Abstract
There are only a few examples of microbial conversion of picric acid (2,4,6-trinitrophenol). None of the organisms that have been described previously is able to use this compound as a sole source of carbon, nitrogen, and energy at high rates. In this study we isolated and characterized a strain, strain CB 22-2, that was able to use picric acid as a sole source of carbon and energy at concentrations up to 40 mM and at rates of 1.6 mmol · h−1 · g (dry weight) of cells−1 in continuous cultures and 920 μmol · h−1 · g (dry weight) of cells−1 in flasks. In addition, this strain was able to use picric acid as a sole source of nitrogen at comparable rates in a nitrogen-free medium. Biochemical characterization and 16S ribosomal DNA analysis revealed that strain CB 22-2 is a Nocardioides sp. strain. High-pressure liquid chromatography and UV-visible light data, the low residual chemical oxygen demand, and the stoichiometric release of 2.9 ± 0.1 mol of nitrite per mol of picric acid provided strong evidence that complete mineralization of picric acid occurred. During transformation, the metabolites detected in the culture supernatant were the [H−]-Meisenheimer complexes of picric acid and 2,4-dinitrophenol (H−-DNP), as well as 2,4-dinitrophenol. Experiments performed with crude extracts revealed that H−-DNP formation indeed is a physiologically relevant step in picric acid metabolism.
Nitroaromatic compounds are very common environmental pollutants which are used as dyes, pesticides, and explosives (9). Many sites of factories that produce such chemicals are highly contaminated with these substances, including picric acid (2,4,6-trinitrophenol [2,4,6-TNP]) (33). Microbial degradation of these compounds depends on the number of nitro groups on the aromatic ring. Usually, the first step in aromatic degradation is electrophilic attack. Due to the electron-withdrawing effect of the nitro group, this step becomes more difficult with an increase in the number of nitro group substituents. Consequently, very few examples of microbial metabolism of trinitroarenes have been described.
In contrast to studies of 2,4,6-trinitrotoluene transformation (2, 5, 21, 32), there have been few investigations of the metabolism of picric acid (4, 8, 11, 16). The first description of microbial attack on this compound was the description of Erikson (4). Only two recent investigations dealing with picric acid metabolism have been described. Lenke et al. characterized a Rhodococcus erythropolis strain that is able to convert this substance with partial dead-end production of 2,4,6-trinitrocyclohexanone; picric acid is used as a sole source of nitrogen by this organism (11). The only example of complete mineralization of this compound was described by Rajan et al. The Nocardioides strain of these authors was able to grow on picric acid as a sole source of carbon and energy without dead-end product formation (16). The only intermediate metabolite detected in the culture supernatant was 2,4-dinitrophenol (2,4-DNP).
The first step in picric acid metabolism by R. erythropolis HL PM-1 is formation of a [H−]-Meisenheimer complex. Generation of Meisenheimer complexes of nitroaromatic compounds is a well-known chemical reaction (13, 22–24). In contrast, there are only two examples of microbial conversion of nitroaromatic compounds to Meisenheimer complexes; both of these involve trinitroarenes (picric acid [11] and trinitrotoluene [31]).
In this communication we describe the first isolation and characterization of a strain which completely mineralizes picric acid with intermediate formation of the [H−]-Meisenheimer complexes H−-TNP and H−-DNP. As this is the first description of a H−-DNP complex in microbial metabolism, the complex was characterized in more detail.
MATERIALS AND METHODS
Chemicals.
Picric acid, 2,4-DNP, 2-nitrophenol, 4-nitrophenol, 4-nitrocatechol, phenol, 2,4-dichloro-6-nitrophenol, 2,6-dichloro-4-nitrophenol, and dry acetonitrile were obtained from Fluka (Neu-Ulm, Germany); 2,6-DNP, 2,4-dinitrotoluene, and 2,6-dinitrotoluene were obtained from Aldrich (Steinheim, Germany); picramic acid was obtained from Tokyo Kasei (Tokyo, Japan); and 2,4,6-trinitrotoluene was a gift from Chemiewerk Schönebeck, Schönebeck, Germany. All other chemicals were A-grade purity and were obtained from Merck (Darmstadt, Germany). Quartz bidistilled water was used throughout this study.
Media and growth conditions.
One liter of R2A medium (17) (pH 7.0) contained 0.5 g of yeast extract, 0.5 g of proteose peptone no. 3, 0.5 g of Casamino Acids, 0.5 g of glucose, 0.5 g of soluble starch, 0.3 g of sodium pyruvate, 0.3 g of K2HPO4, and 0.05 g of MgSO4 · 7H2O. The glucose was autoclaved separately. One liter of mineral salts medium A (pH 7.0) consisted of 1.53 g of Na2HPO4 · 2H2O, 0.76 g of KH2PO4, 0.5 g of (NH4)2SO4, 0.2 g of MgSO4 · 7H2O, 0.05 g of CaCl2, and 10 ml of trace element solution SL-4. Trace element solution SL-4 contained 0.5 g of EDTA, 0.2 g of FeSO4 · 7H2O, 100 ml of trace element solution SL-6, and 900 ml of water. Trace element solution SL-6 contained 0.1 g of ZnSO4 · 7H2O, 0.03 g of MnCl2 · 4H2O, 0.3 g of H3BO3, 0.2 g of CoCl2 · 6H2O, 0.01 g of CuCl2 · 2H2O, 0.02 g of NiCl2 · 6H2O, 0.03 g of Na2MoO4 · 2H2O, and 1,000 ml of water. Mineral salts medium B was the same as mineral salts medium A except that it lacked ammonium sulfate. All media were sterilized by heating them at 121°C for 30 min. Agar plates contained 15 g of agar per liter of medium. Cells were grown at 25°C; cultures in Erlenmeyer flasks were incubated on a rotary shaker at 200 rpm. Additional experiments were performed in a 1.5-liter Biostad B fermentor (Braun, Melsungen, Germany).
