Abstract
Humans are ubiquitously exposed to neurotoxicants in air pollution, causing increased risk for psychiatric outcomes. Effects of prenatal exposure to air pollution on early emerging behavioral phenotypes that increase risk of psychopathology remain understudied. We review animal models that represent analogues of human behavioral phenotypes that are risk markers for internalizing and externalizing problems (behavioral inhibition, behavioral exuberance, irritability), and identify commonalities among the neural mechanisms underlying these behavioral phenotypes and the neural targets of three types of air pollutants (polycyclic aromatic hydrocarbons, traffic-related air pollutants, fine particulate matter < 2.5 microns). We conclude that prenatal exposure to air pollutants increases risk for behavioral inhibition and irritability through distinct mechanisms, including altered dopaminergic signaling and hippocampal morphology, neuroinflammation, and decreased brain-derived neurotrophic factor expression. Future studies should investigate these effects in human longitudinal studies incorporating complex exposure measurement methods, neuroimaging, and behavioral characterization of temperament phenotypes and neurocognitive processing to facilitate efforts aimed at improving long-lasting developmental benefits for children, particularly those living in areas with high levels of exposure.
Keywords: air pollution, behavioral phenotypes, animal models, neural mechanisms
1. Introduction
Humans are ubiquitously exposed to air pollutants. Although there are many studies documenting deleterious physical health outcomes associated with exposure to air pollution (Cohen et al., 2017; Genc et al., 2012; Landrigan, 2017), a growing number of epidemiologic studies also link exposure to risk for a range of psychiatric and neurodevelopmental outcomes (Khan et al., 2019; Lu, 2020; Volk et al., 2021). Concerningly, exposure during pregnancy to even low levels of air pollution – levels that may have no adverse effects on an adult – may disrupt rapid and foundational fetal brain development, potentially leading to life-long functional impairments (Adams et al., 2000; Barker, 2004; Grandjean & Landrigan, 2014; Landrigan & Goldman, 2011; Lanphear, 2015; Rice & Barone, 2000). Prenatal exposure to air pollution has been linked with internalizing/anxiety symptoms (Loftus et al., 2020; Perera et al., 2011, 2012) and externalizing/ADHD symptoms (Alemany et al., 2018; Loftus et al., 2020; Min & Min, 2017; Newman et al., 2013; Perera et al., 2011, 2012; Siddique et al., 2011; Yorifuji et al., 2017), sometimes within the same participants (Loftus et al., 2020; Perera et al., 2011, 2012). Although most studies of prenatal exposures have focused on psychiatric outcomes in childhood or adolescence, a small and growing literature has linked prenatal exposure to neurotoxicants, including air pollution, with early behavioral phenotypes (Cowell et al., 2019; Gartstein & Skinner, 2018; Stroustrup et al., 2016), such as behavioral inhibition (BI), behavioral exuberance (BE), and irritability, that are known risk factors for later internalizing and externalizing psychopathology (Fox et al., 2021; Hirshfeld-Becker et al., 2002; Sjöwall et al., 2017). These behavioral phenotypes have distinct neural mechanisms (Kircanski et al. 2018) that may be vulnerable to prenatal exposure to air pollution. Although the effects of stress and genetics on these behavioral phenotypes (i.e., behavioral inhibition) and later psychiatric risk are well known (McGrath et al. 2012; Monk, Lugo-Candelas, and Trumpff 2019; Smoller 2016; Wiggins et al. 2014; Pagliaccio et al. 2018), the effects of the chemical environment on these early life markers remains understudied. Importantly, these prenatal exposures represent modifiable risk factors amenable to public health policy that can improve children’s mental health outcomes.
In this review, we explore the premise that prenatal exposure to air pollution may contribute to early emerging behavioral phenotypes that increase risk of internalizing and externalizing problems. We first briefly discuss three human behavioral phenotypes: behavioral inhibition, behavioral exuberance, and irritability and decompose, or back-translate, these phenotypes into constituent behavioral analogs measured in animal experimental paradigms. Table 1 presents examples of the applied methods, paradigms, and measured behaviors that correspond to each human behavioral phenotype. Given that behavioral inhibition and behavioral exuberance are often measured via the same paradigm, we discuss them jointly. Although there is a small literature examining the neural correlates of prenatal exposure to air pollution in humans (Guxens et al., 2018; Mortamais et al., 2019; Peterson et al., 2015), these studies are limited by their observational designs, whereas animal models allow for the experimental manipulation of air pollution types, levels, and timing. Thus, we next review findings from animal models of prenatal exposure to three commonly studied air pollutants (polycyclic aromatic hydrocarbons [PAH], traffic-related air pollutants [TRAP], fine particulate matter < 2.5 microns [PM2.5]) and identify commonalities among the neural targets of air pollution and neural mechanisms underlying the animal analogs of behavioral inhibition, behavioral exuberance, and irritability. Overall, this literature points to environmental contributions to behaviorally inhibited and irritable phenotypes that increase risk for later psychopathology, with less evidence of contributions to a behaviorally exuberant phenotype. We conclude with a discussion of directions for future research.
Table 1.
Examples of animal behaviors analogous to human behavioral phenotypes (BI/BE and irritability) for psychiatric risk
| Human behavioral phenotype | Method/paradigm | Model/strain | Measured behavior in animal model | Example References |
|---|---|---|---|---|
| BI/BE | Novel social/nonsocial arena + juvenile reciprocal social interaction | Rats/Sprague-Dawley (outbred) | Longer latency to approach novelty, lower locomotion in both a novel social and nonsocial arena, more exploration of arena rather than following or sniffing unfamiliar rat (BI) Shorter latency to approach and higher locomotion in both a novel social and nonsocial arena, following, chasing, or sniffing unfamiliar rat (BE) |
(Caruso et al., 2014; Cavigelli et al., 2007; Michael et al., 2020; Terranova et al., 1993) |
| Novel social/nonsocial arena + open field, light/dark box, elevated plus maze, elevated zero maze | Mice/C57BL6 (inbred) | Longer latency to approach novelty, lower locomotion, less time spent in anxiogenic and interaction parts of the test apparatus (BI) Shorter latency to approach novelty, higher locomotion, more time spent in anxiogenic and interaction parts of the test apparatus (BE) |
(Miao et al., 2019) | |
| Predator exposure paradigm | Rats/Sprague-Dawley (outbred) | Sum of freezing and hypervigilance: absence of locomotion (BI) | (Nanda et al., 2008; Qi et al., 2010) | |
| Non-spatial cue-based learned approach-avoidance paradigm | Rats/Long-Evans | High avoidance of conflict (BI) High approach of conflict (BE) |
(Schumacher et al., 2018) | |
| Eyeblink conditioning | Rats/Wistar-Kyoto; Sprague Dawley | Wistar-Kyoto rats, an animal model of BI, show facilitated acquisition of the classically conditioned eyeblink response (CCER) | (Janke et al., 2015) | |
| Ultrasonic Vocalizations (USV) | Rats/Wistar | Increased isolation-induced ultrasonic vocalizations (BI) Decreased isolation-induced ultrasonic vocalizations (BE) |
(Wöhr & Schwarting, 2008) | |
| Selective breeding (open field, light/dark box, elevated plus maze) | Rats/Bred “low responders” & bred “high responders” | Bred low responders (LR); decreased locomotion, increased latency to approach novelty, less time spent in anxiogenic parts of the test apparatus (BI) Bred high responders (HR); increased locomotion, decreased latency to approach novelty, more time spent in the anxiogenic sections (BE) |
(Clinton et al., 2011, 2021; Flagel et al., 2010; McCoy et al., 2019; Piazza et al., 1991; Sanna et al., 2015, 2017; Simmons et al., 2012; Thiel et al., 1999) | |
| Irritability | Frustrative non-reward (FNR); omission of expected reward | Fish/Atlanic Salmon Smolts (commercial strain, Aquagen AS) | Aggressive acts like charges, nips and chases elicited by delayed feeding | (Vindas et al., 2014) |
| Frustrative non-reward (FNR); omission of expected reward | Mice/C57BL/6J | Increase in operant responding (lever presses/minute) | (Martín-García et al., 2015) | |
| Frustrative non-reward & threat/opponent confrontation | Rats/Lister, Long-Evans, Sprague-Dawley, Wister | Mice emit USVs in the same frequency in response to both frustrative non-reward and threat | (Knutson et al., 2002) |
2. Behavioral inhibition and behavioral exuberance
BI and BE are conceptualized in terms of negative and positive reactivity to novel stimuli (for a full discussion see Perez-Edgar & Fox, 2018), which can be reliably measured even in infancy and predict later BI/BE traits (Dollar et al., 2017; Filippi, Sachs, et al., 2020; Fox et al., 2001; Kagan et al., 1984, 2007). BI is characterized by high negative affect and avoidance behaviors such as fearfulness or wariness when faced with novel people, objects, or challenging situations (Capitanio, 2018; Fox et al., 2005; Kagan et al., 1984). In contrast, BE is characterized by high positive affect and high approach behaviors, lack of fear, impulsivity, and high motor activity (Fox et al., 2001; Tarullo et al., 2011). In addition, BI has been characterized by increased monitoring of the environment and higher levels of reactive control, that is, the tendency to react to an event with corrective behavior (Buzzell et al., 2018). BI/BE positive/negative reactivity phenotypes exist on a continuum, such that children exhibiting neither high negative nor high positive affect are characterized as low reactive and considered normative along the BI-BE continuum (Fox et al., 2001; see Filippi, Sachs, et al., 2020 for a description of a non-inhibited sample). Prior literature documents sex differences in BI; however, the findings are mixed. One set of findings suggests that high negative reactivity in infancy predicted BI in toddlerhood and early childhood only for boys, but not for girls (Fox et al., 2015; Henderson et al., 2001). However, it was also reported that girls are more inhibited than boys at 14, 21, and 52 months (Kagan et al., 1994; Majdandzić & van den Boom, 2007). Boys have also been reported as being more exuberant than girls in early childhood (Gross & Stright, 2019; Majdandzić & van den Boom, 2007; Tarullo et al., 2011).
Longitudinal studies show that BI and BE are risk markers for future psychopathology. BI in childhood is associated with withdrawal and reticence in social environments, and represents one of the most robust early predictors of future anxiety disorders (Frenkel et al., 2015; Rubin et al., 2002) and specifically social anxiety disorder (Buzzell et al., 2017; Fox et al., 2021). BE is associated with adaptive outcomes, including higher levels of social competence and positive affect (Degnan et al., 2011; Dollar et al., 2017; Fox et al., 2001), but also impaired outcomes, including increased anger, frustration, impulsivity, and externalizing problems (Degnan et al., 2011; Morales et al., 2016), attention-deficit/hyperactivity disorder (ADHD; Bunford et al., 2015; Forslund et al., 2016; Salari et al., 2017), disruptive behavior disorder with or without comorbid mood disorder, and conduct disorder (Degnan et al., 2011; Egger & Angold, 2006; Forbes et al., 2017; Hirshfeld-Becker et al., 2002).
The neurobiology of BI/negative reactivity has been reviewed recently (Clauss, 2019; Clauss et al., 2015; Fox et al., 2021). A recent meta-analysis of task-related functional activation found that relative to non-inhibited individuals, those with BI show greater functional activation in the amygdala, basal ganglia, and prefrontal cortex during a range of tasks (Clauss et al., 2015). Structural imaging studies of the neural correlates of BI in children are lacking (Kircanski et al., 2018), but in one study, children with BI had reduced amygdala volume change from age 10 to 12 years relative to those with BE (Filippi, Sachs, et al., 2020). In addition to the amygdala and frontostriatal circuits, some models suggest that the hippocampus may be important in BI (Villard et al., 2021), but that it has been overlooked in research (Clauss et al., 2015). Recent work also points to the possible role of the bed nucleus of the stria terminalis in BI (Clauss, 2019). In addition, electrophysiology studies show that right resting frontal asymmetry appears to be a biomarker of BI (Fox et al. 2001; R. Liu, Calkins, and Bell 2020; Rutherford and Lindell 2011). Relatedly, BE infants were found to exhibit left frontal asymmetry that persisted at age 4 (Fox et al., 2001; Hane et al., 2008). Though research is limited, BE/positive reactivity has similar yet distinct neural correlates when compared to BI.
Animal analogs of BI and BE-related behaviors can be found in two types of studies: experimental designs that elicit inhibited or exuberant behavior or selective breeding of animals at either end of the BI-BE spectrum (see Table 1). To measure animal behavior, which we argue parallels BI and BE, behavioral paradigms typically expose rodents to novel stimuli (e.g., novel social/non-social arena and exposure to predator cues) or use an approach-avoidance paradigm. In these tasks, longer latency to approach, decreased locomotion, avoidance of a conflict arm (presenting simultaneously learned appetitive i.e., sucrose, and aversive i.e., foot shock, cues), increased hypervigilance, and freezing are all characteristic of BI phenotypes (Schumacher et al., 2018; Caruso et al., 2014; Cavigelli et al., 2007; Michael et al., 2020; Nanda et al., 2008; Qi et al., 2010). On the other hand, shorter latency to approach (including to conflict arm versus a neutral arm) and increased locomotion reflect BE-like behavior. In addition, in selective breeding experiments, “low-responder” mice show BI-like behaviors, such as decreased locomotion, increased latency to approach novelty, and less time spent and less entry frequency into anxiogenic regions (i.e., in the middle of an open field or in the open arms of an elevated plus maze). “High-responder” mice show BE-like behavior, manifesting as increased exploration and interaction with novelty, decreased latency to approach novelty, and more time spent in and times entered into anxiogenic areas (Clinton et al., 2011, 2021; Flagel et al., 2010; Simmons et al., 2012; McCoy et al., 2019; Thiel et al., 1999; Giorgi et al., 2019; Klein et al., 2014; Sabariego et al., 2019; Sanna et al., 2015, 2017).
