Abstract
The apparent efficacy of d-cycloserine (DCS) for enhancing exposure treatment for anxiety disorders appears to have declined over the past 14 years. We examined whether variations in how DCS has been administered can account for this “declining effect”. We also investigated the association between DCS administration characteristics and treatment outcome to find optimal dosing parameters. We conducted a secondary analysis of individual participant data obtained from 1047 participants in 21 studies testing the efficacy of DCS-augmented exposure treatments. Different outcome measures in different studies were harmonized to a 0-100 scale. Intent-to-treat analyses showed that, in participants randomized to DCS augmentation (n = 523), fewer DCS doses, later timing of DCS dose, and lower baseline severity appear to account for this decline effect. More DCS doses were related to better outcomes, but this advantage leveled-off at nine doses. Administering DCS more than 60 minutes before exposures was also related to better outcomes. These predictors were not significant in the placebo arm (n = 521). Results suggested that optimal DCS administration could increase pre-to-follow-up DCS effect size by 50%. In conclusion, the apparent declining effectiveness of DCS over time may be accounted for by how it has been administered. Optimal DCS administration may substantially improve outcomes.
Registration:
The analysis plan for this manuscript was registered on Open Science Framework (https://osf.io/c39p8/).
Keywords: d-cycloserine, augmentation, exposure, dosing, decline effect
1. INTRODUCTION
Cognitive-behavior therapy (CBT) is a treatment of choice for anxiety and related disorders (Carpenter et al., 2018). Accordingly, strategies to enhance the efficacy of CBT have garnered substantial interest. One strategy is the use of d-cycloserine (DCS) administered in conjunction with exposure therapy sessions (Mataix-Cols et al., 2017; Otto et al., 2015). DCS is a partial agonist at the N-methyl-D-aspartate (NMDA) glutamatergic receptor, and its administration is presumed to improve memory consolidation (Davis et al., 2006). Initial small-scale investigations showed large effects for the addition of DCS to exposure therapy (Hofmann et al., 2006; Otto et al., 2015; Ressler, Rothbaum, Tannenbaum, & et al, 2004). Yet, the apparent efficacy of DCS as an augmentation strategy seems to have declined over the years. Sequential meta-analytic reviews documented effect sizes of d = .60 for 8 clinical trials in 2008 (Norberg et al., 2008), d = .46 for 9 trials in 2014 (Bontempo et al., 2012), d = .34 for 13 studies in 2014 (Rodrigues et al., 2014), and g = .12-.27 for 23 trials in 2017 (Bürkner et al., 2017). We contributed to this documentation with our own recent meta-analysis in 2017 (Mataix-Cols et al., 2017), this time utilizing individual-participant data (IPD) from over 1,000 participants across 21 studies (ds = .21-.27). In that meta-analysis, we found that the augmentation effect of DCS (CBT + DCS vs. CBT + placebo) decreased significantly over time (from the initial study in 2004 through the submission of our meta-analysis in 2016). This trend was also reported in Bürkner et al. (2017). Given that the data collected for our IPD meta-analysis included a range of variables, including both individual characteristics (from demographics to how many DCS doses each person actually received) and characteristics of the study (e.g., timing of the administration of DCS), we have a unique opportunity to carefully examine a wide range of possible factors that might explain the apparent declining effectiveness of DCS across the time frame covered by the meta-analysis. Thus, the purpose of the present study was to investigate both individual level and study level variables that might account for this declining effectiveness of DCS that was observed in our meta-analysis (Mataix-Cols et al., 2017).
Declining effects over time are common in biopsychosocial research (Gehr et al., 2006; Monsarrat & Vergnes, 2018; Trikalinos et al., 2004), but the explanations of these declining effects are elusive (Gehr et al., 2006). In this article, we examine some potential explanations of the apparent declining effect size of DCS over time, with our primary focus on how DCS has been administered. In particular, investigators may have unwittingly trended away from optimal dosing strategies over time. For example, an early meta-analysis (Norberg et al., 2008) suggested that DCS may be more effective in limited doses. This may have led to fewer doses in more recent studies. This is an especially apt issue for DCS given the lack of dose-finding and dose-timing studies (Hofmann et al., 2015; Hofmann et al., 2011; Otto et al., 2015).
Using data from our previous IPD meta-analysis (Mataix-Cols et al., 2017), we were primarily interested whether the DCS “decline effect” observed in that meta-analysis was accounted for by the manner in which DCS has been administered across the time frame covered by the studies in that meta-analysis (DCS dose, number of DCS doses, and timing of the doses). Our focus was not on how the administration of DCS has changed over the years, since subtle changes across a number of different dimensions may account for the decline effect. Instead, we focused on whether controlling for these factors accounts for the decline effect that was evident in the sequence of meta-analyses and which was found in our previous IPD meta-analysis (Mataix-Cols et al., 2017). Hence, we believed it was appropriate and important to conduct a secondary analysis of the same dataset rather than perform a new analysis of an updated data set including new studies published since the last meta-analysis was published. A second, equally important goal of this paper is to determine which variables are related to outcome for participants that are treated with DCS-augmented CBT. The third goal of this paper is to ascertain the optimal DCS administration parameters. Determining the optimal DCS dosing parameters could help maximize patient outcomes in real-world application of CBT augmented with DCS (CBT + DCS).
