Abstract
Parkinson’s disease, and other neurodegenerative disorders, are primarily characterized by pathological accumulation of proteins, inflammation, and neuron loss. Although there are some known genetic risk factors, most cases cannot be explained by genetics alone. Therefore, it is important to determine the environmental factors that confer risk and the mechanisms by which they act. Recent epidemiological studies have found that exposure to air pollution is associated with an increased risk of developing Parkinson’s disease, although not all results are uniform. The variability between these studies is likely due to differences in what components of air pollution are measured, timing and methods used to determine exposures, and correction for other variables. There are several potential mechanisms by which air pollution could act to increase the risk of developing Parkinson’s disease, including direct neuronal toxicity, induction of systemic inflammation leading to CNS inflammation, and alterations in gut physiology and the microbiome. Taken together, air pollution is an emerging risk factor in the development of Parkinson’s disease. A number of potential mechanisms have been implicated by which it promotes neuropathology providing biological plausibility and these mechanisms are likely relevant to the development of other neurodegenerative disorders such as Alzheimer’s disease. This field is in its early stages, but a better understanding of how environmental exposures influence the pathogenesis of neurodegeneration is essential for reducing the incidence of disease and finding disease-modifying therapies.
Graphical Abstract
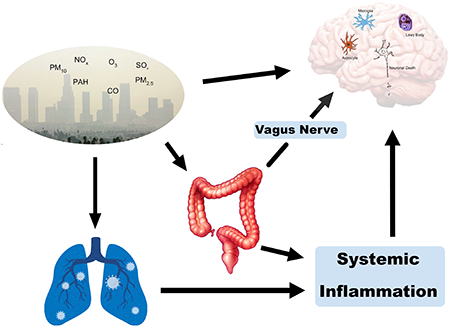
Air pollution is the leading environmental cause of mortality and is the fifth overall cause of mortality worldwide (World Health Organization 2016). There is evidence that air pollution exposure is linked with respiratory disease, heart disease, stroke, lung cancer, and diabetes1. Ambient air pollution, or outdoor air pollution, is primarily composed of gases such as ozone, carbon monoxide, nitrogen dioxide, and sulfur dioxide2. It also contains respirable particulate matter (PM), defined by its aerodynamic diameter (PM10, <10 μm; PM2.5 < 2.5 μm; ultrafine (UF), PM0.1, < 0.1 μm). It has been well-established that PM2.5 can move through the respiratory tract and reach the lung alveoli; PM0.1 has been seen to pass through the alveolar-capillary membrane and enter the bloodstream, allowing it to access various organs3. According to data available in 2017, 92% of the world’s population lived in areas that exceeded the WHO guideline for PM2.51. The ubiquitous nature of air pollution exposure necessitates further research regarding its effects on the brain and the role it plays in development of neurodegenerative diseases such as Parkinson’s disease (PD).
Air Pollution and PD Risk
Although there is a large body of literature linking air pollution exposure to shortened life expectancy and cardiovascular disease, much less is known about the impact of air pollution on the brain. Recent studies suggest that air pollution can affect the brain in a variety of ways, resulting in an increased risk of neurodegenerative disease and stroke4. However, studying environmental influencers of neurodegenerative disease risk is challenging, particularly because of the likely relatively long latency period between disease initiation and diagnosis, and the difficulty with assessing individual exposure over a lifetime. For many neurodegenerative disorders including PD, pre-symptomatic disease initiation and diagnosis are thought to be separated by many years, or possibly decades5,6. Thus, it has been hypothesized that exposure to environmental contributors to disease risk occurs years prior to the disease diagnosis7. This concept is supported by observations with pesticide exposure and PD risk8. For example, paraquat and maneb exposure with a long lag time to diagnosis was associated with an increased risk of PD while short lag time was not9. It is also possible that environmental exposures can accelerate a disease process that is already established. Both of these possibilities need to be considered when interpreting associations with a chronic and progressive disorder such as PD.
For this review, PubMed and the Cochrane databases were searched for English-language studies regarding air pollution and neurodegeneration through June 1, 2021, including epidemiological, basic science studies, meta-analyses, and reviews. Search terms included Parkinson’s disease, air pollution, diesel, traffic, neurodegeneration, and inflammation. Most epidemiological studies examining the role of air pollution in neurodegenerative disease risk have been focused on PD and indicate an association between exposure to certain components of traffic- related air pollution and PD risk, although some studies have not found significant associations (recent studies since 2015 summarized in Table 110–22).
Table 1.