3. Irritability
Irritability is defined as a low threshold for anger in response to frustration. In children, irritability can include a persistently angry/grumpy mood, termed tonic irritability, and/or temper outbursts (physical or verbal aggression), termed phasic irritability (Brotman et al., 2017). Normative in toddlerhood, irritability typically declines from childhood into adolescence (Copeland, Brotman, and Costello 2015; Wiggins et al. 2014; Pagliaccio et al. 2018). Persistent irritability over childhood or into adolescence, however, may be a marker of current or future psychopathology (Pagliaccio et al., 2018) and severe irritability has been conceptualized as Disruptive Mood Dysregulation Disorder in the Diagnostic and Statistical Manual 5th edition (“Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5)” 2021). Findings suggest that children with irritability are at greater risk for future disorders, such as anxiety, depression, ADHD, conduct disorder, and oppositional defiant disorder (Dougherty et al., 2013, 2015; Galera et al., 2021; Vidal-Ribas et al., 2016). Previous research suggests mixed findings regarding sex differences in the prevalence of irritability. Although some researchers have reported higher levels of irritability in adolescent girls (Leibenluft et al., 2006), others failed to find sex differences in irritability (Copeland et al., 2015). The association between irritability and later psychopathology may also vary by sex. For example, irritability was more strongly related to externalizing than to internalizing problems in boys, whereas the association was equally likely in girls (Humphreys et al., 2019). The neurobiology and cognitive mechanisms underlying irritability have also been reviewed recently and include deficient reward learning, elevated sensitivity to receipt or omission of reward, and impaired threat processing and cognitive control (Brotman et al., 2017; Leibenluft, 2017).
In animal models, analogs of irritability have been examined as aberrant emotional and behavioral responding, such as increased motor activity and aggressive behaviors in response to frustrative non-reward (FNR) (Brotman et al., 2017). In FNR paradigms (Table 1) an animal receives repeated reinforcement of a target behavior, e.g., an expectation of a reward is established by associating the reward with a signal, placing it at the end of a runway or maze, or providing it after the push of a lever. This is followed by the omission of the expected reward, which then elicits aggression or increased motor activity and reflects a negative prediction error (de Almeida & Miczek, 2002; Vindas et al., 2014).
4. Measuring air pollutants
Monitoring or estimating human exposures to air pollutants, including PAH, TRAP, and PM, from indoor and outdoor sources can be conducted using personal air sampling, regional monitoring stations, or via computer modeling using combinations of station data, satellite images, and meteorological variables. Portable personal air monitors can measure real-time exposure with high spatial and temporal resolution. Air monitors rely on filters that accumulate particles over a set period of time or measure particles in space through light-based continuous real-time monitoring of particulate exposures. Critical to the utility of these devices is their ability to capture diurnal and seasonal alterations in pollutants as well as the ability to impute values using monitor networks. Satellite imaging-based models continue to prove valuable in air pollution epidemiology (for a contemporary review, see Holloway et al., 2021). Additionally, PAH, TRAP, and PM are broad categories of pollutants made of constituent parts. Over 100 PAHs are known (e.g., benzo[a]pyrene, chrysene, benzo[b]fluoranthene) as are various TRAP components (e.g., black carbon, nitrogen oxides, tire dust, etc.) and PM that includes any particles from a variety of natural and human origins within nanometer size categories.
Studies linking air pollutant exposures to adverse neuropsychiatric outcomes must account for toxicokinetic and toxicodynamic properties of the pollutants and individual differences in susceptibility based on polymorphisms in xenobiotic metabolism genes (e.g., GSTM1; (Yang et al. 2009) and the distinctions between toxicity of parent chemicals and toxic primary and secondary metabolites. For instance, some PAH congeners, such as benzo[a]pyrene, are more toxic as a reactive intermediate metabolite than the original PAH ((+)-Benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide; Miller & Ramos, 2001). However, distinctions in parent compound vs. metabolite toxicities likely differ when considering genotoxic carcinogenic effects relative to other toxicological influences that might disrupt neurodevelopment. Individual and combined toxicological features of air pollutants, including routes of exposure, metabolism, mechanisms of action, and systemic elimination, as well as exposure science factors, including sampling and analytical approaches, are critical in linking exposures to health outcomes in humans and animal models. The paradigm for exposure in animal models also impacts outcomes. Particulate exposures, including ultrafine particle chambers using particle condensers (e.g., the Harvard ultrafine concentrated ambient particle system; Gupta, Demokritou, & Koutrakis, 2004), and other forms of ambient exposure in rodent models (e.g., housing rats adjacent to air pollutants of interest such as automobile exhaust; Patten et al., 2020) facilitate major strides in resolving mechanisms of neuropsychiatric toxicity of air pollution. Technical reviews of experimental approaches in particulate and inhalation toxicity are provided elsewhere (Costa et al., 2014; Shang & Sun, 2018; Zavala et al., 2020).
5. Convergence of neural targets of air pollution and mechanisms of behavioral phenotypes for psychiatric risk
We highlight three potential areas of overlap between the neural consequences of prenatal air pollution exposure and the neural mechanisms of behavioral phenotypes: 1) changes in dopamine signaling in the prefrontal cortex, dorsal striatum, and ventral striatum (nucleus accumbens); 2) neuroinflammatory responses; and 3) reduced brain-derived neurotrophic factor expression in the hippocampus (Table 2). For each of these mechanisms, we present the exposure-related neural consequences and the overlapping neural mechanism of behavioral phenotypes. When present, we also highlight behavioral effects that are consistent with animal behaviors analogous to human behavioral phenotypes (BI, BE, irritability). When available, we include relevant findings from human neuroimaging studies of BI, BE, and irritability.
Table 2.
Overlap between neural correlates of prenatal exposure to air pollution and behavioral phenotypes
| Publication | Model | Air pollutant | Behavioral phenotype (paradigm) | Target of air pollution exposure | Corresponding neural correlate of behavioral phenotype |
|---|---|---|---|---|---|
| Disturbances in the dopamine system | |||||
| (Yokota et al., 2009) | Mice* | TRAP | BI (spontaneous motor activity) DE-exposed mice had decreased spontaneous locomotor activity. |
Decreased dopamine turnover in dorsal striatum and nucleus accumbens | BI: rats have decreased dopamine activity in nucleus accumbens and dorsal striatum (Flagel et al., 2010; Sanna et al., 2015, 2017; Thiel et al., 1999) |
| (Suzuki et al., 2010) | Mice* | TRAP | BI (spontaneous locomotor activity) DE-exposed mice had decreased spontaneous locomotor activity. |
Decreased dopamine turnover in dorsal striatum Increased dopamine, dopamine turnover and noradrenaline in PFC; decreased noradrenaline turnover in PFC | BI: rats have decreased dopamine activity in dorsal striatum (Flagel et al., 2010; Sanna et al., 2015, 2017; Thiel et al., 1999), and increased dopamine in PFC (Piazza et al., 1991) |
| (Gao et al., 2017) | Zebrafish | PAH | BI (novel tank test, T-maze test) Benzo-a-Pyrene-exposed adult fish had decreased locomotor activity, learning, and memory. | Decreased dopamine, DOPAC, and NE in adulthood, and induced loss of dopaminergic neurons that resulted in neurodegeneration. Increased DNA methylation level of DRD4-related sequence, causing significant decrease in DRD4-related sequence mRNA levels in the whole brain. | BI: rats have decreased dopamine activity in nucleus accumbens and dorsal striatum (Flagel et al., 2010; Sanna et al., 2015, 2017; Thiel et al., 1999) |
| (Yokota et al., 2016) | Mice* | TRAP | Irritability (resident-intruder test) DE-exposed mice showed great social isolation-induced territorial aggression behavior. No significant differences in locomotion. | Increased levels of dopamine and its metabolites in PFC and nucleus accumbens | Irritability: enriched dopamine-DARPP-32 signaling pathway in frontal cortex and ventral striatum genes (Martín-García et al., 2015) |
| (Cui et al., 2019) | Mice | PM2.5 | BE (open field test) PM2.5-exposed mice had increased locomotor and exploratory behavior. | Upregulation of l-dopa (precursor of dopamine) and DRD4 expression levels in the whole brain. DRD4 antagonism ameliorated locomotor activities of mice to levels indistinguishable from controls. | BE: rats have increased dopaminergic activity in dorsal and ventral striatum, including the nucleus accumbens (Piazza et al., 1991; Sanna et al., 2015, 2017; Thiel et al., 1999) |
| Decrease in hippocampal BDNF expression | |||||
| (Zhou et al., 2020) | Mice** | PM2.5 | No relevant tests. | Reduced expression of BDNF in hippocampus | Irritability: fish have downregulated BDNF in telencephalon (Vindas et al., 2014) BI: rats have decreased hippocampal BDNF (Janke et al., 2015; Miao et al., 2019) |
| Hippocampal neuroinflammation and structure | |||||
| (Nephew et al., 2020) | Rats*behavior testing | PM2.5 | BI (elevated platform task, predation test, marble burying task) PM2.5-exposed rats had lower levels of whole cage social play and allogrooming as well as increased anxiety-like behaviors. | Decreased expression of proinflammatory interleukin-18 (IL-18) Ex vivo brain MRI analysis revealed disrupted neural integrity in the hippocampus of PM exposed juveniles |
BI: individual differences are correlated with the activation of various stressresponsive brain regions, including CA3 region of the hippocampus (Qi et al., 2010). |
| (Tseng et al., 2019) | Rats | PM2.5 | BI (MWM test, novel object and location recognition test. PM2.5-exposed rats had decreased recognition, decreased long-term, working, and spatial memory, and decreased exploratory behavior (though not significantly different). | Exposed rats had decreased dendritic branches and terminals in the CA1 and CA3 of the hippocampus. Increased expression of proinflammatory cytokines (TNF-α, monocyte chemoattractant protein-1 (MCP-1), interleukin 1 beta (IL-1β), interleukin-15 (IL-15), and vascular endothelial growth factor |
BI: inactivation of ventral CA1 (Schumacher et al., 2018) Irritability: CA1 area of hippocampus activated (Panagiotaropoulos et al., 2009) BI: heightened proinflammatory responses (glucocorticoid and IL-6) (Michael et al., 2020) Irritability: increased proinflammatory cytokines (Takahashi et al., 2018) |
| (Woodward et al., 2018) | Mice* | TRAP | No effects on BI, BE or irritability (novel object in context recognition; elevated zero maze test) TRAP-exposed rats had decreased discrimination of novel object but no effect on object exploration. No differences in elevated zero maze test. | Increased levels of pro-inflammatory cytokines, and reduced levels of anti-inflammatory cytokines (IL-4, IL-10, IL-13) Microglia were activated in DG and CA1 subfields (35% more Iba1 [inflammatory marker]). |
BI: heightened proinflammatory responses (glucocorticoid and IL-6) (Michael et al., 2020) Irritability: increased proinflammatory cytokines (Takahashi et al., 2018)) BI: inactivation of ventral CA1 (Schumacher et al., 2018) |
| (Berg et al., 2020; Patten et al., 2020) | Mice†,† | TRAP | Mixed BI/BE in females (pup ultrasonic vocalizations, reciprocal social interaction, open field, novel object recognition, fear conditioning) TRAP-exposed female pups had decreased USV calls, spent more time following/chasing in social interaction, and preferred novel objects (BE), but had decreased exploratory behavior (BI). |
Increased microglial infiltration in CA1 (inflammation), reduced astrogliosis in DG, increased hippocampal anti-inflammatory IL-10 in females | BI: inactivation of ventral CA1 produces avoidance (Schumacher et al., 2018) |
| (Bolton et al., 2013) | Mice† | TRAP | BI (fear conditioning, elevated zero-maze) TRAP-exposed males had increased freezing and decreased exploratory behavior. | Increased proinflammatory interleukin (IL)-1β and decreased anti-inflammatory IL-10 in males | BI: heightened proinflammatory responses (glucocorticoid and IL-6) (Michael et al., 2020) Irritability: increased proinflammatory cytokines (Takahashi et al., 2018) |
Note.
indicates only male offspring included in study
indicates only female offspring included in study
indicates sex-specific effect.
5.1. Dopaminergic signaling
In animal models, prenatal exposure to air pollution alters levels of dopamine and its metabolites across several key regions: the prefrontal cortex, the ventral striatum, including the nucleus accumbens, and the dorsal striatum (Table 2; Haber, 2014). These regions belong to parallel frontostriatal loops which are implicated in limbic (e.g., error monitoring and reward processing), associative (e.g., cognitive control), and sensorimotor (e.g., locomotion; Alexander et al., 1986; Botvinick et al., 2004; Shenhav et al., 2016; Tremblay et al., 2015) processes. In the striatum, specifically, there is a ventromedial to dorsolateral gradient, with the former striatal subregions subserving limbic functions, the latter subserving motor functions, and those in between subserving mostly associative functions (Tremblay et al., 2015).
The two extant whole brain analyses exploring the effects of prenatal air pollution on dopaminergic signaling in animal models have yielded equivocal findings. Prenatal exposure to PAH, specifically benzo-[a]-pyrene (BAP), reduced dopamine levels and induced dopaminergic neuron loss across the zebrafish brain (Gao et al., 2017). In contrast, prenatal PM2.5 exposure in mice upregulated a dopamine precursor, l-dopa, and DRD4 receptor expression across the whole brain (Cui et al., 2019). Further study is required to better understand different mechanisms of action associated with these different exposure types and how effects may be localized to specific dopaminergic pathways. Different air pollutants might not exert the same influences in select neurotransmitter systems or in shared brain regions.