Thus, we used Multilevel modeling (MLM) to analyze IPD from the 21 studies which compared CBT + DCS to CBT + Placebo over the last 14 years, using raw data from our previous IPD meta-analysis (Mataix-Cols et al., 2017). Our primary analyses included only the participants in the CBT + DCS treatment condition. These analyses determined if 1) outcome severity is worse in CBT + DCS in more recent studies, and 2) if that effect was eliminated when putative predictors are controlled by adding them as additional independent variables (predictors) in the MLM model. This latter analysis also provided important information on which DCS-related predictors are significantly associated with outcomes and suggests how to optimize DCS treatment outcome. We examined predictors of outcome in CBT + DCS, rather than moderators of the difference between the CBT + DCS and CBT + Placebo groups, because 1) the effect of DCS dose cannot be examined as a moderator in a sample which includes CBT + Placebo as well as CBT + DCS since dose = 0 for all CBT + Placebo participants, 2) there is considerable variability in placebo responsivity (Furukawa et al., 2016), which can cloud the interpretation of moderators of between-group effects thereby masking such effects, and 3) interaction effects are smaller and involve more variability than main effects, which could result in missing a factor that might be a significant predictor of outcome for participants receiving DCS. However, we repeated our analyses in the CBT + Placebo group. If it is DCS that is responsible for the effects that we observe in the CBT + DCS analyses, and not some spurious variable, we would expect that the predictive variables would not be significant in the placebo group.
2. Material and methods
2.1. Participants
Details of the method can be found in our previous paper (Mataix-Cols et al., 2017). Briefly, an IPD meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses of Individual Participant Data protocol. Inclusion criteria were: (1) double-blind, randomized, placebo-controlled trials examining DCS augmentation of CBT which incorporated exposure; and (2) studying either social anxiety disorder (SAD), panic disorder with or without agoraphobia (PD/A), specific phobia (SP), obsessive-compulsive disorder (OCD), or posttraumatic stress disorder (PTSD).
The search (see (Mataix-Cols et al., 2017)) yielded 22 studies, from which IPD from 21 studies was obtained (total N = 1,047: N = 523 in CBT + DCS, N = 521 in CBT + Placebo, and N = 3 without treatment assignment information). These studies were published between January 2004 and February, 20161. Participant data included sex, age, diagnosis, number of DCS (or placebo) doses received, timing of pill administration (number of minutes before/after the exposure sessions), DCS dose (in mg), number of CBT sessions completed, and the primary outcome at major treatment time-points (baseline, mid-treatment, post-treatment, and final follow-up, as measured by the primary outcome for each study). Because different studies had different outcome measures, we transformed outcomes into ranked data to ensure a common metric across studies (these “harmonized” scores ranged 0-100; higher numbers indicated worse symptoms; see Mataix-Cols et al. (2017) for details).
2.2. Data analysis
The analysis plan was registered on Open Science Framework (https://osf.io/c39p8/). The distributions of the variables were examined and appropriate transformations were used to improve non-normal distributions and to address outliers. Three-level multilevel modeling (MLM) was used to analyze the data (repeated assessments nested within individuals, who were nested within studies). These analyses were conducted using the mixed effects models in SPSS 24. Mixed effects models in SPSS use the conservative Satterthwaite approximation to calculate degrees of freedom for the t-tests of the regression coefficients. This approach results in degrees of freedom that are different for every regression coefficient.
MLM is intent-to-treat, including all participants regardless of missing data, thereby improving power and generalizability. Level-1 of the MLM analysis modeled outcome severity for participants over the course of their study. Assessments were at baseline, mid-treatment, post-treatment, and follow-up. Since inaccurate models for change over time can produce misleading results, various growth curve models (GCMs) for the change in outcome from baseline to follow-up for each participant (linear, quadratic, logarithmic, hyperbolic, categorical time, and piecewise) were examined to determine the model that best fit the data (based on Akaike’s Information Criterion [AIC] and Schwartz’s Bayesian Criteria [BIC]). The best-fitting and hence final GCM was hyperbolic time (referred to hereafter as Time), indicating that participants’ outcomes from baseline to follow-up showed rapid improvement followed by rapid leveling-off. The primary endpoint was the final follow-up. P was set at .05, two-tailed. Approximate effect sizes were calculated using the t to d conversion.
To assess the decline effect, we added “publication year” and the Time by publication year interaction to the basic GCM (which included only Time). A significant positive regression coefficient for the Time by publication year interaction will suggest that pre-to-follow-up improvement is smaller in more recent studies. Because a meta-analysis (Gehr et al., 2006) showed that the decline effect for certain drugs was partially explained by differences in baseline severity, we next examined whether baseline severity, and the interaction between Time and baseline severity, accounted for the decline effect. In the final model, we added the balance of our putative predictors of improvement to the model. To avoid unnecessarily inflating Type 1 error, we limited the number of predictors in this analysis to the variables that were either 1) significant predictors of outcome in our previous analysis (Mataix-Cols et al., 2017), those predictors being sex, diagnosis, and number of treatment sessions, as well as 2) the three predictors that reflected characteristics of DCS administration (DCS dose, number of doses, and timing of the doses). These variables, and their interactions with Time, were added to the model that already included publication year and baseline severity. We included quadratic terms for all continuous predictors (curvilinear relations were not examined in our previous meta-analysis), but dropped non-significant quadratic terms. Finally, since baseline severity might moderate the effect of number of treatment sessions on outcome (i.e., more sessions might be especially helpful for participants with greater baseline severity), we included the baseline severity by number of sessions interaction, and the baseline severity by number of sessions by Time interaction in the analysis. Continuous predictors were standardized. We will infer that the variables in the model account for much of the declining effectiveness of DCS if publication year is not significant in this final analysis (assuming that it is significant in the previous analysis). This analysis was designed to show which variables were significant predictors of outcome in DCS treatment, and potentially show optimal dosing parameters for reducing outcome severity in CBT + DCS.
Power analyses, using Optimal Design (Spybrook et al., 2019), indicated power > .80 to detect small effect sizes (Cohen’s d = .20) for individual-level predictors of outcome. For study-level predictors, we had power > .60 to detect a large effect size (d = .80).