Recent Epidemiological Studies Investigating the Association of PD and Air Pollution
| Author | Study Design | Population | Exposure Range | Length of Exposure/Lag time to Ds | OR | Strengths | Weaknesses |
|---|---|---|---|---|---|---|---|
| Kirrane 201514 | Case-Control | 301 cases in US |
O3 (ppb): • IA mean: 39.0, max: 41.5 • NC mean 40.6, max 46.5 PM2.5 (μg/m3): • IA mean 8.9, max 11.5 • NC mean 12.6, max 17.7 |
4 years |
O3: OR = 1.39 (.98-1.98) PM2.5: OR = 1.34 (.93–1.93) |
Adjusted for multiple variables including pesticides | Short exposure time, small population |
| Ritz 2016 19 | Case-Control | 1696 PD cases in Denmark |
NO2: 9.8-43.26 ug/m3 NOx: 13.46-181.55 ug/m3 CO: 0.36-2.34 mg/m3 |
31 years |
NO2: 1.09 per 2.97 μg/m of exposure NOx: 1.06 per 7.10 ppb of exposure CO: 1.13 per 0.12 ppm of exposure |
Large population, long exposure, adjusted for multiple variables | Low air pollution in Denmark |
| Lee 2016 15 | Case-Control | 11,117 incident PD cases in Taiwan |
PM10: 29.3-86.8 ug/m3 NOx: 5.2-77.6 ppb O3: 19.0-39.2 ppb CO: 0.2-1.5 ppm |
11 years |
CO: OR = 1.37 (1.23–1.52) Multi-pollutant models: OR = 1.17 (1.07–1.27) |
Large population, adjusted for age, year of Dx | Short exposure time, adjusted for only a few variables |
| Lee 2016 16 | Case-Control | 408 incident PD cases in Denmark (subset of Ritz 2016) |
NO2: 9.8-43.26 ug/m3 NOx: 13.46-181.55 ug/m3 CO: 0.36-2.34 mg/m3 |
31 years | AA allele of the interleukin-1β) gene with high NO exposure: OR = 3.1 | Long exposure, adjusted for multiple variables, gene interaction with air pollution | Small population, low air pollution in Denmark, |
| Liu 2016 17 | Case-Control | 1,556 cases in US | Quintiles PM2.5 (μg/m3): • Q1:4.4- < 10.8 • Q2: 10.8- < 12.3 • Q3: 12.3- < 13.8 • Q4: 13.8- < 15.4 • Q5: 15.4–26.9 PM10 • Q1:4.3- < 22.9 • Q2: 22.9- < 25.1 • Q3: 25.1- < 27.9 • Q4: 27.9- < 33.8 • Q5: 33.8–65.4 NO2 (ppb): • Q1: 1.0- < 7.7 • Q2: 7.7- < 10.4 • Q3: 10.4- < 13.1 • Q4: 13.1- < 16.6 • Q5: 16.6–34.2 |
Average of 1990 and 2000, very short to no lag to disease onset. |
PM2.5: OR = 1.02 per 3.8 μg/m3 PM10: OR = 1.02 per 8.4 μg/m3 NO2: OR = 1.01 per 7.3 ppb of exposure Female: PM10: Q5 vs. Q1: OR = 1.65 Never smokers: PM2.5: Q5 vs. Q1: OR = 1.29 |
Adjusted for multiple variables, large population. Preplanned subgroup analysis | Short exposure time, little to no lag time |
| Palacios 2017 18 | Prospective | N =50,352 550 PD cases in US |
PM2.5: 3.1-29.2 ug/m3 PM10: 7.4-81.3 ug/m3 |
9 years | 0.99 (0.96-1.01) | Prospective, Adjusted for age, tobacco | Short exposure time to disease. |
| Chen 2017 13 | Case-Control | 1060 incident PD cases in Taiwan |
PM10 Tertiles (μg/m3): • T1 ≤ 54 • 54 < T2 ≤ 65 • T3 > 65 SO2, O3, CO, NOx, NO, NO2, THC, CH4, and NMHC not specified. |
1-18 years |
PM10 T3 v T1: OR = 1.35 (1.12-1.62) Others increased only with comorbidity |
Large population of incident cases. Considered comorbid conditions. | Variable exposure and lag time. Did not have data on PM2.5. Adjusted for age and gender only. |
| Shin 2018 21 | Population-based cohort | 38 745 incident cases in Ontario, Canada |
PM2.5: 1.3-20.0 ug/m3 NO2: 2.2-53.2 ppb O3: 24.3-64.1 ppb |
2, 5 and 10 year lag. |
PM2.5: 4% increase risk per interquartile increment of 3.8 ug/m3 Similar results for NO2 and O3. |
Large population, Considered comorbid conditions. | Relatively short exposure lag, did not adjust for smoking, physical activity or pesticide exposure. |
| Cerza 2018 12 | Prospective cohort | 13,104 cases in Rome, Italy |
PM2.5: 9.8-31.4 ug/m3 PM10: 29.6-58.2 ug/m3 NO2: 13.2-84.9 ug/m3 NOx: 14.3-173.4 ug/m3 O3: 54.5-112.8 ug/m3 |
0-3 year lag |
PM abs, NO2: HR = 0.97 per 10 μg/m3 increase NOx: HR = 0.97 per 20 μg/m3 increase O3: HR = 1.02 per 10 μg/m3 increase |
Large prospective study. Adjusted for gender and age | Short to no lag time, Did not adjust for other exposures, occupation, limited adjustment for smoking. |
| Salimi 2019 20 | Cross sectional | 1,428 cases in New South Wales, Australia |
PM2.5: 0.1-10.3 ug/m3 NO2: 4.21-71.2 ug/m3 |
0-2 year lag |
PM2.5: OR = 1.01 per 1 ug/m3 NO2: OR = 1.03 (all) and 1.11 (past smokers) per 5 ug/m3 |
Adjusted for multiple potential confounds including smoking | Short to no lag time, Self-reported cases. Limited statistical power. Relatively low range PM2.5 range. |
| Yuchi 2020 22 | Database Cohort | 4,201 cases in Vancouver, Canada |
PM2.5: 0.1-10.4 ug/m3 NO2: 12-57.7 ppb NO: 8.2-101.0 ppb |
5-9 years |
PM2.5: HR = 1.09 per interquartile range (IQR) NO2: HR = 1.12 per IQR NO: HR = 1.03 per IQR |
Large number of cases. Multiple co-variants reported. | Relatively low range PM2.5 range. Did not adjust for smoking or other behavioral risks. |
| Jo 2021 10 | Retrospective cohort study | 338 newly diagnosed cases in South Korea |
PM2.5: 18.0-44.4 ug/m3 PM10: 41.0-79.0 NO2: 0.026-0.045 ppm O3: 0.013-0.025 ppm SO2: 0.0036-0.0074 ppm CO: 0.40-0.82 ppm. |
5 years |
NO2: HR = 1.41 highest v lowest quartile. No significance for other measures |
Measured several pollution components, Adjusted for multiple co-variants. | Low number of cases. Relatively low range PM2.5 and PM10 range. Short lag. |
One potential reason for the disparities is that the relative risk scores from the positive studies are relatively modest. For example, in the Ritz study, they found odds ratios (OR) of 1.08 for exposures in the 25-75th percentile and an OR of 1.22 (95%CI: 0.99, 1.51) above the 75th percentile for NO2 as compared to the <25th percentile. NO2 exposures above the 95th percentile had the strongest association (OR=1.92). The effect size poses some important implications. 1) Since so many people are exposed to air pollution, it could account for a significant percentage of PD cases. For example, if we extrapolate the risk of PD in the Ritz study to the air pollution levels (i.e. NO2, NOx and CO levels) in Los Angeles, CA during the 1970s and 80s, air pollution could account for up to 70% of the cases19. 2) Small relative risk scores make it very challenging to document an effect in epidemiological studies of relatively rare disorders like PD.