Prenatal TRAP exposure more consistently affects dopamine signaling. Two early studies show that prenatal TRAP yields decreased dorsal striatal dopamine turnover (Suzuki et al., 2010; Yokota et al., 2009) and increased levels of dopamine in the prefrontal cortex (Suzuki et al., 2010). In a later study using environmentally relevant levels of exposure (roughly half that of Suzuki et al., 2010), exposed animals continued to show increased dopamine in the prefrontal cortex (Yokota et al., 2016). Notably, equivocal evidence links prenatal air pollution exposure with dopamine and its metabolites in the nucleus accumbens with one study reporting decreased (Yokota et al., 2009) and a second reporting increased levels (Yokota et al., 2016). In sum, prenatal TRAP exposure yielded decreased dorsal striatal dopamine turnover and increased prefrontal dopamine.
The effects of air pollution on dopamine signaling converge with the neural mechanisms of animal behaviors consistent with BI, suggesting that air pollution exposure may increase risk for this behavioral phenotype. For example, low-responder (behaviorally inhibited) rats demonstrate lower striatal dopamine responses to novelty (Thiel et al., 1999) and reduced expression of functionally active (high affinity) D2 receptors in the dorsal striatum (Flagel et al., 2010), which parallel the decreased dorsal striatal dopamine turnover evident in rats prenatally exposed to TRAP (Suzuki et al., 2010; Yokota et al., 2009). Behaviorally, exposure-related striatal or whole brain reductions in dopamine (or in its turnover) are possibly accompanied by decreased spontaneous locomotion (Gao et al., 2017; Suzuki et al., 2010; Yokota et al., 2009) (but see Cui et al., 2019; Yokota et al., 2016), a behavioral pattern characteristic of low-responder rat strains (Clinton et al., 2011, 2021; Flagel et al., 2010; Simmons et al., 2012). Additionally, increased monitoring associated with BI (Buzzell et al., 2018) may be paralleled by increased prefrontal (including the cingulate) dopamine associated with TRAP exposure (Suzuki et al., 2010; Yokota et al., 2009; 2016). We thus propose that prenatal air pollution exposure may increase risk for BI in infancy via disruptions across frontostriatal circuits.
Likewise prenatal air pollution exposure may also increase risk for irritable temperament. Negative prediction errors elicited by FNR are signaled by phasic reductions in striatal dopamine (Chang et al., 2016), and TRAP-induced dopamine signaling disruptions may alter prediction error coding in rats (Suzuki et al., 2010; Yokota et al., 2009). TRAP exposure also increases prefrontal dopamine (Suzuki et al., 2010; Yokota et al., 2016), which is implicated in prediction error processing under state uncertainty (Starkweather et al., 2018) as well as in cognitive control (Ott & Nieder, 2019). Similarly, TRAP-related increases in prefrontal dopamine have been shown to increase aggression (shorter attack latency) in the residential intruder paradigm (Yokota et al., 2016), a behavioral pattern consistent with FNR outcomes and irritability. Given TRAP-related dopaminergic effects, we propose that increased prenatal TRAP exposure may disrupt negative prediction error processing and impair cognitive control, thereby increasing risk for irritability.
5.2. Neuroinflammatory responses and hippocampal structure
Prenatal exposure to PM 2.5 and TRAP results in systemic neuroinflammatory responses. Prenatal PM 2.5 exposure increased serum levels of proinflammatory cytokines (TNF-α, monocyte chemoattractant protein-1 [MCP-1], interleukin 1 beta [IL-1β], IL-15, and vascular endothelial growth factor) (Tseng et al., 2019) but notably, in a separate study, it decreased proinflammatory IL-18 in plasma (Nephew et al., 2020). In addition to increased levels of pro-inflammatory cytokines caused by PM2.5 exposure, reduced serum levels of anti-inflammatory cytokines (IL-4, IL-10, IL-13) were observed in animals exposed to ecologically valid levels of prenatal TRAP, taken from roadways and tunnels (Woodward et al., 2018). Thus, further study is required to resolve some of the conflicting findings of PM2.5- and TRAP-related effects on pro-inflammatory and anti-inflammatory cytokine responses. However, studies generally suggest that exposure causes systemic increased proinflammatory and decreased anti-inflammatory cytokine expression.
One study has specifically examined neuroinflammatory responses across the whole brain and two have examined neuroinflammatory responses in hippocampal subfield regions. Whole brain analysis points to sex-specific effects of prenatal TRAP exposure on increased proinflammatory IL-1β and decreased anti-inflammatory IL-10 in male but not female fetal brains (Bolton et al., 2013). In contrast, female TRAP-exposed offspring showed increased hippocampal anti-inflammatory IL-10 (Patten et al., 2020). Prenatal TRAP also resulted in activated microglia as marked by increased immunofluorescence (Iba1) in the dentate gyrus (DG) polymorphic layer and in the neuropil layer of the CA1 subregion (Woodward et al., 2018) in both males and females; whereas, only female TRAP-exposed offspring showed increased microglial infiltration in the CA1 region, as well as reduced astrogliosis in the DG in a second study (Patten et al., 2020). Notably, prenatal PM 2.5 exposure also resulted in altered hippocampal morphology, including decreases in the number of dendritic branches and terminals in the CA1 and CA3 subregions (Tseng et al., 2019) and disrupted neural integrity in the hippocampus (Nephew et al., 2020). Additionally, TRAP exposure resulted in reduced neurogenesis in the DG (Woodward et al., 2018). Findings linking the neuroinflammatory processes subsequent to prenatal viral infection with altered morphology in the dentate gyrus (Li et al., 2014) suggest that the neuroinflammation secondary to prenatal air pollution exposure may cause TRAP-related morphologic changes in the hippocampus.
The broad neuroinflammatory effects of PM2.5 and TRAP converge with neural mechanisms that underlie behaviors in animal models consistent with BI and irritability. Compared to non-inhibited rats, behaviorally inhibited rats show heightened proinflammatory -- glucocorticoid and IL-6 -- responses to stress (Michael et al., 2020) similar to increases in proinflammatory cytokines caused by prenatal PM2.5 and TRAP exposure. Human studies also show associations between frontal EEG asymmetry, a marker of BI and avoidance (Rutherford & Lindell, 2011), and IL-6 (Shields & Moons, 2016). Increased proinflammatory cytokines are also associated with increased aggressive behavior in animal models (Takahashi et al., 2018) paralleling human irritability. Human findings also point to associations between TNF-related inflammatory cytokine gene expression and neural activity associated with FNR and aggressive behaviors in pediatric bipolar disorder (Barzman et al., 2014).
The effects of prenatal PM2.5 and TRAP exposure on hippocampal structure converge with neural mechanisms that underlie BI and irritability. In one rodent model, ventral CA1 and CA3 subregions had distinct roles in processing approach versus avoidance behavior to conflict (Schumacher et al., 2018). CA1 is implicated in avoidance behaviors and is affected by TRAP and PM2.5 exposure. TRAP- and PM2.5-exposed animals also showed behaviors consistent with BI: lower center time in an open field test (Berg et al., 2020), greater latency to climb down from an elevated platform, increased fecal boli and risk assessment behavior in a cricket predation task, and less whole-cage social play and allogrooming (Nephew et al., 2020). In contrast, prenatally exposed animals showed reduced neonatal pup ultrasonic vocalizations and altered juvenile reciprocal social interactions, and preferred a novel versus familiar object (Berg et al., 2020), which, although traditionally considered a marker of memory, may instead reflect an increased interest in novelty, which is more consistent with BE than BI. Moreover, TRAP exposure altered CA3 morphology, which has been shown to underlie approach behavior (Schumacher et al., 2018). Finally, adults with higher BI, as measured by sensitivity to punishment, show greater hippocampal volumes (Barrós-Loscertales et al., 2006; Cherbuin et al., 2008; Levita et al., 2014). With respect to irritable temperament, the FNR paradigm yielded a higher number of cFos immunopositive cells in the CA1 subregion (Panagiotaropoulos et al., 2009) that is targeted by TRAP and PM2.5. Given these findings, exposure-related effects on CA1 may increase risk for BI and irritable temperament in infancy and effects on CA3 may increase risk for BE.
5.3. Brain-derived neurotrophic factor expression in the hippocampus
Prenatal PM2.5 exposure caused reductions in brain-derived neurotrophic factor (BDNF) expression in the hippocampus (Zhou et al., 2020). Tasks measuring behavioral phenotypes were not investigated. Although there is only a single study documenting effects of prenatal exposure on hippocampal BDNF expression, a model of postnatal exposure showed a similar pattern of results such that PM2.5 exposure on postnatal day 3–15 resulted in decreased hippocampal BDNF expression (Liu et al., 2019). In humans, PAH-DNA adducts, a marker of exposure, and BDNF in umbilical cord blood were compared in two successive birth cohorts after a highly polluting, coal-fired power plant in Tongliang County, China ceased operation. Infants born after the closing of the coal burning factory had decreased PAH-DNA adducts and increased levels of mature BDNF (Tang et al., 2014).
The effects of air pollution on hippocampal BDNF also converge with the neural mechanisms of BI and irritability. For example, hippocampal BDNF is negatively correlated with avoidant behavior (Miao et al., 2019). Facilitated eye-blink, another marker of BI, is related to reductions in hippocampal BDNF (Janke et al., 2015), which also arises from prenatal PM2.5 exposure (Zhou et al., 2020). In addition, postnatal PM2.5 exposure caused reductions in hippocampal BDNF and accompanying anxiety-like symptoms as measured by lower number of entries on open arms in an elevated maze task (Liu et al., 2019). However, reductions in BDNF have been linked with increased spontaneous locomotion (Monteggia et al., 2004; Rios et al., 2001), a back-translated marker of BE, but not in studies of exposure to air pollutants. Despite some conflicting evidence in locomotion measures, given direct observations of BI-like behaviors in postnatally exposed animals, we propose that reductions in hippocampal BDNF may increase risk for BI. With respect to irritability, down-regulated BDNF expression in the telencephalon of Atlantic salmon has also been observed after omission of expected reward (i.e., FNR; Vindas et al., 2014), paralleling effects of exposure on reduced BDNF, although not localized to the hippocampus (fish do not have an anatomical hippocampus). PM2.5 exposure may alter BDNF expression through hypermethylation of the BDNF promoter IV (Zhou et al., 2020), but further study is required to understand the mechanisms underlying PM2.5 exposure, decreased BDNF expression, and behavior in FNR paradigms.
6. Discussion
We have reviewed animal models of prenatal exposure to three types of air pollutants and identified overlapping neural circuits, regions, and processes that are both targets of exposure and putative mechanisms underlying behavioral phenotypes that confer risk for later life psychopathology, specifically internalizing and externalizing problems. Additionally, we discuss animal models that represent analogues of human phenotypes which are predictive of later psychopathology. Although these pollutants have also been shown in animal models to affect other processes in the brain such as decreased cytochrome oxidase activity (Crépeaux et al. 2012), the behavioral phenotypic consequences of such exposures are yet to be explored. The convergence of evidence reviewed herein points to neural and cognitive pathways through which novel exposure-related behavioral phenotypes may emerge. Although much of the evidence converges, there are inconsistencies and many understudied areas.
Across different air pollutant exposure models, evidence points to an increased risk for BI and irritable phenotypes. Prenatal TRAP exposure alters levels of dopamine and its metabolites in regions critical for dopamine signaling. TRAP-related reductions in striatal dopamine turnover and increases in prefrontal dopamine (Suzuki et al., 2010; Yokota et al., 2009, 2016) may increase risk for BI and irritability as evidenced by reduced locomotion (Gao et al., 2017; Suzuki et al., 2010; Yokota et al., 2009) and increased aggression in animals (Yokota et al., 2016), respectively. Further, findings from human studies of prenatal exposure to PAH point to associations between exposure and reduction in caudate volumes (Mortamais et al. 2017), consistent with altered dopamine functioning reported in animal models. Prenatal TRAP and PM2.5 also increase risk for BI and irritability through neuroinflammatory effects across the brain (Nephew et al., 2020; Tseng et al., 2019; Woodward et al., 2018) and through effects in the hippocampus localized in the CA1 subregion (Patten et al., 2020; Woodward et al., 2018), which has been theorized to subserve avoidance behaviors and shows higher cFos reactivity after FNR (Panagiotaropoulos et al., 2009). Moreover, in vitro findings point to links between TRAP exposures, neuroinflammatory processes (phagocytic activation of microglial NADPH oxidase), and selective damage to dopamine cells, including cell death (Block et al., 2004). Finally, PM2.5 exposure increases risk for BI and irritability through reduced BDNF in the hippocampus, which has been previously linked with BI and anxiety (Janke et al., 2015; Miao et al., 2019) and with omission of reward in FNR (Vindas et al., 2014). Less convincing evidence points to potential associations between exposure to these three air pollutants and BE, suggesting that this phenotype may not be pollution-related and pollution-related risk for ADHD may not depend on early-life BE. Further, in some cases, exposures have been shown to confer an advantage such as when cigarette smoking which contains PAH is protective against Parkinson symptoms (Mappin-Kasirer et al. 2020). Thus, we propose that prenatal exposure to these air pollutants increases risk for BI and irritable phenotypes and suggest that future studies investigate these effects in longitudinal designs incorporating complex exposure measurement methods, neuroimaging, and behavioral characterization of temperament and neurocognitive processing from infancy through childhood.
