Abstract
Background
Focused ultrasound (FUS) is an emerging technology, offering the capability of tuning and prescribing thermal and mechanical treatments within the brain. While early works in utilizing this technology have mainly focused on maximizing the delivery of therapeutics across the blood–brain barrier (BBB), the potential therapeutic impact of FUS-induced controlled thermal and mechanical stress to modulate anti-tumor immunity is becoming increasingly recognized.
Objective
To better understand the roles of FUS-mediated thermal and mechanical stress in promoting anti-tumor immunity in central nervous system tumors, we performed a comprehensive literature review on focused ultrasound-mediated immunomodulation and immunotherapy in brain tumors.
Methods
First, we summarize the current clinical experience with immunotherapy. Then, we discuss the unique and distinct immunomodulatory effects of the FUS-mediated thermal and mechanical stress in the brain tumor-immune microenvironment. Finally, we highlight recent findings that indicate that its combination with immune adjuvants can promote robust responses in brain tumors.
Results
Along with the rapid advancement of FUS technologies into recent clinical trials, this technology through mild-hyperthermia, thermal ablation, mechanical perturbation mediated by microbubbles, and histotripsy each inducing distinct vascular and immunological effects, is offering the unique opportunity to improve immunotherapeutic trafficking and convert immunologically “cold” tumors into immunologically “hot” ones that are prone to generate prolonged anti-tumor immune responses.
Conclusions
While FUS technology is clearly accelerating concepts for new immunotherapeutic combinations, additional parallel efforts to detail rational therapeutic strategies supported by rigorous preclinical studies are still in need to leverage potential synergies of this technology with immune adjuvants. This work will accelerate the discovery and clinical implementation of new effective FUS immunotherapeutic combinations for brain tumor patients.
Keywords: Focused ultrasound, Immunomodulation, Immunotherapy, Brain tumors
Introduction
Focused ultrasound (FUS) is an emerging clinical technology for applying thermal and mechanical stress deep in the brain [1]. Thermal stress occurs at relatively high ultrasound intensities (e.g., > 10 W/cm2 [2, 3]) depending on the temperature and is caused by friction at the molecular level within tissues as the molecules become out of phase with the propagating pressure wave (i.e., viscous heating). By changing the excitation amplitude, pulse duration, and frequency, different levels of heat can be deposited in the tissue, from mild hyperthermia (< 43 °C) to tissue ablation (~ 60 °C for 1 Sec). This mode of FUS operation in humans has already been employed in glioblastoma patients [4], although, in the clinic, it is currently only used for non-invasive thalamotomy (i.e. thermal ablation) in patients with Essential or Parkinsonian tremor [5–7].
The mechanical stress is primarily mediated by ultrasound (vascular) contrast agents called microbubbles (MBs) that are administered in the vasculature at concentrations of 107–109 MBs/ml (e.g., 10–20 μl/kg for Definity microbubbles) concurrently with the FUS exposures (sonication) [8, 9]. When these vascular agents pass through the FUS focal region they undergo high frequency vibrations (expand in peak negative and contract in peak positive pressures). The bubble oscillation is categorized into two regimes: stable cavitation and inertial cavitation [10]. Stable cavitation is induced at lower intensities compared to ablation (i.e., pressures below 400 kPa peak negative pressure) and is associated with circumferential or shear stresses (stretch, pull, or perturb behavior). Extended research has demonstrated that stable cavitation can transiently open the blood brain barrier (BBB), composed of specialized neurovascular units (NVU) only present in the central nervous system and with unique properties to maintain brain homeostasis. The FUS-mediated BBB opening may last up to 24 h post sonication [11–13] and can improve the uptake, penetration and efficacy of a range of drugs, including small molecular weight chemotherapeutics, antibodies, and nanoparticles, in the brain and brain tumors [14]. As a result, multiple clinical trials have been initiated that have already demonstrated the feasibility and safety of this mode of operation across different diseases and provided evidence of its efficacy using small chemotherapeutic agents in patients with glioblastomas [15–18].
The onset of inertial cavitation typically occurs at higher applied focal pressures (mechanical index near 0.4; , provides an indicator of the likelihood of adverse mechanical effects from cavitation that accounts for frequency related responses), wherein overexpansion and subsequent collapse of the microbubble due to the surrounding fluid’s inertia gives rise to more violent mechanical forces, such as jetting and shock wave formation [19–21]. Although MB collapse can lead to dramatic changes in NVU structure and function [22, 23], several investigations have demonstrated that MB type, dose, and exposure settings can be optimized to limit undesired effects and toxicity [8, 24–28]. Despite safety concerns (e.g., tissue and vascular damage), recent investigations have explored the potential of inertial cavitation to promote mechanical tissue ablation and facilitate therapy [29–31], providing another mode of operation to target primary and metastatic brain tumors. Finally, very high intensity ultrasound pulses (< 100 μsec, > 20 MPa peak negative pressure) can generate cavitation bubble clouds to promote tissue fractionation, a method also known as histotripsy (i.e., mechanical acoustic ablation) [32]. This mode of operation does not require microbubbles and is currently under clinical evaluation for a variety of indications [33].
Increasing evidence suggest that the above modes of FUS operation can not only directly impact tissues and drug delivery, but also modulate the brain tumor-immune microenvironment (Fig. 1). Such mechano- and thermo-biological effects offer the opportunity to improve immunotherapeutic trafficking and convert immunologically “cold” (lowly infiltrated by immune cells) tumors into immunologically “hot” (highly infiltrated) ones that are potentially more likely to generate prolonged anti-tumor immune responses. In this review we (i) briefly summarize the current clinical experience with immunotherapy, (ii) discuss the immunomodulatory effects of the thermal (i.e., hyperthermia) and mechanical (i.e., microbubble mediated) stress on brain tumor-immune microenvironment, and (iii) highlight recent advances in the combination of this technology with immunotherapy. For extended discussion on the abilities of microbubble-enhanced FUS (MB-FUS) to promote targeted drug delivery in brain tumors, we direct the reader to recent review papers on the topic [14, 23].
Fig. 1.
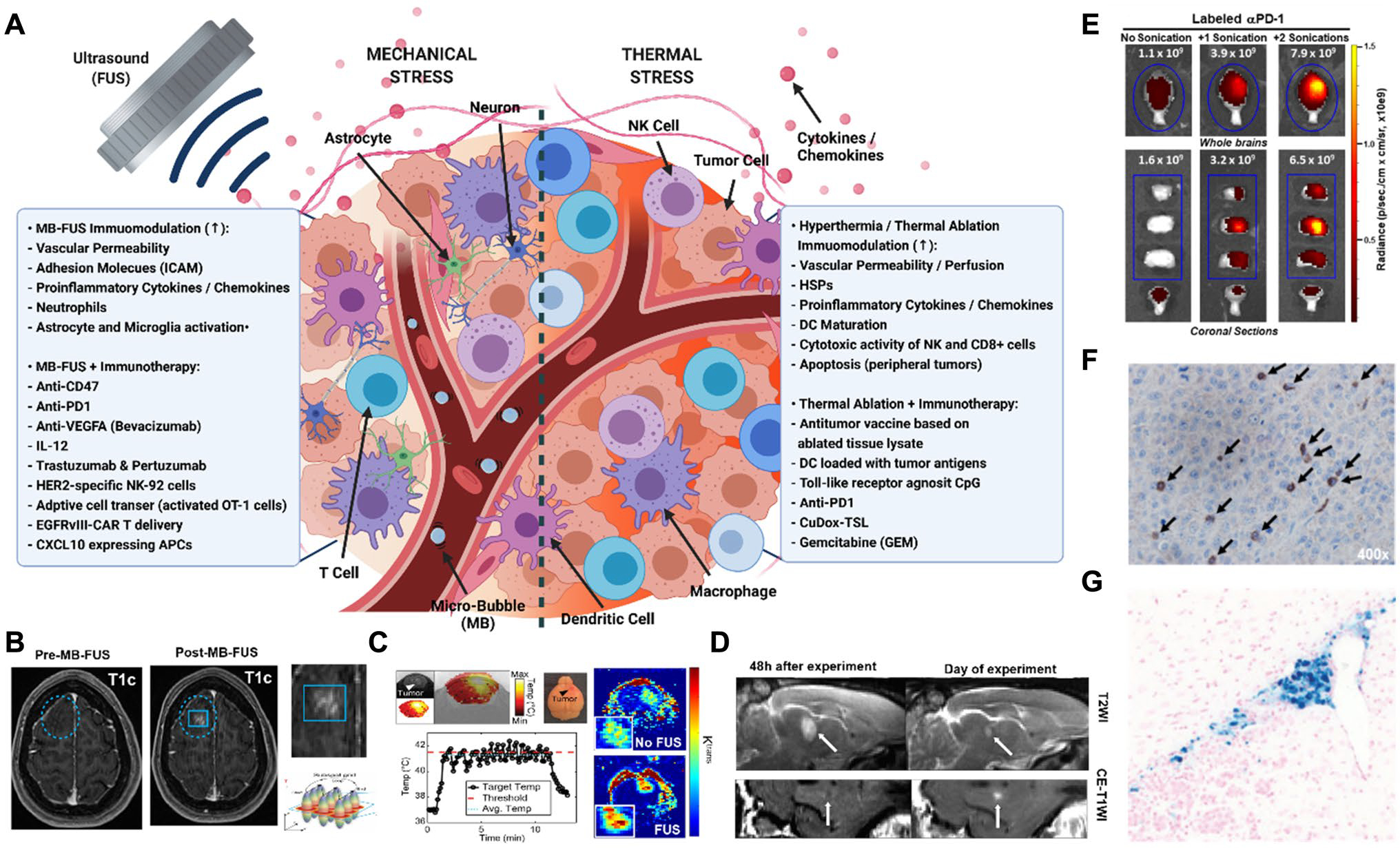
A Schematic highlighting the immunomodulatory mechanism of thermal and mechanical stress and the most promising combinations with immune adjuvants to treat brain tumors (created with BioRender). B-D FUS thermal and mechanical stress mediated changes the BBB permeability. B MB-FUS BBB opening in patient with glioma [34]. C FUS mediated mild hyperthermia increases the KTrans in orthotopic GL261 brain tumors [35]. D FUS-mediated thermal ablation increases BBB leakiness in the brain of healthy rabbit. T2WI images show evidence of inflammation followed the sonication. White arrow indicates the sonicated region [36]. E Increased delivery of Anti-PD-1 after MB-FUS in GL261 glioma bearing mice [37]. F MB-FUS in combination with intravenously administered EGFRvIII CAR T cells lead to increase accumulation and cytotxic activity in EGFRvIII-expressing U87 tumors [37]. G MB-FUS leads to greater accumulation of intravenously administered HER-2 specific NK-92 cells in a model of HER2 + breast cancer brain metastasis [38]
Current clinical experience with immunotherapy
The recent discovery of functional lymphatic vessels in the meninges along with increasing evidence of T cell entry and immunosurveillance within the brain [39–41], indicating that the brain is immunologically distinct rather than “privileged”, created hope that immune adjuvants that have led to breakthrough results in peripheral tumors could also be beneficial against brain tumors. Below, we present current clinical findings and briefly discuss challenges towards improving antitumor immunity in brain tumors and in particular glioblastoma (GBM).