The variability between studies could also be due to methodological differences. Some studies do not control for other factors known to associate with altered risk of PD. These include age, smoking history, pesticide exposure and time between exposures and disease onset. As mentioned previously, the pre-symptomatic lag time to disease is likely long, so exposures might need to be determined decades prior to diagnosis. The sources and composition of air pollution are often not considered, and the components measured vary considerably between studies. No one component can be used as a proxy for air pollution. For example, PM2.5 is often used as a proxy for all particulate matter (PM), even though there are other sizes and chemical compositions that are not reflected by this one value. Furthermore, novel PM, trace metals and nanoparticles, which are not routinely measured, should be considered when studying the health effects of air pollution23,24.
Despite these methodological challenges, recent meta-analyses support the association between air pollution exposure and risk of developing PD25. Since air pollution is generally associated with an urban environment, its association with PD would appear to contradict a widely-believed association of PD with rural living. The epidemiology of PD is likely more nuanced. For example, in a large registry in Thailand, elevated PD risk was associated with rural living primarily in regions where pesticides were heavily used, but was also associated with urban areas26. Furthermore, Wright Willis et al found that the prevalence and incidence of PD in urban counties were greater than in rural ones within the US27. Since associations do not necessarily indicate causality, animal and mechanistic studies are necessary to establish biological plausibility of a causal connection.
Modeling Air Pollution Exposure and Neurotoxicity
Altered risk of disease from environmental factors depends to some degree on the concentration of the toxicant, duration of exposure, and timing of exposure. Based on epidemiologic and animal studies, it is likely that long-term exposure to air pollution prior to diagnosis is necessary to alter risk of PD. Therefore, it is very difficult to model in animal and human studies. One approach to overcome this problem is to identify alterations in biological pathways that, over time, can lead to disease. This approach, referred to as “adverse outcome pathways (AOP)”, utilizes molecular and biochemical endpoints as biomarkers to investigate disease pathogenesis28. The AOP approach has been extensively utilized in investigating the potentially causal link between environmental toxins and PD. For example, ziram, a fungicide, was identified to be an inhibitor of the ubiquitin proteasome system (UPS) in a screen and subsequently was found to increase the risk of developing PD29–31. In the context of air pollution, diesel exhaust has been shown to increase α-syn levels and induce neuroinflammation, both thought to be AOP for PD (see below)32.
The Pathophysiology of PD
The primary pathology in PD involves dopaminergic neuron loss, particularly in the substantia nigra, and inflammation. Most clinically defined PD patients have abnormally misfolded and aggregated α-synuclein (α-syn) in the form of Lewy Bodies at autopsy. Pathological α-syn aggregates appear to spread throughout the nervous system in a predictable manner which determines the clinical symptomology33. For example, the prodromal symptoms of constipation and olfactory dysfunction that can occur many years, or even decades, before disease diagnosis, likely reflect underlying α-syn pathology. This is supported by studies describing α-syn aggregates in the enteric nervous system of the gut and the olfactory bulb several years before motor symptoms develop, and it has been proposed that these aggregates can then spread to the CNS via the vagus nerve33–37. Later, the cardinal features of PD develop (e.g. tremor, rigidity etc.) when the substantia nigra becomes involved.
The molecular mechanisms by which these pathologies occur are still being uncovered, but aggregation and propagation of misfolded α-syn appear central to the pathogenesis of most PD cases38. Insight into the pathological pathways in sporadic PD have come from patients with relatively rare PD-associated gene mutations and include disrupted proteostasis, inflammation, and mitochondrial dysfunction33. Increased levels of α-syn can promote the formation of toxic oligomers and fibrils and can result from increased gene expression (e.g. SNCA gene duplication) or by reduced degradation39,40,41. Both the UPS and autophagy degrade α-syn and dysfunction in both processes has been implicated in the pathogenesis of PD through mutations in the Parkin and GBA genes42,43.
In addition to alterations in proteostasis, mitochondrial dysfunction has also been implicated in PD and can lead to increased oxidative stress and neuronal loss44. Dysfunction of mitochondria promote the generation of reactive oxygen species that can result in α-syn aggregation or decreased α-syn degradation45. Aggregated α-syn can then bind to mitochondria and further its dysfunction, creating a positive feedback loop46. This is supported by the fact that mutations in mitochondrial-associated genes (e.g. PINK1, Parkin) or exposure to mitochondrial toxins (e.g. rotenone, trichloroethylene) markedly increase the risk of PD47,48.
Another pathway implicated in PD pathogenesis is inflammation. Neuroinflammation (e.g. microglial and astrocyte activation) is a universal pathological finding in PD brains and recent studies suggest that it contributes to neuronal damage and is not simply a response to injury49. Genetic alterations in several immune-related genes (e.g., DJ-1, leucine-rich repeat protein kinase-2 and HLA-DR) can markedly increase the risk of developing PD, and provide support for the hypothesis that inflammation contributes to the pathogenesis of sporadic PD49.
Mechanisms by which Air Pollution May Increase the Risk of Developing PD
There are number of ways by which air pollution can affect the brain and contribute to the pathogenesis of PD and we hypothesize that it by disrupting proteostasis, injuring mitochondria, and/or inducing inflammation (Figure 1). Air pollution is a complex mixture of gases, PM, and smaller chemical moieties. Some of these components gain access to the brain via the bloodstream and/or by direct diffusion through the olfactory system (Fig 1A). Several potential mechanisms of toxicity have been proposed once these chemicals enter the brain. Air pollution may also affect brain health more indirectly through systemic mechanisms such as inducing the release of inflammatory cytokines or other chemicals from the lungs (Fig. 1B). Components of air pollution can also accumulate in the GI tract and alter gut mucosal physiology, promoting α-syn pathology (Fig 1C) and/or altering the microbiome (Fig. 1D), both of which have been implicated in the pathogenesis of PD. It is likely that multiple mechanisms are involved in altering the risk of PD and the evidence for each is reviewed below.
Figure 1. Proposed mechanisms by which air pollution may promote Parkinson’s disease pathology.
