Abstract
Background:
Children with severe asthma have substantial morbidity and healthcare utilization. Pediatric severe asthma is a heterogeneous disease, and a multidisciplinary approach can improve the diagnosis and management of these children.
Methods:
We reviewed the electronic health records for patients seen in the Severe Asthma Clinic (SAC) at UPMC Children’s Hospital of Pittsburgh between August 2012 and October 2019.
Results:
Of the 110 patients in whom we extracted data, 46% were female, 48% were Black/African American, and 41% had ≥1 admission to the pediatric intensive care unit (PICU) for asthma. Compared to patients without a PICU admission, those with ≥1 PICU admission were more likely to be non-White (64.4% vs 41.5%, P=0.031) and more atopic (eosinophil count geometric mean= 673 vs 319 cells/mm3, P=0.002; total IgE geometric mean=754 vs 303 KU/L, P=0.003), and to have lower pre-bronchodilator FEV1 (58.6% [±18.1%] vs 69.9% [±18.7%], P=0.002) and elevated FeNO (60% vs 22%, P=0.02). In this cohort, 84% of patients were prescribed high-dose ICS/LABA and 36% were on biologics. Following enrollment in the SAC, severe exacerbations decreased from 3.2/year to 2.2/year (P<0.0001); compared to the year prior to joining the SAC, in the following year the group had 106 fewer severe exacerbations.
Conclusions:
This large cohort of children with severe asthma had a high level of morbidity and healthcare utilization. Patients with a history of PICU admissions for asthma were more likely to be non-White and highly atopic, and to have lower lung function. Our data supports a positive impact of a multidisciplinary clinic on patients with severe childhood asthma.
Keywords: Severe asthma, severe childhood asthma, multidisciplinary clinic
INTRODUCTION
Pediatric severe asthma is a heterogenous disease phenotype that affects a relatively small but vulnerable population at high-risk for adverse outcomes. Most definitions of severe asthma include poor symptom control despite high dose treatment1. Severe asthma is generally classified as difficult to treat vs. truly severe therapy-resistant asthma. The former includes cases in which symptoms are poorly controlled because of non-adherence, comorbidities, poor inhaler technique, or other “extrinsic causes”, rather than the severe underlying pathobiology of the latter2,3. The prevalence of severe asthma is estimated to be up to 5-10% within the asthmatic population1,3, but the accuracy of this estimate is limited by the multiple definitions of the phenotype.
Patients with severe asthma incur higher health care utilization and costs, and thus improving the diagnosis and management of this condition is a critical healthcare need4,5. Multidisciplinary care for complex medical issues has been well described in the pain literature6 but has yet to be widely adopted for severe childhood asthma despite some supportive findings7-12. A multidisciplinary clinic provides a standardized approach for the management of children with severe asthma, as recommended by current guidelines1,7,13.
To better understand and care for our pediatric patients with difficult-to-treat and severe asthma, we created a multidisciplinary Severe Asthma Clinic (SAC) by bringing together an Allergist and a Pediatric Pulmonologist. Herein we describe the SAC population and show the significant burden this disease represents to patients, their families and the health system. We also evidence some of the disparities that exist in terms of disease severity and control, and identify potential risk factors for more severe disease. We thereby highlight the need for a better understanding of this heterogeneous disease and the need to develop both standardized yet personalized evaluation and treatment approaches, which can be achieved through a multidisciplinary clinic.
METHODS
The SAC at the University of Pittsburgh Medical Center (UPMC) Children’s Hospital of Pittsburgh (CHP) was started in 2012. We reviewed the Electronic Health Records (EHR) for all patients seen in the SAC between August 2012 and October 2019. The SAC includes patients referred by pulmonologists, allergists, and other providers in the system due to asthma severity, frequent severe exacerbations, and/or frequent acute health care utilization, usually including patients with ≥1 hospitalization or emergency department (ED) visit for asthma in the previous year. Most patients meet the ATS/ERS criteria for severe asthma1, and they may also be referred due to difficult-to-treat disease that requires close follow-up. As such, the clinic reflects a real-life, vulnerable patient population. The Institutional Review Board of the University of Pittsburgh waived informed consent and approved the retrospective analysis/chart review of patients in the clinic.
We recorded the age, sex, race/ethnicity, and body mass index percentile (BMI), categorized as underweight (<5th percentile), healthy (5th-85th percentile), overweight (85th-95th percentile) and obese (>95th percentile) of SAC patients at their first visit. Height and weight are measured in the clinic by trained technicians and respiratory therapists using standard equipment (Seca-216 wall-mounted stadiometer, Seca North America, Mount Pleasant, SC; and Befour MX810 scale, Befour Inc., Saukville, WI) following standard protocols as per ATS/ERS guidelines14. Therapists ensure patients are not wearing shoes or head pieces, or heavy clothing and other apparel that may alter height or weight measurements. Certified respiratory therapists perform lung function testing following ATS/ERS guidelines14. Spirometry is performed in clinic using Pneumotrac 6800 spirometers (Vitalograph Inc., Lenexa, KS). For the period covered in this report, our clinical pulmonary function test (PFT) lab used Hankinson / NHANES III reference equations for children >8 years old15 and Wang / Dockery equations for 5-7 year-olds16. Per standard PFT lab protocol, bronchodilator response (BDR) was evaluated 15 minutes after the administration of 2 puffs (180mcg) of albuterol (salbutamol), and expressed as percent change of baseline FEV1. For each patient, we recorded the lowest FEV1 percent predicted (FEV1pp) before and after bronchodilator administration, their BDR, and the fractional exhaled nitric oxide (FeNO, obtained at the same clinic visit as the FEV1pp) during the period of the SAC follow-up. A positive BDR was defined as an increase in FEV1 of ≥12% from baseline. If the FeNO and FEV1pp were measured at different times, the highest FeNO was chosen. FeNO was categorized as low (<20 ppb), intermediate (21-35 ppb), and high (>35 ppb) as per ATS standards17. The uppermost values for total IgE and absolute eosinophil count were collected for each child, which in some cases preceded the start of the SAC; however, all those patients were being followed by an Allergist or Pulmonologist within CHP. We abstracted data from chest CT scans and flexible bronchoscopies performed prior to or as part of the referral to the SAC. We also abstracted from the EHR data on trials of biologics (omalizumab, mepolizumab, benralizumab, and dupilumab), systemic (oral or intramuscular) steroids, severe asthma exacerbations (defined as an ED visit, hospitalization, or oral steroid course, two years before and one year after the patient’s first SAC visit), admissions to the pediatric intensive care unit (PICU) for asthma (along with the type of respiratory support required), other subspecialty care, and comorbid diagnoses.
