Abstract
With the rapidly growing population of older adults, an improved understanding of brain and cognitive aging is critical, given the impacts on health, independence, and quality of life. To this point, we have a well-developed literature on the cortical contributions to cognition in advanced age. However, while this work has been foundational for our understanding of brain and behavior in older adults, subcortical contributions, particularly those from the cerebellum, have not been integrated into these models and frameworks. Incorporating the cerebellum into models of cognitive aging is an important step for moving the field forward. There has also been recent interest in this structure in Alzheimer’s dementia, indicating that such work may be beneficial to our understanding of neurodegenerative disease. Here, I provide an updated overview of the cerebellum in advanced age and propose that it serves as a critical source of scaffolding or reserve for cortical function. Age-related impacts on cerebellar function further impact cortical processing, perhaps resulting in many of the activation patterns commonly seen in aging.
Keywords: cerebellum, aging, cognition, neuroimaging
Introduction
A comprehensive understanding of the aging brain and behavior continues to elude researchers, though certainly substantial advances have been made. Given the rapidly aging population across the majority of the globe, improved understanding of brain aging and its subsequent impacts on behavior, health, and independence, are increasingly important. To this point, we now have several decades of literature investigating the brain in aging with respect to both structure and function due to the advent of brain imaging methodologies such as magnetic resonance imaging (MRI) and positron emission tomography (PET). Thanks to this foundational work, we now have a better understanding of age differences and longitudinal changes in brain structure (e.g., Raz et al. 2010, 2012; Walhovd et al. 2011), function (e.g., Reuter-Lorenz et al. 1999; Cabeza 2002; Cabeza et al. 2002, 2018; Reuter-Lorenz and Cappell 2008), and both structural and functional connectivity (e.g., Sullivan and Pfefferbaum 2006; Andrews-Hanna et al. 2007; Damoiseaux et al. 2008; Salat 2011; Bernard et al. 2013a, 2021a; Ferreira and Busatto 2013; Ferreira et al. 2016). This has also extended to further advance our understanding of neurodegenerative diseases associated with aging, in particular Alzheimer’s dementia. However, while our understanding of the aging brain and behavior has expanded greatly in the past several decades, the ability to predict behavioral outcomes for aging individuals, particularly those related to neurodegenerative disease, remains largely elusive. To this point, the primary focus of research on aging, with the exception of extensive work looking at the hippocampus (e.g., Daselaar et al. 2006; Woodruff-Pak et al. 2010; Carr et al. 2017) has focused on the cerebral cortex. While this work has been foundational for our understanding of brain and behavioral aging, it has also left a hole in our understanding of brain processes, and in turn, in our understanding of the factors that contribute to age-related behavioral differences and changes. Often overlook and relatively understudied is the cerebellum, or “little brain”.
In the human brain, the cerebellum represents approximately 10% of total brain volume, and recent estimates suggests that the surface area of the cerebellum is 78% of that in the cerebral cortex (Sereno et al. 2020), highlighting the computational power of this structure. Further, the posterior cerebellum, which is both structurally and functionally linked to the prefrontal cortex (PFC) (e.g., Kelly and Strick 2003; Krienen and Buckner 2009; Strick et al. 2009; Bernard et al. 2012), has increased in relative volume in proportion with the evolution of the PFC (Balsters et al. 2010). Given the interconnectedness of this cerebellar territory with the PFC, it is perhaps not surprising to find that functional task activation is seen in this region during the performance of cognitive tasks (Stoodley and Schmahmann 2009; Stoodley et al. 2012; Keren-Happuch et al. 2014; King et al. 2019). In addition to these posterior regions that are volumetrically larger as compared to other non-human primates (Balsters et al. 2010), there are also regions associated with motor function, having disynaptic connections with the motor cortex via the thalamus (e.g., Kelly and Strick 2003; Salmi et al. 2010; Stoodley et al. 2012; Bernard et al. 2016; King, Hernandez-castillo, Poldrack, et al. 2019). Because there are known differences in both motor and cognitive function that are experienced in older adults (OA) (e.g., Seidler et al. 2010; Cabeza et al. 2018), understanding how the cerebellum differs in this population, and changes across adulthood is of a great deal of interest and importance.
Current Perspectives on the Aging Brain and Behavior
At this point, the Literature on the cognitive neuroscience of aging has taken off and produced a variety of descriptive frameworks and hypotheses related to cortical function and activation patterns in OA. While an overview will be provided here to provide important context, a more detail review and comparison across these frameworks is presented by Festini and colleagues (Festini et al. 2018). Arguably, the most well-known of these patterns is that of bilateral cortical activation in advanced age. First described by Reuter-Lorenz and colleagues (Reuter-Lorenz et al. 1999), this was subsequently conceptualized as hemispheric asymmetry reduction in older adults (HAROLD; Cabeza 2002). The HAROLD pattern has been robustly replicated, and though it was first focused on during cognitive task performance, similar patterns are seen in the motor cortex as well (Mattay et al. 2002; Seidler et al. 2010). In parallel to these bilateral patterns, a shift in activation from more posterior cortical regions to more anterior regions in the prefrontal cortex has also been described (Davis et al. 2008). While HAROLD and the posterior-to-anterior shift are both largely descriptive, and provide excellent benchmarks for our understanding of functional activation in the aging brain, additional frameworks have been subsequently developed to generate theories and predictions with respect to brain activation patterns, and to better understand the individual differences in behavioral outcomes for older adults.
The compensation-related utilization of neural circuits hypothesis (CRUNCH; Reuter-Lorenz and Cappell 2008) postulates that the additional activation seen in OA in the prefrontal cortex is compensatory. That is, OA use this activation to maintain performance as tasks become increasingly difficult. Most notably CRUNCH allows for the generation of testable predictions with respect to prefrontal activation patterns in OA in the context of increasing task difficulty. Initial work looking at this idea was consistent with the hypothesis. That is, as working memory load increased, OA showed increased activation in the lateral PFC; however, they could only do this to a point and when the task became too difficult, this activation decreased again (Cappell et al. 2010). More recently, it has been demonstrated that training can improve the degree to which individuals can bring on these resources, but in aging, this occurs only to a point (Iordan et al. 2020). Notably, while there is some evidence in support of the CRUNCH hypothesis, recent work has suggested that these predictions do not always hold, and that there is, in general, not enough work testing this directly to make any firm conclusions related to the compensatory nature of this bilateral activation in older adults (Jamadar 2020).
While CRUNCH focuses on functional activation patterns in the PFC, the scaffolding theory of aging and cognition, along with its revised version (STAC, STAC-R; Park and Reuter-Lorenz 2009; Reuter-Lorenz and Park 2014) focuses instead on the brain, environmental, and behavioral factors that contribute to behavioral outcomes in later life. In its revised form STAC-R (Reuter-Lorenz and Park 2014) also considers the impact of plasticity, and life experience at all points, recognizing that functional outcomes in OA are not static. In both instantiations, STAC notes that scaffolding may be recruited in an effort to compensate for changes in brain structure and networks in advanced age, but this scaffolding could be in multiple forms (Park and Reuter-Lorenz 2009; Reuter-Lorenz and Park 2014). That is, in individuals that better maintain white matter for example, they may be better able to rely on other network connections for performance if there is volumetric loss in the PFC. Similarly, bilateral activation patterns could be considered scaffolding of a sort, as they too are recruited to compensate for other brain differences and changes and help to maintain some degree of functional performance. Finally, cognitive training, exercise, or nutritional interventions could also influence this scaffolding process, further mitigating age-related changes, and contributing to the variability in performance in OA (Park and Reuter-Lorenz 2009; Kennedy et al. 2014). Here, again, predictions related to brain structure and networks with respect to their impact on behavioral performance and brain activation patterns can be made. Notably, in the context of STAC and STAC-R, many things can serve as scaffolding, further opening up new avenues for research to better understand factors (biological, psychological, environmental, etc.) that may scaffold function across older adulthood. Critically, with this in mind, I have adopted the scaffolding term henceforth throughout this review.
In a recent review on the cognitive neuroscience of aging, Cabeza and colleagues (Cabeza et al. 2018) sought to clarify several key concepts, that are cross-cutting themes with respect to the perspectives and frameworks just described. As they note, differences in terminology and the definitions used in research in aging, can be a barrier to the needed advances in the field, critical for better understand the variable trajectories that can be experienced in later life (Cabeza et al. 2018). In their work, they provide definitions of reserve, maintenance, and compensation based on work focused on aging in healthy OA. Reserve refers to neurocognitive resources that can help buffer the effects of neural decline seen in aging. Maintenance refers to the preservation of neural resources, with a particular focus on bringing resources back to a baseline level, and not improvement beyond that point. Finally, they refer to compensation as the recruitment of neural resources in the face of increasing cognitive demands to help with performance (Cabeza et al. 2018). While these definitions were not presented without controversy, particularly with respect to reserve (Stern et al. 2019), they represent a helpful framework moving forward for research in this domain. This terminology encompasses the ideas outlined above in existing frameworks for function. Moving forward, I will be focusing on the idea of reserve and use this term interchangeably with the notion of scaffolding. Ultimately, the concepts are the same and are used in reference to the same idea. That is, what are the mechanisms that an individual may rely upon to buffer against the changes experienced in older adulthood?
With all that said, what are the sources of reserve? What mechanisms are used to compensate for changes in neural function in advanced age? While some of the theoretical frameworks described above such as CRUNCH and STAC are agnostic as to the brain regions involved (Reuter-Lorenz and Cappell 2008; Park and Reuter-Lorenz 2009; Reuter-Lorenz and Park 2014), the majority of the work to this point has been focused on the cerebral cortex. While this has been foundational for the development of these frameworks and brought forth key insights into the aging brain, a portion of the puzzle is missing. With the exception of the hippocampus, the integration of subcortical structures and circuits into our broader conceptualizations of aging has been limited. Specifically, the cerebellum may serve as a key region of reserve and may help scaffold function and performance in the healthy brain. As individuals age, the cerebellum is impacted by the aging process (MacLullich et al. 2004; Woodruff-Pak et al. 2010; Bernard and Seidler 2013a, 2014; Bernard et al. 2013a; Miller et al. 2013), weakening this reserve, and in turn impacting behavioral performance and cortical activation patterns. Indeed, loss of gray matter in the cerebellum has been demonstrated to be second only to that in the hippocampus, and similar in degree as losses in the PFC (Jernigan et al. 2001), while work in rodent models indicates that cerebellar senescence may occur earlier relative to other brain regions (Woodruff-Pak et al. 2010), and as such these losses may be particularly important for our understanding of behavior.
