Abstract
Purpose
CHHiP is a randomized trial evaluating moderately hypofractionated radiation therapy for treatment of localized prostate cancer. Of all participants, 97% of them had concurrent short-course hormone therapy (HT), either luteinizing hormone-releasing hormone analog (LHRHa) or 150 mg of bicalutamide daily. This exploratory analysis compares efficacy and side effects in a nonrandomized comparison.
Methods and Materials
In our study, 2700 patients received LHRHa and 403 received bicalutamide. The primary endpoint was biochemical/clinical failure. Groups were compared with Cox regression adjusted for various prognostic factors and stratified by radiation therapy dose. A key secondary endpoint was erectile dysfunction (ED) assessed by clinicians (using scores from Late Effects on Normal Tissues: Subjective/Objective/Management [LENT-SOM] subjective erectile function for vaginal penetration) and patients (single items within the University of California-Los Angeles Prostate Cancer Index [UCLA PCI] and Expanded Prostate Cancer Index Composite [EPIC]-50 questionnaires) at 2 years and compared between HT regimens by χ2 trend test.
Results
Bicalutamide patients were significantly younger (median 67 vs 69 years LHRHa). Median follow-up was 9.3 years. There was no difference in biochemical or clinical failure with an adjusted hazard ratio or 0.97 (95% confidence interval, 0.77-1.23; P = .8). At 2 years, grade ≥2 LENT-SOM ED was reported in significantly more LHRHa patients (313 out of 590; 53%) versus bicalutamide (17 out of 68; 25%) (P < .0001). There were no differences in ED seen with UCLA-PCI and EPIC-50 questionnaires.
Conclusions
In this nonrandomized comparison, there was no evidence of a difference in efficacy according to type of HT received. Bicalutamide preserved clinician assessed (LENT-SOM) erectile function at 2 years but patient-reported outcomes were similar between groups.
Introduction
Neoadjuvant or adjuvant hormone therapy (HT) given with radiation therapy improves both biochemical progression-free survival and overall survival for men with localized prostate cancer.1, 2, 3 Most trials have used luteinizing hormone-releasing hormone analogs (LHRHa) to achieve this effect. Bicalutamide is an oral nonsteroidal antiandrogen which acts as a competitive antagonist at the androgen receptor. Bicalutamide is sometimes preferred to LHRHa due to perceived reduced cardiovascular risk or the patient's wish to preserve sexual function.
In the metastatic disease setting, bicalutamide results in inferior overall and progression-free survival compared with LHRHa although there is a suggestion that side effects might be reduced.4,5 However for patients with nonmetastatic disease, older studies showed no difference in overall survival or clinical progression in those receiving antiandrogens versus LHRH or orchidectomy.6,7 In the salvage radiation therapy setting, bicalutamide has been shown to reduce overall mortality.8
To the best of our knowledge, there are no large randomized studies which have compared bicalutamide with LHRHa combined with radiation therapy. It is not known whether bicalutamide has equivalent efficacy to LHRHa and whether it preserves sexual function after curative radiation therapy.
The CHHiP trial is a multicenter, randomized, phase 3, noninferiority trial comparing 2 Gy per fraction (74 Gy in 37 fractions) with 3 Gy per fraction (either 60 Gy in 20 fractions or 57 Gy in 19 fractions) in men with localized prostate cancer. The trial showed noninferiority of 60 Gy in 20 fractions compared with 74 Gy in 37 fractions.9 The trial protocol permitted bicalutamide monotherapy or LHRHa. In these exploratory analyses, we compare the efficacy, clinician-reported, and patient-reported outcomes (PRO) of men receiving bicalutamide with those receiving LHRHa.
Materials and Methods
Trial design
The CHHiP trial design has been described elsewhere.9 Briefly, men with histologically proven, T1b-T3a N0M0 prostate cancer with a maximum Gleason score of 7 were eligible. Men were randomized (1:1:1) to receive 74 Gy in 37 fractions over 7.4 weeks or 60 Gy in 20 fractions over 4 weeks or 57 Gy in 19 fractions over 3.8 weeks. Randomization was stratified by National Comprehensive Cancer Network risk classification and treatment center. HT was nonrandomized. The trial was registered (ISRCTN97182923), approved by the London Multicenter Research Ethics Committee (04/MRE02/10), and conducted in accordance with principles of good clinical practice.