Due to its success in peripheral tumors, immune checkpoint blockade targeting the PD1 ligand 1 (PDL1) axis and/or cytotoxic T lymphocyte- associated antigen 4 (CTLA4) was among the first to be tested in the clinic against brain tumors. Most notably, the anti-PD1 therapy nivolumab or its combination with anti-CTLA-4 ipilimumab led to remarkable responses in melanoma brain metastases, supporting the use of immune checkpoint blockade for the treatment of brain tumors [42, 43]. Contrary to promising preclinical investigations [44], nivolumab alone or in combination with bevacizumab (antivascular endothelial growth factor A (VEGFA)) led to no overall survival benefit in the treatment of recurrent glioblastoma in phase III clinical trials [45, 46], highlighting the challenges to elicit robust responses in aggressive brain tumors, such as GBM. Interestingly, recent clinical trials (Phase II) that employed a single neoadjuvant administration of the anti-PD1 agent pembrolizumab prior to resection of recurrent GBM were able to significantly extended the median survival to 417 days, compared with 228.5 days in patients receiving typical adjuvant dosing of pembrolizumab [47, 48], suggesting that refined treatment protocols in combination with neurosurgical interventions can significantly impact antitumor immunity.
Adoptive T cell therapy has also been tested in the clinic against brain tumors. Most notably, a recent pilot study that employed Chimeric Antigen Receptor (CAR) T cells (CART) targeting the EGFRvIII mutant receptor in GBM patients, found that all patients have had significant expansion of CART-EGFRvIII cells in the peripheral blood [49]. Importantly, tissue analysis following CART infusion revealed some trafficking of CART-EGFRvIII cells to GBM and decreased EGFRvIII levels in five of the seven patients that were enrolled in this study. Moreover, a case study in a GBM patient that employed CART cells targeting the IL-13 receptor α2 (IL-13Rα2), showed remarkable early responses, although a few months later the tumor relapsed, presumably due to IL-13Rα2-negative tumor cells [50]. CART cells targeting the human epidermal growth factor receptor 2 (EGFR2) also demonstrated a clinical benefit for some patients with progressive GBM [51]. Similarly encouraging results were also obtained in four patients with H3K27M-mutant diffuse intrinsic pontine glioma (DIPG) and diffuse midline gliomas (DMG) that were treated with GD2-CAR T cells [52].
Despite these encouraging findings, many challenges remain towards attaining clinically effective treatments with immune adjuvants across all brain cancer sub-types. Most notably, the BBB is considered to play a major role in regulating therapeutic trafficking from the circulation to the brain and brain tumor microenvironment (TME) [53]. While the BBB is considered compromised in many brain tumors its permeability is characterized by significant heterogeneity that varies not only between different brain tumors, but also within brain tumors [53–56]. Due to these characterstics, the BBB has been implicated not only to limited delivery of therapeutics (e.g., anti-PD1) but also to limited antigen presentation [39, 57]. While the barriers to (and impact of) local delivery of anti-PD1 in brain tumors is currently under intense investigations, intensive treatment protocols (i.e., multiple and frequent dosing) that led to improved outcomes [58], suggest that improved delivery may be beneficial. Interestingly, recent preclinical investigations indicated that anti–PD-1 can support the development of an anti-tumorigenic M1 phenotype and/or increase the phagocytic capacity of tumor associated macrophages/microglia (TAMs) [58, 59]. These findings further support the importance of localized delivery and penetration of immune checkpoint inhibitors in the GBM TME. Such strategies may also reduce immune-related adverse events associated with immune checkpoint blockade toxicity [60, 61].
Morover, while T cells have the capacity to transmigrate across the BBB [40], most tumors are characterized by limited T cell infiltration [62]. Detailed analysis of T cell trafficking dynamics at different time points and tumor types is needed to better understand if this is due to limited transmigration across the BBB [63], limited residence time in the brain TME [64], or other reasons [65]. Another major impediment for effective anti-tumor immunity is related to the immunosuppressive nature of the tumor microenvironment. For instance, tumors by secreting soluble factors, including C–C-motif chemokine ligand 2 (CCL2) and C-X-C-motif chemokine ligand 8 (CXCL8), among others, can promote the recruitment of immunosuppressive cells, including immature dendritic cells (DCs), regulatory T (Treg) cells, myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs) with pro-tumor phenotypes [66, 67]. Unfortunately, many biologics designed to enhance T cell activity (e.g., IL-2, IL-15 superagonist) by overcoming immunosuppression [68–72] affect both antitumor as well as endogenous T cell populations, leading to systemic toxicities including off-target cell killing, and reduced overall patient response rates [73].
Together the above observations suggest that identifying and optimizing methods that could change the TME phenotype to become more amenable to immune cell infiltration and homing along with increasing the ability to promote multi-faceted immune-stimulator effects, may lead to prolonged anti-tumor immune responses in brain tumors. Below we highlight the recent advances in FUS technology and its combination with immunotherapy towards accomplishing this goal.
FUS mediated immunomodulation and immunotherapy
Mechanical stress
Immunomodulation
Several investigations in the healthy rodent brain have implicated downstream effects from mechano-responsive and acute inflammation processes following MB-FUS mechanical stress, in addition to promoting transient BBB opening (Fig. 1B). Most notably, the observed responses have demonstrated increased expression of pro-inflammatory cytokines, chemokines, and adhesion molecules [24, 74], followed by infiltration of immune cells (e.g. neutrophils) in the brain parenchyma [75], and finally by the activation of astrocytes and microglia, which can last up to several days [22, 76] (Table 1). Due to these profound changes, it has been hypothesized that MB-FUS may be able to improve antigen presentation either within the brain TME or at the level of the meninges and cervical lymph nodes. This hypothesis was tested by Curley et al. using the B16F1Zs-GreenOVA melanoma cancer cell line, which was stably transfected to express the tumor-associated antigen ovalbumin (OVA) [77]. In their investigations, they found significant changes in proinflammatory cytokines and chemokine signaling, among others, confirming the impact of MBFUS in the bran TME. Unfortunately, these changes failed to increase antigen drainage to the lymph nodes and elicit dendritic cell activation and maturation. Consequently, they concluded that the responses to MB-FUS mechanical stress are mild, transient, and unlikely to elicit a systemic response via DC priming and activation independent of administration of immune adjuvants. These findings are consistent with numerous investigations that assessed the impact of MB-FUS on survival studies in immunocompetent murine brain tumor models (i.e., the control arm in drug delivery investigations) that did not report a statistically significant improvement in survival [78–85].
Table 1.
Representative data on immune responses in (healthy) brain under mechanical stress (MB-FUS)
| Model | Exposure | Cytokines and Chemokines | Proteins | Immune cells | References |
|---|---|---|---|---|---|
|
| |||||
| Melanoma Metastasis (B16F1cOVA) | 1.1 MHz, 0.5 MPa | TNF, IL6, CCL2, CCL12, CXCL16, CXCL10, CCL7, and CXCL1 (6 h) | ICAM-1, VCAM-1 no change | No impact on DCs in tumor & lymph nodes | [77] |
| Mouse Brain (Healthy) | 1.1 MHz, 0.4 MPa | IL1α, IL1β, TNF, IFNγ, CCL2, CXCL16, CCL3 (6 h) | [93] | ||
| Mouse Brain (Healthy) | 1.1 MHz, 0.4 MPa | CCL3, CCL12, CCL4, CCL6, CCL9 (6–24 h) | – | – | [94] |
| Rat Brain (Healthy) | 0.59 MHz, 0.3 MPa | TNFα, IL1α, IL1β, IL18, IFNγ CCL12, CXCL1, CXCL3 (< 24 h) | pAkt, pGSK3β, HSP70, ICAM-1 | Macrophages (CD68+) | [74] |
| Rat Brain (Healthy) | 0.55 MHz, ~ 0.2 MPa | TNF, IL1b, CCL2, CCL5 (6 h) | ICAM-1 | – | [24] |
| Rat Brain (Healthy) | 0.55 MHz, 0.18 MPa | Sele, Cxcl1, Ccl3, Ccl2 (return to baseline at 24 h) | [95] | ||
| Rat Brain (Healthy)) | 0.55 MHz, 0.18 MPa | – | – | Microglial & Astrocyte activation | [76] |
Some of the entries in the table display a subset of the molecules and transcripts reported in the papers (see original publications for additional info). The reported responses indicate an increase in expression, unless otherwise stated
Although most of the studies so far have been conducted in combination with MBs, investigations in extracranial malignancies have demonstrated that histotripsy can also activate the adaptive immune system and promote immune responses [86] (Table 2). Such responses include but are not limited to, recruiting of dendritic cells, macrophage, NK cells, CD4 +, CD8 + T cells and leading to robust immune responses, including protection against tumor re-challenge [87–92]. While these preliminary data are promising in support of the potential for histotripsy as an immunomodulatory strategy, additional investigations in brain tumors are needed to demonstrate their impact on the distinct brain TME.