Statistical Analysis
Our outcomes of interest were: 1) having at least one PICU admission for asthma; 2) the lowest pre-bronchodilator FEV1 measured at the SAC; and 3) the number of severe asthma exacerbations. Categorical variables are shown as count (percentage) and continuous variables are shown as mean (standard deviation) or median [interquartile range (IQR)], as appropriate. For continuous variables, bivariate analyses were performed using two-tailed t-tests (for normally distributed variables) or Mann-Whitney two-sample rank sum tests (for non-normally distributed variables). Consistent with prior reports in the literature, total IgE and eosinophil counts were log10-transformed for analysis; however, for clarity we report geometric means and show the original values in the figures (rather than log10-transformed values). Categorical variables were compared using Pearson Chi-square tests or, if cell counts <5, using Fisher’s Exact tests. The difference in severe exacerbations before and after joining the clinic was assessed using the Wilcoxon signed rank test. Multivariable analyses were performed using logistic regression for binary outcomes (e.g., PICU admission) or linear regression for continuous outcomes (e.g., FEV1), adjusting for age, sex, and race/ethnicity. For PICU admissions, we additionally constructed a second multivariable model that included all covariates associated with PICU admission, and retained those that remained significant at P<0.05. All analyses were performed using STATA v.15 (StataCorp, College Station, TX).
RESULTS
The Severe Asthma Clinic (SAC)
The SAC is staffed by a pediatric pulmonologist and a pediatric allergist, two nurse practitioners, two nurses with a primary interest in asthma, and a team of respiratory therapists. The group meets every month to review cases and quality improvement initiatives. Children must be 5 years or older to be referred into the clinic, though exceptions are made for severely ill younger patients. The clinic takes place four half-days per month, but some patients are additionally followed in the pulmonary and allergy clinics as needed. As described, most patients meet ATS/ERS criteria for severe asthma while others are referred due to the need for very frequent follow-up. New patients are seen by both the pulmonologist and allergist; return patients typically have alternating visits between specialists.
General characteristics
A total of 110 patients were followed in the SAC from August 2012 to October 2019 and had EHR data available for extraction (Table 1). Of these 110 patients, 51 (46%) were female; 54 (49%) were White, 53 (48%) Black/African American, and 3 (3%) of other races/ethnicities; 52 (47%) were overweight or obese; and 17 (15.6%) were born at ≤36 weeks gestational age (including five born at ≤32 weeks). The average age of patients at their first SAC visit was 10 (SD=4.1) years.
Table 1 –
Baseline characteristics of the Severe Asthma Clinical Cohort
| N= 110 | |
|---|---|
| Age at first clinic visit | 10.4 ± 4.1 |
| Sex | |
| - Female | 51 (46.4%) |
| - Male | 59 (53.6%) |
| Race | |
| - White | 54 (49.1%) |
| - Black/ African American | 53 (48.2%) |
| - Other | 3 (2.7%) |
| History of PICU admission | 45 (40.9%) |
| BMI percentile | 71.4 ± 29.0 |
| Weight category | |
| - Underweight (BMI <5th pct) | 6 (5.5%) |
| - Healthy weight (BMI 5th to <85th pct) | 52 (47.3%) |
| - Overweight (BMI 85th to <95th pct) | 22 (20.0%) |
| - Obese (BMI ≥ 95th pct) | 30 (27.3%) |
| Gestational age | |
| - < 32 weeks | 5 (4.5%) |
| - 32-36 weeks | 12 (10.9%) |
| - ≥ 37 weeks | 89 (80.9%) |
| - Unknown | 4 (3.6%) |
| Comorbidities | |
| - Eczema | 60 (54.6%) |
| - Allergic rhinitis | 94 (85.5%) |
| - Food allergy | 30 (27.3%) |
| - GERD | 17 (15.5%) |
| - VCD | 14 (12.7%) |
| - Anxiety | 21 (19.1%) |
| - Depression | 10 (9.1%) |
| - ADHD | 9 (8.2%) |
| - OSA | 8 (7.3%) |
| Pulmonary function | |
| - Pre-bronchodilator FEV1pp | 65.3 ± 19.1 |
| - Post-bronchodilator FEV1pp | 78.5 ± 21.3 |
| - Bronchodilator response (BDR) | 20.1 ± 10.4 |
| - FeNO (ppb)* | 28.5 [16-56] |
| Atopy biomarkers | |
| - Total IgE (IU/mL)* | 556 [214-1325] |
| - Eosinophil counts (cells/mm3)* | 440 [200-810] |
Median [IQR]. ADHD: Attention deficit and hyperactivity disorder. BDR: Bronchodilator response. BMI: Body mass index. FeNO: Fractional exhaled nitric oxide. GERD: Gastroesophageal reflux disease. OSA: Obstructive sleep apnea. PICU: Pediatric intensive care unit. VCD: Vocal cord dysfunction.
