Abstract
Background & Aims
Inflammatory bowel diseases are multifactorial diseases commonly treated with either immunomodulatory drugs or anti–tumor necrosis factor (TNF). Currently, failure to respond to anti-TNF therapy (assessed no earlier than 8–12 weeks after starting treatment) occurs in 20%–40% of patients enrolled in clinical trials and in 10%–20% in clinical practice. Murine models of inflammatory bowel disease provide important tools to better understand disease mechanism(s). In this context and among the numerous models available, Winnie–TNF–knockout (KO) mice recently were reported to show characteristics of ulcerative colitis (UC) that are independent of TNF, and with increased interleukin (IL)1β production.
Methods
Herein, the efficacy of recombinant IL1-receptor antagonist (anakinra) administration was evaluated in Winnie-TNF-KO mice, used as a UC model of primary anti-TNF nonresponders.
Results
We analyzed gut mucosal biopsy specimens and circulating cytokine profiles of a cohort of 30 UC patients; approximately 75% of primary nonresponders were characterized by abundant IL1β in both the serum and local intestinal tissues. In Winnie-TNF-KO mice, administration of anakinra efficiently reduced the histologic score of the distal colon, which represents the most common site of inflammation in Winnie mice. Furthermore, among lamina propria and mesenteric lymph node–derived T cells, interferon γ–expressing CD8+ T cells were reduced significantly after anakinra administration.
Conclusions
Our study provides new insight and alternative approaches to treat UC patients, and points to anti-IL1 strategies (ie, anakinra) that may be a more effective therapeutic option for primary nonresponders to anti-TNF therapy.
Keywords: Ulcerative Colitis, Cytokines, TNF
Abbreviations used in this paper: APC, Allophycocyanin; BMDC, bone marrow–derived dendritic cell; BSA, bovine serum albumin; CCL, C-C motif chemokine ligand; DAI, disease activity index; DC, dendritic cell; DPBS, Dulbecco's PhosphateBuffered Saline; ELISA, enzyme-linked immunosorbent assay; Foxp3, forkhead box P3; GM-CSF, granulocyte-macrophage colony-stimulating factor; IBD, inflammatory bowel disease; IFN, interferon; IFX, infliximab; IHC, immunohistochemistry; IL, interleukin; IP, intraperitoneal; KO, knockout; LP, lamina propria; LPS, lipopolysaccharide; MLN, mesenteric lymph node; N-GAL, neutrophil gelatinase-associated lipocalin; PE, phycoerythrin; TNF, tumor necrosis factor; T0, first infusion; T1, 12 weeks after the first infusion; UC, ulcerative colitis; WT, wild type
Graphical abstract
Summary.
Anakinra administration represents a therapeutic option for tumor necrosis factor–independent ulcerative colitis subjects. Circulating interleukin 1β can predict the candidate subpopulation of ulcerative colitis patients non responder to the anti-tumor necrosis factor therapy, who may benefit from anakinra administration.
Inflammatory bowel diseases (IBDs) are gastrointestinal disorders characterized by dysregulated immune responses and prominent dysbiosis, which in turn leads to chronic relapsing inflammation of the gut. Environmental factors, as well as a genetic predisposition, also are important contributors to disease pathogenesis. IBDs include ulcerative colitis (UC), which manifests as inflammation restricted to the mucosal surface of the colon, and Crohn’s disease, which is more transmural in nature and can affect the entire length of the gastrointestinal tract. For both, one main immunopathogenic cause is the dysregulated equilibrium between proinflammatory and regulatory cytokines. Several cytokines have been reported to play a pivotal role in the activation of innate and adaptive immune responses, of which tumor necrosis factor (TNF) has been found in the sera, colonic mucosa, and stool of UC patients.1, 2, 3 The development of anti-TNF biologics with the ability to block this key proinflammatory cytokine represents a significant improvement in the quality of life for IBD patients, reducing the need for surgical interventions and improving, and often inducing, persistent clinical remission.4 Nonetheless, variability of disease involvement, as well as response to therapy, is a commonplace occurrence for patients with IBD.5
Anti-TNF agents are not a magic bullet for all IBD cases. First, side effects can be severe and life-threatening in some patients, mainly owing to infective events and then to immunogenicity, with the formation of antibodies to anti-TNF and consequent loss of response to these drugs over time. In fact, primary nonresponse to anti-TNF induction therapy occurs in 20%–40% of patients in a clinical trial setting. Secondary loss of response is also a common clinical problem, with the incidence ranging between 23% and 46% at 12 months after anti-TNF initiation.6, 7, 8 Using a cohort of 955 patients treated with infliximab (IFX), Kennedy et al9 reported a 23.8% incidence of primary nonresponse. A suboptimal drug concentration at week 14 was associated with primary nonresponse, but this could be only partially explained by the development of antidrug antibodies.9, 10, 11 In these cases, the clinical management of this second group of patients implies the use of alternative drugs blocking different molecules with different rates of success.
In a recent study, interleukin (IL)1β was identified as one of the most important biomarkers associated with the failure of anti-TNF therapy.12 IL1β is produced by limited types of cells, including macrophages, dendritic cells (DCs), and monocytes, and requires a series of intracellular events to occur before becoming completely active and ready to trigger inflammation.13 The IL1 axis, which includes IL1α and IL1β binding to the same receptor (IL1R1: Interleukin 1 Receptor Type 1), has been identified repeatedly as a crucial mediator for the onset of UC inflammation.14,15 IL1-receptor antagonist (IL1Ra) can inhibit the biological effects of IL1, acting as a natural decoy for IL1β.16 Anakinra (Kineret, Swedish Orphan Biovitrum AB, Stockholm, Sweden), a recombinant form of IL1Ra, is able to inhibit the effects of IL1β17 and has been used successfully for the treatment of patients with rheumatoid arthritis18, 19, 20 and severe sepsis.21
Recently, the association of IL1β with primary nonresponse to anti-TNF was shown.22,23 In the present study, we analyzed circulating cytokine profiles and intestinal biopsy specimens of a cohort of UC patients. Significant differences in IL1β were associated with primary nonresponse to anti-TNF administration. A similar cytokine profile was detected in our murine model of TNF-independent UC (Winnie–TNF–knockout [KO]).24 Thus, we evaluated whether anakinra administration to Winnie-TNF-KO mice could reduce intestinal signs of chronic inflammation. Our results highlight the beneficial effects of anakinra administration, characterized by a significant decrease in interferon (IFN)γ production by mucosal CD8+ T cells. Mice affected by TNF-independent UC resemble a subpopulation of UC patients that currently are defined as primary nonresponders after months of ineffective IFX administration; they represent a novel model to study this important patient population. The results of the present study pave the way to clinical trials based on anakinra administration to a subpopulation of primary nonresponder UC patients, characterized by high IL1β in sera and colonic biopsy specimens.
Results
Increased IL1β in IBD Patients Defined as Primary Nonresponders
A cohort of 30 UC patients was enrolled and started IFX therapy. Patients’ sera were collected and stored before the first infusion (T0) and 12 weeks after the first infusion (T1). At T1, patients’ response to IFX was assessed by routine endoscopic examination. All patients had similar serum levels of C-reactive protein; 18 patients were classified as responders to the biologic, while 12 were nonresponders.