Procedures
We mandated HT in men with National Comprehensive Cancer Network intermediate and high-risk disease. HT was given in monthly depot injections of LHRH agonists with initial cyproterone acetate to prevent “flare” phenomenon, or alternatively 150 mg of bicalutamide was given daily if preferred by the patient and physician. The duration of HT was at least 3 months (maximum 6 months) before start of radiation therapy and continued until the end of radiation therapy. The last monthly depot injection was to be given within 1 week of the start or during radiation therapy. Bicalutamide was continued for 2 months after the end of radiation therapy to mimic the duration of action of monthly depot LHRHa injections.10
PSA concentrations were recorded pre-HT and preradiation therapy; then at weeks 10, 18, and 26 after start of radiation therapy; and then at intervals of 6 months for 5 years thereafter annually. We assessed acute and late toxicity using clinician-reported outcome grading systems and PRO questionnaires. We conducted sexual function assessments pre-HT; pre-radiation therapy; and then at 6, 12, 18, 24, 36, 48, and 60 months, and graded the assessments according to the Late Effects on Normal Tissues: Subjective/Objective/Management (LENT-SOM)11 and the Royal Marsden (RMH)12 scoring systems. Men participating in a PRO substudy received questionnaires at baseline if they had not yet started androgen deprivation therapy (ADT), and all men received questionnaires preradiation therapy and at 10 weeks and 6, 12, 18, and 24 months after the start of radiation therapy thereafter annually until 5 years. Full details of the PRO substudy have been published previously.13 Initially, we used the University of California-Los Angeles Prostate Cancer Index,14 including Short Form 36 and Functional Assessment of Cancer Therapy-Prostate,15 but after a protocol amendment in 2009, we used the Expanded Prostate Cancer Index Composite (EPIC)16,17 and Short Form 1218 instead. We used the Radiation Therapy Oncology Group (RTOG) system19 to score late bladder and bowel toxicity.
Outcomes
Efficacy was the primary endpoint, which we evaluated as biochemical or clinical failure (BCF) and overall survival. We used the Phoenix consensus definition20 of a prostate-specific antigen concentration > nadir + 2ng/mL to define biochemical failure; clinical failure events included recommencement of HT, local recurrence, lymph node, or pelvic recurrence and distant metastases. Key secondary endpoints for this analysis were the proportion of patients with LENT-SOM grade ≥2 erectile dysfunction (ED) for vaginal penetration at 2 years; we assessed erectile functioning over time by clinician-assessed LENT-SOM and RMH scales (Appendix E1); individual patient-reported sexual functioning scores (UCLA PCI/EPIC questionnaire); general quality of life measures including hot flushes, fatigue, breast tenderness, low mood, and general health scores; testosterone levels pre-HT and at 12 months; and RTOG bladder and bowel toxicity. We report disease-free survival and recommencement of hormone therapy as exploratory endpoints.
Statistical considerations
All analyses presented are exploratory in nature, but a statistical analysis plan was written before conducting the analyses. Because this was a nonrandomized comparison of LHRHa with bicalutamide, we made statistical comparisons for the baseline demographic data by HT group (t tests, Mann-Whitney, χ2 tests and χ2 trend tests were used as appropriate). We used Kaplan-Meier methods to analyze time-to-event data stratified by treatment regimen. We compared HT groups using the log-rank test. An adjusted Cox model included age (continuous), National Comprehensive Cancer Network risk group (low vs intermediate v high), Gleason score (≤6 vs 7 & 8), clinical T-stage (T1 vs T2 vs T3a), prehormone prostate-specific antigen concentration (<10 vs 10-20 vs >20 ng/mL), and the proportion of core biopsies that were positive (≤50 vs >50%).21, 22, 23 We used stratified log-rank tests to compare HT groups for baseline variables that were imbalanced. The proportional hazards assumption held for all time-to-event analyses. Hazard ratios (HR) less than 1 favored bicalutamide. We conducted competing risks analysis for BCF with death from any cause as the competing event.
We restricted analysis for the sexual functioning secondary endpoints to patients with preserved sexual function pre-HT. For the RMH scale, we analyzed data separately for patients with normal erections (grade 0) and decreased erections (grade 1) pre-HT. We used χ2 trend tests to compare hormone groups at 2 years. Due to the nonrandomized comparisons being made, we used multivariable logistic models for analysis of some secondary endpoints. We created binary variables for grade ≥2 (poor/very poor for PRO endpoints) at 2 years and adjusted models for age, pre-HT symptom score, and pre-HT testosterone level.
To account for multiple testing, we used a significance level of .5% for the primary endpoints of efficacy and key secondary endpoints (LENT-SOM erectile function for vaginal penetration and UCLA/EPIC question on ability to have an erection). For all other secondary endpoints, we used a significance level of .1%. We based analyses on a data snapshot taken on October 9, 2019, for the efficacy and clinician assessments (median follow-up 9.3; interquartile range [IQR], 8.2-11.0 years) and on August 26, 2018, for the PRO data (final data set, follow-up completed at 5 years for PRO). We undertook all analyses with STATA v15.1.
Results
Baseline demographics
Baseline demographics for patients in the LHRHa (n = 2700) and bicalutamide (n = 403) groups are shown in Table 1. All but 29 patients had started hormones before randomization. HT was omitted in 3% of men who are excluded from all analyses. Patients receiving bicalutamide were younger, with a median age of 67 years (IQR, 63-72) compared with 69 years (IQR, 65-73) for LHRHa. Men receiving bicalutamide had a shorter time between diagnosis and randomization and a lower burden of core involvement (see Table 1). Patients treated with LHRHa received HT for a median of 5.3 months (IQR, 4.5-6.2) (measured from date of first monthly injection to last injection plus 4 weeks) and bicalutamide patients for a median 6.3 months (IQR 5.7-7.1) months.
