Abstract
Copper (Cu) metal and alloys are used in cookware and other food contact surfaces due to their desirable properties for various applications. However, Cu metal can ionise and subsequently transfer to food and beverages under certain conditions. Here, we tested how pH and temperature affected Cu release kinetics using model systems utilising Cu metal foil and commercially available copperware. Cu foil and copperware were exposed to food simulants composed of 3% (w:w) aqueous solutions of citric acid, malic acid, acetic acid, or deionised water (DI) water at temperatures ranging from 4–60 °C. An additional pilot experiment tested how simulated long-term cleaning affected subsequent Cu release from lined and unlined copperware to 3% citric acid. Food simulants were then analysed by ICP-MS for total Cu. After 180 min, incubation of Cu metal foil with acid-containing food simulants at 4 °C resulted in Cu release ranging from 8.7–14.0 μg·cm−2, while 21.5–38.1 μg·cm−2 was released at 60 °C. In contrast, Cu transfer from metal foil to DI water was relatively low, with <0.6 μg·cm−2 released after 180 min at 60 °C. With citric acid food simulant, lined copperware released between 0.6–3.0 μg Cu·cm−2 over 180 min at the set temperatures, while unlined copperware released approximately 25–45 fold higher amounts of Cu (26.9–74.6 μg·cm−2) over this same time period. In contrast, use of DI water food simulant resulted in Cu release of <0.1 μg·cm−2 for the lined copperware and <2 μg·cm−2 for the unlined type. No significant effect of simulated long-term cleaning on Cu release from copperware was observed. These data indicate that Cu release is affected by temperature and pH, and that specific steps can be taken to limit Cu metal release from food contact surfaces to foods and beverages.
Keywords: Copper, food simulants, organic acids, ICP-MS, copperware, acrylic coatings
INTRODUCTION
Copper (Cu) metal is often a component of food contact materials, including plumbing, cookware, and drinking vessels. Use of copperware has gained in popularity due, in part, to the desirable thermal conductivity, aesthetic appearance, and antimicrobial properties of Cu (Simonson; Sovich; Vincent et al. 2016). When used as a food contact surface, metallic Cu is capable of ionizing under certain conditions and can subsequently transfer to food or beverages. Although Cu has been well defined as an essential micronutrient, an upper intake limit for this metal has been established, since chronic elevated ingestion of this element can result in liver and kidney damage (NRC 2000). In addition, there is ongoing research that is characterizing whether elevated dietary Cu exposure may be related to increased risk for the development of neurological diseases (Chen et al. 2019). Besides these more critical outcomes, acute Cu toxicity can induce symptoms of gastrointestinal (GI) distress that mimic foodborne illness (Barceloux and Barceloux 1999; Gaetke and Chow 2003). In fact, there have been several case reports of GI distress occurring after consuming acidic beverages left in contact with copperware (Wyllie 1957; Gill and Bhagat 1999). As such, it is important to understand the factors that drive transfer of Cu from copperware food contact surfaces to food and beverages in order to help prevent overexposure to this element.
There are several governmental organizations that have established guidelines and regulations that limit the major sources of dietary Cu. For drinking water, the U.S. Environmental Protection Agency (EPA) has in place an action level of 1.3 mg·L−1 Cu (2019), while Health Canada’s maximum acceptable concentration (MAC) aligns with the World Health Organization’s (WHO) recommended limit of 2.0 mg·L−1 (WHO 2003; Health Canada 2018). Although there is a lack of information on the amount of Cu that can induce symptoms of GI distress, data suggest that these adverse events can occur after consumption of water with Cu concentrations in excess of 4.0 mg·L−1, which is twice the WHO recommended limit (Araya et al. 2003).
In addition to regulations surrounding drinking water, other recommendations are in place to prevent excess dietary copper intake. For example, a Nordic guidance document for industry notes that acidic foods are able to react with Cu metal and therefore recommends that the food contact area of copperware should be lined with a non-reactive material (Cederberg et al. 2015). The Food Code, published every four years by the U.S. Food and Drug Administration (US FDA), specifically recommends that a food or beverage with a pH less than 6.0 should not contact Cu and Cu alloy materials due to high risk of leaching from copperware (US FDA 2017). Moreover, a technical guide from the Council of Europe sets a release limit for copper at 4 mg·kg−1 foodstuffs (Directorate for the Quality of Medicines & HealthCare of the Council of Europe 2013). To prevent excessive Cu exposure, a number of manufacturers line the food-contact surfaces of copperware with a less reactive metal or a lacquer. Regardless of these efforts, there is limited published information on the factors that influence Cu release to food materials and how best to limit such transfer.
As noted, previous experiments have found that acidic conditions will enhance release of Cu from copperware to food and beverages, and to a certain extent even water alone can ionise Cu metal and lead to release of the metal (Hopper and Adams 1958; Ishiwata et al. 1986; Sharda and Bhandari 1986; Thomas et al. 2014). Still, detailed kinetics of Cu release from copperware have not been completely elucidated, in addition to other factors that may influence such release, including as temperature and acid type, have not been evaluated. In the present study, we first evaluated release of Cu from metal foil and then from commercially available copperware (drinking vessels) with and without a food-grade lacquer lining to food simulants. We also evaluated the impact of simulated multiple washing treatments on a set of commercially available lined and unlined copperware on Cu transfer to food simulants.
MATERIALS AND METHODS
Chemicals
Optima ultra trace-metal grade nitric acid and hydrochloric acid, and toluene (certified ACS grade, >99.5%) were purchased from Thermo Fisher Scientific (Waltham, MA). Deionised (DI) water (18.2 MΩ·cm at 25 °C) for experiments was obtained from a Milli-Q system (Millipore-Sigma, Burlington, MA). Citric acid (>99.5%), acetic acid (>99.99% trace metals basis), malic acid (>99% trace metals basis), and poly(methyl methacrylate) (PMMA) with an average Mw ≈120,000 (density = 1.188 g·mL−1 at 25 °C) was purchased from Sigma-Aldrich (St. Louis, MO). Cu standard was from Inorganic Ventures (Blacksburg, VA). Calibration tuning mix and pulse/analog solutions were purchased from Agilent Technologies (Santa Clara, CA). Trace metal quality plastic tubes were purchased from SCP Sciences (Champlain, NY). Trace Elements in Natural Water standard reference material (SRM 1640a) was obtained from the National Institute of Standards and Technology (NIST; Gaithersburg, MD). Cu metal sheet foil (0.1 × 200 × 1000 mm; 99.9% Cu), copperware drinking vessels advertised as “100% copper,” and copperware drinking vessels advertised as coated with a food-grade lacquer were purchased from an online retailer.
Food simulants
Test food simulants were formulated to contain organic acids commonly found in foods/juices and those used as ingredients in fruit-flavored beverages (Shui and Leong 2002; Yoshikawa et al. 2007). Food simulants consisted of DI water (control), 3% citric acid (w:w), 3% malic acid (w:w), and 3% acetic acid (w:w). Acid concentration of the food simulants (3%) was based on guidance from governmental bodies for testing migration of chemical contaminants from food packaging materials (US Food and Drug Administration 2007; European Commission 2011). Measurement of food simulant pH was performed using a calibrated S20 SevenEasy pH meter with InLab Routine Pro pH electrode (Mettler Toledo, Columbus, OH).
Cu release from sheet metal foil
Cu release experiments were first conducted in a model system using Cu sheet metal foil. Circular sections of metal foil (42 mm × 0.1 mm; surface area of 27.8 cm2) were cut using a 42 mm diameter hole punch and then washed with DI water to remove any extraneous material on the surface of the metal foil. Test food simulants (30 g) were dispensed into a trace-metal free tube, and Cu metal foil sections were submerged in the simulant. Independent trials (n = 3) were conducted at 0 (metal foil submerged in liquid and immediately removed), 15, 30, 90, and 180 min timepoints. A blank control was prepared where food simulant was incubated in the trace-metal free tubes without the metal foil to confirm the absence of background Cu. Experimental timepoints were selected to approximate similar food contact time occurring in a retail or in-home setting. For release studies at 4 ± 1 °C, samples were placed in a refrigerator (VWR; Radnor, PA), while an Isotemp circulating oven (Fisher Scientific; Hampton, NH) was used for studies at 30 ± 2 and 60 ± 2 °C. The specified temperature range was used to simulate cold- or hot-served beverages (Brown and Diller 2008). Food simulants were either pre-heated or pre-cooled to match the set incubation temperature before beginning the experimental trial.
