Abstract
Bacterial strain LW1, which belongs to the family Comamonadaceae, utilizes 1-chloro-4-nitrobenzene (1C4NB) as a sole source of carbon, nitrogen, and energy. Suspensions of 1C4NB-grown cells removed 1C4NB from culture fluids, and there was a concomitant release of ammonia and chloride. Under anaerobic conditions LW1 transformed 1C4NB into a product which was identified as 2-amino-5-chlorophenol by 1H and 13C nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry. This transformation indicated that there was partial reduction of the nitro group to the hydroxylamino substituent, followed by Bamberger rearrangement. In the presence of oxygen but in the absence of NAD, fast transformation of 2-amino-5-chlorophenol into a transiently stable yellow product was observed with resting cells and cell extracts. This compound exhibited an absorption maximum at 395 nm and was further converted to a dead-end product with maxima at 226 and 272 nm. The compound formed was subsequently identified by 1H and 13C NMR spectroscopy and mass spectrometry as 5-chloropicolinic acid. In contrast, when NAD was added in the presence of oxygen, only minor amounts of 5-chloropicolinic acid were formed, and a new product, which exhibited an absorption maximum at 306 nm, accumulated.
Chlorinated nitroaromatic compounds, which are important building blocks for synthesis of industrial chemicals, are present in industrial wastes (19) and are serious environmental pollutants (23). In 1988 the worldwide production of 1-chloro-4-nitrobenzene (1C4NB) for use as an intermediate in the industrial production of azo and sulfur dyes and as a raw material in the production of drugs and pesticides was 120,000 tons (5, 51). The European Economic Community has declared that 1C4NB is a compound that is particularly harmful and persistent in the environment and is one of the priority pollutants (16). 1C4NB causes methemoglobinemia in humans and animals (26) and reportedly is weakly mutagenic (37) and carcinogenic (49). Steinwandter, reporting on pollution found in the River Main (Germany), detected 1C4NB, along with other polychlorinated nitrobenzene compounds, in fish (42). Therefore, waste management and detoxification of this compound are important for protecting the environment and human health.
Little is known about the microbial metabolism of chloronitrobenzenes which are realcitrant to biological breakdown. Some authors have observed biological transformation rather than mineralization of these compounds. Several Pseudomonas species have been reported to be able to reduce mononitro compounds to the corresponding anilines under aerobic conditions (34). Resting cells of Pseudomonas sp. strain CBS3 convert 1C4NB to 4-chloroaniline, N-acetyl-4-chloroaniline, and 4-chloronitrosobenzene at low rates without any further degradation (34). Corbett and Corbett described metabolism of 1C4NB by the yeast Rhodosporidium sp. via a reductive pathway which resulted in the formation of 4-chloroacetanilide and 4-chloro-2-hydroxyacetanilide as the major final metabolites (12). Complete reduction of the nitro group is the first step in anoxic transformation of chloronitrobenzenes, resulting in the formation of the corresponding chloroanilines (46). Abiotic reduction of 1C4NB to 4-chloroaniline in a dissimilatory iron-reducing enrichment culture has also been reported (21). Stockinger et al. reported that 1C4NB was removed from wastewater by an initial chemical ozonation, followed by biotreatment (43). To our knowledge, a report of biological mineralization of the isomer 1-chloro-3-nitrobenzene is the only report thus far of mineralization by a mixed culture during treatment of industrial wastewater in a membrane reactor (27). However, mineralization of 1C4NB or its isomer by a single bacterium has not been described previously. Bacterial strain LW1 is the first strain that uses 1C4NB as a sole source of carbon, nitrogen, and energy.
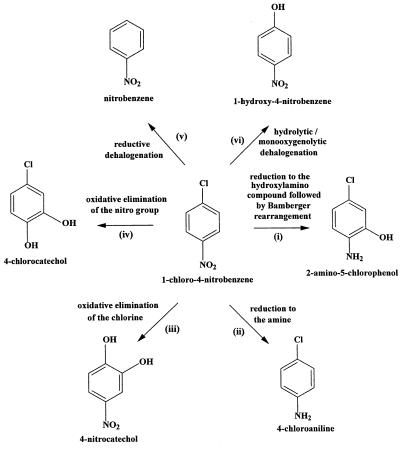
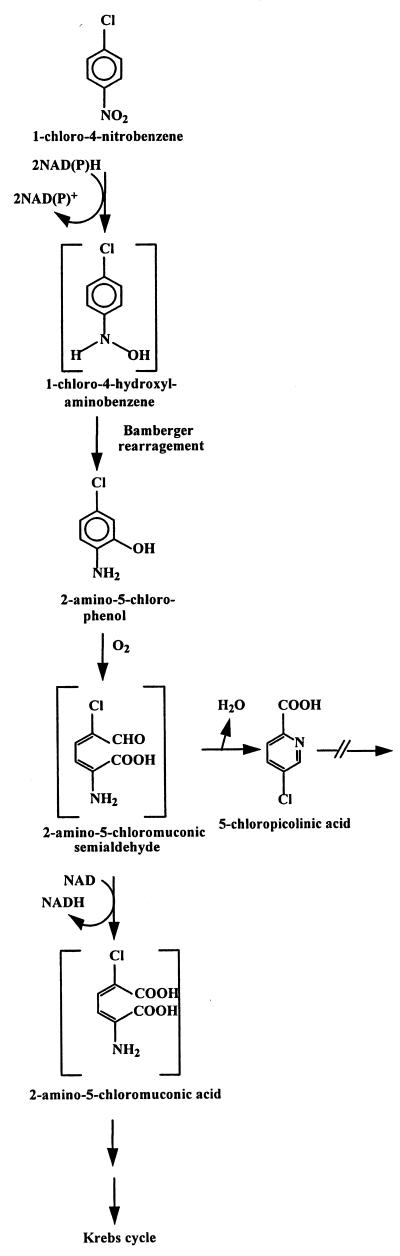
As a starting point for biochemical analysis, six distinct potential pathways for 1C4NB mineralization were considered because of their occurrence in bacterial strains that degrade halogen group-containing aromatic compounds (6, 13, 17, 28, 31, 32) and nitro group-containing aromatic compounds (2, 25, 31, 40) (Fig. 1). To elucidate the initial steps of the degradative pathway in LW1, we investigated each of the following possibilities (Fig. 1): reduction of the nitro group to the hydroxylamino compound, followed by Bamberger rearrangement to form 2-amino-5-chlorophenol (Fig. 1, reaction i); complete reduction of the nitro substituent to form 4-chloroaniline (Fig. 1, reaction ii); oxidative elimination of the chloride to form 4-nitrocatechol (Fig. 1, reaction iii); oxidative elimination of the nitro group to form 4-chlorocatechol (Fig. 1, reaction iv); reductive dehalogenation to form 4-nitrobenzene (Fig. 1, reaction v); and dehalogenation by a monooxygenolytic or hydrolytic activity (Fig. 1, reaction vi).
FIG. 1.
Putative initial reactions with 1C4NB based on studies of nitrobenzene degradation via catechol (40) or 2-aminophenol (30), nitrobenzene transformation into aniline (34), reductive dehalogenation (13), and dehalogenation due to hydrolytic (25) or dioxygenolytic (32) activity.
MATERIALS AND METHODS
Chemicals.