Table 1.
Baseline Demographics by Hormone Therapy Received
| Demographics | LHRHa (n = 2700) |
Bicalutamide (n = 403) |
P Value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Treatment group | |||||
| 74Gy/37 fractions | 881 | 33 | 144 | 36 | .4* |
| 60Gy/20 fractions | 910 | 34 | 133 | 33 | |
| 57Gy/19 fractions | 909 | 33 | 126 | 31 | |
| NCCN risk group | |||||
| High-risk | 332 | 12 | 50 | 12 | .4† |
| Intermediate risk | 2006 | 74 | 308 | 76 | |
| Low Risk | 362 | 13 | 45 | 11 | |
| Age (y) | |||||
| Median (IQR) | 69 (65-73) | 67 (63-72) | <.001‡ | ||
| Range | 44-85 | 50-83 | |||
| Age category | |||||
| ≤69 y | 1445 (54) | 256 (64) | <.001* | ||
| ≥70 y | 1255 (46) | 147 (36) | |||
| Gleason score | |||||
| ≤6 | 918 | 34 | 114 | 28 | .04† |
| 3 + 4 | 1179 | 44 | 189 | 47 | |
| 4 + 3 | 515 | 19 | 89 | 22 | |
| 8 | 87 | 3 | 11 | 3 | |
| Clinical T stage | |||||
| T1 | 945 | 35 | 158 | 39 | 0.4§ |
| T2 | 1520 | 56 | 203 | 50 | |
| T3 | 232 | 9 | 42 | 11 | |
| TX | 1 | <1 | 0 | 0 | |
| MRI T stage | |||||
| T1 | 158 | 8 | 51 | 16 | .044§ |
| T2 | 1235 | 66 | 184 | 59 | |
| T3 | 445 | 24 | 75 | 24 | |
| TX | 44 | 2 | 4 | 1 | |
| Months from histologic confirmation of prostate cancer to randomization | <.001‡ | ||||
| N | 2697 | 403 | |||
| Median (IQR) | 5 (3-6) | 4 (3-5) | |||
| Range | 0-177 | 1-102 | |||
| Prehormone PSA (ng/mL) | |||||
| N | 2676 | 401 | .8‡ | ||
| Median (IQR) | 10.3 (7.2, 14.6) | 10.0 (7.2, 14.6) | |||
| Range | 0.2, 33.6 | 1.3, 28.8 | |||
| Number of core biopsies taken‖, ¶ | |||||
| N | 2006 | 261 | .3‡ | ||
| Median (IQR) | 11 (10-12) | 11 (8-13) | |||
| Range | 2-20 | 3-20 | |||
| Number of positive core biopsies‖ | |||||
| N | 1892 | 247 | <.001‡ | ||
| Median (IQR) | 4 (3-7) | 4 (2-6) | |||
| Range | 0-16 | 0-12 | |||
| Proportion of positive core biopsies‖ | |||||
| N | 1862 | 234 | .001* | ||
| <50% | 973 (52) | 150 (64) | |||
| ≥50% | 889 (48) | 84 (36) | |||
| Maximum length of core involvement (%) | |||||
| N | 1426 | 259 | .001‡ | ||
| Median (IQR) | 40 (16-70) | 30 (10-60) | |||
| Range | 1-100 | 1-100 | |||
| Maximum length of core involvement¶ (mm) | |||||
| N | 383 | 79 | <.001‡ | ||
| Median (IQR) | 10 (5-16) | 6 (3-9) | |||
| Range | 0.4-20 | 0.7-20 | |||
Abbreviations: IQR = interquartile range; MRI = magnetic resonance imaging; NCCN = National Comprehensive Cancer Network; PSA = prostate-specific antigen.
χ2.
Test for trend.
Mann-Whitney.
Test for trend excluding TX.
Number of core biopsies taken/positive was capped at 20 as part of central data cleaning with values >20 discarded as errors/implausible in an era when template biopsies were not used.
Maximum length of core involvement capped at 20 mm as part of central data cleaning with values >20 discarded as errors/implausible given cutting length of biopsy needle.
