Abstract
Background
Advances in embryo culture media have led to a shift in in vitro fertilisation (IVF) practice from cleavage‐stage embryo transfer to blastocyst‐stage embryo transfer. The rationale for blastocyst‐stage transfer is to improve both uterine and embryonic synchronicity and enable self selection of viable embryos, thus resulting in better live birth rates.
Objectives
To determine whether blastocyst‐stage (day 5 to 6) embryo transfer improves the live birth rate (LBR) per fresh transfer, and other associated outcomes, compared with cleavage‐stage (day 2 to 3) embryo transfer.
Search methods
We searched the Cochrane Gynaecology and Fertility Group Specialised Register of controlled trials, CENTRAL, MEDLINE, Embase, PsycINFO, and CINAHL, from inception to October 2021. We also searched registers of ongoing trials and the reference lists of studies retrieved.
Selection criteria
We included randomised controlled trials (RCTs) which compared the effectiveness of IVF with blastocyst‐stage embryo transfer versus IVF with cleavage‐stage embryo transfer.
Data collection and analysis
We used standard methodological procedures recommended by Cochrane. Our primary outcomes were LBR per fresh transfer and cumulative clinical pregnancy rates (cCPR). Secondary outcomes were clinical pregnancy rate (CPR), multiple pregnancy, high‐order multiple pregnancy, miscarriage (all following first embryo transfer), failure to transfer embryos, and whether supernumerary embryos were frozen for transfer at a later date (frozen‐thawed embryo transfer). We assessed the overall quality of the evidence for the main comparisons using GRADE methods.
Main results
We included 32 RCTs (5821 couples or women).
The live birth rate following fresh transfer was higher in the blastocyst‐stage transfer group (odds ratio (OR) 1.27, 95% confidence interval (CI) 1.06 to 1.51; I2 = 53%; 15 studies, 2219 women; low‐quality evidence). This suggests that if 31% of women achieve live birth after fresh cleavage‐stage transfer, between 32% and 41% would do so after fresh blastocyst‐stage transfer.
We are uncertain whether blastocyst‐stage transfer improves the cCPR. A post hoc analysis showed that vitrification could increase the cCPR. This is an interesting finding that warrants further investigation when more studies using vitrification are published.
The CPR was also higher in the blastocyst‐stage transfer group, following fresh transfer (OR 1.25, 95% CI 1.12 to 1.39; I2 = 51%; 32 studies, 5821 women; moderate‐quality evidence). This suggests that if 39% of women achieve a clinical pregnancy after fresh cleavage‐stage transfer, between 42% and 47% will probably do so after fresh blastocyst‐stage transfer.
We are uncertain whether blastocyst‐stage transfer increases multiple pregnancy (OR 1.05, 95% CI 0.83 to 1.33; I2 = 30%; 19 studies, 3019 women; low‐quality evidence) or miscarriage rates (OR 1.12, 95% CI 0.90 to 1.38; I2 = 24%; 22 studies, 4208 women; low‐quality evidence). This suggests that if 9% of women have a multiple pregnancy after fresh cleavage‐stage transfer, between 8% and 12% would do so after fresh blastocyst‐stage transfer. However, a sensitivity analysis restricted only to studies with low or 'some concerns' for risk of bias, in the subgroup of equal number of embryos transferred, showed that blastocyst transfer probably increases the multiple pregnancy rate.
Embryo freezing rates (when there are frozen supernumerary embryos for transfer at a later date) were lower in the blastocyst‐stage transfer group (OR 0.48, 95% CI 0.40 to 0.57; I2 = 84%; 14 studies, 2292 women; low‐quality evidence). This suggests that if 60% of women have embryos frozen after cleavage‐stage transfer, between 37% and 46% would do so after blastocyst‐stage transfer.
Failure to transfer any embryos was higher in the blastocyst transfer group (OR 2.50, 95% CI 1.76 to 3.55; I2 = 36%; 17 studies, 2577 women; moderate‐quality evidence). This suggests that if 1% of women have no embryos transferred in planned fresh cleavage‐stage transfer, between 2% and 4% probably have no embryos transferred in planned fresh blastocyst‐stage transfer.
The evidence was of low quality for most outcomes. The main limitations were serious imprecision and serious risk of bias, associated with failure to describe acceptable methods of randomisation.
Authors' conclusions
There is low‐quality evidence for live birth and moderate‐quality evidence for clinical pregnancy that fresh blastocyst‐stage transfer is associated with higher rates of both than fresh cleavage‐stage transfer. We are uncertain whether blastocyst‐stage transfer improves the cCPR derived from fresh and frozen‐thawed cycles following a single oocyte retrieval. Although there is a benefit favouring blastocyst‐stage transfer in fresh cycles, more evidence is needed to know whether the stage of transfer impacts on cumulative live birth and pregnancy rates. Future RCTs should report rates of live birth, cumulative live birth, and miscarriage. They should also evaluate women with a poor prognosis to enable those undergoing assisted reproductive technology (ART) and service providers to make well‐informed decisions on the best treatment option available.
Plain language summary
When trying to have a baby through assisted conception, is it better to transfer the embryo to the womb on day 3 or day 5?
Background
Many women and couples are unlikely to get pregnant and have a baby without medical treatment, due to infertility. Doctors have developed a variety of assisted reproductive technologies (ARTs), such as in vitro fertilisation (IVF), which involve the manipulation of eggs and sperm outside a woman's body, to try to increase her chances of getting pregnant.
Typically, in assisted conception, doctors collect eggs from a woman and fertilise them in a laboratory, leading to the formation of embryos. An embryo is the early stage of human development. Doctors commonly transfer one or several embryos into a woman’s womb (uterus) at one of two stages of embryo development: either the cleavage stage, which is 2 or 3 days after egg collection when an embryo typically consists of between 2 and 128 cells; or the blastocyst stage, which is 5 or 6 days after egg collection when an embryo consists of between 70 and 100 cells.
Until recently, doctors usually transferred embryos at the earlier, cleavage, stage. However, there has been a trend to transferring embryos at the later, blastocyst, stage. Researchers believe that only those embryos capable of surviving make it to the blastocyst stage; in other words, viable embryos will self‐select. So, it is thought that transferring embryos at the later stage may improve a woman's chances of becoming pregnant and having a healthy baby.
Review question
We wanted to find out if transferring embryos into a woman's womb at cleavage stage (day 2 to 3) or blastocyst stage (day 5 to 6) is better, in terms of:
– number of babies born alive (live birth rate) following embryo transfers using only 'fresh' embryos; that is, embryos that have not been frozen and subsequently thawed;
– total number of pregnancies achieved following embryo transfers using both 'fresh' and frozen then thawed embryos, collected from a single egg collection procedure (cumulative clinical pregnancy rate);
– multiple pregnancy rate (when a woman is carrying more than one baby at a time);
‐ miscarriage rate (the loss of a pregnancy before the 20th week of development in the womb).
Study characteristics
We included 32 randomised controlled trials (studies in which participants are assigned randomly to 2 or more treatment groups), which included 5821 women or couples. The evidence is current to October 2021.
Key results
– Transferring 'fresh' embryos at the blastocyst stage (day 5 to 6) may lead to more live births than when 'fresh' embryos are transferred at the cleavage stage (day 2 to 3). This suggests that if 31% of women achieve live birth after 'fresh' cleavage‐stage embryo transfer, between 32% and 41% would do so after 'fresh' blastocyst‐stage transfer.
– Transferring 'fresh' embryos at the blastocyst stage probably leads to more clinical pregnancies – defined as evidence of fetal heart activity on an ultrasound scan – than when 'fresh' embryos are transferred at the cleavage stage. This suggests that if 39% of women achieve a clinical pregnancy after 'fresh' cleavage‐stage transfer, between 42% and 47% will probably do so after 'fresh' blastocyst‐stage transfer.
– We are uncertain whether blastocyst‐stage transfer favors cumulative clinical pregnancy rates (i.e. pregnancies from both fresh and thawed cycles deriving from a single egg collection procedure).
– We are uncertain whether blastocyst‐stage transfer increases multiple pregnancy rates compared to cleavage‐stage transfer, when we consider all the studies that reported information on this.
– When we consider evidence only from higher‐quality studies and studies that transferred the same number of embryos in both embryo stages, we found that multiple pregnancy rate is probably higher in the blastocyst‐stage transfer group.
– We are uncertain whether blastocyst‐stage transfer increases miscarriage rates compared to cleavage‐stage transfer.
Future studies should report rates of live birth, cumulative live birth, and miscarriage, to enable women, couples and their doctors to make well‐informed decisions on the best treatment option available.
Quality of the evidence
We have low to moderate confidence in the quality of the evidence for most outcomes. The main limitation was the failure of some studies to describe acceptable methods of assigning women or couples at random to treatment groups.
Summary of findings
Summary of findings 1. Blastocyst‐stage versus cleavage‐stage embryo transfer for assisted reproductive technology.
| Blastocyst‐stage versus cleavage‐stage embryo transfer for assisted reproductive technology | ||||||
| Population: women and couples with subfertility Settings: assisted reproductive technology Intervention: blastocyst‐stage embryo transfer Comparison: cleavage‐stage embryo transfer | ||||||
| Outcomes per couple | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Cleavage‐stage embryo transfer | Blastocyst‐stage embryo transfer | |||||
| Live birth rate per fresh transfer | 312 per 1000 |
365 per 1000 (324 to 406) |
OR 1.27 (1.06 to 1.51) | 2219 (15 studies) |
⊕⊕⊝⊝ Lowa,c | When sensitivity analysis restricted to 9 studies with low or some concerns of overall risk of bias, it results in a similar effect (OR 1.26, 95% CI 1.04 to 1.54). |
| Cumulative pregnancy rate (slow freezing) | 565 per 1000 |
472 per 1000 (384 to 562) |
OR 0.69 (0.48 to 0.99) |
512 (4 studies) |
⊕⊕⊝⊝ Lowa,c | |
| Cumulative pregnancy rate (vitrification) | 333 per 1000 |
550 per 1000 (369 to 719) |
OR 2.44 (1.17 to 5.12) |
120 (1 study) |
⊕⊕⊕⊝ Moderatea | |
| Clinical pregnancy rate | 390 per 1000 | 444 per 1000 (417 to 470) | OR 1.25 (1.12 to 1.39) | 5821 (32 studies) | ⊕⊕⊝⊝ Moderatea | When sensitivity analysis restricted to 17 studies with low risk or some concerns of overall risk of bias, it results in a similar effect (OR 1.24, 95% CI 1.08 to 1.41). |
| Multiple pregnancy rate | 89 per 1000 | 99 per 1000 (81 to 119) | OR 1.12 (0.90 to 1.38) | 4208 (22 studies) | ⊕⊕⊝⊝ Lowa,b,c | When sensitivity analysis restricted to 15 studies with low risk or some concerns of overall risk of bias, it results in an increase of multiple pregnancy rate (OR 1.33, 95% CI 1.04 to 1.70). |
| Miscarriage rate | 67 per 1000 | 82 per 1000 (68 to 111) | OR 1.24, (0.98 to 1.57) | 4106 (21 studies) | ⊕⊕⊝⊝ Lowa,c | |
| Embryo freezing rate | 594 per 1000 |
412 per 1000 (369 to 455) |
OR 0.48 (0.40 to 0.57) |
2292 (14 studies) |
⊕⊕⊝⊝ Lowa,b | I2 = 84%. Direction of effect largely consistent |
| Failure rate to transfer any embryos | 11 per 1000 |
26 per 1000 (19 to 37) |
OR 2.50 (1.76 to 3.55) |
2577 (17 studies) |
⊕⊕⊕⊝ Moderatea | I2 = 36% |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level for serious risk of bias: most studies have some concerns of overall risk of bias, mainly due to the randomisation process, deviations from the intended intervention and selective reporting bDowngraded one level for serious inconsistency cDowngraded one level for serious imprecision: findings compatible with benefit or minimal effect
Background
Description of the condition
Worldwide, 15% of reproductive‐aged couples are affected by infertility (WHO 2022). The World Health Organization estimates that, globally, between 48 million couples and 186 million individuals live with infertility (WHO 2021). Assisted reproductive technologies (ARTs), such as in vitro fertilisation (IVF), intracytoplasmic sperm injection (ICSI), and embryo freezing, are considered beneficial for many couples and women who are unlikely to conceive without treatment, and for whom less invasive forms of treatment have failed or are unlikely to be effective. The fledgling era of IVF, from 1980 to the mid‐1990s, was characterised by relatively static successful pregnancy rates of around 20%. The past decade, however, has given rise to advances in ovarian stimulation, cell culture, embryo transfer, and new cryopreservation techniques as well as freeze‐all cycles, that have culminated in significant overall improvements in successful pregnancies (Wirleitner 2016; Roque 2015). This is evident in the annual statistical reports from different areas of the globe. One such report, for example, has demonstrated a doubling of the pregnancy rate per embryo transfer cycle from 1994 to 2003, despite a decrease in the mean number of embryos transferred (Waters 2006).
Description of the intervention
IVF involves the use of hormones to stimulate the ovaries to produce many eggs (oocytes), followed by egg collection (oocyte retrieval), addition or injection of sperm, fertilisation, embryo culture, and lastly, the return of a few selected embryos to the uterus (embryo transfer).
Conventionally, embryos have been transferred on either day 2 or day 3 when the embryos were two to eight cells, or 'cleavage stage', because the uterus was thought to provide the best environment for the survival of the embryo (Laverge 2001). The question of optimal timing for embryo transfer arises when examining the differences between IVF procedures and what happens naturally in vivo. Day 2 is an early time at which morphological grading of the embryos is possible, allowing selection of the 'best' embryos for transfer. Embryo morphology, along with other factors, is thought to be highly indicative of pregnancy outcome (De Placido 2002). Early replacement in the uterus may be advantageous for the embryos by limiting the time spent in the in vitro environment of the embryology laboratory.
Over the past decade, there has been a steady shift in practice to the transfer of embryos on day 5 or 6, when the embryos are 'blastocysts'. With the introduction of a variety of commercial preparations of sequential media in the late 1990s, the ART service sector witnessed an explosion of worldwide interest in blastocyst culture, with most clinics conducting research into its application in their own settings. As a result, a substantial volume of publications followed. These documented trials with conflicting results and reflected debates about the merits and drawbacks of extended culture (Sfontouris 2021; Sunde 2016; Sunde 2021).
One of the benefits of blastocyst‐stage transfer could be the potential for an improved implantation rate, which could lead to a change in policy about the number of embryos to be transferred (Kamath 2020). The higher the implantation rate is, the lower the number of embryos are transferred.
The fact that many blastocyst‐stage transfer trials were not prospectively randomised or were underpowered has contributed to the lack of a strong consensus about best practice for blastocyst culture. The need for an evidence‐based approach using meta‐analysis of small trials was, therefore, required to assist in deciphering the overall effect of blastocyst culture to help identify participant subsets and practices that might best benefit from this approach.
How the intervention might work
Blastocyst culture is not novel; indeed, the very first report of an IVF pregnancy was from a transferred blastocyst (Edwards 1995). Despite this, cleavage‐stage transfer was adopted as standard global practice early in the history of IVF for two reasons: the low developmental rate of embryos cultured past this stage; and the observation that, unlike other primates, human embryos have an unusual propensity to survive when replaced prematurely into the uterus (Marston 1977). However, as knowledge of embryo metabolic requirements expanded, so did the range of more advanced culture media (Scholtes 1996), and co‐culture techniques, culturing endometrial cells in co‐culture with the embryo (Menezo 1990; Van Blerkom 1993; Yeung 1992). One important finding was that the in vitro environment in which a cleavage‐stage embryo grows best is different from that for a blastocyst. This led to the evolution of stage‐specific (or sequential) media (G1/G2) by Gardner in 1998 (Gardner 1998b): embryos are transferred on day 3 from a medium containing low concentrations of glucose and one or more amino acids to a medium containing higher concentrations of glucose and a wider range of amino acids (Gardner 1996). At this stage, the embryo undergoes cell compaction and genomic activation so that the embryo is no longer under the control of transcripts and ribonucleic acid (RNA) messages of maternal origin (Braude 1998). With the application of stage‐specific media, there have been reports of blastocyst development rates as high as 60% to 65% (Schoolcraft 2001). Interestingly, with the development of time‐lapse systems (TLS), stage‐specific media is no longer considered essential (Armstrong 2019). Time‐lapse systems take frequent digital images of embryos, allowing embryologists to assess their quality without physically removing them from the incubator.
There are two central arguments why blastocyst culture has possible advantages over traditional cleavage‐stage transfer. Firstly, it is considered to be physiologically premature to expose early‐stage embryos to the uterine environment, particularly one that has been subjected to superovulation and thus high levels of oestrogen (Valbuena 2001). In vivo, embryos travel through the fallopian tubes and do not reach the uterus before the morula (16‐cell compacted) stage (Croxatto 1972), which equates to at least day 4 of in vitro culture. The uterus provides a different nutritional environment from the oviduct. Therefore, it is postulated that the uterine environment may cause stress on the embryo, if transferred at cleavage stage (Baart 2006; Munne 2002), and result in reduced implantation potential (Fanchin 2001; Gardner 1996).
The second argument for blastocyst‐stage transfer is the reported higher implantation potential compared with cleavage‐stage embryos. As a consequence of self selection, it is postulated that only the most viable embryos are expected to develop into blastocysts. It is widely acknowledged that the morphological criteria used for selection of the best embryos on day 2 to 3 are limited. Many published studies that debate the correlation of morphological features with pregnancy rates can be found in the literature (Palmstierna 1998; Puissant 1987; Roseboom 1995; Scott 2000; Sjoblom 2006; Steer 1992). It is now understood that a disturbingly large proportion of morphologically‐normal day 3 embryos are chromosomally abnormal or mosaic, thus contributing to the 80% to 90% rate of implantation failure post‐transfer that is observed in cleavage‐stage protocols (Magli 1998). While the transfer of day 5 blastocysts cannot ensure the absence of chromosomal abnormality (Magli 2000), Staessen 2004 demonstrated that, at least in women older than 36 years, the incidence can be reduced from 59% in day 3 embryos to 35% in day 5 blastocysts. The question that this review aims to answer is whether the higher implantation rates do translate into higher live birth rates.
Arguments against blastocyst culture are largely related to this process of self selection. Women undergoing blastocyst culture are expected to have a higher incidence of cycle cancellation due to failed embryo development (Marek 1999), and to have fewer embryos cryopreserved (frozen) (Tsirigotis 1998).
Overall utilisation rates have previously been described as the total number of embryos transferred plus the embryos thawed divided by the number of fertilised eggs. Whilst this approach presents information about the comparative number of pregnancy opportunities that each treatment approach can provide a woman or couple, it does not take into account the implantation potential for fresh and thawed embryos. Cumulative live birth rate is the only outcome that can assess this. An alternative efficacy formula was developed in the Schoolcraft 2001 study that does take cumulative pregnancy rate into account. Using the formula (mean number of embryos transferred multiplied by implantation rate) plus (mean number of embryos cryopreserved multiplied by implantation rate) minus (1 minus cancellation rate), this group of researchers was able to demonstrate a 19% greater efficiency in blastocyst culture compared to cleavage‐stage transfers. Disappointingly, such a utilisation and efficiency analysis is not possible in the majority of RCTs due to the lack of reporting of frozen‐thawed cycle outcomes within a reasonable time frame for trials. A frozen‐thawed cycle is an embryo transfer that is performed at a later date with embryos that were frozen a few days after the oocyte retrieval. We would argue that a superior approach to both of the foregoing methods is to report the live birth rates for both fresh and frozen‐thawed cycles following a single oocyte retrieval in women randomised to either cleavage‐stage or blastocyst‐stage transfers.
Why it is important to do this review
This is an update of a Cochrane Review first published in 2002, and previously updated in 2005, 2007, 2012, and 2016.
Advocates of blastocyst culture are confident that only the most viable embryos survive the extended culture to day 5 or 6. They argue that this results in a higher probability of implantation and requires fewer embryos to be transferred, thereby lowering the costly multiple birth rate (Gardner 1998b; Jones 1999). It is important to be aware that clinic policies may differ on the minimum criteria for blastocyst culture and the day on which this decision is made (for example, number of follicles, fertilised eggs, 8‐cell embryos on day 3) (Milki 1999). It is also yet to be clarified if there are patient groups for whom blastocyst culture is disadvantageous. Most importantly, does blastocyst culture achieve the primary aim of providing the subfertile couple with a normal, healthy baby? Methods for identifying viable blastocysts are a popular research focus, involving a range of approaches which include identification of chromosomally‐normal blastocysts by polar‐body and blastomere, trophectoderm genetic analysis (using microarrays or next‐generation sequencing known as pre‐implantation genetic screening (Jones 2008)), and metabolomic analysis of culture media (Nel‐Themaat 2011).
Critics of the blastocyst culture approach express concern at the increased incidence of women failing to have embryos available for transfer (Marek 1999), although the day of participant recruitment into the blastocyst programme is crucial to this argument.Other negative outcomes reported to be associated with blastocyst culture include a higher incidence of monozygotic twinning and altered sex ratio in favour of males (Menezo 1999; Spangmose 2020). Monozygotic twinning is frequently reported at above 1% in ART cycles (Sills 2000), whilst the background rate of monozygotic twins in spontaneous conceptions is in the order of 1 in 330. This twinning is associated with miscarriage, serious structural congenital anomalies, growth discrepancy and twin‐to‐twin transfusion syndrome. Extended culture of an embryo has been implicated as one of the interventions associated with an increase in monozygotic twinning (Behr 2000; Cohen 1990; De Felici 1982; Jain 2004), but a recent report suggests that improvements in cell culture techniques over time can result in a significant decrease in its incidence (Moayeri 2007). Similarly, as the underlying mechanisms that lead to an altered sex ratio are elucidated, whether it be media constituents or simply the morphological selection criteria (Luna 2007), the imbalance may also be rectified.
The aim of this review is to determine whether the number of days between oocyte retrieval and embryo transfer (that is, the embryo stage) has any effect on the success of ART treatment, and in particular, the live birth rate, the most important outcome for couples or women undergoing treatment as well as for service providers.
Objectives
To determine whether blastocyst‐stage (day 5 to 6) embryo transfer improves the live birth rate (LBR) per fresh transfer, and other associated outcomes, compared with cleavage‐stage (day 2 to 3) embryo transfer.
Methods
Criteria for considering studies for this review
Types of studies
We included only individually‐randomised parallel‐group trials (RCTs). We excluded quasi‐RCTs and cluster‐randomised trials. We also excluded cross‐over trials unless pre‐cross‐over data were available.
Types of participants
Inclusion criteria
We included couples or women affected by subfertility undergoing in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) for therapeutic reasons or for oocyte donation within all patient prognosis groups.
'Patient prognosis groups' (participant subsets or populations) is a term used to describe the categories that couples or women are assigned to based on several factors such as their age, type of subfertility, ovarian response to the superovulation drugs, and number of previous attempts. See the Subgroup analysis and investigation of heterogeneity section below for the categories.
Exclusion criteria
We excluded couples or women whose IVF or ICSI cycle, or both, involved in vitro matured oocytes or pre‐implantation genetic screening. We also excluded couples or women whose frozen‐thawed cycle results were shown, but where no data were available from the fresh cycle.
Types of interventions
Inclusion criteria
We included studies comparing blastocyst‐stage (day 5 to 6) transfers to cleavage‐stage (day 2 to 3) transfers in settings using single and sequential media culture methods for IVF and ICSI, where the embryos were grown for between 2 and 6 days in vitro prior to embryo transfer.
Exclusion criteria
We excluded studies using co‐culture methods as an intervention.
We also excluded studies comparing blastocyst‐stage transfers to cleavage‐stage transfers in frozen‐thawed cycles, but where no data were available from the fresh cycle.
Types of outcome measures
Primary outcomes
Live birth rate per couple or woman (number of live births after week 20 of pregnancy per couple or woman) following fresh transfer
Cumulative pregnancy rate (cCPR) per couple or woman (from both fresh and thawed cycles deriving from a single egg collection procedure)
Secondary outcomes
Clinical pregnancy rate per couple or woman: number of couples or women achieving a clinical pregnancy following fresh transfer (defined by the demonstration of foetal heart activity on ultrasound scan)
Multiple pregnancy rate per couple or woman following fresh transfer: number of multiple pregnancies per couple or woman
High‐order multiple pregnancy rate per couple or woman following fresh transfer: three or more foetal heartbeats per couple or woman
Miscarriage rate for fresh transfer: number of occurrences per couple or woman and per pregnant woman
Embryo freezing rates per couple or woman: number of couples or women that had supernumerary embryos for transfer at a later date per couple or woman
Failure rate to transfer embryos (per couple or woman): percentage of couples or women that did not have an embryo transfer
Additional outcomes not appropriate for statistical pooling
We were unable to pool data per cycle or per embryo transfer or per oocyte pick up (OPU) (Vail 2003). If a study included multiple cycles, transfers, or OPUs per woman, then results reported per cycle/transfer/OPU cannot be validly pooled. If the study reported results per cycle/transfer/OPU and this did not coincide with the point of randomisation (i.e. if the denominator did not coincide with the point in the treatment where randomisation occurred), we calculated new results using randomised participants as the denominator and treated those excluded as having negative outcomes. However, due to the frequency that this form of data is reported in the literature, we have entered them into the 'Data and analyses tables' for the following outcomes.
Live births per OPU and embryo transfer.
Clinical pregnancy rate per OPU and embryo transfer.
Implantation rate: the number of foetal sacs divided by the number of embryos transferred.
Search methods for identification of studies
We obtained all reports that described (or might have described) RCTs comparing cleavage‐stage embryo transfer and blastocyst‐stage transfer in the treatment of subfertility, using IVF or ICSI, using the search strategy developed by the Gynaecology and Fertility Group.
Electronic searches
We searched the following databases.
The Cochrane Gynaecology and Fertility Group Specialised Register of Controlled Trials, searched 20 October 2021, ProCite platform (Appendix 1).
CENTRAL via the Cochrane Register of Studies Online (CRSO), searched 20 October 2021, Web platform (Appendix 2).
MEDLINE, searched from 1946 to 20 October 2021, Ovid platform (Appendix 3).
Embase, searched from 1980 to 20 October 2021, Ovid platform (Appendix 4).
PsycINFO, searched from 1806 to 20 October 2021, Ovid platform (Appendix 5).
CINAHL (Cumulative Index to Nursing and Allied Health Literature), searched from 1961 to 4 April 2020, EBSCO platform (Appendix 6). Any later CINAHL search output is contained in the 2021 CENTRAL search output.
We combined the MEDLINE search with the Cochrane highly sensitive search strategy for identifying randomised trials, which appears in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019a, hereafter referred to as the Cochrane Handbook).
We combined the Embase search with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/what-we-do/methodology/search-filters/). We did not impose any language restrictions in these searches.
Searching other resources
We searched the National Research Register, a register of ongoing and recently completed research projects funded by, or of interest to, the United Kingdom's National Health Service (NHS); entries from the Medical Research Council Clinical Trials Register; and details on reviews in progress that are collected by the NHS Centre for Reviews and Dissemination.
We also searched ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (Appendix 7; Appendix 8).
We performed the search on titles, abstracts, and keywords of the listed articles. We also searched the citation lists of relevant publications, review articles, and included studies. We handsearched relevant conference abstracts and contacted experts in the field.
We conduct a search for new trials bi‐annually and update the review as and when we find new trials to be incorporated.
Data collection and analysis
Selection of studies
Three review authors (DG, CAS, and AQR) performed the selection of trials for inclusion in the review after employing the search strategy described previously. Two review authors independently viewed each record. We resolved any disagreements about eligibility through discussion. We have presented details of the included studies in the Characteristics of included studies tables, which provide a context for assessing the reliability of results. We have described excluded studies in the Characteristics of excluded studies table.
Data extraction and management
Two of three review authors (DG, CAS, and AQR) extracted data from eligible studies using a data extraction form designed and pilot‐tested by the authors. Two review authors independently viewed each record. We resolved any disagreements about data extraction through discussion. Data extracted included study characteristics and outcome data. Where studies had multiple publications, we used the main trial report as the primary reference and derived additional details from secondary papers. We corresponded with study investigators for further data, as required.
Assessment of risk of bias in included studies
Two of three review authors (DG, SC, and AC) independently assessed the included studies for risk of bias using the Cochrane risk of bias 2 (RoB 2) assessment tool (Higgins 2019b; Sterne 2019). We assessed: bias arising from the randomisation process; bias due to deviations from intended interventions; bias due to missing outcome data; bias in measurement of the outcome; and bias in selection of the reported result. The effect of interest was the effect of assignment to the intervention at baseline, regardless of whether the interventions were received as intended (the 'intention‐to‐treat effect').
A potential deviation from the intended protocols is an adherence issue, such as moving to the cleavage transfer arm if there were few available embryos on day 3. Another potential deviation is a difference that could be found in the number of transferred embryos in each of the arms. Although both of these deviations could occur in the real world, they could also occur as a result of participating in a trial.
The outcomes we selected to be assessed for risk of bias are the same as those reported in the summary of findings table: live birth, cumulative pregnancy, clinical pregnancy, multiple pregnancy, miscarriage, embryo freezing, and failure to transfer any embryo. We describe the measurement methods and time points in the Measures of treatment effect section.
Judgements were classified as 'low' or 'high' risk of bias, or as expressing 'some concerns', assigned as recommended in the Cochrane Handbook, Chapter 8 (Higgins 2019a). We resolved any disagreements about risk of bias judgements through discussion. We described all judgements fully and presented the consensus judgements in the main review document (e.g. as a table, or a figure, or within a forest plot of the results).
These domain‐level judgements provided the basis for an overall risk of bias judgement for the specific trial result being assessed.
We used the RoB 2 Excel tool (available on the riskofbiasinfo.org website) to process the use of RoB 2 and to store data for presentation (Sterne 2019). We made risk of bias data available by providing detailed appendices.
We sought additional information on trial methodology or actual original trial data from the principal author of trials that appeared to meet eligibility criteria but were unclear in aspects of methodology, or where the data were in a form unsuitable for meta‐analysis. We sent reminder correspondence when a reply was not received within three weeks.
Measures of treatment effect
For dichotomous data (for example, clinical pregnancy rate), we expressed results for each study as odds ratios (ORs) with 95% confidence intervals (CIs) and combined them for meta‐analysis with RevMan Web 2020 (RevMan Web 2020).
Unit of analysis issues
The primary analysis was per woman randomised. We counted multiple live births (for example, twins or triplets) as one live birth event.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible (i.e. including all randomised participants in analysis, in the groups to which they were randomised). We attempted to obtain missing data from the original trialists. Where these were unobtainable, we imputed individual values to all the outcomes: they were assumed not to have occurred in participants without a reported outcome.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by the measure of the I2 statistic. An I2 measurement greater than 50% was taken to indicate substantial heterogeneity. When we detected substantial heterogeneity, we explored possible explanations in subgroup analyses. We took statistical heterogeneity into account when interpreting the results.
We examined heterogeneity between the results of different studies by inspecting the scatter of data points, the overlap in their confidence intervals, and more formally, by checking the results of the Chi2 tests. A priori, we had planned to look at the possible contribution of differences in trial design to the heterogeneity identified. Where possible, we pooled the outcomes statistically.
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert to duplication of data. If there were ten or more studies in an analysis, we used a funnel plot to explore the possibility of small study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
We performed statistical analyses in accordance with the Cochrane Handbook (Higgins 2019a). The primary analyses included all studies.
We pooled data for meta‐analysis with RevMan Web 2020 (RevMan Web 2020), using the fixed‐effect Mantel‐Haenszel model method. We entered the data on the graphs so that, for beneficial outcomes (for example, pregnancy), data are displayed to the right of the line of no effect, and in detrimental outcomes (for example, miscarriage), to the left of the line of no effect.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses for the outcomes live birth, cumulative pregnancy, clinical pregnancy, and multiple pregnancy.
Studies that actively selected for good prognosis participants (for example, four or more zygotes, first two cycles, more than 10 follicles, young population, no male‐factor individuals) versus participants with poor prognostic factors (for example, previous failed ART cycles or poor response to ovulation stimulation) versus studies with unselected participants.
Studies that randomised at the start of the cycle (that is, prior to ovarian stimulation) were compared with those that randomised at the days immediately prior to and post‐OPU (that is, day of final ultrasound scan and prior to human chorionic gonadotropin trigger up to and including the day of fertilisation check, when numbers of oocytes are anticipated).
Studies where the policy for the number of embryos replaced was equal in both blastocyst‐stage and cleavage‐stage groups versus studies where fewer blastocyst‐stage than cleavage‐stage embryos were replaced.
We also included a post hoc analysis to investigate substantial heterogeneity for one of the primary outcomes: studies where freezing technique was slow freezing versus studies where vitrification was used.
-
We made a subgroup analysis for time‐lapse s ystem s election (TLS selection ) with algorithm which was not stated in the protocol, as TLS is a technology that was unavailable when the protocol was written. Subgroups would be:
Conventional cleavage stage versus conventional blastocyst stage;
TLS selection (with algorithm) cleavage stage versus conventional blastocyst stage;
TLS selection (with algorithm) cleavage stage versus TLS selection (with algorithm) blastocyst stage.
We planned to perform an overall assessment of risk of bias for subgroups.
Sensitivity analysis
Primary analysis pooled the data of all the studies. The selected outcomes assessed by a sensitivity analysis are live birth, cumulative pregnancy, clinical pregnancy, and embryo freezing.
Eligibility was restricted to studies with outcomes with 'low' or 'some concerns for' overall risk of bias.
Summary of findings and assessment of the certainty of the evidence
We prepared a summary of findings table using GRADEpro and Cochrane methods (GRADEpro GDT; Higgins 2019a). This table evaluated the overall quality of the body of evidence for the main review outcomes (live birth rate, cumulative pregnancy rate, clinical pregnancy rate, multiple pregnancy rate, miscarriage rate, embryo freezing rate and failure rate to transfer any embryos) for the main review comparison (blastocyst‐stage transfer versus cleavage‐stage transfer). We assessed the quality of the evidence using GRADE criteria: overall risk of bias (fed by RoB2 tool assessment), consistency of effect, imprecision, indirectness, and publication bias. Two review authors working independently made judgements about evidence quality (high, moderate, low, or very low), resolving any disagreements through discussion. We justified, documented, and incorporated our judgements into the reporting of results for each outcome.
We planned to extract study data, format our comparisons in data tables, and prepare a summary of findings table before writing the results and conclusions of our review.
Results
Description of studies
Results of the search
At the 2022 update we identified 1651 articles as potentially relevant for comparing blastocyst‐stage versus cleavage‐stage embryo transfer, and we retrieved 26 new articles in full text. In the 2022 update we included five new studies (Hatirnaz 2017; Kaser 2017; Levi‐Setti 2018; Singh 2017; Yang 2018), and there were 3 newly excluded studies (Cornelisse 2018; Green 2016; Holden 2017). Including studies retrieved in the latest update, we now have 32 studies (47 articles) in the review. See Figure 1.
1.
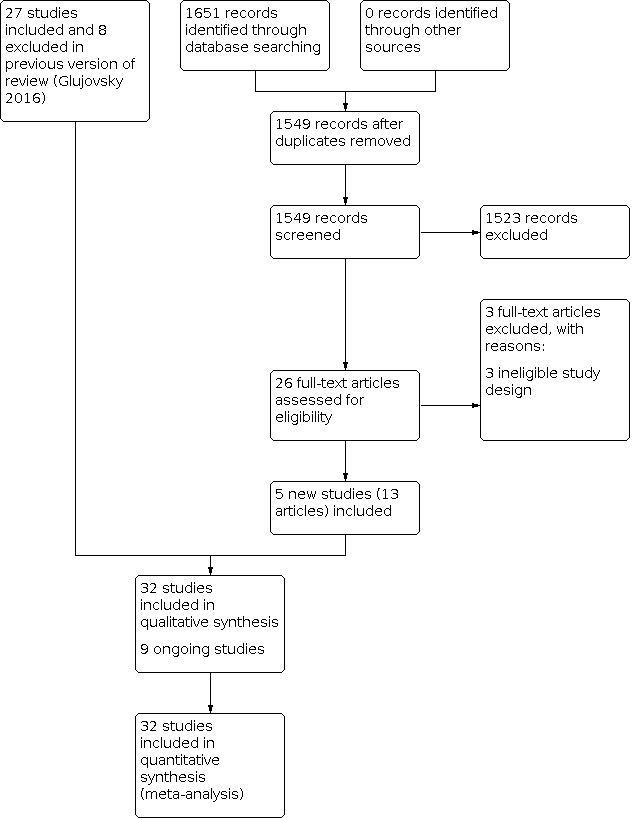
Study flow diagram: results of search from review inception to 2020 (update of the flow diagram published in 2016)
We also found 7 new ongoing studies. In total, there are 9 ongoing studies: one each from China (ChiCTR‐ICR‐15006184), Iran (NCT01107002), and the USA (Neuhausser 2020); two from Italy (ISRCTN48090543; NCT02639000), and the Netherlands (Cornelisse 2021); and three from Egypt (NCT04210414; PACTR201402000773124; PACTR201709002592834).
We attempted to contact study authors for information regarding methodology and outcome data. We received replies from 11 contact authors (Bungum 2003; Fernandez‐Shaw 2015; Frattarelli 2003; Hreinsson 2004; Karaki 2002; Levitas 2004; Levron 2002; Livingstone 2002, Papanikolaou 2005; Papanikolaou 2006; Rienzi 2002). Cumulative live birth data were provided by Fernandez‐Shaw 2015.
Included studies
Study design and setting
We included 32 parallel‐design RCTs in this review (5821 participants). The size of trials ranged from 20 in Fisch 2007 to 460 couples or women in Kolibianakis 2004, including both comparison groups.
The majority of trials were carried out in less than six months, except for the two largest studies. All studies were reported to have been performed at single private clinics or university‐based clinics. Twelve countries were represented in the included studies, with Belgium being the most prolific, providing six studies. The countries represented were: Australia (Livingstone 2002); Belgium (Devreker 2000; Emiliani 2003; Kolibianakis 2004; Papanikolaou 2005; Papanikolaou 2006; Van der Auwera 2002); Brazil (Motta 1998); China (Yang 2018); Denmark (Bungum 2003); Egypt (Elgindy 2011; Gaafar 2015); France (Brugnon 2010); Greece (Pantos 2004); India (Kaur 2014; Singh 2017); Iran (Aziminekoo 2015); Israel (Coskun 2000; Levitas 2004; Levron 2002); Italy (Levi‐Setti 2018; Rienzi 2002; Schillaci 2002); Jordan (Karaki 2002); Spain (Fernandez‐Shaw 2015; Ten 2011); Sweden (Hreinsson 2004); Turkey (Hatirnaz 2017); and the USA (Fisch 2007; Frattarelli 2003; Gardner 1998a; Kaser 2017).
Participants
Participant selection criteria comprised three main groups: unselected participants (Emiliani 2003; Fernandez‐Shaw 2015; Gaafar 2015; Hatirnaz 2017; Karaki 2002; Kolibianakis 2004; Motta 1998; Pantos 2004; Schillaci 2002; Van der Auwera 2002); good prognostic factors where participants were positively selected; that is, those who would be expected to do well with blastocyst culture (Brugnon 2010; Bungum 2003; Coskun 2000; Elgindy 2011; Fisch 2007; Frattarelli 2003; Gardner 1998a; Hreinsson 2004; Kaser 2017; Kaur 2014; Levi‐Setti 2018; Levron 2002; Livingstone 2002; Papanikolaou 2005; Papanikolaou 2006; Rienzi 2002; Singh 2017; Ten 2011; Yang 2018); and poor prognostic factors, where couples or women were selected who had experienced multiple failures with conventional treatment or had a poor response to ovulation induction (Aziminekoo 2015; Devreker 2000; Levitas 2004). Most studies recruited women under 40 years of age, except for Fernandez‐Shaw 2015, Gardner 1998a, and Gaafar 2015, which had no age limit. The mean age across all the studies varied from 29 years to 34 years.
We found no studies of participants using donor eggs.
Interventions
Twenty trials used sequential media, of which 13 used Vitrolife G1/G2, while the remaining media were combinations of brands or made in‐house. Five did not state the media used (Table 2).
1. Culture techniques of included studies.
| Trial | Culture technique day 2/3 | Culture technique day 5/6 |
| Aziminekoo 2015 | Sydney IVF cleavage medium, Cook | Sydney IVF blastocyst medium |
| Brugnon 2010 | G series™ medium (Vitrolife, Sweden) | G series™ medium (Vitrolife, Sweden) |
| Bungum 2003 | Sequential G1 Vitrolife | Sequential G1/G2 Vitrolife |
| Coskun 2000 | Sequential MediCult | Sequential G1/G2 Vitrolife |
| Devreker 2000 | NS | NS |
| Elgindy 2011 | NS | NS |
| Emiliani 2003 | In‐house sequential (based on G1/G2) | In‐house sequential (based on G1/G2) |
| Fernandez‐Shaw 2015 | Sequential G1 Vitrolife | Sequential G1/G2 Vitrolife |
| Fisch 2007 | NS | NS |
| Frattarelli 2003 | NS | NS |
| Gaafar 2015 | NS | NS |
| Gardner 1998a | Single Ham's F10 In‐house | Sequential G1/G2 In‐house |
| Hatirnaz 2017 | Standard culture medium | Sequential G1/G2 Scandinavian IVF Sciences |
| Hreinsson 2004 | Vitrolife IVF | Sequential G1/G2 or CCM Vitrolife |
| Karaki 2002 | MediCult | Sequential G1/G2 Vitrolife |
| Kaser 2017 | Global total with HSA LifeGlobal | Global total with HSA LifeGlobal |
| Kaur 2014 | Cleavage medium | G2 Plus media |
| Kolibianakis 2004 | Sequential G1 Vitrolife | Sequential G1/G2 Vitrolife |
| Levi‐Setti 2018 | NS | NS |
| Levitas 2004 | NS | Sequential ‐ G1/G2 Vitrolife |
| Levron 2002 | NS | NS |
| Livingstone 2002 | Sequential ‐ Sydney IVF Cook | Sequential ‐ Sydney IVF Cook |
| Motta 1998 | Sequential ‐ Irvines P1 | Sequential ‐ Irvines P1 then Blast media |
| Pantos 2004 | ||
| Papanikolaou 2005 | Sequential ‐ Vitrolife G1/G2 GII or GIII | Sequential ‐ Vitrolife G1/G2 GII or GIII |
| Papanikolaou 2006 | Assume sequential ‐ Vitrolife G1/G2 | Assume sequential ‐ Vitrolife G1/G2 |
| Rienzi 2002 | Sequential G1 Vitrolife | Sequential G1/G2 Vitrolife |
| Schillaci 2002 | NS | NS |
| Singh 2017 | NS | NS |
| Ten 2011 | NS | NS |
| Van der Auwera 2002 | Sequential both Cook and Vitrolife | Sequential both Cook and Vitrolife |
| Yang 2018 | Sequential media (G1.5, Vitrolife) in a Primo Vision time‐lapse system (Vitrolife) | Sequential G1/G2 Vitrolife |
CCM: a Vitrolife trademarked medium for blastocyst culture IVF: in vitro fertilisation G1/G2:sequential media from Vitrolife NS: not stated
Freezing of embryos in both experimental groups was reported in 14 of the 32 included trials (Brugnon 2010; Bungum 2003; Fernandez‐Shaw 2015; Gardner 1998a; Hreinsson 2004; Karaki 2002; Kolibianakis 2004; Levron 2002; Motta 1998; Pantos 2004; Papanikolaou 2006; Rienzi 2002; Ten 2011; Van der Auwera 2002). Coskun 2000 reported no provision for day 5 freezing. Levitas 2004 stated that most of the remaining embryos were not suitable for freezing. Other interventions, such as assisted hatching, were either not provided or not reported on for the majority of trials. Gardner 1998a was the only trial that practised assisted hatching, but only for the day 3 embryo transfer group.
All the studies compared blastocyst‐stage versus cleavage‐stage embryo transfers. For the cleavage‐stage transfer groups, most transfers were on day 3, except for five trials that did the embryo transfers on day 2 (Devreker 2000; Emiliani 2003; Gaafar 2015; Motta 1998; Van der Auwera 2002), and one study that had a policy of transferring on day 2 or 3 (Levitas 2004).
The trials that provided details on the ovarian stimulation regimen mostly reported using a similar gonadotropin‐releasing hormone pituitary down‐regulation protocol prior to human menopausal gonadotropin (HMG) and follicle stimulating hormone (FSH) administration. However, in some trials (Kolibianakis 2004; Levi‐Setti 2018; Papanikolaou 2005; Papanikolaou 2006; Singh 2017), gonadotropin‐releasing hormone antagonists were used in varying degrees.
Two studies (Kaser 2017; Yang 2018) evaluated the effects of adding time‐lapse system (TLS) to cleavage‐stage transfer.
Outcomes
15/32 studies reported live birth rate per fresh embryo transfer
5/32 studies reported cumulative pregnancy rate
32/32 studies reported clinical pregnancy rate
22/32 studies reported multiple pregnancy rate
13/32 studies reported high‐order multiple pregnancy rate
21/32 studies reported miscarriage rate
14/32 studies reported embryo freezing rate (when there are supernumerary embryos for transfer at a later date)
17/32 studies reported failure rate to transfer embryos
Excluded studies
We excluded 10 studies from the review for the following reasons. Six were not truly randomised studies (Cornelisse 2018; Green 2016; Holden 2017; Levron 2001; Utsonomiya 2004; Zech 2007). Three studies used co‐culture (Bungum 2002; Guerin 1991; Menezo 1992). In the remaining study, fresh embryo transfers on day 5 or 6 were not the main intervention (Loup 2009).
One study (Clua Obrado 2020) randomised Spanish recipients between 18 and 50 years old in their first or second synchronous cycle to D3 or D5 embryo transfer. The authors will not provide the missing information until its publication, therefore it awaits classification.
Risk of bias in included studies
We attempted to obtain additional information regarding all aspects of randomisation, blinding, power analysis, and intention‐to‐treat from all trial authors.
We accessed the RoB 2 tool on 20 August 2020. Risk of bias assessments for each outcome, including all domain judgements and support for the judgement, are located within each included study, and at the side of all forest plots. To access further detailed risk of bias assessment data, please use the following link.
The risk of bias judgements for outcomes across all studies were predominantly of 'some concerns' and 'high'. The most common reason for 'some concerns' of overall risk of bias was the lack of publication of the protocol in a trial registry, while overall high risk of bias was mainly due to lack of reporting of allocation concealment.
Detailed risk of bias assessment data (with consensus responses to the signalling questions) are available upon reasonable request to the authors.
Effects of interventions
See: Table 1
Blastocyst‐stage versus cleavage‐stage transfer
For an overview of our main analyses, please see Table 1.
Primary outcomes
1. Live birth rate per couple or woman
The live birth rate per fresh embryo transfer was higher in the fresh blastocyst transfer group (odds ratio (OR) 1.27, 95% confidence interval (CI) 1.06 to 1.51; I2 = 53%; 15 studies, 2219 women; low‐quality evidence; Analysis 1.1; Figure 2). This suggests that if 31% of women achieve live birth after fresh cleavage‐stage transfer, between 32% and 41% would do so after fresh blastocyst‐stage transfer.
1.1. Analysis.