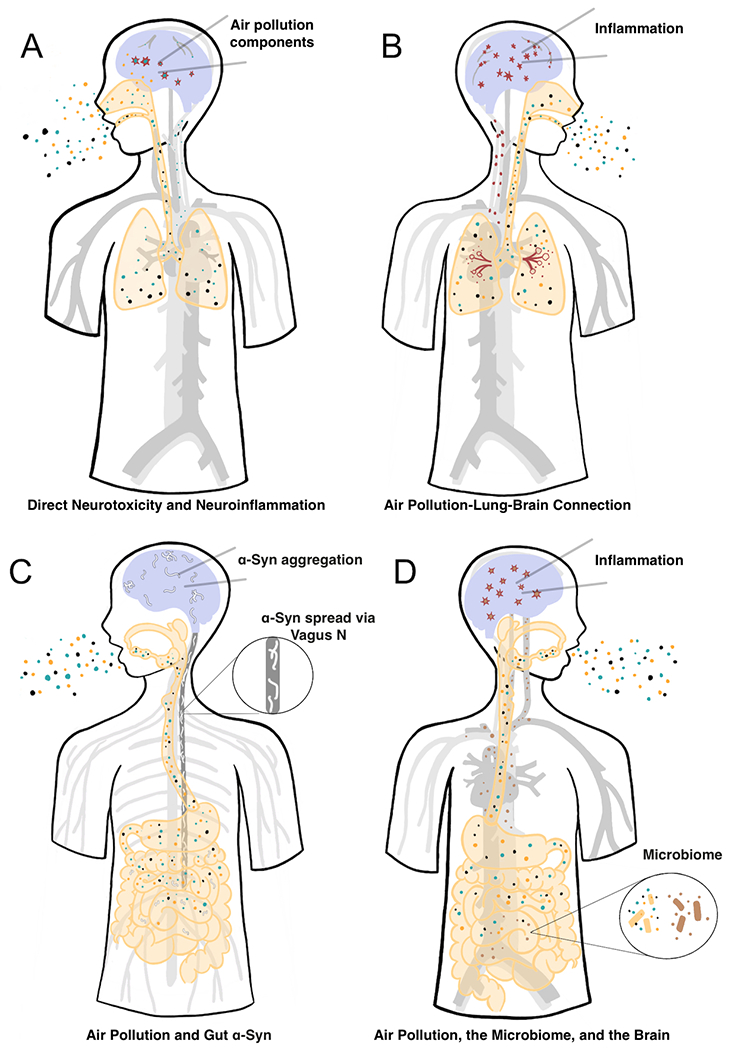
1A: Neurotoxicity and Neuroinflammation by Air Pollution. Components of air pollution reach the brain either through the blood stream and/or through olfactory mucosa. Once in the brain, some moieties are neurotoxic and cause neuroinflammation. 1B: The Air Pollution-Lung-Brain Connection. Air pollution induces pulmonary and systemic inflammation, which induces CNS inflammation. 1C: Air Pollution and Gut α-Syn. Air pollution causes gut inflammation and leakiness, which promotes local accumulation of α-syn. α-Syn (white) can then spread to the brainstem via the vagus nerve (enlarged). 1D: Air Pollution, the Microbiome, and the Brain. Air pollution alters gut microbiome (enlarged), which can lead to systemic inflammation, release of neuroactive molecules and neuroinflammation.
Neurotoxicity and Neuroinflammation by Air Pollution Components
Most mechanistic studies on air pollution to date have focused on inflammation50. CNS inflammation and oxidative stress are important findings in PD brains and air pollution appears to increase both. Observations from human autopsy studies and experiments in rodents support the hypothesis that air pollution increases inflammation in the CNS51–53.
It is clear that many of the components of air pollution do reach the brain and therefore can contribute to the pathogenesis of PD by direct neurotoxicity and/or inducing neuroinflammation (Fig. 1A). Ultrafine particles can gain access to the brain either through the blood stream and/or through olfactory mucosa57,58. Of particular interest with regard to air pollution are the potent and biologically active compounds known as polycyclic aromatic hydrocarbons (PAHs), which can reach the brain through the circulation and accumulate in the CNS59,60. In fact, the levels of some PAHs found in human brains were very high and support the concept that components of air pollution can bioaccumulate in the nervous system, posing significant risk through direct toxicity59. Furthermore, exposure to air pollution can weaken the integrity of the blood brain barrier (BBB)61, making it more permeable to toxicants that enter circulation.
In vitro, DE particles (DEP) are toxic to dopaminergic neurons, but this is less evident when animals are exposed to DE in vivo. Block et al reported that DEP caused selective dopaminergic neurotoxicity in primary mesencephalic cultures. They demonstrated that toxicity of DEP was dependent on microglial activation and 20 phagocytosis54. They later showed that phagocytosis of the particulate was responsible for activating microglia and was necessary for neurotoxicity62. Particles clearly activate microglia, but only very small quantities reach the brain63. DE exposure for weeks in vivo does not result in dopaminergic neuron loss, but does result in an increase in α-syn levels in the midbrain and enhanced dopaminergic toxicity of LPS54–56. Accumulation of α-syn is an important AOP in the pathogenesis of PD and this evidence further implicates air pollution in the disruption of α-syn proteostasis.
Inhaled moieties that are soluble in organic solvents, such as PAHs, reach the brain much more readily than particulate matter57. Therefore, many researchers utilize organic extracts of DE (DEPe) for experiments where inhalation is not the primary means of exposure. We have utilized zebrafish to study direct neurotoxicity of DEPe containing concentrations of PAHs similar to those found in human brains59,64. A brief exposure to DEPe resulted in abnormal swimming and loss of dopaminergic neurons, although the neuronal loss was not selective64. Importantly, there was an accumulation of zebrafish synuclein in this model, which appeared to be caused by dysfunction of neuronal autophagic flux, and stimulation of autophagy was neuroprotective. We also exposed zebrafish embryos to DEPe and performed proteomic and transcriptomic analyses on brain tissue to identify altered pathogenic pathways. DEPe treatment altered several AOPs relevant to PD and other neurodegenerative disorders, including xenobiotic metabolism, phagosome maturation, and amyloid processing65. Others have reported CNS inflammation in rodent models similar to that seen in human PD brains. Of interest are the findings that DE increases expression of some inflammatory genes in the olfactory bulb (OB) of mice, a brain region where PD pathology is seen very early in the disease55,56,66. Inflammatory changes are also seen in the brains of dogs and people living in urban areas, as compared to those living in rural areas, and the authors speculated that these changes are due to high levels of air pollution51–53. There are clear limitations in many of these studies, but they support a causal link between air pollution and increased risk of PD through direct neurotoxicity and/or neuroinflammation.
