In their article in this issue of the Journal, Zhu and colleagues1 have identified another role for their ex vivo cardiac simulator, previously developed and reported on by Dr Woo’s research team.1-8 Their simulator includes a pulsatile piston pump with an impedance adapter, compliance chambers (“ventricular” and “aortic” chambers), and an adjustable peripheral resistance. It is equipped with high-speed videographic and echocardio-graphic capability, thus allowing for both visual and hemodynamic “aortic” or “mitral” valvular assessments within the similator.2-8 In this latest article from Zhu and colleagues,1 the group reports on the development of a moderately regurgitant “bicuspid” aortic valve model. They have mimicked the most common bicuspid aortic valve (BAV) morphotype, the Sievers type 1 L/R morphotype,9 by sewing the apposing leading edges of the right and left coronary cusps of a bovine aortic valve to one another and reducing the R/L commissural height by detaching and repositioning it 10 mm below its original height. The bovine valve was then implanted into a porcinesized outflow tract mounted within their simulator between its “ventricular” and “aortic” chambers. This valve construct rendered a moderately insufficient valve with demonstrated R/L cusp prolapse. The group then showed that this modified bovine valve could be successfully “repaired” by shortening the free margin of their conjoined R/L cusp with David’s free margin reinforcement technique.10
The current state-of-the-art of bicuspid aortic valve repair (and aortic valve repair in general) is fraught with inadequacies. The degree of nuance often required to determine what cusp repair maneuvers are most appropriate for use with a given construct and pathology can be baffling, even futile. Despite many advances in the field, the reality is that we still have a long way to go toward packaging a consistently durable and reproducible repair paradigm that can be broadly applied. Consequently, the concept of developing a tunable aortic valve repair simulator to study valve constructs and repair techniques could be helpful to our community. Zhu and colleagues1 have demonstrated proof of concept toward development of such a simulator, albeit with several limitations related specifically to aortic valve repair that may limit its overall impact.
Notably, Zhu and colleagues1 appropriately recognize that both cusp prolapse and cusp restriction play important roles in development of regurgitation in the context of type 1 L/R BAV—the most common form of BAV. The difficulty in truly mimicking these pathologic human valves with a normal bovine tricuspid aortic valve cannot be overemphasized. Leading edge fibrosis and cusp sclerosis are very important components of type 1 BAVs that impart restriction and dysfunction that are not easily reproduced ex vivo from normal animal valve constructs—as they are not in this current study of Zhu and colleagues.1 Similarly, the R/L cusp depth (the cusp excursion distance between the leading edge at the raphe to its basilar attachment) in type 1 BAV is foreshortened and markedly thickened relative to the opposing “nonraphe’d” cusps, further imparting restriction in addition to the variable commissural height (Figure 1)—this is another difficult pathoanatomy to mimic accurately ex vivo.11 In addition to refining pathologic valve mimicry to be more clinically relevant, expanding the capability of interrogating flow characteristics across the valve within their simulator (eg, quantifying and localizing laminar, turbulent, and vortical flow12) to allow for more detailed analyses of cusp biomechanical stress maps could further broaden its utility. Finally, some validation of durability indices will be necessary when evaluating valve repair techniques for distinct pathoanatomic findings. Notwithstanding these limitations, this simulator may provide a practical means of studying valve repair paradigms.
FIGURE 1.
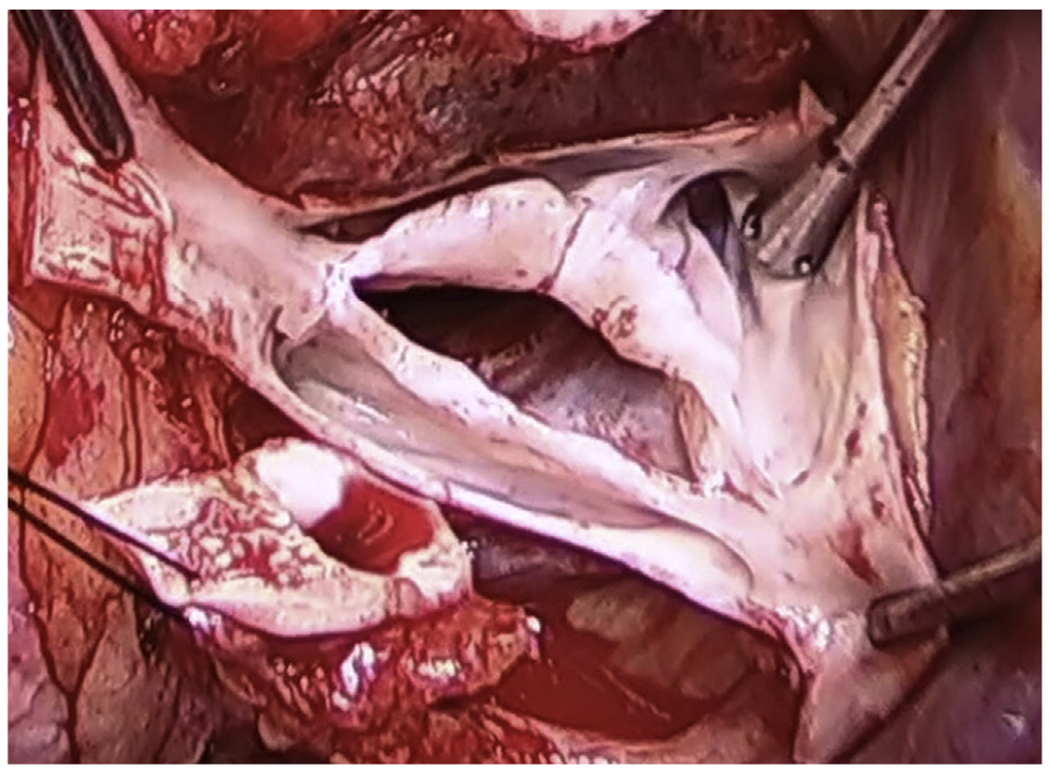
Note the degree of sclerosis and fibrosis of this type 1 R/N bicuspid aortic valve rendering the raphe’d cusp both restricted and prolapsing below the coaptation plane. This valve was severely regurgitant.