Comparison 1: Blastocyst‐ versus cleavage‐stage transfer: live birth rate following fresh transfer, Outcome 1: Live birth per couple
2.

Forest plot of comparison: 1 Blastocyst‐ versus cleavage‐stage transfer: live birth rate, outcome: 1.1 live birth per couple
Subgroup and sensitivity analyses
We did not find evidence that the treatment effect differed between fresh cleavage‐stage and blastocyst‐stage transfer based either on number of embryos transferred (test for subgroup differences: P = 0.48, I² = 0%; Analysis 1.2) or on the prognosis (test for subgroup differences: P = 0.73, I² = 0%; Analysis 1.3).
1.2. Analysis.

Comparison 1: Blastocyst‐ versus cleavage‐stage transfer: live birth rate following fresh transfer, Outcome 2: Live birth per couple: grouped by number of embryos transferred
1.3. Analysis.

Comparison 1: Blastocyst‐ versus cleavage‐stage transfer: live birth rate following fresh transfer, Outcome 3: Live birth rate per couple: grouped by prognosis
Sensitivity analysis including only those studies with low or some concerns of overall risk of bias found a higher live birth rate in the blastocyst group (OR 1.26, 95% CI 1.04 to 1.54; I2 = 69%; 9 studies, 1821 women; moderate‐quality evidence).
2. Cumulative clinical pregnancy rate per couple or woman (following fresh and frozen‐thawed transfer)
We are uncertain whether blastocyst‐stage transfer improves cumulative clinical pregnancy rate (OR 0.89, 95% CI 0.64 to 1.22; I2 = 71%; 5 studies, 632 women; very low‐quality evidence; Analysis 2.1). There was substantial heterogeneity for this outcome, with differing directions of effect. The heterogeneity was largely attributable to two studies (Fernandez‐Shaw 2015; Rienzi 2002). We investigated statistical heterogeneity by conducting a post hoc subgroup analysis according to the method of freezing. The test for subgroup differences showed a significant difference between the subgroups (Chi² = 9.12, degrees of freedom (df) = 1 (P = 0.003), I² = 89.0%). The only study using vitrification showed evidence of higher cumulative pregnancy rate in blastocyst transfers (OR 2.44, 95% CI 1.17 to 5.12; moderate‐quality evidence; Fernandez‐Shaw 2015), whilst the four studies with slow freezing showed that the confidence interval is too wide to know if blastocyst transfer decreases the cumulative pregnancy rate (OR 0.69, 95% CI 0.48 to 0.99; low‐quality evidence). This is an interesting finding which should be investigated further when more studies using vitrification are published.
2.1. Analysis.

Comparison 2: Blastocyst‐ versus cleavage‐stage transfer: cumulative pregnancy rate following fresh and frozen transfer, Outcome 1: Cumulative pregnancy rate from fresh and frozen transfers
Subgroup and sensitivity analyses
We did not find evidence that the treatment effect differed between fresh cleavage‐stage and blastocyst‐stage transfer based on number of embryos transferred (test for subgroup differences: P = 0.89, I² = 0%) or day of randomisation (test for subgroup differences: P = 0.42, I² = 0%), and no conclusive evidence of a difference based on prognosis (test for subgroup differences: P = 0.05, I² = 73.6%; Analysis 2.2; Analysis 2.3; Analysis 2.4).
2.2. Analysis.

Comparison 2: Blastocyst‐ versus cleavage‐stage transfer: cumulative pregnancy rate following fresh and frozen transfer, Outcome 2: Cumulative pregnancy rate per couple: grouped by number of embryos transferred
2.3. Analysis.

Comparison 2: Blastocyst‐ versus cleavage‐stage transfer: cumulative pregnancy rate following fresh and frozen transfer, Outcome 3: Cumulative pregnancy rate per couple: grouped by prognosis
2.4. Analysis.

Comparison 2: Blastocyst‐ versus cleavage‐stage transfer: cumulative pregnancy rate following fresh and frozen transfer, Outcome 4: Cumulative pregnancy rate: grouped by day of randomisation
We are also uncertain whether blastocyst‐stage transfer improves the cumulative pregnancy rate when we run a sensitivity analysis including only those studies with low or some concerns of overall risk of bias (OR 1.10, 95% CI 0.74 to 1.61; I2 = 73%; 3 studies, 427 women; very low‐quality evidence).
Secondary outcomes
3. Clinical pregnancy rate per couple or woman
The clinical pregnancy rate was higher in the fresh blastocyst‐transfer group (OR 1.25, 95% CI 1.12 to 1.39; I2 = 51%; 32 studies, 5821 women; moderate‐quality evidence; Figure 3). This suggests that if 39% of women achieve a clinical pregnancy after fresh cleavage‐stage transfer, between 42% and 47% would do so after fresh blastocyst‐stage transfer.
3.

Forest plot of comparison: 2 Blastocyst‐ versus cleavage‐stage transfer: clinical pregnancy rate, outcome: 2.1 clinical pregnancy rate per couple
Subgroup and sensitivity analyses
We did not find evidence that the treatment effect differed between fresh cleavage‐stage and blastocyst‐stage transfer for clinical pregnancy rate. However, the separate analysis of subgroups shows that clinical pregnancy rate may be higher in the fresh blastocyst‐stage transfer group when the number of transferred embryos is equal (OR 1.31, 95% CI 1.16 to 1.48; I2 = 48%; 20 studies; 4434 women) and for single embryo transfers (OR 1.31, 95% CI 1.04 to 1.65; I2 = 43; 5 studies; 1241 women). We also found that good prognosis participants (OR 1.25, 95% CI 1.09 to 1.43; I2 = 51%; 9 studies, 3645 women) and unselected participants (OR 1.22, 95% CI 1.01 to 1.46; I2 = 64%; 10 studies, 1981 women) may have a higher clinical pregnancy rate if fresh embryo transfer was done at blastocyst stage. When analysing a fresh blastocyst transfer without TLS versus TLS cleavage‐stage transfer, the blastocyst transfer group also may have a higher pregnancy rate (OR 1.41, 95% CI 1.04 to 1.90; I2 =0%; 2 studies, 709 women). However, when analysing TLS blastocyst‐stage transfer versus TLS cleavage‐stage transfer, no differences were found (OR 0.91, 95% CI 0.43 to 1.96; 1 study, 110 women).
Sensitivity analysis including only those studies with low risk of bias for allocation concealment did not substantially influence our findings (OR 1.52, 95% CI 1.19 to 1.94; I2 = 68%; 8 studies, 1097 women), though heterogeneity was high. However, we found that transferring at fresh blastocyst stage had a higher clinical pregnancy rate when randomisation was done on day 2 or 3 after oocyte pick up (OPU) (OR 1.59, 95% CI 1.13 to 2.23; I2 = 63%; 4 studies, 537 women), but no differences were found when randomisation was performed earlier.
4. Multiple pregnancy rate per couple or woman
We are uncertain of the effect of the embryo stage on the multiple pregnancy rate in fresh cycles (OR 1.12, 95% CI 0.90 to 1.38; I2 = 24%; 22 studies, 4208 women; low‐quality evidence). This suggests that if 9% of women have a multiple pregnancy after fresh cleavage‐stage transfer, between 8% and 12% would do so after fresh blastocyst‐stage transfer.
Subgroup and sensitivity analyses
We did not find evidence that the treatment effect differed between fresh cleavage‐stage and blastocyst‐stage transfer based on number of embryos transferred, prognosis, or day of randomisation (Analysis 4.2; Analysis 4.3; Analysis 4.4).
4.2. Analysis.

Comparison 4: Blastocyst‐ versus cleavage‐stage transfer: multiple pregnancy following fresh transfer, Outcome 2: Multiple pregnancy rate per couple: grouped by number of embryos transferred
4.3. Analysis.

Comparison 4: Blastocyst‐ versus cleavage‐stage transfer: multiple pregnancy following fresh transfer, Outcome 3: Multiple pregnancy rate per couple: grouped by prognosis
4.4. Analysis.

Comparison 4: Blastocyst‐ versus cleavage‐stage transfer: multiple pregnancy following fresh transfer, Outcome 4: Multiple pregnancy rate per couple: grouped by day of randomisation
Sensitivity analysis including only studies with low or some concerns of overall risk of bias showed that transferring at blastocyst stage probably increases the multiple pregnancy rate (OR 1.33, 95% CI 1.04 to 1.70; moderate‐quality evidence).
We are uncertain of the effect of the embryo stage on the high‐order multiple pregnancy rate in fresh cycles (OR 0.45, 95% CI 0.18 to 1.15; I2 = 0%; 13 studies, 2335 women).
5. Miscarriage rate for fresh transfer
We are uncertain of the effect of the embryo stage on the miscarriage rate per couple or woman in fresh cycles (OR 1.24, 95% CI 0.98 to 1.57; I2 = 0%; 21 studies, 4106 women; low‐quality evidence).
6. Embryo freezing rate per couple or woman
Rates of embryo freezing when there are supernumerary embryos for transfer at a later date per couple or woman were lower in the blastocyst transfer group (OR 0.48, 95% CI 0.40 to 0.57; I2 = 84%; 14 studies, 2292 women; low‐quality evidence). There was very high heterogeneity, but the direction of effect was consistent in most studies.
7. Failure rate to transfer embryos
Rates of failure to transfer any embryos were higher in the blastocyst transfer group (OR 2.50, 95% CI 1.76 to 3.55; I2 = 36%; 17 studies, 2577 women; moderate‐quality evidence).
8. Other data
Blastocyst formation rates
As reported in Table 3, blastocyst formation rates, which show the proportion of 2PN embryos that get to blastocyst stage (day 5 to 6 transfer only) ranged from 22.4% in Aziminekoo 2015 to 60.3% in Schillaci 2002.
2. Blastocyst formation and implantation rate (in day 5 to 6 transfers).
| Study | Blastocyst formation rate | Implantation D2/3 | Implantation D5/6 | Other |
| Aziminekoo 2015 | 22.4% | 21/173; 12.1% | 22/152; 14.5% | |
| Brugnon 2010 | Not stated | 24/52; 46.2% | 23/55; 41.8% | |
| Bungum 2003 | 55.2% | 50/114; 43.9% | 44/120; 36.7% | 2/61 participants had only 1 blastocyst |
| Coskun 2000 | 28% | 50/235; 21.3% | 52/218; 23.9% | 77% participants had at least 1 blastocyst |
| Devreker 2000 | Not stated | 1/34; 2.9% | 8/19; 42.1% | |
| Elgindy 2011 | 97% | 71/197; 36% | 53/280; 19% | |
| Emiliani 2003 | 48% | 57/197; 28.9% | 50/168; 29.8% | |
| Fernandez‐Shaw 2015 | 67.7 % | 20/71; 28.1% | 36/84; 42.8% | |
| Fisch 2007 | Not stated | 11/12; 92% | 4/8; 50% | |
| Frattarelli 2003 | Not stated | 18/69; 26.1% | 23/53; 43.4% | |
| Gaafar 2015 | Not stated | Not stated | Not stated | |
| Gardner 1998a | 46.5% | 64/174; 36.8% | 53/95; 55.8% | 85% women had at least 2 blastocysts |
| Hatirnaz 2017 | 52.6% | 45/95; 47.4% | 43/95; 45.3% | |
| Hreinsson 2004 | 33% | 29/139; 20.9% | 24/114; 21.1% | 2 morula replaced (one implanted). 60% pregnancy rate when top‐quality blasts transferred |
| Karaki 2002 | 33% | 37/291 12.7% | 37/142; 26.1% | 9/80 cancelled due to lack of blastocysts (unselected) |
| Kaser 2017 | Not stated | 23/56; 41.1% | 26/53; 49.1% | |
| Kaur 2014 | Not stated | 66/309; 21.4% | 102/290; 35.2% | |
| Kolibianakis 2004 | 50.7% | 96/234; 41.0% | 94/226; 41.6% | |
| Levi‐Setti 2018 | Not stated | 25.67% | 28.37% | |
| Levitas 2004 | 43% | 4/56; 7.1% | 10/24; 4.2% | Day 5‐7 26% cancelled due to lack of blastocysts (poor prognosis) |
| Levron 2002 | 34.2% | 53/137; 38.7% | 20/99; 20.2% | 6.5% cancelled due to lack of blastocysts (good prognosis) |
| Livingstone 2002 | Not stated | |||
| Motta 1998 | Not stated | 51/262; 19.5% | 36/120; 30.0% | 6/58 cycles cancelled D5 no blastocysts |
| Pantos 2004 | 44.6% | 15.8% | 15.8% | |
| Papanikolaou 2005 | Not stated | 35/170; 20.6% | 59/158; 37.3% | 4/158 women had only 1 blast transferred due to lack of availability and 1 had it on request |
| Papanikolaou 2006 | Not stated | 38/156; 24% | 58/149; 38.9% | Number of participants with no embryos available D3: 8 and D5: 11 |
| Rienzi 2002 | 44.8% | 34/96; 35.4% | 38/100; 38.0% | Good prognosis |
| Schillaci 2002 | 60.3% | 23/168; 13.7% | 26/110; 23.6% | Unselected population nil cancellations D5 |
| Singh 2017 | Not stated | Not stated | Not stated | |
| Ten 2011 | Not stated | 21/54; 38.9% | 26/56; 46.4% | Good prognosis |
| Van der Auwera 2002 | 44.7% | 31/106; 29.2% | 41/90; 45.6% | 27% cancellation D5 (unselected population) |
| Yang 2018 | Not stated | 80/290; 62.1% | 22/306; 72.5% |
Implantation data
For blastocyst‐stage transfer, the implantation rate varied from 4.2% to 55.8%. For cleavage‐stage transfer, the implantation rate varied from 3% to 43.9% (see Table 3).
Assessment of publication bias
We generated funnel plots for the outcomes of live birth and clinical pregnancy. They did not suggest publication bias (see Figure 4; Figure 5).
4.

Funnel plot of comparison: 1 Blastocyst‐ versus cleavage‐stage transfer: live birth rate, outcome: 1.1 live birth per couple
5.