Efficacy
There was no evidence of a difference in BCF between the 2 HT groups with an unadjusted HR 0.97 (95% CI, 0.77-1.23, P = .8) (Fig. 1A). In the LHRHa group, the 5 year BCF-free rate was 88% (95% CI, 87-89) and 86% (95% CI, 82-89) in the bicalutamide group. In view of the imbalances in baseline characteristics we performed log-rank tests stratified for age (≤69 v >69) and proportion of positive core biopsies (≤50 v >50%). These indicated no difference between HT groups for BCF. The adjusted HR was 0.98 (95% confidence interval [CI], 0.70-1.36; P = .9) (Appendix E2). Competing risks analysis indicated no evidence of a difference between HT groups (Gray's test P = .9) (Appendix E3). An exploratory analysis restricted to unfavorable intermediate and high-risk patients (as defined by Zumsteg et al)21 gave similar results (Appendix E4). There was no evidence of a difference in overall survival between HT groups with an adjusted HR of 0.87 (95% CI, 0.60-1.26; P = .5). (Fig. 1B). Time to recommencement of hormone therapy and disease free survival also showed no evidence of a difference between the HT groups (Figs. 1C and 1D; also see Appendix E2).
Fig. 1.
Kaplan-Meier curves for (A) biochemical and/or clinical failure, (B) overall survival, (C) recommencing hormone treatment, and (D) disease free survival by hormone therapy received.
Clinician-reported sexual functioning (LENT-SOM and RMH)
Before starting HT, 607 out of 786 patients (77%) receiving LHRHa and 73 out of 89 patients (82%) receiving bicalutamide had preserved erectile function (LENT-SOM grade ≤2) and were included in subsequent analyses (Appendix E5). At 2 years, grade ≥2 LENT-SOM ED (intermittently insufficient for vaginal penetration or worse) was reported in significantly more LHRHa patients (313 out of 590; 53%) compared with 17 out of 68 patients (25%) receiving bicalutamide patients (P <.0001). At 2 years, grade 3 to 4 ED (erections insufficient for penetration or impotent) was reported in 220 out of 585 patients (38%) receiving LHRHa and 14 out of 68 patients (21%) receiving bicalutamide. A similar pattern was seen throughout follow-up with fewer bicalutamide patients assessed as having severe symptoms according to the LENT-SOM sexual dysfunction scale (Figs. 2A-D). A multivariable logistic model including pre-HT erectile function score, age and pre-HT testosterone level also indicated reduced erectile dysfunction in the bicalutamide patients (odds ratio, 0.30; 99 % CI, 0.10-0.90; P = .005) (Appendix E6).
Fig. 2.
LENTSOM sexual dysfunction items: distribution of grade at each time point assessed by hormone therapy received. (A) Subjective: erectile function for vaginal penetration. (B) Subjective worse grade. (C) Objective worse grade. (D) Management worse grade. Abbreviations: PH = prehormones; PR = preradiation therapy.
Using the RMH scale prehormones, 378 out of 761 patients (50%) and 44 out of 80 patients (55%) reported normal erections in the LHRHa and bicalutamide groups, respectively; and 259 out of 761 patients (34%) and 23 out of 80 patients (29%) reported decreased erections in the LHRHa and bicalutamide groups, respectively (Appendix E5). Of those with preserved sexual function at baseline, a lower proportion of the patients receiving bicalutamide developed ED defined using the RMH scale, but this did not reach statistical significance. At 2 years, there was no evidence of a difference between HT groups for either patients with normal erections (P = .1) or decreased erections (P = .6) prehormones (Appendix E7).
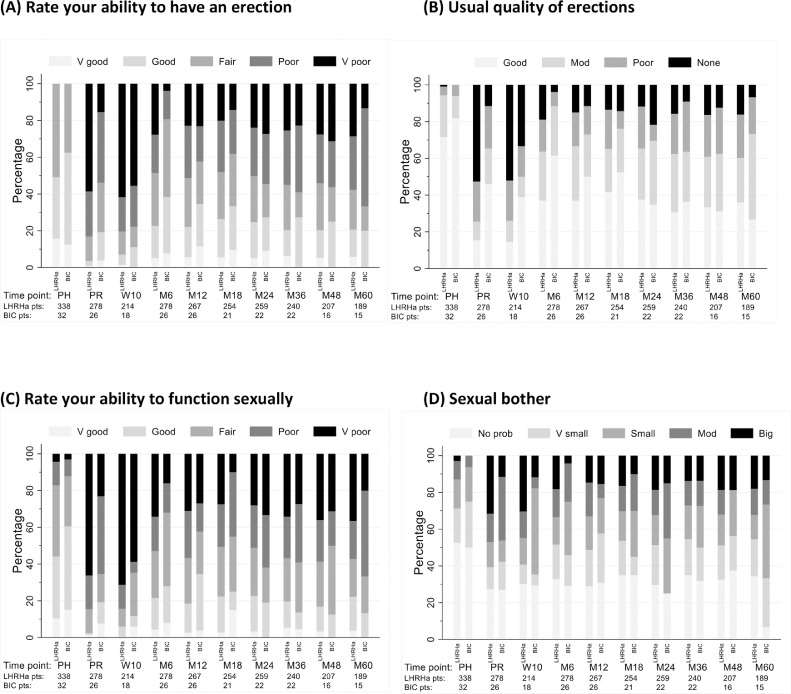
Patient-reported sexual functioning (UCLA-PCI)
Before starting hormones, 215 out of 553 patients (39%) and 17 out of 49 patients (34%) of LHRHa and bicalutamide patients reported very poor or poor ability to have an erection (see Appendix E5). At 2 years, there was no evidence of a difference between HT groups for ability to have an erection (P > .9). Multivariable models also indicate no difference between hormone treatments (odds ratio, 0.77; 99% CI, 0.15-3.85; P = .676) (Appendix E8). Patients reported worst erectile function at 10 weeks from the start of radiation therapy (Fig. 3A). UCLA/EPIC sexual function scores appeared better in bicalutamide patients before starting radiation therapy and at the week 10 and month 6 assessments (no statistical comparisons made). From 12 months onwards the distribution of scores remained similar for each HT group (Appendix E9). This pattern was also seen when all patients were included in the analysis, including those without erectile function documented prehormonal therapy (Appendix E10).