As imaging methods have advanced in recent years, getting good coverage of the whole brain to include the cerebellum in particular has advanced our knowledge of this structure, particularly in the context of its contributions to cognition (Schmahmann and Sherman 1998; Stoodley and Schmahmann 2009; Stoodley et al. 2012; Keren-Happuch et al. 2014; King et al. 2019), further underscoring the utility of its inclusion in our frameworks and discussions of cognitive aging. Here, I will first provide an overview of cerebellar function and processing, followed by an overview of the literature on the cerebellum and in advanced age. I posit that the cerebellum is a critical form of reserve and that its functions serve to scaffold cortical processing. Specifically, I argue that based on what is known to date about cerebellar functions in behavior, this is a key region that contributes to automatic processing and performance (Ramnani 2006, 2014; Ito 2008). In healthy young adults, cortical processing can be offloaded to the cerebellum during task performance, freeing up cortical processing resources so they can be further allocated if a task becomes more challenging. Based on current work on the cerebellum in advanced age, I argue that this offloading cannot occur as effectively, resulting in an increase in cortical processing, consistent with what is seen and proposed in many of the existing frameworks (e.g., HAROLD, CRUNCH) (Cabeza 2002; Reuter-Lorenz and Lustig 2005; Reuter-Lorenz and Cappell 2008; Cappell et al. 2010; Cabeza et al. 2018). This hypothesis also results in testable predictions with respect to cerebellar activation patterns in conjunction with those in the cerebral cortex, as well as in behavior. Notably, while the discussion throughout focuses primarily on the PFC, this idea is applicable to all cortical areas, given that the cerebellum is connected to distinct regions of the cortex through parallel closed-loop circuits (described further below) (Jissendi et al. 2008; Strick et al. 2009; Salmi, Pallesen, Neuvonen, et al. 2010). Thus, while the discussion is often somewhat specific to the PFC, the idea would generalize more broadly across the cortex.
Cerebellar processing and internal models
Before covering the cerebellum in aging in more detail, I will first provide a very brief overview of cerebellar function. With that said, this is not meant to be exhaustive, but primarily to serve as a touch point for readers less familiar with cerebellar function. For readers interested in learning more about this topic there are many reviews available, some more classic and many more recent that provide comprehensive accounts of this functionality (Ramnani 2006, 2014; Ito 2008; Sokolov et al. 2017; Raymond and Medina 2018; Diedrichsen et al. 2019; Schmahmann et al. 2019) and more details about these models. Broadly speaking, the cerebellum is thought to process internal models of behavior. When considering internal models, it is recognized that they are important in the smooth performance of motor tasks, and this has been adapted to include thought and cognitive processes as well, given the closed-loop circuits linking the cerebellum to the cerebral cortex (Ramnani 2006, 2014; Ito 2008; Sokolov et al. 2017; Schmahmann et al. 2019). In the motor domain, over the course of learning new tasks, the cerebellum is involved and activation becomes more refined (Imamizu et al. 2000) as the internal model becomes more well-formed. Because the cytoarchitecture is consistent across the cerebellum, and the main differences in the source of input, this argument and approach would extend to the cognitive domain as well. Once an individual has well-formed and refined internal models, they may be relied upon automatically to produce fluid behavior or thought. Further, with this reliance upon learned internal models, individuals may then be able to free up cortical resources for other processing. Think for example about riding a bike, procedural knowledge that many individuals have. Once this is well-learned, bike riding becomes fairly automatic, and during a bike ride an individual may be able to think about other things or maintain a conversation with a friend, all while keeping an eye on the path or road ahead. This is because automatic procedural systems are allowing for the bike riding to occur while freeing up cortical processing. If the resources allowing for that automatic bike riding, in this example the internal models, are less reliable and functioning poorly (or, communicating less well with the cortex) then the task will become more demanding and requires more cortical resources. Indeed, we previously suggested that the structural and connectivity differences that impact the cerebellum in advanced age (described briefly in the next section) negatively impact processing related to internal models (Bernard and Seidler 2014).
The cerebellum in aging
Relatively recently, we reviewed the extant literature on the cerebellum in advanced age (Bernard and Seidler 2014). In the interest of brevity, a brief overview of that work is provided here, and an overview of notable updates to the literature are provided. As reviewed by Bernard and Seidler (2014), there are significant age differences in cerebellar volume, functional connectivity, and structural connectivity. This work proposed that as a result of these differences in advanced age, cerebellar processing is negatively impacted. As such, in the brain of older adults it is increasingly difficult to process efference copies of task commands (be it motor or cognitive) due to the differences in connectivity with the cortex (both structural and functional) and smaller volume which may relate to reduced processing capacity (Bernard and Seidler 2014). Since this publication, additional evidence supporting age differences in regional cerebellar volume has emerged, and it further supports findings suggesting that regional cerebellar volume is associated with behavioral performance (Koppelmans et al. 2015, 2017) Further, recent work from Han and colleagues (Han et al. 2020) has advanced our understanding of regional cerebellar volume in aging by demonstrating longitudinal changes in volume over time. While earlier work had demonstrated changes in cerebellar volume in aging, this was done at a more gross anatomical level (Raz et al. 2005, 2013). This new investigation looks at the lobular level and has provided a more nuanced understanding of changes in the cerebellum in advanced age (Han et al. 2020). In addition to this longitudinal work, we investigated lobular volumetric associations with age from adolescence through middle age (Bernard et al. 2015) and Romero and colleagues (Romero et al. 2021) conducted an extensive analysis of lobular cerebellar volume in a large sample (n>2,500) ranging in age from 1 to 94 years old. The trajectories presented by Romero and colleagues (2021) indicate that during childhood and adolescence there is a rapid increase in lobular volume when looking at raw volumes, and when normalized, this decreases with age. They also found that cerebellar white matter shows an inverted-u shaped relationship with age. The findings from Romero and colleagues (2021) are broadly consistent with our work looking at adolescence and adulthood through middle age (Bernard et al. 2015), though the relationships with age differed based on cerebellar region. That is, in the lateral posterior aspects of the cerebellum, we demonstrated an inverted-u relationship with age, but in anterior regions (e.g., Lobule V), the relationships were negative linear associations (Bernard et al. 2015). Furthermore, in a large investigation using the OASIS data set, additional age differences in lobular cerebellar volume have been reported (Uwisengeyimana et al. 2020), while Cui and colleagues (Cui et al. 2020) found that volume was smaller in Lobules VI, X, Crus I and Crus II bilaterally in addition to unilateral lobular differences when comparing old adults to young. Furthermore, they found that lobular volume was related to memory recall (Cui et al. 2020). Thus, while individual studies may show some differences regarding the specificity of patterns of lobular volume differences, together however, the literature on cerebellar structure in advanced age is quite clear. In OA, cerebellar grey matter volume is smaller, and decreases over time when investigated longitudinally.
In parallel to these structural differences, cerebellar functional connectivity and activation are also different in OA. Since my prior review (Bernard and Seidler 2014), additional work characterizing cerebellar resting state connectivity in advanced age has been published. de Dieu Uwisengeyimana and colleagues (2020) demonstrated within cerebellar age differences, as well as age differences in cerebello-cortical connectivity impacting the medial temporal lobes, default mode network, and basal ganglia, which is nearly identical to what we first reported (Bernard et al. 2013a). Furthermore, work investigating earlier differences in connectivity, with a focus on middle age demonstrated that connectivity in the motor network to the anterior cerebellum is lower when compared to young adults (Siman-Tov et al. 2017). This work suggests that differences, at least in connectivity of motor regions of the cerebellum, may begin to emerge earlier in adulthood. Finally, work from my own group investigating resting state connectivity in the cerebellum has added to this literature. We took advantage of a large-data set (Cambridge Center for Aging and Neuroscience) (Shafto et al. 2014; Taylor et al. 2017) to investigate relationships between age and cerebellar dentate connectivity across adulthood. Work in both non-human primates and in humans has demonstrated that the dorsal and ventral sub-regions of the cerebellar dentate nucleus are associated with motor and frontal/association cortices, respectively (Dum and Strick 2003; Strick et al. 2009; Bernard et al. 2014; Steele et al. 2017). As this is a key output region of the cerebellum, it was of great interest to understand how connectivity relates to age. Given what is known about lobular cerebellar connectivity, it is perhaps not surprising that in this sample of nearly 600 adults, there were negative correlations with age (Bernard et al. 2021a). For seeds in both the dorsal and ventral dentate, with older age, connectivity from the dentate nucleus to the cerebral cortex was lower. Furthermore, the associations between connectivity and age were investigated for both males and females. Different patterns of connectivity and age relationships were revealed between the two sexes, and several significant interactions emerged, suggesting that the experience of aging may impact connectivity of the dentate nucleus differently in females and males (Bernard et al. 2021a).
With that said, patterns reflecting multi-directionality in advanced age are not uncommon. Thus, while there is a substantial amount of evidence demonstrating lower connectivity between the cerebellum and cortex in advanced age, this is not exclusively the case. In an investigation of dynamic network segregation in advanced age, while most networks showed lower segregation in advanced age, this was not the case for the cerebellum (He et al. 2020). Furthermore, Seidler and colleagues (Seidler et al. 2015) investigated lobular cerebellar connectivity in the context of motor function and demonstrated both higher and lower connectivity in older individuals depending upon the cerebellar seed. Cerebellar lobule VIII showed higher connectivity with the putamen with increasing age, while Lobule V showed lower connectivity with cortical regions (Seidler et al. 2015). Certainly, differences in sample size, region, and analysis method could all result in somewhat diverging patterns. On average, across studies there are noted age differences in cerebellar connectivity when comparing young and older adults, wherein connectivity is generally lower in older adults. However, aging is characterized by multi-dimensionality and there may be limited instances where resting state cerebellar networks do not differ in advanced age.
While resting state connectivity provides insights into the functional interactions and dynamics of the cerebellum in the context of the cortex and other subcortical regions, functional activation during task performance in older adults is also of interest and importance. Briefly, though the cerebellum is active during task performance across domains (e.g., Stoodley et al. 2012; Keren-Happuch et al. 2014; King, Hernandez-Castillo, Poldrack, et al. 2019), there are very few direct investigations of cerebellar functional activation in advanced age. Though this literature is relatively limited, the work to date has led to key insights. Using a predictive motor timing task Filip and colleagues (Filip et al. 2019) were the first to directly investigate cerebellar function in older adults. Interestingly, the authors found no major negative impacts of age on performance, even in the presences of robust age differences in cerebellar structure (Filip et al. 2019). In parallel, they were the first to suggest the idea of cerebellar scaffolding of function in advanced age, as they also demonstrated increased functional activation in the cerebellum during task performance in older adults that was associated with better performance. As such, they argued that this increased activation was in order to scaffold and maintain performance.
