Abstract
The mature lactococcal cell envelope proteinase (CEP) consists of an N-terminal subtilisin-like proteinase domain and a large C-terminal extension of unknown function whose far end anchors the molecule in the cell envelope. Different types of CEP can be distinguished on the basis of specificity and amino acid sequence. Removal of weakly bound Ca2+ from the native cell-bound CEP of Lactococcus lactis SK11 (type III specificity) is coupled with a significant reversible decrease in specific activity and a dramatic reversible reduction in thermal stability, as a result of which no activity at 25°C (pH 6.5) can be measured. The consequences of Ca2+ removal are less dramatic for the CEP of strain Wg2 (mixed type I-type III specificity). Autoproteolytic release of CEP from cells concerns this so-called “Ca-free” form only and occurs most efficiently in the case of the Wg2 CEP. The results of a study of the relationship between the Ca2+ concentration and the stability and activity of the cell-bound SK11 CEP at 25°C suggested that binding of at least two Ca2+ ions occurred. Similar studies performed with hybrid CEPs constructed from SK11 and Wg2 wild-type CEPs revealed that the C-terminal extension plays a determinative role with respect to the ultimate distinct Ca2+ dependence of the cell-bound CEP. The results are discussed in terms of predicted Ca2+ binding sites in the subtilisin-like proteinase domain and Ca-triggered structural rearrangements that influence both the conformational stability of the enzyme and the effectiveness of the catalytic site. We argue that distinctive primary folding of the proteinase domain is guided and maintained by the large C-terminal extension.
Production of a catalytically active cell envelope proteinase (CEP) in Lactococcus lactis subsp. cremoris AM1 and SK11 during growth in a chemically defined medium depends on the presence of calcium ions in the medium; calcium cannot be replaced by other bivalent cations present in the medium to obtain a stable, active CEP (6). A similar dependence on Ca2+ ions has been observed with strain Wg2 (19). The CEPs of strains SK11 and Wg2 have been characterized extensively both genetically and biochemically (22, 29). These enzymes represent two of the several types of lactococcal CEPs which have been distinguished on the basis of their specificity towards peptides, namely, a CEP with type III specificity (CEPIII) and a CEP with mixed type I-type III specificity (CEPI/III) (14). The mature CEPs of strains SK11 and Wg2 are large molecules that are 1,775 or 1,715 amino acid residues long. If the repeat sequence in the C-terminal part is not included, the sequence of the SK11 CEP differs at 44 positions from the sequence of the Wg2 CEP (33). The CEP N-terminal domain consisting of 500 residues exhibits significant sequence similarities with subtilisins and related serine proteinases (the subtilases). One difference between the CEPs and the subtilases is the large C-terminal extension in each CEP whose extreme C terminus anchors the enzyme in the cell envelope. In the subtilases four Ca-binding sites have been recognized; the association of Ca2+ ions with these sites is known to influence the activity and thermal stability of the enzymes and to protect the enzymes from autoproteolytic degradation (16, 30, 35). It has been predicted that there are three corresponding Ca2+-binding sites in CEP (31). In view of the role of Ca2+ in the subtilases, the observed dependence of the production of an active CEP on Ca2+ ions during growth may be interpreted either in terms of an active conformation that can be adopted only if Ca2+ ions are involved in the folding process or in terms of a requirement for Ca2+ ions that are needed to stabilize the active conformation.
Ca2+ ions protect cell-bound CEP from detachment from the cells as well. In the absence of Ca2+ ions in the suspending buffer, CEP activity can be released (10, 26). It has been suggested that this release occurs following the removal of bound calcium and that, as a result of this removal, local molecular unfolding outside the actual proteinase domain, in the large C-terminal extension, exposes a sequence which is highly susceptible to autoproteolytic attack (23, 24). Consequently, prevention of release of CEP by relatively high concentrations of calcium in the buffer should be due to preservation of the resistant form of the bound enzyme. Complete release of the proteinase from the cells occurs only after repeated resuspension of the cells in fresh Ca-free buffer (10, 24). In fact, this suggests that each subsequent treatment in Ca-free buffer results in the establishment of an equilibrium between free Ca2+ and bound Ca2+ which causes the release process to slow down and eventually to halt. Only resuspension in fresh Ca-free buffer results in continuation of this process.
In order to better understand the role of Ca2+ ions in the protection and functioning of the cell-bound CEP, we used dilute suspensions of cells of strains SK11 and Wg2 which were thoroughly washed with ice-cold Ca-free buffer and investigated the effects of temperature and Ca2+ concentration on cell-bound CEP. Significant differences between the two strains with respect to activity, stability, and release of the cell-bound CEPs were observed. As these differences were apparently related to the few substitutions inside and/or outside the proteinase domains of the two CEP molecules, we investigated the possible involvement of different segments of the CEP molecule in the Ca2+-related features of the wild-type enzymes by studying recombinant strains which produced hybrid CEPs constructed from the wild types. These experiments established that the C-terminal extension plays an essential role.
(A preliminary account of some of the results has been published in the proceedings of the International Dairy Lactic Acid Bacteria Conference held in Palmerston North, New Zealand [9].)
MATERIALS AND METHODS
Organisms.
