Abstract
Background
There is extensive interest in understanding how neighborhood socioeconomic status (nSES) may affect cancer incidence or survival. However, variability regarding items included and approaches used to form a composite nSES index presents challenges in summarizing overall associations with cancer. Given recent calls for standardized measures of neighborhood sociodemographic effects in cancer disparity research, the objective of this systematic review was to identify and compare existing nSES indices studied across the cancer continuum (incidence, screening, diagnosis, treatment, survival/mortality) and summarize associations by race/ethnicity and cancer site to inform future cancer disparity studies.
Methods
Using PRISMA guidelines, peer‐reviewed articles published between 2010 and 2019 containing keywords related to nSES and cancer were identified in PubMed.
Results
Twenty‐four nSES indices were identified from 75 studies. In general, findings indicated a significant association between nSES and cancer outcomes (n = 64/75 studies; 85.33%), with 42/64 (65.63%) adjusting for highly‐correlated individual SES factors (e.g., education). However, the direction of association differed by cancer site, race/ethnicity, and nSES index.
Conclusions
This review highlights several methodologic and conceptual issues surrounding nSES measurement and potential associations with cancer disparities. Recommendations pertaining to the selection of nSES measures are provided, which may help inform disparity‐related disease processes and improve the identification of vulnerable populations in need of intervention.
Keywords: cancer incidence, cancer mortality, cancer survival, health disparities, neighborhood deprivation index, socioeconomic status, systematic review
After a comprehensive systematic review, 24 unique nSES indices were identified with significant associations only observed between nSES, cancer incidence among non‐Hispanic Whites, and cancer survival for select sites. Findings highlight the complex association between nSES and cancer control outcomes and a need for standardized approaches to nSES measurement and variable selection in future studies.
1. INTRODUCTION
In the United States, approximately 40% of men and 38% of women will develop cancer in their lifetime. 1 Abundant research has focused on identifying biological and individual‐level exposures and risk factors for cancer; but recently, there is increasing emphasis on understanding how neighborhood‐level factors, notably neighborhood socio‐economic environment, impact cancer incidence, and mortality. 2 Neighborhood socioeconomic status (nSES), often defined in terms of the economic (e.g., employment, income), physical (e.g., housing/transportation), and social (e.g., poverty, education) characteristics of a place where a person lives, 3 , 4 has been associated with risk for chronic diseases including stroke, 5 coronary heart disease, 6 and select cancer outcomes. 7 Some multilevel conceptual frameworks have been developed to illustrate the various pathways by which nSES can impact cancer outcomes. 2 , 8 , 9 , 10 For instance, low SES neighborhoods often lack adequate health care resources, which can influence behavioral pathways associated with cancer outcomes, including timely receipt of cancer screening or access to quality care related to risk‐reducing interventions (e.g., smoking cessation). 2 , 8 , 9 , 11 , 12 , 13 , 14 , 15 , 16 In the context of these frameworks, nSES has also been shown to impact biologic pathways implicated in cancer under a chronic stress hypothesis. Specifically, studies show that residents from disadvantaged neighborhoods experience greater emotional stress and constant “wear and tear” on the body that can affect cancer initiation and progression 17 , 18 and biologic markers associated with cancer, such as telomere length. 19 Furthermore, these frameworks consider nSES to be an important contributor to race/ethnic disparities often noted in cancer outcomes, given patients of color often disproportionately live in low resource, disadvantaged areas compared to non‐Hispanic White patients (NHW). 2 , 8 , 9 , 20 , 21 Thus, empirical evidence indicates that nSES is important to assess in order to fully understand and ultimately better address cancer health disparities. To evaluate the impact of nSES in relation to various cancer outcomes, researchers often create indices to both define and characterize overall nSES in a single measure. These indices are composite measures that provide a summary score of a neighborhood’s overall employment, education, income, housing, etc. 22 However, numerous nSES indices exist, and the approaches used to operationalize nSES frequently differ across studies. Although nSES indices often comprise similar domains (e.g., income, employment, education, housing) and utilize similar geographic boundaries (i.e., census tracts), the variables used to represent domains differ enough so that one neighborhood may be considered highly deprived by one index but not another. 10 Thus, using different nSES indices complicates the ability to draw meaningful conclusions about nSES as a common risk factor for cancer. Given the lack of consensus regarding appropriate measures of disparity, several national and federal organizations have called for a standardized approach to measuring neighborhood and sociodemographic effects. 23
This review aimed to summarize existing nSES indices in the literature and characterize their associations with outcomes across the cancer continuum (incidence, diagnosis, treatment, mortality) overall, and where possible, by cancer site. In light of studies reporting interactions of race/ethnicity with socioeconomic status on cancer outcomes, 24 associations between nSES indices and cancer outcomes were also examined by race/ethnicity and individual‐level SES to determine if associations vary by race or index used, and to further identify gaps in the literature. Findings from this review will help clarify the potential role of nSES in contributing to cancer outcomes and inform nSES variable selection in future studies.
2. METHODS
We conducted a literature review of peer‐reviewed studies to identify nSES measures that were studied in relation to various cancer outcomes. We used the National Library of Medicine’s PubMed search engine, searching articles published from 2010 to 2019. Boolean operator “AND” was used to identify combinations of search terms including neighborhood, neighborhood environment, social environment, contextual, neighborhood deprivation, neighborhood socioeconomic status, neighborhood SES, area‐based SES, macro environment, and segregation (first terms) combined with terms from the cancer control continuum, cancer + risk, incidence, screening, diagnosis, stage, treatment, survival, and mortality (second terms). A manual search of reference lists from reviews and related articles supplemented the electronic search. We followed Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines for this review (Figure 1). Duplicate manuscripts were deleted. Articles were excluded if they: (1) Did not include a cancer outcome (e.g., studied cancer risk behaviors like smoking); (2) were reviews or theory‐based papers; (3) used nSES measures as a surrogate for individual‐level SES (e.g., used the median household income to represent SES when individual income data were not available); (4) investigated built environment, pollution, or environmental contaminants, not nSES; (5) focused on access to care or supportive care; (6) reported all‐cause mortality, not cancer mortality; (7) were conducted outside the U.S.; (8) investigated broader geographies than census tracts (e.g., county‐level), given prior studies suggest areas larger than a census tract are more susceptible to the modified area unit problem (MAUP) and are likely to result in different associations compared to geographies smaller than a census tract 25 ; or (9) did not report relevant statistics (e.g., effect sizes). We identified 1140 articles through the database search and an additional 10 from reference lists. After a preliminary abstract review, 127 full‐text articles were assessed for eligibility; 52 did not meet inclusion criteria, resulting in 75 studies included in this review. Two primary reviewers collected data from the studies, followed by two secondary reviewers for quality assurance; discrepancies were resolved through discussion with the principal investigator and co‐authors.
FIGURE 1.
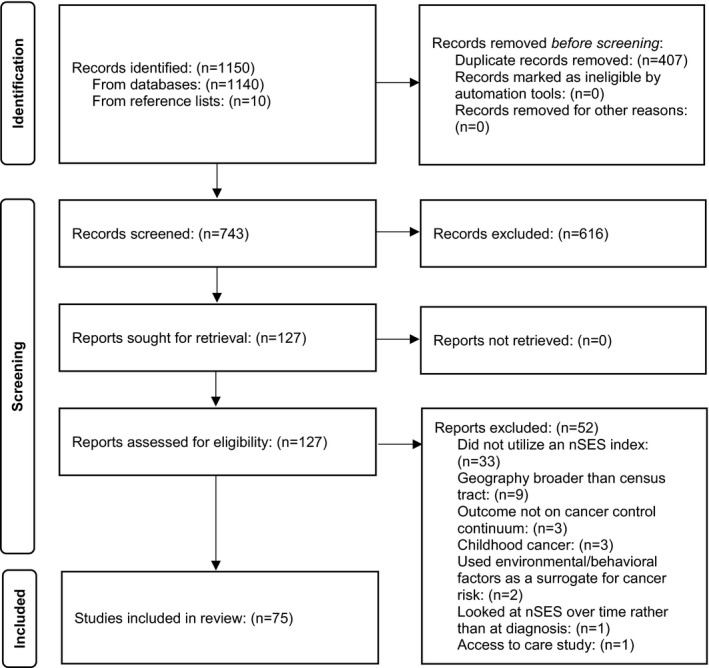
PRISMA flowchart
The studies were categorized based on cancer outcome(s) studied. The overall incidence of each cancer primary was summarized, however, if results were provided for cancer subtypes only (e.g., the incidence of the cardia and non‐cardia gastric cancer rather than overall gastric cancer incidence), the incidence of each subtype was reported. 26 Only two cancer screening studies were identified 27 , 28 across three cancer sites (prostate, colorectal, cervical). Given the small, heterogeneous sample, these results are not shown. Diagnosis studies examined cancer stage, grade, aggressiveness (i.e., in prostate cancer), or hormone receptor status (i.e., in breast cancer). This category was defined, as appropriate for each cancer type, based on the study definition. 3 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 Treatment studies assessed comprehensiveness and time to treatment. Survival studies included an outcome of cancer survival or mortality. nSES indices were defined by either the name given by the original author (e.g., Concentrated Affluence 43 ) or the name of the author who first published a study using that index (e.g., Yost Index 44 ).
Associations between cancer outcomes and nSES were defined as positive, no association, or inverse using study‐reported effect estimates (i.e., odds ratios, incidence rate ratios, hazards ratios, and p‐values (<0.05)). The majority of prior nSES and cancer outcome studies largely rely on reporting statistical significance in terms of p‐values, where p < 0.05 signify statistical significance. Thus, we used this definition, along with the original authors’ own interpretation or designation of statistical significance, to categorize associations as positive, no association or inverse in this study. We summarized only the results of the final multivariate model presented. Effect sizes are summarized in a publicly available database (Table S1; https://github.com/ksorice/nSES‐Systematic‐Review.) We were unable to conduct a meta‐analysis, given differing coding schemes of nSES across studies (i.e., different indices, quartiles vs. quintiles, etc.).
Indices measuring deprivation and disadvantage were reported inversely so all associations reflected nSES consistently (e.g., low neighborhood deprivation/disadvantage = high nSES). Positive associations between nSES and cancer outcomes include higher nSES being significantly associated with higher cancer incidence, more favorable diagnosis (e.g., lower stage/grade), receiving more comprehensive treatment (e.g., lumpectomy plus radiation vs. lumpectomy alone 45 ), and better survival. Because papers often presented multiple sets of results stratified by race, sex, or primary cancer site, and these factors can confound or impact associations between nSES and cancer, studies were additionally assessed for the total number of positive associations, no associations, and inverse associations found overa ll, by cancer site, and by race/ethnic group (White, Black, Asian, Hispanic) summed together to further evaluate nSES associations with cancer outcomes (Tables S2–S6). Most studies are reported in terms of the independent association between nSES and an outcome (i.e., incidence/risk, diagnosis, treatment, survival). If an independent association between nSES and cancer outcome was not available, associations between cancer outcomes and nSES combined with individual SES measures (e.g., nSES/race/education) 46 or other area‐level measures (e.g., nSES/ethnic enclave) 45 were assessed. This study was conducted under Protocol #18‐9015 approved by the Institutional Review Board at Fox Chase Cancer Center.