All of the chemicals used were analytical grade (≥99% pure) or very pure (≥97% pure). All of the water used was ultrapure double-distilled water. 1C4NB, 4-chlorophenol, chlorobenzene, 4-nitrocatechol, and 4-chloroaniline were obtained from Fluka (Buchs, Switzerland); 4-nitrophenol was obtained from Sigma Chemical Co. (St. Louis, Mo.); NAD+, NADH, and NADPH were obtained from Boehringer Mannheim (Mannheim, Germany); nitrobenzene and hydroquinone were obtained from E. Merck (Darmstadt, Germany); and 1,2,4-trihydroxybenzene and 4-chlorocatechol were obtained from TCI, Tokyo Kasei Kogyo Co., LRD (Tokyo, Japan). The trimethylsulfonium hydroxide (TMSH) reagent was obtained from Macherey-Nagel (Düren, Germany). 2-Amino-5-chlorophenol and 5-chloropicolinic acid were prepared from 1C4NB by using 1C4NB-grown resting cells of strain LW1 as described below.
Bacterial strain and culture conditions.
Strain LW1 was grown aerobically in a mineral salts medium (15) that was supplemented with 1C4NB as the carbon source and was incubated at 30°C on a rotary shaker at 150 rpm in baffled Erlenmeyer flasks. Due to its relatively low water solubility (20 mg/liter), 1C4NB was incubated with the mineral salts medium at 30°C and 150 rpm for 24 h, and the medium was filtered before inoculation to remove the undissolved crystals. Mineral salts medium containing dissolved 1C4NB (1.5 mM) was used to determine the degradation kinetics and mass balances. Scaled-up cultures were grown with an amount of 1C4NB that, if completely dissolved, corresponded to a concentration of 5 mM. The mineral salts medium was prepared as described previously (15). For growth experiments performed with 1C4NB as the sole nitrogen source, Ca(NO3)2 was replaced by CaCl2, Fe-ammonium citrate was replaced by FeCl2 and (NH4)SO4 was replaced by Na2SO4. Cultures were inoculated (10%, vol/vol) with precultures in the late exponential phase.
Preparation of cell extracts and preparation of resting cells.
Cells of strain LW1 were harvested at the end of the exponential growth phase (optical density at 546 nm [OD546], 0.3). Cells were pelleted by centrifugation at 27,500 × g for 10 min at 4°C, washed twice with 50 mM sodium phosphate buffer (pH 7.2), and resuspended in a small volume of the same buffer. The suspended cells were disrupted by four passages through a French pressure cell (Aminco, Silver Spring, Md.) operated at 18,000 lb/in2. Intact cells and insoluble debris were removed by centrifugation at 40,000 × g for 45 min at 4°C. The supernatant fluid was designated the cell extract and was used for enzyme assays. Experiments with resting cells were carried out with washed cells as described above; these cells were resuspended in 10 ml of 50 mM sodium phosphate buffer (pH 7.2) to a final OD546 of 5. Resting cells were incubated with 1.5 mM dissolved 1C4NB in a water bath shaker at 30°C.
Extraction, purification, and identification of metabolites.
The metabolites 2-amino-5-chlorophenol and 5-chloropicolinic acid were purified and characterized as follows. 2-Amino-5-chlorophenol was prepared biologically from 1C4NB under anaerobic conditions in a glove box (Coy Laboratory Products, Grass Lake, Mich.) filled with oxygen-free nitrogen. The reaction mixture initially contained 50 mM sodium phosphate buffer (pH 7.2), 1.5 mM 1C4NB completely dissolved in the buffer, and 1C4NB-grown resting cells of strain LW1. The reaction was carried out at 30°C with continuous stirring, and the progress of the reaction was monitored by monitoring the formation of 2-amino-5-chlorophenol by high-performance liquid chromatography (HPLC). Anaerobic conditions were necessary not only during the biotransformation but also during the work-up. After 6 h of incubation and complete substrate turnover, the resting cells were pelleted by centrifugation, and the pH of the supernatant was adjusted to 12.0 by adding 5 N NaOH. Extraction was performed twice with ethyl acetate. The organic phase containing 2-amino-5-chlorophenol was concentrated by evaporation of the organic solvent under a vacuum after it was dried over Na2SO4, and it was analyzed without further purification by nuclear magnetic resonance (NMR) and gas chromatography-mass spectrometry (GC-MS). Cell suspensions containing 2-amino-5-chlorophenol were aerated for 5 min for preparation of 5-chloropicolinic acid. After complete conversion, as determined by HPLC, the cells were pelleted by centrifugation. The pH of the yellow supernatant was adjusted to 2.0 with HCl. The water phase lost its yellow color and was extracted twice with ethyl acetate. The organic phases were pooled, 5-chloropicolinic acid was concentrated by evaporation of the organic solvent under a vacuum, and the preparation was analyzed without further purification by NMR and GC-MS.
Enzyme assays.
2-Amino-5-chlorophenol 1,6-dioxygenase activity was determined as previously described for the 2-aminophenol 1,6-dioxygenase of Pseudomonas pseudoalcaligenes (30) by measuring the formation of the ring cleavage product of 2-amino-5-chlorophenol at 395 nm. The molar extinction coefficient (E395 = 21 mM−1 cm−1) was estimated by assuming that all of the 2-amino-5-chlorophenol was converted to the ring cleavage product under excess enzyme conditions as described previously (30). 4-Nitrocatechol monooxygenase activities were measured as previously described (18). Nitrobenzene reductase activity was measured as described previously (38) by monitoring the decrease in absorption at 340 nm due to conversion of NADPH to NADP. Catechol 1,2-dioxygenase (EC 1.13.11.1), catechol 2,3-dioxygenase (EC 1.13.11.2), muconate cycloisomerase (EC 5.5.1.1), and maleylacetate reductase (EC 1.3.1.32) activities were measured as previously described (36). 1,2,4-Trihydroxybenzene 1,2-dioxygenase activity was determined qualitatively by monitoring spectral changes between 220 and 400 nm as described by Stolz and Knackmuss (44). The reaction mixture contained 1,2,4-trihydroxybenzene (200 μM) and cell extract. Controls containing 50 mM phosphate buffer (pH 7.2) instead of cell extract were used to correct for autoxidation of 1,2,4-trihydroxybenzene.
The oxygen uptake rates of resting cell suspensions washed twice with 50 mM sodium phosphate buffer (pH 7.2) were determined polarographically by using a type LTD CB1D electrode (Hansatech, Kings Lynn, Great Britain). The rates were corrected for endogenous respiration.
One unit of specific activity was defined as 1 μmol of substrate converted per min per gram of protein or 1 μmol of product formed per min per gram of protein at 25°C.
The protein contents of cell extracts were determined by using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, Calif.), which is based on the colorimetric dye-binding procedure of Bradford (7). The total protein content of LW1 cells was determined after lysis in the presence of 0.15 M NaOH at 100°C for 10 min and centrifugation at 27,500 × g for 10 min to remove the cell debris; the protein assay described above was used.
Analytical methods.
The chloride ion concentration was measured with a chloride sensor integrated into a flow-injection system (developed by M. Otto, Fraunhofer Gesellschaft für Grenzflächen- und Bioverfahrenstechnik, Stuttgart, Germany) as described previously (4). The nitrite concentration was determined colorimetrically as described previously (29). The ammonium ion concentration was measured colorimetrically by using a Spectroquant kit (E. Merck).
