Abstract
Cardiovascular disease is the leading cause of death worldwide. New therapeutic strategies are aimed to modulate the athero-inflammatory process that partially orchestrates underlying vascular damage. Peripheral blood circulating cells include different immune cells with a central role in the development of the atherogenic inflammatory response. The anti-aging protein α-Klotho has been related to protective effects against CVD. KL is expressed in monocytes, macrophages, and lymphocytes where it exerts anti-inflammatory effects. In this work, we analyse the relationships of the levels of inflammatory markers with the expression of the KL gene in PBCCs and with the serum levels of soluble KL in atherosclerotic vascular disease. For this, we conducted a cross-sectional single-center case–control study including a study group of 76 CVD patients and a control group of 16 cadaveric organ donors without medical antecedent or study indicating CVD. Vascular artery fragments and whole blood and serum samples were obtained during elective or organ retrieval surgery. Serum levels of sKL, TNFα and IL10, and gene expression levels of KL, TNF, IL10, NFKB1, DNMT1, and DNMT3A in PBCCs were measured. In these cells, we also determined KL promoter methylation percentage. Histological and immunohistochemical analyses were employed to visualize atherosclerotic lesions and to measure IL10 and TNFα levels in vascular fragments. Patients with CVD presented higher values of proinflammatory markers both at systemic and in the vasculature and in the PBCCs, compared to the control group. In PBCCs, CVD patients also presented lower gene expression levels of KL gene (56.4% difference, P < 0.001), higher gene expression levels of DNMT1 and DNMT3A (P < 0.0001, for both) and a higher methylation status of in the promoter region of KL (34.1 ± 4.1% vs. 14.6 ± 3.4%, P < 0.01). In PBCCs and vasculature, KL gene expression correlated inversely with pro-inflammatory markers and directly with anti-inflammatory markers. sKL serum levels presented similar associations with the expression levels of pro- and anti-inflammatory markers in PBCCs. The differences in KL expression levels in PBCCs and in serum sKL levels with respect to control group was even greater in those CVD patients with macroscopically observable atheromatous plaques. We conclude that promoter methylation-mediated downregulation of KL gene expression in PBCCs is associated with the pro-inflammatory status in atherosclerotic vascular disease.
Subject terms: Atherosclerosis, Ageing
Introduction
Atherosclerosis underlies most of cardiovascular diseases (CVD), such as coronary artery disease (CAD), stroke and peripheral artery disease (PAD)1. The pathophysiology of CVD constitutes a slow, progressive and chronic inflammatory process2 that results of the systemic influence of diverse cardiovascular risk factors (CVRF) -dyslipidemia, smoking, obesity, diabetes, and hypertension, among others-. These CVRF cause endothelial dysfunction and, thereby, permeabilization of low-density lipoproteins (LDL) and various inflammatory cells into subintimal space, ultimately leading to the atheroma plaque formation. Although conventional therapeutic strategies have been focused on managing these CVRF, new alternatives have been proposed focused on modulating the athero-inflammatory process3,4. Macrophages, monocytes, lymphocytes, and other peripheral blood circulating cells (PBCCs) have a central role in the development of the inflammatory response associated to the atherogenic process. Their contribution to this state range from the low-grade systemic inflammation that accompanies CVD (secretion of pro- or anti-inflammatory factors into the systemic circulation) to the resolution of the local response of the vascular wall (environmental signal transduction, uptake of LDL or LDLox particles, engulfment of dead cells, secretion of inflammatory cytokines or pro-resolving molecules, etc.)2,5–7. Immunomodulation of these cells constitutes an interesting approach to the development of new therapeutic strategies in CVD.
Deficits in the antiaging factor α-Klotho (KL) are observed in diverse pathologies related to human aging8–13. Reductions in the soluble form (sKL) have been associated with the appearance of CAD, PAD, heart failure or stroke, as well as with poor results in vascular functionality tests14–17. Furthermore, experimental evidences from animal studies or in vitro models have shown that KL has protective effects against different forms of cardiovascular damage (vascular calcification, endothelial dysfunction or heart failure)18–23. One of the proposed mechanisms for the effects of KL on the vasculature is the modulation of the inflammatory response. Anti-inflammatory effects of KL already described include the reduction in the expression of pro-inflammatory cytokines24 and the increased secretion of anti-inflammatory factors25,26, the reduction of oxidative stress molecules27,28, the inhibition of the NFkB or NLRP3 inflammasome signaling pathways29–32, and the inhibition of the expression of endothelial adhesion molecules (ICAM1 and VCAM1)33. Furthermore, proinflammatory processes also have the ability to negatively modulate KL expression, thus shutting down a hypothetical feedback control system. KL is mainly expressed in the kidneys, but also in other tissues and cell types including monocytes, macrophages and lymphocytes26,34,35. In these cells, KL displays various anti-inflammatory effects26,34–39; therefore, the modulation of its production could represent a potential pathway for the regulation of the inflammatory response. However, there is a lack of knowledge about KL expression profile in PBCCs and its relationships with the inflammatory process during CVD. In this work, we aim to fill this gap by analyzing different markers of systemic and vascular inflammation and their relationships with KL gene expression in PBCCs and with serum levels of sKL in a group of patients with diagnosed atherosclerosis.
Methods
Patients and samples
This study follows a cross-sectional single center case–control design. Participants were consecutively enrolled from November 2014 to September 2017 in the Vascular Surgery and Transplant Coordination services of the University Hospital Nuestra Señora de Candelaria (UHNSC). The study protocol was approved by the UHNSC Ethics Committee and complied with ethical standards of the Declaration of Helsinki. Written informed consent was obtained from all participants.
Case group consisted in 76 patients older than 18 years which underwent an elective open vascular surgery procedure due to clinical atherosclerotic vascular disease. Exclusion criteria included hemodynamic instability during the surgical procedure (defined as a systolic blood pressure lower than 90 mmHg or the need for inotropes or vasopressors); history of chronic inflammatory, immunologic, or tumoral disease; positive serology to hepatitis B, hepatitis C, or HIV; acute inflammatory or infectious intercurrent episodes in the previous month; renal insufficiency, defined as an estimated glomerular filtration rate (eGFR) lower than 60 mL/min/1.73m2; institutionalization; receipt of immunotherapy or immunosuppressive treatment; and inability or unwillingness to provide informed consent. The distribution of clinical diagnosis was: 45 patients with PAD and intermittent claudication, 30 patients with transient ischemic attack (TIA), and 16 patients with abdominal aortic aneurysm (AAA). Two or more of these diagnoses were present in 14 patients. In all cases, the presence of established atherosclerotic vascular disease was confirmed with imaging studies that included computing tomography, magnetic resonance, and/or angiography procedures. During surgery, a sample of the carotid, aorta, femoral artery, or peripheral territories, according to the affected vessel, was obtained from the participants.
The control group consisted of 16 cadaveric organ donors, without any medical history or study indicating the presence of CVD. All organ donors underwent monitoring in the hospital prior to death. This was carried out by the Transplant Coordination Unit, which was responsible for the diagnosis of death, organs extraction, and vascular samples retrieval. Control subjects suffered brain death (10) or were controlled asystole donors (6). In order to minimize the inflammatory cascade occurring in the decease process, a corticosteroid treatment is routinely administered to donors (1 single bolus of methylprednisolone, 15 mg/kg) at the time of diagnosis of death (in cases of brain death) or before extubation (in asystole). Vascular samples were retrieved at the time of organ harvesting (4–6 h after time of death).
Whole blood samples (2.5 mL) were collected in either PAXgene Blood RNA tubes (BD, Franklin Lakes, NJ) and routine blood-collection tubes (BD serum separation transport tube—BD, Franklin Lakes, NJ). In case group, the collection was made at the time of surgery. In control subjects, samples were obtained at the moment of diagnosis (brain death) or while the patient was still alive (asystole). Serum fractions were isolated, aliquoted and immediately frozen at -80ºC. For methylation analysis, a blood-collection tube (BD K2EDTA tube—BD, Franklin Lakes, NJ) was drawn from 44 CVD patients and form 15 control subjects.
Serum determinations
Routine biochemical and hematological parameters were determined using standardized tests at the UHNSC Clinical Analysis Service. Serum C-reactive protein (CRP) levels were measured using a highly sensitive automated immunoturbidimetric test on a Cobas 6000 analyzer (Roche Diagnostics GmbH), with a sensitivity of 0.3 mg/L and intra- and inter-assay coefficients of variation of 1.6 and 8.4%, respectively. Serum levels of the inflammatory cytokines TNFα and IL10 were measured using commercial ELISA assay kits (Human TNFα Quantikine HS ELISA HSTA00D and Human IL10 Quantikine D100B, R&D Systems) according to manufacturer's instructions. Samples with serum IL10 values below the kit's sensitivity limit (< 3.9 pg/mL) were reanalyzed with the commercial high sensitivity assay Human IL10 Quantikine HS HS100C (R&D Systems). Concentrations of serum sKL protein were measured by solid phase sandwich ELISA (human soluble α-Klotho assay kit, Immuno-Biological Laboratories) according to manufacturer’s instructions. The assay sensitivity for this kit was 6.15 pg/mL and the intra- and inter-assay coefficients of variation were 2.7–3.5% and 2.9–11.4%, respectively.
