Abstract
The green synthesis of silver nanoparticles (AgNPs) and their applications have attracted many researchers as the AgNPs are used effectively in targeting specific tissues and pathogenic microorganisms. The purpose of this study is to synthesize and characterize silver nanoparticles from fully expanded leaves of Eugenia roxburghii DC., as well as to test their effectiveness in inhibiting biofilm production. In this study, at 0.1 mM concentration of silver nitrate (AgNO3), stable AgNPs were synthesized and authenticated by monitoring the color change of the solution from yellow to brown, which was confirmed with spectrophotometric detection of optical density. The crystalline nature of these AgNPs was detected through an X-Ray Diffraction (XRD) pattern. AgNPs were characterized through a high-resolution transmission electron microscope (HR-TEM) to study the morphology and size of the nanoparticles (NPs). A new biological approach was undertaken through the Congo Red Agar (CRA) plate assay by using the synthesized AgNPs against biofilm production. The AgNPs effectively inhibit biofilm formation and the biofilm-producing bacterial colonies. This could be a significant achievement in contending with many dynamic pathogens.
Subject terms: Biotechnology, Nanoscience and technology
Introduction
Most of the plants found in the family Myrtaceae are medicinally important. The secondary metabolites found in these plants can be utilized to cure different diseases. Among the different genera, Eugenia is an important taxon in the family having active principles which have pharmaceutical importance. Eugenia species produce delicious edible fruits with high vitamin and mineral content. Eugenia roxburghii DC. is one such wild edible fruit-producing plant under the family Myrtaceae. It is also known as Roxburgh’s Cherry due to the deliciousness of its fruits. This plant species is mostly found in the coastal and tropical areas of India and Sri Lanka. This species, containing the various secondary metabolites, has anticancer and antibacterial activity1–7. Though there is no such systematic study on the medicinal utilization of the species, the plant is used to treat diseases associated with diabetes, arthritis, hypertension, etc., as revealed by the local people8,9.
The bioavailability of the active principle is drastically reduced when it is supplied in the form of crude extract, but this can be enhanced when the crude extract is supplied in a modified form like nanomaterial10. A nanoparticle (NP) is a microscopic particle having a high surface area. Synthesis of NPs has picked up most attention in recent years due to their vast application in areas like catalysis, optics, electronics11–13, antibacterial, and antimicrobial activity14–16. The physical and chemical properties of metallic nanoparticles are remarkably different from their corresponding bulk form and can be used as an anti-microbial agent17. Plant and plant parts can be used for the reduction of metal to prepare respective metal nanoparticles18,19. Among the different metallic NPs, silver nanoparticles (AgNPs) have enormous applications in the medical and biotechnological fields20. The synthesis of AgNPs can be achieved both chemically and physically. Physicochemical approaches, on the other hand, include drawbacks such as high running costs, the use of toxic chemicals, and increased energy limits. Physical operations are complex procedures that fail to regulate particle sizes in the nanoscale range. The biggest drawbacks are that they create irregularly sized particles and have a high manufacturing cost21. Chemically synthesized NPs are not cost-effective and harm the environment with high energy requirements22. This is when biological approaches employing less expensive sources are exploited as AgNPs precursors. The green synthesis of nanoparticles has gained a lot of attraction since it uses non-toxic phytochemicals and avoids the dangerous ingredients that would otherwise be used in chemical synthesis23. Green synthesis methods use extracts from diverse plant parts, microbial cells, and biopolymers, and are so classified as such. The nanoparticles created are biocompatible and have the correct level of efficacy for the purpose for which they were created24. Metallic NPs can be synthesized biologically using various plants and their extracts which are easily available in huge quantities. The plants and their extracts are safe to handle, less toxic and eco-friendly.
From the leaf extract of Eugenia jambolana, silver nanoparticle synthesis was carried out and their phytochemical screening was evaluated25. Earlier, reports are available regarding the formation of AgNPs and their biological applications from Syzygium cumini 26, Eugenia caryophyllata27. From the leaf extract of Eugenia uniflora, silver nanoparticle formation was carried out and their antibacterial and antidiabetic potential were evaluated28.
Biofilm is a very fine extracellular polymer fibril that helps the bacteria adhere to the surface29. The bacterial community secrets an extracellular polymeric substance after adherence to a matrix or substratum which results in an alternation of phenotype and genetic change with the growth rate30. Bacteria forming biofilms possess great resistance to numerous stress conditions including some antibiotics, high salt concentration, acidic conditions, and many oxidizing agents, which results in increasing their pathogenicity31. Biofilm formation is seen in most medical devices, catheters, and other implants32.
Silver nanoparticle formation has already been reported from different plant extracts such as Azadirachta indica (Neem), Aloe vera, Emblica Officinalis (Amla), Cinnamomum camphora19,33–36. However, there is no such information about the synthesis of silver nanoparticles and any of their biological applications from the plant Eugenia roxburghii. Hence, in this study, an attempt was made to synthesize silver nanoparticles from the leaf extract and their activity against microbes. In our previous study, we found that leaf extract is highly effective in inhibiting the growth of microbes37. To enhance the antimicrobial activity of the leaf extract we have tried to prepare AgNPs from the extract to access the effect of the nanoparticles, we have used it to inhibit the growth of biofilms by S. aureus. As it has been seen that nanomaterial is better at combating microbes than normal crude extracts, our present investigation will help evaluate the antimicrobial effect of Eugenia AgNPs.
