Abstract
Amphibians are threatened globally with at least 43% of species declining and the most important stressor being habitat loss or degradation. Amphibians inhabiting highly biodiverse tropical regions are disproportionately threatened; however, the effects of landscape alterations on amphibian health are virtually unknown. In this study, we utilised non-destructive techniques to compare size (weight, snout-vent length [SVL]), body condition, male secondary sexual features (forelimb width, nuptial pad length) and breeding success (egg number, fertility [percentage fertilised eggs], hatching success) in túngara frogs (Engystomops pustulosus) collected from reference (n = 5), suburban (n = 6) and agricultural (n = 4) sites in Trinidad; characterised by presence/absence of crops/houses. All measured endpoints were negatively impacted in frogs collected from agricultural sites. The largest effect was observed for hatching success (2.77-fold lower) and egg number (2.5-fold lower). Less pronounced effects were observed on male frogs (weight: 1.77-fold lower; SVL: 1.18-fold lower; forelimb width: 1.33-fold lower; nuptial pad length: 1.15-fold lower). Our findings demonstrate negative impacts of agricultural sites on túngara frog health, with the number of viable offspring reduced by almost one third. The methods outlined here are technically simple and low-cost and thereby have potential for application to other species in order to investigate the potential impacts of habitat degradation on amphibian health. Furthermore, as these methods are non-destructive, they could be used to investigate the potential contribution of frog size and/or reproductive capability as a causative factor contributing to population declines in threatened species, which is particularly pressing in tropical regions.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11356-022-20105-4.
Keywords: Amphibian, Freshwater, Pollution, Pesticides, Ecotoxicology, Endocrine disruption, Reproduction, Biodiversity
Introduction
Amphibians are the most threatened vertebrate group, with at least 43% of species declining (Díaz et al. 2019). Several anthropogenic drivers are indicated in causing declines, including habitat loss and degradation, pollution, disease, climate change and invasive species. Habitat loss or degradation and pollution are listed as the first and second most important causes of amphibian declines globally, However, these stressors often co-occur as agricultural intensification and urbanisation cause both habitat alterations, as well as pollutant discharges (Díaz et al. 2019). Tropical regions encompass highly biodiverse amphibian assemblages and are disproportionately impacted by drivers of decline (Hof et al. 2011); however, little is known regarding the effects of habitat loss, habitat degradation or pollution, on tropical amphibians. Apart from mortality caused by chytridiomycosis (e.g. Latin America: Lips et al. 2008), the impacts of other stressors—for example habitat loss/alteration and pollution—on the health of tropical amphibian species are poorly defined (Ghose et al. 2014). To date, only a handful of studies have investigated amphibian biodiversity differences between degraded (agricultural) versus ‘reference’ (non-agricultural) areas, with all reporting lower biodiversity in the agricultural areas (Mexico: Lips et al. 2004; Guatemala: Mendelson et al. 2004; Brazil: Ferrante and Fearnside 2020; Sri Lanka: Rajakaruna et al. 2007; India: Rathod and Rathod 2013). Investigation into intraspecies differences between polluted and non-polluted sites have also been occasionally reported, for example lower acetylcholinesterase activity and genotoxicity in tadpoles (Brazil: Santos et al. 2015; Gonçalves et al. 2019) and in adult frogs (Hegde and Krishnamurthy 2014; Nigeria: Taiwo et al. 2014; India: Hegde et al. 2019) was reported in individuals collected from polluted environments. Additionally, there are reports of undersized frogs from agricultural versus reference environments in India (Hegde and Krishnamurthy 2014) and Thailand (Thammachoti et al. 2012).
There is a greater depth of research into health of wild amphibians from temperate regions, and these have indicated a range of effects on morphology of individuals collected from degraded/polluted versus reference/unpolluted environments. For example, the toads Spea multiplicata, Spea bombifrons and Bufo cognatus were smaller in cultivated versus uncultivated wetlands in the USA (Gray and Smith 2005), and common toads (Bufo bufo) were smaller in an agricultural versus a non-agricultural site in the UK (Orton et al. 2014), although natterjack toads (Epidalea calamita) were reported to be larger from agricultural sites (Zamora-Camacho and Comas 2017). Less commonly, secondary sexual features in males, such as nuptial pad size and/or number, and forelimb width, have been reported to differ in wild amphibians collected from polluted versus reference populations, and both smaller (McCoy et al. 2008; Orton et al. 2014) and larger (Zamora-Camacho and Comas 2017) size has been reported. These features have been shown to have importance to breeding success in laboratory exposed (Silurana tropicalis: Orton et al. 2020) and as well as in wild (Rana luteiventris: Greene and Funk 2009) frogs. In addition, toadspawn from Bufo bufo (Orton and Routledge 2011; Bókony et al. 2018) or Anaxyrus terrestris (Metts et al. 2013) collected from polluted environments (agricultural or coal combustion (metal) contamination) and reared in laboratory ‘clean’ water displayed lower hatching success (Orton and Routledge 2011; Metts et al. 2013) or reduced offspring fitness, characterised by reduced development rates and lower body mass (Bókony et al. 2018). To the authors’ knowledge, neither breeding success (egg number, fertility, hatching success) nor male secondary sexual characteristics (forelimb width, nuptial pad) have been investigated in wild tropical frogs to date.
In this study, we set out to investigate the impacts of agricultural and suburban sites on body size, male secondary sexual characteristics and reproductive success in túngara frogs (Engystomops pustulosus) utilising non-destructive sampling methods. Túngara frogs are leptodactylid frogs (Duellman and Trueb 1994) that are widely distributed across Latin America and the Caribbean (Weigt et al. 2005). They are found in small water bodies where amplectant pairs lay eggs in foam nests (Heyer and Rand 1977), which typically hatch in ~ 48 h (Duellman and Trueb 1994). Túngara frogs are prolonged breeders and exhibit female mate choice resulting in individual males and females forming amplectant pairs (Ryan 1985). They are a well-studied species (e.g. Ryan 1983, 1985, 2010), facilitating comparisons of morphological and reproductive endpoints in new study populations.