Cu release from commercial copperware vessels
To test Cu release from commercially available copperware, 200 g food simulant consisting of either 3% citric acid (w:w) or DI water (control) was dispensed into copperware drinking vessels (n = 3) that had been rinsed with DI water. The surface area in contact with the food simulants was 150.8 cm2. Citric acid was the only acid selected for use in these experiments as a food simulant to minimise material costs and due to citric acid having the lowest pKa value of the acids tested in the previously described model system experiments. One copperware set was advertised as treated with a food-safe lacquer lining (lined), while the other set advertised was not treated with a lacquer (unlined). For both lined and unlined copperware, 5 g food simulant sample was removed and replaced with fresh food simulant at each of the following timepoints: 0 (food simulant dispensed into vessel and immediately collected), 15, 30, 90, and 180 min, with each experimental temperature set at 4 ± 1, 30 ± 2, or 60 ± 2 °C. Timepoint samples were analyzed for Cu concentrations using the ICP-MS method described below.
Simulated repeated cleaning treatment
To assess the effect of abrasion from repeated cleaning on Cu release from commercially available copperware, both copperware lined with a food-grade lacquer (n = 3) and unlined copperware (n = 3) drinking vessels were subjected to a procedure that simulated multiple cleaning treatments that could occur in either a retail or in-home setting (Brede et al. 2003). A DeWalt electric drill (Towson, MD) was fitted with a bristle bottle brush obtained from an online retailer (RotoScrub Bottle Brush) and set to 430 rpm. The copperware vessel was filled with 200 g DI water and the inner surfaces of the vessel were subjected to brushing for 5 min. Copperware was rinsed with DI water after the cleaning treatment and then Cu release from each set of copperware vessels was subsequently evaluated using 3% citric acid (w:w) at 60 °C. Due to the exploratory nature of this experiment, only the most extreme conditions were selected. Experimental timepoints were as indicated in the previous section.
Characterization of copperware coating
Since the identity of the coating of the set of lined copperware was not available from the manufacturer or retailer, a series of analytical characterisations were performed.
Attenuated total reflectance/Fourier transform-infrared (ATR/FT-IR) spectroscopy
ATR/FT-IR spectroscopy was performed on a Frontier IR Dual-Range (MIR/NIR) spectrometer (Perkin-Elmer, Waltham, MA) using the universal ATR sampling accessory with a single-bounce top plate containing a composite ZnSe and diamond crystal. Background and sample scans were recorded between 4000 and 650 cm−1 with 32 scans. Samples were scanned at a resolution of 4.00 cm−1. Samples were placed in contact with the crystal using an ATR shoe with a 1.5-mm diameter tip. A constant force setting of 120 was applied to ensure good sample contact with the ATR crystal due to the thin polymer coating on the metal substrate. A section from the bottom of each copperware vessel type was cut in order to analyze the inner food-contact surface of the drinking vessel. The ID Expert application (version 18.3.111.0) within the KnowItAll Vibrational Spectroscopy Edition (Bio-Rad, Hercules, CA) was used to search unknown coating samples within reference ATR-IR spectral databases. Spectral matches are conducted using a Hit Quality Index (HQI) value on a 0 to 100% scale, with an HQI value of 100% indicating a perfect match between the query spectrum and a given reference database spectrum.
Solvent extraction of acrylic coating
Since a preliminary ATR/FT-IR analysis of the copperware coating indicated an acrylic compound, toluene was selected as a solvent to solubilise the lining (Verleye et al. 2001; Patra et al. 2011). Toluene (50 mL) was added to the lined copperware vessel (n = 3) and stirred using a glass stir bar at room temperature for 60 min. Cast films of the acrylic coating were prepared by drop casting the solutions onto a glass Petri dish surface and let to dry overnight at room temperature. Films were further dried in a vacuum oven without heat for 24 h to remove any residual toluene. Dry coating extract was scraped from the surface for further characterization.
Coating thickness measurement
An MDH series 293 digimatic micrometer (Mitutoyo, Aurora, IL) with a constant-force mechanism was used to measure thickness at eight locations around the perimeter of the coated surface at the bottom of each cup (n = 3). Coating thickness was estimated by calculating the difference between the thickness of the copperware before and after removing the coating with toluene.
Differential scanning calorimetry (DSC)
A DSC Q2000 instrument (TA Instruments, New Castle, DE) was used to determine the glass transition temperature (Tg) of polymer samples. A heat/cool/heat method was used: (1) equilibration at 0 °C followed by heating to 200 °C at a rate of 10 °C·min−1; (2) cooling from 200–0 °C at 10 °C·min−1; and (3) heating from 0–200 °C at 10 °C·min−1. An N2 sample purge flow was used at 50 mL·min−1. A Tzero aluminum sample pan was used to hold samples (n = 3) with weights of 1.20 ± 0.17 mg. Since residual toluene and water can act as plasticizers in acrylic polymers, only vacuum dried sample powders were used. TA Universal Analysis 2000 software (version 4.5A) was used to perform Tg analysis and to calculate the midpoint Tg of the second heating curve.
Thermogravimetric Analysis (TGA)
A TGA Q500 thermobalance (TA Instruments, New Castle, DE) was used to measure polymer sample weight loss to determine thermal decomposition temperature profiles. The thermobalance was calibrated with an alumel alloy and nickel for temperature settings and with a 100 mg standard for weight accuracy. Samples (6 mg coating and 10 mg PMMA) (n = 2) were placed on a tared platinum balance pan and transferred to the furnace at room temperature, where the exact sample weight was determined. The temperature program increased the temperature at a rate of 20 °C·min−1 from 25–600 °C under dry N2. Universal Analysis 2000 software was used to evaluate sample decomposition temperatures at 10% mass loss.
Inductively coupled plasma-mass spectrometry (ICP-MS) analysis
In preparation for elemental analysis, food simulant samples were diluted at minimum 10X with 2% HNO3 (v:v). Diluted samples were then analysed for total Cu using an Agilent 8800 ICP-MS/MS using previously reported elemental analysis methods that were adapted for Cu analysis (Gray and Cunningham 2019; Redan et al. 2019). ICP-MS was set in single quadrupole mode with research grade helium (99.999%) from Airgas (Radnor, PA) as the collision gas (4.3 mL·min−1) and argon as the carrier gas (0.95 L·min−1) and makeup gas (0.20 L·min−1). Cu was monitored as isotopes 63Cu and 65Cu and is reported as 65Cu. Internal standard solution was mixed in line with the sample stream using a mixing tee before entering the nebuliser (sample tubing 1.02 mm inner diameter; internal standard tubing 0.25 mm inner diameter). ICP-MS Internal Standard Mix (Agilent Technologies) was prepared in aqueous 2% HNO3 (v:v), with internal standard set as rhodium (Rh). Quality control measures included validating each analytical sequence using NIST Trace Elements in Natural Water reference material and by analyzing a calibration verification standard of Cu every 10 samples. Method detection and quantitation limits were estimated by analyzing blank samples over 7 nonconsecutive days and calculating 3X and 10X the standard deviation (σ) of the analyte responses for detection (LOD) and quantitation limits (LOQ), respectively. Data processing was conducted using MassHunter Workstation software version 4.2 (Agilent Technologies) with results exported as Excel worksheets (Microsoft Office; Microsoft Co., Redmond, WA).
Statistical analysis
All trials were repeated three times, and data are presented as means ± SD. Statistical analysis was performed using JMP 12 (SAS Institute, Cary, NC). Cu concentrations were normalised based on total macroscopic surface area in contact with the food simulant and are expressed as mass Cu per cm2 surface area. Cu release rate was determined by plotting Cu concentrations versus time and then using linear regression to obtain a value for the slope parameter. One-way or two-way ANOVA followed by pairwise mean comparisons using the Tukey-Kramer post-hoc correction was used to determine significant differences (P<0.05) between treatments. Data with residuals not normally distributed underwent a Box-Cox transformation before statistical analysis.