L. lactis subsp. cremoris SK11 (producing CEPIII) and Wg2 (producing CEPI/III) (14) were used in this study. Recombinant L. lactis MG 1363 strains containing plasmid-located hybrid proteinase genes constructed from the SK11 (ABCD) and Wg2 (abcd) wild-type proteinase genes (34) were obtained from the Netherlands Institute for Dairy Research and from the Department of Genetics, University of Groningen, Groningen, The Netherlands. The hybrid proteinase genes contained one or more interchanged DNA fragments: fragment A or a, encoding amino acid residues 1 to 173; fragment B or b, encoding amino acid residues 174 to 496; fragment C or c, encoding amino acid residues 497 to 1089; and fragment D or d, encoding amino acid residues 1090 to 1775 (1715).
Growth and treatment of cells.
Strains were grown in reconstituted skim milk (100 ml) at 25°C until the early stationary phase. In the case of the recombinant MG 1363 strains the milk was supplemented with 1% (wt/vol) glucose and either chloramphenicol (10 μg · ml−1) or erythromycin (5 μg · ml−1) as an additional selection factor (34).
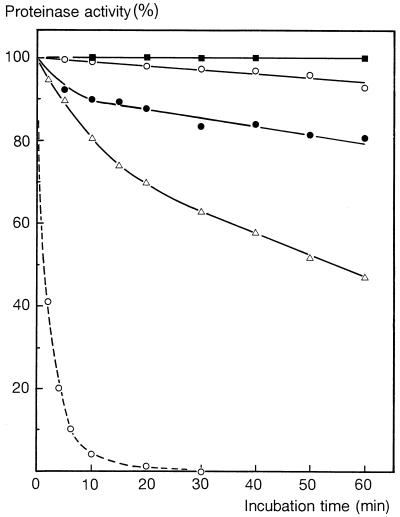
Cells were harvested after the milk culture was cleared with 1% (wt/vol) trisodium citrate at pH 6.5 (7); then they were routinely washed twice with 40 ml of ice-cold 50 mM imidazole buffer (pH 6.5) prepared with double-distilled water and finally resuspended in 40 ml of the same buffer (optical density at 650 nm after 10-fold dilution, 0.5 to 0.6) and kept on ice. This standard procedure completely removed weakly bound Ca2+ from the cell-bound CEP (“Ca-free” CEP) (see below) (Fig. 1).
FIG. 1.
Influence of treatment of milk-grown cells of L. lactis subsp. cremoris SK11 (○ and ●) and Wg2 (□ and ■) with ice-cold Ca2+-free 50 mM imidazole buffer (pH 6.5) on the activity of the cell-bound CEP measured in the absence (solid symbols) or in the presence (open symbols) of 10 mM Ca2+. The activities at zero (no wash) are the activities of cells harvested from the milk culture and resuspended in the Ca-free buffer. Activities are expressed as percentages of the activity of unwashed cells of each strain measured in the presence of Ca2+.
Proteinase assay.
To 8 ml of 50 mM imidazole buffer (pH 6.5) containing the appropriate final concentration of Ca2+ we added 1 ml of a 10 mM solution in buffer of the substrate succinyl-alanyl-glutamyl-prolyl-phenylalanyl-p-nitroanilide (S-Glu) (Bachem AG, Bubendorf, Switzerland) or methoxysuccinyl-arginyl-prolyl-tyrosyl-p-nitroanilide (MS-Arg) (Chromogenix AB, Mölndal, Sweden). The mixture was warmed to 25°C, and then 1 ml of a cell suspension was added. Hydrolysis was monitored by stopping the reaction with 0.3 ml of 80% acetic acid per ml of incubation mixture. The extinction at 410 nm was measured after filtration through a 0.22-mm-pore-size filter (Millex GV; Millipore, Bedford, Mass.). The progress curve was linear for at least 60 min; all activity assays were performed by using this initial linearity.
Specificity of the CEP.
Enzymatic hydrolysis of as1-casein fragment f1-23 [αs1-CN(f1-23)] by the (hybrid) CEP in situ (whole cells) at pH 6.5 in 50 mM imidazole containing 0, 0.1, 1, or 10 mM Ca2+, Mn2+, or Cd2+ followed by identification of the products by reversed-phase high-performance liquid chromatography (HPLC) was performed as described previously (12, 13). This peptide has been shown to have excellent characteristics which enable workers to distinguish small changes in the specificity of CEP (14).
RESULTS
Loss of CEP activity due to treatment of cells with ice-cold calcium-free buffer.
When milk-grown cells of L. lactis SK11 containing CEPIII were harvested and then resuspended (no wash) and washed twice with ice-cold Ca-free imidazole buffer (pH 6.5) (standard procedure), a nearly complete loss of cell-bound CEP activity at 25°C with the substrate S-Glu was observed, which was not accompanied by the appearance of activity in the supernatant.
The activity of the cell-bound proteinase of washed cells (referred to below as the “Ca-free” CEP) could be regained if Ca2+ ions were added (optimal activity occurred at a concentration of 10 mM [see below]; referred to below as potential activity of the Ca-free CEP), although to a decreasing extent depending on the number of washes in Ca-free buffer. With no wash the activity was only 45% of the maximal activity measured with the cells if Ca2+ ions were included in the assay solution (maximal potential activity, 100%). After one wash the activity was further reduced to 3 to 4%, and there was a 10% reduction in the potential activity as well. Additional treatments of the cells resulted in progressive reductions in this potential activity (Fig. 1). At 25°C restoration of the Ca-free SK11 CEP activity by Ca2+ took place without any detectable delay; a linear activity progress curve intersecting the x axis at zero was obtained when washed cells were added to the buffered substrate solution containing Ca2+ (data not shown).