Funnel plot of comparison: 2 Blastocyst‐ versus cleavage‐stage transfer: clinical pregnancy rate, outcome: 2.1 clinical pregnancy rate per couple
Discussion
Summary of main results
In the 15 RCTs that reported live birth rates, there was low‐quality evidence of a benefit in live birth rate per couple or woman in the fresh blastocyst transfer group and there was no evidence of a difference in the miscarriage rate. Clinical pregnancy rates were also higher in the fresh blastocyst transfer group, based on moderate‐quality evidence. When the comparator was TLS cleavage‐stage transfer, the benefit favoured blastocyst transfer as well.
We did not find any evidence of a difference when pooling the five RCTs that reported cumulative pregnancy rates (derived from fresh and thawed cycles from a single oocyte retrieval cycle): three were published in 2002/2003, one in 2010, and one in 2015. However, a post hoc analysis showed an interesting finding in which a blastocyst‐stage transfer may improve the cumulative pregnancy rate when the method of freezing was vitrification, but not when slow freezing was used (low‐quality evidence). This finding should be regarded cautiously as it resulted from a post hoc subgroup analysis.
Embryo freezing rates and failure rates to transfer embryos favoured early cleavage‐stage transfers. We did not find any evidence of a difference between fresh blastocyst‐stage and cleavage‐stage transfers for rates of miscarriage, multiple pregnancies, or high‐order multiple pregnancies.
Overall completeness and applicability of evidence
The data in this review of 32 RCTs are incomplete. Of 32 RCTs, only five reported cumulative pregnancy rates. Three of the five new studies (for the 2022 update) failed to report live birth data and all five failed to report cumulative pregnancy rates. This lack of data from the subsequent frozen‐thawed cycles and, therefore, the lack of data on cumulative pregnancy rates, means that couples and women are not adequately informed about the outcomes.
For a well‐informed decision between a cleavage‐stage or blastocyst‐stage embryo transfer policy, professionals, couples and women consider multiple variables, such as the chance of pregnancy, the time to pregnancy, the safety of the treatment, its burden, and the costs involved. The fact that the freezing rate is higher in cleavage‐stage transfer highlights the importance of getting the cumulative pregnancy rates data, in order to draw more complete conclusions (Cornelisse 2018).
The applicability of the evidence to everyday practice is somewhat limited by the many variables present in an in vitro fertilisation (IVF) cycle, which directly result from different clinic policies. Although the 32 studies all compared cleavage‐stage with blastocyst‐stage embryo transfer, they differed with respect to media used, freezing protocols, policies about the numbers of embryos transferred, the use of time‐lapse systems for embryo selection, and embryo quality scores. However, the most serious limitation was the lack of data from the frozen‐thawed cycles, as alluded to in the previous paragraph. For the most part, the variation in the different protocols can be a strength, as it means that in spite of the variation in cycles, the effect is still seen. With so few studies reporting the most useful outcome (cumulative pregnancy rate), it is difficult to report the applicability of the evidence.
Blastocyst culture is expected to result in higher implantation rates (number of fetal sacs observed divided by the number of embryos transferred). However, pooling of implantation data could not be included in the meta‐analysis as this would not generate valid estimates or confidence intervals due to the unit of analysis used (Vail 2003). Implantation rate is also no longer considered a useful outcome for a number of methodological reasons (Griesinger 2016).
There was a significantly higher failure rate to transfer any embryos in the blastocyst‐stage group, leading to cycle cancellation. The increased rates of failure to transfer with blastocyst stage is largely because of embryos with arrested development prior to the day of embryo transfer. Indeed, many of the studies that transferred fewer blastocysts than cleavage‐stage embryos did so from a lack of options rather than by policy. Only the reporting of fresh and frozen‐thawed cycle embryos can overcome this challenge. Although it could be better for some women to learn that their embryos failed to develop by day 5 than go through with a transfer at cleavage stage with embryos that had a low potential for success, there has been little research into the emotional status of couples or women given such choices (Borg 2000). Avoiding unnecessary embryo transfer needs to be balanced against the need for an additional oocyte retrieval. The possibility that extended culture may cause harm to viable embryos, through suboptimal culture conditions, must be considered, particularly when there are large variations between trials in blastocyst development rates.
There are no good‐quality studies showing the data of women with poor prognosis, such as few available embryos, or women at advanced reproductive age. Both scenarios are extremely common, yet the evidence to make an educated decision in these scenarios is still missing. We found one ongoing study Neuhausser 2020) that intends to analyse these subgroups. Thus, the next update of this review may be able to assess this information.
The varying embryo transfer policies between the two experimental groups was also a concern: a significant number of the studies had a policy to transfer fewer embryos in the blastocyst‐stage group than in the cleavage‐stage group (Table 4). There are two primary reasons for this difference. First, there is a reduced survival rate of day 5 to 6 blastocysts. Second, many clinics worried about the high incidence of multiple pregnancies with blastocysts will have a policy to transfer no more than two blastocyst‐stage embryos. Some clinics state that by employing blastocyst culture, they have been able to reduce the multiple pregnancy rate whilst maintaining the pregnancy rate. In this review, many of the studies were still transferring two to three embryos. Single‐embryo transfers for selected patient groups are now considered standard practice in many clinics throughout the world (Hamberger 2005).
3. Mean number of embryos transferred.
| Study ID | Day 2/3 | Day 5/6 |
| Aziminekoo 2015 | 2.8 ± 1.1 | 2.6 ± 0.6 |
| Brugnon 2010 | 1.0 ± 0 | 1.0 ± 0 |
| Bungum 2003 | 2.0 ± NS | 2.0 ± NS |
| Coskun 2000 | 2.3 ± 0.6 | 2.2 ± 0.5 |
| Devreker 2000 | 2.8 ± NS | 1.7 ± NS |
| Elgindy 2011 | 2.8 ± 0.4 | 2.0 ± 0.2 |
| Emiliani 2003 | 2.1 ± 0.4 | 1.9 ± 0.3 |
| Fernandez‐Shaw 2015 | 1.5 ± 0.5 | 1.4 ± 0.5 |
| Fisch 2007 | 1.0 ± 0 | 1.0 ± 0 |
| Frattarelli 2003 | 3.0 ± 0.5 | 2.0 ± 0.2 |
| Gaafar 2015 | NS | NS |
| Gardner 1998a | 3.7 ± 0.1 | 2.2 ± 0.1 |
| Hatirnaz 2017 | 1.4 ± 0.6 | 1.4 ± 0.4 |
| Hreinsson 2004 | 1.8 ± NS | 1.9 ± NS |
| Karaki 2002 | 3.5 ± 0.6 | 2.0 ± 0.1 |
| Kaser 2017 | 1.0 ± 0 | 1.0 ± 0 |
| Kaur 2014 | 2.0 ± 0.7 | 1.9 ± 0.5 |
| Kolibianakis 2004 | 1.9 ± 0.1 | 1.8 ± 0.1 |
| Levi‐Setti 2018 | 1.9 ± 0.4 | 1.8 ± 0.6 |
| Levitas 2004 | 3.4 ± NS | 1.9 ± NS |
| Levron 2002 | 3.1 ± 0.6 | 2.3 ± 0.8 |
| Livingstone 2002 | 2.0 ± NS | 1.0 ± NS |
| Motta 1998 | 4.6 ± NS | 2.3 ± NS |
| Pantos 2004 | 4.0 ± 1.5 | 3.4 ± 1.1 |
| Papanikolaou 2005 | 2.0 ± 0 | 2.0 ± 0.5 |
| Papanikolaou 2006 | 1.0 ± 0 | 1.0 ± 0 |
| Rienzi 2002 | 2.0 ± 0 | 2.0 ± 0 |
| Schillaci 2002 | 2.8 | 1.8 |
| Singh 2017 | NS | NS |
| Ten 2011 | 2.0 ± NS | 2.0 ± NS |
| Van der Auwera 2002 | 1.9 ± 0.3 | 1.9 ± 0.2 |
| Yang 2018 | 1.0 ± 0 | 1.0 ± 0 |
NS ‐ not stated
The importance of selecting the single most viable embryo for transfer has intensified the search to improve assessment of the quality of embryos. Performing blastocyst culture may offer one of those mechanisms (Gardner 2004; Milki 2004). In this meta‐analysis, significantly fewer embryos were transferred in the blastocyst‐stage group than in the cleavage‐stage group. When we performed a subgroup analysis for trials where equal numbers of embryos were transferred (including single‐embryo transfers), the clinical pregnancy rate remained unchanged. It could be argued that this is the most valid comparison, because trials with a greater number of cleavage‐stage embryos being transferred are probably advantaged inappropriately.
Regardless of the embryo transfer policy, for many women, there is simply a lack of choice, as only one, if any, embryo reaches the blastocyst stage. Only three studies in this review had a policy for single‐blastocyst transfer, although only one reported the live birth rate. None of the new studies added to this updated review had a single‐embryo transfer policy.
Studies have shown that women with a high oocyte yield and good‐quality 8‐cell embryos on day 3 are more likely to have blastocysts by day 5 to 6 than poor responders and those with no 8‐cell embryos by day 3. Therefore, we considered whether outcomes might be influenced by the time of randomisation. In subgroup analysis for live birth and pregnancy outcomes, we compared studies that randomised couples or women prior to the start of the treatment cycle (at a time when neither the number of oocytes retrieved nor fertilised nor the number of 8‐cell embryos could be anticipated) versus studies that randomised women at a later stage. We found no evidence of a statistically significant difference between the subgroups.
Miscarriage rates were reported in just over half of the included trials. Theoretically, the rate of miscarriage might be expected to be lowest with the transfer of highly selected embryos into a better synchronous uterine environment, such as in blastocyst culture. However, the results to date reveal little change from earlier reviews that showed no evidence of a difference in miscarriage rates for couples or women randomised (odds ratio (OR) 1.15, 95% confidence interval (CI) 0.88 to 1.50; 21 studies). Only seven of the included trials reported on the presence or absence of monozygotic twinning, so this analysis remains underpowered to comment meaningfully on monozygotic twin rates. A total of three sets of monozygotic twins were reported, two with cleavage‐stage embryo transfers and only one set of monozygotic twins from blastocyst transfer. Monozygotic twin rates in assisted reproductive technologies (ARTs) are thought to be underestimated, with up to one‐third being missed without genetic testing (Vitthala 2009).
Overall, this review found that women in the blastocyst group were less likely to have any embryos frozen, but there was no clear evidence of a difference in cumulative pregnancy rate (fresh and frozen‐thawed cycle transfers). The number of embryos frozen is an important consideration when assessing the effectiveness of a treatment, as it may offer women an additional opportunity to achieve a pregnancy. When considering an alteration in treatment procedure from cleavage‐stage to blastocyst‐stage transfer, the benefits of possible higher implantation rates are weighed up against the disadvantages of not only higher failure rates to transfer, but also lower cryopreservation rates.
Five trials reported data on pregnancies following transfer of frozen‐thawed embryos in both groups (Brugnon 2010; Emiliani 2003; Fernandez‐Shaw 2015; Rienzi 2002; Van der Auwera 2002). Van der Auwera 2002 reported a cumulative live birth rate of 47% in the blastocyst‐stage transfer group and 52% in the cleavage‐stage transfer group. That is, the added benefit of a higher cryopreservation rate in the cleavage‐stage group cancelled out the higher implantation rates of the fresh day 5 to 6 transfers. Similarly, Rienzi 2002 and Brugnon 2010 reported no evidence of a difference in cryo‐augmented pregnancy rates when at least one thawed cycle was carried out in the cleavage‐stage group. Emiliani 2003, on the other hand, reported significantly higher cumulative pregnancy rates in the cleavage‐stage group, presumably correlating to the much lower cryosurvival rate they reported in their blastocyst group (cleavage stage: 46%; blastocyst stage: 27%). However, Fernandez‐Shaw 2015 reported significantly higher cumulative pregnancy rates in the blastocyst‐stage group (day 3: 43.3%; day 5: 56.8%). This study was the only study to use vitrification, and also included women with a good prognosis. The pooled data for cumulative clinical pregnancy has 71% heterogeneity and this appears to be explained by the differences in freezing methods. This is supported by other reports of improved blastocyst outcomes with vitrification (Edgar 2012; Gardner 2003; Iwayama 2011; Richter 2016).
Although the evidence is limited, there is a growing trend for freeze‐only cycles to further reduce the risk of ovarian hyperstimulation (OHSS) and improve pregnancy outcomes if the progesterone levels are elevated (Roque 2015; Zaat 2021). This trend will also have an impact on the decision about cleavage‐stage or blastocyst‐stage transfers and should be considered in the next update.
Quality of the evidence
The overall quality of the evidence in this review was low for most outcomes and moderate for clinical pregnancy rate and failure to transfer any embryos. The main limitations in evidence quality were serious imprecision and serious risk of bias. Blinding is not possible with this intervention. Only half of the included studies adequately described their method of sequence generation, and less than one‐third reported their allocation concealment method. For some outcomes, we also downgraded the quality of evidence for imprecision. The large imprecision impacts most of the outcomes: both in cases in which we cannot say if the intervention increases or decreases the outcome rates, and also in some cases in which we cannot say if the intervention increases those rates a little or a lot.
Live birth rates were reported in 15 trials, providing low‐quality evidence (serious risk of bias and imprecision). Data for cumulative pregnancy rates after fresh and frozen‐thawed embryo transfer were only reported in five trials, in which heterogeneity was found. The most likely explanation for the heterogeneity was the difference in freezing protocols, with one study using vitrification and reporting benefit for blastocyst‐stage transfer for the cumulative pregnancy rate (Fernandez‐Shaw 2015), and the other four studies using slow freezing and reporting benefit for cleavage‐stage transfer.
Potential biases in the review process
As far as possible, we adhered to the protocol methodology in order to limit any potential biases. However, we added one new outcome – cumulative pregnancy rates (derived from fresh and thawed cycles) – in the 2016 update. This outcome reflects the policy of fresh embryo transfer and freezing remaining embryos for transfer later and more correctly reflects modern IVF practice than a single cycle transfer. Although we contacted study authors to try to obtain data for cumulative pregnancy rates, most did not collect this information.
There is an important distinction between the outcomes 'live birth rate following fresh transfer' and ‘cumulative pregnancy rate’. In the last decade, many studies only reported the outcome live birth rate following the first transfer, and trialists are generally used to reporting this outcome. Due to the extended embryo culture in blastocyst‐stage transfers, the selected embryos are potentially of higher quality, but the number available for transfer is reduced, which could potentially affect the cumulative pregnancy outcomes. Still, low‐quality evidence showed that cumulative pregnancy rate, when using vitrification, may benefit blastocyst transfer as well.
The issue of publication bias is important in systematic reviews as it may result in incorrect conclusions being reached. For example, it might be expected that the pressure for clinics to obtain high implantation rates with blastocyst culture could lead to a bias in publication towards those that do achieve this. However, the funnel plots for live birth and clinical pregnancy did not suggest publication bias.
Another point to consider is the widely varying policies for assessing minimal quality of embryos for transfer that may have existed amongst trials. Some trials accepted transfer of developmentally‐delayed embryos in blastocyst stage, whilst other trials were more selective and denied transfer of embryos that had not reached the late morula or early blastocyst stage. A strict selection policy for blastocyst transfer could have a potential bias in favor of the cleavage stage arm for two outcomes, embryo freezing rate per couple and failure rate to transfer embryos per couple. The freezing rate can be higher and the failure rate to transfer embryos lower in the cleavage stage group.
Blastocyst formation rates may also influence the pregnancy rate per embryo transfer for each trial. They ranged from 22.4% in the Aziminekoo 2015 trial to 60% in the Schillaci 2002 trial. Of the included trials that provided information on the media used, all used sequential media for the culture of blastocysts. However, while the majority reported using various versions of Vitrolife G1/G2, others used a combination of different brands, or made the media in‐house. This highlights the possibility that different brands and formulations are likely to influence the blastulation rates and subsequent outcomes. Improvements in culture media and embryo laboratory procedures should prompt new studies.
We note that there are six ongoing studies that started more than five years ago, and which have not yet published findings. These may raise a concern about publication bias.
Finally, although we took steps to conduct an exhaustive search for eligible trials, it is possible that our search strategy failed to find some studies, resulting in bias.
Agreements and disagreements with other studies or reviews
This Cochrane Review is in agreement with the Papanikolaou 2008 systematic review, which reported that cycles where equal numbers of embryos were transferred had higher live birth rates in blastocyst‐stage transfers than in cleavage‐stage tranfers. Our review also includes the cumulative pregnancy rate. Another systematic review was published by Wang 2014, which only included RCTs published after 2004. Wang 2014 shows similar results with respect to live birth rates, but reported lower miscarriage rates in the blastocyst group, which was not observed in our review. Two published reviews that cited the previous version of this review are in agreement that better studies are required (Maheshwari 2016; Martins 2016), and argue that analysing obstetric and neonatal outcomes results is critical in drawing clearer conclusions. Another systematic review by Martins 2017 emphasises that there is still too much uncertainty to conclude that transferring at one stage is better than the other.
Alviggi 2018 published a systematic review of retrospective studies, analysing perinatal outcomes. They showed that blastocyst transfers may slightly increase the preterm and very preterm birth rates but may decrease the small‐for‐gestational‐age rates in fresh cycles. However, these associations were not seen in frozen transfers.
Busnelli 2019 published a systematic review including mostly retrospective studies, analysing the risk factors for monozygotic twins. They showed that blastocyst transfer may be one of the factors to increase the risk of monozygotic twins.
Spangmose 2020 published a large retrospective cohort study analysing obstetric and perinatal outcomes, after fresh blastocyst or cleavage transfer. They showed that fresh blastocyst transfer increases the risk of placenta previa, preterm birth, and monozygotic twins. Furthermore, an altered male‐female ratio with more males following blastocyst transfer was seen.
The same study group published a large retrospective cohort study (Ginström 2019), analysing obstetric and perinatal outcomes, after frozen‐thawed transfer of vitrified blastocysts or slow‐frozen cleavage‐stage embryos. They showed a higher risk of preterm birth after blastocyst‐stage transfer. For maternal outcomes, no significant differences were found, although the precision was limited.
Authors' conclusions
Implications for practice.
There is low‐quality evidence that blastocyst‐stage transfer, in comparison to cleavage‐stage transfer, is associated with higher rates of live birth, and moderate‐quality evidence showing that clinical pregnancy rate is also higher in blastocyst‐stage transfer. We are uncertain whether blastocyst‐stage transfer improves the cumulative pregnancy rate (derived from fresh and thawed cycles). Given that the embryo freezing rate may be higher in cleavage‐stage transfer, more evidence about cumulative pregnancy rate and a cost‐effectiveness analysis are needed to draw firmer conclusions. Finally, there is low‐quality evidence showing that the addition of a time‐lapse system (TLS) to a cleavage‐stage transfer is still less effective than a blastocyst‐stage transfer without TLS in terms of pregnancy rate.
Implications for research.
Although this review provides evidence that there is a significant difference in live birth rates from fresh cycles in favour of blastocyst‐stage transfer compared to cleavage‐stage transfer cycles, the findings for cumulative clinical pregnancy rates (derived from fresh and thawed cycles) still raise questions. Further new studies of blastocyst cycles must report live birth, cumulative live birth rates (especially when vitrification is used), and miscarriage rates to enable consumers and service providers to make well‐informed decisions on the best treatment option. Also, more studies evaluating poor prognosis women will help to obtain more information about this specific subgroup. Finally, more studies with single‐embryo transfer are also needed in order to identify the best option with the fewest adverse event rates.
Based on the results of this review, we recommend the following to ensure valuable data are produced.
Adherence to Consolidated Standards of Reporting Trials (CONSORT) recommendations for randomised controlled trials (RCTs), especially methods of concealment (Begg 1996).
Research into the best participant selection and inclusion criteria; consider prognostic factors such as age and outcomes from previous cycles.
Same media composition and brand for both groups up to the cleavage stage.
Explicit prespecified embryo transfer policies for both groups; preferably single‐embryo transfer.
Long‐term follow‐up reports of cumulative live birth rates (including embryo thaws) presented as a survival analysis. A minimum of a one‐year time period after randomisation for follow‐up of cumulative live birth rates within studies (as part of study protocol), and a possible follow‐up of results of transfers outside this time window in a later report (if applicable, i.e. if not all transfers have been performed within one year).
Research into improved blastocyst cryopreservation techniques, e.g. vitrification.
Reporting of miscarriage, live birth, and cumulative pregnancy rates.
What's new
| Date | Event | Description |
|---|---|---|
| 21 June 2022 | Amended | Correction of study data in absract and summary of findings table |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2002
| Date | Event | Description |
|---|---|---|
| 11 May 2020 | New citation required but conclusions have not changed | The addition of five new RCTs has not led to a change in the conclusions of this review. |
| 11 May 2020 | New search has been performed | Updated with five new RCTs (Hatirnaz 2017; Kaser 2017; Levi‐Setti 2018; Singh 2017; Yang 2018). Cindy Farquhar has been removed from the list of authors and Agustin Ciapponi and Simone Cornelisse have been added to the list of authors for this update. |
| 5 May 2016 | New citation required and conclusions have changed | Updated May‐June 2016 |
| 3 May 2016 | New search has been performed | Updated with four new RCTs (Aziminekoo 2015; Fernandez‐Shaw 2015; Gaafar 2015; Kaur 2014) |
| 28 May 2013 | Amended | Correction of author order |
| 21 February 2012 | New search has been performed | History of this review: the review was first published in The Cochrane Library in 2000 with 10 RCTs, and a journal paper version was published in Human Reproduction in 2003 with an additional four RCTs. The authors were Debbie Blake, Michelle Proctor, Neil Johnson and David Olive. There was no evidence for a difference in pregnancy rate and only one trial reported live birth rates In the 2005 update, seven RCTs from the 2000 review were excluded (for quasi‐randomisation or per cycle data only or other study design problems) and 13 new RCTs were added. In addition, the outcomes in Metaview were reconfigured. Cindy Farquhar and Quirine Lamberts assisted with the update and were added as authors. Some protocol changes were made to the outcome measures and to the sensitivity analysis (day of randomisation) and subgroup analyses (prognosis). More included trials reported live birth outcomes but there was still no evidence of a difference in success rates The 2007 update had two new trials added, to bring the total to 18. There was a new subcategory for single embryo transfer. This update was performed by Debbie Blake, Neil Johnson and Cindy Farquar. It reported for the first time a significant difference in live birth and pregnancy outcomes in favour of blastocyst culture In the 2012 update led by Demian Glujovsky with the assistance of Cindy Farquhar and Debbie Blake, five new studies were added (Brugnon 2010; Elgindy 2011; Fisch 2007; Pantos 2004; Ten 2011) |
| 21 February 2012 | New citation required but conclusions have not changed | 5 new studies have been added. There are no changes to the conclusions reported in the 2007 update |
| 29 August 2011 | Amended | Plain Language Summary corrected |
| 17 November 2010 | Amended | New search strategies performed |
| 11 November 2010 | Amended | New author |
| 23 July 2007 | New citation required and conclusions have changed | Substantive amendment |
Notes
Conflicts of interest added.
Risk of bias
Risk of bias for analysis 1.1 Live birth per couple.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Brugnon 2010 | Some concerns | Sequence generated but there was no information about concealment. No table of patients' baseline characteristics reported | High risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No information about the deviations or the analysis | High risk of bias | information. Only proportions were reported | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk due to deviation from the intended interventions |
| Devreker 2000 | High risk of bias | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Elgindy 2011 | Some concerns | The number of embryos per transfer was significantly higher in group than the other group | Some concerns | This open‐label, prospective, randomized, controlled trial. No information about a deviation from the intended protocols | Low risk of bias | All randomised women were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process, deviations from intended interventions and selection of reported results. |
| Emiliani 2003 | Some concerns | Concealment was not stated. Only minor differences in age | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Not reported but inappropriate methods are unlikely. It is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Fernandez‐Shaw 2015 | Some concerns | No statement about allocation concealment. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, in both groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Frattarelli 2003 | Low risk of bias | Randomization was accomplished using a computer‐generated randomization table. The sequences of randomization were concealed until intervention was assigned. The groups were balanced. Concealment method was not described. | High risk of bias | Those patients undergoing a day 5 ET had a significantly higher implantation rate than those undergoing a day 3, these deviations likely have affected the outcome | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, no different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk of bias due to deviations from the intended protocol and some concerns due to bias in selection of the reported result. |
| Hatirnaz 2017 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | 95 out of 100 women of each arm had data to analyze | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Levi‐Setti 2018 | Some concerns | Allocation sequence was not concealed but no baseline clinical and statistical differences between groups were found in a table that described more than 15 different variables. | Low risk of bias | Participants and embryologists were aware of their assigned intervention. No deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not informed. However, given the nature of the outcome and the methods to measure it, it was probably not different between groups and, as it is an objective outcome, it is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Data that produced this result was analysed in accordance with a pre‐specified analysis plan that was finalised before unblinded outcome data were available for analysis | Some concerns | There is some concerns of risk of bias due to randomisation process. |
| Levitas 2004 | Low risk of bias | Allocation sequence was random and concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. Analysis was done by ITT | Low risk of bias | Although there are missing outcome data, the missingness in the outcome probably does not depend on its true value. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to devitations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Levron 2002 | Some concerns | No information if the allocation sequence was random and concealed but no baseline differences between groups. | Low risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | Only 3 out of 46 in the blastocyst group did not have embryo transfer | Low risk of bias | Method of measuring the outcome was not stated, but it is not probable that it was measured different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Livingstone 2002 | Low risk of bias | Allocation sequence was random and concealed | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the selection of reported results. |
| Papanikolaou 2005 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Papanikolaou 2006 | Some concerns | There was not enough information to evaluate the method of allocation sequence concealment. | Low risk of bias | Participants were probably aware of their assigned intervention. No deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Rienzi 2002 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols and no information about the total number of randomised women. | High risk of bias | No information about neither the total number of randomized women nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions, and there are some concerns of risk of bias due to randomisation process, missing outcomes and selection of reported results. |
| Van der Auwera 2002 | Low risk of bias | No information available to know if the allocation sequence was random. However, allocation sequence was concealed and no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Quote: “Three patients were excluded for analysis from group 1 because they wanted an elective blastocyst culture while four patients were excluded from group 2 because they wanted an elective day 2 transfer.” | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions and selection of reported results. |
Risk of bias for analysis 1.2 Live birth per couple: grouped by number of embryos transferred.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.2.1 More cleavage‐stage than blastocyst embryos transferred | ||||||||||||
| Devreker 2000 | High risk of bias | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Elgindy 2011 | Some concerns | The number of embryos per transfer was significantly higher in group than the other group | Some concerns | This open‐label, prospective, randomized, controlled trial. No information about a deviation from the intended protocols | Low risk of bias | All randomised women were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process, deviations from intended interventions and selection of reported results. |
| Frattarelli 2003 | Low risk of bias | Randomization was accomplished using a computer‐generated randomization table. The sequences of randomization were concealed until intervention was assigned. The groups were balanced. Concealment method was not described. | High risk of bias | Those patients undergoing a day 5 ET had a significantly higher implantation rate than those undergoing a day 3, these deviations likely have affected the outcome | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, no different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk of bias due to deviations from the intended protocol and some concerns due to bias in selection of the reported result. |
| Levitas 2004 | Low risk of bias | Allocation sequence was random and concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. Analysis was done by ITT | Low risk of bias | Although there are missing outcome data, the missingness in the outcome probably does not depend on its true value. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to devitations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Levron 2002 | Some concerns | No information if the allocation sequence was random and concealed but no baseline differences between groups. | Low risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | Only 3 out of 46 in the blastocyst group did not have embryo transfer | Low risk of bias | Method of measuring the outcome was not stated, but it is not probable that it was measured different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Livingstone 2002 | Low risk of bias | Allocation sequence was random and concealed | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the selection of reported results. |
| Subgroup 1.2.2 Single embryo transfer | ||||||||||||
| Brugnon 2010 | Some concerns | Sequence generated but there was no information about concealment. No table of patients' baseline characteristics reported | High risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No information about the deviations or the analysis | High risk of bias | information. Only proportions were reported | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk due to deviation from the intended interventions |
| Papanikolaou 2006 | Some concerns | There was not enough information to evaluate the method of allocation sequence concealment. | Low risk of bias | Participants were probably aware of their assigned intervention. No deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Subgroup 1.2.3 Equal number of embryos transferred | ||||||||||||
| Brugnon 2010 | Some concerns | Sequence generated but there was no information about concealment. No table of patients' baseline characteristics reported | High risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No information about the deviations or the analysis | High risk of bias | information. Only proportions were reported | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk due to deviation from the intended interventions |
| Emiliani 2003 | Some concerns | Concealment was not stated. Only minor differences in age | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Not reported but inappropriate methods are unlikely. It is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Fernandez‐Shaw 2015 | Some concerns | No statement about allocation concealment. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, in both groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Hatirnaz 2017 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | 95 out of 100 women of each arm had data to analyze | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Levi‐Setti 2018 | Some concerns | Allocation sequence was not concealed but no baseline clinical and statistical differences between groups were found in a table that described more than 15 different variables. | Low risk of bias | Participants and embryologists were aware of their assigned intervention. No deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not informed. However, given the nature of the outcome and the methods to measure it, it was probably not different between groups and, as it is an objective outcome, it is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Data that produced this result was analysed in accordance with a pre‐specified analysis plan that was finalised before unblinded outcome data were available for analysis | Some concerns | There is some concerns of risk of bias due to randomisation process. |
| Papanikolaou 2005 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Papanikolaou 2006 | Some concerns | There was not enough information to evaluate the method of allocation sequence concealment. | Low risk of bias | Participants were probably aware of their assigned intervention. No deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Rienzi 2002 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols and no information about the total number of randomised women. | High risk of bias | No information about neither the total number of randomized women nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions, and there are some concerns of risk of bias due to randomisation process, missing outcomes and selection of reported results. |
| Van der Auwera 2002 | Low risk of bias | No information available to know if the allocation sequence was random. However, allocation sequence was concealed and no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Quote: “Three patients were excluded for analysis from group 1 because they wanted an elective blastocyst culture while four patients were excluded from group 2 because they wanted an elective day 2 transfer.” | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions and selection of reported results. |
Risk of bias for analysis 1.3 Live birth rate per couple: grouped by prognosis.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.3.1 good prognostic factors | ||||||||||||
| Brugnon 2010 | Some concerns | Sequence generated but there was no information about concealment. No table of patients' baseline characteristics reported | High risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No information about the deviations or the analysis | High risk of bias | information. Only proportions were reported | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk due to deviation from the intended interventions |
| Elgindy 2011 | Some concerns | The number of embryos per transfer was significantly higher in group than the other group | Some concerns | This open‐label, prospective, randomized, controlled trial. No information about a deviation from the intended protocols | Low risk of bias | All randomised women were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process, deviations from intended interventions and selection of reported results. |
| Frattarelli 2003 | Low risk of bias | Randomization was accomplished using a computer‐generated randomization table. The sequences of randomization were concealed until intervention was assigned. The groups were balanced. Concealment method was not described. | High risk of bias | Those patients undergoing a day 5 ET had a significantly higher implantation rate than those undergoing a day 3, these deviations likely have affected the outcome | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, no different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk of bias due to deviations from the intended protocol and some concerns due to bias in selection of the reported result. |
| Levi‐Setti 2018 | Some concerns | Allocation sequence was not concealed but no baseline clinical and statistical differences between groups were found in a table that described more than 15 different variables. | Low risk of bias | Participants and embryologists were aware of their assigned intervention. No deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not informed. However, given the nature of the outcome and the methods to measure it, it was probably not different between groups and, as it is an objective outcome, it is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Data that produced this result was analysed in accordance with a pre‐specified analysis plan that was finalised before unblinded outcome data were available for analysis | Some concerns | There is some concerns of risk of bias due to randomisation process. |
| Levron 2002 | Some concerns | No information if the allocation sequence was random and concealed but no baseline differences between groups. | Low risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | Only 3 out of 46 in the blastocyst group did not have embryo transfer | Low risk of bias | Method of measuring the outcome was not stated, but it is not probable that it was measured different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Livingstone 2002 | Low risk of bias | Allocation sequence was random and concealed | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the selection of reported results. |
| Papanikolaou 2005 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Papanikolaou 2006 | Some concerns | There was not enough information to evaluate the method of allocation sequence concealment. | Low risk of bias | Participants were probably aware of their assigned intervention. No deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Rienzi 2002 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols and no information about the total number of randomised women. | High risk of bias | No information about neither the total number of randomized women nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions, and there are some concerns of risk of bias due to randomisation process, missing outcomes and selection of reported results. |
| Subgroup 1.3.2 poor prognostic factors | ||||||||||||
| Devreker 2000 | High risk of bias | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Levitas 2004 | Low risk of bias | Allocation sequence was random and concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. Analysis was done by ITT | Low risk of bias | Although there are missing outcome data, the missingness in the outcome probably does not depend on its true value. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to devitations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Subgroup 1.3.3 unselected group | ||||||||||||
| Emiliani 2003 | Some concerns | Concealment was not stated. Only minor differences in age | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Not reported but inappropriate methods are unlikely. It is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Fernandez‐Shaw 2015 | Some concerns | No statement about allocation concealment. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, in both groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Hatirnaz 2017 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | 95 out of 100 women of each arm had data to analyze | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Van der Auwera 2002 | Low risk of bias | No information available to know if the allocation sequence was random. However, allocation sequence was concealed and no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Quote: “Three patients were excluded for analysis from group 1 because they wanted an elective blastocyst culture while four patients were excluded from group 2 because they wanted an elective day 2 transfer.” | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions and selection of reported results. |
Risk of bias for analysis 1.4 Live birth rate: grouped by day of randomisation.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.4.1 randomisation at start of cycle | ||||||||||||
| Brugnon 2010 | Some concerns | Sequence generated but there was no information about concealment. No table of patients' baseline characteristics reported | High risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No information about the deviations or the analysis | High risk of bias | information. Only proportions were reported | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk due to deviation from the intended interventions |
| Emiliani 2003 | Some concerns | Concealment was not stated. Only minor differences in age | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Not reported but inappropriate methods are unlikely. It is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Levi‐Setti 2018 | Some concerns | Allocation sequence was not concealed but no baseline clinical and statistical differences between groups were found in a table that described more than 15 different variables. | Low risk of bias | Participants and embryologists were aware of their assigned intervention. No deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not informed. However, given the nature of the outcome and the methods to measure it, it was probably not different between groups and, as it is an objective outcome, it is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Data that produced this result was analysed in accordance with a pre‐specified analysis plan that was finalised before unblinded outcome data were available for analysis | Some concerns | There is some concerns of risk of bias due to randomisation process. |
| Levitas 2004 | Low risk of bias | Allocation sequence was random and concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. Analysis was done by ITT | Low risk of bias | Although there are missing outcome data, the missingness in the outcome probably does not depend on its true value. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to devitations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Papanikolaou 2006 | Some concerns | There was not enough information to evaluate the method of allocation sequence concealment. | Low risk of bias | Participants were probably aware of their assigned intervention. No deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Van der Auwera 2002 | Low risk of bias | No information available to know if the allocation sequence was random. However, allocation sequence was concealed and no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Quote: “Three patients were excluded for analysis from group 1 because they wanted an elective blastocyst culture while four patients were excluded from group 2 because they wanted an elective day 2 transfer.” | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions and selection of reported results. |
| Subgroup 1.4.2 randomised on day of OPU and day 1 after OPU | ||||||||||||
| Fernandez‐Shaw 2015 | Some concerns | No statement about allocation concealment. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, in both groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Frattarelli 2003 | Low risk of bias | Randomization was accomplished using a computer‐generated randomization table. The sequences of randomization were concealed until intervention was assigned. The groups were balanced. Concealment method was not described. | High risk of bias | Those patients undergoing a day 5 ET had a significantly higher implantation rate than those undergoing a day 3, these deviations likely have affected the outcome | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, no different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk of bias due to deviations from the intended protocol and some concerns due to bias in selection of the reported result. |
| Hatirnaz 2017 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | 95 out of 100 women of each arm had data to analyze | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Levron 2002 | Some concerns | No information if the allocation sequence was random and concealed but no baseline differences between groups. | Low risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | Only 3 out of 46 in the blastocyst group did not have embryo transfer | Low risk of bias | Method of measuring the outcome was not stated, but it is not probable that it was measured different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Rienzi 2002 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols and no information about the total number of randomised women. | High risk of bias | No information about neither the total number of randomized women nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions, and there are some concerns of risk of bias due to randomisation process, missing outcomes and selection of reported results. |
| Subgroup 1.4.3 randomised day 2 to 3 post‐OPU | ||||||||||||
| Elgindy 2011 | Some concerns | The number of embryos per transfer was significantly higher in group than the other group | Some concerns | This open‐label, prospective, randomized, controlled trial. No information about a deviation from the intended protocols | Low risk of bias | All randomised women were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process, deviations from intended interventions and selection of reported results. |
| Papanikolaou 2005 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Subgroup 1.4.4 day of randomisation unstated | ||||||||||||
| Devreker 2000 | High risk of bias | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Livingstone 2002 | Low risk of bias | Allocation sequence was random and concealed | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the selection of reported results. |
Risk of bias for analysis 2.1 Cumulative pregnancy rate from fresh and frozen transfers.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Brugnon 2010 | Some concerns | Sequence generated but there was no information about concealment. No table of patients' baseline characteristics reported | High risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No information about the deviations or the analysis | High risk of bias | information. Only proportions were reproted | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk due to deviation from the intended interventions |
| Emiliani 2003 | Some concerns | Concealment was not stated. Only minor differences in age | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Not reported but inappropriate methotds are unlikely. It is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Fernandez‐Shaw 2015 | Some concerns | No statement about allocation concealment. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, in both groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Rienzi 2002 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols and no information about the total number of randomised women. | High risk of bias | No information about neither the total number of randomized women nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions, and there are some concerns of risk of bias due to randomisation process, missing outcomes and selection of reported results. |
| Van der Auwera 2002 | Low risk of bias | No information available to know if the allocation sequence was random. However, allocation sequence was concealed and no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Quote: “Three patients were excluded for analysis from group 1 because they wanted an elective blastocyst culture while four patients were excluded from group 2 because they wanted an elective day 2 transfer.” | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions and selection of reported results. |
Risk of bias for analysis 2.2 Cumulative pregnancy rate per couple: grouped by number of embryos transferred.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 2.2.1 single embryo transfer | ||||||||||||
| Brugnon 2010 | Some concerns | Sequence generated but there was no information about concealment. No table of patients' baseline characteristics reported | High risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No information about the deviations or the analysis | High risk of bias | information. Only proportions were reproted | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk due to deviation from the intended interventions |
| Subgroup 2.2.2 equal number of embryos transferred | ||||||||||||
| Brugnon 2010 | Some concerns | Sequence generated but there was no information about concealment. No table of patients' baseline characteristics reported | High risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No information about the deviations or the analysis | High risk of bias | information. Only proportions were reproted | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk due to deviation from the intended interventions |
| Emiliani 2003 | Some concerns | Concealment was not stated. Only minor differences in age | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Not reported but inappropriate methotds are unlikely. It is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Fernandez‐Shaw 2015 | Some concerns | No statement about allocation concealment. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, in both groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Rienzi 2002 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols and no information about the total number of randomised women. | High risk of bias | No information about neither the total number of randomized women nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions, and there are some concerns of risk of bias due to randomisation process, missing outcomes and selection of reported results. |
| Van der Auwera 2002 | Low risk of bias | No information available to know if the allocation sequence was random. However, allocation sequence was concealed and no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Quote: “Three patients were excluded for analysis from group 1 because they wanted an elective blastocyst culture while four patients were excluded from group 2 because they wanted an elective day 2 transfer.” | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions and selection of reported results. |
Risk of bias for analysis 2.3 Cumulative pregnancy rate per couple: grouped by prognosis.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 2.3.1 good prognostic factors | ||||||||||||
| Brugnon 2010 | Some concerns | Sequence generated but there was no information about concealment. No table of patients' baseline characteristics reported | High risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No information about the deviations or the analysis | High risk of bias | No information. Only proportions were reported | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk due to deviation from the intended interventions |
| Rienzi 2002 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols and no information about the total number of randomised women. | High risk of bias | No information about neither the total number of randomized women nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions, and there are some concerns of risk of bias due to randomisation process, missing outcomes and selection of reported results. |
| Subgroup 2.3.2 unselected group | ||||||||||||
| Emiliani 2003 | Some concerns | Concealment was not stated. Only minor differences in age | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Not reported but inappropriate methotds are unlikely. It is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Fernandez‐Shaw 2015 | Some concerns | No statement about allocation concealment. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, in both groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Van der Auwera 2002 | Low risk of bias | No information available to know if the allocation sequence was random. However, allocation sequence was concealed and no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Quote: “Three patients were excluded for analysis from group 1 because they wanted an elective blastocyst culture while four patients were excluded from group 2 because they wanted an elective day 2 transfer.” | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions and selection of reported results. |
Risk of bias for analysis 2.4 Cumulative pregnancy rate: grouped by day of randomisation.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 2.4.1 randomisation at start of cycle | ||||||||||||
| Brugnon 2010 | Some concerns | Sequence generated but there was no information about concealment. No table of patients' baseline characteristics reported | High risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No information about the deviations or the analysis | High risk of bias | No information. Only proportions were reported | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk due to deviation from the intended interventions |
| Emiliani 2003 | Some concerns | Concealment was not stated. Only minor differences in age | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Not reported but inappropriate methods are unlikely. It is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Van der Auwera 2002 | Low risk of bias | No information available to know if the allocation sequence was random. However, allocation sequence was concealed and no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Quote: “Three patients were excluded for analysis from group 1 because they wanted an elective blastocyst culture while four patients were excluded from group 2 because they wanted an elective day 2 transfer.” | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions and selection of reported results. |
| Subgroup 2.4.2 randomised on day of OPU and day 1 after OPU | ||||||||||||
| Fernandez‐Shaw 2015 | Some concerns | No statement about allocation concealment. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, in both groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Rienzi 2002 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols and no information about the total number of randomised women. | High risk of bias | No information about neither the total number of randomized women nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions, and there are some concerns of risk of bias due to randomisation process, missing outcomes and selection of reported results. |
Risk of bias for analysis 2.5 Cumulative pregnancy rate from fresh and frozen transfers: grouped by vitrification or slow freezing.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 2.5.1 slow freezing | ||||||||||||
| Brugnon 2010 | Some concerns | Sequence generated but there was no information about concealment. No table of patients' baseline characteristics reported | High risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No information about the deviations or the analysis | High risk of bias | No information. Only proportions were reported | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk due to deviation from the intended interventions |
| Emiliani 2003 | Some concerns | Concealment was not stated. Only minor differences in age | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Not reported but inappropriate methotds are unlikely. It is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Rienzi 2002 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols and no information about the total number of randomised women. | High risk of bias | No information about neither the total number of randomized women nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions, and there are some concerns of risk of bias due to randomisation process, missing outcomes and selection of reported results. |
| Van der Auwera 2002 | Low risk of bias | No information available to know if the allocation sequence was random. However, allocation sequence was concealed and no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Quote: “Three patients were excluded for analysis from group 1 because they wanted an elective blastocyst culture while four patients were excluded from group 2 because they wanted an elective day 2 transfer.” | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions and selection of reported results. |
| Subgroup 2.5.2 vitrification | ||||||||||||
| Fernandez‐Shaw 2015 | Some concerns | No statement about allocation concealment. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, in both groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
Risk of bias for analysis 3.1 Clinical pregnancy rate per couple.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Aziminekoo 2015 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All 118 randomised women were available. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Brugnon 2010 | Some concerns | Sequence generated but there was no information about concealment. No table of patients' baseline characteristics reported | High risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No information about the deviations or the analysis | High risk of bias | No information. Only proportions were reported | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | High risk of bias | High risk due to deviation from the intended interventions and bias due to missing outcome data |
| Bungum 2003 | Low risk of bias | There was not a complete explanation about concealment and there are some concerns about baseline differences | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. All 118 randomised women were analysed. | Low risk of bias | All 118 randomised women were analysed. | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results |
| Coskun 2000 | Low risk of bias | Randomisation process apparently has no problems. Patients were randomized to receive embryo transfer either at day 3 or day 5. Quote " An equal number of ‘day 3' or ‘day 5' labels placed in sealed envelopes in a block of 14 was kept in a box and an envelope was drawn by the embryologist as soon as a patient qualified for the study. The label was attached with adhesive to the patients’ data collection sheet." | Some concerns | Participants and carers and people delivering the intervention were probably aware of their assigned intervention. | Low risk of bias | All 201 randomised women were available. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns due to deviation from intended interventions and selection of reported outcomes |
| Devreker 2000 | High risk of bias | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidence suggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | High risk of bias | No protocol registration was found. | High risk of bias | There is high risk of bias due to the randomisation process, deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Elgindy 2011 | Some concerns | The number of embryos per transfer was significantly higher in group than the other group | Some concerns | This open‐label, prospective, randomized, controlled trial. No information about a deviation from the intended protocols | Low risk of bias | All ranomised women were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process, deviations from intended interventions and selection of reported results. |
| Emiliani 2003 | Some concerns | Concealment was not stated. Only minor differences in age | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Not reported but inappropriate methotds are unlikely. It is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Fernandez‐Shaw 2015 | Some concerns | No statement about allocation concealment. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. All women randomised were analysed | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, in both groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Fisch 2007 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Although the number of missing outcomes is high, there is no evidence of bias about it | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There is some risk of bias due to the randomisation process, to deviations from the intended interventions and selection of reported results. |
| Frattarelli 2003 | Low risk of bias | Randomization was accomplished using a computer‐generated randomization table. The sequences of randomization were concealed until intervention was assigned. The groups were balanced. Concealment method was not described. | High risk of bias | Those patients undergoing a day 5 ET had a significantly higher implantation rate than those undergoing a day 3, these deviations likely have affected the outcome | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, no different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk of bias due to deviations from the intended protocol and some concerns due to bias in selection of the reported result. |
| Gaafar 2015 | Low risk of bias | Quote "Randomization was done by computer permuted blocks size of 4, allocation by closed envelops, parallel technique" No mention about opaque envelopes | Some concerns | Not evidence of potential of concerns generating a substantial impact | High risk of bias | A large proportion of missing outcomes: 326 were eligible. 126 cases had day 2/3 transfer and 126 had blastocyst transfer. | Low risk of bias | Clinical pregnancy with cardiac pulsation four weeks after ET. PACTR201308000581376 Quote "Masking Care giver/Provider,Outcome assessors,Participants" | Some concerns | There is only a difference in outcome reporting regarding multiple pregnancies | High risk of bias | High bias due to missing outcome data and some concerns due to deviations from intended interventions and bias in selection of the reported result |
| Gardner 1998a | Some concerns | Concealment was not reported, there was a difference in Previous no. of cycles | High risk of bias | No effort for blindness is described. There was a difference No. of embryos transferred and proportion of embryo freezing that likely have affected the outcome | Low risk of bias | All women randomised were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Low risk of bias | ClinicalTrials.gov Identifier: NCT03764865 | High risk of bias | High risk of bias due to deviations from intended intervention |
| Hatirnaz 2017 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | 95 out of 100 women of each arm had data to analyze | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Hreinsson 2004 | Low risk of bias | Allocation sequence was random and concealed. Quote:"Sealed envelopes were used for randomisation of the patients for each group". Groups were balanced. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No differences in embryos per transfer bat there was for cycles with embryo freezing. All women randomised were analyses by assignment | Low risk of bias | All women randomised were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results. |
| Karaki 2002 | Low risk of bias | No information if the allocation sequence was random, but it was concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Kaser 2017 | Low risk of bias | Allocation sequence was random and concealed | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | The observed number of events is much greater than the number of participants with missing outcome data. | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Quote:"NCT02218255" | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions. |
| Kaur 2014 | Some concerns | No information if the allocation sequence was random and/or concealed but no baseline differences between groups. | Low risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Kolibianakis 2004 | High risk of bias | Allocation sequence was random but it was not concealed. No baseline differences between groups were found. | High risk of bias | Participants, carers and people delivering the intervention were aware of their assigned intervention. No information about a deviation from the intended protocols. No information if there was a substantial impact of the failure to analyse participants in the group to which they were randomized. | Low risk of bias | All randomised women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There are high of risk of bias due to the randomisation process and deviations from the intended interventions, and some concerns of risk of bias due to selection of reported results. |
| Levi‐Setti 2018 | Some concerns | Allocation sequence was not concealed but no baseline clinical and statistical differences between groups were found in a table that described more than 15 different variables. | Low risk of bias | Participants and embryologists were aware of their assigned intervention. No deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not informed. However, given the nature of the outcome and the methods to measure it, it was probably not different between groups and, as it is an objective outcome, it is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Data that produced this result was analysed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis | Some concerns | There is some concerns of risk of bias due to randomisation process. |
| Levitas 2004 | Low risk of bias | Allocation sequence was random and concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. Analysis was done by ITT | Low risk of bias | Although there are missing outcome data, the missingness in the outcome probably does not depend on its true value. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to devitations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Levron 2002 | Some concerns | No information if the allocation sequence was random and concealed but no baseline differences between groups. | Low risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | Only 3 out of 46 in the blastocyst group did not have embryo transfer | Low risk of bias | Method of measuring the outcome was not stated, but it is not probable that it was measured different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Livingstone 2002 | Low risk of bias | Allocation sequence was random and concealed | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidence suggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the selection of reported results. |
| Motta 1998 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Pantos 2004 | Some concerns | No information about the allocation sequence random and concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | There is no information about the method of measuring the outcome, but it is probable that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Papanikolaou 2005 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Papanikolaou 2006 | Some concerns | There was not enough information to evaluate the method of allocation sequence concealment. | Low risk of bias | Participants were probably aware of their assigned intervention. No deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Rienzi 2002 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols and no information about the total number of randomised women. | High risk of bias | No information about neither the total number of randomized women nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions, and there are some concerns of risk of bias due to randomisation process, missing outcomes and selection of reported results. |
| Schillaci 2002 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Singh 2017 | Some concerns | No information about the allocation sequence concealment and no information about the baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the number of randomised women per arm and it is not possible to know how the analysis was performed. | High risk of bias | It is not possible to know if there were missing outcome data. | Low risk of bias | Method of measuring the outcome was not informed, but it was probably not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is a high risk of bias due to deviations from the intended interventions and missing outcomes data. And some concerns of risk of bias due to randomisation process and selection of reported results. |
| Ten 2011 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the analysis that was performed. | High risk of bias | No information about neither the absolute number of events nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to the randomisation process, deviations from the intended interventions and missing outcomes, and some concerns of risk of bias due to randomisation process and selection of reported results. |
| Van der Auwera 2002 | Low risk of bias | No information available to know if the allocation sequence was random. However, allocation sequence was concealed and no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Quote: “Three patients were excluded for analysis from group 1 because they wanted an elective blastocyst culture while four patients were excluded from group 2 because they wanted an elective day 2 transfer.” | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions and selection of reported results. |
| Yang 2018 | Low risk of bias | Allocation sequence was random and concealed | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the number of randomised women per arm and it is not possible to know how the analysis was performed. | Low risk of bias | The observed number of events is much greater than the number of participants with missing outcome data, but it is probably not related with the true value | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Quote" ChiCTR‐ICR‐15006600" | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions. |
Risk of bias for analysis 3.2 Clinical pregnancy rate per couple: grouped by number of embryos transferred.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 3.2.1 equal number of embryo transfers | ||||||||||||
| Aziminekoo 2015 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All 118 randomised women were available. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Brugnon 2010 | Some concerns | Sequence generated but there was no information about concealment. No table of patients' baseline characteristics reported | High risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No information about the deviations or the analysis | High risk of bias | No information. Only proportions were reported | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | High risk of bias | High risk due to deviation from the intended interventions and bias due to missing outcome data |
| Bungum 2003 | Low risk of bias | There was not a complete explanation about concealment and there are some concerns about baseline differences | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. All 118 randomised women were analysed. | Low risk of bias | All 118 randomised women were analysed. | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results |
| Coskun 2000 | Low risk of bias | Randomisation process apparently has no problems. Patients were randomized to receive embryo transfer either at day 3 or day 5. Quote " An equal number of ‘day 3' or ‘day 5' labels placed in sealed envelopes in a block of 14 was kept in a box and an envelope was drawn by the embryologist as soon as a patient qualified for the study. The label was attached with adhesive to the patients’ data collection sheet." | Some concerns | Participants and carers and people delivering the intervention were probably aware of their assigned intervention. | Low risk of bias | All 201 randomised women were available. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns due to deviation from intended interventions and selection of reported outcomes |
| Fernandez‐Shaw 2015 | Some concerns | No statement about allocation concealment. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. All women randomised were analysed | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, in both groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Fisch 2007 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Although the number of missing outcomes is high, there is no evidence of bias about it | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to the randomisation process, due to deviations from the intended interventions and selection of reported results. |
| Gaafar 2015 | Low risk of bias | Quote "Randomization was done by computer permuted blocks size of 4, allocation by closed envelops, parallel technique" No mention about opaque envelopes | Some concerns | Not evidence of potential of concerns generating a substantial impact | High risk of bias | A large proportion of missing outcomes: 326 were eligible. 126 cases had day 2/3 transfer and 126 had blastocyst transfer. | Low risk of bias | Clinical pregnancy with cardiac pulsation four weeks after ET. PACTR201308000581376 Quote "Masking Care giver/Provider,Outcome assessors,Participants" | Some concerns | There is only a difference in outcome reporting regarding multiple pregnancies | Some concerns | High bias due to missing outcome data and some concerns due to deviations from intended interventions and bias in selection of the reported result |
| Hatirnaz 2017 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | 95 out of 100 women of each arm had data to analyze | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Hreinsson 2004 | Low risk of bias | Allocation sequence was random and concealed. Quote:"Sealed envelopes were used for randomisation of the patients for each group". Groups were balanced. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No differences in embryos per transfer bat there was for cycles with embryo freezing. All women randomised were analyses by assignment | Low risk of bias | All women randomised were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results. |
| Kaser 2017 | Low risk of bias | Allocation sequence was random and concealed | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | The observed number of events is much greater than the number of participants with missing outcome data. | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Quote:"NCT02218255" | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions. |
| Kaur 2014 | Some concerns | No information if the allocation sequence was random and/or concealed but no baseline differences between groups. | Low risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Kolibianakis 2004 | High risk of bias | Allocation sequence was random but it was not concealed. No baseline differences between groups were found. | High risk of bias | Participants, carers and people delivering the intervention were aware of their assigned intervention. No information about a deviation from the intended protocols. No information if there was a substantial impact of the failure to analyse participants in the group to which they were randomized. | Low risk of bias | All randomised women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There are high of risk of bias due to the randomisation process and deviations from the intended interventions, and some concerns of risk of bias due to selection of reported results. |
| Levi‐Setti 2018 | Some concerns | Allocation sequence was not concealed but no baseline clinical and statistical differences between groups were found in a table that described more than 15 different variables. | Low risk of bias | Participants and embryologists were aware of their assigned intervention. No deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not informed. However, given the nature of the outcome and the methods to measure it, it was probably not different between groups and, as it is an objective outcome, it is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Data that produced this result was analysed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis | Some concerns | There is some concerns of risk of bias due to randomisation process. |
| Papanikolaou 2005 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Papanikolaou 2006 | Some concerns | There was not enough information to evaluate the method of allocation sequence concealment. | Low risk of bias | Participants were probably aware of their assigned intervention. No deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Rienzi 2002 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols and no information about the total number of randomised women. | High risk of bias | No information about neither the total number of randomized women nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions, and there are some concerns of risk of bias due to randomisation process, missing outcomes and selection of reported results. |
| Singh 2017 | Some concerns | No information about the allocation sequence concealment and no information about the baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the number of randomised women per arm and it is not possible to know how the analysis was performed. | High risk of bias | It is not possible to know if there were missing outcome data. | Low risk of bias | Method of measuring the outcome was not informed, but it was probably not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is a high risk of bias due to deviations from the intended interventions and missing outcomes data. And some concerns of risk of bias due to randomisation process and selection of reported results. |
| Ten 2011 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the analysis that was performed. | High risk of bias | No information about neither the absolute number of events nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and missing outcomes, and some concerns of risk of bias due to randomisation process and selection of reported results. |
| Van der Auwera 2002 | Low risk of bias | No information available to know if the allocation sequence was random. However, allocation sequence was concealed and no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Quote: “Three patients were excluded for analysis from group 1 because they wanted an elective blastocyst culture while four patients were excluded from group 2 because they wanted an elective day 2 transfer.” | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions and selection of reported results. |
| Yang 2018 | Low risk of bias | Allocation sequence was random and concealed | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the number of randomised women per arm and it is not possible to know how the analysis was performed. | Low risk of bias | The observed number of events is much greater than the number of participants with missing outcome data, but it is probably not related with the true value | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Quote" ChiCTR‐ICR‐15006600" | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions. |
| Subgroup 3.2.2 more cleavage stage than blastocyst embryos transferred | ||||||||||||
| Devreker 2000 | High risk of bias | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidence suggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Elgindy 2011 | Some concerns | The number of embryos per transfer was significantly higher in group than the other group | Some concerns | This open‐label, prospective, randomized, controlled trial. No information about a deviation from the intended protocols | Low risk of bias | All ranomised women were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process, deviations from intended interventions and selection of reported results. |
| Emiliani 2003 | Some concerns | Concealment was not stated. Only minor differences in age | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Not reported but inappropriate methotds are unlikely. It is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Frattarelli 2003 | Low risk of bias | Randomization was accomplished using a computer‐generated randomization table. The sequences of randomization were concealed until intervention was assigned. The groups were balanced. Concealment method was not described. | High risk of bias | Those patients undergoing a day 5 ET had a significantly higher implantation rate than those undergoing a day 3, these deviations likely have affected the outcome | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, no different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk of bias due to deviations from the intended protocol and some concerns due to bias in selection of the reported result. |
| Gardner 1998a | Some concerns | Concealment was not reported, there was a difference in Previous no. of cycles | High risk of bias | No effort for blindness is described. There was a difference No. of embryos transferred and proportion of embryo freezing that likely have affected the outcome | Low risk of bias | All women randomised were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Low risk of bias | ClinicalTrials.gov Identifier: NCT03764865 | High risk of bias | High risk of bias due to deviations from intended intervention |
| Karaki 2002 | Low risk of bias | No information if the allocation sequence was random, but it was concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Levitas 2004 | Low risk of bias | Allocation sequence was random and concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. Analysis was done by ITT | Low risk of bias | Although there are missing outcome data, the missingness in the outcome probably does not depend on its true value. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to devitations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Levron 2002 | Some concerns | No information if the allocation sequence was random and concealed but no baseline differences between groups. | Low risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | Only 3 out of 46 in the blastocyst group did not have embryo transfer | Low risk of bias | Method of measuring the outcome was not stated, but it is not probable that it was measured different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Livingstone 2002 | Low risk of bias | Allocation sequence was random and concealed | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidence suggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the selection of reported results. |
| Motta 1998 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Pantos 2004 | Some concerns | No information about the allocation sequence random and concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | There is no information about the method of measuring the outcome, but it is probable that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Schillaci 2002 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Subgroup 3.2.3 single embryo transfer | ||||||||||||
| Brugnon 2010 | Some concerns | Sequence generated but there was no information about concealment. No table of patients' baseline characteristics reported | High risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No information about the deviations or the analysis | High risk of bias | No information. Only proportions were reported | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | High risk of bias | High risk due to deviation from the intended interventions and bias due to missing outcome data |
| Fisch 2007 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Although the number of missing outcomes is high, there is no evidence of bias about it | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to the randomisation process, due to deviations from the intended interventions and selection of reported results. |
| Kaser 2017 | Low risk of bias | Allocation sequence was random and concealed | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | The observed number of events is much greater than the number of participants with missing outcome data. | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Quote:"NCT02218255" | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions. |
| Papanikolaou 2006 | Some concerns | There was not enough information to evaluate the method of allocation sequence concealment. | Low risk of bias | Participants were probably aware of their assigned intervention. No deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Yang 2018 | Low risk of bias | Allocation sequence was random and concealed | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the number of randomised women per arm and it is not possible to know how the analysis was performed. | Low risk of bias | The observed number of events is much greater than the number of participants with missing outcome data, but it is probably not related with the true value | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Quote" ChiCTR‐ICR‐15006600" | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions. |
Risk of bias for analysis 3.3 Clinical pregnancy rate per couple: grouped by prognosis.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 3.3.1 good prognostic factors | ||||||||||||
| Brugnon 2010 | Some concerns | Sequence generated but there was no information about concealment. No table of patients' baseline characteristics reported | High risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No information about the deviations or the analysis | High risk of bias | No information. Only proportions were reported | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | High risk of bias | High risk due to deviation from the intended interventions and bias due to missing outcome data |
| Bungum 2003 | Low risk of bias | There was not a complete explanation about concealment and there are some concerns about baseline differences | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. All 118 randomised women were analysed. | Low risk of bias | All 118 randomised women were analysed. | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results |
| Coskun 2000 | Low risk of bias | Randomisation process apparently has no problems. Patients were randomized to receive embryo transfer either at day 3 or day 5. Quote " An equal number of ‘day 3' or ‘day 5' labels placed in sealed envelopes in a block of 14 was kept in a box and an envelope was drawn by the embryologist as soon as a patient qualified for the study. The label was attached with adhesive to the patients’ data collection sheet." | Some concerns | Participants and carers and people delivering the intervention were probably aware of their assigned intervention. | Low risk of bias | All 201 randomised women were available. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns due to deviation from intended interventions and selection of reported outcomes |
| Elgindy 2011 | Some concerns | The number of embryos per transfer was significantly higher in group than the other group | Some concerns | This open‐label, prospective, randomized, controlled trial. No information about a deviation from the intended protocols | Low risk of bias | All ranomised women were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process, deviations from intended interventions and selection of reported results. |
| Fisch 2007 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Although the number of missing outcomes is high, there is no evidence of bias about it | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to the randomisation process, due to deviations from the intended interventions and selection of reported results. |
| Frattarelli 2003 | Low risk of bias | Randomization was accomplished using a computer‐generated randomization table. The sequences of randomization were concealed until intervention was assigned. The groups were balanced. Concealment method was not described. | High risk of bias | Those patients undergoing a day 5 ET had a significantly higher implantation rate than those undergoing a day 3, these deviations likely have affected the outcome | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, no different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk of bias due to deviations from the intended protocol and some concerns due to bias in selection of the reported result. |
| Gardner 1998a | Some concerns | Concealment was not reported, there was a difference in Previous no. of cycles | High risk of bias | No effort for blindness is described. There was a difference No. of embryos transferred and proportion of embryo freezing that likely have affected the outcome | Low risk of bias | All women randomised were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Low risk of bias | ClinicalTrials.gov Identifier: NCT03764865 | High risk of bias | High risk of bias due to deviations from intended intervention |
| Hreinsson 2004 | Low risk of bias | Allocation sequence was random and concealed. Quote:"Sealed envelopes were used for randomisation of the patients for each group". Groups were balanced. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No differences in embryos per transfer bat there was for cycles with embryo freezing. All women randomised were analyses by assignment | Low risk of bias | All women randomised were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results. |
| Kaser 2017 | Low risk of bias | Allocation sequence was random and concealed | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | The observed number of events is much greater than the number of participants with missing outcome data. | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Quote:"NCT02218255" | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions. |
| Kaur 2014 | Some concerns | No information if the allocation sequence was random and/or concealed but no baseline differences between groups. | Low risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Levi‐Setti 2018 | Some concerns | Allocation sequence was not concealed but no baseline clinical and statistical differences between groups were found in a table that described more than 15 different variables. | Low risk of bias | Participants and embryologists were aware of their assigned intervention. No deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not informed. However, given the nature of the outcome and the methods to measure it, it was probably not different between groups and, as it is an objective outcome, it is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Data that produced this result was analysed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis | Some concerns | There is some concerns of risk of bias due to randomisation process. |
| Levron 2002 | Some concerns | No information if the allocation sequence was random and concealed but no baseline differences between groups. | Low risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | Only 3 out of 46 in the blastocyst group did not have embryo transfer | Low risk of bias | Method of measuring the outcome was not stated, but it is not probable that it was measured different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Livingstone 2002 | Low risk of bias | Allocation sequence was random and concealed | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidence suggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the selection of reported results. |
| Papanikolaou 2005 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Papanikolaou 2006 | Some concerns | There was not enough information to evaluate the method of allocation sequence concealment. | Low risk of bias | Participants were probably aware of their assigned intervention. No deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Rienzi 2002 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols and no information about the total number of randomised women. | High risk of bias | No information about neither the total number of randomized women nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions, and there are some concerns of risk of bias due to randomisation process, missing outcomes and selection of reported results. |
| Singh 2017 | Some concerns | No information about the allocation sequence concealment and no information about the baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the number of randomised women per arm and it is not possible to know how the analysis was performed. | High risk of bias | It is not possible to know if there were missing outcome data. | Low risk of bias | Method of measuring the outcome was not informed, but it was probably not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is a high risk of bias due to deviations from the intended interventions and missing outcomes data. And some concerns of risk of bias due to randomisation process and selection of reported results. |
| Ten 2011 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the analysis that was performed. | High risk of bias | No information about neither the absolute number of events nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and missing outcomes, and some concerns of risk of bias due to randomisation process and selection of reported results. |
| Yang 2018 | Low risk of bias | Allocation sequence was random and concealed | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the number of randomised women per arm and it is not possible to know how the analysis was performed. | Low risk of bias | The observed number of events is much greater than the number of participants with missing outcome data, but it is probably not related with the true value | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Quote" ChiCTR‐ICR‐15006600" | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions. |
| Subgroup 3.3.2 poor prognostic factors | ||||||||||||
| Aziminekoo 2015 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All 118 randomised women were available. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Devreker 2000 | High risk of bias | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidence suggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Levitas 2004 | Low risk of bias | Allocation sequence was random and concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. Analysis was done by ITT | Low risk of bias | Although there are missing outcome data, the missingness in the outcome probably does not depend on its true value. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to devitations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Subgroup 3.3.3 unselected group | ||||||||||||
| Emiliani 2003 | Some concerns | Concealment was not stated. Only minor differences in age | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Not reported but inappropriate methotds are unlikely. It is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Fernandez‐Shaw 2015 | Some concerns | No statement about allocation concealment. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. All women randomised were analysed | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, in both groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Gaafar 2015 | Low risk of bias | Quote "Randomization was done by computer permuted blocks size of 4, allocation by closed envelops, parallel technique" No mention about opaque envelopes | Some concerns | Not evidence of potential of concerns generating a substantial impact | High risk of bias | A large proportion of missing outcomes: 326 were eligible. 126 cases had day 2/3 transfer and 126 had blastocyst transfer. | Low risk of bias | Clinical pregnancy with cardiac pulsation four weeks after ET. PACTR201308000581376 Quote "Masking Care giver/Provider,Outcome assessors,Participants" | Some concerns | There is only a difference in outcome reporting regarding multiple pregnancies | Some concerns | High bias due to missing outcome data and some concerns due to deviations from intended interventions and bias in selection of the reported result |
| Hatirnaz 2017 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | 95 out of 100 women of each arm had data to analyze | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Karaki 2002 | Low risk of bias | No information if the allocation sequence was random, but it was concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Kolibianakis 2004 | High risk of bias | Allocation sequence was random but it was not concealed. No baseline differences between groups were found. | High risk of bias | Participants, carers and people delivering the intervention were aware of their assigned intervention. No information about a deviation from the intended protocols. No information if there was a substantial impact of the failure to analyse participants in the group to which they were randomized. | Low risk of bias | All randomised women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There are high of risk of bias due to the randomisation process and deviations from the intended interventions, and some concerns of risk of bias due to selection of reported results. |
| Motta 1998 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Pantos 2004 | Some concerns | No information about the allocation sequence random and concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | There is no information about the method of measuring the outcome, but it is probable that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Schillaci 2002 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Van der Auwera 2002 | Low risk of bias | No information available to know if the allocation sequence was random. However, allocation sequence was concealed and no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Quote: “Three patients were excluded for analysis from group 1 because they wanted an elective blastocyst culture while four patients were excluded from group 2 because they wanted an elective day 2 transfer.” | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions and selection of reported results. |
Risk of bias for analysis 3.4 Clinical pregnancy rate per couple: grouped by day of randomisation.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 3.4.1 randomised start of cycle | ||||||||||||
| Brugnon 2010 | Some concerns | Sequence generated but there was no information about concealment. No table of patients' baseline characteristics reported | High risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No information about the deviations or the analysis | High risk of bias | No information. Only proportions were reported | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | High risk of bias | High risk due to deviation from the intended interventions and bias due to missing outcome data |
| Emiliani 2003 | Some concerns | Concealment was not stated. Only minor differences in age | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Not reported but inappropriate methotds are unlikely. It is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Gardner 1998a | Some concerns | Concealment was not reported, there was a difference in Previous no. of cycles | High risk of bias | No effort for blindness is described. There was a difference No. of embryos transferred and proportion of embryo freezing that likely have affected the outcome | Low risk of bias | All women randomised were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Low risk of bias | ClinicalTrials.gov Identifier: NCT03764865 | High risk of bias | High risk of bias due to deviations from intended intervention |
| Kolibianakis 2004 | High risk of bias | Allocation sequence was random but it was not concealed. No baseline differences between groups were found. | High risk of bias | Participants, carers and people delivering the intervention were aware of their assigned intervention. No information about a deviation from the intended protocols. No information if there was a substantial impact of the failure to analyse participants in the group to which they were randomized. | Low risk of bias | All randomised women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There are high of risk of bias due to the randomisation process and deviations from the intended interventions, and some concerns of risk of bias due to selection of reported results. |
| Levi‐Setti 2018 | Some concerns | Allocation sequence was not concealed but no baseline clinical and statistical differences between groups were found in a table that described more than 15 different variables. | Low risk of bias | Participants and embryologists were aware of their assigned intervention. No deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not informed. However, given the nature of the outcome and the methods to measure it, it was probably not different between groups and, as it is an objective outcome, it is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Data that produced this result was analysed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis | Some concerns | There is some concerns of risk of bias due to randomisation process. |
| Levitas 2004 | Low risk of bias | Allocation sequence was random and concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. Analysis was done by ITT | Low risk of bias | Although there are missing outcome data, the missingness in the outcome probably does not depend on its true value. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to devitations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Papanikolaou 2006 | Some concerns | There was not enough information to evaluate the method of allocation sequence concealment. | Low risk of bias | Participants were probably aware of their assigned intervention. No deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Van der Auwera 2002 | Low risk of bias | No information available to know if the allocation sequence was random. However, allocation sequence was concealed and no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Quote: “Three patients were excluded for analysis from group 1 because they wanted an elective blastocyst culture while four patients were excluded from group 2 because they wanted an elective day 2 transfer.” | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions and selection of reported results. |
| Subgroup 3.4.2 randomised on day of OPU or day 1 | ||||||||||||
| Aziminekoo 2015 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All 118 randomised women were available. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Coskun 2000 | Low risk of bias | Randomisation process apparently has no problems. Patients were randomized to receive embryo transfer either at day 3 or day 5. Quote " An equal number of ‘day 3' or ‘day 5' labels placed in sealed envelopes in a block of 14 was kept in a box and an envelope was drawn by the embryologist as soon as a patient qualified for the study. The label was attached with adhesive to the patients’ data collection sheet." | Some concerns | Participants and carers and people delivering the intervention were probably aware of their assigned intervention. | Low risk of bias | All 201 randomised women were available. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns due to deviation from intended interventions and selection of reported outcomes |
| Fernandez‐Shaw 2015 | Some concerns | No statement about allocation concealment. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. All women randomised were analysed | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, in both groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Fisch 2007 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Although the number of missing outcomes is high, there is no evidence of bias about it | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to the randomisation process, due to deviations from the intended interventions and selection of reported results. |
| Frattarelli 2003 | Low risk of bias | Randomization was accomplished using a computer‐generated randomization table. The sequences of randomization were concealed until intervention was assigned. The groups were balanced. Concealment method was not described. | High risk of bias | Those patients undergoing a day 5 ET had a significantly higher implantation rate than those undergoing a day 3, these deviations likely have affected the outcome | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, no different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk of bias due to deviations from the intended protocol and some concerns due to bias in selection of the reported result. |
| Hatirnaz 2017 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | 95 out of 100 women of each arm had data to analyze | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Hreinsson 2004 | Low risk of bias | Allocation sequence was random and concealed. Quote:"Sealed envelopes were used for randomisation of the patients for each group". Groups were balanced. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No differences in embryos per transfer bat there was for cycles with embryo freezing. All women randomised were analyses by assignment | Low risk of bias | All women randomised were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results. |
| Karaki 2002 | Low risk of bias | No information if the allocation sequence was random, but it was concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Kaser 2017 | Low risk of bias | Allocation sequence was random and concealed | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | The observed number of events is much greater than the number of participants with missing outcome data. | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Quote:"NCT02218255" | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions. |
| Levron 2002 | Some concerns | No information if the allocation sequence was random and concealed but no baseline differences between groups. | Low risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | Only 3 out of 46 in the blastocyst group did not have embryo transfer | Low risk of bias | Method of measuring the outcome was not stated, but it is not probable that it was measured different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Rienzi 2002 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols and no information about the total number of randomised women. | High risk of bias | No information about neither the total number of randomized women nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions, and there are some concerns of risk of bias due to randomisation process, missing outcomes and selection of reported results. |
| Schillaci 2002 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Yang 2018 | Low risk of bias | Allocation sequence was random and concealed | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the number of randomised women per arm and it is not possible to know how the analysis was performed. | Low risk of bias | The observed number of events is much greater than the number of participants with missing outcome data, but it is probably not related with the true value | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Quote" ChiCTR‐ICR‐15006600" | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions. |
| Subgroup 3.4.3 randomised on day 2 to 3 | ||||||||||||
| Bungum 2003 | Low risk of bias | There was not a complete explanation about concealment and there are some concerns about baseline differences | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. All 118 randomised women were analysed. | Low risk of bias | All 118 randomised women were analysed. | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results |
| Elgindy 2011 | Some concerns | The number of embryos per transfer was significantly higher in group than the other group | Some concerns | This open‐label, prospective, randomized, controlled trial. No information about a deviation from the intended protocols | Low risk of bias | All ranomised women were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process, deviations from intended interventions and selection of reported results. |
| Kaur 2014 | Some concerns | No information if the allocation sequence was random and/or concealed but no baseline differences between groups. | Low risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Papanikolaou 2005 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Singh 2017 | Some concerns | No information about the allocation sequence concealment and no information about the baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the number of randomised women per arm and it is not possible to know how the analysis was performed. | High risk of bias | It is not possible to know if there were missing outcome data. | Low risk of bias | Method of measuring the outcome was not informed, but it was probably not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is a high risk of bias due to deviations from the intended interventions and missing outcomes data. And some concerns of risk of bias due to randomisation process and selection of reported results. |
| Ten 2011 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the analysis that was performed. | High risk of bias | No information about neither the absolute number of events nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and missing outcomes, and some concerns of risk of bias due to randomisation process and selection of reported results. |
| Subgroup 3.4.4 day of randomisation unstated | ||||||||||||
| Devreker 2000 | High risk of bias | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidence suggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Gaafar 2015 | Low risk of bias | Quote "Randomization was done by computer permuted blocks size of 4, allocation by closed envelops, parallel technique" No mention about opaque envelopes | Some concerns | Not evidence of potential of concerns generating a substantial impact | High risk of bias | A large proportion of missing outcomes: 326 were eligible. 126 cases had day 2/3 transfer and 126 had blastocyst transfer. | Low risk of bias | Clinical pregnancy with cardiac pulsation four weeks after ET. PACTR201308000581376 Quote "Masking Care giver/Provider,Outcome assessors,Participants" | Some concerns | There is only a difference in outcome reporting regarding multiple pregnancies | Some concerns | High bias due to missing outcome data and some concerns due to deviations from intended interventions and bias in selection of the reported result |
| Livingstone 2002 | Low risk of bias | Allocation sequence was random and concealed | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidence suggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the selection of reported results. |
| Motta 1998 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Pantos 2004 | Some concerns | No information about the allocation sequence random and concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | There is no information about the method of measuring the outcome, but it is probable that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
Risk of bias for analysis 3.5 Clinical pregnancy rate per couple: TLS (with algorithm) cleavage stage versus conventional blastocyst stage.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kaser 2017 | Low risk of bias | Allocation sequence was random and concealed | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | The observed number of events is much greater than the number of participants with missing outcome data. | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Quote:"NCT02218255" | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions. |
| Yang 2018 | Low risk of bias | Allocation sequence was random and concealed | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the number of randomised women per arm and it is not possible to know how the analysis was performed. | Low risk of bias | The observed number of events is much greater than the number of participants with missing outcome data, but it is probably not related with the true value | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Quote" ChiCTR‐ICR‐15006600" | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions. |
Risk of bias for analysis 3.6 Clinical pregnancy rate per couple: TLS (with algorithm) cleavage stage versus TLS (with algorithm) blastocyst stage.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kaser 2017 | Low risk of bias | Allocation sequence was random and concealed | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | The observed number of events is much greater than the number of participants with missing outcome data. | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Quote:"NCT02218255" | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions. |
Risk of bias for analysis 4.1 Multiple pregnancy rate per couple.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Aziminekoo 2015 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All 118 randomised women were available. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Bungum 2003 | Low risk of bias | There was not a complete explanation about concealment and there are some concerns about baseline differences | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All 118 randomised women were analysed. | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results |
| Coskun 2000 | Low risk of bias | Randomisation process apparently has no problems. Patients were randomized to receive embryo transfer either at day 3 or day 5. Quote " An equal number of ‘day 3' or ‘day 5' labels placed in sealed envelopes in a block of 14 was kept in a box and an envelope was drawn by the embryologist as soon as a patient qualified for the study. The label was attached with adhesive to the patients’ data collection sheet." | Some concerns | Participants and carers and people delivering the intervention were probably aware of their assigned intervention. | Low risk of bias | All 201 randomised women were available. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns due to deviation from intended interventions and selection of reported outcomes |
| Elgindy 2011 | Some concerns | The number of embryos per transfer was significantly higher in group than the other group | Some concerns | This open‐label, prospective, randomized, controlled trial. No information about a deviation from the intended protocols | Low risk of bias | All ranomised women were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process, deviations from intended interventions and selection of reported results. |
| Emiliani 2003 | Some concerns | Concealment was not stated. Only minor differences in age | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Not reported but inappropriate methotds are unlikely. It is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Fernandez‐Shaw 2015 | Some concerns | No statement about allocation concealment. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, in both groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Frattarelli 2003 | Low risk of bias | Randomization was accomplished using a computer‐generated randomization table. The sequences of randomization were concealed until intervention was assigned. The groups were balanced. Concealment method was not described. | High risk of bias | Those patients undergoing a day 5 ET had a significantly higher implantation rate than those undergoing a day 3, these deviations likely have affected the outcome | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, no different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk of bias due to deviations from the intended protocol and some concerns due to bias in selection of the reported result. |
| Hatirnaz 2017 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | 95 out of 100 women of each arm had data to analyze | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Hreinsson 2004 | Low risk of bias | Allocation sequence was random and concealed. Quote:"Sealed envelopes were used for randomisation of the patients for each group". Groups were balanced. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No differences in embryos per transfer bat there was for cycles with embryo freezing. All women randomised were analyses by assignement | Low risk of bias | All women randomised were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results. |
| Karaki 2002 | Low risk of bias | No information if the allocation sequence was random, but it was concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to devitations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Kaur 2014 | Some concerns | No information if the allocation sequence was random and/or concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Kolibianakis 2004 | High risk of bias | Allocation sequence was random but it was not concealed. No baseline differences between groups were found. | High risk of bias | Participants, carers and people delivering the intervention were aware of their assigned intervention. No information about a deviation from the intended protocols. No information if there was a substantial impact of the failure to analyse participants in the group to which they were randomized. | Low risk of bias | All randomised women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There are high of risk of bias due to the randomisation process and deviations from the intended interventions, and some concerns of risk of bias due to selection of reported results. |
| Levi‐Setti 2018 | Some concerns | Allocation sequence was not concealed but no baseline clinical and statistical differences between groups were found in a table that described more than 15 different variables. | Low risk of bias | Participants and embryologists were aware of their assigned intervention. No deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not informed. However, given the nature of the outcome and the methods to measure it, it was probably not different between groups and, as it is an objective outcome, it is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Data that produced this result was analysed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis | Some concerns | There is some concerns of risk of bias due to randomisation process. |
| Levitas 2004 | Low risk of bias | Allocation sequence was random and concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. Analysis was done by ITT | Low risk of bias | Although there are missing outcome data, the missingness in the outcome probably does not depend on its true value. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to devitations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Levron 2002 | Some concerns | No information if the allocation sequence was random and concealed but no baseline differences between groups. | Low risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | Only 3 out of 46 in the blastocyst group did not have embryo transfer | Low risk of bias | Method of measuring the outcome was not stated, but it is not probable that it was measured different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Livingstone 2002 | Low risk of bias | Allocation sequence was random and concealed | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the selection of reported results. |
| Motta 1998 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Papanikolaou 2005 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Papanikolaou 2006 | Some concerns | There was not enough information to evaluate the method of allocation sequence concealment. | Low risk of bias | Participants were probably aware of their assigned intervention. No deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Rienzi 2002 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols and no information about the total number of randomised women. | High risk of bias | No information about neither the total number of randomized women nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions, and there are some concerns of risk of bias due to randomisation process, missing outcomes and selection of reported results. |
| Van der Auwera 2002 | Low risk of bias | No information available to know if the allocation sequence was random. However, allocation sequence was concealed and no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Quote: “Three patients were excluded for analysis from group 1 because they wanted an elective blastocyst culture while four patients were excluded from group 2 because they wanted an elective day 2 transfer.” | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions and selection of reported results. |
| Yang 2018 | Low risk of bias | Allocation sequence was random and concealed | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the number of randomised women per arm and it is not possible to know how the analysis was performed. | Low risk of bias | The observed number of events is much greater than the number of participants with missing outcome data, but it is probably not related with the true value | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Quote" ChiCTR‐ICR‐15006600" | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions. |
Risk of bias for analysis 4.2 Multiple pregnancy rate per couple: grouped by number of embryos transferred.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 4.2.1 equal number of embryos transferred | ||||||||||||
| Aziminekoo 2015 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All 118 randomised women were available. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Bungum 2003 | Low risk of bias | There was not a complete explanation about concealment and there are some concerns about baseline differences | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All 118 randomised women were analysed. | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results |
| Coskun 2000 | Low risk of bias | Randomisation process apparently has no problems. Patients were randomized to receive embryo transfer either at day 3 or day 5. Quote " An equal number of ‘day 3' or ‘day 5' labels placed in sealed envelopes in a block of 14 was kept in a box and an envelope was drawn by the embryologist as soon as a patient qualified for the study. The label was attached with adhesive to the patients’ data collection sheet." | Some concerns | Participants and carers and people delivering the intervention were probably aware of their assigned intervention. | Low risk of bias | All 201 randomised women were available. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns due to deviation from intended interventions and selection of reported outcomes |
| Fernandez‐Shaw 2015 | Some concerns | No statement about allocation concealment. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, in both groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Hatirnaz 2017 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | 95 out of 100 women of each arm had data to analyze | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Hreinsson 2004 | Low risk of bias | Allocation sequence was random and concealed. Quote:"Sealed envelopes were used for randomisation of the patients for each group". Groups were balanced. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No differences in embryos per transfer bat there was for cycles with embryo freezing. All women randomised were analyses by assignement | Low risk of bias | All women randomised were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results. |
| Kaur 2014 | Some concerns | No information if the allocation sequence was random and/or concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Kolibianakis 2004 | High risk of bias | Allocation sequence was random but it was not concealed. No baseline differences between groups were found. | High risk of bias | Participants, carers and people delivering the intervention were aware of their assigned intervention. No information about a deviation from the intended protocols. No information if there was a substantial impact of the failure to analyse participants in the group to which they were randomized. | Low risk of bias | All randomised women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There are high of risk of bias due to deviations from the intended interventions and randomisation process, and some concerns of risk of bias due to selection of reported results. |
| Levi‐Setti 2018 | Some concerns | Allocation sequence was not concealed but no baseline clinical and statistical differences between groups were found in a table that described more than 15 different variables. | Low risk of bias | Participants and embryologists were aware of their assigned intervention. No deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not informed. However, given the nature of the outcome and the methods to measure it, it was probably not different between groups and, as it is an objective outcome, it is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Data that produced this result was analysed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis | Some concerns | There is some concerns of risk of bias due to randomisation process. |
| Papanikolaou 2005 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Papanikolaou 2006 | Some concerns | There was not enough information to evaluate the method of allocation sequence concealment. | Low risk of bias | Participants were probably aware of their assigned intervention. No deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Rienzi 2002 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols and no information about the total number of randomised women. | High risk of bias | No information about neither the total number of randomized women nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions, and there are some concerns of risk of bias due to randomisation process, missing outcomes and selection of reported results. |
| Van der Auwera 2002 | Low risk of bias | No information available to know if the allocation sequence was random. However, allocation sequence was concealed and no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Quote: “Three patients were excluded for analysis from group 1 because they wanted an elective blastocyst culture while four patients were excluded from group 2 because they wanted an elective day 2 transfer.” | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions and selection of reported results. |
| Yang 2018 | Low risk of bias | Allocation sequence was random and concealed | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the number of randomised women per arm and it is not possible to know how the analysis was performed. | Low risk of bias | The observed number of events is much greater than the number of participants with missing outcome data, but it is probably not related with the true value | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Quote" ChiCTR‐ICR‐15006600" | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions. |
| Subgroup 4.2.2 more cleavage stage than blastocyst embryos transferred | ||||||||||||
| Elgindy 2011 | Some concerns | The number of embryos per transfer was significantly higher in group than the other group | Some concerns | This open‐label, prospective, randomized, controlled trial. No information about a deviation from the intended protocols | Low risk of bias | All ranomised women were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process, deviations from intended interventions and selection of reported results. |
| Emiliani 2003 | Some concerns | Concealment was not stated. Only minor differences in age | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Not reported but inappropriate methotds are unlikely. It is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Frattarelli 2003 | Low risk of bias | Randomization was accomplished using a computer‐generated randomization table. The sequences of randomization were concealed until intervention was assigned. The groups were balanced. Concealment method was not described. | High risk of bias | Those patients undergoing a day 5 ET had a significantly higher implantation rate than those undergoing a day 3, these deviations likely have affected the outcome | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, no different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk of bias due to deviations from the intended protocol and some concerns due to bias in selection of the reported result. |
| Karaki 2002 | Low risk of bias | No information if the allocation sequence was random, but it was concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Levitas 2004 | Low risk of bias | Allocation sequence was random and concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. Analysis was done by ITT | Low risk of bias | Although there are missing outcome data, the missingness in the outcome probably does not depend on its true value. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to devitations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Levron 2002 | Some concerns | No information if the allocation sequence was random and concealed but no baseline differences between groups. | Low risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | Only 3 out of 46 in the blastocyst group did not have embryo transfer | Low risk of bias | Method of measuring the outcome was not stated, but it is not probable that it was measured different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Livingstone 2002 | Low risk of bias | Allocation sequence was random and concealed | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the selection of reported results. |
| Motta 1998 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Subgroup 4.2.3 single embryo transfer | ||||||||||||
| Papanikolaou 2006 | Some concerns | There was not enough information to evaluate the method of allocation sequence concealment. | Low risk of bias | Participants were probably aware of their assigned intervention. No deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Yang 2018 | Low risk of bias | Allocation sequence was random and concealed | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the number of randomised women per arm and it is not possible to know how the analysis was performed. | Low risk of bias | The observed number of events is much greater than the number of participants with missing outcome data, but it is probably not related with the true value | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Quote" ChiCTR‐ICR‐15006600" | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions. |
Risk of bias for analysis 4.3 Multiple pregnancy rate per couple: grouped by prognosis.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 4.3.1 good prognostic factors | ||||||||||||
| Aziminekoo 2015 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All 118 randomised women were available. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Bungum 2003 | Low risk of bias | There was not a complete explanation about concealment and there are some concerns about baseline differences | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All 118 randomised women were analysed. | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results |
| Coskun 2000 | Low risk of bias | Randomisation process apparently has no problems. Patients were randomized to receive embryo transfer either at day 3 or day 5. Quote " An equal number of ‘day 3' or ‘day 5' labels placed in sealed envelopes in a block of 14 was kept in a box and an envelope was drawn by the embryologist as soon as a patient qualified for the study. The label was attached with adhesive to the patients’ data collection sheet." | Some concerns | Participants and carers and people delivering the intervention were probably aware of their assigned intervention. | Low risk of bias | All 201 randomised women were available. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns due to deviation from intended interventions and selection of reported outcomes |
| Elgindy 2011 | Some concerns | The number of embryos per transfer was significantly higher in group than the other group | Some concerns | This open‐label, prospective, randomized, controlled trial. No information about a deviation from the intended protocols | Low risk of bias | All ranomised women were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process, deviations from intended interventions and selection of reported results. |
| Frattarelli 2003 | Low risk of bias | Randomization was accomplished using a computer‐generated randomization table. The sequences of randomization were concealed until intervention was assigned. The groups were balanced. Concealment method was not described. | High risk of bias | Those patients undergoing a day 5 ET had a significantly higher implantation rate than those undergoing a day 3, these deviations likely have affected the outcome | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, no different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk of bias due to deviations from the intended protocol and some concerns due to bias in selection of the reported result. |
| Hreinsson 2004 | Low risk of bias | Allocation sequence was random and concealed. Quote:"Sealed envelopes were used for randomisation of the patients for each group". Groups were balanced. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No differences in embryos per transfer bat there was for cycles with embryo freezing. All women randomised were analyses by assignement | Low risk of bias | All women randomised were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results. |
| Kaur 2014 | Some concerns | No information if the allocation sequence was random and/or concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Levi‐Setti 2018 | Some concerns | Allocation sequence was not concealed but no baseline clinical and statistical differences between groups were found in a table that described more than 15 different variables. | Low risk of bias | Participants and embryologists were aware of their assigned intervention. No deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not informed. However, given the nature of the outcome and the methods to measure it, it was probably not different between groups and, as it is an objective outcome, it is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Data that produced this result was analysed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis | Some concerns | There is some concerns of risk of bias due to randomisation process. |
| Levron 2002 | Some concerns | No information if the allocation sequence was random and concealed but no baseline differences between groups. | Low risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | Only 3 out of 46 in the blastocyst group did not have embryo transfer | Low risk of bias | Method of measuring the outcome was not stated, but it is not probable that it was measured different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Livingstone 2002 | Low risk of bias | Allocation sequence was random and concealed | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidence suggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the selection of reported results. |
| Motta 1998 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Papanikolaou 2005 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Papanikolaou 2006 | Some concerns | There was not enough information to evaluate the method of allocation sequence concealment. | Low risk of bias | Participants were probably aware of their assigned intervention. No deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Rienzi 2002 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols and no information about the total number of randomised women. | High risk of bias | No information about neither the total number of randomized women nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions, and there are some concerns of risk of bias due to randomisation process, missing outcomes and selection of reported results. |
| Yang 2018 | Low risk of bias | Allocation sequence was random and concealed | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the number of randomised women per arm and it is not possible to know how the analysis was performed. | Low risk of bias | The observed number of events is much greater than the number of participants with missing outcome data, but it is probably not related with the true value | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Quote" ChiCTR‐ICR‐15006600" | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions. |
| Subgroup 4.3.2 poor prognostic factors | ||||||||||||
| Levitas 2004 | Low risk of bias | Allocation sequence was random and concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. Analysis was done by ITT | Low risk of bias | Although there are missing outcome data, the missingness in the outcome probably does not depend on its true value. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to devitations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Subgroup 4.3.3 unselected | ||||||||||||
| Emiliani 2003 | Some concerns | Concealment was not stated. Only minor differences in age | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Not reported but inappropriate methotds are unlikely. It is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Fernandez‐Shaw 2015 | Some concerns | No statement about allocation concealment. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, in both groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Hatirnaz 2017 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | 95 out of 100 women of each arm had data to analyze | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Karaki 2002 | Low risk of bias | No information if the allocation sequence was random, but it was concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to devitations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Kolibianakis 2004 | High risk of bias | Allocation sequence was random but it was not concealed. No baseline differences between groups were found. | High risk of bias | Participants, carers and people delivering the intervention were aware of their assigned intervention. No information about a deviation from the intended protocols. No information if there was a substantial impact of the failure to analyse participants in the group to which they were randomized. | Low risk of bias | All randomised women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There are high of risk of bias due to deviations from the intended interventions and randomisation process, and some concerns of risk of bias due to selection of reported results. |
| Van der Auwera 2002 | Low risk of bias | No information available to know if the allocation sequence was random. However, allocation sequence was concealed and no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Quote: “Three patients were excluded for analysis from group 1 because they wanted an elective blastocyst culture while four patients were excluded from group 2 because they wanted an elective day 2 transfer.” | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions and selection of reported results. |
Risk of bias for analysis 4.4 Multiple pregnancy rate per couple: grouped by day of randomisation.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 4.4.1 randomised start of cycle | ||||||||||||
| Emiliani 2003 | Some concerns | Concealment was not stated. Only minor differences in age | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Not reported but inappropriate methotds are unlikely. It is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Hreinsson 2004 | Low risk of bias | Allocation sequence was random and concealed. Quote:"Sealed envelopes were used for randomisation of the patients for each group". Groups were balanced. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No differences in embryos per transfer bat there was for cycles with embryo freezing. All women randomised were analyses by assignement | Low risk of bias | All women randomised were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results. |
| Kolibianakis 2004 | High risk of bias | Allocation sequence was random but it was not concealed. No baseline differences between groups were found. | High risk of bias | Participants, carers and people delivering the intervention were aware of their assigned intervention. No information about a deviation from the intended protocols. No information if there was a substantial impact of the failure to analyse participants in the group to which they were randomized. | Low risk of bias | All randomised women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There are high of risk of bias due to deviations from the intended interventions and randomisation process, and some concerns of risk of bias due to selection of reported results. |
| Levi‐Setti 2018 | Some concerns | Allocation sequence was not concealed but no baseline clinical and statistical differences between groups were found in a table that described more than 15 different variables. | Low risk of bias | Participants and embryologists were aware of their assigned intervention. No deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not informed. However, given the nature of the outcome and the methods to measure it, it was probably not different between groups and, as it is an objective outcome, it is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Data that produced this result was analysed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis | Some concerns | There is some concerns of risk of bias due to randomisation process. |
| Levitas 2004 | Low risk of bias | Allocation sequence was random and concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. Analysis was done by ITT | Low risk of bias | Although there are missing outcome data, the missingness in the outcome probably does not depend on its true value. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to devitations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Papanikolaou 2006 | Some concerns | There was not enough information to evaluate the method of allocation sequence concealment. | Low risk of bias | Participants were probably aware of their assigned intervention. No deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Van der Auwera 2002 | Low risk of bias | No information available to know if the allocation sequence was random. However, allocation sequence was concealed and no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Quote: “Three patients were excluded for analysis from group 1 because they wanted an elective blastocyst culture while four patients were excluded from group 2 because they wanted an elective day 2 transfer.” | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions and selection of reported results. |
| Subgroup 4.4.2 randomised on day of OPU or day 1 | ||||||||||||
| Aziminekoo 2015 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All 118 randomised women were available. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Coskun 2000 | Low risk of bias | Randomisation process apparently has no problems. Patients were randomized to receive embryo transfer either at day 3 or day 5. Quote " An equal number of ‘day 3' or ‘day 5' labels placed in sealed envelopes in a block of 14 was kept in a box and an envelope was drawn by the embryologist as soon as a patient qualified for the study. The label was attached with adhesive to the patients’ data collection sheet." | Some concerns | Participants and carers and people delivering the intervention were probably aware of their assigned intervention. | Low risk of bias | All 201 randomised women were available. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns due to deviation from intended interventions and selection of reported outcomes |
| Fernandez‐Shaw 2015 | Some concerns | No statement about allocation concealment. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, in both groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Frattarelli 2003 | Low risk of bias | Randomization was accomplished using a computer‐generated randomization table. The sequences of randomization were concealed until intervention was assigned. The groups were balanced. Concealment method was not described. | High risk of bias | Those patients undergoing a day 5 ET had a significantly higher implantation rate than those undergoing a day 3, these deviations likely have affected the outcome | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, no different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk of bias due to deviations from the intended protocol and some concerns due to bias in selection of the reported result. |
| Hatirnaz 2017 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | 95 out of 100 women of each arm had data to analyze | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Karaki 2002 | Low risk of bias | No information if the allocation sequence was random, but it was concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Levron 2002 | Some concerns | No information if the allocation sequence was random and concealed but no baseline differences between groups. | Low risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | Only 3 out of 46 in the blastocyst group did not have embryo transfer | Low risk of bias | Method of measuring the outcome was not stated, but it is not probable that it was measured different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Rienzi 2002 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols and no information about the total number of randomised women. | High risk of bias | No information about neither the total number of randomized women nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions, and there are some concerns of risk of bias due to randomisation process, missing outcomes and selection of reported results. |
| Yang 2018 | Low risk of bias | Allocation sequence was random and concealed | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the number of randomised women per arm and it is not possible to know how the analysis was performed. | Low risk of bias | The observed number of events is much greater than the number of participants with missing outcome data, but it is probably not related with the true value | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Quote" ChiCTR‐ICR‐15006600" | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions. |
| Subgroup 4.4.3 randomised on day 2 to 3 | ||||||||||||
| Bungum 2003 | Low risk of bias | There was not a complete explanation about concealment and there are some concerns about baseline differences | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All 118 randomised women were analysed. | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results |
| Elgindy 2011 | Some concerns | The number of embryos per transfer was significantly higher in group than the other group | Some concerns | This open‐label, prospective, randomized, controlled trial. No information about a deviation from the intended protocols | Low risk of bias | All ranomised women were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process, deviations from intended interventions and selection of reported results. |
| Kaur 2014 | Some concerns | No information if the allocation sequence was random and/or concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Papanikolaou 2005 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Subgroup 4.4.4 day of randomisation unstated | ||||||||||||
| Livingstone 2002 | Low risk of bias | Allocation sequence was random and concealed | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the selection of reported results. |
| Motta 1998 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
Risk of bias for analysis 4.5 High‐order pregnancies (more than 2 gestational sacs) per couple.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Bungum 2003 | Low risk of bias | There was not a complete explanation about concealment and there are some concerns about baseline differences | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All 118 randomised women were analysed. | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results |
| Coskun 2000 | Low risk of bias | Randomisation process apparently has no problems. Patients were randomized to receive embryo transfer either at day 3 or day 5. Quote " An equal number of ‘day 3' or ‘day 5' labels placed in sealed envelopes in a block of 14 was kept in a box and an envelope was drawn by the embryologist as soon as a patient qualified for the study. The label was attached with adhesive to the patients’ data collection sheet." | Some concerns | Participants and carers and people delivering the intervention were probably aware of their assigned intervention. | Low risk of bias | All 201 randomised women were available. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns due to deviation from intended interventions and selection of reported outcomes |
| Frattarelli 2003 | Low risk of bias | Randomization was accomplished using a computer‐generated randomization table. The sequences of randomization were concealed until intervention was assigned. The groups were balanced. Concealment method was not described. | High risk of bias | Those patients undergoing a day 5 ET had a significantly higher implantation rate than those undergoing a day 3, these deviations likely have affected the outcome | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, no different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk of bias due to deviations from the intended protocol and some concerns due to bias in selection of the reported result. |
| Hreinsson 2004 | Low risk of bias | Allocation sequence was random and concealed. Quote:"Sealed envelopes were used for randomisation of the patients for each group". Groups were balanced. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No differences in embryos per transfer bat there was for cycles with embryo freezing. All women randomised were analyses by assignement | Low risk of bias | All women randomised were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results. |
| Karaki 2002 | Low risk of bias | No information if the allocation sequence was random, but it was concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to devitations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Kaur 2014 | Some concerns | No information if the allocation sequence was random and/or concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Kolibianakis 2004 | High risk of bias | Allocation sequence was random but it was not concealed. No baseline differences between groups were found. | High risk of bias | Participants, carers and people delivering the intervention were aware of their assigned intervention. No information about a deviation from the intended protocols. No information if there was a substantial impact of the failure to analyse participants in the group to which they were randomized. | Low risk of bias | All randomised women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There are high of risk of bias due to randomisation process, deviations from the intended interventions, and some concerns of risk of bias due to selection of reported results. |
| Levitas 2004 | Low risk of bias | Allocation sequence was random and concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. Analysis was done by ITT | Low risk of bias | Although there are missing outcome data, the missingness in the outcome probably does not depend on its true value. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to devitations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Levron 2002 | Some concerns | No information if the allocation sequence was random and concealed but no baseline differences between groups. | Low risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | Only 3 out of 46 in the blastocyst group did not have embryo transfer | Low risk of bias | Method of measuring the outcome was not stated, but it is not probable that it was measured different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Papanikolaou 2005 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Papanikolaou 2006 | Some concerns | There was not enough information to evaluate the method of allocation sequence concealment. | Low risk of bias | Participants were probably aware of their assigned intervention. No deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Rienzi 2002 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols and no information about the total number of randomised women. | High risk of bias | No information about neither the total number of randomized women nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions, and there are some concerns of risk of bias due to randomisation process, missing outcomes and selection of reported results. |
| Van der Auwera 2002 | Low risk of bias | No information available to know if the allocation sequence was random. However, allocation sequence was concealed and no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Quote: “Three patients were excluded for analysis from group 1 because they wanted an elective blastocyst culture while four patients were excluded from group 2 because they wanted an elective day 2 transfer.” | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions and selection of reported results. |
Risk of bias for analysis 4.6 Multiple pregnancy rate per couple: TLS (with algorithm) cleavage stage versus conventional blastocyst stage.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Yang 2018 | Low risk of bias | Allocation sequence was random and concealed | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the number of randomised women per arm and it is not possible to know how the analysis was performed. | Low risk of bias | The observed number of events is much greater than the number of participants with missing outcome data, but it is probably not related with the true value | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Quote" ChiCTR‐ICR‐15006600" | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions. |
Risk of bias for analysis 5.1 Miscarriage rate per couple.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Aziminekoo 2015 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All 118 randomised women were available. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns due to randomization process and selection of the reported result |
| Bungum 2003 | Low risk of bias | There was not a complete explanation about concealment and there are some concerns about baseline differences | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All 118 randomised women were analysed. | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results |
| Coskun 2000 | Low risk of bias | Randomisation process apparently has no problems. Patients were randomized to receive embryo transfer either at day 3 or day 5. Quote " An equal number of ‘day 3' or ‘day 5' labels placed in sealed envelopes in a block of 14 was kept in a box and an envelope was drawn by the embryologist as soon as a patient qualified for the study. The label was attached with adhesive to the patients’ data collection sheet." | Some concerns | Participants and carers and people delivering the intervention were probably aware of their assigned intervention. | Low risk of bias | All 201 randomised women were available. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns due to deviation from intended interventions and selection of reported outcomes |
| Devreker 2000 | High risk of bias | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | No protocol registration was found. | High risk of bias | There is high risk of bias due to the randomisation process, deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Elgindy 2011 | Some concerns | The number of embryos per transfer was significantly higher in group than the other group | Some concerns | This open‐label, prospective, randomized, controlled trial. No information about a deviation from the intended protocols | Low risk of bias | All randomised women were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process, deviations from intended interventions and selection of reported results. |
| Fernandez‐Shaw 2015 | Some concerns | No statement about allocation concealment. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, in both groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Frattarelli 2003 | Low risk of bias | Randomization was accomplished using a computer‐generated randomization table. The sequences of randomization were concealed until intervention was assigned. The groups were balanced. Concealment method was not described. | High risk of bias | Those patients undergoing a day 5 ET had a significantly higher implantation rate than those undergoing a day 3, these deviations likely have affected the outcome | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, no different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk of bias due to deviations from the intended protocol and some concerns due to bias in selection of the reported result. |
| Gaafar 2015 | Low risk of bias | Quote "Randomization was done by computer permuted blocks size of 4, allocation by closed envelops, parallel technique" No mention about opaque envelopes | Some concerns | Not evidence of potential of concearns generating a substantial impact | High risk of bias | A large proportion of missing outcomes: 326 were eligible. 126 cases had day 2/3 transfer and 126 had blastocyst transfer. | Low risk of bias | PACTR201308000581376 Quote "Masking Care giver/Provider,Outcome assessors,Participants" | Some concerns | There is only a difference in outcome reporting regarding multiple pregnancies | High risk of bias | High bias due to missing outcome data and some concerns due to deviations from intended interventions and bias in selection of the reported result |
| Hatirnaz 2017 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | 95 out of 100 women of each arm had data to analyze | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Hreinsson 2004 | Low risk of bias | Allocation sequence was random and concealed. Quote:"Sealed envelopes were used for randomisation of the patients for each group". Groups were balanced. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No differences in embryos per transfer bat there was for cycles with embryo freezing. All women randomised were analyses by assignement | Low risk of bias | All women randomised were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results. |
| Karaki 2002 | Low risk of bias | No information if the allocation sequence was random, but it was concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to devitations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Kaur 2014 | Some concerns | No information if the allocation sequence was random and/or concealed but no baseline differences between groups. | Low risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, and selection of reported results. |
| Kolibianakis 2004 | High risk of bias | Allocation sequence was random but it was not concealed. No baseline differences between groups were found. | High risk of bias | Participants, carers and people delivering the intervention were aware of their assigned intervention. No information about a deviation from the intended protocols. No information if there was a substantial impact of the failure to analyse participants in the group to which they were randomized. | Low risk of bias | All randomised women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There are high of risk of bias due to deviations from the intended interventions and randomisation process, and some concerns of risk of bias due to selection of reported results. |
| Levi‐Setti 2018 | Some concerns | Allocation sequence was not concealed but no baseline clinical and statistical differences between groups were found in a table that described more than 15 different variables. | Low risk of bias | Participants and embryologists were aware of their assigned intervention. No deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not informed. However, given the nature of the outcome and the methods to measure it, it was probably not different between groups and, as it is an objective outcome, it is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Data that produced this result was analysed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis | Some concerns | There is some concerns of risk of bias due to randomisation process. |
| Levitas 2004 | Low risk of bias | Allocation sequence was random and concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. Analysis was done by ITT | Low risk of bias | Although there are missing outcome data, the missingness in the outcome probably does not depend on its true value. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to devitations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Livingstone 2002 | Low risk of bias | Allocation sequence was random and concealed | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the selection of reported results. |
| Papanikolaou 2005 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Papanikolaou 2006 | Some concerns | There was not enough information to evaluate the method of allocation sequence concealment. | Low risk of bias | Participants were probably aware of their assigned intervention. No deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Rienzi 2002 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols and no information about the total number of randomised women. | High risk of bias | No information about neither the total number of randomized women nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions, and there are some concerns of risk of bias due to randomisation process, missing outcomes and selection of reported results. |
| Van der Auwera 2002 | Low risk of bias | No information available to know if the allocation sequence was random. However, allocation sequence was concealed and no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Quote: “Three patients were excluded for analysis from group 1 because they wanted an elective blastocyst culture while four patients were excluded from group 2 because they wanted an elective day 2 transfer.” | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions and selection of reported results. |
| Yang 2018 | Low risk of bias | Allocation sequence was random and concealed | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the number of randomised women per arm and it is not possible to know how the analysis was performed. | Low risk of bias | The observed number of events is much greater than the number of participants with missing outcome data, but it is probably not related with the true value | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Low risk of bias | Quote" ChiCTR‐ICR‐15006600" | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions. |
Risk of bias for analysis 6.1 Embryo freezing per couple.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Brugnon 2010 | Some concerns | Sequence generated but there was no information about concealment. No table of patients' baseline characteristics reported | High risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No information about the deviations or the analysis | High risk of bias | No information. Only proportions were reported | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk due to deviation from the intended interventions |
| Bungum 2003 | Low risk of bias | There was not a complete explanation about concealment and there are some concerns about baseline differences | Low risk of bias | No effort for blindness is described. All 118 randomised women were analysed. | Low risk of bias | All 118 randomised women were analysed. | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results |
| Fernandez‐Shaw 2015 | Some concerns | No statement about allocation concealment. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, in both groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Gardner 1998a | Some concerns | Concealment was not reported, there was a difference in Previous no. of cycles | High risk of bias | No effort for blindness is described. There was a difference No. of embryos transferred and proportion of embryo freezing that likely have affected the outcome | Low risk of bias | All women randomised were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Low risk of bias | ClinicalTrials.gov Identifier: NCT03764865 | High risk of bias | High risk of bias due to deviations from intended intervention |
| Hreinsson 2004 | Low risk of bias | Allocation sequence was random and concealed. Quote:"Sealed envelopes were used for randomisation of the patients for each group". Groups were balanced. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No differences in embryos per transfer bat there was for cycles with embryo freezing. All women randomised were analyses by assignement | Low risk of bias | All women randomised were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results. |
| Karaki 2002 | Low risk of bias | No information if the allocation sequence was random, but it was concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to devitations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Kolibianakis 2004 | High risk of bias | Allocation sequence was random but it was not concealed. No baseline differences between groups were found. | High risk of bias | Participants, carers and people delivering the intervention were aware of their assigned intervention. No information about a deviation from the intended protocols. No information if there was a substantial impact of the failure to analyse participants in the group to which they were randomized. | Low risk of bias | All randomised women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There are high of risk of bias due to deviations from the intended interventions and randomisation process, and some concerns of risk of bias due to selection of reported results. |
| Levron 2002 | Some concerns | No information if the allocation sequence was random and concealed but no baseline differences between groups. | Low risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | Only 3 out of 46 in the blastocyst group did not have embryo transfer | Low risk of bias | Method of measuring the outcome was not stated, but it is not probable that it was measured different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Motta 1998 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidence suggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Pantos 2004 | Some concerns | No information about the allocation sequence random and concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | There is no information about the method of measuring the outcome, but it is probable that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Papanikolaou 2006 | Some concerns | There was not enough information to evaluate the method of allocation sequence concealment. | Low risk of bias | Participants were probably aware of their assigned intervention. No deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Rienzi 2002 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols and no information about the total number of randomised women. | High risk of bias | No information about neither the total number of randomized women nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions, and there are some concerns of risk of bias due to randomisation process, missing outcomes and selection of reported results. |
| Ten 2011 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. And no information about the analysis that was performed. | High risk of bias | No information about neither the absolute number of events nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and missing outcomes, and some concerns of risk of bias due to randomisation process and selection of reported results. |
| Van der Auwera 2002 | Low risk of bias | No information available to know if the allocation sequence was random. However, allocation sequence was concealed and no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Quote: “Three patients were excluded for analysis from group 1 because they wanted an elective blastocyst culture while four patients were excluded from group 2 because they wanted an elective day 2 transfer.” | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions and selection of reported results. |
Risk of bias for analysis 7.1 Failure to transfer any embryos (per couple).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Gardner 1998a | Some concerns | Concealment was not reported, there was a difference in Previous no. of cycles | High risk of bias | No effort for blindness is described. There was a difference No. of embryos transferred and proportion of embryo freezing that likely have affected the outcome | Low risk of bias | All women randomised were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Low risk of bias | ClinicalTrials.gov Identifier: NCT03764865 | High risk of bias | High risk of bias due to deviations from intended intervention |
| Aziminekoo 2015 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All 118 randomised women were available. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Bungum 2003 | Low risk of bias | There was not a complete explanation about concealment and there are some concerns about baseline differences | Low risk of bias | No effort for blindness is described. All 118 randomised women were analysed. | Low risk of bias | All 118 randomised women were analysed. | Low risk of bias | Method of measuring the outcome was probably appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results |
| Coskun 2000 | Low risk of bias | Randomisation process apparently has no problems. Patients were randomized to receive embryo transfer either at day 3 or day 5. Quote " An equal number of ‘day 3' or ‘day 5' labels placed in sealed envelopes in a block of 14 was kept in a box and an envelope was drawn by the embryologist as soon as a patient qualified for the study. The label was attached with adhesive to the patients’ data collection sheet." | Some concerns | Participants and carers and people delivering the intervention were probably aware of their assigned intervention. | Low risk of bias | All 201 randomised women were available. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | Some concerns due to deviation from intended interventions and selection of reported outcomes |
| Devreker 2000 | High risk of bias | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidencesuggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions and there are some concerns of risk of bias due to the selection of reported results. |
| Emiliani 2003 | Some concerns | Concealment was not stated. Only minor differences in age | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. | Low risk of bias | All women randomised were analysed | Low risk of bias | Not reported but inappropriate methotds are unlikely. It is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention | Some concerns | No protocol registration was found. | Some concerns | There some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Frattarelli 2003 | Low risk of bias | Randomization was accomplished using a computer‐generated randomization table. The sequences of randomization were concealed until intervention was assigned. The groups were balanced. Concealment method was not described. | High risk of bias | Those patients undergoing a day 5 ET had a significantly higher implantation rate than those undergoing a day 3, these deviations likely have affected the outcome | Low risk of bias | All women randomised were analysed | Low risk of bias | Method of measuring the outcome was probably appropriate, no different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | High risk of bias due to deviations from the intended protocol and some concerns due to bias in selection of the reported result. |
| Hreinsson 2004 | Low risk of bias | Allocation sequence was random and concealed. Quote:"Sealed envelopes were used for randomisation of the patients for each group". Groups were balanced. | Low risk of bias | No effort for blindness is described. Considering the nature of the intervention, it is very unlikely that participants and carers and people delivering the intervention were not aware of their assigned intervention. No differences in embryos per transfer bat there was for cycles with embryo freezing. All women randomised were analyses by assignement | Low risk of bias | All women randomised were analysed | Low risk of bias | Knowledge of the assigned intervention is unlikely to influence this objective outcome assessment. | Some concerns | No protocol registration was found. | Some concerns | Some concerns of risk of bias due to selection of reported results. |
| Karaki 2002 | Low risk of bias | No information if the allocation sequence was random, but it was concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to devitations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Kolibianakis 2004 | High risk of bias | Allocation sequence was random but it was not concealed. No baseline differences between groups were found. | High risk of bias | Participants, carers and people delivering the intervention were aware of their assigned intervention. No information about a deviation from the intended protocols. No information if there was a substantial impact of the failure to analyse participants in the group to which they were randomized. | Low risk of bias | All randomised women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There are high of risk of bias due to deviations from the intended interventions and randomisation process, and some concerns of risk of bias due to selection of reported results. |
| Levitas 2004 | Low risk of bias | Allocation sequence was random and concealed and no baseline differences were found between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was probably a deviation from the intended protocols. Analysis was done by ITT | Low risk of bias | Although there are missing outcome data, the missingness in the outcome probably does not depend on its true value. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to devitations of the protocol and some concerns of risk of bias due to selection of reported results. |
| Levron 2002 | Some concerns | No information if the allocation sequence was random and concealed but no baseline differences between groups. | Low risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. Analysis was performed by intention‐to‐treat. | Low risk of bias | Only 3 out of 46 in the blastocyst group did not have embryo transfer | Low risk of bias | Method of measuring the outcome was not stated, but it is not probable that it was measured different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Motta 1998 | Some concerns | No information available to know if the allocation sequence was random or the allocation sequence was concealed. And no information about baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There was evidence suggesting deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | No lost to follow up | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to the randomisation process, deviations from the intended interventions and there are some concerns of risk of bias due to the randomisation process and selection of reported results. |
| Papanikolaou 2005 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed. | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process, deviations from the intended interventions and selection of reported results. |
| Papanikolaou 2006 | Some concerns | There was not enough information to evaluate the method of allocation sequence concealment. | Low risk of bias | Participants were probably aware of their assigned intervention. No deviation from the intended protocols. All randomised women were analysed. | Low risk of bias | All the randomized women were analysed | Low risk of bias | Method of measuring the outcome was appropriate, was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to randomisation process and selection of reported results. |
| Rienzi 2002 | Some concerns | No information about the allocation sequence concealed but no baseline differences between groups. | High risk of bias | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. No information about a deviation from the intended protocols and no information about the total number of randomised women. | High risk of bias | No information about neither the total number of randomized women nor the missing outcome data. | Low risk of bias | Method of measuring the outcome was appropriate, it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | High risk of bias | There is high risk of bias due to deviations from the intended interventions, and there are some concerns of risk of bias due to randomisation process, missing outcomes and selection of reported results. |
| Van der Auwera 2002 | Low risk of bias | No information available to know if the allocation sequence was random. However, allocation sequence was concealed and no baseline differences between groups. | Some concerns | Participants, carers and people delivering the intervention were probably aware of their assigned intervention. There were only few with deviation from the intended protocols, and no imbalance was found. All randomised women were analysed. | Low risk of bias | Quote: “Three patients were excluded for analysis from group 1 because they wanted an elective blastocyst culture while four patients were excluded from group 2 because they wanted an elective day 2 transfer.” | Low risk of bias | Method of measuring the outcome was not described. However, it is unlikely that it was not different between groups and it is an objective outcome that is unlikely to be influenced by the knowledge of the assigned intervention. | Some concerns | No protocol registration was found. | Some concerns | There are some concerns of risk of bias due to deviations from the intended interventions and selection of reported results. |
Acknowledgements
The authors acknowledge the helpful comments of those who refereed previous versions of this review, in particular, Mr Andy Vail and Dr Gayle Jones. Thanks to Dr Plachot, Dr Huisman, Dr Utsunomiya, Dr Hreinsson, Dr Rienzi, Dr Levron, Dr Levitas, Dr Bungum, Dr Papanikolaou, Dr Karaki, Dr Frattarelli, Dr Brugnon, Dr Vanderzwalmen and Dr Fernández‐Shaw for supplying additional information. Thanks to the librarian Daniel Comandé. Finally, special thanks to the highly supportive team at the Cochrane office in Auckland.
Dr Neil Johnson was a review author for the previous version of this review and made a significant contribution to the interpretation of results and performed some data extraction. David Olive, for the initial review, commented on drafts of the protocol and review. Michelle Proctor, for the initial review, was involved in selecting trials for inclusion, performed independent data extraction and quality assessment of the included trials, contributed to the discussion and interpretation of results. Quirine Lamberts, for the 2005 update, checked the data and study information extracted. Ariel Bardach, for the 2012 update, was involved in selecting trials for inclusion, performed independent data extraction, and performed quality assessment of the included trials.
The authors of the 2022 update acknowledge the contributions of Professor Cindy Farquhar to previous versions of this review. They thank also Dr Katie Stocking, Associate Professor Vanessa Jordan, Dr Selma Mourad and Assistant Professor Sebastiaan Mastenbroek for providing peer review comments.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility specialised register search strategy
ProCite platform
Searched 20 October 2021
Keywords CONTAINS "day 2"or"day 3"or "day 3 embryo transfer" or "day 4 embryo transfer" or "cleavage stage" or "cleavage transfer" or "pronuclear morphology" or "early cleavage assessment" or "early cleavage medium" or "early cleavage status" or Title CONTAINS "day 2"or"day 3"or "day 3 embryo transfer" or "day 4 embryo transfer" or "cleavage stage" or "cleavage transfer" or "pronuclear morphology" or "early cleavage assessment" or "early cleavage medium" or "early cleavage status"
AND
Keywords CONTAINS "day 5" or "day 5 transfer" or "day 6 transfer" or "Blastocyst" or "blastocyst culture technique" or "blastocyst media" or "blastocyst stage" or "blastocyst transfer" or "morula" or "morula formation "or Title CONTAINS "day 5" or "day 5 transfer" or "day 6 transfer" or "Blastocyst" or "blastocyst culture technique" or "blastocyst media" or "blastocyst stage" or "blastocyst transfer" or "morula" or "morula formation"
(226 records)
Appendix 2. CENTRAL via Cochrane Register of Studies Online (CRSO) search strategy
Web platform
Searched 20 October 2021
#1 MESH DESCRIPTOR Embryo Transfer EXPLODE ALL TREES 1131
#2 MESH DESCRIPTOR Fertilization in Vitro EXPLODE ALL TREES 2134
#3 MESH DESCRIPTOR Sperm Injections, Intracytoplasmic EXPLODE ALL TREES 564
#4 MESH DESCRIPTOR Oocyte Donation EXPLODE ALL TREES 72
#5 (embryo transfer*):TI,AB,KY 4062
#6 (in vitro fertili?ation):TI,AB,KY 3629
#7 (intracytoplasmic sperm injection*):TI,AB,KY 2073
#8 ((ivf or icsi)):TI,AB,KY 7008
#9 ET:TI,AB,KY 33956
#10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 41991
#11 (day 2):TI,AB,KY 7060
#12 (day 3):TI,AB,KY 7980
#13 48*:TI,AB,KY 106489
#14 72*:TI,AB,KY 67552
#15 cleav*:TI,AB,KY 1616
#16 pronuclear:TI,AB,KY 78
#17 day2:TI,AB,KY 162
#18 day3:TI,AB,KY 141
#19 ((early adj3 embryo*)):TI,AB,KY 160
#20 ((day two or day three)):TI,AB,KY 1005
#21 MESH DESCRIPTOR Cleavage Stage, Ovum EXPLODE ALL TREES 70
#22 #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 174669
#23 MESH DESCRIPTOR Blastocyst EXPLODE ALL TREES 182
#24 Blastocyst*:TI,AB,KY 1432
#25 ((day 5 or day 6)):TI,AB,KY 7676
#26 ((day5 or day6)):TI,AB,KY 81
#27 ((day five or day six)):TI,AB,KY 520
#28 #23 OR #24 OR #25 OR #26 OR #27 9313
#29 #10 AND #22 AND #28 801
Appendix 3. MEDLINE search strategy
OVID platform
Searched from 1946 to 20 October 2021
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ or exp oocyte donation/ (45499) 2 embryo transfer$.tw. (13414) 3 in vitro fertili?ation.tw. (24970) 4 intracytoplasmic sperm injection$.tw. (7866) 5 (ivf or icsi).tw. (29787) 6 ET.tw. (296630) 7 or/1‐6 (353408) 8 day 2.tw. (23962) 9 day3.tw. (83) 10 48$.tw. (766982) 11 72$.tw. (508121) 12 cleav$.tw. (209100) 13 pronuclear.tw. (2442) 14 day 3.tw. (32480) 15 day2.tw. (60) 16 (early adj3 embryo$).tw. (29813) 17 (day two or day three).tw. (3430) 18 exp Cleavage Stage, Ovum/ (2311) 19 or/8‐18 (1478615) 20 exp Blastocyst/ (28404) 21 Blastocyst$.tw. (24051) 22 (day 5 or day 6).tw. (37664) 23 (day5 or day6).tw. (46) 24 (day five or day six).tw. (2006) 25 or/20‐24 (79108) 26 7 and 19 and 25 (5619) 27 randomized controlled trial.pt. (546666) 28 controlled clinical trial.pt. (94462) 29 randomized.ab. (537227) 30 placebo.tw. (228288) 31 clinical trials as topic.sh. (197761) 32 randomly.ab. (367850) 33 trial.ti. (249362) 34 (crossover or cross‐over or cross over).tw. (90920) 35 or/27‐34 (1433750) 36 exp animals/ not humans.sh. (4899579) 37 35 not 36 (1318057) 38 26 and 37 (406)
Appendix 4. Embase search strategy
OVID platform
Searched from 1980 to 20 October 2021
1 exp embryo transfer/ (33960) 2 exp fertilization in vitro/ (77081) 3 exp intracytoplasmic sperm injection/ (22837) 4 exp oocyte donation/ (4375) 5 embryo transfer$.tw. (21741) 6 in vitro fertili?ation.tw. (32941) 7 intracytoplasmic sperm injection$.tw. (10530) 8 (ivf or icsi).tw. (51536) 9 ET.tw. (702743) 10 or/1‐9 (797670) 11 day 2.tw. (38612) 12 day3.tw. (700) 13 48$.tw. (1191910) 14 72$.tw. (802373) 15 cleav$.tw. (240374) 16 pronuclear.tw. (3034) 17 day 3.tw. (52695) 18 day2.tw. (455) 19 (early adj3 embryo$).tw. (32753) 20 (day two or day three).tw. (6490) 21 exp oocyte cleavage/ (3044) 22 or/11‐21 (2197261) 23 exp BLASTOCYST/ (29149) 24 Blastocyst$.tw. (32419) 25 (day 5 or day 6).tw. (57687) 26 (day5 or day6).tw. (455) 27 (day five or day six).tw. (3242) 28 or/23‐27 (95325) 29 10 and 22 and 28 (10987) 30 Clinical Trial/ (1006996) 31 Randomized Controlled Trial/ (676265) 32 exp randomization/ (92118) 33 Single Blind Procedure/ (44016) 34 Double Blind Procedure/ (185761) 35 Crossover Procedure/ (68302) 36 Placebo/ (358800) 37 Randomi?ed controlled trial$.tw. (268420) 38 Rct.tw. (43809) 39 random allocation.tw. (2224) 40 randomly allocated.tw. (39286) 41 allocated randomly.tw. (2694) 42 (allocated adj2 random).tw. (831) 43 Single blind$.tw. (27376) 44 Double blind$.tw. (217076) 45 ((treble or triple) adj blind$).tw. (1411) 46 placebo$.tw. (326910) 47 prospective study/ (718257) 48 or/30‐47 (2418660) 49 case study/ (81550) 50 case report.tw. (453208) 51 abstract report/ or letter/ (1166106) 52 or/49‐51 (1688536) 53 48 not 52 (2360524) 54 29 and 53 (1466)
Appendix 5. PsycINFO search strategy
OVID platform
Searched from 1806 to 20 October 2021
1 exp reproductive technology/ (1991) 2 embryo transfer$.tw. (131) 3 in vitro fertili?ation.tw. (814) 4 intracytoplasmic sperm injection$.tw. (61) 5 (ivf or icsi).tw. (651) 6 ET.tw. (150085) 7 or/1‐6 (152389) 8 day 2.tw. (2055) 9 day3.tw. (10) 10 48$.tw. (78525) 11 72$.tw. (48856) 12 cleav$.tw. (4014) 13 pronuclear.tw. (15) 14 day 3.tw. (1826) 15 day2.tw. (11) 16 (early adj3 embryo$).tw. (693) 17 (day two or day three).tw. (397) 18 exp embryo/ (1818) 19 or/8‐18 (133288) 20 Blastocyst$.tw. (91) 21 (day 5 or day 6).tw. (2055) 22 (day5 or day6).tw. (2) 23 (day five or day six).tw. (205) 24 or/20‐23 (2350) 25 7 and 19 and 24 (16)
Appendix 6. CINAHL search strategy
EBSCO platform
Searched from 1961 to 4 April 2020. Later CINAHL output is included in the CENTRAL 20 October 2021 search output.
| # | Query | Results |
| S20 | S9 AND S14 AND S19 | 373 |
| S19 | S15 OR S16 OR S17 OR S18 | 44,772 |
| S18 | TX(day five or day six) | 42,508 |
| S17 | TX blastocyst* | 2,417 |
| S16 | TX morula | 45 |
| S15 | (MM "Blastocyst") | 1,015 |
| S14 | S10 OR S11 OR S12 OR S13 | 79,180 |
| S13 | TX pronuclear | 61 |
| S12 | (MM "Cleavage Stage, Ovum") | 53 |
| S11 | TX (day two or day three) | 76,416 |
| S10 | TX cleavage | 2,862 |
| S9 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 | 11,987 |
| S8 | TX intracytoplasmic sperm injection* | 1,032 |
| S7 | TX embryo* N3 transfer* | 3,446 |
| S6 | TX ovar* N3 hyperstimulat* | 963 |
| S5 | TX ovari* N3 stimulat* | 1,149 |
| S4 | TX IVF or TX ICSI | 5,682 |
| S3 | (MM "Fertilization in Vitro") | 3,898 |
| S2 | TX vitro fertilization | 7,938 |
| S1 | TX vitro fertilisation | 7,938 |
Appendix 7. ClinicalTrials.gov search strategy
Web platform
Searched 4 April 2020
"embryo" and "day" and "transfer" (262 hits)
OR
"blastocyst" and "transfer" (87 hits)
Appendix 8. WHO portal (ICTRP) search strategy
Web platform
Searched 4 April 2020
"embryo" and "day" and "transfer" (3 hits)
OR
"blastocyst" and "transfer" (24 hits)
Data and analyses
Comparison 1. Blastocyst‐ versus cleavage‐stage transfer: live birth rate following fresh transfer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Live birth per couple | 15 | 2219 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.06, 1.51] |
| 1.2 Live birth per couple: grouped by number of embryos transferred | 15 | 2677 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.10, 1.53] |
| 1.2.1 More cleavage‐stage than blastocyst embryos transferred | 6 | 483 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.03, 2.22] |
| 1.2.2 Single embryo transfer | 2 | 458 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.98, 2.20] |
| 1.2.3 Equal number of embryos transferred | 9 | 1736 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.99, 1.47] |
| 1.3 Live birth rate per couple: grouped by prognosis | 15 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 good prognostic factors | 9 | 1514 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.04, 1.59] |
| 1.3.2 poor prognostic factors | 2 | 77 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.53, 7.96] |
| 1.3.3 unselected group | 4 | 628 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.86, 1.66] |
| 1.4 Live birth rate: grouped by day of randomisation | 15 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 randomisation at start of cycle | 6 | 1207 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.84, 1.38] |
| 1.4.2 randomised on day of OPU and day 1 after OPU | 5 | 566 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.82, 1.65] |
| 1.4.3 randomised day 2 to 3 post‐OPU | 2 | 364 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.42, 3.33] |
| 1.4.4 day of randomisation unstated | 2 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.67, 4.39] |
1.4. Analysis.