Methods
Site characteristics
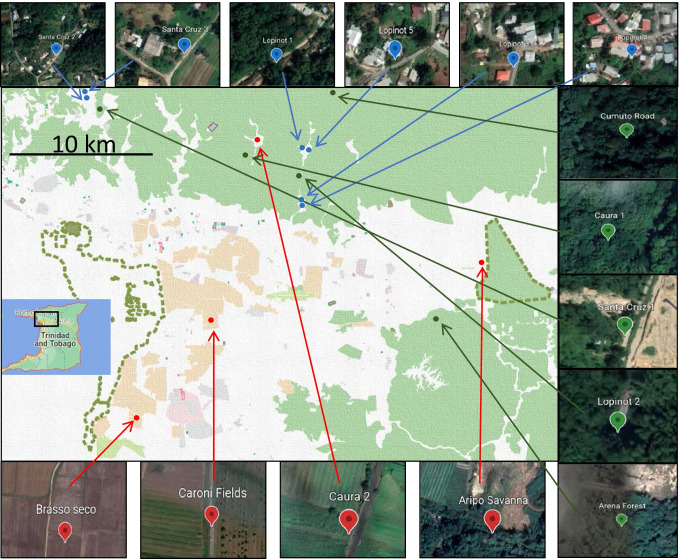
Sites were defined as individual ponds and selection was based on the existence of a túngara frog population, practical considerations (no more than a 2-h drive from laboratory, safety, access), as well as attempting to select approximately equal numbers of each site type (see Fig. 1 for map of sites). As a first step in site characterisation, visual inspection was used (i.e. suburban—ponds in hamlets or outskirts of towns [< 50 m from nearest dwelling]; agricultural—ponds in close proximity to crop fields [< 50 m from nearest crop field]; reference—no houses or crops in close proximity to the pond [> 200 m distance]). Additionally, in 2019, Google Maps was used to investigate whether initial visual site designation tallied with satellite images. For each site, the format of GPS coordinates were converted for compatibility with Google Maps (https://www.gps-coordinates.net/gps-coordinates-converter) and a satellite photo was used to characterise the surrounding environment (presence/absence of crops and houses). All sites were deemed independent, with the two closest sites 559 m apart from each other with a road and/or forest separating the two sites (Santa Cruz 2 and Santa Cruz 3). No differences between initial visual characterisation and that using mapping were observed (see Supplemental Table S1 for site coordinates and Supplemental Figure S1 for individual site images).
Fig. 1.
Map showing site locations and land use (white = buildings, brown = farming land, green = forested, pink/grey = large settlement). Green points = reference sites (2016: Cumuto road (R.CR), Caura 1 (R.C1), Arena Building (R.AB), Lopinot 2 (R.L2); 2018: Santa Cruz 1 (R.SC1)). Blue points = suburban sites (2016: Lopinot 3, 4 (U.L3, U.L4), Lopinot 1 (U.L1); 2018: U.L1, Lopinot 5 (U.L5), Santa Cruz 2, 3 (U.SC2, U.SC3)). Red points = agricultural sites (2016: Brasso Seco (A.BS), Aripo Savanna (A.AS), Caura 2 (A.C2); 2018: Caroni Fields (A.CF))
Since car access was required to carry equipment and samples, all of the sites, including the reference sites, were situated in close proximity to roads (range: < 1–20 m) with varying amounts of traffic. Therefore, the term ‘reference’ is not synonymous with ‘undisturbed’. Data were collected from 15 sites (one site was visited in 2016 and 2018), comprising reference (5 sites: 2016—Cumuto road (R.CR), Caura 1 (R.C1), Arena Building (R.AB), Lopinot 2 (R.L2); 2018—Santa Cruz 1 (R.SC1)), suburban (6 sites: 2016—Lopinot 3 (U.L3), Lopinot 4 (U.L4), Lopinot 1 (U.L1); 2018—U.L1, Lopinot 5 (U.L5), Santa Cruz 2 (U.SC2), Santa Cruz 3 (U.SC3)) and agricultural (4 sites: 2016—Brasso Seco (A.BS), Aripo Savanna (A.AS), Caura 2 (A.C2); 2018—Caroni Fields (A.CF)).
Experimental design
Sampling took place during the rainy season and amplectant pairs of frogs were collected in both years on warm nights when breeding activity was high (June–October/November). On arrival at each sampling site (between 4:00 and 5:00 pm), water quality (temperature, pH, dissolved oxygen [DO], salinity, conductivity, total dissolved solids [TDS]—Hach multimeter) and nitrogenous compounds to assess potential agricultural inputs (nitrate, ammonia—API test kit) basic habitat features (pond description, surrounding vegetation, proximity to houses, crops) and location (GPS) were recorded. All amplectant pairs of túngara frogs that were observed were then collected. Upon collection, each pair was placed in a plastic tub filled with site pond water. Amplectant pairs were transported to the laboratory (University of the West Indies) to deposit foam nests overnight and kept at ambient temperature (i.e. equivalent to outside air temperature, not recorded). The next day, all pairs had separated, and adult males and females were photographed, weighed and snout-vent length (SVL) measured. Forelimb width (FLW) of males was measured using photographs of the forelimb (2016/2018: Adobe Photoshop, see Supplemental Text S1). In 2016, nuptial pad length in males was measured with callipers and in 2018 nuptial pad length was measured from photos (Adobe Photoshop, different methods were used due to an unforeseen error in the data collection: see Supplemental Text S1). Nests were relocated to new tanks containing natal pond water and placed over a white background to facilitate photographs of hatched tadpoles. The foam nests were monitored until hatching of tadpoles (24–48 h after collection). All adults and tadpoles were returned to their natal ponds after breeding status metrics had been recorded, and within 60 h of collection.
Breeding success
After hatching of tadpoles had occurred (24–48 h after collection), the foam nest was removed and gently shaken before being dissected to release any remaining tadpoles. Tanks containing tadpoles were then photographed and the number of tadpoles determined using the cell count function of ImageJ (National Institutes of Health, Bethesda, MD). Comparison of visual and ImageJ tadpole counting was carried out with 10 randomly selected tank photos, with an R2 = 0.96, indicating high similarity between the two methods. Hatching success was determined by counting the number of hatched tadpoles. The number of unhatched eggs was also counted, and egg number (total number of eggs) was calculated by adding together the number of unhatched eggs and the number of hatched tadpoles. Fertility (percentage fertilised) was calculated by dividing the number of hatched tadpoles by egg number (× 100 for percentage).