RESULTS
ICP-MS method linearity and detection limits
Cu in standard solution exhibited a highly linear response using this ICP-MS method (R2 > 0.9999). The 3σ LOD and 10σ LOQ of the method were estimated to be 0.02 and 0.07 μg·L−1, respectively.
Cu release from sheet metal foil to food simulants
Mean pH values of food simulants were 1.9, 2.0, 2.4, and ≈5.5 for citric acid, malic acid, acetic acid, and DI water, respectively. Figure 1 displays a plot of the effect of time, temperature, and acid type on Cu release from the metal foil. The results overall indicated that compared to the DI water control, incubation of the metal foil with the acidic food simulant resulted in an approximately linear increase in Cu release over the 180 min incubation period. In addition, increasing temperature and decreasing pH generally corresponded to greater Cu release. After 180 min, incubation of Cu metal foil with acidic food simulants at 4 °C resulted in release values ranging from 8.7 μg Cu·cm−2 (acetic acid) to 14.0 μg Cu·cm−2 (malic acid). At the highest temperature tested (60 °C), incubation of Cu metal foil after 180 min resulted in 21.5 μg Cu·cm−2 release for the acetic acid food simulant and increased 44% to 38.1 μg Cu·cm−2 when incubated with the citric acid food simulant. In contrast, Cu transfer to DI water was relatively low, with no greater than 0.6 μg Cu·cm−2 released after 180 min at 60 °C. Two-way ANOVA indicated a significant effect (P<0.01) of main factors temperature and acid type on the rate of Cu release and a significant (P<0.01) interaction between the main factors (Table 1). Post-hoc pairwise mean comparisons revealed a significant (P<0.05) increase in Cu release rate with respect to temperature for all food simulants except DI water. Figure 2 shows a plot of Cu release kinetics to food simulants using commercially available lined and unlined copperware. Using citric acid as the food simulant, lined copperware released between 0.6–3.0 μg Cu·cm−2 over 180 min when incubated at the experimental temperatures, while unlined copperware released approximately 25–45 fold higher amounts of Cu (26.9–74.6 μg·cm−2) over this same time period. To determine if saturation of Cu release from unlined copperware would occur, 24 h timepoints were collected and it was found that 78.7 ± 8.5, 141.9 ± 15.6, and 407.4 ± 96.2 μg Cu·cm−2 was released at 4, 30, and 60 °C, respectively, indicating that Cu release was still occurring after 180 min. Use of DI water as the food simulant released much lower amounts of Cu, with all values <0.1 μg Cu·cm−2 for the lined copperware and <2 μg Cu·cm−2 for the unlined type over 180 min. Cu release from unlined copperware to DI water at 24 h was found to be <3 μg Cu·cm−2 at all experimental temperatures, suggesting equilibrium was reached at 180 min (data not shown). Two-way ANOVA of Cu release rates (Tables 2 and 3) from copperware showed a significant (P<0.01) effect of main factors temperature and lining. Post-hoc mean comparisons revealed that at 4 °C, the Cu release rate from unlined copperware to citric acid food simulant was 41-fold greater (P<0.01) compared to the lined vessel. At the highest temperature (60 °C), the unlined copperware released Cu to citric acid food simulant at a rate 21-fold greater (P<0.01) than the lined vessel. Results of two-way ANOVA with DI water as a food simulant only indicated a significant effect (P<0.01) of main factor lining, with post-hoc comparisons indicating no difference in Cu release rate due to temperature.
Figure 1. Effect of temperature and pH on Cu release kinetics from sheet metal foil to food simulants.
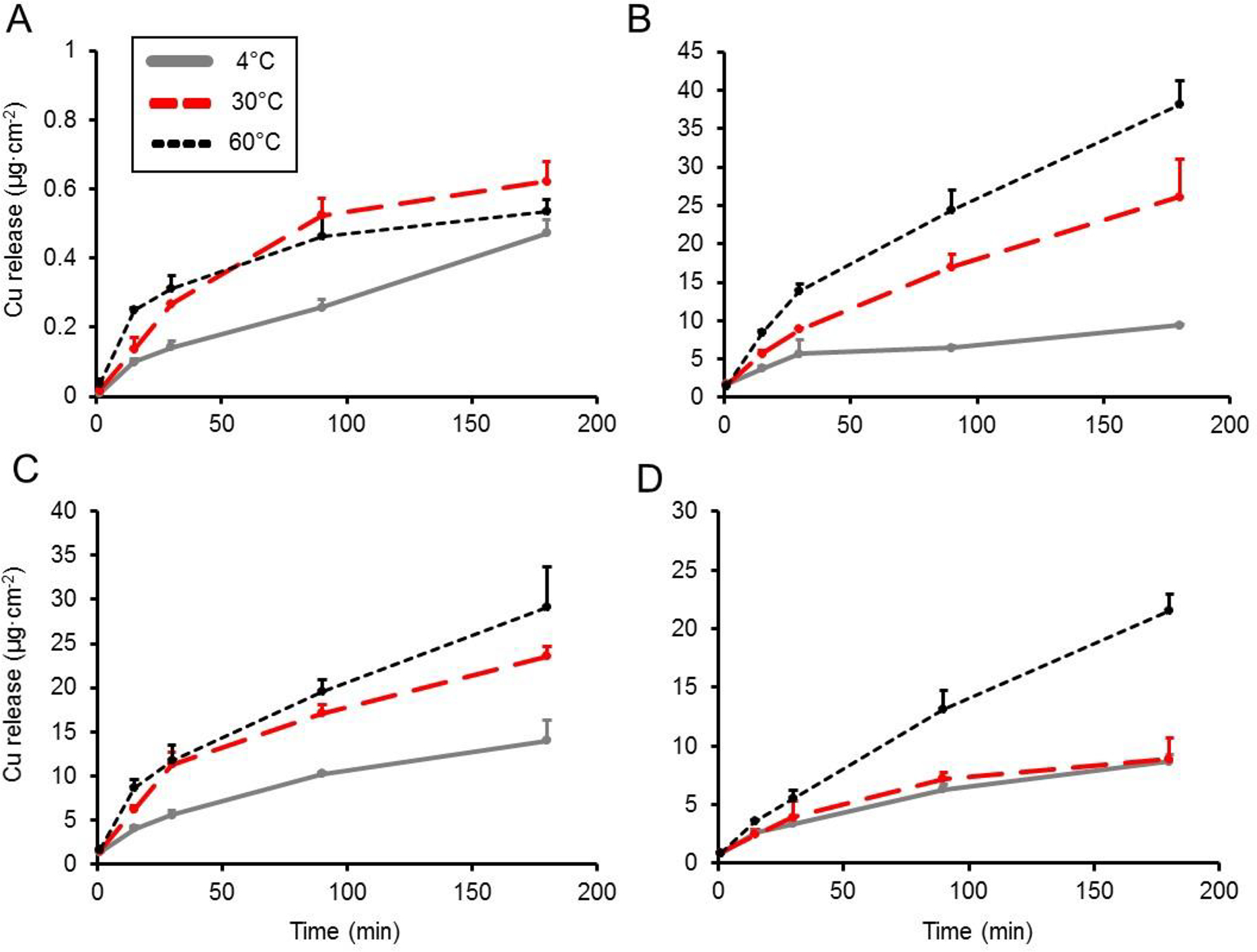
Cu release (μg·cm-2) at 4, 30, and 60 °C to food simulants composed of (A) DI water, (B) 3% citric acid, (C) 3% malic acid, and (D) 3% acetic acid. Values are plotted as means ± SD.
Table 1.
Cu release rates over 180 min from metal foil to food simulants.a
| DI Water | Citric acid (3%) | Malic acid (3%) | Acetic acid (3%) | |
|---|---|---|---|---|
| (ng·cm−2·min−1) | ||||
| 4°C | 2.40 ± 0.27 D | 36.3 ± 1.62 CD | 66.8 ± 11.8 C | 41.2 ± 2.55 C |
| 30°C | 3.23 ± 0.31 D | 131 ± 24.9 B | 113 ± 6.60 B | 43.0 ± 10.5 C |
| 60°C | 2.33 ± 0.21 D | 191 ± 14.3 A | 139 ± 25.5 B | 113 ± 9.31 B |
Values are presented as means ± SD. Two-way ANOVA indicated a significant (p<0.01) effect of main factors acid type and temperature, in addition to an interaction effect. Different letters indicate significant (p<0.05) pairwise difference with Tukey-Kramer post-hoc correction.
