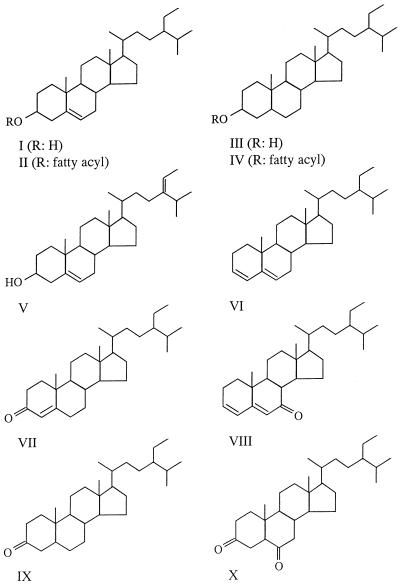
Pitch deposits were obtained after TCF and ECF bleaching of E. globulus wood. These industrial processes include the use of H2O2 and ClO2, respectively, as bleaching agents (20). Two types of organic fractions were distinguished on the basis of acetone solubility. The acetone-insoluble fractions contained mainly salts of fatty acids and minor amounts of ellagic acid salts (4). The compositions of the acetone-soluble fractions of the different pitch deposits analyzed by GC and GC-MS are summarized in Table 1, which also shows the results for extractives obtained from E. globulus wood. This analysis was possible because a method for analysis of this type of compounds was optimized previously (13). The results obtained showed that many of the chemical species found in eucalypt wood extracts survive the pulping and bleaching processes, since they were identified in pitch deposits. In all of the samples (wood and pitch deposits), the different steroid compounds identified (Fig. 1) accounted for more than 70% of the total lipophilic compounds in the acetone extracts. Several steroid ketones (compounds VII to X), which have been reported to be sterol oxidation products previously (16), were present at relatively high levels in pitch deposits, but they were also detected in wood. Triglycerides were absent from both types of deposits since they were hydrolyzed during Kraft cooking. The composition of the pitch deposits produced after TCF bleaching was very similar to the composition of E. globulus wood extractives. The deposits produced after ECF bleaching had a very different composition. No sitosterol, the main sterol present in E. globulus wood, was found in either the free form or the esterified form, whereas only a saturated sterol (stigmastanol) remained in the deposits because of its higher resistance to oxidation. The same thing happened with unsaturated fatty acids, which were absent after ClO2 treatment. Based on the results described above, during the screening of fungi to determine whether they remove extractives from E. globulus wood prior to Kraft cooking special emphasis was placed on the biological removal of free and esterified sterols.
TABLE 1.
Hydrocarbons, fatty acids, waxes, sterols, ketones, sterol esters, and triglycerides in extracts from E. globulus wood and pitch deposits after ECF and TCF bleachinga
| Compound(s)a | % in:
|
||
|---|---|---|---|
| Wood | ECF | TCF | |
| Alkanes | 3.0 | <0.1 | 0.5 |
| Steroid hydrocarbons (compound VI)b | 5.3 | 20.2 | 9.6 |
| Saturated fatty acids | 7.6 | 13.3 | 19.7 |
| Unsaturated fatty acids | 5.8 | NDc | 6.3 |
| Waxes (C20–C40) | 3.0 | <0.1 | 1.6 |
| Sitosterol (compound I) | 24.6 | <0.1 | 21.0 |
| Stigmastanol (compound III) | 3.0 | 20.8 | 5.4 |
| Fucosterol (compound V) | 1.2 | ND | 1.2 |
| Other sterols | 3.2 | <0.1 | 1.4 |
| Aliphatic ketones | ND | 1.1 | 0.1 |
| Stigmastan-3-one (compound IX) | 0.6 | 10.7 | 1.0 |
| Stigmast-4-en-3-one (compound VII) | 4.8 | 7.9 | 1.2 |
| Stigmasta-3,5-dien-7-one (compound VIII) | 4.4 | 12.6 | 3.7 |
| Stigmastane-3,6-dione (compound X) | 1.0 | 7.9 | 0.5 |
| Sitosterol esters (compound II)d | 17.3 | <0.1 | 11.0 |
| Stigmastanol esters (compound IV)d | 3.5 | 4.5 | 5.0 |
| Other sterol estersd | 5.2 | 1.0 | 11.0 |
| Triglycerides | 6.5 | ND | ND |
See Fig. 1 for the chemical structures of steroid compounds.