Chemical analysis
Analysis of agricultural chemicals was undertaken utilising destructive methods. This was justified from an ethical perspective as typically, few tadpoles survive the larval period to complete metamorphosis (e.g. 5%: Calef 1973), and therefore, sacrificing a small proportion of anurans from their larval life stage is unlikely to impact populations. For 5 out of the 10 sites surveyed in 2016 (R.C1 and R.AB (reference), U.L1 and U.L4 (suburban), A.AS (agricultural)), chemical analysis of a pooled sample of whole wild caught tadpoles was undertaken (283 chemicals, detection limits: 0.01 mg/kg). Due to financial constraints, the analysis of chemicals in was not possible in 2018. From each site sampled in 2016, 30 to 50 g of wild caught tadpoles (~ 50–100 tadpoles per site) were collected using a net, with the total sample placed in a 50-ml falcon tube. Tadpole samples were collected approximately 2 weeks after adult pairs had been caught for measurement of the other endpoints. The falcon tubes containing each tadpole sample were immediately placed on dry ice on site, stored in − 80 °C freezer and transported to Almeria, Spain (Laboratorio Analitico Bioclinico) for chemical analysis using gas chromatography–mass spectrometry or liquid chromatography-tandem mass spectroscopy (depending on the analyte). Sampling of biota, instead of water sampling, was chosen in order to detect only the bioavailable fraction of chemicals. In addition, sampling of tadpoles reflected ‘early-life exposure’, which is well known to be critically important to long-term health of vertebrate organisms (e.g. Coe et al. 2010). All analytical methods were optimised for chemicals testing in the tadpole matrix. See Supplemental Text S2 for more details of the analytical methods and Supplemental Tables S2 and S3 for the list of chemicals analysed.
Statistical analysis
Some data were removed prior to analysis: in one case a female escaped (reference: weight and SVL not measured), in another case the female SVL was not measured in error (suburban) and for 12 pairs of frogs, no nest was made (5 reference, 5 suburban, 2 agricultural). The condition of individuals was calculated using a ratio-based condition index (CI: body weight/SVL), which has been shown to be effective for comparing condition between amphibian populations (Labocha et al. 2013). Data ranges for all endpoints in 2016 versus 2018 were compared, and as they showed a high degree of overlap (Table S3), data were pooled in order to maximise sample size. Pooled data were then tested for normality (Kolmogorov–Smirnov test). For data that were not normally distributed (all endpoints except for CI and FLW), analyses for differences between site types were conducted using a Kruskal–Wallis test, followed by a Dunn’s post hoc test (reference compared to suburban or agricultural, multiplicity adjusted p value is reported). For the forelimb width data, a one-way ANOVA was conducted, followed by a Holm-Sidak post hoc test (Bonferroni multiplicity adjusted p value is reported). Additionally, in order to identify if observed differences between sites were confounded by the size of frogs, analyses of covariance (using non-linear regression) were conducted on endpoints where confounding effects may be expected according to the published literature. For females, differences in egg number between sites were analysed with female weight as the covariate (Poisson fit (appropriate for count data), followed by a likelihood ratio test) as these measurements are sometimes reported to be correlated (e.g. Prado and Haddard, 2005). For males, differences in forelimb width/nuptial pad length between sites were analysed with male weight as the covariate (least squares fit, followed by an extra sum of squares F test), as overall male size could influence FLW or nuptial pad length (e.g. Orton et al. 2020). Apart from temperature and nitrate (pooled between years, not normally distributed), differences in water quality between site types were not analysed statistically due to low sample size. All analyses were carried out using GraphPad Prism. p values of < 0.05 were deemed significant.
Results
Field sampling
In total, data were collected from 15 sites (reference: 5 sites—R.CR, R.C1, R.AB, R.L2, R.SC1; suburban: 6 sites—U.L3, U.L4, U.L1, U.L5, U.SC2, U.SC3; agricultural: 4 sites—A.BS, A.AS, A.C2, A.CF), with site U.L1 visited in both years; and water bodies comprised either puddles or drainage ditches (Table 1). Conductivity, salinity and TDS levels were higher at suburban and agricultural sites, compared to the reference site where these measurements were taken, whereas pH values were lower (not analysed statistically, Table 1). Temperature and nitrate levels did not differ between site categories (Kruskal–Wallis, p = 0.15; Table 1).
Table 1.
Characteristics of field sites
| Site | Year | Temp | pH | DO | Sal | Cond | TDS | Nitr | NH3 | Description |
|---|---|---|---|---|---|---|---|---|---|---|
| R.CR | 2016 | 24.5 | 5 | 1 m2 puddle in drainage ditch in side of road. Short grassland on slope above | ||||||
| R.C1 | 2016 | 23.8 | 5 | 2 × 3 m puddle next to abandoned house. Short grassland, 10 m above river | ||||||
| R.AB | 2016 | 24.7 | 10 | Several puddles from heavy machinery tracks in forest opening. Grassland/edge of forest | ||||||
| R.L2 | 2016 | 24.6 | 0 | Along ditch in roadside, ~ 3 m long, narrow puddle. Steep cliffs either side of road, short grass around puddle | ||||||
| R.SC1 | 2018 | 23.45 | 10.3 | 2.38 | 0.06 | 123 | 88 | 2.5 | 2.0 | Long drainage ditch, 30 m along side of road. Grassland/edge of forest |
| Reference sites | 24.21a | 10.3 | 2.38 | 0.06 | 123 | 88 | 4.5a | 2.0 | ||
| U.L3 | 2016 | 24.5 | 5 | 0.5 m2 puddles in roadside drainage ditch. Short grass nearby | ||||||
| U.L4 | 2016 | 23.9 | 0 | Drainage ditch on roadside plus large grass area with 2 m2 puddle. Tall grasses | ||||||
| U.L1 | 2016 | 24.3 | 15 | 4 × 2 m puddle on small road, next to construction. Tall grasses and trees behind | ||||||
| U.L1 | 2018 | 24.2 | 8.67 | 3.26 | 0.06 | 126 | 87 | 0.5 | 0.75 | 4 × 2 m puddle on small road, next to construction. Tall grasses and trees behind |
| U.L5 | 2018 | 25.1 | 9.9 | 2.49 | 0.13 | 280 | 184 | 2.5 | 0.5 | Several 0.5 m2 puddles within a small residential area. Tall grasses nearby |
| U.SC2 | 2018 | 24.9 | 7.2 | 1.86 | 0.12 | 261 | 170 | 0 | 2.0 | 2 × 1 m puddle within 5 m of two residential dwellings. Short grass nearby |
| U.SC3 | 2018 | 24.2 | 6.16 | 1.32 | 0.19 | 405 | 264 | 0 | 0.5 | 2 × 1 m puddle within 5 m of a residential dwellings. Short grass nearby. Trees nearby |
| Urban sites | 24.4a | 7.98a | 2.23a | 0.13a | 268a | 176a | 3.29a | 0.94a | ||
| A.BS | 2016 | 24.1 | 0 | 0.5 m long puddle in shallow dip in short grassland. Crop fields and hedge behind | ||||||
| A.AS | 2016 | 24.5 | 0 | 2 × 1 m puddle, off main road on track. Grassland going into forest, crop fields observed within 5 m | ||||||
| A.C2 | 2016 | 25.0 | 0 | 4 × 1.5 m puddle at the end of a driveway on roadside. At the bottom of a crop fields | ||||||
| A.CF | 2018 | 23.9 | 5.7 | 3.36 | 0.14 | 190 | 123 | 1 | 2.5 | 1 m long drainage ditch beside the road. Crop fields and hedge behind |
| Agricultural sites | 24.4a | 5.7 | 3.36 | 0.14 | 190 | 123 | 0.25a | 2.5 | ||
aMean values. Abbreviations: R.CR, Cumuto road; R.C1, Caura 1; R.AB, Arena Building; R.L2, Lopinot 2; R.SC1, Santa Cruz 1; U.L3, Lopinot 3; U.L4, Lopinot 4; U.L1, Lopinot 1; U.L5, Lopinot 5; U.SC2, Santa Cruz 2; U.SC3, Santa Cruz 3; A.BS, Brasso Seco; A.AS, Aripo Savannah; A.C2, Caura 2; A.CF, Caroni Fields; Sal., salinity; Cond., conductivity; Nitr., nitrate. See Supplemental Table S1 for map of sites and coordinates
In total, 205 frog pairs were collected (2016—63 pairs; 2018—142 pairs, Table 2) from 15 sites (reference = 5, suburban = 6, agricultural = 4, Table 2, Fig. 1); 9 of these sites were visited in 2016 and 5 sites in 2018, with one site visited in both years (U.L1—Lopinot 1). Fewer pairs overall were collected in 2016 (63 out of 205; 31%) versus 2018 (142 out of 205; 69%). The proportion of pairs sampled was broadly similar between different site categories in both 2016 (reference = 27%, suburban = 43%, agricultural = 30%) and in 2018 (reference = 37%, suburban = 39%, agricultural = 25%), as were the total number of amplecting pairs collected in the different site categories (reference = 69; suburban = 82; agricultural = 54).