Comparison 1: Blastocyst‐ versus cleavage‐stage transfer: live birth rate following fresh transfer, Outcome 4: Live birth rate: grouped by day of randomisation
Comparison 2. Blastocyst‐ versus cleavage‐stage transfer: cumulative pregnancy rate following fresh and frozen transfer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Cumulative pregnancy rate from fresh and frozen transfers | 5 | 632 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.64, 1.22] |
| 2.2 Cumulative pregnancy rate per couple: grouped by number of embryos transferred | 5 | 739 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.65, 1.18] |
| 2.2.1 single embryo transfer | 1 | 107 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.39, 1.79] |
| 2.2.2 equal number of embryos transferred | 5 | 632 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.64, 1.22] |
| 2.3 Cumulative pregnancy rate per couple: grouped by prognosis | 5 | 632 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.64, 1.22] |
| 2.3.1 good prognostic factors | 2 | 205 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.30, 0.98] |
| 2.3.2 unselected group | 3 | 427 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.74, 1.61] |
| 2.4 Cumulative pregnancy rate: grouped by day of randomisation | 5 | 632 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.64, 1.22] |
| 2.4.1 randomisation at start of cycle | 3 | 414 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.54, 1.20] |
| 2.4.2 randomised on day of OPU and day 1 after OPU | 2 | 218 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.61, 1.83] |
| 2.5 Cumulative pregnancy rate from fresh and frozen transfers: grouped by vitrification or slow freezing | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.5.1 slow freezing | 4 | 512 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.48, 0.99] |
| 2.5.2 vitrification | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.17, 5.12] |
2.5. Analysis.