Results
Characterization by UV–Vis spectrophotometer
UV–Vis spectrophotometric analysis was carried out for the primary investigation of silver nanoparticle synthesis. A color change has been observed in the mixture of plant extract and AgNPs. The color of the mixture gradually changes from green to yellowish-brown confirming the production of E. roxburghii AgNPs. The absorbance of the solution was investigated for one week. From the spectral analysis, it is observed that the AgNPs peak was obtained at 417 nm with the highest peak (Fig. 1) and was stable thereafter for a few days as there was no increase in the absorption.
Figure 1.
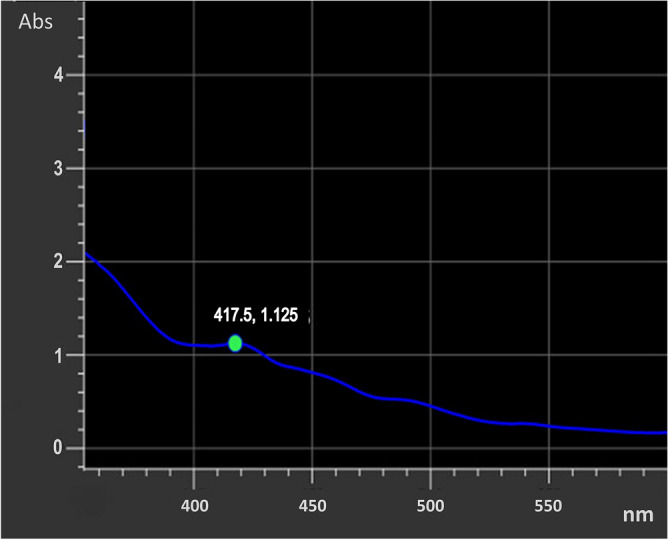
UV–Vis absorption spectra of synthesized AgNPs from E. roxburghii leaf extract.
Characterization by XRD
The crystallinity of the synthesized silver nanoparticle using E. roxburghii leaf extract was examined through X-ray diffraction (XRD) (Fig. 2). The size of the nanoparticles was calculated based on the Debye–Scherrer equation: (D = kλ/βcosθ).
Figure 2.
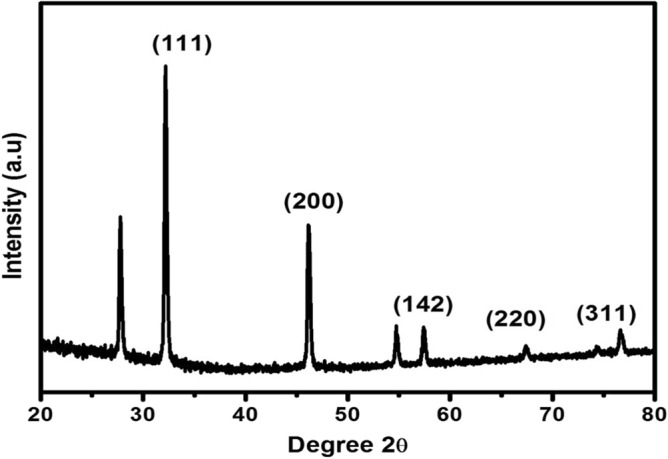
XRD pattern of AgNPs synthesized from E. roxburghii leaf extract.
In the above equation: D represents particle diameter size, K: a constant with a value of 0.9, λ: X-ray source wavelength (0.1541 nm), β and θ represent the FWHM (full width at half maximum), and diffraction angle concerning the (111) lattice planes respectively. The average crystalline size was found to be approximately 35 nm. The lattice parameters for the synthesized AgNPs were determined to be a = 0.4086 nm, b = 0.4086 nm, c = 0.4086 nm respectively. The calculated lattice value was 0.4086 nm, which was nearly identical to the normal lattice parameter of 0.4073 nm for silver38.
Characterization by HR-TEM
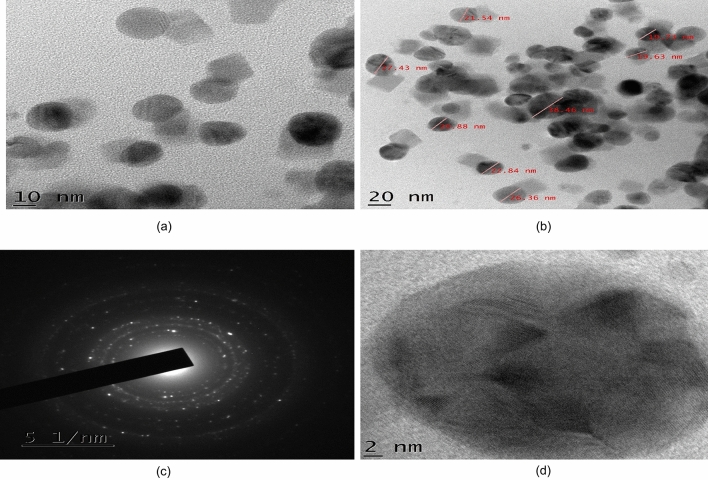
The resulted colloidal particles were characterized to determine their shape and size by high-resolution transmission electron microscopy (TEM). For the preparation of the TEM grid, a carbon-coated copper grid was used. A drop of the particle solution was placed over the grid and dried at room temperature. Different TEM micrograph images including SAED pattern and HR-TEM images of the synthesized AgNPs were obtained which are displayed in Fig. 3a–d. The estimated average particle size was approximately 24 nm whereas particle sizes ranged from approximately 19–39 nm.