In our own work looking at second-order rule learning, we found mixed results with respect to cerebellar activation patterns in older adults relative to young adults. In this investigation, modelled after prior work demonstrating that the cerebellum can encode higher-order rules in young adults (Balsters et al. 2013), we demonstrated that while older adults did learn these higher-order rules, there were age differences in this learning. Older adults never reached the same degree of performance as young adults (Jackson et al. 2020). When investigating cerebellar activation, analyses of effect sizes suggested significantly greater activation in the cerebellum during early learning in young adults when compared to older adults (Jackson et al. 2020). Unlike Filip and colleagues (Filip et al. 2019), when older participants were learning the higher-order rules, they did not recruit the cerebellum as extensively as young adults. However, when receiving performance feedback older adults did show some cerebellar regions where activation effect sizes were larger than those seen in young adults (Jackson et al. 2020). Thus, in this cognitive task, activation of the cerebellum during performance is seemingly more complex.
In an attempt to synthesize across the existing literature, we also computed a large-scale activation likelihood-estimation meta-analysis (Bernard et al. 2020). While direct investigations of cerebellar functional activation in older adults are limited to those described above, some degree of cerebellar activation in many, if not most, commonly used tasks in cognitive and motor aging result in cerebellar activation (Stoodley and Schmahmann 2009; Stoodley et al. 2012; Keren-Happuch et al. 2014; King et al. 2019). We took advantage of this and synthesized these findings to compare young and older adults. After collecting activation foci from 175 studies, we had data from 1,710 young adults and 2,160 older adults, across behavioral domains (Bernard et al. 2020). This investigation revealed several key findings. First, when examining the functional topography of activation in the cerebellum, the general patterns of overlap in older adults were greatly similar to what has previously been reported in young adults (Stoodley and Schmahmann 2009; Keren-Happuch et al. 2014; King et al. 2019; Bernard et al. 2020). Second, when we contrasted young and older adults we saw that for cognitive tasks, activation overlap was substantially lower in older relative to younger adults. However, when we looked at motor tasks this pattern was reversed and activation overlap was higher in older relative to younger (Bernard et al. 2020). This latter finding in the motor domain is consistent with what was observed by Filip and colleagues (Filip et al. 2019) during predictive motor timing. Thus, their suggestion of scaffolding of performance in the motor domain is consistent with our meta-analytic findings. However, this was not the case for cognition. Indeed, it seems as though the scaffolding is “shaky”, as older adults are bringing on cerebellar resources to help with performance during cognitive tasks. One possibility that we suggested for these domain differences is in the context of internal models (e.g., Ramnani 2006; Ito 2008; Bernard and Seidler 2014; Bernard et al. 2020), as older adults have differing input and feedback loops for information about ongoing cognitive versus motor performance relative to younger adults. That is, cognitive performance information will have feedback coming from the cortex, whereas feedback about motor behaviors may be more peripheral and via spinal circuits, and these pathways may be differentially impacted in advanced age (Bernard et al. 2020). In this context however, this work provides an indication that cerebellar function during cognitive performance may be altered in older adults, and contributes, at least in part to the differences and declines experienced in advanced age.
Cerebello-Subcortical Interactions in Advanced Age
As described above, the cerebellum is structurally and functionally different in advanced age. And, while connectivity measures imply interactions with the cortex also differ, there are two subcortical circuits that are notable and worth further discussion, particularly when considering whether and how the cerebellum contributes to age-related behavioral differences and changes. In our exploratory resting state work investigating lobular connectivity, we consistently saw differences in connectivity with both the striatum and medial temporal lobes (Bernard et al. 2013a), and additional recent work has replicated this (Uwisengeyimana et al. 2020). Given the suggested importance of subcortical structures and interactions, further consideration of the basal ganglia and medial temporal lobes in relation to the cerebellum is of importance for our conceptualization of the role of this structure in cognitive aging.
Cerebellum and Basal Ganglia
The basal ganglia, like the cerebellum are often considered in the context of motor behavior, given their prominent role in movement disorders such as Parkinson’s Disease. However, the connectivity patterns of the basal ganglia indicate interactions with the cortex more broadly, including cognitive and associative regions (Di Martino et al. 2008; Draganski et al. 2008; Gordon et al. 2021; Liu et al. 2021). Furthermore, there is longstanding evidence to suggest that the basal ganglia are important for cognitive processing (e.g., Graybiel 1997; Middleton and Strick 2000). A more recent meta-analysis has also provided strong support for the basal ganglia in cognitive processing, and further demonstrates a degree of functional topography that is consistent with the known network topography and organization (Di Martino et al. 2008; Draganski et al. 2008; Bernard et al. 2017; Gordon et al. 2021; Liu et al. 2021). Thus, much like the cerebellum there are both cognitive and motor contributions of the basal ganglia, though a complete overview of this literature is beyond the scope of this review.
The most recent investigations of connectivity patterns of the basal ganglia have demonstrated that there is a great deal of evolutionary conservation with respect to the connections with the cortex, as compared to non-human primates; however, there has also been the emergence of circuits that are seemingly uniquely human and relate to language (Liu et al. 2021). Furthermore, when mapping function using meta-analysis, activation overlap across unique task domains parallels the relative organization of circuits mapped using diffusion tensor imaging (Bernard et al. 2017). Notably, this work broadly suggests a degree of topographical organization within the basal ganglia, while also providing evidence that these nuclei are involved in a wide range of task domains beyond just motor function.
In advanced age, function of the basal ganglia is also notably impacted. Much of the work in this domain has focused on dopamine. Differences in dopaminergic function in the basal ganglia of aged individuals have been reported across mammal species (Morgan and Finch, 1988). In the human brain, in advanced age there is a decrease in dopaminergic receptor density, a decline in the dopamine transporter, and a decrease in dopaminergic cells in the substantia nigra resulting in an overall downregulation of the dopaminergic system (Bäckman et al. 2006). It has also been suggested that the dopaminergic system may be under responsive during cognitive performance (Bäckman et al. 2010). Finally, dopamine release may be decreased in advanced age (Karlsson et al. 2009). Meta-analytic data supports this, though age-effects on dopamine release are not as clear as there is less data in this regard (Karrer et al. 2017). Critically however, these age-differences in dopamine associated with the basal ganglia are related to cognitive performance (Bäckman et al. 2006, 2010; Karrer et al. 2017) in large part due to dopaminergic receptors in the prefrontal cortex. Motor behaviors have also been implicated as well (Noohi et al. 2014). Furthermore, both structural and functional networks connecting the basal ganglia and cortex have been implicated in cognitive performance in older adults (Ystad et al. 2010, 2011). While this aspect of the literature is not as robust as that focused on the dopaminergic system, and much is still to be learned about the basal ganglia in healthy normative aging, it is certainly the case that this region is impacted in advanced age, and in ways that impact behavioral performance, contributing to the normative age-related declines seen in motor and cognitive function.
Though the cerebellum and basal ganglia are often investigated separately, particularly in investigations of connectivity, both regions are not independent from one another. Behaviorally, in motor learning in particular, these structures have been recognized as complementary, and together play a key role in learning and performance (e.g., Doyon et al. 2018). However, anatomically, these regions also share connections. In non-human primates, bi-directional circuits connecting the basal ganglia to the cerebellum have been delineated (Hoshi et al. 2005; Bostan et al. 2010; Bostan and Strick 2018). While in the human brain it can be difficult to resolve the dense white matter between these two regions, at least one investigation has confirmed the structural connections between these key subcortical regions (Pelzer et al. 2013). Furthermore, at rest, there are robust interactions between the cerebellum and basal ganglia in the human brain (Hausman et al. 2020). The resting state signal in subregions that share functional contributions (that is areas associated with motor tasks, and those associated with non-motor tasks) are strongly correlated with one another (Hausman et al., 2020) further supporting the interactions between these key subcortical structures. As such, together, these regions may further act as a broader subcortical scaffolding for function in advanced age.
Indeed, as alluded to above when investigating cerebellar connectivity in older adults, a pattern wherein connectivity between the cerebellum and basal ganglia was consistently lower in older relative to young adults was revealed (Bernard et al. 2013a). To follow-up on this finding we completed a targeted investigation of connectivity between the cerebellum and basal ganglia in older adults in comparison to young adults. This work demonstrated that older individuals show significantly lower connectivity between the cerebellum and basal ganglia when compared to young (Hausman et al. 2020). In addition, not only is connectivity lower, but it is different in direction. That is, in older adults, we saw anti-correlations between the cerebellum and basal ganglia. The overall consistency of this targeted investigation with our prior whole-brain exploratory work provided strong additional evidence to suggest age differences in the communication between these two important sub-cortical regions (Bernard et al. 2013; Hausman et al. 2020). Furthermore, it suggests that age differences in the cerebellum, along with its interactions with the basal ganglia may have wide-reaching impacts on cortical function as a form of scaffolding in advanced age.
Cerebellum and Hippocampus
When thinking about aging, the structure perhaps most often mentioned is the hippocampus, given its prominent role in memory. An exhaustive overview of the role of the hippocampus in memory, spatial navigation, and cognition more generally is beyond the scope of this review, as is a detailed overview of age differences in changes in this structure and its function. In brief, we know that the hippocampus is smaller in older adults (e.g., Raz et al. 2004), and hippocampal function is different (e.g., Daselaar et al. 2006; Carr et al. 2017), which has impacts for memory, spatial navigation, and cognition more generally (e.g., O’Shea et al. 2016). Furthermore, there are hippocampal contributions to motor learning (Schendan et al. 2003; Doyon et al. 2018) which could in turn have impacts for rehabilitation in advanced age. The relative importance of the hippocampus, coupled with its purported role in Alzheimer’s disease (Halliday 2017) have resulted in a large literature focused on the structure in advanced age; however, consideration of its interactions with the cerebellum are of interest, particularly given the important role both of these structures play in cognition.
