Abstract
An organophosphorus-catalyzed C–N bond-forming reductive coupling of nitroalkanes with arylboronic acids and esters is reported. The method shows excellent chemoselectivity for the nitro/boronic acid substrate pair, allowing synthesis of N-(hetero)arylamines rich in functionalization. The identification of a sterically-reduced phosphetane catalyst capable of productive coupling in the P(III)/P(V)=O redox manifold is the key enabling development. Combined experimental kinetics and computational mechanistic studies show that the sterically-reduced catalyst affects post-rate limiting steps to enable the C–N coupling event in preference to deleterious side-paths.

1. INTRODUCTION
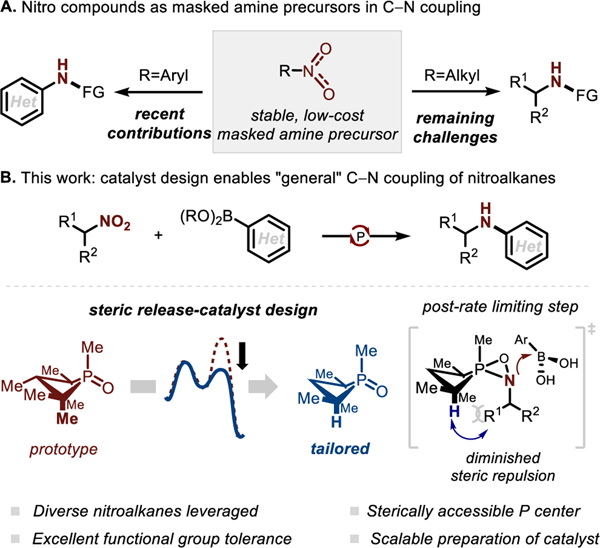
The use of nitro compounds—specifically nitroarenes—as direct partners in catalytic reductive C–N coupling represents an important emerging mode of direct N-functionalization for these common substrates (Figure 1A).1–2,3,4,5,6,7 By contrast, the catalytic reductive N-functionalization of nitroalkanes has been substantially less developed; notable exceptions include a B2pin2-mediated reductive N-alkylation of nitroalkanes with organozinc nucleophiles, 8 and a triphenylphosphine-mediated reductive N-arylation of nitroalkanes catalyzed by an oxomolybdenum(VI) compound under microwave irradiation.9 In order to more generally reveal the reactivity of readily-accessible nitroalkanes as convenient and easy-to-handle masked amine precursors for C–N coupling chemistry, we report here a new catalytic method—driven by redox cycling in the P(III)/P(V)=O manifold—that results in selective C–N bond formation between highly functionalized nitroalkanes and arylboronic acids (Figure 1B). We show that a seemingly peripheral alteration to a phosphetane-based organophosphorus catalyst dramatically improves the productive C–N coupling by retaining the biphilic electronic structure (i.e. low HOMO-LUMO gap) of the small-ring phosphacycle and subverting unwanted side reactions that otherwise plague the use of nitroalkanes as amination reagents. These results point the way to a more general use of nitroalkanes as direct coupling partners in reductive N-functionalization, and illustrate the versatility of the phosphetane core as a platform for biphilic catalyst tailoring.
Figure 1.
A) Nitroarene (left) vs nitroalkane (right) reductive N-functionalization. B) This work: steric considerations enable P(III)/P(V)=O-catalyzed reductive C–N coupling of nitroalkanes and boronic acids.
2. RESULTS AND DISCUSSION
2.1. Development and Optimization.
The challenges associated with the development of a P(III)/P(V)=O-catalyzed reductive coupling of nitroalkanes and boronic acids are exemplified in the context of the model coupling reaction between secondary nitroalkane 1 and boronic acid 2 (Table 1). Applying P(III)/P(V)=O-catalytic conditions previously established for methylamination of arylboronic acids (15 mol % of P1·[O] and 4.0 equiv of Ph2SiH2 in CPME (0.5 M) at 120 °C),6e the formation of the desired product 3 only in 10% yield (entry 1). The culmination of extensive solvent, hydrosilane, and additive investigations improved the reaction yield, but only modestly (24%, entry 2, see SI for more details). Under these conditions, in situ 31P NMR spectroscopy indicated persistence of intact P(III) catalyst P1 (δ 29.1 (anti), 16.0 (syn) ppm; Figure S1) after 24 h, but 19F NMR showed complete consumption of the nitroalkane starting material 1. Evidently, the low observed yield of 3 suggested that a nonproductive pathway was competing with desired reductive P(III)/P(V)=O-catalyzed N-arylation reaction.
Table 1.