Figure 3.
(a) TEM micrographs image of the synthesized AgNPs, (b) TEM image of different sized AgNPs, (c) SAED image of AgNPs, (d) HR-TEM image of AgNPs.
Characterization by Zeta sizer
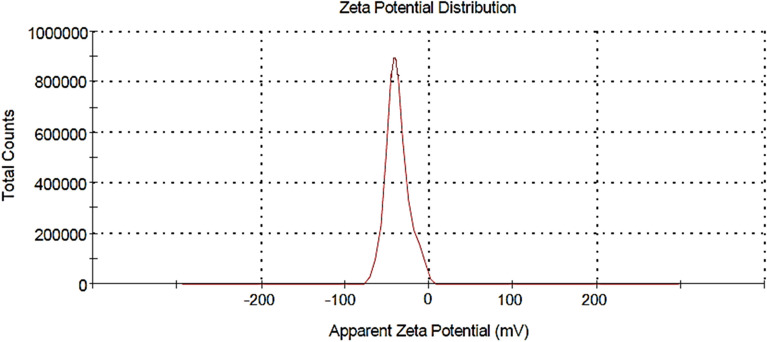
The surface potential of nanoparticles is the potential difference between the medium where nanoparticles are dispersed and the accessible surface of dispersed nanoparticles, which can be analyzed using a zeta sizer. Figure 4 demonstrates the zeta potential of the biosynthesized AgNPs which was found to be − 37.8 mV. This shows that the AgNPs synthesized from the leaf extract of E. roxburghii are highly stable.
Figure 4.
Zeta potential of synthesized AgNPs.
Analysis of antimicrobial activity
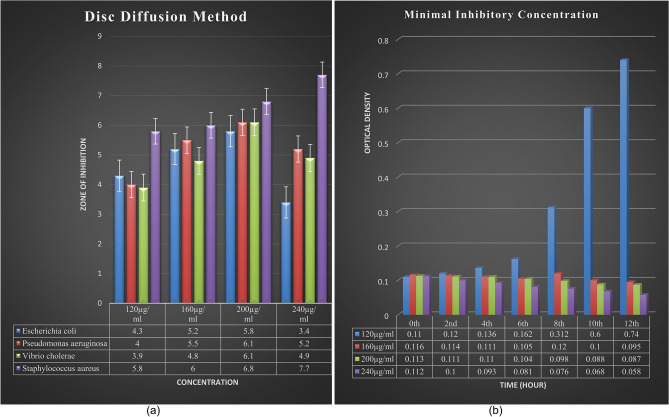
An antibacterial activity assay was carried out by using disc diffusion and the MIC method. It was observed that among all the bacteria taken, the AgNPs extract of E. roxburghii showed maximum effectiveness towards S. aureus (Fig. 5a). So, the MIC experiment was continued with the selection of S. aureus bacterium and the MIC test revealed that there was a continuous increase in absorbance at 120 µg/ml concentration whereas, at 240 µg/ml concentration of extract, there was a continuous decrease in absorbance. However, there was no such change in absorbance observed in other concentrations of the extract (Fig. 5b).
Figure 5.
(a) Antimicrobial activity test by Disc Diffusion Method against different bacterial strains, (b) Minimal Inhibitory Concentration test on S. aureus.
Examine the effect on biofilm
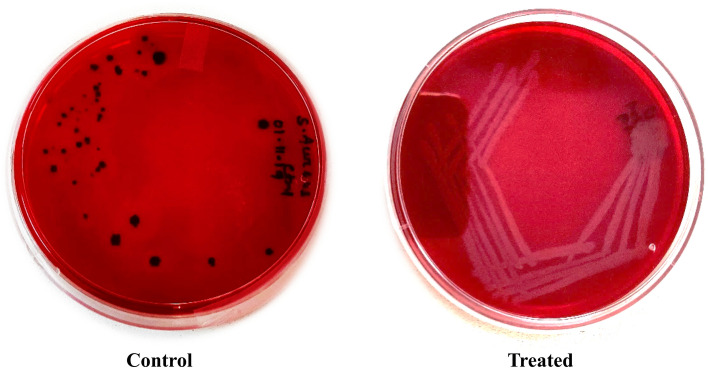
In this study, it was observed that bacteria changed their color in the control plate (CRA plate without AgNPs) whereas there was no change in the color of bacteria in AgNPs treated CRA plate (Fig. 6). This confirmed the direct inhibition of the biofilm production of bacteria by AgNPs.
Figure 6.