The total organic carbon (TOC) contents of aqueous samples were determined by using a TOC analyzer (model TOC-5050; Shimadzu Corporation, Kyoto, Japan). Samples were centrifugated at 27,500 × g for 10 min to remove the biomass and then acidified to pH 2.0 with a 2 N hydrochloric acid solution and degassed for 5 min with sparge nitrogen gas at a flow rate of 150 ml/min to remove the inorganic carbon dioxide. The TOC content was measured by the nonpurgeable organic carbon method. The inorganic carbon was first removed by catalytic conversion of all inorganic carbon to carbon dioxide, followed by nitrogen sparging. Calibration curves were obtained by using potassium hydrogen phthalate as the organic carbon standard.
The HPLC analysis was carried out as follows. Water-soluble substrates and metabolites were analyzed with a Shimadzu HPLC system equipped with a type SC reversed-phase column (125 by 4.6 mm) filled with Lichrospher 100 RP8 5.0 μm (Bischoff, Leonberg, Germany). An aqueous solvent system containing 36 to 63% (vol/vol) methanol and 0.1% (vol/vol) ortho-phosphoric acid in Milli Q water was used as the mobile phase (flow rate, 1 ml/min). The column effluent was monitored simultaneously at 210 and 270 nm.
The GC-MS analysis was carried out as follows. A model GC-17A gas chromatograph (Shimadzu) equipped with a type XTI-5 column obtained from Resteck (Bellefonte, Pa.) was used. A model QP-5000 quadrupole mass spectrometer was operated in the electron impact mode at 70 eV with an ion source temperature of 320°C. Helium was used as the carrier gas at a flow rate of 1.0 ml/min. The oven temperature was maintained at 60°C for 2 min, and then it was increased to 150°C at a rate of 20°C/min and finally to 320°C at a rate of 6°C/min. Samples (1.0 μl) were injected into the gas chromatography operating in the splitless mode with an injector temperature of 270°C. Methylation of metabolites was carried out with the TMSH reagent. One hundred microliters of the TMSH reagent was added to 50 μl of sample diluted in methanol, which was then boiled for 10 min (11).
Spectroscopic methods.
High-resolution one-dimensional NMR spectra (1H, 13C) and two-dimensional NMR spectra (1H-detected long-range 13C-1H correlations) were recorded with a Bruker model DPX 300 NMR spectrometer locked onto the major deuterium resonance of the solvent, CD3OD. Chemical shifts (in parts per million) were determined relative to tetramethylsilane, and coupling constants (in hertz) were also determined.
Nucleotide sequence accession number.
A 16S rRNA sequence of strain LW1 has been deposited in the EMBL database under accession no. AJ130765.
RESULTS
Isolation, characterization, and growth of 1C4NB-degrading strain LW1.
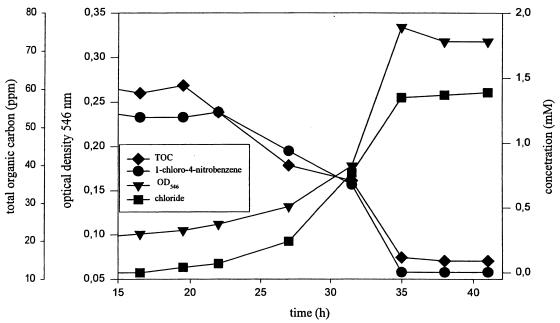
Strain LW1 was isolated from the River Spittelwasser (Germany), a highly contaminated tributary of the River Elbe, by using a previously described enrichment technique (50) and 1C4NB as the sole carbon source. The taxonomy of this strain is currently under investigation. Results based on 16S rRNA sequencing showed that strain LW1 belongs to a new subgroup in the family Comamonadaceae. Investigations by workers at the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany) revealed that this isolate cannot be assigned to the genus Xylophilus or the genus Acidovorax but represents a new species belonging to a new genus in the family Comamonadaceae. Strain LW1 utilized 1C4NB as a sole carbon, nitrogen, and energy source. A growth curve obtained with 1C4NB as the only substrate is shown in Fig. 2. The initial concentration of the dissolved carbon source in the culture fluid was 1.3 mM. Doubling times of 4 to 6 h were observed during the exponential growth phase. The amount of chloride released (1.3 mM) indicates that stoichiometric elimination occurred. Accumulation of ammonium or nitrite was not observed, as determined by the colorimetric assay, nor was metabolite accumulation, as determined by HPLC analysis. The initial amount of TOC was reduced by 75%.
FIG. 2.
Growth of bacterial strain LW1 with 1C4NB as the sole carbon source. The initial concentration of the dissolved carbon source in the culture fluid was 1.2 mM. The culture was inoculated (10%, vol/vol) with a preculture grown with 1C4NB and harvested during the exponential phase. Substrate depletion, formation of chloride, and TOC values were determined as described in Materials and Methods. Growth was monitored by monitoring the increase in OD546.
Growth of strain LW1 with substrate analogues, such as nitrobenzene and chlorobenzene, and with several hypothetical pathway intermediates, such as 2-amino-5-chlorophenol (which was biologically prepared in this work), 4-chloroaniline, 4-nitrophenol, 4-nitrocatechol, 4-chlorophenol, and 4-chlorocatechol, was tested in liquid cultures by using each compound at a concentration of 1 mM, as well as on diffusion gradient agar plates, which should have prevented toxic effects on bacterial cells. Only two of these compounds, nitrobenzene and 2-amino-5-chlorophenol, were utilized as carbon sources by LW1.
Aerobic transformation of 1C4NB and of hypothetical pathway intermediates.
In order to elucidate the pathway of 1C4NB degradation in strain LW1, oxygen uptake experiments were performed with growth substrates and several hypothetical intermediates (Table 1). 1C4NB-grown resting cells had an oxygen uptake rate of 86 U/g of protein with 1C4NB, and the oxygen uptake rate with 4-chlorocatechol was approximately fourfold lower (23 U/g of protein). Negligible oxygen consumption occurred with 4-chlorophenol, 4-nitrocatechol, 4-nitrophenol, hydroquinone, and 4-chloroaniline.
TABLE 1.
Relative oxygen uptake rates and conversion rates for possible pathway intermediates in 1C4NB-grown resting cells
| Substrate | Reactiona | Specific rate of oxygen consumption (U/g of protein) after growth with 1C4NBb | Initial conversion rate (U/g of protein)c |
|---|---|---|---|
| 1C4NB | 86 | 23 | |
| 2-Amino-5-chlorophenol | i | 450 | 820 |
| 2-Aminophenol | 160 | 290 | |
| 4-Chloroaniline | ii | <1 | 0 |
| 4-Nitrocatechol | iii | 6 | 0 |
| 4-Nitrophenol | iii | <1 | 1.3 |
| Hydroquinone | iii | 1 | 0.7 |
| 4-Chlorocatechol | iv | 23 | 18 |
| 4-Chlorophenol | iv | 1 | NDd |
| Nitrobenzene | v | 43 | ND |
| Chlorobenzene | 7 | ND |
Catabolic pathway in which the compound is a possible intermediate. See Fig. 1.