6.1. Pollution-related BI versus irritable phenotypes
In human clinical settings, anxiety has been reported to co-occur with irritability (Cornacchio et al., 2016; Shimshoni et al., 2020), and in animal models FNR has been shown to elicit irritable behavior and anxiety (Cuenya et al., 2012; Taylor et al., 2019). However, in human infants, BI and irritable phenotypes are uncorrelated and have distinct neural underpinnings (Filippi, Subar, et al., 2020). Our review suggests that in animal models, prenatal exposure to air pollution increases risk for each phenotype via effects on their neural correlates. Future studies could examine why some infants manifest one versus the other phenotype and how this contributes to risk for later psychopathology. Yet unidentified factors, including genetic vulnerability to effects of air pollution, may moderate effects of exposure.
6.2. Proposed cognitive mechanisms underlying exposure-related BI and irritability
Cognitive pathways to BI and irritability arise via altered cognitive control, specifically error monitoring (Buzzell et al., 2018), and disrupted prediction error processing (Chang et al., 2016). Increased prefrontal (including in the cingulate) dopamine associated with TRAP exposure (Suzuki et al., 2010; Yokota et al., 2009) may cause increased environmental monitoring which is associated with BI (Buzzell et al., 2018). TRAP-induced decreases in striatal dopamine turnover (Suzuki et al., 2010; Yokota et al., 2009) may lead to abnormal negative prediction error processing associated with FNR (Chang et al., 2016). Human studies have shown that irritability may derive from impaired prediction error processing during reward learning (Brotman et al., 2017; Meyers et al., 2017). Future studies may be able to better understand effects of pollution on irritability using computational models, such as Q learning. For example, since prenatal TRAP exposure yielded decreased dorsal striatal dopamine turnover and increased prefrontal dopamine, we would expect to see decreased learning from positive prediction errors and increased learning from negative prediction errors.
The role of prenatal or concurrent exposure to air pollution on error monitoring and reinforcement learning has yet to be explored. Future studies can examine the impact of exposure both in animal models and in human cognitive and neuroimaging paradigms. In animal models, equivocal evidence links prenatal air pollution exposure to dopamine levels in the nucleus accumbens (Yokota et al., 2009, 2016) and whole brain dopamine (Cui et al., 2019; Gao et al., 2017). Human neuroimaging studies can now study effects of PAH exposure on negative prediction errors using model-based fMRI reinforcement-learning tasks (O’Doherty et al., 2007; Maia & Frank, 2011; Series, 2020).
6.3. Prenatal exposure to air pollution magnifies risk pathways in developmental models of anxiety
Air pollution exposure has been associated with increased risk for internalizing disorders and anxiety (Volk et al., 2021), as well as reductions in working memory (Rivas et al., 2019), attention (Chiu et al., 2016; Cowell et al., 2015), self-regulation (Margolis et al., 2016), and inhibitory control capacities (Margolis et al., 2021), which modulate the pathway from BI in young children to anxiety symptoms and disorders in older children (Fox et al., 2021; White et al., 2011). Importantly, these executive capacities are modulated by dopamine signaling (Ott & Nieder, 2019), which also is associated with anxiety symptoms, genetic risk, and treatment response, and as such has been hypothesized to play a critical role in the interaction between BI and inhibitory control on the manifestation of anxiety (Gunther & Pérez-Edgar, 2021). We propose that prenatal exposure to air pollution disrupts dopamine signaling thus contributing to BI and reductions in cognitive control which ultimately increases risk for anxiety. Human imaging studies of dopamine function in individuals with BI have been challenged by the lack of noninvasive methods for imaging dopamine, but studies of dopamine receptor gene expression and pharmacologic manipulation of dopamine levels suggest that dopamine plays a role in social anxiety symptoms (Cervenka et al., 2012; Hood et al., 2010; Schneier et al., 2000). Innovative noninvasive magnetic resonance imaging pulse sequences now allow for visualization of neuromelanin, a catabolite of dopamine, which binds to iron and is thus available for imaging in the substantia nigra and the ventral tegmental area (Sulzer et al., 2018), opening new avenues for the study of the dopaminergic effects of prenatal exposure to air pollution in humans.
6.4. Chemical and social stressors interact
Recent models have investigated the interacting effects of early life exposure to neurotoxicants including social stress and chemical exposures such as air pollution on neurodevelopment (Padula et al., 2020). For example, prenatal exposure to PAH moderated associations between early life stress and attention and thought problems (Pagliaccio et al., 2020). The mechanisms underlying such synergy remain understudied. We propose that effects of prenatal exposure to air pollution on hippocampal BDNF can increase vulnerability to stress, given that higher levels of hippocampal BDNF buffer the deleterious effects of stress (Taliaz et al., 2011). Thus, pollution-related reductions in hippocampal BDNF may provide one mechanism by which air pollution and stress have synergistic effects on behavioral and psychiatric outcomes. In addition, stress has significant effects on dopaminergic signaling, such that dopamine is released in the striatum in response to noxious stressors (Belujon and Grace 2015; Carvalheiro et al. 2021), which can further potentiate the deleterious effects of air pollution on brain function. Importantly, developmental models should consider mixtures of chemical and social exposures to identify modifiable risk factors.
6.5. Sex-specific effects of prenatal exposure to air pollution
Studies of the outcomes associated with prenatal exposures in animal models have primarily focused on male offspring. However, it is evident that there are sex-specific effects and sex differences in outcomes associated with prenatal exposure to air pollution. In females, prenatal TRAP-exposure resulted in increased microglial infiltration in the CA1, reduced astrogliosis in the DG, and increased hippocampal anti-inflammatory cytokines (Patten et al., 2020). In males, prenatal TRAP-exposure resulted in increased neurogenesis in the DG (Patten et al., 2020) as well as increased proinflammatory but decreased anti-inflammatory cytokines (Bolton et al., 2013). In both studies, prenatal TRAP-exposure resulted in sex-specific effects on behaviors. Exposed female mice exhibited BE-like behaviors including decreased USV calls, more time following an unfamiliar mouse, and preference for a novel object, but also exhibited BI-like behavior such as decreased exploratory behavior in an open field task (Berg et al., 2020). Affected male mice exhibited BI with increased freezing and decreased exploratory behavior compared to females (Bolton et al., 2013). High levels of IL-10 are protective against behavioral changes due to microglial-driven neuroinflammation (Schwarz et al., 2011), which may explain why the male mice with decreased IL-10 were more vulnerable to BI-like impairments compared to the females with normative IL-10 levels. In contrast, exposed females showed increased IL-10 (Patten et al., 2020) and increases in BE-like behavior (Berg et al., 2020), suggesting a behavioral continuum for effects of IL-10. Sex-specific effects of air pollution on neurodevelopment have also been exhibited in humans; associations between prenatal PM2.5 exposure and adverse memory performance have been reported in females, but associations between exposure and lower IQ and poor attention have been reported in males (Chiu et al., 2016). Additionally, some studies have identified sex-specific effects of prenatal air pollutant exposure on child executive functions (e.g., attention, memory) that contribute to self-regulatory capacity in boys (Chiu et al., 2013, 2016; Cowell et al., 2015; Rivas et al., 2019). These findings suggest that increased prenatal air pollution exposure may have sex-specific behavioral and cognitive effects and underscore the need for further study of these sex-specific effects in human and animal studies. When evaluating the effects of air pollution exposure on females, it is particularly critical to evaluate the contribution of the estrous cycle. Studies have demonstrated that high-estrogen phases are associated with less depression and anxiety-like behavior relative to low-estrogen phases (Jaric et al., 2019; Marcondes et al., 2001; Walf & Frye, 2006). Therefore, failure to observe sex-specific effects may reflect natural hormonal variation due to the estrous cycle that was not considered in the design or analysis.
6.6. Conflicting findings from animal models: Proposed systems to explore
Much remains unknown about the effects of prenatal exposure to air pollution. There is an urgent need for future studies to examine whole brain processes across mechanisms rather than testing only for hypothesized or likely areas. Whole brain analyses of exposure have been rare. Only one study has examined whole brain effects of exposure-related neuroinflammatory processes (Bolton et al., 2013). Further study is required to resolve conflicting findings of sex-specific effects of PM2.5 and TRAP on pro-inflammatory and anti-inflammatory cytokine responses. In some cases, extant studies were conducted in different species making comparisons challenging. For example, prenatal PAH exposure reduced dopamine levels and induced dopaminergic neuron loss across the zebrafish brain (Gao et al., 2017), but in a mouse model prenatal PM2.5 exposure upregulated a dopamine precursor, l-dopa, and DRD4 receptor expression across the brain (Cui et al., 2019). Similar challenges exist in the human neuroimaging literature. For example, much work has focused on the role of the amygdala in BI and anxiety with a general lack of exploration of the role of the hippocampus (Clauss et al., 2015), although recent work in non-human primates also points to hippocampal involvement in BI (Villard et al., 2021). A recent study in humans documents links between prenatal exposure to PAH and reductions in hippocampal volumes (Lubczyńska et al. 2021). Whole brain analyses require large samples to achieve adequate power, and prenatal exposure studies require prospective birth cohorts or retrospective biomarkers, such as can be accomplished with dentine (Petrick et al., 2020). Large sample sizes with decades of prospective data are challenging to find; however, some studies are acquiring appropriate samples such as in the Environmental influences on Children’s health Outcomes (ECHO) study or the Columbia Center for Children’s Environmental Health studies.
Studies that examine particular brain regions based on prior findings or outcomes of interest limit what we know. Alternate pathways from prenatal air pollution exposure to behavioral phenotypes for psychiatric risk through neuroinflammation likely exist; however, we have not discussed such findings because direct evidence is lacking. For example, links between air pollution and neuroinflammation (Bolton et al., 2013; Nephew et al., 2020; Patten et al., 2020; Tseng et al., 2019; Woodward et al., 2018), neuroinflammation and central amygdala morphology (Althammer et al., 2020), central amygdala and BI (Kalin et al., 2016; Kalin & Shelton, 1989; Rogers et al., 2013) have all been separately shown; however, experiments have not yet examined the effects of air pollution on central amygdala structure. Future studies must profile effects of exposure comprehensively, rather than focusing on specific endpoints of interest based on prior findings or specific laboratory expertise.
Conflicting findings using spontaneous locomotion as a measure of the effects of air pollution on BI and BE suggest that further study is required. For example, reduced BDNF has been associated with both increased (Monteggia et al., 2004; Rios et al., 2001) and decreased (Liu et al., 2019) locomotion. Similarly, prenatal TRAP exposure caused reduced striatal dopamine and reduced spontaneous locomotion in one study (Yokota et al., 2009) but not in a second study (Yokota et al., 2016). TRAP exposed animals also preferred a novel versus familiar object (Berg et al., 2020), possibly pointing to increased BE with TRAP exposure. Finally, TRAP exposure altered CA1 morphology (Tseng et al., 2019), which is thought to underlie avoid behavior, but it also affected CA3 morphology which is thought to underlie approach behavior (Schumacher et al., 2018). Notably, some rodent and human studies document animals and infants who show features of both BI and BE, depending on the context. For example, rodents display both BI and BE in social and non-social experimental contexts (Cavigelli et al., 2007; Michael et al., 2020). Similarly, a child with BI is more likely to feel distress and anxiety upon encountering a new social environment and simultaneously be driven to interact with others to lessen these feelings (Degnan & Fox, 2007; Tarullo et al., 2011). This motivation is associated with left frontal asymmetry which typically indicates approach tendencies and mitigates the relation between BI and social wariness (Fox et al., 2001; Harmon-Jones & Gable, 2018). Furthermore, shy (BI) individuals have enhanced reward sensitivity and respond faster to incentives than non-shy individuals, reflecting increased approach behaviors in particular contexts (Barker et al., 2019; Hardin et al., 2006).
We also encourage basic studies to look at specific behavioral tasks. For example, studies examining effects of exposure on reinforcement learning and specifically on negative prediction errors are needed to understand mechanisms through which exposure yields altered outcomes in the FNR paradigm. Tasks that are designed and refined to define the phenotype will enhance our understanding of the neural targets of air pollution. Finally, well designed animal models can identify underlying biological mechanisms through which prenatal exposure to air pollutants affect offspring brain development; recent work suggests that exposure may act on gut microbiota (Liu et al. 2020) or maternal immune activation (Volk et al. 2020) potentially via effects on microglia which have been implicated in psychiatric illness (Frick and Pittenger 2016; Tay et al. 2017; Rahimian et al. 2021). More work is required to understand how these processes affect humans and thus may be prevented.
6.7. Measuring Air Pollution
The exposures in the studies reviewed herein can be measured in different ways in human epidemiologic studies (see Volk et al., 2021 for an in-depth description). PAHs are typically measured through personal exposure, for example a backpack with a filter that captures chemicals for analysis. PM2.5 and TRAP are more frequently measured with geocoded data based on a participant’s address and satellite data regarding air quality, or distance from a roadway or traffic on one’s street of residence. Studies using geocoded rather than personal exposure measures may be more at risk of conflating more general neighborhood level effects with effects of neurotoxicant exposure. Future studies could aim to disentangle these issues by collecting personal measures of neighborhood level variables to use in conjunction with geocoded data.