The Air Pollution-Lung-Brain Connection
In addition to direct particle translocation, exposure of the airway to air pollution also leads to peripheral or systemic inflammation, which in turn, can affect the CNS (Fig. 1B). Epithelial cells lining the airway can physically block larger inhaled particles and can secrete cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β), which promote synthesis of other cytokines and chemokines and results in immune cell activation67. Peripheral inflammation from the lungs and other tissues is thought to contribute to CNS neuroinflammation68,69. The BBB is weakened by systemic inflammation-derived pro-inflammatory cytokines and chemokines, which can allow inflammatory cells and pro-inflammatory cytokines to pass into the brain. Once in the brain, these factors from the periphery, along with brain-derived cytokines, chemokines, β-amyloid, α-syn, and amyloid precursor proteins can activate CNS immune cells and lead to downstream effects such as neuronal injury69,70. Air pollution exposure is well-accepted as a significant risk to pulmonary health and contributor to systemic inflammation, but its downstream effects on the brain may be important mechanisms by which it increases the risk of developing PD and neurodegenerative diseases.
Systemic inflammation, which has been observed in PD patients, may contribute to CNS neuroinflammation. Blood cytokines representing a proinflammatory state (e.g. TNF-α, IL-1β, IL-2 and −6, CRP) are elevated in PD patients, and are associated not only with an increased risk of developing PD71 also with faster progression72. There is strong evidence in animal models that systemic inflammation can lead to neuroinflammation and loss of dopaminergic neurons, especially in combination with elevated α-syn levels73. Taken together, air pollution induces a systemic inflammatory response that may lead to neuroinflammation and an elevated risk of developing PD (Fig 1B).
Air Pollution and Gut α-Syn
Evidence from animal and human studies supports the hypothesis that pathological forms of α-syn can accumulate in the gut, spread to the brainstem via the vagus nerve, and eventually lead to neuronal loss in the substantia nigra (SN; Fig. 1C). This ordered progression of α-syn pathology was first described in autopsy specimens33 and later supported by the finding that α-syn aggregates are present in colonic biopsy specimens years before PD diagnosis34. Furthermore, prior vagotomy is associated with a reduced risk of developing PD35,36. In animals, α-syn preformed fibrils (PFFs) injected into the duodenum results in α-syn spread into brainstem nuclei and eventually to the SN37. Vagotomy blocked this spread, as well as dopaminergic neuron loss. Relevant to this review, environmental toxins administered into the GI tract can lead to the formation of pathological α-syn in the gut that spreads to the CNS and induces dopaminergic neuron loss74,75. There is little direct evidence that air pollution can induce α-syn aggregates in the gut that spreads to the CNS, but there is an increasing body of literature demonstrating that it can induce changes in the gut mucosa that are thought to promote α-syn pathology. Air pollution exposure has been reported to induce gut inflammation and leakiness and alters the risk of developing inflammatory bowel disease (IBD)76. It has been hypothesized that gut inflammation and increased permeability may be the trigger for α-syn aggregation, which eventually spreads to the CNS. Interestingly, IBD has also been associated with an increased risk of developing PD77. It is unclear how air pollution exerts these changes in the gut, but it may act, in part, by altering the microbiome.
Air Pollution, the Microbiome, and the Brain
The microbiome is an emerging topic of interest related to human health. Its role and importance in the development of neurodegenerative disease is not well-understood, but recent studies in rodents and PD patients raise the possibility that alterations in the gut microbiome may be an important means by which the environment can alter risk of developing CNS diseases. This is particularly relevant for PD, since α-syn pathology may first appear in the GI system in close proximity to the gut microbiome (Fig. 1D)78–80. Studies in rodent models of PD have reported dramatic changes in behavior and CNS pathology resulting from alterations in the microbiome81. Several groups have found altered microbiota in PD patients, further supporting its pathogenic role in the development of PD82.
Exposure to air pollution has recently been linked to alterations in the microbiome, but primarily in animals. “Gut leakiness”, a result of imbalances in the microbiome leading to disruption of the epithelial barrier of the gut, can allow various bacterial metabolites and virulence factors to travel through the intestinal lining, into the bloodstream, and subsequently across the BBB83–85. Some examples of molecules that can be produced, suppressed, and overused by strains of microbiota include synaptogenic proteins, short-chained fatty acids, L-DOPA, GABA, serotonin and dopamine, Such changes can have a direct effect on neurological function86. Mice exposed to ambient PM2.5 exhibited significant changes in gut microbial diversity. Significant increases were found in Bacteriodales which likely involve degradation of the mucus layer and increased gut permeability, as well as the depletion of Lactobacillus, a strain known to help maintain homeostasis in the gut87. Furthermore, PM10 exposure in mice led to changes in microbiota composition and an intestinal inflammatory response88. These findings have not been reproduced in all studies, and the mouse microbiome is very different from the human microbiome.
Alterations in the microbiome in PD patients have been reported by several investigators89. Although some changes are common among these studies, there are significant inconsistencies and few controls for confounds, such as constipation. Some studies have investigated the effect of air pollution on the microbiome in humans and they have found associations between air pollution and changes in gut microbiome, but there are several potential confounds. Taken together, these studies raise the possibility that air pollution might alter disease risk by changing the microbiome, but this field is in very early stages.