Comparison 2: Blastocyst‐ versus cleavage‐stage transfer: cumulative pregnancy rate following fresh and frozen transfer, Outcome 5: Cumulative pregnancy rate from fresh and frozen transfers: grouped by vitrification or slow freezing
Comparison 3. Blastocyst‐ versus cleavage‐stage transfer: clinical pregnancy following fresh transfer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Clinical pregnancy rate per couple | 32 | 5821 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.12, 1.39] |
| 3.2 Clinical pregnancy rate per couple: grouped by number of embryos transferred | 32 | 7062 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.14, 1.38] |
| 3.2.1 equal number of embryo transfers | 20 | 4434 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.16, 1.48] |
| 3.2.2 more cleavage stage than blastocyst embryos transferred | 12 | 1387 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.86, 1.33] |
| 3.2.3 single embryo transfer | 5 | 1241 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.04, 1.65] |
| 3.3 Clinical pregnancy rate per couple: grouped by prognosis | 32 | 5821 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.12, 1.39] |
| 3.3.1 good prognostic factors | 19 | 3645 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.09, 1.43] |
| 3.3.2 poor prognostic factors | 3 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.84, 3.10] |
| 3.3.3 unselected group | 10 | 1981 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.01, 1.46] |
| 3.4 Clinical pregnancy rate per couple: grouped by day of randomisation | 32 | 5821 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.12, 1.39] |
| 3.4.1 randomised start of cycle | 8 | 1759 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.92, 1.37] |
| 3.4.2 randomised on day of OPU or day 1 | 13 | 2094 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.00, 1.42] |
| 3.4.3 randomised on day 2 to 3 | 6 | 1275 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.19, 1.88] |
| 3.4.4 day of randomisation unstated | 5 | 693 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.98, 1.80] |
| 3.5 Clinical pregnancy rate per couple: TLS (with algorithm) cleavage stage versus conventional blastocyst stage | 2 | 709 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.04, 1.90] |
| 3.6 Clinical pregnancy rate per couple: TLS (with algorithm) cleavage stage versus TLS (with algorithm) blastocyst stage | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.43, 1.96] |
3.1. Analysis.