7. Conclusion
Human studies show associations between prenatal exposure to air pollution and internalizing and externalizing behaviors as well as cognitive problems that act as effect modifiers of associations between BI/BE and internalizing and externalizing behaviors. Our review of animal models of both the neural targets of exposure and the neural mechanisms of these behavioral phenotypes suggests that these effects may arise from several distinct mechanisms. One may operate through hippocampal effects, specifically effects of prenatal air pollution exposure on hippocampal morphology, inflammation, and BDNF expression, suggesting that the role of the hippocampus in BI, irritability, and anxiety should be further investigated, as others have recently argued (Clauss et al., 2015; Villard et al., 2021). A second putative mechanism is through dopamine and prediction error processing which may increase the likelihood of BI and irritable phenotypes. Even small effect sizes at the individual level translate to costly consequences at the population level making the effects of prenatal exposure to air pollution a significant public health problem with respect to poor developmental outcomes. These findings taken together point to the need to study BI and irritable phenotypes associated with prenatal exposure to air pollution and to search for appropriate treatment targets that may have long-lasting developmental benefits for children, particularly those living in areas with high levels of exposure.
Figure 1. Convergence of evidence from animal models of prenatal exposure to air pollution and altered behavioral phenotypes with developmental models of human psychiatric risk.
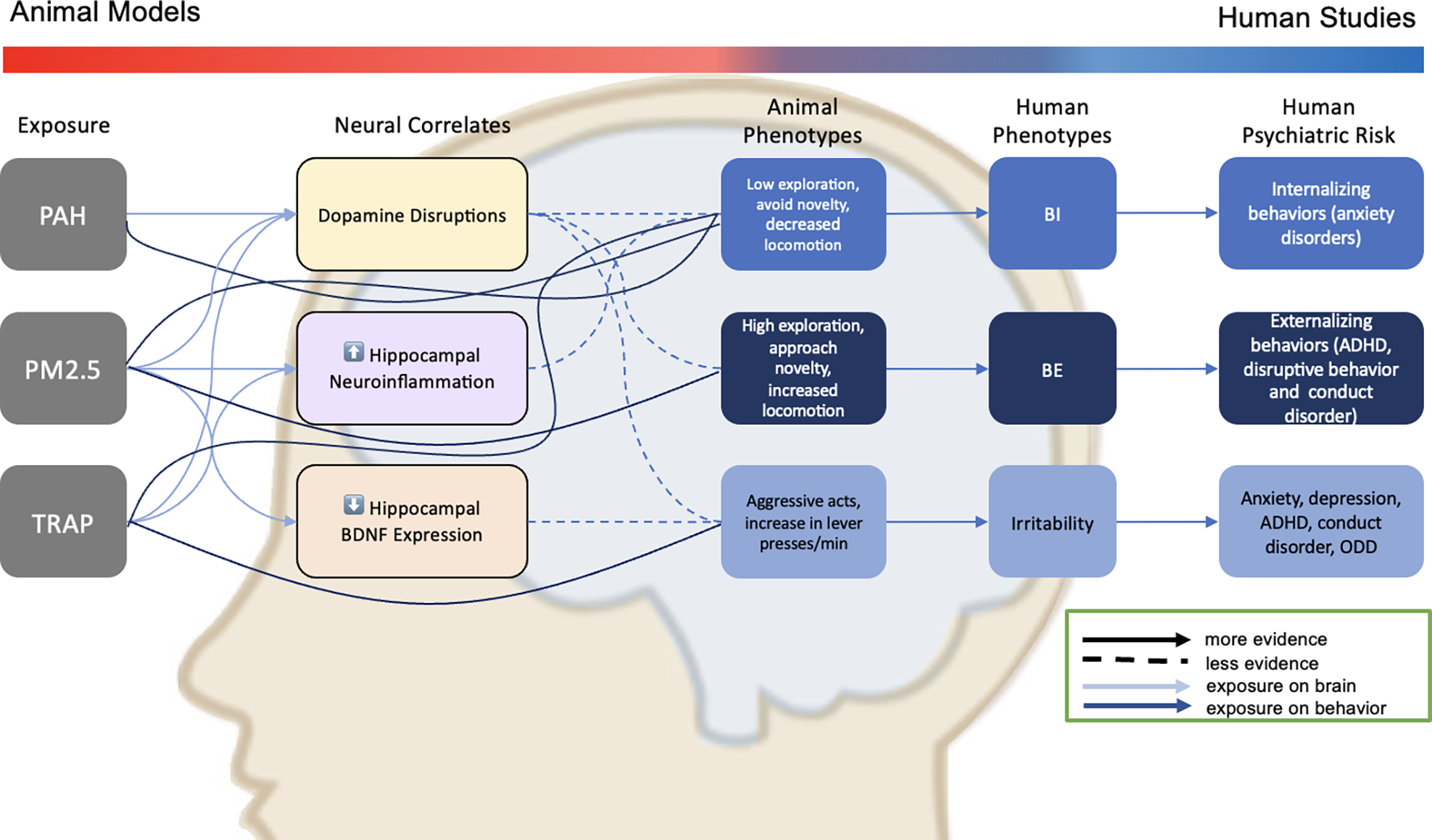
The gradient bar illustrates how the studies reviewed herein cover animal models (red) and human studies (blue) and where these overlap conceptually (cross-species phenotypes; shown in purple). Light blue arrows from “exposure” to “neural correlates” and dark blue lines from “exposure” to analogous – back-translated – ”animal phenotypes” show potential mechanisms of exposure on human psychiatric risk. Solid versus dashed lines indicate more versus less persuasive evidence (Table 2). Observational studies linking human prenatal exposure to air pollution and alterations in brain structure are described in the text (see Discussion). Animal phenotypes akin to behavioral inhibition (BI) and behavioral exuberance (BE) were assessed in animal models of anxiety (elevated plus maze test, open field test, etc. see Table 1), whereas those akin to irritability were assessed using frustrative non-reward tasks (Table 1). Arrows from “exposure” to “neural correlates” to “animal phenotypes” and from “human phenotypes” to “human psychiatric risk” represent the hypothesized causal model positing that exposure increases psychiatric risk via effects on the brain and behavioral reactivity. ADHD = Attention Deficit hyperactivity Disorder; ODD=Oppositional Defiant Disorder.
Highlights.
Air pollution alters dopamine, BDNF, and hippocampal structure in animal models.
Neural mechanisms of risk markers of psychopathology are vulnerable to exposure.
Exposure may increase risk for social anxiety and irritability via these mechanisms.
Funding:
This work was supported by the National Institutes of Environmental Health Sciences R01ES032296 and K23026239 to A.E.M. and Whole Communities Whole Health, a University of Texas at Austin Grand Challenge Initiative (OVPR) to F.A.C.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams J, Barone S Jr, LaMantia A, Philen R, Rice DC, Spear L, & Susser E (2000). Workshop to identify critical windows of exposure for children’s health: neurobehavioral work group summary. Environmental Health Perspectives, 108 Suppl 3, 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemany S, Vilor-Tejedor N, García-Esteban R, Bustamante M, Dadvand P, Esnaola M, Mortamais M, Forns J, van Drooge BL, Álvarez-Pedrerol M, Grimalt JO, Rivas I, Querol X, Pujol J, & Sunyer J (2018). Traffic-related air pollution, APOEε4 status, and neurodevelopmental outcomes among school children enrolled in the BREATHE project (Catalonia, Spain). Environmental Health Perspectives, 126(8), 087001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, & Strick PL (1986). Parallel Organization of Functionally Segregated Circuits Linking Basal Ganglia and Cortex. Annual Review of Neuroscience, 9(1), 357–381. [DOI] [PubMed] [Google Scholar]
- Althammer F, Ferreira-Neto HC, Rubaharan M, Roy RK, Patel AA, Murphy A, Cox DN, & Stern JE (2020). Three-dimensional morphometric analysis reveals time-dependent structural changes in microglia and astrocytes in the central amygdala and hypothalamic paraventricular nucleus of heart failure rats. Journal of Neuroinflammation, 17(1), 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP (2004). The developmental origins of chronic adult disease. Acta Paediatrica. Supplement, 93(446), 26–33. [DOI] [PubMed] [Google Scholar]
- Barker TV, Buzzell GA, & Fox NA (2019). Approach, avoidance, and the detection of conflict in the development of behavioral inhibition. New Ideas in Psychology, 53, 2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrós-Loscertales A, Meseguer V, Sanjuán A, Belloch V, Parcet MA, Torrubia R, & Avila C (2006). Behavioral Inhibition System activity is associated with increased amygdala and hippocampal gray matter volume: A voxel-based morphometry study. NeuroImage, 33(3), 1011–1015. [DOI] [PubMed] [Google Scholar]
- Barzman D, Eliassen J, McNamara R, Abonia P, Mossman D, Durling M, Adler C, DelBello M, & Lin P-I (2014). Correlations of inflammatory gene pathways, corticolimbic functional activities, and aggression in pediatric bipolar disorder: a preliminary study. Psychiatry Research, 224(2), 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, & Grace AA (2015). Regulation of dopamine system responsivity and its adaptive and pathological response to stress. Proceedings. Biological Sciences / The Royal Society, 282(1805). 10.1098/rspb.2014.2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg EL, Pedersen LR, Pride MC, Petkova SP, Patten KT, Valenzuela AE, Wallis C, Bein KJ, Wexler A, Lein PJ, & Silverman JL (2020). Developmental exposure to near roadway pollution produces behavioral phenotypes relevant to neurodevelopmental disorders in juvenile rats. Translational Psychiatry, 10(1), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Wu X, Pei Z, Li G, Wang T, Qin L, Wilson B, Yang J, Hong JS, & Veronesi B (2004). Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 18(13), 1618–1620. [DOI] [PubMed] [Google Scholar]
- Bolton JL, Huff NC, Smith SH, Mason SN, Foster WM, Auten RL, & Bilbo SD (2013). Maternal stress and effects of prenatal air pollution on offspring mental health outcomes in mice. Environmental Health Perspectives, 121(9), 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, & Carter CS (2004). Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences, 8(12), 539–546. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Kircanski K, & Leibenluft E (2017). Irritability in Children and Adolescents. Annual Review of Clinical Psychology, 13, 317–341. [DOI] [PubMed] [Google Scholar]
- Bunford N, Evans SW, & Wymbs F (2015). ADHD and Emotion Dysregulation Among Children and Adolescents. Clinical Child and Family Psychology Review, 18(3), 185–217. [DOI] [PubMed] [Google Scholar]
- Buzzell GA, Troller-Renfree SV, Barker TV, Bowman LC, Chronis-Tuscano A, Henderson HA, Kagan J, Pine DS, & Fox NA (2017). A Neurobehavioral Mechanism Linking Behaviorally Inhibited Temperament and Later Adolescent Social Anxiety. Journal of the American Academy of Child and Adolescent Psychiatry, 56(12), 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzell GA, Troller-Renfree SV, Morales S, & Fox NA (2018). Relations between Behavioral Inhibition, Cognitive Control, and Anxiety: Novel Insights Provided by Parsing Subdomains of Cognitive Control. In Pérez-Edgar K & Fox NA (Eds.), Behavioral Inhibition: Integrating Theory, Research, and Clinical Perspectives (pp. 213–235). Springer International Publishing. [Google Scholar]
- Capitanio JP (2018). Behavioral Inhibition in Nonhuman Primates: The Elephant in the Room. In Pérez-Edgar K & Fox NA (Eds.), Behavioral Inhibition: Integrating Theory, Research, and Clinical Perspectives (pp. 17–33). Springer International Publishing. [Google Scholar]
- Caruso MJ, McClintock MK, & Cavigelli SA (2014). Temperament moderates the influence of periadolescent social experience on behavior and adrenocortical activity in adult male rats. Hormones and Behavior, 66(3), 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalheiro J, Conceição VA, Mesquita A, & Seara-Cardoso A (2021). “Acute Stress Blunts Prediction Error Signals in the Dorsal Striatum during Reinforcement Learning.” BioRxiv. bioRxiv. 10.1101/2021.02.11.430640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavigelli SA, Stine MM, Kovacsics C, Jefferson A, Diep MN, & Barrett CE (2007). Behavioral inhibition and glucocorticoid dynamics in a rodent model. Physiology & Behavior, 92(5), 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka S, Hedman E, Ikoma Y, Djurfeldt DR, Rück C, Halldin C, & Lindefors N (2012). Changes in dopamine D2-receptor binding are associated to symptom reduction after psychotherapy in social anxiety disorder. Translational Psychiatry, 2, e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CY, Esber GR, Marrero-Garcia Y, Yau H-J, Bonci A, & Schoenbaum G (2016). Brief optogenetic inhibition of dopamine neurons mimics endogenous negative reward prediction errors. Nature Neuroscience, 19(1), 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbuin N, Windsor TD, Anstey KJ, Maller JJ, Meslin C, & Sachdev PS (2008). Hippocampal volume is positively associated with behavioural inhibition (BIS) in a large community-based sample of mid-life adults: the PATH through life study. Social Cognitive and Affective Neuroscience, 3(3), 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y-HM, Bellinger DC, Coull BA, Anderson S, Barber R, Wright RO, & Wright RJ (2013). Associations between traffic-related black carbon exposure and attention in a prospective birth cohort of urban children. Environmental Health Perspectives, 121(7), 859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y-HM, Hsu H-HL, Coull BA, Bellinger DC, Kloog I, Schwartz J, Wright RO, & Wright RJ (2016). Prenatal particulate air pollution and neurodevelopment in urban children: Examining sensitive windows and sex-specific associations. Environment International, 87, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss J (2019). Extending the neurocircuitry of behavioural inhibition: a role for the bed nucleus of the stria terminalis in risk for anxiety disorders. General Psychiatry, 32(6), e100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Avery SN, & Blackford JU (2015). The nature of individual differences in inhibited temperament and risk for psychiatric disease: A review and meta-analysis. Progress in Neurobiology, 127–128, 23–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Shupe EA, Glover ME, Unroe KA, McCoy CR, Cohen JL, & Kerman IA (2021). Modeling heritability of temperamental differences, stress reactivity, and risk for anxiety and depression: Relevance to research domain criteria (RDoC). The European Journal of Neuroscience. 10.1111/ejn.15158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Stead JDH, Miller S, Watson SJ, & Akil H (2011). Developmental underpinnings of differences in rodent novelty-seeking and emotional reactivity. The European Journal of Neuroscience, 34(6), 994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, Feigin V, Freedman G, Hubbell B, Jobling A, Kan H, Knibbs L, Liu Y, Martin R, Morawska L, … Forouzanfar MH (2017). Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. The Lancet, 389(10082), 1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Brotman MA, & Costello EJ (2015). Normative Irritability in Youth: Developmental Findings From the Great Smoky Mountains Study. Journal of the American Academy of Child and Adolescent Psychiatry, 54(8), 635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornacchio D, Crum KI, Coxe S, Pincus DB, & Comer JS (2016). Irritability and Severity of Anxious Symptomatology Among Youth With Anxiety Disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 55(1), 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Coburn J, Chang YC, Dao K, & Roque P (2014). Neurotoxicants are in the air: convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. BioMed Research International, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell WJ, Bellinger DC, Coull BA, Gennings C, Wright RO, & Wright RJ (2015). Associations between Prenatal Exposure to Black Carbon and Memory Domains in Urban Children: Modification by Sex and Prenatal Stress. PloS One, 10(11), e0142492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell WJ, Brunst KJ, Malin AJ, Coull BA, Gennings C, Kloog I, Lipton L, Wright RO, Enlow MB, & Wright RJ (2019). Prenatal exposure to PM2.5 and cardiac vagal tone during infancy: Findings from a multiethnic birth cohort. Environmental Health Perspectives, 127(10), 107007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crépeaux G, Bouillaud-Kremarik P, Sikhayeva N, Rychen G, Soulimani R, & Schroeder H (2012). “Late Effects of a Perinatal Exposure to a 16 PAH Mixture: Increase of Anxiety-Related Behaviours and Decrease of Regional Brain Metabolism in Adult Male Rats.” Toxicology Letters 211 (2): 105–13. [DOI] [PubMed] [Google Scholar]