Figure 2. Effect of temperature and pH on Cu release kinetics from two types of copperware to food simulants.
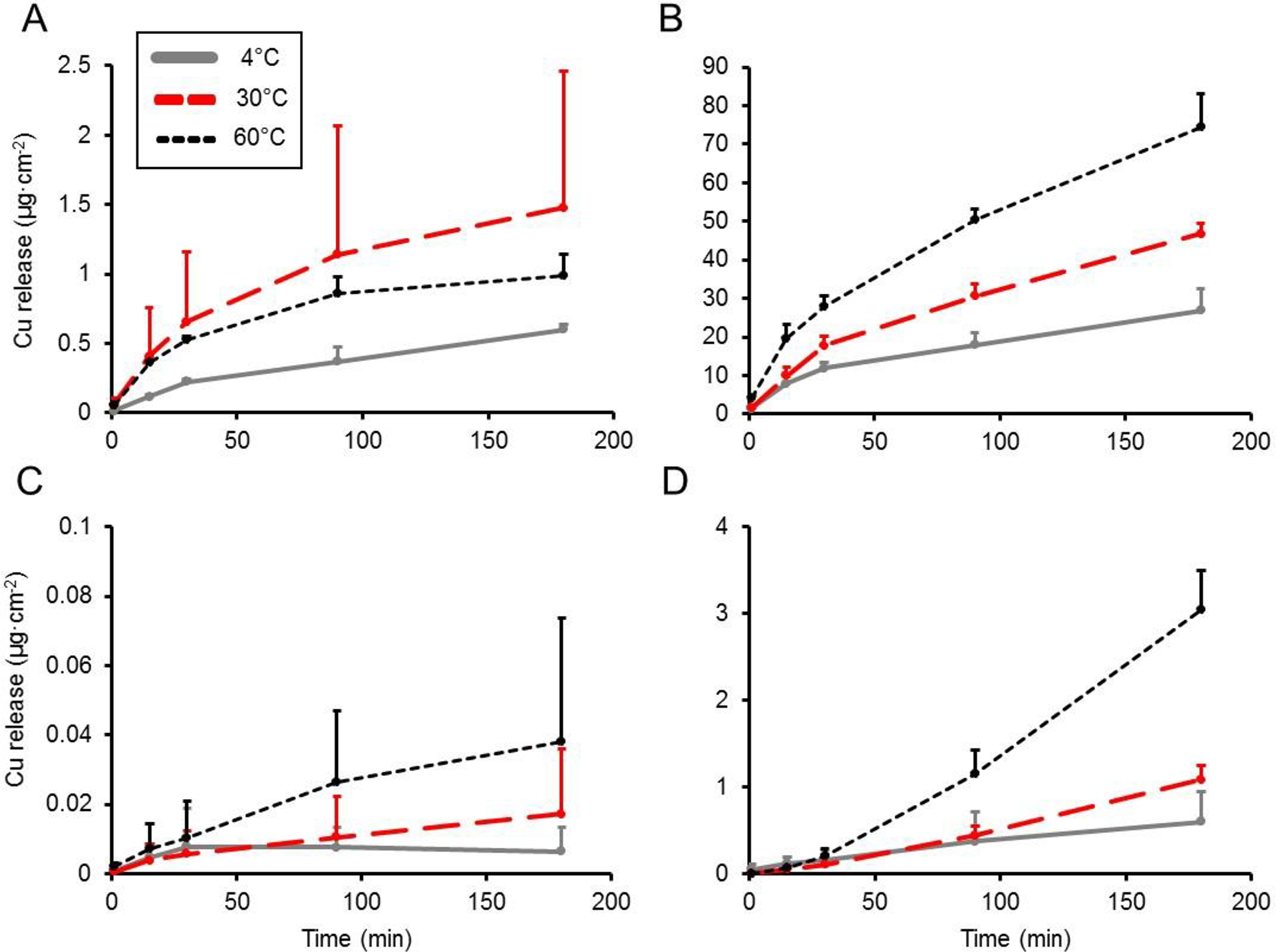
Cu release (μg·cm-2) at 4, 30, and 60 °C from (A) unlined copperware to DI water, (B) unlined copperware to 3% citric acid, (C) lined copperware to DI water, and (D) lined copperware to 3% citric acid. Values are plotted as means ± SD.
Table 2.
Cu release rates over 180 min from lined and unlined copperware to DI water food a simulant.a
| Lined copperware | Unlined copperware | |
|---|---|---|
| Cu release (ng·cm−2·min−1) | ||
| 4°C | 0.0433 ± 0.0321 A | 3.03 ± 0.208 B |
| 30°C | 0.0897 ± 0.0989 A | 7.30 ± 7.40 B |
| 60°C | 0.201 ± 0.198 A | 4.60 ± 1.00 B |
Values are presented as means ± SD. Two-way ANOVA indicated a significant effect (p<0.01) of main factor lining. Different letters indicate significant (p<0.01) pairwise difference with Tukey-Kramer post-hoc correction.
Table 3.
Cu release rates over 180 min from lined and unlined copperware to 3% citric acid.a
| Lined copperware | Unlined copperware | |
|---|---|---|
| Cu release (ng·cm−2·min−1) | ||
| 4°C | 3.07 ± 1.74 D | 127 ± 27.7 B |
| 30°C | 6.13 ± 0.71 D | 250 ± 32.8 A |
| 60°C | 17.3 ± 0.23 C | 366 ± 56.9 A |
Values are presented as means ± SD. Two-way ANOVA indicated a significant (p<0.01) effect of main factors temperature and lining within citric acid food simulant columns. Different letters indicate significant (p<0.01) pairwise difference with Tukey-Kramer post-hoc correction within food simulant type.
Cu release from commercial copperware after simulated multiple cleaning treatments
In Figure 3, Cu release versus time is shown from commercial copperware after simulated multiple cleaning treatments. After 180 min of incubation with citric acid food simulant at 60 °C, there was no significant (P>0.05) difference in the amount of Cu release after the cleaning treatments for either the lined or unlined copperware. The Cu release rate of cleaned copperware vessels was also not significantly different from untreated copperware (data not shown). Though not statistically significant, there was interestingly greater Cu release after cleaning treatment in both lined (3.0 versus 4.0 μg Cu·cm−2) and unlined copperware drinking vessels (74.6 versus 80.6 μg Cu·cm−2).
Figure 3. Effect of simulated long-term cleaning on Cu release kinetics from two types of copperware.