RT-PCR and gene expression analysis
Vascular segments were completely homogenized in liquid nitrogen with a pestle and mortar. Total RNA was extracted using RNAzol RT according to manufacturer's instructions (Sigma Aldrich, MO, USA) and stored at − 80 °C. Total RNA from blood samples was isolated using PAXgene Blood RNA Kit (PreAnalytiX, Switzerland) following the manufacturer's guidelines. RNA was retrotranscribed to cDNA using a High Capacity RNA-to-cDNA kit (Applied Biosystems, CA, USA) for further use in quantitative RT-PCR (qRT-PCR).
KL gene cDNA was amplified by RT-PCR (KLcDNA-F:5'ACTCCCCCAGTCAGGTGGCG G3', KLcDNA-R:5'TGGGCCCGGGAAACCATTGCT3') to confirm its expression in PBCCs with the following conditions: 1.5 μL KAPA Taq Buffer B 10X, 0.75 μL MgCl2 25 mM, 1.5 μL dNTPs 2 mM, 0.3 μL forward and reverse primer 20 μM, 0.06 μL KAPA Taq DNA pol 5U/μL and 2 μL DNA sample in a total volume of 15 μL. Thermal cycling conditions were 94 °C, 3 min; (94 °C, 30 s; 55 °C, 30 s; 72 °C, 30 s) × 35 cycles; 72 °C, 1 min. The result was confirmed by the presence of a 350 bp band in 1% agarose electrophoresis and sequencing.
Transcripts encoding for KL, TNF, IL10, NFKB1, DNMT1, DNMT3A and GAPDH genes were measured by TaqMan qRT-PCR with PerfeCTa FastMix II Low ROX (QuantaBio, MA, USA) in a 7500 Fast Real-Time PCR System (Applied Biosystems, CA, USA). TaqMan gene expression assays employed were: Hs00183100_m1 [KL], Hs00174128 m1 [TNF], Hs00961622_m1 [IL10], Hs00765730 m1 [NFKB1], Hs00945875_m1 [DNMT1], Hs00173377_m1 [DNMT3A] and Hs99999905_m1 [GAPDH] The level of target mRNA was estimated by relative quantification using the 2-ΔΔCt method and GAPDH as housekeeping gene. Quantification of each cDNA sample was tested in triplicate.
Promoter methylation analysis
DNA was extracted from PBCCs using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. DNA quantity and purity were determined using a Nanodrop Lite Spectrophotometer (Thermo Fisher Scientific, MA, USA). 500–1000 ng of genomic DNA were subjected to conversion with sodium bisulfite using the EpiTect® Fast DNA Bisulfite kit (QIAGEN) according to the supplier's instructions.
Posteriorly, KL gene promoter was amplified by methylation independent PCR (KL-MIP). Firstly, the − 1363 to + 74 region was pre-amplified (KL-MIPouterF: 5'GGGTAGGGAGGTAGGGATATTAG3’, KL-MIPouterR: 5'CCCAACAACACCAACAACAAC3’) and the region of interest (− 827 to − 258) was subsequently amplified using a 1/10–1/100 dilution of the previous PCR product as template (KL-MIPinnerF: 5’AATTTGGTGTTTGGTTTTTTAGGAG3’, KL-MIPinnerR: 5’CACCTATTTCTCCCAACTCCC3’). PCR reactions were performed with the KAPA2G Robust HotStart PCR kit (KAPA Biosystems), using the following conditions: 3 μL KAPA2G Buffer B 5X, 3 μL KAPA Enhancer 5X, 0.3 μL dNTPs 10 mM, 0.3 μL forward and reverse primer 20 μM, 0.12 μL KAPA2G Robust HotStart DNA pol 5U/μL and 2 μL DNA sample in a total volume of 15 μL. Thermal cycling conditions were 95 °C, 5 min; (95 °C, 30 s; 55 °C, 30 s; 72 °C, 90 s) × 25 cycles; 72 °C, 5 min for MIPouter, and 95 °C, 3 min; (95 °C, 15 s; 60 °C, 15 s; 72 °C, 15 s) × 35 cycles; 72 °C, 1 min for MIPinner. PCR products were sequenced and the methylation status of 11 CpG positions in the region between -648 and -560 was analyzed. Methylation levels were calculated as percentages: (number of methylated CpG positions/total number of CpG positions) × 100.
Immunohistochemistry
Immunohistochemical and histological analyses of the vascular wall were performed in 37 patients and 10 controls. Sections of blood vessels were fixed in 4% buffered formalin for 24 h and subsequently dehydrated in ascending concentrations of ethanol, cleared in xylene and embedded in paraffin. Blocks were trimmed and 3 µm sections were processed for histology and immunohistochemistry. Hematoxylin and eosin (HE) and Masson's trichrome staining were performed. Primary antibodies used for immunohistochemistry were mouse monoclonal anti-IL10, 1:300 dilution (Santa Cruz Biotechnology Inc., Dallas, TX, USA) and rabbit monoclonal anti-TNFα, 1:100 dilution (Santa Cruz Biotechnology Inc.). For quantification analysis, a total of 5 images, that included intima and media layers, of each slide were captured and processed with a high-resolution video camera (Sony, DF-W-X710, Kōnan, Japan) connected to a light microscope (Nikon Eclipse 50i). Stained areas were quantified using ImageJ software (Rasband, W.S., ImageJ, National Institutes of Health, Bethesda, MD, USA). Results are expressed in square microns (µm2).
Statistics
Continuous variables are reported as mean ± standard deviation (SD) or medians and interquartile ranges (IQR). Categorical data are presented as percentages. Continuous variables were assessed for normal distribution by D’Agostino-Pearson test, and those variables that presented non-normal distribution were log-transformed for statistical analysis. Differences among groups were analyzed by unpaired t test, Mann–Whitney test or one-way analysis of variance with Tukey's post hoc test. Categorical variables were compared between groups using Fisher's exact test. Correlation analysis was evaluated by Spearman correlation test. Multiple lineal regression analysis was performed using KL gene expression in PBCCs as dependent variable and eGFR, CRP, phosphorus, total cholesterol, vascular TNF/IL10 ratio, PBCCs TNF/IL10 ratio, serum TNFα/IL10 ratio and serum sKL levels were introduced as covariates. Pair correlations between these covariates and KL gene expression are shown in supplemental material. Multiple logistic regression analyses were performed to assess independent predictors of the presence of CVD and of atherosclerotic plaque. For this purpose, we adopted three models: in model 1, we introduced conventional risk factors as covariates (age, sex, smoking, hypertension (HT), diabetes mellitus (DM)); in model 2, we additionally included TNF/IL10 gene expression ratio in PBCCs and serum TNFα/IL10 ratio; in model 3, we adjusted the analysis by including KL gene expression in PBCCs and sKL serum concentrations. Values of P < 0.05 were considered significant. Statistical analyses were performed using IBM SPSS Statistics V.19 (IBM Corporation, NY, USA) and GraphPad Prism 6.01 software (GraphPad Software, CA, USA).
Results
Clinical data
The group of CVD patients tended to a lower body mass index (27.5 ± 3.6 vs. 29.4 ± 3.2, P = 0.06), a higher prevalence of HT (77.6% vs. 56.3%, P = 0.11), with no differences in the frequency of DM (43.4% vs. 31.3%, P = 0.42). Regarding laboratory data, CVD patients presented lower serum LDL (88.2 ± 37.2 vs. 121.0 ± 26.1, P < 0.01) and calcium (9.1 ± 0.5 vs. 9.3 ± 0.9, P < 0.05) (Table 1). The use of antiplatelet agents (91% vs. 16.7%, P < 0.0001), angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists (51.3% vs. 25.0%, P < 0.05) and statins (85.5% vs. 8.3%, P < 0.0001) was significantly higher in the CVD group (Table 1).
Table 1.