Effect of AgNPs on biofilm production by S. aureus on Congo Red Agar plates. *Control = without AgNPs, Treated = With AgNPs.
Discussion
The essential enzyme for nitrogen assimilation in a variety of species is nitrate reductase (NR)39, which catalyses the conversion of nitrate to nitrite in the cytoplasm of plant cells40. An enzymatic pathway involving NADPH-dependent reductase was shown to be responsible for the bioreduction of silver ions. Silver ions exposed to nitrate reductase resulted in the formation of very stable silver NPs and NADPH was found to be the cofactor of the nitrate reductase enzyme41. From a previous study, the absorption spectra of synthesized AgNPs from Syzygium Jambola was found to be 460 nm and its particle size from TEM analysis was found to be ranging from 6 to 23 nm42, similarly in Syzygium cumini the UV spectra of synthesized nanoparticle was observed at ~ 450 nm with particle size 3.5 nm from the XRD analysis43 and also in Eugenia uniflora UV spectra of synthesized nanoparticle was observed at 440 nm having its particle size was ranging from 25 to 50 nm44.
In this study, after mixing of extract and silver nitrate solutions a color change of extract was observed over the progression of time which may be due to the reduction of the silver ions leading to the excitation of Surface Plasmon Resonance (SPR) of the AgNPs45. To confirm this, UV spectra analysis was carried out and a peak was observed at 417 nm which showed a stable range for nanoparticle formation.
From the XRD pattern of the silver nanoparticle, the structure obtained to be a face-centered cubic one46. Four Bragg’s reflections conforming to (111), (200), (220), and (311) planes of metallic silver with FCC crystal structures are understood clearly from the XRD plot (JCPDS No. 89-3722)47. So, in the present study, the average crystalline nanoparticle size was measured to be approximately 35 nm. The extra peak obtained at 2θ nearly equal to 28 may be due to the bio-organic phase crystallization over silver nanoparticles surface48.
From the resulting images of HR-TEM analysis of synthesized AgNPs, it was observed that there was a presence of few agglomerated AgNPs in some places (Fig. 3a) which may be an indication of further sedimentation. Mostly spherical-shaped particles were observed with variations in their size (Fig. 3b). The average particle size was measured to be approximately 24 nm and the overall particle size ranged between 19 and 39 nm. The electron beam was directed perpendicular to one of the spheres to obtain the SAED (selected area electron diffraction) pattern and the crystallinity of the synthesized AgNO3 was confirmed through this pattern (Fig. 3c) which was recorded from one of the nanoparticles. The morphology of a single AgNO3 was obtained from the HR-TEM (high-resolution transmission electron microscopy) image and found to be spherical (Fig. 3d).
The antimicrobial activity of silver nanoparticle extract of E. roxburghii was tested against four different types of bacteria viz. E. coli, P. aeruginosa, V. cholera and S. aureus. In the disc diffusion method, the nanoparticle extract showed a significant effect towards S. aureus among the above four bacteria for that reason MIC experiment was conducted by taking S. aureus bacteria against which different concentrations (120 µg/ml, 160 µg/ml, 200 µg/ml and 240 µg/ml) of nanoparticle extract were treated. In this experiment, while measuring OD, a continuous increase in absorbance at 120 µg/ml concentration of extract may suggest that at a low concentration the bacteria get dominant over the activity of the extract while a continued decrease in absorbance at 240 µg/ml concentration of extract may suggest that at this concentration the extract is efficient enough to remove the bacterial colony.
As the bacteria, S. aureus itself is a biofilm-producing bacterium, confirmed biofilm production was observed in the control plate as the plate contains Congo Red media turns into a back color. It was reported that the biofilm-producing capacity of pathogenic bacteria was due to the secretion of exopolysaccharides (EPS)49. The change of color from red to black in CRA plates is due to EPS secretion by bacteria50. Because of the clinical approach, nowadays biofilm production by the microbes and their growth on the surfaces of medicating instruments and disposable products are the major paths through which microbes enter into the body51,52. The biofilms are extremely resistant to host defense mechanisms and also to antibiotic treatment. Adhesion or attachment of microorganisms to a substrate is the first step towards colonization and this strategy has been used for microbial biofilm production53. In this study, a new approach was undertaken by synthesizing nanoparticles from biomaterial and using them against biofilm-producing microorganisms to test their effects on them.