The oxygen uptake rates were corrected for endogenous respiration. Most substrates were added at an initial concentration of 100 μM; the exceptions were 1C4NB, chlorobenzene, and nitrobenzene, which were added at an initial concentration of 1.0 mM.
In most cases depletion was measured by HPLC for 6 h; for 2-aminophenol and 2-amino-5-chlorophenol depletion was measured for 30 min. Experiments were carried out under aerobic conditions. Most substrates were added at an initial concentration of 200 μM; 1C4NB was added at an initial concentration of 1.3 mM. With the exception of 4-chlorocatechol transformation, no products were detected by HPLC.
ND, not determined.
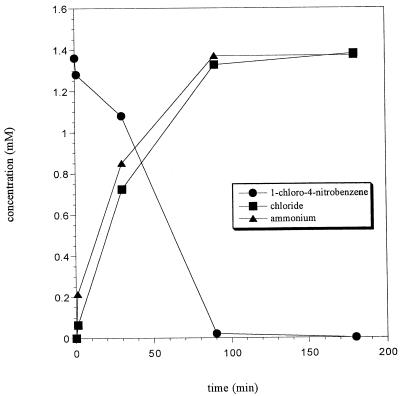
In contrast, the specific transformation rate (23 U/g of protein) for 1C4NB (1.3 mM) obtained with 1C4NB-grown resting cells (OD546, 6.0), as determined by HPLC, was lower than the specific oxygen uptake rate. Again, there was no indication that significant amounts of pathway intermediates accumulated, as determined by HPLC. Stoichiometric amounts of both chloride (1.4 mM) and ammonium (1.4 mM) accumulated, as determined by colorimetric assays (Fig. 3). Similar results were obtained with acetate-grown cells, which transformed 1C4NB at a rate of 20 U/g of protein.
FIG. 3.
Time course for conversion of 1C4NB by resting cells of strain LW1 grown with 1C4NB under aerobic conditions. Cell suspensions at an OD546 of 6.0 were resuspended in 50 mM sodium phosphate buffer (pH 7.2), and substrate was added to a final concentration of 1.36 mM. Substrate depletion was determined by HPLC.
4-Chlorocatechol was transformed by resting cells at a rate of 18 U/g of protein, which was similar to the measured oxygen uptake rate. Transformation was accompanied by transient yellow coloration of the medium. HPLC analysis of the supernatant revealed that there was transient formation of a metabolite, the in situ UV spectrum (after HPLC) of which exhibited an absorption maximum at 332 nm. Formation of small amounts of a new product from 4-chlorocatechol by both 1C4NB-grown resting cells and acetate-grown resting cells was detected by GC-MS of methylated ethyl acetate extracts; this new product was later identified as 5-chloropicolinic acid.
When 4-nitrophenol and hydroquinone were used, about 20% of the substrates were removed from the resting cell suspensions in 6 h, but no products were detected by HPLC. No significant conversion was observed with 4-chloroaniline or 4-nitrocatechol. While there was no significant decrease in the initial concentration of 4-nitrocatechol (200 μM), a quick change in the color of the reaction mixture from yellow to orange took place. No reaction was observed with controls that did not contain cells or controls that contained cells that had been heat inactivated (10 min, 60°C).
The activities of various possible pathway enzymes in cell extracts are shown in Table 2. Low nitrobenzene reductase activities, 2 and 22 U/g of protein with nitrobenzene and 1C4NB as the substrates, respectively, were observed. In contrast to the observed transformation by whole cells, the cell extracts exhibited neither catechol 1,2-dioxygenase activity nor catechol 2,3-dioxygenase activity when 4-chlorocatechol or catechol was the substrate. No muconate cycloisomerase and chloromuconate cycloisomerase activities were observed when muconate and 2-chloromuconate were the substrates, respectively. No 4-nitrocatechol monooxygenase activity was observed. The spectral changes observed with cell extracts when 1,2,4-trihydroxybenzene was the substrate indicated that a 1,2,4-trihydroxybenzene 1,2-dioxygenase activity was present. However, no activity of the potential subsequent pathway enzyme maleylacetate reductase was observed.
TABLE 2.
Specific activities of catabolic enzyme activities in cell extracts of strain LW1
| Enzymea | Reaction(s)b | Substrate | Sp act after growth with 1C4NB (U/g of protein) |
|---|---|---|---|
| Nitrobenzene reductase | i | Nitrobenzene | 2 |
| 1C4NB | 22 | ||
| 2-Amino-5-chlorophenol 1,6-dioxygenase | i | 2-Amino-5-chlorophenol | 3,570 |
| 2-Aminophenol 1,6-dioxygenase | 2-Aminophenol | 3,020 | |
| 4-Nitrocatechol monooxygenase | iii | 4-Nitrocatechol | <0.01 |
| Maleylacetate reductase | iii, iv | Maleylacetate | <0.01 |
| Catechol 1,2-dioxygenase | iv | Catechol | <0.01 |
| 4-Chlorocatechol | <0.01 | ||
| Catechol 2,3-dioxygenase | iv | Catechol | <0.01 |
| 4-Chlorocatechol | <0.01 | ||
| Chloromuconate cycloisomerase | iv | 2-Chloromuconate | <0.01 |
| Muconate cycloisomerase | iv | Muconate | <0.01 |
Cell extract was added to give final protein concentrations of 0.1 to 0.15 mg. Enzyme assays were performed as described in Materials and Methods.
See Fig. 1.
Anaerobic transformation of 1C4NB by 1C4NB-grown resting cells.
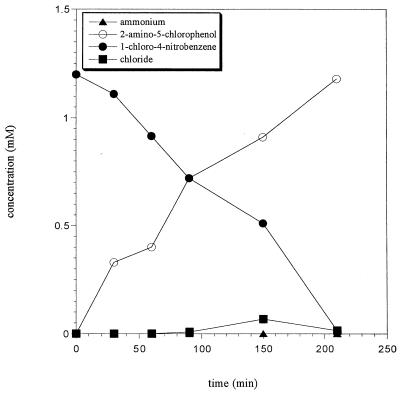
Previous results indicated that the nitro substituent was reduced, that ammonium rather than nitrite was eliminated, and that a nitrobenzene reductase was present; therefore, transformation of 1C4NB in the absence of oxygen should have led to the accumulation of pathway intermediates. Under anaerobic conditions, LW1 cells grown on 1C4NB converted 1C4NB into a single product (Fig. 4), later identified as 2-amino-5-chlorophenol. The elimination of chloride or ammonium under these conditions was negligible. Acetate-grown resting cells of LW1 converted 1C4NB under anaerobic conditions at the same conversion rate.
FIG. 4.
Time course for conversion of 1C4NB by resting cells of strain LW1 grown with 1C4NB under anaerobic conditions. Cell suspensions at an OD546 of 5.0 were resuspended in 50 mM sodium phosphate buffer (pH 7.2). Substrate was added to a final concentration of 1.2 mM. Substrate depletion and product formation were determined by HPLC.
The metabolite mentioned above had a retention volume of 3.5 ml when an HPLC solvent system containing 36% (vol/vol) methanol was used and had an in situ UV absorption spectrum with maxima at 202, 217, and 277 nm at pH 2.0. The mass spectrum of its methyl ether had a molecular ion at m/z = 158 (M+), indicative of C7H8C1NO, and major fragments due to the loss of −CH3 (m/z = 142) and −CH3, −CO (m/z = 114).