3. RESULTS
3.1. Overview of nSES Indices
Seventy‐five studies evaluated associations between nSES and cancer control outcomes. A searchable summary of these studies is available online (Table S1). This database allows for comparisons of studies, particularly related to methods and study design that could influence potential associations. Briefly, the majority of studies were cross‐sectional, meaning analysis of nSES was conducted at a single time point (e.g., at the date of diagnosis) (n = 70/75 studies). Five studies were longitudinal, comparing cancer outcomes at different nSES periods (e.g., 1998–2002 vs. 2008–2012). The majority of cross‐sectional studies utilized Surveillance, Epidemiology, and End Result Program (SEER) registry data (n = 55); 24 studies utilized more detailed individual‐level data (beyond age, race/ethnicity, e.g., smoking history, physical activity, alcohol intake, income). In 37 of 75 studies, the main outcome was cancer survival/mortality; cancer incidence was the outcome in 26 studies. Studies ranged across different US states, but the majority were from California (n = 54). Twenty‐four nSES indices were identified (Tables S1 and S2); all were calculated at the census tract level of geography or lower. The Yost Index was most commonly utilized (n = 40 studies), 26 , 31 , 32 , 34 , 35 , 37 , 41 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 followed by the Concentrated Disadvantage Index (n = 6), 33 , 40 , 42 , 78 , 79 , 80 Messer Index (n = 4), 3 , 15 , 36 , 81 and Yang Index (n = 4). 82 , 83 , 84 , 85 Indices were developed using methods including principal components analysis, 3 , 15 , 26 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 40 , 41 , 42 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 factor analysis, 27 , 39 , 91 , 92 , 93 , 94 , 95 principal components analysis plus factor analysis, 96 a priori selection, 29 , 38 , 40 , 78 , 80 , 97 , 98 , 99 and weighted quantile sums 28 that generally characterized indices by eight main domains: income, education, employment, housing, transportation, family structure, demographic data, and other (Table 1). All indices included the income domain, and variables used to represent this domain were relatively consistent (i.e., poverty (n = 18 indices), median household income (n = 11 indices)). Eleven of 24 indices included variables to represent education, employment, and housing, but the variables selected to represent these domains differed. 3 , 15 , 26 , 27 , 29 , 31 , 32 , 34 , 35 , 36 , 37 , 39 , 41 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 81 , 82 , 83 , 84 , 85 , 87 , 88 , 89 , 91 , 92 , 93 , 96 Thirteen indices included family structure, often represented by variables related to female‐headed households or single head of households with children. 3 , 15 , 27 , 33 , 36 , 38 , 39 , 40 , 42 , 78 , 79 , 80 , 81 , 86 , 87 , 88 , 89 , 91 , 94 , 95 , 96 , 98 Eight indices included transportation, which was consistently represented by variables related to vehicle ownership. 27 , 86 , 87 , 88 , 89 , 90 , 91 , 98 , 99
TABLE 1.
Summary of nSES indices identified
| Index/Author a (variable selection method) | Domains | |||||||
|---|---|---|---|---|---|---|---|---|
| Income | Education | Employment | Housing | Transportation | Family structure | Demographic | Other | |
| Area deprivation index 131 (factor analysis) |
(1) median family income; (2) income disparity; (3) % families below the poverty level; (4) % population <150% of the poverty threshold |
(1) population aged >25 years with <9 years of education; (2) population aged >25 years with at least a high school diploma |
(1) employed persons aged >16 years in white collar occupations; (2) civilian labor force population aged >16 years unemployed |
(1) median home value; (2) median gross rent; (3) median monthly mortgage; (4) owner occupied housing units; (5) % households with more than one person per room |
(1) % households without a motor vehicle | (1) % single‐parent households with children aged <18 years | — | — |
| Banegas index 29 (a priori) |
(1) household income; (2) poverty |
(1) education |
(1) occupation; (2) unemployment |
(1) rent; (2) house values |
— | — | — | — |
| Beyer index 97 (a priori) | (1) median household income | (1) proportion without a high school diploma | (1) proportion unemployed | — | — | — | — | — |
| Concentrated affluence 43 (a priori) | (1) % families with incomes above $75,000 (2000 Census period) or $50,000 (1990 Census period) | (1) % adults with college education | (1) % civilian labor force employed in professional/ managerial occupations | — | — | — | — | — |
|
Concentrated disadvantage (2 variables) 30 (PCA) b |
(1) % below the poverty line | — | (1) % unemployed | — | — | — | — | — |
| Concentrated disadvantage (6 variables) 132 (PCA) c |
(1) % below the poverty line; (2) % receiving public assistance income |
— | (1) % unemployed | — | — | (1) % female‐headed families |
(1) % aged <18 years; (2) % Black |
— |
| Coogan index 133 (PCA + factor analysis) |
(1) median household income; (2) % households receiving interest, dividend or net rental income |
(1) % adults aged ≥25 years that have completed college | (1) % employed persons aged ≥16 years that are in occupations classified as managerial, executive, or professional specialty | (1) median housing value | — | (1) % families with children not headed by single female | — | — |
| Diez‐Roux index 134 (factor analysis) |
(1) log of median household income; (2) % households receiving net rental, interest or dividend income |
(1) % aged ≥25 years who completed high school and who completed college | (1) % employed aged ≥16 years in professional and managerial occupations | (1) log of median value of owner‐occupied housing units | — | — | — | — |
| Doubeni index 86 (PCA) |
(1) % below 1999 federal poverty levels; (2) % on public assistance; (3) % annual income of <$30,000 |
(1) % less than high school education |
(1) % unemployed; (2) % men in managerial jobs; (3) % women in managerial jobs |
— | (1) % no car | (1) % headed by a female with dependent children | (1) % non‐Hispanic Black | — |
| Dubowitz index 135 (factor analysis) |
(1) % below the poverty line; (2) % receiving public assistance; (3) median household income |
(1) % aged ≥25 years with less than a high school education | (1) % male unemployment | — | — | (1) % households with children that are headed only by a female | — | — |
| ICE ‐ Income 136 , 137 | (1) (n of persons in high‐income households)—(n of persons in low—income households)/total population with household income data | — | — | — | — | — | — | — |
| Johnson economic deprivation index 94 (factor analysis) |
(1) % below the poverty level; (2) % on public assistance |
— | — | — | — |
(1) % female head of house with children; (2) % married |
— | — |
| Lian index 88 (PCA) |
(1) % receiving public assistance; (2) % low income; (3) % income no less than 400% of the US median household income; (4) median household income in 1999; (5) % below federal poverty line |
(1) % less than a high school education; (2) % with a college degree |
(1) % unemployed males aged ≥20 years; (2) % unemployed females aged ≥20 years; (3) % white collar; (4) % with low social class |
(1) % households with ownership; (2) % vacant households; (3) % no less than 1 person per room; (4) median value of all owner‐occupied households; (5) % living in the same residence since 1995 |
(1) % households without a car | (1) % female‐headed households with dependent children |
(1) % non‐Hispanic Black; (2) % Hispanic; (3) % residents aged ≥65 years |
— |
| Material deprivation index 138 (a priori) | (1) % living below the poverty level | — | (1) % aged ≥16 years unemployed | (1) % living in a crowded residence (more than 1 person per room) | (1) % households with no vehicle available | — | — | (1) % households with no telephone available |
| Messer index 22 (PCA) |
(1) % poverty; (2) % on public assistance; (3) households earning $30,000 per year estimating poverty |
(1) % earning less than a high school education |
(1) % males in management/ professional occupations; (2) % unemployed |
(1) % crowded housing | — | (1) % female headed households with dependents | — | — |
|
Mojica index 38 (a priori) |
(1) population receiving public assistance | (1) % without a high school diploma | (1) male population aged ≥16 who are unemployed | — | — | (1) households with children headed by females | — | — |
| Neighborhood deprivation index 139 (PCA) |
(1) % with income below the 1999 poverty status; (2) % income <$30 000 per year (3) % on public assistance income |
(1) % did not graduate high school (age ≥25 years) | (1) % males and females who are unemployed;(2) % males in professional occupations |
(1) % housing units with ≥1 occupant per room; (2) % occupied housing units with renter/owner costs >50% of income; (3) median household value |
(1) % households with no car | (1) % female headed households with dependent children | — | — |
| Palmer index 39 (factor analysis) |
(1) median household income; (2) % households receiving interest, dividend, or net rental income |
(1) % aged ≥25 years that have completed college | (1) % employed aged ≥16 years that are in occupations classified as managerial, executive, or professional specialty | (1) median housing value | — | (1) % families with children not headed by a single female | — | — |
| Reitzel index 98 (a priori) | (1) % income below the poverty level in 1999 | (1) % aged ≥25 years with less than high school degree/GED | (1) % aged ≥16 years unemployed | — | (1) % households with no vehicle available for use | (1) % single parent households | — | — |
| Social deprivation index 140 (factor analysis) | (1) % in poverty | (1) % less than high school diploma | (1) % nonemployed |
(1) % crowding; (2) % renter‐occupied housing |
(1) % no car ownership | (1) % single parent households | — | — |
| Wheeler index 28 (weighted quantile sum regression) |
(1) median household income; (2) per capita income; (3) % households not on public assistance; (4) % families with children <18 years not in poverty; (5) Gini index of income equality |
(1) % aged ≥25 years with a bachelor's degree | — |
(1) % owner occupied housing; (2) % not vacant housing units; (3) median gross rent; (4) % households with mortgages |
— | — | (1) % White | — |
| Yang index 141 (PCA) |
(1) % above 200% poverty line; (2) median household income |
(1) Liu Education Index (% aged ≥25 years with college, high school and less than high school) |
(1) % persons with a blue collar job; (2) % persons employed |
(1) median rent; (2) median value of owner‐occupied housing units |
— | — | — | — |
| Yost index 44 (PCA d ) |
(1) median household income; (2) % below 200% of the poverty line |
(1) Liu Education Index (% aged ≥25 years with college, high school and less than high school) |
(1) proportion with a blue collar job; (2) % aged ≥16 years in the workforce without a job |
(1) median rent; (2) median house value |
— | — | — | — |
| Zhang index 90 (PCA) |
(1) % income below poverty; (2) % income <$22,500 (1990) or <$30,000 (2000); (3) % on public assistance |
(1) % with less than a high school education | (1) % unemployed | — | (1) % households without a car | — | — | — |
Indices that were created for use in one study only are named after the first author of the article in this table.