3. RESULTS
3.1. Baseline characteristics
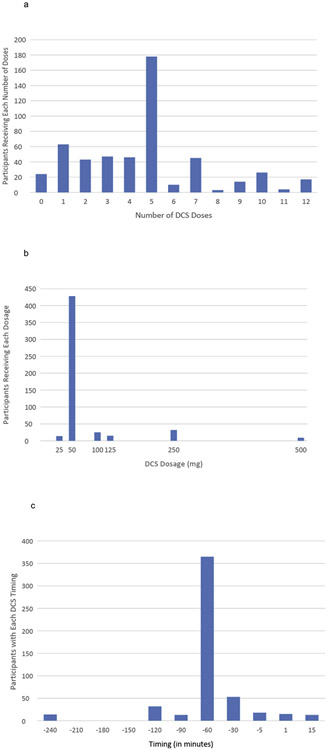
Information on baseline characteristics of the full sample can be found in our previous paper (Mataix-Cols et al., 2017). The present analysis included the 523 individuals in the CBT + DCS treatment condition in the 21 studies included from the previous IPD meta-analysis. The sample included 254 males (48.6%). Mean age was 32.3 years (SD = 13.7, range: 7-70). There were 145 participants with SAD, 142 with OCD, 131 with PTSD, 66 with SP, and 39 with PD/A. The average number of DCS doses was 4.61 (SD = 2.85, range: 0-12; see Fig. 1a). The DCS dose ranged from 25-500 mg (Fig. 1b), and was highly skewed (skewness = 3.90). Log-transforming dosage did not sufficiently reduce skewness (skewness = 2.34). However, an inverse transformation did reduced skewness to acceptable levels (skewness = .31). Thus, the inverse transformed dosage variable was used in analyses. The timing of administration of DCS (Fig. 1c) varied from 240 minutes before exposure (coded −240) to 15 minutes after exposure (coded 15), with almost 70% of cases receiving DCS 60 minutes before exposure (coded −60). No transformation of the dose timing data reduced skewness to acceptable levels. Thus, we recoded DCS timing into 3 groups: more than 60 minutes before exposure (11.3% of cases), exactly 60 minutes before exposure (69.8% of cases), and less than 60 minutes before exposure (including administration after exposure) (18.8%). We dummy coded the three categories with 2 dummy variables. Sensitivity analyses using dose timing as a linear predictor yielded the same results as reported below (but this model using linear time was a worse fit to the data [p = .03] than our model using categorical DCS timing).
Fig. 1.
Distributions of the DCS administration-related Variables. 1a: Number of DCS doses received; 1b: DCS Dosage (in mg); 1c: Timing of the administration of the DCS dose in relation to the beginning of the exposure session.
3.2. Testing the decline effect
To test the decline effect, we added the following variables to our basic growth curve model (which included only Time): year published, Time by year published, year published squared, and Time by year published squared (in these analyses only, year published was not z-scored to enhance interpretation). The quadratic effects for year published were not significant, so they were dropped. Consistent with the decline effect, results revealed that studies published more recently showed less pre-to-follow-up improvement than did studies published earlier, b = 5.59, t(673) = 3.58, p < .001, d = .28. Despite the fact that year published was highly related to improvement in outcome, year published was not significantly related to outcome severity at follow-up (p = .094). This finding was likely due to more recent studies having lower baseline severity (see below).
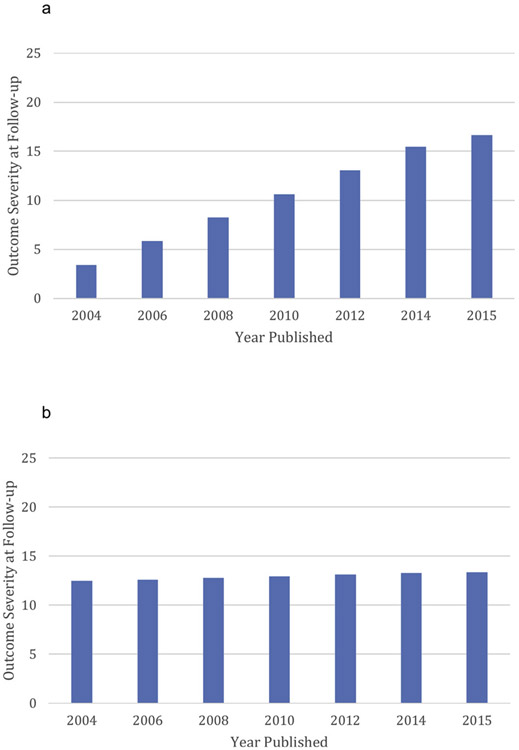
To determine if the smaller improvement in more recent studies was a result of changes in baseline severity over time, we controlled for baseline severity by including it as a predictor and moderator in this analysis. Still, more recent studies continued to evidence the decline effect, with more recent studies showing lower pre-to-follow-up improvement than earlier studies, b = 4.06, t(672) = 2.83, p = .005, d = .22, and showing higher severity at follow-up, b = 3.39, t (26) = 2.43, p = .022, d = .95 (Fig. 2a). However, the regression coefficient for the year published by Time interaction did decrease by 27% when baseline severity was controlled, suggesting that a portion of the decline effect is due to lower baseline severity in more recent studies. Indeed, baseline severity in studies conducted before 2010 averaged 57.2 while baseline severity in studies conducted in 2010 and thereafter averaged 49.3. Because lower severity was related to slower improvement, b = −6.39, t(686) = 18.18, p < .001, d = 1.39, lower baseline severity in more recent studies may partially account for the lessor improvement in those studies.
Fig. 2.
Outcome Severity at Follow-up as a Function of the Year of Publication. 2a: Before controlling for DCS administration-related variables and other predictors; 2b: After controlling for DCS administration-related variables and other predictors.