Buffers.
Buffer A contained 50 mM potassium phosphate, 1 mM dithioerythritol, and 1 mM EDTA, and the pH was adjusted to 7.0. Buffer B contained 20 mM potassium phosphate, 5 mg of lysozyme per g of cells, 12.5 mg of deoxycholic acid (sodium salt) per g of cells, 1 mM dithioerythritol, 1 mM phenylmethylsulfonyl fluoride, and 1 mM EDTA, and the pH was adjusted to 7.0.
Isolation and selection of picric acid-degrading bacteria.
A mixture of soil samples from former German trinitrotoluene production sites contaminated with various nitroaromatic compounds was incubated in mineral salts medium A supplemented with 0.44 mM picric acid for 2 days (1 g of soil per 100 ml of medium). Decolorized cultures were transferred into fresh mineral medium containing 0.44 mM picric acid. After five serial transfers, cultures were streaked onto agar plates containing mineral salts medium A and 0.44 mM picric acid as the only carbon source. Single colonies were picked. In order to obtain pure cultures, the cells were alternately plated onto R2A agar or mineral salts medium A agar supplemented with different concentrations of picric acid (0.44 to 2.2 mM). Only one type of colonies, designated strain CB 22-1, was obtained.
Strain CB 22-1 was also able to use 2,4-DNP as a sole source of carbon and energy, whereas phenol, 2-nitrophenol, 4-nitrophenol, and 4-nitrocatechol were not metabolized or cometabolized. Furthermore, 4-nitrocatechol inhibited the transformation of picric acid.
To further improve the strain, cultures of CB 22-1 were grown under sterile conditions in mineral salts medium A containing 2 mM picric acid. During a 15-day period the picric acid concentration was determined at different times and was brought up to 2 mM, which led to an increase in the nitrite concentration. Subsequently, one-tenth of the culture was transferred into fresh medium and treated as described above. Following each step the cells were streaked onto agar plates containing mineral salts medium B lacking a nitrogen source. After 3 months strain CB 22-2 was isolated; this strain grew in a liquid culture containing picric acid as the only source of carbon, nitrogen, and energy.
Taxonomic assignment of colonies.
Systematic microbiological assays of CB 22-1 and CB 22-2, including Gram staining, were performed as described previously (30). Strain CB 22-1 was classified by performing biochemical tests and a 16S ribosomal DNA analysis and by determining the composition of the cellular fatty acids, which was done by workers at the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany). The similarity of CB 22-1 and CB 22-2 was confirmed by biochemical and morphological tests.
Determination of cell density.
Bacterial growth was monitored spectrophotometrically by measuring the optical density at 600 nm. As the culture supernatant turned red during fermentation, a cell-free sample of the corresponding supernatant was used as the blank.
Nitrite analysis.
The nitrite concentration was determined as previously described (14), with the following modifications. Two hundred microliters of a sample diluted so that the nitrite concentration was up to 0.1 mM was added to 50 μl of 20 mM sulfanilic acid. The reaction mixture was incubated for 5 min at room temperature, and then 50 μl of a 0.04% (wt/vol) N-(1-naphthyl)ethylenediamine hydrochloride solution was added. After 20 min of incubation at room temperature, 700 μl of water was added, and the absorption at 550 nm was determined. Nitrite concentrations were calculated by using nitrite standards.
COD analysis.
The chemical oxygen demand (COD) was determined by using a SPEKTROQUANT test (Merck) for a COD range of 4 to 40 mg of O2. For the COD analysis cells were grown in flasks in mineral salts medium B supplemented with 1 mM picric acid until complete degradation was achieved. Subsequently, each suspension was centrifuged at 5,000 × g, and the nitrite concentration of the supernatant was determined. Various dilutions of the supernatants were analyzed by using the instructions provided with the COD test. Mineral salts medium B supplemented with nitrite at the appropriate concentration was used as the blank.
Preparation of H−-TNP and H−-DNP.
H−-TNP was prepared by adding 20 μl of 0.525 M sodium borohydride to 458 μl of 44 mM picric acid in aqueous solution (pH 7.0). H−-DNP was synthesized as follows: 5 mmol of dry 2,4-DNP was dissolved in 10 ml of dry acetonitrile and heated to 50°C. At this temperature 4 mmol of dry sodium borohydride was added over a period of 10 s. The reaction mixture spontaneously turned red, and an orange-red solid formed at the bottom of the flask. After 3 min of incubation at 50°C, the supernatant was removed. The remaining solid was washed with 5 ml of cold acetonitrile and dried.