Stigmasta-3,5-diene (compound VI) is the main compound.
ND, not detected.
Esters of sterols and several unidentified fatty acids (including linoleic acid).
FIG. 1.
Main free sterols (sitosterol [compound I], stigmastanol [compound III], and fucosterol [compound V]), esterified sterols (sitosterol esters [compound II], and stigmastanol esters [compound IV]), steroid hydrocarbon (stigmasta-3,5-diene [compound VI]), and ketones (stigmast-4-en-3-one [compound VII], stigmasta-3,5-dien-7-one [compound VIII], stigmastan-3-one [compound IX], and stigmastane-3,6-dione [compound X]) in acetone extracts from E. globulus wood and pitch deposits during manufacture of Cl2-free eucalypt Kraft pulp. See Table 1 for compound abundance values.
Fungal treatment of E. globulus wood.
The first screening for extractive removal by treatment of E. globulus wood with fungi under sterile solid-state fermentation conditions was performed by examining different species of basidiomycetes, ascomycetes, and conidial fungi (18). The ascomycetes included strains belonging to eight Ophiostoma species, five Ceratocystis species, and two Mollisia species (the latter were isolated from eucalypt wood). The basidiomycetes included members of three Pleurotus species and two Phlebia species, as well as strains of Funalia trogii, Bjerkandera adusta, P. chrysosporium, Crepidotus variabilis, and Melanotus hepatochrous (the two latter organisms were isolated from eucalypt wood). Finally, strains of Paecilomyces sp., Penicillium megasporum (isolated from resin of Eucalyptus tereticornis), and several lipase-producing Fusarium species were among the conidial fungi investigated.
Wide differences were observed in the extent of wood extractive removal by the different fungi. While some of the fungi, such as Pleurotus eryngii, Paecilomyces sp., F. trogii, Ophiostoma valdivianum, and Mollisia melaleuca, reduced the total extractive content by 50 to 70%, a significant increase in the extractive content was observed in wood treated with Coniophora puteana, C. variabilis, P. subvermispora, and other organisms. However, close examination of the acetone extracts obtained from the biotreated woods revealed that some of the fungi that decreased the total extractive content reduced only the polar fraction, while the lipophilic fraction (the main fraction responsible for pitch deposition, as shown above) remained unchanged. The opposite occurred with other fungi which increased the total acetone extract content. The results obtained with two representative fungi are shown in Table 2. In this experiment the acetone extracts obtained from the biotreated woods were fractionated into lipophilic (chloroform-soluble) and polar (chloroform-insoluble) fractions. O. valdivianum reduced the total acetone extract content due to a drastic decrease in the polar fraction content; however, the lipophilic fraction content was barely modified. In contrast, C. variabilis reduced the lipophilic fraction content, although it increased the total acetone extract content due to an increase in the polar compound content (Table 2). The origins of the polar compounds were determined by thermochemolysis. We found that the acid/aldehyde ratio of vanillyl compounds, an indicator of lignin oxidative degradation (14), was higher in the polar fraction from wood treated with C. variabilis. This, together with the decrease in lignin content estimated by the Klason method, suggested that the polar compounds described above probably arose from fungal alteration of lignin. We deduced from this study that the abilities of some fungi to control pitch should be evaluated by specifically analyzing compounds involved in deposit generation and not simply estimating the decrease in the total amount of extractives.
TABLE 2.
Effects of two fungi on total extractive, lipophilic and polar fraction, and lignin contents
| Prepn | Amt (g/100 g) of:
|
Vanillyl acid/alde-hyde ratioa | |||
|---|---|---|---|---|---|
| Wood extractives | Lipophilic compounds | Polar compounds | Lignin | ||
| Control | 0.75 | 0.31 | 0.44 | 15.8 | 2.5 |
| C. variabilis | 1.04 (−26)b | 0.14 (55) | 0.90 (−104) | 10.8 (38) | 9.5 |
| O. valdivianum | 0.33 (56) | 0.27 (13) | 0.06 (86) | 16 (0) | 3.5 |
Vanillyl acid/aldehyde ratio after thermochemolysis of polar compounds.