Fig. 3.
Patient-reported outcomes of sexual function assessed using the University of California Los Angeles Prostate Cancer Index and Expanded Prostate Cancer Index Composite questionnaires—distribution of grade at each time point assessed by hormone therapy received. (A) Rate your ability to have an erection. (B) Usual quality of erections. (C) Rate your ability to function sexually. (D) How big a problem has sexual function been (sexual bother). Abbreviations: PH = prehormones; PR = preradiation therapy.
Patient-reported general items
Hot flushes were worse in the LHRHa group up to 6 months, but untroublesome thereafter (Fig. 4A) with no difference between HT groups at 2 years (P = .4). Lack of energy was reported similarly between HT groups, and there was little improvement in scores over time (Fig. 4B), with no evidence of difference at 2 years (P = .3). Breast tenderness was reported more often in patients receiving bicalutamide across all time points assessed (Fig. 4C). At week 10, moderate or worse breast tenderness was seen in 10 out of 39 patients (26%) on bicalutamide and 6 out of 214 patients (2.8%) on LHRHa. More bicalutamide patients (3 out of 40; 7.5%) remained with moderate or big problems with breast tenderness at 2 years than the LHRHa group (2 out of 299; 0.7%) (P = .002). General health assessments were similar in the HT groups over time (Fig. 4D) with no difference at 2 years (P = .5). Reported levels of depression were low, and there was no difference between HT groups at 2 years (P = .6) (Appendix E11).
Fig. 4.
Patient-reported outcomes of general quality of life items—distribution of scores at each time point assessed by hormone therapy received (A) Hot flushes, (B) Lack of energy (C) Breast tenderness (D) General health score. Abbreviations: PH = prehormones; PR = preradiation therapy.
Before starting HT, there was no difference in the testosterone levels between patients receiving LHRHa or bicalutamide with median values of 12.6 and 11.9 nmol/L respectively (P >.9) (Fig. 5 and Appendix E12). By 12 months, the majority of patients who received LHRHa (1170 out of 1553; 75%) had testosterone levels within the normal range (>8 nmol/L), as did 181 out of 213 (85%) of bicalutamide patients (Appendix E12).
Fig. 5.
Boxplots illustrating testosterone levels at baseline and 12 months.
Late RTOG bladder toxicity was reported by very few patients, with similar distributions for those receiving LHRHa or bicalutamide (Appendix E13). Small numbers of patients had predominantly grade 1 RTOG bowel toxicity, which was similar for both HT groups (see Appendix E13).
Discussion
This nonrandomized comparison of short course bicalutamide and LHRHa with prostate radiation therapy showed similar efficacy with a median follow-up over 8 years. Initially, sexual function declined less preradiation therapy using bicalutamide with some evidence of reduced ED 2 years after treatment. Hot flushes were reduced using bicalutamide. Gynaecomastia and breast discomfort were more common in the bicalutamide group (25.6% at 6 months) but less marked that in other studies which have reported rates of about 70%,24 likely due to the protocol recommendation to use tamoxifen if gynaecomastia developed and the shorter course of bicalutamide.25 Prophylactic use of Tamoxifen may be more effective. By 12 months, testosterone levels had recovered in most patients in both groups but there was no difference in levels of ongoing fatigue. Interventions using structured exercise might be of value.26,27
HT using LHRHa with radiation therapy is well established for the treatment of localized prostate cancer.2,3,28,29 Bicalutamide has compared favorably with placebo in locally advanced and recurrent disease,8,30 but no phase 3 comparisons with LHRHa have been performed. Single-center series,31,32 albeit with short follow-up, have reported similar efficacy between bicalutamide and LHRHa. However, concerns about the efficacy of bicalutamide monotherapy have persisted. One randomized trial did not show the expected improvement in biochemical control with bicalutamide compared with radiation therapy alone,33 and, for earlier disease, overall survival with bicalutamide appeared to be worse than watchful waiting in another study.34
Because bicalutamide is less effective in metastatic disease,4,5 standard practice is to offer LHRHa concomitantly with radiation therapy, and our data does not change that. There is some concern relating to cardiovascular side effects with LHRHa and a suggestion that antiandrogens may have a more favorable profile, although this remains controversial,35 and higher cardiac events were noted in the bicalutamide arm of the RTOG 9601 study.36 For men wishing to minimize the chance of erectile dysfunction, our analysis suggests that bicalutamide is a safe option but any advantages are modest.