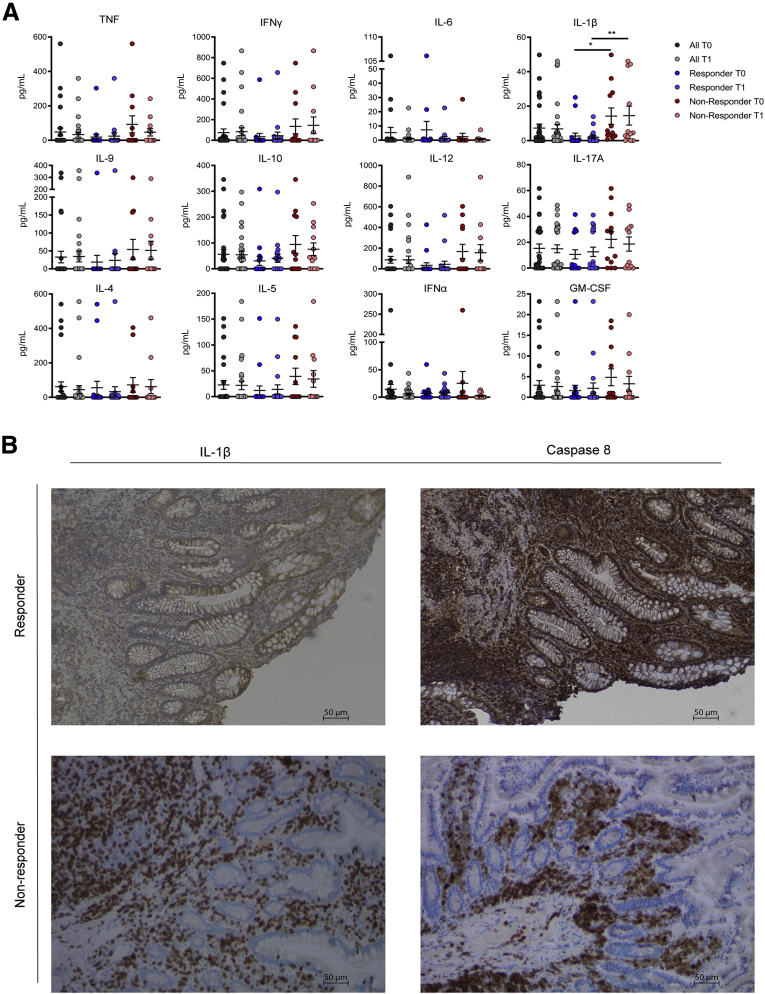
Patients’ sera were analyzed by multiplex for 13 target cytokines. As shown in Figure 1A, the nonresponder group was characterized by a significantly higher concentration of circulating IL1β compared with responders, even at the T0 time point, suggesting a role for IL1β as a predictor for IFX primary nonresponders. Specifically, the IL1β mean concentration in sera was 14.23 ± 4.75 pg/mL in nonresponder patients compared with 2.66 ± 1.73 pg/mL in the responder group at T0, and 14.53 ± 5.51 pg/mL in nonresponders vs 1.98 ± 0.87 pg/mL in responders were measured at T1 (Figure 1A). Results obtained from the analysis of 12 other cytokines, including TNF and IL6, did not support any predictive role regarding primary nonresponder patients. Serum levels of IL2 were below the standard limit of detection in all of the analyzed samples.
Figure 1.
(A) Assessment of cytokine levels in sera of patients before and after IFX infusion. All data sets from each time point (T0 and T1) are grouped and shown as "All" (in grey, N=30), the responder group is shown in blue (N = 18), and the nonresponder group is shown in red (N = 12). T0 refers to before IFX infusion; T1 refers to 12 weeks after the first IFX infusion. (B) IHC analysis for IL1β and caspase 8 in formalin-fixed, paraffin-embedded tissues obtained from responder and nonresponder patients at T0. Magnification, 10×. ∗P < .05, ∗∗P < .01.
To better understand the differences observed between responder and nonresponder patients, and to determine if a correlation exists among all cytokines, we performed a linear regression analysis between circulating levels of IL1β vs all other cytokines analyzed in both the responder and nonresponder groups. No correlation was detected among all circulating cytokines in the responder group at T0 and T1 (Tables 1 and 2, respectively), as well as in the nonresponders at T0 (Table 3), while a positive correlation was detected for 9 analyzed cytokines (TNF, IFNγ, IL9, IL12p70, IL10, IL17A, IL4, IL5, and granulocyte-macrophage colony-stimulating factor [GM-CSF]) at T1 only in the nonresponder group (Table 4).
Table 1.
Correlation Between the Concentrations of Circulating IL1β and All Other Cytokines in the Responder Group at T0
| Parameter | IFNα | IL9 | TNF | IFNγ | IL6 | IL12p70 | IL10 | IL17A | IL4 | IL5 | GM-CSF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of XY pairs | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 |
| Pearson r | -0.04885 | -0.09036 | -0.1001 | -0.09567 | -0.1099 | -0.1210 | -0.06357 | -0.2456 | -0.1330 | -0.1207 | -0.1133 |
| 95% CI | -0.5043 to 0.4279 | -0.5348 to 0.3932 | -0.5417 to 0.3849 | -0.5386 to 0.3887 | -0.5487 to 0.3764 | -0.5565 to 0.3667 | -0.5152 to 0.4157 | -0.6393 to 0.2500 | -0.5649 to 0.3560 | -0.5563 to 0.3670 | -0.5511 to 0.3734 |
| P value (2-tailed) | .8474 | .7214 | .6927 | .7057 | .6642 | .6325 | .8021 | .3259 | .5987 | .6334 | .6544 |
| P value summary | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Is the correlation significant? (α = .05) | No | No | No | No | No | No | No | No | No | No | No |
| R2 | 0.002386 | 0.008166 | 0.01002 | 0.009153 | 0.01208 | 0.01463 | 0.004041 | 0.06033 | 0.01770 | 0.01456 | 0.01284 |
NOTE. All data were analyzed using the Pearson correlation test.
Table 2.
Correlation Between the Concentrations of Circulating IL1β and All Other Cytokines in the Responder Group at T1
| Parameter | IFNα | IL9 | TNF | IFNγ | IL6 | IL12p70 | IL10 | IL17A | IL4 | IL5 | GM-CSF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of XY pairs | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 |
| Pearson r | -0.04982 | -0.1702 | -0.1730 | -0.1228 | -0.1628 | -0.1927 | -0.02136 | -0.3663 | -0.1353 | -0.2177 | -0.2260 |
| 95% CI | -0.5050 to 0.4271 | -0.5902 to 0.3224 | -0.5922 to 0.3198 | -0.5578 to 0.3651 | -0.5853 to 0.3292 | -0.6052 to 0.3014 | -0.4835 to 0.4501 | -0.7116 to 0.1214 | -0.5665 to 0.3540 | -0.6215 to 0.2775 | -0.6268 to 0.2694 |
| P value (2-tailed) | .8444 | .4996 | .4923 | .6275 | .5187 | .4435 | .9329 | .1349 | .5925 | .3855 | .3672 |
| P value summary | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Is the correlation significant? (α = .05) | No | No | No | No | No | No | No | No | No | No | No |
| R2 | 0.002482 | 0.02896 | 0.02994 | 0.01507 | 0.02650 | 0.03715 | 0.0004563 | 0.1342 | 0.01831 | 0.04739 | 0.05107 |
NOTE. All data were analyzed using the Pearson correlation test.
Table 3.
Correlation Between the Concentrations of Circulating IL1β and All Other Cytokines in the Nonresponder Group at T0
| Parameter | IFNα | IL9 | TNF | IFNγ | IL6 | IL12p70 | IL10 | IL17A | IL4 | IL5 | GM-CSF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of XY pairs | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 |
| Pearson r | -0.2126 | 0.1876 | -0.02277 | 0.3847 | -0.1919 | 0.2611 | 0.2071 | 0.2167 | 0.2219 | 0.3949 | 0.3015 |
| 95% CI | -0.7011 to 0.4116 | -0.4330 to 0.6876 | -0.5891 to 0.5585 | -0.2430 to 0.7853 | -0.6899 to 0.4294 | -0.3681 to 0.7262 | -0.4165 to 0.6981 | -0.4080 to 0.7033 | -0.4035 to 0.7060 | -0.2316 to 0.7899 | -0.3295 to 0.7464 |
| P value (2-tailed) | .5071 | .5592 | .9440 | .2169 | .5501 | .4125 | .5185 | .4987 | .4883 | .2039 | .3408 |
| P value summary | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Is the correlation significant? (α = .05) | No | No | No | No | No | No | No | No | No | No | No |
| R2 | 0.04520 | 0.03521 | 0.0005185 | 0.1480 | 0.03684 | 0.06815 | 0.04287 | 0.04697 | 0.04922 | 0.1559 | 0.09093 |
NOTE. All data were analyzed using the Pearson correlation test.
Table 4.