NMR spectra.
Samples of the supernatant H−-TNP were prepared by using thin-layer chromatography. Samples of H−-DNP were prepared as described above. Nuclear magnetic resonance (NMR) spectra were recorded with a Bruker 400-MHz spectrometer by using solutions in D2O–0.1 M NaOD and tetramethylsilane as the external standard.
HPLC.
High-pressure liquid chromatography (HPLC) was performed with a Shimadzu instrument equipped with a photodiode array UV-visible light detector. A C18 reversed-phase column (ET 250/8/4, 10-μm-diameter particles in packing; Macherey-Nagel, Düren, Germany) was used. The solvent systems used were water–1 M potassium phosphate (pH 7.0)–1 M sodium azide (979:20:1, vol/vol/vol) (solvent A) and methanol (solvent B). Compounds were eluted at a flow rate of 1 ml min−1 by using a gradient starting with 15% solvent B (0 to 5 min), followed by a gradual increase to 90% solvent B (5 to 15 min) and 90% solvent B (15 to 17.5 min). Commercially available picric acid, 2,4-DNP, 4-nitrophenol, 2-nitrophenol, 2-amino-4-nitrophenol, 4-amino-2-nitrophenol, and picramic acid were used as references. For quantification of H−-DNP, a linear extinction coefficient at 420 nm (ɛ420) (9 mM−1 cm−1) was estimated by using the UV spectrum of the synthetic substance.
Preparation of cell extracts.
Cell extracts were prepared as follows. Cells were harvested by centrifugation at 5,000 × g for 20 min, washed with buffer B, and resuspended in 2 ml of buffer B per g of cells. After 30 min of gentle stirring at room temperature, the mixture was centrifuged at 30,000 × g for 30 min. The concentration of the resulting crude cell extract was determined as described by Bradford by using bovine serum albumin as the protein standard (2).
Preparation of substrates for enzymatic conversions.
As the synthetic Meisenheimer complexes of picric acid and 2,4-DNP contained traces of sodium borohydride, the substrates used for transformation studies were prepared as follows. One hundred milliliters of culture supernatant from an incomplete batch fermentation containing H−-TNP and H−-DNP was applied to 1 ml of Q-Sepharose (Pharmacia, Uppsala, Sweden) previously equilibrated with buffer A. Ten-milliliter portions of the supernatant were subsequently added to the same 1 ml of Q-Sepharose, and each preparation was shaken until total decolorization was achieved. The saturated Q-Sepharose was applied to 4 ml of fresh Q-Sepharose (previously equilibrated with buffer A) in a 10-ml column connected to a fast-protein liquid chromatography system. Elution was accomplished by using a linear 0 to 2 M NaCl gradient in buffer A. The Meisenheimer complexes of 2,4-DNP and picric acid eluted in separate fractions at NaCl concentrations of 0.5 and 1.4 M, respectively.
Enzyme assays.
The H−-TNP-synthesizing enzyme was assayed by monitoring the increase in absorbance at 490 nm in buffer A containing 75 μM NADPH and 5 to 20 μl of sample in a total volume of 950 μl. The assay was started by adding 50 μl of 1 mM picric acid. Specific activities were calculated by using an ɛ490(H−-TNP) of 14 mM−1 cm−1. The linear extinction coefficient was determined as follows. A 50 μM picric acid solution was enzymatically converted to H−-TNP by using partially purified H−-TNP-synthesizing enzyme. An HPLC analysis was performed to confirm the purity of the resulting H−-TNP. The extinction coefficient was calculated by using the difference between the absorption of the completed reaction and the original absorption at 490 nm. The enzyme that converts H−-TNP to 2,4-DNP was assayed by monitoring the decrease in absorbance at 490 nm in buffer A containing the Meisenheimer complex at a concentration of 50 μM and 20 μl of sample. The H−-DNP-synthesizing enzyme was assayed by determining the absorbance at 460 nm in buffer A containing 75 μM NADPH and 5 to 20 μl of sample in a 950-μl (total volume) reaction mixture. The assay was started by adding 50 μl of 1 mM 2,4-DNP. H−-DNP transformations were measured by monitoring the changes in the UV spectra in buffer A containing 50 μM Meisenheimer complex, 20 μl of sample, and 0.1 mM NADPH.
Partial purification of the H−-TNP-synthesizing enzyme.
Eight milliliters of crude cell extract with a protein concentration of 2 mg/ml was applied to a 10-ml DEAE Trisacryl column (Pharmacia) previously equilibrated with buffer A. The column was washed with 30 ml of buffer A. Elution was accomplished by using a linear gradient of sodium chloride (0 to 2 M) in 60 ml of buffer A at a concentration of 1.4 M of NaCl.
RESULTS
Taxonomic assignment of colonies.
Cells of the strain that was isolated initially, strain CB 22-1, and its derivative, strain CB 22-2, were gram-positive, strictly aerobic, motile, nonsporulating, club-shaped rods. They were catalase positive. No acid or gas was produced from glucose. The peptidoglycan type of CB 22-1 was A3γ, ll-diaminopimelic acid–Gly. The 16S ribosomal DNA analysis of the section with the highest variability revealed that this strain exhibited 94.3% similarity to the type strain of Nocardioides simplex (the highest value obtained). As data for all previously described Nocardioides strains were available for comparison, we concluded that strain CB 22-1 represents a new species in this genus. Therefore, it was designated a Nocardioides sp. strain.