Table 2.
Representative data on immune responses in extracranial malignancies under FUS histotripsy
| Model | Exposure | Cytokines Chemokines | Proteins | Immune Cells | Key observations | References |
|---|---|---|---|---|---|---|
|
| ||||||
| N2A – Neuroblastoma (not orthotopic tumor) | 1.5 MHz 14 MPa (P−) | IL2, GM-CSF, VEGF-A (decrease), IL6, IFN-γ, IL10 (decrease) | PD-L1, Hspb7, lipocalin 2 (Lcn2), Hsp70, Cd72 | CD4+ T cells, CDS+ T cells, CD68 +, NK, DC (CDllc +) | FUS in combination with aCTLA-4 ϸ aPD-L1 enhances antitumor response and cures neuroblastoma tumors in the flank | [87] |
| MC-38 colon adenocarcinoma | 3.3 MHz 12.5 MPa (P−) | IFN-³ | – | DC (CD1 lc +; increased infiltration in tumor) | FUS treatment caused a reduction in the growth of primary tumors and provided protection against subcutaneous tumor re-challenge | [89] |
| RM-9 Prostate Cancer | 3.3 MHz 10 MPa (P−) | IFN-γ | − | DC, CDS + | FUS combined with surgery significantly inhibits the growth of rechallenged tumors, and down-regulates intra-tumoral STAT3 activities | [90] |
| B16GP33 Melanoma / Hepal-6Hepatocellular carcinoma | 1 MHz 30 MPa (P−) | HMGB1 | – | NK, DC, neutrophils, macrophages, B cells, CD4 + T cells, and antigen-specific CDS + T cells (infiltration) | Histotripsy augments the efficacy of checkpoint inhibition immunotherapy (anti-CTLA-4 mAb) | [91] |
| Renal cell carcinoma (RCC) | 1.5 MHz 17–20 MPa (P") | HMGB1 TNF IL6, IL10 |
– | CDS + T cells (infiltration) | No trends were observed in plasma levels in any of the other cytokines analyzed (IL-lα and β, IL-5, IL-8 and IL-13) | [92] |
While these findings suggest that this ultrasound mode is unlikely to be sufficient to promote robust immune responses, one should also keep in mind that the immune responses are very sensitive to sonication pressure, MB type and dose, and type of anesthesia, among others [24, 28, 77, 96], and current studies have tested only a fraction of the possible settings. Refining our understanding on the role of MB type and dose, which seems to have a significant impact on the expression of several key genes involved in acute inflammation and immune activation, including TNF, BIRC3, and CCL2 [24], is the next critical step towards identifying settings that produce robust immune responses. It is also conceivable that sonicating the meninges and cervical lymph nodes, in addition to brain tumors, might lead to improved antigen presentation and DC maturation. Therefore, more systematic investigations, using different tumor models, sonication parameters and sites should be considered to fully assess the impact of MB-FUS towards improving antigen presentation and eliciting robust immune responses. Investigations in extracranial malignancies using mechanical acoustic ablation to promote tissue fractionation (i.e., histotripsy), where increased numbers of CD8 + cells have been observed in the targeted tumors, suggest that the best responses should be attained at or above the threshold for inertial cavitation [90–92]. However, the capability of excessive mechanical stress (i.e., inertial cavitation) to induce tissue damage or generate vigorous immune responses in the brain must be carefully considered.
Immunotherapy
While MB-FUS mechanical stress alone may be insufficient for promoting antitumor immunity, recent investigations suggest that the combination of MB-FUS with immune checkpoint inhibitors and/or adoptive cell therapy, among others, can lead to robust responses (Table 3). In particular, MB-FUS combined with anti-PD1 was able to significantly increase the accumulation of anti-PD1 in GL261 gliomas, with best anti-PD1 delivery achieved by employing multiple sonications [37]. The improved delivery also led to increased survival, as compared to control (anti-PD1 alone). While it is not clear what was the relative contribution of anti-PD1 in reprogramming immune cells within the brain tumors versus systemically, it is possible that the therapeutic activity of anti-PD-1 is mediated by M1-like polarization of the microglia in the brain TME [97]. Perhaps a more direct way to assess the impact of MB-FUS in combination antibody-based immunotherapy was proposed by Sheybani et al. where, using the same tumor model, they targeted phagocytic cells (e.g., macrophages) using the mCD47 antibody [82]. This antibody is directed against the CD47 antiphagocytosis cell surface protein, which is highly expressed in this tumor model. In addition to demonstrating improved survival as compared to anti-mCD47 alone, this study also suggested that the timing of anti-mCD47 administration with respect to the sonication can be another significant variable to consider, with post sonication administration being the best. To explain these findings the authors suggested that BBB opening before mCD47 administration may ensure that the BBB is open as mCD47 makes its first pass through the circulation, thereby leading to improved uptake. Additional studies, potentially with different tumors and antibodies with different affinities to cancer and stroma cells, may allow to better understand the principle behind these observations. Nevertheless, considering that CD47 + macrophages constitute a significant fraction of cell population in gliomas in humans, this combination is one of the most promising to translate to the clinic, especially if the drug has the same level of activity in humans.
Table 3.
Summary of studies on MB-FUS mediated mechanical stress in combination with immunotherapy
| Therapy | Model | Key observations | References |
|---|---|---|---|
|
| |||
| Anti-CD47 | GL261 glioma | mCD47 delivery across the BBB/BTB with repeat sessions of FUS can significantly constrain tumor outgrowth and extend survival in glioma-bearing mice The timing of antibody injection relative to FUS BBB/BTB disruption is a critical determinant of mCD47 access, with post-FUS injection conferring superlative antibody delivery to gliomas |
[82] |
| Anti-PD1 | GL261 glioma | Mice treated with anti-PD-1 and MB-FUS had a median survival duration of 58 days compared to 39 days for mice treated with anti-PD-1 alone (P = 0.6226) | [37] |
| Anti-PD1 | GL261 glioma (not orthotopic tumor) | This study employed mechanical ablation mediated by perfluorocarbon (PFC) liquid filled silica microshells. Significant temperature elevation was observed during the sonications Significant tumor infiltrating lymphocytes and IFN-γ levels were observed at the targeted site Only the combined treatment (mechanical/thermal ablation + anti-PDl) improved outcomes |
[99] |
| Anti-VEGFA (Bevacizumab) | U87 glioma | The combined MB-FUS and bevacizumab administration strategy resulted in a significantly improved median survival (73 days), which was 2.35-fold higher than the median survival in the control group (P < 0.0001) and 1.58-fold higher than that in the bevacizumab-alone group (P > 0.01) | [114] |
| IL-12 | C6 glioma | MB-FUS combined with IL-12 treatment increased IL-12 brain deposition, generated the highest CD8 + /T-reg ratio, inhibited brain tumor growth, and improved survival rate | [115] |
| Trastuzumab & pertuzumab | MDA-MB-361 HER2+ Breast Brain Met | A subset of animals in the FUS + antibody (trastuzimab and pertuzumab) showed slower tumor growth rate (responders) | [116] |
| HER2-specific NK-92 cells | MDA-MB-231-HER2+ breast brain metastasis | Cytolytic activity of the targeted NK-92 cell line was confirmed. Early (front-loaded) intensive treatment protocol reduced tumor growth and improved survival Administration of the NK cells before MB-FUS provided the best data |
[38, 100] |
| Adoptive cell transfer of activated OT-1 cells (TCR transgenic CD8 + T cells specific for OVA) | B16F1cOVA, melanoma brain metastasis | There were no differences in the number of transferred OT-1 cells in the tumor or meninges in MB-FUS treated versus sham animals (18 h post treatment) MB-FUS did not elicit significant systemic response |
[77] |
| Delivery of EGFRvIII-CAR T | EGFRvIII-U87 glioma | CAR T-cell administration with MB-FUS resulted in significant increases in CAR T-cell delivery to the CNS after 24 (P < 0.005) and 72 (P < 0.001) hours and increased median survival by greater than 129%, in comparison with CAR T cells alone | [37] |
| Delivery of CXCL10 expressing APCs | GL261 glioma | Local deposition of CXCL10-secreting APCs in the glioma microenvironment with MB-FUS enhanced T-cell glioma infiltration during the therapeutic window (p = 0.004) and enhanced survival (p < 0.05) | [37] |
More invasive approaches based on local microbubble administration and FUS-mediated destruction to promote local transfection of cancer and stromal cells with plasmid DNA encoding inflammatory markers (e.g. IFN-β) and have shown to augment anti-PD1 efficacy in peripheral tumors, should also be considered [98]. Moreover, mechanical ablation mediated by perfluorocarbon (PFC) liquid filled silica micro-shells (directly injected to the tumor) in combination with systemically administered anti-PD1 can lead to significant tumor infiltrating lymphocytes and IFN-γ levels in GL261 tumor inoculated in the right flank [99]. Interestingly, in this study improved outcomes observed only in the combination group. However, during the sonications significant temperature rise was also observed, hence the reported responses cannot be solely attributed to mechanical effects. Also, ectopic tumors, as the one used in this study, are not able to capture the TME and therapeutic trafficking that characterizes brain (orthotopic) tumors, hence the observed immune responses might not translate well to the brain.
