Abstract
Immature motor response inhibition in adolescence is considered contributory to adolescent risk-taking and externalizing behaviors. We review studies reporting age-related variations in motor response inhibition and MRI measurements from typically-developing adolescents. Reviewed studies measured response inhibition using one of three tasks—the Stop Signal Task, Go/No-Go, and Antisaccade Task. Task reliability appears to be particularly strong for the SST. Across tasks and study designs, results indicate that inhibitory control improves markedly through early adolescence. The trajectory of change in later adolescence and into young adulthood (i.e., linear or plateauing) varies depending on the task design. Neuroimaging studies identify adult-like response inhibition networks that are involved in behavioral development. The pros and cons of each task are discussed, including recommendations to guide future studies. Ongoing studies in large longitudinal datasets offer opportunities for further exploration of the shape of change in response inhibition, related neural regions, and associations with other affective and cognitive processes to identify potential impacts of motor response inhibition immaturities or individual differences on adolescent risk-taking behaviors.
Keywords: Response Inhibition, Action Inhibition, Adolescent Development, Inhibitory Control Neural Activity, Inhibitory Control Measurement
1. Introduction
Adolescence is a developmental period marked by changes in decision making and engagement in risky behaviors such as reckless driving, delinquency, and experimentation with alcohol and drugs. One theorized common vulnerability for the observed increase in risk-taking behavior has been referred to as behavioral disinhibition, represented by difficulties in inhibiting goal-inconsistent automatic behaviors in favor of more effortful, appropriate responses (Young et al., 2009). While behavioral disinhibition may index a variety of cognitive processes (Frost & McNaughton, 2017) and may have particular relevance to temperamental dispositions (Nigg, 2017), this review focuses more narrowly on motor response inhibition, which represents a critical and foundational mechanism involved in the inhibition of action (Eagle et al., 2008). Through the use of laboratory tasks, motor or action-based inhibition can be directly observed and analyzed at a nuanced level. Difficulties in controlling momentary urges or impulses to act not only characterize many of the psychopathologies that emerge from adolescence into early adulthood, including attention deficit hyperactivity disorder (ADHD) and externalizing disorders like substance abuse (Barkley, 1997; Castellanos, 2006; Stevens et al., 2014), but also may characterize some phases of typical development (Constantinidis & Luna, 2019; Steinberg, 2010). These behavioral patterns are accompanied by neural alterations. To understand the neural underpinnings of motor response inhibition, it is vital to clarify how existing measures theoretically relate to the construct and how they are operationalized.
Laboratory measures of motor response inhibition provide valuable opportunities to measure in-vivo performance and to explore neural activations related to engagement in cognitive processes (Cyders & Coskunpinar, 2011; King et al., 2014; Sharma et al., 2014). In particular, motor response inhibition tasks—Stop Signal, Go/No-Go, and the Antisaccade Task—have been broadly used to measure adolescents’ inhibitory control (Young et al., 2009). Despite their widespread implementation, inconsistencies in task designs and analytic techniques threaten reliability, and different neuroimaging contrasts in event-related designs isolate unique aspects of task performance that have not generalized broadly across studies (Mostofsky & Simmonds, 2008; Swick et al., 2011; Criaud & Boulinguez, 2013). Moreover, like many executive tasks, these measures may index variance beyond motor response inhibition alone; they may also measure abilities such as working memory (Criaud & Boulinguez, 2013), error monitoring (Dupuis et al., 2019), or emotion and reward processing (Constantinidis & Luna, 2019). Given the clinical importance of achieving successful motor response inhibition in adolescents, a thorough understanding of the trajectory and range of typical development of this process is fundamental to our understanding of when and how adverse outcomes may emerge (Cicchetti, 1989; Atherton 2020).
Accordingly, this narrative review will clarify common patterns of neural and motor response inhibition development in adolescent development by exploring the existing empirical literature. First, the construct of motor response inhibition in adolescent development will be introduced followed by a review of the three most commonly researched motor response inhibition tasks—the Stop Signal Task, Go/No-Go, and Antisaccade Tasks. For each task, the underlying models, explanatory variables, task variants, and task reliability as it relates to the construct of motor response inhibition will be described. Next, age-related patterns of behavioral performance will be described. The common patterns in each task’s MRI literature will be contrasted to identify findings that seem to generalize broadly, those that appear specific to particular tasks, and existing gaps in the literature that can inform future work.
This review is based on a literature search using Google Scholar and PubMed with search terms “response inhibition,” “action restraint,” “action cancellation,” “inhibitory control,” “stop signal task,” “stop signal,” “go/no-go,” ”antisaccade,” “inhibitory control,” independently and combined with “adolescent,” “developmental,” “maturation,” and/or “fMRI,” “MRI,” “neuroimaging,” “activation.” Given the intended focus on developmental aspects of inhibitory control, only studies that included participants between the ages of 10–17 were considered. Additional studies, including those focused on adult samples and meta-analyses, were identified through the same searches and reviews of Tables of Contents (e.g., Constantinidis & Luna, 2019) or studies that have cited relevant articles (e.g., Young et al., 2009). Empirical studies must have included a version of the Stop Signal Task, Go/No-Go, or Antisaccade Task. Selected studies included either only typically developing adolescents or provided results regarding age-related variations in a healthy adolescent control group (as in case-control studies).
Response inhibition, specifically motor response inhibition or what has been termed “action inhibition” (Eagle et al, 2008), refers to the ability to voluntarily suppress a prepotent response (Verbruggen & Logan, 2008b, Constantinidis & Luna, 2019). Within the context of this review, “response inhibition” refers to motor response inhibition where a reactive response that is inappropriate or inconsistent with an individual’s goals is restrained in favor of maintaining or initiating a more appropriate response (Luna et al., 2010; Thomsen et al., 2018). Response inhibition is a foundational component of cognitive control (Miyake et al., 2000; Lambek & Shevlin, 2011). Poor motor response inhibition may characterize the impulsivity that has been observed in the context of some psychopathologies as well as typical adolescent development (Nigg, 2017).
High rates of comorbidities and common behavioral manifestations between disorders like ADHD, Oppositional Defiant or Conduct Disorder, and Substance Abuse or Dependence have led researchers to hypothesize that there is a fundamental risk phenotype that underpins these disorders (Iacono et al., 2008; Young et al., 2009; Castellanos-Ryan et al., 2014). Specifically, these various psychopathologies are considered related but distinct syndromes on the spectrum of externalizing, a dimensional construct representing undercontrolled behavior that characterizes childhood disruptive disorders, substance use disorders, and Antisocial Personality Disorder (Krueger et al., 2002; Iacono et al., 2008) and that relates to personality characteristics like sensation seeking, impulsivity, and low constraint (McGue et al., 2001; Johnson et al., 2018). Meta-analyses of response inhibition have found significant effect sizes in distinguishing cases from controls across various forms of psychopathology (Lipszyc & Schachar, 2010; Wright et al., 2014).
Twin studies suggest that the risk for emergence of externalizing behaviors during adolescence is genetically driven (Hicks et al., 2007; Young et al., 2009). Several different lines of research, including common pathway (e.g., Iacono et al., 2008) and dual process (e.g., Nigg, 2003) models, have posited that traitwise behavioral disinhibition, measured in vivo by response inhibition tasks, is a biologically-based component of the risk phenotype for adolescent risk-taking behaviors and externalizing psychopathologies. These studies employed latent variable analyses to pool variance across similar measures that are conceptually related rather than examining pair-wise relationships between measures (Miyake et al., 2000; Friedman et al., 2008; Young et al., 2009). This approach can be particularly helpful in exploring executive tasks because these tend to be influenced by various factors, such as different strategies to approaching the task, error monitoring, or motor speed (Miyake et al., 2000).
2. Measurement of Motor Response Inhibition
Although each task has evolved over time, forms of the Stop Signal Task (SST), Go/No-Go (GNG), and Antisaccade Task (AST) have dominated the laboratory-based motor response inhibition literature for over two decades. In a landmark study on executive function, Miyake et al. found that the AST and SST, along with the Stroop task, loaded well onto an “Inhibition” factor in a confirmatory model (2000; Miyake & Friedman, 2012). Another study exploring the structure of response inhibition and working memory in 240 healthy adolescents (ages 14–19) provided support for the interchangeability of these tasks, with moderate loading of the AST and small loadings of both the GNG and SST (Malagoli & Usai, 2018). However, this finding has not yet been replicated (e.g., Aichert et al., 2012; Gärtner & Strobel, 2021), and few studies report reliability estimates for these tasks in adolescent samples (Table 1).
Table 1.
Reliability estimates from selected studies.
| Author | Year | Age range (N) | Variable | Reliability type | Statistic |
|---|---|---|---|---|---|
|
| |||||
| Stop Signal Task | |||||
| Williams | 1999 | 9–12 (41) | SSRT | Split half | SB2 r = .86 |
| 13–17(50) | SB2 r = .91 | ||||
| 18–29 (47) | SB2 r = .91 | ||||
| Bedard | 2002 | 6–82 (317) | SSRT | IC | α = .93 |
| Nigg | 2006 | 12–17 (498) (two waves) | SSRT | IC | α = .93 |
| Meisel | 2015 | T1: 12.6 (373) T2: 13.6 (370) |
SSRT | IC | α = .70 |
| IC | α = .71 | ||||
| Malagoli | 2018 | 14–19 (240) | SSRT | Split-half | SB2 r = .85 |
| Tiego | 2020 | 11–12 (136) | SSRT | Split-half | R = 0.94 |
| Go/No-Go | |||||
| Kuntsi | 2005 | 12–13 (47) | CE | Test-retest of 2 weeks | ICC = .70 |
| McAuley & White | 2011 | 6–24 (153) | CE | IC | α = .07 |
| Malagoli | 2018 | 14–19 (240) | CE | IC | α = .70 |
| Tiego | 2020 | 11–12 (136) | CE | Split-half | R = 0.82 |
| Antisaccade Task | |||||
| Klein & Fischer | 2005 | 6–18 (117) | AS accuracy | Test-retest of 18.9(1.2) months | ICC = .68 |
| 6–88 (327) | AS accuracy | Odd-even | R = .96 | ||
| Split-half | R = .87 | ||||
| Ordaz | 2013 | 9–26 (123) | AS latency | Test-retest (∼12 months) | R = .53 |
| IC | ICC = .61 | ||||
| AS accuracy | Test-retest (∼12 months) | R = .76 | |||
| IC | ICC = .65 | ||||
Research exploring task performance on all three tasks within single samples is limited. The GNG and SST, which require similar peripheral, button presses instead of more rapid saccade-based responses, have been studied in conjunction and in hybridized tasks (e.g., Rubia et al., 2001; Schachar et al., 2007; Goghari & MacDonald, 2009; Schachar et al., 2010). Studies with data on two of these tasks (e.g., Malagoli & Usai, 2018; rtner Strobel, 2021) provide relatively consistent evidence of shared variance between these response inhibition tasks, but the vast majority of studies, particularly those considering neurobehavioral development of motor response inhibition, used only one of these three tasks in isolation. As they are interchangeably used to represent the construct of motor response inhibition, it is vital to understand how these tasks are designed and interpreted and how behavioral and neural correlates change throughout adolescent development. Next, we will consider these features for each task.
2.1. Stop Signal Task Design, Estimation, and Reliability
In the typical SST, participants are seated before a computer screen and given a press pad or keyboard with left and right options. Participants typically first complete a brief Go task to encourage development of a prepotent response to task stimuli. The subsequent Stop task involves similar Go stimulus presentations, but on a subset of trials (usually 25%), the Go stimulus is followed, after a varying delay interval, by a Stop signal. When confronted by the Stop signal, the participant attempts to restrain the button press (Figure 1a; Logan & Cowan, 1984; Verbruggen & Logan, 2008b).
Figure 1.
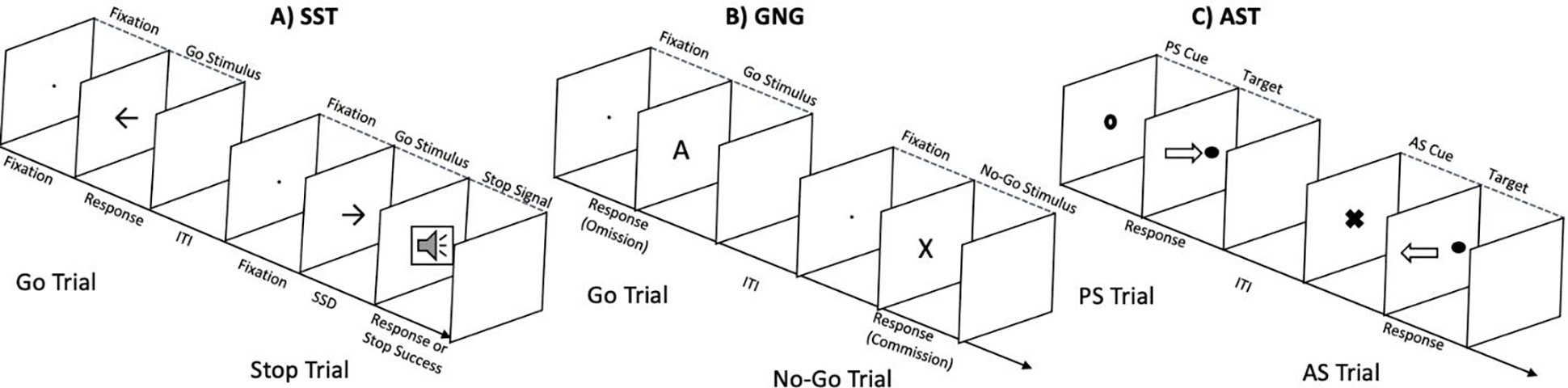
Depiction of typical trial course in A) Stop Signal Task (adapted from Verbruggen et al., 2008). Two visual stimuli are paired with left and right responses during a Go task. During a Stop Trial (25% frequency), the Go signal is followed by an auditory Stop Signal after a delay, SSD. B) Depiction of a typical trials course in Go/No-Go, where individuals respond to Go stimuli (~75% frequency, e.g., all letters except “X”) but refrain from responding to No-Go stimuli (e.g., “X”). C) Depiction of a trial course of a prosaccade trial, where instructions are to look in the direction of the target (filled circle) after a PS signal (open circle) and away from the direction of the target after an AS signal (X, adapted from Velanova et al., 2008).
SST scoring is based on the independent horse race model, which characterizes task performance as a competition between two independent processes—labeled Go and Stop (Logan & Cowan, 1984). This model provides simple calculations and representations of action cancellation based on the speed of going (GoRT), speed of stopping (Stop Signal Reaction Time; SSRT), and the delay between the two (Stop Signal Delay; SSD; Verbruggen & Logan, 2008b; Logan et al., 2014; Verbruggen & Logan, 2017). By dynamically manipulating the SSD, task difficulty shifts so that each participant’s probability of inhibiting a response when given a stop signal is approximately 50%. This design allows for the most reliable estimation of the main variable of interest, the SSRT (Ridderinkhof et al., 1999; van Boxtel et al., 2001; Band et al., 2003; Verbruggen & Logan, 2009a, Verbruggen et al, 2019).
Recently published guidelines described several factors that should be considered when designing or evaluating research using the SST (Verbruggen et al., 2019). Recommendations included using two-choice reaction time tasks (such as left/right arrows) to establish a reliable prepotent response, which is consistent with most, but not all, of the reviewed studies (e.g., Rubia et al., 2001). Most reviewed studies complied with the recommendation to use a salient Stop signal, although many studies used visual Stop signals instead of more standard auditory stimuli (e.g., Weathers et al., 2012; Rubia et al., 2013; van de Laar et al., 2014; Wang et al., 2016; Cai et al., 2019). To maximize the prepotent response, consensus guidelines recommend using a 25% frequency of Stop signals. Most studies reviewed here have 15–30% Stop signal frequencies (Table 2). The consensus recommends encouraging speed over accuracy and frequently providing feedback. Guidelines also recommend including a sufficient number of trials (e.g., ≥ 50 Stop signals) as simulations show increased reliability with greater numbers of trials (Verbruggen et al., 2019). Some of the reviewed studies (e.g., Rubia et al., 2007; Madsen et al., 2010; Cai et al., 2019; Dupuis et al., 2019) did not meet this criterion (Table 2).
Table 2.