Pulmonary function
All patients had reproducible and interpretable spirometry data. Values for lowest pre-bronchodilator and post-bronchodilator FEV1 (as % predicted) are shown in Figure 1, mean lowest FEV1pp was 65.3% (SD=19.1%). Ninety-two percent of patients had BDR data, with mean post-bronchodilator FEV1pp=78.5% (SD=21.3%); average BDR was 20.1% (SD=10.4%), and seventy-three percent of children had a positive BDR. Forty-two children had FeNO data; median was 28.5 ppb [IQR=16-55 ppb], and ~35% had high FeNO.
Figure 1 – Baseline lung function of Severe Asthma Clinic (SAC) cohort.
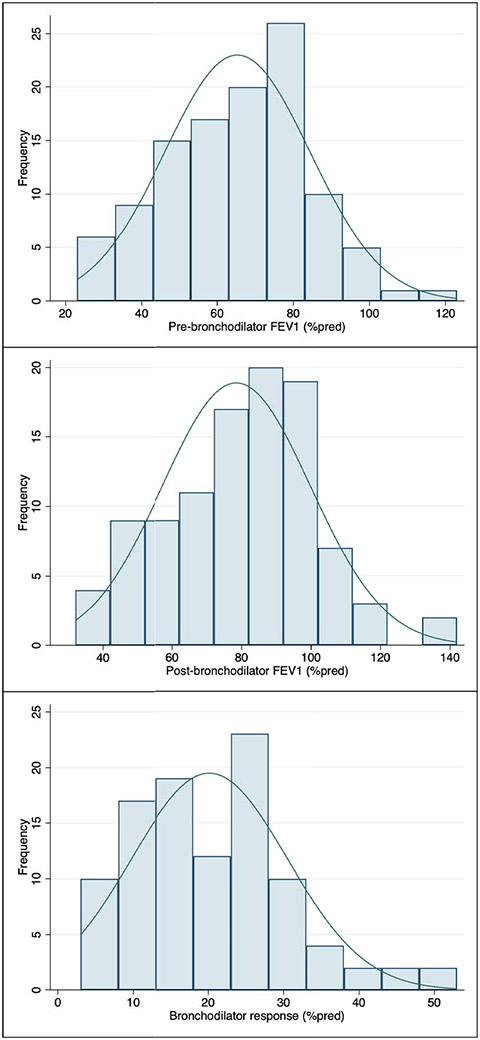
Figure shows the baseline lowest FEV1 as percent-of-predicted (FEV1pp) upon clinic entry, before and after bronchodilator administration. Bronchodilator response (BDR) defined as percent change from pre-bronchodilator FEV1.
Atopy and allergic sensitization
In our cohort, 86% of patients had concurrent allergic rhinitis and 55% had eczema. Median eosinophil count was 440 cells/mm3 (IQR=200-810) (Figure 2). Median total IgE was 556 IU/mL (IQR=214-1325), and 39% of patients had IgE levels >700 IU/mL. Of the 110 children, 77 (70%) had ≥3 positive skin tests or specific IgEs to environmental allergens, while 16 (15%) children were not sensitized to any environmental allergen. Three children had an elevated total IgE without any positive allergen-specific IgE, prompting testing for parasitic disease; two of them had positive antibodies for Toxocara and one for Strongyloides, and were treated accordingly.
Figure 2 – Atopy biomarkers in SAC cohort.
Eosinophil counts (cells/μL) and total IgE (IU/mL) were highly skewed, as seen in the figure, and therefore were log10-transformed for analysis. Results in the text are reported as medians and interquartile ranges (IQR) or as geometric means.
Other comorbidities
In addition to eczema, allergic rhinitis, and obesity, the most common comorbidities were food allergies (27%), gastroesophageal reflux disease (GERD, 15.5%), vocal cord dysfunction (VCD, 12.7%), anxiety (19%), depression (9%), attention deficit hyperactivity disorder (ADHD, 8%), and obstructive sleep apnea (OSA, 7%) (Table 1). Forty one percent of the children saw ENT for sinus disease and or adenoid/tonsillar evaluation, and 10% saw Endocrinology for chronic steroid use (including 11 patients with adrenal insufficiency). One child had selective IgA deficiency, one had steroid-resistant nephrotic syndrome and pulmonary embolism, and one had leukopenia and neutropenia. Finally, one child had clubbing on exam but had a negative sweat chloride test.
Asthma severity
We assessed the history of prior PICU admissions as a marker of life-threatening disease severity. Of the 110 patients, 45 (41%) had ≥1 PICU admission for asthma. Twelve children required non-invasive ventilatory support (4 required CPAP and 8 required BiPAP), 5 were intubated and required mechanical ventilation, and 1 was placed on ECMO during an asthma exacerbation. Compared to patients without PICU admissions, those who required PICU admission were more likely to be non-White (64.4% vs 41.6%), were more atopic (i.e, a higher prevalence of allergic rhinitis or eczema, higher total IgE and eosinophil count), and had lower pre-bronchodilator FEV1 and higher FeNO (Table 2). There was also a non-significant trend towards higher BDR (22.5% vs 18.4%, P=0.053). Moreover, children with ≥1 PICU admission for asthma were more likely to be on chronic oral steroids and on mepolizumab than those without a PICU admission.