The combination of MB-FUS with adoptive immune cell therapy is another promising therapeutic strategy. Early evidence suggested that MB-FUS leads to greater accumulation of HER-2 specific NK-92 cells in a model of HER2 + breast cancer brain metastasis [38]. These investigators also demonstrated that the treatment protocol and administration of NK cells with respect to sonication is critical, with front-loaded treatment and NK-92 administration before sonication providing the best responses [38, 100]. While the exact mechanism in which the order of BBB opening and cell injection impacts delivery of the immune cells is not understood, the authors suggested that inter-endothelial clefts may be larger during the sonication, which can facilitate the extravasation of effector cells [100]. They also noted that the expression of proteins involved in immune cell adhesion to the endothelium might be more pronounced shortly after exposure, hence circulating HER2-specific NK-92 cells might adhere to endothelium more effectively. It is also conceivable that the brief microbubble—NK-92 cell interaction during the sonication might trigger the transmigration process across the BBB of circulating or already adhered the endothelium NK-92 cells. Additional investigations, possibility in combination with intravital microscopy for direct visualization of transmigration, are needed to elucidate the underlying mechanisms.
More recent investigations using CART EGFRvIII cells and similar treatment protocol have also demonstrated increased homing and improved median survival in NSG mice (immunodeficient) with EGFRvIII-U87 gliomas [37]. While these findings are favorable for combining MB-FUS with cell-based immunotherapy, other investigations showed limited homing and cytotoxicity of activated T cells to melanoma metastasis (B16F1ZsGreenOVA in immunocompetent mice) brain tumors [77]. While it is not clear why the former approaches work and the latter not, these data suggest that better understanding of the lymphocyte and endothelial interactions within the brain TME along with more detailed characterization of the (immunosuppressive) TME and effectiveness of the immune adjuvant employed is needed. In addition, closed-loop control and advanced MB formulations [24, 28, 101] should allow to further refine this combination and develop effective therapeutic strategies based on highly tuned immuno-mechano-biological mechanisms. In addition to exploring the combination of MB-FUS with immune adjuvants, utilizing the excellent capabilities of this technology for targeted gene delivery [102], may create new possibilities for modifying the brain TME and promote antitumor immunity in combination with immune adjuvants [103].
Thermal stress
Immunomodulation
Locally applied thermal stress or local hyperthermia is broadly separated into two different regimens: mild hyperthermia that utilizes mildly elevated physiologic temperatures (38 – 42 °C for up to one hour; sub-ablative fibrillar heating) that can promote heat-dependent physiological changes and high temperature focal hyperthermia, also referred to as thermal ablation (> 45 °C for a few seconds; ablative heating), which can directly kill cancer cells and reduce tumor burden [104]. Ablative heating is characterized by the ablation zone and the periablative or transition zone (i.e., the zone between direct coagulative cytotoxic effects and more reversible thermal exposures), where cells receive a sublethal thermal dose, but instead experience thermal stresses that ultimately give rise to alternative routes of cellular response, such as apoptosis, immunogenic cell death, and others [105]. For pulses with high amplitude and short duration (< 3 s) the heat is primarily dissipated at the ablation zone, resulting in the thinnest possible transition zone (i.e., limited heat diffusion) [106].
Mild hyperthermia creates unique opportunities to promote mass transport across the physical barriers and interfaces of brain tumors. Recent investigations, using a MRg-FUS system, designed to safely apply mild hyperthermia in rodents through the intact skull, and Dynamic Contrast Enhanced MRI (DCE-MRI) showed that ultrasound-mediated thermal stress (≈ 41 °C for 10 min) led to increased Ktrans values ( Ktrans reflects the combined effects of blood flow, vascular permeability, and capillary surface area [107]) in the brain TME [35]. Improved perfusion can have immunostimulatory effect by reducing tumor hypoxia and improving therapeutic trafficking and effectiveness [66]. Localized thermal stress at non-ablative thermal doses has also been shown to reduce the interstitial fluid pressure (IFP) in peripheral tumor models and increase cell membrane fluidity and heat shock protein production (HSP), which can mediate a range of immune responses [108–110]. Additional responses to mild hyperthermia that have been observed in extracranial tumors include the up-regulation of proinflammatory cytokines and chemokines, DC maturation, and increased cytotoxic activity of NK and CD8 + T cells [111, 112].
Despite the many and different ways that FUS mediated mild hyperthermia can modulate the TME, its application in brain tumors remains unexplored. This is partially due to challenges associated with applying hyperthermia safely in the brain using FUS technology. One of the main challenges is presence of the skull bone, which can attenuate, reflect, and distort the ultrasound beam, resulting in inefficient delivery and off-target effects [1]. However, as we alluded to recent systems using finely tuned trans-skull FUS systems to maximize acoustic energy deposition in the brain (as compared to skull) combined with closed-loop methods based on MR temperature imaging (MRTI) can attain and sustain sub-ablative thermal stress in the brain (i.e., below tissue damage) [35]. In the clinical setting, the Exablate system with central frequency of 220 kHz, should be able to safely apply sub-ablative thermal stress. While extended heating durations and treatment of large volumes might still be challenging, exploring thermal dose fractionation protocols, which have shown to augment antitumor responses and improve survival in extracranial malignancies [113], along with different sonication schemes (e.g., beam steering, spherical sonication trajectories, etc.) can potentially address these challenges, thereby allowing to assess the still unexplored immunomodulatory potential of FUS-meditated mild hyperthermia in brain tumors.
The relationship between thermal ablation and the expression of local or systemic inflammatory responses has also been primarily studied in extracranial malignancies. Besides irreversible cell damage, thermal ablation leads to the release of tumor antigens and multiple bioactive molecules, such as damage-associated molecular patterns (DAMPs; e.g., HSP), which can lead to DC maturation and the secretion of cytokines such as TNF-α and IFN-γ by CD8 + T lymphocytes, among others [111, 123–125]. Changes in BBB permeability has also been observed both under FUS in healthy brains [36] and other thermo-ablative technologies in brain tumors [126, 127]. However, it is not clear whether the observed BBB leakiness is driven by direct effects of hyperthermia on the vasculature or by other effects of thermal ablation, such as heat-induced (neuro) inflammation or other mechanisms. Responses that have also been observed in the clinic, include increased infiltration of DCs, macrophages and CD3 +, CD4 + and CD8 + lymphocytes in the margins of breast tumors, albeit in a subset of patients [128, 129], along with a small but statistically significant decrease in the immunosuppressive cytokines, including VEGF, TGF-β1 and -β2 [130]. Despite these encouraging findings thermal ablation may also shut down perfusion and trafficking of therapeutic agents, while there is a concern that premature cell death could trigger immunosuppressive responses, which are consistent with the induction of a physiological wound healing response, that ultimately may hinder its potential to promote anti-tumor immunity [125]. Overall, there is a consent that thermal ablation does significantly influence the immune system, but the overall antitumor immune response is not durable and to some extent inconsistent, presumably due to the stimulation of a mixture of pro- and anti-inflammatory responses [64, 111, 124, 125].
Despite the potential limitations of hyperthermia to produce robust antitumor immune responses, establishing thermal dose dependent physiological responses along with detailed assessment of its impact on the BBB phenotype (e.g., permeability, upregulation of adhesion molecules, etc.) and heat-responsive and acute inflammation processes in the brain TME (e.g., HSP production, release of cytokines and chemokines, neutrophil-to-lymphocyte ratio [131], etc.), it is expected to provide sufficient information to determine if and how hyperthermia and immune adjuvants should be combined to treat brain tumors. Moreover, targeting a fraction of the tumor, especially at ablative temperatures, will preserve perfusion and presentation of bioactive molecules that may in turn boost responses. For example, sparse-scan FUS mediated ablation treatment regime was able to provide increased DC infiltration and maturation in MC-38 adenocarcinoma and B16 melanoma tumors, as compared to a dense-scan regime [132]. It is also possible to elicit different responses by targeting selectively the tumor core or infiltrating margin under different pulse amplitudes and durations.
Immunotherapy
Investigations combining hyperthermia stimuli with immune adjuvants in brain tumors are currently limited in the literature, thus only extrapolation from extracranial malignancies is possible (Table 4). Most notably, the combination of hyperthermia (thermal ablation) with Toll-like receptor agonist CpG and anti-PD1 was shown to upregulate innate receptors across multiple families and alter the polarization of infiltrating macrophages, leading to enhanced systemic immune responses [120]. This combination also amplified antigen cross-presentation in the draining lymph node, increased type I interferon (IFN) release from tumor cells, and polarized macrophages and dendritic cells towards a CD169 subset [119]. Interestingly, the investigators also demonstrated that seven days of anti-PD-1 before ablation reduced macrophages and myeloid-derived suppressor cells and enhanced IFN-γ–producing CD8 + T cells and PD-L1 expression on CD45 + cell, indicating that the timing of immunotherapy and thermal ablation is critical for attaining robust responses [118, 121]. Another study combined FUS mediated thermal ablation with gemcitabine (GEM), which attenuates MDSC function, to treat triple-negative breast cancer (4T1 tumors) [122]. This combination significantly attenuated tumor growth and extended overall survival that also correlated with increased circulating antigen-experienced T cells. Interestingly, the ability of FUS + GEM to control tumor growth was moderately enhanced by either neoadjuvant or adjuvant treatment with anti-PD1, suggesting that PD-L1 was not significantly expressed in MDSCs treated with GEM. While FUS-mediated hyperthermia in combination with immune adjuvants has not been tested in brain tumors yet, several clinical investigations using different thermo-ablative technologies (e.g. LITT) are currently testing this approach in gliomas [64]. Beyond exploring the ability of thermal stress to facilitate immune cell trafficking and cytotoxic activity in the brain TME, mild hyperthermia can also be used to directly promote anti-tumor immunity, for example, by remotely controlling genetically engineered T cells with thermal gene switches to promote expression of tumor-targeting receptors, among others [133, 134].