Clinical characteristics and biochemical assessments of the patients included in the study.
| CVD (n = 76) | Non CVD (n = 16) | P value | |
|---|---|---|---|
| Age (years) | 65.3 ± 7.4 | 63.2 ± 9.3 | 0.38 |
| Sex (M/F) | 59 / 17 | 10/6 | 0.22 |
| Smoker (%) | 77.6 | 75.0 | 0.75 |
| BMI (kg/m2) | 27.5 ± 3.6 | 29.4 ± 3.2 | 0.06 |
| HT (%) | 77.6 | 56.3 | 0.11 |
| DM (%) | 43.4 | 31.3 | 0.42 |
| Pharmacological treatment | |||
| Antiaggregants (%) | 91.0 | 16.7 | < 0.0001 |
| Beta-blockers (%) | 27.6 | 16.7 | 0.34 |
| ACEI/ARA2 (%) | 51.3 | 25.0 | < 0.05 |
| CCB (%) | 25.0 | 16.7 | 0.35 |
| Statins (%) | 85.5 | 8.3 | < 0.0001 |
| Laboratory data | |||
| eGFR (mL/min/1.73 m2) | 89.6 ± 13.2 | 89.8 ± 26.7 | 0.61 |
| Creatinine (mg/dL) | 0.84 ± 0.2 | 0.93 ± 0.5 | 0.48 |
| Albumin (g/dL) | 3.8 ± 0.5 | 3.9 ± 0.6 | 0.43 |
| Calcium (mg/dL) | 9.1 ± 0.5 | 9.3 ± 0.9 | < 0.05 |
| Phosphorus (mg/dL) | 3.6 ± 0.5 | 3.5 ± 0.9 | 0.67 |
| Uric acid (mg/dL) | 5.9 ± 1.4 | 6.1 ± 1.5 | 0.63 |
| Glucose (mg/dL) | 117.3 ± 37.1 | 131.2 ± 39.4 | 0.21 |
| Cholesterol (mg/dL) | 165.4 ± 48.7 | 190.2 ± 29.9 | 0.12 |
| HDL (mg/mL) | 43.2 ± 11.2 | 47.5 ± 14.6 | 0.67 |
| LDL (mg/dL) | 88.2 ± 37.2 | 121.0 ± 26.1 | < 0.01 |
| Neutrophils (/mL) | 7494 ± 5081 | 8287 ± 5815 | 0.50 |
| Lymphocytes (/mL) | 2020 ± 998.2 | 1894 ± 729.9 | 0.91 |
| Serum inflammatory markers | |||
| NLR | 3.8 ± 1.9 | 2.8 ± 1.2 | 0.21 |
| CRP (mg/L) | 2.9 ± 2.6 | 1.9 ± 0.9 | 0.38 |
| TNFα (pg/mL) | 1.04 (0.80–1.44) | 1.37 (0.90–1.98) | 0.11 |
| IL10 (pg/mL) | 3.93 (0.61–10.13) | 10.38 (7.52–30.20) | < 0.001 |
| TNFα/IL10 | 0.28 (0.10–1.62) | 0.11 (0.02–0.27) | < 0.01 |
| sKL (pg/mL) | 507.7 (361.4–656.6) | 1007 (590.4–1883) | < 0.01 |
ACEI/ARA2 Angiotensin converting enzyme inhibitor/angiotensin receptor antagonist 2; BMI Body mass index; CCB Calcium channels blockers; CRP C-reactive protein; DM Diabetes mellitus; eGFR Estimated glomerular filtration rate; HDL High-density lipoprotein; HT hypertension; IL10 Interleukin 10; LDL Low-density lipoprotein; NLR Neutrophil-to-lymphocyte ratio; TNFα Tumor necrosis factor alpha.
Inflammatory markers in serum, PBCCs and vascular wall
We evaluated systemic inflammation by determining serum levels of the cytokines TNFα (pro-inflammatory) and IL10 (anti-inflammatory), and also of CRP and the neutrophil-to-lymphocyte ratio (NLR). The CVD group showed no significant differences for the systemic levels of TNFα [1.04 (0.80 to 1.41) vs. 1.37 (0.90 to 1.98); P = 0.11], while IL10 concentrations were reduced compared to the control group [3.93 (0.61 to 10.13) versus 10.38 (7.52 to 30.20), P < 0.001]. In order to assess the global inflammatory status, values for the TNFα/IL10 ratio were also calculated for each sample. This parameter was significantly higher in the CVD group [0.28 (0.10 to 1.62) vs. 0.11 (0.02 to 0.27), P < 0.01]. CRP levels (3.8 ± 1.9 vs. 2.8 ± 1.2; P < 0.21) and the NLR (2.9 ± 2.6 vs. 1.9 ± 0.9; P < 0.38) were higher in the CVD group but differences did not reach statistical significance. Serum sKL levels were significantly diminished in the CVD group [507.7 (361.4 to 656.6) vs. 1007 (590.4 to 1883), P < 0.01] (Table 1).
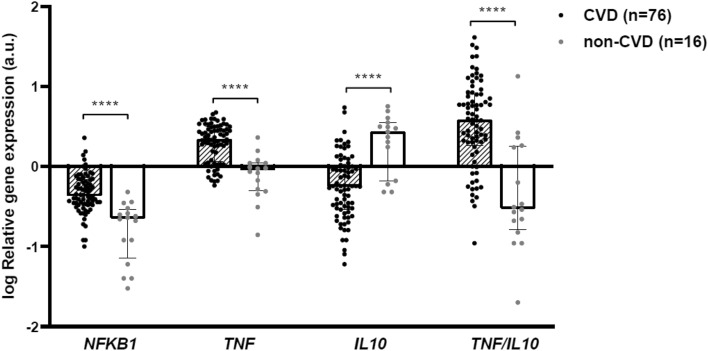
Inflammatory markers were also investigated in PBCCs. Gene expression levels of NFKB1, which codifies for the nuclear factor-kappa-B (NFκB) p105 subunit implied in the pro-inflammatory NFκB pathway, were significantly higher in PBCCs of the CVD group [log RQ: − 0.37 (− 0.47 to − 0.17) versus − 0.65 (− 1.15 to − 0.54), P < 0.0001]. Similarly, TNF gene expression levels were significantly higher in PBCCs of the CVD group [log RQ: 0.34 (0.06 to 0.49) versus − 0.05 (− 0.30 to 0.04), P < 0.0001], while transcript levels of IL10 gene were higher in the control group [log RQ: − 0.25 (− 0.53 to 0.01) vs. 0.43 (− 0.18 to 0.55), P < 0.0001]. Thus, the TNF/IL10 expression ratio presented significantly higher values in the CVD group compared to control individuals [log: 0.58 (0.26 to 0.90) versus − 0.52 (− 0.79 to 0.26), P < 0.0001] (Fig. 1).
Figure 1.
Inflammatory markers in PBCCs of CVD and non-CVD subjects. Relative gene expression levels of NFKB1, TNF and IL10 loci and their ratio. a.u.: arbitrary units. Bars and range represent median and IQR. ***: P < 0.001; ****: P < 0.0001.
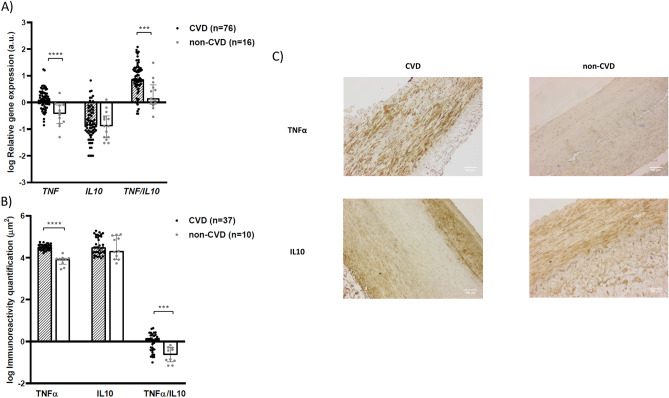
Vascular gene expression analysis revealed that patients with CVD had higher expression levels of TNF [log RQ: 0.09 (− 0.08 to 0.34) versus − 0.43 (− 0.78 to − 0.09), P < 0.0001] with no differences for IL10 expression [log RQ: − 0.85 (− 1.16 to − 0.33) versus − 0.89 (− 1.33 to -0.50), P = 0.64]. A more pronounced pro-inflammatory state was observed in patients with CVD compared to non-CVD subjects when assessing the TNF/IL10 ratio [log: 0.87 (0.64 to 1.24) versus 0.15 (− 0.07 to 0.66), P < 0.001] (Fig. 2A). Inmmunohistochemical analysis (Fig. 2C) revealed higher vascular immunoreactivity levels for TNFα protein in patients with established CVD compared to control subjects [log μm2: 4.51 (4.38 to 4.61) versus 3.94 (3.68 to 3.97), P < 0.0001], while immunoreactivity levels for IL10 were similar in both groups [log μm2: 4.49 (4.22 to 5.00) versus 4.32 (3.92 to 5.07), P = 0.66]. Values for the TNFα/IL10 ratio were also significantly higher in CVD patients compared to control group [log: 0.09 (− 0.40 to 0.28) versus − 0.64 (− 0.98 to − 0.30), P < 0.001] (Fig. 2B).
Figure 2.
Inflammatory markers in the vascular wall of CVD and non-CVD subjects. (A) Relative gene expression levels and (B) immunoreactivity levels for TNF, IL10 and their ratio. (C) Immunohistochemistry images for TNFα and IL10. a.u.: arbitrary units. Bars and range represent median and IQR. ***: P < 0.001; ****: P < 0.0001.