PCA, principal components analysis.
One paper utilized the six‐variable Concentrated Disadvantage Index but removed two of the variables (% households receiving public assistance income and % Black). 42
The ICE—Income Index is described above. Additional ICE indices include ICE—Race/Ethnicity (n of “White non‐Hispanic” persons)‐(n of “black non‐Hispanic” persons)/n of persons with race/ethnicity data and ICE—Income + Race/Ethnicity (n of “White non‐Hispanic” high‐income persons)−(n of “black alone” low income persons)/n of persons with race/ethnicity and household income data.
3.2. nSES associations by cancer outcomes and cancer sites
We first investigated whether statistically significant positive or inverse associations were reported for each index and cancer control outcome (incidence, diagnosis, treatment, survival/mortality). However, this analysis was limited because many indices were investigated in only one study. For indices used in more than one study and/or for more than one cancer outcome, there did not appear to be a consistent association between nSES and any of the cancer outcomes (Table S1). To further investigate whether potential patterns exist, associations were summed across all indices for each cancer outcome (Figure 2). No consistent association between nSES and overall cancer incidence/risk was observed. In contrast, studies predominately reported either a positive or no association for cancer diagnosis, treatment, and survival. These patterns generally remained: (1) Even when nSES indices with similar domains (n = 11 indices with income, education, employment, and housing domains) were summed and compared across cancer outcomes; (2) when restricting the analysis to only studies conducted in California (Table S3); or (3) when stratifying by only those studies that controlled for individual SES factors (e.g., education) or covariates such as smoking. Figures S1 and S2 show associations for incidence and survival by individual‐level adjustment (e.g., age), individual SES adjustment (e.g., race/ethnicity), and adjustments with covariates outside those included in SEER data (e.g., income, smoking).
FIGURE 2.
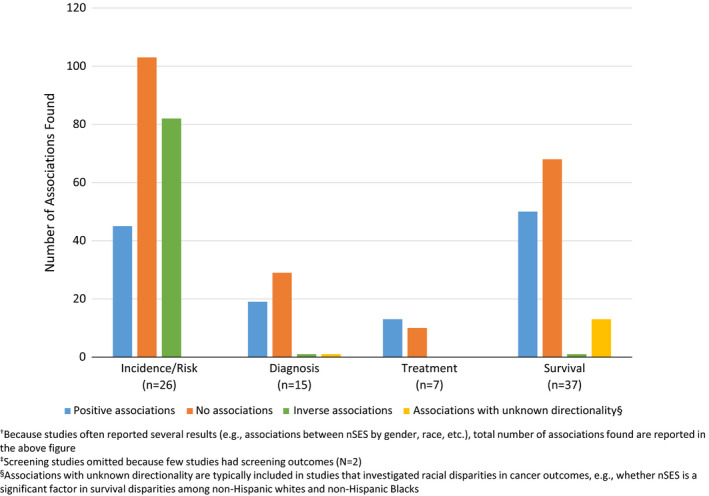
Number of associations† found across studies by cancer control outcome‡
A closer analysis of nSES associations with cancer incidence was conducted by cancer site. For breast cancer (n = 6 studies), 34 , 39 , 52 , 53 , 74 , 92 thyroid cancer (n = 1), 60 and melanoma (n = 1), 31 positive associations with nSES were commonly reported (breast: 11 positive associations/16 total 34 , 52 , 53 , 74 ; melanoma/thyroid: 2 positive associations/2 total 31 , 60 ) (Table S4). Inverse associations between nSES and incidence of cervical (n = 3 studies; 20 inverse associations/27 total) 51 , 58 , 74 and lung (n = 7 studies; 70 inverse associations/123 total) 73 , 74 , 82 , 85 , 89 , 91 , 92 cancer were more likely to be reported. No consistent associations emerged for prostate (n = 3 studies), 36 , 74 , 92 colorectal (n = 6), 67 , 74 , 79 , 86 , 90 , 92 gastric (n = 2), 26 , 76 head and neck (n = 2), 51 , 57 and liver (n = 1) 47 cancers (Table S4). No association between nSES indices and anal (n = 1 study; 9 no associations/10 total) 51 and lymphoid (n = 2 studies; 62 no associations/86 total) 50 , 75 cancers were reported, but study number and sample sizes were low (Table S1).
Next, we explored associations of nSES with cancer diagnosis characteristics (e.g., stage, grade, aggressiveness, hormone receptor status). No consistent pattern emerged for any cancer, except colorectal cancer, where no association was most commonly reported (n = 1 study; 5 no associations/5 total). 38 For cancer treatment, positive associations for lymphoid (n = 1 study; 4 positive associations/4 total) 61 and lung (n = 1 study; 2 positive associations/2 total) 94 cancers were observed. No clear patterns emerged in breast (n = 3 studies), 45 , 54 , 99 ovarian (n = 1), 80 and prostate (n = 1) 95 cancers.
nSES was positively associated with survival in liver (n = 1 study; 4 positive associations/5 total), 66 lymphoid (n = 2 studies; 2 positive associations/2 total), 62 , 71 head and neck (n = 2 studies; 5 positive associations/6 total), and ovarian (n = 1 study; 2 positive associations/2 total) 78 cancers. No association between nSES and cancer survival/mortality was commonly reported in breast (n = 17 studies; 39 no associations/59 total) 10 , 29 , 33 , 35 , 37 , 41 , 46 , 48 , 54 , 59 , 64 , 65 , 66 , 70 , 77 , 83 , 87 and kidney (n = 1 study; 4 no associations/5 total) 66 cancers. No clear pattern of association between nSES and cancer survival/mortality was observed for prostate (n = 4 studies), 56 , 66 , 69 , 83 lung (n = 5), 66 , 68 , 83 , 84 , 94 colorectal (n = 4), 66 , 72 , 83 , 88 and thyroid (n = 1) 63 cancers (Table S4).
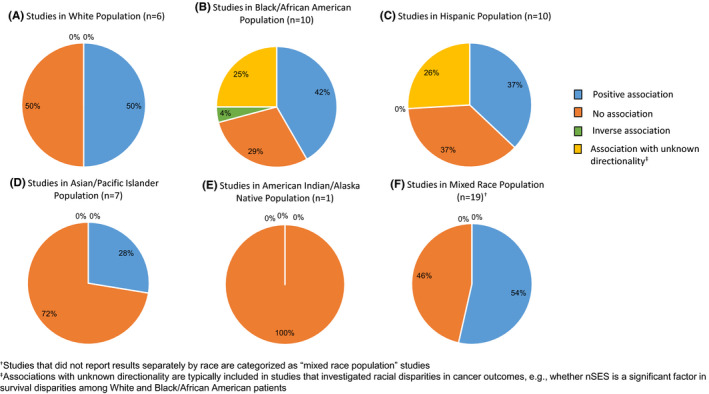
3.3. n SES associations by race/ethnicity
A number of studies have observed that nSES and race/ethnicity are correlated and may independently and jointly impact cancer outcomes. Further, racial disparities often exist and continue to persist, even across low and high nSES. 24 , 100 This suggests that factors other than nSES play a role in contributing to minority health and health outcomes. Therefore, we examined nSES associations separately by racial/ethnic group to ensure associations were not being missed. Sixty percent (45/75 studies) reported associations by race/ethnicity. 46 , 66 , 85 Among non‐Hispanic White cases (NHW; n = 12 studies), a trend emerged showing a clear inverse association between nSES and cancer incidence/risk (25 inverse associations, 5 no associations, 5 positive associations) (Figure 3). However, the protective benefits of nSES in relation to cancer incidence are diminished among other racial/ethnic groups.
FIGURE 3.
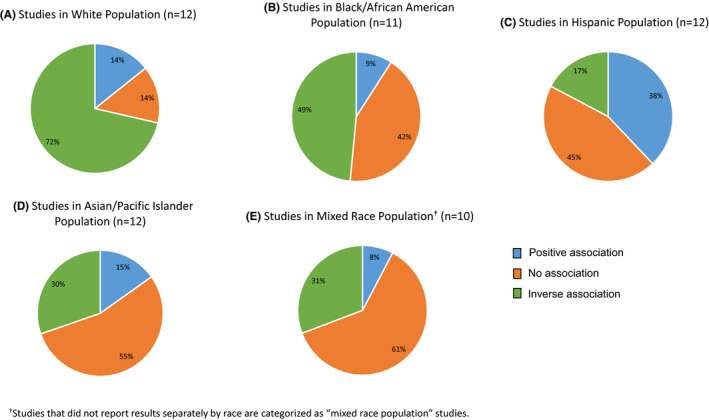
Associations between nSES and cancer risk/incidence by racial/ethnic group
A similar analysis was conducted for cancer survival, but a consistent pattern of association was not observed (Figure 4; Table S5). Across each race/ethnic group, approximately half of the findings reported no association and slightly fewer than half reported a positive association of higher nSES/improved survival.
FIGURE 4.
Associations between nSES and cancer survival by racial/ethnic group
We also conducted additional sub‐analyses to examine associations between nSES and cancer site‐specific outcomes by race/ethnicity (Table S6). The number of studies was generally too small to analyze for most cancers, but the following trends were observed. Among Hispanic cases, nSES was positively associated with breast cancer incidence (n = 4 studies; 5 positive associations/5 total) but inversely associated with cervical cancer incidence (n = 3 studies; 5 inverse associations/5 total). For lung cancer incidence (n = 7 studies), inverse associations were consistently reported for NHW cases (n = 12 inverse associations/14 total), Black cases (n = 10 inverse associations/14 total), and Asian cases (n = 7 inverse associations/12 total). For colorectal cancer incidence (n = 6 studies), nSES was inversely associated in NHW cases (4 inverse associations/4 total) but positively associated in Hispanic cases (2 positive associations/2 total). For head and neck cancer incidence, inverse associations were reported in NHW cases (n = 1 study; 5 inverse associations/6 total); whereas no associations were found among Asian cases (n = 2 studies; 11 no associations/18 total).
4. DISCUSSION
In this review, we found the relationship between nSES indices and cancer outcomes varied by cancer site and race/ethnicity. In general, nSES was inversely associated with cancer incidence/risk among NHW cases, but this association was less consistent in other race/ethnic groups. nSES was positively associated with cancer survival only for select cancers. Among more common cancers (prostate, colorectal, lung), no clear patterns emerged. These findings highlight the complex association between nSES and cancer control outcomes.