The 1H and 13C NMR data (Table 3) were unambiguously assigned on the basis of the two-dimensional 1H-detected long-range 13C-1H correlation. The 13C chemical shift data were compatible only with the substitution pattern of 2-amino-5-chlorophenol, as shown by calculations of the shifts when the known shifts of 2-aminophenol (8) and the chlorosubstituent chemical shifts (SCS) (45) were used, and they were incompatible with the substitution pattern of 2-chloro-5-aminophenol (with known shifts of 4-chloroaniline [9] and SCS of an aromatic hydroxyl group [45]).
TABLE 3.
1H and 13C NMR data for 2-amino-5-chlorophenol and 5-chloropicolinic acid in CD3OD
| 2-Amino-5-chlorophenol
|
5-Chloropicolinic acid
|
|||||
|---|---|---|---|---|---|---|
| 1H chemical shifts (ppm) | 1H-1H coupling constants (Hz) | 13C chemical shifts (ppm) | 1H chemical shifts (ppm) | 1H-1H coupling constants (Hz) | 13C chemical shifts (ppm) | One-bond 13C-1H coupling constants (Hz)a |
| H-3 6.70 | (3-4) 8.3 | C-1 147.2 | H-3 8.18 | (3-4) 8.4 | C-2 147.6 | |
| H-4 6.64 | (4-6) 2.3 | C-2 135.5 | H-4 8.08 | (3-6) 0.7 | C-3 127.3 | 168.0 |
| H-6 6.72 | C-3 117.6 | H-6 8.70 | (4-6) 2.4 | C-4 138.7 | 170.5 | |
| C-4 120.5 | C-5 137.1 | |||||
| C-5 123.8 | C-6 149.4 | 186.3 | ||||
| C-6 115.4 | C-7 166.6 | |||||
The values of the one-bond 13C-1H coupling constants were estimated from the residual cross peaks in the 1H-detected long-range 13C-1H correlation.
Assuming that the new product was 2-amino-5-chlorophenol, we calculated that transformation of 1C4NB was stoichiometric and occurred at a rate of 4.5 U/g of protein.
Aerobic transformation of 2-amino-5-chlorophenol.
2-Amino-5-chlorophenol (initial concentration, 0.575 mM) was rapidly converted into a yellow product by 1C4NB-grown resting cells at a conversion rate of 820 U/g of protein. An HPLC analysis revealed that a single product was formed, and this product had a retention volume of 4.2 ml and an in situ UV spectrum with absorption maxima at 201, 234, and 274 nm. This compound was extracted and derivatized as described above. The mass spectrum of the methyl ester had a molecular ion at m/z = 171 (M+), indicative of C7H6C1NO2. Major fragments appeared at m/z = 141 (M+ −OCH2) and at m/z = 113 (M+ −CO2CH2). Loss of HCl from the mass at 112 yielded a mass of 76, which is consistent with the presence of a pyridine nucleus. These results are similar to the previously published mass spectrum of the methyl ester of 2-chloropicolinic acid (14).
Again, unambiguous NMR assignments (Table 3) were provided by the two-dimensional 1H-detected long-range 13C-1H correlation, and both 13C shift values (with picolinic acid [10] and chloro SCS) and the magnitude of the one-bond 13C-1H coupling constants were compatible only with the proposed structure.
The transformation of 2-amino-5-chlorophenol into 5-chloropicolinic acid was not stoichiometric; only 50% of the substrate was recovered as 5-chloropicolinic acid (0.267 mM). Despite the fact that no other products were detected by HPLC, the accumulation of significant amounts of ammonium (0.124 mM) but negligible amounts of chloride (0.043 mM) indicated that additional products were formed. 1C4NB-grown resting cells of LW1 had an oxygen uptake rate of 450 U/g of protein when 2-amino-5-chlorophenol was the substrate, which was fivefold higher than the oxygen uptake rate observed with 1C4NB. Resting cells had very low oxygen uptake rate (8 U/g of protein) when 5-chloropicolinic acid was the substrate.
2-Amino-5-chlorophenol 1,6-dioxygenase activity.
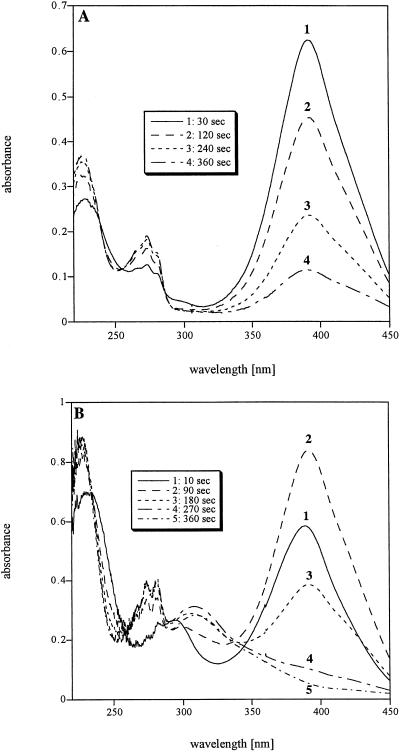
Cell extracts catalyzed transformation of both 2-aminophenol and 2-amino-5-chlorophenol, and yellow intermediates with absorption maxima at 380 and 395 nm, respectively, appeared and disappeared rapidly. Figure 5A shows the changes in the UV-visible light spectra during enzyme reactions when 2-amino-5-chlorophenol was the substrate. When the initial maximum absorption at 395 nm decreased, absorption at 226 and 272 nm increased. The final absorption spectrum was identical to that of 5-chloropicolinic acid.
FIG. 5.
Spectral changes due to 2-amino-5-chlorophenol 1,6-dioxygenase activity in extracts of cells pregrown with 1C4NB. (A) Reaction mixture containing 2-amino-5-chlorophenol (100 μM) and LW1 cell extract (0.1 mg of protein) in 1 ml of 50 mM sodium phosphate buffer (pH 7.2). (B) Reaction mixture also containing NAD (100 μM). Overlay spectra were recorded for 6 min from 220 to 450 nm as indicated.
Cell extracts of LW1 catalyzed transformation of 2-amino-5-chlorophenol and 2-aminophenol with specific activities of 3,570 and 3,020 U/g of protein, respectively. Figure 5B shows the changes in the UV-visible light spectra during transformation by cell extracts of 2-amino-5-chlorophenol in the presence of NAD, which differed from the results of spectrophotometric assays in which NAD was not present by the presence of a new absorption maximum at 306 nm.