KL expression and epigenetic regulation in PBCC
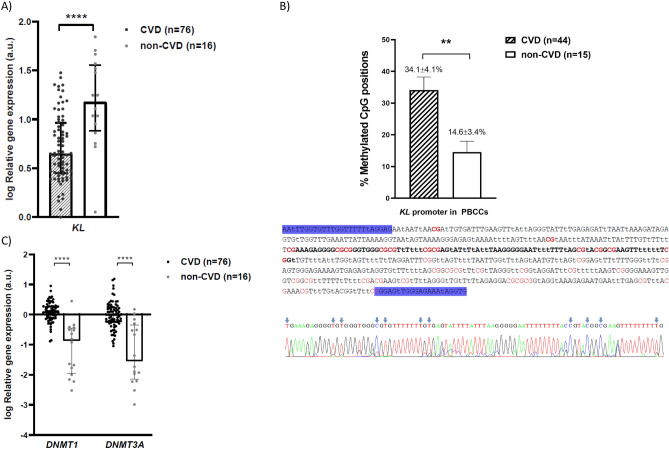
Quantitative analysis of KL gene expression in PBCCs showed that patients with established CVD presented significantly lower levels of the mRNA compared to control subjects [log RQ: 0.65 (0.45 to 0.97) versus 1.18 (0.88 to 1.57), P < 0.0001; a reduction of 56.4%] (Fig. 3A). We did not observe significant differences in KL gene expression in PBCCs when we stratified the patients according to their co-morbidities (Supplementary Fig. S1). KL gene promoter has two CpG islands between − 848 and + 88 positions, and their hypermethylation is associated with gene expression downregulation. We investigated the methylation status of 11 positions in the promoter region (between − 648 and − 560 positions) of the KL gene promoter in PBCCs from the study subjects. The analysis showed that the group of CVD patients had a higher degree of methylation of the interrogated positions compared to the control group (34.1 ± 4.1% vs. 14.6 ± 3.4%; P < 0.01) (Fig. 3B). Moreover, this same group of patients presented higher expression levels of two genes codifying for DNA-methyltransferases responsible for genome methylation: DNMT1 [log RQ: 0,10 (− 0.11 to 0.28) versus − 0.86 (− 1.94 to − 0.45); P < 0,0001] and DNMT3A [log RQ: − 0.03 (− 0.26 to 0.24) versus − 1.54 (− 2.15 to − 0.35); P < 0.0001] (Fig. 3C).
Figure 3.
KL gene expression and epigenetic regulation in PBCCs of CVD and non-CVD subjects. (A) Relative gene expression levels of KL locus, (B) methylation levels of 11 CpG positions (red) in the − 648 and − 560 regions (bold) of KL gene promoter (lowercase “t” represents unmethylated cytosines after conversion with sodium bisulfite), (C) relative gene expression levels of DNMT1 and DNMT3A loci. a.u.: arbitrary units. Bars and range represent median and IQR (A and C) or mean and SEM (B). **: P < 0.01; ****: P < 0.0001.
Associations between inflammatory markers and KL expression in PBCCs and serum sKL
We developed correlation analyses to study the bivariate associations of different vascular, PBCCs and serum inflammatory parameters with KL expression in PBCCs and sKL serum concentrations (Table 2). Pro-inflammatory markers in PBCCs were significantly and inversely associated with the gene expression of KL in these cells (NFKB1: r = − 0.281, P < 0.01; TNF: r = − 0.310, P < 0.01; TNF/IL10 ratio: r = − 0.509, P < 0.0001), while IL10 expression levels was directly correlated (r = 0.500, P < 0.0001). Regarding serum inflammatory parameters, we only found a significant direct association for IL10 circulating levels and KL expression in PBBCs (r = 0.253, P < 0.05). Interestingly, vascular TNF/IL10 ratio presented a significant inverse correlation with KL expression in PBCCs (r = − 0.337, P < 0.01). KL and DNMT1 expressions were significantly and inversely correlated (r = − 0.257, P < 0.05), while association between KL and DNMT3A expressions did not reach statistical significance (r = − 0.166, P = 0.12). Curiously, pro-inflammatory markers in PBCCs were directly associated with both DNMT1 and DNMT3A, while IL10 expression in these cells was inversely correlated with both genes encoding for DNA-methyltransferases (Table 2). Serum levels of sKL presented significant correlations with inflammatory markers in PBCCs, being direct with pro-inflammatory markers (TNF: r = − 0.295, P < 0.01; r = − 0.294, P < 0.01 for TNF/IL10) and inverse with IL10 gene expression (IL10: r = 0.234, P < 0.05). We also observed that circulating levels of sKL tended to be positively associated with KL expression in these cells (r = 0.173, P = 0.11), but it did not present significant associations with vascular markers of inflammation (Table 2).
Table 2.
Bivariate correlation analysis between PBCCs expression, vascular tissue expression and serum levels parameters.
| PBCCs expression (n = 92) | ||||||
|---|---|---|---|---|---|---|
| KL (a.u.) | DNMT1 (a.u.) | DNMT3A (a.u.) | ||||
| r | P value | r | P value | r | P value | |
| PBCCs expression | ||||||
| KL (a.u.) | 1.000 | |||||
| DNMT1 (a.u.) | − 0.257* | < 0.05 | 1.000 | |||
| DNMT3A (a.u.) | − 0.166 | 0.12 | 0.692* | < 0.0001 | 1.000 | |
| NFKB1 (a.u.) | − 0.281* | < 0.01 | 0.580* | < 0.0001 | 0.484* | < 0.0001 |
| TNF (a.u.) | − 0.310* | < 0.01 | 0.487* | < 0.0001 | 0.308* | < 0.01 |
| IL10 (a.u.) | 0.500* | < 0.0001 | − 0.253* | < 0.05 | − 0.235* | < 0.05 |
| TNF/IL10 | − 0.509* | < 0.0001 | 0.397* | < 0.001 | 0.306* | < 0.01 |
| Serum levels | ||||||
| sKL (pg/mL) | 0.173 | 0.11 | − 0.186 | 0.08 | − 0.041 | 0.70 |
| TNFα (pg/mL) | 0.162 | 0.12 | − 0.198 | 0.16 | 0.283* | < 0.01 |
| IL10 (pg/mL) | 0.253* | < 0.05 | − 0.403* | < 0.0001 | − 0.155 | 0.14 |
| TNFα/IL10 | − 0.186 | 0.08 | 0.334 | < 0.01 | 0.069 | 0.52 |
| Vascular tissue expression (n = 92) | ||||||
|---|---|---|---|---|---|---|
| TNF (a.u.) | IL10 (a.u.) | TNF/IL10 | ||||
| r | P value | r | P value | r | P value | |
| PBCCs expression | ||||||
| KL (a.u.) | − 0.168 | 0.13 | 0.159 | 0.15 | − 0.337* | < 0.01 |
| DNMT1 (a.u.) | 0.353* | < 0.01 | 0.069 | 0.53 | 0.236* | < 0.05 |
| DNMT3A (a.u.) | 0.309* | < 0.01 | 0.104 | 0.35 | 0.184 | 0.10 |
| NFKB1 (a.u.) | 0.379* | < 0.001 | − 0.006 | 0.95 | 0.302* | < 0.01 |
| TNF (a.u.) | 0.275* | < 0.05 | 0.037 | 0.74 | 0.196 | 0.08 |
| IL10 (a.u.) | − 0.061 | 0.58 | 0.143 | 0.19 | − 0.270* | < 0.05 |
| TNF/IL10 | 0.166 | 0.13 | − 0.125 | 0.26 | 0.311* | < 0.01 |
| Serum levels | ||||||
| sKL (pg/mL) | − 0.179 | 0.11 | − 0.078 | 0.49 | − 0.070 | 0.54 |
| TNFα (pg/mL) | − 0.185 | 0.10 | − 0.021 | 0.85 | − 0.144 | 0.20 |
| IL10 (pg/mL) | − 0.244* | < 0.05 | 0.137 | 0.22 | − 0.302* | < 0.01 |
| TNFα/IL10 | 0.166 | 0.14 | − 0.137 | 0.22 | 0.233* | < 0.05 |
We performed a multiple regression analysis using KL gene expression in PBCCs as the dependent variable to analyze how different clinical and inflammatory parameters are able to predict its levels. The eGFR, CRP, phosphorus, and cholesterol serum levels, vascular and PBCC gene expression ratio TNF/IL10, serum TNFα/IL10 ratio and sKL levels were used as covariates (Table 3). The analysis showed that TNF/IL10 ratio in PBCCs (β = − 0.234, P < 0.05) and systemic sKL concentrations (β = 0.282, P < 0.05) were significantly associated with KL expression in circulating cells (adjusted R2 = 0.287, P < 0.01).
Table 3.