Overview of reviewed study methodology and behavioral findings
| Author | Year | Variant | Age Range (N) | Design | Age-Related Patterns of Response Inhibition | Related Findings |
| Standard Version | ||||||
| Stop Signal Task | ||||||
| Williams | 1999 | SST | 6 – 8 (29) 9 – 12 (41) 13 – 17 (50) 18 – 29 (47) 30 – 44 (55) 45 – 59 (28) 60 – 81 (25) |
C | SSRT-MN: quadratic + linear age effects over entire sample |
GoRT: linear improvement within 6–17 year-olds; linear + quadratic age effects over entire sample SD(GoRT): adulthood > adolescence > mid childhood |
| Rubia | 2000 | SST-NoT | 12 – 19 (9) 20 – 40 (8) |
C | P(Inh|Stop): n.s. age effect | GoRT: n.s. age effect |
| Bedard | 2002 | SST-S | 6 – 8 (40) 9 – 12 (62) 13 – 17 (54) 18 – 29 (48) 30 – 44 (65) 45 – 59 (23) 60 – 82 (25) |
C | SSRT-MN: quadratic + linear age effects over entire sample | GoRT: quadratic age effect over entire sample |
| Johnstone | 2007 | GNG/SST Hybrid | 7 – 11 (24) | C |
SSRT-MN: n.s. age effect CE: linear improvement |
GoRT: linear improvement |
| Rubia | 2007 | SST | 10 – 17 (26) 20 – 42 (21) |
C | SSRT-MN: n.s. age effect | GoRT: n.s. age effect |
| Cohen | 2010 | SST | 9 – 19 (27) 25 – 30 (9) |
C | SSRT-INT: older > younger; linear improvement | GoRT: n.s. age effect |
| Madsenc | 2010 | SST-C | 7 – 12 (65) | C | SSRT-MD: linear improvement | Sex n.s. |
| Wong | 2010 | SST | 15 – 17 (386) | C | SSRT-MN: linear improvement | Males < Females |
| Schachar | 2011 | GNG/SST Hybrid | 8.3 (SD = 1.3; 234) | C | SSRT: n.s. age effect | GoRT: n.s. age effect |
| Weathers | 2012 | SST | 13.8 (SD = 2.0; 21) 35.2 (SD = 8.1; 29)* |
C | SSRT-INT: n.s. age effect | |
| Rubia | 2013 | SST | 13 – 45 (66) | C |
P(Inh): linear improvement SSRT-MN: n.s. age effect |
For ages < 18, SSRT-MN: females>males |
| Van de Laar | 2014 | SST-S | 7.8 (SD = 0.5; 16) 11.8 (SD = 1.2;17) 20.7 (SD = 3.1; 17) |
C | SSRT-INT: oldest > middle > youngest | GoRT: oldest > middle > youngest |
| Meisel | 2015 | SST | 12.5 (373) | L (2:1 yr) | SSRT-MN: n.s. age effect | |
| Ware | 2015 | SST-NoT | 13 – 16 (21)* | C | P(inh) on Hard trials: linear improvement | GoRT: linear decline |
| Author | Year | Variant | Age range (N) | Design | Age-Related Patterns of Response Inhibition | Related Findings |
| Curley | 2018 | SST-C | 6.9 (SD = 1.6; 110) | L (4:1 yr) | log(SSRT-MD): linear longitudinal improvement | log(SSRT-MD): Female > male |
| Murphy | 2018 | SST-C | 9 – 17 (130) | C |
SSRT-MN: linear improvement P(Inh): n.s. age effect |
|
| Dupuis | 2019 | SST | 6 – 17 (13,709) | C | SSRT-INT: linear improvement |
GoRT: linear improvement Performance monitoring adjustments: plateau by 10 years old |
| Madsenc | 2020 | SST-C | 7 – 12 (88) | L (9:0.5–2 yrs) | SSRT-INT: longitudinal improvement, plateau around 13–14 y.o; n.s. quadratic age effect |
SD(GoRT): linear longitudinal improvement Male only SSRT-INT: linear longitudinal improvement Female only SSRT-INT: curvilinear longitudinal improvement plateaus around 13–14 y.o. |
| Mürner-Lavancy | 2020 | SST-C | 9 – 12 (125) | L (3:1 yr) | SSRT-MN: linear longitudinal improvement; n.s. quadratic age effect | SSRT-MN: n.s. age by sex effect |
| Ogilvie | 2020 | SST-C | 17 – 22 (129) | C | SSRT: linear improvement | GoRT: n.s. age effect |
| Tiego | 2020 | SST GNG | 11 – 12 (136) | C |
SSRT-MN: linear improvements CE: n.s. age effect |
|
| Go/No-Go | ||||||
| Casey | 1997 | GNG | 7 – 12 (9) 21 – 24 (9) |
C | CE: older > younger | RT: n.s. age effect |
| Booth | 2002 | GNG-EQ | 9 – 11 (12) 20 – 30 (12) |
C | CE: older > younger | RT: older > younger |
| Bunge | 2002 | GNG-F | 8 – 12 (16) 19 – 33 (16) |
C | CE: older > younger | |
| Durston | 2002 | GNG | 6 – 10 (10) Adults (10) |
C | CE + OE: older > younger; linear improvement | |
| Tamm | 2002 | GNG-EQ | 8 – 20 (19) | C | CE: n.s. age effect |
OE: n.s. age effect RT: linear improvement |
| Hooper | 2004 | GNG | 9 – 17 (145) | C | d’: linear improvement | OE: F > M |
| Johnstone | 2005 | GNG | ∼9 – 40 (60) | C |
CE: older > younger CE + OE: older > younger |
RT: quadratic + linear age effects |
| Durston | 2006 | GNG-SL | 8 – 14 (14) | L (2:2 yrs) | CE: n.s. age effects |
OE: n.s. age effect RT: longitudinal improvement |
| Eigsti | 2006 | GNG | 11 – 22 (34) | C | CE: linear improvement |
OE: linear improvement RT: n.s. age effect |
| Liston | 2006 | GNG | 7 – 15 (9) 18 – 31 (6) |
C | CE + OE controlling for RT: older > younger | RT: linear improvement |
| Rubia | 2006 | GNG | 10 – 17 (29) 20 – 43 (23) |
C |
CE + OE: older > younger CE: linear improvement |
|
| Author | Year | Variant | Age range (N) | Design | Age-Related Patterns of Response Inhibition | Related Findings |
| Johnstone | 2007 | GNG/SST Hybrid | 7 – 11 (24) | C |
SSRT: n.s. age effect CE: linear improvement |
GoRT: linear improvement |
| Cragg | 2008 | GNG | 5 – 7 (44) 9 – 11 (44) |
C | CE: older > younger |
OE: older > younger RT: older > young |
| McAuley | 2011 | GNG | 6 – 8 (38) 9 – 12 (38) 13 – 17 (39) 18 – 24 (38) |
C |
CE: linear improvement RT:linear improvement |
|
| Schachar | 2011 | GNG/SST Hybrid | 8.3 (SD = 1.3; 234) | C | CE: n.s. age effect | |
| Barber | 2013 | GNG-WM | 8 – 12 (24) 18 – 47 (27) |
C | CE: older > younger |
RT: n.s. age effect Working memory effects: n.s. age effect |
| Bezdijan | 2013 | GNG | 9 – 10 (560) | L (5:2 yrs) | CE: longitudinal improvement | OE: longitudinal improvement, females > males |
| Brydges | 2013 | GNG-F | 8 – 11 (13) 18 (13) |
C | CE: older > younger | |
| Silveri | 2013 | GNG | 12 – 14 (20) 18 – 24 (30) |
C | CE: older > younger | OE: older > younger |
| Wetherill | 2013 | GNG | 11 – 16 (40)* | L (2:3 yrs) | CE: older > younger | |
| Vara | 2014 | GNG | 13–17 (15) 20 – 35 (15) |
C | CE: n.s. age effect |
OE: n.s. age effect RT: n.s. age effect |
| Spielberg | 2015 | GNG | Females 11.3 (SD = 0.7; 28) Males 12.2 (SD = 0.6; 35) |
L (2:2 yrs) | d’: decreases for low SES females over time | RT: sex by age interaction (female-specific improvement) |
| Burwell | 2016 | GNG | 14.4 (SD = 0.3; 32) | L (3:1 yr) | d’: longitudinal improvement | RT: longitudinal decline |
| Humphrey | 2016 | GNG-WM | 11 – 12 (28) 14 – 15 (39) 16 – 18 (32) |
C | CE: older > younger |
OE: older > younger RT: older > younger |
| McCormick | 2016 | GNG | 14.4 (SD = 0.3; 20) | L (2:1 yr) | CE: n.s. age effect | |
| Bodmer | 2017 | GNG-V | 10 – 14 (24) 20 – 29 (24) |
C | CE: older > younger | RT: older > younger |
| Motes | 2018 | GNG | 7 – 8 (26) 10 – 11 (23) 12 – 15 (68) 18 – 25 (58) 54 – 80 (58) |
C | CE: older > younger (children to young adults) |
OE: older > younger (children to young adults) RT: older > younger (children to young adults) |
| Chung | 2020 | GNG | 12 – 25 (130) | C | CE: linear improvement |
OE: linear improvements RT: linear improvement |
| Cope | 2020 | GNG | 7 – 12 (117) | L (9:1.5 yrs) | CE: longitudinal linear improvement; n.s. quadratic age effect |
OE: longitudinal linear improvement RT: longitudinal linear improvement |
| Ludyga | 2020 | GNG | 9 – 13 (92) | C | CE: n.s. age effect | OE: linear improvement |
| Nelson | 2020 | GNG | 14 – 16 (208) | C | CE: n.s. age effect | RT: n.s. age effect |
| Author | Year | Variant | Age range (N) | Design | Age-Related Patterns of Response Inhibition | Related Findings |
| Tiego | 2020 | SST GNG | 11 – 12 (136) | C |
SSRT-MN: linear improvement CE: n.s. age effect |
|
| Andrade | 2021 | GNG | 13 – 15 (30) 20 – 22 (30) |
C | CE: older > younger | OE: older > younger |
| Antisaccade | ||||||
| Fischera | 1997 | AS-G | 5 – 75 (280) | C |
AS ACC: inverse age effect AS RT: inverse age effect |
PS RT: inverse age effect |
| Munoz | 1998 | AS-G | 5 – 79 (168) | C |
AS ACC: plateau in young adulthood AS RT: plateau in mid-adolescence AS RT – PS RT: plateau in mid-adolescence |
SD(RT): plateau in young adulthood |
| Kleina | 2001 | AS-G | 6 – 28 (199) | C |
AS ACC: inverse age effect AS RT: inverse age effect |
SD(PS RT): inverse age effect Gap effect: decreases with age |
| Malone | 2002 | AS | 11 (674) 17 (616) |
C | AS ACC: older > younger | |
| Luna | 2004 | AS | 8 – 30 (245) | C |
AS ACC: inverse age effect AS RT: inverse age effect |
|
| Kleina | 2005 | AS-G | 9 – 27 (159) 28 – 88 (168) |
C |
AS ACC: inverse age effect AS RT: inverse age effect |
SD(RT):inverse age effect |
| Velanovab | 2008 | AS | 8 – 12 (35) 13 – 17 (35) 18 – 27 (28) |
C | AS ACC: older > younger; linear improvement | PS ACC: n.s. age effect |
| Ordaz | 2010 | AS | 8 – 12 (67) 13 – 17 (62) 18 – 31 (31) |
C |
AS ACC: inverse age effect AS RT: inverse age effect |
Preparation time: n.s. age effect |
| Tamnes | 2010 | AS | 8 – 10 (20) 11 – 13 (24) 14 – 16 (26) 17 – 19 (28) |
C | AS ACC: quadratic + linear age effects | AS ACC: positively related to IQ, n.s. sex effect |
| Kleina | 2011 | AS-G | 6 – 18 (117) | L (2:1.5 yrs) |
AS ACC: longitudinal improvement AS RT: longitudinal curvilinear improvement |
Gap effect: age interaction |
| Ordazb | 2013 | AS | 8 – 27 (98) | L (6:1 yr) |
AS ACC: longitudinal inverse age effect AS RT: longitudinal inverse age effect |
|
| Alahyane | 2014 | AS | 8 – 12 (31) 13 – 17 (25) 18 – 25 (23) |
C |
AS ACC: older > younger AS RT: linear improvement |
|
| Marekd | 2015 | AS | 10 – 12 (41) 13 – 15 (41) 16 – 19 (53) 20 – 26 (57) |
C | AS RT: inverse age effect | |
| Author | Year | Variant | Age range (N) | Design | Age-Related Patterns of Response Inhibition | Related Findings |
| Hwang | 2016 | AS | 14 – 16 (17) 20 – 30 (20) |
C |
AS ACC: older > younger AS RT: n.s. age effect |
|
| West | 2016 | AS | 6 – 16 (15) | C |
AS ACC: linear improvement AS RT: linear improvement |
PS n.s. age effect |
| Ordaz | 2018 | AS | Males 12–14 (44) Females 11–13 (34) |
C |
Female only AS ACC: linear improvement AS RT: n.s. age effect |
|
| Emotional and Rewarded Task Variants | ||||||
| Stop Signal Task | ||||||
| Sinopoli | 2011 | GNG/SST-R | 7–12 (22) 13–17 (22) |
C |
SSRT: older > younger CE: older > younger |
Reward: n.s. age effect |
| Fosco | 2019 | SST-R | 11 – 13 (384) | L (3:1 yr) | SSRT-INT: quadratic + linear age effects |
GoRT: n.s. age effect Lower baseline SSRT: steeper improvement Sex: n.s. effect |
| Go/No-Go | ||||||
| Hare | 2008 | GNG-E, SL | 7 – 12 (12) 13 – 18 (24) 19 – 32 (24) |
C | d’: older > younger |
Emotion: age by RT interaction RT: older > younger |
| Kohls | 2009 | GNG-R | 8 – 12 (65) | C | CE: linear improvement |
OE: linear improvement Reward: n.s. age interaction |
| Sinopoli | 2011 | GNG/SST-R | 7 – 12 (22) 13 – 17 (22) |
C |
SSRT: older > younger CE: older > younger |
Reward: n.s. age effect |
| Somerville | 2011 | GNG-E, SL | 6 – 12 (18) 13 – 17 (19) 18 – 29 (25) |
C | CE: Adolescent-specific peak | OE: n.s. age effect |
| Tottenham | 2011 | GNG-E | 5 – 12 (53) 13 – 18 (24) 19 – 28 (23) |
C |
CE: oldest > middle > youngest d’: n.s. quadratic age effect |
d’:female > male Emotional RT: quadratic age effect (adolescent peak) Neutral RT: n.s. quadratic age effect OE: n.s. age effect |
| Cohen-Gilbert | 2013 | GNG-E | 11 – 12 (20) 13 – 14 (20) 15 – 16 (20) 18 – 19 (20) 20 – 25 (20) |
C |
CE: older > younger, descriptively plateauing at 16 d’: older > younger |
Emotion × d’: significant effect in all but oldest group; 15–16 y.o. only sex effect RT: n.s. age × emotion interaction, descriptively plateauing around 15–16 |
| Schel | 2013 | GNG-E | 6 – 25 (96) | C | CE: older > younger |
Emotion × CE: n.s. age effects RT: older > younger |
| Cohen Kadosh | 2014 | GNG-E | 11.5 (0.5; 30) 17 (0.4; 30) |
C | CE: n.s. age effect |
OE: n.s. age effect RT: older > younger, emotion × age effects |
| Author | Year | Variant | Age range (N) | Design | Age-Related Patterns of Response Inhibition | Related Findings |
| Dreyfuss | 2014 | GNG-E | 6 – 12 (18) 13 – 17 (19) 18+ (20) |
C | CE during threat: adolescents < others | |
| Cohen | 2016 | GNG-E | 13 – 17 (41) 18 – 21 (35) 22 – 25 (34) |
C |
d’ in during negative emotions: oldest > others d’: quadratic + linear age effects |
|
| Kray | 2020 | GNG-E | 9 – 10 (30) 11 – 12 (37) 13 – 14 (42) 15 – 16 (32) 17 – 18 (44) |
C |
CE: linear improvement (plateauing in oldest two groups); positive emotion effect in oldest two groups d’: linear improvement; improvement greater for negative emotions |
OE: linear improvement (plateauing in oldest two groups) RT: emotion × age effects; n.s. age effect overall |
| Antisaccade | ||||||
| Jazbec | 2006 | AST-RP | 13 – 18 (23) 19 – 40 (30) |
C |
AS ACC: older > younger AS RT during neutral: older > younger AS RT during reward: n.s. age effectts |
|
| Geier | 2010 | AST-R | 13 – 17 (22) 18 – 30 (16) |
C |
AS ACC during reward: specific effect for only 13–17 y.o.s AS-RT: n.s. age effect |
|
| Padmanabhan | 2011 | AST-R | 8 – 13 (10) 14 – 17 (10) 18 – 25 (10) |
C |
AS ACC during neutral: older> younger AS ACC during reward: n.s. age effect AS RT: n.s. age effect |
|
| Geierd | 2012 | AST-RP | 13 – 15 (32) 15 – 17 (32) 18 – 29 (42) |
C |
AS ACC overall: youngest < others AS RT: quadratic + linear age effects |
AS ACC × reward valence: adolescent variability AS RT × reward valence: adolescent only quadratic relationship |
| Tervo-Clemmense | 2017 | AST-R | 12 – 21 (116) | L (2:1 yr) |
AS ACC: longitudinal improvement AS RT: longitudinal improvement |
|
| Hallquist | 2018 | AST-R | 10 – 25 (140) | C | AS ACC: inverse age effect | AS ACC by reward: n.s. age effect |
| Quache | 2020 | AST-R | 12 – 21 (94) | L (3:1 yr) |
AS ACC: longitudinal improvement AS RT: longitudinal improvement |
|
| Ravindranath | 2020 | AST - E | 14 – 31 (66) | C |
AS ACC: n.s. age effect AS RT: n.s. age effects; improvement in silent condition |
|
Note: For all measures, table reflects performance, not raw scores. > implies relatively superior performance (lower RT, less errors).
Findings reflect data from only the healthy subsample of larger studies
refers to studies with same samples
Variant: C: CANTAB version, E: Emotional variant, EQ: equiprobable Go/No-Go, F: Flanker stimuli, G: gap condition (AST), NoT: without tracking procedure (SST), R: reward variant, RP: reward and punishment variant, S: selective, SL: slow (ISI > 4000 ms for GNG), V: vibrotactile, WM: working memory variant
Design: C: Cross-sectional; L(a:b): longitudinal where a = timepoints and b = years apart
Age Related Patterns of Response Inhibition & Related Findings: CE: commission error, d’: discrimination index, GoRT: reaction time on Go trials, Log: log transform, OE: omission error, PRT: prosaccade reaction time, RT: reaction time, SSRT-INT: SSRT Integration, SSRT-MD: SSRT Median, SSRT-MN: SSRT Mean
Studies also differ in their estimation of the primary variable of response inhibition, SSRT, which can influence conclusions (Boehler et al., 2012). Three common estimation methods have been used to calculate the SSRT—the mean, median, and integration methods. The mean method assumes that for each individual, p(respond|signal) = .50 and the distribution of GoRT is normal, and the mean SSRT can be estimated as the difference between the mean SSD and the mean of correct trial GoRTs. A slightly less biased method is the median method, which uses the mean SSD and the median of correct GoRTs, and this method is a more robust indicator of central tendency given the expected skew of response time data (Verbruggen et al., 2019). The recommended method is the integration method, which includes two steps—replacement of Go omission trials with the maximum reaction time across the test on a Go trial and an approximation of the integral of the GoRT. Instead of assuming that the median GoRT represents task performance, the integration method uses the nth reaction time, where n represents the reaction time at the quantile corresponding with the accuracy of stopping. While the mean and median methods are commonly used in the literature (see Table 2), simulation studies demonstrate that the integration method provides more reliable and less biased SSRT estimates (Verbruggen et al., 2019). Theoretically, if dynamic adjustments perform perfectly (p(respond|signal) = 0.50) and there are no omission errors, then the mean, median, and integration methods would all produce the same estimation of motor response inhibition. However, most designs include some level of pre-set SSDs at least at the beginning of blocks, and some variability in accuracy in the task can be best accounted for by the integration method. Lastly, guidance is provided noting possible violations of the independent race model; most studies in this review did not indicate whether investigators considered such violations while screening their data. Notably, many studies were published before the 2019 consensus guidelines were derived to represent best practices.
Studies of adult samples have found variable reliability of the SST. In one study of 128 healthy adults (ages 18–30) who were tested twice (retest interval M = 8.6 days, SD = 7.8), the SSRT showed moderate retest reliability (r = 0.65, p < .001) and good discriminant validity as SSRT was not associated with mood (Weafer et al., 2013). A separate study with 23 adult participants found that at baseline, the internal consistency reliability of SSRT was poor (Cronbach’s α = 0.29), was moderately acceptable on retest (Cronbach’s α = 0.61) and showed poor test-retest reliability after 28–105 days (r = −0.03; test-retest interval M = 77.7 days, SD = 26.0; ICC = 0.03; Wöstmann et al., 2013). In the same study, reliability of the mean and standard deviation of the GoRT was more acceptable (Cronbach’s α = 0.93 for each; Wöstmann et al., 2013). A simulation study found that task performance under guideline-recommended parameters and lenient outlier exclusion criteria had good split-half reliability (ICC = 0.71; Congdon et al., 2012).
Among studies that quantified SST performance changes during adolescent development, several included specific information regarding reliability (see Table 1 for summary). In a large, community-based sample, SSRT split-half reliability was excellent for late childhood/early adolescence (ages 9–12, n = 41, SB2 r = 0.86) and for mid-adolescence (ages 13–17, n = 50, SB2 r = 0.91) and young adulthood (ages 18–29, n = 47, SB2 r = 0.91; Williams et al., 1999). In a similar study with a selective version of the SST, researchers found excellent internal consistency reliability coefficients for both GoRT and SSRT across the total sample (Cronbach’s α = 0.93 and 0.97, respectively) and within specific age groups (Bedard et al., 2002). In a large community-sample longitudinal study of adolescents, internal consistency was good (Cronbach’s α = 0.70 and 0.71) at various timepoints (Meisel et al., 2015). In a longitudinal community sample with information from two waves (ages 12–14 and ages 15–17, respectively), the composite SSRT showed excellent reliability (Cronbach’s α = 0.93; Nigg et al., 2006). In another study of healthy adolescents and young adults (ages 14 – 19; N = 240), split-half reliability was excellent (SB2 r = 0.85; Malagoli & Usai, 2018). Split-half reliability of the log-transformed SSRT was also excellent (r = 0.94) in another study of healthy young adolescents (ages 11–12, N = 136; Tiego et al., 2020). Thus, overall, reliability of the SST appears to be strong.
2.2. Go/No-Go Design, Estimation, and Reliability
The Go/No-Go (GNG) paradigm involves presentation of a series of simple, usually visual, stimuli one at a time. Participants respond, typically with button presses, as quickly as possible to presented target stimuli (Go) and withhold responses to non-target stimuli (No-Go; Donders et al., 1969; Figure 2B). Following each trial, a new stimulus appears after a designated waiting time (inter-stimulus interval or ISI; Ratcliff & McKoon, 2008). Task difficulty parameters that can be manipulated include frequencies of No-Go stimuli, the number of Go stimuli between instances of No-Go stimuli, and the inter-stimulus-interval (ISI). Infrequent stimuli and a faster pace render the task more difficult and may more consistently recruit response inhibition (Wessel, 2018).
Unlike the SST, estimation of GNG task scores does not rely upon a specified model, and there have been fewer explorations of how aspects of task design and estimation affect validity or reliability. The most commonly-analyzed outcome variable is commission errors (e.g., false alarms; CE), with higher rates reflecting poorer motor response inhibition (Aron & Poldrack, 2005; Wright et al., 2014). Some studies also operationalize inhibition as d’, derived from signal detection theory, which accounts for both CEs and omission errors (OEs) to represent the overall ability to discriminate between Go and No-Go stimuli (Green & Swets, 1966; Wetherill et al., 2013).
Various task manipulations have provided information about the processes underlying GNG performance. First, the frequency of No-Go trials can vary; less frequent No-Go trials are assumed to lead to greater prepotent response as it becomes a beneficial strategy to initiate Go responses for every trial (Menon et al., 2001; Wessel, 2018). In addition to the frequency of No-Go stimuli, variation in the latency between trials has important effects on interpretation. Shorter interstimulus intervals and latencies require faster responses, theoretically increasing the prepotency of the response (Garavan et al., 1999). Other researchers consider the discriminability between stimuli (e.g., nonwords/words as more difficult than vowels/consonants; Gomez et al., 2007), where simpler associations, especially spatially-mapped ones, rely less on working memory maintenance and may provide a more pure representation of response inhibition (Sebastian et al., 2013a). Further, some tasks maintain stimulus-response pairings throughout the experimental sessions, while others may switch stimulus-response mappings across task blocks or otherwise increase working memory demands by increasing the number of total stimuli in the task (Simmonds et al., 2008). Overall, there is a wide variety of versions of the GNG task, and it is important that the task parameters, especially the frequency of No-Go stimuli and pace, be considered in any interpretations or generalizations.
The GNG paradigm has generally shown fair-to-good reliability in adult and adolescent samples. In a study of 23 adults, commission errors, mean RT, and SD of RT all showed good to excellent test-retest reliability over an interval of 28–105 days (ICC = 0.84, 0.78, 0.75, 0.74, respectively; Wöstmann et al., 2013). Among studies reviewed for specific developmental aspects of motor response inhibition, internal consistency was very poor for CE (Cronbach’s α=.07) and excellent for mean RT (Cronbach’s α = .87) among 153 healthy participants aged 6–24 (McAuley & White, 2011, Table 1). In a study that measured healthy children’s and young adolescents’ ( 2–13 years old; n= 44–47 depending on conditions) performance on slow, fast, and rewarded GNG tasks, test-retest reliability was good to excellent for all measures in the most standard, fast condition (commission errors ICC = 0.70; mean RT ICC = 0.88; SD(RT) ICC = 0.83); while test-retest reliability of the same variables in the other conditions, after excluding outliers, range from fair to excellent (ranging from 0.54 for CEs during an incentive condition to 0.76 for mean RT in a slow condition; Kuntsi et al., 2005, Table 1). In another study of 240 healthy adolescents and young adults (ages 14–19), internal consistency was good (Cronbach’s α = 0.70; Malagoli & Usai, 2018, Table 1). Split-half reliability of square-root transformed commission errors was excellent (r = 0.82) in a sample of healthy young adolescents (ages 11–12, N = 136; Tiego et al., 2020). Overall, reliability of the GNG appears good to excellent in most adolescent samples.