Discovery and control experiments for organophosphorus-catalyzed C(sp2)–N cross coupling with nitroalkane 1.a

| |||
|---|---|---|---|
|
| |||
| Entry | P•[O] | Deviation from sth. cond. | Yield (%)b |
|
| |||
| 1 | P1•[O] | in CPME (0.5 M) w/o MgO | 10 |
| 2 | P1•[O] | -- | 24 |
| 3 | P2•[O] | -- | 73 |
| 4 | P3•[O] | -- | 69 |
| 5 | P4•[O] | -- | 90 |
| 6 | none | -- | 0 |
| 7 | P4•[O] | omit hydrosilane | 0 |
Reactions were carried out with P4•[O] (0.027 mmol, 15 mol %), diphenylsilane (0.730 mmol, 4.0 equiv), boronic acid 2 (0.274 mmol, 1.5 equiv) and nitroalkane 1 (0.182 mmol, 1.0 equiv) in CPME:PivCN = 6:4 (0.3 M) at 120 °C for 24 h, unless noted otherwise.
Yields were determined by 1H NMR integration with the aid of an internal standard.
The energy gap of HOMO-LUMO was measured from P(III) state.
The foregoing results indicated that a direct extension of prior conditions P(III)/P(V)=O-catalytic conditions to the challenge of reductive N-functionalization of nitroalkanes would not be viable and that alteration of the catalyst structure might be required. In this vein, the reaction yield was found to respond significantly to changes in the methyl substitution pattern on the phosphetane core. Indeed, reaction yields were markedly improved with catalysts P2•[O] (73%, entry 3) and P3•[O] (69%, entry 4) that omit geminal dimethylation at one of the α-positions of the phosphetane ring. Despite these improved yields, in situ 31P NMR studies indicated complete nonspecific decomposition of these two catalysts during the course of the reaction (Figure S1).
Hypothesizing that the decreased steric profile of P2•[O] and P3•[O] might improve catalyst performance at the expense of unwanted ring-cleavage10 that limits catalyst lifetime, we prepared P4•[O] containing a single methyl group at the α-position of the phosphetane ring. Interestingly, an optimized reaction yield of 3 was observed using P4•[O] as catalyst (90%, entry 5), and 31P NMR spectroscopy showed retention of intact P(III) phosphetane P4 (δ 25.3 (anti), 13.0 (syn) ppm) at the end of reaction.11,12 Control experiments confirm that this reductive coupling reaction requires the presence of both the organophosphorus catalyst and hydrosilane (entries 6 and 7), consistent with the notion of P(III)/P(V)=O catalytic cycling.13
2.2. Comparative Initial Rate Studies.
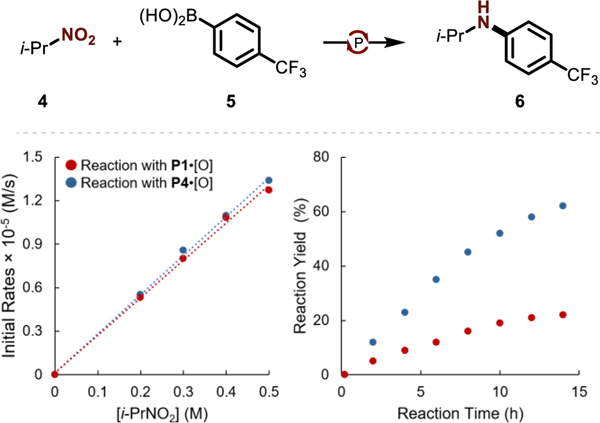
The superior performance of P4•[O] over P1•[O] can be traced to an improved selectivity for C–N coupling rather than an inherent increase in reactivity, as illustrated by the comparative kinetic analyses summarized in Figure 2. Under optimized catalytic conditions with both P4•[O] and P1•[O], the initial rates of reductive C–N coupling of 2- nitropropane (4) and 4-trifluoromethylphenylboronic acid (5) vary linearly with substrate concentration in the range 0.2 M ≤ [i-PrNO2] ≤ 0.5 M (Figure 2, left). This observation indicates that the reaction is first-order with respect to nitro substrate 4. Notably, the slopes of initial rate vs substrate concentration plot for both catalysts P1•[O] and P4•[O] are similar, demonstrating that 2-nitropropane is consumed at nearly identical rates by both organophosphorus catalysts. Indeed, these findings are consistent with the previously established mechanistic picture of involving a rate-limiting engagement of nitro substrate with biphilic P(III) catalyst in a (3+1) cheletropic addition;6b both P1 and P4 exhibit nearly identical frontier electronic structure (ΔE(HOMO-LUMO): P1=6.51 eV, P4=6.52 eV; Figure S12) and would be expected to react similarly in the FMO pericyclic reaction.
