Abstract
The renin-angiotensin system (RAS) is a highly complex hormonal cascade that spans multiple organs and cell types to regulate solute and fluid balance along with cardiovascular function. Much of our current understanding of the functions of the RAS has emerged from a series of key studies in genetically-modified animals. Here, we review key findings from ground-breaking transgenic models, spanning decades of research into the RAS, with a focus on their use in studying blood pressure. We review the physiological importance of this regulatory system as evident through the examination of mouse models for several major RAS components: angiotensinogen, renin, ACE, ACE2, and the type 1A angiotensin receptor. Both whole-animal and cell-specific knockout models have permitted critical RAS functions to be defined and demonstrate how redundancy and multiplicity within the RAS allow for compensatory adjustments to maintain homeostasis. Moreover, these models present exciting opportunities for continued discovery surrounding the role of the RAS in disease pathogenesis and treatment for cardiovascular disease and beyond.
Keywords: Mouse models, ACE, AT1R, ACE2, cell-specific knockout mice
1.1. Introduction.
The renin angiotensin system (RAS)* is a tightly regulated homeostatic system with a key role in fluid balance and blood pressure regulation, with dysregulation of the RAS frequently occurring in disease states such as hypertension, kidney disease, and heart failure. Our current understanding of the functions of the RAS has emerged from a series of key studies in genetically-modified animals, several of which are highlighted in this chapter. These studies have supported the development and use of pharmacologic compounds to block the RAS such as angiotensin converting enzyme (ACE)† inhibitors and angiotensin receptor blockers (ARB)‡ which are some of the most routinely prescribed medications in the world.1–4
The classical RAS initiates with the production of angiotensinogen (Agt)§ and concludes with the actions of angiotensin II (Ang II)** mediated by its major receptor (AT1R).†† Additional elements comprise the non-classical RAS and include peptide products (Ang1–7) of alternative degradative pathways (prolyloligopeptidase,5 polycarboxypeptidase6) and receptor binding (MAS receptor)7 which can lead to largely opposing effects.8 Insights into the physiological relevance of these components were elucidated from some of the earliest transgenic mouse models created and remain a hallmark of the power of transgenic tools over the past few decades.9 Initially, individual RAS components would be targeted globally, meaning to eliminate expression from the entire organism, typically a mouse. However, many RAS components are expressed in multiple cell types present in different organs, ultimately leading researchers to question whether systemic or local production played the larger role in driving their physiological functions.10 Development of newer technologies for cell-specific gene targeting emerged, such as cre-loxP methodology, which allowed investigators to define gene function within specific cell populations.11 Examination of the cell-specific actions of RAS components has advanced the field by illustrating the complexity of this homeostatic system.
Our review focuses on insights into the RAS gained from gene targeting in mice, with a focused update to include cell-specific models. We have organized this review by RAS component starting with the generation of Agt and concluding with the receptor responsible for transducing the majority of RAS signaling, the type 1 angiotensin receptor (AT1R). Our review highlights the use of transgenic mouse models for the study of blood pressure, in addition to examining effects within the cardiovascular system. Our review focuses on the production and action of Ang II, and does not cover bradykinin, chymase, or aldosterone. Additional details are found throughout this volume, and we include a more comprehensive table for further reference (Table 1).
Table 1.
Mouse models to study the renin angiotensin system
| RAS Component | Gene Species | Mouse Strain | Notes | |
|---|---|---|---|---|
|
| ||||
| AGT | ||||
|
| ||||
| Knockout | Agt KO | Murine | C57BL/6 | Whole animal knockout19 |
| Agt KO | Murine | C57BL/6 | Whole animal knockout14 | |
| KAP-Agt-KO | Murine | C57BL/6N | Proximal tubule knockout122 | |
| Alb-Agt-Cre | Murine | C57BL/6N | Hepatocyte knockout22 | |
| KAP-Cre:Alb-Cre-Agt | Murine | C57BL/6N | Proximal tubule and hepatocyte knockout22 | |
| NEP25-KAP-Agt-KO | Murine | Podocyte injury model2 | ||
| Agt Copy Number Variants | Murine | 129xC57BL/6 | Changes in the level of Agt expression14 | |
| Agt+/−, Agt−/− | Murine | C57BL/6 | ||
| Overexpression | hRen+/hAng+ | Human | Mixed C57BL/6 | Double transgenic: human renin and human angiotensinogen16 |
| hAgt | Human | C57BL/6 × SJL/J | Whole animal overexpression17 | |
| Renin | ||||
|
| ||||
| Knockout | Ren2 KO | Murine | 129/Ola | Normal resting BP in presence of Renld alone30 |
| Ren-1 d KO | Murine | 129 | Abnormalities in the macula densa and juxtaglomerular cells31 | |
| Ren-1 c KO | Murine | Mixed C57:CBA Inbred C57 | Early death, significant hypotension, inability to concentrate urine33,34 | |
| AQP2-Cre | Murine | C57BL/6 | Collecting duct knockout36 | |
| Hox-b7-Cre | Murine | C57BL/6 | Collecting duct knockout of prorenin receptor109 | |
| Prorenin receptor KO | Murine | C57BL/6 | Renal tubular-specific prorenin receptor knockout38 | |
| Pax8-Cre | Murine | C57BL/6 | Nephron-specific knockout of Atp6ap2 pro-renin receptor39 | |
| nNOS/CreERT2 MD PRR KO | Murine | C57BL/6 | Macula densa knockout of pro-renin receptor40 | |
| Ren1d-Cre | Murine | C57BL6/J 129SvEv | Crossed to R26R or Z/EG reporter lines expressing β- galactosidase or GFP.42 Ren-1dCre mouse crossed to mT/mG reporter mice43 |
|
| ACE | ||||
|
| ||||
| Knockout | ACE1/1 | Murine | 129 × C57BL/6 | Low blood pressure, renal pathology50 |
| ACE N-KO | Murine | Mice lack N-domain of ACE53 | ||
| ACE C-KO | Murine | 129 × C57BL/6 | Mice lack C-domain of ACE54 | |
| ACE3/3 | Murine | 129 × C57BL/6 | Endothelial ACE knockout; ACE expression swapped to Albumin promoter58 | |
| ACE 8/8 | Murine | 129/C57BL/6 | Cardiac-restricted expression via a-myosin heavy chain promoter64 | |
| ACE 9/9 | Murine | 129 × C57BL/6 | ACE expression limited to the renal tubular epithelium63 | |
| ACE10/10 | Murine | 129 × C57BL/6 | ACE expression under control of c-fms promoter cassette: targeted to macrophage and macrophage- lineage cells 23 | |
| NeuACE | Murine | C57BL/6 | ACE expression under the control of the cfms promoter: targeted to neutrophils124 | |
| Overexpression | ACE copy number variants | Murine | C57BL/6 × 129/SvEv | One, two, or three functional copies of ACE gene68 |
| ACE2 | ||||
|
| ||||
| Knockout | ACE2−/y | Murine | Mixed 129 × C57BL/6 | Severe cardiac contractility disruption (phenotype lost in later studies)72 |
| ACE2−/y | Murine | Mixed 129 × C57BL/6 | Normal cardiac function and morphology73 | |
| ACE2−/y | Murine | Mixed 129/SvEv × C57BL/6 | Normal cardiac function and morphology74 | |
| ACE2−/y | Murine | 129/SvEv | No change in baseline BP74 | |
| ACE2−/y | Murine | C57BL/6 | Modest increase in baseline BP (~7 mmHg), no cardiac hypertrophy74 | |
| ACE2−/− | Murine | C57BL/6 | CRISPR-mediated frameshift mutation resulting ACE2 knock out125 | |
| Overexpression | ACE2-S680D Knock-in | Murine | C57BL/6 | A phosphomimic (S680D) mutant of ACE2125 |
| Synapsin-hACE2 | Human | C57BL/6 | Neuron-specific overexpression of ACE284 | |
| Synapsin-LoxP-hACE2 | Human | C57BL/6 × SJL/J | Cre-mediated neuron-specific overexpression of hACE2126 | |
| ROSA26 Ace2/Ace2 | Murine | Mixed 129/BL6 | Global ACE2 overexpression127 | |
| Nephrin-ACE2 | Human | FVB | Podocyte-specific overexpression of ACE282 | |
| hACE2 | Human | ICR | Driven by mouse ACE2 promoter87 | |
| hACE2 | Human | C57BL/6 × BALB/c mixed | Driven by CAG promoter; several lines with differing ACE2 expression91 | |
| K18-hACE2 | Human | C57BL/6J × SJL/J | Keratin 18 promoter; epithelial-specific expression88 | |