Table 2 –
Clinical characteristics associated with PICU admission
| PICU (n=45) | No PICU (n=65) | P-value | |
|---|---|---|---|
| Race | 0.031 | ||
| - White | 16 (35.6%) | 38 (58.5%) | |
| - Black/ African American | 28 (62.2%) | 25 (38.5%) | |
| - Other | 1 (2.2%) | 2 (3.1%) | |
| Atopic comorbidities | |||
| - Eczema | 30 (66.7%) | 30 (46.2%) | 0.034 |
| - Allergic rhinitis | 42 (93.3%) | 52 (80.0%) | 0.05 |
| Lowest pre-bronchodilator FEV1pp | 58.6 ±18.7 | 69.9 ±18.1 | 0.002 |
| Total IgE* | 754 [487-1167] | 303 [199-460] | 0.003 |
| Highest eosinophil count (cells/mm3)* | 673 [509-890] | 319 [231-442] | 0.002 |
| FeNO category ^ | 0.016 | ||
| - Low (≤20 ppb) | 1 (6.7%) | 12 (44.4%) | |
| - Intermediate (21-35 ppb) | 5 (33.3%) | 9 (33.3%) | |
| - High (≥35 ppb) | 9 (60.0%) | 6 (22.2%) | |
| Medications | |||
| - Mepolizumab | 7 (15.6%) | 1 (1.5%) | 0.008 |
| - Chronic oral steroids | 11 (24.4%) | 6 (9.2%) | 0.035 |
Geometric means [95% confidence interval]; analyzed using t-tests after log10-transformation to minimize skewness. Similar results obtained when analyzed using Mann-Whitney rank sum test (p=0.001 for total IgE, p=0.038 for eosinophil count).
Only N=42 patients had FeNO (n=15 in the PICU group, n=27 in the no PICU group).
In the multivariable analysis adjusting for sex and age at the first SAC visit, Black/African Americanpatients were ~3-fold more likely to have had ≥1 PICU admission than White patients (adjusted OR=2.96, 95%CI=1.30-6.73; Table 3 Model 1). In addition, baseline FEV1, VCD, total IgE, and eosinophil count were each independently associated with having had ≥1 PICU admission for asthma (Table 3 Model 2); each IQR increment in total IgE level or eosinophil count (i.e., total IgE 1325 vs 214 IU/mL, or eosinophil count 810 vs 200 cells/mm3) was associated with ~15% higher odds of PICU (aOR for IgE=1.16 [95%CI=1.01-1.33]; aOR for eosinophils=1.15 [1.02-1.30]); while each IQR increment in FEV1pp (i.e., 78% vs 50%) was associated with lower ~17% odds of PICU admission (aOR=0.83, 95% CI=0.72-0.96).
Table 3 –
Multivariable analysis of risk factors associated with PICU admissions
| Model 1 (N=110) |
Model 2 (N=84) |
|
|---|---|---|
| Race | ||
| - White | Ref. | Ref. |
| - Black/ African American | 2.96 (1.30-6.73), p=0.01 | 4.03 (1.25-12.98), p=0.02 |
| Sex | ||
| - Female | Ref. | Ref. |
| - Male | 0.97 (0.44-2.15), p=0.94 | 0.85 (0.27-2.66), p=0.78 |
| Age at first SAC visit | 0.93 (0.84-1.03), p=0.16 | 0.84 (0.73-0.98), p=0.027 |
| Pre-bronchodilator FEV1pp | --- | 0.96 (0.94-0.99), p=0.014 |
| Vocal cord dysfunction (VCD) | --- | 4.62 (1.06-20.1), p=0.041 |
| Total IgE (log10) | --- | 2.74 (1.05-7.16), p=0.039 |
| Total eosinophil counts (log10) | --- | 5.16 (1.19-7.16), p=0.028 |
Shown are adjusted odds ratios (aOR) and 95% confidence intervals (95%CI). In model 2, 26 subjects had missing data for total IgE (n=10), eosinophil counts (n=11), or both (n=5).
Finally, we evaluated clinical characteristics associated with lower baseline lung function, as a marker for disease severity. Variables that were significantly associated with lower pre-bronchodilator FEV1pp included: age (~1% lower FEV1 per year older age, p=0.03); Black/African American race (~7.9% lower FEV1 compared to White race, p=0.03); elevated FeNO (~15% lower FEV1, p=0.04); and a history of PICU admission (~11.3% lower FEV1, p=0.002).
Additional diagnostic evaluation
As part of SAC evaluation, thirty-five children (32%) underwent sweat chloride tests to rule out cystic fibrosis; all were normal. Thirty-three children (30%) had chest CTs and 17 were abnormal: seven reported focal atelectasis in the setting of poorly controlled asthma, five had normal follow-up radiologic images, and two did not have follow-up. Other CT findings included single ≤5 mm nodules; pectus excavatum with slight compression of right ventricle but no airway compression (one patient); tree-in-bud pattern (one patient); and lobar hypoattenuation (one patient). Other CTs were obtained due to acute illness: one patient had multifocal infiltrates (right upper lobe and left lower lobe) found incidentally as the patient had no fever or coughing; follow-up chest x-ray two months later showed resolution and chest CT several years later was normal. One had right lower lobe pneumonia with bilateral pleural effusions, found while acutely ill during a hospital admission, with follow-up chest x-ray showing resolution. One had pneumomediastinum with soft tissue emphysema found during asthma exacerbation at an outside hospital.