Figure 2.
Kinetic studies for reductive C–N coupling of 2-nitropropane (4) and (4-(trifluoromethyl)phenyl)boronic acid (5). Conditions: P4•[O] (25 mol %), diphenylsilane (4.0 equiv), boronic acid 5 (1.5 equiv) and 2-nitropropane 4 (1.0 equiv) in CPME:PivCN = 6:4 (0.3 M) at 120 °C. (left) Plots of initial consumption rate of 4 vs concentration of 4 for organophosphorus catalysts P1•[O] (•) and P4•[O] (•). (right) Plot of the formation of product 6 vs time organophosphorus catalysts P1•[O] (•) and P4•[O] (•).
Despite the similarity in initial rates of substrate consumption, there is however a significant difference in yields of the desired C–N coupling product 6 (Figure 2, right), in which P4•[O] outperforms P1•[O] over the entire time course. A major inference from these data is that the superior performance of catalyst P4•[O] in the title reductive C–N coupling arises not because of an inherently greater rate of reaction with the nitroalkane substrate, but rather because the catalyst impacts post-rate limiting steps to enable the C–N coupling event in preference deleterious side-paths (vide infra).
2.3. Reaction Scope.
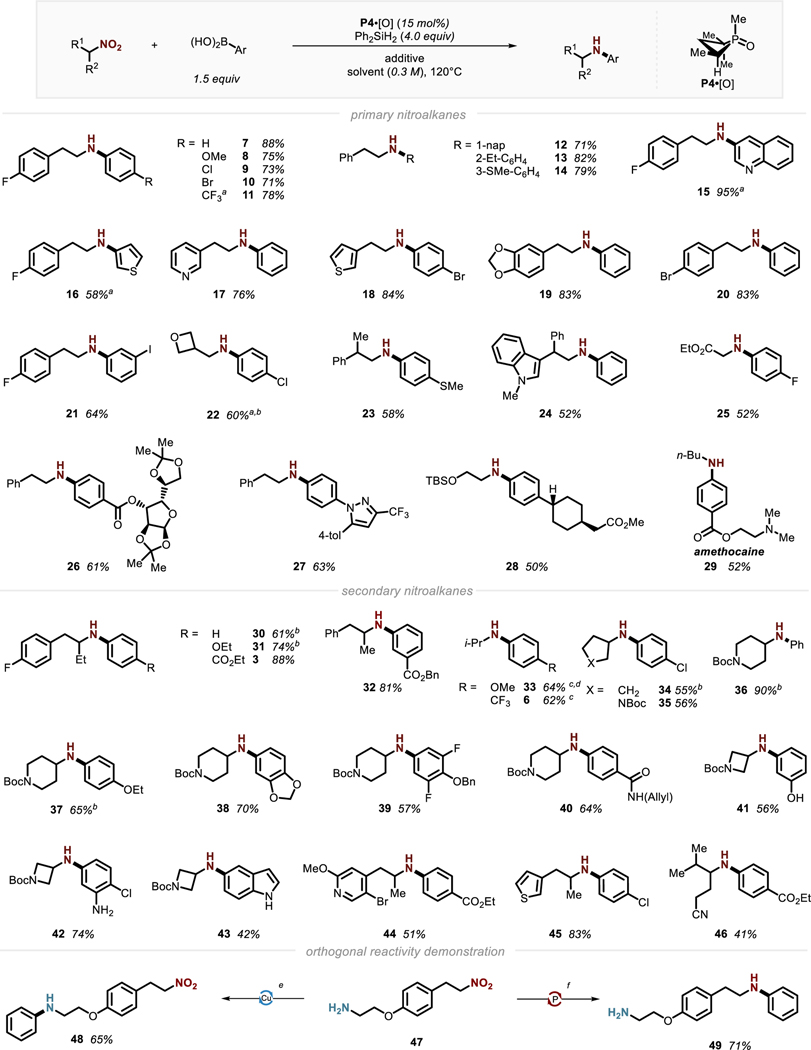
On the basis of this knowledge of the optimized reaction, substrate generality was investigated. A variety of both primary (7-29)14 and secondary (3, 6, 30-46) nitroalkanes afforded the desired N-arylated amine products efficiently (Figure 3). A diverse array of substituents is tolerated, including heterocylic moieties such as pyridine (17), thiophene (18 and 45) and indole (24). Notably, reductive coupling on ethyl nitroacetate afforded N-aryl glycine 25 in serviceable yield, notwithstanding the presence of highly acidic α-proton in the starting material (pKa < 6 in H2O).15 Among secondary cyclic structures, pyrrolidine- (35), piperidine- (36-40), and azetine-derived (41-43) nitro substrates are all tolerated and illustrate the application of the current method to the diversification of saturated heterocycles. Attempts to extend the catalytic conditions to tertiary nitroalkanes have not proven successful to date, representing a target for future reaction development.16
Figure 3.