4.1. n SES indices
Twenty‐four nSES indices were identified, and the construction of nSES indices varied across studies. The majority included domains related to income, education, employment, and housing and utilized similarly representative variables. Notably, the relationship between nSES and cancer outcomes did not necessarily change by nSES variable selection. When we compared cancer incidence and survival findings of nSES indices that only included these four domains to those that also included transportation, family structure, additional demographics, etc., we did not see different associations across indices. This suggests nSES measures are similar in what they capture, and thus it might not matter which nSES measure is used in association studies. However, the majority of studies did not compare across nSES indices within the same study and selection of standard nSES measures may be preferred moving forward to allow for consistency and comparability across studies. Furthermore, the majority of studies did not consider the geographic distribution of the disease to account for the possibility that nearby neighborhoods are more likely to be similar to one another. Recent geospatial cluster analyses show some nSES measures may be more effective than others at explaining the geospatial distribution of cancer within a particular state, 10 and that differences in nSES by neighborhood or geographic location, including living in an urban versus rural area, 101 could affect cancer mortality. 102 This suggests additional studies that consider spatial associations, urbanicity, and evaluate more than one existing nSES index are warranted before particular nSES measures can be recommended as a standard (Table 3).
TABLE 3.
Recommendations for future association studies in cancer to aid in variable selection and studies of health disparities
|
4.2. Cancer incidence
In the aggregate, there were no consistent relationships between nSES indices and overall cancer incidence. Findings for cancer incidence remained unchanged even when restricting analyses to studies with additional adjustments for individual‐level factors, including education and income, which are known to be associated with both nSES and cancer outcomes (Table S1). 36 , 39 , 52 , 53 , 79 , 92 Because combining all cancer types could be masking potential associations, site‐specific analyses subsequently revealed positive associations of nSES with breast, thyroid, and melanoma cancer incidence. Analyses by race/ethnicity also demonstrated an inverse association of nSES with cancer incidence for NHW cases, reflecting a protective role in this population. This is consistent with prior research demonstrating that neighborhood disadvantage can adversely impact cancer risk through various pathways, including limited access to high‐quality diet, fewer opportunities for outdoor recreation and physical activity, and environmental exposures. 103 , 104
Although the sample size and the number of studies were limited for race/ethnicity‐specific analyses, our findings revealed positive associations of nSES with breast and colorectal cancer incidence among Hispanic cases. Living in higher SES neighborhoods may reflect greater acculturation and adoption of U.S. lifestyle behaviors. Acculturation has been correlated with key breast cancer risk factors including later age at first birth, having fewer children, shorter duration of breastfeeding, and increased alcohol consumption. 105 , 106 In contrast, an inverse association emerged for nSES and cervical cancer incidence among Hispanic women. In this context, greater acculturation may be beneficial as it has been associated with greater cervical cancer screening uptake. 107
An inverse association was also observed between nSES and lung cancer incidence among NHW, Black, and Asian cases. Given the impact of environmental factors (e.g., smoking, 89 air pollution exposure 108 ) on this cancer, it is likely that low nSES correlates with greater exposure to these risk factors, but additional studies are warranted to tease these associations apart.
Overall, the stratified findings indicate the benefits of higher nSES for cancer incidence can be attenuated for racial and ethnic minoritized groups, particularly by cancer site. There may be several explanations for this finding. First, it is well‐documented that systemic and structural racism and discrimination against members of racial/ethnic minoritized groups occurs in healthcare, 109 , 110 , 111 , 112 regardless of the patient’s socioeconomic status or neighborhood residence. These biases, in turn, impact patient‐provider interactions, decision making and access to treatments, and healthcare utilization, 109 , 110 , 111 , 112 all of which have subsequent downstream effects on health outcomes. Further, discrimination against race/ethnic groups can also influence place of residence. Choice of residence is not always voluntary and may be driven by financial resources 113 , 114 or policies. Over several decades, policies on various scales intentionally created racial segregation through housing development and financial programs and shaped the demographics of the neighborhoods (e.g., red‐lining). 115 These red‐lining policies are examples of structural racism that could be contributing to the attenuation of cancer incidence outcomes in race/ethnic groups from both high and low SES areas. 116 , 117
Second, nativity may play an important role as well. Ethnically dense neighborhoods (e.g., ethnic enclaves), some of which are low‐SES neighborhoods with large immigrant populations, report better health behaviors associated with cancer, including diet. 118 As a result, the maintenance of healthy behaviors may help offset some of the adverse effects of low nSES, particularly among immigrant and poorer populations. Third, the frequency of nSES variables or domains used to represent common components in nSES indices may differ across race/ethnic groups, subgroups, and geographic location in a way that can impact disease associations. For instance, a higher proportion of Black and Hispanic patients compared to NHW patients often live in neighborhoods with lower income and higher poverty; thus, the impact of nSES indices may be attenuated when comparing within versus across race/ethnic groups. As such, it should be noted that studies of minority populations often have smaller sample sizes than studies of NHW populations, which could adversely influence the ability to detect statistically significant associations. Finally, previous studies have shown that different nSES domains may have differential effects by race/ethnicity. For instance, an empiric study of independent measures of nSES found that economic (e.g., income, poverty) and transportation measures were associated with advanced prostate cancer in White men, whereas housing measures were associated with advanced disease in Black men. 119 These findings suggest indices that equally‐weight nSES domains may be masking important neighborhood effects in racial/ethnic minoritized populations; 120 however, additional studies are needed. In particular, standardized methodologic assessments of existing indices are warranted, particularly before creating potentially new indices. More specifically, pooled analyses from multiple states (to increase sample size) that evaluate more than one nSES index within a single study AND that evaluate individual domains within that index, overall and by race/ethnicity, would be suggested to determine what indices or domains may be impacting observed associations. These analyses should further include adjustments for nativity when these data are available to help elucidate true nSES effects, which will aid in the standardized selection of nSES indices, as well as provide insights into drivers of disparities in cancer incidence (Table 2).
TABLE 2.
Summary of geographic locations and cancer control outcomes studied within nSES indices
| Index/author | States/Regions | Total studies (N) a | Number of studies by cancer control outcome | ||||
|---|---|---|---|---|---|---|---|
| Risk/incidence (N) | Screening (N) | Diagnosis (N) | Treatment (N) | Survival/mortality (N) | |||
| Yost index 44 | CA (n = 37); SEER‐18 participating regions (n = 1); National (n = 1) | 40 | 16 | — | 6 | 3 | 22 |
| Concentrated disadvantage (6 variables) 132 | IL (n = 4); LA (n = 2) | 6 | 1 | — | 3 | 1 | 2 |
| Messer index 22 | AR, KY, MS, SC, TN, VA, WV (n = 1 each); CA, MI, NJ (n = 2 each); FL, GA, LA, NC, PA (n = 3 each) | 4 | 1 | — | 2 | — | 2 |
| Yang index 141 | CA | 4 | 2 | — | — | — | 2 |
| Concentrated affluence 43 | IL | 3 | — | — | 1 | 1 | 1 |
| Diez‐Roux index 134 | WA | 2 | 1 | — | — | — | 1 |
| Lian index 88 | CA, FL, GA, LA, MI, MO, NC, NJ, PA (n = 1 each) | 2 | — | — | — | — | 2 |
| Area deprivation index 131 | OH | 1 | 1 | — | — | — | — |
| Banegas index 29 | CA | 1 | — | — | 1 | — | 1 |
| Beyer index 97 | National (100 metropolitan areas) | 1 | — | — | — | — | 1 |
| Concentrated disadvantage (2 variables) 30 | IL | 1 | — | — | 1 | — | — |
| Coogan index 133 | Southeastern US (AL, AR, FL, GA, KY, LA, MS, NC, SC, TN, VA, WV) | 1 | — | — | — |
— |
1 |
| Doubeni index 86 | 6 US states (CA, FL, LA, NJ, NC, PA) or 2 metropolitan areas (Atlanta, Georgia; Detroit, Michigan) | 1 | 1 | — | — | — | — |
| Dubowitz index 135 | PA | 1 | — | — | — | 1 | — |
| ICE ‐ income 136 , 137 | NJ | 1 | — | — | — | — | 1 |
| Johnson economic deprivation index 94 | GA | 1 | — | — | — | 1 | 1 |
| Material deprivation index 138 | MI | 1 | — | — | — | 1 | — |
| Mojica index 38 | CA | 1 | — | — | 1 | — | — |
| Neighborhood deprivation index 139 | AL, AR, FL, GA, KY, LA, MS, NC, SC, TN, VA, WV | 1 | 1 | — | — | — | — |
| Palmer index 39 | CA, GA, IL, MA, NJ, NY, VA, Washington DC | 1 | 1 | — | 1 | — | — |
| Reitzel index 98 | LA; TX | 1 | — | — | — | — | 1 |
| Social deprivation index 140 | VA | 1 | — | 1 | — | — | — |
| Wheeler index 28 | MN; WI | 1 | — | 1 | — | — | — |
| Zhang index 90 | CA, FL, GA, LA, MI, NJ, NC, PA | 1 | 1 | — | — | — | — |
Because several studies utilized the same nSES index for multiple cancer control outcomes, the number of studies listed across the cancer control outcomes may not add up to the total studies.
4.3. Cancer survival
Overall, no clear pattern of associations of nSES with diagnosis or treatment emerged, perhaps due to the heterogeneity of disease staging approaches across cancer sites. nSES was positively associated with cancer survival for selected cancers (liver, lymphoid, head, and neck, ovarian). Previous literature has reported higher individual‐level SES (e.g., education, income, insurance coverage) often correlates with higher nSES. 13 As a result, residents of higher SES neighborhoods may have greater access to health care resources and healthier foods, which could lead to positive associations with cancer outcomes because individuals from high SES backgrounds and environments are more likely to receive timely cancer treatment and follow‐up care. 121 , 122
On the other hand, no association between nSES and cancer survival was observed for breast cancer, and a clear association could not be established for prostate, lung, and colorectal cancers. This could be due to the small number of studies conducted within these sites, or it could be reflective of studies being conducted across multiple‐year ranges (e.g., breast cancer survival studies ranged from 1988 to 2014) or in a single state (e.g., 14/17 breast cancer survival studies were conducted in California). Notably, there was no clear association between nSES and different cancer outcomes within one cancer. For instance, nSES was positively associated with breast cancer incidence, but generally not breast cancer survival. This suggests that nSES exerts differential effects, not just by cancer site, but within a cancer site, across the disease continuum. Thus, future studies are needed to investigate the longitudinal trajectory of nSES on outcomes from cancer incidence to survival.