Bronchoscopy and bronchoalveolar lavage (BAL) were performed in 30 children (27%), and one additional patient had a bronchoscopy without BAL. Four children had two bronchoscopies done at different points. Four children had moderate-high numbers of lipid laden macrophages on BAL. Viral pathogens (rhinovirus, parainfluenza type II) were identified in six children; bacterial pathogens (H. influenzae, M. catarrhalis) grew in three children, two of whom received treatment. One child had a variation in their left lower lobe (superior segment), one had four segments of RUL and another had left main stem bronchomalacia; all other children had normal structure of their airways, with 8 children in this group having a combination of mucosal edema and diffusely increased secretions.
Initial management and outcomes
Most of our patients met ATS/ERS criteria for severe asthma (88.2%), as 84% were on high-dose inhaled corticosteroids and long-acting beta agonists (ICS/LABA); 14 (12.7%) received IM triamcinolone, usually as part of steroid responsiveness evaluation; 17 (15.5%) were on chronic oral steroids at some point; 7 (6.4%) received tiotropium; and 40 (36.4%) were placed on biologics. Prescribed biologics were as follows: 34 (31%) omalizumab, 8 (7%) mepolizumab, 5 (5%) dupilumab, and 2 (2%) benralizumab; and five patients were placed on ≥2 biologics (at different times). Compared to the rest of patients, those prescribed biologics had lower FEV1 (57.2% vs 69.9%, p<0.001), higher BDR (24.5% vs 17.2%, p<0.001), and higher total IgE (geometric mean 712 vs 342 IU/mL); but similar eosinophil counts (p=0.17) and FeNO (p=0.64).
As a result of pharmacologic and non-pharmacologic interventions, the average rate of severe asthma exacerbations per patient decreased from 3.2/year (95%CI=2.8-3.6) to 2.2/year (1.8-2.7) (p<0.0001; Figure 3). Results were similar when including only patients who had a full year of follow-up prior to the onset of the COVID-19 pandemic in Allegheny County (n=97). Overall, the whole cohort had 106 fewer severe exacerbations in the year after joining the SAC compared to the year prior: 77 patients (70%) had fewer severe exacerbations, 6 (5.5%) had the same number of events, and 27 (27%) had more events than the prior year. Among those who improved, mean reduction was 2 fewer events per year. The only characteristic associated with improvement was younger age at start of clinic (9.9 vs 11.9 years, p=0.037).
Figure 3 – Change in annual rate of severe asthma exacerbations.
Numbers represent the difference in severe asthma exacerbations between the year after joining the SAC, and the average of the two years prior to joining the clinic (e.g., −5 means the patient had 5 fewer events in the year after their index clinic visit, compared to the average of the two years before that date).
DISCUSSION
In this study, we describe a cohort of patients with severe and difficult-to-treat asthma followed in our multidisciplinary asthma clinic, and we report sociodemographic and other characteristics associated with exacerbations requiring admission to the critical care unit. In addition, we describe the complexity of these patients, their overall initial evaluation and the management changes made in the clinic, and the impact of the program in reducing severe exacerbations in this vulnerable pediatric population.
Several baseline characteristics of SAC patients must be highlighted. First, Black/African American children constitute 48% of the SAC cohort, much higher than the ~13.5% Black/African American proportion of the population in Allegheny County18. Second, while 32% of children in Allegheny County are overweight or obese19, nearly half of the SAC patients were overweight or obese. Going forward, our program is engaging on quality improvement initiatives to address these concerning trends and thus existing disparities in asthma severity and control20-24.
All children in the SAC cohort had serial lung function testing and over 90% had BDR data. While most children with asthma have normal baseline lung function, in our cohort we found broad variability in FEV1 (from normal to severely reduced), which has also been described in children enrolled in the Severe Asthma Research Program (SARP)25. Although the utility of BDR in the management of pediatric asthma is unclear26,27, a large proportion of our patients had a BDR ≥12%. Unlike reports in adults, most children in our SAC were atopic and had an elevated FeNO, a marker of eosinophilic airway inflammation. We did not find that FeNO measurements correlated well with lung function, yet high FeNO levels were significantly associated with PICU admissions, highlighting the role of allergic airway inflammation in pediatric severe asthma. Although there has been significant debate regarding the utility of FeNO in the diagnosis and management of asthma1,28, FeNO measurements are recommended in the 2020 update to the NHLBI’s National Asthma Education and Prevention Program (NAEPP) guidelines as part of the comprehensive evaluation of patients with asthma ≥5 years old29.
In 2019, the Global Initiative for Asthma (GINA) published its first guide on the diagnosis and management of difficult-to-treat and severe asthma30. While the period analyzed here preceded those guidelines, the principles followed in the SAC largely overlapped with those recommended by GINA. Most of our patients had atopic disease (eczema, allergic rhinitis, food allergies, or atopic sensitization) and many had other comorbidities such as obesity, GERD, VCD, and anxiety/depression. Addressing these comorbidities is key to managing patients with severe asthma. As part of their evaluation, roughly 1/3 of patients each had chest CT imaging and sweat chloride testing, and slightly over 1/4 underwent bronchoscopy with BAL. Interestingly, thus far none of these tests have led to a change in management, but they are important when the diagnosis is unclear, symptoms are uncharacteristic, or the patient is not responding adequately to treatment1,13,30, especially before committing them to long-term medications with potential side effects.