3.3. Predictors of improvement
The other putative predictors of DCS treatment outcome (sex, diagnosis, number of treatment sessions, DCS dose, DCS timing, and number of DCS doses) and their interactions with Time were simultaneously added to the GCM (which already included baseline severity and year published with their interactions with Time). In addition, quadratic terms for the continuous predictors (number of sessions, dosage, number of doses) and their interactions with Time were also included in the analysis, along with the baseline severity by number of treatment sessions interaction. The quadratic terms for number of sessions and dosage were nonsignificant. Thus, they were dropped and the model recomputed. Finally, since sex and dosage were not significantly related to outcome, they were also dropped and the final analysis was computed.
3.3.1. DCS administration-related predictors
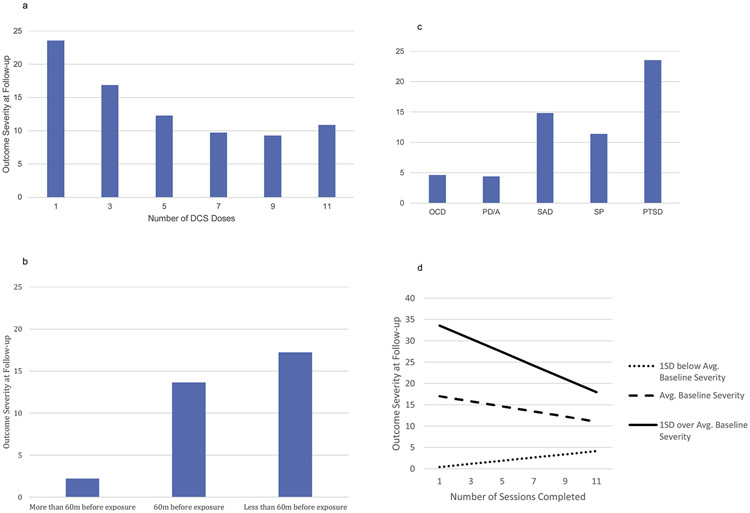
The final model showed that participants who were administered more DCS doses showed more pre-to-follow-up improvement, b = −9.03, t(715) = −2.67, p = .008, d = .20, and lower follow-up severity, b = −5.61, t(447) = −2.84, p = .005, d = .27. However, significant quadratic effects for DCS doses on both the slope, b = 3.58, t(746) = 2.75, p = .006, d = .20, and on severity at follow-up, b = 2.13, t(276) = 2.77, p = .006, d = .33, showed that the impact of number of doses leveled off at about 9 doses (Fig. 3a).
Fig. 3.
Effect of DCS Administration-related Variables and other Significant Predictors on Outcome Severity at Follow-up. 3a: Effect of Number of DCS Doses on Outcome Severity at Follow-up; 3b: Effect of the Timing of the DCS Dose Relative to the Beginning of the Exposure Session on Outcome Severity at Follow-up; 3c: Outcome Severity at Follow-up as a Function of Diagnosis; 3d. Effect of Number of Sessions on Outcome Severity at Follow-up as a Function of Baseline Severity.
Dose timing also emerged as a significant predictor of outcome. Those who received DCS more than 60 minutes prior to exposure showed significantly more improvement, b = −5.98, t(714) = −3.02, p = .003, d = .23, and lower severity scores at follow-up, b = −3.62, t(48) = −2.98, p = .005, d = .86, than those who were dosed 60 minutes prior to exposure. However, it should be noted that only about 11% of participants received their DCS dose more than 60 minutes before exposure. Those who were dosed less than 60 minutes prior to exposure (including dosing after exposure) did not differ from those dosed 60 minutes prior (Fig. 3b).
3.3.2. Predictors not related to DCS administration
Diagnosis (a categorical predictor) was significantly related to outcome, χ2(8) = 51.37, p < .001. Those with OCD and PD/A had the lowest follow-up severity, followed by those with SAD and SP (Fig. 3c). Those with PTSD had the highest severity at follow-up.
Total number of sessions attended was not directly related to treatment outcome for the average participant. However, the number of sessions x initial severity interaction showed that for those with high initial severity (1SD above the mean), more sessions were related to more improvement and to lower follow-up severity, b = −6.95, t(721) = −4.82, p < .001, d = .36 and b = −4.24, t(1036) = −5.44, p < .001, d = .34, respectively (Fig. 3d).
In this final model, which included (and therefore controlled for) our proposed predictors of outcome, year of publication was no longer significantly related to either slope of improvement, b = −60, t(670) = −0.23, p = .821, d = .02, or to follow-up severity, b = .24, t(56) = 0.14, p = .886, d = .04 (Fig. 2b). Thus, our predictors together accounted for virtually all of the decline effect. To determine if the elimination of the decline effect was primarily due to the DCS administration variables or to the non-DCS factors, we removed the DCS administration variables, and recomputed the model. In this analysis, which did not control for the DCS administration variables but did control for all the other predictors (e.g., diagnosis, baseline severity, number of sessions x initial severity, etc.), year of publication was significantly related to both the slope of improvement, b = 6.19, t(696) = 4.01, p < .001, d = .30, and to outcome severity at follow-up, b = 4.59, t(34) = 4.11, p < .001, d = 1.41. Then, when the DCS predictors were added to these non-DCS predictors, the relation between publication year and outcome severity decreased by about 95% (e.g., the effect of publication year on severity at follow-up decreased from b = 4.59 to b = .24). These results suggest that variables related to DCS administration may account for a very substantial portion of the decline effect.
3.4. Additional analyses
We used the regression equation from the final model to predict what the follow-up severity score would be for the average participant if she/he had received nine DCS doses administered more than 60 minutes before exposure. The predicted value was 0.16 (scale range: 0-100), suggesting that optimal dosing possibly would lead to negligible severity at follow-up. Additionally, we calculated that the predicted effect size for the within-subjects change from pre-to-follow-up would have been d = 1.92 if all patients had received 9 DCS doses administered more than 60 minutes before exposure, whereas the actual within-subjects pre-to-follow-up effect size for the average participant in the studies as conducted was only d = 1.26.