The effect of the washing procedure on the activity of cell-bound Wg2 CEP (which could be measured only with MS-Arg as the substrate [14]) was relatively small. An initial reduction of about 30% was observed (with no wash); the remaining activity of the Ca-free cell-bound CEP and its potential activity (viz., the activity in the presence of Ca2+) were only slightly decreased after repeated treatments with cold buffer (Fig. 1). The reductions in the activities with S-Glu of strains producing hybrid CEPs containing fragment A (harboring part of the SK11 substrate-binding site) were similar to or slightly less than those of the wild-type SK11 CEP (data not shown).
Thermal stability and release of the Ca-free cell-bound CEP.
The Ca-free cell-bound SK11 CEP appeared to be extremely unstable at pH 6.5 and 25°C; within 20 min 99% of its potential activity was lost (Fig. 2a). Even storage on ice resulted in a gradual loss of potential activity (there was a 20% reduction in activity after 4 h) (data not shown). This relatively low rate of inactivation cannot explain the reduction in potential activity after each wash with ice-cold buffer (Fig. 1), indicating that other factors are responsible as well. The instability of the Ca-free CEP may be mainly responsible for the failure to measure activity at 25°C. However, Ca-free CEP activity could be measured at 10°C showing linear initial progress; the rate was 50 to 60% of the potential activity rate at 10°C. Temperature-related changes in relative affinities which result in complete inhibition of the cleavage of S-Glu by competitive autoproteolysis at 25°C and almost no inhibition at 10°C could indeed explain these observations but do not seem very likely. At this stage, therefore, we believe that removal of weakly bound Ca2+ destabilizes the enzyme against thermal denaturation rather than against autoproteolytic inactivation and that destabilization is coupled with a decline in specific activity.
FIG. 2.
(a) Stability of the cell-bound and released Ca-free SK11 CEP at pH 6.5 and 25°C. Low-density cell suspensions in 50 mM imidazole (pH 6.5) (1:10 dilution of the standard suspension) were incubated at 25°C in the absence of Ca2+ (cells containing the Ca-free CEP) (○) or in the presence of 10 mM Ca2+ (●). At different times residual activities were measured in the presence of 10 mM Ca and expressed as percentages of the initial activity. For comparison, the results obtained for Ca-free cell-bound hybrid proteinases ABCd and Abcd are also shown, as are the results obtained for released Ca-free SK11 CEP in the absence of Ca2+ (□) or in the presence of 10 mM Ca2+ (▵). For details see the text. (b) Stability of cell-bound Ca-free Wg2 CEP in 50 mM imidazole (pH 6.5) at 25°C. Residual activities associated with the cell fraction (○) and in the supernatant (●) of the cell suspension were assayed in the absence of Ca2+ and were expressed as percentages of the initial activity. For comparison the activities associated with the cell fraction after incubation of a cell suspension in buffer supplemented with 0.2 mM Ca2+ are also shown (▴).
Release of the catalytically active Ca-free CEP from cells (11) occurred at high cell densities (200 times the standard density) but not in dilute suspensions. After 20 min of incubation of a dense suspension at 25°C and pH 6.5, all residual activity was recovered in the supernatant; however, the amount of activity recovered was less than 0.2% of the initial potential activity. The released Ca-free SK11 CEP appeared to be relatively stable compared to the cell-bound Ca-free enzyme (Fig. 2a) and could be activated to a maximum of 135% and further stabilized by 10 mM Ca2+ (Fig. 2a).
The cell-bound Ca-free Wg2 CEP appeared to be more stable than the cell-bound Ca-free SK11 CEP; a linear initial progress curve for MS-Arg conversion versus time was obtained at 25°C (data not shown). This means that in this case the initial reduction of ca. 25%, observed when Ca2+ was removed (Fig. 1), was due to a decline in specific activity rather than to instability. During incubation of a dilute standard suspension at 25°C and pH 6.5, the Ca-free Wg2 CEP was efficiently released into the supernatant; complete release was obtained within 30 min (Fig. 2b). In view of the stability of the enzyme, the reduction in the activity of the Ca-free Wg2 CEP over this period (approximately 35%) probably reflects a further decrease in specific activity upon release. A Ca2+ concentration as low as 0.2 mM almost completely protected the enzyme from release (Fig. 2b).
With cells of strains harboring the fragment A-containing Ca-free hybrid CEPs the rates of reduction in potential activity towards S-Glu in dilute suspensions during incubation at 25°C were all less than the rate of reduction in potential activity of the wild-type SK11 CEP. In all cases final residual activities ranging from 4% (hybrid ABCd) to 16% (hybrid Abcd) (Fig. 2a) were recovered in the soluble fraction. Each of these released Ca-free hybrid CEPs was relatively stable in the absence of Ca2+ at 25°C compared to its Ca-free cell-bound counterpart.
Effect of divalent cations on the Ca-free cell-bound CEP.
Strain SK11 or Wg2 cells that were washed with ice-cold Ca-free buffer in order to obtain the Ca-free form of the cell-bound CEP were resuspended in buffers containing different concentrations of Ca2+ ions.