4.4. Limitations and additional recommendations for future studies
Several limitations should be noted (Table 3). First, given the heterogeneity in defining nSES and cancer outcomes themselves, potential publications may have been missed, despite our comprehensive search strategy. Second, publication bias can result from the tendency of authors to only publish studies with significant results and is a cited limitation of most systematic reviews. Although the inclusion of grey literature is sometimes suggested to address this bias and aid in validating the results of a literature search of published research, there are also disadvantages in that these sources are often not peer‐reviewed, may not report relevant information, and have the potential for introducing additional bias. To address the question of when grey literature should be included, Benzies et al provided a checklist to help guide authors’ decisions. 123 Using this checklist, we determined that the availability of studies on the impact of nSES on cancer outcomes is of high volume and similar quality, with studies often utilizing similar data resources (e.g., cancer registry and U.S. Census variables for the general of nSES indices) for study measures. Through these steps, we concluded that the focus on published, peer‐reviewed data was justified. Further, the results of this systematic review showing variation in associations overall (including many reports of null associations) and by factors known to affect associations with nSES and cancer (e.g., race/ethnicity in SEER registry studies), suggest publication bias may be minimized in this body of literature. Notably, in studies where more detailed risk factors for cancer were available (e.g., studies that utilized cohort data with detailed smoking data), patterns and associations with cancer overall and by race/ethnicity continued to remain. Third, the majority of nSES indices were constructed at the census tract level, which is considered to be an adequate geographic level with which to look for associations with disease; 25 but research on the incorporation of daily activity spaces, of how people in a particular neighborhood move and interact with their local geographies, is also needed. 124 , 125 This is because while the use of administrative boundaries, like census tracts, allows for consistency in reporting across US studies, they may not adequately represent where people spend their time 7 or what neighborhood environments they are exposed to. Fourth, the majority of studies reviewed were conducted in California, a national leader in the evaluation of nSES and cancer outcomes which can serve as a model for other states. 126 However, in order to move towards standardized nSES index measures, studies across more US states are needed, given that the variation in nSES indices and their associated variables likely differ by geography. 102 Fifth, the majority of reviewed studies utilized State Cancer Registries. Increasing the number of state‐specific analyses within and across cancer sites could help clarify the role of nSES in cancer outcomes. Given cancer registry and U.S. Census data are readily available, efforts to support investigations into the role of nSES in cancer outcomes within and across states are warranted and in line with initiatives to incorporate standardized disparity measures in future cancer studies. More accessible mechanisms for investigators to link their own custom nSES indices to multistate cancer registry datasets like SEER or North American Association of Central Cancer Registries (NAACCR) data would help advance investigations into the role of nSES in cancer outcomes.
To further elucidate potential etiologic effects, additional studies are needed to investigate nSES associations with cancer screening and diagnosis. This is crucial for determining where along the continuum nSES may exert effects. For example, it is possible that nSES may have more of an impact on cancer development and stage at diagnosis, due to differential exposures and access to care, but it may have less of an impact on survival, particularly among patients diagnosed with metastatic disease. 127 More generally, studies that investigate the role of nSES across the continuum for specific cancers and that can incorporate relevant individual‐level behaviors, race/ethnicity, and clinical factors are needed. This would involve the extension of current nSES research beyond just the use of registry data to incorporate nSES data with electronic medical records, and other available cohort and case‐control studies.
To expand on etiologic work, studies investigating the effect of residential history (e.g., change in nSES over time) are beginning to emerge. The role of residential history may be particularly relevant to the Hispanic paradox and socio‐spatial mobility (i.e., movement between neighborhoods with foreign‐born and U.S.‐born residents or various nSES). Prior studies have just utilized nSES at the time of diagnosis, without consideration of lifetime nSES exposures, which may change over time. 128 , 129 More recent studies have shown that not only does residential history vary by race/ethnicity, but also that the pattern of nSES moves (e.g., moving from high to low nSES areas) may influence cancer survival outcomes. 128 , 129 , 130 Many of these studies utilize poverty as the main measure of nSES change, but the differential pattern of findings by race/ethnicity in the present study suggests that other measures, including change in segregation or movement in/out of ethnic enclaves over time, should also be explored. This suggestion is consistent with our recommendation to evaluate multiple nSES indices in state/national studies going forward, given the differences noted by race/ethnicity and geographic levels, in order to aid in more standardized variable selection. To further support etiologic investigations, changes in nSES and length of time spent in an unfavorable nSES environment should continue to be explored, given that chronic exposure to unfavorable circumstances over a long time period (10 years or more) may be needed for chronic disease development, like cancer. 128 , 129
5. CONCLUSION
This comprehensive review yielded a searchable, publically available database that can be used by researchers designing future studies centered on nSES and cancer. These findings highlight methodologic and conceptual approaches surrounding the measurement of nSES that can inform nSES variable selection in future studies and help clarify its role in contributing to cancer disparities. The use of different nSES indices (and different variables to form these indices) across geographic locations, study cohorts, cancer sites, and outcomes complicates the field’s ability to draw meaningful conclusions about nSES as a standard risk factor for cancer outcomes. Given the lack of consensus regarding appropriate measures of disparity, including optimal variables to include in index construction, this study has recommended approaches for evaluating different nSES measures within and across cancer sites, overall and by race/ethnic group, utilizing additional state/national cancer registries to help standardize variable selection in future studies. Utilization of a standard nSES index would aid in etiologic and intervention research related to cancer health disparities. Furthermore, this study highlights the need for additional studies in population and clinical datasets that couple nSES measures with more detailed clinical and behavioral variables to enable the evaluation of the true impact of nSES on cancer health disparities.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DATA SHARING AND ACCESSIBILITY
The data underlying this article are available in Supporting Files.
Supporting information
Figure S1.
Figure S2.
Table S1.
Table S2.
Table S3.
Table S4.
Table S5.
Table S6
Sorice KA, Fang CY, Wiese D. Systematic review of neighborhood socioeconomic indices studied across the cancer control continuum. Cancer Med. 2022;11:2125–2144. doi: 10.1002/cam4.4601
Funding information
This research was supported by grants to SML from NCI/NIH (P30CA06927), ACS (131618‐MRSG‐18‐098‐01‐CPHPS), and DOD (W81XWH‐17‐1‐0276).
DATA AVAILABILITY STATEMENT
The data underlying this article are available in Supporting Files (https://github.com/ksorice/nSES‐Systematic‐Review).
REFERENCES
- 1. American Cancer Society . Cancer facts & figures 2018. American Cancer Society; 2018. [Google Scholar]
- 2. Lynch SM, Rebbeck TR. Bridging the gap between biologic, individual, and macroenvironmental factors in cancer: a multilevel approach. Cancer Epidemiol Biomarkers Prev. 2013;22(4):485‐495. doi: 10.1158/1055-9965.epi-13-0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zeigler‐Johnson C, Tierney A, Rebbeck TR, Rundle A. Prostate cancer severity associations with neighborhood deprivation. Prostate Cancer. 2011;2011: 9. doi: 10.1155/2011/8462639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010;1186(1):125‐145. doi: 10.1111/j.1749-6632.2009.05333.x [DOI] [PubMed] [Google Scholar]
- 5. Howard VJ, McClure LA, Kleindorfer DO, et al. Neighborhood socioeconomic index and stroke incidence in a national cohort of blacks and whites. Neurology. 2016;87(22):2340‐2347. doi: 10.1212/wnl.0000000000003299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pollack CE, Slaughter ME, Griffin BA, Dubowitz T, Bird CE. Neighborhood socioeconomic status and coronary heart disease risk prediction in a nationally representative sample. Public Health. 2012;126(10):827‐835. doi: 10.1016/j.puhe.2012.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gomez SL, Shariff‐Marco S, DeRouen M, et al. The impact of neighborhood social and built environment factors across the cancer continuum: Current research, methodological considerations, and future directions. Cancer. 2015;121(14):2314‐2330. doi:10.1002/cncr.29345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alvidrez J, Castille D, Laude‐Sharp M, Rosario A, Tabor D. The National Institute on minority health and health disparities research framework. Am J Public Health. 2019;109(S1):S16‐S20. doi: 10.2105/AJPH.2018.304883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Warnecke RB, Oh A, Breen N, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98(9):1608‐1615. doi: 10.2105/AJPH.2006.102525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wiese D, Stroup AM, Crosbie A, Lynch SM, Henry KA. The impact of neighborhood economic and racial inequalities on the spatial variation of breast cancer survival in New Jersey. Cancer Epidemiol Biomarkers Prev. 2019;28(12):1958‐1967. doi: 10.1158/1055-9965.Epi-19-0416 [DOI] [PubMed] [Google Scholar]
- 11. Byers TE, Wolf HJ, Bauer KR, et al. The impact of socioeconomic status on survival after cancer in the United States: findings from the National Program of Cancer Registries Patterns of Care Study. Cancer. 2008;113(3):582‐591. doi: 10.1002/cncr.23567 [DOI] [PubMed] [Google Scholar]
- 12. Carpenter WR, Howard DL, Taylor YJ, Ross LE, Wobker SE, Godley PA. Racial differences in PSA screening interval and stage at diagnosis. Cancer Causes Control. 2010;21(7):1071‐1080. doi: 10.1007/s10552-010-9535-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hussein M, Diez Roux AV, Field RI. Neighborhood socioeconomic status and primary health care: usual points of access and temporal trends in a major US urban area. J Urban Health. 2016;93(6):1027‐1045. doi: 10.1007/s11524-016-0085-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stock CE, Ellaway A. Neighbourhood structure and health promotion: an introduction. In: Stock C., Ellaway A. eds Neighbourhood Structure and Health Promotion. Springer; 2013:1‐7. doi: 10.1007/978-1-4614-6672-7_1 [DOI] [Google Scholar]