DISCUSSION
The accumulation of ammonia but not of nitrite in conversion experiments performed with resting cells grown with 1C4NB and the observed 1C4NB reductase activity in LW1 cell extracts suggested that the initial attack on the nitro group was reductive. The complete conversion of 1C4NB to 2-amino-5-chlorophenol in the absence of oxygen indicated that the nitro group was partially reduced. In the proposed pathway, the nitro group is partially reduced to a hydroxylamino group; this is followed by an enzyme-catalyzed Bamberger rearrangement to form 2-aminophenol (30), whereas a nonenzymatic Bamberger rearrangement results in the formation of the corresponding 4-aminophenol (39). Formation of 2-aminophenol as the only intermediate has also been reported for nitrobenzene degradation by Pseudomonas pseudoalcaligenes JS45 (30) and for 4-nitrotoluene degradation by a Mycobacterium strain (41). Similarly, 3-nitrophenol was transformed by Ralstonia eutropha JMP 134 into aminohydroquinone but not into 4-aminocatechol (35). In contrast to the high regioselectivity of the rearrangement observed during transformation of the growth-supporting substrate 3-nitrophenol, nitrobenzene, which does not serve as a growth substrate, was transformed into both 2-aminophenol and 4-aminophenol by this strain (35). Such rearrangements of the hydroxyl substituents at both the ortho and para positions have also been reported for 4-chlorohydroxylamino benzene transformation by Rhodosporidium sp. (12). It has been assumed that a similar reaction occurs during transformation of 2,4,6-trinitrotoluene by Clostridium acetobutylicum (22). 2-Amino-5-chlorophenol, but not 4-amino-5-chlorophenol, was formed as an intermediate during degradation of 1C4NB by LW1. Evidently, in microorganisms able to mineralize nitroaromatic compounds via a pathway involving Bamberger rearrangement, this rearrangement leads exclusively to the formation of 2-aminophenols, which are subject to meta cleavage.
The transformation of 2-aminophenol by LW1 cell extracts was similar to the transformation of 2-aminophenol by P. pseudoalcaligenes JS45 1,6-dioxygenase (30). The ring cleavage product cyclized spontaneously to form picolinic acid. Similarly, the ring cleavage product of 2-amino-5-chlorophenol with a UV maximum at 395 nm (probably 2-amino-5-chloromuconic semialdehyde) intramolecularly condensed to give 5-chloropicolinic acid. As this compound was not a growth substrate and was not transformed further by whole cells or cell extracts, a degradative pathway involving this compound as an intermediate is highly unlikely. Such abiotic chemical rearrangements may take place when an unstable intermediate is formed and the activity of the subsequent pathway enzyme is insufficient under the conditions used. When 2-amino-5-chlorophenol was the substrate, high transformation rates were observed. A pathway bottleneck presumably prevented the complete conversion of the semialdehyde formed, which led to the accumulation of 5-chloropicolinic acid. In contrast, when 1C4NB was the substrate, the rate-limiting initial reduction (low 1C4NB reductase activity) suggests that the rate of formation of the semialdehyde was low, so that additional pathway enzymes could quantitatively channel the intermediate into the productive route. In accordance with this hypothesis, only traces of 5-chloropicolinic acid (<50 μM) accumulated in both growing and resting LW1 cultures when 1C4NB was used as the substrate.
Formation of picolinic acid as a dead-end product has been reported in many studies, and this compound has been shown to arise from abiotic intramolecular condensation of enzymatically formed 2-aminomuconic semialdehyde derivatives (1, 24, 41). Asano et al. described the chemical synthesis of various picolinic acid derivatives from ammonia and 2-hydroxymuconic semialdehyde derivatives which had been biologically prepared from various catechols by using catechol 2,3-dioxygenase (3). Similarly, Davison et al. described the abiotic formation of halopicolinic acids from substituted 2-halohydroxymuconic semialdehydes formed during degradation of chloro- and bromobiphenyls by Sphingomonas paucimobilis BPSI-3 (14). Riegert et al. used this abiotic transformation to determine the structure of the ring fission product of 3-chlorocatechol obtained during 2,3-dihydroxybiphenyl 1,2-dioxygenase activity of Sphingomonas sp. strain BN6 (33). The nitrogens present in picolinic acid formed from 2-aminophenol by P. pseudoalcaligenes JS45 (30) and in 5-chloropicolinic acid formed from 2-amino-5-chlorophenol by LW1, however, clearly originated from the substrates.
The high activities observed with both 2-aminophenol and 2-amino-5-chlorophenol in LW1 cell extracts indicated that a 2-amino-5-chlorophenol 1,6-dioxygenase with high substrate specificity was present. Neither catechol nor 4-chlorocatechol was transformed by cell extracts. Similar substrate specificity was observed for the 6-amino-m-cresol 1,6-dioxygenase of a Mycobacterium strain which was shown to be active against 2-aminophenol and the pathway intermediate 6-amino-m-cresol but not against catechol or 4-methylcatechol (41). In contrast, the 2-aminophenol 1,6-dioxygenase of P. pseudoalcaligenes JS45 exhibited significant activity with catechol (24). Additional substituents in both the 2-aminophenol structure and the catechol structure severely diminished or abolished activity. 2-Aminophenol 1,6-dioxygenase of Pseudomonas sp. strain AP-3 also catalyzed the catechol reaction (1). The amino acid sequence of b-subunit AmnB of purified 2-aminophenol 1,6-dioxygenase of Pseudomonas sp. strain AP-3 exhibited similarities to the amino acid sequences of extradiol dioxygenases (48). Lendenmann and Spain suggested that the purified 2-aminophenol 1,6-dioxygenase of P. pseudoalcaligenes JS45 is also related to catechol 2,3-dioxygenases (24). How much differences in substrate specificity are reflected by evolutionary relationships still must be elucidated.
The catabolic pathway responsible for transformation of 1C4NB (Fig. 6) seems to be constitutively expressed in LW1, as similar conversion rates for 1C4NB and 2-amino-5-chlorophenol were obtained with 1C4NB-grown cells and acetate-grown cells (data not shown). This contrasts with the situation in recently described 3-nitrophenol- and 4-nitrotoluene-degrading organisms (35, 37, 43), whose cells did not transform the respective substrates when they were grown under noninducing conditions.
FIG. 6.
Proposed pathway for catabolism of 1C4NB by bacterial strain LW1. The ring cleavage product 2-amino-5-chloromuconic semialdehyde can be subject to dehydrogenation (probably producing 2-amino-5-chloromuconic acid) or intramolecular condensation to form the dead-end product 5-chloropicolinic acid as previously reported for 2-aminophenol transformation by P. pseudoalcaligenes JS45 (20) or by Pseudomonas sp. strain AP-3 (48).
As described above, extracts of 1C4NB-grown cells of LW1, like extracts of nitrobenzene-grown cells of P. pseudoalcaligenes JS45, transformed 2-aminophenol into picolinic acid (30). In both cases addition of NAD to reaction mixtures containing 2-aminophenol and cell extracts reduced the formation of picolinic acid and resulted in transient accumulation of NADH and formation of 2-aminomuconate (20). In P. pseudoalcaligenes JS45, 2-aminomuconate was further transformed by 2-aminomuconate deaminase into 2-oxohex-3-ene-1,6-dioate, which, in turn, can be assumed to be degraded to Krebs cycle intermediates by enzymes of the classical meta-cleavage pathway (20). It has recently been postulated that there is an alternative pathway for 2-aminophenol degradation in Pseudomonas sp. strain AP-3 (47). Takenaka et al. postulated that there is an initial decarboxylation of the intermediate 2-aminomuconic acid, based on identification of 2-aminopenta-2,4-dienoic acid as an intermediate, which is subject to deamination, resulting in 2-oxopent-4-enoic acid (47).