CENTRAL MESSAGE.
Cardiac simulators with tunable morphologic valve phenotype and pathophysiologic lesion capability may be a platform for testing valve repair strategies. Validation for clinical relevance remains.
Footnotes
Disclosures: Dr Gleason serves on a Medical Advisory Board for Abbott. Aranki has reported no conflicts of interest.
References
- 1.Zhu Y, Imbrie-Moore AM, Paulsen MJ, Priromprintr B, Wang H, Lucian HJ, et al. Novel bicuspid aortic valve model with aortic regurgitation for hemodynamics analysis using an ex vivo simulator. J Thorac Cardiovasc Surg. 2022;163:e161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imbrie-Moore AM, Paullin CC, Paulsen MJ, Grady F, Wang H, Hironaka CE, et al. A novel 3D-printed preferential posterior mitral annular dilation device delineates regurgitation onset threshold in an ex vivo heart simulator. Med Eng Phys. 2020;77:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imbrie-Moore AM, Paulsen MJ, Thakore AD, Wang H, Hironaka CE, Lucian HJ, et al. Ex vivo biomechanical study of apical versus papillary neochord anchoring for mitral regurgitation. Ann Thorac Surg. 2019;108:90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imbrie-Moore AM, Paulsen MJ, Zhu Y, Wang H, Lucian HJ, Farry JM, et al. A novel cross-species model of Barlow’s disease to biomechanically analyze repair techniques in an ex vivo left heart simulator. J Thorac Cardiovasc Surg. 2021; 161:1776–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paulsen MJ, Bae JH, Imbrie-Moore A, Wang H, Hironaka C, Farry JM, et al. Development and ex vivo validation of novel force-sensing neochordae for measuring chordae tendineae tension in the mitral valve apparatus using optical fibers with embedded Bragg gratings. J Biomech Eng. 2019;142:0145011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulsen MJ, Imbrie-Moore AM, Wang H, Bae JH, Hironaka CE, Farry JM, et al. Mitral chordae tendineae force profile characterization using a posterior ventricular anchoring neochordal repair model for mitral regurgitation in a threedimensional-printed ex vivo left heart simulator. Eur J Cardiothorac Surg. 2020;57:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paulsen MJ, Kasinpila P, Imbrie-Moore AM, Wang H, Hironaka CE, Koyano TK, et al. Modeling conduit choice for valve-sparing aortic root replacement on biomechanics with a 3-dimensional–printed heart simulator. J Thorac Cardiovasc Surg. 2019;158:392–403. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y, Imbrie-Moore AM, Paulsen MJ, Priromprintr B, Park MH, Wang H, et al. A novel aortic regurgitation model from cusp prolapse with hemodynamic validation using an ex vivo left heart simulator. J Cardiovasc Transl Res. 2021;14:283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. 2007;133:1226–33. [DOI] [PubMed] [Google Scholar]
- 10.Alsoufi B, Borger MA, Armstrong S, Maganti M, David TE. Results of valve preservation and repair for bicuspid aortic valve insufficiency. J Heart Valve Dis. 2005;14:752–8; discussion 758-9. [PubMed] [Google Scholar]
- 11.Gleason TG. Bicuspid aortic valve repair by complete conversion from “raphe’d” (type 1) to “symmetric” (type 0) morphology. J Thorac Cardiovasc Surg. 2014; 148:2862-8.e1-2. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Weale P, Reiter G, Kino A, Dill K, Gleason T, et al. Breathhold time-resolved three-directional MR velocity mapping of aortic flow in patients after aortic valve-sparing surgery. J Magn Reson Imaging. 2009;29:569–75. [DOI] [PubMed] [Google Scholar]