Table 2.
Summary of sampling effort
| Site | Year | Type | Month | No. visits | Pairs |
|---|---|---|---|---|---|
| R.CR | 2016 | Ref | Jun | 1 | 2 |
| R.C1 | 2016 | Ref | Jun | 1 | 1 |
| R.AB | 2016 | Ref | Jun–Jul | 3 | 8 |
| R.L2 | 2016 | Ref | Jun | 2 | 6 |
| Summary 2016 | Jun–Jul | 7 | 17 | ||
| R.SC1 | 2018 | Ref | Aug–Oct | 5 | 52 |
| Summary 2018 | Aug–Oct | 5 | 52 | ||
| Reference summary | Jun–Oct | 12 | 69 | ||
| U.L3 | 2016 | Urb | Jun–Jul | 2 | 7 |
| U.L4 | 2016 | Urb | Jun–Jul | 4 | 11 |
| U.L1 | 2016 | Urb | Jun–Jul | 3 | 9 |
| Summary 2016 | Jun–Jul | 9 | 27 | ||
| U.L1 | 2018 | Urb | Jul | 2 | 21 |
| U.L5 | 2018 | Urb | Jul | 1 | 1 |
| U.SC2 | 2018 | Urb | Aug–Nov | 3 | 5 |
| U.SC3 | 2018 | Urb | Aug–Nov | 4 | 28 |
| Summary 2018 | Jul–Nov | 10 | 55 | ||
| Suburban summary | Jun–Nov | 19 | 82 | ||
| A.BS | 2016 | Agr | Jun–Jul | 2 | 3 |
| A.AB | 2016 | Agr | Jun–Jul | 5 | 15 |
| C.C2 | 2016 | Agr | Jun | 1 | 1 |
| Summary 2016 | Jun–Jul | 8 | 19 | ||
| A.CF | 2018 | Agr | Oct | 4 | 35 |
| Summary 2018 | Oct | 4 | 35 | ||
| Agricultural summary | Jun–Oct | 12 | 54 | ||
| Complete sampling effort | Jun–Nov | 43 | 205 | ||
Abbreviations: Ref, reference; Urb, suburban; Agr, agricultural; R.CR, Cumuto road; R.C1, Caura 1; R.AB, Arena Building; R.L2, Lopinot 2; R.SC1, Santa Cruz 1; U.L3, Lopinot 3; U.L4, Lopinot 4; U.L1, Lopinot 1; U.L5, Lopinot 5; U.SC2, Santa Cruz 2; U.SC3, Santa Cruz 3; A.BS, Brasso Seco; A.AS, Aripo Savannah; A.C2, Caura 2; A.CF, Caroni Fields
Morphology and breeding success
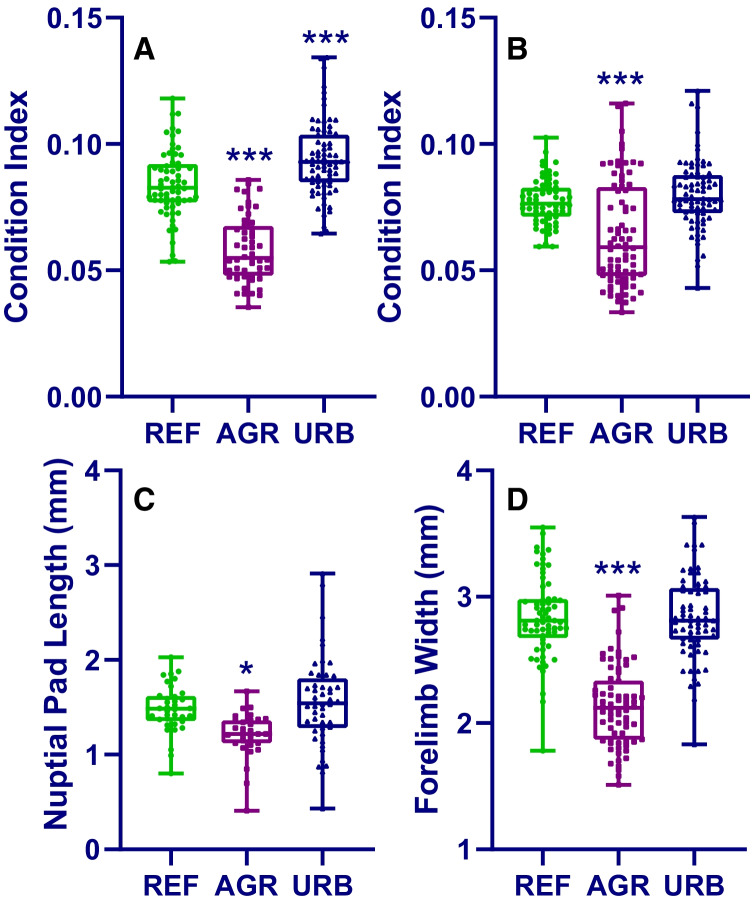
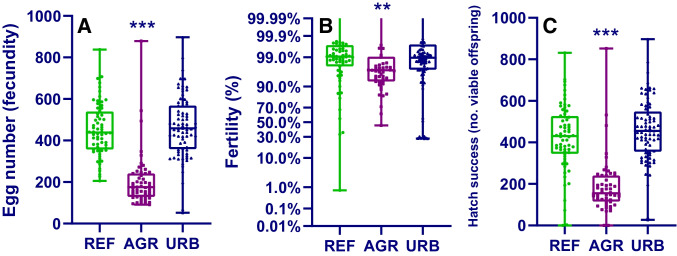
Few differences were observed between frogs collected from reference or suburban site types, with the only significant differences being larger females collected from suburban sites (female weight: 1.15-fold higher; female CI: 1.13-fold higher: Fig. 2, 3 and Table S4). In contrast, all measured endpoints were significantly reduced in frogs collected from agricultural site types compared to those collected from reference site types (Figs. 2, 3 and Table S4, Kruskal–Wallis/ANOVA, Dunn’s/Holm-Sidak, p < 0.026). For morphological characteristics, the magnitude of difference between frogs collected from reference versus agricultural site types ranged from 1.15-fold lower for nuptial pad length to 2.16-fold lower for female weight (Table S4). For female frog morphology, effects ranged from 1.3-fold lower (CI) to 2.16-fold lower (weight), and for male frog morphology, effects ranged from 1.15-fold lower (nuptial pad length) to 1.77-fold lower (weight). For breeding success, the magnitude of difference between frogs collected from reference versus agricultural site types ranged from 1.06-fold lower (fertility) to 2.77-fold lower (hatching success). Therefore, effects were the most pronounced for breeding success, in particular, the number of hatched tadpoles (2.77-fold lower) and fecundity (2.5-fold lower), compared to morphological endpoints (Table S4).