Whereas spectrophotometric monitoring of the progress of 2-aminophenol transformation by LW1 cell extracts in the presence of NAD gave no indication that pathway intermediates were formed, a product exhibiting an absorption maximum at 306 nm accumulated during 2-amino-5-chlorophenol transformation. This product interfered with spectrophotometric monitoring of the accumulating NADH. If it is assumed that there is a pathway in LW1 for the degradation of 2-amino-5-chlorophenol similar to the pathway characterized by He and Spain (20) and Takenaka et al. (47) for 2-aminophenol degradation, it can be assumed that 2-amino-5-chloromuconate and 5-chloro-2-oxohex-3-ene-1,6-dioate or 2-aminopenta-2,4-dienoate are intermediates. 2-Aminomuconate has been reported to exhibit an absorption maximum at 326 nm (20). Whether the new absorption maximum at 306 nm can be attributed to transient accumulation of 2-amino-5-chloromuconate or transient accumulation of 5-chloro-2-hydroxymuconate will be the subject of further investigation. Although the growth substrate 1C4NB is transformed by LW1 at a low rate, the pathway intermediate 2-amino-5-chlorophenol is transformed very rapidly, so that subsequently, active pathway enzymes cannot adequately turn over the intermediates. In addition to the accumulation of 5-chloropicolinic acid, the increase in absorbance at 306 nm mentioned above indicates that there is a second pathway bottleneck. Moreover, the observed elimination of larger amounts of ammonium than of chloride during resting cell-mediated transformation of 2-amino-5-chlorophenol could indicate that ammonium elimination precedes dechlorination. The next steps of the pathway, including those responsible for elimination of ammonium and chloride in LW1, are currently under investigation.
ACKNOWLEDGMENTS
This work was supported by grant ENV4 CT 93-0081 from the European Union Environment and Climate Program.
Michael Klemba kindly helped prepare the manuscript and proofread it. We thank Margit Mau for 16S rRNA sequencing of strain LW1 and Tilmann Spiess and Andreas Schenzle for helpful discussions concerning the anaerobic biotransformations.
REFERENCES
- 1.Aoki K, Takenaka S, Murakami S, Shinke R. Partial purification and characterization of a bacterial dioxygenase that catalyzes the ring fission of 2-aminophenol. Microbiol Rev. 1997;152:33–38. [Google Scholar]
- 2.Arensdorf J J, Focht D. A meta-cleavage pathway for 4-chlorobenzoate, an intermediate in the metabolism of 4-chlorobiphenyl by Pseudomonas cepacia P166. Appl Environ Microbiol. 1995;61:443–447. doi: 10.1128/aem.61.2.443-447.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asano Y, Yamamoto Y, Yamada H. Catechol 2,3-dioxygenase-catalyzed synthesis of picolinic acids from catechols. Biosci Biotechnol Biochem. 1994;58:2054–2056. [Google Scholar]
- 4.Beil S, Happe B, Timmis K N, Pieper D H. Genetic and biochemical characterization of the broad-spectrum chlorobenzene dioxygenase from Burkholderia sp. strain PS12: dechlorination of 1,2,4,5-tetrachlorobenzene. Eur J Biochem. 1997;247:190–199. doi: 10.1111/j.1432-1033.1997.00190.x. [DOI] [PubMed] [Google Scholar]
- 5.Beratergremium für umweltrelevante Altstoffe der Gesellschaft Deutscher Chemiker. m-Chlornitrobenzol, p-Chlornitrobenzol. BUA-Stoffbericht 11 (Februar 1988). Weinheim, Germany: VCH Verlagsgesellschaft; 1988. [Google Scholar]
- 6.Blasco R, Wittich R-M, Mallavarapu M, Timmis K N, Pieper D H. From xenobiotic to antibiotic, formation of protoanemonin from 4-chlorocatechol by enzymes of the 3-oxoadipate pathway. J Biol Chem. 1995;270:29229–29235. doi: 10.1074/jbc.270.49.29229. [DOI] [PubMed] [Google Scholar]
- 7.Bradford M M. A rapid and sensitive method for the quantitation of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 8.Bremser W. CNMR no. 4945. CNMR spectral data. Weinheim, Germany: VCH; 1986. [Google Scholar]
- 9.Bremser W. CNMR no. 5601. CNMR spectral data. Weinheim, Germany: VCH; 1986. [Google Scholar]
- 10.Bremser W. CNMR no. 28624. CNMR spectral data. Weinheim, Germany: VCH; 1986. [Google Scholar]
- 11.Butte W. Rapid method for the determination of fatty acid profiles from fats and oils using trimethylsulphonium hydroxide for transesterification. J Chromatogr. 1983;261:142–145. [Google Scholar]
- 12.Corbett M D, Corbett B R. Metabolism of 4-chloronitrobenzene by the yeast Rhodosporidium sp. Appl Environ Microbiol. 1981;41:942–949. doi: 10.1128/aem.41.4.942-949.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cozza C L, Woods S L. Reductive dechlorination pathways for substituted benzenes: a correlation with electronic properties. Biodegradation. 1992;2:265–278. [Google Scholar]
- 14.Davison A D, Karuso P, Jardine D R, Veal D A. Halopicolinic acids, novel products arising through the degradation of chloro- and bromobiphenyl by Sphingomonas paucimobilis BPSI-3. Can J Microbiol. 1996;42:66–71. doi: 10.1139/m96-009. [DOI] [PubMed] [Google Scholar]
- 15.Dorn E, Hellwig M, Reineke W, Knackmuss H-J. Isolation and characterization of a 3-chlorobenzoate degrading pseudomonad. Arch Microbiol. 1974;99:61–70. doi: 10.1007/BF00696222. [DOI] [PubMed] [Google Scholar]
- 16.European Economic Community. Communication from the commission to the council on dangerous substances which might be included in list I of council directive 76/464/EEC. Brussels, Belgium: European Economic Community; 1982. [Google Scholar]
- 17.Fetzner S, Lingens F. Bacterial dehalogenases: biochemistry, genetics, and biotechnological applications. Microbiol Rev. 1994;58:641–685. doi: 10.1128/mr.58.4.641-685.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haigler B E, Nishino S F, Spain J C. Biodegradation of 4-methyl-5-nitrocatechol by Pseudomonas sp. strain DNT. J Bacteriol. 1994;176:3433–3437. doi: 10.1128/jb.176.11.3433-3437.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harttner D R. Toxicity of nitroaromatic compounds. Washington, D.C: Hemisphere Publishing Corp.; 1985. [Google Scholar]
- 20.He Z, Spain J C. A novel 2-aminomuconate deaminase in the nitrobenzene degradation pathway of Pseudomonas pseudoalcaligenes JS45. J Bacteriol. 1998;180:2502–2506. doi: 10.1128/jb.180.9.2502-2506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heijman C G, Hollinger C, Claus M A, Schwarzenbach R P, Zeyer J. Abiotic reduction of 4-chloronitrobenzene to 4-chloroaniline in a dissimilatory iron-reducing enrichment culture. Appl Environ Microbiol. 1993;59:4350–4353. doi: 10.1128/aem.59.12.4350-4353.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes J B, Wang C, Yesland K, Richardson A, Bhadra R, Bennett G, Rudolph F. Bamberger rearrangement during TNT metabolism by Clostridium acetobutylicum. Environ Sci Technol. 1998;32:494–500. [Google Scholar]