Comparison 3: Blastocyst‐ versus cleavage‐stage transfer: clinical pregnancy following fresh transfer, Outcome 1: Clinical pregnancy rate per couple
3.2. Analysis.

Comparison 3: Blastocyst‐ versus cleavage‐stage transfer: clinical pregnancy following fresh transfer, Outcome 2: Clinical pregnancy rate per couple: grouped by number of embryos transferred
3.3. Analysis.

Comparison 3: Blastocyst‐ versus cleavage‐stage transfer: clinical pregnancy following fresh transfer, Outcome 3: Clinical pregnancy rate per couple: grouped by prognosis
3.4. Analysis.

Comparison 3: Blastocyst‐ versus cleavage‐stage transfer: clinical pregnancy following fresh transfer, Outcome 4: Clinical pregnancy rate per couple: grouped by day of randomisation
3.5. Analysis.

Comparison 3: Blastocyst‐ versus cleavage‐stage transfer: clinical pregnancy following fresh transfer, Outcome 5: Clinical pregnancy rate per couple: TLS (with algorithm) cleavage stage versus conventional blastocyst stage
3.6. Analysis.

Comparison 3: Blastocyst‐ versus cleavage‐stage transfer: clinical pregnancy following fresh transfer, Outcome 6: Clinical pregnancy rate per couple: TLS (with algorithm) cleavage stage versus TLS (with algorithm) blastocyst stage
Comparison 4. Blastocyst‐ versus cleavage‐stage transfer: multiple pregnancy following fresh transfer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Multiple pregnancy rate per couple | 22 | 4208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.90, 1.38] |
| 4.2 Multiple pregnancy rate per couple: grouped by number of embryos transferred | 22 | 5159 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.91, 1.38] |
| 4.2.1 equal number of embryos transferred | 14 | 3399 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.01, 1.65] |
| 4.2.2 more cleavage stage than blastocyst embryos transferred | 8 | 809 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.49, 1.13] |
| 4.2.3 single embryo transfer | 2 | 951 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.51, 2.91] |
| 4.3 Multiple pregnancy rate per couple: grouped by prognosis | 22 | 4208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.90, 1.38] |
| 4.3.1 good prognostic factors | 15 | 2904 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.89, 1.46] |
| 4.3.2 poor prognostic factors | 1 | 54 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.14, 5.81] |
| 4.3.3 unselected | 6 | 1250 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.71, 1.59] |
| 4.4 Multiple pregnancy rate per couple: grouped by day of randomisation | 22 | 4208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.90, 1.38] |
| 4.4.1 randomised start of cycle | 7 | 1704 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.71, 1.46] |
| 4.4.2 randomised on day of OPU or day 1 | 9 | 1647 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.73, 1.52] |
| 4.4.3 randomised on day 2 to 3 | 4 | 682 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.14, 2.56] |
| 4.4.4 day of randomisation unstated | 2 | 175 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.06, 0.70] |
| 4.5 High‐order pregnancies (more than 2 gestational sacs) per couple | 13 | 2335 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.18, 1.15] |
| 4.6 Multiple pregnancy rate per couple: TLS (with algorithm) cleavage stage versus conventional blastocyst stage | 1 | 600 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.61, 4.17] |
4.1. Analysis.