We report several characteristics associated with PICU admission for asthma, as a proxy for life-threatening events, among patients with severe asthma. Most notably, Black/African American were almost threefold more likely to require PICU admissions than whites. Similarly, lower baseline lung function, VCD, and higher atopy markers were each associated with PICU admissions, as previously described31-33. In contrast to prior reports for severe asthma exacerbations31,34, overweight or obesity was not associated with PICU admissions in our SAC, perhaps due to limited statistical power or selection bias (as all SAC patients have poorly controlled asthma). Additional risk factors include the use of chronic oral steroids and mepoluzimab, which likely represent therapeutic interventions in more severe patients, rather than being true risk factors for critical care hospitalization.
Severe asthma exacerbations were reduced among patients who joined the SAC: roughly 70% of patients had fewer severe exacerbations, and on average each of them had two fewer events in the year after starting SAC. This is likely due to stepping up pharmacological management, non-pharmacological interventions including education, and close follow-up by a multidisciplinary team. As part of their initial evaluation, some patients were placed on IM triamcinolone after failing inhaled therapies, with improved asthma symptoms, lung function, and decreased asthma attacks. In our experience, brief courses of IM triamcinolone were helpful in the management of these patients, but it is important to closely monitor for side effects and wean as tolerated. Our observations correlate with the limited literature on triamcinolone use in severe asthma35,36.
Over one third of our patients were placed on “biologics” to improve asthma control, and that number has increased in the period after the current analysis. A small number of patients failed therapy with one type of biologic and required change to another agent. The reasons for variable therapeutic response are unclear but may include severe asthma heterogenicity and poor understanding of underlying mechanisms in pediatric patients37. With more biologics available, identifying biomarkers and clinical predictors of therapeutic responses to each drug is imperative38-40. Some patients in our SAC may not have therapy resistant asthma but rather “difficult to treat” disease, based largely on their comorbidities and problems with adherence or technique for medication use7,10. In these children, monthly follow up (alternating between the pulmonologist and allergist) has positively impacted their asthma care through education and improved adherence. Overall, the cohort had ~100 fewer severe exacerbations in the year after joining the multidisciplinary clinic, suggesting that this approach may reduce healthcare utilization at a system-wide level.
The current report has several limitations. First, this was a retrospective chart review, and thus we were limited by the type and completion of data available from the EHR. Second, our sample size is relatively small, which limited our ability to analyze certain subgroups and led to somewhat imprecise estimates, particularly in the fully saturated regression model. However, results were consistent in the unadjusted and the two adjusted models, and given the vulnerability of this patient population we believe it is important to report these findings. While the number of patients seen in the program continues to grow, we excluded patients seen in 2020 to minimize confounding from the impact of the COVID-19 pandemic on asthma control and clinical practice; in addition, our sensitivity analysis including only patients with a full year of follow-up prior to the pandemic yielded similar reductions in severe exacerbations.
Finally, the SAC cohort reflects the experience at a single center, and thus we cannot evaluate the impact of approaches that differ from those at our hospital. Nonetheless, the current report contributes to the very small body of literature on the diagnosis and management of severe, therapy resistant asthma in pediatrics.
Multidisciplinary asthma clinics have been shown to reduce visits to the emergency room, decrease hospitalizations, and improve asthma severity and quality of life8,9,11,12. We are currently working to create spaces where families and patients can meet, receive education, share their experiences, and provide peer support and empowerment. Thus far, we have had three virtual support group sessions that have been well attended. We are also planning to reach out to community partners and create platforms to address existing health disparities in asthma by facilitating access to care and disease education. Moving forward, we are partnering with several large pediatric programs to establish a Severe Pediatric Asthma Consortium, with the goals of sharing approaches in the management of these patients, working together to advance our knowledge in the field through research and collaboration, and reducing childhood asthma disparities.
Acknowledgments:
The authors would like to thank the Children’s Hospital of Pittsburgh Foundation for their support of the CHP Pediatric Asthma Center and the severe asthma program.
Funding:
Dr. Forno’s effort was partly funded by grant HL149693 from the US National Institutes of Health (NIH).
Footnotes
Disclosures: Dr. Celedón has received Asmanex® from Merck, Flovent® from Glaxo Smith Kline, and vitamin D and placebo capsules from Pharmavite to provide to participants as part of other NIH-funded studies; all unrelated to the current work. The other authors report no conflicts of interest. An abstract for this study was presented at the American Thoracic Society (ATS) International Conference 2021.