| HFH4-hACE2 | Human | C3H × C57BL/6 (C3B6) | HFH4 promoter—expression in multiple tissues90 | |
| PRCP & POP | ||||
|
| ||||
| KST302 | Murine | C57BL/6 | Global PRCP deficiency6 | |
| GST090 | Murine | FVB/N | Global PRCP deficiency6 | |
| ACE2 −/− /PRCP −/− | Murine | Global deficiency of ACE2 and PRCP5 | ||
| POP−/− | Murine | Global POP deficiency5 | ||
| AT1AR | ||||
|
| ||||
| Knockout | AT1AR KO | Murine | C57BL/6J | Severe hypotension97 |
| Pepck-Cre | Murine | 129/SvEv | Proximal tubule knockout; hypotensive103 | |
| KAP2-Cre | Murine | C57BL/6 | Proximal tubule knockout; hypotensive at baseline and with Ang II infusion107 |
|
| KAP2-AT1AR-N11G | Rat | C57BL/6J | Constitutively active form (N111G) of the rat AT1AR; active in the absence of Ang II107 | |
| Pod-Cre | Murine | Double knockout of AT1AR and Dyn1 and Dyn2110 | ||
| Hoxb7-Cre | Murine | 129/SvEv | Collecting duct knockout using Hoxb7-Cre promoter 109 | |
| AQP2-Cre | Murine | 129/SvEv | Principal cells of the collecting duct knockout108 | |
| AVP-Cre | Murine | C57BL/6J | Vasopressin-producing cells111 | |
| Nefh-Cre | Murine | Neuron-specific knockout128 | ||
| LepR-Cre | Murine | C57BL/6J | Leptin receptor-expressing cells113 | |
| ARP-Cre | Murine | C57BL/6J | Neurons expressing agouti-related peptide113 | |
| TH-Cre | Murine | Catacholaminergic cells114 | ||
| CRF-Cre | C57BL/6 | Cortocotropin-releasing factor neurons115 | ||
| CD4-Cre | Murine | 129/SvEv | T Lymphocytes117 | |
| LysM-Cre | Murine | 129/SvEv | Macrophages118 | |
| Lck-Cre | 129/SvEv | T Lymphocytes | ||
| OT-1-Cre | Murine | C57BL/6 | Antigen-specific CD8+ cells120 | |
| KISm22α-Cre | Murine | 129/SvEv | Vascular smooth muscle96 | |
RAS: Renin angiotensin system
Agt: Angiotensinogen
ACE: Angiotensin converting enzyme
ACE2: Angiotensin converting enzyme 2
PRCP: Prolylcarboxypeptidase
POP: Prolyloligopeptidase
AT1AR: Type 1 angiotensin receptor
2.1. Angiotensinogen
Angiotensinogen (Agt) is the only known substrate for the enzyme renin in the rate-limiting reaction that generates angiotensin I (Ang I)‡‡ as the initial step of the RAS.12 Unlike other components of the RAS, the cleavage of Agt by renin is species-specific,13 and this feature has been leveraged to design molecular genetic studies by Sigmund and others.14–17 For example, double transgenic mice with expression of both human renin and angiotensinogen genes in the central nervous system (CNS)§§ were moderately hypertensive.18 In contrast, blood pressures (BP)*** in global Agt KO††† mice were markedly reduced and mice had increased mortality associated with abnormal renal structure. Total genetic deletion of Agt resulted in a severe phenotype characterized by diminished RAS activity from loss of this early substrate as well as other subsequent critical RAS components (described below).14,19 Conversely, mice with additional copies of the Agt gene had a nearly dose-dependent linear increase in BP related to Agt gene copy number.20
These studies examining Agt clearly demonstrate the contribution of Agt to BP control under normal and pathological states. More recent work by Ichikawa and colleagues examined the cellular source of Agt under different conditions.21–23 Kidney-specific Agt KO mice (KAP-Cre)22 had reduced mRNA expression of Agt but unaffected Agt protein levels in the kidney, compared with control animals. In contrast, liver-specific Agt KO mice (Alb-Cre)22 had the expected reduction in liver Agt mRNA and this was associated with reduced Agt protein in the kidney (intact mRNA) and lower renal Ang II levels. Dual liver and kidney Agt KO mice resembled liver Agt KO animals. This work suggested that liver was the primary source of Agt in the kidney and that Agt was incorporated in renal proximal tubule cells via a megalin-dependent pathway which was increased with disruption of the glomerular filtration barrier. Follow-up studies using a model of glomerular disease induced with podocyte-specific immunotoxin (NEP25 mice injected with LMB2)21 demonstrated increased renal Agt protein and Ang II, which was attenuated with either liver disruption of Agt (reduction in source of substrate) or megalin disruption (reduction in Agt internalization).23 Thus, liver-derived Agt does appear to play a key role in renal Ang II generation and intra-renal RAS regulation.
3.1. Renin
Catalyzing the first step in the RAS cascade, renin levels are a key determinant of overall RAS tone and are subject to complex regulation.24 Active renin possesses enzymatic activity against angiotensinogen whereas its precursor, prorenin, does not. Putative receptors for prorenin (PRR)‡‡‡ have been described and are believed to play a role in activating renin; data regarding this component of the RAS continue to emerge.25 In the adult, the major source of circulating renin is the kidney, specifically the specialized juxtaglomerular cells (JG cells)§§§ near the macula densa.26 Renin is also produced by the distal nephron and may serve physiological and pathophysiological roles there.26–28
Mice have 1 or 2 renin genes per haploid genome depending on strain (eg., C57 have 1; 129Sv have 2); some strains have a conserved Ren1c or modified Ren1d allele for Ren1 in addition to the Ren2 gene.29 Ren2-deficient mice30 had normal resting blood pressures in the presence of Ren1d alone. In contrast, deletion of Ren1d in female mice led to reduced renin levels and lower baseline blood pressures.31 Ren1d KO mice of both sexes had significant morphological abnormalities in macula densa cells and absence of secretory granules in JG cells of the renal afferent arterial. Modulating the two separate renin genes delineated non-overlapping roles for these renin genes as well as potential for phenotypic rescue with complementation with human renin.32 Even more pronounced was the phenotype resulting from Ren1c deletion in mixed C57:CBA and inbred C57 mice.33,34 Homozygous C57-Ren1c null mice had early death, significant hypotension, inability to concentrate urine in the setting of hydronephrosis and accelerated renal pathologic changes.34
Cell-specific genetic models have helped to illustrate the role of renin produced outside of the macula densa. Ramkumar et al generated mice with collecting duct overexpression of renin (under control of AQP2 promoter).35 Collecting duct-derived renin contributed to hypertension (on high salt diet), urinary excretion of renin, and suppression of plasma renin concentration. The same group also generated a collecting duct-specific knockout model36 which demonstrated complementary findings with reduced kidney and urinary excretion of renin, higher plasma renin concentration, and attenuated hypertensive response to Ang II infusion. Similarly, models targeting the prorenin receptor have been utilized to further examine renin and precursors37–40 and are included in Table 1. Together, these studies highlight the contributions of tubule-derived renin and renin precursors to the RAS and BP regulation.41
To understand the plasticity of renin-producing cells, fate-mapping studies were performed using cre-LoxP technology to identify the location of renin expression.42 A novel Ren1d-Cre mouse line was generated and crossed with an R26R or Z/Eg reporter line expressing βgal or GFP respectively, which demonstrated that multiple cell types originate from renin-producing precursors. Treatment of Ren1d-Cre;R26R mice with a low sodium diet and an ACE inhibitor led to recruitment of renin-expressing cells within glomerular and extraglomerular mesangium. Fate-mapping demonstrated that cells recruited for renin expression under stress are retransformed cells that previously expressed renin and can also de-transform after the stress has ceased. Thus, renin production is recruited to compensate for homeostatic changes. Subsequent use of Ren1d-Cre crossed to mT/mG reporter mice in streptozotocin-induced diabetes examined the renin lineage in a diabetic environment.43 In diabetic mice, increased urinary renin resulted from glomerular hyperfiltration coupled with impaired proximal tubule renin reclamation while the renin cell distribution was preserved. These studies highlight the complex mechanisms by which renin can respond to shifts in homeostasis.