Just as much of our initial understanding of cerebello-thalamo-cortical circuits was based on work in non-human animal models (Dum and Strick 2003; Kelly and Strick 2003; Hoshi et al. 2005; Strick et al. 2009; Bostan et al. 2010), such is also the case with respect to interactions between the cerebellum and hippocampus. As reviewed by Yu and Krook-Magnuson (Yu and Krook-Magnuson 2015), in animal models, largely rodent, there are bidirectional interactions between the cerebellum and hippocampus, with evidence largely coming from work investigating spatial and temporal processing. They also highlight that these interactions could be due to direct connections between the two regions; but, multi-synaptic pathways would also allow for the emergence of the cerebello-hippocampal circuits. In subsequent empirical work focused on goal-directed behavior in the context of navigation, there was prominent involvement of the cerebellum and hippocampus together once animals learned a sequence of movements, moving these interactions beyond just spatial and temporal processing (Babayan et al. 2017). In a detailed study investigating both structural connections and functional interactions between the cerebellum and hippocampus, Watson and colleagues (Watson et al. 2019) found that there are indeed multi-synaptic connections linking the cerebellum and hippocampus, as evidenced using rabies tracers. Most notably, they demonstrated three main inputs to the hippocampus from the cerebellum: from the vestibulo-cerebellum, the central region of the structure via Vermis VI, and finally from Crus I via the dentate nucleus (Watson et al. 2019). Furthermore, they also found functional interactions between the regions, as quantified by local field potentials during a spatial learning task (Watson et al. 2019). Finally, further showing the interactions between the regions, and providing a degree of relative causality with respect to these interactions, work using optogenetic stimulation to the cerebellum resulted in disrupted performance on a spatial memory task but also influenced the local field potentials recorded from the hippocampus, relative to a control condition (Zeidler et al. 2020). With this work, the authors coined the idea of the “hippobellum” given the interactions between these regions, and the notion that modulation of the cerebellum can in turn influence the hippocampus and associated cognitive functions (Zeidler et al. 2020). Notably however, this is not an exhaustive overview of this literature (see Yu & Krook-Magnuson 2015 for a review), but this highlights the interactions between these important regions as investigated in non-human animal models.
Given the vibrant literature on interactions between the cerebellum and hippocampus in animal models and the known important contributions of these structures to cognition, there is also work investigating these structures and their interactions in the human brain. As noted above, we have long known that both the cerebellum and hippocampus are involved in motor sequence learning (Schendan et al. 2003; Doyon et al. 2018). However more recently, as our knowledge and understanding of cerebellar involvement in varying domains of cognitive function has grown (e.g., Stoodley et al. 2012; King, Hernandez-Castillo, et al. 2019), there is also an interest in its contributions to domains like spatial processing, an area where the hippocampus has been heavily implicated. Using a virtual reality navigation task in the neuroimaging environment Iglói and colleagues (Iglói et al. 2015) specifically investigated the cerebellum in this domain. Further supporting the existence of interactions between the cerebellum and hippocampus they suggested that there are two functional circuits that engage Crus I of the cerebellum and the hippocampus for place-based and sequence-based navigation (Iglói et al. 2015). This work highlights the functional interactions between these regions, and extends our understanding of cerebellar and hippocampal involvement in function beyond motor sequence learning. Finally, structural connectivity between the two regions has been mapped in the human brain using diffusion imaging (Arrigo et al. 2014). While this work has a small sample and replication is needed, it suggests the possibility of a direct connection from the hippocampus to the cerebellum, via the cerebellar peduncles may be present in the human brain (Arrigo et al. 2014). With that said, it is also worth highlighting that indirect pathways, such as those outlined by Yu and Crook-Magnuson (2015), may support cerebello-hippocampal interactions in the human brain.
Work from both animal models and the human brain suggest that the cerebellum and hippocampus interact in dynamic ways, and perturbations of these circuits can impact behavior. As we consider the role of the cerebellum in aging, recognizing these interactions is critical, particularly given the role of hippocampal function for memory and cognition, and the known differences in hippocampal structure and function in advanced age. To this point, there has been some suggestion that the functional connections between the regions are impacted in advanced age. In our own work investigating cerebello-cortical connectivity in older adults, one of the prominent patterns that emerged across cerebellar seeds was decreased connectivity with regions of the medial temporal lobe including the hippocampus and parahippocampal gyrus (Bernard et al. 2013a). Notably many of the cerebellar seed regions that showed these age differences correspond to the regions showing structural connections with the hippocampus in animal models (Watson et al. 2019). Furthermore, this resting state connectivity finding was recently replicated in a larger sample (Uwisengeyimana et al. 2020) further highlighting potential disruptions in this subcortical circuit. This could in turn have impacts on cognitive function and other cortical processing streams, though certainly more targeted research on this circuit and age differences therein is warranted. Here, I suggest that again, the cerebellum contributes to further scaffolding of cortical functions via this additional subcortical circuit with the hippocampus.
Conceptualizing the Cerebellum in the Aging Brain: Shaky Scaffolding
To this point, there is clear and compelling evidence to suggest that the cerebellum is impacted in advanced age with respect to structure, function and connectivity patterns. However, it is also critical to integrate the cerebellum and cerebellar function into current models of the aging brain and aging brain function. The current frameworks for understanding cognitive aging are centered around cortical systems, and have been defined largely based on patterns of cortical atrophy and activation patterns. As noted above, these frameworks and conceptualizations have been critical for advancing our understanding of behavior in advanced age, and for providing insights into age-related disease. However, these cortically-focused theories have left a lot on the table, in terms of cerebellar contributions. Its surface area alone nearly matches that of the cortex (Sereno et al. 2020), and as outlined above, there are well-categorized differences in the structure, its networks, and seemingly function in advanced age (e.g., MacLullich et al. 2004; Bernard and Seidler 2013b, 2014; Miller et al. 2013; Koppelmans et al. 2015; Bernard et al. 2020; Hausman et al. 2020; Jackson et al. 2020; Uwisengeyimana et al. 2020). With our increased understanding of the contributions of the cerebellum to numerous behavioral domains (Stoodley and Schmahmann 2009; Stoodley et al. 2012; King et al. 2019), it is imperative that we begin to incorporate the cerebellum into frameworks and theories of cognitive (and motor) aging.
The question then becomes, how does the cerebellum contribute to the differences seen in advanced age in both brain and behavior? Recently, there have been several reviews that address the cerebellum in the context of compensation (Liang and Carlson 2020), and in terms of cerebellar reserve (Mitoma et al. 2020; Bordignon et al. 2021). Notably however, this concept of “cerebellar reserve” is distinct from reserve in the context of the cortex and cognitive aging (no matter the definition -- whether it is one broader concept, or if brain and cognitive reserve are considered separately) (Cabeza et al. 2018; Stern et al. 2019). It is however a concept rooted in this same framework. As argued by Mitoma and colleagues (2020) cerebellar reserve is indeed specific to the cerebellum, and relates to the ability of the cerebellum to compensate for infarct and lesion (Bordignon and colleagues (2021) further reiterate this idea). Most notably, Mitoma and colleagues (2020) highlight the capabilities of the cerebellum to recover after both acute injury as well as in the context of congenital differences/damage. Further, cerebellar reserve in the context of aging and disease are discussed. They note that differences in cerebellar structure seen across some disease states may in fact be a form of compensation for cortical differences (Mitoma et al. 2020), though notably in many instances volume is in fact smaller making this somewhat less likely (e.g. Bottmer et al. 2005; Tabatabaei-Jafari et al. 2017; Moberget et al. 2018; Lin et al. 2020). Notably, the reorganization of internal models is highlighted, as are the many interconnected networks with the cortex (Mitoma et al. 2020). Both of these factors are likely critical components when considering the functional role of the cerebellum in aging.
Liang and Carlson (Liang and Carlson 2020) sought to describe and define instances of cerebellar compensation with a focus on pathology. In particular, they worked to define the degree to which an intact cerebellum may compensate to help maintain cognitive function, and they highlight and reiterate the importance of studying this structure for understanding cognition and disease (Liang and Carlson 2020). They argue that the cerebellum is a key component of brain networks, and framed their arguments in the context of machine learning. However, while these reviews have provided insights highlighting the cerebellum more broadly, particularly as a potential source of compensation, integrated frameworks with the cortex are still lacking.
To advance our understanding of cerebellar function and cerebello-cortical interactions in the aging brain, I have proposed a framework, building on prior work investigating the cerebral cortex (Park and Reuter-Lorenz 2009; Cappell et al. 2010; Reuter-Lorenz and Park 2014; Cabeza et al. 2018), and generating testable hypotheses that can drive future work to better understand this important subcortical structure and its role in aging. I propose that the cerebellum is a critical element of scaffolding for function in advanced age, using terminology and ideas proposed by Park and Reuter-Lorenz (Park and Reuter-Lorenz 2009; Reuter-Lorenz and Park 2014). Consistent with their concept of scaffolding, and their update that notes the capacity for change and modification to the various mechanisms for compensatory scaffolding (Reuter-Lorenz and Park 2014), the cerebellum and its function over the course of the lifespan may serve as scaffolding for cortical function. In the terminology discussed recently by Cabeza and colleagues (Cabeza et al. 2018), the cerebellum may also be considered a form of reserve. Notably however, in this context the cerebellum is acting as a source of reserve for the cortex, and this is distinct from cerebellar reserve as defined by Mitoma and colleagues (2020) (though cerebellar reserve may of course allow for the structure to better serve as a more general source of reserve for cortical function and processing). Similarly, Filip and colleagues (2019) suggested that cerebellar function may be able to scaffold cortical motor processing.
As outlined above in advanced age there are differences in cerebellar structure (Bernard and Seidler 2013b; Miller et al. 2013; Koppelmans et al. 2015; Han et al. 2020), networks (Bernard et al. 2013b, 2021b; Uwisengeyimana et al. 2020), and function (Filip et al. 2019; Bernard et al. 2020). Previously we had suggested that in advanced age, due to the smaller structure of the cerebellum and degraded connections (both structural and functional), internal models are less efficient and effectively used, contributing at least in part, to the behavioral differences and declines experienced in advanced age (Bernard and Seidler 2014). Here, I build on this idea to suggest that the cerebellum is critical scaffolding for optimal cortical functioning in advanced age, and alterations in cerebellar function result in many of the cortical activation patterns that have been classically described in advanced age (Reuter-Lorenz et al. 1999; Cabeza 2002; Cabeza et al. 2002). I suggest that the cerebellum is a critical site for offloading cortical processing due to its processing of internal models (described and discussed earlier in this review), and when that is not possible (Bernard and Seidler 2014), additional cortical resources are needed.