REFERENCES
- 1.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. European Respiratory Journal. 2014;43(2):343–373. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 2.Guilbert TW, Bacharier LB, Fitzpatrick AM. Severe Asthma in Children. Journal of Allergy and Clinical Immunology: In Practice. 2014;2(5):489–500. 10.1016/j.jaip.2014.06.022. doi: 10.1016/j.jaip.2014.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed H, Turner S. Severe asthma in children—a review of definitions, epidemiology, and treatment options in 2019. Pediatric Pulmonology. 2019;54(6):778–787. 10.1002/ppul.24317. doi: 10.1002/ppul.24317 [DOI] [PubMed] [Google Scholar]
- 4.Perry R, Braileanu G, Palmer T, Stevens P. The Economic Burden of Pediatric Asthma in the United States: Literature Review of Current Evidence. PharmacoEconomics. 2019;37(2):155–167. 10.1007/s40273-018-0726-2. doi: 10.1007/s40273-018-0726-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan SD, Rasouliyan L, Russo PA, Kamath T, Chipps BE. Extent, patterns, and burden of uncontrolled disease in severe or difficult-to-treat asthma. Allergy: European Journal of Allergy and Clinical Immunology. 2007;62(2):126–133. doi: 10.1111/j.1398-9995.2006.01254.x [DOI] [PubMed] [Google Scholar]
- 6.Odell S, Logan DE. Pediatric pain management: The multidisciplinary approach. Journal of Pain Research. 2013;6:785–790. doi: 10.2147/JPR.S37434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bush A, Fleming L, Saglani S. Severe asthma in children. Respirology. 2017;22(5):886–897. doi: 10.1111/resp.13085 [DOI] [PubMed] [Google Scholar]
- 8.Chan DS, Callahan CW, Moreno C. Multidisciplinary education and management program for children with asthma. American Journal of Health-System Pharmacy. 2001;58(15):1413–1417. https://academic.oup.com/ajhp/article/58/15/1413/5150154. doi: 10.1093/ajhp/58.15.1413 [DOI] [PubMed] [Google Scholar]
- 9.Condren M, Boger JA. Impact of a Pediatric Clinic-Based Multidisciplinary Asthma Education and Management Program. The Journal of Pediatric Pharmacology and Therapeutics. 2005;10(4):254–258. http://www.jppt.org/doi/abs/10.5863/1551-6776-10.4.254. doi: 10.5863/1551-6776-10.4.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook J, Beresford F, Fainardi V, Hall P, Housley G, Jamalzadeh A, Nightingale M, Winch D, Bush A, Fleming L, et al. Managing the pediatric patient with refractory asthma: A multidisciplinary approach. Journal of Asthma and Allergy. 2017;10:123–130. doi: 10.2147/JAA.S129159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krupp NL, Weist A, Fiscus CD, Slaven J, Harner A, Montgomery GS, Howenstine MS. Efficacy, cost effectiveness, and sustainability of a pediatric high risk asthma clinic. Pediatric Pulmonology. 2018;53(5):538–543. doi: 10.1002/ppul.23967 [DOI] [PubMed] [Google Scholar]
- 12.Smith WG, Downey K, Frampton B, Collings A, Fletcher J. Regional paediatric asthma centre: An intervention model. Paediatrics and Child Health. 2004;9(3):159–162. doi: 10.1093/pch/9.3.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barsky EE, Giancola LM, Baxi SN, Gaffin JM. A practical approach to severe asthma in children. Annals of the American Thoracic Society. 2018;15(4):399–408. doi: 10.1513/AnnalsATS.201708-637FR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham BL, Steenbruggen I, Barjaktarevic IZ, Cooper BG, Hall GL, Hallstrand TS, Kaminsky DA, McCarthy K, McCormack MC, Miller MR, et al. Standardization of spirometry 2019 update an official American Thoracic Society and European Respiratory Society technical statement. American Journal of Respiratory and Critical Care Medicine. 2019;200(8):E70–E88. doi: 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. Population. American Journal of Respiratory and Critical Care Medicine. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108 [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG. Pulmonary function between 6 and 18 years of age. Pediatric Pulmonology. 1993;15(2):75–88. doi: 10.1002/ppul.1950150204 [DOI] [PubMed] [Google Scholar]
- 17.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin A, Plummer AL, Taylor DR, Thoracic A, et al. American Thoracic Society Documents An Official ATS Clinical Practice Guideline : Interpretation of Exhaled Nitric Oxide Levels ( F E NO ) for Clinical Applications. 2011;184:602–615. doi: 10.1164/rccm.912011ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Census Bureau. State and County QuickFacts: Allegheny County, Pennsylvania 2019. http://quickfacts.census.gov/qfd/states/42/42003.html [Google Scholar]
- 19.Pennsylvania Department of Health, Bureau of Community Health Systems D of SH. Teens who are Overweight or Obese 2017-2018. http://www.miamidadematters.org/indicators/index/view?indicatorId=460&localeId=414&localeChartIdxs=1%7C2%7C3%7C4 [Google Scholar]