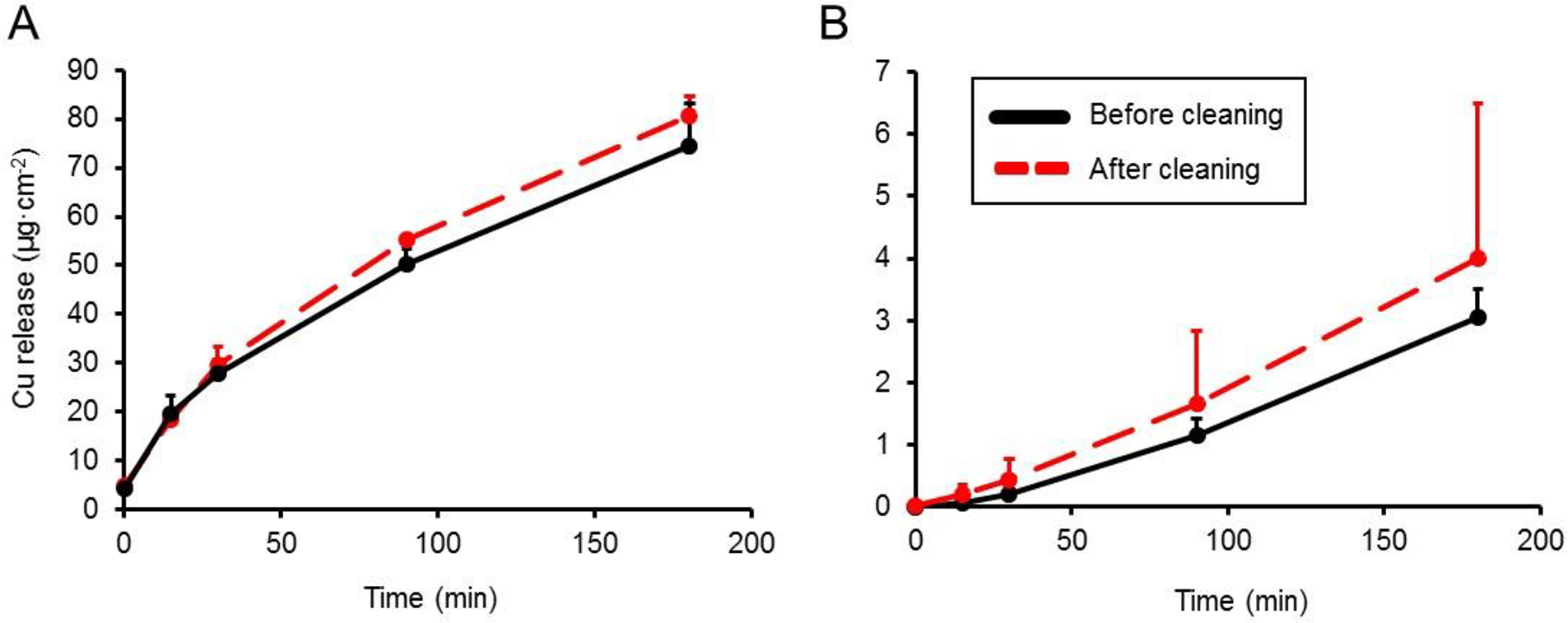
Cu release (μg·cm-2) to 3% citric acid at 60 °C from (A) unlined copperware, and (B) lined copperware. Values are plotted as means ± SD.
Correlation between Cu release models
Figure 4 compares the Cu release kinetics from the metal foil and the unlined copperware using DI water or 3% citric acid as the food simulant. Overall, normalised Cu release values from the metal foil were lower than that of the copperware under all conditions. With citric acid food simulant, Cu release over 180 min from the copperware was 80–190% greater compared to the metals foils, while use of DI water as a food simulant resulted in Cu release that was 30–150% greater from the copperware compared to the metals foils. While the absolute values for Cu release differed between the two models, the correlation between the release curves was high. Correlation values (R) between the two models with citric acid food simulant were greater than 0.94 (P<0.01). Release curves with DI water food simulant had identical correlation values for incubation temperatures at 4 and 60 °C (R=0.96; P<0.01), while incubation at 30 °C had a lower but nonetheless statistically significant correlation (R=0.62; P<0.05).
Figure 4. Comparison of Cu release kinetics from sheet metal foil and unlined copperware model systems.
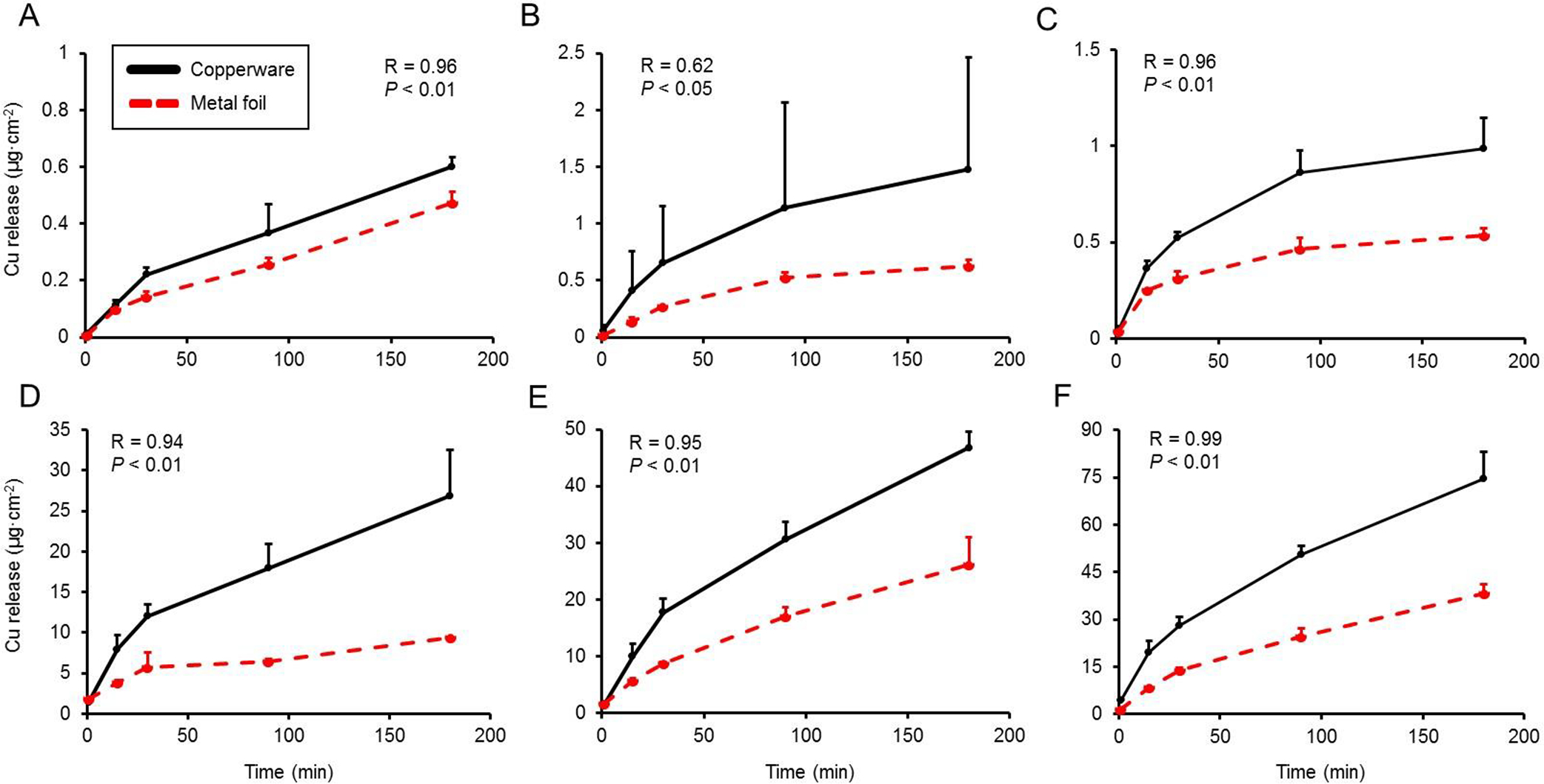
Cu release (μg·cm-2) to (A) DI water at 4 °C, (B) DI water at 30 °C, (C) DI water at 60 °C, (D) 3% citric acid at 4 °C, (E) 3% citric acid at 30 °C, and (F) 3% citric acid at 60 °C. Values are plotted as means ± SD.
Analysis of copperware lining
The thickness of the copperware coating was 3.0 ± 0.9 μm. In Figure 5A, the ATR/FT-IR spectrum of the copperware coating contains the following characteristic structural features of the acrylic class polymer, PMMA: the peaks of stretching vibration of –CH2 and –CH3 groups at 3000–2840 cm−1; the stretching peak of C=O group at 1724 cm−1; the peaks of the bend vibration of –CH2 and –CH3 groups at 1449 and 1387 cm−1, respectively; and the peaks of the stretching vibration of the C(=O)O at 1240 cm−1 (Verleye et al. 2001). The native coating measured on the copperware and the extracted coating had HQI values of 98.31 and 98.89, respectively, with PMMA as the reference spectrum. However, there were slight peak differences within the 3000–2840 cm−1 range between the copperware coating and PMMA. The reference spectrum of poly(butyl methacrylate-co-methyl methacrylate) (MMA/BMA copolymer) exhibited a closer spectral match in the 3000–2840 cm−1 region and had comparable overall HQI values (97.79 and 98.07 for the native coating and extracted coating, respectively) with the reference spectrum of MMA/BMA copolymer. Analysis of Cu metal foil and unlined copperware showed lack of the absorption bands indicative of the presence of the coating.
Figure 5. Characterization of lined copperware coating.