Fig. 2.
Condition index (females: A; males: B), nuptial pad length (C) and forelimb width (D) in túngara frogs collected from reference (REF), agricultural (AGR) and suburban (URB) sites. Box and whisker plots, showing data range (whiskers) and all data values, line represents median value. Statistical significance asterisks are for suburban or agricultural site types, compared to reference site types (*p < 0.05, **p < 0.01, ***p < 0.001)
Fig. 3.
Egg number (A), fertility (B: probability scale on y axis) and hatching success (C) in pairs of túngara frogs collected from reference (REF), agricultural (AGR) and suburban (URB) sites. Box and whisker plots, showing data range (whiskers) and all data values, line represents median value. Statistical significance asterisks are for suburban or agricultural site types, compared to reference site types (*p < 0.05, **p < 0.01, ***p < 0.001)
Analyses of covariance
Analyses of egg number with female weight as the covariate showed significant differences between site types (semi-log, Poisson fit, likelihood ratio test p < 0.0001), indicating that differences in fecundity between site types occurred independently of female size. Analyses of forelimb width and nuptial pad length with male weight as the covariate showed significant differences between site types for forelimb width (linear, least squares fit, extra sum of squares F test p < 0.0001), indicating that this effect occurred independently of male size; but not for nuptial pad length (linear, least squares fit, extra sum of squares F test p = 0.395), indicating that differences between site types for this endpoints were not independent of male weight (for graphical representation of correlations see Supplemental Figure S2).
Chemistry
None of the chemicals analysed in tadpole tissue was detected from any of the sampling sites.
Discussion
The aim of this study was to investigate morphology and reproductive health in túngara frogs collected from reference, suburban and agricultural sites utilising non-destructive methods. Despite the lack of chemicals detection in tadpole tissue, we found that morphology, male secondary sexual characteristics and breeding success were negatively impacted in frogs from agricultural compared to reference site types. Egg number (2.5-fold lower) and hatching success (2.77-fold lower) were the most severely impacted endpoints, with the reduced egg number occurring independently of the smaller female size which was also observed in frogs collected from agricultural sites (2.16-fold lower). Differences observed between reference and agricultural populations were unlikely to be an artefact of sampling effort since data collected from the different site characterisations had similar temporal (sampled during the same months) and numerical (number of sampled frogs) characteristics (Table 2). Since we did not age sampled frogs as this is an invasive process that causes harm and was inconsistent with aims of this work, we cannot rule out that different age of individuals contributed to effects observed. However, túngara frogs do not normally live for longer than 1 year (Ryan, 2010), and therefore, similar demographics between populations are expected. To the authors’ knowledge, this is the first time that male secondary sexual characteristics, egg number, hatching success or fertility in frogs collected from different types of sites have been reported in a tropical amphibian species. The methods outlined here have wide applicability since they are technically simple and cheap, and, importantly, have applicability for use in threatened/declining species since they are non-destructive.
Reduced hatching success has been demonstrated in several temperate species, for example in southern toads (Anaxyrus terrestris) from coal combustion sites in the USA (Metts et al. 2013) and in common toads (Bufo bufo) from an agricultural site in the UK (Orton and Routledge 2011). However, in contrast to our study, hatching success was reported as the proportion of a pre-determined number of collected eggs that successfully hatched, rather than the total reproductive output from a pair of amplecting frogs; so, our results are not directly comparable. Reports of total egg number in temperate species collected from polluted versus unpolluted environments are much more scarce and to the authors’ knowledge no differences have been reported to date (Anaxyrus terrestris, Canada: Metts et al. 2013; Bufo bufo, Hungary: Bókony et al. 2018; Bufo raddei, China: Zhang et al. 2018). With just one known publication that has recorded egg number in agricultural versus reference sites (Bókony et al. 2018), comparisons with the present study are difficult. Furthermore, in that study the difference between the females pre-spawning and post-spawning body mass was used to estimate egg number (rather than counting the number of eggs) so the results are not directly comparable to those presented here. Overall, much more research is needed on egg number in wild amphibians inhabiting a range of different environments to be able to make conclusions regarding the effects of habitat degradation and/or pollution on female reproductive health.
Hatching success was highly correlated with egg number (egg number versus number hatched tadpoles R2 values: reference = 0.97; suburban = 0.99; agricultural = 0.91), strongly indicating that reduced oviposition likely accounted for the reduced hatching success, rather than reduced fertilisation. Albeit significantly reduced in frogs collected from agricultural sites, fertilisation success in all groups was high (reference and suburban = 99%, agricultural = 97%), further indicating that impacts on male reproductive fitness may be comparatively small. Females are classically considered to be the limiting sex in populations, since they require higher levels of investment to produce gametes compared to males (Bateman 1948). It is perhaps surprising, therefore, that egg number has not been analysed more often in studies investigating reproductive health in wild amphibians. Particularly, since this endpoint is commonly used to investigate the reproductive health of wild fish (e.g. Benejam et al. 2010) and the methods for both types of organisms are similar. It was also surprising that egg number was lower in frogs collected from agricultural sites independently from female weight, since these endpoints are normally assumed to be highly correlated. This lack of correlation may be an artefact of the study design, as weights used for correlations were post-breeding values (it was not possible to record pre-spawning female weight without disrupting breeding). On the other hand, the lack of correlation between female weight and fecundity has previously been reported for another foam building species in the Leptodactylidae family (Leptodactylus fuscusa: Prado and Haddad 2005), providing tentative evidence that for foam building species, female size may not be an important determinator for egg number; especially as this correlation was observed for the other 6 species investigated in that study (Prado and Haddad 2005).