Synthetic scope and representative examples of arylamines via PIII/PV=O catalyzed C(sp2)–N coupling. See SI for full experimental details and conditions. a The corresponding boronic acid glycol ester was used. b The reaction was run for 48 h. c25 mol% organophosphorus catalyst. d1.5 equiv of nitroalkane and 1.0 equiv of arylboronic acid. eReaction conditions: (a) Cu2O (10 mol%) PhB(OH)2 (1.0 equiv), 47 (1.2 equiv), MeOH (0.3 M), rt; f Reaction conditions: PhB(OR)2 (1.0 equiv), 47 (1.5 equiv), Ph2SiH2 (4.0 equiv), P4·[O] (15 mol %), CPME, 120 °C.
With respect to the boronic acid coupling partner, both electron-rich (8, 33, 31, 38) and electron-poor boronic acids (3, 6, 44, 46) are transformed smoothly to the desired products with good efficiency. Substituents on ortho, meta or para position of boronic acids show no influence on the reaction yields (12-14). A variety of five- and six-membered heterocyclic boronic acids (esters) such as quinoline (15), thiophene (16), pyrazole (27) and indole (43), were successfully transformed to the corresponding C–N coupling products. Boronic acids derived from celecoxib and acetonide-protected D-glucofuranose could be converted to aminated derivatives efficiently (26 and 27). An intermediate en route to a DGAT-1 inhibitor (28)17 could be prepared from the corresponding nitro alcohol. Local anesthetic amethocaine (29) could be prepared directly from nitrobutane and the corresponding boronic acid.18
In terms of overall chemoselectivity and compatibility, this reductive C–N cross coupling shows an excellent functional group tolerance toward reductively labile functional groups, such as esters (26, 28–29, 32) and amides (29, 40). Furthermore, aryl halides (9, 10, 18, 21 and 45) are inert in this reaction due to the nature of organophosphorus catalysis, allowing for subsequent functionalization with alternative coupling chemistries. Common protecting groups for heteroatoms including silyl ethers (28), benzyl ethers (39), alkylidene ketals (19, 26, 38), and N-Boc carbamates (36-43), were all fully preserved. Indeed, even free O–H (41) and N–H moieties (42, 43) were tolerated without competing arylation of the heteroatom.
A further demonstration of the complementarity of the present transformation with respect to existing methods of C–N coupling is exemplified by bifunctional substrate 47. While C–N coupling under Cu-catalyzed protocol chemoselectively functionalized the free amine position (48), this organophosphorus-catalyzed method selectively functionalized the nitro moiety (49).19 These results suggest a strategic orthogonality between organophosphorus-catalyzed and transition metal-catalyzed C–N coupling methods, providing new chemical flexibility to synthesize valuable secondary amine products from diverse building blocks.
2.4. Computational Modelling of the Reaction Sequence.
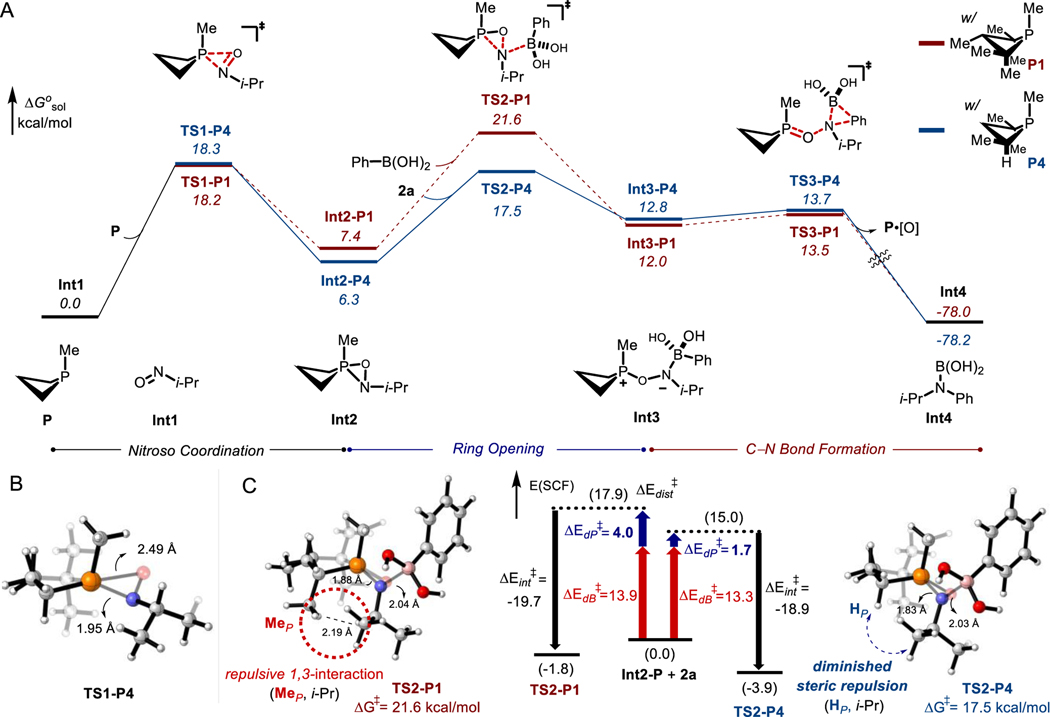