- 20.Peters U, Dixon AE, Forno E. Obesity and asthma. The Journal of allergy and clinical immunology. 2018;141(4):1169–1179. http://www.ncbi.nlm.nih.gov/pubmed/29627041. doi: 10.1016/j.jaci.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forno E, Celedón JC. The effect of obesity, weight gain, and weight loss on asthma inception and control. Current opinion in allergy and clinical immunology. 2017;17(2):123–130. http://www.ncbi.nlm.nih.gov/pubmed/28030376. doi: 10.1097/ACI.0000000000000339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzpatrick AM, Gillespie SE, Mauger DT, Phillips BR, Bleecker ER, Israel E, Meyers DA, Moore WC, Sorkness RL, Wenzel SE, et al. Racial disparities in asthma-related health care use in the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. The Journal of allergy and clinical immunology. 2019;143(6):2052–2061. http://www.ncbi.nlm.nih.gov/pubmed/30635198. doi: 10.1016/j.jaci.2018.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guilbert T, Zeiger RS, Haselkorn T, Iqbal A, Alvarez C, Mink DR, Chipps BE, Szefler SJ. Racial Disparities in Asthma-Related Health Outcomes in Children with Severe/Difficult-to-Treat Asthma. The journal of allergy and clinical immunology. In practice. 2019;7(2):568–577. http://www.ncbi.nlm.nih.gov/pubmed/30172020. doi: 10.1016/j.jaip.2018.07.050 [DOI] [PubMed] [Google Scholar]
- 24.Mitchell SJ, Bilderback AL, Okelo SO. Racial Disparities in Asthma Morbidity among Pediatric Patients Seeking Asthma Specialist Care. Academic Pediatrics. 2016;16(1):64–67. doi: 10.1016/j.acap.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 25.Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, Wenzel SE, Aujla S, Castro M, Bacharier LB, et al. Heterogeneity of severe asthma in childhood: Confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. Journal of Allergy and Clinical Immunology. 2011;127(2):382–389.e13. 10.1016/j.jaci.2010.11.015. doi: 10.1016/j.jaci.2010.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schifano ED, Hollenbach JP, Cloutier MM. Mismatch between asthma symptoms and spirometry: Implications for managing asthma in children. Journal of Pediatrics. 2014;165(5):997–1002. 10.1016/j.jpeds.2014.07.026. doi: 10.1016/j.jpeds.2014.07.026 [DOI] [PubMed] [Google Scholar]
- 27.Tse SM, Gold DR, Sordillo JE, Hoffman EB, Gillman MW, Rifas-Shiman SL, Fuhlbrigge AL, Tantisira KG, Weiss ST, Litonjua AA. Diagnostic accuracy of the bronchodilator response in children. Journal of Allergy and Clinical Immunology. 2013;132(3):554–559.e5. 10.1016/j.jaci.2013.03.031. doi: 10.1016/j.jaci.2013.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korevaar DA, Westerhof GA, Wang J, Cohen JF, Spijker R, Sterk PJ, Bel EH, Bossuyt PMM. Diagnostic accuracy of minimally invasive markers for detection of airway eosinophilia in asthma: A systematic review and meta-analysis. The Lancet Respiratory Medicine. 2015;3(4):290–300. doi: 10.1016/S2213-2600(15)00050-8 [DOI] [PubMed] [Google Scholar]
- 29.Cloutier MM, Dixon AE, Krishnan JA, Lemanske RF, Pace W, Schatz M. Managing Asthma in Adolescents and Adults: 2020 Asthma Guideline Update From the National Asthma Education and Prevention Program. JAMA. 2020;324(22):2301–2317. http://www.ncbi.nlm.nih.gov/pubmed/33270095. doi: 10.1001/jama.2020.21974 [DOI] [PubMed] [Google Scholar]
- 30.Global Initiative for Asthma. Global strategy for asthma management and prevention,2019. Global Initiative for Asthma. https://ginasthma.org/ [Google Scholar]
- 31.Puranik S, Forno E, Bush A, Celedón JC. Predicting severe asthma exacerbations in children. American Journal of Respiratory and Critical Care Medicine. 2017;195(7):854–859. doi: 10.1164/rccm.201606-1213PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silvers SK, Lang DM. Asthma in African Americans: What can we do about the higher rates of disease? Cleveland Clinic Journal of Medicine. 2012;79(3):193–201. doi: 10.3949/ccjm.79a.11016 [DOI] [PubMed] [Google Scholar]
- 33.Belessis Y, Dixon S, Thomsen A, Duffy B, Rawlinson W, Henry R, Morton J. Risk Factors for an Intensive Care Unit Admission in Children with Asthma. Pediatric Pulmonology. 2004;37(3):201–209. doi: 10.1002/ppul.10443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gross E, Lee DS, Hotz A, Ngo KC, Rastogi D. Impact of Obesity on Asthma Morbidity During a Hospitalization. Hospital pediatrics. 2018;8(9):538–546. doi: 10.1542/hpeds.2017-0265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koo S, Gupta A, Fainardi V, Bossley C, Bush A, Saglani S, Fleming L. Ethnic Variation in Response to im Triamcinolone in Children with Severe Therapy-Resistant Asthma. Chest. 2016;149(1):98–105. doi: 10.1378/chest.14-3241 [DOI] [PubMed] [Google Scholar]
- 36.Panickar JR, Kenia P, Silverman M, Grigg J. Intramuscular triamcinolone for difficult asthma. Pediatric Pulmonology. 2005;39(5):421–425. doi: 10.1002/ppul.20176 [DOI] [PubMed] [Google Scholar]
- 37.Licari A, Manti S, Castagnoli R, Marseglia A, Foiadelli T, Brambilla I, Marseglia GL. Immunomodulation in pediatric asthma. Frontiers in Pediatrics. 2019;7(JULY):1–9. doi: 10.3389/fped.2019.00289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duffey H, Anderson WC. It’s Time to Start Phenotyping Our Patients with Asthma. Immunology and Allergy Clinics of North America. 2019;39(4):561–572. 10.1016/j.iac.2019.07.009. doi: 10.1016/j.iac.2019.07.009 [DOI] [PubMed] [Google Scholar]
- 39.Holguin F, Cardet JC, Chung KF, Diver S, Ferreira DS, Fitzpatrick A, Gaga M, Kellermeyer L, Khurana S, Knight S, et al. Management of severe asthma: A European Respiratory Society/American Thoracic Society guideline. European Respiratory Journal. 2020;55(1). 10.1183/13993003.00588-2019. doi: 10.1183/13993003.00588-2019 [DOI] [PubMed] [Google Scholar]
- 40.Vijverberg SJH, Brinkman P, Rutjes NWP, Maitland-Van Der Zee AH. Precision medicine in severe pediatric asthma: Opportunities and challenges. Current Opinion in Pulmonary Medicine. 2019;0. doi: 10.1097/MCP.0000000000000633 [DOI] [PMC free article] [PubMed] [Google Scholar]