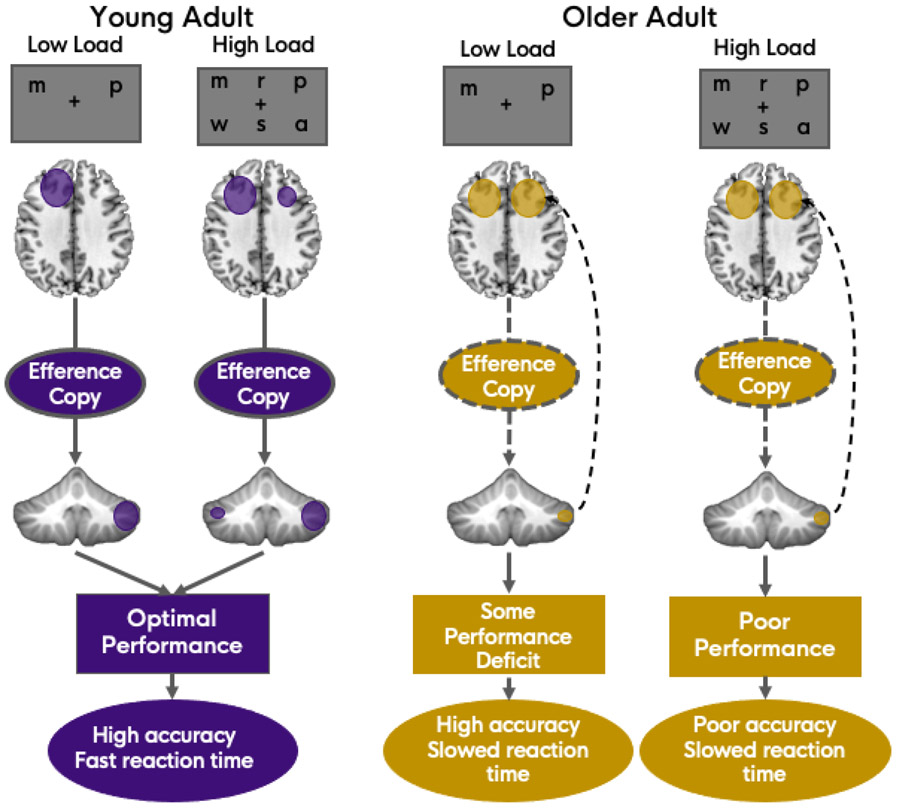
Having a well-functioning cerebellum that is active during task performance is key in this framework. Meta-analytic findings from my group suggest that across cognitive task domains, there is less activation convergence in older adults as compared to young adults in the cerebellum (Bernard et al. 2020). One interpretation is that older adults are not showing as much activation in the cerebellum as young adults, resulting in less overlap across studies. While there is no direct evidence to support this given the meta-analytic approach used in the work, imaging of second-order rule learning in young and older adults seems to support this idea (Jackson et al. 2020). With fewer cerebellar resources being recruited, we would in turn expect an increase in cortical activation (Figure 1). Older adults are not able to effectively process the efference copy (of a given thought process) due to connectivity differences between the cerebellum and cortex, and in turn, are less able to offload processing to this system that allows for automaticity. Notably, we speculate that in young adults, as we increase task difficulty, we would see an increase in cerebellar activation, and eventually, cortical activation increases as well (Figure 1). However, in the healthy young adult brain, compensation via more automatic cerebellar processes would occur first, before recruiting additional cortical processing. This is broadly consistent with the compensation related utilization of neural circuits hypothesis (CRUNCH; Reuter-Lorenz and Cappell 2008). In older adults, the ability to initially bolster resources and compensate for increasing task difficulty via the cerebellum is not available. Thus, I suggest that for the maintenance of cognitive performance and function, cerebellar resources are key as a form of reserve and scaffolding. As these resources begin to deteriorate through the process of aging, and are no longer able to rely upon their distinct cerebellar reserve capacity (Mitoma et al. 2020), additional cortical resources are needed. The impact of this is seen in behavioral performance on cognitive tasks, as well as in brain imaging in the form of increased cortical activation. Critically, this would apply to, and impact more generalized accounts of cognitive aging as well, such as those related to processing speed (e.g., Kail and Salthouse 1994; Salthouse 1996). Being less able to use automatic resources for processing will cause an additional bottleneck, compounded by differences in sensory systems and the increased need for cortical resources. This could in part contribute to age-related slowing in processing speed. Finally, it is also notable that this framework is agnostic to the specifics of internal model processing, as multiple hypotheses as to the nature of this process and the underlying cerebellar computations have been proposed (Ramnani 2006, 2014; Ito 2008; Sokolov et al. 2017; Raymond and Medina 2018; Schmahmann et al. 2019).
Figure 1.
Proposed model of cortico-cerebellar interactions under low and high task demands in young and older adults. Here, a verbal working memory task (variation on the Sternberg task) is depicted with a load of 2 (low) and 6 (high) as an example. However, any task domain where challenge can be increased based on number of items used or level of complexity would result in similar predictions based on this model. In young adults (left), there are effective cortico-cerebellar connections, as well as intact cerebellar tissue allowing for the efficient communication of efference copies, and the ability to rely upon internal models of behavior. As tasks become more difficult, cortical activation increases, along with some concomitant bilateral cerebellar activation. Under high load, this compensatory activation allows for optimal performance, here defined as high accuracy with fast reaction times. Connections in older adults (right) are degraded (dashed lines) and as such they are less able to offload processing and rely upon efference copies. This results in a need to recruit compensatory cortical resources (curved dashed arrow), and some performance deficits under low task demands. In this instance this may be manifest as relatively high accuracy but slowed reaction times. As tasks get harder, the cerebellum is still under-recruited, and cortical resources reach their maximum, causing more pronounced performance deficits, here defined as poor accuracy as well as slower responses. Please note, the dashed arrows back to the cortex in the older adult panel are illustrative only, reflecting the need for additional cortical compensation. The white matter tracts connecting the cerebellum to the cortex (via the thalamus) are contralateral (right cerebellum is connected to the left cortical hemisphere).
To this point, this framework remains generally theoretical, and direct evidence in older adults is needed. However, I suggest that the idea itself as outlined in Figure 1 results in testable hypotheses and clear predictions as to what might be expected in subsequent research. Indirect evidence using non-invasive brain stimulation in young adults has however been provided. Maldonado and colleagues used cerebellar transcranial direct current stimulation (tDCS) just prior to fMRI to investigate how cortical activation differs after cerebellar inhibition relative to sham or excitatory stimulation (Maldonado et al. 2021). Given the framework outlined here, the inhibitory condition was of particular interest. Participants performed both an explicit sequence learning task and the Sternberg verbal working memory task during functional scanning. When investigating cortical activation during performance of the explicit sequence learning task, there was bilateral parietal lobe activation when comparing inhibitory relative to excitatory stimulation. When compared to sham stimulation, there was greater activation in the prefrontal cortex. During performance of the verbal working memory task, Maldonado and colleagues found robust prefrontal cortical activation after inhibitory stimulation to the cerebellum (Maldonado et al. 2021). Together, this is consistent with the idea proposed here. That is, if the cerebellum is not functioning optimally, the cortex needs to compensate. When individuals are less able to offload processing to the cerebellum, more cortical resources are needed. In this example, task difficulty was relatively low. However, the current framework would suggest that when the cerebellum is inhibited, young adults would more quickly begin to show declines in task performance as well, as task difficulty increases.
The current framework for understanding the cerebellum and cerebello-cortical interactions is focused predominantly on the prefrontal cortex. However, interactions between the cerebellum and other cortical and subcortical regions are also impacted by cerebellar functional differences. This in turn, can further impact function in advanced age. Closed-loop circuits have been demonstrated linking the cerebellum to both the motor and prefrontal cortex, and the parietal cortex is also a known target of these projections (Clower et al. 2001; Kelly and Strick 2003; Strick et al. 2009). While our predictions in the context of this framework are outlined with respect to the prefrontal cortex, such predictions would hold when considering other cortical regions, though of course this is an open empirical question.
As outlined earlier, there are robust connections between the cerebellum and basal ganglia seen in both the human and non-human primate brains (Hoshi et al. 2005; Bostan et al. 2010; Bostan and Strick 2018; Hausman et al. 2020), and in advanced age, connectivity is lower (Hausman et al. 2020). Furthermore, animal work in particular has revealed interactions between the hippocampus and cerebellum (e.g., Yu and Krook-Magnuson 2015; Zeidler et al. 2020) and stimulation to the cerebellum impacts hippocampal firing, and hippocampally-mediate behavior (Zeidler et al. 2020). If functional processing in the cerebellum is impacted in advanced age as proposed here, there are also likely additional impacts on behavior, across domains, via these interactions with the basal ganglia and hippocampus. More generally, this may be indicative of weakened subcortical scaffolding across systems that gives rise to differences in cortical functional activation differences and behavioral deficits. Moving forward, it will be of great interest to create more inclusive frameworks that also incorporate the contributions of the basal ganglia and hippocampus in relation to the role of the cerebellum in advanced age.
Implications for Age Related Disease: Alzheimer’s Dementia, Mild Cognitive Impairment, and Other Dementias
The proposed framework above has been developed with typical aging in mind. That is, aging in the absence of disease or infarct. With that in mind, this conceptualization of the cerebellum and its relationships with the cerebral cortex may also better inform our understanding of pathological aging. While this framework and conceptualization is based on typical aging and normative cognitive/motor differences, one can also extrapolate based on these ideas to consider how the cerebellum may also serve as a critical form of scaffolding in age-related neurodegenerative disease. The literature to this point is relatively emergent, but existing evidence further points to a role for the cerebellum in function here as well.
In parallel to work investigating the cerebellum in healthy aging, there is also a growing literature investigating the cerebellum in Alzheimer’s dementia (AD) and mild cognitive impairment (MCI) (Guo et al. 2016; Jacobs, Hopkins, Mayrhofer, Bruner, Van Leeuwen, et al. 2018; Toniolo et al. 2018; Olivito et al. 2020). Notably, MCI is often conceptualized as a prodromal phase of AD, as though not all individuals with MCI go on to develop AD, these individuals are at substantially higher risk (Jessen et al. 2014). In the context of the discussion here, MCI and AD are often discussed separately due to the nature of the literature, though in actuality they are highly related. While the primary focus of the literature in this area has been cortical (much like the work on healthy cognitive aging), with an emphasis on hippocampal and frontal regions, as well as tau pathology, emerging evidence highlights the role of the cerebellum in MCI and subsequently in AD. There seems to be a pattern of atrophy across disease progression wherein more midline and anterior regions are implicated early on, and atrophy progresses to the lateral and posterior regions, such as Crus I (Toniolo et al. 2018). Further, atrophy in the cerebellum is correlated with that in the cortex, in regions known to be part of the same functional networks (Guo et al. 2016).