4.1. Angiotensin Converting Enzyme
Angiotensin converting enzyme (ACE) is one of the central enzymes in the RAS due to its ability to convert Ang I into the potent vasoconstrictor Ang II. As such, the pharmacologic inhibition of ACE is one of the best-proven therapeutic strategies in cardiovascular disease.2–4,44–47 Tissue expression of ACE is nearly ubiquitous, with high expression in endothelial cells and the kidney.48 The importance of ACE within the RAS has been delineated by the expansive work of Bernstein and colleagues. For example, generation of ACE knockout mice (ACE KO or ACE1/1) using targeted homologous recombination yielded a powerful tool for studying the varied actions of ACE. ACE KO mice had consistent phenotypic abnormalities also seen in mice lacking renin, angiotensinogen, or the AT1Rs, including profound hypotension, hypoplastic renal medullae, and an inability to concentrate urine. ACE KO mice also exhibit hematologic abnormalities such as anemia.49 The consistency of the renal abnormalities suggests that a lack of Ang II formation or action underlies these changes.33,49–52
ACE is comprised of two homologous, independent catalytic domains, the N-domain and the C-domain, which have been studied separately for their unique contributions to blood pressure regulation. Bernstein and colleagues studied these domains independently by introducing mutations to disrupt the catalytic activity of the N or C domain—generating ACE N-KO53 or ACE C-KO54 mice, respectively. These studies demonstrated that mice lacking the N domain possess an unhindered ability to process Ang I, whereas mice lacking the C domain maintain blood pressure through compensatory increases in renin and plasma Ang I levels. Thus, the C domain appears to be the predominant site for the conversion of Ang I to Ang II.54 The N-domain has activity against N-acetyl-seryl-aspartyl-lysyl-proline (AcSDKP) which has anti-inflammatory and anti-fibrotic effects. Diabetic ACE N-KO had increased sodium excretion and protection against renal injury. These studies underscore the multifaceted functions of ACE to include substrates other than angiotensin I.55,56
4.2. Endothelial ACE.
ACE is highly expressed in the vascular endothelium, where it facilitates the conversion of Ang I to Ang II throughout the circulation.57 To examine the function of endothelial ACE, mice lacking endothelial ACE (termed ACE.3 mice), were generated by directing expression of ACE under the albumin promoter, yielding mice which produce ACE by hepatocytes to maintain normal levels of circulating ACE.58 Mice lacking endothelial ACE were able to maintain normal blood pressures, pointing to the relative flexibility of the RAS, wherein origin of ACE may vary without impacting blood pressure. One potential explanation for the blood pressure preservation is the compensatory expression of renin, which can compensate up to a 90% reduction of ACE.49,59 Thus, a lack of endothelial ACE alone appeared insufficient to destabilize the RAS.
4.3. Renal ACE.
As mentioned, ACE, angiotensinogen, renin, and AT1R knockout mice all display renal developmental abnormalities.33,49–52 In contrast, mice which lack renal ACE but have normal systemic ACE have preserved kidney organogenesis, suggesting that renal expression of ACE is not necessary during development if systemic ACE is present.60 Moreover, studies indicate that mice lacking renal ACE are able to maintain renal function if expression of ACE in other tissues is preserved. Heterozygous mice with one null ACE allele also maintained normal blood pressures, renal filtration rates and urine concentrating abilities, despite a reduction in tubuloglomerular feedback, owing to increases in renin expression.61 The brush border of the proximal tubule displays robust ACE expression relative to other organs.62 Mice in which expression is limited to only the renal tubular epithelium (ACE9/9 mice) had a reduction in total ACE levels and were phenotypically similar to ACE knockout mice with hypotension, an inability to concentrate urine, and medullary thinning.63 Taken together, this work highlights the importance of the cellular sources of ACE, suggesting that systemic ACE activity is necessary for preservation of renal structure, while renal tubular ACE is important for blood pressure regulation.
ACE9/9 mice which lack systemic ACE developed sustained hypertension during chronic Ang I infusion, likely due to locally-generated increased intra-renal Ang II levels.63 While previous studies demonstrated that systemic ACE is necessary for renal function under normal conditions, this study points to the importance of the intra-renal RAS system in the regulation of blood pressure, particularly in pathological states.60,63 Moreover, the hypertensive response to Ang II was similarly blunted in mice with ACE expression restricted to the liver (ACE 3/3) or myelomonocytic cells (ACE 10/10) which had no or minimal renal ACE levels.60 This response was coincident with changes in natriuresis and nephron transporter activation in the ACE3/3 and ACE 10/10 mice. From these studies in mice with tissue-specific targeting of ACE expression, we see that renal ACE and intrarenal Ang II play an integral role in sodium handling by the kidney and responding to hypertensive stimuli.
4.4. Cardiac ACE.
Overexpression of ACE within cardiomyocytes—with concomitant absence of ACE in kidney or vascular endothelium—lead to a severe phenotype characterized by atrial enlargement, arrythmia, and sudden death. In these mice, termed ACE 8/8 mice,64 ventricular size and function were preserved despite increased cardiac Ang II levels. The conduction defects seen in these mice were associated with changes in cardiac connexins, suggesting a possible mechanism of increased arrythmia risk in heart failure and other pathological changes associated with increased RAS activation.65 Moreover, ACE 8/8 mice treated with an ACE inhibitor or ARB had improved conductivity, associated with a reduction in connexin43.66 A more tempered model of cardiac-restricted ACE expression was also developed (ACE1/8 mice) by combining the ACE KO (ACE 1/1) and cardiac overexpression mouse lines (ACE 8/8).67 ACE1/8 mice had normal blood pressures and intact renal concentrating function, suggesting that cardiac ACE expression is sufficient to maintain major homeostatic mechanisms.67
4.5. Gene titration studies.
Gene titration studies from the Smithies group helped to identify key compensatory and regulatory features of the RAS by examining mice with differing ACE copy numbers. Blood pressures were normal in mice with 1–3 copies of the ACE gene, largely due to a compensatory increase in kidney renin levels in mice possessing only one copy of the ACE gene.68 Indeed in mice with only one ACE copy, increases in renin expression are sufficient to rescue blood pressure to normal levels,68 in contrast to an earlier study by Esther et al. in which mice homozygous for an ACE mutant allele— thus deficient in ACE—were markedly hypotensive due to incomplete compensation by renin.50 Taken together, these studies highlight the multiplicity of the RAS in regulating blood pressure. The RAS possesses adaptive flexibility that helps to maintain homeostatic mechanisms in the absence of additional pathological forces.69 Beyond the scope of this review, transgenic mouse models to study ACE in the immune system are included in Table 1.
The complexity and multiplicity of ACE within organ systems is demonstrated by the relative abundance of transgenic mouse models dedicated to its study. Together, these studies support a fundamental role for ACE in the maintenance of cardiovascular homeostasis, among other functions.
5.1. Angiotensin Converting Enzyme 2
Identification of ACE2,**** an ACE homolog, from ventricular tissue of a heart failure patient in 200070 sparked new inquiry in cardiovascular physiology. Differences between the homologs—namely that ACE2 is expressed in a more limited pattern in the kidney and heart and has substrate specificity to degrade Ang II preferentially over Ang I—implicated ACE2 in the non-classical actions of the RAS. Degradation of Ang II by ACE2 leads to the formation of Angiotensin 1–7 (Ang 1–7)††††, which has vasodilatory actions that oppose Ang II.71 Over the past 20 years, our understanding of ACE2 has grown, and much of this research has hinged on genetic manipulation of ACE2 in rodents. Presently, ACE2 is being intensely studied as it also serves as the receptor for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV2)‡‡‡‡ and there may be overlap between its cardiovascular functions and viral receptor pathways.
Initial studies in ACE2 knockout mice raised questions about the role of ACE2 in cardiac structure and function, with divergent phenotypes resulting from deletion of ACE2 in mice by different groups. The first ACE2 knockout mouse line created by Crackower et al. displayed a severe contractility defect associated with elevated renal, cardiac, and plasma Ang II levels.72 However, independently-generated ACE2 knockout mice by Yamamoto et al. and Gurley et al. had normal cardiac function and morphology at baseline.73,74 Explanation for these disparities are unknown but may be attributable to factors such as genetic background or environment. Further work by Gurley et al. led to inbred ACE2 knockout mouse lines backcrossed for six generations onto parental strains. Inbred C57 or 129 mice had no differences in cardiac contractility compared to wild type littermates.75 Of note, a later publication using the original ACE2 knockout line generated by Crackower and colleagues reported normal cardiac function, indicating that the phenotype first reported was lost,76 though it remains unclear why.