Conclusions
The etiology of PD is complex, but undoubtedly involves a combination of genetic and environmental factors. Air pollution is emerging as an important risk factor for many diseases including PD, and may account for a significant percentage of cases worldwide. Recent epidemiological studies support an association of PD and air pollution and investigations are beginning to determine if this association is causal. There are several potential mechanisms by which air pollution may promote neurodegeneration and it is likely that several disease-related pathways are involved. A better understanding of this field is important given the number of people affected worldwide and the fact that many pathological pathways implicated are likely common to other disorders, such as Alzheimer’s disease. Ultimately, determining the pathogenesis of PD and the factors that alter risk can inform efforts to lower incidence and improved therapies targeted at underlying causes.
Acknowledgement:
We would like to acknowledge Ena Bronstein for creating the figure.
Funding Sources:
The Levine Foundation (JMB), grants from National Institute of Environmental Health Sciences, NIEHS T32ES015457 (LMB), and The Parkinson’s Alliance (JMB).
Financial Disclosures:
This work was supported by The Levine Foundation (JMB), grants from National Institute of Environmental Health Sciences, NIEHS T32ES015457 (LMB), and The Parkinson’s Alliance (JMB). JMB also received funding as site investigator from Abbvie Inc, Alexion Pharmaceuticals, Ultragenix Inc, and UC WorldMeds which are unrelated to the current work. JMB has also received funding from the NIEHS (R01 ES031106 01A1) for an unrelated study.
Footnotes
Financial Disclosures/Conflicts: None
References
- 1.Institute HE. State of Global Air 2019: A Special Report on Global Exposure to Air Pollution And Its Disease Burden. Boston, MA: Health Effects Institute;2019. [Google Scholar]
- 2.Sun Z, Zhu D. Exposure to outdoor air pollution and its human health outcomes: A scoping review. PLOS ONE. 2019;14(5):e0216550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geiser M, Rothen-Rutishauser B, Kapp N, et al. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect. 2005;113(11):1555–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu P, Guo X, Cheung FMH, Yung KKL. The association between PM2.5 exposure and neurological disorders: A systematic review and meta-analysis. Sci Total Environ. 2019;655:1240–1248. [DOI] [PubMed] [Google Scholar]
- 5.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging. 2003;24(2):197–211. [DOI] [PubMed] [Google Scholar]
- 6.Berg D, Borghammer P, Fereshtehnejad SM, et al. Prodromal Parkinson disease subtypes - key to understanding heterogeneity. Nature reviews Neurology. 2021;17(6):349–361. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Burton EA, Ross GW, et al. Research on the premotor symptoms of Parkinson’s disease: clinical and etiological implications. Environ Health Perspect. 2013;121(11-12):1245–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, Ritz B. The Search for Environmental Causes of Parkinson’s Disease: Moving Forward. Journal of Parkinson’s disease. 2018;8(s1):S9–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson’s disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. American Journal of Epidemiology. 2009;169(8):919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jo S, Kim YJ, Park KW, et al. Association of NO2 and Other Air Pollution Exposures With the Risk of Parkinson Disease. JAMA Neurol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Block ML, Elder A, Auten RL, et al. The outdoor air pollution and brain health workshop. Neurotoxicology. 2012;33(5):972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerza F, Renzi M, Agabiti N, et al. Residential exposure to air pollution and incidence of Parkinson’s disease in a large metropolitan cohort. Environmental Epidemiology. 2018;2(3):e023. [Google Scholar]
- 13.Chen CY, Hung HJ, Chang KH, et al. Long-term exposure to air pollution and the incidence of Parkinson’s disease: A nested case-control study. PLoS One. 2017;12(8):e0182834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirrane EF, Bowman C, Davis JA, et al. Associations of Ozone and PM2.5 Concentrations With Parkinson’s Disease Among Participants in the Agricultural Health Study. J Occup Environ Med. 2015;57(5):509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee PC, Liu LL, Sun Y, et al. Traffic-related air pollution increased the risk of Parkinson’s disease in Taiwan: A nationwide study. Environment international. 2016;96:75–81. [DOI] [PubMed] [Google Scholar]
- 16.Lee PC, Raaschou-Nielsen O, Lill CM, et al. Gene-environment interactions linking air pollution and inflammation in Parkinson’s disease. Environ Res. 2016;151:713–720. [DOI] [PubMed] [Google Scholar]
- 17.Liu R, Young MT, Chen JC, Kaufman JD, Chen H. Ambient Air Pollution Exposures and Risk of Parkinson Disease. Environ Health Perspect. 2016;124(11):1759–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palacios N, Fitzgerald KC, Hart JE, et al. Air Pollution and Risk of Parkinson’s Disease in a Large Prospective Study of Men. Environ Health Perspect. 2017;125(8):087011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritz B, Lee PC, Hansen J, et al. Traffic-Related Air Pollution and Parkinson’s Disease in Denmark: A Case-Control Study. Environ Health Perspect. 2016;124(3):351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salimi F, Hanigan I, Jalaludin B, et al. Associations between long-term exposure to ambient air pollution and Parkinson’s disease prevalence: A cross-sectional study. Neurochem Int. 2020;133:104615. [DOI] [PubMed] [Google Scholar]
- 21.Shin S, Burnett RT, Kwong JC, et al. Effects of ambient air pollution on incident Parkinson’s disease in Ontario, 2001 to 2013: a population-based cohort study. Int J Epidemiol. 2018;47(6):2038–2048. [DOI] [PubMed] [Google Scholar]
- 22.Yuchi W, Sbihi H, Davies H, Tamburic L, Brauer M. Road proximity, air pollution, noise, green space and neurologic disease incidence: a population-based cohort study. Environ Health. 2020;19(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamanaka RB, Mutlu GM. Particulate Matter Air Pollution: Effects on the Cardiovascular System. Front Endocrinol (Lausanne). 2018;9:680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. Environmental and Health Impacts of Air Pollution: A Review. Front Public Health. 2020;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu CY, Fang Y, Li FL, et al. Association between ambient air pollution and Parkinson’s disease: Systematic review and meta-analysis. Environ Res. 2019;168:448–459. [DOI] [PubMed] [Google Scholar]