- 23.Keither L H, Telliard W A. Priority pollutants. I. A perspective view. Environ Sci Technol. 1979;13:416–423. [Google Scholar]
- 24.Lendenmann U, Spain J C. 2-Aminophenol 1,6-dioxygenase: a novel aromatic ring cleavage enzyme purified from Pseudomonas pseudoalcaligenes JS45. J Bacteriol. 1996;178:6227–6232. doi: 10.1128/jb.178.21.6227-6232.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenke H, Knackmuss H-J. Initial hydrogenation and extensive reduction of substituted 2,4-dinitrophenols. Appl Environ Microbiol. 1996;62:784–790. doi: 10.1128/aem.62.3.784-790.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linch A L. Biological monitoring for industrial exposure to cyanogenic aromatic nitro and amino compounds. Am Ind Hyg Assoc J. 1974;35:426–432. doi: 10.1080/0002889748507055. [DOI] [PubMed] [Google Scholar]
- 27.Livingston A G. A novel membrane bioreactor for detoxifying industrial waste-water. I. Biodegradation of 3-chloronitrobenzene in an industrially produced waste-water. Biotechnol Bioeng. 1993;41:927–936. doi: 10.1002/bit.260411003. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy D L, Louie D F, Copley S D. Identification of a covalent intermediate between glutathion and cysteine13 formed during catalysis by tetrachlorohydroquinone dehalogenase. J Am Chem Soc. 1997;119:11337–11338. [Google Scholar]
- 29.Montgomery H A C, Dymock D F. The determination of nitrite in water. Analyst. 1961;86:414–416. [Google Scholar]
- 30.Nishino S F, Spain J C. Degradation of nitrobenzene by a Pseudomonas pseudoalcaligenes. Appl Environ Microbiol. 1993;59:2520–2525. doi: 10.1128/aem.59.8.2520-2525.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pieper D H, Timmis K N, Ramos J L. Designing bacteria for the degradation of nitro- and chloroaromatic pollutants. Naturwissenschaften. 1996;83:201–213. [Google Scholar]
- 32.Reineke W, Knackmuss H-J. Microbial degradation of haloaromatics. Annu Rev Microbiol. 1988;42:263–287. doi: 10.1146/annurev.mi.42.100188.001403. [DOI] [PubMed] [Google Scholar]
- 33.Riegert U, Heiss G, Fischer P, Stolz A. Distal cleavage of 3-chlorocatechol by an extradiol dioxygenase to 3-chloro-2-hydroxymuconic semialdehyde. J Bacteriol. 1998;180:2849–2853. doi: 10.1128/jb.180.11.2849-2853.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schackmann A, Müller R. Reduction of nitroaromatic compounds by different Pseudomonas species under aerobic conditions. Appl Microbiol Biotechnol. 1991;34:809–813. [Google Scholar]
- 35.Schenzle A, Lenke H, Fischer P, Williams P A, Knackmuss H-J. Catabolism of 3-nitrophenol by Ralstonia eutropha JMP 134. Appl Environ Microbiol. 1997;63:1421–1427. doi: 10.1128/aem.63.4.1421-1427.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlömann M, Schmidt E, Knackmuss H-J. Different types of dienelactone hydrolase in 4-fluorobenzoate-utilizing bacteria. J Bacteriol. 1990;172:5112–5118. doi: 10.1128/jb.172.9.5112-5118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimizu M, Yasui T, Matsumoto N. Structural specificity of aromatic compounds with special reference to mutagenic activity in Salmonella typhimurium—a series of chloro- or fluoro-nitrobenzene derivatives. Mutat Res. 1983;116:217–238. doi: 10.1016/0165-1218(83)90060-5. [DOI] [PubMed] [Google Scholar]
- 38.Somerville C C, Nishino S F, Spain J C. Purification and characterization of nitrobenzene nitroreductase from Pseudomonas pseudoalcaligenes JS45. J Bacteriol. 1995;177:3837–3842. doi: 10.1128/jb.177.13.3837-3842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sone T, Tokuda Y, Sakai T, Shinkai S, Manabe O. Kinetics and mechanisms of the Bamberger rearrangement. 3. Rearrangement of phenylhydroxylamines to p-aminophenols in aqueous sulphuric acid solutions. J Chem Soc Perkin Trans 2. 1981;1981:298–302. [Google Scholar]
- 40.Spain J C. Biodegradation of nitroaromatic compounds. New York, N.Y: Plenum Press; 1995. [DOI] [PubMed] [Google Scholar]
- 41.Spiess T, Desiere F, Fischer P, Spain J C, Knackmuss H-J, Lenke H. A new 4-nitrotoluene degradation pathway in a Mycobacterium strain. Appl Environ Microbiol. 1998;64:446–452. doi: 10.1128/aem.64.2.446-452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinwandter H. Research in environmental pollution. II. Determination of polychlorinated nitrobenzenes (PCNB’s) in Main River fish. Fresenius Z Anal Chem. 1987;326:139–141. [Google Scholar]
- 43.Stockinger H, Heinzle E, Kut O M. Removal of chloro- and nitro-aromatic waste-water pollutants by ozonation and biotreatment. Environ Sci Technol. 1995;29:2016–2022. doi: 10.1021/es00008a021. [DOI] [PubMed] [Google Scholar]
- 44.Stolz A, Knackmuss H-J. Degradation of 2,4-dihydroxybenzoate by Pseudomonas sp. BN9. FEMS Microbiol Lett. 1993;108:219–224. doi: 10.1111/j.1574-6968.1993.tb06102.x. [DOI] [PubMed] [Google Scholar]
- 45.Stothers J B. Carbon-13 NMR spectroscopy. New York, N.Y: Academic Press; 1972. [Google Scholar]
- 46.Susarla S, Masunaga S, Yonezawa Y. Transformations of chloronitrobenzenes in anaerobic sediment. Chemosphere. 1996;32:967–977. [Google Scholar]
- 47.Takenaka S, Murakami S, Shinke R. Metabolism of 2-aminophenol by Pseudomonas sp. AP-3: modified meta-cleavage pathway. Arch Microbiol. 1998;170:132–137. doi: 10.1007/s002030050624. [DOI] [PubMed] [Google Scholar]
- 48.Takenaka S, Murakami S, Shinke R, Hatakeyama K, Yukawa H, Aoki K. Novel genes encoding 2-aminophenol 1,6-dioxygenase from Pseudomonas species AP-3 growing on 2-aminophenol and catalytic properties of the purified enzyme. J Biol Chem. 1997;272:14727–14732. doi: 10.1074/jbc.272.23.14727. [DOI] [PubMed] [Google Scholar]
- 49.Weisburger E K, Russfield F, Homburger J, Weisburger J H, Boger E, Van Dongen C G, Chu K C. Testing of twenty-one environmental aromatic amines or derivatives for long-term toxicity or carcinogenicity. Environ Pathol Toxicol. 1978;2:325–356. [PubMed] [Google Scholar]
- 50.Wittich R-M, Wilkes H, Sinnwell V, Francke W, Fortnagel P. Metabolism of dibenzo-p-dioxin by Sphingomonas sp. strain RW1. Appl Environ Microbiol. 1992;58:1005–1010. doi: 10.1128/aem.58.3.1005-1010.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshida T. Determination of p-chloronitrobenzene and its metabolites in urine by reversed-phase high-performance liquid chromatography. J Chromatogr. 1993;613:79–88. doi: 10.1016/0378-4347(93)80199-e. [DOI] [PubMed] [Google Scholar]