Picric acid degradation.
In experiments performed in flasks, picric acid was completely transformed by Nocardioides sp. strain CB 22-2 at concentrations up to 5 mM in mineral salts medium A, and transformation was accompanied by stoichiometric nitrite release (Table 1). The COD of culture supernatants after degradation of 1 mM picric acid was 5 ± 2 mg of O2 liter−1, which corresponded to an average of 3% of the initial COD plus the COD due to nitrite.
TABLE 1.
Growth and picric acid degradation rates of Nocardioides sp. strain CB 22-2
| Mineral salts medium | Growth conditions | Growth rate (h−1) | Degradation rate (μmol/h/g [dry wt] of cells) | Yield (g [dry wt] of cells/g of picric acid) | Amt of nitrite released (mol/mol of picric acid) |
|---|---|---|---|---|---|
| A | Flask | 0.058 ± 0.003 | 920 | 0.27 ± 0.02 | 2.9 ± 0.1 |
| A | Fermentor | 0.087 ± 0.001 | 1,600 | 0.29 ± 0.02 | 2.9 ± 0.1 |
| B | Flask | 0.035 ± 0.002 | 850 | 0.19 ± 0.02 | 2.2 ± 0.2 |
In batch fermentations picric acid was metabolized at starting concentrations up to 40 mM. Even at a concentration of 40 mM significant cell growth was observed. Independent of the picric acid concentration, 5 to 6 mM picric acid was mineralized. Subsequently, cell death occurred. Complete degradation of picric acid took place at concentrations up to 6 mM.
In order to clarify whether the cell death that occurred after constant picric acid consumption was due to nitrite sensitivity, the nitrite dependence of picric acid degradation was examined. The degradation rate decreased dramatically as the nitrite concentration increased. Total transformation of 2 mM picric acid was observed at initial nitrite concentrations up to 10 mM. Initial nitrite concentrations of 15 and 20 mM resulted in incomplete degradation and residual picric acid concentrations of 0.38 and 0.72 mM, respectively.
In addition, strain CB 22-2 was able to utilize picric acid as the sole nitrogen source in mineral salts medium B lacking any other nitrogen source. Therefore, it is possible that the lower nitrite release leads to prolonged picric acid degradation. Nevertheless, the results of batch fermentation experiments revealed that picric acid degradation was complete only at concentrations up to 6 mM.
Characterization of metabolites.
During growth on picric acid a change in color from yellow to orange-red was observed. A bathochrome effect was detected in the corresponding UV-visible light spectra (data not shown).
During fermentations in continuous chemostatic cultures the following three compound were detected with the HPLC connected to a diode array detector (230 to 600 nm): picric acid, nitrite, and a third metabolite. To find out whether the third metabolite was H−-TNP as reported by Lenke and Knackmuss (11), some of its properties were compared with the properties of a synthetic complex. The UV spectra at a retention time of 2.73 min in the HPLC for both the culture supernatant and the synthetic preparation were identical. The UV spectrum and the NMR data [δ(H3) = 4.00 (singlet); δ(H5) = 8.81 (singlet)] were similar to the spectrum and NMR data described by Rieger for H−-TNP (18).
Near the end of some batch fermentations with preparations containing more than 3 mM picric acid, three additional metabolites were detected by the HPLC–UV-visible light analysis. The first metabolite (retention time, 9.50 min) was identified as 2,4-DNP; the second metabolite, compound X (retention time, 1.96 min) was characterized by performing additional experiments (see below); and the third metabolite, compound Y (retention time, 4.70 min; λmax, 480 nm), could not be identified. We eliminated the possibility that the compound Y peak belonged to aminonitrophenols or mononitrophenols. A typical culture supernatant from an incomplete batch fermentation contained 100 μM picric acid, 80 μM H−-TNP, 50 μM 2,4-DNP, about 5 μM compound X, and traces of compound Y.
Examination of cell extracts.
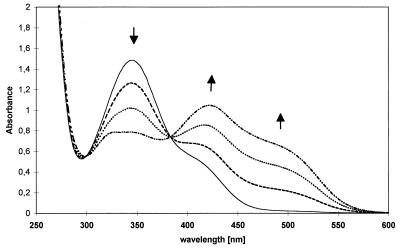
Figure 1 shows the time-dependent UV-visible light spectra related to the conversion of picric acid after addition of NADPH and cell extract. The characteristic increases in absorbance at 420 and 490 nm indicate that H−-TNP was formed, as described previously for R. erythropolis HL PM-1 (11, 18). HPLC analysis confirmed this result. No activity was detected in the controls which did not contain either crude extract, picric acid, or NADPH. NADH could not be used instead of NADPH. The specific activities were 0.15 μmol min−1 mg of protein−1.
FIG. 1.
[H−]-Meisenheimer complex formation during conversion of picric acid by cell extracts of Nocardioides sp. strain CB 22-2. UV spectra were recorded at zero time (——), 5 min (––––), 15 min (····), and 25 min (–··–).