Table 4.
Summary of studies on FUS mediated thermal stress in combination with immunotherapy. All studies are from peripheral tumors
| Therapy | Model | Key observations | References |
|---|---|---|---|
|
| |||
| DCs loaded with tumor antigens | H22 hepatocellular carcinoma | DCs treated with thermal-ablated tumor debris lead to increased magnitude of mature DCs and greater IL-12 and IFN-y secretion DCs loaded with HIFU-ablated tumor inhibit tumor growth in vivo and provide slightly longer survival |
[117] |
| Toll-like receptor agonist CpG & anti-PDl | Orthotopic neu exon deletion line (NDL) model of mammary adenocarcinoma | 7 days of immunotherapy, prior to thermal ablation, reduced macrophages and myeloid-derived suppressor cells and enhanced IFN-γ-producing CD8 + T cells, the Ml macrophage fraction, and PD-L1 expression on CD45 + cell Immunotherapy begun before ablation can be curative and can enhance efficacy in the presence of a high tumor burden |
[118] |
| CpG & anti-PD-1 administration | neu exon deletion line (NDL), B16-F10, and B 16-OVA | Combined ablation-immunotherapy treatment amplified cross-presentation in the draining lymph node, increased type I interferon (IFN) release from tumor cells, and polarized macrophages and dendritic cells towards a CD169 subset Directly treated tumors were skewed to high expression of F4/S0, Cell lb and Tnf and the distant tumors to enhanced Cd11c, Cd3 and Ifng |
[119] |
| CpG and αPD-1 administration | neu exon deletion line (NDL) | The combined ablation-immunotherapy protocol upregulates innate receptors across multiple families and alters the polarization of infiltrating macrophages, thereby facilitating a strong systemic immune response Further upregulated expression of Nod2, Oas2, RhoA, Pycard, Tlr1/2 and Il12, and enhanced T-cell number and activation while polarizing macrophages to an anti-tumor phenotype |
[120] |
| Temperature-sensitive liposomal Dox (CuDox- TSL), Toll-like receptor agonist CpG and anti-PDl | neu-deletion (NDL) tumor, B16-F10/B16-OVA melanoma, and MMTV-PyMT | Hyperthermia therapy combining activatable drug delivery (CuDox-TSL) and immunotherapy (CpG + anti-PDl) or immunotherapy alone enhanced tumor infiltrating CDS +T-cells and myeloid recruitment in treated and distant lesions Complete response rate was greatest (90%) when 1 week of immunotherapy priming preceded a single activatable chemotherapeutic administration |
[121] |
| Gemcitabine (GEM), anti-PDl | 4T1 Triple-negative breast cancer (TNBC) | The combination of FUS thermal ablation and GEM significantly controlled primary TNBC tumor outgrowth and extended overall survival of mice (correlated with increased circulating antigen-experienced T cells) The ability of FUS thermal ablation and GEM to control primary tumor outgrowth was moderately enhanced by either neoadjuvant or adjuvant treatment with anti-PDl |
[122] |
Combination with other modalities
Focused ultrasound in the form of either thermal or mechanical stress may also act as a radiosensitizer [135, 136]. An early clinical study reported the feasibility of ultrasound-mediated hyperthermia combined with radiotherapy for the treatment of primary malignant tumors of the brain [137]. In that study, fifteen patients with a histological diagnosis of primary malignant tumors of a cerebral hemisphere received the combined therapy repeatedly. Despite the difficulty with applying FUS hyperthermia in the brain at the time, this study was able to achieve hyperthermic temperatures (≥ 42.5 °C) demonstrating the feasibility of this treatment combination. Interestingly, recent investigations exploring the interaction of radiation with MB-FUS, indicated that both radiation can augment the effects of MB-FUS BBB opening [138] and MB-FUS can sensitize tumors to radiotherapy. These combined effects have been shown to modulate vascular function and further increase tumor cell death [139, 140]. Although additional investigations are needed these studies highlight the potential synergies between these two modalities.
Summary and perspective
It is increasingly apparent that most CNS tumors, especially infiltrating gliomas, are immunologically cold. Hence combination strategies that activate the tumor-immune environment will likely be a key component of effective, durable treatments. Major barriers to treatment efficacy include this unique tumor-immune environment and the BBB. The role and potential therapeutic impact of controlled thermal and mechanical stress in tumor-immune modulation is increasingly being recognized. Focused ultrasound is an emerging clinical tool which offers the capability of tuning and prescribing thermal and mechanical treatments non-invasively within the brain. In addition, FUS can open critical barriers to drug delivery and augment drug transport in the brain tumor microenvironment. Furthermore, focused ultrasound can directly modify the tumor microenvironment through mechanisms such as modulating critical immunosuppressive myeloid cells and promoting a proinflammatory milieu. Taken together, this technological approach offers new opportunities to extend and combine with immunotherapies for transformative treatment of brain tumors.
The rapid advancement of FUS technologies into clinical trials and reimbursed patient treatments has accelerated concepts for new immunotherapeutic combinations. With the first-in-human trial combining MB-FUS with nivolumab in melanoma brain metastases enrolling patients (NCT04021420) more studies are expected to follow. While this is exciting period to explore FUS technology for other tumors, such as GBMs that are characterized by low lymphocytic infiltration and complex adaptive mechanisms of immunosuppression, among others, additional parallel efforts to detail rational therapeutic strategies supported by rigorous preclinical studies to identify unique synergies between the two technologies will be critical. These parallel efforts will accelerate the discovery and clinical implementation of new and effective FUS immunotherapeutic combinations for brain tumor patients.
Acknowledgements
Costas Arvanitis research in this area is supported by the NIH (National Institutes of Health) Grant R37CA239039 (National Cancer Institute) and the Ian’s Friends Foundation. Graeme Woodworth’s research in this area is supported by NIH Grant R21NS113016 and the Focused Ultrasound Foundation
Funding
Costas Arvanitis research in this area is supported by NIH Grant R37CA239039 and the Ian’s Friends Foundation. Graeme Woodworth’s research in this area is supported by NIH Grant R21NS113016 and the Focused Ultrasound Foundation.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Declarations
Ethical approval This article does not contain any studies with human participants or animals performed by any of the authors.