The values in parentheses are relative degradation values.
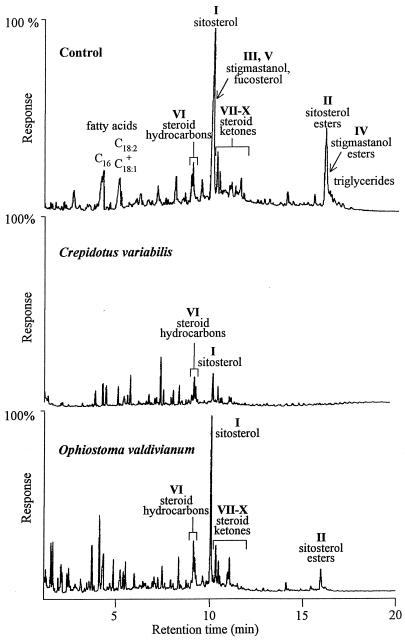
Next, the compositions of lipophilic extractives from E. globulus wood treated with the different fungal species were analyzed by GC and GC-MS. Two representative chromatograms are shown in Fig. 2. A total of 73 species were investigated initially, and the species that significantly degraded total extractives and/or significantly decreased the content of problematic lipophilic compounds, as well as caused a limited loss of wood weight (18), were selected for more detailed quantitative study (Table 3). Different patterns of extractive degradation were observed with the fungal strains used. Some of the fungi, including O. piliferum and O. valdivianum, reduced the sterol ester content but simultaneously increased the content of free sterols, mainly sitosterol, which was probably related to the fatty acyl-sterol esterase activity detected (unpublished results). A similar pattern has been found previously during pine wood treatment with Ophiostoma ainoae (19). O. piliferum (Cartapip strain from Clariant) has been reported to be useful for reducing pitch problems in mechanical pulping of pine wood (6), as well as in spruce sulfite pulping (8). However, this strain proved to have limited utility in the case of eucalypt wood because it was not able to degrade the free sterols released, which, as shown above, are among the problematic compounds in this type of wood. This fungus and other ascomycetous fungi have also been reported to remove 60 to 70% of resin acids from pine wood (12, 23), whereas some basidiomycetes can completely degrade these compounds (19). Some of the basidiomycetes assayed, such as P. chrysosporium, degraded both sterols and sterol esters but significantly increased the content of triglycerides, which were probably derived from fungal metabolism (24). The ability of liquid cultures of P. chrysosporium to degrade sterol esters from aspen (Populus tremuloides), which also cause pitch problems during pulping of this type of wood (3), has been described by Leone and Breuil (17). During the present study we found for the first time that a number of fungi, including Phlebia radiata, F. trogii, B. adusta, P. subvermispora, and C. variabilis, efficiently degrade the lipophilic compounds that have been identified as the compounds responsible for pitch deposition during manufacture of Cl2-free pulp from eucalypt wood. One of these fungi, P. subvermispora, has been reported to be an efficient degrader of resin acids in pine wood (8). Further experiments are being carried out to establish the time course of extractive removal and to scale up wood treatment with the most promising strains that remove problematic lipophilic compounds.
FIG. 2.
Gas chromatograms for lipophilic extracts from E. globulus wood after treatment with two fungi and the corresponding control. The same sample volume for the lipophilic fraction obtained from the same amount of wood was injected in each case, and the relative chromatographic responses are shown. Figure 1 and Table 3 show the chemical structures and abundance values for the different steroids (compounds I to X).
TABLE 3.