Material and methods
Preparation of plant extract
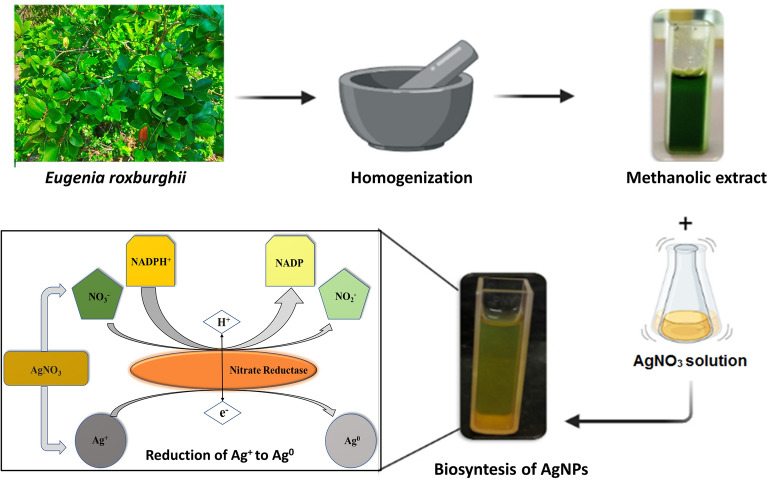
Fresh (disease-free) and fully expanded leaves of Eugenia roxburghii were collected with the permission of local authorities from the coastal area of Konark, Odisha (latitude 19.878 and longitude 86.101). The plant was taxonomically identified and authenticated by Dr. Laxmikanta Acharya (Associate professor, Centre for Biotechnology, Siksha ‘O’ Anusandhan University, Odisha, India) and a voucher specimen (SOAU/CBT/2020/ER/01) was retained in the department for future reference and the plant has been maintained in an environmentally controlled greenhouse. Experimental research on the plant used for the study complies with relevant institutional, national, and international guidelines and legislation. For the experiment, fresh and healthy leaves were taken and washed three times with distilled water. After washing, the methanolic extract was prepared by finely grinding 25 g of leaves with liquid nitrogen in a mortar and pestle followed by the addition of 250 ml of methanol. The debris from the leaf extract was separated with filter paper (Whatman No 1). The filtrate was collected and preserved at − 20 °C.
Preparation of AgNPs with Eugenia roxburghii leaf extract
One molar silver nitrate (AgNO3) stock solution was prepared. From that stock solution, 0.1 mM AgNO3 solution was taken along with the leaf extract in a 5:1 proportion for the preparation of AgNPs. 20 ml of leaf extract was mixed with 100 ml of 0.1 mM AgNO3 solution and incubated in a shaker incubator at 300 rpm at 37 °C for 48 h. Gradually the deep green color solution changed to yellowish-brown color which indicated the conversion of Ag+ to Ag0 (Fig. 7). The effect of this synthesis of silver nanoparticles was monitored in UV–VIS spectrophotometer. The spectrophotometric reading was taken at different time intervals.
Figure 7.
Synthesis of AgNPs from E. roxburghii leaf extract.
Characterization of AgNPs
Various analytical techniques were used for the characterization of green synthesized silver nanoparticles from E. roxburghii leaf extract. Constant monitoring of the reaction for the reduction of Ag+ ion by taking the OD (optical density) from 200–700 nm in a double beam UV–Vis spectrophotometer (Hitachi, UH5300). Further characterization of AgNPs was carried out through XRD (Rigaku, Ultima IV, Japan) equipped with Cu-Kα radiation; a crystal monochromator employing wavelengths of 0.1541 nm in a 2θ range from 20° to 80°. HR-TEM analysis of derived nanoparticles was carried out on a JEM 2100 (Jeol), operated at 200 kV.
Antimicrobial activity test
By disc diffusion method
To check the antimicrobial activity of E. roxburghii AgNPs extract, a disc diffusion method was carried out. For this test, different strains of bacteria such as E. coli (ATCC-443), P. aeruginosa (Clinically isolated from SCB medical college, Microbiology department, Cuttack, Odisha, India), V. cholera (ATCC-3906), and S. aureus (ATCC-96) were used which were identified and confirmed at the Centre for Biotechnology, Siksha O’ Anusandhan (Deemed to be University), Odisha, India. Active bacterial cultures were revived by inoculating a loop full of bacterial culture in nutrient broth from the stock maintained at 4 °C and incubated overnight at 37 °C in a shaker incubator at 800 rpm. Nutrient agar plates were prepared and spreading of 60 µl of each bacterial culture was carried out. Different concentrations such as 120 µg/ml, 160 µg/ml, 200 µg/ml, and 240 µg/ml of AgNPs extract infused discs were prepared and placed over the bacterial spread plates followed by incubation overnight at 37 °C. the observed zone of inhibition was measured in mm against the commercially available antibiotic ciprofloxacin.
By minimal inhibitory concentration (MIC)
To evaluate the minimal inhibitory concentration, different concentrations (120 µg/ml, 160 µg/ml, 200 µg/ml and 240 µg/ml) of AgNPs extract were tested against S. aureus. For this experiment, 25 ml of nutrient broth was added to four different conical flasks containing different concentrations of the AgNPs extract mentioned above followed by the addition of 100 µl of bacterial culture. After the addition of bacterial culture, OD was measured at 600 nm in every 2 h interval of time from 0 to 12 h followed by incubation at 37 °C at 800 rpm.
Effect of AgNPs on Biofilm synthesis
In this study, the main target was to evaluate the effectiveness of AgNPs extract of E. roxburghii against biofilm production and this experiment was carried out by selecting the S. aureus bacterium. A Congo Red Agar (CRA) plate assay was carried out to investigate the activity of AgNPs on Biofilm production54. Two media plates named control and treated were taken in which the control plate was incorporated with Congo Red Dye mixed nutrient agar streaked with S. aureus bacteria and the treated plate was incorporated with a mixture of nutrient agar, Congo Red Dye, and AgNPs extract (0.065 g/ml) streaked with S. aureus. Then the plates were incubated at 34 °C for 3 days.