Multiple regression analysis for KL expression in PBCCs as dependent variable.
| PBCCs KL expression (n = 92) | |||||
|---|---|---|---|---|---|
| Adjusted R2 | Standarized β | t | Tolerance | P value | |
| Model | 0.287 | < 0.01 | |||
| eGFR (mL/min/1.73 m2) | 0.178 | 10.58 | 0.96 | 0.12 | |
| CRP (mg/L) | − 0.153 | − 10.40 | 0.88 | 0.17 | |
| Phosphorus (mg/dL) | 0.019 | 0.17 | 0.95 | 0.86 | |
| Cholesterol (mg/dL) | 0.145 | 10.32 | 0.94 | 0.19 | |
| Vascular TNF/IL10 | 0.148 | 10.36 | 0.96 | 0.18 | |
| PBCCs TNF/IL10 | − 0.234 | − 20.08 | 0.90 | < 0.05 | |
| Serum TNFα/IL10 | 0.024 | 0.22 | 0.89 | 0.83 | |
| Serum sKL (pg/mL) | 0.282 | 20.46 | 0.86 | < 0.05 | |
KL expression in PBCCs and serum sKL levels according to the presence of developed atherosclerotic damage
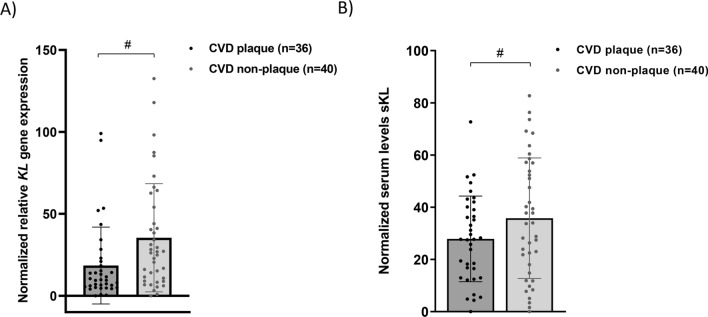
In the CVD group, 36 patients showed a developed atherosclerotic plaque (identifiable by macroscopic visualization) in the vascular tissue samples, while 40 showed no obvious signs of vascular damage. After normalizing the data against the non-CVD group (mean value for the different variables of this group was considered as 100%), we stratified the patients in the CVD group according to the presence of these plaques. We found that KL gene expression levels in PBCCs were significantly lower in those individuals with vascular damage [Normalized RQ: 9.66 (5.18 to 20.33) versus 26.53 (9.46 to 51.56), P < 0.05]. Similarly, serum sKL concentrations in patients with developed atherosclerotic plaque in the tissue sample were significantly lower [Normalized serum levels: 27.87 ± 16.43 versus 35.78 ± 23.10, P < 0.05] (Fig. 4).
Figure 4.
KL levels according to the presence of developed atherosclerotic plaque in CVD patients. (A) Relative gene expression levels of KL locus in PBCCs, (B) Serum levels of sKL (pg/mL). a.u.: arbitrary units. Bars and range represent median and IQR. **: P < 0.01; ****: P < 0.0001 vs non-CVD group; #: P < 0.05.
To study the association of these KL elements with the presence or absence of CVD and of atherosclerotic damage, we developed multiple logistic regression analyses with iterative models that included classic cardiovascular risk factors (model 1), PBCCs and serum inflammatory markers (model 2), and KL expression in leukocytes and serum sKL levels (model 3). In a final adjusted model, we observed that expression in PBCCs of KL gene and serum sKL concentration were protective factors for the presence of both CVD and developed atherosclerotic plaque (Table 4, Supplementary Fig. S3).
Table 4.
Logistic regression analysis for presence of CVD or presence of atherosclerotic plaque.
| Presence of CVD (n = 92) | Presence of atherosclerotic plaque (n = 92) | |||
|---|---|---|---|---|
| OR (CI 95%) | P value | OR (CI 95%) | P value | |
| Model 1 | ||||
| Age | 1.04 (0.94–1.15) | 0.47 | 1.01 (0.95–1.08) | 0.72 |
| Sex | 0.44 (0.12–1.68) | 0.23 | 0.70 (0.24–2.04) | 0.52 |
| Smoking | 1.21 (0.23–6.26) | 0.82 | 1.82 (0.56–5.88) | 0.32 |
| HT | 0.96 (0.21–4.28) | 0.95 | 0.76 (0.26–2.18) | 0.60 |
| DM | 1.33 (0.35–5.02) | 0.67 | 1.21 (0.50–2.95) | 0.68 |
| Model 2 | ||||
| Age | 1.06 (0.95–1.18) | 0.31 | 1.01 (0.95–1.08) | 0.79 |
| Sex | 0.29 (0.05–1.51) | 0.14 | 0.78 (0.26–2.39) | 0.67 |
| Smoking | 2.42 (0.27–21.46) | 0.43 | 1.78 (0.53–5.96) | 0.35 |
| HT | 2.85 (0.46–17.54) | 0.26 | 0.72 (0.24–2.11) | 0.53 |
| DM | 0.78 (0.17–3.57) | 0.75 | 1.40 (0.55–3.56) | 0.48 |
| PBCCs TNF/IL10 | 1.44 (0.98–2.11) | 0.06 | 0.99 (0.93–1.06) | 0.78 |
| Serum TNFα/IL10 | 3.29 (0.52–20.89) | 0.21 | 1.12 (0.95–1.32) | 0.17 |
| Model 3 | ||||
| Age | 1.16 (0.96–1.40) | 0.12 | 1.03 (0.96–1.11) | 0.39 |
| Sex | 0.12 (0.01–1.30) | 0.08 | 0.60 (0.18–2.03) | 0.41 |
| Smoking | 5.06 (0.21–120.38) | 0.32 | 2.01 (0.54–7.45) | 0.30 |
| HT | 4.65 (0.42–51.31) | 0.21 | 0.84 (0.24–2.93) | 0.78 |
| DM | 0.36 (0.03–4.08) | 0.41 | 1.14 (0.40–3.25) | 0.80 |
| PBCCs TNF/IL10 | 1.09 (0.79–1.51) | 0.62 | 0.95 (0.88–1.02) | 0.16 |
| Serum TNFα/IL10 | 1.87 (0.33–10.63) | 0.48 | 1.10 (0.93–1.31) | 0.25 |
| PBCCs KL | 0.86 (0.75–0.97) | < 0.05 | 0.89 (0.81–0.99) | < 0.05 |
| Serum sKL | 0.99 (0.98–0.99) | < 0.05 | 0.99 (0.98–0.99) | < 0.05 |
Discussion
Circulating monocytes, macrophages, and lymphocytes play a central role in the development of the atherosclerotic lesion. Although endothelial dysfunction is the main prerequisite to unleash the atherogenic process, activation and adhesion of these PBCCs to the vascular wall are the starting point. In addition, these circulating cells are mainly responsible for the resolution of the inflammatory response at local level in the vasculature, establishing a balance between pro-inflammatory stimuli and inflammation-resolving processes. In atherosclerotic plaques we can find subpopulations of these cells that will produce pro-inflammatory mediators with functions of chemotaxis, infiltration and proliferation of monocytes/macrophages in the damaged area, development of foam cells by accumulation of LDLox in macrophages40, phenotypic modulation of resident vascular cells or destabilization of the plaque and thrombogenesis41. Beyond the vascular wall, these circulating immune cells contribute to the systemic meta-inflammatory state that usually accompanies CVD which is originated in the adipose tissue as a response to metabolic alterations associated with cardiovascular risk factors (CVRF)42,43. KL is an anti-aging factor that can be found as a transmembrane glycoprotein or as a soluble factor (sKL) produced by shedding of the first one or directly secreted by cells (this one correspond to a protein produced from an alternative splicing transcript of KL gene)44. This factor is mainly expressed in the kidneys, parathyroid glands or brain, and to a lesser extent in reproductive organs, skeletal muscle or vasculature16,45,46. In humans, the involvement of KL deficiency in various pathological processes associated to aging has been stated, such as chronic kidney disease (CKD)9, cancer10, CVD12, or defective angiogenesis in scleroderma disease13. Several mechanisms have been described for the role of KL in these diseases, one of interest being its various anti-inflammatory activities24–26,29–33. The ability of pro-inflammatory mediators to repress KL expression suggests a relationship where the balance between both elements may affect the outcome of the inflammatory response45,47–49. Regarding CVD, multiple studies have shown that KL develop protective effects in the vasculature, being able to prevent vascular calcification18,19, endothelial dysfunction20–22 or heart failure23. Gene expression of this factor has been observed in different PBCCs, such as monocytes, macrophages26,35–39 and CD4 + lymphocytes34. Its presence in these cells should also be considered, albeit indirectly, as a possible actor involved in the preservation of vascular function.