In addition to effects on breeding success, body condition of male and female frogs collected from agricultural sites were smaller compared to those collected from reference sites (fold-difference: female − 1.3/male − 1.16). For both male and females, the recorded SVL of frogs collected from the reference and suburban sites were within the range of those reported previously from forested areas (e.g. Ryan 1983), suggesting that frogs collected from the agricultural sites were undersized. Similarly to our results, in other tropical amphibians, common frogs (Fejervarya limnocharis) from agricultural sites in India (Hegde and Krishnamurthy 2014) and Thailand (Thammachoti et al. 2012) were reported to have a lower condition factor than frogs from a reference environment. By contrast, body size did not differ in the temperate species Bufo raddei (China: Zhang et al. 2018) or Anaxyrus terrestris (USA: Metts et al. 2013) in heavy metal contaminated versus reference sites, whereas natterjack toads (Epidalea calamita) from agricultural sites were larger than those from reference sites (Zamora-Camacho and Comas 2017). It is difficult to hypothesise regarding this apparent difference in size and/or condition between tropical and temperate species due to the limited data set, large range of different landscape types investigated and large geographic area across these studies. Male secondary sexual characteristics were also smaller in frogs from agricultural sites, and for forelimb width, this occurred independently from male weight. To date, we could find no reports of measuring these features in tropical species, however, forelimb width is controlled by androgens (Dorlochter et al. 1994) and has been shown to be reduced upon laboratory exposure to an anti-androgenic herbicide (linuron: Orton et al. 2018), and therefore, the smaller forelimb width in these frogs could possibly have been due to the presence of endocrine disrupting pollutants (for review see Orton and Tyler 2015). However, in the absence of detection of any suspected endocrine disrupting contaminants in tadpole samples, it is unknown if this explanation is likely or not.
We cannot assign observed effects to chemicals exposure, since we did not detect any of the selected chemicals in tadpole tissue. We could find no examples in the literature of chemicals being analysed in tadpoles for comparison; however, studies in adult frogs have reported relatively low detection rates of chemicals (17 out of 98: Smalling et al. 2015; 4 out of 98: Swanson et al. 2018), perhaps due to biotransformation. There is evidence that some of the pesticides we measured (paraquat, cypermethrin, glyphosate/aminomethylphosphonic acid (AMPA), profenofos) have been used in the area of Trinidad where our field sites were located (Hroudova 2012), so we may expect these chemicals to be detected. For glyphosate/AMPA, it has previously been reported that simultaneous analysis of water and frog tissue resulted in detection in the water sample only (Smalling et al. 2015); therefore, at least for these chemicals absence of detection may not accurately represent absence from the water body. Chemicals may also have been present at levels that were below the detection limit of the instruments (our detection limits were relatively high (10–50 µg/kg versus, e.g. 0.5–4.2 µg/kg: Smalling et al. 2015; Swanson et al. 2018)) and it is well known that the combined effects of low concentrations of chemical mixtures elicit effects on biota, including in amphibians (Relyea 2004; Hayes et al. 2006). However, in the absence of detected chemicals this is a hypothesis only. Beyond chemical contamination, agricultural environments are likely to have larger temperature fluctuations due to shallower pools and less shading, altered abiotic factors such as pH, dissolved oxygen and total dissolved solids, reduction in food availability and/or alterations to community dynamics (Mann et al. 2009) and interactions between environmental stressors are known to negatively impact amphibian health (Relyea 2003). Finally, since our morphological data derived from adult frogs, a temporal mismatch occurred between the biological collected from adult frogs and the tadpoles collected for chemical analysis. Therefore, it is not known if the sampled adult frogs experienced a distinct early life exposure (i.e. the previous year) compared to the sampled tadpoles (i.e. due to low site fidelity or changes in sites over time).
In conclusion, we observed significant differences between frogs collected in agricultural and reference sites with respect to morphological and reproductive endpoints, the first time that this has been reported in a tropical species. Since tropical amphibians are under threat on a global scale, this finding has significance for conservation of amphibians as species producing fewer offspring over a given time span are fundamentally at increased risk of decline (Owens and Bennett 2000). Despite evidence linking reduced reproduction and population decline in other species associated with freshwater environments (piscivorous birds, freshwater fish and alligators: see Bernanke and Köhler 2009), this evidence is currently lacking for amphibians. The methods outlined here have potential to be utilised to investigate these linkages as they are simple, cheap, informative and in the case of reproductive success, can be directly linked to population stability. Finally, tropical species may be well suited to these types of analyses since the assessment of breeding success is relatively undemanding due to the lower time required for hatching in many species compared to temperate species (< 72 h vs. 10–20 days: Duellman and Trueb 1994), resulting in reduced time and cost requirements.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to acknowledge the key contributions made by Gervonne Barran and Kent Kadoo as field assistants for the collection of the 2018 datasets, and the University of the West Indies for providing lab space in which to conduct our study. We would like to thank Prof Roger Downie of Glasgow University for facilitating the data collection in 2016 and Nigel Noriega of Sustainable Innovation Initiatives for facilitating the data collection in 2018. Finally, we would like to thank the comments of two anonymous reviewers who contributed to the improvement of this manuscript.
Author contribution
F. Orton conceived and designed experiments, carried out statistical analyses and wrote the manuscript. S. Mangan (2016) and A. Marianes (2018) collected the data. L. Newton organised the data and performed analyses of photos. All authors commented on the draft.