Phenotypic differences between mice on different genetic backgrounds were further evident in studies measuring blood pressure. ACE2-deficiency had an inconsistent effect on blood pressure in mice with mixed genetic background (C57BL/6 and 129), whereas inbred C57BL/6 ACE2-deficient mice had a 7 mmHg increase in blood pressures compared to those of controls at baseline.74 As a comparison, ACE2 deficiency on a 129/SvEv background yielded no significant change in baseline blood pressures compared to their control animals. Chronic infusion of Ang II into ACE2-deficient mice (129/SvEv 74 and C57BL/6, unpublished data Gurley Lab) led to significant increases in blood pressures consistent with impaired renal metabolism of Ang II contributing to hypertension. Further evidence that ACE2 does not significantly impact blood pressure under normal conditions is seen in mice treated with recombinant ACE2 (rACE2),77 whereas in Ang II-hypertension, rACE2 is able to normalize blood pressure. Importantly, these studies highlight how strain differences can impact cardiovascular phenotypes.
Use of ACE2 null mice has elucidated important cardiac protective roles for ACE2. ACE2 deficiency impairs degradation of Ang II in the circulation, thus leaving tissues exposed to increased Ang II stimulation. Elevations in Ang II in the setting of ACE2 deficiency were associated with worsened aortic and myocardial inflammation and oxidative stress78,79, and an increased susceptibility to heart disease80. Within the kidney, deficiency of ACE2 was associated with worsened histopathological markers of diabetic kidney injury 76 and elevated markers of oxidative stress.81 More recent studies have begun to focus on the physiologic roles of ACE2 using cell-specific targeting approaches. Overexpression of ACE2 within the glomerular podocyte was protective of renal function and structure in a model of streptozotocin-induced diabetes,82 in part attributed to lower renal Ang II levels. In a model of cell-specific ACE2 depletion, adipocyte-specific ACE2 deletion compounded obesity-induced changes in systolic blood pressure in female but not male mice, supporting sex differences in the regulation of hypertension by the RAS.83 While our understanding of the physiologic role of ACE2 continues to grow, disease modeling and cell-specific ACE2 models point to the importance of the non-canonical RAS.
Using mice with ACE2 overexpression targeted to the central nervous system (Syn-hACE2), studies by Lazartigues and colleagues have examined the role of ACE2 in neurogenic hypertension.84 CNS-overexpression of ACE2 in these animals protected against the development of hypertension and increased drinking behavior following chronic administration of Ang II85 or high salt diet.86 However, shedding of the catalytically-active ectodomain of ACE2 by a disintegrin and metalloproteinase (ADAM17) effectively removed ACE2 from key areas in the brain that are involved in regulation of blood pressure and resulted in enhanced hypertension. The finding that ACE2 cleavage by ADAM17 effectively removes ACE2 from its point of action within the CNS calls attention to the complex nature of hypertension regulation, with inflammatory proteins such as ADAM17 playing a superimposing role on the RAS.86 Together, these studies using transgenic models suggest that regulation of neurogenic hypertension is a highly dynamic process which is impacted by various pathological forces.86
5.2. Humanized ACE2 mice.
A more recent approach to generating relevant animal models of human disease include generation of humanized mice, which involves mice expressing a functional human gene.87,88 This approach allows for more clinically-relevant studies in mimicking human disease and evaluating pharmacological therapies for disease treatment.
For example, humanized mice serve as great resources for further study into the roles of ACE2. Beyond its cardiovascular effects, ACE2 serves as the receptor for both SARS-CoV and SARS-CoV2. SARS coronaviruses have greater binding affinity for human ACE2 (hACE2) as compared to mouse ACE2 (mACE2)89; as such, humanized ACE2 mice have been particularly important in the study of these emerging infections.
Several groups developed hACE2 mice during the original SARS-1 outbreak using differing genetic approaches, thus allowing for the study of SARS-CoV in whole-animal vs. cell-specific approaches.87,88,90,91 In 2007, Yang et al. generated mice expressing hACE2 driven by the mouse ACE2 promoter which maintained normal physiologic ACE2 expression patterns. 87 In another model, expression of hACE2 under the CAG promoter was utilized in three mouse lines that had differing susceptibility to SARS-CoV1.91 Expression of hACE2 under control of either mACE2 promoter or the CAG promoter led to mice with robust hACE2 expression that were susceptible to SARS-CoV1 infection, though with some differences in severity and mortality. Cell-specific approaches for studying SARS-CoV1 infection in hACE2 mice have been developed as well. Mice expressing high levels of ACE2 within epithelial cells specifically, K18-hACE2 mice, have increased hACE2 within the lungs, kidneys, liver, and colon.88 K18-hACE2 mice developed lung pathology upon inhalation of SARS-CoV1 that was rapidly fatal. In a second cell-specific model, airway-targeted expression of hACE2 under the HFH4 promoter yielded mice with broader expression than anticipated—with hACE2 levels in the brain, liver, kidney, and gastrointestinal tract.90 These mice were infected with SARS-like WIV1-coronavirus and developed a severe illness associated with 100% mortality after developing respiratory illness with extrapulmonary manifestations. Each of these studies presented a new model to investigate ACE2 and SARS infections, yet there was not a clear association between tissue-ACE2 expression and viral infectivity as several groups have documented viral antigen in tissues expressing relatively low levels of hACE2.88,91,92
Within the past year, hACE2 mice have been successfully used to study the pathogenesis of SARS-CoV2. K18-hACE2 mice inoculated intranasally with SARS-CoV2 developed high viral loads in lung tissue and other organs, thus providing a mouse model of COVID-19.93 Infection with SARS-CoV2 led to histopathologic evidence of pulmonary and extrapulmonary injury, along with an excessive inflammatory response. Similarly, mice expressing hACE2 downstream of HFH4 promoter developed interstitial pneumonia associated with high viral load in the lungs, along with evidence of viral RNA in the brain and heart.94 Together, these studies demonstrate how hACE2 mice are essential to studying SARS-CoV2.
The differences in susceptibility to lung pathology and extrapulmonary manifestations, along with recovery potential of these lines provides a range of resources for studying viral infectivity and infection severity. Moreover, broader application of humanized ACE2 mouse lines provides new opportunities for understanding the non-canonical RAS pathway in other human-relevant disease models.
In addition to ACE2, prolylcarboxypeptidase (PRCP)§§§§ and prolyloligopeptidase (POP)***** both can degrade Ang II to form Ang 1–7. ACE2/PRCP double knockout mice treated with Ang II were able to convert Ang II to Ang 1–7, whereas POP knockout mice had a blunted conversion of Ang II to Ang 1–7 in plasma, suggesting that circulating Ang II is degraded primarily by POP.5 In contrast, PRCP deficiency does not disrupt degradation of circulating Ang II but instead acts at the kidneys to metabolize Ang II and regulate BP.5,6
6.1. Type 1 Angiotensin Receptor (AT1R)
The actions of Ang II are dictated by the receptors that bind the Ang II peptide and propagate and amplify the Ang II signal into cells. In Homo sapiens, there are two well defined receptors that bind to Ang II: the type 1 angiotensin receptor (Gene name: Agtr1, Protein name: AT1R) and the type 2 angiotensin receptor (Gene name: Agtr2, Protein name: AT2R). In Mus musculus a gene duplication event of the Agtr1 gene lead to two separate genes that are expressed in mice, Agtr1a and Agtr1b.8 Activation of the AT1R (or AT1AR in mice) signaling can cause vastly different downstream effects within different cell-types. For example, in proximal tubule cells it causes changes in the expression of electrolyte transporters thereby altering natriuresis,95 while in vascular smooth muscle cells it causes vasoconstriction leading to acute changes in blood pressure.96 How these diverse functions are regulated is still not completely understood but support cell-specific functionality to preserve homeostasis.