In exploratory analyses, we next examined additional predictors or moderators of the DCS effects. First, we investigated whether participants in studies with larger sample sizes showed lesser improvement in outcome severity. Sample size was not related to improvement (ps > .84) when it was added as an additional predictor in our final model above. Second, we examined whether sex, diagnosis, or age moderated the effect of the DCS variables by adding the interaction of those variables with the DCS variables to our final model. None of these variables (sex, diagnosis, age) moderated the effects of the DCS measures on outcome. Lastly, we added all the two-way interactions between the DCS variables to our final model (e.g., dose x number of doses). None of these interactions was significant.
Because dose timing was significantly related to outcome, yet early dose timing (more than 60 minutes before the exposure session) was only experienced by about 11% of the sample, we investigated whether these 11% were biasing the results by performing a sensitivity analysis which excluded these “early dose timing” participants. Results from this sensitivity analysis (excluding the early dose timing participants) were very similar to the results for the entire sample. In particular, as in the full sample, the following effects were significantly related to slope of improvement and severity of outcome at follow-up in the sensitivity analysis: baseline severity, the linear and quadratic effects of number of DCS doses, diagnosis, and the baseline severity x number of sessions interaction. Even the effect of the timing of the administration of DCS was similar to the full sample: as in the analysis with the full sample, there was no significant difference between those receiving DCS 60 minutes before exposure and those receiving DCS less than 60 minutes before exposure in the sensitivity analysis (which excluded participants receiving DSC more than 60 minutes before exposure). And, similar to the full sample, the proportion of the decline effect that was accounted for by our model in the sensitivity analysis was 93%, comparable to the 95% in the full sample.
Finally, the model that was used to predict outcome for the DCS participants was then applied to the placebo participants (n = 521). The DCS administration variables were not significantly related to outcome for the placebo participants. However, the non-DCS related predictors (diagnosis and number of sessions x initial severity) had similar relations with outcome in the CBT + Placebo group as they did in the CBT + DCS group.
4. DISCUSSION
We observed a linear decline over time in the efficacy of DCS augmentation for 21 studies published across a 14-year period, and found that characteristics of DCS administration (e.g., the number of DCS doses, the timing of dosing), along with baseline severity, accounted for almost all of this declining effect over time. We did not observe the same results in patients randomized to placebo, suggesting that these findings are related to DCS, rather than to other nonspecific or unmeasured factors. The DCS declining effect may well be related to the unique way in which DCS progressed to clinical trials. For example, the 2008 meta-analysis by Nordberg et al. (2008) suggested that fewer DCS doses might lead to better results because of the rapid development of tolerance to DCS (Otto et al., 2015). Perhaps because of this publication, our data shows that the number of DCS doses received by participants peaked in 2007 with an average of 9.19 DCS doses in studies conducted that year, and then declined to an average of only 4.32 doses in the years after Nordberg et al. (2008) was published. As for the timing of the DCS administration, Ressler and associates (2004) administered DCS 2-4 hours prior to the exposure session. This is consistent with the estimated time to peak concentrations in the brain for oral administration (D’Souza et al., 2000). Nonetheless, many (88.7%) subsequent trials administered the study drug just one hour or less before exposure sessions, based on the assumption that DCS would be active by the conclusion of the exposure session, in accordance with the animal literature indicating that delivery of DCS during memory consolidation produced extinction augmentation effects (Davis et al., 2006).
As compared to these somewhat ad-hoc strategies adopted over time, empirically derived recommendations can now be suggested based on the current meta-analysis. Our data indicates that participants might benefit most from about 9 doses of DCS, and from administering the doses more than 60 minutes prior to exposure, although the latter recommendation is based on a small sample of participants (11%). Our data do not suggest that a dosage greater than 50 mg is more effective than a 50 mg dose. On the other hand, we cannot speculate on whether the DCS effect may weaken with smaller doses since we had few participants (N = 14) who received a dose less than 50 mg. Our analysis suggests that had more studies adopted these parameters for the delivery of DCS augmentation, the declining efficacy of DCS may never have occurred. Indeed, using our MLM regression equation, we were able to calculate the predicted level of outcome severity at follow-up for participants if they had received the optimal dosing regimen of nine doses administered more than 60 minutes before exposure therapy. This predicted level of severity was virtually zero on the harmonized 0-100 severity scale, where zero represents the lowest severity achieved by any participant in any of the studies. Also, the projected effect size for within-subjects improvement (baseline-to-follow-up) if the studies had administered 9 DCS doses more than 60 minutes before exposure therapy was much larger (d = 1.92) than the within-subjects, baseline-to-follow-up effect size for DCS in the studies as they were actually conducted (d = 1.26).
As noted, the decline effect is common in both the psychiatric and general medical literature. The phenomenon of declining effect sizes over time has been documented for risk/protective variables (Monsarrat & Vergnes, 2018) as well as for the efficacy of drugs for both non-psychiatric (Gehr et al., 2006) and psychiatric conditions (Trikalinos et al., 2004). Explanations for these effects have been rare, and may be specific to the variable/drug under study. For example, Gehr and associates (2006) found that baseline severity, but not study size, partially explained the decline effect of certain cholesterol drugs, with less severe patients being randomized over time and less severe patients improving less. Still, for several drugs that they studied, year published continued to account for significant variance in effect sizes even after controlling for baseline severity. Our results for baseline severity were similar to the findings in Gehr et al. (2006). Baseline severity was a significant predictor of clinical improvement during DCS augmentation, baseline severity was lower in more recent trials, and baseline severity accounted for some (27%), but not most, of the prediction afforded by year of publication. Further, as in Gehr et al. (2006), baseline severity did not account for all the decrease in effectiveness of DCS over the years. However, we were able to explain virtually all (95%) of the remaining unexplained decline effect using variables related to the administration of DCS.