- 15. Warren Andersen S, Blot WJ, Shu XO, et al. Associations between neighborhood environment, health behaviors, and mortality. Am J Prevent Med. 2018;54(1):87‐95. doi: 10.1016/j.amepre.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zeigler‐Johnson C, Weber A, Glanz K, Spangler E, Rebbeck TR. Gender‐ and ethnic‐specific associations with obesity: individual and neighborhood‐level factors. J Nat Medi Assoc. 2013;105(2):173‐182. doi: 10.1016/s0027-9684(15)30107-3 [DOI] [PubMed] [Google Scholar]
- 17. Geronimus AT, Hicken M, Keene D, Bound J. "Weathering" and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96(5):826‐833. doi: 10.2105/AJPH.2004.060749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171‐179. doi: 10.1056/nejm199801153380307 [DOI] [PubMed] [Google Scholar]
- 19. Lynch SM, Mitra N, Ravichandran K, et al. Telomere length and neighborhood circumstances: evaluating biological response to unfavorable exposures. Cancer Epidemiol Biomarkers Prev. 2017;26(4):553‐560. doi: 10.1158/1055-9965.Epi-16-0554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lynch SM, Sorice K, Tagai EK, Handorf EA. Use of empiric methods to inform prostate cancer health disparities: comparison of neighborhood‐wide association study "hits" in black and white men. Cancer. 2020;126(9):1949‐1957. doi: 10.1002/cncr.32734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Churchwell K, Elkind MSV, Benjamin RM, et al. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory From the American Heart Association. Circulation. 2020;142(24):e454‐e468. doi:10.1161/cir.0000000000000936 [DOI] [PubMed] [Google Scholar]
- 22. Messer LC, Laraia BA, Kaufman JS, et al. The development of a standardized neighborhood deprivation index. J Urban Health. 2006;83(6):1041‐1062. doi: 10.1007/s11524-006-9094-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Polite BN, Adams‐Campbell LL, Brawley OW, et al. Charting the future of cancer health disparities research: a position statement from the American Association for Cancer Research, the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute. Cancer Res. 2017;77(17):4548‐4555. doi: 10.1158/0008-5472.can-17-0623 [DOI] [PubMed] [Google Scholar]
- 24. Kim J, Artinyan A, Mailey B, et al. An interaction of race and ethnicity with socioeconomic status in rectal cancer outcomes. Ann Surg. 2011;253(4):647‐654. doi: 10.1097/sla.0b013e3182111102 [DOI] [PubMed] [Google Scholar]
- 25. Krieger N, Chen JT, Waterman PD, Soobader M‐J, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area‐based measure and geographic level matter?: The public health disparities geocoding project. Am J Epidemiol. 2002;156(5):471‐482. doi: 10.1093/aje/kwf068 [DOI] [PubMed] [Google Scholar]
- 26. Chang ET, Gomez SL, Fish K, et al. Gastric cancer incidence among Hispanics in California: patterns by time, nativity, and neighborhood characteristics. Cancer Epidemiol Biomarkers Prev. 2012;21(5):709‐719. doi: 10.1158/1055-9965.Epi-11-1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liaw W, Krist AH, Tong ST, et al. Living in "Cold Spot" communities is associated with poor health and health quality. J Am Board Fam Med. 2018;31(3):342‐350. doi: 10.3122/jabfm.2018.03.170421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wheeler DC, Czarnota J, Jones RM. Estimating an area‐level socioeconomic status index and its association with colonoscopy screening adherence. PLoS One. 2017;12(6):e0179272. doi: 10.1371/journal.pone.0179272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Banegas MP, Tao L, Altekruse S, et al. Heterogeneity of breast cancer subtypes and survival among Hispanic women with invasive breast cancer in California. Breast Cancer Res Treat. 2014;144(3):625‐634. doi: 10.1007/s10549-014-2882-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cho YI, Johnson TP, Barrett RE, Campbell RT, Dolecek TA, Warnecke RB. Neighborhood changes in concentrated immigration and late stage breast cancer diagnosis. J Immigr Minor Health. 2011;13(1):9‐14. doi: 10.1007/s10903-010-9339-3 [DOI] [PubMed] [Google Scholar]
- 31. Clarke CA, McKinley M, Hurley S, et al. Continued increase in melanoma incidence across all socioeconomic status groups in California, 1998‐2012. J Investigat Dermatology. 2017;137(11):2282‐2290. doi: 10.1016/j.jid.2017.06.024 [DOI] [PubMed] [Google Scholar]
- 32. Gomez N, Guendelman S, Harley KG, Gomez SL. Nativity and neighborhood characteristics and cervical cancer stage at diagnosis and survival outcomes among Hispanic women in California. Am J Public Health. 2015;105(3):538‐545. doi: 10.2105/ajph.2014.302261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hossain F, Danos D, Prakash O, et al. Neighborhood social determinants of triple negative breast cancer. Front Public Health. 2019;7:18. doi: 10.3389/fpubh.2019.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keegan TH, John EM, Fish KM, Alfaro‐Velcamp T, Clarke CA, Gomez SL. Breast cancer incidence patterns among California Hispanic women: differences by nativity and residence in an enclave. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1208‐1218. doi: 10.1158/1055-9965.Epi-10-0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Keegan TH, Quach T, Shema S, Glaser SL, Gomez SL. The influence of nativity and neighborhoods on breast cancer stage at diagnosis and survival among California Hispanic women. BMC Cancer. 2010;10:603. doi:10.1186/1471‐2407‐10‐603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Major JM, Norman Oliver M, Doubeni CA, Hollenbeck AR, Graubard BI, Sinha R. Socioeconomic status, healthcare density, and risk of prostate cancer among African American and Caucasian men in a large prospective study. Cancer Causes Control. 2012;23(7):1185‐1191. doi: 10.1007/s10552-012-9988-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martinez ME, Gomez SL, Tao L, et al. Contribution of clinical and socioeconomic factors to differences in breast cancer subtype and mortality between Hispanic and non‐Hispanic white women. Breast Cancer Res Treat. 2017;166(1):185‐193. doi: 10.1007/s10549-017-4389-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mojica CM, Glenn BA, Chang C, Bastani R. The relationship between neighborhood immigrant composition, limited english proficiency, and late‐stage colorectal cancer diagnosis in California. BioMed Res Int. 2015;2015:460181. doi: 10.1155/2015/460181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Palmer JR, Boggs DA, Wise LA, Adams‐Campbell LL, Rosenberg L. Individual and neighborhood socioeconomic status in relation to breast cancer incidence in African‐American women. Am J Epidemiol. 2012;176(12):1141–1146. doi: 10.1093/aje/kws211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peterson CE, Rauscher GH, Johnson TP, et al. The association between neighborhood socioeconomic status and ovarian cancer tumor characteristics. Cancer Causes Control. 2014;25(5):633‐637. doi: 10.1007/s10552-014-0357-7 [DOI] [PubMed] [Google Scholar]
- 41. Tao L, Chu L, Wang LI, et al. Occurrence and outcome of de novo metastatic breast cancer by subtype in a large, diverse population. Cancer Causes Control. 2016;27(9):1127‐1138. doi: 10.1007/s10552-016-0791-9 [DOI] [PubMed] [Google Scholar]
- 42. Warnecke RB, Campbell RT, Vijayasiri G, Barrett RE, Rauscher GH. Multilevel examination of health disparity: the role of policy implementation in neighborhood context, in patient resources, and in healthcare facilities on later stage of breast cancer diagnosis. Cancer Epidemiol Biomarkers Prev. 2019;28(1):59‐66. doi: 10.1158/1055-9965.Epi-17-0945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sampson RJ, Morenoff JD, Earls F. Beyond social capital: spatial dynamics of collective efficacy for children. Am Sociol Rev. 1999;64(5):633‐660. doi: 10.2307/2657367 [DOI] [Google Scholar]
- 44. Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703‐711. [DOI] [PubMed] [Google Scholar]
- 45. Gomez SL, Press DJ, Lichtensztajn D, et al. Patient, hospital, and neighborhood factors associated with treatment of early‐stage breast cancer among Asian American women in California. Cancer Epidemiol Biomarkers Prev. 2012;21(5):821‐834. doi: 10.1158/1055-9965.Epi-11-1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shariff‐Marco S, Yang J, John EM, et al. Intersection of race/ethnicity and socioeconomic status in mortality after breast cancer. J Community Health. 2015;40(6):1287‐1299. doi: 10.1007/s10900-015-0052-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chang ET, Yang J, Alfaro‐Velcamp T, So SK, Glaser SL, Gomez SL. Disparities in liver cancer incidence by nativity, acculturation, and socioeconomic status in California Hispanics and Asians. Cancer Epidemiol Biomarkers Prev. 2010;19(12):3106‐3118. doi: 10.1158/1055-9965.Epi-10-0863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cheng I, Shariff‐Marco S, Koo J, et al. Contribution of the neighborhood environment and obesity to breast cancer survival: the California Breast Cancer Survivorship Consortium. Cancer Epidemiol Biomarkers Prev. 2015;24(8):1282‐1290. doi: 10.1158/1055-9965.Epi-15-0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chu KP, Shema S, Wu S, Gomez SL, Chang ET, Le QT. Head and neck cancer‐specific survival based on socioeconomic status in Asians and Pacific Islanders. Cancer. 2011;117(9):1935‐1945. doi: 10.1002/cncr.25723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Clarke CA, Glaser SL, Gomez SL, et al. Lymphoid malignancies in U.S. Asians: incidence rate differences by birthplace and acculturation. Cancer Epidemiol Biomarkers Prev. 2011;20(6):1064‐1077. doi: 10.1158/1055-9965.Epi-11-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Colevas AD. Population‐based evaluation of incidence trends in oropharyngeal cancer focusing on socioeconomic status, sex, and race/ethnicity. Head Neck. 2014;36(1):34‐42. doi: 10.1002/hed.23253 [DOI] [PubMed] [Google Scholar]