In mice lacking expression of either the AT1AR or AT1BR isoforms, it has been shown that blood pressure control is dominated by the AT1AR isoform. While baseline blood pressure was significantly decreased in mice lacking AT1AR97, AT1BR-deficient animals were normotensive.98 Double null homozygotes (AT1AR and AT1BR double KO) had severe hypotension and abnormal renal structure, mimicking several of the other total RAS component KO phenotypes.98 Interestingly, mice without AT2R expression showed an increase in blood pressure under basal conditions.99 AT1AR, AT1BR, and AT2R have been deleted in combination with one another to demonstrate that these are the only 3 receptors that respond to Ang II and that AT1AR is the receptor that drives most of the physiological response to Ang II.100,101
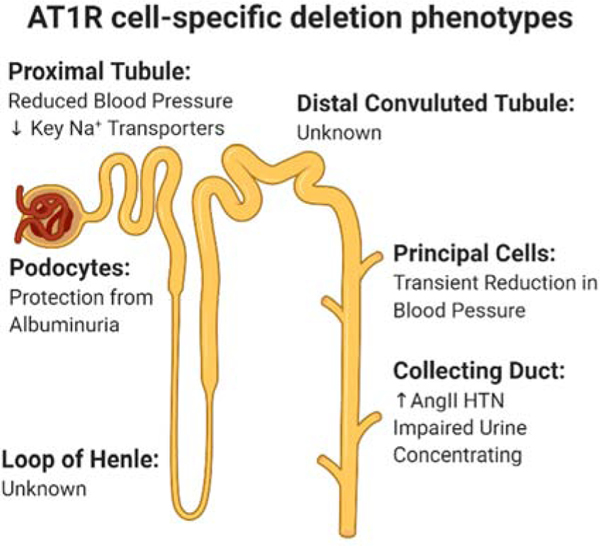
Building on seminal work showing that global loss of AT1R leads to severe hypotension, impaired urinary concentration, and renal developmental defects,97 more recent studies have focused on defining the organs and cell types that are driving different physiological aspects of RAS signaling. The majority of these studies have focused on cell populations in the kidney (Figure 1), central nervous system, and immune system. Another chapter in this collection is going to focus on the role of AT1AR in the vasculature.
Figure 1.
Description of AT1AR cell-specific deletion models and phenotypes along the nephron. Created with Biorender.com
The dominance of the kidney in regulating blood pressure-associated pathologies was clearly demonstrated by kidney cross-transplantation experiments between global AT1AR knockout and wild type animals.102 Since then, researchers have sought to determine which cell types within the kidney were driving various aspect of Ang II signaling.
To facilitate cell-specific deletion of the AT1AR, the Coffman Lab (Duke University) generated a mouse line with a conditional Agtr1a allele to allow targeting of specific cell populations in dissecting mechanisms of hypertension.103 As the renal proximal tubule arguably has the highest abundance of AT1AR among epithelial cell types, it was selected as one of the first cell populations to apply cell-specific deletion of AT1AR.104,105To target the renal proximal tubule (PT), two different cre lines have been utilized to delete AT1AR: Pepck-cre103,106 and Kap2-cre.107 The Pepck-cre mediated deletion resulted in a significant hypotensive phenotype that was maintained during Ang II hypertension. Additionally, mice lacking AT1AR from the renal PT had an altered pressure-natriuresis curve which was associated with alterations in key sodium transporters.103 The Kap2-cre mediated deletion resulted in a similar phenotype with the mice being hypotensive at baseline with blood pressure differences maintained during Ang II-induced hypertension.107 The consistency in phenotype of these two distinct genetic lines provides strong evidence thatAT1ARs in the PT play a critical role in regulating blood pressure in vivo.
The AT1AR has also been deleted from distal segments of the nephron by using the AQP2-Cre which targets principal cells108 and Hoxb7-cre109 which targets the entire collecting duct, both principal and intercalating cells. Within principal cells, loss of angiotensin signaling results in a transient hypotensive phenotype early within Ang II-induced hypertension that resolves after 10 days.108 This transient hypotensive phenotype is accompanied by a slight protection from cardiac hypertrophy and decrease in the abundance of the sodium channel alpha-ENaC. In contrast, deleting AT1AR from the entire collecting duct causes an exaggerated hypertensive response to Ang II.109 This unexpected finding was associated with diminished COX2 expression in CD KO mice and offered a new pathway in RAS-mediated hypertension pathogenesis. These series of seminal experiments have demonstrated the diverse role that AT1AR has throughout the nephron and furthered our understanding of the physiological relevance of RAS signaling in the kidney.
AT1AR has also been deleted from podocytes using the Pod-cre mouse line.110 The authors deleted AT1AR on a background that was also deficient in two proteins involved in clathrin-mediated endocytosis (Dyn1 and Dyn2). They found that deletion of AT1AR in this context improved albuminuria and kidney function compared to the mice that only lacked Dyn1 and Dyn2.
Outside of the kidney, another cardiovascular regulatory center with important RAS activity is the central nervous system (CNS). Within the brain, AT1AR has been deleted from multiple neuronal populations using the following cre recombinase mouse lines; AVP-cre111, Nefh-cre112, LepR-cre113, AgRP-cre113, TH-cre114, and CRF-cre115. These studies have added to our understanding of how the RAS functions in the CNS. Deletion of AT1AR using the AVP-cre resulted in mice having increased plasma and serum osmolality without changes in fluid or salt-intake behaviors, hematocrit, or total body water. Deletion of AT1AR using the Nefh-cre demonstrated that the receptor promotes Adam17 upregulation, a metalloprotease responsible for the cleavage of TNFalpha and Ace2. In the arcuate nucleus, AT1AR was shown to play a role in Leptin receptor (LepR) and agouti-related peptide (AgRP) expressing neurons to regulate the resting metabolic rate in response to a high-fat diet and deoxycorticosterone acetate-salt (DOCA-salt) treatments. Deletion of AT1AR using TH-cre demonstrated that catecholaminergic cells play a role in the maximal response that mice have to Ang II-induced hypertension and the development of cardiac hypertrophy. AT1AR has even been shown to be important for the regulating the behavior of mice as deletion of AT1AR with CRF-cre altered fear conditioning in mice. Additionally, AT1AR was deleted from astrocytes using GFAP-cre116. This study showed that angiotensin signaling in astrocytes plays a role in enhancing central sympathetic outflow in heart failure. Within the CNS, AT1R is expressed in multiple cell types that are important for maintaining homeostasis.
Work from the Crowley Lab has selectively targeted AT1AR in multiple immune cell populations, including macrophages and T-lymphocytes, using mouse lines driving cre recombinase with CD4117, LysM118, Lck119, and OT-1120 promoters in combination with the conditional Agtr1a allele. In contrast to many of the other cell-types in which AT1AR activation drives hypertensive organ damage (nephrosclerosis, fibrosis, proteinuria),121 these experiments have compellingly demonstrated a protective role for AT1AR receptor activation in immune cells that limits fibrosis in the hypertensive kidney.117–120 This opposing role highlights the complexity of RAS signaling in which the context of RAS activation is relevant and suggests that targeted inactivation of RAS signals may offer more therapeutic potential over current approaches that inhibit the RAS globally.
7.1. Concluding Remarks
The RAS is a complex signaling cascade that spans multiple organs and cell types. The development of new and improved methodologies to generate transgenic animals has significantly contributed to our understanding of how the RAS contributes to physiology. For example, cell-specific deletion of Agt has demonstrated how synthesis of Agt in the liver plays in regulating blood pressure. We also now know that renin synthesis outside of the JGA plays an important role in regulating RAS expression and activation. Redundancy and multiplicity within the RAS allow for compensatory adjustments to maintain cardiovascular homeostasis. We see this exemplified in studies in which mice lacking renal ACE are protected by systemic ACE circulation at baseline, whereas in the presence of hypertensive stimuli the imbalance of the system is made evident. Moreover, systematic generation of cell-specific deletion models of the major receptor for Ang II (AT1AR) have uncovered how the RAS modulates epithelial transport, urinary concentration, and a connection with the prostaglandin system, summarily emphasizing the intra-renal RAS in BP regulation.
Studies in ACE2 functions have highlighted an important lesson in mouse physiology: namely, that mouse strain can have significant impact on phenotype. Such work provides unique opportunities to study differences in phenotype across multiple knockout strains thereby generating a more robust understanding of the role of ACE2 in cardiovascular biology. The emerging role of ACE2 in viral pathogenesis will also lead to advances in the field of the RAS, which should inform BP and cardiovascular disease more broadly. Indeed, exciting opportunities are found in studies that expand our understanding of the RAS into new territories.
Acknowledgments
Funding
The following funding sources supported this work: Medical Scientist Training Program of Oregon Health and Science University T32 GM 109835 (JME), The Collins Medical Trust (JWN), The National Institutes of Health R01DK098382 (SBG), K01DK121737 (JWN), The American Heart Association 20CDA35320169 (JWN).