Prior mechanistic work on nitroarene N-functionalization by P(III)/P(V)=O-catalyzed reductive C–N coupling with arylboronic acids6b has established the overall pathway evolves in two sequential deoxygenative stages—an initial reduction of the nitro substrate to the formal nitroso oxidation state, followed by a second deoxygenation of a putative nitroso compound that results in N-arylation. Concerning the differential performance of catalysts P1 and P4, DFT modeling (PCM(PivCN)-B3LYP-D3/6–311++g(d,p)//6–31g(d,p)) was employed in effort to trace the impact of the α-methyl deletion on the phosphetane.
In accord with kinetic data in Figure 2, transition states corresponding to an initial (3+1) cheletropic addition of 2-nitropropane either P1 or P4 are found to be the highest energy barrier along the predicted stepwise C–N coupling reaction coordinate (Figure 4). However, they are similar in respective energies (ΔΔGǂ = 0.6 kcal/mol, see also Figure S7) and cannot account for the different catalytic efficiency. In effect, while the initial (3+1) cheletropic addition is turnover-limiting for catalysis, it is not determinative of the apparent differential performance of catalysts P1 and P4 in the overall reductive C–N coupling reaction.
Figure 4.
Proposed two-stage catalytic cycle for P(III)/P(V)=O-catalyzed reductive C−N coupling. (3+1) Transition state energies in kcal/mol.
The investigation turned therefore to post-rate limiting steps in the C–N bond forming sequence stemming from the 2-nitrosopropane intermediate (Int1, Figure 5A). Here, capture of the Int1 by either P1 or P4 takes place via (2+1) cycloaddition again with virtually identical free energy barriers of 18.2 kcal/mol for TS1-P1 and 18.3 kcal/mol for TS1-P4 (Figure 5B). Interestingly, though, a modest energy difference is evident in the resulting oxazaphosphirane intermediates, where Int2-P4 is predicted to be 1.1 kcal/mol more stable than Int2-P1. This energy difference is further accentuated in the subsequent step, which involves heterolytic ring-opening of Int2 by engagement of PhB(OH)2 (2a). Here, the transition state TS2-P4 is found to be substantially lower in energy (ΔΔG╪ = –4.1 kcal/mol) compared with TS2-P1. This relative energy difference between TS2-P4 and TS2-P1 proves critical for understanding the differential reactivity of the two phosphetane catalysts. Namely, the low energy of TS2-P4 poises it below TS1-P4, meaning that forward reaction to afford the C–N coupling products proceeds (by ring-opening via TS2-P4 followed by 1,2-migration via TS3-P4) without the back-formation of Int1 from Int2-P4.20 By contrast, in the case of P1 congener, TS2-P1 is higher in energy than TS1-P1, such that reversion of Int2-P1 to Int1 and P1 is favorable.
Figure 5.
(A) Mechanistic proposal for second deoxygenation and C–N bond formation supported by density functional theory (DFT) calculations at the PCM(PivCN)-B3LYP-D3/6–311++g(d,p)//6–31g(d,p) level of theory. Relative free energies (italics) are given in kcal/mol. (B) Computed model of TS1-P4. (C) Transition structures and distortion/interaction analyses for TS2-P: Oxazaphosphirane distortion energy (ΔEdP⧧) in blue, phenylboronic acid distortion energy (ΔEdB⧧) in red, fragment interaction energy (ΔEint⧧) in black.