Restoration of the cell-bound Ca-free SK11 CEP activity at 25°C proceeded in an essentially biphasic manner up to concentrations of 1 to 2 mM, although at concentrations of Ca2+ up to 10 mM an additional increase in activity was still observed; concentrations greater than 10 mM appeared to be inhibitory (Fig. 3). The biphasic character of the saturation curve was also demonstrated by constructing a semilogarithmic plot (Fig. 4). The specific activity of the enzyme (measured at 10°C) of the first phase (at 0.1 mM Ca2+) was increased by 55% of the maximal increase observed at 10 mM Ca2+ (data not shown). Other divalent cations (Zn2+, Mg2+, Ba2+, Ni2+, Co2+, Cu2+, Sn2+, Mn2+, and Cd2+) were tested, and only Cd2+ could replace Ca2+, while Mn2+ was much less efficient (Fig. 3); all other cations had no significant effect.
FIG. 3.
Restoration of Ca-free cell-bound SK11 CEP as a function of the concentration of Ca2+ (●), Cd2+ (□), or Mn2+ (○). Two different scales were used, 0 to 1 mM (solid lines) and 0 to 10 mM (dashed lines). The dotted lines indicate shifts in the scale at 0.1 mM in the cases of Ca2+ and Mn2+.
FIG. 4.
Modulation of cell-bound CEP activity by Ca2+ ions. Plasmid-free strain L. lactis MG 1363 into which the wild-type plasmid or a hybrid proteinase gene-containing plasmid was introduced was used. Activities associated with washed cells containing the Ca-free enzyme were determined at 25°C as a function of the Ca2+ concentration. Symbols: ▵, wild-type SK11 CEP (ABCD); □, hybrid CEP Abcd; ○, hybrid CEP AbCd; ●, hybrid CEP ABCd; ◬, hybrid CEP ABcD; ■, hybrid CEP AbCD.
The stability of the Ca-free cell-bound SK11 enzyme at different concentrations of Ca2+ was measured. At each concentration of Ca2+ below the optimum concentration (10 mM) equilibria involving enzyme molecules without or with (one or more) bound Ca2+ ions might be expected to exist, with each of these enzyme fractions having its own rate constant of inactivation. The enzyme of the first phase (at 0.125 mM Ca2+) was dominated by a fraction having increased stability at 25°C compared to that of the Ca-free enzyme (Fig. 5). The latter enzyme seemed to be still represented since there was an initial relatively rapid reduction in activity of about 20% on a semilogarithmic plot. A similar rapid reduction (of 5%) occurred for the enzyme of the second phase (at 0.8 mM Ca2+), which exhibited further increased stability. At 2 mM Ca2+ only a slight improvement in stability was detected, and at 10 mM 100% of the activity was recovered even after incubation for 6 h at 25°C (Fig. 5).
FIG. 5.
Stabilization of the Ca-free cell-bound SK11 CEP by Ca2+ ions. Washed cells in 50 mM imidazole (pH 6.5) were incubated at 25°C in the absence of Ca2+ (dashed line) or in the presence of 0.125 mM Ca2+ (▵), 0.8 mM Ca2+ (●), 2 mM Ca2+ (○), or 10 mM Ca2+ (□) (solid lines). At different times residual activities were measured in the presence of 10 mM Ca2+ and expressed as percentages of the initial activity.
Hybrid SK11-Wg2 CEPs.
In order to reveal the possible involvement of different segments of the SK11 CEP molecule in the ultimate specific relationships among Ca2+ binding, stability, and activity, the activities of Ca-free cell-bound, fragment A-containing hybrid CEPs were measured at 25°C and pH 6.5 as a function of the Ca2+ concentration. Highly reproducible, distinct Ca saturation curves were obtained (Fig. 4).
The SK11 hybrid CEP in which only the N-terminal half of the C-terminal extension (viz., residues 497 to 1089) originated from the Wg2 CEP (viz., hybrid ABcD) exhibited a clearly lower response on Ca2+ than the wild-type SK11 (ABCD) CEP. However, the Wg2 CEP in which the N-terminal segment of the proteinase domain (residues 1 to 173) was replaced by the N-terminal segment of the SK11 CEP (viz., hybrid Abcd) exhibited the lowest response, indicating that the affinity for Ca2+ was relatively low. If the hybrid proteinase also contained segment C (viz., hybrid AbCd), the response on Ca2+ was significantly improved. Further improvement in the effect of Ca2+ was observed if the hybrid CEP contained SK11 CEP segment D as well (viz., AbCD). In this case the response on Ca2+ at low concentrations indeed indicated that the affinity was lower, but the steepness of the curve suggests that the specific activity of the bound enzyme in both the first and second phases was higher than the corresponding specific activity of the wild-type ABCD. Hybrid ABCd exhibited a Ca2+ dependency which was only slightly different from that of hybrid AbCd.
Specificity of the Ca-free cell-bound CEP.