Conclusion
The silver nanoparticle prepared from E. roxburghii leaf extract were observed under UV–Vis Spectroscopy monitored at 417 nm and their crystallinity nature was confirmed from their XRD study. AgNPs are found to be very effective against biofilm production by bacteria. However, an experiment must be carried out to find the effect of the NPs on the animal model as well as on human beings for the evaluation of efficacy. Toxicological studies are also required to eradicate any kind of intoxication in a mouse model or human being. Once the NP is found nontoxic or safe in vivo studies, the nanoparticle can be utilized for the treatment of various diseases such as diabetes, arthritis, hypertension, etc. AgNPs play a major role in inhibiting bacterial colonies and biofilm formation. This study springs a new approach for synthesizing nanoparticles from the leaves of E. roxburghii which is found out to be inhibiting biofilm production and bacterial colonies can be a significant achievement in contending many dynamic pathogens. Other nanoparticles besides AgNPs can also be prepared from the leaf extract and their medicinal properties can be exploited for the remedy of various diseases. So, the present work can be considered an attempt to exploit the active principle present in the leaf of E. roxburghii to cure various ailments.
Acknowledgements
The President and Vice-Chancellor of SOA (Deemed to be University) are highly acknowledged for the infrastructure facility.
Abbreviations
- AgNPs
Silver nanoparticles
- NPs
Nanoparticles
- AgNO3
Silver nitrate
- XRD
Xray Diffraction
- TEM
Transmission Electron Microscope
- SAED
Selected Area Electron Diffraction
- FWHM
Full Width at Half Maximum
- FCC
Face Centered Cubic
- CRA
Congo Red Agar
- EPS
Exopolysaccharide
- Units
G, ml, M
Author contributions
Sample collection and experiment were performed by G.A.K., J.B., and B.B. Data analysis was carried out by P.A.K., A.M., and A.S.. Final manuscript was written by G.A.K. Overall experiment was designed and guided by A.L. All authors have contributed to this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nassar MI, et al. Chemical constituents of clove (Syzygium aromaticum, Fam. Myrtaceae) and their antioxidant activity. Rev. Latinoam. Quim. 2007;35:47–57. [Google Scholar]
- 2.Park MJ, et al. Antifungal activities of the essential oils in Syzygium aromaticum (L.) Merr. Et Perry and Leptospermum petersonii bailey and their constituents against various dermatophytes. J. Microbiol. 2007;45:460–465. [PubMed] [Google Scholar]
- 3.Djipa CD, Delmée M, Quetin-Leclercq J. Antimicrobial activity of bark extracts of Syzygium jambos (L.) Alston (Myrtaceae) J. Ethnopharmacol. 2000;71:307–313. doi: 10.1016/S0378-8741(99)00186-5. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, et al. Anti-diabetic activity of Syzygium cumini and its isolated compound against streptozotocin-induced diabetic rats. J. Med. Plants Res. 2008;2:246–249. [Google Scholar]
- 5.Ayoola G, et al. Chemical analysis and antimicrobial activity of the essential oil of Syzigium aromaticum (clove) Afr. J. Microbiol. Res. 2007;2(7):162–166. [Google Scholar]
- 6.Chaudhuri AKN, Pal S, Gomes A, Bhattacharya S. Anti-inflammatory and related actions of Syzygium cuminii seed extract. Phyther. Res. 1990;4:5–10. doi: 10.1002/ptr.2650040103. [DOI] [Google Scholar]
- 7.Nonaka G, Aiko Y, Aritake K, Nishioka I. Tannins and related compounds. CXIX. Samarangenins A and B, novel proanthocyanidins with doubly bonded structures, from Syzygium samarangens and S. aqueum. Chem. Pharm. Bull. Tokyo. 1992;40:2671–2673. doi: 10.1248/cpb.40.2671. [DOI] [Google Scholar]
- 8.Chopra, R. N. et al. Glossary of Indian Medicinal Plants; [with] Supplement. (Council of Scientific and Industrial Research, 1956).
- 9.Dymock W, Warden CJH, H. D. Pharmacographia Indica. (Srishti Book Distributors, 2005).