- Cuenya L, Fosacheca S, Mustaca A, & Kamenetzky G (2012). Effects of isolation in adulthood on frustration and anxiety. Behavioural Processes, 90(2), 155–160. [DOI] [PubMed] [Google Scholar]
- Cui J, Fu Y, Lu R, Bi Y, Zhang L, Zhang C, Aschner M, Li X, & Chen R (2019). Metabolomics analysis explores the rescue to neurobehavioral disorder induced by maternal PM2.5 exposure in mice. Ecotoxicology and Environmental Safety, 169, 687–695. [DOI] [PubMed] [Google Scholar]
- de Almeida RMM, & Miczek KA (2002). Aggression Escalated by Social Instigation or by Discontinuation of Reinforcement (“Frustration”) in Mice: Inhibition by Anpirtoline: A 5-HT 1B Receptor Agonist. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 27(2), 171–181. [DOI] [PubMed] [Google Scholar]
- Degnan KA, & Fox NA (2007). Behavioral inhibition and anxiety disorders: multiple levels of a resilience process. Development and Psychopathology, 19(3), 729–746. [DOI] [PubMed] [Google Scholar]
- Degnan KA, Hane AA, Henderson HA, Moas OL, Reeb-Sutherland BC, & Fox NA (2011). Longitudinal stability of temperamental exuberance and social-emotional outcomes in early childhood. Developmental Psychology, 47(3), 765–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- “Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5).” (2021). In Encyclopedia of Autism Spectrum Disorders, 1401–1401. Cham: Springer International Publishing. [Google Scholar]
- Dollar JM, Stifter CA, & Buss KA (2017). Exuberant and inhibited children: Person-centered profiles and links to social adjustment. Developmental Psychology, 53(7), 1222–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty LR, Smith VC, Bufferd SJ, Kessel E, Carlson GA, & Klein DN (2015). Preschool irritability predicts child psychopathology, functional impairment, and service use at age nine. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 56(9), 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty LR, Smith VC, Bufferd SJ, Stringaris A, Leibenluft E, Carlson GA, & Klein DN (2013). Preschool irritability: longitudinal associations with psychiatric disorders at age 6 and parental psychopathology. Journal of the American Academy of Child and Adolescent Psychiatry, 52(12), 1304–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger HL, & Angold A (2006). Common emotional and behavioral disorders in preschool children: presentation, nosology, and epidemiology. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 47(3–4), 313–337. [DOI] [PubMed] [Google Scholar]
- Filippi CA, Sachs JF, Phillips D, Winkler A, Gold AL, Leibenluft E, Pine DS, & Fox NA (2020). Infant behavioral reactivity predicts change in amygdala volume 12 years later. Developmental Cognitive Neuroscience, 42, 100776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi CA, Subar AR, Sachs JF, Kircanski K, Buzzell G, Pagliaccio D, Abend R, Fox NA, Leibenluft E, & Pine DS (2020). Developmental pathways to social anxiety and irritability: The role of the ERN. Development and Psychopathology, 32(3), 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PEM, & Akil H (2010). An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 35(2), 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes MK, Wright AGC, Markon KE, & Krueger RF (2017). Further evidence that psychopathology networks have limited replicability and utility: Response to Borsboom et al. (2017) and Steinley et al. (2017) [Review of Further evidence that psychopathology networks have limited replicability and utility: Response to Borsboom et al. (2017) and Steinley et al. (2017)]. Journal of Abnormal Psychology, 126(7), 1011–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forslund T, Brocki KC, Bohlin G, Granqvist P, & Eninger L (2016). The heterogeneity of attention-deficit/hyperactivity disorder symptoms and conduct problems: Cognitive inhibition, emotion regulation, emotionality, and disorganized attachment. The British Journal of Developmental Psychology, 34(3), 371–387. [DOI] [PubMed] [Google Scholar]
- Fox NA, Buzzell GA, Morales S, Valadez EA, Wilson M, & Henderson HA (2021). Understanding the Emergence of Social Anxiety in Children With Behavioral Inhibition. Biological Psychiatry, 89(7), 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, & Ghera MM (2005). Behavioral inhibition: linking biology and behavior within a developmental framework. Annual Review of Psychology, 56, 235–262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, & Schmidt LA (2001). Continuity and Discontinuity of Behavioral Inhibition and Exuberance: Psychophysiological and Behavioral Influences across the First Four Years of Life. Child Development, 72(1), 1–21. [DOI] [PubMed] [Google Scholar]
- Fox NA, Snidman N, Haas SA, Degnan KA, & Kagan J (2015). The relation between reactivity at 4 months and Behavioral Inhibition in the second year: Replication Across Three Independent Samples. Infancy: The Official Journal of the International Society on Infant Studies, 20(1), 98–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel TI, Fox NA, Pine DS, Walker OL, Degnan KA, & Chronis-Tuscano A (2015). Early childhood behavioral inhibition, adult psychopathology and the buffering effects of adolescent social networks: a twenty-year prospective study. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 56(10), 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick L, & Pittenger C (2016). “Microglial Dysregulation in OCD, Tourette Syndrome, and PANDAS.” Journal of Immunology Research 2016 (December): 8606057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galera C, Orri M, Vergunst F, Melchior M, Van der Waerden J, Bouvard MP, Collet O, Boivin M, Tremblay RE, & Côté SM (2021). Developmental profiles of childhood attention-deficit/hyperactivity disorder and irritability: association with adolescent mental health, functional impairment, and suicidal outcomes. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 62(2), 232–243. [DOI] [PubMed] [Google Scholar]
- Gao D, Wang C, Xi Z, Zhou Y, Wang Y, & Zuo Z (2017). Early-Life Benzo[a]Pyrene Exposure Causes Neurodegenerative Syndromes in Adult Zebrafish (Danio rerio) and the Mechanism Involved. Toxicological Sciences: An Official Journal of the Society of Toxicology, 157(1), 74–84. [DOI] [PubMed] [Google Scholar]
- Gartstein MA, & Skinner MK (2018). Prenatal influences on temperament development: The role of environmental epigenetics. Development and Psychopathology, 30(4), 1269–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc S, Zadeoglulari Z, Fuss SH, & Genc K (2012). The adverse effects of air pollution on the nervous system. Journal of Toxicology, 2012, 782462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi O, Corda MG, & Fernández-Teruel A (2019). A Genetic Model of Impulsivity, Vulnerability to Drug Abuse and Schizophrenia-Relevant Symptoms With Translational Potential: The Roman High- vs. Low-Avoidance Rats. Frontiers in Behavioral Neuroscience, 13, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, & Landrigan PJ (2014). Neurobehavioural effects of developmental toxicity. Lancet Neurology, 13(3), 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross RA, & Stright AD (2019). Does exuberance moderate the relation between effortful control at 54 months and first grade achievement? Interaction of a regulatory and a reactive temperament dimension. Early Childhood Research Quarterly, 48, 295–302. [Google Scholar]
- Gunther KE, & Pérez-Edgar K (2021). Dopaminergic associations between behavioral inhibition, executive functioning, and anxiety in development. Developmental Review: DR, 60, 100966. [Google Scholar]
- Gupta T, Demokritou P, & Koutrakis P (2004). Development and performance evaluation of a high-volume ultrafine particle concentrator for inhalation toxicological studies. Inhalation Toxicology, 16(13), 851–862. [DOI] [PubMed] [Google Scholar]
- Guxens M, Lubczyńska MJ, Muetzel RL, Dalmau-Bueno A, Jaddoe VWV, Hoek G, van der Lugt A, Verhulst FC, White T, Brunekreef B, Tiemeier H, & El Marroun H (2018). Air Pollution Exposure During Fetal Life, Brain Morphology, and Cognitive Function in School-Age Children. Biological Psychiatry, 84(4), 295–303. [DOI] [PubMed] [Google Scholar]
- Haber SN (2014). The place of dopamine in the cortico-basal ganglia circuit. Neuroscience, 282, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane AA, Fox NA, Henderson HA, & Marshall PJ (2008). Behavioral reactivity and approach-withdrawal bias in infancy. Developmental Psychology, 44(5), 1491–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin MG, Perez-Edgar K, Guyer AE, Pine DS, Fox NA, & Ernst M (2006). Reward and punishment sensitivity in shy and non-shy adults: Relations between social and motivated behavior. Personality and Individual Differences, 40(4), 699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, & Gable PA (2018). On the role of asymmetric frontal cortical activity in approach and withdrawal motivation: An updated review of the evidence. Psychophysiology, 55(1). 10.1111/psyp.12879 [DOI] [PubMed] [Google Scholar]
- Henderson HA, Fox NA, & Rubin KH (2001). Temperamental contributions to social behavior: the moderating roles of frontal EEG asymmetry and gender. Journal of the American Academy of Child and Adolescent Psychiatry, 40(1), 68–74. [DOI] [PubMed] [Google Scholar]
- Hirshfeld-Becker DR, Biederman J, Faraone SV, Violette H, Wrightsman J, & Rosenbaum JF (2002). Temperamental correlates of disruptive behavior disorders in young children: preliminary findings. Biological Psychiatry, 51(7), 563–574. [DOI] [PubMed] [Google Scholar]
- Hood SD, Potokar JP, Davies SJC, Hince DA, Morris K, Seddon KM, Nutt DJ, & Argyropoulos SV (2010). Dopaminergic challenges in social anxiety disorder: evidence for dopamine D3 desensitisation following successful treatment with serotonergic antidepressants. Journal of Psychopharmacology, 24(5), 709–716. [DOI] [PubMed] [Google Scholar]
- Holloway T, Miller D, Anenberg S, Diao M, Duncan B, Fiore AM, … & Zondlo MA (2021). Satellite monitoring for air quality and health. Annual Review of Biomedical Data Science, 4, 417–447. [DOI] [PubMed] [Google Scholar]
- Humphreys KL, Schouboe SNF, Kircanski K, Leibenluft E, Stringaris A, & Gotlib IH (2019). Irritability, Externalizing, and Internalizing Psychopathology in Adolescence: Cross-Sectional and Longitudinal Associations and Moderation by Sex. Journal of Clinical Child and Adolescent Psychology: The Official Journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53, 48(5), 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke KL, Cominski TP, Kuzhikandathil EV, Servatius RJ, & Pang KCH (2015). Investigating the Role of Hippocampal BDNF in Anxiety Vulnerability Using Classical Eyeblink Conditioning. Frontiers in Psychiatry / Frontiers Research Foundation, 6, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaric I, Rocks D, Cham H, Herchek A, & Kundakovic M (2019). Sex and Estrous Cycle Effects on Anxiety- and Depression-Related Phenotypes in a Two-Hit Developmental Stress Model. Frontiers in Molecular Neuroscience, 12, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Clarke C, Snidman N, & Garcia-Coll C (1984). Behavioral Inhibition to the Unfamiliar. Child Development, 55(6), 2212–2225. [Google Scholar]
- Kagan J, Snidman N, Arcus D, & Reznick JS (1994). Early Predictors of the Two Types. Galen’s Prophecy: Temperament in Human Nature. [Google Scholar]
- Kagan J, Snidman N, Kahn V, Towsley S, Steinberg L, & Fox NA (2007). The Preservation of Two Infant Temperaments into Adolescence. Monographs of the Society for Research in Child Development, 72(2), i–95. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Fox AS, Kovner R, Riedel MK, Fekete EM, Roseboom PH, Tromp DPM, Grabow BP, Olsen ME, Brodsky EK, McFarlin DR, Alexander AL, Emborg ME, Block WF, Fudge JL, & Oler JA (2016). Overexpressing Corticotropin-Releasing Factor in the Primate Amygdala Increases Anxious Temperament and Alters Its Neural Circuit. Biological Psychiatry, 80(5), 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, & Shelton SE (1989). Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science, 243, 1718+. [DOI] [PubMed] [Google Scholar]
- Khan A, Plana-Ripoll O, Antonsen S, Brandt J, Geels C, Landecker H, Sullivan PF, Pedersen CB, & Rzhetsky A (2019). Environmental pollution is associated with increased risk of psychiatric disorders in the US and Denmark. PLoS Biology, 17(8), e3000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircanski K, White LK, Tseng W-L, Wiggins JL, Frank HR, Sequeira S, Zhang S, Abend R, Towbin KE, Stringaris A, Pine DS, Leibenluft E, & Brotman MA (2018). A Latent Variable Approach to Differentiating Neural Mechanisms of Irritability and Anxiety in Youth. JAMA Psychiatry, 75(6), 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AB, Ultved L, Adamsen D, Santini MA, Tobeña A, Fernandez-Teruel A, Flores P, Moreno M, Cardona D, Knudsen GM, Aznar S, & Mikkelsen JD (2014). 5-HT(2A) and mGlu2 receptor binding levels are related to differences in impulsive behavior in the Roman Low- (RLA) and High- (RHA) avoidance rat strains. Neuroscience, 263, 36–45. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, & Panksepp J (2002). Ultrasonic vocalizations as indices of affective states in rats. Psychological Bulletin, 128(6), 961–977. [DOI] [PubMed] [Google Scholar]