Correlation Between the Concentrations of Circulating IL1β and All Other Cytokines in the Nonresponder Group at T1
| Parameter | IFNα | IL9 | TNF | IFNγ | IL6 | IL12p70 | IL10 | IL17A | IL4 | IL5 | GM-CSF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of XY pairs | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 |
| Pearson r | -0.1790 | 0.5854 | 0.7472 | 0.7156 | 0.5305 | 0.7483 | 0.7225 | 0.6496 | 0.6983 | 0.7386 | 0.6570 |
| 95% CI | -0.6828 to 0.4403 | 0.01722–0.8678 | 0.3033–0.9246 | 0.2403–0.9141 | -0.06261 to 0.8467 | 0.3056–0.9250 | 0.2537–0.9164 | 0.1206–0.8913 | 0.2075–0.9083 | 0.2857–0.9218 | 0.1333–0.8939 |
| P value (2-tailed) | .5778 | .0455 | .0052 | .0089 | .0760 | .0051 | .0080 | .0222 | .0115 | .0061 | .0203 |
| P value summary | NS | ∗ | ∗∗ | ∗∗ | NS | ∗∗ | ∗∗ | ∗ | ∗ | ∗∗ | ∗ |
| Is the correlation significant? (α = .05) | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| R2 | 0.03203 | 0.3427 | 0.5583 | 0.5121 | 0.2814 | 0.5600 | 0.5220 | 0.4220 | 0.4877 | 0.5455 | 0.4317 |
NOTE. All data were analyzed using the Pearson correlation test. ∗P < .05, ∗∗P < .01
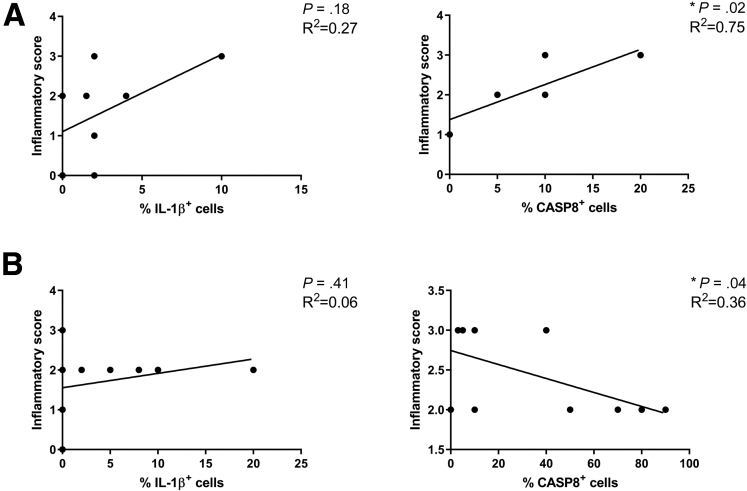
In further support of the predictive role of IL1β in nonresponder patients, we obtained intestinal biopsy specimens from the same cohort used for cytokine assessment (Figure 1B). Immunohistochemistry (IHC) staining showed that IL1β and caspase 8–positive cells were detectable in 75% and 83% of nonresponder patients, respectively, while a dramatic reduction of IL1β-positive (46.2%), but not caspase 8–positive (91.7%), cells were observed in biopsy specimens from responder patients. A significant correlation was observed between the inflammatory score and the percentage of caspase 8–positive cells in nonresponder as well as in the responder patients (Figure 2).
Figure 2.
Linear regression between inflammatory score and the percentage of IL1β or caspase 8 (CASP8)-positive cells in the (A) nonresponder (N = 8) and (B) responder (N = 12) groups at T0.
Increased IL1β Production by DCs in the Absence of TNF
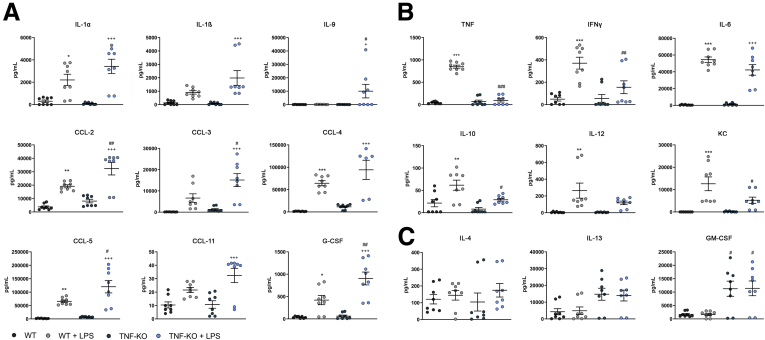
To investigate a possible correlation between IL1β and TNF secretion in the inflammatory response, we isolated and cultured bone marrow–derived dendritic cells (BMDCs) from wild-type (WT) and TNF-KO mice. In the TNF-KO BMDC media, we detected significantly higher concentrations of IL1α, IL1β, IL9, C-C motif chemokine ligand (CCL)-2, CCL-3, CCL-4, CCL-5, CCL-11, and granulocyte colony-stimulating factor (Figure 3A), while we observed TNF, IFNγ, IL6, IL10, IL12, and keratinocyte-derived chemokine to be reduced significantly (Figure 3B). In both groups, lipopolysaccharide (LPS) administration did not result in increased secretion of IL4, IL13, and GM-CSF within each genotype, but TNF-KO BMDCs produced higher quantities of IL4 and GM-CSF (significantly) than WT BMDCs, even in the absence of LPS (Figure 3C).
Figure 3.
Murine BMDC cytokine (CK) and chemokine (CC) profiles after LPS exposure determined by multiplex assay. BMDCs from WT and TNF-KO mice were exposed to LPS for 24 hours (N = 8 for each group). Dark grey and blue circles represent the CK or CC level from WT and TNF-KO BMDCs, respectively; light grey and light blue circles show the CK and CC levels after LPS exposure. (A) CK and CC were increased in the TNF-KO BMDCs. (B) CK and CC were decreased in the TNF-KO BMDCs. (C) There was no effect in CK and CC secretion mediated by LPS stimulation. ∗/+/#P < .05, ∗∗/++/##P < .01, and ∗∗∗/+++/P < .001. ∗WT versus WT+LPS; +TNF-KO versus TNF-KO +LPS; #WT+LPS versus TNF-KO +LPS. G-CSF, granulocyte colony-stimulating factor; KC, keratinocyte-derived chemokine.
Anakinra Administration Successfully Suppresses Colonic IL1β Production
We hypothesize that a percentage of primary nonresponder UC patients are characterized by a TNF-independent disease and abundant IL1β secretion. To better explore this hypothesis, we took advantage of our previously generated murine model of TNF-independent UC, obtained by crossing the progressive and spontaneous UC model Winnie25,26 with the commercial strain TNF-KO (Winnie-TNF-KO).24 Immediately after weaning, we observed significantly increased expression of IL1β by measuring the messenger RNA extracted from colonic tissues of Winnie-TNF-KO compared with controls (WT mice and parental strains).24
All of these experimental observations indicated that, in the Winnie model, the absence of TNF does not suppress colonic inflammation, and vice versa this is fostered through the production of the inflammatory mediator IL1β. Therefore, our hypothesis is that blocking IL1 could be an effective therapeutic option for TNF-independent UC patients.