2.3. Antisaccade Task Design, Estimation, and Reliability
Computerized ASTs usually involve three core elements—fixation, Prosaccade, and Antisaccade targets. In fixation between trials, participants focus their gaze on a central point. During Prosaccade (PS) trials, which are either indicated by instructions for a block or a characteristic of the stimulus itself, a target appears adjacent to the fixation point. The participant is instructed to direct their gaze as quickly as possible to the target (e.g., Munoz et al., 1998; Hutton & Ettinger, 2006). In Antisaccade (AS) trials, when the target appears, the participant is instructed through a pre-target cue to avoid directing their gaze to it and instead must direct a saccade to a point opposite of the target (Figure 1C). Key variables include the length of the fixation interval, the initial cue onset, the target onset, and the latency of target presentation (Hutton & Ettinger). Eye tracking equipment is typically utilized to measure fixation locations and saccade latencies (e.g., Geier et al., 2010).
The most common measurement of motor response inhibition in the AST is antisaccade errors, the frequency of the reflexive first saccade in the incorrect direction (i.e., toward the target). Saccade latency is determined by the length of time it takes for eye velocity to increase beyond a certain degree (typically 30°/s; Gitelman, 2002) from fixation. It is considered a measurement of executive processing and attention in prosaccade conditions and motor response inhibition in correctly-performed antisaccade trials (Hallett, 1978; Luna et al., 2008).
The estimation of AST performance is based on models assuming that the appearance of the visual target triggers a prepotent, reflexive motor response and that errors occur when effortful processes fail to inhibit that reactive response (Hutton & Ettinger, 2006). Some researchers have argued that the process can be modeled similarly to the independent race model with two parallel and competing processes (pro- and anti-saccade; Massen, 2004). While this theoretical model is supported by consistent findings that increased latencies for correct antisaccades are related to increased errors, it still relies on reaction times and does not translate such a process into a computational model (Hutton & Ettinger, 2006).
Perhaps due to relatively fewer studies using the AST than the GNG and SST, there are fewer discussed considerations regarding its design. One primary difference between tasks is the inclusion of a “gap” between the fixation/instruction cue (usually indicating whether the trial will be a prosaccade or antisaccade) and the presentation of the target stimulus. The instruction cue enables the preparation of the type of motor response to be executed prior to target stimulus presentation. The ability to engage in response preparation appears to be a critical AST component, given that the existence and duration of the gap are known to have effects on performance accuracy, and the gap effect may compound developmental findings as it appears to have differential age-related effects (Fischer & Weber, 1997). Among the studies reviewed for developmental aspects of AST performance, earlier studies (Fischer & Weber, 1997; Munoz et al., 1998; Klein, 2001; Klein, et al., 2005) included a gap between fixation and target, however it should be noted that more recent studies excluded the gap in order to avoid confounding effects in developmental samples (e.g., Velanova et al., 2008).
Dedicated studies exploring reliability of executive tasks in adult samples have found variable reliabilities for AST measures. In a test-retest study of 23 adults over an interval of 23–105 days, antisaccade latency and accuracy showed excellent internal consistency at baseline (Cronbach’s α= 0.92 and 0.94, respectively) and retest (Cronbach’s α = 0.97 and 0.94, respectively; Wöstmann et al., 2013). Test-retest reliability in the same sample showed excellent reliability for AS latency and accuracy (ICC = 0.88 and 0.92, respectively). Test-retest reliability over a period of 19 months for 117 children and adolescents aged 6–18 ranged from fair (0.46 for proportion of express saccades during PS task and 0.48 for error reaction time for ASs) to good/excellent for most commonly used measures (0.77 for AS latency and 0.74 for PS latency; Klein & Fischer, 2005; Table 1). In the same study, split-half reliabilities for a larger (N = 327) lifespan sample (ages 9–88) were excellent (SB2 r = 0.87 for AS latency and SB2 r = 0.92 for PS latency), and those reliability estimates were not significantly changed when accounting for age (Klein & Fischer, 2005). Among studies presently reviewed, one cohort-sequential study of 123 participants aged 9–26 years old found poor to good to excellent test-retest reliability of antisaccade latency and accuracy (ICC = 0.53, 0.76 respectively) during the AST (Ordaz et al., 2013, Table 1).
2.4. Large-Scale Studies Using Motor Response Inhibition Tasks
The Stop Signal Task is employed in several major ongoing longitudinal studies of adolescent development. The IMAGEN consortium, based in eight sites throughout the UK, Germany, France, and Ireland, has evaluated 2,000 young people from the age of 14 with assessments at age 16, 19, and 22 and employed the SST during 3T functional scans. Initial studies based on this sample have delineated networks related to SST performance at baseline (Whelan et al., 2012) and changes over time (Wang et al., 2020). The SST is also part of the fMRI battery in the ongoing Adolescent Brain Cognitive Development (ABCD) study (Casey et al., 2018) but see Bisset et al (2021) and Garavan et al. (2020) for discussions of its implementation. The GNG task has been included in many individual studies as well as several longitudinal efforts. An event-related GNG paradigm with fMRI was included in a cohort-sequential study of 290 participants covering an age range between 7.6 to 28.5 (e.g., Cope et al., 2020). For the AST, many of the reviewed studies come from the same group based in Pittsburgh, Pennsylvania. In addition to several smaller longitudinal studies, this group frequently publishes developmental antisaccade articles based on data from the National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA) study (e.g., Tervo-Clemmens et al., 2017; Quach et al., 2020).
2.5. Common Variations of Motor Response Inhibition Tasks
Among reviewed studies, several task variants have been devised to explore motor response inhibition in the context of other attentional, working memory, and emotional or reward processes. Several studies included a third type of trial that captures attention without demanding actual inhibition and thus requires selective stopping (Bedard et al., 2002; Rubia et al., 2006; Geier et al., 2010; Alahyane et al., 2014; van de Laar et al., 2014). By varying the number of preceding Go trials and varying stimulus-response mappings, researchers have studied impacts of working memory load manipulations on adolescents’ motor response inhibition (e.g., Johnstone et al., 2005; Liston et al., 2006). Incorporation of emotional stimuli as either the primary stimuli or as background have been most common in GNG literature (e.g., Tottenham et al., 2011; Somerville et al., 2011; Cohen Kadosh et al., 2014; Dreyfuss et al., 2014; Cohen et al., 2016; Kray et al., 2020), but have been used recently using the AST (Ravindranath et al., 2020). Finally, while most common in the AST literature (e.g., Geier & Luna, 2012), reward and punishment have been incorporated into developmental studies using both the SST (e.g., Sinopoli et al., 2011; Fosco et al., 2019) and GNG (e.g., Kohls et al., 2009) to clarify how value-driven cognitive control develops in adolescence (Davidow et al., 2018). Although infrequently reported in publications (but see Wang et al., 2016, West & Lippé, 2016; Madsen et al., 2020), standard feedback on performance and interaction with test administrators between task blocks of the SST may provide social reinforcement that has non-negligible effects on performance.
2.6. Distinctions between Motor Response Inhibition Tasks
While each of these three tasks primarily measures the construct of motor response inhibition (Tiego et al., 2020), findings spanning non-human primate research to human neuroimaging studies indicate that each task elicits some unique cognitive and neural processes that could represent different processes or aspects of inhibition as a broader construct. Some researchers distinguish the SST and GNG as measures of action cancellation and action restraint, respectively. The SST demands cancellation of an already initiated response, while the GNG requires that participants refrain from initiating the prepotent response altogether (Eagle et al., 2008; Chambers et al., 2009). This distinction is supported by differences in the patterns of neural activation, including the greater lateralization of activity on the SST compared to the GNG (e.g., Mostofsky & Simmonds, 2008, Raud et al., 2020). In contrast to the GNG and SST, during which participants are unaware of the upcoming stimulus demands, in the AST, participants are cued of the stimulus demand prior to the actual stimulus presentation and thus are able to anticipate and prepare to inhibit their dominant response (Munoz & Everling, 2004). The unique measurement of preparation for response inhibition in the AST is supported by neuroimaging studies that find performance on the task is related to greater involvement in preparatory regions like the FEF during that anticipatory phase (e.g., Hwang et al., 2016). While these tasks share a number of similar overall demands that cohere to represent a unitary construct of motor response inhibition that relies on frontal suppression of a response (Malagoli & Usai, 2018), the distinctions among them are also important to consider especially if task-unique processes have different developmental patterns (e.g., Ordaz et al., 2013).
3. Adolescent Development of Motor Response Inhibition Measured by the Stop Signal, Go/No-Go, and Antisaccade Tasks
3.1. Age-Related Variations in Stop Signal Task Performance
While SST studies have found a general pattern of improving motor response inhibition across adolescence, the shape and extent of this change has varied between studies and may have been influenced by their samples, task designs, and estimation methods (Table 2). Two community-based studies found that adolescent performance was better than that of children on both a standard (Williams et al., 1999) and selective version of the SST (Bedard et al., 2002). Studies comparing adolescents to adults generally found that adults had faster SSRTs than adolescents (Bedard et al., 2002; Cohen, 2010; van de Laar et al., 2014). Linear associations between SSRT and age indicates steady improvements in performance across the adolescent period (Schachar et al., 2007; Cohen, 2010; Madsen et al., 2010; Wong et al., 2010; Murphy et al., 2018; Ogilvie et al., 2020; Tiego et al., 2020). Some studies have not found significant age-related changes in SSRT, though design variations and small sample sizes may limit the confidence in the validity of null findings (e.g., Rubia et al., 2000; 2007; 2013; Meisel et al., 2015; Thompson et al., 2021; Table 2).
Longitudinal studies and estimations of nonlinear age-functions have provided more information about the shape of the developmental trajectory in addition to individual differences in baseline performance and change over time. For example, in a longitudinal study of 4–13 year-olds and including up to 3 annual follow-ups, transformed (log) SSRT became faster over time and females showed better performance than males (Curley et al., 2018). The best fitting model of motor response inhibition in a longitudinal sample (baseline ages 11–13, N = 387 at baseline, up to 3 annual assessments) included both linear and quadratic effects of age, as well as significant variance of the intercept and slope, indicating that improvements in SSRT level off around age 13 and that there is significant variability in both baseline performance and individual differences in the rate of development of response inhibition (Fosco et al., 2019). In an older longitudinal sample (baseline N = 88, overall age range 7 – 19 years old) with up to nine assessments over six years, SSRT (and log(SSRT)) improved linearly (but not quadratically) with age, though performance appeared to plateau around age 14 (Madsen et al., 2020). Two of these studies found that individuals who performed worse at baseline showed steeper improvements until reaching equivalent performance around the age of 14 (Curley et al., 2018; Madsen et al., 2020) However, the best fitting linear model in a cohort-sequential design of 112 healthy adolescents (ages 9 – 16) indicated that accelerations in SSRT continue until age 16 (Mürner-Lavancy et al., 2020). Using a subset of individuals (n = 326, baseline age 14, follow-up age 19) from the IMAGEN study (Whelan et al., 2012), researchers unexpectedly found an overall slowing effect across time, though additional analyses based on a reliable change index found considerable variability across individuals such that some became faster (17.48%), some became slower (29.45%), and some remained stable (53.07%; Wang et al., 2020). These longitudinal studies have not, to date, included an age range extending into young adulthood or tested nonlinear age-based trajectories (e.g., inverse age). Furthermore, most studies have not controlled for sex, and many studies may have been underpowered to detect interactions between age and sex, especially in early puberty (but see Curley et al., 2018; Ordaz et al., 2018).
Lastly, a few studies have explored motor response inhibition as measured by inhibition accuracy on the SST. Despite use of an adjusting algorithm to approximate equivalent inhibition success rates across all individuals, the likelihood of correct inhibition increased with age in a sample of healthy 13–38 year olds completing a standard version of the SST (Rubia et al., 2013). In another study that included a subset of 21 typically-developing adolescents (ages 13–16) with pre-set SSDs, age was positively correlated with higher rates of successful inhibition, especially on difficult trials (Ware et al., 2015). Three studies found non-significant associations between age and accuracy using variants of the SST, though each had methodological concerns (Rubia et al., 2000; Murphy et al., 2018; Roe et al., 2021; Table 2). One study utilized a task design with equiprobable presentations of Stop and Go signals (Rubia et al., 2000). Another used an altered tracking procedure to purposefully limit the variance in percent of successful inhibitions (Murphy et al., 2018). The most reliable SST versions control for individual differences in accuracy (Verbruggen et al., 2019).
Overall, the existing SST literature indicates that there are improvements in motor response inhibition during adolescence, but different sampled age ranges and statistical tests make it difficult to directly compare findings across studies. Studies with younger age ranges (e.g., Meisel et al., 2015; Curley et al., 2018; Fosco et al., 2019) have indicated a plateau in performance around age 13–14. However, longitudinal studies and samples with broader age ranges have shown linear age effects (e.g., Madsen et al., 2020; Mürner-Lavancy et al., 2020) that suggest improvements may extend beyond early adolescence into young adulthood. Moreover, over time, the range of performances between individuals appears to decrease with increasing age (Curley et al., 2018; Wang et al., 2020).
3.2. Age-Related Variations in Go/No-Go Performance
Similar to the SST literature, GNG studies have found a general pattern of improving motor response inhibition across adolescence that may extend into young adulthood (Table 2). Across studies, direct comparisons show that adults committed fewer GNG CEs than adolescents (e.g., Casey et al., 1997b; Bunge et al., 2002; Durston et al., 2002; Johnstone et al., 2005; Barber et al., 2013). Adolescents committed fewer CEs than children (Cragg & Nation, 2008; Humphrey & Dumontheil, 2016; Motes et al., 2018). Many studies found linear age-related decreases in CEs across variants of the GNG paradigm (Rubia et al., 2006; Eigsti et al., 2006; Kohls et al., 2009; McAuley & White, 2011; Silveri et al., 2013; Bodmer et al., 2018; Chung et al., 2020; Cope et al., 2020; Andrade & Raposo, 2021). Longitudinal studies also have also shown decreases in CEs with increasing age (Wetherill et al., 2013; Bezdijan et al., 2014). Studies that have reported non-significant age-related variations in OEs or overall accuracy have potential limitations; many included too-frequent No-Go stimuli that weaken the prepotent response (e.g., Tamm et al., 2002; Cohen Kadosh et al., 2014; Vara et al., 2014), lengthy periods between stimuli (i.e., longer than 4000 ms; Durston et al., 2006; Somerville et al., 2011), incorporation of Flanker stimuli (e.g., Brydges et al., 2013), limited age ranges (McCormick et al., 2016; Nelson et al., 2020), small sample sizes (Liston et al., 2006; Lock et al., 2011) or novel (e.g., vibrotactile) paradigms that require further validation (Bodmer et al., 2018). Nonetheless, another study (ages 9–13, N = 92) found no relationship between CEs and age on a well-designed GNG task (Ludyga et al., 2021).
Using discriminability (d’) or overall accuracy, better performance has been reported in adolescence versus childhood (Rubia et al., 2006; Humphrey & Dumontheil, 2016) as well as adulthood versus adolescence (e.g., Booth et al., 2003; Hooper et al., 2004; Brydges et al., 2013). Performance was negatively correlated with age across a variety of different age spans within adolescence and young adulthood (e.g., Durston et al., 2002; Johnstone et al., 2005). One longitudinal study of 48 pairs of 14–16 year old twins tested twice over a period of one year found that overall accuracy improved with age (Burwell et al., 2016). To date, only one study reported non-linear (quadratic) modeling of GNG performance (Cope et al., 2020), and no studies reviewed tested a plateauing trajectory of age-related change.
3.3. Age-Related Variations in Antisaccade Task Performance
Adolescent motor response inhibition maturation as measured by AST errors has yielded different findings from what has been reported for the GNG and SST (Table 2), which may reflect different analytic approaches or differences in the constructs measured by the tasks. Researchers comparing group performance on AST trials found that adolescents are faster and more accurate than children (e.g., Malone & Iacono, 2002; Velanova et al., 2008; Ordaz et al., 2013; Alahyane et al., 2014) and less accurate than healthy adults (Velanova et al., 2008; Padmanabhan et al., 2011, Geier & Luna, 2012). Across both gap and overlap conditions of the task and through multiple reports focused on the same dataset, researchers found that between the ages of 6 and 28, errors decreased with age, and this trajectory was best modeled by an inverse function reflecting initial steep improvements that level off over time (Fischer et al., 1997; Munoz et al., 1998; Klein, 2001; Klein et al., 2005; Table 2). One longitudinal study found that a linear age function best fit performance of 117 participants between baseline (6–18 years old) and follow-up (17–21 months later; Klein et al., 2011). Other studies have found negative linear correlations between age and direction errors on AS trials in both standard (Velanova et al., 2008; Tamnes et al., 2010; Klein et al., 2011; West & Lippé, 2016) and rewarded task versions (Tervo-Clemmens et al., 2017). Similarly, linear negative relationships between age and latency have been reported across task versions (standard: Fischer et al., 1997; West & Lippé., 2016; rewarded: Tervo-Clemmens et al., 2017; Quach et al., 2020). Inverse or curvilinear (quadratic) age models have generally provided the best fit to the data, indicating monotonic improvement in latency with steepest gains in early adolescence (Klein et al., 2001; Klein et al., 2005; Ordaz et al., 2010; Klein et al., 2011; Ordaz et al., 2013; Marek et al., 2015). One study with puberty-matched males (ages 12–14, n = 44) and females (ages 11–13, n = 34) found that a negative linear correlation between age and direction errors was significant only for girls, indicating possible sex differences in early development (Ordaz et al., 2018). When tested against linear models, researchers within the Pittsburgh group have reported that the inverse age function best fits the data across several independent cross-sectional and longitudinal datasets (Luna et al., 2004; Ordaz et al., 2010; 2013; Marek et al., 2015). Longitudinal analyses suggested that while individuals varied in their baseline antisaccade accuracies and latencies on AST trials, variability around the mean inverse age curve was significant only for AST latency (Ordaz et al., 2013). While the development of motor response inhibition as measured by AST trial errors is robust, four reviewed studies across independent datasets found insignificant age-relationships with AST latencies during adolescence, though these may have been impacted by low sample sizes or small age ranges (Jazbec et al., 2006; Padmanabhan et al., 2011; Ordaz et al., 2018; Ravindranath et al., 2020).
3.4. Other Age-Related Findings from Motor Response Inhibition Tasks
The three tasks discussed here are not necessarily pure measures of motor response inhibition. Processing speed is reflected by the average reaction time to Go trials (GoRT) in the SST (e.g., Williams et al., 1999), mean reaction time in the GNG (Vaurio et al., 2009; Tottenham et al., 2011; Wessel, 2018), and mean PS latency in the AST (Klein et al., 2005). Recently, consideration has been given to measures of variability and inattention, including standard deviation of reaction times or saccade latencies (Madsen et al., 2020; Johnstone et al., 2007) as well as GNG OEs (Trommer et al., 1988; Wright et al., 2014). Lastly, some studies have examined performance monitoring via post-error slowing in the SST (Dupuis et al., 2019) or preparation (Ordaz et al., 2010) and Correction Reaction Time in the AST (Klein et al., 2005). Age-related variations in these processes may impact interpretations regarding inhibitory control.
Although less commonly reported than inhibition measures, several reviewed studies included information about how executive processing speed changes over time. Within the independent race model, this process is critical because the speed of the “ o” process as compared to the speed of the “ top” process determines actual behavior (Verbruggen et al., 2019). However, among the three tasks, GNG studies most frequently reported results of executive processing speed. While not a universal finding (e.g., insignificant age relationships in Eigsti et al., 2006; Vara et al., 2014; Kray et al., 2020), most studies reviewed report decreases in Go RTs (e.g., faster responses) across adolescence (e.g., Casey et al., 1997a; 1997b; Liston et al., 2006; McAuley & White, 2011; Chung et al., 2020). Notably, while some have found negative linear correlations between speed and age (e.g., McAuley & White, 2011; Chung et al., 2020), others have noted curvilinear change with plateauing performance in later adolescence (Johnstone et al., 2005; Cohen-Gilbert & Thomas, 2013). Furthermore, some have reported that adults have slower Go RTs than adolescents (e.g., Bodmer et al., 2018; Rubia et al., 2006), suggesting a possible peak in adolescence. Studies reporting the GoRT from the SST generally found that speed accelerates linearly across adolescence (Williams et al., 1999; Bedard et al., 2002; van de Laar et al., 2014; Ware et al., 2015; Dupuis et al., 2019). One longitudinal study spanning over 9 years found developmental sex differences, with females showing curvilinear improvements (plateaus) and males showing linear improvements across the tested age range (7–18 years old; Madsen et al., 2020). Yet, the pattern of improvement in SST-based executive reaction times was less consistent than in the GNG as several well-designed studies reported non-significant age effects on Go RTs (e.g., Cohen, 2010; Rubia et al., 2007; 2013). As in many SST studies, when reported, AST-based PS RTs decrease through adolescence and into early adulthood either linearly (e.g., Fischer et al., 1997; Ordaz et al., 2013) or curvilinearly (i.e., plateauing; Klein & Fischer, 2005). One reviewed study reported a non-significant correlation between age and PS latency, but the sample size was very small (n = 15; West & Lippé, 2016). Another study using an incentivized AST also found nonsignificant age effects on PS latencies, suggesting that the potential for reward receipt elevates adolescents’ performance to adult levels (Jazbec et al., 2006).
How reward affects motor response inhibition and related processes has been studied most consistently with the AST (Table 2). In the first study utilizing a rewarded AST paradigm, reward feedback affected latency equally in adults and adolescence, but only in adolescents did reward feedback improve accuracy (Geier et al., 2010). Reward feedback exerted different effects on latency and accuracy (e.g., Geier et al., 2010; Padmanabhan et al., 2011), and there were apparent interactions between incentive magnitudes and age (e.g., Geier & Luna, 2012), suggesting that adolescents were capable of adult levels of response inhibition but variably engaged in the behavior depending on context (Contantinidis & Luna, 2019). Yet, a relatively large (ages 10–25, N = 140) cross-sectional study by the same group failed to find age by incentivized condition interactions (e.g., Hallquist et al., 2018). Overall, GNG and SST studies have shown modest ability to elicit improved performance with reward, but methodological concerns (e.g., frequent No-Go stimuli, application of SST models to estimate performance on GNG/SST hybrid tasks) limit the interpretations to be drawn (Kohls et al., 2009; Fosco et al., 2019; Castellanos-Ryan et al., 2011; Sinopoli et al., 2011).