The curtailed accumulation of Int1 as given by the operative pathway with P4 is important for the success of the desired C–N coupling reaction because a nitrosoalkane such as Int1 would otherwise be expected to undergo isomerization to the corresponding oxime.21 However, oximes are not viable substrates for the reductive coupling; independent subjection of acetone oxime to standard reaction conditions does not provide product 6 (Figure S2). Consequently, nitroso-oxime tautomerization of Int1 would be a nonproductive dead end in this organophosphorus-catalyzed reaction sequence.
The energy difference between transition states TS2-P1 and TS2-P4 can be traced to a key repulsive interaction between the catalyst and the substrate. As visualized in Figure 5C, the ring-opening transition state (TS2-P1) clearly displays a destabilizing syn-pentane-like interaction between catalyst methyl (MeP) and substrate isopropyl (i-Pr) groups; the HMe…Hi-Pr distance is only 2.19 Å. However, this destabilizing interaction is mitigated in TS2-P4, where the offending methyl substituent is replaced with a hydrogen atom (HP). Distortion/interaction analysis further evidences this destabilizing effect (Figure 5C).22 The computed interaction energy (ΔEint⧧) of fragments Int2 and 2a is quite similar for both TS2-P1 (ΔEint⧧ = −19.7 kcal/mol) and TS2-P4 (ΔEint⧧ = −18.9 kcal/mol). By contrast, a larger difference is found for the distortion energy required for the fragments to access geometries as in the transition state (ΔEdist⧧ = −17.9 kcal/mol for TS2-P1; ΔEdist⧧ = −15.0 kcal/mol for TS2-P4). The greater energetic cost to the oxazaphosphirane fragment in TS2-P1 reflects the penalty arising from impingement of the N-alkyl moiety on the phosphetane α-methyl group (Mep). Absent this penalty, TS2-P4 is lower than TS2-P1 in overall electronic energy by 2.1 kcal/mol. These data substantiate the assignment of a steric perturbation on the phosphorus catalyst as a key factor enabling the desired reaction trajectory in reductive P(III)/P(V)=O-catalyzed C–N coupling with nitroalkanes as a viable amination source.
3. CONCLUSION
In summary, we have found that a new organophosphorus catalyst facilitates the reductive C–N cross coupling of functionalized primary and secondary nitroalkanes with arylboronic acids. Various functional group-rich substrates could be employed as coupling partners, showcasing the practicality, generality, and robustness of the present method. The success of nitroalkanes as direct synthons in intermolecular C–N coupling chemistry provides an expanded retrosynthetic approach for the streamlined synthesis of bioactive lead molecules, especially in view of the exceptional chemoselectivity inherent to this main group-catalyzed approach. The mechanistic insights reported herein highlight the centrality of oxazaphosphirane intermediates in P(III)/P(V)=O-catalyzed reductive nitro N-functionalization, and suggest further opportunities for catalyst optimization that support ongoing expansion of scope.
Supplementary Material
ACKNOWLEDGMENT
Financial support for this work was provided by NIH (GM114547), G. L. thanks Bristol Myers Squibb for a graduate fellowship. Y. K. thanks Uehara memorial foundation for a predoctoral fellowship. S.Y.H. thanks National Research Foundation of Korea (NRF) for a postdoctoral fellowship (2021R1A6A3A03044649). The authors thank Z. Qin for preparation of select substrates. We are grateful to Drs. P. Mueller, A. Tanushi and M. Drance (MIT) for assistance with crystallographic data collection and refinement.
Footnotes
Supporting Information Placeholder
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
General methods and synthetic procedures (.pdf)
1H, 2H, 13C, 19F and 31P NMR spectra (.pdf).
Contributor Information
Gen Li, Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, United States.
Yuzuru Kanda, Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, United States.
Seung Youn Hong, Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, United States.
Alexander T. Radosevich, Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, United States.