Data Availability All the associated data in this review paper are included in the paper (Tables) or can be found in the cited literature.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Meng Y, Hynynen K, Lipsman N (2021) Applications of focused ultrasound in the brain: from thermoablation to drug delivery. Nat Rev Neurol 17:7–22. 10.1038/s41582-020-00418-z [DOI] [PubMed] [Google Scholar]
- 2.ter Haar >Gail, Coussios C, (2007) High intensity focused ultrasound: Physical principles and devices. Int J Hyperthermia 23:89–104. 10.1080/02656730601186138 [DOI] [PubMed] [Google Scholar]
- 3.ter Haar G (2007) Therapeutic applications of ultrasound. Prog Biophys Mol Biol 93:111–129 [DOI] [PubMed] [Google Scholar]
- 4.McDannold N, Clement GT, Black P et al. (2010) Transcranial magnetic resonance imaging- guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery 66:323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elias WJ, Huss D, Voss T et al. (2013) A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med 369:640–648. 10.1056/NEJMoa1300962 [DOI] [PubMed] [Google Scholar]
- 6.Elias WJ, Lipsman N, Ondo WG et al. (2016) A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med 375:730–739. 10.1056/NEJMoa1600159 [DOI] [PubMed] [Google Scholar]
- 7.Martínez-Fernández R, Máñez-Miró JU, Rodríguez-Rojas R et al. (2020) Randomized trial of focused ultrasound subthalamotomy for parkinson’s disease. N Engl J Med 383:2501–2513. 10.1056/NEJMoa2016311 [DOI] [PubMed] [Google Scholar]
- 8.Song K-H, Harvey BK, Borden MA (2018) State-of-the-art of microbubble-assisted blood-brain barrier disruption. Theranostics 8:4393–4408. 10.7150/thno.26869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dauba A, Delalande A, Kamimura HAS et al. (2020) Recent advances on ultrasound contrast agents for blood-brain barrier opening with focused ultrasound. Pharmaceutics 12:1125. 10.3390/pharmaceutics12111125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stride E, Coussios C (2019) Nucleation, mapping and control of cavitation for drug delivery. Nat Rev Phys. 10.1038/s42254-019-0074-y [DOI] [Google Scholar]
- 11.Aryal M, Arvanitis CD, Alexander PM, McDannold N (2014) Ultrasound-mediated blood–brain barrier disruption for targeted drug delivery in the central nervous system. Adv Drug Deliv Rev 72:94–109. 10.1016/j.addr.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA (2001) Noninvasive MR imaging–guided focal opening of the blood-brain barrier in rabbits1. Radiology 220:640–646. 10.1148/radiol.2202001804 [DOI] [PubMed] [Google Scholar]
- 13.Meairs S (2015) Facilitation of drug transport across the blood-brain barrier with ultrasound and microbubbles. Pharmaceutics 7:275–293. 10.3390/pharmaceutics7030275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoen S, Kilinc MS, Lee H et al. (2022) Towards controlled drug delivery in brain tumors with microbubble-enhanced focused ultrasound. Adv Drug Deliv Rev 180:114043. 10.1016/j.addr.2021.114043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carpentier A, Canney M, Vignot A et al. (2016) Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med 8:3432. 10.1126/scitranslmed.aaf6086 [DOI] [PubMed] [Google Scholar]
- 16.Mainprize T, Lipsman N, Huang Y et al. (2019) Blood-brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: a clinical safety and feasibility study. Sci Rep 9:321. 10.1038/s41598-018-36340-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipsman N, Meng Y, Bethune AJ et al. (2018) Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat Commun 9:2336. 10.1038/s41467-018-04529-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Idbaih A, Canney M, Belin L et al. (2019) Safety and Feasibility of Repeated and Transient Blood-Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma. Clin Cancer Res Clincanres. 10.1158/1078-0432.CCR-18-3643 [DOI] [PubMed] [Google Scholar]
- 19.Miller MW, Miller DL, Brayman AA (1996) A review of in vitro bioeffects of inertial ultrasonic cavitation from a mechanistic perspective. Ultrasound Med Biol 22:1131–1154. 10.1016/S0301-5629(96)00089-0 [DOI] [PubMed] [Google Scholar]
- 20.Cleve S, Inserra C, Prentice P (2019) Contrast Agent Micro-bubble Jetting during Initial Interaction with 200-kHz Focused Ultrasound. Ultrasound Med Biol 45:3075–3080. 10.1016/j.ultrasmedbio.2019.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Kreider W, Brayman AA et al. (2011) Blood vessel deformations on microsecond time scales by ultrasonic cavitation. Phys Rev Lett 106:034301. 10.1103/PhysRevLett.106.034301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Todd N, Angolano C, Ferran C et al. (2020) Secondary effects on brain physiology caused by focused ultrasound-mediated disruption of the blood–brain barrier. J Controlled Release 324:450–459. 10.1016/j.jconrel.2020.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon D, O’Reilly MA, Hynynen K (2021) Therapeutic agent delivery across the blood-brain barrier using focused ultrasound. Annu Rev Biomed Eng. 10.1146/annurev-bioeng-062117-121238 [DOI] [PubMed] [Google Scholar]
- 24.McMahon D, Hynynen K (2017) Acute inflammatory response following increased blood-brain barrier permeability induced by focused ultrasound is dependent on microbubble dose. Theranostics 7:3989–4000. 10.7150/thno.21630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovacs ZI, Kim S, Jikaria N et al. (2016) Disrupting the blood–brain barrier by focused ultrasound induces sterile inflammation. Proc Natl Acad Sci. 10.1073/pnas.1614777114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arvanitis CD, Livingstone MS, Vykhodtseva N, McDannold N (2012) Controlled ultrasound-induced blood-brain barrier disruption using passive acoustic emissions monitoring. PLoS ONE 7:e45783. 10.1371/journal.pone.0045783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baseri B, Choi JJ, Tung Y-S, Konofagou EE (2010) Multi-modality safety assessment of blood-brain barrier opening using focused ultrasound and definity microbubbles: a short-term study. Ultrasound Med Biol 36:1445–1459. 10.1016/j.ultrasmedbio.2010.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMahon D, Lassus A, Gaud E et al. (2020) Microbubble formulation influences inflammatory response to focused ultrasound exposure in the brain. Sci Rep 10:21534. 10.1038/s41598-020-78657-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arvanitis CD, Vykhodtseva N, Jolesz F et al. (2015) Cavitation-enhanced nonthermal ablation in deep brain targets: feasibility in a large animal model. J Neurosurg 124:1450–1459. 10.3171/2015.4.JNS142862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDannold N, Zhang Y, Vykhodtseva N (2016) Nonthermal ablation in the rat brain using focused ultrasound and an ultrasound contrast agent: long-term effects. J Neurosurg 125:1539–1548. 10.3171/2015.10.JNS151525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng C, Sun T, Vykhodtseva N et al. (2019) Intracranial nonthermal ablation mediated by transcranial focused ultrasound and phase-shift nanoemulsions. Ultrasound Med Biol 45:2104–2117. 10.1016/j.ultrasmedbio.2019.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sukovich JR, Cain CA, Pandey AS et al. (2018) In vivo histotripsy brain treatment. J Neurosurg. 10.3171/2018.4.JNS172652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hendricks-Wenger A, Hutchison R, Vlaisavljevich E, Allen IC (2021) Immunological effects of histotripsy for cancer therapy. Front Oncol 11:681629. 10.3389/fonc.2021.681629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anastasiadis P, Gandhi D, Guo Y, Ahmed A-K, Bentzen SM, Arvanitis C, Woodworth GF (2021) Localized blood–brain barrier opening in infiltrating gliomas with MRI-guided acoustic emissions–controlled focused ultrasound. Proc Natl Acad Sci 118(37):e2103280118. 10.1073/pnas.2103280118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim C, Guo Y, Leisen J et al. (2021) Closed-loop trans-skull ultrasound hyperthermia leads to improved drug delivery from thermosensitive drugs and promotes changes in vascular transport dynamics in brain tumors. Theranostics 11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDannold N, Vykhodtseva N, Jolesz FA, Hynynen K (2004) MRI investigation of the threshold for thermally induced blood-brain barrier disruption and brain tissue damage in the rabbit brain. Magn Reson Med 51:913–923. 10.1002/mrm.20060 [DOI] [PubMed] [Google Scholar]
- 37.Sabbagh A, Beccaria K, Ling X et al. (2021) Opening of the blood-brain barrier using low-intensity pulsed ultrasound enhances responses to immunotherapy in preclinical glioma models. Clin Cancer Res. 10.1158/1078-0432.CCR-20-3760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alkins R, Burgess A, Ganguly M et al. (2013) Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res 73:1892–1899. 10.1158/0008-5472.CAN-12-2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sampson JH, Gunn MD, Fecci PE, Ashley DM (2020) Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer 20:12–25. 10.1038/s41568-019-0224-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engelhardt B, Ransohoff RM (2012) Capture, crawl, cross: the T cell code to breach the blood–brain barriers. Trends Immunol 33:579–589. 10.1016/j.it.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 41.Schläger C, Körner H, Krueger M et al. (2016) Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature 530:349–353. 10.1038/nature16939 [DOI] [PubMed] [Google Scholar]
- 42.Tawbi HA, Forsyth PA, Algazi A et al. (2018) Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med 379:722–730. 10.1056/NEJMoa1805453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Long GV, Atkinson V, Lo S et al. (2018) Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol 19:672–681. 10.1016/S1470-2045(18)30139-6 [DOI] [PubMed] [Google Scholar]
- 44.Zeng J, See AP, Phallen J et al. (2013) Anti-PD-1 Blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol 86:343–349. 10.1016/j.ijrobp.2012.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Filley AC, Henriquez M, Dey M (2017) Recurrent glioma clinical trial, CheckMate-143: the game is not over yet. Oncotarget 8:91779–91794. 10.18632/oncotarget.21586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reardon DA, Brandes AA, Omuro A et al. (2020) Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma. JAMA Oncol 6:1–8. 10.1001/jamaoncol.2020.1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cloughesy TF, Mochizuki AY, Orpilla JR et al. (2019) Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med 25:477–486. 10.1038/s41591-018-0337-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R et al. (2019) Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med 25:470–476. 10.1038/s41591-018-0339-5 [DOI] [PubMed] [Google Scholar]
- 49.O’Rourke DM, Nasrallah M, Morrissette JJ et al. (2016) Pilot study of T cells redirected to EGFRvIII with a chimeric antigen receptor in patients with EGFRvIII+ glioblastoma. J Clin Oncol 34:2067–2067. 10.1200/JCO.2016.34.15_suppl.2067 [DOI] [Google Scholar]