Emotionally salient stimuli have also been utilized within motor response inhibition tasks (Table 2). Adolescents were especially sensitive to the detrimental effects of negative emotion on GNG (Hare et al., 2008; Somerville et al., 2011; Tottenham et al., 2011; Cohen Kadosh et al., 2014; Dreyfuss et al., 2014; Kray et al., 2020) and AST (Ravindranath et al., 2020) performance. Several studies have examined age effects on accuracy in emotional GNG paradigms using either inherently emotional stimuli such as happy, sad, or neutral faces (e.g., Dreyfuss et al., 2014) or neutral stimuli presented against emotional backgrounds (e.g., Cohen-Gilbert & Thomas, 2013). Results are mixed; some studies showed that heightened emotional states facilitated performance in adolescents (e.g., Dreyfuss et al., 2014) and some suggest that emotions negatively impacted performance only in some age groups (Cohen et al., 2016; Kray et al., 2020). Negative emotions tended to affect performance more than positive ones (e.g., Cohen et al., 2016), especially in early adolescence (Cohen-Gilbert & Thomas, 2013). However, the specific patterns (e.g., effects of happy vs. fearful emotional stimuli) have not been consistently replicated (e.g., Schel & Crone, 2013).
All three tasks require attention and performance monitoring. Reviewed studies indicated that typical adolescent development is characterized by decreases in OEs in both the GNG and SST. Adolescents committed fewer OEs than children (e.g., Cragg & Nation, 2008; Humphrey & Dumontheil, 2016) and more than adults (e.g., Silveri et al., 2013; Andrade & Raposo, 2021). OE rates in the two tasks are also negatively correlated with age within groups of adolescents who vary in their developmental stage (Eigsti et al., 2006; Kohls et al., 2009; Chung et al., 2020; Cope et al., 2020; Ludyga et al., 2020). Furthermore, reaction time variability decreased across adolescence in the GNG (Barber et al., 2013; Bodmer et al., 2018), SST (Williams et al., 1999; Madsen et al., 2020; Thompson et al., 2021), and AST (Munoz et al., 1998; Klein et al., 2001; Klein et al., 2005; Klein et al., 2011). In a hybrid GNG/SST, reaction time variability similarly decreased across adolescence and was greater under GNG versus SST conditions (Johnstone et al., 2007). Overall, across all three tasks, OEs and variability in executive response speed decrease with age, which may facilitate response inhibition.
4. Neural Regions Involved in Motor Inhibitory Control and Adolescent Neurodevelopment
Different methods of isolating neural activity related to motor response inhibition have implications for our understanding of this basic process as well as its development (Criaud & Boulinguez, 2013). Several of the earliest studies in the field incorporated block-based designs that measured activity during overall task performance (Rubia et al., 2001; Tamm et al., 2002; Booth et al., 2003; Velanova et al., 2008), but activation in these studies is not necessarily specific to inhibition as it could relate to concurrent attention, action selection, or motor processes (Simmonds et al., 2008). Several researchers explored the effects of AST inhibition on the shape of the hemodynamic response (Geier et al., 2010; Padmanabhan et al., 2011), but most studies explored the overall magnitude of activity related to an epoch instead of the trajectory. In event-related studies, researchers isolate and compare activity between the fixation periods, the “Go” periods (“Go” trials in SST or GNG, PS trials in AST), and inhibition periods (Stop Trials in SST, No-Go in GNG, AS trials in AST). Further analyses can isolate successfully inhibited responses or failures to inhibit. Some studies may additionally assess the epochs of “Continue” or “Preparation” trials to identify activity related to processing infrequent stimuli or anticipating rewards, respectively (e.g., Alahyane et al., 2014; Tervo-Clemmens et al., 2017). While the exact form of the trials differs, almost all reviewed fMRI studies use these basic events to explore the neural activity related to developmental changes in motor response inhibition.
The broadest comparison, Inhibition – Go (NoGo – Go; Stop – Go; AS – PS) theoretically isolates the general processes related to viewing infrequent stimuli and attempting to inhibit the dominant motor response, but activity is generalized across successful and failed inhibition trials (e.g., Rubia et al., 2007; Spielberg et al., 2015; Ware et al., 2015; Cai et al., 2019). Comparing Inhibition trials against fixation trials, as is frequent in the AST literature, similarly accounts for the general processes of inhibition but also represents perceptual, attentional, and preparatory processes as well as task engagement (Cohen, 2010; Weathers et al., 2012; Rubia et al., 2013; Cai et al., 2019; Cope et al., 2020). More specific queries assess failed Inhibition trials against Go trials, controlling for the motor activity related to execution of the dominant response while isolating activity supporting attention processes related to the infrequent stimulus and attempting to inhibit the response. Comparing successful Inhibition trials against failed Inhibition trials is theoretically more likely to identify inhibitory processes, though the motor component of the failed Inhibition trials may confound results (Rubia et al., 2007; Cohen, 2010; Criaud & Boulinguez, 2013). More specific contrasts utilizing task variants may have isolated activation related to inhibitory processes while controlling for attention (e.g., Unsuccessful Stop – Continue) or various reward processes (Padmanabhan et al., 2011; Quach et al., 2020). Other functional neuroimaging studies explored functional connectivity related to specific epochs through ICA (e.g., Stevens et al., 2007; Hwang et al., 2010; Hallquist et al., 2018; Chung et al., 2020; Wang et al., 2020; Thompson et al., 2021). Lastly, researchers have used diffusion tensor imaging (DTI), cortical thickness, and cortical surface area to assess how neural architecture relates to development of motor response inhibition (e.g., Liston et al., 2006; Madsen et al., 2010; Curley et al., 2018; Fosco et al., 2019; Murner-Lavanchy et al., 2020).
Despite ongoing debate regarding the neurobiological mechanisms of motor response inhibition, several general patterns are evident from the adult literature. The most commonly studied region is the right inferior frontal gyrus (rIFG), which appears to play a critical role in enacting motor response inhibition through striatal activation (e.g., Aron et al., 2003; Aron et al., 2004; Chambers et al., 2009; Hampshire, 2015; Wessel & Aron, 2017; Puiu et al., 2020). Within the rIFG, the anterior and posterior regions appear to play specific roles in preparing and enacting response inhibition, respectively (Chikazoe et al., 2009). The pre-supplemental motor area (preSMA) seems to prepare the motor response that is then enacted by the IFG (Mostofsky & Simmonds, 2008). Other regions of interest include the parietal cortex, dorsolateral prefrontal cortex (dlPFC), insula, and anterior cingulate (ACC). These areas enable preparation, performance monitoring, and salience detection, which may influence inhibitory control (Erika-Florence et al., 2014; Hampshire, 2015; Puiu et al., 2020). While neural activity is observed to be generally right-lateralized in the SST literature (e.g., Boehler et al., 2010), the same patterns are seen to a lesser extent in the GNG literature (e.g., Rubia et al., 2001; Dambacher et al., 2014). In addition to the aforementioned regions like prefrontal cortex, striatum, and thalamus, regions association with fixation, pursuit and reflexive systems like the frontal and supplementary eye fields are activated during AST performance (Munoz & Everling, 2004; Brown et al., 2006; Luna et al., 2008).
Neural maturation in adolescence is generally reflected by changes in the structural configurations of several tissue classes as well as changes in functional activity and connectivity. In terms of structural change, decreased gray matter volume and thickness and increased cortical surface area are observed as unused synapses are pruned along a posterior-to-anterior gradient (Gogtay et al., 2004; Gogtay & Thompson, 2010). DTI measures can estimate white matter maturity. Changes in myelination, thickness, and directional organization of white matter tracts in cortical and subcortical regions have been observed throughout adolescence (Lebel et al., 2016). Neural changes in either gray or white matter may drive changes in functional connectivity and regional activations, which can then be measured through functional MRI via the BOLD signal.
While comparisons to adult activity can reveal immaturities in neural activation, it is challenging to interpret developmental differences in BOLD activity as it could be related to a variety of factors including neural maturation, resource allocation and efficiency, or strategy use (Luna et al., 2010). Researchers can address these concerns through use of tracking procedures within tasks to approximate equivalent task difficulty (e.g., Verbruggen et al., 2019), tasks that employ rapid reactions that are resistant to use of strategy (Constantinidis & Luna, 2019), or use a trial-specific approach in contrasts (Luna et al., 2010). Lastly, fMRI during the task or at rest can provide functional connectivity estimates that reflect the strength of various networks or, in the case of causality analysis, the relative effect of each network on the other (e.g., Stevens et al., 2007). While network organization is generally intact by the beginning of adolescence, regional involvement in networks and between-network connectivity appear to change during adolescence (Stevens, 2016).
4.1. Neural Correlates of Age-Related Variations in Stop Signal Task Performance
Initial studies from large ongoing projects (ABCD, IMAGEN) offer opportunities for groundbreaking advancements in the understanding of SST performance changes in adolescence. A major study from the IMAGEN consortium created a foundation for future work exploring neural activity during response inhibition in adolescents. Independent component analysis (ICA) of activity during successful Stop trials and unsuccessful Stop trials from 1,896 14-year-olds identified separate subcortical, frontal, parietal, and motor networks (Whelan et al., 2012). These networks are similar to those identified in the more extensive literature of adult SST performance (Zhang & Li, 2012). A recent IMAGEN study identified how differences in development of SSRT over a five-year period was related to the functional connectivity subcortical-ventral attention network (Wang et al., 2020).
Several studies explored gray and white matter correlates of adolescent response inhibition development (Table 3). In a study of 227 healthy emerging adults (ages 18–24), linear declines in SSRT were related to increased gray matter volume in the rIFG and SMA and decreased gray matter volume in the anterior cingulate cortex (Wang et al., 2016). In a longitudinal study of healthy children and adolescents (up to four timepoints, baseline N = 110; ages 4–13), age-related change in SSRT was related to the surface area, but not cortical thickness, of the bilateral pars opercularis (IFG), and this effect was maximal in the right hemisphere (Curley et al., 2018). While this study provided evidence that IFG pruning was associated with developmental changes in response inhibition, effects may have been primarily influenced by the youngest participants. Two research groups have explored white matter changes related to response inhibition as measured by the SST in adolescent development. In a cross-sectional evaluation of their baseline cohort (ages 7–13, N = 92), Madsen et al. found that SSRT was negatively correlated with the fractional anisotropy (FA) of the rIFG and right preSMA, but only the FA of the left IFG mediated the negative association between age and SSRT (2010). Low FA reflects relative decreases in the directional organization of white matter. Longitudinal analysis of the same cohort after up to nine assessments across 5.5 years (total age range 7–18; 576 total assessments) found different age-related patterns from the initial cross-sectional findings (Madsen et al., 2020). Longitudinal analysis showed that the age-related improvements in SSRT were related to maturational increases in the FA of the right preSMA. Moreover, the baseline FA of the preSMA was related to the rate of change, with lower baseline preSMA FA predicting faster improvements before a plateau around 13–14 years old (Madsen et al., 2020). In a separate longitudinal study of healthy adolescents (ages 9–16, baseline N = 112, up to three annual assessments), no relationships were found between FA or mean diffusivity of tested white matter pathways and age despite linear decreases in SSRT with age (Mürner-Lavanchy et al., 2020).
Table 3.
Overview of reviewed study methodology and neuroimaging findings.
| Author | Year | Variant | Age Range (N) | Design | Imaging Method | Contrasts | Imaging Age Increases | Imaging Age Decreases |
| Standard Versions | ||||||||
| Stop Signal Task | ||||||||
| Rubia | 2000 | SST-NoT | 12 – 19 (9) 20 – 40 (8) |
C | b-fMRI | Stop – Control | L MFG, L IFG/INS | R caudate, R IFG |
| Rubia | 2007 | SST | 10 – 17 (26) 20 – 42 (21) |
C | e-fMRI | Stop – Fail Fail - Go |
B IPC, L MdPFC, L STG, THA, INS, caudate, cerebellum dACC, R premotor, frontal pole, R PCC |
R INS/premotor, R STG, L THA/MTG/PCC, putamen, R precuneus/PCC, B IPL L THA, B BG, B PCC, R PL |
| Cohen | 2010 | SST | 9 – 19 (27) 25 – 30 (9) |
C | e-fMRI | Go – fixation Stop – Go Stop - Fail |
n.s n.s. n.s. |
n.s. L MdPFC, L dACC n.s. |
| Madsenc | 2010 | SST-C | 7 – 12 (65) | C | DTI | L IFG FA ∼ SSRT | ||
| Weathers | 2012 | SST | 13.8 (SD = 2.0; 21) 35.2 (SD = 8.1; 29)* |
C | e-FMRI | Fail – Go | R ACC | |
| Rubia | 2013 | SST | 13–45 (66) | C | e-fMRI | Stop – Go | RIFC, R vmPFC/ACC, R premotor, L MFG, B THA, B BG, L temporal, R PL, L precuneus, L OCC/precuneus/PCC, B Cerebellum (lateral) | B OFC, B vmPFC, R SFC/SMA, P INS/VS, B temporal lobe, PCC, R precuneus, medial cerebellum |
| Ware | 2015 | SST-NoT | 13–16 (21)* | C | e-fMRI | All Stop – Go | L BG | |
| Curley | 2018 | SST-C | 6.9 (SD = 1.6; 110) | L (4:1 yr) | Brain Structure | Cortical Thickness Cortical Surface Area |
n.s. SSRT or age effects L pars opercularis ∼ SSRT) |
|
| Cai | 2019 | SST | 9 – 12 (38) 18 – 39 (42) |
C | e-fMRI | Stop – Go | R STN ∼ SSRT | |
| Madsenc | 2020 | SST-C | 7 – 12 (88) | L (9:0.5–2 yrs) | DTI | FA of R preSMA ∼ SSRT | ||
| Mürner-Lavancy | 2020 | SST-C | 9 – 12 (125) | L (3:1 yr) | DTI | n.s. age effects related to SSRT | ||
| Roe | 2021 | SST | 8 – 17 (232) | C | e-fMRI | Stop - Fail | R preSMA | |
| Author | Year | Variant | Age Range (N) | Design | Imaging Method | Contrasts | Imaging Age Increases | Imaging Age Decreases |
| Go/No-Go | ||||||||
| Casey | 1997 | GNG | 7 – 12 (9) 21 – 24 (9) |
C | e-fMRI; volume of activity | No-Go - Go | dlPFC, vlPFC | |
| Booth | 2002 | GNG-EQ | 9 – 11 (12) 20 – 30 (12) |
C | b-fMRI | No-Go - Go | R SFG, L BG, cingulate, HP/AMY, R INS, R MdFG, L THA | |
| Bunge | 2002 | GNG-F | 8 – 12 (16) 19 – 33 (16) |
C | e-fMRI | No-Go - neutral | B IFG, L MFG, R SFG, ACC, PCC, L AG, R MTG, R STG | R MdFG |
| Durston | 2002 | GNG | 6 – 10 (10) Adults (10) |
C | e-fMRI | No-Go - Go | n.s.age effects | B vlPFC, R dlPFC, R PL |
| Tamm | 2002 | GNG-EQ | 8 – 20 (19) | C | b-fMRI | GNG – Go | L IFG/INS, L OFG | L SFG, L MFG, cingulate |
| Durston | 2006 | GNG-SL | 8 – 14 (14) | L (2:2 yrs) | e-fMRI | No-Go - Go | R IFG | R dlPFC |
| Liston | 2006 | GNG | 7 – 15 (9) 18 – 31 (6) |
C | DTI | Frontostriatal rRD, ∼ RT | ||
| Rubia | 2006 | GNG | 10 – 17 (29) 20 – 43 (23) |
C | e-fMRI | No-Go – Go | R ACC, R OFG, R caudate | |
| Stevens | 2007 | GNG | 11 – 17 (25) 18 – 37 (25) |
C | e-fMRI | ICA | Frontostriatal network strength + influence | |
| Barber | 2013 | GNG-WM | 8 – 12 (24) 18 – 47 (27) |
C | e-fMRI + rs-fMRI | Seeded | Within-network conn of L dlPFC in TPN; MdPFC and R PHG in TNN Between-network anticorrelation of: R AI/IFG, B IPL, B SPL, PCC ∼ CE |
|
| Silveri | 2013 | GNG | 12 – 14 (20) 18 – 24 (30) |
C | MRS | GABA in ACC, ∼ CE | ||
| Spielberg | 2015 | GNG | Female: 11.3 (SD = 0.7; 28) Male: 12.2 (SD = 0.6; 35) |
L (2:1 yr) | b-fMRI | No-Go - Go | dACC, L dlPFC Low SES females: R ACC ∼ CE |
Low SES F: ACC-dlPFC conn. |
| McCormick | 2016 | GNG | 14.4 (SD = 0.3; 20) | L (2:1 yr) | e-fMRI | No-Go trials × time | Neg family relationship × time ∼ L vlPFC | |
| Author | Year | Variant | Age Range (N) | Design | Imaging Method | Contrasts | Imaging Age Increases | Imaging Age Decreases |
| Chung | 2020 | GNG | 12 – 25 (130) | C | e-fMRI | No-Go ICA | R MFG within-network conn. | R SPL within-network conn. Females only: B SPL within-network |
| Cope | 2020 | GNG | 7 – 12 (117) | L (9:1.5 yrs) | e-fMRI | No-Go – fixation | B OCC, B MdFG, B IFG, L STG, B MFG, B PCG, L SPL | n.s. |
| Antisaccade Task | ||||||||
| Velanovab | 2008 | AS | 8 – 12 (35) 13 – 17 (35) 18 – 27 (28) |
C | b-fMRI e-fMRI |
AS errors – other trials | Posterior PL and OCC | dACC |
| Velanovab | 2009 | AS | 8 – 12 (35) 13 – 17 (35) 18 – 27 (28) |
C | b-fMRI + e-fMRI | Sustained activity AS - Fixation |
B MFG, R STG, B lingual gyrus 13–17 peak in R IPL/postcentral gyrus |
B precuneus, OCC, MTG, B PHG, R THA R FG, paracentral lobe |
| Hwangb | 2010 | AS | 8 – 12 (35) 13 – 17 (35) 18 – 27 (28) |
C | e-fMRI | Granger causality | Short-range connections in parietal cortex Frontal influencing connections ACC—R IFG rMFG—R INS |
B SMG—R IPS R IPS—B SPL |
| Tamnes | 2010 | AS | 8 – 10 (20) 11 – 13 (24) 14 – 16 (26) 17 – 19 (28) |
C | Brain Structure | Cortical Thickness | L pericalcarine, R cuneus | |
| Ordazb | 2013 | AS | 8 – 27 (98) | L (6:1 yr) | e-fMRI | Trial × time | dACC during corrected errors ∼ accuracy | |
| Alahyane | 2014 | AS | 8 – 12 (31) 13 – 17 (25) 18 – 25 (23) |
C | e-fMRI | Correct AS - baseline | Prep only: PEF, ACC | |
| Marekd | 2015 | AS | 10 – 12 (41) 13 – 15 (41) 16 – 19 (53) 20 – 26 (57) |
C | rs-fMRI | CO/SN strength ∼ AS RT; 1/age Frontoparietal network, visual network: quadratic + linear age relationships |
||
| Author | Year | Variant | Age Range (N) | Design | Imaging Method | Contrasts | Imaging Age Increases | Imaging Age Decreases |
| Emotional and Rewarded Task Variants | ||||||||
| Go/No-Go | ||||||||
| Hare | 2008 | GNG-E, SL | 7 – 12 (12) 13 – 18 (24) 19 – 32 (24) |
C | e-fMRI | Emotion-specific No-Go – Go |
AMY (Adolescent Peak) | |
| Somerville | 2011 | GNG-E, SL | 6 – 12 (18) 13 – 17 (19) 18 – 29 (25) |
C | e-fMRI | Emotion × No-Go – Go PPI |
VS (Adolescent Peak) DS- R IFG |
Right IFG VS – R IFG |
| Dreyfuss | 2014 | GNG-E | 6 – 12 (18) 13 – 17 (19) 18 + (20) |
C | e-FMRI | Age × emotion × trial | R IFG, R ACC, L PM | 13–17 specific: L OFC and mdPFC to fear |
| Cohen | 2016 | GNG-E | 13 – 17 (41) 18 – 21 (35) 22 – 25 (34) |
C | b-fMRI e-fMRI |
Parietal in threat + excitement conditions | vmPFC in threat condition | |
| Antisaccade Task | ||||||||
| Geier | 2010 | AS-R | 13 – 17 (22) 18 – 30 (16) |
C | e-fMRI | Reward – Neutral | Reward-specific VS | |
| Padmanabhan | 2011 | AS-R | 8 – 13 (10) 14 – 17 (10) 18 – 25 (10) |
C | e-fMRI | Reward - Neutral (correct trials) | IPS, putamen, VS | |
| Tervo-Clemmense | 2017 | AS-R | 12 – 21 (116) | L (2:1 yr) | e-fMRI | Task – fixation | NAcc during preparation | |
| Hallquist | 2018 | AS-R | 10 – 25 (140) | C | e-fMRI | ICA | Cue phase: DAN | Cue phase: SN Prep: visual network Prep: DMN/valuation network conn |
| Quache | 2020 | AS-R | 12 – 21 (94) | L (3:1 yr) | e-fMRI | Epochs (cue, prep, response) | General ability × age ∼ L precuneus, R INS (during response phase) SES × age ∼ L AG (during cue phase) |
IQ × age ∼ rMFG, ∼ acc SES × age ∼ IPL Sex × age ∼ PL (during prep phase) General ability × age ∼ precuneus (during prep phase) |
| Ravindranath | 2020 | AS – E | 14 – 31 (66) | C | e-fMRI rs-fMRI |
F. Conn. | AMY – L ACC, B precuneus, B mPFC, B dlPFC, R INS, R THA, PL AMY ∼ OCC, Cerebellum | |
Findings reflect data from only the healthy subsample of larger studies
refers to studies with same samples
Variant: C: CANTAB version, E: Emotional variant, EQ: equiprobable Go/No-Go, F: Flanker stimuli; NoT: without tracking procedure (SST only), R: reward conditions, RP: reward and punishment conditions, SL: slow (ISI > 4000 ms for GNG), WM: working memory adaptation
Design: C: Cross-sectional; L (a:b) where a- timepoints and b- years apart
Imaging methods: b-fMRI: block-based fMRI, e-fMRI: event-related fMRI, DTI: diffusion tensor imaging, GMV: Gray matter volume, MRS: magnetic resonance spectroscopy, rs-fMRI: resting state fMRI
Contrasts: Fail: Failure on Stop/No-Go Trial, ICA: Independent Component Analysis, Stop: (Successful) Stop trial
Brain Regions: ∼: related to behavioral performance, ACC: anterior cingulate cortex, AG: angular gyrus, AI: anterior insula, AMY: amygdala, B: bilateral, BG: basal ganglia, BN: between-network, DAN: dorsal attention network, HP: hippocampus, IFG: inferior frontal gyrus, INS: insula, IPL: inferior parietal lobe, IPS: intraparietal sulcus, L: left, MFG: middle frontal gyrus, MdFG: medial frontal gyrus, MdPFC: medial prefrontal cortex, MTG: middle temporal gyrus, NAcc: Nucleus Accumbens, OCC: Occipital cortex, OFG: orbitofrontal gyrus, PCC: posterior cingulate cortex, PHG: parahippocampal gyrus, PL: parietal lobe, PM: premotor cortex, R: right, S: superior, SFC: superior frontal cortex, SFG: superior frontal gyrus, SN: salience network, STG: superior temporal gyrus, THA: thalamus, TNN: task-negative network, TPN: task-positive network, vmPFC: ventromedial prefrontal cortex, VS: ventral striatum, WN: within-network
The most common contrast used in the reviewed fMRI studies compared failed Stop trials against correct Go trials to isolate effects associated with response inhibition versus the processing of a salient stimulus. One study observed that compared to adults (ages 20–42, n = 21), adolescents (ages 10–17, n = 26) showed decreased activity in the dACC (Rubia et al., 2007). Age was positively correlated with activity in the frontal pole and posterior cingulate cortex, while age was negatively correlated with activity in the left thalamus, basal ganglia, and parietal cortex (Rubia et al., 2007). In another small study, there were no activation differences observed between healthy adults (ages 25–30, n = 9) and adolescents (ages 9–19, n = 27) as both showed activity in several right-lateralized frontal, parietal and temporal regions (though not the rIFG; Cohen, 2010). While adolescents (ages 8 – 17, N = 232) generally showed typical adult-like patterns of activity during the SST based on literature-derived maps (Neta et al., 2015), right preSMA activity significantly increased with age (Roe et al., 2021).