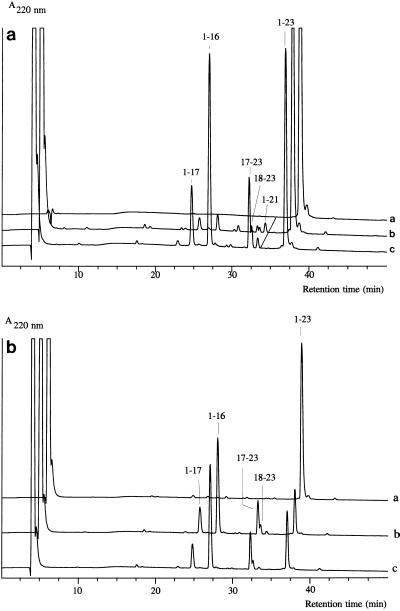
The specificities of the Ca-free cell-bound SK11 CEP towards αs1-CN(f1-23) in the presence of different concentrations of Ca2+ (corresponding to different phases of activity [Fig. 4]) or in the presence of Cd2+ (data not shown) or 10 mM Mn2+ were determined (see Materials and Methods) and were found to be identical (Fig. 6). Ca-free CEP activity in the absence of Ca2+ could be detected at 25°C (albeit at a very low rate) with this peptide as a substrate and with a relatively dense all suspension. Its specificity was distinguished from that of the Ca-loaded CEP only by a slightly increased relative cleavage rate at bond 17-18 compared to the Ca2+-loaded enzyme (Fig. 6a). With the wild-type Wg2 CEP no shift was observed, and with the hybrid CEPs either no shift or only a slight shift in the preference for peptide bonds was observed (data not shown), which again mainly concerned the occurrence of cleavage or an increased relative cleavage rate of bond 17-18 in the absence of Ca2+.
FIG. 6.
(a) Analytical reversed-phase HPLC of αs1-CN(f1-23) (line a) and of the products of degradation of αs1-CN(f1-23) by the cell-bound Ca-free CEP of strain SK11 at pH 6.5 in the absence of Ca2+ (120 min, 25°C) (line b) or in the presence of 0.1 mM Ca2+ (30 min, 25°C) (line c). (b) Analytical reversed-phase HPLC of the products of αs1-CN(f1-23) degradation (25°C, pH 6.5) by the cell-bound Ca-free CEP of strain SK11 in the absence of Ca2+ (line a), at 10 mM Ca2+ (line b), or at 10 mM Mn2+ (line c).
DISCUSSION
The results of this study suggest that removal of relatively weakly bound calcium in cell-bound CEP initiates a (local) structural rearrangement in the proteinase domain, resulting in an enzyme which not only is susceptible to autoproteolytic release (10, 26) but also has a lower specific activity and, in the case of the SK11 CEP, is significantly destabilized against thermal denaturation, as a result of which no activity can be detected at 25°C and pH 6.5. The Ca-free CEP still can regain its native stable conformation if Ca2+ ions are added. Ca-triggered local rearrangements which may be essential to stabilization apparently extend to the substrate-binding site and affect substrate binding and/or the catalytic process itself. The suggestion that destabilization upon Ca2+ removal is against denaturation rather than against autoproteolytic attack is supported by results showing that the autoproteolytically released proteinase fraction obtained under conditions in which the enzyme is unstable (pH 6.5, 25°C) migrates under denaturing conditions in a polyacrylamide gel as a major 145-kDa band together with minor 80- to 130-kDa bands. All of these C-terminally truncated products that are larger than 100 kDa in the release fraction of SK11 (4, 11, 31) or the release fractions of other strains (5, 24, 27) are still proteolytically active. These results indicate that no serious autoproteolytic inactivation of the Ca-free cell-bound SK11 CEP occurs; otherwise, an inactive component would have been detected, which, in view of the very small percentage of active CEP released, should have been present at a relatively very high concentration.
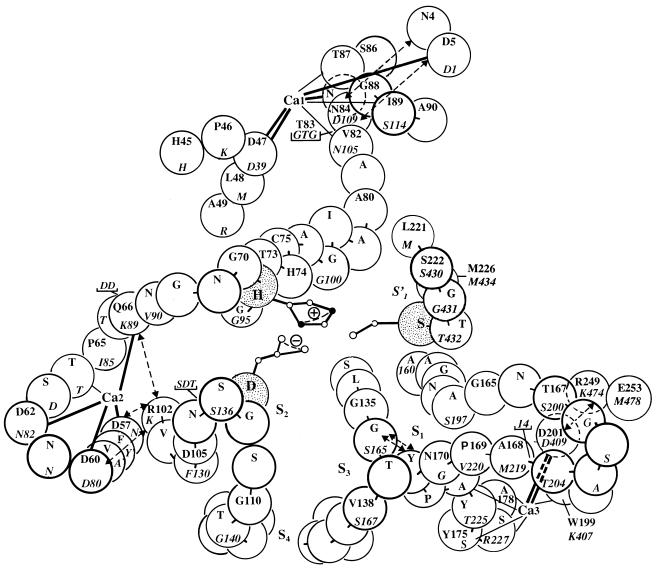
Lactococcal CEPs are predicted, on the basis of their sequence similarity to subtilisin and thermitase, to possess at least three Ca-binding sites in their proteinase domains (30) (Fig. 7), and one of these, Ca1, might be expected to bind Ca2+ even more tightly than the corresponding site in thermitase (3, 17, 18) due to an additional negative charge on residue 85 (i.e., D-110 in the CEP). The Ca2 and Ca3 binding sites are even weaker than the corresponding weak sites in related proteinases, and Ca3 is the weakest (9). These binding sites are located in external loops close to the substrate recognition site (Fig. 7). The binding of Ca2+, which supposedly occurs first predominantly to Ca2 and subsequently to Ca3, may trigger sequential concerted movements within the proteinase domain which exert long-range effects on the geometry of the catalytic triad in particular. The underlying local structural rearrangements at the Ca sites are thus reflected in altered specific activities which are responsible for the biphasic dependence of activity on Ca2+ concentration, in the increasing stability of the enzyme, and to some extent in the specificity of the enzyme.