REFERENCES
- 1.(a) Ferretti F; Ramadan DR; Ragaini F Transition Metal Catalyzed Reductive Cyclization Reactions of Nitroarenes and Nitroalkenes. ChemCatChem 2019, 11, 4450–4488. [Google Scholar]; (b) Gao Y; Yang S; Huo Y; Hu XQ Recent Progress on Reductive Coupling of Nitroarenes by Using Organosilanes as Convenient Reductants. Adv. Synth. Catal. 2020, 362, 3971–3986. [Google Scholar]; (c) Moshapo PT; Simelane SB Advances in Nitroarene Reductive Amidations. Arkivoc 2021, 2020, 190–215. [Google Scholar]
- 2.Srivastava RS; Nicholas KM Kinetics of the Allylic Amination of Olefins by Nitroarenes Catalyzed by [CpFe(CO)2]2. Organometallics 2005, 24, 1563–1568. [Google Scholar]
- 3.Gui J; Pan C-M; Jin Y; Qin T; Lo JC; Lee BJ; Spergel SH; Mertzman ME; Pitts WJ; La Cruz TE; Schmidt MA; Darvatkar N; Natarajan SR; Baran PS Practical olefin hydroamination with nitroarenes. Science 2015, 348, 886–891. [DOI] [PubMed] [Google Scholar]
- 4.(a) Cheung CW; Hu X Amine synthesis via iron-catalysed reductive coupling of nitroarenes with alkyl halides. Nat. Commun. 2016, 7, 12494. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cheung CW; Ploeger ML; Hu X Nickel-Catalyzed Reductive Transamidation of Secondary Amides with Nitroarenes. ACS Catal. 2017, 7, 7092–7096. [Google Scholar]
- 5.Xiao J; He Y; Ye F; Zhu S Remote Sp3 C–H Amination of Alkenes with Nitroarenes. Chem 2018, 4, 1645–1657. [Google Scholar]
- 6.(a) Nykaza TV; Cooper JC; Li G; Mahieu N; Ramirez A; Luzung MR; Radosevich AT Intermolecular Reductive C−N Cross Coupling of Nitroarenes and Boronic Acids by PIII/PV=O Catalysis. J. Am. Chem. Soc. 2018, 140, 15200–15205. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Li G; Nykaza TV; Cooper JC; Ramirez A; Luzung MR; Radosevich AT An Improved PIII/PV=O-Catalyzed Reductive C−N Coupling of Nitroaromatics and Boronic Acids by Mechanistic Differentiation of Rate- and Product-Determining Steps. J. Am. Chem. Soc. 2020, 142, 6786–6799. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Nykaza TV; Li G; Yang J; Luzung MR; Radosevich AT PIII/PV=O-Catalyzed Cascade Synthesis of N-Functionalized Azaheterocycles. Angew. Chem., Int. Ed. 2020, 59, 4505–4510. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Li G; Qin Z; Radosevich ATP (III)/P(V)-Catalyzed Methylamination of Arylboronic Acids and Esters: Reductive C−N Coupling with Nitromethane as a Methylamine Surrogate. J. Am. Chem. Soc. 2020, 142, 16205–16210. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Li G; te Grotenhuis C; Radosevich AT Reductive Csp2−N Coupling by PIII/PV=O−Catalysis. Trends Chem. 2021, 3, 72–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li G; Miller SP; Radosevich AT PIII/PV=O-Catalyzed Intermolecular N–N Bond Formation: Cross-Selective Reductive Coupling of Nitroarenes and Anilines J. Am. Chem. Soc. 2021, 143, 14464–14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reductive C–N coupling with nitroalkane mediated by stoichiometric main-group metal approaches include. ; (a) Rauser M; Ascheberg C; Niggemann M Direct Reductive N-Functionalization of Aliphatic Nitro Compounds. Chem. Eur. J. 2018, 24, 3970–3974. [DOI] [PubMed] [Google Scholar]; (b) Rauser M; Eckert R; Gerbershagen M; Niggemann M Catalyst-Free Reductive Coupling of Aromatic and Aliphatic Nitro Compounds with Organohalides. Angew. Chem. Int. Ed. 2019, 58, 6713–6717. [DOI] [PubMed] [Google Scholar]; (c) Roscales S; Csáky AG Transition-Metal-Free Three-Component Synthesis of Tertiary Aryl Amines from Nitro Compounds, Boronic Acids, and Trialkyl Phosphites. Adv. Synth. Catal. 2020, 362, 111–117. [Google Scholar]
- 9.Suárez-Pantiga S; Hernández-Ruiz R; Virumbrales C; Pedrosa MR; Sanz R Reductive Molybdenum-Catalyzed Direct Amination of Boronic Acids with Nitro Compounds. Angew. Chem., Int. Ed. 2019, 58, 2129–2133. [DOI] [PubMed] [Google Scholar]
- 10.(a) Cremer SE; Chorvat RJ Syntheses of substituted phosphetanes and related derivatives. J. Org. Chem. 1967, 32, 4066–4070. [Google Scholar]; (b) Marinetti A; Carmichael D Synthesis and Properties of Phosphetanes. Chem. Rev. 2002, 102, 201–230. [DOI] [PubMed] [Google Scholar]
- 11.The non-nucleophilic base MgO curtails catalyst degradation, but has a minor effect. Reductive C–N coupling between nitroalkane 1 and boronic acid 2 in the absence of MgO under otherwise same reaction conditions could still generate the desired product in 75% NMR yield (Table S1).