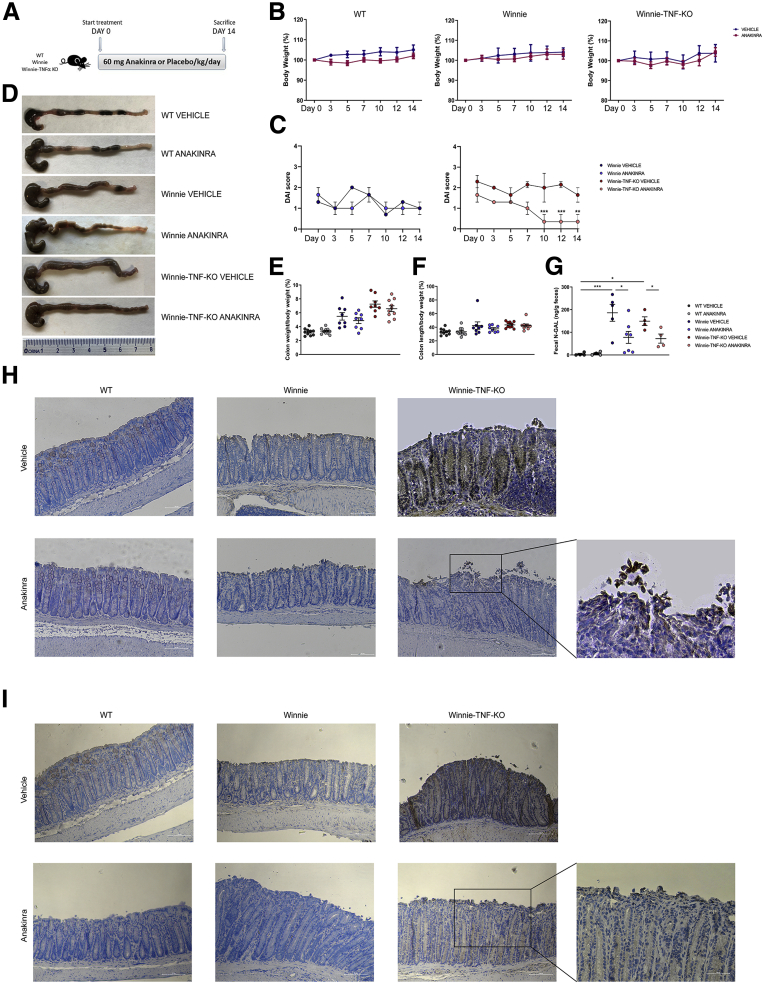
To evaluate whether blocking IL1 could protect Winnie-TNF-KO mice from UC-like colitis, we used an experimental protocol of anakinra administration, a recombinant IL1-receptor antagonist. Winnie and WT mice were used as control. As shown in Figure 4A, we treated adult mice that were 16 weeks of age (N = 9 for each group) with daily intraperitoneal injections of 60 mg/kg anakinra or vehicle (phosphate-buffered saline [PBS]) for 2 weeks. Before the treatment and every 2 days, we recorded mice body weight and the disease activity index (DAI) score. As expected, no toxic side effects were observed with anakinra administration; indeed, mice gained weight regularly during the 2 weeks of treatment (Figure 4B), with a similar trend to vehicle administration. The absence of any adverse side effects related to anakinra administration was shown previously.27 Moreover, in our previous studies, Winnie mice showed a reduced weight at weaning compared with their WT counterparts, and this difference persisted also at adult age; nevertheless, Winnie mice were able to gain weight during life.24,26 The DAI score recorded was almost 0 in the WT mice at each time point. Instead, in Winnie mice treated with vehicle, the initial DAI score was 1.3 ± 0; at the end of treatment, it decreased to 1.0 ± 0.3. Similarly, Winnie mice in the anakinra group had an initial DAI score of 1.6 ± 0.3 that decreased to 1.0 ± 0.3. Lastly, for vehicle-treated Winnie-TNF-KO mice, the DAI score was 2.3 ± 0.3 at day 0; at the end of treatment, it decreased to 1.6 ± 0.3, while the anakinra group started from a DAI score of 1.6 ± 0.3, which decreased to 0.3 ± 0.3 at the end of treatment (Figure 4C).
Figure 4.
Macroscopic features and measurements of colonic parameters at the end of treatment with anakinra. (A) Schematic representation of the experimental design. WT, Winnie and Winnie-TNF-KO mice were treated with IP injection of anakinra or vehicle for 14 days (N = 9 for each group). (B) Effect of anakinra or vehicle on mice body weight from day 0 until the end of treatment. (C) DAI score recorded for Winnie and Winnie-TNF-KO mice treated with anakinra or vehicle. (D) Representative images of whole colons for each experimental group. (E and F) Measurement of colon weight/body weight and colon length/body weight indices (%), respectively. (G) Detection of N-GAL level in feces of WT, Winnie, and Winnie-TNF-KO mice treated with anakinra or vehicle. (H and I) IHC analysis for IL1β and caspase 8, respectively, in formalin-fixed, paraffin-embedded tissues obtained from WT, Winnie, and Winnie-TNF-KO mice treated with anakinra or vehicle. (10× and magnified 20× on the right). ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
At the end of treatment, mice were killed, and colons were harvested for subsequent histologic analysis. As shown in Figure 4D, the colon length in WT mice was comparable after treatment with anakinra or vehicle, as well as stool consistency. Both Winnie and Winnie-TNF-KO mice treated with vehicle showed colonic fibrosis and watery stool. After anakinra injections, the colon length increased slightly. The treatment with anakinra also ameliorated the ratio between colon weight and total body weight (Figure 4E), even if the differences observed did not reach statistical significance. Instead, no differences were observed in the ratio between colon length and total body weight (Figure 4F).
Anakinra efficiency in Winnie-TNF-KO mice was confirmed by quantitative determination of neutrophil gelatinase-associated lipocalin (N-GAL) in feces. As shown in Figure 4G, the fecal N-GAL level was almost 0 for WT mice treated with vehicle or anakinra. On the contrary, in anakinra-treated Winnie mice, the fecal N-GAL was 77.7 ± 26.2 ng/g feces, which was significantly lower than vehicle-treated Winnie mice (186.0 ± 37.4 ng/g feces). Similarly, in Winnie-TNF-KO mice the level of fecal N-GAL was significantly lower in the anakinra group compared with vehicle (72.2 ± 20.2 vs 149.2 ± 19.1 ng/g feces, respectively).
IHC of the distal colon showed positive IL1β staining in Winnie-TNF-KO mice, while it was absent in all control strains (WT and Winnie mice) (Figure 4H). IL1β decreased significantly after anakinra administration in Winnie-TNF-KO mice. However, the signal for IL1β still was present and well defined as ubiquitous positivity of the intestinal endoluminal border (Figure 4H, enlargement). In vehicle-treated Winnie-TNF-KO, IL1β IHC showed a diffuse positivity in crypts, lymphoplasma cellular inflammatory cells, and surface desquamating cells (Figure 4H). Likewise, the expression of caspase 8 was decreased significantly in the intestinal tissues of anakinra-treated Winnie-TNF-KO mice compared with controls (Figure 4I). Positive cells were located only on the endoluminal secretory side. In vehicle-treated Winnie-TNF-KO, caspase 8 immunolocalization showed strong positivity in the cytoplasm and/or nucleus of colonic epithelial cells, with the highest expression in the crypts and at the tip of erosion areas (Figure 4I).
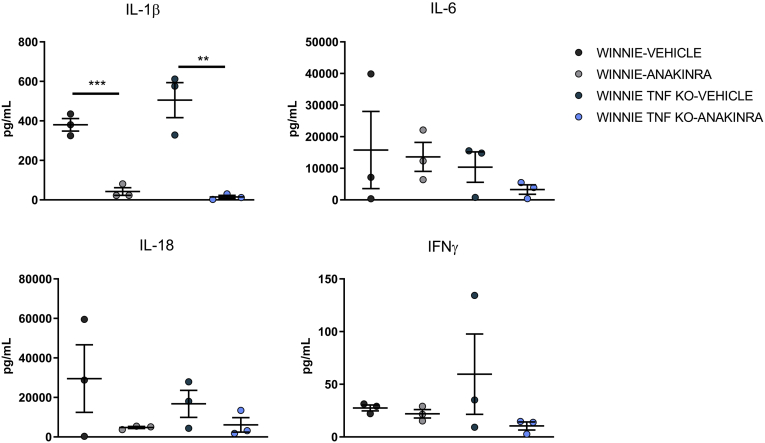
Approximately 0.5 cm of tissue from the terminal colon was cultured in vitro, the supernatant was collected 24 hours later, and analyzed by enzyme-linked immunosorbent assay (ELISA). Supernatants from the colon culture of Winnie-TNF-KO mice were enriched in IL1β and IFNγ if compared with the parental strain Winnie (Figure 5). When the colon was obtained from anakinra-treated mice, a decrease in both cytokines could be observed, significant only for IL1β. Similarly, IL6 and IL18 appear differently secreted in our experimental groups, even though there was no significance (Figure 5).
Figure 5.
Cytokine profile of colonic tissue culture from Winnie and Winnie-TNF-KO mice treated with vehicle or anakinra (N = 3 for each group). ∗∗P < .005, ∗∗∗P < .001.