- Korzan WJ, Forster GL, Watt MJ, & Summers CH (2006). Dopaminergic activity modulation via aggression, status, and a visual social signal. Behavioral Neuroscience, 120(1), 93–102. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ (2017). Air pollution and health. The Lancet Public Health, 2(1), e4–e5. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, & Goldman LR (2011). Children’s vulnerability to toxic chemicals: a challenge and opportunity to strengthen health and environmental policy. Health Affairs, 30(5), 842–850. [DOI] [PubMed] [Google Scholar]
- Lanphear BP (2015). The impact of toxins on the developing brain. Annual Review of Public Health, 36, 211–230. [DOI] [PubMed] [Google Scholar]
- Leibenluft E (2017). Pediatric Irritability: A Systems Neuroscience Approach. Trends in Cognitive Sciences, 21(4), 277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Cohen P, Gorrindo T, Brook JS, & Pine DS (2006). Chronic versus episodic irritability in youth: a community-based, longitudinal study of clinical and diagnostic associations. Journal of Child and Adolescent Psychopharmacology, 16(4), 456–466. [DOI] [PubMed] [Google Scholar]
- Levita L, Bois C, Healey A, Smyllie E, Papakonstantinou E, Hartley T, & Lever C (2014). The Behavioural Inhibition System, anxiety and hippocampal volume in a non-clinical population. Biology of Mood & Anxiety Disorders, 4(1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W-Y, Chang Y-C, Lee LJ-H, & Lee L-J (2014). Prenatal infection affects the neuronal architecture and cognitive function in adult mice. Developmental Neuroscience, 36(5), 359–370. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang C, Yang J, Song X, Han W, Xie M, Cheng L, Xie L, Chen H, & Jiang L (2019). Effects of early postnatal exposure to fine particulate matter on emotional and cognitive development and structural synaptic plasticity in immature and mature rats. Brain and Behavior, 9(12), e01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Calkins SD, & Bell MA (2020). Frontal EEG asymmetry moderates the associations between negative temperament and behavioral problems during childhood. Development and Psychopathology, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Zhou Y, Li Y, Qin Y, Yu L, Li R, Chen Y, & Xu Y (2020). “Effects of PM2.5 Exposure during Gestation on Maternal Gut Microbiota and Pregnancy Outcomes.” Chemosphere 247 (125879): 125879. [DOI] [PubMed] [Google Scholar]
- Loftus CT, Ni Y, Szpiro AA, Hazlehurst MF, Tylavsky FA, Bush NR, Sathyanarayana S, Carroll KN, Young M, Karr CJ, & LeWinn KZ (2020). Exposure to ambient air pollution and early childhood behavior: A longitudinal cohort study. Environmental Research, 183, 109075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JG (2020). Air pollution: A systematic review of its psychological, economic, and social effects. Current Opinion in Psychology, 32, 52–65. [DOI] [PubMed] [Google Scholar]
- Lubczyńska Małgorzata J., Muetzel RL, El Marroun H, Hoek G, Kooter IM, Thomson EM, Hillegers M, et al. (2021). “Air Pollution Exposure during Pregnancy and Childhood and Brain Morphology in Preadolescents.” Environmental Research 198 (110446): 110446. [DOI] [PubMed] [Google Scholar]
- Maia TV, & Frank MJ (2011). From reinforcement learning models to psychiatric and neurological disorders. Nature Neuroscience, 14(2), 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdandzić M, & van den Boom DC (2007). Multimethod longitudinal assessment of temperament in early childhood. Journal of Personality, 75(1), 121–168. [DOI] [PubMed] [Google Scholar]
- Mappin-Kasirer B, Pan H, Lewington S, Kizza J, Gray R, Clarke R, & Peto R (2020). “Tobacco Smoking and the Risk of Parkinson Disease: A 65-Year Follow-up of 30,000 Male British Doctors.” Neurology 94 (20): e2132–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes FK, Miguel KJ, Melo LL, & Spadari-Bratfisch RC (2001). Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiology & Behavior, 74(4–5), 435–440. [DOI] [PubMed] [Google Scholar]
- Margolis AE, Herbstman JB, Davis KS, Thomas VK, Tang D, Wang Y, Wang S, Perera FP, Peterson BS, & Rauh VA (2016). Longitudinal effects of prenatal exposure to air pollutants on self-regulatory capacities and social competence. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 57(7), 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis AE, Ramphal B, Pagliaccio D, Banker S, Selmanovic E, Thomas L, Factor-Litvak P, Perera F, Peterson BS, Rundle A, Herbstman J, Goldsmith J, & Rauh V (2021). Prenatal exposure to air pollution is associated with childhood inhibitory control and adolescent academic achievement. Environmental Research, 111570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-García E, Fernández-Castillo N, Burokas A, Gutiérrez-Cuesta J, Sánchez-Mora C, Casas M, Ribasés M, Cormand B, & Maldonado R (2015). Frustrated expected reward induces differential transcriptional changes in the mouse brain. Addiction Biology, 20(1), 22–37. [DOI] [PubMed] [Google Scholar]
- McCoy CR, Sabbagh MN, Huaman JP, Pickrell AM, & Clinton SM (2019). Oxidative metabolism alterations in the emotional brain of anxiety-prone rats. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 95, 109706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath LM, Weill S, Robinson EB, Macrae R, & Smoller JW (2012). Bringing a developmental perspective to anxiety genetics. Development and Psychopathology, 24(4), 1179–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers E, DeSerisy M, & Roy AK (2017). Disruptive Mood Dysregulation Disorder (DMDD): An RDoC perspective. Journal of Affective Disorders, 216, 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Z, Zhang J, Li Y, Li X, Song W, Sun ZS, & Wang Y (2019). Presence of the pregnant partner regulates microRNA-30a and BDNF levels and protects male mice from social defeat-induced abnormal behaviors. Neuropharmacology, 159, 107589. [DOI] [PubMed] [Google Scholar]
- Michael KC, Bonneau RH, Bourne RA, Godbolt L, Caruso MJ, Hohmann C, & Cavigelli SA (2020). Divergent immune responses in behaviorally-inhibited vs. non-inhibited male rats. Physiology & Behavior, 213, 112693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KP, & Ramos KS (2001). Impact of cellular metabolism on the biological effects of benzo[a]pyrene and related hydrocarbons. Drug Metabolism Reviews, 33(1), 1–35. [DOI] [PubMed] [Google Scholar]
- Min J-Y, & Min K-B (2017). Exposure to ambient PM10 and NO2 and the incidence of attention-deficit hyperactivity disorder in childhood. Environment International, 99, 221–227. [DOI] [PubMed] [Google Scholar]
- Monk C, Lugo-Candelas C, & Trumpff C (2019). Prenatal Developmental Origins of Future Psychopathology: Mechanisms and Pathways. Annual Review of Clinical Psychology, 15, 317–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, & Nestler EJ (2004). Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proceedings of the National Academy of Sciences of the United States of America, 101(29), 10827–10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales S, Pérez-Edgar K, & Buss K (2016). Longitudinal relations among exuberance, externalizing behaviors, and attentional bias to reward: the mediating role of effortful control. Developmental Science, 19(5), 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortamais M, Pujol J, Martínez-Vilavella G, Fenoll R, Reynes C, Sabatier R, Rivas I, Forns J, Vilor-Tejedor N, Alemany S, Cirach M, Alvarez-Pedrerol M, Nieuwenhuijsen M, & Sunyer J (2019). Effects of prenatal exposure to particulate matter air pollution on corpus callosum and behavioral problems in children. Environmental Research, 178, 108734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortamais M, Pujol J, van Drooge BL, Macià D, Martínez-Vilavella G, Reynes C, Sabatier R, et al. (2017). “Effect of Exposure to Polycyclic Aromatic Hydrocarbons on Basal Ganglia and Attention-Deficit Hyperactivity Disorder Symptoms in Primary School Children.” Environment International 105 (August): 12–19. [DOI] [PubMed] [Google Scholar]
- Nanda SA, Qi C, Roseboom PH, & Kalin NH (2008). Predator stress induces behavioral inhibition and amygdala somatostatin receptor 2 gene expression. Genes, Brain, and Behavior, 7(6), 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Nemeth A, Hudda N, Beamer G, Mann P, Petitto J, Cali R, Febo M, Kulkarni P, Poirier G, King J, Durant JL, & Brugge D (2020). Traffic-related particulate matter affects behavior, inflammation, and neural integrity in a developmental rodent model. Environmental Research, 183, 109242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman NC, Ryan P, Lemasters G, Levin L, Bernstein D, Hershey GKK, Lockey JE, Villareal M, Reponen T, Grinshpun S, Sucharew H, & Dietrich KN (2013). Traffic-related air pollution exposure in the first year of life and behavioral scores at 7 years of age. Environmental Health Perspectives, 121(6), 731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’doherty JP, Hampton A, & Kim H (2007). Model-based fMRI and its application to reward learning and decision making. Annals of the New York Academy of Sciences, 1104(1), 35–53. [DOI] [PubMed] [Google Scholar]
- Ott T, & Nieder A (2019). Dopamine and Cognitive Control in Prefrontal Cortex. Trends in Cognitive Sciences, 23(3), 213–234. [DOI] [PubMed] [Google Scholar]
- Padula AM, Monk C, Brennan PA, Borders A, Barrett ES, McEvoy CT, Foss S, Desai P, Alshawabkeh A, Wurth R, Salafia C, Fichorova R, Varshavsky J, Kress A, Woodruff TJ, Morello-Frosch R, & program collaborators for Environmental influences on Child Health Outcomes. (2020). A review of maternal prenatal exposures to environmental chemicals and psychosocial stressors-implications for research on perinatal outcomes in the ECHO program. Journal of Perinatology: Official Journal of the California Perinatal Association, 40(1), 10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D, Herbstman JB, Perera F, Tang D, Goldsmith J, Peterson BS, Rauh V, & Margolis AE (2020). Prenatal exposure to polycyclic aromatic hydrocarbons modifies the effects of early life stress on attention and Thought Problems in late childhood. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 61(11), 1253–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D, Pine DS, Barch DM, Luby JL, & Leibenluft E (2018). Irritability Trajectories, Cortical Thickness, and Clinical Outcomes in a Sample Enriched for Preschool Depression. Journal of the American Academy of Child and Adolescent Psychiatry, 57(5), 336–342.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotaropoulos T, Diamantopoulou A, Stamatakis A, Dimitropoulou M, & Stylianopoulou F (2009). Learning of a T-maze by rat pups when contact with the mother is either permitted or denied. Neurobiology of Learning and Memory, 91(1), 2–12. [DOI] [PubMed] [Google Scholar]
- Patten KT, González EA, Valenzuela A, Berg E, Wallis C, Garbow JR, Silverman JL, Bein KJ, Wexler AS, & Lein PJ (2020). Effects of early life exposure to traffic-related air pollution on brain development in juvenile Sprague-Dawley rats. Translational Psychiatry, 10(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Series P (2020). Computational Psychiatry: A Primer. MIT Press. [Google Scholar]
- Perera FP, Tang D, Wang S, Vishnevetsky J, Zhang B, Diaz D, Camann D, & Rauh V (2012). Prenatal Polycyclic Aromatic Hydrocarbon (PAH) Exposure and Child Behavior at Age 6–7 Years. In Environmental Health Perspectives (120(6), 921–926). 10.1289/ehp.1104315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Wang S, Vishnevetsky J, Zhang B, Cole KJ, Tang D, Rauh V, & Phillips DH (2011). Polycyclic aromatic hydrocarbons-aromatic DNA adducts in cord blood and behavior scores in New York city children. Environmental Health Perspectives, 119(8), 1176–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K & Fox NA (2018). Behavioral Inhibition: Integrating Theory, Research, and Clinical Perspectives, (1). [Google Scholar]
- Peterson BS, Rauh VA, Bansal R, Hao X, Toth Z, Nati G, Walsh K, Miller RL, Arias F, Semanek D, & Perera F (2015). Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry, 72(6), 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrick LM, Arora M, & Niedzwiecki MM (2020). Minimally Invasive Biospecimen Collection for Exposome Research in Children’s Health. Current Environmental Health Reports, 7(3), 198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Rougé-Pont F, Deminière JM, Kharoubi M, Le Moal M, & Simon H (1991). Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Research, 567(1), 169–174. [DOI] [PubMed] [Google Scholar]
- Qi C, Roseboom PH, Nanda SA, Lane JC, Speers JM, & Kalin NH (2010). Anxiety-related behavioral inhibition in rats: a model to examine mechanisms underlying the risk to develop stress-related psychopathology. Genes, Brain, and Behavior, 9(8), 974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimian R, Wakid M, O’Leary LA, & Mechawar N (2021). “The Emerging Tale of Microglia in Psychiatric Disorders.” Neuroscience and Biobehavioral Reviews 131 (December): 1–29. [DOI] [PubMed] [Google Scholar]