In our study we showed that KL gene expression in PBCCs is reduced in patients with clinical diagnosis of atherosclerotic vascular disease in relation to healthy subjects. Previous studies have stated that different forms of CVD are associated with deficiency of KL expression in the vasculature12,45 or circulating levels of sKL11,12,14,15,17. These results would extend the range of tissues/cells whose expression of this factor is compromised during CVD to the PBCCs. Although a reasonable proportion of CVD patients had other co-morbidities (HT, 77.6% and DM, 43.4%), no differences were observed in KL expression levels in PBCCs between patients who had these and those who did not (Supplementary Fig. S1). One of the epigenetic mechanisms that regulates KL gene expression is hypermethylation of the promoter in the − 1200 bp region upstream50. This region is extremely rich in GC (65.9% G + C), with two CpG islands located between positions − 848 and + 8851. Methylation of this promoter is responsible for KL predominant expression in the kidney compared to other tissues, and it has been associated with decreased expression levels in the kidney and peripheral blood mononuclear cells during CKD51–53. Furthermore, different pharmacological approaches have shown the ability to recover KL expression through demethylation of its promoter54,55. In our study, analysis of the methylation status of KL gene promoter in PBCCs revealed a higher methylation degree of the interrogated CpG positions in the CVD group. Moreover, we observed that this same group presented higher expression levels of DNMT1 and DNMT3A genes (which encode for DNA-methyltransferases implicated in genome methylation). Besides, we detected an inverse significant correlation between KL expression and DNMT1 expression. All these observations would suggest that this epigenetic mechanism is likely to modulate the observed KL downregulation in PBCCs during CVD. To our knowledge, this is the first work in which KL expression and methylation of its promoter has been assessed in blood circulating cells in the context of atherosclerotic vascular disease.
The expression of KL by different blood-circulating cells has been related to repression of the inflammatory response induced by lipopolysaccharide through proteolysis of TLR4 in the plasmatic membrane37, suppression of the stress response of the Golgi apparatus and endoplasmic reticulum, reduction of oxidative stress and pro-inflammatory cytokines, as well as to increased production of anti-inflammatory cytokines and preservation of immune function26,38. It also participates in the polarization of macrophages towards an M2 anti-inflammatory phenotype39. The exogenous protein has also been shown to have a modulating effect on circulating blood cells through repression of pro-inflammatory cytokines secretion36,56. All these mechanisms exerted by leukocytes play key roles in the atherosclerotic process and, therefore, make KL expression in these cells an interesting target in such scenario. Although it is necessary to study in depth what are the consequences of the loss of KL in these blood-circulating cells, it is presumable to hypothesize that a lack of its anti-inflammatory activity would play some role in the progression of vascular damage (at least, it would be expected that leukocyte downregulation of KL gene may predispose to decompensation of the inflammatory response in a pro-inflammatory sense).
In this study we found that KL expression in PBCCs was inversely associated with markers of pro-inflammatory response in these same cells, while it presented a direct correlation with the expression of the anti-inflammatory gene IL10. Interestingly, DNMT1 and DNMT3A gene expression were also associated with all these inflammatory markers, but in an opposite direction (directly with pro-inflammatory markers and inversely with anti-inflammatory). Moreover, we observed similar associations between the expression in PBCCs of KL, DNMT1 and DNMT3A genes and parameters of systemic inflammation (serum concentrations of TNFα, IL10 and their ratio). Together, these results highlight the existence of a negative relationship between the inflammatory process and the expression of KL in PBCCs. Other studies have appreciated similar associations between the development of diseases characterized by a pro-inflammatory profile and the reduction of KL gene expression in these cells34,36,57. Our observations here support such findings in the frame of CVD. In addition, our results also suggest that the increase in the pro-inflammatory systemic response would be associated with a greater presence of the elements responsible for DNA methylation and, therefore, with higher methylation processes.. According with this, previous reports have already observed that low-grade systemic inflammation is related to changes in DNA methylation patterns implicated in macrophage polarization towards pro-inflammatory phenotypes58 or with cardiometabolic phenotypes59. In vitro and in vivo experimental models have clearly stated that pro-inflammatory mediators (such as TNFα, TWEAK, or IFNγ among others) can repress KL expression45,47–49. Uremic toxins also have the ability to decrease KL expression, and it has been experimentally shown to occur through hypermethylation of its promoter53. Although to the best of our knowledge, it has not yet been demonstrated that this is the mechanism by which pro-inflammatory cytokines are capable to downregulate KL expression, it is plausible to assume that this is possible. In this paper, we only point to the existence of links between such processes, but taken together, our results could suggest that negative modulation of KL expression in PBCCs by the CVD pro-inflammatory environment might occur by hypermethylation of its promoter. However, experimental in vitro validation in leukocytes is necessary to determine if pro-inflammatory cytokines have such direct effect on KL promoter.
Regarding the vascular wall, we also observed that higher values of the TNF/IL10 ratio are inversely associated with KL expression in PBCCs and, again, directly related to DNA-methyltransferases loci expression (only significant for DNMT1). This might indicate that a loss of KL expression in leukocytes is also linked to the inflammatory state of the vessel, which would extend the role of leukocyte KL in the modulation of athero-inflammation In the systemic circulation, sKL protein is the form mainly associated with its vasculoprotective effects. Different studies have linked decreased serum or plasma concentrations of sKL with the appearance of various forms of CVD12–17. In the present study, we have analysed how circulating sKL concentrations are associated with the inflammatory response in PBCCs or in the vascular wall. We found only significant associations that point to a negative relationship between sKL levels and the pro-inflammatory response in leukocytes. These results provide another point of connection between the inflammatory phenomenon underlying CVD and this anti-aging molecule.
We also showed that during CVD occurs a reduction of KL gene expression in circulating leukocytes. To assess this, we analysed KL expression levels in PBCCs and sKL serum levels according to the presence or absence of atherosclerotic plaque in an advanced stage of development (macroscopically visible in the vascular fragments). We observed that patients with advanced vascular lesion presented lower levels of expression for this gene in PBCCs and serum concentration of the soluble protein compared to those without observable plaque. Furthermore, in a multiple logistic regression model for the presence of atherosclerotic plaque as dependent variable, which included classical CVRF and inflammatory parameters as predictors, we observed that only KL gene expression in PBCCs or serum sKL levels acted as protective factors. Altogether, these results would indicate that the systemic KL (either the serum protein or the factor produced by leukocytes) might play a role in the development of the atherosclerotic lesion. The appearance of atherosclerotic plaques in specific regions of the vasculature (such as carotid or aorta) is directly related to the total atherosclerotic burden in the entire vascular tree60. Thus, it is possible to broadly assume that the observation of atheromatous plaques in our vascular tissue samples recovered from the surgeries of the CVD group implies a high degree of atherosclerotic burden in the patient. However, we are aware that this approach is not the most accurate to assess this parameter.
We realize that the present study has a series of limitations that must be taken into account when considering its conclusions, including: (1) small sample size (as a consequence, the ability to describe certain associations is limited), (2) nature of the control group (since this group consists of cadaveric organ donors and samples were retrieved in the early stages after death, it is presumable to consider that some inflammatory targets of the study may be affected by the decease process. and therefore, future studies are needed in living subjects free of CVD to validate our results), (3) impossibility to infer causality (this is a cross-sectional observational study that only allows to detect associations between the analysed variables, not their causes. Such associations need to be validated in experimental models), (4) influence of confounding variables not considered (it is plausible the existence of potential confounding factors not considered that could influence the observed associations), (5) lack of measurement of other inflammatory parameters (systemic and local inflammatory response in atherosclerosis involves other inflammatory mediators with significant roles in the process, such as IL1β, IL6, or TGFβ), (6) lack of measurement of mineral metabolism parameters (KL plays a relevant role in calcium and phosphorus metabolism, interacting with other molecules with a potential impact on CVD, such as FGF23 or vitamin D) and (7) limited assessment of the degree of methylation of KL gene promoter (this regulatory region includes two large CpG islands in a space of 936 bp and of which we only analysed 11 CpG positions, so we cannot conclude the exact influence of the overall methylation state of the promoter region).
Conclusions
On the whole, the results presented here allow us to establish a profile of KL expression in PBCCs during atherosclerotic vascular disease, which is mediated by its promoter methylation. In addition, various associations with the inflammatory process are pointed out that reinforce the notion of a negative relationship between this anti-aging factor and a pro-inflammatory response, both at the systemic and vascular levels. These relationships allow to propose KL as a potential anti-inflammatory modulator that acts at different stages of the atherosclerotic damage, being necessary to investigate in depth the mechanisms underlying such associations in future studies.
Supplementary Information
Acknowledgements
The authors thank to all the participants of the study, as well as to the nurse staff implicated that made the logistical work possible. This study was supported by Instituto de Salud Carlos III (ISCIII, PI13/01726, PI16/00024, PI19/00035, PI21/01037 and RD16/0009/0022) and co-funded by Fondo Europeo de Desarrollo Regional, Unión Europea (“Una forma de hacer Europa”). EMN is funded by a research contract from REDinREN-ISCIII (RD16/0009/0022). CF is recipient of a fellowship from Agencia Canaria de Investigación, Innovación y Sociedad de la Información of Consejería de Economía, Industria, Comercio y Conocimiento, Gobierno de Canarias (ACIISI, TESIS2018010110) co-funded by Fondo Social Europeo (FSE) Programa Operativo Integrado de Canarias 2014-2020, Eje 3 Tema Prioritario 74 (85%). VGT is supported by Cabildo de Tenerife, TF Innova, FDCAN and MEDII, in the Agustín de Betancourt programme. JDC is recipient of a contract from Miguel Servet Programme-ISCIII (CP20/00122). AGL is recipient of a contract from Agencia Canaria de Investigacion, Innovacion y Sociedad de la Informacion del Gobierno de Canarias (ACIISI) (TESIS2021010045).