Jacobs and colleagues (Jacobs, Hopkins, Mayrhofer, Bruner, Leeuwen, et al. 2018) have suggested that the cerebellum may in fact be a contributor to the functional decline associated with dementia, and both cognitive and affective deficits may result from cerebellar deficits in the modulation of broader cortical circuits. Work investigating the conversion of individuals from MCI to AD has also implicated the cerebellum, wherein volume in Crus I and II is reduced in those that converted (Kim et al. 2021). Further, looking at resting state, in both MCI and AD there are some areas of lower connectivity relative to controls, though in MCI there are also some areas of higher connectivity (Tang et al. 2021). The authors argue this may be indicative of a reliance on cerebellar resources to compensate for cortical declines. This is consistent with the proposed notion of cerebellar scaffolding or reserve, and together this highlights the potential utility of the cerebellum as a possible marker of further decline. That is, if individuals with MCI lose cerebellar resources, they are less able to compensate for cortical pathology, and thus transition to a dementia diagnosis. Together, this suggests that the cerebellum could potentially serve as a marker of dementia transitions and underscores the importance of targeted cerebellar investigations in MCI. It has been suggested that the cerebellum is not just a “silent bystander” in AD (Schmahmann 2016). In the proposed framework, the cerebellum can be situated relative to the cortex and its contributions can be considered in concert with cortical regions. Critically, an improved understanding of cerebellar contributions may open up new avenues for diagnosis, treatment, and assessment. Incorporating measures of cerebellar function may provide revealing insights related to compensation and the potential for transition to later disease. Indeed, if early on patients are better able to use cerebellar resources to compensate for cortical pathology, careful measurement of cerebellar function stands to be especially useful and revealing. Quantification of early signs of cerebellar decline may suggest that the structure is no longer compensating as well for cortical dysfunction (e.g., Tang et al., 2021), and may indicate the potential for worsening disease, given work indicating the cerebellum may relate to conversion (Kim et al., 2021).
With all that said, the above discussion is limited to AD and MCI. The degree to which the cerebellum contributes to other forms of dementia is less clear, but worthy of further investigation. As the framework and model proposed here are more generalized, the notion of potential cerebellar compensation for vascular damage or declines specific to frontal and temporal cortices would apply. However, potential relative sparing of cerebellar resources is less known, meaning the extent to which the structure can scaffold cortical functions is not clear. Despite this, we can expect that general cerebellar function would, at minimum, be subject to the typical impacts of aging, negatively impacting its compensatory power, and in turn contributing to the greater declines in function experienced by these patients.
Caveats and Limitations
Here, I have proposed a framework for how the cerebellum may serve as a form of scaffolding or reserve that is critical for optimal cortical functioning and in turn, behavior. However, there are several caveats to consider. First, this is a speculative framework. While there is some initial evidence in support of this hypothesis using non-invasive brain stimulation in young adults (Maldonado et al. 2021), further targeted work in older populations is warranted. With that said, the proposed framework does allow for the generation of clear testable hypotheses and can serve as a driver for future investigations in this regard. Second, this framework targets the cerebellum. As reviewed here, there are also connections between the cerebellum and basal ganglia, and the latter may also contribute to the automaticity of behavior. Broader declines in basal ganglia function in advanced age may also serve as a parallel and integrated system of scaffolding and reserve in older adulthood. Targeted investigations of both of these critical subcortical systems together in older adults will further increase our understanding of the brain systems and processes that contribute to age-related cognitive differences and declines.
Highlights.
Current frameworks for understanding cognitive aging are cortically-focused
Cerebellar structure, function, and connectivity are different in older adults
The cerebellum may be a key source of reserve and scaffolding in advanced age
Cerebellar functional differences may contribute to bilateral cortical activation
Acknowledgments
This work was supported by R01 AG064010-01 to J.A.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. 2007. Disruption of large-scale brain systems in advanced aging. Neuron. 56:924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo A, Mormina E, Anastasi GP, Gaeta M, Calamuneri A, Quartarone A, De Salvo S, Bruschetta D, Rizzo G, Trimarchi F, Milardi D. 2014. Constrained spherical deconvolution analysis of the limbic network in human, with emphasis on a direct cerebello-limbic pathway. Front Hum Neurosci. 8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babayan BM, Watilliaux A, Viejo G, Paradis AL, Girard B, Rondi-Reig L. 2017. A hippocampo-cerebellar centred network for the learning and execution of sequence-based navigation. Sci Rep. 7:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L, Lindenberger U, Li S-C, Nyberg L. 2010. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: Recent data and future avenues. Neurosci Biobehav Rev. 34:670–677. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li SC, Farde L. 2006. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neurosci Biobehav Rev. 30:791–807. [DOI] [PubMed] [Google Scholar]
- Balsters JH, Cussans E, Diedrichsen J, Phillips KA, Preuss TM, Rilling JK, Ramnani N. 2010. Evolution of the cerebellar cortex: The selective expansion of prefrontal-projecting cerebellar lobules. Neuroimage. 49:2045–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsters JH, Whelan CD, Robertson IH, Ramnani N. 2013. Cerebellum and cognition: evidence for the encoding of higher order rules. Cereb Cortex. 23:1433–1443. [DOI] [PubMed] [Google Scholar]
- Bernard J a, Peltier SJ, Wiggins JL, Jaeggi SM, Buschkuehl M, Fling BW, Kwak Y, Jonides J, Monk CS, Seidler RD. 2013a. Disrupted cortico-cerebellar connectivity in older adults. Neuroimage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Ballard HK, Jackson TB. 2021a. Cerebellar Dentate Connectivity across Adulthood: A Large-Scale Resting State Functional Connectivity Investigation. Cereb Cortex Commun. 2:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Ballard HK, Jackson TB. 2021b. Cerebellar Dentate Connectivity across Adulthood: A Large-Scale Resting State Functional Connectivity Investigation. Cereb Cortex Commun. tgab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Leopold DR, Calhoun VD, Mittal VA. 2015. Regional Cerebellar Volume and Cognitive Function From Adolescence to Late Middle Age. Hum Brain Mapp. 1120:1102–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Nguyen AD, Hausman HK, Maldonado T, Ballard HK, Jackson TB, Eakin SM, Lokshina Y, Goen JRM. 2020. Shaky scaffolding: Age differences in cerebellar activation revealed through activation likelihood estimation meta-analysis. Hum Brain Mapp. 41:5255–5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Orr JM, Mittal VA. 2016. Differential motor and prefrontal cerebello-cortical network development: Evidence from multimodal neuroimaging. Neuroimage. 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Peltier SJ, Benson BL, Wiggins JL, Jaeggi SM, Buschkuehl M, Jonides J, Monk CS, Seidler RD. 2014. Dissociable functional networks of the human dentate nucleus. Cereb Cortex. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Peltier SJ, Wiggins JL, Jaeggi SM, Buschkuehl M, Fling BW, Kwak Y, Jonides J, Monk CS, Seidler RD. 2013b. Disrupted cortico-cerebellar connectivity in older adults. Neuroimage. 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Russell CE, Newberry RE, Goen JRM, Mittal VA. 2017. Patients with schizophrenia show aberrant patterns of basal ganglia activation: Evidence from ALE meta-analysis. NeuroImage Clin. 14:450463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD. 2013a. Relationships between regional cerebellar volume and sensorimotor and cognitive function in young and older adults. Cerebellum. 12:721–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD. 2013b. Relationships between regional cerebellar volume and sensorimotor and cognitive function in young and older adults. Cerebellum. 12:721–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD. 2014. Moving forward: Age effects on the cerebellum underlie cognitive and motor declines. Neurosci Biobehav Rev. 42:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD, Hassevoort KM, Benson BL, Welsh RC, Wiggins JL, Jaeggi SM, Buschkuehl M, Monk CS, Jonides J, Peltier SJ. 2012. Resting state cortico-cerebellar functional connectivity networks: a comparison of anatomical and self-organizing map approaches. Front Neuroanat. 6:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordignon A, Devita M, Sergi G, Coin A. 2021. “Cerebellar cognitive reserve”: a possible further area of investigation. Aging Clin Exp Res. 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL. 2010. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A. 107:8452–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan AC, Strick PL. 2018. The basal ganglia and the cerebellum: Nodes in an integrated network. Nat Rev Neurosci. 19:338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottmer C, Bachmann S, Pantel J, Essig M, Amann M, Schad LR, Magnotta V, Schröder J. 2005. Reduced cerebellar volume and neurological soft signs in first-episode schizophrenia. Psychiatry Res - Neuroimaging. 140:239–250. [DOI] [PubMed] [Google Scholar]
- Cabeza R 2002. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging. 17:85–100. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Albert M, Belleville S, Craik FIM, Duarte A, Grady CL, Lindenberger U, Nyberg L, Park DC, Reuter-lorenz PA, Rugg MD, Steffener J. 2018. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci. 19:701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. 2002. Aging Gracefully: Compensatory Brain Activity in High-Performing Older Adults. Neuroimage. 17:1394–1402. [DOI] [PubMed] [Google Scholar]