Anakinra Reduces Gene Expression and Secretion of Inflammatory Cytokines
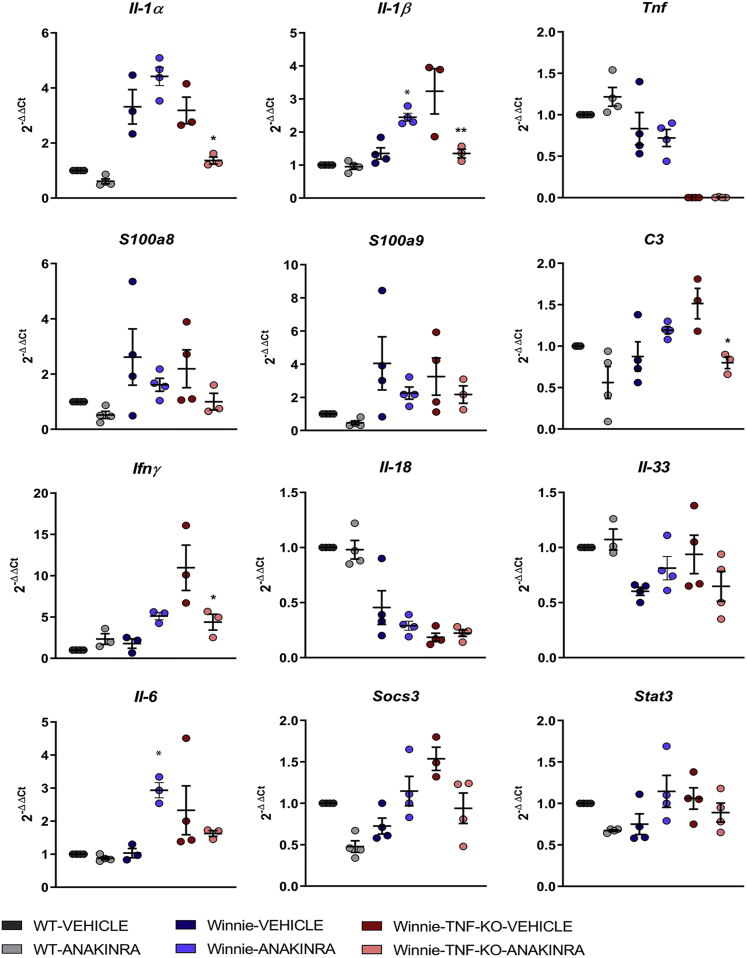
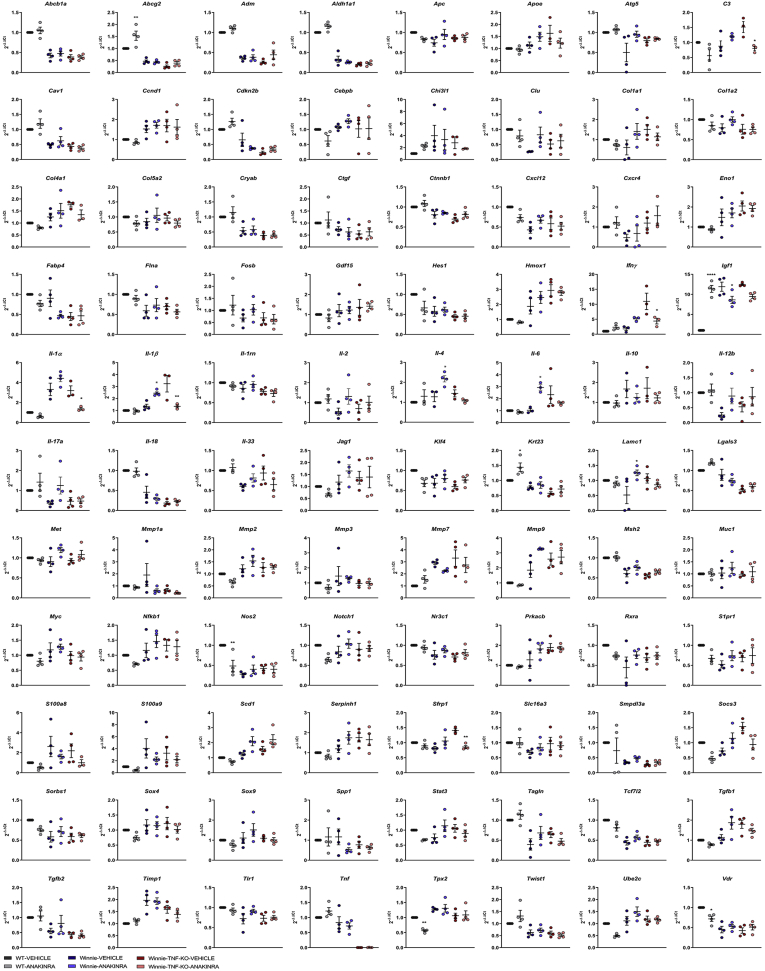
Gene expression analysis by Quantitative Polymerase Chain Reaction of the distal colon of treated mice confirmed significant Il1α and Il1β reduction in Winnie-TNF-KO after anakinra administration. Moreover, inflammatory UC markers S100a8 and S100a9 and C3 expression levels decreased after anakinra administration in Winnie-TNF-KO mice (Figures 6 and 7). Inflammatory genes related to the IL1 pathway, Il18 and Il33, were not affected by anakinra administration, while Ifnγ expression was reduced significantly in Winnie-TNF-KO. Similarly, the Socs3/Stat3/Il6 pathway expression was reduced in Winnie-TNF-KO after anakinra administration. It is important to notice that the expression of numerous genes, including Il1α, Il1β, Socs3, Stat3, and Il6, was elicited in Winnie mice after anakinra administration (Figures 6 and 7).
Figure 6.
Relative expression of Il1α, Il1β, Tnf, S100a8, S100a9, C3, Ifnγ, Il18, Il33, Il6, Socs3, and Stat3 in WT (N = 3), Winnie (N = 3), and Winnie-TNF-KO (N = 4) mice treated with vehicle or anakinra. ∗P < .05 (anakinra vs vehicle).
Figure 7.
Gene expression analysis of the 88 genes expressed in the distal colon of WT (N = 3), Winnie (N = 3), and Winnie-TNF-KO (N = 4) mice treated with vehicle or anakinra. ∗p < 0.05 (Anakinra vs vehicle)
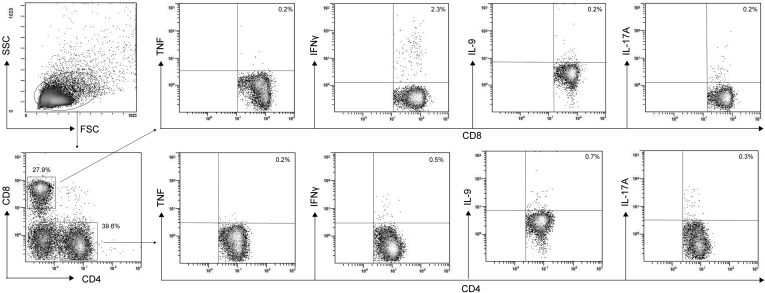
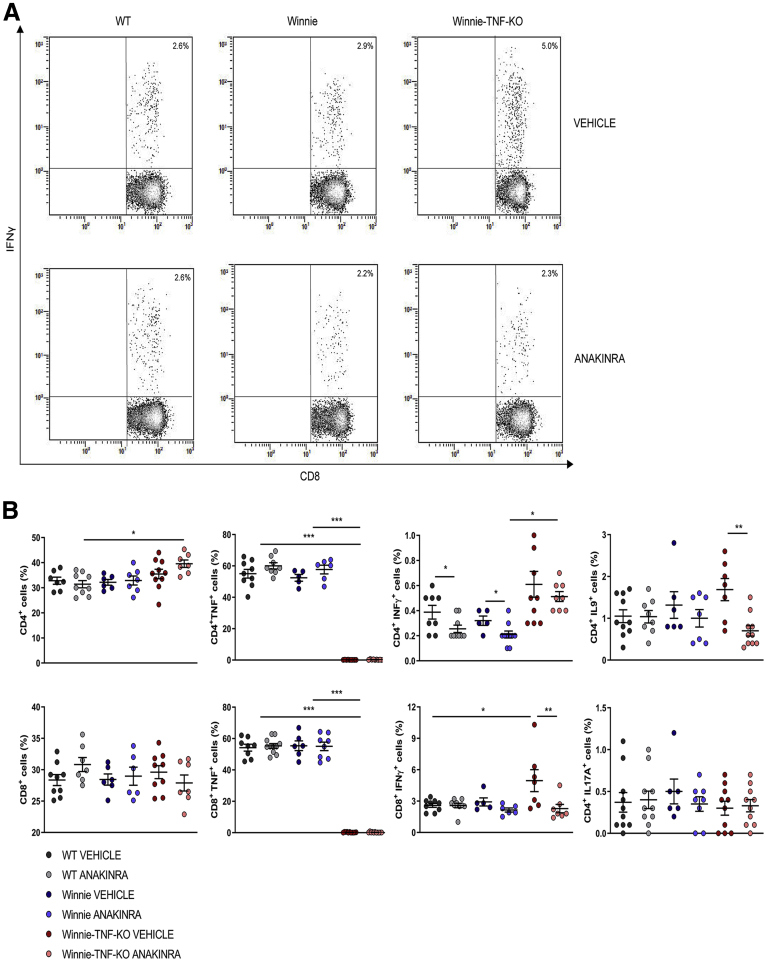
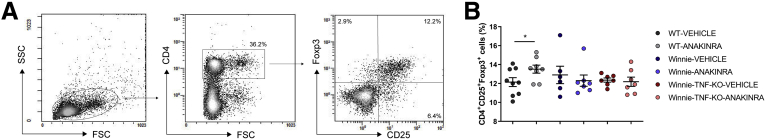
We then performed fluorescence-activated cell sorting analysis of mesenteric lymph node (MLN) and lamina propria (LP) cells to evaluate the effects of anakinra administration on lymphocyte polarization. In particular, we analyzed the production of TNF, IFNγ, IL9, and IL17A by CD4+ and CD8+ cells. Figure 8 shows the experimental scheme used. In MLN of Winnie-TNF-KO mice treated with anakinra, there was an increased percentage of CD4+ cells (39.6% ± 1.5%) compared with the WT group treated with anakinra (31.4% ± 1.4%), while the percentages of CD8+ cells were similar in all experimental conditions (Figure 9B). Treatment with anakinra significantly reduced secretion of IFNγ from both CD4+ T cells in all of the experimental groups and, to a greater extent, from CD8+ T cells in Winnie-TNF-KO mice (Figure 9). Interestingly, in Winnie-TNF-KO mice, the percentage of CD8+ IFNγ+ cells decreased from 5.0% ± 1.1% with the vehicle to 2.3% ± 0.4% with anakinra. Moreover, in Winnie-TNF-KO mice, anakinra significantly reduced the percentage of CD4+ IL9+ T cells (from 1.7% ± 0.3% with the vehicle to 0.7% ± 0.1% with anakinra) (Figure 9B). Lastly, in anakinra-treated Winnie mice, the percentage of CD4+ IL17A+ cells decreased, while no relevant differences were observed for TNF in both CD4+ and CD8+ cells from WT and Winnie mice (Figure 9B). Lastly, we detected significantly increased percentages of CD4+ CD25+ forkhead box P3 (Foxp3+) regulatory T cells only in WT mice MLNs after anakinra administration (12.1% ± 0.5% with the vehicle vs 13.5% ± 0.4% with anakinra) (Figure 10).