Abbreviations
- AAA
Abdominal aortic aneurysm
- CAD
Coronary artery disease
- CRP
C-reactive protein
- CVD
Cardiovascular disease
- CVRF
Cardiovascular risk factors
- DM
Diabetes mellitus
- DNMT1
DNA Methyltransferase 1
- DNMT3A
DNA Methyltransferase 3A
- eGFR
Estimated glomerular filtration rate
- HT
Hypertension
- IL10
Interleukin 10
- KL
α-Klotho
- LDL
Low density lipoprotein
- NFKB1
Nuclear Factor Kappa B Subunit 1
- NLR
Neutrophil-to-lymphocyte ratio
- PAD
Peripheral artery disease
- PBCC
Peripheral blood circulating cell
- sKL
Soluble α-Klotho
- TIA
Transient ischemic attack
- TNFα
Tumor necrosis factor α
Author contributions
E.M.N, J.D.C., C.M.F., and J.F.N.G. designed the study, performed data analysis and wrote the manuscript; E.M.N., A.P.C., C.F., V.G.T., C.H.C. and N.P.D. planned the experiments and conducted them; S.R.M., P.C.L., A.L.P., A.D.M., V.C.L., and M.A.A.G. participated in patient and donor recruitment and critically reviewed the manuscript; A.G.L., A.M.O. and C.C.M.E assisted with laboratory tests. All authors approved the final version of the manuscript.
Data availability
DNA sequences obtained by bisulfite sequencing are deposited in DDBJ (DNA Data Bank of Japan) with entry ID 621f54273a01a500641c43f1. Methylated C positions are represented as “m”.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Carmen Mora-Fernández, Javier Donate-Correa and Juan F. Navarro-González.
Contributor Information
Javier Donate-Correa, Email: jdonatecorrea@gmail.com.
Juan F. Navarro-González, Email: jnavgon@gobiernodecanarias.org
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-12548-z.
References
- 1.Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000-2019. Geneva, World Health Organization; (2020). https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death.
- 2.Gisterå A, Hansson GK. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017;13(6):368–380. doi: 10.1038/nrneph.2017.51. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Luscher TF. Anti-inflammatory therapies for cardiovascular disease. Eur. Heart J. 2014;35(27):1782–1791. doi: 10.1093/eurheartj/ehu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 5.Chávez-Sánchez L, Espinosa-Luna JE, Chávez-Rueda K, Legorreta-Haquet MV, Montoya-Díaz E, Blanco-Favela F. Innate immune system cells in atherosclerosis. Arch. Med. Res. 2014;45(1):1–14. doi: 10.1016/j.arcmed.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circ. Res. 2016;118(4):653–667. doi: 10.1161/CIRCRESAHA.115.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.López-Candales A, Hernández Burgos P, Hernandez-Suarez D, Harris D. Linking chronic inflammation with cardiovascular disease: From normal aging to the metabolic syndrome HHS public access. J Nat Sci. 2017;3(4):e341. [PMC free article] [PubMed] [Google Scholar]
- 8.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Su W, Shen Z, Wang R. Correlation between soluble α-klotho and renal function in patients with chronic kidney disease: A review and meta-analysis. Biomed. Res. Int. 2018;12(2018):1–12. doi: 10.1155/2018/9481475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang X, Wang Y, Fan Z, Ji G, Wang M, Lin J, et al. Klotho: A tumor suppressor and modulator of the Wnt/β-catenin pathway in human hepatocellular carcinoma. Lab. Invest. 2015;96(2):197–205. doi: 10.1038/labinvest.2015.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, et al. Plasma klotho and cardiovascular disease in adults. J. Am. Geriatr. Soc. 2011;59(9):1596–1601. doi: 10.1111/j.1532-5415.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navarro-González JF, Donate-Correa J, Muros de Fuentes M, Pérez-Hernández H, Martínez-Sanz R, Mora-Fernández C. Reduced Klotho is associated with the presence and severity of coronary artery disease. Heart. 2013;100(1):34–40. doi: 10.1136/heartjnl-2013-304746. [DOI] [PubMed] [Google Scholar]
- 13.Mazzotta C, Manetti M, Rosa I, Romano E, Blagojevic J, Bellando-Randone S, et al. Proangiogenic effects of soluble α-Klotho on systemic sclerosis dermal microvascular endothelial cells. Arthritis Res. Ther. 2017;19(1):27. doi: 10.1186/s13075-017-1233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Ogawa A, et al. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS ONE. 2013;8(2):e56695. doi: 10.1371/journal.pone.0056695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keles N, Caliskan M, Dogan B, Keles NN, Kalcik M, Aksu F, et al. Low serum level of klotho is an early predictor of atherosclerosis. Tohoku J. Exp. Med. 2015;237(1):17–23. doi: 10.1620/tjem.237.17. [DOI] [PubMed] [Google Scholar]
- 16.Martín-Núñez E, Donate-Correa J, López-Castillo Á, Delgado-Molinos A, Ferri C, Rodríguez-Ramos S, et al. Soluble levels and endogenous vascular gene expression of KLOTHO are related to inflammation in human atherosclerotic disease. Clin. Sci. 2017;131(21):2601–2609. doi: 10.1042/CS20171242. [DOI] [PubMed] [Google Scholar]
- 17.Pan H-C, Chou K-M, Lee C-C, Yang N-I, Sun C-Y. Circulating Klotho levels can predict long-term macrovascular outcomes in type 2 diabetic patients. Atherosclerosis. 2018;276:83–90. doi: 10.1016/j.atherosclerosis.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, Zhao M-M, Cai Y, Zheng M-F, Sun W-L, Zhang S-Y, et al. Mammalian target of rapamycin signaling inhibition ameliorates vascular calcification via Klotho upregulation. Kidney Int. 2015;88(4):711–721. doi: 10.1038/ki.2015.160. [DOI] [PubMed] [Google Scholar]
- 19.Chang JR, Guo J, Wang Y, Hou YL, Lu WW, Zhang JS, et al. Intermedin1–53 attenuates vascular calcification in rats with chronic kidney disease by upregulation of α-Klotho. Kidney Int. 2016;89(3):586–600. doi: 10.1016/j.kint.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 20.Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, et al. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem. Biophys. Res. Commun. 2000;276(2):767–772. doi: 10.1006/bbrc.2000.3470. [DOI] [PubMed] [Google Scholar]
- 21.Six I, Okazaki H, Gross P, Cagnard J, Boudot C, Maizel J, et al. Direct, acute effects of Klotho and FGF23 on vascular smooth muscle and endothelium. PLoS ONE. 2014;9(4):e93423. doi: 10.1371/journal.pone.0093423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusaba T, Okigaki M, Matui A, Murakami M, Ishikawa K, Kimura T, et al. Klotho is associated with VEGF receptor-2 and the transient receptor potential canonical-1 Ca2+ channel to maintain endothelial integrity. Proc. Natl. Acad. Sci. 2010;107(45):19308–19313. doi: 10.1073/pnas.1008544107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen K, Wang S, Sun QW, Zhang B, Ullah M, Sun Z. Klotho deficiency causes heart aging via impairing the Nrf2-GR pathway. Circ. Res. 2021;128(4):492–507. doi: 10.1161/CIRCRESAHA.120.317348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu F, Wu S, Ren H, Gu J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat. Cell Biol. 2011;13(3):254–262. doi: 10.1038/ncb2167. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Fan J, Wang S, Sun Z. Secreted Klotho attenuates inflammation-associated aortic valve fibrosis in senescence-accelerated mice P1. Hypertension. 2018;71(5):877–885. doi: 10.1161/HYPERTENSIONAHA.117.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mytych J, Romerowicz-Misielak M, Koziorowski M. Klotho protects human monocytes from LPS-induced immune impairment associated with immunosenescent-like phenotype. Mol. Cell. Endocrinol. 2018;470:1–13. doi: 10.1016/j.mce.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Cui W, Leng B, Wang G. Klotho protein inhibits H2O2-induced oxidative injury in endothelial cells via regulation of PI3K/AKT/Nrf2/HO-1 pathways. Can. J. Physiol. Pharmacol. 2019;97(5):370–376. doi: 10.1139/cjpp-2018-0277. [DOI] [PubMed] [Google Scholar]
- 28.Romero A, San Hipólito-Luengo Á, Villalobos LA, Vallejo S, Valencia I, Michalska P, et al. The angiotensin-(1–7)/Mas receptor axis protects from endothelial cell senescence via klotho and Nrf2 activation. Aging Cell. 2019;18(3):e12913. doi: 10.1111/acel.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang N, Ma J, Ren Y, Xiang S, Jia R. Secreted klotho from exosomes alleviates inflammation and apoptosis in acute pancreatitis. Am. J. Transl. Res. 2019;11(6):3375–3383. [PMC free article] [PubMed] [Google Scholar]