Fungal degradation of E. globulus wood extractives: percentage of total extract and main lipophilic fraction contents in wood treated with different fungi and a control, as determined by GCa
| Prepn | % of total extract | Extractive fraction contents (mg/100 g of wood)
|
|||
|---|---|---|---|---|---|
| Fatty acids | Sterols | Sterol esters | Triglyc-erides | ||
| Control | 0.75 | 17.2 | 41.8 | 33.6 | 8.6 |
| Bjerkandera adusta | 0.48 | 2.1 (88)b | 9.6 (77) | 8.1 (76) | 1.9 (78) |
| Crepidotus variabilis | 0.76 | 2.4 (86) | 7.5 (82) | 3.7 (89) | 4.9 (43) |
| Funalia trogii | 0.62 | 4.3 (75) | 4.6 (89) | 3.7 (89) | 1.4 (84) |
| Melanotus hepato-chrous | 0.89 | 13.6 (21) | 49.3 (−18) | 34.0 (0) | 3.2 (63) |
| Mollisia sp. | 0.46 | 10.3 (40) | 47.2 (−13) | 25.2 (25) | 5.2 (39) |
| Ophiostoma piliferum | 0.74 | 9.1 (47) | 76.1 (−82) | 7.4 (78) | 0 (100) |
| Ophiostoma piliferum Cartapip | 0.43 | 10.0 (42) | 51.4 (−23) | 10.8 (68) | 3.0 (65) |
| Ophiostoma valdi-vianum | 0.39 | 15.7 (8) | 64.8 (−55) | 18.5 (45) | 6.5 (25) |
| Paecilomyces sp. | 0.63 | 62.4 (−263) | 32.2 (23) | 20.8 (38) | 14.8 (−72) |
| Phanerochaete chryso-sporium | 0.80 | 3.2 (81) | 11.3 (73) | 7.1 (79) | 15.8 (−84) |
| Phlebia radiata | 1.01 | 1.7 (90) | 1.7 (96) | 0 (100) | 0 (100) |
| Pleurotus eryngii | 0.42 | 5.1 (70) | 35.9 (14) | 21.2 (37) | 2.7 (69) |
| Pleurotus pulmonarius | 0.43 | 4.6 (73) | 25.5 (39) | 8.1 (76) | 1.7 (80) |
| Poria subvermisporac | 1.34 | 0.6 (97) | 0.8 (98) | 0 (100) | 0 (100) |
In all cases the standard deviation for replicates (including variability from chromatographic analysis, acetone extraction, fungal growth, and extractive degradation) was less than 15% of the mean. Figure 2 shows representative gas chromatograms for lipophilic extracts.
The values in parentheses are percentages of degradation.
Synonym, Ceriporiopsis subvermispora.
ACKNOWLEDGMENTS
We thank J. M. Barrasa (University of Alcalá, Madrid, Spain) for fungal strains, Javier Romero (Centro de Investigación, ENCE, Pontevedra, Spain) for samples of eucalypt wood and pitch deposits, and Clariant (Barcelona, Spain) for a sample of Cartapip (O. piliferum white strain).
This research was carried out with the financial support of the European project “Wood Extractives in Pulp and Paper Manufacture: Technical and Environmental Implications and Biological Removal” (FAIR contract CT95-560) and the Spanish Biotechnology Programme.
REFERENCES
- 1.Allen J, Sitholé B B, MacLeod J M, Lapointe C, McPhee F J. The importance of seasoning and debarking in the Kraft pulping of aspen. J Pulp Paper Sci. 1991;17:J85–J91. [Google Scholar]
- 2.Behrendt C J, Blanchette R A. Biological processing of pine logs for pulp and paper production with Phlebiopsis gigantea. Appl Environ Microbiol. 1997;63:1995–2000. doi: 10.1128/aem.63.5.1995-2000.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen T, Wang Z, Zhou Y, Breuil C, Aschim O K, Yee E, Nadeau L. Using solid-phase extraction to assess why aspen causes more pitch problems than softwoods in Kraft pulping. TAPPI (Tech Assoc Pulp Pap Ind) J. 1995;78:143–149. [Google Scholar]
- 4.del Río J C, Gutiérrez A, González-Vila F J, Martín F, Romero J. Characterization of organic deposits produced in the kraft pulping of Eucalyptus globulus wood. J Chromatogr. 1998;823:457–465. [Google Scholar]
- 5.Dreisbach D D, Michalopoulos D L. Understanding the behavior of pitch in pulp and paper mills. TAPPI (Tech Assoc Pulp Pap Ind) J. 1989;72:129–134. [Google Scholar]