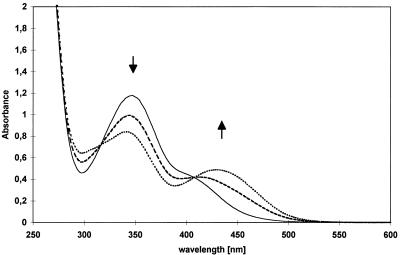
It has been shown for R. erythropolis HL PM-1 that the H−-TNP complex is enzymatically transformed to 2,4-DNP (18). As shown in Fig. 2, H−-TNP was converted by crude extracts of Nocardioides sp. strain CB 22-2 to 2,4-DNP with a specific activity of 0.10 μmol min−1 mg of protein−1. HPLC analysis proved that 2,4-DNP was formed almost exclusively. In contrast, experiments performed with thermally denatured extracts yielded 90% picric acid and only 10% 2,4-DNP after a reaction time of about 20 h.
FIG. 2.
Degradation of the [H−]-Meisenheimer complex of picric acid by cell extracts of Nocardioides sp. strain CB 22-2 with formation of 2,4-DNP. UV spectra were recorded at zero time (——), 2 min (––––), 4 min (····), and 8 min (–··–).
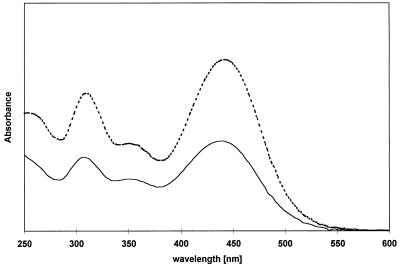
During conversion of 2,4-DNP by cell extracts of Nocardioides sp. strain CB 22-2 in the presence of NADPH, a bathochrome effect was detected in the UV-visible light spectrum (Fig. 3). A comparison of the UV-visible light spectra revealed that the compound (compound X) was also present in the supernatants of some incomplete batch fermentations. As the bathochrome effect in the UV-visible light spectra and the retention time in the HPLC analysis were similar to the bathochrome effect and retention time of H−-TNP, it is possible that compound X is a [H−]-Meisenheimer complex of 2,4-DNP. To confirm this, a synthetic analog of compound X was prepared. As the HPLC and UV-visible light spectrum data were the same (Fig. 4), synthetic compound X was characterized by performing a NMR analysis, and the data were compared with the data obtained for two compounds with great structural similarities (Table 2). ![]() The data verified that compound X was the 3-[H−]-Meisenheimer complex of 2,4-DNP (H−-DNP).
The data verified that compound X was the 3-[H−]-Meisenheimer complex of 2,4-DNP (H−-DNP).
FIG. 3.
Formation of the [H−]-Meisenheimer complex of 2,4-DNP during conversion of 2,4-DNP by cell extracts of Nocardioides sp. strain CB 22-2. UV spectra were recorded at zero time (——), 4 min (––––), and 12 min (····).
FIG. 4.
UV-visible light spectra after HPLC analysis for a retention time of 2.07 min, showing the curves for the Meisenheimer complex of 2,4-DNP. The dashed line is the curve for the synthetic complex, and the solid line is the curve for the culture supernatant.
TABLE 2.
1H NMR chemical shift and coupling constants for H−-DNP, 1,5-dinitro-3-methyl-3-aza-bicyclo[3.3.1]-nonene-(6)-one-(8) (a derivative of H−-DNP) (compound 1), and 3-[H−]-2,4-dinitroaniline (compound 2)
| Position | H−-DNP in D2Oa | H−-DNP in DMSO-d6a,b | Compound 1 in CDCl3c | Compound 2 in DMSO-d6b,d |
|---|---|---|---|---|
| H3, H3′ | 3.84 ppm (s)e | 3.34 ppm (s) | 3.57 ppm (s) | |
| H5 | 7.50 ppm (d)f | 7.33 ppm (d) | 7.42 ppm (dd) | 7.39 ppm (d) |
| H6 | 5.90 ppm (d) | 5.08 ppm (d) | 6.45 ppm (d) | 5.16 ppm (d) |
| 3J(H5H6) = 10.2 Hz | 3J(H5H6) = 9.2 Hz | 3J(H5H6) = 10.5 Hz | 3J(H5H6) = 10 Hzg |
Experiments performed with H−-DNP as the substrate resulted in the formation of 2,4-DNP if no NADPH was added to the assay mixture. The same result was obtained when we used extracts that were previously desalted with a NAP-10 column (Pharmacia) to remove NADP+, which would be necessary for the reverse reaction of H−-DNP formation. Moreover, addition of NADP+ did not enhance the degradation rate. In contrast, addition of NADPH resulted in a fourfold increase in the degradation rate of H−-DNP without generation of a new maximum absorbance, as would be expected for the formation of 2,4-DNP, 2-nitrophenol, or 4-nitrophenol. Furthermore, the nitrophenols were not converted by cell extracts of Nocardioides sp. strain CB 22-2.
Partial purification of the H−-TNP-synthesizing enzyme.