- 10.Gunasekaran T, Haile T, Nigusse T, Dhanaraju MD. Nanotechnology: An effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac. J. Trop. Biomed. 2014;4:S1–S7. doi: 10.12980/APJTB.4.2014C980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vorobyova SA, Lesnikovich AI, Sobal NS. Preparation of silver nanoparticles by interphase reduction. Colloids Surf. A Physicochem. Eng. Asp. 1999;152:375–379. doi: 10.1016/S0927-7757(98)00861-9. [DOI] [Google Scholar]
- 12.Liu YC, Lin LH. New pathway for the synthesis of ultrafine silver nanoparticles from bulk silver substrates in aqueous solutions by sonoelectrochemical methods. Electrochem. commun. 2004;6:1163–1168. doi: 10.1016/j.elecom.2004.09.010. [DOI] [Google Scholar]
- 13.Bae CH, Nam SH, Park SM. Formation of silver nanoparticles by laser ablation of a silver target in NaCl solution. Appl. Surf. Sci. 2002;197–198:628–634. doi: 10.1016/S0169-4332(02)00430-0. [DOI] [Google Scholar]
- 14.Saravanan M, Nanda A. Extracellular synthesis of silver bionanoparticles from Aspergillus clavatus and its antimicrobial activity against MRSA and MRSE. Colloids Surf. B Biointerfaces. 2010;77:214–218. doi: 10.1016/j.colsurfb.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 15.Saravanan M, Vemu AK, Barik SK. Rapid biosynthesis of silver nanoparticles from bacillus megaterium (ncim 2326) and their antibacterial activity on multi drug resistant clinical pathogens. Colloids Surf. B Biointerfaces. 2011;88:325–331. doi: 10.1016/j.colsurfb.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Kumar V, Yadav S, Yadav S. Syzygium cumini leaf and seed extract mediated biosynthesis of silver nanoparticles and their characterization. J. Chem. Technol. Biotechnol. 2010;85:1301–1309. doi: 10.1002/jctb.2427. [DOI] [Google Scholar]
- 17.Daniel MC. Gold nanoparticles: assembly, supramolecularchemistry, quantum-size-related properties, and applications toward. Chem. Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 18.Vilchis-Nestor AR, et al. Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater. Lett. 2008;62:3103–3105. doi: 10.1016/j.matlet.2008.01.138. [DOI] [Google Scholar]
- 19.Shankar SS, Rai A, Ahmad A, Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004;275:496–502. doi: 10.1016/j.jcis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Becker RO. Silver ions in the treatment of local infections. Met. Based. Drugs. 1999;6:311–314. doi: 10.1155/MBD.1999.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vinayagam R, et al. Structural characterization of green synthesized magnetic mesoporous Fe3O4NPs@ME. Mater. Chem. Phys. 2021;262:124323. doi: 10.1016/j.matchemphys.2021.124323. [DOI] [Google Scholar]
- 22.Suman TY, Rajasree SRR, Kanchana A, Elizabeth SB. Biosynthesis, characterization and cytotoxic effect of plant mediated silver nanoparticles using Morinda citrifolia root extract. Colloids Surf. B Biointerfaces. 2013;106:74–78. doi: 10.1016/j.colsurfb.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 23.Selvaraj R, et al. Green synthesis of magnetic α–Fe2O3 nanospheres using Bridelia retusa leaf extract for Fenton-like degradation of crystal violet dye. Appl. Nanosci. 2021;11:2227–2234. doi: 10.1007/s13204-021-01952-y. [DOI] [Google Scholar]
- 24.Varadavenkatesan T, Pai S, Vinayagam R, Selvaraj R. Characterization of silver nano-spheres synthesized using the extract of Arachis hypogaea nuts and their catalytic potential to degrade dyes. Mater. Chem. Phys. 2021;272:125017. doi: 10.1016/j.matchemphys.2021.125017. [DOI] [Google Scholar]
- 25.Gomathi S, et al. Phytochemical screening of silver nanoparticles extract of eugenia jambolana using fourier infrared spectroscopy. Int. J. Res. Pharm. Sci. 2017;8:383–387. [Google Scholar]
- 26.de Carvalho Bernardo WL, et al. Antimicrobial effects of silver nanoparticles and extracts of Syzygium cumini flowers and seeds: Periodontal, cariogenic and opportunistic pathogens. Arch. Oral Biol. 2021;125:105101. doi: 10.1016/j.archoralbio.2021.105101. [DOI] [PubMed] [Google Scholar]
- 27.Barbinta-Patrascu ME, Badea N, Bacalum M. Novel bio-friendly nanomaterials based on artificial cell membranes, chitosan and silver nanoparticles phytogenerated. Rom. Rep. 2020;601:1–14. [Google Scholar]
- 28.Dugganaboyana, G. K., Mukunda, C. & Inakanally, S. D. Evaluation of Antibacterial and Antidiabetic Potential of Silver Nanoparticles using Eugenia Uniflora L. Seed Extract. Int. J. Pharm. Sci. Nanotechnol.13, (2020).
- 29.Marshall KC. Interfaces in microbial ecology. 1976. Soil Sci. 1977;123:344. doi: 10.1097/00010694-197705000-00012. [DOI] [Google Scholar]
- 30.Donlan RM, Costerton JW, Donlan RM, Costerton JW. Biofilms survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002;15(2):167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fux CA, Stoodley P, Hall-Stoodley L, Costerton JW. Bacterial biofilms: a diagnostic and therapeutic challenge. Expert Rev. Anti. Infect. Ther. 2003;1:667–683. doi: 10.1586/14787210.1.4.667. [DOI] [PubMed] [Google Scholar]
- 32.Götz F. MicroReview Staphylococcus and biofilms Mol. Microbiol. 2002;43:1367–1378. doi: 10.1046/j.1365-2958.2002.02827.x. [DOI] [PubMed] [Google Scholar]
- 33.Canizal G, Ascencio JA, Gardea-Torresday J, José-Yacamán M. Multiple twinned gold nanorods grown by bio-reduction techniques. J. Nanopart. Res. 2001;3:475–481. doi: 10.1023/A:1012578821566. [DOI] [Google Scholar]
- 34.Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog. 2006;22:577–583. doi: 10.1021/bp0501423. [DOI] [PubMed] [Google Scholar]
- 35.Ankamwar B, Damle C, Ahmad A, Sastry M. Biosynthesis of gold and silver nanoparticles using Emblica Officinalis fruit extract, their phase transfer and transmetallation in an organic solution. J. Nanosci. Nanotechnol. 2005;5:1665–1671. doi: 10.1166/jnn.2005.184. [DOI] [PubMed] [Google Scholar]
- 36.Krishnaraj C, et al. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B Biointerfaces. 2010;76:50–56. doi: 10.1016/j.colsurfb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Giri, A. K., Jena, B., Biswal, B., Pradhan, A. K. & Acharya, L. GC-MS profiling for the detection of bio-active compounds in Eugenia roxburghii and their activity against different microbes. Plant Arch.20, (2020).