FIG. 7.
Model of part of the substrate-binding region and of the calcium-binding sites in CEPIII of L. lactis SK11, predicted on the basis of a close sequence similarity with subtilisin and thermitase. Residues of the catalytic triad (Asp-30, His-94, and Ser-433 in CEP) are stippled. Insertions relative to subtilisin are indicated either by an underlined one-letter code or by the number of residues involved. The numbering is thermitase numbering (roman type) (25) and SK11 CEP numbering (italic type) (33). S′1, S1, S2, S3, and S4 are substrate-binding sites, and Ca1, Ca2, and Ca3 are predicted calcium-binding sites deduced from sequence similarities. Thick lines represent known side chain ligands, and thin lines represent main chain ligands for Ca2+ in subtilisin and thermitase. Dashed arrows indicate stabilizing side chain or main chain interactions. For details see the text.
The association of Ca2+ with binding sites in other subtilisinlike serine proteinases has been shown to induce structural changes in the direct surroundings of these sites (18) and in the substrate-binding site as well (1, 2), to protect the enzymes from autoproteolytic degradation, and to contribute to thermal stabilization by reducing the flexibility of the molecules (16, 30, 35). These previously described examples may support the view concerning the impact of Ca2+ binding to the predicted sites on CEP described above.
One of the cations tested, Cd2+, can replace Ca2+ most efficiently, probably because its ion radius (0.97 Å) is very similar to that of Ca2+ (0.99 Å). In addition, only Mn2+ (ion radius, 0.80 Å) is able to restore the Ca-free enzyme. All other cations have ion radii that are either smaller than 0.80 Å or much larger than 0.99 Å. The inefficacy of these cations may be explained in terms of the rigidity of the area of the Ca site where the protein is not able to either collapse around small ions or expand to admit large ions (32). The relatively poor efficacy of Mn2+ could reflect a much lower capacity of the Ca sites involved to bind this small ion and/or a less efficient ultimate effect of binding on stability and activity.
Autoproteolytic release of active CEP at 25°C in a Ca-free buffer at pH 6.5 appeared to be restricted to the Ca-free form. However, the stability of the enzyme against denaturation may determine the relative amount of CEP released and thus, together with a lower specific activity of the released CEP, the total activity in the final soluble fraction. Unlike the release of the relatively stable Ca-free Wg2 CEP, no release of Ca-free CEP from SK11 cells was detected in dilute suspensions, perhaps because of its highly unstable character. An increase in the incidence of intercellular autoproteolytic action may explain the detection of release when cells are treated at very high densities. The released Ca-free SK11 CEP was relatively stable compared to the bound Ca-free enzyme, suggesting that there was conformational stabilization which was independent of Ca2+ ions in the weak binding sites.
The binding affinity of a Ca-binding site is determined by the relevant residues delivering the formal ligands, by residues which, owing to their location and charge, can influence the electrostatic potential at the site and thus can affect binding (20, 28), and by the geometry of the site, which determines Ca2+ coordination. The binding to the Ca-binding sites in the Ca-free CEP and/or the ultimate consequences of Ca2+ binding for the enzyme seem to be influenced by one or more of the substitutions which have been established by comparing the sequences of the SK11 and Wg2 CEPs. All substituted residues within the proteinase domain are outside the predicted Ca-binding regions and are not likely to influence Ca binding directly. Moreover, the present results show that not only substitutions in the proteinase domain but also substitutions in other domains of the molecule are essential for Ca binding and activity. Therefore, if substituted residues have an influence on the affinities of Ca sites, then they do so because the substitutions are connected with modulation of the geometry of these sites. If there is no such influence, apparently only Ca2+-triggered specific conformations are responsible for the characteristic Ca2+ dependencies of the activities of the hybrid CEPs. Of all of the substitutions, the substitutions in the C-terminal extension (fragments c and d) of the Wg2 CEP, especially the substitutions in fragment c, have the most distinct negative effect on Ca2+-dependent restoration of the hybrid proteinase. The results suggest that the C-terminal extension has an impact on the final conformation of the Ca-free proteinase domain and is involved in the folding process which leads to the final, completely Ca2+-loaded, optimally active, stable conformation of the proteinase domain. It might guide this domain to adopt an active, Ca1-loaded specific primary conformation, the Ca-free CEP, with specific additional Ca2+-binding characteristics. Only in the case of the wild-type combination abcd (Wg2 CEP) is this conformation relatively stable. The final conformation of the proteinase domain (and thus its specific activity) depends on the continued occupation of Ca sites in the enzyme by Ca2+ ions. The proposed identity of the Ca-free CEP, containing a Ca2+ ion on Ca1, was supported by the effect of EDTA treatment at 2°C on this form of the enzyme. A complete loss of potential activity (in the case of the SK11 CEP) or a significant additional irreversible decrease in activity (in the case of the Wg2 CEP) was sustained (15). In this heuristic view the reversibility of a specific Ca2+ dependency of the native cell-bound enzyme indicates that the C-terminal extension is permanently involved in maintaining the basic, Ca1-loaded conformation. Therefore, our hypothesis is that one function of the large C-terminal extension is to act as a template on which the proteinase domain finds and maintains its initial conformation, which is then modulated by Ca2+. The extreme C terminus is assumed to function as a membrane anchor which stops translocation of the polypeptide over the membrane; the molecule may then undergo cell wall sorting involving C-terminal processing at the sorting motif which flanks the transmembrane domain at the N-terminal side (9). The sequence preceding this cell wall sorting signal has typical hydrophilic, flexible features of a transwall domain (9); this leaves the middle domain to act, either as a whole or only in part, as the proposed template.