- 12.All catalysts in this survey, including catalyst P4•[O], are easily prepared on a decagram scale in a single batch by McBride synthesis (Nykaza TV; Cooper JC; Radosevich, A. T. anti-1,2,2,3,4,4-Hexamethylphosphetane 1-Oxide. Org. Synth. 2019, 96, 418–435). See SI for details. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.For recent reviews about P(III)/P(V)=O redox catalysis, see: ; (a) Lao Z; Toy PH Catalytic Wittig and aza-Wittig Reactions. Beilstein J. Org. Chem. 2016, 12, 2577–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Guo H; Fan YC; Sun Z; Wu Y; Kwon O Phosphine Organocatalysis. Chem. Rev. 2018, 118, 10049–10293. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lipshultz JM; Li G; Radosevich AT Main Group Redox Catalysis of Organopnictogens: Vertical Periodic Trends and Emerging Opportunities in Group 15. J. Am. Chem. Soc. 2021, 143, 1699–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Xie C; Smaligo AJ; Song XR; Kwon O PhosphorusBased Catalysis. ACS Cent. Sci. 2021, 7, 536–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Similar reaction yields were obtained for substrates 7–9 with P1•[O] under otherwise identical conditions. See SI for full experimental details and conditions.
- 15.Young AJ; White MC Catalytic Intermolecular Allylic C−H Alkylation. J. Am. Chem. Soc. 2008, 130, 14090–14091. [DOI] [PubMed] [Google Scholar]
- 16.Reductive C–N coupling between 1-fluoro-4-((1-nitrocyclohexyl)methyl)benzene (0.2 mmol, 1.0 equiv) and phenyl boronic acid (0.3 mmol, 1.5 equiv) under standard conditions generated the desired product in 18% NMR yield. [Google Scholar]
- 17.(a) McCoull W; Addie MS; Birch AM; Birtles S; Buckett LK; Butlin RJ; Bowker SS; Boyd S; Chapman S; Davies RDM; Donald CS; Green CP; Jenner C; Kemmitt PD; Leach AG; Moody GC; Morentin Gutierrez P; Newcombe NJ; Nowak T; Packer MJ; Plowright AT; Revill J; Schofield P; Sheldon C; Stokes S; Turnbull AV; Wang SJY; Whalley DP; Wood JM Identification, Optimisation and in Vivo Evaluation of Oxadiazole DGAT-1 Inhibitors for the Treatment of Obesity and Diabetes. Bioorganic Med. Chem. Lett. 2012, 22, 3873–3878. [DOI] [PubMed] [Google Scholar]; (b) Zhou G; Ting PC; Wishart G; Zorn N; Aslanian RG; Lin M; Smith M; Walker SS; Cook J; Van Heek M; Lachowicz J Discovery of Novel Quinoline Carboxylic Acid Series as DGAT1 Inhibitors. Bioorganic Med. Chem. Lett. 2014, 24, 1790–1794. [DOI] [PubMed] [Google Scholar]
- 18.(a) Vilallonga FA; Phillips EW Surface Activities of Procaine, Lidocaine, and Tetracaine and Their Interaction Energies with Phospholipid Monolayers. J. Pharm. Sci. 1979, 68, 314–316. [DOI] [PubMed] [Google Scholar]; (b) Haque SJ; Poddar MK Amethocaine‐induced Inhibition of Mitochondrial Monoamine Oxidase Activity. J. Pharm. Pharmacol. 1986, 38, 858–860. [DOI] [PubMed] [Google Scholar]; (c) Miller KJ; Goodwin SR; Westermann-Clark GB; Shah DO J. Pharm. Pharmacol 1993, 82, 1123–1125. [DOI] [PubMed] [Google Scholar]
- 19.Sreedhar B; Venkanna GT; Shiva Kumar KB; Balasubrahmanyam V Copper(I) Oxide Catalyzed N-Arylation of Azoles and Amines with Arylboronic Acid at Room Temperature under Base-Free Conditions. Synthesis 2008, 2008, 795–799. [Google Scholar]
- 20.DFT pathways using either P2 and P3 as catalysts similarly reproduce the relative energetic ordering of TS1 and TS2. See (see Figure S11–12).
- 21.Yamamoto H; Momiyama N Rich Chemistry of Nitroso Compounds. Chem. Commun. 2005, 3514–3525 [DOI] [PubMed] [Google Scholar]
- 22.(a) Ess DH; Houk KN Distortion/interaction energy control of 1,3-dipolar cycloaddition reactivity. J. Am. Chem. Soc. 2007, 129, 10646–10647. [DOI] [PubMed] [Google Scholar]; (b) Fernandez I; Bickelhaupt FM The activation strain model and molecular orbital theory: understanding and designing chemical reactions. Chem. Soc. Rev. 2014, 43, 4953–4967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.