- 38.Vinayagam R, Varadavenkatesan T, Selvaraj R. Green synthesis, structural characterization, and catalytic activity of silver nanoparticles stabilized with Bridelia retusa leaf extract. Green Process. Synth. 2018;7:30–37. doi: 10.1515/gps-2016-0236. [DOI] [Google Scholar]
- 39.Chamizo-Ampudia A, Sanz-Luque E, Llamas A, Galvan A, Fernandez E. Nitrate reductase regulates plant nitric oxide homeostasis. Trends Plant Sci. 2017;22:163–174. doi: 10.1016/j.tplants.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Kolbert Z, Bartha B, Erdei L. Exogenous auxin-induced NO synthesis is nitrate reductase-associated in Arabidopsis thaliana root primordia. J. Plant Physiol. 2008;165:967–975. doi: 10.1016/j.jplph.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 41.Kumar SA, et al. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO 3. Biotechnol. Lett. 2007;29:439–445. doi: 10.1007/s10529-006-9256-7. [DOI] [PubMed] [Google Scholar]
- 42.Ghareeb MA, et al. Antioxidant, antimicrobial, cytotoxic activities and biosynthesis of silver and gold nanoparticles using Syzygium jambos leaves growing in Egypt. Der Pharma Chem. 2016;8:107–116. [Google Scholar]
- 43.Banerjee J. (L.) Seed extract and evaluation of their in vitro antioxidant activities. Evaluation. 2011;6:961–968. [Google Scholar]
- 44.Dugganaboyana GK, Eranna CK. Green synthesis of silver nanoparticles by using Simarouba amara aubl. Fruit extract and their antioxidant and antibacterial activities. Int. J. Drug Deliv. Technol. 2017;7:137–145. [Google Scholar]
- 45.Mulvaney P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir. 1996;12:788–800. doi: 10.1021/la9502711. [DOI] [Google Scholar]
- 46.Shameli K, Ahmad MB, Yunus WZW, Ibrahim NA, Darroudi M. Synthesis and characterization of silver/talc nanocomposites using the wet chemical reduction method. Int. J. Nanomed. 2010;5:743–751. doi: 10.2147/IJN.S13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baker C, Pradhan A, Pakstis L, Pochan DJ, Shah SI. Synthesis and antibacterial properties of silver nanoparticles. J. Nanosci. Nanotechnol. 2005;5:244–249. doi: 10.1166/jnn.2005.034. [DOI] [PubMed] [Google Scholar]
- 48.Sathyavathi R, Krishna MB, Rao SV, Saritha R, Narayana Rao D. Biosynthesis of silver Nanoparticles using Coriandrum Sativum leaf extract and their application in nonlinear optics. Adv. Sci. Lett. 2010;3:138–143. doi: 10.1166/asl.2010.1099. [DOI] [Google Scholar]
- 49.Bosch A, et al. Characterization of Bordetella pertussis growing as biofilm by chemical analysis and FT-IR spectroscopy. Appl. Microbiol. Biotechnol. 2006;71:736–747. doi: 10.1007/s00253-005-0202-8. [DOI] [PubMed] [Google Scholar]
- 50.Ferreira AA, Tette PAS, Mendonça RCS, De Souza Soares A, De Carvalho MM. Detection of exopolysaccharide production and biofilm-related genes in staphylococcus spp. Isolated from a poultry processing plant. Food Sci. Technol. 2015;34:710–716. doi: 10.1590/1678-457X.6446. [DOI] [Google Scholar]
- 51.Donlan RM. Biofilm formation: a clinically relevant microbiological process. Clin. Infect. Dis. 2001;33:1387–1392. doi: 10.1086/322972. [DOI] [PubMed] [Google Scholar]
- 52.Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 53.Dror N, Mandel M, Hazan Z, Lavie G. Advances in microbial biofilm prevention on indwelling medical devices with emphasis on usage of acoustic energy. Sensors (Basel). 2009;9:2538–2554. doi: 10.3390/s90402538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mariana NS, Salman SA, Neela V, Zamberi S. Evaluation of modified Congo red agar for detection of biofilm produced by clinical isolates of methicillinresistance Staphylococcus aureus. Afr. J. Microbiol. Res. 2009;3:330–338. [Google Scholar]