Funding
This work was funded by the University of the West of Scotland.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
As no adult animals were harmed in the collection of these data, ethical approval was not required to carry out the analyses of breeding/morphology. For the tadpole collection, ethical approval was not sought, but not required under Trinidadian/University of the West Indies guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bateman AJ. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- Benejam L, Benito J, García-Berthou E. Decreases in condition and egg number of freshwater fishes in a highly polluted reservoir. Water Air Soil Pollut. 2010;210:231–242. doi: 10.1007/s11270-009-0245-z. [DOI] [Google Scholar]
- Bernanke J, Köhler H-R (2009) The impact of environmental chemicals on wildlife vertebrates. In: Whitacre D (ed) Reviews of environmental contamination and toxicology. Vol 198-Reviews of environmental contamination and toxicology. Springer New York, pp 1–47 [DOI] [PubMed]
- Bókony V, Üveges B, Ujhegyi N, Verebélyi V, Nemesházi E, Csíkvári O, Hettyey A. Endocrine disruptors in breeding ponds and reproductive health of toads in agricultural, urban and natural sites. Sci Total Environ. 2018;634:1335–1345. doi: 10.1016/j.scitotenv.2018.03.363. [DOI] [PubMed] [Google Scholar]
- Calef GW. Natural mortality of tadpoles in a population of Rana aurora. Ecology. 1973;54(4):741–758. doi: 10.2307/1935670. [DOI] [Google Scholar]
- Coe TS, Soffker MK, Filby AL, Hodgson D, Tyler CR. Impacts of early life exposure to estrogen on subsequent breeding behavior and reproductive success in zebrafish. Environ Sci Technol. 2010;44:6481–6487. doi: 10.1021/es101185b. [DOI] [PubMed] [Google Scholar]
- Díaz SJ, Settele ES, Brondízio E.S., Ngo HT, Guèze M, Agard J, Arneth A, Balvanera P, Brauman KA, Butchart SHM, Chan KMA, Garibaldi LA, Ichii K, Liu J, Subramanian SM, Midgley GF, Miloslavich P, Molnár Z, Obura D, Pfaff A, Polasky S, Purvis A, Razzaque J, Reyers B, Roy Chowdhury R, Shin YJ, Visseren-Hamakers IJ, Willis KJ, Zayas CN (2019) IPBES: global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Status and Trends - Drivers of change, Bonn, Germany
- Dorlochter M, Astrow SH, Herrera AA. Effects of testosterone on a sexually dimorphic frog muscle: repeated in vivo observations and androgen receptor distribution. J Neurobiol. 1994;25:897–916. doi: 10.1002/neu.480250802. [DOI] [PubMed] [Google Scholar]
- Duellman WE, Trueb L. Biology of amphibians. Baltimore and London: John Hopkins University Press; 1994. [Google Scholar]
- Ferrante L, Fearnside PM. Evidence of mutagenic and lethal effects of herbicides on Amazonian frogs. Acta Amazon. 2020;50:363–366. doi: 10.1590/1809-4392202000562. [DOI] [Google Scholar]
- Ghose SL, Donnelly MA, Kerby J, Whitfield SM. Acute toxicity tests and meta-analysis identify gaps in tropical ecotoxicology for amphibians. Environ Toxicol Chem. 2014;33:2114–2119. doi: 10.1002/etc.2665. [DOI] [PubMed] [Google Scholar]
- Gonçalves MW, de Campos CBM, Godoy FR, Gambale PG, Nunes HF, Nomura F, Bastos RP, da Cruz AD, de Melo e Silva D. Assessing genotoxicity and mutagenicity of three common amphibian species inhabiting agroecosystem environment. Arch Environ Contam Toxicol. 2019;77:409–420. doi: 10.1007/s00244-019-00647-4. [DOI] [PubMed] [Google Scholar]
- Gray MJ, Smith LM. Influence of land use on postmetamorphic body size of playa lake amphibians. J Wildlife Management. 2005;69:515–524. doi: 10.2193/0022-541x(2005)069[0515:ioluop]2.0.co;2. [DOI] [Google Scholar]
- Greene AE, Funk WC. Sexual selection on morphology in an explosive breeding amphibian, the Columbia spotted frog (Rana luteiventris) J Herpetol. 2009;43:244–251. doi: 10.1670/08-112R.1. [DOI] [Google Scholar]
- Hayes TB, Case P, Chui S, Duc C, Haeffele C, Haston K, Lee M, Vien Phoung M, Marjuoa Y, Parker J, Tsui M. Pesticide mixtures, endocrine disruption, and amphibian declines: are we underestimating the impact? Environ Health Perspect. 2006;114:40–50. doi: 10.1289/ehp.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde G, Krishnamurthy SV. Analysis of health status of the frog Fejervarya limnocharis (Anura: Ranidae) living in rice paddy fields of Western Ghats, using body condition factor and AChE content. Ecotoxicol Environ Contam. 2014;9:69–76. [Google Scholar]
- Hegde G, Krishnamurthy SV, Berger G. Common frogs response to agrochemicals contamination in coffee plantations, Western Ghats, India. Chem Ecol. 2019;35:397–407. doi: 10.1080/02757540.2019.1584613. [DOI] [Google Scholar]
- Heyer WR, Rand AS. Foam nest construction in the leptodactylid frogs Leptodactylus pentadactylus and Physalaemus pustulosus (Amphibia, Anura, Leptodactylidae) J Herpetol. 1977;11:4. doi: 10.2307/1563148. [DOI] [Google Scholar]
- Hof C, Araújo M, Jetz W, Rahbek C. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature. 2011;480:516–519. doi: 10.1038/nature10650. [DOI] [PubMed] [Google Scholar]
- Hroudova K (2012) Assessing residents’ attitude and practices of pesticide use in Arip sub-catchment, Trinidad and Tobago. University of the West indies, Trinidad and Tobago. http://hdl.handle.net/2139/43853
- Labocha MK, Schutz H, Hayes JP. Which body condition is best? Oikos. 2013;123:111–119. doi: 10.1111/j.1600-0706.2013.00755.x. [DOI] [Google Scholar]
- Lips KR, Diffendorfer J, Mendelson JR, Sears MW. Riding the wave: reconciling the roles of disease and climate change in amphibian declines. PLoS Biol. 2008;6:441–454. doi: 10.1371/journal.pbio.0060072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips KR, Mendelson JR, Munoz-Alonso A, Canseco-Marquez L, Mulcahy DG. Amphibian population declines in montane southern Mexico: resurveys of historical localities. Biol Cons. 2004;119:555–564. doi: 10.1016/j.biocon.2004.01.017. [DOI] [Google Scholar]
- Mann RM, Hyne RV, Choung CB, Wilson SP. Amphibians and agricultural chemicals: review of the risks in a complex environment. Environ Poll. 2009;157:2903–2927. doi: 10.1016/j.envpol.2009.05.015. [DOI] [PubMed] [Google Scholar]