Figure 8.
Gating strategy for intracellular staining. Representative density plot analysis of intracellular staining of TNF, IFNγ, IL9, and IL17A from CD4+ and CD8+ cells from MLN of Winnie-TNF-KO mice treated with anakinra (N = 9 for each group). SSC, side scatter.
Figure 9.
(A) Representative density plot analysis of intracellular staining of IFNγ from CD8+T cells in MLN of WT, Winnie, and Winnie-TNF-KO mice, treated with vehicle or anakinra. (B) Frequencies of CD4+ and CD8+ T cells and intracellular staining of TNF, IFNγ, IL9, and IL17A from CD4+ and CD8+ T cells, in the MLNs of WT, Winnie, and Winnie-TNF-KO mice treated with anakinra or vehicle. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Figure 10.
Regulatory T cell (Treg) staining of CD4+cells isolated from MLNs. (A) Representative density plot of Treg gating strategy. (B) Frequencies of CD4+CD25+Foxp3+ cells in MLNs of WT, Winnie, and Winnie-TNF-KO mice treated with anakinra or vehicle (N = 9 for each group). ∗P < .05. FSC, forward scatter; SSC, side scatter.
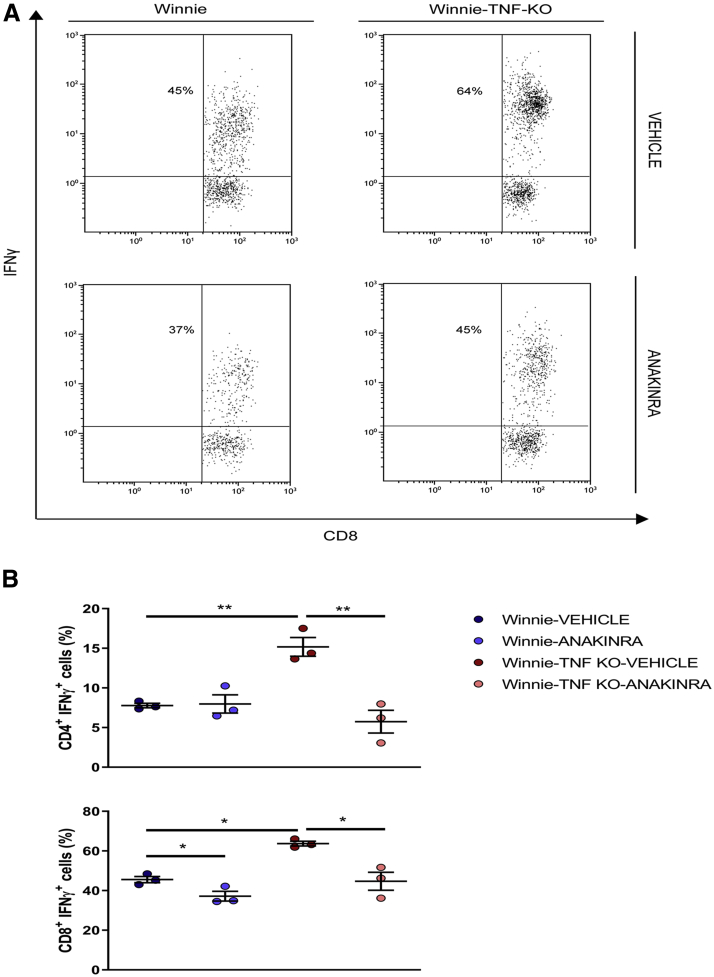
In the LP, the absence of TNF resulted in a sharp increase in the percentage of IFNγ+ CD8+ cells, from 45.6% ± 1.6% in Winnie to 63.7% ± 1.2% in Winnie-TNF-KO mice (Figure 11). Anakinra administration was able to reduce IFNγ+ CD8+ cells in both models. In Winnie mice, the percentage decreased to 37.2% ± 2.5%, while in Winnie-TNF-KO mice it decreased to 44.7% ± 4.5%. In LP of Winnie mice, the percentage of CD4+ IFNγ+ cells was similar in both the vehicle and anakinra groups (7.8% ± 0.3% vs 8.0% ± 1.2%, respectively). In Winnie-TNF-KO mice, instead, it decreased significantly from 15.2% ± 1.2% in the vehicle group to 5.7% ± 1.4% in the anakinra-treated mice (Figure 11B).
Figure 11.
(A) Representative density plot analysis of intracellular staining of IFNγ from CD8+T cells in LP of Winnie and Winnie-TNF-KO mice, treated with vehicle or anakinra. (B) Frequencies of IFNγ+ from CD4+ and CD8+ T cells in the LP of Winnie and Winnie-TNF-KO mice treated with anakinra or vehicle. ∗P < .05, ∗∗P < .01.
Discussion
Despite the introduction of numerous drugs and biological treatments for UC, primary nonresponders to anti-TNF represent a major concern for gastroenterologists concerning an appropriate approach to effectively treat this patient population. The absence of reliable predictors for driving the most effective therapeutic choice leaves no other options than conventional drug administration and frequent follow-up evaluation with serologic and endoscopic evaluation. Besides this, the increasing presence in the IBD pipeline of innovative biological therapies targeting different cytokines and immune processes raises a clear need for predictors of efficacy for anti-TNF treatment.
Indeed, the right drug and specific treatment strategy for each patient is the end goal for personalized medicine.8
In the present study, we analyzed the circulating cytokine profiles of a cohort of 30 UC patients including 18 responders and 12 primary nonresponders. Significant differences in IL1β were associated with primary nonresponse to IFX administration, confirming the previous report describing IL1β as a possible predictive factor to identify a subpopulation of primary nonresponders.12,22,23 IL1β and caspase 8 were detectable by IHC in biopsy specimens from a percentage of nonresponder patients. Importantly, IL1β-positive cells were absent in biopsy specimens from 25% of nonresponder patients, independently from the inflammatory score. These results indicate that IHC staining for IL1β-positive cell assessment may be an important predictive test for deciding the most appropriate biological drug administration.
In this context, in our recent study, we created an animal model of TNF-independent intestinal inflammation (Winnie-TNF-KO) in which we observed a significant increase in colonic IL1β.24 Notably, this model introduced the concept of TNF-independent colitis, which may represent a significant number of primary nonresponder patients. Nonetheless, this does not infer that primary nonresponders are TNF negative, but underlines the possibility that TNF may not be the most appropriate target in a subpopulation of UC patients.