- 50.Brown CE, Alizadeh D, Starr R et al. (2016) Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med 375:2561–2569. 10.1056/NEJMoa1610497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahmed N, Brawley V, Hegde M et al. (2017) HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol 3:1094–1101. 10.1001/jamaoncol.2017.0184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Majzner RG, Ramakrishna S, Yeom KW et al. (2022) GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. 10.1038/s41586-022-04489-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arvanitis CD, Ferraro GB, Jain RK (2020) The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat Rev Cancer 20:26–41. 10.1038/s41568-019-0205-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarkaria JN, Hu LS, Parney IF et al. (2018) Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-Oncol 20:184–191. 10.1093/neuonc/nox175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phoenix TN, Patmore DM, Boop S et al. (2016) Medulloblastoma genotype dictates blood brain barrier phenotype. Cancer Cell 29:508–522. 10.1016/j.ccell.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vanan MI, Eisenstat DD (2015) DIPG in children – what can we learn from the past? Front Oncol. 10.3389/fonc.2015.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lim M, Xia Y, Bettegowda C, Weller M (2018) Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol 15:422–442. 10.1038/s41571-018-0003-5 [DOI] [PubMed] [Google Scholar]
- 58.Rao G, Latha K, Ott M et al. (2020) Anti–PD-1 Induces M1 Polarization in the glioma microenvironment and exerts therapeutic efficacy in the absence of CD8 Cytotoxic T Cells. Clin Cancer Res 26:4699–4712. 10.1158/1078-0432.CCR-19-4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gordon SR, Maute RL, Dulken BW et al. (2017) PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545:495–499. 10.1038/nature22396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morad G, Helmink BA, Sharma P, Wargo JA (2021) Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 10.1016/j.cell.2021.09.020 [DOI] [PubMed] [Google Scholar]
- 61.Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378:158–168. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 62.Thorsson V, Gibbs DL, Brown SD et al. (2018) The immune landscape of cancer. Immunity 48:812–830.e14. 10.1016/j.immuni.2018.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown CE, Aguilar B, Starr R et al. (2018) Optimization of IL13Rα2-targeted chimeric antigen receptor T cells for improved anti-tumor efficacy against glioblastoma. Mol Ther 26:31–44. 10.1016/j.ymthe.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Srinivasan ES, Sankey EW, Grabowski MM et al. (2020) The intersection between immunotherapy and laser interstitial thermal therapy: a multipronged future of neuro-oncology. Int J Hyperthermia 37:27–34. 10.1080/02656736.2020.1746413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jackson CM, Kochel CM, Nirschl CJ et al. (2016) Systemic tolerance mediated by melanoma brain tumors is reversible by radiotherapy and vaccination. Clin Cancer Res Off J Am Assoc Cancer Res 22:1161–1172. 10.1158/1078-0432.CCR-15-1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fukumura D, Kloepper J, Amoozgar Z et al. (2018) Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 15:325–340. 10.1038/nrclinonc.2018.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quail DF, Joyce JA (2017) The Microenvironmental Landscape of Brain Tumors. Cancer Cell 31:326–341. 10.1016/j.ccell.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stoklasek TA, Schluns KS, Lefrancois L (2006) Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol 177:6072–6080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stephan MT, Moon JJ, Um SH et al. (2010) Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med 16:1035–1041. 10.1038/nm.2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dubois S, Patel HJ, Zhang M et al. (2008) Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J Immunol 180:2099–2106 [DOI] [PubMed] [Google Scholar]
- 71.Rubinstein MP, Kovar M, Purton JF et al. (2006) Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha}. Proc Natl Acad Sci U A 103:9166–9171. 10.1073/pnas.0600240103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Waldmann TA (2006) The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol 6:595–601. 10.1038/nri1901 [DOI] [PubMed] [Google Scholar]
- 73.Kaehler KC, Piel S, Livingstone E et al. (2010) Update on immunologic therapy with anti-CTLA-4 antibodies in melanoma: identification of clinical and biological response patterns, immune-related adverse events, and their management. Semin Oncol 37:485–498. 10.1053/j.seminoncol.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 74.Kovacs ZI, Kim S, Jikaria N et al. (2017) Disrupting the blood–brain barrier by focused ultrasound induces sterile inflammation. Proc Natl Acad Sci U S A 114:E75–E84. 10.1073/pnas.1614777114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Poon C, Pellow C, Hynynen K (2021) Neutrophil recruitment and leukocyte response following focused ultrasound and micro-bubble mediated blood-brain barrier treatments. Theranostics 11:1655–1671. 10.7150/thno.52710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sinharay S, Tu T-W, Kovacs ZI et al. (2019) In vivo imaging of sterile microglial activation in rat brain after disrupting the blood-brain barrier with pulsed focused ultrasound: [18F]DPA-714 PET study. J Neuroinflammation 16:155. 10.1186/s12974-019-1543-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Curley CT, Stevens AD, Mathew AS et al. (2020) Immunomodulation of intracranial melanoma in response to blood-tumor barrier opening with focused ultrasound. Theranostics 10:8821–8833. 10.7150/thno.47983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen P-Y, Hsieh H-Y, Huang C-Y et al. (2015) Focused ultrasound-induced blood-brain barrier opening to enhance interleukin-12 delivery for brain tumor immunotherapy: a preclinical feasibility study. J Transl Med 13:93. 10.1186/s12967-015-0451-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kovacs Z, Werner B, Rassi A et al. (2014) Prolonged survival upon ultrasound-enhanced doxorubicin delivery in two syngenic glioblastoma mouse models. J Controlled Release 187:74–82. 10.1016/j.jconrel.2014.05.033 [DOI] [PubMed] [Google Scholar]
- 80.Liu H-L, Hua M-Y, Chen P-Y et al. (2010) Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology 255:415–425. 10.1148/radiol.10090699 [DOI] [PubMed] [Google Scholar]
- 81.Liu H-L, Huang C-Y, Chen J-Y et al. (2014) Pharmacodynamic and Therapeutic Investigation of Focused Ultrasound-Induced Blood-Brain Barrier Opening for Enhanced Temozolomide Delivery in Glioma Treatment. PLoS ONE. 10.1371/journal.pone.0114311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sheybani ND, Breza VR, Paul S et al. (2021) ImmunoPET-informed sequence for focused ultrasound-targeted mCD47 blockade controls glioma. J Controlled Release 331:19–29. 10.1016/j.jconrel.2021.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Treat LH, McDannold N, Zhang Y et al. (2012) Improved antitumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med Biol 38:1716–1725. 10.1016/j.ultrasmedbio.2012.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wei H-J, Upadhyayula PS, Pouliopoulos AN et al. (2021) Focused Ultrasound-mediated blood-brain barrier opening increases delivery and efficacy of etoposide for glioblastoma treatment. Int J Radiat Oncol 110:539–550. 10.1016/j.ijrobp.2020.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao G, Huang Q, Wang F et al. (2018) Targeted shRNA-loaded liposome complex combined with focused ultrasound for blood brain barrier disruption and suppressing glioma growth. Cancer Lett 418:147–158. 10.1016/j.canlet.2018.01.035 [DOI] [PubMed] [Google Scholar]
- 86.Joiner JB, Pylayeva-Gupta Y (1950) Dayton PA (2020) Focused ultrasound for immunomodulation of the tumor microenvironment. J Immunol Baltim Md 205:2327–2341. 10.4049/jimmunol.1901430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eranki A, Srinivasan P, Ries M et al. (2020) High-intensity focused Ultrasound (HIFU) triggers immune sensitization of refractory murine neuroblastoma to checkpoint inhibitor therapy. Clin Cancer Res Off J Am Assoc Cancer Res 26:1152–1161. 10.1158/1078-0432.CCR-19-1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khokhlova V, Fowlkes J, Roberts W et al. (2015) Histotripsy methods in mechanical disintegration of tissue: toward clinical applications. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group 31:145–162. 10.3109/02656736.2015.1007538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu Z, Yang XY, Liu Y et al. (2007) Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. J Transl Med 5:34. 10.1186/1479-5876-5-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang X, Yuan F, Liang M et al. (2012) M-HIFU inhibits tumor growth, suppresses STAT3 activity and enhances tumor specific immunity in a transplant tumor model of prostate cancer. PLoS ONE 7:e41632. 10.1371/journal.pone.0041632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qu S, Worlikar T, Felsted AE et al. (2020) Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. J Immunother Cancer 8:e000200. 10.1136/jitc-2019-000200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schade GR, Wang Y-N, D’Andrea S et al. (2019) Boiling histotripsy ablation of renal cell carcinoma in the EKER rat promotes a systemic inflammatory response. Ultrasound Med Biol 45:137–147. 10.1016/j.ultrasmedbio.2018.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mathew AS, Gorick CM, Thim EA et al. (2020) Transcriptomic response of brain tissue to focused ultrasound-mediated blood–brain barrier disruption depends strongly on anesthesia. Bioeng Transl Med 6:e10198. 10.1002/btm2.10198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gorick CM, Mathew AS, Garrison WJ et al. (2020) Sonoselective transfection of cerebral vasculature without blood–brain barrier disruption. Proc Natl Acad Sci 117:5644–5654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McMahon D, Bendayan R, Hynynen K (2017) Acute effects of focused ultrasound-induced increases in blood-brain barrier permeability on rat microvascular transcriptome. Sci Rep 7:45657. 10.1038/srep45657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kovacs ZI, Tu T-W, Sundby M et al. (2018) MRI and histological evaluation of pulsed focused ultrasound and microbubbles treatment effects in the brain. Theranostics 8:4837–4855. 10.7150/thno.24512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rao G, Latha K, Ott M et al. (2020) Anti-PD-1 induces M1 polarization in the glioma microenvironment and exerts therapeutic efficacy in the absence of CD8 cytotoxic t cells. Clin Cancer Res Off J Am Assoc Cancer Res 26:4699–4712. 10.1158/1078-0432.CCR-19-4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ilovitsh T, Feng Y, Foiret J et al. (2020) Low-frequency ultrasound-mediated cytokine transfection enhances T cell recruitment at local and distant tumor sites. Proc Natl Acad Sci 117:12674–12685. 10.1073/pnas.1914906117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang J, Huang C-H, Echeagaray OH et al. (2019) Microshell enhanced acoustic adjuvants for immunotherapy in glioblastoma. Adv Ther 2:1900066. 10.1002/adtp.201900066 [DOI] [Google Scholar]