- Cappell KA, Gmeindl L, Reuter-Lorenz PA. 2010. Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex. 46:462–473> [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr VA, Bernstein JD, Favila SE, Rutt BK, Kerchner GA, Wagner AD. 2017. Individual differences in associative memory among older adults explained by hippocampal subfield structure and function. Proc Natl Acad Sci U S A. 114:12075–12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clower DM, West RA, Lynch JC, Strick PL. 2001. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J Neurosci. 21:6283–6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui D, Zhang L, Zheng F, Wang H, Meng Q, Lu W, Liu Z, Yin T, Qiu J. 2020. Volumetric reduction of cerebellar lobules associated with memory decline across the adult lifespan. Quant Imaging Med Surg. 10:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJS, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts S a RB. 2008. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 18:1856–1864. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. 2006. Effects of healthy aging on hippocampal and rhinal memory functions: An event-related fMRI study. Cereb Cortex. 16:1771–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. 2008. Qué PASA? the posterior-anterior shift in aging. Cereb Cortex. 18:1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. 2008. Functional connectivity of human striatum: A resting state fMRI study. Cereb Cortex. 18:2735–2747. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, King M, Hernandez-castillo C, Sereno M, Ivry RB. 2019. Universal Transform or Multiple Functionality ? Understanding the Contribution of the Human Cerebellum across Task Domains. Neuron. 102:918–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Gabitov E, Vahdat S, Lungu O, Boutin A. 2018. Current issues related to motor sequence learning in humans. Curr Opin Behav Sci. 20:89–97. [Google Scholar]
- Draganski B, Kherif F, Klöppel S, Cook PA, Alexander DC, Parker GJM, Deichmann R, Ashburner J, Frackowiak RSJ. 2008. Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J Neurosci. 28:7143–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. 2003. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol. 89:634–639. [DOI] [PubMed] [Google Scholar]
- Ferreira LK, Busatto GF. 2013. Resting-state functional connectivity in normal brain aging. Neurosci Biobehav Rev. 37:384–400. [DOI] [PubMed] [Google Scholar]
- Ferreira LK, Regina ACB, Kovacevic N, Martin MDGM, Santos PP, Cameiro CDG, Kerr DS, Amaro E, Mcintosh AR, Busatto GF. 2016. Aging effects on whole-brain functional connectivity in adults free of cognitive and psychiatric disorders. Cereb Cortex. 26:3851–3865. [DOI] [PubMed] [Google Scholar]
- Festini SB, Zahodne L, Reuter-Lorenz PA. 2018. Theoretical Perspectives on Age Differences in Brain Activation: HAROLD, PASA, CRUNCH—How Do They STAC Up? Oxford Res Encycl Psychol. 1–24. [Google Scholar]
- Filip P, Gallea C, Lehéricy S, Lungu O, Bareš M. 2019. Neural Scaffolding as the Foundation for Stable Performance of Aging Cerebellum. Cerebellum. 18:500–510. [DOI] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Marek S, Newbold DJ, Hampton JM, Seider NA, Montez DF, Nielsen AM, Van AN, Zheng A, Miller R, Siegel JS, Kay BP, Snyder AZ, Greene DJ, Schlaggar BL, Petersen SE, Nelson SM, Dosenbach NUF. 2021. Individualized Functional Subnetworks Connect Human Striatum and Frontal Cortex. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. 1997. The basal ganglia and cognitive pattern generators. Schizophr Bull. 23:459–469. [DOI] [PubMed] [Google Scholar]
- Guo CC, Tan R, Hodges JR, Hu X, Sami S, Hornberger M. 2016. Network-selective vulnerability of the human cerebellum to Alzheimer’s disease and frontotemporal dementia. Brain. 139:1527–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday G 2017. Pathology and hippocampal atrophy in Alzheimer’s disease. Lancet Neurol. 16:862–864. [DOI] [PubMed] [Google Scholar]
- Han S, An Y, Carass A, Prince JL, Resnick SM. 2020. Neuroimage Longitudinal analysis of regional cerebellum volumes during normal aging. Neuroimage. 220:117062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman HK, Jackson TB, Goen JRM, Bernard JA. 2020. From Synchrony to Asynchrony: Cerebellar-Basal Ganglia Functional Circuits in Young and Older Adults. Cereb Cortex. 30:718–729. [DOI] [PubMed] [Google Scholar]
- He L, Wang X, Zhuang K, Qiu J. 2020. Decreased Dynamic Segregation but Increased Dynamic Integration of the Resting-state Functional Networks During Normal Aging. Neuroscience. 437:54–63. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Féger J, Carras PL, Strick PL. 2005. The cerebellum communicates with the basal ganglia. Nat Neurosci. 8:1491–1493. [DOI] [PubMed] [Google Scholar]
- Iglói K, Doeller CF, Paradis AL, Benchenane K, Berthoz A, Burgess N, Rondi-Reig L. 2015. Interaction between hippocampus and cerebellum crus i in sequence-based but not place-based navigation. Cereb Cortex. 25:4146–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Pütz B, Yoshioka T, Kawato M. 2000. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature. 403:192–195. [DOI] [PubMed] [Google Scholar]
- Iordan AD, Cooke KA, Moored KD, Katz B, Buschkuehl M, Jaeggi SM, Polk TA, Peltier SJ, Jonides J, Reuter-Lorenz PA. 2020. Neural correlates of working memory training: Evidence for plasticity in older adults. Neuroimage. 217:116887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M 2008. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 9:304–313. [DOI] [PubMed] [Google Scholar]
- Jackson TB, Maldonado T, Eakin SM, Orr JM, Bernard JA. 2020. Cerebellar and prefrontal-cortical engagement during higher-order rule learning in older adulthood. Neuropsychologia. 148:107620. [DOI] [PubMed] [Google Scholar]
- Jacobs HIL, Hopkins DA, Mayrhofer HC, Bruner E, Van Leeuwen FW, Raaijmakers W, Schmahmann JD. 2018. The cerebellum in Alzheimer ’s disease : evaluating its role in cognitive decline. 37–47. [DOI] [PubMed] [Google Scholar]
- Jacobs HIL, Hopkins DA, Mayrhofer HC, Bruner E, Van Leeuwen FW, Raaijmakers W, Schmahmann JD. 2018. The cerebellum in Alzheimer’s disease: Evaluating its role in cognitive decline. Brain. 141:37–47. [DOI] [PubMed] [Google Scholar]
- Jamadar SD. 2020. The CRUNCH model does not account for load-dependent changes in visuospatial working memory in older adults. Neuropsychologia. 142:107446. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. 2001. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 22:581–594. [DOI] [PubMed] [Google Scholar]
- Jessen F, Wolfsgruber S, Wiese B, Bickel H, Mösch E, Kaduszkiewicz H, Pentzek M, Riedel-Heller SG, Luck T, Fuchs A, Weyerer S, Werle J, Van Den Bussche H, Scherer M, Maier W, Wagner M. 2014. AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimer’s Dement. 10:76–83. [DOI] [PubMed] [Google Scholar]
- Jissendi P, Baudry S, Balériaux D. 2008. Diffusion tensor imaging (DTI) and tractography of the cerebellar projections to prefrontal and posterior parietal cortices: A study at 3T. J Neuroradiol. 35:42–50. [DOI] [PubMed] [Google Scholar]
- Kail R, Salthouse TA. 1994. Processing speed as a mental capacity. Acta Psychol (Amst). 86:199–225. [DOI] [PubMed] [Google Scholar]
- Karlsson S, Nyberg L, Karlsson P, Fischer H, Thilers P, MacDonald S, Brehmer Y, Rieckmann A, Halldin C, Farde L, Bäckman L. 2009. Modulation of striatal dopamine D1 binding by cognitive processing. Neuroimage. 48:398–404. [DOI] [PubMed] [Google Scholar]
- Karrer TM, Josef AK, Mata R, Morris ED, Samanez-Larkin GR. 2017. Reduced dopamine receptors and transporters but not synthesis capacity in normal aging adults: a meta-analysis. Neurobiol Aging. 57:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. 2003. Cerebellar Loops with Motor Cortex and Prefrontal Cortex of a Nonhuman Primate. J Neurosci. 23:8432–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Rodrigue KM, Bischof GN, Hebrank AC, Reuter-Lorenz P a, Park DC. 2014. Age Trajectories of Functional Activation Under Conditions of Low and High Processing Demands: An Adult Lifespan fMRI Study of the Aging Brain. Neuroimage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren-Happuch E, Chen S-HA, Ho M-HR, Desmond JE. 2014. A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Hum Brain Mapp. 35:593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Cheong E, Jo S, Lee S, Shim W, Kwon M, Kim JS, Kim BJ, Lee J. 2021. The cerebellum could serve as a potential imaging biomarker of dementia conversion in patients with amyloid-negative amnestic mild cognitive impairment. Eur J Neurol. 1–8. [DOI] [PubMed] [Google Scholar]
- King M, Hernandez-Castillo CR, Poldrack RA, Ivry RB, Diedrichsen J. 2019. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat Neurosci. 22:1371–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, Hirsiger S, Susan M, Seidler RD. 2015. Cerebellar Gray and White Matter Volume and Their Relation With Age and Manual Motor Performance in Healthy Older Adults. 2363:2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, Hoogendam YY, Hirsiger S, Mérillat S, Jäncke L, Seidler RD. 2017. Regional cerebellar volumetric correlates of manual motor and cognitive function. Brain Struct Funct. 222:1929–1944. [DOI] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL. 2009. Segregated Fronto-Cerebellar Circuits Revealed by Intrinsic Functional Connectivity. Cereb Cortex. 19:2485–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KJ, Carlson ES. 2020. Resistance, vulnerability and resilience: A review of the cognitive cerebellum in aging and neurodegenerative diseases. Neurobiol Learn Mem. 170:106981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Chen CH, Tom SE, Kuo SH. 2020. Cerebellar Volume Is Associated with Cognitive Decline in Mild Cognitive Impairment: Results from ADNI. Cerebellum. 19:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Eickhoff SB, Caspers S, Wu J, Genon S, Hoffstaedter F, Mars RB, Sommer IE, Eickhoff CR, Chen J, Jardri R, Reetz K, Dogan I, Aleman A, Kogler L, Gruber O, Caspers J, Mathys C, Patil KR. 2021. Functional parcellation of human and macaque striatum reveals human-specific connectivity in the dorsal caudate. Neuroimage. 235:118006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLullich AMJ, Edmond CL, Ferguson KJ, Wardlaw JM, Starr JM, Seckl JR, Deary IJ. 2004. Size of the neocerebellar vermis is associated with cognition in healthy elderly men. Brain Cogn. 56:344–348. [DOI] [PubMed] [Google Scholar]
- Maldonado T, Jackson TB, Bernard JA. 2021. Inhibitory cerebellar stimulation increases cortical activation: evidence for cerebellar scaffolding of cortical processing. bioRxiv. 1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore a., Hariri a. R, Das S, Callicott JH, Weinberger DR. 2002. Neurophysiological correlates of age-related changes in human motor function. Neurology. 58:630–635. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. 2000. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 42:183–200. [DOI] [PubMed] [Google Scholar]
- Miller TD, Ferguson KJ, Reid LM, Wardlaw JM, Starr JM, Seckl JR, Deary IJ, Maclullich AMJ. 2013. Cerebellar vermis size and cognitive ability in community-dwelling elderly men. Cerebellum. 12:68–73. [DOI] [PubMed] [Google Scholar]