IL1β can be efficiently blocked by anakinra, a recombinant form of IL1Ra, successfully used for the treatment of rheumatoid arthritis.17 Numerous studies have reported the importance of IL1β and IL1Ra genetic polymorphism in IBD,28,29 but only recently was a clinical trial based on anakinra administration to patients affected by a severe form of acute severe ulcerative colitis approved.30 Administration of anakinra to UC patients may result in nonsignificant disease remission if the patient cohort is not preliminarily selected. Our results indicate that only a percentage of primary nonresponder UC patients is characterized by increased IL1β in sera and colonic biopsy specimens. Thus, these parameters should be used as inclusion criteria for patient enrollment in a new clinical trial based on anakinra administration to primary nonresponder UC patients.
The Winnie model is a mild but progressive model of UC-like colitis, showing several features of the human disease. Although the histologic features of UC-like colitis are modest, the presence of inflammation can be detected by gene expression, cytokine profile, and dysbiosis.25,26 The Winnie inflammatory pathway is not triggered by TNF. Indeed, the intestinal tract of Winnie-TNF-KO mice is affected by pathologic features similarly to Winnie.24 We noted potential negative feedback between TNF and IL1β, suggesting an inverse correlation between TNF and IL1β secretion.
With the intent of providing a rationale for a new therapeutic option suited for a subgroup of anti-TNF primary nonresponder UC patients, characterized by increased circulating levels of IL1β, we evaluated the effects of anakinra administration to Winnie-TNF-KO mice.
Anakinra injections significantly reduced the DAI score in Winnie-TNF-KO mice compared with their vehicle-treated counterparts and Winnie mice, this provided useful insights into the positive effects that IL1β blockade has on colitis. Moreover, immunohistology examination showed that IL1β could be detected only in the colonic tissue of Winnie-TNF-KO mice, reinforcing the idea of TNF-mediated negative feedback for IL1β secretion. Intraperitoneal administration of anakinra efficiently blocked intestinal IL1β and caspase 8 in Winnie-TNF-KO.
Caspase 8 activity and function are regulated through shuttling between the nucleus and the cytoplasm.31, 32, 33 The nuclear active form of caspase 8 mediates the transcription of a broad array of genes involved in cell-cycle inhibition and apoptosis.34, 35, 36 Recently, it was shown that caspase 8 is crucially involved in the induction of pro-IL1β synthesis and processing via both noncanonical and canonical pathways.37
In the present study, in anakinra-treated Winnie-TNF-KO mice, caspase 8 expression was detected only in the cytoplasm, while in vehicle-treated Winnie-TNF-KO mice, caspase 8 also was present in the nucleus. In line with these results, IL1β was detected ubiquitously in vehicle-treated Winnie-TNF-KO mice, while only present at the desquamating tip of anakinra-treated Winnie-TNF-KO mice, suggesting initiation of homeostatic reepithelization.
The intracellular staining of MLN CD4+ and CD8+ T cells shows that Winnie-TNF-KO mice are characterized by a higher frequency of IFNγ+ and IL9+ CD4+ T cells when compared with Winnie, suggesting a relevant role for T helper 9 (Th9) cells in this TNF-independent model of UC.38 Anakinra administration was able to reduce the percentage of IFNγ+ CD4+ T cells in Winnie, while the percentage of TNF+ CD4+ T cells was unchanged. These data suggest that, at least under these experimental conditions, TNF can influence IL1β production, but the opposite is not true, or that in TNF competent models, IL1β is not expressed sufficiently in the mucosal tissues.
When compared with the parental strain Winnie, the percentage of IFNγ+ CD8+ T cells was higher in Winnie-TNF-KO mice, suggesting a pivotal role for cytotoxic T cells in the TNF-independent intestinal inflammation. The percentage of IFNγ+ CD8+ T cells sharply decreases after anakinra administration, revealing the importance of IL1β in sustaining this crucial inflammatory pathway. As reported by Corridoni et al,39 there is extensive heterogeneity of CD8+ T cells playing a primary role in UC. These results are even more dramatic in the lamina propria, where approximately 64% of CD8 cells are IFNγ+ in Winnie-TNF-KO mice. Anakinra administration was able to reduce IFNγ+ CD8+ cells to 45%, the same percentage of IFNγ+ CD8+ observed in Winnie mice.
Although we did not characterize the presence of IL26+ CD8+ T cells in the intestinal mucosa, the axis between IL1β and IL26 already is known and potentially may contribute and explain the effects of anakinra administration in TNF-independent UC.40
ELISA assays of the colonic tissue culture showed that, in the absence of TNF, the inflammatory pathway can switch to an IL1β-and IFNγ-dependent inflammation. Anakinra administration was able to reduce IL1β release both in Winnie and Winnie-TNF-KO. Finally, analysis of the distal colon showed a reduction of several inflammatory markers, including Il1α, Il1β, Ifnγ, and C3, in Winnie-TNF-KO mice. The results also indicated increased expression of these genes in Winnie mice after anakinra administration. In the absence of TNF, the IL1β inflammatory pathway becomes prevalent, thus the IL1 sequestration strategy efficiently blocks the inflammatory cascade. Surprisingly, the expression of some inflammatory mediators is elicited by anakinra in Winnie mice. This is particularly evident for the Socs3/Stat3/Il6 expression pathway41 that is induced by IL1 sequestration in Winnie. This may be owing to the need to compensate for the blockade of activity of both IL1α and IL1β by the IL1-receptor antagonist. Overall, the gene expression pathway obtained from the distal colon indicates a reduced intestinal inflammation in anakinra-treated mice.
Our results highlight the beneficial effects of anakinra administration, particularly in mice affected by TNF-independent UC, a feature that may characterize a subpopulation of UC patients. Precision medicine relies on efficient patient stratification. Adjustments to the therapeutic strategies may be driven by the objective assessment of circulating inflammatory markers, even if in our mice models this analysis was inconsistent owing to the numerous samples below detection levels. Another important approach may be represented by the noninvasive analysis of patients’ fecal material, which could be considered a reliable option to adjust patients’ therapies. In our model, N-GAL assessment was able to indicate the mouse response to anakinra. UC patients share similar effects triggered by different genetic and environmental factors. TNF is clearly among the most important factor involved in the inflammatory cascade causing uncontrolled chronic inflammation. Nonetheless, TNF-independent UC is an event likely unnoticed, even for primary nonresponders, to anti-TNF therapy. Our results indicate that circulating levels of IL1β can be used as a predictor for primary nonresponder patients, who may be enrolled in clinical trials. Our data also highlight the need for a thorough patient selection in future clinical trials based on IL1 sequestration to primary nonresponder UC patients and routine analysis of fecal material inflammatory markers to allow real-time adjustments to the therapeutic strategy.
The use of anakinra in inflammatory disorders related to TNF dysfunction already has produced promising responses, including for the treatment of TNF Receptor-Associated Periodic Syndrome, an autoinflammatory disorder caused by a mutant TNF receptor that is not transported to the cell surface efficiently.42,43 TNF Receptor-Associated Periodic Syndrome is characterized by increased inflammatory cytokine secretion, including IL1, underscoring the potential negative feedback mechanism(s) between TNF and IL1.44,45 Although the axis between TNF and IL1β may require further investigation, our results lay the foundation for a new concept of TNF-independent UC that requires innovative approaches to suppress chronic inflammation favoring UC remission.
Materials and Methods
Ethical Considerations
The study protocols on human subjects were conducted in accordance with the principles of the Declaration of Helsinki and approved by the local ethics committee (ID: 2383 Comitato Etico Fondazione Policlinico Universitario “A. Gemelli” Istituto di Ricovero e Cura a Carattere Scientifico and number 333 National Institute of Gastroenterology “S. de Bellis”).
Our investigations were performed under the relevant animal protocol, which was approved by the Institutional Animal Care Committee of the National Institute of Gastroenterology “S. de Bellis” (Organism engaged for compliance of Animal Wellbeing: Organismo Preposto al Benessere degli Animali [OPBA]). All of the animal experiments were performed according to the national guidelines of Italian Directive 26/2014 and approved by the Italian Animal Ethics Committee of Ministry of Health–General Directorate of Animal Health and Veterinary Drugs (Direzione generale della sanita' animale e dei farmaci veterinari [DGSAF] - Protocollo 768/2015-PR 27/07/2015). All animals were maintained in a controlled environment (20°C–22°C, 12-hour light and 12-hour dark cycle, and 45%–55% relative humidity).
Murine Models
WT mice were purchased from Jackson Laboratories, (Bar Harbor, ME; C57BL/6, stock no.: 000664).