- 6.Farrell R L, Blanchette R A, Brush T S, Hadar Y, Iverson S, Krisa K, Wendler P A, Zimmerman W. CartapipTm: a biopulping product for control of pitch and resin acid problems in pulp mills. J Biotechnol. 1993;30:115–122. [Google Scholar]
- 7.Fengel D, Wegener G. Wood: chemistry, ultrastructure, reactions. Berlin, Germany: De Gruyter; 1984. [Google Scholar]
- 8.Fischer K, Akhtar M, Blanchette R A, Burnes T A, Messner K, Kirk T K. Reduction of resin content in wood chips during experimental biological pulping processes. Holzforschung. 1994;48:285–290. [Google Scholar]
- 9.Fischer K, Messner K. Reducing troublesome pitch in pulp mills by lipolytic enzymes. TAPPI (Tech Assoc Pulp Pap Ind) J. 1992;75:130–135. [Google Scholar]
- 10.Fischer K, Puchinger L, Schloffer K. Enzymatic pitch control of sulfite pulp on pilot scale. J Biotechnol. 1993;27:341. [Google Scholar]
- 11.Fujita Y, Awaji H, Taneda H, Matsukura M, Hata K, Shimoto H, Sharyo M, Sakaguchi H, Gibson K. Recent advances in enzymic pitch control. TAPPI (Tech Assoc Pulp Pap Ind) J. 1992;75:117–122. [Google Scholar]
- 12.Gao Y, Breuil C, Chen T. Utilization of triglycerides, fatty acids and resin acids in lodgepole pine wood by a sapstaining fungus Ophiostoma piceae. Mater Org (Berlin) 1994;28:105–118. [Google Scholar]
- 13.Gutiérrez A, del Río J C, González-Vila F J, Martín F. Analysis of lipophilic extractives from wood and pitch deposits by solid-phase extraction and gas chromatography. J Chromatogr. 1998;823:449–455. [Google Scholar]
- 14.Hatcher P G, Nanny M A, Minard R D, Dible S D, Carson D M. Comparison of two thermochemolytic methods for the analysis of lignin in decomposing gymnosperm wood: the CuO oxidation method and the method of thermochemolysis with tetramethylammonium hydroxyde (TMAH) Org Geochem. 1995;23:881–888. [Google Scholar]
- 15.Hillis W E, Sumimoto M. Effect of extractives on pulping. In: Rowe J W, editor. Natural products of woody plants II. Berlin, Germany: Springer-Verlag; 1989. pp. 880–920. [Google Scholar]
- 16.Jansson M B, Wormald P, Dahlman O. Reactions of wood extractives during ECF and TCF bleaching of Kraft pulp. Pulp Pap Can. 1995;96:42–45. [Google Scholar]
- 17.Leone R, Breuil C. Filamentous fungi can degrade aspen steryl esters and waxes. Int Biodeterior Biodegrad. 1998;41:133–137. [Google Scholar]
- 18.Martínez M J, Barrasa J M, Gutiérrez A, del Río J C, Martínez A T. Proceedings of the 7th International Conference on Biotechnology in the Pulp and Paper Industry. Quebec, Canada: Canadian Pulp and Paper Associations; 1998. Biological depitching of eucalypt wood with ascomycetous and basidiomycetous fungi; pp. B37–B44. [Google Scholar]
- 19.Martínez-Iñigo, M. J., P. Immerzeel, A. Gutiérrez, J. C. del Río, and R. Sierra Alvarez. Biodegradability of extractives in sapwood and heartwood from Scots pine by sapstain and white-rot fungi. Holzforschung, in press.
- 20.Sjöström E. Wood chemistry. Fundamentals and applications. San Diego, Calif: Academic Press; 1993. [Google Scholar]
- 21.Technical Association of the Pulp and Paper Industry. Test methods, 1992–1993. Atlanta, Ga: TAPPI; 1993. [Google Scholar]
- 22.Viikari L, Kantelinen A, Sundquist J, Linko M. Xylanases in bleaching—from an idea to the industry. FEMS Microbiol Rev. 1994;13:335–350. [Google Scholar]
- 23.Wang Z, Chen T, Gao Y, Breuil C, Hiratsuka Y. Biological degradation of resin acids in wood chips by wood-inhabiting fungi. Appl Environ Microbiol. 1995;61:222–225. doi: 10.1128/aem.61.1.222-225.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wassef M K. Fungal lipids. Adv Lipid Res. 1977;15:159–232. [Google Scholar]