Comparing successful inhibition trials and correct Go trials theoretically isolates activation related to processing infrequent stimuli and inhibiting motor responses. A small study using an equiprobable Stop Signal frequency found that compared to adults (ages 20–40, n = 8), adolescents (ages 12–19, n = 9) showed less activity in the left middle and inferior frontal gyri and insula and greater activity in the right caudate and rIFG (Rubia et al., 2000). In a study that utilized a more standard SST, both adults (ages 25–30, n = 9) and adolescents (ages 9–19, n = 27) showed activation in frontal, striatal, parietal, and temporal regions that related to SSRT, and the only significant age relationships were decreasing activity in the left medial prefrontal cortex and left dACC during successful inhibition (Cohen, 2010). Using a broader age range of participants (ages 13–45, N = 66), Rubia et al. found that when controlling for sex, age was positively related to activity in the inferior and medial prefrontal cortices, right ACC, striatum, parietal and temporal regions, and lateral cerebellum (2013). Age was negatively correlated with activity in the bilateral OFC and vmPFC, right superior frontal lobe, supplementary motor cortex, and posterior insula extending to the ventral striatum (Rubia et al., 2013). In a study that compared all Stop trials against Go trials, Ware et al. (2015) found that among healthy adolescents (ages 13–16, N = 21), age was negatively related to basal ganglia activity. Overall, results of these studies that examined either successful and failed inhibition trials are mixed and even conflicting at times, which may relate to difference in the choice of contrast, variations in age ranges tested, and small sample sizes.
While less studied, comparing successful Stop trials against failed Stop trials may also isolate activation related to successful response inhibition. Rubia et al. found that healthy adults (ages 20–42, n = 21) had greater activation in the rIFC than adolescents (ages 10–17, n = 26) and that age was related positively to activity in the bilateral inferior PFC, thalamus, insula, and caudate and negatively related to activity in more posterior regions of the insula, the putamen, and posterior cingulate cortex (2007). In a similar study, Cohen did not find any significant age relationships with the same contrast (ages 9–30, N = 76; 2010). That study also examined Go trial activation by comparing it to a fixation state. While activation in frontal regions related to GoRT and response time variability, Go trial activation was unrelated to age (Cohen, 2010). In another study (Thompson et al., 2021), age-related decreases in reaction time variability were associated with increasing variance in bilateral thalamus activity among young adolescents (ages 10–12, n = 19) and adults (ages 18–26, n = 26), indicating that BOLD signal variability may be more developmentally sensitive than overall signal magnitude. Researchers have also looked at functional connectivity during the entire task and found that the strength of correlation between a ventral attention network and a subcortical network predicted improvements in SSRT from age 14 to 19 (n = 326; Wang et al., 2020).
Overall, results from functional neuroimaging studies using the SST to explore maturation of response inhibition in adolescence are mixed. On the other hand, studies using structural and longitudinal methods have identified common patterns, such as development of gray matter and white matter tracts of the right inferior frontal cortex that relate to changes in SSRT in adolescence (Curley et al., 2018; Madsen et al., 2020). While event-related fMRI findings are also mixed, regions involved in attention, motor planning, and reward, such as the ACC, parietal cortex, striatum, insula, and prefrontal cortex, including bilateral IFG, appear to be promising targets for future research.
4.2. Neural Correlates of Age-Related Variations in Go/No-Go Performance
While many studies have looked at neural correlates of GNG performance (e.g., Criaud & Boulinguez, 2013; see Table 3), only a subset specifically explored age-related associations or how relationships among neural properties reflect task performance in healthy adolescents. The majority of reviewed studies use task-based fMRI, but two employed other MRI methods. In a small (ages 7–31, N = 15) DTI study, radial diffusivity of frontostriatal tracts was negatively correlated with Go RTs and positively related to age (Liston et al., 2006), indicating that myelination of the frontostriatal circuits is associated with improvements in executive processing speed. In a spectroscopy study of healthy adolescents and adults (ages 12–25, N = 70), GABA levels in the anterior cingulate cortex (ACC) were lower in adolescents than adults, providing further evidence that frontal development may be associated with improvements in adolescent response inhibition (Silveri et al., 2013).
Early block-based fMRI studies of the GNG measured averaged signals across the task (Table 3). In a study comparing children and young adolescents (ages 7–12, n = 9) with young adults (ages 21–24, n = 9), activity differences between No-Go and Go blocks were seen in the ACC, inferior frontal gyrus (IFG), and other regions of the frontal lobe (Casey et al., 1997b). Children and young adolescents showed different patterns of magnitude and spatial extent of activation in ACC, IFG, and other frontal regional activity during No-Go and Go blocks, and the extent of activation in the frontal cortex during the faster task increased with age (Casey et al., 1997b). A similar block-based study of a typically developing sample (ages 8 – 20, N = 19) found inhibition-related activation in bilateral middle and inferior frontal gyri, frontal regions, the insula, and ACC. Activity in the lIfG extending to the insula and the orbital frontal cortex increased with age, while activity in the left superior and middle frontal gyrus decreased with age (Tamm et al., 2002). Across blocks of a GNG, adolescents (ages 9–11, n = 12) showed greater activity in frontal and subcortical regions than adults (ages 20–30, n = 12) and a similar overall pattern, which may reflect increasing specialization with age (Booth et al., 2002). In an event-related study comparing children (ages 7–12, n = 12), adolescents (ages 13–18, n = 24), and adults (ages 19–32, n = 24), amygdala activity and connectivity with the prefrontal cortex showed specific adolescent peaks and associations with the processing of negative emotional stimuli (Hare et al., 2008). Using the same design in an expanded sample, Somerville et al. found that compared to children (ages 6–12, n = 18) and adults (ages 18–29, n = 25), adolescents (ages 13–17, n = 19) demonstrated a peak in ventral striatal activity when evaluating negative stimuli. Moreover, connectivity of the rIFG shifted from the dorsal to ventral striatum with age (Somerville et al., 2011). However, each of these studies used small sample sizes and task designs with equiprobable Go and No-Go stimuli (Tamm et al., 2002; Booth et al., 2003) as well as relatively slow rates of stimulus presentation (mean ISI = 5.2 sec in Somerville et al. and Hare et al., 2008), potentially impacting the extent to which the tasks strongly demanded inhibitory processes.
Another event-related study found that activation in children (ages 8–12, n = 16) was limited to the middle frontal gyrus and rIFG. Adults (ages 19–33, n = 16) showed greater activation than children in frontal regions including the bilateral IFG and ACC, while children showed greater activation in the right pre-SMA and diffuse areas of activity that related to CEs (Bunge et al., 2002). In a comparison of healthy adolescents (ages 10–17, n = 29) and adults (ages 20–43, n = 23), Rubia et al. found that adults had greater activity in the ACC and caudate, which could be related to better preparation and performance monitoring (2006). More recent event-related studies found similar mean activations across healthy young adolescents (age M = 11.3, SD = 0.72 years, N = 63) in the dACC, left IFG, right dlPFC, and bilateral PFC regions (Spielberg et al., 2015). A longitudinal study comparing activation between baseline and a two-year follow-up found that overall activation increased in the dACC and left dlPFC, with specific sex-interactions showing that in females, coupling between the ACC and dlPFC was related to accuracy and socioeconomic status (Spielberg et al., 2015). In a larger study (ages 13–25, N = 110) using an emotional GNG task, parietal activity was positively correlated with age in threat and excitement (but not neutral) conditions. Ventromedial prefrontal cortex (vmPFC) activity was increased during the threat condition in children and adolescents, but not adults (Cohen et al., 2016).
In another longitudinal study (baseline ages 7 −12, up to 8 follow-ups 1.5 years apart, N = 117), age was positively associated with increased activity in several inhibitory control regions (IFG, preSMA) as well as other regions in the frontal, parietal, temporal, and occipital lobes. Linear models provided better fit than either quadratic or cubic ones for each area (Cope et al., 2020). In a small longitudinal study of healthy young adolescents (Group 1: n = 7, 9.0 (SD=1.2) years old, follow-up interval 2.1 (0.6) years; Group 2: n = 7, 12.1 (SD=2.0) years old), age was associated with decreased dlPFC activity and increased rIFG activity (Durston et al., 2006).
A growing body of research has explored GNG activation in emotional contexts. Adolescents (ages 13–17, n = 19) showed greater activation in the orbitofrontal and middle PFC in response to fearful faces than adults (ages 18 +, n = 20) or children (ages 6–12, n = 18) but otherwise demonstrated adult-like activation patterns across conditions (Dreyfuss et al., 2014). Compared to adults (ages 22–25, n = 34), both adolescents (ages 13–17, n = 41) and younger adults (ages 18–21, n = 35) showed sustained activation of the vmPFC during a threat condition, providing additional evidence of adolescent-specific threat sensitivity that may interfere with cognitive control during emotional contexts (Cohen et al., 2016). In a longitudinal study of 20 adolescents at ages 14 and 15, age-related increases in IFG activation over one year were mediated by poor family relationships (McCormick et al., 2016).
Several studies have explored connectivity measures related to GNG performance. Using task-based ICA with 50 participants, Stevens et al. identified three hierarchical and interdependent networks (fronto-striatal-thalamic, parietal-premotor, frontal-parietal) and found that compared to adults (ages 18 – 37), adolescents (ages 11–17) had less activity in the frontal-parietal network, less engagement of the rIFG, left putamen, and thalamus in the fronto-striatal-thalamic network, greater performance costs of monitoring, greater involvement of the right IFG, and less influence of the frontal regions over the striatum (2007). These results were consistent with event-related findings showing increased activity of the IFG in adolescence as executive functions are not fully mature. In a resting-state study of children (ages 8–12, n = 24) and adults (ages 18–47, n = 27), adults showed greater within-network and between-network connectivity of a task-positive network and the default mode network, and the between-network connectivity predicted GNG commission errors (Barber et al., 2013). In a task-based ICA study with 130 healthy participants (ages 12–25), within-network connectivity of the bilateral superior parietal lobe (SPL) and rostral middle frontal gyrus during correct responses increased with age, while connectivity of the right superior parietal lobe component decreased with age (Chung et al., 2020). Unlike males, females showed age-related change in components involving the left IFG and bilateral SPL, suggesting that mechanisms of development may vary by sex (Chung et al., 2020).
Overall, while heterogeneity between GNG task designs, contrasts, and samples limits generalizability, several patterns are evident. Studies employing a variety of neuroimaging techniques have identified age-related increases in efficiency and activity of frontostriatal regions (Durston et al., 2006; Liston et al., 2006; Rubia et al., 2006; Steven et al., 2007; Cope et al., 2020) and the ACC during motor response inhibition across adolescent development (Bunge et al., 2002; Tamm et al., 2002; Silveri et al., 2013; Spielberg et al., 2015). Several studies found that when confronted by emotional contexts, especially negative ones, adolescents showed relatively greater activation in regions related to sustained attention and emotion processing such as the OFC and vmPFC (Cohen-Gilbert & Thomas, 2013; Dreyfuss et al., 2014; Cohen et al., 2016). Taken together, these patterns suggest greater neural efficiency as motor response inhibition matures across adolescence.
4.3. Neural Correlates of Age-Related Variations in Antisaccade Task Performance
The majority of research exploring adolescent development of response inhibition as measured by the AST has emerged from a single lab at the University of Pittsburgh. In the only structure-based MRI study using the AST, among 98 healthy adolescents (ages 8–19), better accuracy on antisaccade trials was associated with more pronounced cortical thinning in bilateral parietal and occipital regions (though not prefrontal regions; Tamnes et al., 2010). In the first of four studies using a longitudinal sample of 98 healthy individuals (ages 8–27), Velanova et al. explored event-related differences in activation of selected regions of interest across children (ages 8–12, n = 35), adolescents (ages 13–17, n = 35), and young adults (ages 18–27, n = 28; 2008). Across groups, activation during AST performance was observed in the SMA/preSMA, frontal eye field, PPC, and striatum, and correctly executed ASs showed greater activity in the preSMA and less activity in the IFG and ACC than direction error trials, indicating that basic oculomotor circuitry was intact by late childhood (Velanova et al., 2008). Both children and adolescents showed decreased dACC modulation to incorrect AS trials compared to correct AS trials and greater activity in frontal regions overall. Across subsequent block- and event-based analysis of AST performance, it appeared that while transient activation in primary response inhibition regions (IFG, preSMA, striatum) was mostly mature by adolescence, incremental behavioral improvements were supported by greater sustained activity in regions involved in attention and executive control (Velanova et al., 2008; 2009). Granger causality analysis identified that while adolescents showed adult-like short-range effective connectivity, long-distance connectivity was immature. Adolescents showed a relatively decreased influence of frontal regions (ACC, rMFG, rIFG, FEF, left precuneus) on sensorimotor regions (intraparietal sulcus, thalamus, cerebellum) as well as greater effective connectivity from the SMG to the IPS (Hwang et al., 2010), suggesting that short-range connections were mature by adolescence, while long-distance (e.g., ACC-rIFG) effective and functional connectivity increased with age. Longitudinal analysis of the same sample after up to six annual visits (302 total visits) based on event-related data (separated by trial type) with hierarchical linear regression to test the fixed and random effects of various age models (Ordaz et al., 2013) identified three regions that showed developmental changes in activation—the right dlPFC and left frontal eye field during correct AS trials and the dACC during corrected error trials. Asymptotic (1/age) improvements in task performance were mediated by error-related dACC activity (Ordaz et al., 2013). In a separate sample, Alahyane et al. found that while children (ages 8–12, n = 31), adolescents (ages 13–17, n = 25), and adults (ages 18–25, n = 23) showed similar activity during catch trials and AS trials, differences during preparation trials in the parietal eye field (adolescents vs. adults) and the frontal eye field and ACC (children vs. others) indicated that behavioral improvements during adolescence are supported by preparation but not execution of response inhibition (2014).
While the above-referenced work established basic patterns of AST activation during standard neutral conditions, subsequent efforts have focused on how incentivization through reward and punishment contributes to inhibitory control. While overall activation during an AST with reward and neutral conditions was similar between children (ages 8–13, n = 10), adolescents (ages 14–17, n = 10), and adults (ages 18–25, n = 10), adolescents demonstrated relative increases in activation in the ventral striatum, intraparietal sulcus, and putamen to rewarded trials versus neutral ones, while children showed greater activity in the dACC than adults (Padmanabhan et al., 2011). There were no significant group differences in the dACC for the older adolescent group. In a separate study using neutral, rewarded, and punishment conditions of the AS, adolescent (ages 13–17, n = 22) and adult (ages 18–30, n = 16) activity in inhibitory regions was similar during incentive presentations, but only adolescents showed an increase in activity in the ventral striatum in response to the preparatory cue as well as greater activity in the right medial and superior frontal gyri during cue assessment of reward trials (Geier et al., 2010). During neutral trials, adults showed greater activation in the posterior parietal cortex in preparation and decreased activity in the OFC, suggesting that adolescents rely more heavily on frontal regions during response inhibition than adults (Geier et al., 2010). In a larger study using the same reward task completed outside the scanner, graph theory analysis of resting state fMRI data from 192 healthy participants (age 10–26) found that most networks were internally stable by the beginning of adolescence, while cross-network integration of the cingulo-opercular and salience networks was best modeled by an inverse age function (Marek et al., 2015). Further, the integration of the cingulo-opercular and salience networks moderated associations between age and AS latency (Marek et al., 2015). In another sample of 140 healthy participants (ages 10–25) using a graded incentive design, adolescent-specific increases in activation were observed in ventral striatum and vmPFC in reaction to feedback and receipt of reward or punishment. Increases in activity within the dorsal attention network and salience network in response to all types of cues were observed but were not related to task performance. However, activity in salience network regions prior to the target presentation mediated age-related improvements in accuracy on AS trials (Hallquist et al., 2018). Using a variant with emotional sounds presented during AS trials, Ravindranath et al. (2020) found that amygdala connectivity with the dlPFC and ACC during negative emotional contexts increases with age, though the study may have been underpowered to identify effects of specific emotional contexts (ages 14–31, n = 66).
Using the same rewarded AST developed in the previous studies (Geier et al., 2010), the National Consortium on Adolescent Neurodevelopment and Alcohol (NCANDA) study is examining AST performance in a subsample of its larger ongoing study (ages 12–21 at baseline, annual follow-ups, n = 116) with a targeted population at risk for substance abuse (Brown et al., 2015). Using data from the first two timepoints, Tervo-Clemmens et al. found that during preparation, age was positively associated with increased activation of the nucleus accumbens to reward versus neutral cues (2017). Another study explored NCANDA data from the first three timepoints (total visits = 220), and specific main effects of age were not described, behavioral and neuroimaging data indicated that externalizing, SES, and general cognitive ability are related to developmental changes in activity of regions supporting inhibitory control and associated behaviors (e.g., posterior parietal cortex, inferior parietal lobes, and middle frontal gyrus; Quach et al., 2020).
5. Discussion
This narrative review aimed to characterize the trajectory of change in motor response inhibition, the ability to restrain an automatic or prepotent response in favor of a more goal-appropriate one (Young et al., 2009), during typical adolescent development as well as its neural correlates in order to establish a baseline from which we can identify deviations that could indicate vulnerability for impulsivity in the context of risk-taking behaviors (Cicchetti & Rogosch, 1996; Atherton, 2020). We focused on motor response inhibition due to the large behavioral and neuroimaging literature base available for analysis, relative ease of task interpretation as compared to some multifaceted measures such as temporal discounting and gambling tasks that index impulsivity at both cognitive and motor levels, and the salience of motor inhibition for adaptive behavior and momentary instances of decision-making. Our analysis allows us to draw several conclusions related to the adolescent development of motor response inhibition, its neural correlates, individual difference factors that may interact with age-related changes, and psychometrics.