- 26.Bhidayasiri R, Wannachai N, Limpabandhu S, et al. A national registry to determine the distribution and prevalence of Parkinson’s disease in Thailand: implications of urbanization and pesticides as risk factors for Parkinson’s disease. Neuroepidemiology. 2011;37(3-4):222–230. [DOI] [PubMed] [Google Scholar]
- 27.Wright Willis A, Evanoff BA, Lian M, Criswell SR, Racette BA. Geographic and ethnic variation in Parkinson disease: a population-based study of US Medicare beneficiaries. Neuroepidemiology. 2010;34(3):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ankley GT, Bennett RS, Erickson RJ, et al. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2010;29(3):730–741. [DOI] [PubMed] [Google Scholar]
- 29.Wang X-F, Li S, Chou AP, Bronstein JM. Inhibitory effects of pesticides on proteasome activity: Implication in Parkinson’s disease. Neurobiology of Disease. 2006;23(1):198–205. [DOI] [PubMed] [Google Scholar]
- 30.Chou AP, Maidment N, Klintenberg R, et al. Ziram causes dopaminergic cell damage by inhibiting E1 ligase of the proteasome. The Journal of biological chemistry. 2008;283(50):34696–34703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang A, Costello S, Cockburn M, Zhang X, Bronstein J, Ritz B. Parkinson’s disease risk from ambient exposure to pesticides. Eur J Epidemiol. 2011;26(7):547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levesque S, Surace MJ, McDonald J, Block ML. Air pollution & the brain: Subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. J Neuroinflammation. 2011;8:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braak H, Del Tredici K. Invited Article: Nervous system pathology in sporadic Parkinson disease. Neurology. 2008;70(20):1916–1925. [DOI] [PubMed] [Google Scholar]
- 34.Shannon KM, Keshavarzian A, Mutlu E, et al. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov Disord. 2012;27(6):709–715. [DOI] [PubMed] [Google Scholar]
- 35.Liu B, Fang F, Pedersen NL, et al. Vagotomy and Parkinson disease: A Swedish register-based matched-cohort study. Neurology. 2017;88(21):1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Svensson E, Horvath-Puho E, Thomsen RW, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78(4):522–529. [DOI] [PubMed] [Google Scholar]
- 37.Kim S, Kwon SH, Kam TI, et al. Transneuronal Propagation of Pathologic alpha-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron. 2019;103(4):627–641 e627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501(7465):45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chartier-Harlin MC, Kachergus J, Roumier C, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364(9440):1167–1169. [DOI] [PubMed] [Google Scholar]
- 40.Maraganore DM, de Andrade M, Elbaz A, et al. Collaborative analysis of alpha-synuclein gene promoter variability and Parkinson disease. JAMA. 2006;296(6):661–670. [DOI] [PubMed] [Google Scholar]
- 41.Ritz B, Rhodes SL, Bordelon Y, Bronstein J. alpha-Synuclein genetic variants predict faster motor symptom progression in idiopathic Parkinson disease. PLoS One. 2012;7(5):e36199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dennissen FJ, Kholod N, van Leeuwen FW. The ubiquitin proteasome system in neurodegenerative diseases: culprit, accomplice or victim? Prog Neurobiol. 2012;96(2):190–207. [DOI] [PubMed] [Google Scholar]
- 43.Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013;19(8):983–997. [DOI] [PubMed] [Google Scholar]
- 44.Gautier CA, Corti O, Brice A. Mitochondrial dysfunctions in Parkinson’s disease. Rev Neurol (Paris). 2014;170(5):339–343. [DOI] [PubMed] [Google Scholar]
- 45.Musgrove RE, Helwig M, Bae EJ, et al. Oxidative stress in vagal neurons promotes parkinsonian pathology and intercellular alpha-synuclein transfer. J Clin Invest. 2019;129(9):3738–3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Maio R, Barrett PJ, Hoffman EK, et al. alpha-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Sci Transl Med. 2016;8(342):342ra378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanner CM, Kamel F, Ross GW, et al. Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect. 2011;119(6):866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gash DM, Rutland K, Hudson NL, et al. Trichloroethylene: Parkinsonism and complex 1 mitochondrial neurotoxicity. Ann Neurol. 2008;63(2):184–192. [DOI] [PubMed] [Google Scholar]
- 49.Joshi N, Singh S. Updates on immunity and inflammation in Parkinson disease pathology. J Neurosci Res. 2018;96(3):379–390. [DOI] [PubMed] [Google Scholar]
- 50.Jayaraj RL, Rodriguez EA, Wang Y, Block ML. Outdoor Ambient Air Pollution and Neurodegenerative Diseases: the Neuroinflammation Hypothesis. Curr Environ Health Rep. 2017;4(2):166–179. [DOI] [PubMed] [Google Scholar]
- 51.Calderon-Garciduenas L, Maronpot RR, Torres-Jardon R, et al. DNA damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol Pathol. 2003;31(5):524–538. [DOI] [PubMed] [Google Scholar]
- 52.Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol. 2008;36(2):289–310. [DOI] [PubMed] [Google Scholar]
- 53.Calderon-Garciduenas L, Reed W, Maronpot RR, et al. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol. 2004;32(6):650–658. [DOI] [PubMed] [Google Scholar]
- 54.Block ML, Wu X, Pei Z, et al. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2004;18(13):1618–1620. [DOI] [PubMed] [Google Scholar]
- 55.Levesque S, Surace MJ, McDonald J, Block ML. Air pollution & the brain: Subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. J Neuroinflammation. 2011;8:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levesque S, Taetzsch T, Lull ME, et al. Diesel exhaust activates and primes microglia: air pollution, neuroinflammation, and regulation of dopaminergic neurotoxicity. Environ Health Perspect. 2011;119(8):1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bond JA, Ayres PH, Medinsky MA, Cheng YS, Hirshfield D, McClellan RO. Disposition and metabolism of [14C]dibenzo[c,g]carbazole aerosols in rats after inhalation. Fundam Appl Toxicol. 1986;7(1):76–85. [PubMed] [Google Scholar]