Our analyses also revealed other potentially useful information about predictors and non-predictors of outcome in CBT plus DCS. Although one should not make strong conclusions from null findings, neither sex, diagnosis, nor age moderated any of the effects of DCS administration variables on outcome, nor were larger sample sizes related to improvement. Additionally, there were no interactions between DCS dosing variables. Although these null findings must be interpreted cautiously, they may provide some useful information. On the other hand, we did find that participants with high baseline severity benefited more from additional sessions of CBT than those with lower severity, and that improvement was dependent on the disorder being treated. These latter two findings appear to be characteristics of CBT itself, rather than DCS, since they were found to be significant in both the CBT + DCS group and the CBT + Placebo group.
Several limitations deserve comment. First, although we had sufficient power to detect small effects for individual-level factors, statistical power was low for detecting study-level effects. Further, given the small number of studies included in our analyses, the results for the study-level variables may not generalize to a larger population of studies. In addition, it is possible that unmeasured third variables account for the significant relations we observed in this study. Although we included a number of plausible third-variable candidates in our final model, establishing causal effects requires future experimental work. In addition, our finding that earlier administration of DCS may be more beneficial than later administration of DCS was based on a small portion (11%) of our participants, and hence requires replication. Finally, in addition to the treatment of anxiety-related disorders, DCS has been applied as an augmentation agent for exposure-based CBT for substance dependence, eating disorders, and psychotic symptoms (Otto et al., 2015). It is not clear whether the dosage characteristics we recommend have validity for other disorders.
5. Conclusion
The findings from the present study add to the growing literature on DCS augmentation for anxiety disorders and show that DCS efficacy might depend on judicious (and informed) administration of the drug. The findings also suggest that specific dosing characteristics, as well as attending to baseline severity, may largely reverse the apparent DCS decline effect over time. DCS augmentation studies evolved without a specific dose-finding phase; as such, an analysis of optimal DCS administration characteristics represents a needed addition to the DCS literature. Our findings underscore the importance of parametric research in treatment development. Determining the optimal application of any clinical strategy requires careful testing of the dosing and timing of an intervention, which should be guided by theory and empirical evidence about mechanisms (in this case, DCS and CBT). Thus, these suggestive results need confirmation in prospectively designed studies employing the parameters identified as optimal in this reanalysis.
Acknowledgements
All authors with the exception of Drs. Fernández de la Cruz, Frumento, and Pérez-Vigil were investigators on one or more of the original randomized controlled trials that contributed data to the individual patient data and secured grant funding for these trials. Drs. Davis and Ressler hold patents for the use of D-cycloserine and psychotherapy, targeting PAC1 receptor for extinction, targeting tachykinin 2 for prevention of fear, targeting angiotensin to improve extinction of fear. Dr. Davis and Dr. Ressler are also co-founding members of Extinction Pharmaceuticals to develop D-cycloserine to augment the effectiveness of psychotherapy, for which he has received no equity or income within the last three years. Dr. Davis retired in 2012 and has received no financial support from any source since his retirement. Dr. Rosenfield reports funds from the National Institute of Mental Health, the National Institute on Drug Abuse, CPRIT, and Behavior Research and Therapy. Dr. Smits is funded by the NIH, CPRIT and has been a paid consultant for Big Health, Ltd. Ms Pérez-Vigil is supported by a grant from the Alicia Koplowitz Foundation. Dr. Farrell reports funds from the Rotary Mental Health Research Fund Australia. Dr. Geller reports funds from the National Institute of Mental Health. Dr. Hofmann reports funds from the National Center for Complementary and Integrative Health (R01AT007257) and the National Institute of Mental Health (R01MH099021, R34MH099311, R34MH086668, R21MH102646, R21MH101567, and K23MH100259), the James S. McDonnell Foundation 21st Century Science Initiative in Understanding Human Cognition – Special Initiative, and the Department of the Army. Dr. Kushner reports funds from the National Institute on Alcohol Abuse and Alcoholism (R01AA015069). Dr. Levinson reports funds from the National Institutes of Health (5T32DA007261-17). Dr. McConnell reports funds form the Rotary Mental Health Research Fund. Dr. Otto reports current funds from the National Institute of Mental Health (R21MH102646 and R34MH099311), and recent or past support from National Institute of Mental Health (R01MH081116, R21DA030808, and R01MH078308). In addition, Dr. Otto reports serving, in the last three years, as a paid consultant for MicroTransponder Inc., Concert Pharmaceuticals, and ProPhase, providing expert consensus opinion for Otsuka Pharmaceuticals, receiving royalty support for use of the SIGH-A from ProPhase, and receiving book royalties from Oxford University Press, Routledge, and Springer. Dr. Plag serves as a consultant for Pfizer, Inc. Dr. Pollack reports funds from the National Institute of Mental Health, the National Institutes of Health, Janssen, and Edgemont. In addition, Dr. Pollack serves as consultant/advisor for Clintara, Edgemont Pharmaceuticals, and Palo Alto Health Sciences. Dr. Pollack reports the following patents/royalties: SIGH-A, SAFER