Comparison 4: Blastocyst‐ versus cleavage‐stage transfer: multiple pregnancy following fresh transfer, Outcome 1: Multiple pregnancy rate per couple
4.5. Analysis.

Comparison 4: Blastocyst‐ versus cleavage‐stage transfer: multiple pregnancy following fresh transfer, Outcome 5: High‐order pregnancies (more than 2 gestational sacs) per couple
4.6. Analysis.

Comparison 4: Blastocyst‐ versus cleavage‐stage transfer: multiple pregnancy following fresh transfer, Outcome 6: Multiple pregnancy rate per couple: TLS (with algorithm) cleavage stage versus conventional blastocyst stage
Comparison 5. Blastocyst‐ versus cleavage‐stage transfer: miscarriage rate following fresh transfer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Miscarriage rate per couple | 21 | 4106 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.98, 1.57] |
5.1. Analysis.

Comparison 5: Blastocyst‐ versus cleavage‐stage transfer: miscarriage rate following fresh transfer, Outcome 1: Miscarriage rate per couple
Comparison 6. Blastocyst‐ versus cleavage‐stage transfer: embryo freezing rate per couple.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Embryo freezing per couple | 14 | 2292 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.40, 0.57] |
6.1. Analysis.

Comparison 6: Blastocyst‐ versus cleavage‐stage transfer: embryo freezing rate per couple, Outcome 1: Embryo freezing per couple
Comparison 7. Blastocyst‐ versus cleavage‐stage transfer: failure rate to transfer embryos (per couple).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Failure to transfer any embryos (per couple) | 17 | 2577 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.50 [1.76, 3.55] |
7.1. Analysis.