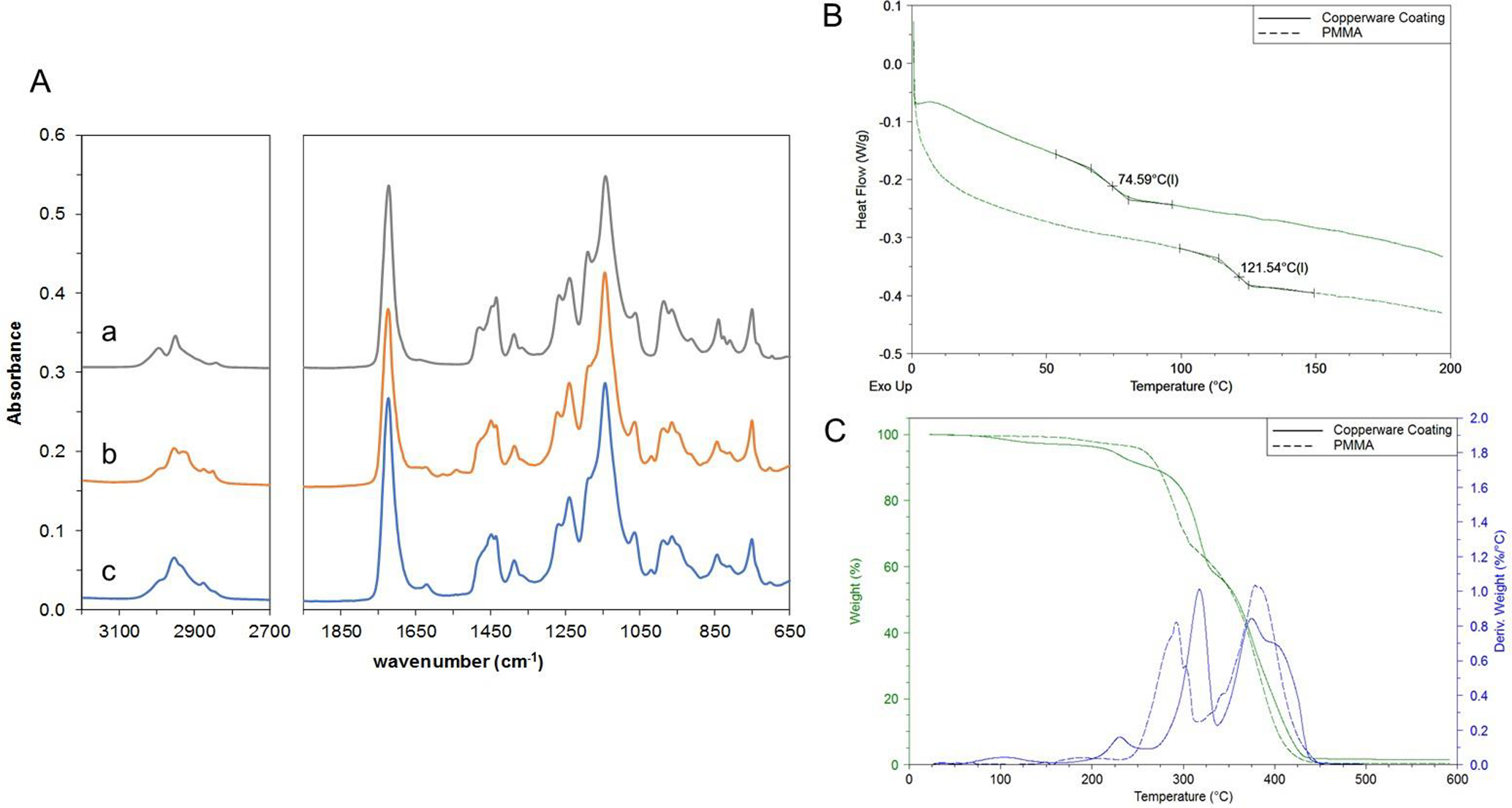
(A) ATR/FT-IR spectra of (a) PMMA powder, (b) copperware coating film, and (c) copperware coating extract as a dry powder. (B) DSC curves indicating the glass transition temperatures of copperware coating extract and PMMA under N2 at a temperature rate of 10 °C·min-1. (C) TGA curves for the thermal decomposition of copperware coating extract and PMMA under N2 at a temperature rate of 20 °C·min-1.
Figure 5B shows the DSC curves of PMMA and the extracted copperware coating. The Tg of PMMA reference was 121.5 ± 0.2 °C, while the copperware coating had a Tg of 75.4 ± 2.2 °C. The considerably lower Tg of the copperware coating complemented the ATR/FT-IR spectral database match indicating that the copperware coating is an MMA/BMA copolymer. Since MMA/BMA copolymers with monomer ratios of 60/40 and 40/60 have reported Tg values of 83 and 63 °C, respectively (Penzel et al. 1997), the measured Tg of the copperware coating would be consistent with an estimated 50/50 monomer ratio of a MMA/BMA copolymer. Analysis by TGA showed that the copperware coating exhibited excellent thermal stability up to 200 °C (Figure 5C). The temperature at 10% mass loss was 257 ± 8.2 °C and 274 ± 1.9 °C for the copperware coating and PMMA, respectively.
DISCUSSION
Although not as prevalent in developed countries, toxicity from overexposure to Cu due to copperware has been documented in the literature. For example, incidence of childhood hepatic cirrhosis in India has been linked to Cu overexposure from the widespread use of copperware to store water and use as a drinking vessel (Sharda and Bhandari 1986). Although copperware use has been reported to have beneficial endpoints in developing countries due to the anti-microbial properties of Cu (Vincent et al. 2016), extensive use of these materials has the potential downside of increasing Cu intake to amounts that can result in toxicological effects.
Several controlled studies have attempted to estimate a threshold of Cu intake required to elicit acute toxicity symptoms. One study in Chile noted adverse events in participants after they consumed water containing >3 mg Cu·L−1, with daily Cu intake totaling approximately 4.8 mg (Pizarro et al. 1999). A multi-site study that tested Cu toxicity symptoms in human female participants found that consuming water containing 4 mg Cu·L−1 could produce symptoms of GI distress within 15 min of intake (Araya et al. 2003). From the amount of water consumed, this concentration corresponded to an acute dose of 0.8 mg Cu. Overall, these data indicate that both concentration and the absolute amount of Cu are important factors affecting toxicological endpoints.
There have been multiple documented cases where use of copperware has resulted in acute Cu toxicity (Hall 1970). In a report from Canada, symptoms of foodborne illness were exhibited in multiple people approximately 30–60 min after they consumed a beverage prepared with lemon juice (Wyllie 1957). It was later determined that this acidic beverage (containing mainly citric acid) had been in contact with a copperware container for 2 h prior to being served. Later analysis of the consumed beverage indicated that those affected ingested >5 mg Cu. In another incident in Australia, multiple children exhibited GI distress only minutes after drinking a beverage prepared with lime juice (Gill and Bhagat 1999). A sample of the beverage was later analysed for pathogens and chemical contaminants, and, although no pathogens were found, the Cu concentration was at the highly elevated level of 300 mg·L−1. It was then determined that the acidic beverage had been stored in a copperware vessel overnight before being consumed. Together, these reports are consistent with literature suggesting that elevated ingestion of Cu can elicit symptoms of GI distress.
In the present experiments, the data indicate that Cu release from commercial copperware could result in Cu concentrations associated with potential toxic effects. Assuming a surface area of 300 cm2 (to approximate the contact area of a typical copperware drinking vessel) the data comprising the citric acid food simulant predict that after 180 min, 8–23 mg Cu would be released at temperatures ranging from 4–60 °C. Even after 30 min, 3.6–8.4 mg Cu would be released at temperatures between 4–60°C with the citric acid food simulant. These are significant amounts that, if consumed, would likely result in adverse GI symptoms. With DI water, 0.2–0.4 mg Cu was released at 4–60 °C after 180 min. Although these amounts are much lower compared to the acidic conditions, they are still a noteworthy source of Cu exposure. In comparison, Ishiwata et al. reported greater release values from a copperware vessel after 30 min using 60 °C water and 4% acetic acid as food simulants (5.7 and 167 μg Cu·cm−2, respectively; values estimated using data provided in publication). Another study found that Cu concentrations in water (pH = 6.0) increased to 0.3 mg·L−1 after water was stored in a copperware vessel over 6 h (Sharda and Bhandari 1986), This value is similar to our experimental range of 0.2–0.4 mg Cu, but it is difficult to compare directly since the authors did not report the actual surface area of the storage vessel. Also, it is important to note that although we chose to use 3% acid solutions tested over 3 h to simulate food contact in a retail or in-home environment, a guidance document from the Council of Europe recommends using a 0.5% citric acid solution over 10 days at 40 °C when considering metal release to foods for use at ambient temperature (Directorate for the Quality of Medicines & HealthCare of the Council of Europe 2013).