5.1. Developmental Trajectory of Motor Response Inhibition
An understanding of the developmental trajectory of motor response inhibition as measured by the SST, GNG, and AST is essential for identifying possible periods of plasticity or vulnerability as well as identifying the degree to which impulsivity in the context of immediate decision-making may be attributable to immaturities as opposed to individual differences. The importance of early adolescence and mid-adolescence as a vulnerability period is supported by findings from all three tasks that suggest rapid rates of development from early- to mid-adolescence. Findings regarding whether development of motor response inhibition extends beyond mid-adolescence are more ambiguous, though rigorously conducted longitudinal studies (e.g., Ordaz et al., 2013, Madsen et al., 2020) provide evidence of nonlinear patterns where motor response inhibition under standard circumstances appears to plateau or show only very slight ongoing improvements from mid-adolescence into young adulthood. This nonlinear pattern of rapid changes in early adolescence and more modest gains in mid- to late-adolescence has also been observed for other aspects of executive functioning and cognitive control (e.g., Luciana & Collins, 2012; Taylor et al. 2013, 2015). The steep early trajectory supports that early adolescence might be considered to be a period of vulnerability for some individuals consistent with the emergence of other oppositional, perhaps impulsive, behaviors (Bjork & Pardini, 2015). Still, mortality concerns about risk taking behaviors tend to focus on late adolescence, which makes sense given that behaviors such as substance misuse, sexual experimentation, and reckless driving are most prevalent during that time (NASEM, 2020). However, based on this integration of existing data, motor response inhibition appears to have largely stabilized by this period, which introduces an interpretive complexity. As late-adolescents appear capable of adult-like motor response inhibition, the construct seems an unlikely candidate for explaining age-related increases in mortality and reactive aspects of decision-making that might characterize risk-taking behaviors during the period in which mortality is highest. It could be the case that age-specific differences in inhibitory control during more emotional or rewarding circumstances are associated with risk-taking (e.g., Constantinidis & Luna, 2019), but this hypothesis has not yet been thoroughly explored outside of the AST. An important future direction will be to examine how individual difference factors interact with age-related variations in performance to influence decision-making and risk-taking vulnerabilities (Bjork & Pardini, 2015). Studying such individual differences requires large, longitudinal samples, and existing large-scale studies such as ABCD and IMAGEN offer promising opportunities to explore these questions.
5.2. Neural Correlates of Motor Response Inhibition Development
Neural correlates of motor response inhibition performance identify similar patterns across the tasks and regions that support motor aspects of inhibitory control as it develops. While event-related neuroimaging studies of response inhibition development in adolescence raise challenges in interpretation (Luna et al., 2010), successful inhibition in the SST, GNG and AST elicits activity in common regions of the inhibitory control networks that have been established in the adult literature (Stevens et al., 2007). Similar to adults, adolescents show activation in the rIFG, preSMA, ventral striatum, and prefrontal regions when engaging in response inhibition (Roe et al., 2021), suggesting that the basic circuitry of inhibitory control is established by adolescence. Changes in activation patterns across these characteristic inhibitory network regions (e.g., Rubia et al., 2013, Velanova et al., 2008) likely reflect maturation and increased efficiency of the basic function of inhibitory control. Other regions that show age-related changes, such as the dACC, vmPFC, and portions of the parietal cortex, may support task engagement by enabling sustained attention, motivation, or error monitoring (e.g., Spielberg et al., 2015; Marek et al., 2015, Curley et al., 2018). While the heterogeneity in tested neural regions, task designs, and contrasts elicit some isolated findings in specific studies, there appears to be a reasonable degree of consistency in identifying regions that are related to age-related change in response inhibition. In parallel with behavioral approaches, exploring various age-related trajectories including nonlinear patterns may be fruitful in neuroimaging analyses as associated regions and tissue classes have shown heterogeneous age-related changes (Sowell et al., 2001; Gogtay & Thompson, 2010).
5.3. Individual Differences and Vulnerabilities in the Context of Psychopathology
This characterization of the trajectory and range of individual differences in the neurobehavioral development of motor response inhibition among healthy individuals provides a framework from which vulnerability for emerging psychopathology can be explored. Within the context of various developmental and externalizing disorders, notably attention-deficit/hyperactivity disorder (ADHD) and substance use disorders, relatively poor motor response inhibition has been observed (Lipsyck & Wright, 2010; Wright al., 2014; Constantinidis & Luna, 2019). One hypothesis is that individual deviations from the typical developmental course, notably delayed development (Shaw et al., 2007; Rubia et al., 2014), may confer vulnerability for pathology. Currently, the field lacks comprehensive norms for performance on measures such as the SST, GNG, and AST. The use of these tasks within large-scale national and international consortium studies as described above might facilitate the generation of such norms. If so, inferences could then be made about the patterns and extent of delayed development, if applicable, that could ultimately be used to guide interpretations in individual cases. Conditions such as ADHD are manifested early in childhood and thus, as affected children age into adolescence, premorbid psychopathology and associated disruptions within cognitive control circuitry may represent risk factors for motor response inhibition difficulties especially in early-to-mid adolescence when this ability is most rapidly maturing and as vulnerable youth are faced with momentary decisions in risk-taking contexts. Whether these youth are those that are most likely to engage in impulsive forms of risk-taking, including substance use, due to poor motor control is debated (Molina & Pelham, 2014) as are the relevant neural mechanisms that contribute to such comorbidities (Paraskevopoulou et al., 2021). To the extent that motor inhibition is dysfunctional, targeted efforts at prevention and intervention, such as cognitive training and psychoeducation, may be most fruitfully applied during this time to provide vulnerable youth with support as they navigate this developmental transition.
5.4. Sex Differences in Motor Response Inhibition Development
Given that motor response inhibition development is most notable in early adolescence, when sex differences in the timing of puberty are well-established, it is reasonable to speculate that there may be sex differences in response inhibition development, though these were not extensively covered in the context of this review. To date, no sex-specific patterns have been established, though sex differences themselves have been infrequently explored or reported outside of recent longitudinal studies (e.g. Curley et al., 2018, Fosco et al., 2019; Madsen et al., 2020, Mürner-Lavancy et al., 2020). While some studies report a lack of significant sex differences (Madsen et al., 2010; Tamnes et al., 2010; Mürner-Lavancy et al., 2020), there is limited evidence that females, especially in early adolescence, perform better and mature more rapidly than males (Curley et al., 2018; Ordaz et al., 2018; Madsen et al., 2020). Several smaller studies have found sex differences in neural processes supporting response inhibition development (Rubia et al., 2013; Chung et al., 2020). Continued assessment of sex effects on motor response inhibition may represent a fruitful focus for future studies.
5.5. Motor Response Inhibition and Common Factor Theories
The similarities between the developmental patterns and related neural regions across the SST, GNG, and AST literatures provides relatively consistent evidence of response inhibition improving rapidly during early adolescence and modest sustained development into young adulthood. Studies across the three tasks also consistently identify that the regions supporting development of response inhibition are those that comprise a well-studied adult-like response inhibition network. While some variability in task designs threaten the ability of the tasks to measure inhibition of an established prepotent response, the consistency in results across a variety of well-designed tasks indicates that the three tasks are robust to measuring developmental change in response inhibition. Different analytic approaches across studies also contributes to variability in findings within and across tasks. Group comparisons and linear analyses may not be as capable of capturing developmental variance as nonlinear functions, which demand greater sample sizes. Nonetheless, the robustness of the developmental findings within specific tasks and the similarities of behavioral and neural results between the three tasks provides some support for a common factor model of response inhibition, though the ideal evidence for such a model requires assessing multiple measures within the same sample. Indeed, empirical evidence in support of a single latent factor of motor response inhibition measured by each of these tasks appears to be minimal. Malagoli & Usai (2018) provided some evidence of a common factor model by gathering data on each task within a single adolescent sample, but only a few studies have adopted a similar approach, examining more than one of these frequently-used response inhibition tasks within discrete adolescent samples (e.g., Miyake & Friedman, 2012).
5.6. Task Reliabilities
As indicated in Table 1, several studies have reported reliabilities of individual tasks in adolescent samples, and while estimates in the reviewed literature appear to be generally acceptable, other dedicated reliability studies in adult samples have reported poor reliability for the same tasks, though antisaccade latency appears to be a reliable measure across samples (e.g., Wöstmann et al., 2013, Hedge et al., 2018). Poor reliability constrains the detection of individual differences and developmental progressions in longitudinal studies.
5.7. Guidelines for Future Research
In the short term, how, then, might researchers approach the decision of selecting which single task to use in assessing the behavioral development of motor inhibitory control? Conclusions drawn from this review do not point to a single task as superior to the others, and each task has its advantages and disadvantages (Table 4). As indicated in Table 2, the literature on adolescent behavioral development is clearly most abundant for GNG. The demand for inhibitory control is distinct across measures given that the SST requires inhibition of an executed response (e.g., response cancellation), while the AST includes anticipatory preparation for inhibition and requires a similar “stop” followed by a reversal or shift. Alternatively, the GNG, which is perhaps the least cumbersome of the tasks to administer, requires full inhibition of motor execution. Thus, each measure captures a distinct phase of the motor response execution cycle. The achievement of high-level control over this motor circuitry may be more difficult once a response has been initiated at the neural level, as demanded by the SST and AST. In terms of ease of implementation and analysis, the AST introduces considerable researcher burden and expense, as many laboratories are not prepared to implement such a measure with appropriate psychophysiological precision. To this end, for ease of implementation and adaptation, the GNG may be most suitable for researchers exploring response inhibition behaviorally, though the SST might be recommended for functional MRI studies in order to control for task difficulty. Furthermore, the SST is the only model with a well-evidenced computational model and consensus guidelines (Verbruggen et al., 2019), which may facilitate consistent analytic approaches and generalizability across studies.
Table 4.
Advantages and disadvantages of using each test to study developmental changes of response inhibition in adolescence.
| Advantages | Disadvantages | |
|---|---|---|
| Stop Signal Task | Evidence for sensitivity to age-related changes in early adolescence Strong reliability in adolescent samples Amenability to neuroimaging-based assessments Most extensive neuroimaging literature Used in large-scale neuroimaging studies (IMAGEN, ABCD) Consensus guidelines for administration and scoring Standardized theoretically-driven analysis based on independent race model Dynamic adjustments to stimuli control for effort/difficulty |
Limited paradigms for assessing impacts of reward/emotion on performance Response inhibition measures may be impacted by post-error slowing and performance monitoring High effort required of participants |
| Go/No-Go | Evidence for sensitivity to age-related changes in early and late adolescence Moderate reliability in adolescent samples Amenability to neuroimaging-based assessments Ease of administration Existing emotion paradigms Moderately extensive neuroimaging literature |
Limited use in large-scale studies Lack of consensus for administration and scoring Response inhibition measures may be impacted by decision-making processes and processing speed |
| Antisaccade | Evidence for sensitivity to age-related changes in early adolescence Moderate reliability in adolescent samples Replicated reward paradigm Neural network underpinning performance is well-understood Used in large-scale neuroimaging study (NCANDA) |
Most expensive Requires technical expertise for eye tracking Limited replication outside of one research group Lack of consensus for administration and scoring |
In summary, this review draws attention to several much-needed future directions. Understanding the neurobehavioral development of response inhibition in adolescence calls for 1) well-designed tasks and consistent analytic strategies, 2) multimodal exploration of neural correlates, 3) replication of emotional and reward variants across tasks, and 4) well-powered longitudinal studies. First, task designs should be mindful of potential barriers to assessing the intended construct (Wessel, 2018; Verbruggen et al., 2019). Consensus guidelines (Verbruggen et al., 2019) empower researchers to use and analyze SST data with confidence in their methodology, and similar guidelines for the AST and GNG would provide a positive foundation for studies using those tasks. In terms of characterizing the shape of developmental change, similar analytic approaches across studies (e.g., testing the same models, inclusion of nonlinear models) would be beneficial. Examining nonlinear patterns and individual differences will require large sample sizes and longitudinal studies, positioning existing studies like ABCD and IMAGEN as opportunities for exploring these questions. Furthermore, given the variable reliability of executive tasks (Hedge et al., 2018), studies would benefit from providing information about reliability estimates as a standard practice in publications. Information on floor and ceiling effects would also be helpful. Second, ongoing neuroimaging efforts can identify common patterns in the regions typically associated with cognitive control as well as regions that may support performance through attentional, motor, or other processes, but developmental patterns associated with changes are unclear. Functional MRI poses challenges in developmental research (e.g., Luna et al., 2010), and structural changes in gray or white matter may provide more insights into the neural mechanisms of change in adolescence that support response inhibition maturation. Third, incorporation of emotion or reward into response inhibition tasks may represent important aspects of development that relate to risk-taking behaviors (e.g., Constantinidis & Luna, 2019), yet reward and emotion paradigms have not been implemented across the tasks. Replication across a variety of sites and samples with similar tasks would provide more evidence for validity and could be a valuable avenue for understanding adolescent risk-taking behaviors. Last, well-powered longitudinal studies are essential for providing information about the developmental trajectories and individual differences in response inhibition development. Ongoing data collection through the NCANDA, ABCD, and IMAGEN consortia represent promising opportunities for understanding these processes in normative development to better characterize within-individual adolescent maturation and risk-taking behaviors. Future work exploring development from early adolescence into young adulthood in both health and high-risk samples will be informative to our understanding of typical adolescent risk-taking behavior as well as risk for psychopathology.
Highlights.
Motor response inhibition measurement in adolescence is heterogeneous.
The trajectory of motor response inhibition development in adolescence is unclear.
Affective & cognitive processes may impact motor response inhibition development.
Links between neural activity and response inhibition development are unclear.
Funding:
This work was supported by a National Science Foundation Predoctoral Fellowship awarded to H. Weiss and by the following grants to M.L. from the National Institutes of Health: 2U01DA041120) and R01MH122473. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aichert DS, Wöstmann NM, Costa A, Macare C, Wenig JR, Möller H-J, Rubia K, & Ettinger U (2012). Associations between trait impulsivity and prepotent response inhibition. Journal of Clinical and Experimental Neuropsychology, 34(10), 1016–1032. 10.1080/13803395.2012.706261 [DOI] [PubMed] [Google Scholar]
- Alahyane N, Brien DC, Coe BC, Stroman PW, & Munoz DP (2014). Developmental improvements in voluntary control of behavior: Effect of preparation in the fronto-parietal network? NeuroImage, 98, 103–117. 10.1016/j.neuroimage.2014.03.008 [DOI] [PubMed] [Google Scholar]
- Andrade MÂ, & Raposo A (2021). Underdeveloped recollection during adolescence: Semantic elaboration and inhibition as underlying mechanisms. Journal of Experimental Child Psychology, 203, 105044. 10.1016/j.jecp.2020.105044 [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, & Robbins TW (2003). Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience, 6(2), 115–116. 10.1038/nn1003 [DOI] [PubMed] [Google Scholar]
- Aron AR, & Poldrack RA (2005). The Cognitive Neuroscience of Response Inhibition: Relevance for Genetic Research in Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry, 57(11), 1285–1292. 10.1016/j.biopsych.2004.10.026 [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, & Poldrack RA (2004). Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences, 8(4), 170–177. 10.1016/j.tics.2004.02.010 [DOI] [PubMed] [Google Scholar]
- Atherton OE (2020). Typical and atypical self-regulation in adolescence: The importance of studying change over time. Social and Personality Psychology Compass, 14(1), e12514. 10.1111/spc3.12514 [DOI] [Google Scholar]
- Band GPH, & van Boxtel GJM (1999). Inhibitory motor control in stop paradigms: Review and reinterpretation of neural mechanisms. Acta Psychologica, 101(2), 179–211. 10.1016/S0001-6918(99)00005-0 [DOI] [PubMed] [Google Scholar]
- Barber AD, Caffo BS, Pekar JJ, & Mostofsky SH (2013). Developmental changes in within- and between-network connectivity between late childhood and adulthood. Neuropsychologia, 51(1), 156–167. 10.1016/j.neuropsychologia.2012.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA, (1997). Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin, 121(1), 65–94. 10.1037/0033-2909.121.1.65 [DOI] [PubMed] [Google Scholar]
- Bedard A-C, Nichols S, Barbosa JA, Schachar R, Logan GD, & Tannock R (2002). The Development of Selective Inhibitory Control Across the Life Span. Developmental Neuropsychology, 21(1), 93–111. 10.1207/S15326942DN2101_5 [DOI] [PubMed] [Google Scholar]
- Bezdjian S, Tuvblad C, Wang P, Raine A, & Baker LA (2014). Motor impulsivity during childhood and adolescence: A longitudinal biometric analysis of the go/no-go task in 9- to 18-year-old twins. Developmental Psychology, 50(11), 2549–2557. 10.1037/a0038037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett PG, Hagen MP, Jones JM, & Poldrack RA (2021). Design issues and solutions for stop-signal data from the Adolescent Brain Cognitive Development (ABCD) study. eLife, 10, 360185. 10.7554/eLife.60185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, & Pardini DA (2015). Who are those “risk-taking adolescents”? Individual differences in developmental neuroimaging research. Developmental Cognitive Neuroscience, 11, 56–64. 10.1016/j.dcn.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer B, Friedrich J, Roessner V, & Beste C (2018). Differences in response inhibition processes between adolescents and adults are modulated by sensory processes. Developmental Cognitive Neuroscience, 31, 35–45. 10.1016/j.dcn.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, & Woldorff MG (2010). Pinning down response inhibition in the brain—Conjunction analyses of the Stop-signal task. NeuroImage, 52(4), 1621–1632. 10.1016/j.neuroimage.2010.04.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehler CN, Appelbaum LG, Krebs RM, Hopf J-M, & Woldorff MG (2012). The influence of different Stop-signal response time estimation procedures on behavior–behavior and brain–behavior correlations. Behavioural Brain Research, 229(1), 123–130. 10.1016/j.bbr.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, & Mesulam MM (2003). Neural development of selective attention and response inhibition. NeuroImage, 20(2), 737–751. 10.1016/S1053-8119(03)00404-X [DOI] [PubMed] [Google Scholar]
- Brown MR, Goltz HC, Vilis T, Ford KA, & Everling S (2006). Inhibition and generation of saccades: rapid event-related fMRI of prosaccades, antisaccades, and nogo trials. Neuroimage, 33(2), 644–659. 10.1016/j.neuroimage.2006.07.002 [DOI] [PubMed] [Google Scholar]