- 58.Oberdorster G, Sharp Z, Atudorei V, et al. Translocation of inhaled ultrafine particles to the brain. Inhalation toxicology. 2004;16(6-7):437–445. [DOI] [PubMed] [Google Scholar]
- 59.Pastor-Belda M, Campillo N, Arroyo-Manzanares N, et al. Bioaccumulation of Polycyclic Aromatic Hydrocarbons for Forensic Assessment Using Gas Chromatography-Mass Spectrometry. Chem Res Toxicol. 2019;32(8):1680–1688. [DOI] [PubMed] [Google Scholar]
- 60.Peiffer J, Cosnier F, Grova N, et al. Neurobehavioral toxicity of a repeated exposure (14 days) to the airborne polycyclic aromatic hydrocarbon fluorene in adult Wistar male rats. PLoS One. 2013;8(8):e71413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oppenheim HA, Lucero J, Guyot AC, et al. Exposure to vehicle emissions results in altered blood brain barrier permeability and expression of matrix metalloproteinases and tight junction proteins in mice. Part Fibre Toxicol. 2013;10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levesque S, Taetzsch T, Lull ME, Johnson JA, McGraw C, Block ML. The role of MAC1 in diesel exhaust particle-induced microglial activation and loss of dopaminergic neuron function. J Neurochem. 2013;125(5):756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takenaka S, Karg E, Roth C, et al. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ Health Perspect. 2001;109 Suppl 4:547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barnhill LM, Khuansuwan S, Juarez D, Murata H, Araujo JA, Bronstein JM. Diesel Exhaust Extract Exposure Induces Neuronal Toxicity by Disrupting Autophagy. Toxicol Sci. 2020;176(1):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jami MS, Murata H, Barnhill LM, Li S, Bronstein JM. Diesel exhaust exposure alters the expression of networks implicated in neurodegeneration in zebrafish brains. Cell Biol Toxicol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yokota S, Moriya N, Iwata M, Umezawa M, Oshio S, Takeda K. Exposure to diesel exhaust during fetal period affects behavior and neurotransmitters in male offspring mice. J Toxicol Sci. 2013;38(1):13–23. [DOI] [PubMed] [Google Scholar]
- 67.Wong J, Magun BE, Wood LJ. Lung inflammation caused by inhaled toxicants: a review. Int J Chron Obstruct Pulmon Dis. 2016;11:1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hopkins SJ. Central nervous system recognition of peripheral inflammation: a neural, hormonal collaboration. Acta Biomed. 2007;78 Suppl 1:231–247. [PubMed] [Google Scholar]
- 69.Kempuraj D, Thangavel R, Selvakumar GP, et al. Brain and Peripheral Atypical Inflammatory Mediators Potentiate Neuroinflammation and Neurodegeneration. Front Cell Neurosci. 2017;11:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takeda S, Sato N, Morishita R. Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: relevance to pathogenesis and therapy. Front Aging Neurosci. 2014;6:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qin XY, Zhang SP, Cao C, Loh YP, Cheng Y. Aberrations in Peripheral Inflammatory Cytokine Levels in Parkinson Disease: A Systematic Review and Meta-analysis. JAMA Neurol. 2016;73(11):1316–1324. [DOI] [PubMed] [Google Scholar]
- 72.Williams-Gray CH, Wijeyekoon R, Yarnall AJ, et al. Serum immune markers and disease progression in an incident Parkinson’s disease cohort (ICICLE-PD). Mov Disord. 2016;31(7):995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao HM, Zhang F, Zhou H, Kam W, Wilson B, Hong JS. Neuroinflammation and alpha-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson’s disease. Environ Health Perspect. 2011;119(6):807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anselmi L, Bove C, Coleman FH, et al. Ingestion of subthreshold doses of environmental toxins induces ascending Parkinsonism in the rat. NPJ Parkinsons Dis. 2018;4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pan-Montojo F, Schwarz M, Winkler C, et al. Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci Rep. 2012;2:898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vignal C, Guilloteau E, Gower-Rousseau C, Body-Malapel M. Review article: Epidemiological and animal evidence for the role of air pollution in intestinal diseases. Sci Total Environ. 2021;757:143718. [DOI] [PubMed] [Google Scholar]
- 77.Campos-Acuna J, Elgueta D, Pacheco R. T-Cell-Driven Inflammation as a Mediator of the Gut-Brain Axis Involved in Parkinson’s Disease. Front Immunol. 2019;10:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shannon KM, Keshavarzian A, Dodiya HB, Jakate S, Kordower JH. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov Disord. 2012;27(6):716–719. [DOI] [PubMed] [Google Scholar]
- 79.Shannon KM, Keshavarzian A, Mutlu E, et al. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2012;27(6):709–715. [DOI] [PubMed] [Google Scholar]
- 80.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396(1):67–72. [DOI] [PubMed] [Google Scholar]
- 81.Sampson TR, Debelius JW, Thron T, et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 2016;167(6):1469–1480 e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rani L, Mondal AC. Unravelling the role of gut microbiota in Parkinson’s disease progression: Pathogenic and therapeutic implications. Neurosci Res. 2021. [DOI] [PubMed] [Google Scholar]
- 83.Rosenfeld CS. Gut Dysbiosis in Animals Due to Environmental Chemical Exposures. Front Cell Infect Microbiol. 2017;7:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alonso C, Vicario M, Pigrau M, Lobo B, Santos J. Intestinal barrier function and the brain-gut axis. Adv Exp Med Biol. 2014;817:73–113. [DOI] [PubMed] [Google Scholar]
- 85.Tan AH, Chong CW, Lim SY, et al. Gut Microbial Ecosystem in Parkinson Disease: New Clinicobiological Insights from Multi-Omics. Ann Neurol. 2021;89(3):546–559. [DOI] [PubMed] [Google Scholar]
- 86.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–712. [DOI] [PubMed] [Google Scholar]
- 87.Mutlu EA, Comba IY, Cho T, et al. Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ Pollut. 2018;240:817–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kish L, Hotte N, Kaplan GG, et al. Environmental particulate matter induces murine intestinal inflammatory responses and alters the gut microbiome. PLoS One. 2013;8(4):e62220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shen T, Yue Y, He T, et al. The Association Between the Gut Microbiota and Parkinson’s Disease, a Meta-Analysis. Front Aging Neurosci. 2021;13:636545. [DOI] [PMC free article] [PubMed] [Google Scholar]