Footnotes
RAS: renin angiotensin system
ACE: angiotensin converting enzyme
ARB: angiotensin receptor blocker
Agt: angiotensinogen
Ang II: angiotensin II
AT1R: type 1 angiotensin receptor
Ang I: Angiotensin I
CNS: Central Nervous System
BP: Blood pressure
KO: Knock out
PRR: Prorenin receptor
JG: Juxtaglomerular
ACE2: Angiotensin Converting Enzyme 2
Ang 1–7: Angiotensin 1–7
SARS-CoV2: Severe Acute Respiratory Syndrome Coronavirus 2
PRCP: Prolylcarboxypeptidase
POP: Prolyloligopeptidase
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jarred G, Kennedy RL. Therapeutic perspective: starting an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker in a diabetic patient. Ther Adv Endocrinol Metab. 2010;1(1):23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329(20):1456–1462. [DOI] [PubMed] [Google Scholar]
- 3.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. [DOI] [PubMed] [Google Scholar]
- 4.Bromfield S, Muntner P. High blood pressure: the leading global burden of disease risk factor and the need for worldwide prevention programs. Curr Hypertens Rep. 2013;15(3):134–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serfozo P, Wysocki J, Gulua G, et al. Ang II (Angiotensin II) Conversion to Angiotensin-(1–7) in the Circulation Is POP (Prolyloligopeptidase)-Dependent and ACE2 (Angiotensin-Converting Enzyme 2)-Independent. Hypertension. 2020;75(1):173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maier C, Schadock I, Haber PK, et al. Prolylcarboxypeptidase deficiency is associated with increased blood pressure, glomerular lesions, and cardiac dysfunction independent of altered circulating and cardiac angiotensin II. J Mol Med (Berl). 2017;95(5):473–486. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi N, Yamamoto K, Ohishi M, et al. The counterregulating role of ACE2 and ACE2-mediated angiotensin 1–7 signaling against angiotensin II stimulation in vascular cells. Hypertens Res. 2010;33(11):1182–1185. [DOI] [PubMed] [Google Scholar]
- 8.Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical Renin-Angiotensin system in kidney physiology. Compr Physiol. 2014;4(3):1201–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukamizu A, Murakami K. Activated and inactivated renin-angiotensin system in transgenic animals: from genes to blood pressure. Lab Anim Sci. 1997;47(2):127–131. [PubMed] [Google Scholar]
- 10.Zhuo JL, Ferrao FM, Zheng Y, Li XC. New frontiers in the intrarenal Renin-Angiotensin system: a critical review of classical and new paradigms. Front Endocrinol (Lausanne). 2013;4:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouabe H, Okkenhaug K. Gene targeting in mice: a review. Methods Mol Biol. 2013;1064:315–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C, Lu H, Cassis LA, Daugherty A. Molecular and Pathophysiological Features of Angiotensinogen: A Mini Review. N Am J Med Sci (Boston). 2011;4(4):183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Celerier J, Cruz A, Lamande N, Gasc JM, Corvol P. Angiotensinogen and its cleaved derivatives inhibit angiogenesis. Hypertension. 2002;39(2):224–228. [DOI] [PubMed] [Google Scholar]
- 14.Kim HS, Krege JH, Kluckman KD, et al. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci U S A. 1995;92(7):2735–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagata M, Tanimoto K, Fukamizu A, et al. Nephrogenesis and renovascular development in angiotensinogen-deficient mice. Lab Invest. 1996;75(5):745–753. [PubMed] [Google Scholar]
- 16.Merrill DC, Thompson MW, Carney CL, et al. Chronic hypertension and altered baroreflex responses in transgenic mice containing the human renin and human angiotensinogen genes. J Clin Invest. 1996;97(4):1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang G, Merrill DC, Thompson MW, Robillard JE, Sigmund CD. Functional expression of the human angiotensinogen gene in transgenic mice. J Biol Chem. 1994;269(51):32497–32502. [PubMed] [Google Scholar]
- 18.Morimoto S, Cassell MD, Sigmund CD. The brain renin-angiotensin system in transgenic mice carrying a highly regulated human renin transgene. Circ Res. 2002;90(1):80–86. [DOI] [PubMed] [Google Scholar]
- 19.Tanimoto K, Sugiyama F, Goto Y, et al. Angiotensinogen-deficient mice with hypotension. J Biol Chem. 1994;269(50):31334–31337. [PubMed] [Google Scholar]
- 20.Smithies O, Kim HS. Targeted gene duplication and disruption for analyzing quantitative genetic traits in mice. Proc Natl Acad Sci U S A. 1994;91(9):3612–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsusaka T, Niimura F, Pastan I, Shintani A, Nishiyama A, Ichikawa I. Podocyte injury enhances filtration of liver-derived angiotensinogen and renal angiotensin II generation. Kidney Int. 2014;85(5):1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsusaka T, Niimura F, Shimizu A, et al. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol. 2012;23(7):1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortiz-Melo DI, Spurney RF. Special deLIVERy: podocyte injury promotes renal angiotensin II generation from liver-derived angiotensinogen. Kidney international. 2014;85(5):1009–1011. [DOI] [PubMed] [Google Scholar]
- 24.Peach MJ. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev. 1977;57(2):313–370. [DOI] [PubMed] [Google Scholar]
- 25.Danser AH, Deinum J. Renin, prorenin and the putative (pro)renin receptor. J Renin Angiotensin Aldosterone Syst. 2005;6(3):163–165. [DOI] [PubMed] [Google Scholar]
- 26.Rohrwasser A, Morgan T, Dillon HF, et al. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34(6):1265–1274. [DOI] [PubMed] [Google Scholar]
- 27.Robben JH, Fenton RA, Vargas SL, et al. Localization of the succinate receptor in the distal nephron and its signaling in polarized MDCK cells. Kidney Int. 2009;76(12):1258–1267. [DOI] [PubMed] [Google Scholar]
- 28.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol. 2005;289(3):F632–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lum C, Shesely EG, Potter DL, Beierwaltes WH. Cardiovascular and renal phenotype in mice with one or two renin genes. Hypertension. 2004;43(1):79–86. [DOI] [PubMed] [Google Scholar]
- 30.Sharp MG, Fettes D, Brooker G, et al. Targeted inactivation of the Ren-2 gene in mice. Hypertension. 1996;28(6):1126–1131. [DOI] [PubMed] [Google Scholar]
- 31.Clark AF, Sharp MG, Morley SD, Fleming S, Peters J, Mullins JJ. Renin-1 is essential for normal renal juxtaglomerular cell granulation and macula densa morphology. J Biol Chem. 1997;272(29):18185–18190. [DOI] [PubMed] [Google Scholar]
- 32.Buckley C, Nelson RJ, Mullins LJ, et al. Phenotypic dissection of the mouse Ren1d knockout by complementation with human renin. J Biol Chem. 2018;293(4):1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanai K, Saito T, Kakinuma Y, et al. Renin-dependent cardiovascular functions and renin-independent blood-brain barrier functions revealed by renin-deficient mice. J Biol Chem. 2000;275(1):5–8. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi N, Lopez ML, Cowhig JE Jr., et al. Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol. 2005;16(1):125–132. [DOI] [PubMed] [Google Scholar]
- 35.Ramkumar N, Ying J, Stuart D, Kohan DE. Overexpression of Renin in the collecting duct causes elevated blood pressure. Am J Hypertens. 2013;26(8):965–972. [DOI] [PubMed] [Google Scholar]
- 36.Ramkumar N, Stuart D, Rees S, Hoek AV, Sigmund CD, Kohan DE. Collecting duct-specific knockout of renin attenuates angiotensin II-induced hypertension. Am J Physiol Renal Physiol. 2014;307(8):F931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trepiccione F, Gerber SD, Grahammer F, et al. Renal Atp6ap2/(Pro)renin Receptor Is Required for Normal Vacuolar H+-ATPase Function but Not for the Renin-Angiotensin System. J Am Soc Nephrol. 2016;27(11):3320–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramkumar N, Stuart D, Mironova E, et al. Renal tubular epithelial cell prorenin receptor regulates blood pressure and sodium transport. Am J Physiol Renal Physiol. 2016;311(1):F186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prieto MC, Reverte V, Mamenko M, et al. Collecting duct prorenin receptor knockout reduces renal function, increases sodium excretion, and mitigates renal responses in ANG II-induced hypertensive mice. Am J Physiol Renal Physiol. 2017;313(6):F1243–F1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riquier-Brison ADM, Sipos A, Prokai A, et al. The macula densa prorenin receptor is essential in renin release and blood pressure control. Am J Physiol Renal Physiol. 2018;315(3):F521–F534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramkumar N, Kohan DE. Role of the Collecting Duct Renin Angiotensin System in Regulation of Blood Pressure and Renal Function. Curr Hypertens Rep. 2016;18(4):29. [DOI] [PubMed] [Google Scholar]