- Mitoma H, Buffo A, Gelfo F, Guell X, Fucà E, Kakei S, Lee J, Manto M, Petrosini L, Shaikh AG, Schmahmann JD. 2020. Consensus Paper. Cerebellar Reserve: From Cerebellar Physiology to Cerebellar Disorders. Cerebellum. 19:131–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberget T, Doan NT, Alnæs D, Kaufmann T, Córdova-Palomera A, Lagerberg TV, Diedrichsen J, Schwarz E, Zink M, Eisenacher S, Kirsch P, Jönsson EG, Fatouros-Bergman H, Flyckt L, Pergola G, Quarto T, Bertolino A, Barch D, Meyer-Lindenberg A, Agartz I, Andreassen OA, Westlye LT. 2018. Cerebellar volume and cerebellocerebral structural covariance in schizophrenia: A multisite mega-analysis of 983 patients and 1349 healthy controls. Mol Psychiatry. 23:1512–1520. [DOI] [PubMed] [Google Scholar]
- MORGAN DG, FINCH CE. 1988. Dopaminergic Changes in the Basal Ganglia A Generalized Phenomenon of Aging in Mammals. Ann N Y Acad Sci. 515:145–160. [DOI] [PubMed] [Google Scholar]
- Noohi F, Boyden NB, Kwak Y, Humfleet J, Burke DT, Muller MLTM, Bohnen NI, Seidler RD. 2014. Association of COMT val158met and DRD2 G>T genetic polymorphisms with individual differences in motor learning and performance in female young adults. J Neurophysiol. 111:628–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea A, Cohen RA, Porges EC, Nissim NR, Woods AJ. 2016. Cognitive aging and the hippocampus in older adults. Front Aging Neurosci. 8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivito G, Serra L, Marra C, Di Domenico C, Caltagirone C, Toniolo S, Cercignani M, Leggio M, Bozzali M. 2020. Cerebellar dentate nucleus functional connectivity with cerebral cortex in Alzheimer’s disease and memory: a seed-based approach. Neurobiol Aging. 89:32–40. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. 2009. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 60:173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzer EA, Hintzen A, Goldau M, von Cramon DY, Timmermann L, Tittgemeyer M. 2013. Cerebellar networks with basal ganglia: Feasibility for tracking cerebello-pallidal and subthalamo-cerebellar projections in the human brain. Eur J Neurosci. 38:3106–3114. [DOI] [PubMed] [Google Scholar]
- Ramnani N 2006. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 7:511–522. [DOI] [PubMed] [Google Scholar]
- Ramnani N 2014. Automatic and Controlled Processing in the Corticocerebellar System. 1st ed, Progress in Brain Research. Elsevier B.V. [DOI] [PubMed] [Google Scholar]
- Raymond JL, Medina JF. 2018. Computational principles of supervised learning in the cerebellum. Annu Rev Neurosci. 41:233–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. 2010. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. Neuroimage. 51:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. 2005. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 15:1676–1689. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. 2004. Differential aging of the medial temporal lobe: A study of a five-year change. Neurology. 62:433–438. [DOI] [PubMed] [Google Scholar]
- Raz N, Schmiedek F, Rodrigue KM, Kennedy KM, Lindenberger U, Lövdén M. 2013. Differential brain shrinkage over 6months shows limited association with cognitive practice. Brain Cogn. 82:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Yang YQ, Rodrigue KM, Kennedy KM, Lindenberger U, Ghisletta P. 2012. White matter deterioration in 15 months: Latent growth curve models in healthy adults. Neurobiol Aging. 33:429.e1–429.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz P a., Cappell K a. 2008. Neurocognitive Aging and the Compensation Hypothesis. Curr Dir Psychol Sci. 17:177–182. [Google Scholar]
- Reuter-Lorenz P a., Stanczak L, Miller a. C. 1999. Neural Recruitment and Cognitive Aging: Two Hemispheres Are Better Than One, Especially as You Age. Psychol Sci. 10:494–500. [Google Scholar]
- Reuter-Lorenz PA, Lustig C. 2005. Brain aging: Reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 15:245–251. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Park DC. 2014. How Does it STAC Up? Revisiting the Scaffolding Theory of Aging and Cognition. Neuropsychol Rev. 24:355–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero JE, Coupe P, Lanuza E, Catheline G, Manjón JV. 2021. Toward a unified analysis of cerebellum maturation and aging across the entire lifespan: A MRI analysis. Hum Brain Mapp. 1287–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH. 2011. The Declining Infrastructure of the Aging Brain. Brain Connect. 1:279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi J, Pallesen K, Neuvonen T. 2010. Cognitive and motor loops of the human cerebro-cerebellar system. J Cogn …. 2663–2676. [DOI] [PubMed] [Google Scholar]
- Salmi J, Pallesen KJ, Neuvonen T, Brattico E, Korvenoja A, Salonen O, Carlson S. 2010. Cognitive and motor loops of the human cerebro-cerebellar system. J Cogn Neurosci. 22:2663–2676. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. 1996. The Processing-Speed Theory of Adult Age Differences in Cognition. Psychol Rev. 103:403–128. [DOI] [PubMed] [Google Scholar]
- Schendan HE, Searl MM, Melrose RJ, Stern CE. 2003. An fMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 37:1013–1025. [DOI] [PubMed] [Google Scholar]
- Schmahmann J, Sherman JC. 1998. The cerebellar cognitive affective syndrome. Brain. 121:561–579. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. 2016. Cerebellum in Alzheimer’s disease and frontotemporal dementia: not a silent bystander. Brain. 139:1312–1324. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Guell X, Stoodley CJ, Halko MA. 2019. The Theory and Neuroscience of Cerebellar Cognition. Annu Rev Neurosci. 42:337–364. [DOI] [PubMed] [Google Scholar]
- Seidler R, Erdeniz B, Koppelmans V, Hirsiger S, Mérillat S, Jäncke L. 2015. Associations between age, motor function, and resting state sensorimotor network connectivity in healthy older adults. Neuroimage. 108:47–59. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. 2010. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 34:721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Diedrichsen J, Tachrount M, Testa-silva G. 2020. The human cerebellum has almost 80 % of the surface area of the neocortex. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafto MA, Tyler LK, Dixon M, Taylor JR, Rowe JB, Cusack R, Calder AJ, Marslen-Wilson WD, Duncan J, Dalgleish T, Henson RN, Brayne C, Bullmore E, Campbell K, Cheung T, Davis S, Geerligs L, Kievit R, McCarrey A, Price D, Samu D, Treder M, Tsvetanov K, Williams N, Bates L, Emery T, Erzinçlioglu S, Gadie A, Gerbase S, Georgieva S, Hanley C, Parkin B, Troy D, Allen J, Amery G, Amunts L, Barcroft A, Castle A, Dias C, Dowrick J, Fair M, Fisher H, Goulding A, Grewal A, Hale G, Hilton A, Johnson F, Johnston P, Kavanagh-Williamson T, Kwasniewska M, McMinn A, Norman K, Penrose J, Roby F, Rowland D, Sargeant J, Squire M, Stevens B, Stoddart A, Stone C, Thompson T, Yazlik O, Barnes D, Hillman J, Mitchell J, Villis L, Matthews FE. 2014. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) study protocol: A cross-sectional, lifespan, multidisciplinary examination of healthy cognitive ageing. BMC Neurol. 14:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman-Tov T, Bosak N, Sprecher E, Paz R, Eran A, Aharon-Peretz J, Kahn I. 2017. Early age-related functional connectivity decline in high-order cognitive networks. Front Aging Neurosci. 8:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov AA, Miall RC, Ivry RB. 2017. The Cerebellum: Adaptive Prediction for Movement and Cognition. Trends Cogn Sci. 21:313–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CJ, Anwander A, Bazin P, Trampel R, Schaefer A, Turner R, Ramnani N, Villringer A. 2017. Human Cerebellar Sub-millimeter Diffusion Imaging Reveals the Motor and Non-motor Topography of the Dentate Nucleus. 4537–548. [DOI] [PubMed] [Google Scholar]
- Stern Y, Cantillon M, Clouston SAP, Elman JA, Gold BT, Jones R, Morbelli S, Okonkwo O, Ossenkoppele R, Pettigrew C. 2019. Mechanisms underlying resilience in ageing. Nat Rev Neurosci. 20:2019. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. 2009. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 44:489–501. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. 2012. Functional topography of the cerebellum for motor and cognitive tasks: An fMRI study. Neuroimage. 59:1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. 2009. Cerebellum and Nonmotor Function. Annu Rev Neurosci. 32:413–434. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A 2006. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 30:749–761. [DOI] [PubMed] [Google Scholar]
- Tabatabaei-Jafari H, Walsh E, Shaw ME, Cherbuin N. 2017. The cerebellum shrinks faster than normal ageing in Alzheimer’s disease but not in mild cognitive impairment. Hum Brain Mapp. 00 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Zhu D, Ma W, Yao Q, Li Q, Shi J. 2021. Differences Changes in Cerebellar Functional Connectivity Between Mild Cognitive Impairment and Alzheimer’s Disease: A Seed-Based Approach. Front Neurol. 12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Williams N, Cusack R, Auer T, Shafto MA, Dixon M, Tyler LK, Cam-CAN, Henson RN. 2017. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) data repository: Structural and functional MRI, MEG, and cognitive data from a cross-sectional adult lifespan sample. Neuroimage. 144:262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniolo S, Serra L, Olivito G, Marra C, Bozzali M. 2018. Patterns of Cerebellar Gray Matter Atrophy Across Alzheimer’ s Disease Progression. 12:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Uwisengeyimana JD, Nguchu BA, Wang Y, Zhang D, Liu Y, Qiu B, Wang X. 2020. Cognitive function and cerebellar morphometric changes relate to abnormal intra-cerebellar and cerebro-cerebellum functional connectivity in old adults. Exp Gerontol. 140:111060. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Fjell AM. 2011. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol Aging. 32:916–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson TC, Obiang P, Torres-Herraez A, Watilliaux A, Coulon P, Rochefort C, Rondi-Reig L. 2019. Anatomical and physiological foundations of cerebello-hippocampal interaction. Elife. 8:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Foy MR, Akopian GG, Lee KH, Zach J, Nguyen KPT, Comalli DM, Kennard J a, Agelan A, Thompson RF. 2010. Differential effects and rates of normal aging in cerebellum and hippocampus. Proc Natl Acad Sci U S A. 107:1624–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystad M, Eichele T, Lundervold AJ, Lundervold A. 2010. Subcortical functional connectivity and verbal episodic memory in healthy elderly-A resting state fMRI study. Neuroimage. 52:379–388. [DOI] [PubMed] [Google Scholar]
- Ystad M, Hodneland E, Adolfsdottir S, Haász J, Lundervold AJ, Eichele T, Lundervold A. 2011. Cortico-striatal connectivity and cognition in normal aging: a combined DTI and resting state fMRI study. Neuroimage. 55:24–31. [DOI] [PubMed] [Google Scholar]
- Yu W, Krook-Magnuson E. 2015. Cognitive collaborations: Bidirectional functional connectivity between the cerebellum and the hippocampus. Front Syst Neurosci. 9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler Z, Hoffmann K, Krook-Magnuson E. 2020. HippoBellum: Acute cerebellar modulation alters hippocampal dynamics and function. J Neurosci. 40:6910–6926. [DOI] [PMC free article] [PubMed] [Google Scholar]