interviews. Dr. Pollack’s equity disclosure includes Doyen Medical, Medavante, Mensante Corporation, Mindsite, and Targia Pharmaceuticals. Dr. Ressler reports current or past funds from the National Institute of Mental Health, the Howard Hughes Medical Institute, the Brain & Behavior Research Foundation (formerly NARSAD), Burroughs Wellcome Fund. In addition, Dr. Ressler is on the Scientific Advisory Boards for Resilience Therapeutics, Sheppard Pratt-Lieber Research Institute, Laureate Institute for Brain Research, The Army STARRS Project, and the Anxiety and Depression Association of America. Dr. Rodebaugh reports current funds from the Behavior Brain Research Foundation (NARSAD Independent Investigator Award) and the McDonnell Center for Systems Neuroscience and past funds from the National Institute of Mental Health (R21MH090308-01A). Dr. Rothbaum reports funds from the National Institute of Mental Health (R01MH70880). In addition, Dr. Rothbaum owns equity in Virtually Better, Inc. that creates virtual environments. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies. Dr. Storch reports funds from National Institute of Mental Health, the National Institutes of Health, the Agency for Healthcare Research and Quality, and All Children's Hospital Research Foundation. In addition, Dr. Storch reports Royalties from Elsevier, the American Psychological Association, Springer, Wiley Inc., and Lawrence Erlbaum, and is consultant for Ruijin Hospital and Rogers Memorial Hospital. Dr. Ströhle reports funds from the German Federal Ministry of Education and Research (BMBF), the German Research Foundation (DFG), the European Commission (FP6), and Lundbeck. In addition, Dr. Ströhle serves as speaker honoraria for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly & Co, Lundbeck, Pfizer, Wyeth, and UCB, and was a consultant for Actelion. Dr. Ströhle’s educational grants were given by the Stifterverband für die Deutsche Wissenschaft, the Berlin Brandenburgische Akademie der Wissenschaften, the Boehringer Ingelheim Fonds, the Eli Lilly International Foundation, Janssen-Cilag, Pfizer, and Eli Lilly & Co. Dr. Tart reports funds from National Institute of Mental Health. Dr. van Minnen reports funds from Stichting Achmea Slachtoffer en Samenleving and Vereniging tot Christelijke Verzorging van Geestes- en Zenuwzieken. Dr. Waters reports funds from the Rotary Mental Health Research Fund Australia. Dr. Weems reports funds from the National Institute of Mental Health (5RC1MH088969-02). Dr. Wilhelm has received research funding and salary support from the NIH, she has also received research support in the form of free medication, and matching placebo from Forest Laboratories for clinical trials funded by the NIH. Dr. Wilhelm is a presenter for the Massachusetts General Hospital Psychiatry Academy in educational programs supported through independent medical education grants from pharmaceutical companies; she has received royalties from Elsevier Publications, Guilford Publications, and New Harbinger Publications from Oxford University Press. Dr. Wilhelm has also received speaking honorarium from various academic institutions and foundations, including the International Obsessive Compulsive Disorder Foundation and the Tourette’s Syndrome Association. In addition, she received payment from the Association for Behavioral and Cognitive Therapies for her role as Associate Editor for the Behavior Therapy journal, as well as from John Wiley & Sons, Inc. for her role as Associate Editor on the journal Depression & Anxiety. Dr. Wilhelm has also received salary support from Novartis. Dr. Rück is supported by a grant from the Swedish Research Council (K2013-61P-22168). Dr. Gutner reports funds from the National Institute of Mental Health (1K23MH103396). No other disclosures were reported.
Footnotes
Subsequent to that time, nine additional studies (Arman et al., 2017; de Leeuw, van Megen, Kahn, & Westenberg, 2017; Farrell et al., 2018; Hofmeijer-Sevink et al., 2017; Otto et al., 2016; Pyrkosch et al., 2018; Rapee et al., 2016; Sheerin et al., 2016; Storch et al., 2016) have been published that would have met inclusion criteria, but they are not included in the present analysis because 1) we did not have individual participant data for those studies and 2) the purpose of the present study was to explain the decline effect observed in our prior meta-analysis.
References
- Arman S, Soheilimehr A, & Maracy MR (2017). The Efficacy of Augment of D-Cycloserine and Cognitive-behavioral Therapy on Adolescent with one Type of Anxiety Disorders: A Double-blind Randomized Controlled Trial. Advanced Biomedical Research, 6, 11. 10.4103/2277-9175.200786.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempo A, Panza KE, & Bloch MH (2012). D-Cycloserine Augmentation of Behavioral Therapy for the Treatment of Anxiety Disorders: A Meta-Analysis. The Journal of Clinical Psychiatry, 73(04), 533–537. 10.4088/JCP.11r07356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürkner P-C, Bittner N, Holling H, & Buhlmann U (2017). D-cycloserine augmentation of behavior therapy for anxiety and obsessive-compulsive disorders: A meta-analysis. PloS One, 12(3), e0173660. 10.1371/journal.pone.0173660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter JK, Andrews LA, Witcraft SM, Powers MB, Smits JAJ, & Hofmann SG (2018). Cognitive behavioral therapy for anxiety and related disorders: A meta-analysis of randomized placebo-controlled trials. Depression and Anxiety, 35(6), 502–514. 10.1002/da.22728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, & Richardson R (2006). Effects of D-Cycloserine on Extinction: Translation From Preclinical to Clinical Work. Biological Psychiatry, 60(4), 369–375. 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- de Leeuw AS, van Megen HJGM, Kahn RS, & Westenberg HGM (2017d). D-cycloserine addition to exposure sessions in the treatment of patients with obsessive-compulsive disorder. European Psychiatry: The Journal of the Association of European Psychiatrists, 40, 38–44. 10.1016/j.eurpsy.2016.06.011. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Gil R, Cassello K, Morrissey K, Abi-Saab D, White J, … Krystal JH (2000). IV glycine and oral D-cycloserine effects on plasma and CSF amino acids in healthy humans. Biological Psychiatry, 47(5), 450–462. [DOI] [PubMed] [Google Scholar]