REFERENCES
- 1.Bajorath J, Hinrichs W, Saenger W. The enzymatic activity of proteinase K is controlled by calcium. Eur J Biochem. 1988;176:441–447. doi: 10.1111/j.1432-1033.1988.tb14301.x. [DOI] [PubMed] [Google Scholar]
- 2.Bajorath J, Raghunathan S, Hinrichs W, Saenger W. Long-range structural changes in proteinase K triggered by calcium ion removal. Nature. 1989;337:481–484. doi: 10.1038/337481a0. [DOI] [PubMed] [Google Scholar]
- 3.Briedigkeit L, Frömmel C. Calcium ion binding by thermitase. FEBS Lett. 1989;253:83–87. [Google Scholar]
- 4.Bruinenberg P G, de Vos W M, Siezen R J. Prevention of C-terminal autoprocessing of Lactococcus lactis SK11 cell-envelope proteinase by engineering of an essential surface loop. Biochem J. 1994;302:957–963. doi: 10.1042/bj3020957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coolbear T, Reid J R, Pritchard G G. Stability and specificity of the cell wall-associated proteinase from Lactococcus lactis subsp. cremoris H2 released by treatment with lysozyme in the presence of calcium. Appl Environ Microbiol. 1992;58:3263–3270. doi: 10.1128/aem.58.10.3263-3270.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Exterkate F A. Accumulation of proteinase in the cell wall of Streptococcus cremoris AM1 and regulation of its production. Arch Microbiol. 1979;120:247–254. [Google Scholar]
- 7.Exterkate F A. Location of peptidases outside and inside the membrane of Streptococcus cremoris. Appl Environ Microbiol. 1984;47:177–183. doi: 10.1128/aem.47.1.177-183.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Exterkate F A. Differences in short peptide-substrate cleavage by two cell-envelope-located serine proteinases of Lactococcus lactis subsp. cremoris are related to secondary binding specificity. Appl Microbiol Biotechnol. 1990;33:401–406. doi: 10.1007/BF00176654. [DOI] [PubMed] [Google Scholar]
- 9.Exterkate F A. The lactococcal cell-envelope proteinases: differences, calcium-binding effects and role in cheese ripening. Int Dairy J. 1995;5:995–1018. [Google Scholar]
- 10.Exterkate F A, de Veer G J C M. Partial isolation of and degradation of caseins by cell wall proteinase(s) of Streptococcus cremoris HP. Appl Environ Microbiol. 1985;49:328–332. doi: 10.1128/aem.49.2.328-332.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Exterkate F A, de Veer G J C M. Characterization of the cell wall proteinase PIII of Lactococcus lactis subsp. cremoris strain AMI and its relationship with the catalytically different cell wall proteinase PI/PII of strain HP. Syst Appl Microbiol. 1989;11:108–115. [Google Scholar]
- 12.Exterkate F A, Alting A C. The conversion of the αs1-casein-(1-23)-fragment by the free and bound form of the cell-envelope proteinase of Lactococcus lactis subsp. cremoris under conditions prevailing in cheese. Syst Appl Microbiol. 1993;16:1–8. [Google Scholar]
- 13.Exterkate F A, Alting A C, Slangen C J. Specificity of two genetically related cell-envelope proteinases of Lactococcus lactis subsp. cremoris towards as1-casein-(1-23)-fragment. Biochem J. 1991;273:135–139. doi: 10.1042/bj2730135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Exterkate F A, Alting A C, Bruinenberg P G. Diversity of cell envelope proteinase specificity among strains of Lactococcus lactis and its relationship to charge characteristics of the substrate-binding region. Appl Environ Microbiol. 1993;59:3640–3647. doi: 10.1128/aem.59.11.3640-3647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Exterkate, F. A. 1996. Unpublished results.