- 30.He T, Xiong J, Huang Y, Zheng C, Liu Y, Bi X, et al. Klotho restrain RIG-1/NF-κB signaling activation and monocyte inflammatory factor release under uremic condition. Life Sci. 2019;231:116570. doi: 10.1016/j.lfs.2019.116570. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Chen W, Chen Y, Zhou Q, Xiao P, Tang R, et al. Neferine attenuates acute kidney injury by inhibiting NF-κB signaling and upregulating klotho expression. Front. Pharmacol. 2019;15:10. doi: 10.3389/fphar.2019.01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Li Z, Li B, Zhu X, Lai X. Klotho improves diabetic cardiomyopathy by suppressing the NLRP3 inflammasome pathway. Life Sci. 2019;234:116773. doi: 10.1016/j.lfs.2019.116773. [DOI] [PubMed] [Google Scholar]
- 33.Maekawa Y, Ishikawa K, Yasuda O, Oguro R, Hanasaki H, Kida I, et al. Klotho suppresses TNF-α-induced expression of adhesion molecules in the endothelium and attenuates NF-κB activation. Endocrine. 2009;35(3):341–346. doi: 10.1007/s12020-009-9181-3. [DOI] [PubMed] [Google Scholar]
- 34.Witkowski JM, Soroczyńska-Cybula M, Bryl E, Smoleńska Ż, Jóźwik A. Klotho—a common link in physiological and rheumatoid arthritis-related aging of human CD4+ lymphocytes. J. Immunol. 2007;178(2):771–777. doi: 10.4049/jimmunol.178.2.771. [DOI] [PubMed] [Google Scholar]
- 35.Bacchetta J, Sea JL, Chun RF, Lisse TS, Wesseling-Perry K, Gales B, et al. Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. J. Bone Miner. Res. 2012;28(1):46–55. doi: 10.1002/jbmr.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Wang Y, Gao W, Yuan C, Zhang S, Zhou H, et al. Klotho reduction in alveolar macrophages contributes to cigarette smoke extract-induced inflammation in chronic obstructive pulmonary disease. J. Biol. Chem. 2015;290(46):27890–27900. doi: 10.1074/jbc.M115.655431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bi F, Chen F, Li Y, Wei A, Cao W. Klotho preservation by Rhein promotes toll-like receptor 4 proteolysis and attenuates lipopolysaccharide-induced acute kidney injury. J. Mol. Med. 2018;96(9):915–927. doi: 10.1007/s00109-018-1644-7. [DOI] [PubMed] [Google Scholar]
- 38.Mytych J, Sołek P, Będzińska A, Rusinek K, Warzybok A, Tabęcka-Łonczyńska A, et al. Towards age-related anti-inflammatory Therapy: klotho suppresses activation of ER and golgi stress response in senescent monocytes. Cells. 2020;9(2):261. doi: 10.3390/cells9020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lv J, Chen J, Wang M, Yan F. Klotho alleviates indoxyl sulfate-induced heart failure and kidney damage by promoting M2 macrophage polarization. Aging. 2020;12(10):9139–9150. doi: 10.18632/aging.103183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bäck M, Yurdagul A, Tabas I, Öörni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019;16(7):389–406. doi: 10.1038/s41569-019-0169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansson GK, Libby P, Tabas I. Inflammation and plaque vulnerability. J. Intern. Med. 2015;278(5):483–493. doi: 10.1111/joim.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chatzigeorgiou A, Karalis KP, Bornstein SR, Chavakis T. Lymphocytes in obesity-related adipose tissue inflammation. Diabetologia. 2012;55(10):2583–2592. doi: 10.1007/s00125-012-2607-0. [DOI] [PubMed] [Google Scholar]
- 43.Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:1–12. doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem. Biophys. Res. Commun. 1998;242(3):626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 45.Lim K, Lu T-S, Molostvov G, Lee C, Lam FT, Zehnder D, et al. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125(18):2243–2255. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 46.Donate-Correa J, Mora-Fernández C, Martínez-Sanz R, Muros-de-Fuentes M, Pérez H, Meneses-Pérez B, et al. Expression of FGF23/KLOTHO system in human vascular tissue. Int. J. Cardiol. 2013;165(1):179–183. doi: 10.1016/j.ijcard.2011.08.850. [DOI] [PubMed] [Google Scholar]
- 47.Thurston RD, Larmonier CB, Majewski PM, Ramalingam R, Midura-Kiela M, Laubitz D, et al. Tumor necrosis Facto8r and interferon-γ down-regulate klotho in mice with colitis. Gastroenterology. 2010;138(4):1384–1394.e2. doi: 10.1053/j.gastro.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreno JA, Izquierdo MC, Sanchez-Niño MD, Suárez-Alvarez B, Lopez-Larrea C, Jakubowski A, et al. The inflammatory cytokines TWEAK and TNFα reduce renal Klotho expression through NFκB. J. Am. Soc. Nephrol. 2011;22(7):1315–1325. doi: 10.1681/ASN.2010101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sastre C, Rubio-Navarro A, Buendía I, Gómez-Guerrero C, Blanco J, Mas S, et al. Hyperlipidemia-associated renal damage decreases Klotho expression in kidneys from ApoE knockout mice. PLoS ONE. 2013;8(12):e83713. doi: 10.1371/journal.pone.0083713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kale A, Sankrityayan H, Anders H-J, Gaikwad AB. Epigenetic and non-epigenetic regulation of Klotho in kidney disease. Life Sci. 2021;264:118644. doi: 10.1016/j.lfs.2020.118644. [DOI] [PubMed] [Google Scholar]
- 51.Azuma M, Koyama D, Kikuchi J, Yoshizawa H, Thasinas D, Shiizaki K, et al. Promoter methylation confers kidney-specific expression of the Klotho gene. FASEB J. 2012;26(10):4264–4274. doi: 10.1096/fj.12-211631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J, Zhang X, Zhang H, Zhang C, Wu Q, et al. Elevated Klotho promoter methylation is associated with severity of chronic kidney disease. PLoS ONE. 2013;8(11):e79856. doi: 10.1371/journal.pone.0079856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun CY, Chang SC, Wu MS. Suppression of Klotho expression by protein-bound uremic toxins is associated with increased DNA methyltransferase expression and DNA hypermethylation. Kidney Int. 2012;81(7):640–650. doi: 10.1038/ki.2011.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen K, Sun Z. Activation of DNA demethylases attenuates aging-associated arterial stiffening and hypertension. Aging Cell. 2018;17(4):e12762. doi: 10.1111/acel.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gu Y, Chen J, Zhang H, Shen Z, Liu H, Lv S, et al. Hydrogen sulfide attenuates renal fibrosis by inducing TET-dependent DNA demethylation on Klotho promoter. FASEB J. 2020;34(9):11474–11487. doi: 10.1096/fj.201902957RR. [DOI] [PubMed] [Google Scholar]
- 56.Sedighi M, Baluchnejadmojarad T, Fallah S, Moradi N, Afshin-Majdd S, Roghani M. Klotho ameliorates cellular inflammation via suppression of cytokine release and upregulation of miR-29a in the PBMCs of diagnosed alzheimer’s disease patients. J. Mol. Neurosci. 2019;69(1):157–165. doi: 10.1007/s12031-019-01345-5. [DOI] [PubMed] [Google Scholar]
- 57.Karami M, Mehrabi F, Allameh A, Pahlevan Kakhki M, Amiri M, Emami Aleagha MS. Klotho gene expression decreases in peripheral blood mononuclear cells (PBMCs) of patients with relapsing-remitting multiple sclerosis. J. Neurol. Sci. 2017;381:305–307. doi: 10.1016/j.jns.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 58.Wang X, Cao Q, Yu L, Shi H, Xue B, Shi H. Epigenetic regulation of macrophage polarization and inflammation by DNA methylation in obesity. JCI Insight. 2016;1(19):e87748. doi: 10.1172/jci.insight.87748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ligthart S, Marzi C, Aslibekyan S, Mendelson MM, Conneely KN, Tanaka T, et al. DNA methylation signatures of chronic low-grade inflammation are associated with complex diseases. Genome Biol. 2016;17(1):255. doi: 10.1186/s13059-016-1119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ibañez B, Pinero A, Orejas M, Badimón JJ. Novel imaging techniques for quantifying overall atherosclerotic burden. Rev. Esp. Cardiol. 2007;60(3):299–309. doi: 10.1157/13100282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DNA sequences obtained by bisulfite sequencing are deposited in DDBJ (DNA Data Bank of Japan) with entry ID 621f54273a01a500641c43f1. Methylated C positions are represented as “m”.