Long-term ED benefit for bicalutamide was apparent using the LENT-SOM assessment but not with the RMH scoring system nor the PRO. We speculate that this might be because patients selecting bicalutamide had higher expectations of retaining sexual function and were consequently more bothered by ED. With both HT options, ED remains a major concern; a combination of radiation therapy and increasing age appear responsible. It has been suggested that dose to the penile bulb may be important.37 The use of image guided radiation therapy facilitates small margins, sparing these structures in most patients.38 In addition, prehabilitation or early treatment with PDE5 after radiation therapy may be beneficial.
Limitations of our work are that it is a nonrandomized comparison, albeit from a large phase 3 trial. Only 13 % of the patient population had HT with bicalutamide. In consequence, there are imbalances in some presenting features; bicalutamide-treated patients were younger, with some more favorable pathologic parameters, although adjusted analyses continued to show similar efficacy between groups. Most patients had started HT before collection of baseline data and randomization (Appendix E14), limiting the number of patients with both baseline and 2-year data for comparison. However, when data for all patients was analyzed (see Appendix E10) similar patterns were seen. Additionally, PRO data collection decreased over time limiting the robustness of data interrogation.
We acknowledge the problems inherent with nonrandomized data, but our analysis should reassure oncologists considering prescribing short-course bicalutamide with radiation therapy and assist appropriate discussion with patients. Bicalutamide may be preferred in patients wishing to maintain sexual function, although the evidence presented here shows no guarantee of avoiding ED. Bicalutamide may also be preferred in patients who experience significant hot flushes, especially if this limits compliance with LHRHa, although this may be achieved at the cost of some breast symptoms, unless prophylactic tamoxifen is given.
Conclusion
In a nonrandomized analysis within the CHHiP trial, patients receiving HT with bicalutamide had similar 5-year BCF compared with those receiving LHRHa. There was some evidence that bicalutamide reduced ED, although this was not seen on all outcome scales. Bicalutamide also reduced hot flushes but global quality of life was unaffected. With appropriate patient counseling about risks and benefits, Bicalutamide can be considered as an option for men wishing to preserve sexual function or for those who have severe hot flushes.
Acknowledgments
We thank the patients and all investigators and research support staff at the participating centers, Trial Management Group members, the Independent Data Monitoring Committee and Trial Steering Committee. We acknowledge support from Cancer Research UK (C46/A3976, C46/A10588, C33589/A19727, C33589/A28284) and the National Institute for Health Research (NIHR) Cancer Research Network. This project represents independent research supported by the National Institute for Health research (NIHR) Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and the Institute of Cancer Research in London. The views expressed are those of the authors and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care.
Footnotes
The CHHiP trial was funded by Cancer Research UK (C8262/A7253, C1491/A9895, C1491/A15955, C1491/A25351), and the Department of Health.
Disclosures: A.T. reports research funding from Elekta, Varian, and Accuray, and honoraria or travel assistance from Accuray, Elekta, and Janssen. J.S. reports honoraria or travel assistance from Janssen, Astrazeneca, Astellas, and Novartis. J.O. reports honoraria or travel assistance from AAA, Bayer, GE Health care, Janssen, Astrazeneca, Astellas, Sanofi, and Novartis. C.P. reports membership on advisory boards for Bayer, Clarity Pharmaceuticals, Myovant, and ITM Oncologics, and speaker fees from Janssen. V.K. reports honoraria or travel assistance, or both, from Accuray, Astellas, Bayer, Boston Scientific, Janssen, and Tolmar. I.S. reports honoraria or travel assistance from Janssen, Astrazeneca, and Bayer. Z.M. reports honoraria or travel assistance, or both, from Astellas, Bayer, Janssen, and Sanofi-Aventis. A.C. reports grants from National Institute of Health Research Manchester Biomedical Research Center; Cancer Research, United Kingdom; the Medical Research Council, United Kingdom; Prostate Cancer, United Kingdom; and Bayer, United Kingdom; personal fees from Janssen Pharmaceutical; nonfinancial support from the American Society of Clinical Oncology; and grants and nonfinancial support from Elekta AB outside the submitted work. D.D. reports personal fees from The Institute of Cancer Research, grants from Cancer Research UK Program Grant, and honoraria for advisory boards from Janssen during the conduct of the study, and has a patent (EP1933709B1) for a stabilization and location device. E.H. reports grants from Accuray Inc, Varian Medical Systems Inc, Merck Sharp & Dohm, Janssen-Cilag, Aventis Pharma Limited (Sanofi), and Roche Products Ltd, and grants and nonfinancial support from Astra Zeneca, Bayer, all outside the submitted work.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2021.12.160.