- 16.Genov N, Filippi B, Dolashka P, Wilson K S, Betzel C. Stability of subtilisins and related proteinases (subtilases) Int J Pept Protein Res. 1995;45:391–400. doi: 10.1111/j.1399-3011.1995.tb01054.x. [DOI] [PubMed] [Google Scholar]
- 17.Gros P, Betzel C, Dauter Z, Wilson K S, Hol W G J. Molecular dynamics refinement of thermitase-eglin-c complex at 1.98 Å resolution and comparison of two crystal forms that differ in calcium content. J Mol Biol. 1989;210:347–367. doi: 10.1016/0022-2836(89)90336-7. [DOI] [PubMed] [Google Scholar]
- 18.Gros P, Kalk K H, Hol W G J. Calcium binding to thermitase. Crystallographic studies of thermitase at 0, 5 and 100 mM calcium. J Biol Chem. 1991;266:2953–2961. doi: 10.2210/pdb3tec/pdb. [DOI] [PubMed] [Google Scholar]
- 19.Hugenholtz J, Veldkamp H, Konings W N. Detection of specific strains and variants of Streptococcus cremoris in mixed cultures by immunofluorescence. Appl Environ Microbiol. 1987;53:149–155. doi: 10.1128/aem.53.1.149-155.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jang J S, Bae K H, Byun S M. Effect of the weak Ca2+-binding site of subtilisin J by site-directed mutagenesis on heat stability. Biochem Biophys Res Commun. 1992;188:184–189. doi: 10.1016/0006-291x(92)92367-7. [DOI] [PubMed] [Google Scholar]
- 21.Kok J. Genetics of the proteolytic system of lactic acid bacteria. FEMS Microbiol Rev. 1990;87:15–42. doi: 10.1111/j.1574-6968.1990.tb04877.x. [DOI] [PubMed] [Google Scholar]
- 22.Kok J, de Vos W M. The proteolytic system of lactic acid bacteria. In: Gasson M J, de Vos W M, editors. Genetics and biotechnology of lactic acid bacteria. Glasgow, United Kingdom: Blackie Academic and Professional; 1994. pp. 169–210. [Google Scholar]
- 23.Kok J, Hill D, Haandrikman A J, de Reuver M J B, Laan H, Venema G. Deletion analysis of the proteinase gene of Streptococcus cremoris Wg2. Appl Environ Microbiol. 1988;54:239–244. doi: 10.1128/aem.54.1.239-244.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laan H, Konings W N. Mechanism of proteinase release from Lactococcus lactis subsp. cremoris. Appl Environ Microbiol. 1989;55:3101–3106. doi: 10.1128/aem.55.12.3101-3106.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meloun B, Baudyš M, Kostka V, Hausdorf G, Frömmel C, Höhne W E. Complete primary structure of thermitase from Thermoactinomyces vulgaris and its structural features related to the subtilisin-like proteinases. FEBS Lett. 1985;183:195–200. [Google Scholar]
- 26.Mills O E, Thomas T D. Release of cell wall-associated proteinase(s) from lactic streptococci. N Z J Dairy Sci Technol. 1978;13:209–215. [Google Scholar]
- 27.Nissen-Meyer J, Sletten K. Purification and characterization of the free form of the lactococcal extracellular proteinase and its autoproteolytic cleavage products. J Gen Microbiol. 1991;137:1611–1618. doi: 10.1099/00221287-137-7-1611. [DOI] [PubMed] [Google Scholar]
- 28.Pantoliano M W, Whitlow M, Wood J F, Rollence M L, Finzel B C, Gilliland G L, Poulos T L, Bryan P N. The engineering of binding affinity at metal ion binding sites for the stabilization of proteins: subtilisin as a test case. Biochemistry. 1988;27:8311–8317. doi: 10.1021/bi00422a004. [DOI] [PubMed] [Google Scholar]
- 29.Pritchard G G, Coolbear T. The physiology and biochemistry of the proteolytic system in lactic acid bacteria. FEMS Microbiol Rev. 1993;12:179–206. doi: 10.1111/j.1574-6976.1993.tb00018.x. [DOI] [PubMed] [Google Scholar]
- 30.Siezen R J, de Vos W M, Leunissen J A M, Dijkstra B W. Homology modelling and protein engineering strategy of subtilases, the family of subtilisin-like serine proteinases. Protein Eng. 1991;4:719–737. doi: 10.1093/protein/4.7.719. [DOI] [PubMed] [Google Scholar]
- 31.Siezen R J, Bruinenberg P G, Vos P, van Alen-Boerrigter I, Nijhuis M, Alting A C, Exterkate F A, de Vos W M. Engineering of the substrate-binding region of the subtilisin-like cell-envelope proteinase of Lactococcus lactis. Protein Eng. 1993;6:927–937. doi: 10.1093/protein/6.8.927. [DOI] [PubMed] [Google Scholar]
- 32.Stuart D I, Acharya K R, Walker N P C, Smith S G, Lewis M, Phillips D C. α-Lactalbumin possesses a novel calcium binding loop. Nature (London) 1986;324:84–87. doi: 10.1038/324084a0. [DOI] [PubMed] [Google Scholar]
- 33.Vos P, Simons G, Siezen R J, de Vos W M. Primary structure and organization of the gene for a procaryotic cell-envelope-located serine proteinase. J Biol Chem. 1989;264:13579–13585. [PubMed] [Google Scholar]
- 34.Vos P, Boerrigter I J, Buist G, Haandrikman A J, Nijhuis M, de Reuver M B, Siezen R J, Venema G, de Vos W M, Kok J. Engineering of the Lactococcus lactis serine proteinase by construction of hybrid enzymes. Protein Eng. 1991;4:479–484. doi: 10.1093/protein/4.4.479. [DOI] [PubMed] [Google Scholar]
- 35.Wells J A, Estell D A. Subtilisin—an enzyme designed to be engineered. Trends Biochem Sci. 1988;13:291–297. doi: 10.1016/0968-0004(88)90121-1. [DOI] [PubMed] [Google Scholar]