- 52. Conroy SM, Clarke CA, Yang J, et al. Contextual impact of neighborhood obesogenic factors on postmenopausal breast cancer: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2017;26(4):480‐489. doi: 10.1158/1055-9965.Epi-16-0941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Conroy SM, Shariff‐Marco S, Koo J, et al. Racial/ethnic differences in the impact of neighborhood social and built environment on breast cancer risk: the neighborhoods and breast cancer study. Cancer Epidemiol Biomarkers Prev. Apr 2017;26(4):541‐552. doi: 10.1158/1055-9965.Epi-16-0935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Derouen MC, Gomez SL, Press DJ, Tao L, Kurian AW, Keegan TH. A population‐based observational study of first‐course treatment and survival for adolescent and young adult females with breast cancer. J Adolescent Young Adult Oncol. 2013;2(3):95‐103. doi: 10.1089/jayao.2013.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. DeRouen MC, Mujahid M, Srinivas S, Keegan TH. Disparities in adolescent and young adult survival after testicular cancer vary by histologic subtype: a population‐based study in California 1988‐2010. J Adolescent Young Adult Oncol Mar 2016;5(1):31‐40. doi: 10.1089/jayao.2015.0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. DeRouen MC, Schupp CW, Koo J, et al. Impact of individual and neighborhood factors on disparities in prostate cancer survival. Cancer Epidemiol. 2018;53:1‐11. doi: 10.1016/j.canep.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Filion EJ, McClure LA, Huang D, et al. Higher incidence of head and neck cancers among Vietnamese American men in California. Head Neck. 2010;32(10):1336‐1344. doi: 10.1002/hed.21330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Froment MA, Gomez SL, Roux A, DeRouen MC, Kidd EA. Impact of socioeconomic status and ethnic enclave on cervical cancer incidence among Hispanics and Asians in California. Gynecol Oncol. 2014;133(3):409‐415. doi: 10.1016/j.ygyno.2014.03.559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gomez SL, Clarke CA, Shema SJ, Chang ET, Keegan TH, Glaser SL. Disparities in breast cancer survival among Asian women by ethnicity and immigrant status: a population‐based study. Am J Public Health. 2010;100(5):861‐869. doi: 10.2105/ajph.2009.176651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Horn‐Ross PL, Lichtensztajn DY, Clarke CA, et al. Continued rapid increase in thyroid cancer incidence in California: trends by patient, tumor, and neighborhood characteristics. Cancer Epidemiol Biomarkers Prev. 2014;23(6):1067‐1079. doi: 10.1158/1055-9965.Epi-13-1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jabo B, Morgan JW, Martinez ME, Ghamsary M, Wieduwilt MJ. Sociodemographic disparities in chemotherapy and hematopoietic cell transplantation utilization among adult acute lymphoblastic and acute myeloid leukemia patients. PLoS One. 2017;12(4):e0174760. doi: 10.1371/journal.pone.0174760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Keegan TH, DeRouen MC, Parsons HM, et al. Impact of treatment and insurance on socioeconomic disparities in survival after adolescent and young adult hodgkin lymphoma: a population‐based study. Cancer Epidemiol Biomarkers Prev. 2016;25(2):264‐273. doi: 10.1158/1055-9965.Epi-15-0756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Keegan TH, Grogan RH, Parsons HM, et al. Sociodemographic disparities in differentiated thyroid cancer survival among adolescents and young adults in California. Thyroid. 2015;25(6):635‐648. doi: 10.1089/thy.2015.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Keegan TH, Kurian AW, Gali K, et al. Racial/ethnic and socioeconomic differences in short‐term breast cancer survival among women in an integrated health system. Am J Public Health. 2015;105(5):938‐946. doi: 10.2105/ajph.2014.302406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Keegan TH, Shariff‐Marco S, Sangaramoorthy M, et al. Neighborhood influences on recreational physical activity and survival after breast cancer. Cancer Causes Control. 2014;25(10):1295‐1308. doi: 10.1007/s10552-014-0431-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kish JK, Yu M, Percy‐Laurry A, Altekruse SF. Racial and ethnic disparities in cancer survival by neighborhood socioeconomic status in Surveillance, Epidemiology, and End Results (SEER) Registries. J Nat Cancer Inst Monographs. 2014;2014(49):236‐243. doi: 10.1093/jncimonographs/lgu020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ladabaum U, Clarke CA, Press DJ, et al. Colorectal cancer incidence in Asian populations in California: effect of nativity and neighborhood‐level factors. Am J Gastroenterol. 2014;109(4):579‐588. doi: 10.1038/ajg.2013.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Patel MI, Schupp CW, Gomez SL, Chang ET, Wakelee HA. How do social factors explain outcomes in non‐small‐cell lung cancer among Hispanics in California? Explaining the Hispanic paradox. J Clin Oncol. 2013;31(28):3572‐3578. doi: 10.1200/jco.2012.48.6217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schupp CW, Press DJ, Gomez SL. Immigration factors and prostate cancer survival among Hispanic men in California: does neighborhood matter? Cancer. 2014;120(9):1401‐1408. doi: 10.1002/cncr.28587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shariff‐Marco S, Yang J, John EM, et al. Impact of neighborhood and individual socioeconomic status on survival after breast cancer varies by race/ethnicity: the Neighborhood and Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2014;23(5):793‐811. doi: 10.1158/1055-9965.Epi-13-0924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tao L, Foran JM, Clarke CA, Gomez SL, Keegan TH. Socioeconomic disparities in mortality after diffuse large B‐cell lymphoma in the modern treatment era. Blood. 2014;123(23):3553‐3562. doi: 10.1182/blood-2013-07-517110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tao L, Ladabaum U, Gomez SL, Cheng I. Colorectal cancer mortality among Hispanics in California: differences by neighborhood socioeconomic status and nativity. Cancer. 2014;120(22):3510‐8. doi:10.1002/cncr.28837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wong ML, Clarke CA, Yang J, Hwang J, Hiatt RA, Wang S. Incidence of non‐small‐cell lung cancer among California Hispanics according to neighborhood socioeconomic status. J Thoracic Oncol. 2013;8(3):287‐294. doi: 10.1097/JTO.0b013e31827bd7f5 [DOI] [PubMed] [Google Scholar]
- 74. Yin D, Morris C, Allen M, Cress R, Bates J, Liu L. Does socioeconomic disparity in cancer incidence vary across racial/ethnic groups? Cancer Causes Control. 2010;21(10):1721‐1730. doi: 10.1007/s10552-010-9601-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Glaser SL, Chang ET, Clarke CA, Keegan TH, Yang J, Gomez SL. Hodgkin lymphoma incidence in ethnic enclaves in California. Leukemia Lymphoma. 2015;56(12):3270‐3280. doi: 10.3109/10428194.2015.1026815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gupta S, Tao L, Murphy JD, et al. Race/ethnicity‐, socioeconomic status‐, and anatomic subsite‐specific risks for gastric cancer. Gastroenterology. 2019;156(1):59‐62.e4. doi: 10.1053/j.gastro.2018.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tao L, Gomez SL, Keegan THM, Kurian AW, Clarke CA. Breast cancer mortality in African‐American and non‐Hispanic white women by molecular subtype and stage at diagnosis: a population‐based study. Cancer Epidemiol Biomark Prev. 2015;24(7):1039‐1045. doi: 10.1158/1055-9965.EPI-15-0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Brewer KC, Peterson CE, Davis FG, Hoskins K, Pauls H, Joslin CE. The influence of neighborhood socioeconomic status and race on survival from ovarian cancer: a population‐based analysis of Cook County, Illinois. Ann Epidemiol. 2015;25(8):556‐563. doi: 10.1016/j.annepidem.2015.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Danos DM, Ferguson TF, Simonsen NR, et al. Neighborhood disadvantage and racial disparities in colorectal cancer incidence: a population‐based study in Louisiana. Ann Epidemiol. 2018;28(5):316‐321.e2. doi: 10.1016/j.annepidem.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Joslin CE, Brewer KC, Davis FG, Hoskins K, Peterson CE, Pauls HA. The effect of neighborhood‐level socioeconomic status on racial differences in ovarian cancer treatment in a population‐based analysis in Chicago. Gynecol Oncol. 2014;135(2):285‐291. doi: 10.1016/j.ygyno.2014.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Major JM, Doubeni CA, Freedman ND, et al. Neighborhood socioeconomic deprivation and mortality: NIH‐AARP diet and health study. PLoS One. 2010;5(11):e15538. doi:10.1371/journal.pone.0015538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. DeRouen MC, Hu L, McKinley M, et al. Incidence of lung cancer histologic cell‐types according to neighborhood factors: a population based study in California. PLoS One. 2018;13(5):e0197146. doi: 10.1371/journal.pone.0197146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol. 2018;36(1):25‐33. doi: 10.1200/jco.2017.74.2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gomez SL, Yang J, Lin SW, et al. Lung cancer survival among Chinese Americans, 2000 to 2010. J Global Oncol. 2016;2(1):30‐38. doi: 10.1200/jgo.2015.000539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Patel MI, McKinley M, Cheng I, Haile R, Wakelee H, Gomez SL. Lung cancer incidence trends in California by race/ethnicity, histology, sex, and neighborhood socioeconomic status: An analysis spanning 28 years. Lung Cancer (Amsterdam, Netherlands). 2017;108:140‐149. doi: 10.1016/j.lungcan.2017.03.014 [DOI] [PubMed] [Google Scholar]
- 86. Doubeni CA, Laiyemo AO, Major JM, et al. Socioeconomic status and the risk of colorectal cancer: an analysis of more than a half million adults in the National Institutes of Health‐AARP Diet and Health Study. Cancer. 2012;118(14):3636‐3644. doi:10.1002/cncr.26677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lian M, Perez M, Liu Y, et al. Neighborhood socioeconomic deprivation, tumor subtypes, and causes of death after non‐metastatic invasive breast cancer diagnosis: a multilevel competing‐risk analysis. Breast Cancer Res Treat. 2014;147(3):661‐670. doi: 10.1007/s10549-014-3135-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lian M, Schootman M, Doubeni CA, et al. Geographic variation in colorectal cancer survival and the role of small‐area socioeconomic deprivation: a multilevel survival analysis of the NIH‐AARP Diet and Health Study Cohort. Am J Epidemiol. 2011;174(7):828‐838. doi: 10.1093/aje/kwr162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sanderson M, Aldrich MC, Levine RS, Kilbourne B, Cai Q, Blot WJ. Neighbourhood deprivation and lung cancer risk: a nested case‐control study in the USA. BMJ Open. 2018;8(9):e021059. doi:10.1136/bmjopen‐2017‐021059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhang D, Matthews CE, Powell‐Wiley TM, Xiao Q. Ten‐year change in neighborhood socioeconomic status and colorectal cancer. Cancer. 2019;125(4):610‐617. doi: 10.1002/cncr.31832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Adie Y, Kats DJ, Tlimat A, et al. Neighborhood disadvantage and lung cancer incidence in ever‐smokers at a safety net health‐care system: a retrospective study. Chest. 2020;157(4):1021‐1029. doi: 10.1016/j.chest.2019.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hastert TA, Beresford SA, Sheppard L, White E. Disparities in cancer incidence and mortality by area‐level socioeconomic status: a multilevel analysis. J Epidemiol Commun Health. 2015;69(2):168‐176. doi: 10.1136/jech-2014-204417 [DOI] [PubMed] [Google Scholar]
- 93. Hastert TA, Ruterbusch JJ, Beresford SA, Sheppard L, White E. Contribution of health behaviors to the association between area‐level socioeconomic status and cancer mortality. Soc Sci Med. 2016;148:52‐58. doi: 10.1016/j.socscimed.2015.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Johnson AM, Johnson A, Hines RB, Bayakly R. The effects of residential segregation and neighborhood characteristics on surgery and survival in patients with early‐stage non‐small cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2016;25(5):750‐758. doi: 10.1158/1055-9965.Epi-15-1126 [DOI] [PubMed] [Google Scholar]