In order to determine if H−-DNP is formed by the first enzyme in a side reaction or is produced by a separate enzyme, a chromatographic fractionation experiment was performed. After this the H−-TNP-forming enzyme was recovered at a low activity. The fractions did not exhibit any H−-DNP-synthesizing or H−-TNP-degrading activity.
DISCUSSION
Nocardioides sp. strain CB 22-2 was enriched by using picric acid as the sole source of carbon, nitrogen, and energy. The release of approximately 3 mol of nitrite per mol of picric acid in mineral salts medium A containing an additional nitrogen source and the lack of dead-end product formation, as verified by the low residual COD, provided strong evidence that mineralization occurred. The yield, 0.29 g (dry weight) of cells per g of picric acid, seemed to be high for the following two reasons: (i) a considerable portion of the molecular weight of picric acid was provided by the three nitro groups; and (ii) at least two NADPH molecules had to be used to remove the nitro groups from the aromatic system.
Compared to the picric acid-degrading strains described previously, which were limited to picric acid concentrations lower than 4.4 mM, the strain which we investigated exhibited nearly 10 times greater picric acid resistance. In addition, the degradation rates during cultivation in flasks were about three times higher than the degradation rates reported by Rajan et al. (16). Therefore, Nocardioides sp. strain CB 22-2 has greater potential for picric acid degradation in waste streams or soils containing high concentrations of picric acid than other strains have.
A problem in this context is picric acid metabolism that is limited to about 15 mM nitrite. The ability of the strain to grow on picric acid without an additional nitrogen source does not solve this problem; the amount of nitrite which is necessary for biomass production is small compared to the amount of nitrite liberated. One possible solution, which would be useful in continuous chemostat fermentations, is adding nitrifying bacteria. We are currently investigating whether Nitrobacter strains can remove nitrite from fermentation broth.
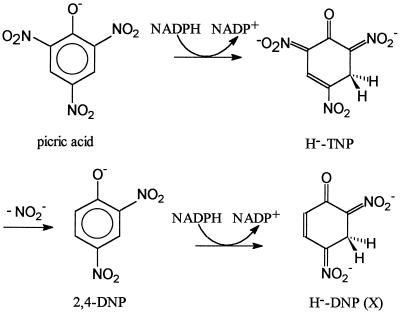
Lenke and Knackmuss (11) postulated and later Rieger and Knackmuss (19) proved that the first two steps in picric acid catabolism by R. erythropolis HL PM-1 are the formation of H−-TNP and the formation of 2,4-DNP (Fig. 5). The second reaction leads to the liberation of nitrite, which is used by the strain as the sole nitrogen source. These first two steps are the same in picric acid metabolism by Nocardioides sp. strain CB 22-2. Consequently, this mechanism seems to be a common mechanism in picric acid degradation.
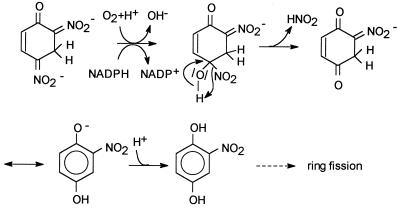
FIG. 5.
First three steps in picric acid metabolism by Nocardioides sp. strain CB 22-2.
Reduction of the aromatic ring was proposed by Lenke et al. for the metabolism of 2,4-DNP (12). A reduced ring fission product containing two nitro groups, 4,6-dinitrohexanoate, was isolated as a dead-end product of 2,4-DNP metabolism by R. erythropolis. To our knowledge, no further details of this reaction are known. In this report we describe additional possible portions of the reductive pathway for catabolism of 2,4-DNP. We found a novel reduced metabolite, the 3-[H−]-Meisenheimer complex of 2,4-DNP, which seems to be physiologically relevant for the following reasons: (i) H−-DNP is produced by cell extracts of Nocardioides sp. strain CB 22-2 and also occurs in the culture supernatant; and (ii) an enzyme, which differs from the H−-TNP-synthesizing activity, seems to be responsible for the formation of H−-DNP.
In contrast to 2,4-DNP, which results from the elimination of nitrite from the H−-TNP complex, no 4- or 2-nitrophenol was detected after transformation of H−-DNP with cell extracts. Additionally, neither 2-nitrophenol nor 4-nitrophenol was used as a carbon source. It has been reported that uptake of nitrophenols into cells limits the reaction rates of these chemicals in Pseudomonas putida B2 (6). Consequently, it is possible that the lack of degradation capacity for mononitrophenols is caused by the lack of a transport mechanism. This explanation could not be confirmed for Nocardioides sp. strain CB 22-2, because the compounds were not transformed by crude cell extracts.