- Brydges CR, Anderson M, Reid CL, & Fox AM (2013). Maturation of Cognitive Control: Delineating Response Inhibition and Interference Suppression. PLoS ONE, 8(7), e69826. 10.1371/journal.pone.0069826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, & Gabrieli JDE (2002). Immature Frontal Lobe Contributions to Cognitive Control in Children: Evidence from fMRI. Neuron, 33(2), 301–311. 10.1016/S0896-6273(01)00583-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell SJ, Malone SM, & Iacono WG (2016). One-year developmental stability and covariance among oddball, novelty, go/no-go, and flanker event-related potentials in adolescence: A monozygotic twin study: One-year developmental stability of ERP measures. Psychophysiology, 53(7), 991–1007. 10.1111/psyp.12646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Duberg K, Padmanabhan A, Rehert R, Bradley T, Carrion V, & Menon V (2019). Hyperdirect insula-basal-ganglia pathway and adult-like maturity of global brain responses predict inhibitory control in children. Nature Communications, 10(1), 4798. 10.1038/s41467-019-12756-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, Soules ME, Teslovich T, Dellarco DV, Garavan H, Orr CA, Wager TD, Banich MT, Speer NK, Sutherland MT, Riedel MC, Dick AS, Bjork JM, Thomas KM, … Dale AM (2018). The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Developmental Cognitive Neuroscience, 32, 43–54. 10.1016/j.dcn.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, Vauss YC, Vaituzis AC, Dickstein DP, Sarfatti SE, & Rapoport JL (1997). Implication of Right Frontostriatal Circuitry in Response Inhibition and Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 36(3), 374–383. 10.1097/00004583-199703000-00016 [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, Dahl RE, & Rapoport JL (1997). A Developmental Functional MRI Study of Prefrontal Activation during Performance of a Go-No-Go Task. Journal of Cognitive Neuroscience, 9(6), 835–847. 10.1162/jocn.1997.9.6.835 [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Milham MP, & Tannock R (2006). Characterizing cognition in ADHD: Beyond executive dysfunction. Trends in Cognitive Sciences, 10(3), 117–123. 10.1016/j.tics.2006.01.011 [DOI] [PubMed] [Google Scholar]
- Castellanos-Ryan N, Rubia K, & Conrod PJ (2011). Response Inhibition and Reward Response Bias Mediate the Predictive Relationships Between Impulsivity and Sensation Seeking and Common and Unique Variance in Conduct Disorder and Substance Misuse. Alcoholism: Clinical and Experimental Research, 35(1), 140–155. 10.1111/j.1530-0277.2010.01331.x [DOI] [PubMed] [Google Scholar]
- Castellanos-Ryan N, Struve M, Whelan R, Banaschewski T, Barker GJ, Bokde ALW, Bromberg U, Büchel C, Flor H, Fauth-Bühler M, Frouin V, Gallinat J, Gowland P, Heinz A, Lawrence C, Martinot J-L, Nees F, Paus T, Pausova Z, … The IMA EN Consortium. (2014). Neural and Cognitive Correlates of the Common and Specific Variance Across Externalizing Problems in Young Adolescence. American Journal of Psychiatry, 171(12), 1310–1319. 10.1176/appi.ajp.2014.13111499 [DOI] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, & Bellgrove MA (2009). Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neuroscience & Biobehavioral Reviews, 33(5), 631–646. 10.1016/j.neubiorev.2008.08.016 [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Asari T, Yamashita K. -i., Morimoto H, Hirose S, Miyashita Y, & Konishi S (2009). Functional Dissociation in Right Inferior Frontal Cortex during Performance of Go/No-Go Task. Cerebral Cortex, 19(1), 146–152. 10.1093/cercor/bhn065 [DOI] [PubMed] [Google Scholar]
- Chung YS, Calhoun V, & Stevens MC (2020). Adolescent sex differences in cortico-subcortical functional connectivity during response inhibition. Cognitive, Affective, & Behavioral Neuroscience, 20(1), 1–18. 10.3758/s13415-019-00718-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D (1989). How research on child maltreatment has informed the study of child development: Perspectives from developmental psychopathology. Child maltreatment: Theory and research on the causes and consequences of child abuse and neglect, 377–431. [Google Scholar]
- Cicchetti D, & Rogosch FA (1996). Equifinality and multifinality in developmental psychopathology. Development and Psychopathology, 8(4), 597–600. 10.1017/S0954579400007318 [DOI] [Google Scholar]
- Cohen AO, Breiner K, Steinberg L, Bonnie RJ, Scott ES, Taylor-Thompson K, Rudolph MD, Chein J, Richeson JA, Heller AS, Silverman MR, Dellarco DV, Fair DA, Galván A, & Casey BJ (2016). When Is an Adolescent an Adult? Assessing Cognitive Control in Emotional and Nonemotional Contexts. Psychological Science, 27(4), 549–562. 10.1177/0956797615627625 [DOI] [PubMed] [Google Scholar]
- Cohen J (2010). Decoding developmental differences and individual variability in response inhibition through predictive analyses across individuals. Frontiers in Human Neuroscience. 10.3389/fnhum.2010.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K, Heathcote LC, & Lau JYF (2014). Age-related changes in attentional control across adolescence: How does this impact emotion regulation capacities? Frontiers in Psychology, 5. 10.3389/fpsyg.2014.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Gilbert JE, & Thomas KM (2013). Inhibitory Control During Emotional Distraction Across Adolescence and Early Adulthood. Child Development, 84(6), 1954–1966. 10.1111/cdev.12085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E, Mumford JA, Cohen JR, Galvan A, Canli T, & Poldrack RA (2012). Measurement and Reliability of Response Inhibition. Frontiers in Psychology, 3. 10.3389/fpsyg.2012.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis C, & Luna B (2019). Neural Substrates of Inhibitory Control Maturation in Adolescence. Trends in Neurosciences, 42(9), 604–616. 10.1016/j.tins.2019.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope LM, Hardee JE, Martz ME, Zucker RA, Nichols TE, & Heitzeg MM (2020). Developmental maturation of inhibitory control circuitry in a high-risk sample: A longitudinal fMRI study. Developmental Cognitive Neuroscience, 43, 100781. 10.1016/j.dcn.2020.100781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg L, & Nation K (2008). Go or no-go? Developmental improvements in the efficiency of response inhibition in mid-childhood. Developmental Science, 11(6), 819–827. ht 10.1111/j.1467-7687.2008.00730.x [DOI] [PubMed] [Google Scholar]
- Criaud M, & Boulinguez P (2013). Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neuroscience & Biobehavioral Reviews, 37(1), 11–23. 10.1016/j.neubiorev.2012.11.003 [DOI] [PubMed] [Google Scholar]
- Curley LB, Newman E, Thompson WK, Brown TT, Hagler DJ, Akshoomoff N, Reuter C, Dale AM, & Jernigan TL (2018). Cortical morphology of the pars opercularis and its relationship to motor-inhibitory performance in a longitudinal, developing cohort. Brain Structure and Function, 223(1), 211–220. 10.1007/s00429-017-1480-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, & Coskunpinar A (2011). Measurement of constructs using self-report and behavioral lab tasks: Is there overlap in nomothetic span and construct representation for impulsivity? Clinical Psychology Review, 31(6), 965–982. 10.1016/j.cpr.2011.06.001 [DOI] [PubMed] [Google Scholar]
- Dambacher F, Sack AT, Lobbestael J, Arntz A, Brugman S, & Schuhmann T (2014). A network approach to response inhibition: Dissociating functional connectivity of neural components involved in action restraint and action cancellation. European Journal of Neuroscience, 39(5), 821–831. 10.1111/ejn.12425 [DOI] [PubMed] [Google Scholar]
- Davidow JY, Insel C, & Somerville LH (2018). Adolescent Development of Value-Guided Goal Pursuit. Trends in Cognitive Sciences, 22(8), 725–736. 10.1016/j.tics.2018.05.003 [DOI] [PubMed] [Google Scholar]
- Donders FC (1969). On the speed of mental processes. Acta psychologica, 30, 412–431. 10.1016/0001-6918(69)90065-1 [DOI] [PubMed] [Google Scholar]
- Dreyfuss M, Caudle K, Drysdale AT, Johnston NE, Cohen AO, Somerville LH, Galván A, Tottenham N, Hare TA, & Casey BJ (2014). Teens Impulsively React rather than Retreat from Threat. Developmental Neuroscience, 36(3–4), 220–227. 10.1159/000357755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis A, Indralingam M, Chevrier A, Crosbie J, Arnold P, Burton CL, & Schachar R (2019). Response Time Adjustment in the Stop Signal Task: Development in Children and Adolescents. Child Development, 90(2), e263–e272. 10.1111/cdev.13062 [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, & Casey BJ (2006). A shift from diffuse to focal cortical activity with development. Developmental Science, 9(1), 1–8. 10.1111/j.1467-7687.2005.00454.x [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman RD, & Casey BJ (2002). A neural basis for the development of inhibitory control. Developmental Science, 5(4), F9–F16. 10.1111/1467-7687.00235 [DOI] [Google Scholar]
- Eigsti I-M, Zayas V, Mischel W, Shoda Y, Ayduk O, Dadlani MB, Davidson MC, Aber JL, & Casey BJ (2006). Predicting Cognitive Control From Preschool to Late Adolescence and Young Adulthood. Psychological Science, 17(6), 478–484. 10.1111/j.1467-9280.2006.01732.x [DOI] [PubMed] [Google Scholar]
- Erika-Florence M, Leech R, & Hampshire A (2014). A functional network perspective on response inhibition and attentional control. Nature Communications, 5(1), 4073. 10.1038/ncomms5073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Biscaldi M, & Gezeck S (1997). On the development of voluntary and reflexive components in human saccade generation. Brain Research, 754(1–2), 285–297. 10.1016/S0006-8993(97)00094-2 [DOI] [PubMed] [Google Scholar]
- Fosco WD, Hawk LW, Colder CR, Meisel SN, & Lengua LJ (2019). The development of inhibitory control in adolescence and prospective relations with delinquency. Journal of Adolescence, 76, 37–47. 10.1016/j.adolescence.2019.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, & Hewitt JK (2008). Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General, 137(2), 201–225. 10.1037/0096-3445.137.2.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost R, & McNaughton N (2017). The neural basis of delay discounting: A review and preliminary model. Neuroscience & Biobehavioral Reviews, 79, 48–65. 10.1016/j.neubiorev.2017.04.022. [DOI] [PubMed] [Google Scholar]
- Garavan H, Chaarani B, Hahn S, Allgaier N, Juliano A, Yuan DK, Orr C, Watts R, Wager TD, Ruiz de Leon O, Hagler DJ, & Potter A (2020). The ABCD Stop Signal Data: Response to Bissett et al. bioRxiv 2020.07.27.223057. 10.1101/2020.07.27.223057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, & Stein EA (1999). Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proceedings of the National Academy of Sciences, 96(14), 8301–8306. 10.1073/pnas.96.14.8301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gärtner A, & Strobel A (n.d.). Individual Differences in Inhibitory Control: A latent Variable Analysis. Journal of Cognition, 4(1). 10.5334/joc.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, & Luna B (2012). Developmental effects of incentives on response inhibition. Child Development, 83(4), 1262–1274. 10.1111/j.1467-8624.2012.01771.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, & Luna B (2010). Immaturities in Reward Processing and Its Influence on Inhibitory Control in Adolescence. Cerebral Cortex, 20(7), 1613–1629. 10.1093/cercor/bhp225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR (2002). ILAB: a program for postexperimental eye movement analysis. Behavior Research Methods, Instruments, & Computers, 34(4), 605–612. 10.3758/BF03195488 [DOI] [PubMed] [Google Scholar]
- Goghari VM, & MacDonald AW (2009). The Neural Basis of Cognitive Control: Response Selection and Inhibition. Brain and Cognition, 71(2), 72–83. 10.1016/j.bandc.2009.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, & Thompson PM (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, 101(21), 8174–8179. 10.1073/pnas.0402680101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, & Thompson PM (2010). Mapping gray matter development: Implications for typical development and vulnerability to psychopathology. Brain and Cognition, 72(1), 6–15. 10.1016/j.bandc.2009.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez P, Ratcliff R, & Perea M (2007). A model of the go/no-go task. Journal of Experimental Psychology: General, 136(3), 389–413. 10.1037/0096-3445.136.3.389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, & Swets JA (1966). Signal detection theory and psycho- physics. New York: Wiley. [Google Scholar]
- Hallett PE (1978). Primary and secondary saccades to goals defined by instructions. Vision Research, 18(10), 1279–1296. 10.1016/0042-6989(78)90218-3 [DOI] [PubMed] [Google Scholar]
- Hallquist MN, Geier CF, & Luna B (2018). Incentives facilitate developmental improvement in inhibitory control by modulating control related networks. NeuroImage, 172, 369–380. 10.1016/j.neuroimage.2018.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A (2015). Putting the brakes on inhibitory models of frontal lobe function. NeuroImage, 113, 340–355. 10.1016/j.neuroimage.2015.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, & Casey BJ (2008). Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry, 63(10), 927–934. 10.1016/j.biopsych.2008.03.015015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedge C, Powell G, & Sumner P (2018). The reliability paradox: Why robust cognitive tasks do not produce reliable individual differences. Behavior Research Methods, 50(3), 1166–1186. 10.3758/s13428-017-0935-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Blonigen DM, Kramer MD, Krueger RF, Patrick CJ, Iacono WG, & McGue M (2007). Gender differences and developmental change in externalizing disorders from late adolescence to early adulthood: A longitudinal twin study. Journal of Abnormal Psychology, 116(3), 433–447. 10.1037/0021-843X.116.3.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper CJ, Luciana M, Conklin HM, & Yarger RS (2004). Adolescents’ Performance on the Iowa Gambling Task: Implications for the Development of Decision Making and Ventromedial Prefrontal Cortex. Developmental Psychology, 40(6), 1148–1158. 10.1037/0012-1649.40.6.1148 [DOI] [PubMed] [Google Scholar]
- Huizinga M, Dolan CV, & van der Molen MW (2006). Age-related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia, 44(11), 2017–2036. 10.1016/j.neuropsychologia.2006.01.010 [DOI] [PubMed] [Google Scholar]
- Humphrey G, & Dumontheil I (2016). Development of Risk-Taking, Perspective-Taking, and Inhibitory Control During Adolescence. Developmental Neuropsychology, 41(1–2), 59–76. ht 10.1080/87565641.2016.1161764 [DOI] [PubMed] [Google Scholar]
- Hutton SB, & Ettinger U (2006). The antisaccade task as a research tool in psychopathology: A critical review. Psychophysiology, 43(3), 302–313. 10.1111/j.1469-8986.2006.00403.x [DOI] [PubMed] [Google Scholar]
- Hwang K, Velanova K, & Luna B (2010). Strengthening of Top-Down Frontal Cognitive Control Networks Underlying the Development of Inhibitory Control: A Functional Magnetic Resonance Imaging Effective Connectivity Study. Journal of Neuroscience, 30(46), 15535–15545. 10.1523/JNEUROSCI.2825-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, & McGue M (2008). Behavioral Disinhibition and the Development of Early-Onset Addiction: Common and Specific Influences. Annual Review of Clinical Psychology, 4(1), 325–348. 10.1146/annurev.clinpsy.4.022007.141157 [DOI] [PubMed] [Google Scholar]
- Jazbec S, Hardin MG, Schroth E, McClure E, Pine DS, & Ernst M (2006). Age-related influence of contingencies on a saccade task. Experimental Brain Research. Experimentelle Hirnforschung. Experimentation Cerebrale, 174(4), 754–762. 10.1007/s00221-006-0520-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AK, Sellbom M, & Glenn AL (2018). Dimensional Personality Traits Broadly and Selectively Associated with Normative Externalizing Behavior. Journal of Psychopathology and Behavioral Assessment, 40(3), 419–430. 10.1007/s10862-018-9665-7 [DOI] [Google Scholar]
- Johnstone SJ, Dimoska A, Smith JL, Barry RJ, Pleffer CB, Chiswick D, & Clarke AR (2007). The development of stop-signal and Go/Nogo response inhibition in children aged 7–12 years: Performance and event-related potential indices. International Journal of Psychophysiology, 63(1), 25–38. 10.1016/j.ijpsycho.2006.07.001 [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Pleffer CB, Barry RJ, Clarke AR, & Smith JL (2005). Development of Inhibitory Processing During the Go/NoGo Task. Journal of Psychophysiology, 19(1), 11–23. 10.1027/0269-8803.19.1.11 [DOI] [Google Scholar]
- King KM, Patock-Peckham JA, Dager AD, Thimm K, & Gates JR (2014). On the Mismeasurement of Impulsivity: Trait, Behavioral, and Neural Models in Alcohol Research among Adolescents and Young Adults. Current Addiction Reports, 1(1), 19–32. 10.1007/s40429-013-0005-4 [DOI] [Google Scholar]
- Klein C (2001). Developmental functions for saccadic eye movement parameters derived from pro- and antisaccade tasks. Experimental Brain Research, 139(1), 1–17. 10.1007/s002210100711 [DOI] [PubMed] [Google Scholar]
- Klein C, & Fischer B (2005). Instrumental and test–retest reliability of saccadic measures. Biological Psychology, 68(3), 201–213. 10.1016/j.biopsycho.2004.06.005 [DOI] [PubMed] [Google Scholar]
- Klein C, Foerster F, Hartnegg K, & Fischer B (2005). Lifespan development of pro- and anti-saccades: Multiple regression models for point estimates. Developmental Brain Research, 160(2), 113–123. 10.1016/j.devbrainres.2005.06.011 [DOI] [PubMed] [Google Scholar]
- Klein C, Rauh R, & Biscaldi M (2011). Patterns of change in ocular motor development. Experimental brain research, 210(1), 33–44. 10.1007/s00221-011-2601-7 [DOI] [PubMed] [Google Scholar]
- Kohls G, Peltzer J, Herpertz-Dahlmann B, & Konrad K (2009). Differential effects of social and non-social reward on response inhibition in children and adolescents. Developmental Science, 12(4), 614–625. 10.1111/j.1467-7687.2009.00816.x [DOI] [PubMed] [Google Scholar]
- Kray J, Ritter H, & Müller L (2020). The interplay between cognitive control and emotional processing in children and adolescents. Journal of Experimental Child Psychology, 193, 104795. 10.1016/j.jecp.2019.104795 [DOI] [PubMed] [Google Scholar]
- Krueger RF (2002). Personality from a realist’s perspective: Personality traits, criminal behaviors, and the externalizing spectrum. Journal of Research in Personality, 36(6), 564–572. 10.1016/S0092-6566(02)00506-8 [DOI] [Google Scholar]
- Kuntsi J, Andreou P, Ma J, Börger NA, & van der Meere JJ (2005). Testing assumptions for endophenotype studies in ADHD: Reliability and validity of tasks in a general population sample. BMC Psychiatry, 5(1), 40. 10.1186/1471-244X-5-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambek R, & Shevlin M (2011). Working memory and response inhibition in children and adolescents: Age and organization issues: Working memory and inhibition in children. Scandinavian Journal of Psychology, 52(5), 427–432. 10.1111/j.1467-9450.2011.00899.x [DOI] [PubMed] [Google Scholar]
- Lebel C, Treit S, & Beaulieu C (2019). A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR in Biomedicine, 32(4), e3778. 10.1002/nbm.3778 [DOI] [PubMed] [Google Scholar]
- Lipszyc J, & Schachar R (2010). Inhibitory control and psychopathology: A meta-analysis of studies using the stop signal task. Journal of the International Neuropsychological Society, 16(6), 1064–1076. 10.1017/S1355617710000895 [DOI] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, & Casey BJ (2006). Frontostriatal Microstructure Modulates Efficient Recruitment of Cognitive Control. Cerebral Cortex, 16(4), 553–560. 10.1093/cercor/bhj003 [DOI] [PubMed] [Google Scholar]
- Lock J, Garrett A, Beenhakker J, & Reiss AL (2011). Aberrant brain activation during a response inhibition task in adolescent eating disorder subtypes. American Journal of Psychiatry, 168(1), 55–64. 10.1176/appi.ajp.2010.10010056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, & Cowan WB (1984). On the ability to inhibit thought and action: A theory on an act of control. Psychological Review, 91(3), 295. [DOI] [PubMed] [Google Scholar]
- Logan GD, van Zandt T, Verbruggen F, & Wagenmarkers EJ (2014). On the Ability to Inhibit Thought and Action: General and Special Theories of an Act of Control. Psychological Review, 121(1), 66. [DOI] [PubMed] [Google Scholar]
- Luciana M (2013). Adolescent brain development in normality and psychopathology. Development and Psychopathology, 25(4pt2), 1325–1345. 10.1017/S0954579413000643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, & Collins PF (2012). Incentive motivation, cognitive control, and the adolescent brain: Is it time for a paradigm shift?. Child development perspectives, 6(4), 392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludyga S, Möhring W, Budde H, Hirt N, Pühse U, & Gerber M (2020). Neurocognitive processes mediate the relation between children’s motor skills, cardiorespiratory fitness and response inhibition: Evidence from source imaging. Psychophysiology, 58(2), e13716. 10.1111/psyp.13716 [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, & Sweeney JA (2004). Maturation of Cognitive Processes From Late Childhood to Adulthood. Child Development, 75(5), 1357–1372. 10.1111/j.1467-8624.2004.00745.x [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, & O’Hearn K (2010). What has fMRI told us about the Development of Cognitive Control through Adolescence? Brain and Cognition, 72(1), 101–113. 10.1016/j.bandc.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Velanova K, & Geier CF (2008). Development of eye-movement control. Brain and Cognition, 68(3), 293–308. 10.1016/j.bandc.2008.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen KS, Baaré WFC, Vestergaard M, Skimminge A, Ejersbo LR, Ramsøy TZ, Gerlach C, Åkeson P, Paulson OB, & Jernigan TL (2010). Response inhibition is associated with white matter microstructure in children. Neuropsychologia, 48(4), 854–862. 10.1016/j.neuropsychologia.2009.11.001 [DOI] [PubMed] [Google Scholar]
- Madsen KS, Johansen LB, Thompson WK, Siebner HR, Jernigan TL, & Baaré WF (2020). Maturational trajectories of white matter microstructure underlying the right presupplementary motor area reflect individual improvements in motor response cancellation in children and adolescents. NeuroImage, 220, 117105. 10.1101/2020.03.13.990507 [DOI] [PubMed] [Google Scholar]
- Malagoli C, & Usai MC (2018a). The effects of gender and age on inhibition and working memory organization in 14- to 19-year-old adolescents and young adults. Cognitive Development, 45, 10–23. 10.1016/j.cogdev.2017.10.005 [DOI] [Google Scholar]
- Malagoli C, & Usai MC (2018b). The effects of gender and age on inhibition and working memory organization in 14- to 19-year-old adolescents and young adults. Cognitive Development, 45, 10–23. 10.1016/j.cogdev.2017.10.005 [DOI] [Google Scholar]
- Malone SM, & Iacono WG (2002). Error rate on the antisaccade task: Heritability and developmental change in performance among preadolescent and late-adolescent female twin youth. Psychophysiology, 39(5), 664–673. 10.1111/1469-8986.3950664 [DOI] [PubMed] [Google Scholar]
- Marek S, Hwang K, Foran W, Hallquist MN, & Luna B (2015). The Contribution of Network Organization and Integration to the Development of Cognitive Control. PLoS Biology, 13(12). 10.1371/journal.pbio.1002328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massen C (2004). Parallel programming of exogenous and endogenous components in the antisaccade task. Quarterly Journal of Experimental Psychology Section A, 57(3), 475–498. 10.1080/02724980343000341 [DOI] [PubMed] [Google Scholar]
- McAuley T, & White DA (2011). A latent variables examination of processing speed, response inhibition, and working memory during typical development. Journal of Experimental Child Psychology, 108(3), 453–468. 10.1016/j.jecp.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick EM, Qu Y, & Telzer EH (2016). Adolescent neurodevelopment of cognitive control and risk-taking in negative family contexts. NeuroImage, 124, 989–996. 10.1016/j.neuroimage.2015.09.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, & Elkins I (2001). Origins and Consequences of Age at First Drink. I. Associations With Substance-Use Disorders, Disinhibitory Behavior and Psychopathology, and P3 Amplitude. Alcoholism: Clinical and Experimental Research, 25(8), 1156–1165. 10.1111/j.1530-0277.2001.tb02330.x [DOI] [PubMed] [Google Scholar]