- 95. Watson M, Grande D, Radhakrishnan A, Mitra N, Ward KR, Pollack CE. Racial differences in prostate cancer treatment: the role of socioeconomic status. Ethnicity Dis. 2017;27(3):201‐208. doi: 10.18865/ed.27.3.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bethea TN, Palmer JR, Rosenberg L, Cozier YC. Neighborhood socioeconomic status in relation to all‐cause, cancer, and cardiovascular mortality in the black women's health study. Ethnicity Dis. 2016;26(2):157‐164. doi: 10.18865/ed.26.2.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Beyer KMM, Laud PW, Zhou Y, Nattinger AB. Housing discrimination and racial cancer disparities among the 100 largest US metropolitan areas. Cancer. 2019;125(21):3818‐3827. doi: 10.1002/cncr.32358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Reitzel LR, Nguyen N, Zafereo ME, Li G, Wei Q, Sturgis EM. Neighborhood deprivation and clinical outcomes among head and neck cancer patients. Health Place. 2012;18(4):861‐868. doi: 10.1016/j.healthplace.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Simon MS, Lamerato L, Krajenta R, et al. Racial differences in the use of adjuvant chemotherapy for breast cancer in a large urban integrated health system. Int J Breast Cancer. 2012;2012:453985. doi: 10.1155/2012/453985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Williams D, Priest N, Anderson N. Understanding associations among race, socioeconomic status, and health: Patterns and prospects. Health Psychol. 2016;35:407‐411. doi:10.1037/hea0000242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural‐urban, and racial inequalities in US cancer mortality: part i—all cancers and lung cancer and part ii—colorectal, prostate breast, and cervical cancers. J Cancer Epidemiol. 2012;2011:107497. doi: 10.1155/2011/107497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Guan A, Lichtensztajn D, Oh D, et al. Breast cancer in San Francisco: disentangling disparities at the neighborhood level. Cancer Epidemiol Biomarkers Prev. 2019;28(12):1968‐1976. doi: 10.1158/1055-9965.Epi-19-0799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prevent Med. 2009;36(1):74‐81. doi: 10.1016/j.amepre.2008.09.025 [DOI] [PubMed] [Google Scholar]
- 104. Algren MH, Bak CK, Berg‐Beckhoff G, Andersen PT. Health‐risk behaviour in deprived neighbourhoods compared with non‐deprived neighbourhoods: a systematic literature review of quantitative observational studies. PLoS One. 2015;10(10):e0139297. doi: 10.1371/journal.pone.0139297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Nodora JN, Cooper R, Talavera GA, et al. Acculturation, behavioral factors, and family history of breast cancer among Mexican and Mexican‐American Women. Women's Health Issues. 2015;25(5):494‐500. doi: 10.1016/j.whi.2015.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. John EM, Phipps AI, Davis A, Koo J. Migration history, acculturation, and breast cancer risk in Hispanic women. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2905‐2913. doi: 10.1158/1055-9965.Epi-05-0483 [DOI] [PubMed] [Google Scholar]
- 107. Shah M, Zhu K, Wu H, Potter J. Hispanic acculturation and utilization of cervical cancer screening in the US. Prevent Med. 2006;42(2):146‐149. doi: 10.1016/j.ypmed.2005.10.002 [DOI] [PubMed] [Google Scholar]
- 108. Ekenga CC, Yeung CY, Oka M. Cancer risk from air toxics in relation to neighborhood isolation and sociodemographic characteristics: A spatial analysis of the St. Louis metropolitan area, USA. Environ Res. 2019;179(Pt B):108844. doi:10.1016/j.envres.2019.108844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Klonoff EA. Disparities in the provision of medical care: an outcome in search of an explanation. J Behav Med. 2009;32(1):48‐63. doi: 10.1007/s10865-008-9192-1 [DOI] [PubMed] [Google Scholar]
- 110. Kressin NR, Raymond KL, Manze M. Perceptions of race/ethnicity‐based discrimination: a review of measures and evaluation of their usefulness for the health care setting. J Health Care Poor Underserved. 2008;19(3):697‐730. doi: 10.1353/hpu.0.0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Paradies Y, Truong M, Priest N. A systematic review of the extent and measurement of healthcare provider racism. J Gen Intern Med. 2014;29(2):364‐387. doi: 10.1007/s11606-013-2583-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Shavers VL, Fagan P, Jones D, et al. The state of research on racial/ethnic discrimination in the receipt of health care. Am J Public Health. 2012;102(5):953‐966. doi: 10.2105/ajph.2012.300773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Clark WAV, Morrison PS. Socio‐spatial mobility and residential sorting: evidence from a Large‐scale survey. Urban Stud. 2012;49(15):3253‐3270. doi: 10.1177/0042098012442418 [DOI] [Google Scholar]
- 114. Hulchanski JD. The three cities within Toronto. Cities Centre; 2010. [Google Scholar]
- 115. Rothstein R. The color of law: A forgotten history of how our government segregated America. Liveright Publishing; 2017. [Google Scholar]
- 116. Diez Roux AV. Neighborhoods and Health. 2nd revised edition ed. Oxford University Press Inc; 2018:392. [Google Scholar]
- 117. Bailey ZD, Feldman JM, Bassett MT. How structural racism works — racist policies as a root cause of U.S. racial health inequities. N Engl J Med. 2020;384(8):768‐773. doi: 10.1056/NEJMms2025396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Osypuk TL, Diez Roux AV, Hadley C, Kandula NR. Are immigrant enclaves healthy places to live? The multi‐ethnic study of atherosclerosis. Soc Sci Med. 2009;69(1):110‐120. doi: 10.1016/j.socscimed.2009.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lynch SM, Mitra N, Ross M, et al. A Neighborhood‐Wide Association Study (NWAS): Example of prostate cancer aggressiveness. PLoS One. 2017;12(3):e0174548. doi: 10.1371/journal.pone.0174548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wheeler DC, Jones RM, Schootman M, Nelson EJ. Explaining variation in elevated blood lead levels among children in Minnesota using neighborhood socioeconomic variables. Science Total Environ. 2019;650(Pt 1):970‐977. doi:10.1016/j.scitotenv.2018.09.088 [DOI] [PubMed] [Google Scholar]
- 121. Wang N, Cao F, Liu F, et al. The effect of socioeconomic status on health‐care delay and treatment of esophageal cancer. J Transl Med. 2015;13:241‐241. doi: 10.1186/s12967-015-0579-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zhang S, Liu Y, Yun S, Lian M, Komaie G, Colditz GA. Impacts of neighborhood characteristics on treatment and outcomes in women with ductal carcinoma in situ of the breast. Cancer Epidemiol Biomarkers Prev. 2018;27(11):1298. doi: 10.1158/1055-9965.EPI-17-1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Benzies KM, Premji S, Hayden KA, Serrett K. State‐of‐the‐evidence reviews: advantages and challenges of including grey literature. Worldviews Evid Based Nurs. 2006;3(2):55‐61. doi: 10.1111/j.1741-6787.2006.00051.x [DOI] [PubMed] [Google Scholar]
- 124. Duncan DT, Kawachi I, Subramanian SV, Aldstadt J, Melly SJ, Williams DR. Examination of how neighborhood definition influences measurements of youths' access to tobacco retailers: a methodological note on spatial misclassification. Am J Epidemiol. 2014;179(3):373‐381. doi: 10.1093/aje/kwt251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Widener M, Farber S, Neutens T, Horner M. Spatiotemporal accessibility to supermarkets using public transit: An interaction potential approach in Cincinnati, Ohio. J Transport Geography. 2015;42:72–83. doi: 10.1016/j.jtrangeo.2014.11.004 [DOI] [Google Scholar]
- 126. Gomez SL, Glaser SL, McClure LA, et al. The California Neighborhoods Data System: a new resource for examining the impact of neighborhood characteristics on cancer incidence and outcomes in populations. Cancer Causes Control. 2011;22(4):631‐647. doi: 10.1007/s10552-011-9736-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Madnick D, Handorf E, Ortiz A, et al. Investigating disparities: the effect of social environment on pancreatic cancer survival in metastatic patients. J Gastrointest Oncol. 2020;11(4):633‐643. doi: 10.21037/jgo-20-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wiese D, Stroup AM, Maiti A, et al. Residential mobility and geospatial disparities in colon cancer survival. Cancer Epidemiol Biomarkers Prev. 2020;29:2119‐2125. doi: 10.1158/1055-9965.Epi-20-0772 [DOI] [PubMed] [Google Scholar]
- 129. Wiese D, Stroup AM, Maiti A, et al. Socioeconomic disparities in colon cancer survival: revisiting neighborhood poverty using residential histories. Epidemiology. 2020;31(5):728‐735. doi: 10.1097/ede.0000000000001216 [DOI] [PubMed] [Google Scholar]
- 130. Shvetsov YB, Shariff‐Marco S, Yang J, et al. Association of change in the neighborhood obesogenic environment with colorectal cancer risk: the multiethnic cohort study. SSM Popul Health. 2020;10:100532. doi: 10.1016/j.ssmph.2019.100532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Singh GK. Area deprivation and widening inequalities in US mortality, 1969‐1998. Am J Public Health. 2003;93(7):1137‐1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Browning CR, Cagney KA. Neighborhood structural disadvantage, collective efficacy, and self‐rated physical health in an urban setting. J Health Social Behav. 2002;43(4):383‐399. [PubMed] [Google Scholar]
- 133. Coogan PF, Cozier YC, Krishnan S, et al. Neighborhood socioeconomic status in relation to 10‐year weight gain in the Black Women's Health Study. Obesity (Silver Spring). 2010;18(10):2064‐2065. doi: 10.1038/oby.2010.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Diez‐Roux AV, Kiefe CI, Jacobs DR Jr, et al. Area characteristics and individual‐level socioeconomic position indicators in three population‐based epidemiologic studies. Ann Epidemiol. 2001;11(6):395‐405. [DOI] [PubMed] [Google Scholar]
- 135. Dubowitz T, Heron M, Bird CE, et al. Neighborhood socioeconomic status and fruit and vegetable intake among whites, blacks, and Mexican Americans in the United States. Am J Clin Nutr. 2008;87(6):1883‐1891. doi: 10.1093/ajcn/87.6.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Massey D. The prodigal paradigm returns: ecology comes back to sociology. In: Booth CA, ed. Does it take a village? Community effects on children, adolescents, and families. Lawrence Erlbaum Associates; 2001:41‐48. [Google Scholar]
- 137. Krieger N, Singh N, Waterman PD. Metrics for monitoring cancer inequities: residential segregation, the Index of Concentration at the Extremes (ICE), and breast cancer estrogen receptor status (USA, 1992‐2012). Cancer Causes Control. 2016;27(9):1139‐1151. doi: 10.1007/s10552-016-0793-7 [DOI] [PubMed] [Google Scholar]
- 138. Klassen AC, Curriero FC, Hong JH, et al. The role of area‐level influences on prostate cancer grade and stage at diagnosis. Prevent Med. 2004;39(3):441‐448. doi: 10.1016/j.ypmed.2004.04.031 [DOI] [PubMed] [Google Scholar]
- 139. Signorello LB, Cohen SS, Williams DR, Munro HM, Hargreaves MK, Blot WJ. Socioeconomic status, race, and mortality: a prospective cohort study. Am J Public Health. 2014;104(12):e98‐e107. doi: 10.2105/ajph.2014.302156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Butler DC, Petterson S, Phillips RL, Bazemore AW. Measures of social deprivation that predict health care access and need within a rational area of primary care service delivery. Health Services Res. 2013;48(2 Pt 1):539‐559. doi: 10.1111/j.1475-6773.2012.01449.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Yang J, Schupp CW, Harrati A, Clarke C, Keegan THM, Gomez SL. Developing an area‐based socioeconomic measure from American Community Survey data. Cancer Prevention Institute of California; 2014: 17. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Figure S2.
Table S1.
Table S2.
Table S3.
Table S4.
Table S5.
Table S6
Data Availability Statement
The data underlying this article are available in Supporting Files (https://github.com/ksorice/nSES‐Systematic‐Review).