An alternative hypothetical pathway for H−-DNP transformation is presented in Fig. 6. The results of the experiments performed with crude extracts imply that NADPH is necessary as a cofactor. The first reaction step is common for monooxygenases. There are some examples of hydroxylation of phenols in the ortho position, such as the phenol hydroxylase reaction (3, 15), and there are other examples of substitution at the para carbon atom, such as the 3-hydroxybenzoate monooxygenase reaction (10). Monooxygenase-catalyzed reactions are known for nitroaromatic compounds as well (25); p-nitrophenol is para-hydroxylated to hydrochinone by an enzyme of a Moraxella sp. strain in an NADPH- and oxygen-dependent reaction with concomitant release of nitrite (26, 27). The nitrophenol oxygenase of P. putida B2 converts 2-nitrophenol (ortho hydroxylation) to catechol in a corresponding reaction (34, 35). In the case of H−-DNP, an analogous mechanism could lead to the following products: para-hydroxylation of H−-DNP could result in 2-nitrohydrochinone, as shown in Fig. 6; and ortho hydroxylation could generate 4-nitrocatechol. As we did not find a 4-nitrocatechol-converting activity in crude extracts (data not shown) and as picric acid metabolism was inhibited by this compound, we postulated that the initial attack occurs at position 4. Compared to the examples described previously, there is the following important difference in our proposed mechanism: a Meisenheimer complex is the substrate for the monooxygenase. We argue that the initial electrophilic attack on 2,4-DNP should be more difficult than the attack on the mononitrophenols described previously, because 2,4-DNP contains one supplementary electron-withdrawing nitro group. To our knowledge, electrophilic attack of 2,4-DNP has not been described. In contrast, 2,4-dinitrotoluene is degraded via an initial dioxygenase reaction (28, 29). However, this reaction should be more facile due to the positive inductive effect of the methyl group, in contrast to the negative inductive effect of the hydroxyl group in 2,4-DNP. The addition of a hydride to the 2,4-DNP aromatic system should facilitate the electrophilic attack, because an additional negative charge is brought into the system. Workers are currently trying to prove or reject these mechanistic considerations.
FIG. 6.
Hypothetical mechanism for NADPH-dependent conversion of H−-DNP.
ACKNOWLEDGMENTS
This work was supported by a Lise-Meitner grant from the Ministerium für Wissenschaft und Forschung des Landes Nordrhein-Westfalen to K.H.-W.
We thank the group of T. N. Mitchell for recording the NMR spectra, the group of U. Pfüller for help with the HPLC analysis, and C. Fetzer, S. Juranek, A. Pfeifer, V. Schulte, and T. Zahn for excellent assistance.
REFERENCES
- 1.Alvarez M A, Kitts C L, Botsford J L, Unkefer P J. Pseudomonas aeruginosa strain MA01 aerobically metabolizes the aminodinitrotoluenes produced by 2,4,6-trinitrotoluene nitro group reduction. Can J Microbiol. 1995;41:984–991. doi: 10.1139/m95-137. [DOI] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Detmer K, Massey V. Effect of substrate and pH on the oxidative half-reaction of phenol hydroxylase. J Biol Chem. 1985;260:5998–6005. [PubMed] [Google Scholar]
- 4.Erikson D. Studies on some lake-mud strains of Micromonaspora. J Bacteriol. 1941;41:277–300. doi: 10.1128/jb.41.3.277-300.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiorella P D, Spain J C. Transformation of 2,4,6-trinitrotoluene by Pseudomonas pseudoalcaligenes JS52. Appl Environ Microbiol. 1997;63:2007–2015. doi: 10.1128/aem.63.5.2007-2015.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folsom B R. Characterization of 2-nitrophenol uptake system of Pseudomonas putida B2. J Ind Microbiol. 1997;19:123–129. [Google Scholar]
- 7.Gold V, Miri A Y, Robinson S R. Sodium borohydride as a reagent for nucleophilic aromatic substitution by hydrogen: the role of hydride Meisenheimer adducts as reaction intermediates. J Chem Soc Perkin Trans I. 1980;1980:243–249. [Google Scholar]
- 8.Gundersen K, Jensen H J. A soil bacterium decomposing organic nitro-compounds. Acta Agric Scand. 1956;6:100–114. [Google Scholar]
- 9.Hartter D R. The use and importance of nitroaromatic chemicals in the chemical industry. In: Rickert D E, editor. Toxicity of nitroaromatic compounds. Chemical Industry Institute of Toxicology Series. New York, N.Y: Hemisphere Publishing; 1985. pp. 1–13. [Google Scholar]
- 10.Jones D C N, Cooper R A. Catabolism of 3-hydroxybenzoate by the gentisate pathway in Klebsiella pneumoniae M5a1. Arch Microbiol. 1990;154:489–495. doi: 10.1007/BF00245233. [DOI] [PubMed] [Google Scholar]