References
- 1.Denham JW, Steigler A, Lamb DS, et al. Short-term androgen deprivation and radiotherapy for locally advanced prostate cancer: Results from the Trans-Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncol. 2005;6:841–850. doi: 10.1016/S1470-2045(05)70348-X. [DOI] [PubMed] [Google Scholar]
- 2.Bolla M, Maingon P, Carrie C, et al. Short androgen suppression and radiation dose escalation for intermediate- and high-risk localized prostate cancer: Results of EORTC trial 22991. J Clin Oncol. 2016;34:1748–1756. doi: 10.1200/JCO.2015.64.8055. [DOI] [PubMed] [Google Scholar]
- 3.Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma—Long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61:1285–1290. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 4.Kunath F, Grobe HR, Rucker G, et al. Non-steroidal antiandrogen monotherapy compared with luteinising hormone-releasing hormone agonists or surgical castration monotherapy for advanced prostate cancer. Cochrane Database Syst Rev. 2014:CD009266. doi: 10.1002/14651858.CD009266.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seidenfeld J, Samson DJ, Hasselblad V, Aronson N, Albertsen PC, Bennect CL. Erratum: Single-therapy androgen suppression in men with advanced prostate cancer: A systematic review and meta-analysis (Ann Intern Med. 2000;132:566-577) Ann Intern Med. 2005;143:764–765. doi: 10.7326/0003-4819-132-7-200004040-00009. [DOI] [PubMed] [Google Scholar]
- 6.Iversen P. Antiandrogen monotherapy: Indications and results. Urology. 2002;60(3 Suppl. 1):64–71. doi: 10.1016/s0090-4295(02)01576-5. [DOI] [PubMed] [Google Scholar]
- 7.Tyrrell CJ, Iversen P, Tammela T, et al. Tolerability, efficacy and pharmacokinetics of bicalutamide 300 mg, 450 mg or 600 mg as monotherapy for patients with locally advanced or metastatic prostate cancer, compared with castration. BJU Int. 2006;98:563–572. doi: 10.1111/j.1464-410X.2006.06275.x. [DOI] [PubMed] [Google Scholar]
- 8.Shipley WU, Seiferheld W, Lukka HR, et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017;376:417–428. doi: 10.1056/NEJMoa1607529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer : 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;2045:1–14. doi: 10.1016/S1470-2045(16)30102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murthy V, Norman AR, Shahidi M, et al. Recovery of serum testosterone after neoadjuvant androgen deprivation therapy and radical radiotherapy in localized prostate cancer. BJU Int. 2006;97:476–479. doi: 10.1111/j.1464-410X.2006.06013.x. [DOI] [PubMed] [Google Scholar]
- 11.LENT SOMA tables. Radiother Oncol. 1995;35:17–60. [PubMed] [Google Scholar]
- 12.Dearnaley DP, Sydes MR, Langley RE, et al. The early toxicity of escalated versus standard dose conformal radiotherapy with neo-adjuvant androgen suppression for patients with localised prostate cancer: Results from the MRC RT01 trial (ISRCTN47772397) Radiother Oncol. 2007;83:31–41. doi: 10.1016/j.radonc.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Wilkins A, Mossop H, Syndikus I, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer : 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;2045:1–12. doi: 10.1016/S1470-2045(15)00280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, Brook RH. The UCLA prostate cancer index: Development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36:1002–1012. doi: 10.1097/00005650-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50:920–928. doi: 10.1016/S0090-4295(97)00459-7. [DOI] [PubMed] [Google Scholar]
- 16.Szymanski KM, Wei JT, Dunn RL, Sanda MG. Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health-related quality of life among prostate cancer survivors. Urology. 2010;76:1245–1250. doi: 10.1016/j.urology.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the Expanded Prostate Cancer Index Composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 18.Ware J, Kosinski M, Keller S.A. 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Pilepich MV, Krall JM, Sause WT, et al. Correlation of radiotherapeutic parameters and treatment related morbidity in carcinoma of the prostate—Analysis of RTOG study 75-06. Int J Radiat Oncol Biol Phys. 1987;13:351–357. doi: 10.1016/0360-3016(87)90008-3. [DOI] [PubMed] [Google Scholar]
- 20.Roach M, Hanks G, Thames H, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 21.Zumsteg ZS, Spratt DE, Pei I, et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol. 2013;64:895–902. doi: 10.1016/j.eururo.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 22.Zumsteg ZS, Zelefsky MJ, Woo KM, Spratt DE. Unification of favourable intermediate-, unfavourable intermediate-, and very high-risk strati fi cation criteria for prostate cancer. BJU Int. 2017;120(5B):E87–E95. doi: 10.1111/bju.13903. [DOI] [PubMed] [Google Scholar]