- 42.Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell. 2004;6(5):719–728. [DOI] [PubMed] [Google Scholar]
- 43.Tang J, Wysocki J, Ye M, et al. Urinary Renin in Patients and Mice With Diabetic Kidney Disease. Hypertension. 2019;74(1):83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403–1419. [DOI] [PubMed] [Google Scholar]
- 45.Investigators S, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293–302. [DOI] [PubMed] [Google Scholar]
- 46.Heart Outcomes Prevention Evaluation Study I, Yusuf S, Sleight P, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342(3):145–153. [DOI] [PubMed] [Google Scholar]
- 47.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. [DOI] [PubMed] [Google Scholar]
- 48.Coates D. The angiotensin converting enzyme (ACE). Int J Biochem Cell Biol. 2003;35(6):769–773. [DOI] [PubMed] [Google Scholar]
- 49.Krege JH, John SW, Langenbach LL, et al. Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature. 1995;375(6527):146–148. [DOI] [PubMed] [Google Scholar]
- 50.Esther CR Jr., Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest. 1996;74(5):953–965. [PubMed] [Google Scholar]
- 51.Oliverio MI, Madsen K, Best CF, et al. Renal growth and development in mice lacking AT1A receptors for angiotensin II. Am J Physiol. 1998;274(1):F43–50. [DOI] [PubMed] [Google Scholar]
- 52.Tsuchida S, Matsusaka T, Chen X, et al. Murine double nullizygotes of the angiotensin type 1A and 1B receptor genes duplicate severe abnormal phenotypes of angiotensinogen nullizygotes. J Clin Invest. 1998;101(4):755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fuchs S, Xiao HD, Cole JM, et al. Role of the N-terminal catalytic domain of angiotensin-converting enzyme investigated by targeted inactivation in mice. J Biol Chem. 2004;279(16):15946–15953. [DOI] [PubMed] [Google Scholar]
- 54.Fuchs S, Xiao HD, Hubert C, et al. Angiotensin-converting enzyme C-terminal catalytic domain is the main site of angiotensin I cleavage in vivo. Hypertension. 2008;51(2):267–274. [DOI] [PubMed] [Google Scholar]
- 55.Eriguchi M, Bernstein EA, Veiras LC, et al. The Absence of the ACE N-Domain Decreases Renal Inflammation and Facilitates Sodium Excretion during Diabetic Kidney Disease. J Am Soc Nephrol. 2018;29(10):2546–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skidgel RA, Erdos EG. The broad substrate specificity of human angiotensin I converting enzyme. Clin Exp Hypertens A. 1987;9(2–3):243–259. [DOI] [PubMed] [Google Scholar]
- 57.Ng KK, Vane JR. Conversion of angiotensin I to angiotensin II. Nature. 1967;216(5117):762–766. [DOI] [PubMed] [Google Scholar]
- 58.Cole J, Quach DL, Sundaram K, Corvol P, Capecchi MR, Bernstein KE. Mice lacking endothelial angiotensin-converting enzyme have a normal blood pressure. Circ Res. 2002;90(1):87–92. [DOI] [PubMed] [Google Scholar]
- 59.Smithies O, Kim HS, Takahashi N, Edgell MH. Importance of quantitative genetic variations in the etiology of hypertension. Kidney Int. 2000;58(6):2265–2280. [DOI] [PubMed] [Google Scholar]
- 60.Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, et al. The absence of intrarenal ACE protects against hypertension. J Clin Invest. 2013;123(5):2011–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hashimoto S, Adams JW, Bernstein KE, Schnermann J. Micropuncture determination of nephron function in mice without tissue angiotensin-converting enzyme. Am J Physiol Renal Physiol. 2005;288(3):F445–452. [DOI] [PubMed] [Google Scholar]
- 62.Alhenc-Gelas F, Baussant T, Hubert C, Soubrier F, Corvol P. The angiotensin converting enzyme in the kidney. J Hypertens Suppl. 1989;7(7):S9–13; discussion S14. [DOI] [PubMed] [Google Scholar]
- 63.Gonzalez-Villalobos RA, Billet S, Kim C, et al. Intrarenal angiotensin-converting enzyme induces hypertension in response to angiotensin I infusion. J Am Soc Nephrol. 2011;22(3):449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiao HD, Fuchs S, Campbell DJ, et al. Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am J Pathol. 2004;165(3):1019–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kasi VS, Xiao HD, Shang LL, et al. Cardiac-restricted angiotensin-converting enzyme overexpression causes conduction defects and connexin dysregulation. Am J Physiol Heart Circ Physiol. 2007;293(1):H182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iravanian S, Sovari AA, Lardin HA, et al. Inhibition of renin-angiotensin system (RAS) reduces ventricular tachycardia risk by altering connexin43. J Mol Med (Berl). 2011;89(7):677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiao HD, Fuchs S, Bernstein EA, Li P, Campbell DJ, Bernstein KE. Mice expressing ACE only in the heart show that increased cardiac angiotensin II is not associated with cardiac hypertrophy. 2008;294(2):H659–667. [DOI] [PubMed] [Google Scholar]
- 68.Krege JH, Kim HS, Moyer JS, et al. Angiotensin-converting enzyme gene mutations, blood pressures, and cardiovascular homeostasis. Hypertension. 1997;29(1 Pt 2):150–157. [DOI] [PubMed] [Google Scholar]
- 69.Smithies O. Theodore Cooper Memorial Lecture. A mouse view of hypertension. Hypertension. 1997;30(6):1318–1324. [DOI] [PubMed] [Google Scholar]
- 70.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275(43):33238–33243. [DOI] [PubMed] [Google Scholar]
- 71.Ferrario CM, Chappell MC, Dean RH, Iyer SN. Novel angiotensin peptides regulate blood pressure, endothelial function, and natriuresis. J Am Soc Nephrol. 1998;9(9):1716–1722. [DOI] [PubMed] [Google Scholar]
- 72.Crackower MA, Sarao R, Oudit GY, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417(6891):822–828. [DOI] [PubMed] [Google Scholar]
- 73.Yamamoto K, Ohishi M, Katsuya T, et al. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension. 2006;47(4):718–726. [DOI] [PubMed] [Google Scholar]
- 74.Gurley SB, Allred A, Le TH, et al. Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J Clin Invest. 2006;116(8):2218–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gurley SB, Coffman TM. Angiotensin-converting enzyme 2 gene targeting studies in mice: mixed messages. Exp Physiol. 2008;93(5):538–542. [DOI] [PubMed] [Google Scholar]
- 76.Wong DW, Oudit GY, Reich H, et al. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol. 2007;171(2):438–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wysocki J, Ye M, Khattab AM, et al. Angiotensin-converting enzyme 2 amplification limited to the circulation does not protect mice from development of diabetic nephropathy. Kidney Int. 2017;91(6):1336–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oudit GY, Kassiri Z, Patel MP, et al. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc Res. 2007;75(1):29–39. [DOI] [PubMed] [Google Scholar]
- 79.Jin HY, Song B, Oudit GY, et al. ACE2 deficiency enhances angiotensin II-mediated aortic profilin-1 expression, inflammation and peroxynitrite production. PLoS One. 2012;7(6):e38502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rabelo LA, Todiras M, Nunes-Souza V, et al. Genetic Deletion of ACE2 Induces Vascular Dysfunction in C57BL/6 Mice: Role of Nitric Oxide Imbalance and Oxidative Stress. PLoS One. 2016;11(4):e0150255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wysocki J, Ortiz-Melo DI, Mattocks NK, et al. ACE2 deficiency increases NADPH-mediated oxidative stress in the kidney. Physiol Rep. 2014;2(3):e00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nadarajah R, Milagres R, Dilauro M, et al. Podocyte-specific overexpression of human angiotensin-converting enzyme 2 attenuates diabetic nephropathy in mice. Kidney Int. 2012;82(3):292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shoemaker R, Tannock LR, Su W, et al. Adipocyte deficiency of ACE2 increases systolic blood pressures of obese female C57BL/6 mice. Biol Sex Differ. 2019;10(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Feng Y, Xia H, Cai Y, et al. Brain-selective overexpression of human Angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ Res. 2010;106(2):373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feng Y, Hans C, McIlwain E, Varner KJ, Lazartigues E. Angiotensin-converting enzyme 2 over-expression in the central nervous system reduces angiotensin-II-mediated cardiac hypertrophy. PLoS One. 2012;7(11):e48910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xia H, Sriramula S, Chhabra KH, Lazartigues E. Brain angiotensin-converting enzyme type 2 shedding contributes to the development of neurogenic hypertension. Circ Res. 2013;113(9):1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang XH, Deng W, Tong Z, et al. Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp Med. 2007;57(5):450–459. [PubMed] [Google Scholar]