- Meisel SN, Colder CR, & Hawk LW (2015). The Moderating Role of Cognitive Capacities in the Association Between Social Norms and Drinking Behaviors. Alcoholism: Clinical and Experimental Research, 39(6), 1049–1056. 10.1111/acer.12710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, & Reiss AL (2001). Error-related brain activation during a Go/NoGo response inhibition task. Human brain mapping, 12(3), 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, & Friedman NP (2012). The Nature and Organization of Individual Differences in Executive Functions: Four General Conclusions. Current Directions in Psychological Science, 21(1), 8–14. 10.1177/0963721411429458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD (2000). The Unity and Diversity of Executive Functions and Their Contributions to Complex “Frontal Lobe” Tasks: A Latent Variable Analysis. Cognitive Psychology, 41(1), 49–100. 10.1006/cogp.1999.0734 [DOI] [PubMed] [Google Scholar]
- Molina BS, & Pelham WE (2014). Attention-deficit/hyperactivity disorder and risk of substance use disorder: Developmental considerations, potential pathways, and opportunities for research. Annual Review of Clinical Psychology, 10, 607–639. 10.1146/annurev-clinpsy-032813-153722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, & Simmonds DJ (2008). Response Inhibition and Response Selection: Two Sides of the Same Coin. Journal of Cognitive Neuroscience, 20(5), 751–761. 10.1162/jocn.2008.20500 [DOI] [PubMed] [Google Scholar]
- Motes MA, Spence JS, Brier MR, Chiang H-S, DeLaRosa BL, Eroh J, … & Hart J (2018). Conjoint differences in inhibitory control and processing speed in childhood to older adult cohorts: Discriminant functions from a Go/No-Go task. Psychology and Aging, 33(7), 1070–1078. 10.1037/pag0000299 [DOI] [PubMed] [Google Scholar]
- Munoz DP, Broughton JR, Goldring JE, & Armstrong IT (1998). Age-related performance of human subjects on saccadic eye movement tasks. Experimental Brain Research, 121(4), 391–400. 10.1007/s002210050473 [DOI] [PubMed] [Google Scholar]
- Munoz DP, & Everling S (2004). Look away: The anti-saccade task and the voluntary control of eye movement. Nature Reviews Neuroscience, 5(3), 218–228. 10.1038/nrn1345 [DOI] [PubMed] [Google Scholar]
- Mürner-Lavanchy IM, Koenig J, Ando A, Henze R, Schell S, Resch F, Brunner R, & Kaess M (2020). Neuropsychological development in adolescents: Longitudinal associations with white matter microstructure. Developmental Cognitive Neuroscience, 45, 100812. 10.1016/j.dcn.2020.100812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy YE, Luke A, Brennan E, Francazio S, Christopher I, & Flessner CA (2018). An Investigation of Executive Functioning in Pediatric Anxiety. Behavior Modification, 42(6), 885–913. 10.1177/0145445517749448 [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. (2020). Promoting positive adolescent health behaviors and outcomes: Thriving in the 21st century. National Academies Press. [PubMed] [Google Scholar]
- Nelson TD, James TD, Nelson JM, Johnson AB, Mason WA, Yaroch AL, & Espy KA (2020). Associations between specific components of executive control and eating behaviors in adolescence: A study using objective and subjective measures. Appetite, 154, 104784. 10.1016/j.appet.2020.104784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M, Schlaggar BL, & Petersen SE (2014). Separable responses to error, ambiguity, and reaction time in cingulo-opercular task control regions. Neuroimage 99, 59–68. 10.1016/j.neuroimage.2014.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT (2003). Response Inhibition and Disruptive Behaviors: Toward a Multiprocess Conception of Etiological Heterogeneity for ADHD Combined Type and Conduct Disorder Early-Onset Type. In Roots of mental illness in children (pp. 170–182). New York Academy of Sciences. [DOI] [PubMed] [Google Scholar]
- Nigg JT (2017). Annual Research Review: On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. Journal of Child Psychology and Psychiatry, 58(4), 361–383. 10.1111/jcpp.12675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Adams KM, Fitzgerald HE, & Zucker RA (2006). Poor Response Inhibition as a Predictor of Problem Drinking and Illicit Drug Use in Adolescents at Risk for Alcoholism and Other Substance Use Disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 45(4), 468–475. 10.1097/01.chi.0000199028.76452.a9 [DOI] [PubMed] [Google Scholar]
- Ogilvie JM, Shum DHK, & Stewart A (2020). Executive Functions in Late Adolescence and Early Adulthood and Their Relationship with Risk-Taking Behavior. Developmental Neuropsychology, 45(7–8), 446–468. 10.1080/87565641.2020.1833885 [DOI] [PubMed] [Google Scholar]
- Ordaz SJ, Foran W, Velanova K, & Luna B (2013). Longitudinal Growth Curves of Brain Function Underlying Inhibitory Control through Adolescence. Journal of Neuroscience, 33(46), 18109–18124. 10.1523/JNEUROSCI.1741-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz SJ, Fritz BL, Forbes EE, & Luna B (2018). The Influence of Pubertal Maturation on Antisaccade Performance. Developmental Science, 21(3), e12568. 10.1111/desc.12568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz S, Stephanie D, & Luna B (2010). Effects of Response Preparation on Developmental Improvements in Inhibitory Control. Acta Psychologica, 134(3), 253–263. 10.1016/j.actpsy.2010.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A, Geier CF, Ordaz SJ, Teslovich T, & Luna B (2011). Developmental changes in brain function underlying the influence of reward processing on inhibitory control. Developmental Cognitive Neuroscience, 1(4), 517–529. 10.1016/j.dcn.2011.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevopoulou M, van Rooij D, Batalla A, Chauvin R, Luijten M, chene AH, … & Schelleken AF (2021). Effects of substance misuse on reward-processing in patients with attention-deficit/hyperactivity disorder. Neuropsychopharmacology, 46(3), 622–631. 10.1038/s41386-020-00896-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puiu AA, Wudarczyk O, Kohls G, Bzdok D, Herpertz-Dahlmann B, & Konrad K (2020). Meta-analytic evidence for a joint neural mechanism underlying response inhibition and state anger. Human Brain Mapping, 41(11), 3147–3160. 10.1002/hbm.25004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach A, Tervo-Clemmens B, Foran W, Calabro FJ, Chung T, Clark DB, & Luna B (2020). Adolescent development of inhibitory control and substance use vulnerability: A longitudinal neuroimaging study. Developmental Cognitive Neuroscience, 42. 10.1016/j.dcn.2020.100771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, & McKoon G (2008). The Diffusion Decision Model: Theory and Data for Two-Choice Decision Tasks. Neural Computation, 20(4), 873–922. 10.1162/neco.2008.12-06-420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindranath O, Ordaz SJ, Padmanabhan A, Foran W, Jalbrzikowski M, Calabro FJ, & Luna B (2020). Influences of affective context on amygdala functional connectivity during cognitive control from adolescence through adulthood. Developmental Cognitive Neuroscience, 45, 100836. 10.1016/j.dcn.2020.100836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Band GP, & Logan GD (1999). A study of adaptive behavior: Effects of age and irrelevant information on the ability to inhibit one’s actions. Acta Psychologica, 101(2–3), 315–337. 10.1016/S0001-6918(99)00010-4 [DOI] [Google Scholar]
- Roe MA, Engelhardt LE, Nugiel T, Harden KP, Tucker-Drob EM, & Church JA (2021). Error-signaling in the developing brain. NeuroImage, 227, 117621. 10.1016/j.neuroimage.2020.117621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Lim L, Ecker C, Halari R, Giampietro V, Simmons A, Brammer M, & Smith A (2013). Effects of age and gender on neural networks of motor response inhibition: From adolescence to mid-adulthood. NeuroImage, 83, 690–703. 10.1016/j.neuroimage.2013.06.078 [DOI] [PubMed] [Google Scholar]
- Rubia K, Alegría AA, & Brinson H (2014). Brain abnormalities in attention-deficit/hyperactivity disorder: A review. Rev Neurol, 58(Supl 1): S3–S18. [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SCR, Simmons A, Andrew C, & Bullmore ET (2000). Functional frontalisation with age: Mapping neurodevelopmental trajectories with fMRI. Neuroscience & Biobehavioral Reviews, 24(1), 13–19. 10.1016/S0149-7634(99)00055-X [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SCR, Giampietro V, Andrew CM, & Taylor E (2001). Mapping Motor Inhibition: Conjunctive Brain Activations across Different Versions of Go/No-Go and Stop Tasks. NeuroImage, 13(2), 250–261. 10.1006/nimg.2000.0685 [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, & Brammer M (2007). Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping, 28(11), 1163–1177. 10.1002/hbm.20347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, & Brammer M (2006). Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping, 27(12), 973–993. 10.1002/hbm.20237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachar RJ, Forget-Dubois N, Dionne G, Boivin M, & Robaey P (2010). Heritability of Response Inhibition in Children. Journal of the International Neuropsychological Society, 17(2), 238–247. 10.1017/S1355617710001463 [DOI] [PubMed] [Google Scholar]
- Schachar R, Logan GD, Robaey P, Chen S, Ickowicz A, & Barr C (2007). Restraint and Cancellation: Multiple Inhibition Deficits in Attention Deficit Hyperactivity Disorder. Journal of Abnormal Child Psychology, 35(2), 229–238. 10.1007/s10802-006-9075-2 [DOI] [PubMed] [Google Scholar]
- Schel MA, & Crone EA (2013). Development of response inhibition in the context of relevant versus irrelevant emotions. Frontiers in Psychology, 4. 10.3389/fpsyg.2013.00383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian A, Baldermann C, Feige B, Katzev M, Scheller E, Hellwig B, Lieb K, Weiller C, Tüscher O, & Klöppel S (2013). Differential effects of age on subcomponents of response inhibition. Neurobiology of Aging, 34(9), 2183–2193. 10.1016/j.neurobiolaging.2013.03.013 [DOI] [PubMed] [Google Scholar]
- Sharma L, Markon KE, & Clark LA (2014). Toward a theory of distinct types of “impulsive” behaviors: A meta-analysis of self-report and behavioral measures. Psychological Bulletin, 140(2), 374–408. 10.1037/a0034418 [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, harp W, Blumenthal J, Lerch JP, Greenstein DEEA, … & Rapoport JL (2007). Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences, 104(49). 19649–19654. 10.1073/pnas.0707741104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Sneider JT, Crowley DJ, Covell MJ, Acharya D, Rosso IM, & Jensen JE (2013). Frontal Lobe γ-Aminobutyric Acid Levels During Adolescence: Associations with Impulsivity and Response Inhibition. Biological Psychiatry, 74(4), 296–304. 10.1016/j.biopsych.2013.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, & Mostofsky SH (2008). Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia, 46(1), 224–232. 10.1016/j.neuropsychologia.2007.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinopoli KJ, Schachar R, & Dennis M (2011). Reward improves cancellation and restraint inhibition across childhood and adolescence. Developmental Psychology, 47(5), 1479–1489. 10.1037/a0024440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, & Casey BJ (2011). Frontostriatal Maturation Predicts Cognitive Control Failure to Appetitive Cues in Adolescents. Journal of Cognitive Neuroscience, 23(9), 2123–2134. 10.1162/jocn.2010.21572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, & Toga AW (2001). Mapping Continued Brain Growth and Gray Matter Density Reduction in Dorsal Frontal Cortex: Inverse Relationships during Postadolescent Brain Maturation. The Journal of Neuroscience, 21(22), 8819–8829. 10.1523/JNEUROSCI.21-22-08819.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, Galarce EM, Ladouceur CD, McMakin DL, Olino TM, Forbes EE, Silk JS, Ryan ND, & Dahl RE (2015). Adolescent development of inhibition as a function of SES and gender: Converging evidence from behavior and fMRI: SES and Inhibition Development in Adolescence. Human Brain Mapping, 36(8), 3194–3203. 10.1002/hbm.22838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L (2010). A dual systems model of adolescent risk-taking. Developmental Psychobiology, 52(3), 216–224. 10.1002/dev.20445 [DOI] [PubMed] [Google Scholar]
- Stevens L, Verdejo-García A, Goudriaan AE, Roeyers H, Dom G, & Vanderplasschen W (2014). Impulsivity as a vulnerability factor for poor addiction treatment outcomes: a review of neurocognitive findings among individuals with substance use disorders. Journal of Substance Abuse Treatment, 47(1), 58–72. 10.1016/j.jsat.2014.01.008 [DOI] [PubMed] [Google Scholar]
- Stevens MC (2016). The contributions of resting state and task-based functional connectivity studies to our understanding of adolescent brain network maturation. Neuroscience & Biobehavioral Reviews, 70, 13–32. 10.1016/j.neubiorev.2016.07.027 [DOI] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, & Calhoun VD (2007). Functional neural networks underlying response inhibition in adolescents and adults. Behavioural Brain Research, 181(1), 12–22. 10.1016/j.bbr.2007.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Ashley V, & Turker AU (2011). Are the neural correlates of stopping and not going identical? A meta-analysis of two response inhibition tasks. Neuroimage, 56, 1655–1665. doi: 10.1016/j.neuroimage.2011.02.070 [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, & Reiss AL (2002). Maturation of Brain Function Associated With Response Inhibition. Journal of the American Academy of Child & Adolescent Psychiatry, 41(10), 1231–1238. 10.1097/00004583-200210000-00013 [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Østby Y, Walhovd KB, Westlye LT, Due-Tønnessen P, & Fjell AM (2010). Neuroanatomical correlates of executive functions in children and adolescents: A magnetic resonance imaging (MRI) study of cortical thickness. Neuropsychologia, 48(9), 2496–2508. 10.1016/j.neuropsychologia.2010.04.024 [DOI] [PubMed] [Google Scholar]
- Taylor SJ, Barker LA, Heavey L, & McHale S (2015). The longitudinal development of social and executive functions in late adolescence and early adulthood. Frontiers in Behavioral Neuroscience, 9. 10.3389/fnbeh.2015.00252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SJ, Barker LA, Heavey L, & McHale S (2013). The typical developmental trajectory of social and executive functions in late adolescence and early adulthood. Developmental Psychology, 49(7), 1253 – 1265. 10.1037/a0029871 [DOI] [PubMed] [Google Scholar]
- Tervo-Clemmens B, Quach A, Luna B, Foran W, Chung T, De Bellis MD, & Clark DB (2017). Neural Correlates of Rewarded Response Inhibition in Youth at Risk for Problematic Alcohol Use. Frontiers in Behavioral Neuroscience, 11, 205. 10.3389/fnbeh.2017.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A, Schel MA, & Steinbeis N (2021). Changes in BOLD variability are linked to the development of variable response inhibition. NeuroImage, 228, 117691. 10.1016/j.neuroimage.2020.117691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen K, Blom Osterland T, Hesse M, & Feldstein Ewing SW (2018). The intersection between response inhibition and substance use among adolescents. Addictive Behaviors, 78, 228–230. 10.1016/j.addbeh.2017.11.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiego J, Bellgrove MA, Whittle S, Pantelis C, & Testa R (2020). Common mechanisms of executive attention underlie executive function and effortful control in children. Developmental Science, 23(3), e12918. 10.1111/desc.12918 [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, & Casey BJ (2011). Behavioral Assessment of Emotion Discrimination, Emotion Regulation, and Cognitive Control in Childhood, Adolescence, and Adulthood. Frontiers in Psychology, 2. 10.3389/fpsyg.2011.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommer BL, Hoeppner J-AB, Lorber R, & Armstrong KJ (1988). The Go/No-Go paradigm in attention deficit disorder. Annals of Neurology, 24(5), 610–614. 10.1002/ana.410240504 [DOI] [PubMed] [Google Scholar]
- van Boxtel GJ, van der Molen MW, Jennings JR, & Brunia CH (2001). A psychophysiological analysis of inhibitory motor control in the stop-signal paradigm. Biological psychology, 58(3), 229–262. 10.1016/S0301-0511(01)00117-X [DOI] [PubMed] [Google Scholar]
- van de Laar MC, van den Wildenberg WPM, van Boxtel GJM, & van der Molen MW (2014). Development of response activation and inhibition in a selective stop-signal task. Biological Psychology, 102, 54–67. 10.1016/j.biopsycho.2014.06.003 [DOI] [PubMed] [Google Scholar]
- Vara AS, Pang EW, Vidal J, Anagnostou E, & Taylor MJ (2014). Neural mechanisms of inhibitory control continue to mature in adolescence. Developmental Cognitive Neuroscience, 10, 129–139. 10.1016/j.dcn.2014.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaurio RG, Simmonds DJ, & Mostofsky SH (2009). Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia, 47(12), 2389–2396. 10.1016/j.neuropsychologia.2009.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, & Luna B (2008). Maturational Changes in Anterior Cingulate and Frontoparietal Recruitment Support the Development of Error Processing and Inhibitory Control. Cerebral Cortex, 18(11), 2505–2522. 10.1093/cercor/bhn012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, & Luna B (2009). The Maturation of Task Set-Related Activation Supports Late Developmental Improvements in Inhibitory Control. Journal of Neuroscience, 29(40), 12558–12567. 10.1523/JNEUROSCI.1579-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Aron AR, Band GP, Beste C, Bissett PG, Brockett AT, Brown JW, Chamberlain SR, Chambers CD, Colonius H, Colzato LS, Corneil BD, Coxon JP, Dupuis A, Eagle DM, Garavan H, Greenhouse I, Heathcote A, Huster RJ, … Boehler CN (2019). A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. ELife, 8, e46323. 10.7554/eLife.46323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Chambers CD, & Logan GD (2013). Fictitious Inhibitory Differences: How Skewness and Slowing Distort the Estimation of Stopping Latencies. Psychological Science, 24(3), 352–362. 10.1177/0956797612457390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, & Logan GD (2008a). Automatic and controlled response inhibition: Associative learning in the go/no-go and stop-signal paradigms. Journal of Experimental Psychology: General, 137(4), 649–672. 10.1037/a0013170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, & Logan GD (2008b). Response inhibition in the stop-signal paradigm. Trends in Cognitive Sciences, 12(11), 418–424. 10.1016/j.tics.2008.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, & Logan GD (2009a). Proactive adjustments of response strategies in the stop-signal paradigm. Journal of Experimental Psychology: Human Perception and Performance, 35(3), 835–854. 10.1037/a0012726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, & Logan GD (2009b). Models of response inhibition in the stop-signal and stop-change paradigms. Neuroscience & Biobehavioral Reviews, 33(5), 647–661. 10.1016/j.neubiorev.2008.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, & Logan GD (2017). Control in Response Inhibition. In Egner T (Ed.), The Wiley Handbook of Cognitive Control (pp. 97–110). John Wiley & Sons, Ltd. 10.1002/9781118920497.ch6 [DOI] [Google Scholar]
- Wang H, Fan L, Song M, Liu B, Wu D, Jiang R, Li J, Li A, Banaschewski T, Bokde ALW, Quinlan EB, Desrivières S, Flor H, Grigis A, Garavan H, Chaarani B, Gowland P, Heinz A, Ittermann B, … Jiang T (2020). Functional Connectivity Predicts Individual Development of Inhibitory Control during Adolescence. Cerebral Cortex, bhaa383. 10.1093/cercor/bhaa383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Chen C, Cai Y, Li S, Zhao X, Zheng L, Zhang H, Liu J, Chen C, & Xue G (2016). Dissociated neural substrates underlying impulsive choice and impulsive action. NeuroImage, 134, 540–549. 10.1016/j.neuroimage.2016.04.010 [DOI] [PubMed] [Google Scholar]
- Ware AL, Infante MA, O’Brien JW, Tapert SF, Jones KL, Riley EP, & Mattson SN (2015). An fMRI study of behavioral response inhibition in adolescents with and without histories of heavy prenatal alcohol exposure. Behavioural Brain Research, 278, 137–146. 10.1016/j.bbr.2014.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Baggott MJ, & de Wit H (2013). Test–retest reliability of behavioral measures of impulsive choice, impulsive action, and inattention. Experimental and Clinical Psychopharmacology, 21(6), 475–481. 10.1037/a0033659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers JD, Stringaris A, Deveney CM, Brotman MA, Zarate CA Jr., Connolly ME, Fromm SJ, LeBourdais SB, Pine DS, & Leibenluft E (2012). A Developmental Study of the Neural Circuitry Mediating Motor Inhibition in Bipolar Disorder. American Journal of Psychiatry, 169(6), 633–641. 10.1176/appi.ajp.2012.11081244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR (2018). Prepotent motor activity and inhibitory control demands in different variants of the go/no-go paradigm. Psychophysiology, 55(3), e12871. 10.1111/psyp.12871 [DOI] [PubMed] [Google Scholar]
- Wessel JR, & Aron AR (2017). On the Globality of Motor Suppression: Unexpected Events and Their Influence on Behavior and Cognition. Neuron, 93(2), 259–280. 10.1016/j.neuron.2016.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West GL, & Lippé S (2016). The development of inhibitory saccadic trajectory deviations correlates with measures of antisaccadic inhibition: NeuroReport, 27(16), 1196–1201. 10.1097/WNR.0000000000000672 [DOI] [PubMed] [Google Scholar]
- Wetherill RR, Squeglia LM, Yang TT, & Tapert SF (2013). A longitudinal examination of adolescent response inhibition: Neural differences before and after the initiation of heavy drinking. Psychopharmacology, 230(4), 663–671. 10.1007/s00213-013-3198-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R, Conrod PJ, Poline J-B, Lourdusamy A, Banaschewski T, Barker GJ, Bellgrove MA, Büchel C, Byrne M, Cummins TDR, Fauth-Bühler M, Flor H, Gallinat J, Heinz A, Ittermann B, Mann K, Martinot J-L, Lalor EC, … Garavan H (2012). Adolescent impulsivity phenotypes characterized by distinct brain networks. Nature Neuroscience, 15(6), 920–925. 10.1038/nn.3092 [DOI] [PubMed] [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, & Tannock R (1999). Development of Inhibitory Control Across the Life Span. 9. [DOI] [PubMed] [Google Scholar]
- Wong MM, Brower KJ, Nigg JT, & Zucker RA (2010). Childhood Sleep Problems, Response Inhibition, and Alcohol and Drug Outcomes in Adolescence and Young Adulthood. Alcoholism: Clinical and Experimental Research, 34(6), 1033–1044. 10.1111/j.1530-0277.2010.01178.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöstmann NM, Aichert DS, Costa A, Rubia K, Möller H-J, & Ettinger U (2013). Reliability and plasticity of response inhibition and interference control. Brain and Cognition, 81(1), 82–94. 10.1016/j.bandc.2012.09.010 [DOI] [PubMed] [Google Scholar]
- Wright L, Lipszyc J, Dupuis A, Thayapararajah SW, & Schachar R (2014). Response inhibition and psychopathology: A meta-analysis of go/no-go task performance. Journal of Abnormal Psychology, 123(2), 429–439. 10.1037/a0036295 [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, & Hewitt JK (2009). Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology, 118(1), 117–130. 10.1037/a0014657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, & Li CSR (2012). Functional networks for cognitive control in a stop signal task: independent component analysis. Human brain mapping, 33(1), 89–104. 10.1002/hbm.21197 [DOI] [PMC free article] [PubMed] [Google Scholar]