- McCoy KA, Bortnick LJ, Campbell CM, Hamlin HJ, Guillette LJ, St. Mary CM (2008) Agriculture alters gonadal form and function in the toad Bufo marinus. Environ Health Perspect 116:1526-1532 [DOI] [PMC free article] [PubMed]
- Mendelson JR, Brodie ED, Malone JH, Acevedo ME, Baker MA, Smatresk NJ, Campbell JA. Factors associated with the catastrophic decline of a cloudforest frog fauna in Guatemala. Rev Biol Trop. 2004;52:991–1000. [PubMed] [Google Scholar]
- Metts BS, Buhlmann KA, Tuberville TD, Scott DE, Hopkins WA. Maternal transfer of contaminants and reduced reproductive success of southern toads (Bufo [Anaxyrus] terrestris) exposed to coal combustion waste. Environ Sci Technol. 2013;47:2846–2853. doi: 10.1021/es303989u. [DOI] [PubMed] [Google Scholar]
- Orton F, Routledge E. Agricultural intensity in ovo affects growth, metamorphic development and sexual differentiation in the common toad (Bufo bufo) Ecotoxicol. 2011;20:901–911. doi: 10.1007/s10646-011-0658-5. [DOI] [PubMed] [Google Scholar]
- Orton F, Baynes A, Clare F, Duffus AJ, Larroze S, Scholze M, Garner TJ (2014) Body size, nuptial pad size and hormone levels: potential non-destructive biomarkers of reproductive health in wild toads (Bufo bufo). Ecotoxicol 1–7. 10.1007/s10646-014-1261-3 [DOI] [PubMed]
- Orton F, Tyler CR. Do hormone-modulating chemicals impact on reproduction and development of wild amphibians? Biol Rev. 2015;90:1100–1117. doi: 10.1111/brv.12147. [DOI] [PubMed] [Google Scholar]
- Orton F, Säfholm M, Jansson E, Carlsson Y, Eriksson A, Fick J, Uren Webster T, McMillan T, Leishman M, Verbruggen B, Economou T, Tyler CR, Berg C. Exposure to an anti-androgenic herbicide negatively impacts reproductive physiology and fertility in Xenopus tropicalis. Sci Reps. 2018;8:9124. doi: 10.1038/s41598-018-27161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orton F, Svanholm JE, Carlsson Y, Eriksson A, Uren Webster T, McMillan T, Leishman M, Verbruggen B, Economou T, Tyler CR, Berg C. A laboratory investigation into features of morphology and physiology for predicting reproductive fitness in male frogs. PLoS ONE. 2020;15:e0241625. doi: 10.1371/journal.pone.0241625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens IPF, Bennett PM. Ecological basis of extinction risk in birds: Habitat loss versus human persecution and introduced predators. PNAS. 2000;97:12144–12148. doi: 10.1073/pnas.200223397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado CPA, Haddad CFB. Size-egg number relationships and reproductive investment in female frogs in the Pantanal, south-western Brazil. Herpetological J. 2005;15:181–189. [Google Scholar]
- Rajakaruna RS, Samarawickrama VAMPK, Ranawana KB. Amphibian declines and possible etiologies: the case for Sri Lanka. J Natl Sci Found. 2007;35:3–8. [Google Scholar]
- Rathod S, Rathod P. CEPF Western Ghats special series: amphibian communities in three different coffee plantation regimes in the Western Ghats, India. J Threatened Tax. 2013;5:4404–4413. doi: 10.11609/JoTT.o3054.4404-13. [DOI] [Google Scholar]
- Relyea RA. Predator cues and pesticides: a double dose of danger for amphibians. Ecol Appl. 2003;13:1515–1521. doi: 10.2307/4134758. [DOI] [Google Scholar]
- Relyea RA. Growth and survival of five amphibian species exposed to combinations of pesticides. Environ Toxico Chem. 2004;23:1737–1742. doi: 10.1897/03-493. [DOI] [PubMed] [Google Scholar]
- Ryan MJ. Sexual selection and communication in a neotropical frog, Physalaemus Pustulosus. Evolution. 1983;37:8. doi: 10.1111/j.1558-5646.1983.tb05536.x. [DOI] [PubMed] [Google Scholar]
- Ryan MJ. The tungara frog: a study in sexual selection and communication. Chicago: The University of Chiago Press; 1985. [Google Scholar]
- Ryan MJ (2010) Túngara frog: a model for sexual selection and communication. In Encyclopedia of animal behavior (second edition), Academic Press. Eds. Breed, Michael D. Moore, Janice. Pages 453–461
- Santos TG, Melo R, Costa-Silva DG, Nunes MEM, Rodrigues NR, Franco JL. Assessment of water pollution in the Brazilian Pampa biome by means of stress biomarkers in tadpoles of the leaf frog Phyllomedusa iheringii (Anura: Hylidae) PeerJ. 2015;3:e1016. doi: 10.7717/peerj.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalling KL, Reeves R, Muths E, Vandever M, Battaglin WA, Hladik ML, Pierce CL. Pesticide concentrations in frog tissue and wetland habitats in a landscape dominated by agriculture. Sci Tot Environ. 2015;502:80–90. doi: 10.1016/j.scitotenv.2014.08.114. [DOI] [PubMed] [Google Scholar]
- Swanson JE, Muths E, Pierce CL, Dinsmore SJ, Vandever MW, Hladik ML, Smalling KL. Exploring the amphibian exposome in an agricultural landscape using telemetry and passive sampling. Sci Reps. 2018;8:1–10. doi: 10.1038/s41598-018-28132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiwo IE, Henry AN, Imbufe AP, Adetoro OO. Heavy metal bioaccumulation and biomarkers of oxidative stress in the wild African tiger frog, Hoplobatrachus occipitalis. Afr J Environ Sci Technol. 2014;8:6–15. doi: 10.5897/AJEST2013.603. [DOI] [Google Scholar]
- Thammachoti P, Knonsue W, Kitana J, Varanusupakul P, Kitana N. Morphometric and gravimetric parameters of the rice frog Fejervarya limnocharis living in areas with different agricultural activity. J Environ Prot. 2012;3:1403–1408. doi: 10.4236/jep.2012.310159. [DOI] [Google Scholar]
- Weigt LA, Crawford AJ, Rand AS, Ryan MJ. Biogeography of the túngara frog, Physalaemus pustulosus: a molecular perspective. Mol Ecol. 2005;14:3857–3876. doi: 10.1111/j.1365-294X.2005.02707.x. [DOI] [PubMed] [Google Scholar]
- Zamora-Camacho FJ, Comas M. Greater reproductive investment, but shorter lifespan, in agrosystem than in natural-habitat toads. PeerJ. 2017;5:e3791. doi: 10.7717/peerj.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Guo R, Ai S, Yang Y, Ding J, Zhang Y. Long-term heavy metal pollution varied female reproduction investment in free-living anura, Bufo raddei. Ecotoxicol Environl Safety. 2018;159:136–142. doi: 10.1016/j.ecoenv.2018.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.