- 23.Cheney MD, Chen MH, Zhang D, Phillips JG, Loffredo MJ, D'Amico AV. Greatest percentage of involved core length and the risk of death from prostate cancer in men with highest gleason score ≥ 7. Clin Genitourin Cancer. 2014;12:234–240. doi: 10.1016/j.clgc.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Tyrrell CJ, Payne H, See WA, et al. Bicalutamide (‘Casodex’) 150 mg as adjuvant to radiotherapy in patients with localised or locally advanced prostate cancer: Results from the randomised Early Prostate Cancer Programme. Radiother Oncol. 2005;76:4–10. doi: 10.1016/j.radonc.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Kunath F, Keck B, Antes G, Wullich B, Meerpohl JJ. Tamoxifen for the management of breast events induced by non-steroidal antiandrogens in patients with prostate cancer: A systematic review. BMC Med. 2012;10:96. doi: 10.1186/1741-7015-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen PL, Alibhai SMH, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67:825–836. doi: 10.1016/j.eururo.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Cormie P, Zopf EM. Exercise medicine for the management of androgen deprivation therapy-related side effects in prostate cancer. Urol Oncol Semin Orig Investig. 2020;38:62–70. doi: 10.1016/j.urolonc.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Bolla M, De Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 29.Joseph D, Denham JW, Steigler A, et al. Radiation dose escalation or longer androgen suppression to prevent distant progression in men with locally advanced prostate cancer: 10-year data from the TROG 03.04 RADAR Trial. Int J Radiat Oncol Biol Phys. 2020;106:693–702. doi: 10.1016/j.ijrobp.2019.11.415. [DOI] [PubMed] [Google Scholar]
- 30.Iversen P, McLeod DG, See WA, Morris T, Armstrong J, Wirth MP. Antiandrogen monotherapy in patients with localized or locally advanced prostate cancer: Final results from the bicalutamide Early Prostate Cancer programme at a median follow-up of 9.7 years. BJU Int. 2010;105:1074–1081. doi: 10.1111/j.1464-410X.2010.09319.x. [DOI] [PubMed] [Google Scholar]
- 31.Buwenge M, Deodato F, Macchia G, et al. Radiotherapy plus GnRH analogue versus high dose bicalutamide: A case control study. Anticancer Res. 2019;39:6373–6378. doi: 10.21873/anticanres.13850. [DOI] [PubMed] [Google Scholar]
- 32.McGivern U, Mitchell DM, McDowell C, O'Hare J, Corey G, O'Sullivan JM. Neoadjuvant hormone therapy for radical prostate radiotherapy: Bicalutamide monotherapy vs. luteinizing hormone-releasing hormone agonist monotherapy: A single-institution matched-pair analysis. Clin Genitourin Cancer. 2012;10:190–195. doi: 10.1016/j.clgc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 33.McPartlin AJ, Glicksman R, Pintilie M, et al. PMH 9907: Long-term outcomes of a randomized phase 3 study of short-term bicalutamide hormone therapy and dose-escalated external-beam radiation therapy for localized prostate cancer. Cancer. 2016;122:2595–2603. doi: 10.1002/cncr.30093. [DOI] [PubMed] [Google Scholar]
- 34.Iversen P, Johansson JE, Lodding P, et al. Bicalutamide (150 mg) versus placebo as immediate therapy alone or as adjuvant to therapy with curative intent for early nonmetastatic prostate cancer: 5.3-year median followup from the Scandinavian Prostate Cancer Group Study Number 6. J Urol. 2004;172(5 I):1871–1876. doi: 10.1097/01.ju.0000139719.99825.54. [DOI] [PubMed] [Google Scholar]
- 35.Scailteux LM, Naudet F, Alimi Q, Vincendeau S, Oger E. Mortality, cardiovascular risk, and androgen deprivation therapy for prostate cancer: A systematic review with direct and network meta-analyses of randomized controlled trials and observational studies. Medicine (Baltimore) 2016;95:e3873. doi: 10.1097/MD.0000000000003873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dess RT, Sun Y, Jackson WC, et al. Association of presalvage radiotherapy PSA levels after prostatectomy with outcomes of long-term antiandrogen therapy in men with prostate cancer. JAMA Oncol. 2020;6:735–743. doi: 10.1001/jamaoncol.2020.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray J, Gulliford S, Griffin C, et al. Evaluation of erectile potency and radiation dose to the penile bulb using image guided radiotherapy in the CHHiP trial. Clin Transl Radiat Oncol. 2020;21:77–84. doi: 10.1016/j.ctro.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray J, Griffin C, Gulliford S, et al. A randomised assessment of image guided radiotherapy within a phase 3 trial of conventional or hypofractionated high dose intensity modulated radiotherapy for prostate cancer. Radiother Oncol. 2020;142:62–71. doi: 10.1016/j.radonc.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]