- 88.McCray PB Jr., Pewe L, Wohlford-Lenane C, et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol. 2007;81(2):813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li W, Greenough TC, Moore MJ, et al. Efficient replication of severe acute respiratory syndrome coronavirus in mouse cells is limited by murine angiotensin-converting enzyme 2. J Virol. 2004;78(20):11429–11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Menachery VD, Yount BL Jr., Sims AC, et al. SARS-like WIV1-CoV poised for human emergence. Proc Natl Acad Sci U S A. 2016;113(11):3048–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tseng CT, Huang C, Newman P, et al. Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human Angiotensin-converting enzyme 2 virus receptor. J Virol. 2007;81(3):1162–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jia HP, Look DC, Tan P, et al. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2009;297(1):L84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Winkler ES, Bailey AL, Kafai NM, et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol. 2020;21(11):1327–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiang RD, Liu MQ, Chen Y, et al. Pathogenesis of SARS-CoV-2 in Transgenic Mice Expressing Human Angiotensin-Converting Enzyme 2. Cell. 2020;182(1):50–58 e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nguyen MT, Lee DH, Delpire E, McDonough AA. Differential regulation of Na+ transporters along nephron during ANG II-dependent hypertension: distal stimulation counteracted by proximal inhibition. Am J Physiol Renal Physiol. 2013;305(4):F510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sparks MA, Stegbauer J, Chen D, et al. Vascular Type 1A Angiotensin II Receptors Control BP by Regulating Renal Blood Flow and Urinary Sodium Excretion. J Am Soc Nephrol. 2015;26(12):2953–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ito M, Oliverio MI, Mannon PJ, et al. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci U S A. 1995;92(8):3521–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oliverio MI, Kim HS, Ito M, et al. Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both AT1A and AT1B receptors for angiotensin II. Proc Natl Acad Sci U S A. 1998;95(26):15496–15501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ichiki T, Labosky PA, Shiota C, et al. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature. 1995;377(6551):748–750. [DOI] [PubMed] [Google Scholar]
- 100.van Esch JH, Gembardt F, Sterner-Kock A, et al. Cardiac phenotype and angiotensin II levels in AT1a, AT1b, and AT2 receptor single, double, and triple knockouts. Cardiovascular research. 2010;86(3):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gembardt F, Heringer-Walther S, van Esch JH, et al. Cardiovascular phenotype of mice lacking all three subtypes of angiotensin II receptors. Faseb j. 2008;22(8):3068–3077. [DOI] [PubMed] [Google Scholar]
- 102.Crowley SD, Gurley SB, Herrera MJ, et al. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103(47):17985–17990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gurley SB, Riquier-Brison AD, Schnermann J, et al. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 2011;13(4):469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee JW, Chou CL, Knepper MA. Deep Sequencing in Microdissected Renal Tubules Identifies Nephron Segment-Specific Transcriptomes. J Am Soc Nephrol. 2015;26(11):2669–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu H, Kirita Y, Donnelly EL, Humphreys BD. Advantages of Single-Nucleus over Single-Cell RNA Sequencing of Adult Kidney: Rare Cell Types and Novel Cell States Revealed in Fibrosis. J Am Soc Nephrol. 2019;30(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rankin EB, Tomaszewski JE, Haase VH. Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res. 2006;66(5):2576–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li H, Weatherford ET, Davis DR, et al. Renal proximal tubule angiotensin AT1A receptors regulate blood pressure. Am J Physiol Regul Integr Comp Physiol. 2011;301(4):R1067–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen D, Stegbauer J, Sparks MA, et al. Impact of Angiotensin Type 1A Receptors in Principal Cells of the Collecting Duct on Blood Pressure and Hypertension. Hypertension. 2016;67(6):1291–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stegbauer J, Chen D, Herrera M, et al. Resistance to hypertension mediated by intercalated cells of the collecting duct. JCI Insight. 2017;2(7):e92720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Inoue K, Tian X, Velazquez H, et al. Inhibition of Endocytosis of Clathrin-Mediated Angiotensin II Receptor Type 1 in Podocytes Augments Glomerular Injury. J Am Soc Nephrol. 2019;30(12):2307–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sandgren JA, Linggonegoro DW, Zhang SY, et al. Angiotensin AT(1A) receptors expressed in vasopressin-producing cells of the supraoptic nucleus contribute to osmotic control of vasopressin. Am J Physiol Regul Integr Comp Physiol. 2018;314(6):R770–r780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu J, Sriramula S, Xia H, et al. Clinical Relevance and Role of Neuronal AT(1) Receptors in ADAM17-Mediated ACE2 Shedding in Neurogenic Hypertension. Circ Res. 2017;121(1):43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Claflin KE, Sandgren JA, Lambertz AM, et al. Angiotensin AT1A receptors on leptin receptor-expressing cells control resting metabolism. J Clin Invest. 2017;127(4):1414–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jancovski N, Bassi JK, Carter DA, et al. Stimulation of angiotensin type 1A receptors on catecholaminergic cells contributes to angiotensin-dependent hypertension. Hypertension. 2013;62(5):866–871. [DOI] [PubMed] [Google Scholar]
- 115.Hurt RC, Garrett JC, Keifer OP Jr., et al. Angiotensin type 1a receptors on corticotropin-releasing factor neurons contribute to the expression of conditioned fear. Genes Brain Behav. 2015;14(7):526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Isegawa K, Hirooka Y, Katsuki M, Kishi T, Sunagawa K. Angiotensin II type 1 receptor expression in astrocytes is upregulated leading to increased mortality in mice with myocardial infarction-induced heart failure. Am J Physiol Heart Circ Physiol. 2014;307(10):H1448–1455. [DOI] [PubMed] [Google Scholar]
- 117.Zhang JD, Patel MB, Song YS, et al. A novel role for type 1 angiotensin receptors on T lymphocytes to limit target organ damage in hypertension. Circ Res. 2012;110(12):1604–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang JD, Patel MB, Griffiths R, et al. Type 1 angiotensin receptors on macrophages ameliorate IL-1 receptor-mediated kidney fibrosis. J Clin Invest. 2014;124(5):2198–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wen Y, Rudemiller NP, Zhang J, et al. Stimulating Type 1 Angiotensin Receptors on T Lymphocytes Attenuates Renal Fibrosis. Am J Pathol. 2019;189(5):981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Silva-Filho JL, Caruso-Neves C, Pinheiro AA. Angiotensin II type-1 receptor (AT(1)R) regulates expansion, differentiation, and functional capacity of antigen-specific CD8(+) T cells. Sci Rep. 2016;6:35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension. 2004;44(5):595–601. [DOI] [PubMed] [Google Scholar]
- 122.Niimura F, Labosky PA, Kakuchi J, et al. Gene targeting in mice reveals a requirement for angiotensin in the development and maintenance of kidney morphology and growth factor regulation. J Clin Invest. 1995;96(6):2947–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shen XZ, Li P, Weiss D, et al. Mice with enhanced macrophage angiotensin-converting enzyme are resistant to melanoma. Am J Pathol. 2007;170(6):2122–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Khan Z, Shen XZ, Bernstein EA, et al. Angiotensin-converting enzyme enhances the oxidative response and bactericidal activity of neutrophils. Blood. 2017;130(3):328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang J, Dong J, Martin M, et al. AMP-activated Protein Kinase Phosphorylation of Angiotensin-Converting Enzyme 2 in Endothelium Mitigates Pulmonary Hypertension. Am J Respir Crit Care Med. 2018;198(4):509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xia H, de Queiroz TM, Sriramula S, et al. Brain ACE2 overexpression reduces DOCA-salt hypertension independently of endoplasmic reticulum stress. Am J Physiol Regul Integr Comp Physiol. 2015;308(5):R370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Qi YF, Zhang J, Wang L, et al. Angiotensin-converting enzyme 2 inhibits high-mobility group box 1 and attenuates cardiac dysfunction post-myocardial ischemia. J Mol Med (Berl). 2016;94(1):37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu J, Sriramula S, Xia H, et al. Clinical Relevance and Role of Neuronal AT1 Receptors in ADAM17-Mediated ACE2 Shedding in Neurogenic Hypertension. Circ Res. 2017;121(1):43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]