- Farrell LJ, Waters AM, Oar EL, Tiralongo E, Garbharran V, Alston-Knox C, … Ollendick TH (2018). D-cycloserine-augmented one-session treatment of specific phobias in children and adolescents. Brain and Behavior, 8(6), e00984. 10.1002/brb3.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa TA, Cipriani A, Atkinson LZ, Leucht S, Ogawa Y, Takeshima N, … Salanti G (2016). Placebo response rates in antidepressant trials: A systematic review of published and unpublished double-blind randomised controlled studies. The Lancet. Psychiatry, 3(11), 1059–1066. 10.1016/S2215-0366(16)30307-8. [DOI] [PubMed] [Google Scholar]
- Gehr BT, Weiss C, & Porzsolt F (2006). The fading of reported effectiveness. A meta-analysis of randomised controlled trials. BMC Medical Research Methodology, 6, 25. 10.1186/1471-2288-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Meuret AE, Smits JAJ, Simon NM, Pollack MH, Eisenmenger K, … Otto MW (2006). Augmentation of Exposure Therapy With D-Cycloserine for Social Anxiety Disorder. Archives of General Psychiatry, 63, 298–304. 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Otto MW, Pollack MH, & Smits JA (2015). D-cycloserine augmentation of cognitive behavioral therapy for anxiety disorders: An update. Current Psychiatry Reports, 17(1), 532. 10.1007/s11920-014-0532-2. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Smits JAJ, Asnaani A, Gutner CA, & Otto MW (2011). Cognitive enhancers for anxiety disorders. Pharmacology, Biochemistry, and Behavior, 99(2), 275–284. 10.1016/j.pbb.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeijer-Sevink MK, Duits P, Rijkeboer MM, Hoogendoorn AW, van Megen HJ, Vulink NC, … Cath DC (2017). No Effects of D-Cycloserine Enhancement in Exposure With Response Prevention Therapy in Panic Disorder With Agoraphobia: A Double-Blind, Randomized Controlled Trial. Journal of Clinical Psychopharmacology, 37(5), 531–539. 10.1097/JCP.0000000000000757. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Fernández de la Cruz L, Monzani B, Rosenfield D, Andersson E, Pérez-Vigil A, … Thuras P (2017). D-Cycloserine Augmentation of Exposure-Based Cognitive Behavior Therapy for Anxiety, Obsessive-Compulsive, and Posttraumatic Stress Disorders: A Systematic Review and Meta-analysis of Individual Participant Data. JAMA Psychiatry, 74(5), 501–510. 10.1001/jamapsychiatry.2016.3955. [DOI] [PubMed] [Google Scholar]
- Monsarrat P, & Vergnes J-N (2018). The intriguing evolution of effect sizes in biomedical research over time: Smaller but more often statistically significant. GigaScience, 7(1), 1–10. 10.1093/gigascience/gix121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg MM, Krystal JH, & Tolin DF (2008). A Meta-Analysis of D-Cycloserine and the Facilitation of Fear Extinction and Exposure Therapy. Biological Psychiatry, 63(12), 1118–1126. 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Otto MW, Kredlow MA, Smits JAJ, Hofmann SG, Tolin DF, de Kleine RA, … Pollack MH (2015). Enhancement of Psychosocial Treatment With d-Cycloserine: Models, Moderators, and Future Directions. Biological Psychiatry. 10.1016/j.biopsych.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, Pollack MH, Dowd SM, Hofmann SG, Pearlson G, Szuhany KL, … Tolin DF (2016). Randomized Trial of D-Cycloserine Enhancement of Cognitive-Behavioral Therapy for Panic Disorder. Depression and Anxiety, 33(8), 737–745. 10.1002/da.22531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrkosch L, Mumm J, Alt I, Fehm L, Fydrich T, Plag J, & Ströhle A (2018). Learn to forget: Does post-exposure administration of d-cycloserine enhance fear extinction in agoraphobia? Journal of Psychiatric Research, 105, 153–163. 10.1016/j.jpsychires.2018.08.016. [DOI] [PubMed] [Google Scholar]
- Rapee RM, Jones MP, Hudson JL, Malhi GS, Lyneham HJ, & Schneider SC (2016). D-Cycloserine does not enhance the effects of in vivo exposure among young people with broad-based anxiety disorders. Behaviour Research and Therapy, 87, 225–231. 10.1016/j.brat.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, et al. (2004). Cognitive enhancers as adjuncts to psychotherapy: Use of d-cycloserine in phobic individuals to facilitate extinctionof fear. Archives of General Psychiatry, 61(11), 1136–1144. 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Rodrigues H, Figueira I, Lopes A, Gonçalves R, Mendlowicz MV, Coutinho ESF, & Ventura P (2014). Does D-Cycloserine Enhance Exposure Therapy for Anxiety Disorders in Humans? A Meta-Analysis. PLoS ONE, 9(7), 10.1371/journal.pone.0093519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheerin CM, Kozak AT, Hale AC, BCBA R, & Spates BKCR (2016). The effect of D-cycloserine on social anxiety treatment using a behavioral outcome measure and a post-session administration strategy. Behavior Analysis (Washington, D.C.), 16(3), 123–134. 10.1037/bar0000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spybrook J, Bloom H, Congdon RT, Hill C, Martinez A, & Raudenbush SW (n.d.). Optimal DesignOptimal Design Retrieved from from http://hlmsoft.net/od/od-manual-20111016-v300.pdf. [Google Scholar]
- Storch EA, Wilhelm S, Sprich S, Henin A, Micco J, Small BJ, … Geller DA (2016). Efficacy of Augmentation of Cognitive Behavior Therapy With Weight-Adjusted D-Cycloserine vs Placebo in Pediatric Obsessive-Compulsive Disorder. JAMA Psychiatry, 73(8), 779–788. 10.1001/jamapsychiatry.2016.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trikalinos TA, Churchill R, Ferri M, Leucht S, Tuunainen A, Wahlbeck K, … EUPSI project (2004). Effect sizes in cumulative meta-analyses of mental health randomized trials evolved over time. Journal of Clinical Epidemiology, 57(11), 1124–1130. [DOI] [PubMed] [Google Scholar]