Within the pH range of the test food simulants (pH 1.9–2.4) and experimental temperatures (4–60 °C), we found a direct relationship between Cu transfer, acidity, and temperature. Several additional studies corroborate these results. In one study where Cu metal plumbing was used, the authors observed a correlation between increased acidity of chlorinated drinking water and elevated Cu release from the material (Hong and Macauley 1998). Authors from two different studies found increased migration of Cu from ceramic and earthenware cookware to acidic food simulants with increased temperature (Belgaied 2003; Demont et al. 2012). In an environmental context, experiments with simulated rain water found that Cu release from brake pads was dependent on pH (Hur et al. 2004). In addition to assessing Cu in its metal form, there has been growing interest in Cu nanoparticles in food applications and how they respond to acidic conditions (Liu F et al. 2016). Although it is well known that Cu and other metals can ionise when exposed to an acidic medium (Liu X et al. 2015), there is a general lack of specific information on the extent to which Cu transfers to foods and the primary factors that drive this release. One proposed mechanism for Cu dissolution in aqueous acidic conditions is the oxidation of metallic Cu0 to Cu2+ through the following reactions (Feng et al. 1997):
| (1) |
| (2) |
In this scenario, metallic Cu is oxidised on the copperware surface by dissolved O2 to form a thin Cu2O passivation layer. Under acidic conditions, this surface layer of Cu2O dissolves, releasing Cu2+ into solution. The present study suggests the proposed reaction, since acidic conditions in our models resulted in much greater Cu release compared to water alone.
Still, other factors aside from food or beverage acidity have also been demonstrated to drive to metal release from copperware. One study found that the protein albumin from egg whites is able to efficiently bind to Cu resulting in a stable complex (Liu X et al. 2015). In addition, Cu can associate with casein, one of the major proteins in milk (O’Neill and Tanner 1989), and storage of milk in copperware can result in a beverage with elevated Cu concentrations (Sharda and Bhandari 1986). These additional components should be more fully explored in future studies.
While the results from the metal foil model system showed a correlation with results from the release experiments using the commercial copperware, use of Cu metal foil underestimated Cu release as compared to the commercial copperware. One reason for this divergence may be due to potential differences in the microscopic surface area of the two materials. The surface of the copperware appeared to be rougher than that of the sheet metal foil, which likely increased the total surface area of the copperware. Another plausible explanation for the difference is that trace impurities in the copperware can enhance Cu release (Meiners et al. 2018). Overall, these differences indicate that proper selection of a model system is crucial to limit error in estimation of metal release.
Container coatings function to protect the metal substrate from its contents and to avoid contamination of the product by metal ions from the container. We estimated the copperware coating had a thickness of approximately 3 μm, which is consistent with literature reporting typical thickness in the 1–10 μm range (Oldring and Nehring 2007; Robertson 2013). The performance of a coating is greatly affected by the coating thickness, with heavier coatings having much lower porosities and fewer defects. Pores in coatings have been previously been reported to allow aqueous solutions to penetrate a coated metal surface (Armstrong et al. 1992). Since we observed Cu release from the lined copperware, this likely indicates a number of pore defects present in the MMA/BMA copolymer coating. Still, this system requires further study to fully elucidate and confirm the proposed mechanism for our data indicating Cu release from the coated copperware to acidic food simulants.
Acrylic coatings are a diverse class of organic coatings comprised of PMMA and copolymer blends used in food contact materials with properties tailored for specific applications dependent upon the percentage of individual monomers in the polymer chain. The exploratory study on the effects of repeated cleaning of the two types of copperware was conducted based on previous research indicating that migration of metals can be influenced by wear from repeated cleaning (Addo Ntim et al. 2018; 2019). There are limited published data on the effect of repeated cleaning treatments in the present experimental paradigm (Mazinanian et al. 2016), but we hypothesised that repeated abrasion to the food-safe lining on the copperware may impact its integrity and result in increased Cu release. However, the results showed no significant difference in copper release with the citric acid food simulant for both the lined and unlined copperware. Acrylic coatings are known to have highly flexible properties (Simal-Gándara 1999), and the lower density reported for MMA/BMA copolymer relative to PPMA (Franz and Brandsch 2013) could provide the copperware coating with properties that limit damage from abrasion.
CONCLUSION
The current study determined the effect of pH and temperature on Cu release kinetics to food simulants from both sheet metal foil and commercially available copperware. Cu release in both models was driven by acidic food simulants, but copperware lined with a food grade lacquer significantly reduced Cu transfer under all conditions. When comparing the two models, use of the Cu metal foil underestimated Cu release as compared to commercially available copperware. Further, Cu release was not significantly different after simulated long-term cleaning of the copperware. Overall, these data contribute to the body of work on how pH and temperature affect metal release from food contact surfaces and, therefore, provide data to help inform safe usage of these materials.
REFERENCES
- Addo Ntim S, Goodwin DG, Sung L, Thomas TA, Noonan GO. 2019. Long-term wear effects on nanosilver release from commercially available food contact materials. Food Addit Contam Part A. 36(11):1757–1768. [DOI] [PubMed] [Google Scholar]
- Addo Ntim S, Norris S, Goodwin DG Jr, Breffke J, Scott K, Sung L, Thomas TA, Noonan GO. 2018. Effects of consumer use practices on nanosilver release from commercially available food contact materials. Food Addit Contam Part A. 35(11):2279–2290. [DOI] [PubMed] [Google Scholar]
- Araya M, Chen B, Klevay LM, Strain J, Johnson L, Robson P, Shi W, Nielsen F, Zhu H, Olivares M. 2003. Confirmation of an acute no-observed-adverse-effect and low-observed-adverse-effect level for copper in bottled drinking water in a multi-site international study. Regul Toxicol Pharmacol. 38(3):389–399. [DOI] [PubMed] [Google Scholar]
- Armstrong R, Wright J, Handyside T. 1992. Impedance studies into the corrosion protective performance of a commercial epoxy acrylic coating formed upon tin plated steel. J Appl Electrochem. 22(9):795–800. [Google Scholar]
- Barceloux DG, Barceloux D. 1999. Copper. J Toxicol Clin Toxicol. 37(2):217–230. [DOI] [PubMed] [Google Scholar]
- Belgaied J-E. 2003. Release of heavy metals from Tunisian traditional earthenware. Food Chem Toxicol. 41(1):95–98. [DOI] [PubMed] [Google Scholar]
- Brede C, Fjeldal P, Skjevrak I, Herikstad H. 2003. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit Contam. 20(7):684–689. [DOI] [PubMed] [Google Scholar]
- Brown F, Diller KR. 2008. Calculating the optimum temperature for serving hot beverages. Burns. 34(5):648–654. [DOI] [PubMed] [Google Scholar]
- Cederberg DL, Christiansen M, Ekroth S, Engman J, Fabech B, Guðjónsdóttir K, Håland JT, Jónsdóttir I, Kostaomo P, Legind C. 2015. Food contact materials-metals and alloys: Nordic guidance for authorities, industry and trade. Copenhagen: Nordisk Ministerråd. [Google Scholar]
- Chen C, Jiang X, Li Y, Yu H, Li S, Zhang Z, Xu H, Yang Y, Liu G, Zhu F et al. 2019. Low-dose oral copper treatment changes the hippocampal phosphoproteomic profile and perturbs mitochondrial function in a mouse model of Alzheimer’s disease. Free Radic Biol Med. 135:144–156. [DOI] [PubMed] [Google Scholar]
- Demont M, Boutakhrit K, Fekete V, Bolle F, Van Loco J. 2012. Migration of 18 trace elements from ceramic food contact material: Influence of pigment, pH, nature of acid and temperature. Food Chem Toxicol. 50(3–4):734–743. [DOI] [PubMed] [Google Scholar]
- Directorate for the Quality of Medicines & HealthCare of the Council of Europe. 2013. Metals and alloys used in food contact materials and articles: A practical guide for manufacturers and regulators. 1st ed. Strasbourg, France: Council of Europe. [Google Scholar]
- [EPA] Environmental Protection Agency. 2019. Lead and copper rule. [accessed 2019 July 07]. https://www.epa.gov/dwreginfo/lead-and-copper-rule.