- Rice D, & Barone S Jr. (2000). Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environmental Health Perspectives, 108 Suppl 3, 511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM, & Jaenisch R (2001). Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Molecular Endocrinology, 15(10), 1748–1757. [DOI] [PubMed] [Google Scholar]
- Rivas I, Basagaña X, Cirach M, López-Vicente M, Suades-González E, Garcia-Esteban R, Álvarez-Pedrerol M, Dadvand P, & Sunyer J (2019). Association between early life exposure to air pollution and working memory and attention. Environmental Health Perspectives, 127(5), 57002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Raveendran M, Fawcett GL, Fox AS, Shelton SE, Oler JA, Cheverud J, Muzny DM, Gibbs RA, Davidson RJ, & Kalin NH (2013). CRHR1 genotypes, neural circuits and the diathesis for anxiety and depression. Molecular Psychiatry, 18(6), 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin KH, Burgess KB, & Hastings PD (2002). Stability and social-behavioral consequences of toddlers’ inhibited temperament and parenting behaviors. Child Development, 73(2), 483–495. [DOI] [PubMed] [Google Scholar]
- Rutherford HJV, & Lindell AK (2011). Thriving and Surviving: Approach and Avoidance Motivation and Lateralization. Emotion Review: Journal of the International Society for Research on Emotion, 3(3), 333–343. [Google Scholar]
- Sabariego M, Rosas M, Piludu MA, Acquas E, Giorgi O, & Corda MG (2019). Active avoidance learning differentially activates ERK phosphorylation in the primary auditory and visual cortices of Roman high- and low-avoidance rats. Physiology & Behavior, 201, 31–41. [DOI] [PubMed] [Google Scholar]
- Salari R, Bohlin G, Rydell A-M, & Thorell LB (2017). Neuropsychological Functioning and Attachment Representations in Early School Age as Predictors of ADHD Symptoms in Late Adolescence. Child Psychiatry and Human Development, 48(3), 370–384. [DOI] [PubMed] [Google Scholar]
- Sanna F, Bratzu J, Piludu MA, Corda MG, Melis MR, Giorgi O, & Argiolas A (2017). Dopamine, noradrenaline and differences in sexual behavior between roman high and low avoidance male rats: A microdialysis study in the medial prefrontal cortex. Frontiers in Behavioral Neuroscience, 11, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna F, Piludu MA, Corda MG, Melis MR, Giorgi O, & Argiolas A (2015). Involvement of dopamine in the differences in sexual behaviour between Roman high and low avoidance rats: an intracerebral microdialysis study. Behavioural Brain Research, 281, 177–186. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Liebowitz MR, Abi-Dargham A, Zea-Ponce Y, Lin SH, & Laruelle M (2000). Low dopamine D(2) receptor binding potential in social phobia. The American Journal of Psychiatry, 157(3), 457–459. [DOI] [PubMed] [Google Scholar]
- Schumacher A, Villaruel FR, Ussling A, Riaz S, Lee ACH, & Ito R (2018). Ventral Hippocampal CA1 and CA3 Differentially Mediate Learned Approach-Avoidance Conflict Processing. Current Biology: CB, 28(8), 1318–1324.e4. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Hutchinson MR, & Bilbo SD (2011). Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(49), 17835–17847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, & Sun Q (2018). Particulate air pollution: major research methods and applications in animal models. Environmental Disease, 3(3), 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Cohen JD, & Botvinick MM (2016). Dorsal anterior cingulate cortex and the value of control. Nature Neuroscience, 19(10), 1286–1291. [DOI] [PubMed] [Google Scholar]
- Shields GS, & Moons WG (2016). Avoidance-related EEG asymmetry predicts circulating interleukin-6. Emotion, 16(2), 150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimshoni Y, Lebowitz ER, Brotman MA, Pine DS, Leibenluft E, & Silverman WK (2020). Anxious-Irritable Children: A Distinct Subtype of Childhood Anxiety? Behavior Therapy, 51(2), 211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique S, Banerjee M, Ray MR, & Lahiri T (2011). Attention-deficit hyperactivity disorder in children chronically exposed to high level of vehicular pollution. In European Journal of Pediatrics (Vol. 170, Issue 7, pp. 923–929). 10.1007/s00431-010-1379-0 [DOI] [PubMed] [Google Scholar]
- Simmons RK, Howard JL, Simpson DN, Akil H, & Clinton SM (2012). DNA methylation in the developing hippocampus and amygdala of anxiety-prone versus risk-taking rats. Developmental Neuroscience, 34(1), 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöwall D, Bohlin G, Rydell A-M, & Thorell LB (2017). Neuropsychological deficits in preschool as predictors of ADHD symptoms and academic achievement in late adolescence. Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence, 23(1), 111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW (2016). The Genetics of Stress-Related Disorders: PTSD, Depression, and Anxiety Disorders. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 41(1), 297–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkweather CK, Gershman SJ, & Uchida N (2018). The Medial Prefrontal Cortex Shapes Dopamine Reward Prediction Errors under State Uncertainty. Neuron, 98(3), 616–629.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroustrup A, Hsu H-H, Svensson K, Schnaas L, Cantoral A, Solano González M, Torres-Calapiz M, Amarasiriwardena C, Bellinger DC, Coull BA, Téllez-Rojo MM, Wright RO, & Wright RJ (2016). Toddler temperament and prenatal exposure to lead and maternal depression. Environmental Health: A Global Access Science Source, 15(1), 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Cassidy C, Horga G, Kang UJ, Fahn S, Casella L, Pezzoli G, Langley J, Hu XP, Zucca FA, Isaias IU, & Zecca L (2018). Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson’s disease. Npj Parkinson’s Disease, 4(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Oshio S, Iwata M, Saburi H, Odagiri T, Udagawa T, Sugawara I, Umezawa M, & Takeda K (2010). In utero exposure to a low concentration of diesel exhaust affects spontaneous locomotor activity and monoaminergic system in male mice. Particle and Fibre Toxicology, 7, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Flanigan ME, McEwen BS, & Russo SJ (2018). Aggression, Social Stress, and the Immune System in Humans and Animal Models. Frontiers in Behavioral Neuroscience, 12, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliaz D, Loya A, Gersner R, Haramati S, Chen A, & Zangen A (2011). Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(12), 4475–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Lee J, Muirhead L, Li TY, Qu L, Yu J, & Perera F (2014). Molecular and neurodevelopmental benefits to children of closure of a coal burning power plant in China. PloS One, 9(3), e91966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarullo AR, Mliner S, & Gunnar MR (2011). Inhibition and exuberance in preschool classrooms: associations with peer social experiences and changes in cortisol across the preschool year. Developmental Psychology, 47(5), 1374–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay TL, Béchade C, D’Andrea I, Henry MS, Roumier A, St-Pierre MK, & Tremblay ME (2017). “Microglia Gone Rogue: Impacts on Psychiatric Disorders across the Lifespan.” Frontiers in Molecular Neuroscience 10: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JE, Ficzere B, Louis JS, & Schoenfeld TJ (2019). Examining the Effects of Exercise on Frustration-Induced Anxiety-like Behavior in Rats. Psi Chi J. Psychol. Res, 24, 210–221. [Google Scholar]
- Terranova ML, Laviola G, & Alleva E (1993). Ontogeny of amicable social behavior in the mouse: gender differences and ongoing isolation outcomes. Developmental Psychobiology, 26(8), 467–481. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Müller CP, Huston JP, & Schwarting RK (1999). High versus low reactivity to a novel environment: behavioural, pharmacological and neurochemical assessments. Neuroscience, 93(1), 243–251. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Worbe Y, Thobois S, Sgambato-Faure V, & Féger J (2015). Selective dysfunction of basal ganglia subterritories: From movement to behavioral disorders. Movement Disorders: Official Journal of the Movement Disorder Society, 30(9), 1155–1170. [DOI] [PubMed] [Google Scholar]
- Tseng C-Y, Yu J-Y, Chuang Y-C, Lin C-Y, Wu C-H, Liao C-W, Yang F-H, & Chao M-W (2019). The Effect of Ganoderma Microsporum immunomodulatory proteins on alleviating PM 2.5-induced inflammatory responses in pregnant rats and fine particulate matter-induced neurological damage in the offsprings. Scientific Reports, 9(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp AM, & Miczek KA (2000). Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 20(24), 9320–9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Ribas P, Brotman MA, Valdivieso I, Leibenluft E, & Stringaris A (2016). The Status of Irritability in Psychiatry: A Conceptual and Quantitative Review. Journal of the American Academy of Child and Adolescent Psychiatry, 55(7), 556–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villard J, Bennett JL, Bliss-Moreau E, Capitanio JP, Fox NA, Amaral DG, & Lavenex P (2021). Structural differences in the hippocampus and amygdala of behaviorally inhibited macaque monkeys. Hippocampus, 31(8), 858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindas MA, Sørensen C, Johansen IB, Folkedal O, Höglund E, Khan UW, Stien LH, Kristiansen TS, Braastad BO, & Øverli Ø (2014). Coping with unpredictability: dopaminergic and neurotrophic responses to omission of expected reward in Atlantic salmon (Salmo salar L.). PloS One, 9(1), e85543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Park B, Hollingue C, Jones KL, Ashwood P, Windham GC, Lurman F, et al. (2020). “Maternal Immune Response and Air Pollution Exposure during Pregnancy: Insights from the Early Markers for Autism (EMA) Study.” Journal of Neurodevelopmental Disorders 12 (1): 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Perera F, Braun JM, Kingsley SL, Gray K, Buckley J, Clougherty JE, Croen LA, Eskenazi B, Herting M, Just AC, Kloog I, Margolis A, McClure LA, Miller R, Levine S, Wright R, & Environmental influences on Child Health Outcomes. (2021). Prenatal air pollution exposure and neurodevelopment: A review and blueprint for a harmonized approach within ECHO. Environmental Research, 196, 110320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, & Frye CA (2006). A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 31(6), 1097–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LK, McDermott JM, Degnan KA, Henderson HA, & Fox NA (2011). Behavioral inhibition and anxiety: the moderating roles of inhibitory control and attention shifting. Journal of Abnormal Child Psychology, 39(5), 735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Mitchell C, Stringaris A, & Leibenluft E (2014). “Developmental Trajectories of Irritability and Bidirectional Associations with Maternal Depression.” Journal of the American Academy of Child and Adolescent Psychiatry 53 (11): 1191–1205.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhr M, & Schwarting RKW (2008). Maternal care, isolation-induced infant ultrasonic calling, and their relations to adult anxiety-related behavior in the rat. Behavioral Neuroscience, 122(2), 310–330. [DOI] [PubMed] [Google Scholar]
- Woodward NC, Haghani A, Johnson RG, Hsu TM, Saffari A, Sioutas C, Kanoski SE, Finch CE, & Morgan TE (2018). Prenatal and early life exposure to air pollution induced hippocampal vascular leakage and impaired neurogenesis in association with behavioral deficits. Translational Psychiatry, 8(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang IA, Fong KM, Zimmerman PV, Holgate ST, & Holloway JW (2009). Genetic susceptibility to the respiratory effects of air pollution. Postgraduate Medical Journal, 85(1006), 428–436. [DOI] [PubMed] [Google Scholar]
- Yokota S, Mizuo K, Moriya N, Oshio S, Sugawara I, & Takeda K (2009). Effect of prenatal exposure to diesel exhaust on dopaminergic system in mice. Neuroscience Letters, 449(1), 38–41. [DOI] [PubMed] [Google Scholar]
- Yokota S, Oshio S, Moriya N, & Takeda K (2016). Social Isolation-Induced Territorial Aggression in Male Offspring Is Enhanced by Exposure to Diesel Exhaust during Pregnancy. PloS One, 11(2), e0149737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorifuji T, Kashima S, Diez MH, Kado Y, Sanada S, & Doi H (2017). Prenatal exposure to outdoor air pollution and child behavioral problems at school age in Japan. Environment International, 99, 192–198. [DOI] [PubMed] [Google Scholar]
- Zavala J, Freedman AN, Szilagyi JT, Jaspers I, Wambaugh JF, Higuchi M, & Rager JE (2020). New approach methods to evaluate health risks of air pollutants: Critical design considerations for in vitro exposure testing. International Journal of Environmental Research and Public Health, 17(6), 2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhang M, Liu W, Li Y, Qin Y, & Xu Y (2020). Transgenerational transmission of neurodevelopmental disorders induced by maternal exposure to PM2.5. Chemosphere, 255, 126920. [DOI] [PubMed] [Google Scholar]