- 100.Alkins R, Burgess A, Kerbel R et al. (2016) Early treatment of HER2-amplified brain tumors with targeted NK-92 cells and focused ultrasound improves survival. Neuro-Oncol 18:974–981. 10.1093/neuonc/nov318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kovacs ZI, Burks SR, Frank JA (2018) Focused ultrasound with microbubbles induces sterile inflammatory response proportional to the blood brain barrier opening: Attention to experimental conditions. Theranostics 8:2245–2248. 10.7150/thno.24181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guo Y, Lee H, Fang Z et al. (2021) Single-cell analysis reveals effective siRNA delivery in brain tumors with microbubble-enhanced ultrasound and cationic nanoparticles. Sci Adv 7:7390. 10.1126/sciadv.abf7390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang N, Wang J, Foiret J et al. (2021) Synergies between therapeutic ultrasound, gene therapy and immunotherapy in cancer treatment. Adv Drug Deliv Rev 178:113906. 10.1016/j.addr.2021.113906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dewhirst MW, Lee C-T, Ashcraft KA (2016) The future of biology in driving the field of hyperthermia. Int J Hyperthermia 32:4–13. 10.3109/02656736.2015.1091093 [DOI] [PubMed] [Google Scholar]
- 105.Hersh DS, Kim AJ, Winkles JA et al. (2016) Emerging applications of therapeutic ultrasound in neuro-oncology: moving beyond tumor ablation. Neurosurgery 79:643–654. 10.1227/NEU.0000000000001399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kennedy JE (2005) High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer 5:321. 10.1038/nrc1591 [DOI] [PubMed] [Google Scholar]
- 107.Cuenod CA, Balvay D (2013) Perfusion and vascular permeability: basic concepts and measurement in DCE-CT and DCE-MRI. Diagn Interv Imaging 94:1187–1204. 10.1016/j.diii.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 108.Csoboz B, Balogh GE, Kusz E et al. (2013) Membrane fluidity matters: hyperthermia from the aspects of lipids and membranes. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group 29:491–499. 10.3109/02656736.2013.808765 [DOI] [PubMed] [Google Scholar]
- 109.Watson KD, Lai C-Y, Qin S et al. (2012) Ultrasound increases nanoparticle delivery by reducing intratumoral pressure and increasing transport in epithelial and epithelial-mesenchymal transition tumors. Cancer Res 72:1485–1493. 10.1158/0008-5472.CAN-11-3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Skitzki JJ, Repasky EA (2000) Evans SS (2009) Hyperthermia as an immunotherapy strategy for cancer. Curr Opin Investig Drugs Lond Engl 10:550–558 [PMC free article] [PubMed] [Google Scholar]
- 111.Skandalakis GP, Rivera DR, Rizea CD et al. (2020) Hyperthermia treatment advances for brain tumors. Int J Hyperthermia 37:3–19. 10.1080/02656736.2020.1772512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Frey B, Weiss E-M, Rubner Y et al. (2012) Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia 28:528–542. 10.3109/02656736.2012.677933 [DOI] [PubMed] [Google Scholar]
- 113.Santos MA, Wu S-K, Regenold M et al. (2020) Novel fractionated ultrashort thermal exposures with MRI-guided focused ultrasound for treating tumors with thermosensitive drugs. Sci Adv 6:5684. 10.1126/sciadv.aba5684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu H-L, Hsu P-H, Lin C-Y et al. (2016) Focused ultrasound enhances central nervous system delivery of bevacizumab for malignant glioma treatment. Radiology 281:99–108. 10.1148/radiol.2016152444 [DOI] [PubMed] [Google Scholar]
- 115.Chen P-Y, Hsieh H-Y, Huang C-Y et al. (2015) Focused ultrasound-induced blood–brain barrier opening to enhance interleukin-12 delivery for brain tumor immunotherapy: a preclinical feasibility study. J Transl Med 13:93. 10.1186/s12967-015-0451-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kobus T, Zervantonakis IK, Zhang Y, McDannold NJ (2016) Growth inhibition in a brain metastasis model by antibody delivery using focused ultrasound-mediated blood-brain barrier disruption. J Control Release Off J Control Release Soc 238:281–288. 10.1016/j.jconrel.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Deng J, Zhang Y, Feng J, Wu F (2010) Dendritic Cells Loaded with Ultrasound-Ablated Tumour Induce in vivo Specific Antitumour Immune Responses. Ultrasound Med Biol 36:441–448. 10.1016/j.ultrasmedbio.2009.12.004 [DOI] [PubMed] [Google Scholar]
- 118.Silvestrini MT, Ingham ES, Mahakian LM et al. (2017) Priming is key to effective incorporation of image-guided thermal ablation into immunotherapy protocols. JCI Insight 2:e90521. 10.1172/jci.insight.90521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chavez M, Silvestrini MT, Ingham ES et al. (2018) Distinct immune signatures in directly treated and distant tumors result from TLR adjuvants and focal ablation. Theranostics 8:3611–3628. 10.7150/thno.25613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fite BZ, Wang J, Kare AJ et al. (2021) Immune modulation resulting from MR-guided high intensity focused ultrasound in a model of murine breast cancer. Sci Rep 11:927. 10.1038/s41598-020-80135-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kheirolomoom A, Silvestrini MT, Ingham ES et al. (2019) Combining activatable nanodelivery with immunotherapy in a murine breast cancer model. J Control Release Off J Control Release Soc 303:42–54. 10.1016/j.jconrel.2019.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sheybani ND, Witter AR, Thim EA et al. (2020) Combination of thermally ablative focused ultrasound with gemcitabine controls breast cancer via adaptive immunity. J Immunother Cancer 8:e001008. 10.1136/jitc-2020-001008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Curley CT, Sheybani ND, Bullock TN, Price RJ (2017) Focused Ultrasound immunotherapy for central nervous system pathologies: challenges and opportunities. Theranostics 7:3608–3623. 10.7150/thno.21225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shin DH, Melnick KF, Tran DD, Ghiaseddin AP (2021) In situ vaccination with laser interstitial thermal therapy augments immunotherapy in malignant gliomas. J Neurooncol 151:85–92. 10.1007/s11060-020-03557-x [DOI] [PubMed] [Google Scholar]
- 125.van den Bijgaart RJ, E, Eikelenboom DC, et al. (2017) Thermal and mechanical high-intensity focused ultrasound: perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunol Immunother 66:247–258. 10.1007/s00262-016-1891-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bredlau AL, Motamarry A, Chen C et al. (2018) Localized delivery of therapeutic doxorubicin dose across the canine blood–brain barrier with hyperthermia and temperature sensitive liposomes. Drug Deliv 25:973–984. 10.1080/10717544.2018.1461280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Salehi A, Paturu MR, Patel B et al. (2020) Therapeutic enhancement of blood–brain and blood–tumor barriers permeability by laser interstitial thermal therapy. Neuro-Oncol Adv. 10.1093/noajnl/vdaa071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lu P, Zhu X-Q, Xu Z-L et al. (2009) Increased infiltration of activated tumor-infiltrating lymphocytes after high intensity focused ultrasound ablation of human breast cancer. Surgery 145:286–293. 10.1016/j.surg.2008.10.010 [DOI] [PubMed] [Google Scholar]
- 129.Xu Z-L, Zhu X-Q, Lu P et al. (2009) Activation of tumor-infiltrating antigen presenting cells by high intensity focused ultrasound ablation of human breast cancer. Ultrasound Med Biol 35:50–57. 10.1016/j.ultrasmedbio.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 130.Zhou Q, Zhu X-Q, Zhang J et al. (2008) Changes in circulating immunosuppressive cytokine levels of cancer patients after high intensity focused ultrasound treatment. Ultrasound Med Biol 34:81–87. 10.1016/j.ultrasmedbio.2007.07.013 [DOI] [PubMed] [Google Scholar]
- 131.Figueroa JM, Semonche A, Magoon S et al. (2020) The role of neutrophil-to-lymphocyte ratio in predicting overall survival in patients undergoing laser interstitial thermal therapy for glioblastoma. J Clin Neurosci 72:108–113. 10.1016/j.jocn.2019.12.057 [DOI] [PubMed] [Google Scholar]
- 132.Liu F, Hu Z, Qiu L et al. (2010) Boosting high-intensity focused ultrasound-induced anti-tumor immunity using a sparse-scan strategy that can more effectively promote dendritic cell maturation. J Transl Med 8:7. 10.1186/1479-5876-8-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gamboa L, Zamat AH, Kwong GA (2020) Synthetic immunity by remote control Theranostics 10:3652–3667. 10.7150/thno.41305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu Y, Liu Y, Huang Z et al. (2021) Control of the activity of CAR-T cells within tumours via focused ultrasound. Nat Biomed Eng. 10.1038/s41551-021-00779-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Meng Y, Pople CB, Budiansky D et al. (2022) Current state of therapeutic focused ultrasound applications in neuro-oncology. J Neurooncol 156:49–59. 10.1007/s11060-021-03861-0 [DOI] [PubMed] [Google Scholar]
- 136.Schneider CS, Woodworth GF, Vujaskovic Z, Mishra MV (2020) Radiosensitization of high-grade gliomas through induced hyperthermia: Review of clinical experience and the potential role of MR-guided focused ultrasound. Radiother Oncol J Eur Soc Ther Radiol Oncol 142:43–51. 10.1016/j.radonc.2019.07.017 [DOI] [PubMed] [Google Scholar]
- 137.Guthkelch AN, Carter LP, Cassady JR et al. (1991) Treatment of malignant brain tumors with focused ultrasound hyperthermia and radiation: results of a phase I trial. J Neurooncol. 10.1007/BF00177540 [DOI] [PubMed] [Google Scholar]
- 138.White PJ, Zhang Y-Z, Power C et al. (2020) Observed effects of whole-brain radiation therapy on focused ultrasound blood-brain barrier disruption. Ultrasound Med Biol 46:1998–2006. 10.1016/j.ultrasmedbio.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang S, Wu C-C, Zhang H et al. (2020) Focused ultrasound induced-blood–brain barrier opening in mouse brain receiving radiosurgery dose of radiation enhances local delivery of systemic therapy. Br J Radiol 93:20190214. 10.1259/bjr.20190214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shi J, Fu C, Su X et al. (2021) Ultrasound-stimulated microbubbles inhibit aggressive phenotypes and promotes radiosensitivity of esophageal squamous cell carcinoma. Bioengineered 12:3000–3013. 10.1080/21655979.2021.1931641 [DOI] [PMC free article] [PubMed] [Google Scholar]