- European Commission. 2011. Commision regulation (EU) No. 10/2011 of 14 January 2011 on plastic materials and articles intneded to come Into contact with food.
- Feng Y, Siow K-S, Teo W-K, Tan K-L, Hsieh A-K. 1997. Corrosion mechanisms and products of copper in aqueous solutions at various pH values. Corrosion. 53(5):389–398. [Google Scholar]
- Franz R, Brandsch R. 2013. Migration of acrylic monomers from methacrylate polymers–establishing parameters for migration modelling. Packag Technol Sci. 26(8):435–451. [Google Scholar]
- Gaetke LM, Chow CK. 2003. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 189(1–2):147–163. [DOI] [PubMed] [Google Scholar]
- Gill JS, Bhagat CI. 1999. Acute copper poisoning from drinking lime cordial prepared and left overnight in an old urn. Med J Aust. 170(10):510. [DOI] [PubMed] [Google Scholar]
- Gray PJ, Cunningham W. 2019. Inductively coupled plasma collision cell quadrupole mass spectrometric determination of extractible arsenic, cadmium, chromium, lead, mercury, and other elements in food using microwave-assisted digestion: Results from an FDA interlaboratory study. J AOAC Int. 102(2):590–604. [DOI] [PubMed] [Google Scholar]
- Hall R 1970. Copper containers for food and drink. Bulletin (National Clearinghouse for Poison Control Centers (US)). Mar-Apr:1–7. [PubMed] [Google Scholar]
- Health Canada. 2018. Copper in drinking water: Guideline technical document for consultation. [accessed 2019 July 08]. https://www.canada.ca/en/health-canada/programs/consultation-copper-drinking-water/document.html.
- Hong PA, Macauley Y-Y. 1998. Corrosion and leaching of copper tubing exposed to chlorinated drinking water. Water Air Soil Poll. 108(3–4):457–471. [Google Scholar]
- Hopper SH, Adams HS. 1958. Copper poisoning from vending machines. Public Health Rep. 73(10):910–915. [PMC free article] [PubMed] [Google Scholar]
- Hur J, Schlautman MA, Yim S. 2004. Effects of organic ligands and pH on the leaching of copper from brake wear debris in model environmental solutions. J Environ Monit. 6(1):89–94. [DOI] [PubMed] [Google Scholar]
- Ishiwata H, Inoue T, Yoshihira K. 1986. Migration of copper and some other metals from copper tableware. Bull Environ Contam Toxicol. 37(1):638–642. [DOI] [PubMed] [Google Scholar]
- Liu F, Hu C-Y, Zhao Q, Shi Y-J, Zhong H-N. 2016. Migration of copper from nanocopper/LDPE composite films. Food Addit Contam Part A. 33(11):1741–1749. [DOI] [PubMed] [Google Scholar]
- Liu X, Hu W, Yang S, Li Z, Pei C, Zhou Y, Yin G. 2015. Severe corrosion of copper in a highly alkaline egg white solution due to a biuret corrosion reaction. Corros Sci. 94:270–274. [Google Scholar]
- Mazinanian N, Herting G, Wallinder IO, Hedberg Y. 2016. Metal release and corrosion resistance of different stainless steel grades in simulated food contact. Corrosion. 72(6):775–790. [Google Scholar]
- Meiners T, Peng Z, Gault B, Liebscher CH, Dehm G. 2018. Sulfur–induced embrittlement in high-purity, polycrystalline copper. Acta Mater. 156:64–75. [Google Scholar]
- [NRC] National Research Council. 2000. Health effects of excess copper. Copper in drinking water. Washington (DC): National Academies Press (US). [Google Scholar]
- O’Neill N, Tanner M. 1989. Uptake of copper from brass vessels by bovine milk and its relevance to Indian childhood cirrhosis. J Pediatr Gastroenterol Nutr. 9(2):167–172. [DOI] [PubMed] [Google Scholar]
- Oldring PK, Nehring U. 2007. Packaging materials: Metal packaging for foodstuffs. ILSI Europe. [Google Scholar]
- Patra N, Salerno M, Diaspro A, Athanassiou A. 2011. Effect of solvents on the dynamic viscoelastic behavior of poly (methyl methacrylate) film prepared by solvent casting. J Mater Sci. 46(15):5044–5049. [Google Scholar]
- Penzel E, Rieger J, Schneider H. 1997. The glass transition temperature of random copolymers: 1. Experimental data and the Gordon-Taylor equation. Polymer. 38(2):325–337. [Google Scholar]
- Pizarro F, Olivares M, Gidi V, Araya M. 1999. The gastrointestinal tract and acute effects of copper in drinking water and beverages. Rev Environ Health. 14(4):231–238. [DOI] [PubMed] [Google Scholar]
- Redan BW, Jablonski JE, Halverson C, Jaganathan J, Mabud MA, Jackson LS. 2019. Factors affecting transfer of the heavy metals arsenic, lead, and cadmium from diatomaceous-earth filter aids to alcoholic beverages during laboratory-scale filtration. J Agric Food Chem. 67(9):2670–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson G. 2013. Metal Packaging Materials. Food packaging: Principles and practice. Boca Raton: CRC Press; p. 49–90. [Google Scholar]
- Sharda B, Bhandari B. 1986. Elimination of brass utensils prevents Indian childhood cirrhosis. Indian J Gastroenterol. 5(3):198–198. [PubMed] [Google Scholar]
- Shui G, Leong LP. 2002. Separation and determination of organic acids and phenolic compounds in fruit juices and drinks by high-performance liquid chromatography. J Chromatogr A. 977(1):89–96. [DOI] [PubMed] [Google Scholar]
- Simal-Gándara J 1999. Selection of can coatings for different applications. Food Rev Int. 15(1):121–137. [Google Scholar]
- Simonson R. 2016. July 18. At age 75, the moscow mule gets its kick back. New York Times; [accessed 8 July 2019];Sect. Food. https://www.nytimes.com/2016/07/20/dining/moscow-mule.html. [Google Scholar]
- Sovich N. 2016. June 7. The cast iron and copper pot comeback. Wall St Journal; [accessed 2019 July 8];Sect. Life & Style. https://www.wsj.com/articles/the-cast-iron-and-copper-pot-comeback-1465328926. [Google Scholar]
- Thomas DR, Sunil B, Latha C, Chacko B. 2014. Microbiological quality of water stored in copper, earthenware and stainless steel vessels. Int J Agric Environ Biotech. 7(1):25. [Google Scholar]
- [US FDA] US Food and Drug Administration. 2007. Guidance for industry: Preparation of premarket submission for food contact substances (chemistry recommendations).
- [US FDA] US Food and Drug Administration. 2017. Food Code. [accessed 2019 July 08]. https://www.fda.gov/food/fda-food-code/food-code-2017.
- Verleye GA, Roeges NP, De Moor MO. 2001. Chapter 3, Thermoplastics. Easy identification of plastics and rubbers. Shawbury, UK: Rapra Publishing; p. 23–106. [Google Scholar]
- Vincent M, Hartemann P, Engels-Deutsch M. 2016. Antimicrobial applications of copper. Int J Hyg Environ Health. 219(7):585–591. [DOI] [PubMed] [Google Scholar]
- [WHO] World Health Organization. 2003. Copper in drinking-water: Background document for preparation of WHO guidelines for drinking-water quality. Geneva: World Health Organization. [Google Scholar]
- Wyllie J. 1957. Copper poisoning at a cocktail party. Am J Public Health. 47(617):1. [Google Scholar]
- Yoshikawa K, Okamura M, Inokuchi M, Sakuragawa A. 2007. Ion chromatographic determination of organic acids in food samples using a permanent coating graphite carbon column. Talanta. 72(1):305–309. [DOI] [PubMed] [Google Scholar]