Comparison 7: Blastocyst‐ versus cleavage‐stage transfer: failure rate to transfer embryos (per couple), Outcome 1: Failure to transfer any embryos (per couple)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aziminekoo 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Clinical pregnancy rate Multiple pregnancy rate Miscarriage rate Cancellation |
|
| Notes | ||
Brugnon 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Cumulative pregnancy rate Clinical pregnancy rate per embryo transfer |
|
| Notes | ||
Bungum 2003.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Clinical pregnancy rate Multiple rate Miscarriage Embryo freezing rate Implantation rate |
|
| Notes | 3 or more 8‐celled D3 Lower blast rate in ICSI than IVF Letter sent and reply received | |
Coskun 2000.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Clinical pregnancy rate Multiple pregnancy rate High‐order multiple pregnancy rate Miscarriage rate |
|
| Notes | Prognosis: good 4 or more zygotes Young women Good ET policy Mixture of media brands Low blast rate High implantation rate considering No dropouts unusual | |
Devreker 2000.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Live birth rate Clinical pregnancy rate Miscarriage rate |
|
| Notes | Prognosis: poor Abstract only Letter sent regarding randomisation | |
Elgindy 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Live birth rate per fresh embryo transfer Clinical pregnancy rate Miscarriage rate Failure rate to transfer embryos |
|
| Notes | ||
Emiliani 2003.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Live birth rate per fresh embryo transfer Cumulative pregnancy rate Clinical pregnancy rate Multiple pregnancy rate Failure rate to transfer embryos |
|
| Notes | Prognosis: mixed unselected Outcome: discontinued blast culture Gives cumulative fresh thawed rates Not all different women ‐ per cycle data only | |
Fernandez‐Shaw 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Cumulative live birth rate* Live birth rate* Cumulative pregnancy rate Clinical pregnancy rate Multiple pregnancy rate Miscarriage rate The percentage of participants with embryos that were vitrified Three participants from the day 3 group and 2 from the day 5 group (with a mean number of 3 vitrified embryos) had not done a frozen embryo transfer cycle at the time of closing our interim analysis. |
|
| Notes | *Additional unpublished data received from contact author May 2016 in personal communication (email) to Demián Glujovsky. This is source of data on live births (analysis 1.1) | |
Fisch 2007.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Clinical pregnancy rate | |
| Notes | ||
Frattarelli 2003.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Live birth rate per fresh embryo transfer Clinical pregnancy rate Multiple pregnancy rate Miscarriage rate Failure rate to transfer embryos |
|
| Notes | Prognosis: good 6 or more high‐grade embryos D3, 1st cycle, young, high numbers of oocytes No dropouts due to lack of blasts High blast implantation rate | |
Gaafar 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Clinical pregnancy rate Miscarriage rate |
|
| Notes | ||
Gardner 1998a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Clinical pregnancy rate Embryo freezing rate Implantation rate |
|
| Notes | Prognosis: good < 1 previous cycle and > 10 follicles Different media used for each arm of study Excluded participants not mentioned 'Number of ET' policy change partway through Unblinded interim analysis: initially participants received three blastocysts. | |
Hatirnaz 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Live birth rate Clinical pregancy rate Miscarriage rate Multiple rate Implantation rate |
|
| Notes | ||
Hreinsson 2004.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Clinical pregnancy rate | |
| Notes | Prognosis: good; excluded poor responders Mixture of media types and ET policy change over course of study Outcome no advantage of blast culture Letter sent and reply received | |
Karaki 2002.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Clinical pregnancy rate Multiple rate Miscarriage rate Failure rate to transfer embryos Implantation rate |
|
| Notes | Prognosis: moderate, young women, moderately high oocyte numbers. Large difference in embryo ET# between groups Sent letter | |
Kaser 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Clinical pregnancy rate Cumulative pregnancy rate was not evaluated as the randomization was broken after the first transfer, and all embryos were cryopreserved at the blastocyst stage. |
|
| Notes | This study was funded by Progyny, Inc, which participated in the initial study design and approved the final embryo selection algorithms. All data handling, statistical analyses, and interpretation was performed independent of Progyny. The sponsor solely provided comments on the manuscript, and did not have editorial control in the manuscript preparation or submission. The trial was terminated prematurely in February 2016 by the sponsor due to a change in funding priorities. Enrolment opened in August 2014 and closed in February 2016. Clinicaltrials.gov NCT02218255 |
|
Kaur 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Clinical pregnancy Multiple pregnancy Miscarriage rate Implantation rate |
|
| Notes | Attempt to contact authors for more details of randomisation methods was not successful | |
Kolibianakis 2004.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Clinical pregnancy rate Miscarriage rate Embryo freezing rate Failure to transfer embryos |
|
| Notes | Prognosis: mixed unselected | |
Levi‐Setti 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Live birth rate Clinical pregnancy rate Multiple pregnancy rate Miscarriage rate Implantation rate |
|
| Notes | ||
Levitas 2004.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Live birth rate Clinical pregnancy rate Multiple pregnancy rate Miscarriage rate Failure rate to transfer embryos Implantation rate |
|
| Notes | Note: young women with large numbers of failed cycles Uneven number in each group Similar figures to the 2001 abstract Letter sent and reply received | |
Levron 2002.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Live birth rate Clinical pregnancy rate Multiple pregnancy rate Embryo freezing rate Failure to transfer emvryos Implantation rate |
|
| Notes | Prognosis: moderate Young women Moderately high numbers of oocytes Clarification letter sent Same ET policy | |
Livingstone 2002.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Live birth rate Clinical pregnancy rate Multiple pregnancy rate Miscarriage rate |
|
| Notes | Prognosis: good prognosis Data from thesis 2002 and not abstract 2001 Low fertilisation rate Aim to reduce twinning | |
Motta 1998.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Clinical pregnancy rate Multiple pregnancy rate Embryo freezing rate Failure to transfer |
|
| Notes | Prognosis: moderate to good. No letter sent | |
Pantos 2004.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Clinical pregnancy rate Embryo freezing rate |
|
| Notes | ||
Papanikolaou 2005.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Live birth rate Clinical pregnancy rate Miscarriage rate Multiple pregnancy rate Failure to transfer |
|
| Notes | Prognosis: high, 4 high‐quality embryos D3, young women Letter sent regarding concealment 100% ET rate | |
Papanikolaou 2006.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Live birth rate Clinical pregnancy rate Multiple pregnancy rate Miscarriage rate Embryo freezing rate Failure to transfer embryos |
|
| Notes | Prognosis: high, young women Letter sent regarding concealment, media and freezing | |
Rienzi 2002.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Live birth rate Clinical pregnancy rate Miscarriage rate Multiple pregnancy rate Embryo freezing rate Failure to transfer embryos |
|
| Notes | Prognosis: good; > 8 zygotes Letter sent and reply received | |
Schillaci 2002.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Clinical pregnancy rate | |
| Notes | Prognosis: unclear Abstract only | |
Singh 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Clinical pregnancy rate | |
| Notes | ||
Ten 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Clinical pregnancy rate but cumulative pregnancy rate later Number of frozen embryos |
|
| Notes | Abstract only | |
Van der Auwera 2002.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Live birth rate Cumulative clinical pregnancy rate Clinical pregnancy rate Multiple pregnancy rate Miscarriage rate Embryo freezing rate Failure to transfer embryos |
|
| Notes | Prognosis: mixed ‐ unselected Aim to reach highest cryoaugmented pregnancy rate Smaller cohort of embryos to choose from in D2 group due to freezing on day 1 New policy women with > 5 zygotes go to D5 transfer with 79% pregnancy rate | |
Yang 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
2PN: two‐pronuclear zygote AHA: assisted hatching ART: assisted reproductive technology Blast: blastocyst Blastocyst rate: number of blastocysts developed divided by number of 2PN embryos available BMI: body mass index BS: blastocyst stage CS: cleavage stage D2: embryo transfer on day 2 post‐OPU (i.e. early cleavage stage) D3: embryo transfer on day 3 post‐OPU D5: embryo transfer on day 5 post‐OPU (i.e. blastocyst stage) ET: embryo transfer FCS: foetal chord serum FSH: follicle stimulating hormone G1/G2: sequential media from Vitrolife GnRH: gonadotropin‐releasing hormone h: hour(s) high‐order: high‐order multiple pregnancy HCG: human chorionic gonadotropin (trigger injection that initiates ovulation and maturation of oocytes) ICSI: intracytoplasmic sperm injection IM: intramuscular injection IU/L: international units per litre IVF: in vitro fertilisation MII: metaphase II mIU/m: milli international units per millilitre morula: embryonic stage prior to blastocysts (usually embryos with delayed development on day 5) NS: not stated OPU: oocyte pick up Ov stim: ovarian stimulation regimen PGD: preimplantation genetic diagnosis rFSH: recombinant follicle stimulating hormone (fertility ovarian stimulation drug) #: number US: ultrasound
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bungum 2002 | Used co‐culture |
| Cornelisse 2018 | Non‐RCT; letter to the editor |
| Green 2016 | Ineligible study design: a retrospective cohort |
| Guerin 1991 | Used co‐culture |
| Holden 2017 | Ineligible study design: retrospective review |
| Levron 2001 | Quasi‐randomised RCT |
| Loup 2009 | Included transfer of embryos on two separate days within the same cycle |
| Menezo 1992 | Used co‐culture |
| Utsonomiya 2004 | Non‐randomised study (sequentially numbered) |
| Zech 2007 | Non‐randomised study; according to even or odd year of birth |
RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Clua Obrado 2020.
| Methods | Single center randomised controlled trial |
| Participants | Recipients between 18 and 50 years old in their first or second synchronous cycle Exclusion criteria: implantation failure and PGT‐A |
| Interventions | D3 or D5 embryo transfer |
| Outcomes | Clinical pregnancy rate and cumulative live birth rate |
| Notes | March 2017 ‐ August 2018 NCT 03088735 Barcelona, Spain The authors were contacted but they did not want to provide the missing information until the study was published in a peer‐reviewed journal. |
PGT‐A: preimplantation genetic testing for aneuploidies
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐ICR‐15006184.
| Study name | Cumulative live birth rates after cleavage‐stage versus blastocyst‐stage embryo transfer: a multicenter, prospective, randomized controlled trial |
| Methods |
|
| Participants |
|
| Interventions | Blastocyst transfer group: extending embryo culture 2‐3 days in vitro to the blastocyst stage Cleavage transfer group: no intervention |
| Outcomes | Cumulative birth rates within one year; embryo freezing rate; cumulative pregnancy rate per woman; miscarriage rate; neonatal outcomes; monozygotic twinning; multiple pregnancy rate per transfer; live birth rate/per embryo |
| Starting date | May 2015 |
| Contact information | Telephone:+86 025 68302608 Email: jyliu_nj@126.com |
| Notes |
ChiCTR‐ICR‐15006184 Recruitment: From2018‐09‐29To 2019‐08‐31 Jiangsu, China |
Cornelisse 2021.
| Study name | Three or Five Trial (ToF Trial) |
| Methods | For randomisation, all cases that subsequently meet all inclusion criteria will be randomised by the local laboratory staff, on the second day after fertilisation using the online software program Castor (V.2018.3.11, Castor Electronic Data Capture, Amsterdam, the Netherlands). Laboratory staff can access the online randomisation program using a unique password for this study. The laboratory staff is unable to access forthcoming random assignments prior to randomisation. Allocation to the cleavage‐stage embryo transfer arm or the blastocyst‐stage embryo transfer arm transfer will be based on a 1:1 randomisation with randomly selected block sizes of 2, 4 and 6 and stratification for age (≥ 36 years or < 36 years). Laboratory staff, clinicians and the participants cannot be blinded, due to the nature of the intervention. Participating clinicians, laboratory staff and investigators will not be able to access the randomisation sequence. |
| Participants | Women between 18 and 43 years of age, aiming to start an IVF treatment, are being selected for inclusion in this study. For inclusion and randomisation, at least four embryos should be available on culture day 2 (an embryo is defined as an oocyte with cell division on day 2 after insemination; ≥ three pronucleus embryos are excluded). A woman can participate in the study in her first, second or third IVF treatment, and can participate in only one treatment cycle. Women are excluded if they meet any of the following criteria: use of preimplantation genetic diagnosis or use of vitrified oocytes. No cycles with preimplantation genetic testing for aneuploidy will be part of this study as this procedure is not allowed in the Netherlands. |
| Interventions | (1) The control group, with embryo transfer on day 3 after oocyte retrieval and with cryopreservation of supernumerary good‐quality embryos on day 3 or 4 according to the local protocol and criteria, or (2) the intervention group, with embryo transfer on day 5 after oocyte retrieval with cryopreservation of supernumerary good‐quality embryos on day 5 or 6. Cryopreserved embryos on day 6 will only be transferred after all frozen–thawed embryo transfer(s) on day 5 have been transferred without an ongoing pregnancy. |
| Outcomes | The primary outcome is the cLBR per oocyte retrieval, which includes the results of the fresh and frozen–thawed embryo transfers. Endpoints of the study are live birth, no pregnancy leading to live birth after transfer of all available embryos or after a follow‐up time of 12 months after the oocyte retrieval. |
| Starting date | 28 August 2018 |
| Contact information | Ms Simone Cornelisse; simone.cornelisse@ radboudumc.nl |
| Notes | Netherlands Inclusions completed, results expected in 2023 |
ISRCTN48090543.
| Study name | Trial comparing blastocyst transfer with cleavage stage transfer in women with increased maternal age |
| Methods | Prospective randomised double‐blinded study |
| Participants | Inclusion criteria
|
| Interventions | Participants will be randomised on the day of ovum pick up by an independent operator to:
1. Blastocyst transfer:
1.1. Ovarian stimulation by agonist or antagonist protocol
1.2. Ovum pick up performed 36 hours after human chorionic gonadotropin (HCG) administration
1.3. In vitro fertilization performed by intracytoplasmic sperm injection (ICSI)
1.4. In vitro culture performed with sequential media in 6% CO2 and 5% O2 atmosphere
1.5. Embryo transfer on day 5 two best quality blastocyst. Remaining blastocyst preserved by vitrification procedure
1.6. Luteal support by progesterone 200 mg vaginally three times a day from oocyte retrieval plus one day 2. Cleavage stage transfer: 2.1. Ovarian stimulation by agonist or antagonist protocol 2.2. Ovum pick up performed 36 hours after human chorionic gonadotropin (HCG) administration 2.3. In vitro fertilization performed by intra‐cytoplasmic sperm injection (ICSI) 2.4. In vitro culture performed with sequential media in 6% CO2 and 5% O2 atmosphere 2.5. Embryo transfer on day 3 two best quality embryos. Remaining embryos preserved by vitrification procedure 2.6. Luteal support by progesterone 200 mg vaginally three times a day from oocyte retrieval plus one day |
| Outcomes | Cumulative live birth rate after blastocyst or cleavage stage strategy including pregnancies from fresh + cryoembryos transferred within 6 months after the end of the treatment |
| Starting date | 01 November 2011 |
| Contact information | Dr. Laura Rienzi: rienzi@generaroma.it |
| Notes |
NCT01107002.
| Study name | Comparison of 5 day embryo transfer with 2‐3 day transfer in patients who failed to conceive in two or more day 2‐3 embryo transfer cycle in Royan Institute |
| Methods | Prospective randomised clinical trial divided into two groups. Random permuted blocks with a block size of 4 was used and complete allocation concealment Single‐blind (outcomes assessor) |
| Participants | 200 participants with infertility Inclusion criteria: two or more previous failed IVF/ICSI cycles; 18 to 40 years |
| Interventions | Experimental: day 5 embryo transfer group Other group (day 2‐3) not described (only mentioned in the title and objectives) |
| Outcomes | Clinical pregnancy rate; live birth rate; implantation rate; miscarriage rate |
| Starting date | July 2008 |
| Contact information | www.royaninstitute.org |
| Notes | Sponsor: Royan Institute Study completion date: August 2010 ClinicalTrials.gov ID: NCT01107002 |
NCT02639000.
| Study name | Effects of blastocyst stage embryo transfer compared with cleavage stage embryo transfer in women ≤ 38 years |
| Methods |
|
| Participants |
|
| Interventions | Experimental: blastocyst: embryo transfer of at maximum 2 embryos at blastocyst stage Active comparator: cleavage: embryo transfer of at maximum 2 embryos at cleavage stage |
| Outcomes | Pregnancy rate; multiple pregnancy rate; implantation rate |
| Starting date | July 2010 |
| Contact information | Paolo Emanuele Levi‐Setti +39‐0282244505 paolo.levi_setti@humanitas.it |
| Notes | Study completion: April 2016 Sponsor: Istituto Clinico Humanitas ClinicalTrials.gov ID: NCT02639000 |
NCT04210414.
| Study name | Cleave‐stage transfer on day 3 versus day 5 transfer when only one embryo available (Cleave‐blast) |
| Methods | Study type: interventional (clinical trial) Estimated enrollment: 1100 participants Allocation: randomised Intervention model: parallel assignment Masking: triple (care provider, investigator, outcomes assessor) |
| Participants | Inclusion criteria: infertile couple with only one embryo available for transfer |
| Interventions | Experimental: day 3 transfer Transfer on day 3 when only one embryo is available Experimental: day 5 transfer Transfer on day 5 when only one embryo is available |
| Outcomes | Ongoing pregnancy rate [Time frame: within 20 weeks of gestation] |
| Starting date | 10 January 2020 |
| Contact information | Muhammad Fawzy drfawzy001@gmail.com |
| Notes |
Neuhausser 2020.
| Study name | Day 3 vs Day 5 Embryo Transfer for Patients With Low Embryo Numbers Going Through in Vitro Fertilization |
| Methods | Phase 2. Allocation: dandomized
Intervention model: parallel assignment
Intervention model description: non‐inferiority Masking: none (open label) Primary purpose: treatment |
| Participants | 18 to 44 years old Inclusion criteria
Exclusion Criteria
|
| Interventions | ‐ uterine transfer of embryo on day 3 after fertilisation (cleavage stage) ‐ uterine transfer of embryo on day 5 after fertilisation (blastocyst stage)
|
| Outcomes | Live birth [Time frame: 9 months] defined as delivery of a live born infant ≥ 22 weeks of gestation |
| Starting date | 1 January 2021 |
| Contact information | Werner Neuhausser, MD PhD (646) 510‐4825 wneuhaus@bidmc.harvard.edu |
| Notes |
PACTR201402000773124.
| Study name | Blastocyst versus day 2 transfer in low responders |
| Methods | Parallel: different groups receive different interventions at same time during study Randomised: permuted block randomisation (block size = 4) and the block size was not variable Sealed opaque envelopes |
| Participants | Inclusion criteria: poor response to ovarian stimulation as defined by European Society of Human Reproduction and Embryology (ESHRE) consensus (the Bologna criteria) (2011) Exclusion criteria: only one immature oocyte retrieve |
| Interventions | Blastocyst versus day 2 embryo transfer |
| Outcomes | Clinical pregnancy with cardiac pulsation |
| Starting date | 25 June 2013 |
| Contact information | elsayedamr@yahoo.com |
| Notes |
PACTR201709002592834.
| Study name | A randomized controlled trial of pregnancy outcome of sequential versus day 3 and day 5 embryo transfer in cases with recurrent implantation failure |
| Methods | RCT: simple randomisation using a randomised table created by a computer software program Sealed opaque envelopes |
| Participants | Age 35 years or less Normal endometrial cavity on hysteroscopy Recurrent (2 or more) implantation failure Negative thrombophilia screening No hydrosalpinx No endometriosis Day 3 FSH < 10 IU/L and Estradiol < 80 pg/mL Antimullerian hormone 1‐3 ng/mL Availability of at least 5 embryos on postfertilisation check |
| Interventions | Day 3 vs day 5 embryo transfer |
| Outcomes | Live birth rate |
| Starting date | 15 April 2015 |
| Contact information | haithamtorky@yahoo.com |
| Notes |
cLBR: cumulative live birth rate FSH: follicle‐stimulating hormone HCG: human chorionic gonadotropin ICSI: intracytoplasmic sperm injection IU: international units IVF: in vitro fertilisation LBR: live birth rate MII: metaphase II NA: not available
Differences between protocol and review
We included only RCTs and excluded quasi‐RCTs (2007 update).
We excluded couples or women where frozen‐thawed cycle results were shown, but no data were available from the fresh cycle (2016 update).
We added cumulative pregnancy rate to the primary outcomes (previously a secondary outcome) (2007 update).
We restricted subgroup and sensitivity analyses to specific clinical outcomes: live birth, cumulative pregnancy, clinical pregnancy, and multiple pregnancy (2007 update).
We added a post hoc subgroup analysis according to freezing technique, to investigate statistical heterogeneity for the outcome cumulative pregnancy rate (2016 update).
We shortened the objectives to the current wording (2012 update).
We included a subgroup analysis comparing time‐lapse system screening (TLS screening) on cleavage‐stage transfer versus blastocyst‐stage transfer (with and without time‐lapse screening), because TLS was not available when this protocol was originally published and the combination of stage of embryo and TLS could provide a different result (2022 update).
We assessed the risk of bias with the Cochrane risk of bias 2 (RoB 2) tool (Sterne 2019, 2022 update).
We excluded cluster‐RCTs because these types of trials are not appropriate in this context (2022 update).
Contributions of authors
Debbie Blake: for the initial review and updates to 2005: took the lead in writing the protocol and review; performed initial searches of databases for trials; involved in selecting trials for inclusion; performed independent data extraction and quality assessment of the included trials; and was responsible for statistical analysis and interpretation of the data. Also contributed to the final analysis and text of the 2012, 2016, and 2022 updates.
Demián Glujovsky: for the 2012, 2016 and 2021 updates: took the lead in writing the update of the review; performed new searches of databases for trials; involved in selecting trials for inclusion; performed independent data extraction and quality assessment of the included trials; and was responsible for statistical analysis and interpretation of the data in the update.
Andrea Quinteiro Retamar: for the 2016 and 2021 updates: involved in selecting trials for inclusion; and performed independent data extraction and quality assessment of the included trials.
Cristian Alvarez Sedo: for the final analysis and text of the 2016 update, and for the 2021 update: involved in selecting trials for inclusion; and performed independent data extraction and quality assessment of the included trials.
Simone Cornelisse: for the 2021 update: involved in the risk of bias assessment, data extraction and final analysis and text.
Agustín Ciapponi: for the 2021 update: involved in the risk of bias assessment, and final analysis and text.
Sources of support
Internal sources
-
Cindy Farquhar, New Zealand
University of Auckland
External sources
-
None, Other
None
Declarations of interest
Demián Glujovsky is part of medical staff of a fertility clinic and undertakes private practice within those premises.
Andrea Quinteiro Retamar is the egg donor coordinator of a fertility clinic and undertakes private practice within those premises.
Simone Cornelisse is the lead author of excluded study Cornelisse 2018 and took no part in assessing it for this review.
Cristian Alvarez Sedo, Agustín Ciapponi, and Deborah Blake have no conflicts of interest to declare.
Edited (no change to conclusions)
References
References to studies included in this review
Aziminekoo 2015 {published data only}
- Aziminekoo E, Mohseni Salehi MS, Kalantari V, Shahrokh Tehraninejad E, Haghollahi F, Hossein Rashidi B, et al. Pregnancy outcome after blastocyst stage transfer comparing to early cleavage stage embryo transfer. Gynecological Endocrinology 2015;31(11):880-4. [DOI] [PubMed] [Google Scholar]
Brugnon 2010 {published data only}
- Brugnon F, Bouraoui Z, Ouchchane L, Gremeau AS, Peikrishvili R, Pouly JL, et al. Cumulative pregnancy rates after single cleavage-stage versus blastocyst-stage embryo transfer: a randomized and prospective study. Human Reproduction 2010;25:i60-1. [Google Scholar]
Bungum 2003 {published data only}
- Bungum M, Bungum L, Humaidan P, Yding Andersen C. Day 3 versus day 5 embryo transfer: a prospective randomized study. Reproductive Biomedicine Online 2003;7(1):98-104. [DOI] [PubMed] [Google Scholar]
Coskun 2000 {published data only}
- Coskun S, Hollanders J, Al-Hassan S, Al-Sufyan H, Al-Mayman H, Jaroudi K. Day 5 versus day 3 embryo transfer: a controlled randomized trial. Human Reproduction 2000;15(9):1947-52. [DOI] [PubMed] [Google Scholar]
Devreker 2000 {published data only}
- Devreker F, Delbaere A, Emiliani S, Van den Bergh M, Biramane J, Englert Y. Prospective and randomized comparison between transfer on day 2 or day 5 for patients with more than four IVF attempts. Human Reproduction 2000;15(Supp 1):151-2. [Google Scholar]
Elgindy 2011 {published data only}
- Elgindy EA, Abou-Setta AM, Mostafa MI. Blastocyst-stage versus cleavage-stage embryo transfer in women with high oestradiol concentrations: randomized controlled trial. Reproductive Biomedicine Online 2011;23(6):789-98. [PMID: ] [DOI] [PubMed] [Google Scholar]
Emiliani 2003 {published data only}
- Emiliani S, Delbaere A, Vannin A, Biramane J, Verdoodt M, Englert Y, et al. Similar delivery rates in a selected group of patients, for day 2 and day 5 embryos both cultured in sequential medium: a randomized study. Human Reproduction 2003;18(10):2145-50. [DOI] [PubMed] [Google Scholar]
Fernandez‐Shaw 2015 {published data only}
- Fernandez-Shaw S, Cercas R, Brana C, Villas C, Pons I. Ongoing and cumulative pregnancy rate after cleavage-stage versus blastocyst-stage embryo transfer using vitrification for cryopreservation: impact of age on the results. Journal of Assisted Reproduction and Genetics 2015;32(2):177-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Shaw S. Unpublished data including live birth and cumulative live birth rates [personal communication]. Email to: Demián Glujovsky 6 May 2016.
Fisch 2007 {published data only}
- Fisch JD, Adamowicz M, Hackworth J, Ginsburg M, Keskintepe L, Sher G. Single embryo transfer (SET) day 3 vs day 5 based on graduated embryo score (GES) and soluble human leukocyte antigen-G (sHLA-G): preliminary results of a prospective, randomized controlled trial. Fertility and Sterility 2007;88(Suppl 1):332-3, abstract no. 679. [Google Scholar]
Frattarelli 2003 {published data only}
- Frattarelli JL, Leondires MP, McKeeby JL, Miller BT, Segars JH. Blastocyst transfer decreases multiple pregnancy rates in in vitro fertilization cycles: a randomized controlled trial. Fertility and Sterility 2003;79(1):228-30. [DOI] [PubMed] [Google Scholar]
Gaafar 2015 {published data only}
- Gaafar SH, El Fourtia I, El Tawil S, El Guiziry D, El Maghraby H. Blastocyst versus day 2-3 transfer in ICSI cycles for male factor infertility: a multicenter randomized controlled trial. Human Reproduction 2015;30(Suppl 1):i217, abstract no. P-223. [Google Scholar]
Gardner 1998a {published data only}
- Gardner DK, Schoolcraft WB, Wagley L, Schlenker T, Stevens J, Hesla J. A prospective randomized trial of blastocyst culture and transfer in in-vitro fertilization. Human Reproduction 1998;13(12):3434-40. [DOI] [PubMed] [Google Scholar]
Hatirnaz 2017 {published data only}
- Hatirnaz S, Pektas MK. Day 3 embryo transfer versus day 5 blastocyst transfers: a prospective randomized controlled trial. Turkish Journal of Obstetrics and Gynecology 2017;14(2):82-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hreinsson 2004 {published data only}
- Hreinsson J, Rosenlund B, Fridstrom M, Ek I, Levkov L, Sjoblom P, et al. Embryo transfer is equally effective at cleavage stage and blastocyst stage: a randomized prospective study. European Journal of Obstetrics, Gynecology and Reproductive Biology 2004;117:194-200. [DOI] [PubMed] [Google Scholar]
Karaki 2002 {published data only}
- Karaki RZ, Samarraie SS, Younis NA, Lahloub TM, Ibrahim MH. Blastocyst culture and transfer: a step toward improved in vitro fertilization outcome. Fertility and Sterility 2002;77(1):114-8. [DOI] [PubMed] [Google Scholar]
Kaser 2017 {published data only}
- Kaser DJ, Bormann CL, Missmer SA, Farland LV, Ginsburg ES, Racowsky C. A pilot randomized controlled trial of Day 3 single embryo transfer with adjunctive time-lapse selection versus Day 5 single embryo transfer with or without adjunctive time-lapse selection. Human Reproduction 2017;32(8):1598-1603. [DOI] [PubMed] [Google Scholar]
- Kaser DJ, Bormann CL, Missmer SA, Farland LV, Ginsburg ES, Racowsky C. Eeva™ pregnancy pilot study: a randomized controlled trial of single embryo transfer (SET) on day 3 or day 5 with or without time-lapse imaging (TLI) selection. Fertility and Sterility 2016;106(3 (suppl 1)):e312. [Google Scholar]
Kaur 2014 {published data only}
- Kaur P, Swarankar ML, Maheshwari M, Acharya V. A comparative study between cleavage stage embryo transfer at day 3 and blastocyst stage transfer at day 5 in in-vitro fertilization/intra-cytoplasmic sperm injection on clinical pregnancy rates. Journal of Human Reproductive Sciences 2014;7(3):194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Swarankar ML, Maheshwari M, Sharma S. Implantation rates after two and five days of embryo culture: a comparative study. JK Science 2013;15(4):185-8. [Google Scholar]
Kolibianakis 2004 {published data only}
- Kolibianakis EM, Zilopoulos K, Verpoest W, Camus M, Joris H, Van Steirteghem AC, et al. Should we advise patients undergoing in-vitro fertilization to start a cycle leading to a day 3 or day 5 transfer? Human Reproduction 2004;19:2550-4. [DOI] [PubMed] [Google Scholar]
Levi‐Setti 2018 {published data only}
- Levi-Setti PE, Cirillo F, Smeraldi A, Morenghi E, Mulazzani GE, Albani E. No advantage of fresh blastocyst versus cleavage stage embryo transfer in women under the age of 39: a randomized controlled study. Human Reproduction 2018;35(3):457-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levitas 2004 {published data only}
- Levitas E, Lunenfeld E, Hackmon Ram R, Sonin Y, Har Vardi I, Potashnik G. A prospective, randomized study comparing blastocyst stage versus 48-72 hr embryo transfer in women failed to conceive three or more in-vitro fertilization treatment cycles. Fertility and Sterility 2001;76(3 (suppl 1)):S118. [DOI] [PubMed] [Google Scholar]
- Levitas E, Lunenfeld E, Har-Vardi I, Albotiano S, Sonin Y, Hackmon-Ram R, et al. Blastocyst-stage embryo transfer in patients who failed to conceive in three or more day 2-3 embryo transfer cycles: a prospective, randomized study. Fertility and Sterility 2004;81(3):567-71. [DOI] [PubMed] [Google Scholar]
Levron 2002 {published data only}
- Levron J, Shulman A, Bider D, Seidman D, Levin T, Dor J. A prospective randomized study comparing day 3 with blastocyst-stage embryo transfer. Fertility and Sterility 2002;77:1300-1. [DOI] [PubMed] [Google Scholar]
Livingstone 2002 {published and unpublished data}
- Livingstone M, Bowman M. Single blastocyst transfer: a prospective randomised trial. In: 17th World Congress on Fertility and Sterility; 2001 Nov 25-30; Melbourne, Australia. Royal (NJ): International Federation of Fertility Societies:218.
Motta 1998 {published data only}
- Motta LA, Alegretti JR, Pico M, Sousa JW, Baracat EC, Serafini P. Blastocyst vs. cleaving embryo transfer: a prospective randomized trial. Fertility and Sterility 1998;70(Suppl 1):17. [Google Scholar]
Pantos 2004 {published data only}
- Pantos K, Makrakis E, Stavrou D, Karantzis P, Vaxevanoglou T, Tzigounis V. Comparison of embryo transfer on day 2, day 3, and day 6: a prospective randomized study. Fertility and Sterility 2004;81(2):454-5. [DOI] [PubMed] [Google Scholar]
Papanikolaou 2005 {published data only}
- Papanikolaou EG, D'haeseleer E, Verheycn G, Van de Velde H, Camus M, Van Steirteghem A, et al. Live birth rate is significantly higher after blastocyst transfer than after cleavage-stage transfer when at least four embryos are available on day 3 of culture. A randomized prospective study. Human Reproduction 2005;20(11):3198-203. [DOI] [PubMed] [Google Scholar]
- Papanikolaou EG Sr, Verheyen G, Camus M, Van Steirteghem A, Devroey P, Tournaye H. Ongoing pregnancy rate is significantly higher with day 5 embryo transfer than after day 3 embryo transfer, when more than three embryos are available on the third day of embryo culture. Fertility and Sterility 2005;84(Suppl 1):s51. [Google Scholar]
Papanikolaou 2006 {published data only}
- Papanikolaou EG, Camus M, Kolibianakis EM, Landuyt LV, Steirteghem AV, Devroey P. In vitro fertilization with single blastocyst-stage versus cleavage-stage embryos. New England Journal of Medicine 2006;354(11):1139-46. [DOI: 10.1056/NEJMoa053524] [DOI] [PubMed] [Google Scholar]
- Papanikolaou EG, Camus M, Kolibianakis EM, Turki H, Van Landuyt L, Van Steirteghem A, et al. Single embryo transfer: comparison of cleavage stage embryo transfer with blastocyst stage embryo transfer. A randomized prospective study. Human Reproduction 2005;20(suppl_1):i144-5. [Google Scholar]
Rienzi 2002 {published data only}
- Rienzi l, Ubaldi F, Lacobelli M, Ferrero S, Minasi MG, Martinez F, et al. Day 3 embryo transfer with combined evaluation at the pronuclear and cleavage stages compares favourably with day 5 blastocyst transfer. Human Reproduction 2002;17:1852-5. [DOI] [PubMed] [Google Scholar]
Schillaci 2002 {published data only}
- Schillaci R, Castelli A, Vassiliadis A, Venezia R, Sciacca GM, Perino A, et al. Blastocyst stage versus day 2 embryo transfer in IVF cycles. Human Reproduction 2002;17(Supp 1):143. [Google Scholar]
Singh 2017 {published data only}
- Singh R, Singh M, Jindal A, Jindal P. A randomised controlled trial comparing the cost-effectiveness of blastocyst (Day 5/6) versus cleavage stage (Day 3) embryo transfers in IVF-ICSI cycles in developing countries. Human Reproduction 2017;32 Suppl 1:i483-4. [Google Scholar]
- Singh R, Singh M. A prospective randomised controlled study comparing the cost effectiveness of IVF-ICSI treatment: cleavage stage (day 3) embryo transfer versus extended culture (day 5/6 blastocyst) transfer. Fertility and Sterility 2013;100(3):S289. [Google Scholar]
Ten 2011 {published data only}
- Ten J, Carracedo MA, Guerrero J, Rodriguez-Arnedo A, Llacer J, Bernabeu R. Day 3 or day 5 embryo transfer? A randomized prospective study. Human Reproduction 2011;26(Suppl 1):i165 Abstract no: P‐109. [Google Scholar]
Van der Auwera 2002 {published data only}
- Van der Auwera I, Debrock S, Spiessens C, Afschrift H, Bakelants E, Meuleman C, et al. A prospective randomized study: day 2 versus day 5 embryo transfer. Human Reproduction 2002;17(6):1507-12. [DOI] [PubMed] [Google Scholar]
Yang 2018 {published data only}
- ChiCTR-ICR-15006600. Using time-lapse technology for single embryo transfer: a prospective randomized controlled study. www.chictr.org.cn/hvshowproject.aspx?id=11293 (first posted 16 June 2015).
- Yang L, Cai S, Zhang S, Kong X, Gu Y, Lu C, et al. Single embryo transfer by Day 3 time-lapse selection versus Day 5 conventional morphological selection: a randomized, open-label, non-inferiority trial. Human Reproduction 2018;33(5):869-76. [DOI] [PubMed] [Google Scholar]
- Yang L, Kong X, Zhang S, Dai J, Gong F, Lu G, Lin G. Single embryo transfer on cleavage-stage (D3) using timelapse selection versus on blastocyst(D5) using traditional morphological selection in patients with good prognosis: a prospective randomized controlled trial. Human Reproduction 2017;32 Suppl 1:i102-3. [Google Scholar]
References to studies excluded from this review
Bungum 2002 {published data only}
- Bungum L, Bungum M, Humaiden P. Blastocyst stage transfer is not better than embryo transfer on day 3: a prospective randomized study. Human Reproduction 2002;17(Suppl 1):53.
Cornelisse 2018 {published data only}
- Cornelisse S, Fleischer K, Repping S, Mastenbroek S. An informed decision between cleavage-stage and blastocyst-stage transfer in IVF requires data on the transfers of frozen-thawed embryos. Human Reproduction 2018;33:1370. [DOI] [PubMed] [Google Scholar]
Green 2016 {published data only}
- Green KA, Patounakis G, DeCherney A, Graham J, Tucker MJ, Widra EA, et al. Day 3 embryo transfer (ET) versus pushing to day 5 in patients with few embryos. Fertility and Sterility 2016;106:e165. [Google Scholar]
Guerin 1991 {published data only}
- Guerin JF, Mathieu C, Pinatel MC, Reginier-Vigouroux G, Lornage J, Boulieu D, et al. Coculture of human embryos with monkey kidney epithelial cells: clinical data concerning transfers delayed at D3 and D5. Contraception, Fertilite, Sexualite 1991;19(7-8):635-8. [Google Scholar]
Holden 2017 {published data only}
- Holden EC, Kashani BN, Morelli S, Alderson D, Jindal SK, McGovern PG. Perinatal outcomes are similar in blastocyst compared to cleavage stage frozen-thawed embryo transfers: a SARTCORS study. Fertility and Sterility 2017;108:e38. [DOI] [PubMed] [Google Scholar]
Levron 2001 {published data only}
- Levron J, Bider D, Shulman A, Rabinovichi Y, Seidman D, Dor J. A randomized prospective study on blastocyst versus day 2-3 embryo transfer. Fertility and Sterility 2001;Suppl 1(3):4. [Google Scholar]
Loup 2009 {published data only}
- Loup V, Anahory T, Reyftmann L, Dechaud H, Hedon B, Hamamah S. Efficiency of consecutive embryos transfer on day 3 and day 5 than replacement of 2 embryos on day 3 for women over 37 years: prospective study. Molecular Human Reproduction 2009;24:i139. [Google Scholar]
Menezo 1992 {published data only}
- Menezo Y, Hazout A, Dumont M, Herbaut N, Nicollet B. Coculture of embryos on Vero cells and transfer of blastocysts in humans. Human Reproduction 1992;7(Suppl 1):101-6. [DOI] [PubMed] [Google Scholar]
Utsonomiya 2004 {published data only}
- Utsunomiya T, Ito H, Nagaki M, Sato J. A prospective, randomised study: day 3 versus hatching blastocyst stage. Human Reproduction 2004;19:1598-1603. [DOI] [PubMed] [Google Scholar]
Zech 2007 {published data only}
- Vanderzwalmen P, Lejeune B, Puissant F, Vanderzwalmen S, Zech H, Zintz M, et al. A prospective evaluation of the optimal time for selecting a single embryo for transfer: day 3 vs. day 5. Human Reproduction 2006;Suppl:i80-1. [DOI] [PubMed] [Google Scholar]
- Zech NH, Lejeune B, Puissant F, Vanderzwalmen S, Zech H, Vanderzwalmen P. Prospective evaluation of the optimal time for selecting a single embryo for transfer: day 3 versus day 5. Fertility and Sterility 2007;88(1):244-6. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Clua Obrado 2020 {published data only}
- Clua Obrado E, Rodriguez I, Arroyo G, Martinez F, Latre L, Coroleu B, et al. Cleavage stage embryo transfer impairs cumulative live birth rates and time to livebirth as compared to blastocyst transfer in oocyte recipients. A randomized controlled trial. Human Reproduction 2020;35(Suppl 1):i2-i3. [Google Scholar]
References to ongoing studies
ChiCTR‐ICR‐15006184 {published data only}
- ChiCTR-ICR-15006184. Cumulative live birth rates after cleavage-stage versus blastocyst-stage embryo transfer: a multicenter, prospective, randomized controlled trial. www.chictr.org.cn/showprojen.aspx?proj=10698 (first received 29 March 2015).
Cornelisse 2021 {published data only}
- Cornelisse S, Ramos L, Arends B, Brink-van der Vlugt JJ, Bruin JP, et al. Comparing the cumulative live birth rate of cleavage-stage versus blastocyst-stage embryo transfers between IVF cycles: a study protocol for a multicentre randomised controlled superiority trial (the ToF trial). BMJ Open 2021;11:e042395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTR7034. ToF-studie Embryo transfer, day Three Or day Five, in good prognosis IVF cycles. www.who.int/trialsearch/Trial2.aspx?TrialID=NTR7034 (first received 19 February 2018).
ISRCTN48090543 {published data only}
- ISRCTN48090543. Trial comparing blastocyst transfer with cleavage stage transfer in women with increased maternal age [Blastocyst stage vs cleavage stage transfer in women with increased maternal age: a prospective randomised controlled trial]. trialsearch.who.int/?TrialID=ISRCTN48090543 (first received 28 December 2011).
NCT01107002 {published data only}
- NCT01107002. Comparison of 5 day embryo transfer with 2-3 day transfer in patients with previous in vitro fertilization failure. clinicaltrials.gov/ct2/show/NCT01107002 (first received 20 April 2010).
NCT02639000 {published data only}
- NCT02639000. Effects of blastocyst stage compared with cleavage stage embryo transfer in women below 39 years. clinicaltrials.gov/show/NCT02639000 (first received 23 December 2015).
NCT04210414 {published data only}
- NCT04210414. Cleave-stage transfer on day 3 versus day 5 transfer when only one embryo available (Cleave-blast). clinicaltrials.gov/show/NCT04210414 (first received 24 December 2019).
Neuhausser 2020 {published data only}
- Neuhausser WM, Vaughan DA, Sakkas D, Hacker MR, Toth T, Penzias A. Non-inferiority of cleavage-stage versus blastocyst-stage embryo transfer in poor prognosis IVF patients (PRECiSE trial): study protocol for a randomized controlled trial. Reproductive Health 2020;17(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
PACTR201402000773124 {published data only}
- PACTR201402000773124. Blastocyst versus day 2 transfer in low responders. www.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201402000773124 (first received 18 February 2014).
PACTR201709002592834 {published data only}
- PACTR201709002592834. A randomized controlled trial of pregnancy outcome of sequential versus day 3 and day 5 embryo transfer in cases with recurrent implantation failure. www.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201709002592834 (first received 7 September 2019).
Additional references
Alviggi 2018
- Alviggi C, Conforti A, Carbone IF, Borrelli R, Placido G, Guerriero S. Influence of cryopreservation on perinatal outcome after blastocyst- vs cleavage-stage embryo transfer: systematic review and meta-analysis. Ultrasound in Obstetrics and Gynecology 2018;51(1):54-63. [DOI] [PubMed] [Google Scholar]
Armstrong 2019
- Armstrong S, Bhide P, Jordan V, Pacey A, Marjoribanks J, Farquhar C. Time-lapse systems for embryo incubation and assessment in assisted reproduction. Cochrane Database of Systematic Reviews 2019, Issue 5. Art. No: CD011320. [DOI: 10.1002/14651858.CD011320.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baart 2006
- Baart EB, Martini E, Van den Berg I, Macklon NS, Galjaard RJ, Fauser BC, et al. Preimplantation genetic screening reveals a high incidence of aneuploidy and mosaicism in embryos from young women undergoing IVF. Human Reproduction 2006;21(1):223-33. [DOI] [PubMed] [Google Scholar]
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials: the CONSORT statement. JAMA 1996;276:637-9. [DOI] [PubMed] [Google Scholar]
Behr 2000
- Behr B, Fisch JD, Racowsky C, Miller K, Pool TB, Milki AA. Blastocyst-ET and monozygotic twinning. Journal of Assisted Reproduction and Genetics 2000;17(6):349-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Borg 2000
- Borg K, Moller A, Hammar M, Blake D, Hillensjo T, Wikland M. Blastocyst culture - more or less stressful for patients? In: European Society of Human Reproduction and Enbryology (ESHRE). Bologna, 2000:48.
Braude 1998
- Braude P, Bolton V, Moore S. Human gene expression first occurs between the four and eight-cell stages of preimplantation development. Nature 1988;332:459-61. [DOI] [PubMed] [Google Scholar]
Busnelli 2019
- Busnelli A, Dallagiovanna C, Reschini M, Paffoni A, Fedele L, Somigliana E. Risk factors for monozygotic twinning after in vitro fertilization: a systematic review and meta-analysis. Fertility and Sterility 2019;111(2):302-17. [DOI] [PubMed] [Google Scholar]
Cohen 1990
- Cohen J, Elsner C, Kort HM. Impairment of hatching process following IVF in the human and improvement of implantation by assisted hatching using micromanipulation. Human Reproduction 1990;5:7-13. [DOI] [PubMed] [Google Scholar]
Cornelisse 2018
- Cornelisse S, Fleischer K, Repping S, Mastenbroek S. An informed decision between cleavage-stage and blastocyst-stage transfer in IVF requires data on the transfers of frozen–thawed embryos. Human Reproduction 2018;33(7):1370. [DOI] [PubMed] [Google Scholar]
Croxatto 1972
- Croxatto HB, Fuentaealba B, Diaz S, Pastene L, Tatum HJ. A simple non-surgical technique to obtain unimplanted eggs from human uteri. American Journal of Obstetrics and Gynecology 1972;112(5):662-8. [DOI] [PubMed] [Google Scholar]
De Felici 1982
- De Felici M, Siracusa G. Spontaneous hardening of the zona pellucida of mouse oocytes during in vitro culture. Gamete Research 1982;6:107-13. [Google Scholar]
De Placido 2002
- De Placido G, Wilding M, Strina I, Alviggi E, Alviggi C, Mollo A, et al. High outcome predictability after IVF using a combined score for zygote and embryo morphology and growth rate. Human Reproduction 2002;17(9):2402-9. [DOI] [PubMed] [Google Scholar]
Edgar 2012
- Edgar DH, Gook DA. A critical appraisal of cryopreservation (slow cooling versus vitrification) of human oocytes and embryos. Human Reproduction Update 2012;18(5):536-54. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Edwards 1995
- Edwards RG, Brody SA. History and ethics of assisted human conception. In: Principles and Practice of Assisted Human Reproduction. Philadelphia (PA): WB Sauders, 1995:17-47. [Google Scholar]
Fanchin 2001
- Fanchin R. Assessing uterine receptivity in 2001: Ultrasonographic glances at the new millennium. Annals of the New York Academy of Sciences 2001/10/01;943:185-202. [DOI] [PubMed] [Google Scholar]
Gardner 1996
- Gardner DK, Lane M, Calderon I, Leeton J. Environment of the preimplantation human embryo in vivo: metabolite analysis of oviduct and uterine fluids and metabolism of cumulus cell. Fertility and Sterility 1996;65(2):349-53. [DOI] [PubMed] [Google Scholar]
Gardner 1998b
- Gardner DK, Vella P, Lane M, Wagley L, Schlenker T, Schoolcraft WB. Culture and transfer of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertility and Sterility 1998;69(1):84-8. [DOI] [PubMed] [Google Scholar]
Gardner 2003
- Gardner DK, Lane M, Stevens J, Schoolcraft WB. Changing the start temperature and cooling rate in a slow-freezing protocol increases human blastocyst viability. Fertility and Sterility 2003;79:407-10. [DOI] [PubMed] [Google Scholar]
Gardner 2004
- Gardner DK, Surry E, Minjarez D, Leitz A, Stevens J, Schoolcraft WB. Single blastocyst transfer a prospective randomised trial. Fertility and Sterility 2004;81:551-5. [DOI] [PubMed] [Google Scholar]
Ginström 2019
- Ginström EE, Spangmose AL, Opdahl S, Henningsen A-K, Romundstad LB, Tiitinen A, et al. Perinatal and maternal outcome after vitrification of blastocysts: a Nordic study in singletons from the CoNARTaS group. Human Reproduction 2019;34(11):2282-9. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 17 April 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Griesinger 2016
- Greisinger G. Beware of the implantation rate. Human Reproducton 2016;31(2):249-51. [DOI] [PubMed] [Google Scholar]
Hamberger 2005
- Hamberger L, Hardarson T, Nygren KG. Avoidance of multiple pregnancy by use of single embryo transfer. Minerva Ginecologica 2005;57:15-9. [PubMed] [Google Scholar]
Higgins 2019a
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Higgins 2019b
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Iwayama 2011
- Iwayama H, Hochi S, Yamashita M. In vitro and in vivo viability of human blastocysts collapsed by laser pulse or osmotic shock prior to vitrification. Journal of Assisted Reproduction and Genetics 2011;28(4):355-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jain 2004
- Jain JK, Boostanfar R, Slater CC, Francis MM, Paulson RJ. Monozygotic twins and triplets in association with blastocyst transfer. Journal of Assisted Reproduction and Genetics 2004;21(4):103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 1999
- Jones GM, Trounson AO. The benefits of extended culture. Human Reproduction 1999;14(6):1405-8. [DOI] [PubMed] [Google Scholar]
Jones 2008
- Jones GM, Cram DS, Song B, Kokkali G, Pantos K, Trounson AO. Novel strategy with potential to identify developmentally competent IVF blastocysts. Human Reproduction 2008;23(8):1748-59. [DOI] [PubMed] [Google Scholar]
Kamath 2020
- Kamath MS, Mascarenhas M, Kirubakaran R, Bhattacharya S. Number of embryos for transfer following in vitro fertilisation or intra-cytoplasmic sperm injection. Cochrane Database of Systematic Reviews 2020, Issue 8. Art. No: CD003416. [DOI: 10.1002/14651858.CD003416.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Laverge 2001
- Laverge H, De Sutter P, Van der Elst J, Dhont M. A prospective, randomized study comparing day 2 and day 3 embryo transfer in human IVF. Human Reproduction 2001;16(3):476-80. [DOI] [PubMed] [Google Scholar]
Luna 2007
- Luna M, Duke M, Copperman A, Grunfeld L, Sandler B, Barritt J. Blastocyst embryo transfer is associated with a sex-ratio imbalance in favor of male offspring. Fertility and Sterility 2007;87:519-23. [DOI] [PubMed] [Google Scholar]
Magli 1998
- Magli MC, Gianaroli L, Munne S, Ferraretti AP. Incidence of chromosomal abnormalities from a morphologically normal cohort of embryos in poor-prognosis patients. Journal of Assisted Reproduction and Genetics 1998;15(5):297-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magli 2000
- Magli MC, Jones GM, Gras L, Gianaroli L, Korman I, Trounson A. Chromosome mosaicism in day 3 aneuploid embryos that develop to morphologically normal blastocysts in vitro. Human Reproduction 2000;15:1781-6. [DOI] [PubMed] [Google Scholar]
Maheshwari 2016
- Maheshwari A, Hamilton M, Bhattacharya S. Should we be promoting embryo transfer at blastocyst stage? Reproductive BioMedicine Online 2016;32(2):142-6. [DOI] [PubMed] [Google Scholar]
Marek 1999
- Marek D, Langley M, Gardner DK, Confer N, Doody KM, Doody KJ. Introduction of blastocyst culture and transfer for all patients in an in vitro fertilization program. Fertility and Sterility 1999;72(6):1035-40. [DOI] [PubMed] [Google Scholar]
Marston 1977
- Marston JH, Penn R, Sivelle PC. Successful autotransfer of tubal eggs in the rhesus monkey (Macaca mulatta). Journal of Reproduction and Fertility 1977;49:175-6. [DOI] [PubMed] [Google Scholar]
Martins 2016
- Martins WP, Nastri CO, Rienzi L, Van der Poel SZ, Gracia CR, Racowsky C. Obstetrical and perinatal outcomes following blastocyst transfer compared to cleavage transfer: a systematic review and meta-analysis. Human Reproduction 2016;31(11):2561-9. [DOI] [PubMed] [Google Scholar]
Martins 2017
- Martins WP, Nastri CO, Rienzi L, Van der Poel SZ, Gracia C, Racowsky C. Blastocyst vs cleavage-stage embryo transfer: systematic review and meta-analysis of reproductive outcomes. Ultrasound in Obstetrics & Gynecology 2017;49(5):583-91. [DOI] [PubMed] [Google Scholar]
Menezo 1990
- Menezo YJ, Guerin JF, Czyba JC. Improvement of human embryo development in vitro by coculture on monolayers of Vero cells. Biology of Reproduction 1990;42(2):301-6. [DOI] [PubMed] [Google Scholar]
Menezo 1999
- Menezo YJ, Chouteau J, Torello J, Girard A, Veiga A. Birth weight and sex ratio after transfer at the blastocyst stage in humans. Fertility and Sterility 1999;72(2):221-4. [DOI] [PubMed] [Google Scholar]
Milki 1999
- Milki AA, Fisch JD, Behr B. Two-blastocyst transfer has similar pregnancy rates and a decreased multiple gestation rate compared with three-blastocyst transfer. Fertility and Sterility 1999;72(2):225-8. [DOI] [PubMed] [Google Scholar]
Milki 2004
- Milki A, Hinckley M, Westphal L, Behr B. Elective single blastoyst transfer. Fertility and Sterility 2004;81:1697-8. [DOI] [PubMed] [Google Scholar]
Moayeri 2007
- Moayeri S, Behr B, Lathi R, Westphal L, Milki A. Risk of monozygotic twinning with blastocyst transfer decreases over time: an 8 year experience. Fertility and Sterility 2007;87(5):1028-32. [DOI] [PubMed] [Google Scholar]
Munne 2002
- Munne S, Sandalinas M, Escudero T, Marquez C, Cohen J. Chromosome mosaicism in cleavage-stage human embryos: evidence of a maternal age effect. Reproductive Biomedicine Online 2002;4(3):223-32. [DOI] [PubMed] [Google Scholar]
Nel‐Themaat 2011
- Nel-Themaat L, Nagy ZP. A review of the promises and pitfalls of oocyte and embryo metabolomics. Placenta 2011;32(3):S257-63. [DOI] [PubMed] [Google Scholar]
Palmstierna 1998
- Palmstierna M, Murkes D, Csemizdy G, Andersson O, Wramsby H. Zona pellucida thickness variation and occurrence of visible mononucleated blastomeres in preembryos are associated with a high pregnancy rate in IVF treatments. Journal of Assisted Reproduction and Genetics 1998;15(2):70-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Papanikolaou 2008
- Papanikolaou EG, Kolibianakis EM, Tournaye H, Venetis CA, Fatemi H, Tarlatzis B, et al. Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in IVF. A systematic review and meta-analysis. Human Reproduction 2008;23(1):91-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Plachot 1999
- Plachot M, Mayenga JM, Chouraqui A, Tesquier L, Serkine AM, Belaisch-Allart J. P-150. A prospective semi-randomized study of blastocyst transfer in an IVF programme. Human Reproduction 1999;14(Suppl_3):215-6. [Google Scholar]
Puissant 1987
- Puissant F, Van Rysselberge M, Barlow P, Deweze J, Leroy F. Embryo scoring as a prognostic tool in IVF treatment. Human Reproduction 1987;2(8):705-8. [DOI] [PubMed] [Google Scholar]
RevMan Web 2020 [Computer program]
- Review Manager Web (RevMan Web). Version 1.22.0. Nordic Cochrane Centre, The Cochrane Collaboration, 2020. Available at https://revman.cochrane.org/.
Roque 2015
- Roque M, Valle M, Guimaraes F, Sampaio M, Geber S. Freeze-all policy: fresh vs. frozen-thawed embryo policy. Fertility and Sterility 2015;103(5):1190-3. [doi: 10.1016/j.fertnstert.2015.01.045. Epub 2015 Mar 4.] [DOI] [PubMed] [Google Scholar]
Roseboom 1995
- Roseboom TJ, Vermeiden JP, Schoute E, Lens JW, Schats R. The probability of pregnancy after embryo transfer is affected by the age of the patient, cause of infertility, number of embryos transferred and the average morphology score, as revealed by multiple logistic regression analysis. Human Reproduction 1995;10(11):3035-41. [DOI] [PubMed] [Google Scholar]
Scholtes 1996
- Scholtes MC, Zeilmaker GH. A prospective, randomized study of embryo transfer results after 3 or 5 days of embryo culture in in vitro fertilization. Fertility and Sterility 1996;65(6):1245-8. [DOI] [PubMed] [Google Scholar]
Schoolcraft 2001
- Schoolcraft WB, Gardner DK. Blastocyst versus day 2 or 3 transfer. Seminars in Reproductive Medicine 2001;19:259-68. [DOI] [PubMed] [Google Scholar]
Scott 2000
- Scott L, Alvero R, Leondires M, Miller B. The morphology of human pronuclear embryos is positively related to blastocyst development and implantation. Human Reproduction 2000;15(11):2394-403. [DOI] [PubMed] [Google Scholar]
Sfontouris 2021
- Sfontouris Ioannis. Timing of Embryo Culture. In: Ahlström, Aisling, Lundin, Kersti, editors(s). Manual of Embryo Culture in Human Assisted Reproduction. Cambridge: Cambridge University Press, 2021:66-74. [Google Scholar]
Sills 2000
- Sills ES, Tucker MJ, Palermo GD. Assisted reproductive technologies and monozygous twins: implications for future study and clinical practice. Twin Research 2000;2:217-23. [DOI] [PubMed] [Google Scholar]
Sjoblom 2006
- Sjoblom P, Menzes J, Cummins L, Mathiyalagan B. Prediction of embryo developmental potential and pregnancy based on early stage morphological characteristics. Fertility and Sterility 2006;86:848-61. [DOI] [PubMed] [Google Scholar]
Spangmose 2020
- Spangmose AL, Ginström EE, Malchau S, Forman J, Tiitinen A, Gissler M, et al. Obstetric and perinatal risks in 4601 singletons and 884 twins conceived after fresh blastocyst transfers: a Nordic study from the CoNARTaS group. Human Reproduction 2020;35(4):805-15. [DOI] [PubMed] [Google Scholar]
Staessen 2004
- Staessen C, Platteau P, Van Assche E, Michiels A, Tournaye H, Camus M. Comparison of blastocyst transfer with or without preimplantation genetic diagnosis for aneuploidy screening in couples with advanced maternal age: a prospective randomised control trial. Human Reproduction 2004;19(12):2849-58. [DOI] [PubMed] [Google Scholar]
Steer 1992
- Steer CV, Mills CL, Tan SL, Campbell S, Edwards RG. The cumulative embryo score: a predictive embryo scoring technique to select the optimal number of embryos to transfer in an in-vitro fertilization and embryo transfer programme. Human Reproduction 1992;7(1):117-9. [DOI] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
Sunde 2016
- Sunde Arne, Brison Daniel, Dumoulin John, Harper Joyce, Lundin Kersti, Magli M Cristina, Van den Abbeel Etienne, Veiga Anna. Time to take human embryo culture seriously†. Hum Reprod 2016/10/01;31(10):2174-2182. [DOI] [PubMed] [Google Scholar]
Sunde 2021
- Sunde Arne, Sturmey Roger G. Culture Media and Embryo Culture. In: Ahlström, Aisling, Lundin, Kersti, editors(s). Manual of Embryo Culture in Human Assisted Reproduction. Cambridge: Cambridge University Press, 2021:42-52. [Google Scholar]
Tsirigotis 1998
- Tsirgotis M. Blastocyst stage transfer: pitfalls and benefits. Too soon to abandon practice? Human Reproduction 1998;13(12):3285-95. [DOI] [PubMed] [Google Scholar]
Vail 2003
- Vail A, Gardener E. Common statistical errors in the design and analysis of subfertility trials. Human Reproduction 2003;18:1000-4. [DOI] [PubMed] [Google Scholar]
Valbuena 2001
- Valbuena D, Martin J, dePablo J, Remohi J, Pellicer A, Simon C. Increasing levels of estradiol are deleterious to embryonic implantation because they affect the embryo. Fertility and Sterility 2001;76(5):962-8. [DOI] [PubMed] [Google Scholar]
Van Blerkom 1993
- Van Blerkom J. Development of human embryos to the hatched blastocyst stage in the presence or absence of a monolayer of Vero cells. Human Reproduction 1993;8:1525-39. [DOI] [PubMed] [Google Scholar]
Vitthala 2009
- Vitthala S, Gelbaya T A, Brison D R, Fitzgerald C T, Nardo L G. The risk of monozygotic twins after assisted reproductive technology: a systematic review and meta-analysis. Hum Reprod Update 2009/01/01;15(1):45-55. [DOI] [PubMed] [Google Scholar]
Wang 2014
- Wang SS, Sun HX. Blastocyst transfer ameliorates live birth rate compared with cleavage-stage embryos transfer in fresh in vitro fertilization or intracytoplasmic sperm injection cycles: reviews and meta-analysis. Yonsei Medical Journal 2014;55(3):815-25. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Waters 2006
- Waters A-M, Dean JH, Sullivan EA. Assisted reproduction technology in Australia and New Zealand 2003; February 2006. Available at www.aihw.gov.au/getmedia/1945b5f3-0566-4f60-8035-761ca876eeb8/artanz03.pdf.aspx?inline=true.
WHO 2021
- World Health Organization (WHO). Infertility - Key facts. www.who.int/news-room/fact-sheets/detail/infertility (accessed 09 April 2021).
WHO 2022
- World Health Organization (WHO). Infertility. www.who.int/health-topics/infertility#tab=tab_1 (accessed 25 January 2022).
Wirleitner 2016
- Wirleitner B, Schuff M, Stecher A, Murtinger M, Vanderzwalmen P. Pregnancy and birth outcomes following fresh or vitrified embryo transfer according to blastocyst morphology and expansion stage, and culturing strategy for delayed development. Human Reproduction 2016;31(8):1685-95. [DOI: 10.1093/humrep/dew127] [DOI] [PubMed] [Google Scholar]
Yeung 1992
- Yeung WS, Ho PC, Lau EY, Chan ST. Improved development of human embryos in vitro by a human oviductal cell co-culture system. Human Reproduction 1992;7:1144-9. [DOI] [PubMed] [Google Scholar]
Zaat 2021
- Zaat T, Zagers M, Mol F, Goddijn M, Wely M, Mastenbroek S. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database of Systematic Reviews 2021 Feb 4, Issue 2. Art. No: CD011184. [DOI: 10.1002/14651858.CD011184.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Blake 2000
- Blake D, Jones G, Johnson NP, Olive D, Wilson ML. Cleavage stage versus blastocyst stage embryo transfer in assisted conception (In vitro fertilisation, Intracytoplasmic sperm injection). Cochrane Database of Systematic Reviews 2000, Issue 2. Art. No: CD002118. [DOI: 10.1002/14651858.CD002118] [DOI] [Google Scholar]
Blake 2002
- Blake D, Proctor M, Johnson N, Olive D. Cleavage stage versus blastocyst stage embryo transfer in assisted conception. Cochrane Database of Systematic Reviews 2002, Issue 2. Art. No: CD002118. [DOI: 10.1002/14651858.CD002118] [DOI] [PubMed] [Google Scholar]
Blake 2005
- Blake D, Proctor M, Johnson N, Olive D. Cleavage stage versus blastocyst stage embryo transfer in assisted conception. Cochrane Database of Systematic Reviews 2005, Issue 4. Art. No: CD002118. [DOI: 10.1002/14651858.CD002118.pub2] [DOI] [PubMed] [Google Scholar]
Blake 2007
- Blake DA, Farquhar CM, Johnson N, Proctor M. Cleavage stage versus blastocyst stage embryo transfer in assisted conception. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD002118. [DOI: 10.1002/14651858.CD002118.pub3] [DOI] [PubMed] [Google Scholar]
Glujovsky 2012
- Glujovsky D, Blake D, Farquhar C, Bardach A. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database of Systematic Reviews 2012, Issue 7. Art. No: CD002118. [DOI: 10.1002/14651858.CD002118.pub4] [DOI] [PubMed] [Google Scholar]