- 11.Lenke H, Knackmuss H-J. Initial hydrogenation during catabolism of picric acid by Rhodococcus erythropolis HL 24-2. Appl Environ Microbiol. 1992;58:2933–2937. doi: 10.1128/aem.58.9.2933-2937.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenke H, Pieper D H, Bruhn C, Knackmuss H-J. Degradation of 2,4-dinitrophenol by two Rhodococcus strains, HL 24-1 and HL 24-2. Appl Environ Microbiol. 1992;58:2928–2932. doi: 10.1128/aem.58.9.2928-2932.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meisenheimer J. Über Reaktionen aromatischer Nitrokörper. Ann Chem. 1902;323:205–246. [Google Scholar]
- 14.Montgomery H A C, Dymock J F. The determination of nitrite in water. Analyst. 1961;35:414–416. [Google Scholar]
- 15.Neujahr H Y, Gaal A. Phenol hydroxylase from yeast. Eur J Biochem. 1973;35:386–400. doi: 10.1111/j.1432-1033.1973.tb02851.x. [DOI] [PubMed] [Google Scholar]
- 16.Rajan J, Valli K, Perkins R E, Sariaslani F S, Barns S M, Reysenbach A-L, Rehm S, Ehringer M, Pace N R. Mineralization of 2,4,6-trinitrophenol (picric acid): characterization and phylogenetic identification of microbial strain. J Ind Microbiol. 1996;16:319–324. doi: 10.1007/BF01570041. [DOI] [PubMed] [Google Scholar]
- 17.Reasoner D J, Geldreich E E. A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol. 1985;49:1–7. doi: 10.1128/aem.49.1.1-7.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rieger P-G. Ph.D. thesis. Stuttgart, Germany: Universität Stuttgart; 1996. [Google Scholar]
- 19.Rieger P-G, Knackmuss H-J. Basic knowledge and perspectives on biodegradation of 2,4,6-trinitrotoluene and related nitroaromatic compounds in contaminated soil. In: Spain J C, editor. Biodegradation of nitrooaromatic compounds. New York, N.Y: Plenum Press; 1995. pp. 1–18. [Google Scholar]
- 20.Schmidt K S, Liaaen-Jensen S, Schlegel H G. Carotinoide der Thiorhodacaceae. Arch Microbiol. 1963;46:117–126. [PubMed] [Google Scholar]
- 21.Schreibner K, Hofrichter M, Herre A, Michels J, Fritsche W. Screening for fungi intensively mineralizing 2,4,6-trinitrotoluene. Appl Microbiol Biotechnol. 1997;47:452–457. doi: 10.1007/s002530050955. [DOI] [PubMed] [Google Scholar]
- 22.Severin T, Adam M. Umsetzung von Nitroaromaten mit Natriumborhydrid, II. Chem Ber. 1963;96:448–452. [Google Scholar]
- 23.Severin T, Schmitz R. Umsetzung von Nitroaromaten mit Natriumborhydrid. Chem Ber. 1962;95:1417–1419. [Google Scholar]
- 24.Severin T, Loske J, Scheel D. Umsetzung von Nitroaromaten mit Natriumborhydrid, V. Chem Ber. 1969;102:3909–3914. doi: 10.1002/cber.19691021135. [DOI] [PubMed] [Google Scholar]
- 25.Spain J C. Biodegradation of aromatic compounds. Annu Rev Microbiol. 1995;49:523–555. doi: 10.1146/annurev.mi.49.100195.002515. [DOI] [PubMed] [Google Scholar]
- 26.Spain J C, Gibson D T. Pathway for biodegradation of p-nitrophenol in a Moraxella sp. Appl Environ Microbiol. 1991;57:812–819. doi: 10.1128/aem.57.3.812-819.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spain J C, Wyss O, Gibson D T. Enzymatic oxidation of p-nitrophenol. Biochem Biophys Res Commun. 1979;88:634–641. doi: 10.1016/0006-291x(79)92095-3. [DOI] [PubMed] [Google Scholar]
- 28.Spangford R J, Spain J C, Nishino S F, Mortelmans K E. Biodegradation of 2,4-dinitrotoluene by Pseudomonas sp. Appl Environ Microbiol. 1991;57:3200–3205. doi: 10.1128/aem.57.11.3200-3205.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suen W-C, Haigler B E, Spain J C. 2,4-Dinitrotoluene dioxygenase from Burkholderia sp. strain DNT: similarity to naphthalene dioxygenase. J Bacteriol. 1996;178:4926–4934. doi: 10.1128/jb.178.16.4926-4934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Süssmuth R, Eberspächer J, Haag R, Springer W. Biochemischmikrobiologisches Praktikum. Stuttgart, Germany: Georg Thieme Verlag; 1987. [Google Scholar]
- 31.Vorbeck C, Lenke H, Fischer P, Knackmuss H-J. Identification of a hydride-Meisenheimer complex as a metabolite of 2,4,6-trinitrotoluene by a Mycobacterium strain. J Bacteriol. 1994;176:932–934. doi: 10.1128/jb.176.3.932-934.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vorbeck C, Lenke H, Fischer P, Spain J C, Knackmuss H-J. Initial reductive reactions in aerobic microbial metabolism of 2,4,6-trinitrotoluene. Appl Environ Microbiol. 1998;64:246–252. doi: 10.1128/aem.64.1.246-252.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wyman J F, Serve M P, Hobson D W, Lee L H, Uddin D. Acute toxicity, distribution, and metabolism of 2,4,6-trinitrophenol (picric acid) in Fischer 344 rats. J Toxicol Environ Health. 1992;37:313–327. doi: 10.1080/15287399209531672. [DOI] [PubMed] [Google Scholar]
- 34.Zeyer J, Kocher H P. Purification and characterization of a bacterial nitrophenol oxygenase which converts ortho-nitrophenol to catechol and nitrite. J Bacteriol. 1988;170:1789–1794. doi: 10.1128/jb.170.4.1789-1794.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeyer J, Kocher H P, Timmis K N. Influence of para substituents on the oxidative metabolism of o-nitrophenols by Pseudomonas putida B2. Appl Environ Microbiol. 1986;52:334–339. doi: 10.1128/aem.52.2.334-339.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]