The murine transgenic line Winnie-TNF-KO was created in our laboratory previously by breeding heterozygous mice from the TNF knockout line and heterozygous Winnie mice.24
The TNF knockout mice were purchased from Jackson Laboratories (B6.129S-Tnftm1Gkl/J, stock no: 005540), while Winnie mice were obtained from the University of Tasmania, Launceston, TAS, Australia.25
Body weight, stool consistency, and rectal bleeding were recorded daily. Mice were killed 14 days after the first intraperitoneal (IP) injection, and the colon was explanted to evaluate the clinical severity of colitis. Colon length and weight were measured as indicators of colonic inflammation. The colon/body weight indices were calculated as the ratio of the colon wet weight and the total body weight and as the ratio of the colon length and the total body weight of each mouse. The DAI was determined by scoring changes in body weight (0–4), stool consistency (0–4), and occult blood (0–4).27
Treatment With IL1-Receptor Antagonist
Adult mice age 16 weeks were treated with anakinra (Kineret; Swedish Orphan Biovitrum AB, Stockholm, Sweden, 60 mg/kg in 0.1 mL PBS) or vehicle (0.1 mL PBS) via IP injections every day for 2 weeks. These doses of anakinra were chosen because they are within the range of doses that have been shown previously to be efficacious in other models of disease in mice.46,47 No signs of any adverse side effects were recorded with these doses of anakinra.
Histologic Examination
Tissue sections from the proximal, medial, and distal colon were fixed in 10% buffered formalin and embedded in paraffin. Sections (3 μm) were deparaffinized in xylene, rehydrated with ethanol series and water, and washed in PBS. H&E staining was performed on the sections using standard techniques. Images were acquired using a Nikon Eclipse Ti2 microscope (Nikon, Tokyo, Japan).
IHC
IHC analyses for IL1β and caspase 8 were performed in the formalin-fixed paraffin-embedded tissues obtained from WT, Winnie, and Winnie-TNF-KO mice treated with anakinra or vehicle, and biopsy specimens from UC patients at the beginning of anti-TNF treatment. Distal colon sections (4 μm) were freshly cut and dried at 60°C for 30 minutes. IHC analysis was performed in sections after deparaffinization for 30 minutes and then rehydration in grades of alcohol. Antigen retrieval was performed at 90°C for 20 minutes with sodium citrate buffer (Sigma-Aldrich, St. Louis, MO). To assess the IL1β and caspase 8 staining used for the present study, the samples were blocked with blocking buffer, incubated with IL1β (Cleaved Asp116, polyclonal, Thermo Fisher Scientific, Waltham, MA) and caspase 8, polyclonal antibody (Thermo Fisher Scientific, Waltham, MA) using a 1:100 dilution (2 h, 22°C), followed by horseradish-peroxidase–conjugated goat anti-rabbit IgG (Heavy and Light chains) secondary antibody at a dilution of 1:500 for 30 minutes at room temperature (Thermo Fisher Scientific). Chromogenic detection was performed using the Metal Enhanced DAB Substrate Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Negative control sections were incubated without the primary antibody in citrate buffer. All sections were counterstained with hematoxylin. Images were taken on a Nikon Eclipse Ti2 microscope (Nikon) at a magnification of 20×. The histologic score was based on the percentage of inflammatory cells present in the mucosa: a score of 0 indicated no inflammatory cells; 1 indicated 1%–25% of cells; 2 indicated 25%–50% of cells; 3 indicated 50%–75% of cells; and 4 indicated 75%–100% of inflammatory cells.
Cytofluorimetric Assay
To obtain LP cells, murine colons were cut into small segments (1-cm long), washed with Dulbecco's Phosphate-Buffered Saline (DPBS) 1× (Gibco, Waltham, MA) + 2.5 mmol/L EDTA (Ambion, Thermo Fisher Scientific) to separate from epithelial cells, and digested with collagenase type IV and DNase I (Sigma Aldrich) for 30 minutes at 37°C after GentleMACS processing. Resulting cell suspensions were pelleted by centrifugation, washed with DPBS 1× + 0.5 mmol/L EDTA, and passed through 100-μm and 30-μm cell strainers (Miltenyi Biotec, Bergisch Gladbach, Germany) and washed with DPBS (Gibco) + 0.5% bovine serum albumin (BSA; Sigma-Aldrich).
MLNs were isolated from mice treated with anakinra or vehicle. MLNs were passed through a 30-μm cell strainer (Miltenyi Biotec) to obtain a single-cell suspension and washed with DPBS (Gibco) + 0.5% BSA (Sigma-Aldrich).
Foxp3 staining
Single-cell suspensions were stained with CD4–FITC (fluorescein isothiocyanate) and CD25-phycoerythrin (PE) (Miltenyi Biotec). Cells were permeabilized with the Foxp3 Fixation/Permeabilization Kit (eBioscience, San Diego, CA) and washed with PERM Buffer (eBioscience). Finally, cells were stained with Foxp3-Allophycocyanin (APC) (Miltenyi Biotec), according to the manufacturer’s instructions.
T-cell intracellular staining
T cells from LP and MLN of mice treated with anakinra or vehicle were cultured with a 500× Cell Stimulation Cocktail (eBioscience) for 12 hours, washed with DPBS + 0.5% BSA, and stained with CD4–FITC and CD8-APC-Vio700 (Miltenyi Biotec). After washing, cells were permeabilized with BD CytoFix/CytoPerm Fixation/Permeabilization Kit (BD Biosciences, Franklin Lakes, NJ), washed with PERM Buffer, and stained with IL9–PE, IL17A–APC, TNF–PE, and IFNγ–APC according to the manufacturer’s instructions (Miltenyi Biotec).
For both stainings, Flow Cytometer acquisition was performed using NAVIOS (Beckman Coulter, Brea, CA). Flow cytometer analysis was performed using Kaluza Software 1.5 (Beckman Coulter).
RNA Extraction and Quantitative Polymerase Chain Reaction Analysis
Total RNA was isolated from the distal colon of mice treated with anakinra or vehicle. The RNA was extracted using TRIzol (Thermo Fisher Scientific) according to the manufacturer’s instructions. Total RNA (1 μg) was reverse-transcribed using an iScript complementary DNA Synthesis kit (Bio-Rad, Hercules, CA) with random primers for complementary DNA synthesis. Gene expression of 88 genes was performed using the Colitis, Ulcerative Tier 1 M96 (Bio-Rad, Hercules, CA). Real-time analysis was performed on a CFX96 Touch System (Bio-Rad), and for the relative expression the Delta Delta Threshold Cycle (ΔΔCt) method was used. Gene cluster analyses were performed with CFX Manager software 3.1 (Bio-Rad).
Colon Cytokine Analysis
Colons were explanted from experimental mice after being killed, then cut, opened, and washed from feces in an antibiotic solution. They then were minced in small pieces (approximately 0.5-cm long) and cultured overnight in RPMI 1640 (Thermo Fisher Scientific) supplemented with 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific) and 100 U/mL penicillin/streptomycin (Thermo Fisher Scientific) at 37°C in a humidified 5% CO2 atmosphere. Supernatants were harvested 24 hours later and used for ELISA.
Feces Analyses
Stool samples of mice were collected on day 0 (before the first IP injection) and day 14 (corresponding to the end of treatment with vehicle or anakinra), and thereafter stored at -80°C. For analysis, frozen feces were diluted in DPBS + 0.1% Tween 20 to a final concentration of 100 mg/mL, homogenized with tissue lyser for 5’ at 30 Hz, then centrifuged at 14,000 × g for 10 minutes at 4°C. The supernatants then were collected and stored at -80°C for ELISA.
Generation and Culture of Murine DCs
BMDCs were obtained from WT or TNF-KO mice. Single-cell suspension of BMDCs from the tibiae and femurs of 8- to 10-week-old male mice were flushed with 0.5 mmol/L EDTA (Thermo Fisher Scientific), and depleted of red blood cells with ACK lysing buffer (Thermo Fisher Scientific). Cells were plated in a 10-mL dish (1 × 106 cells/mL) in RPMI 1640 (Thermo Fisher Scientific) supplemented with 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific), 100 U/mL penicillin/streptomycin (Thermo Fisher Scientific), 25 ng/mL mouse recombinant GM-CSF (rmGM-CSF) and 25 ng/mL rmIL4 (Miltenyi Biotec) at 37°C in a humidified 5% CO2 atmosphere. Five days after the isolation, all nonadherent cells were gently harvested and plated on a 24-well culture plate at a concentration of 1 × 106 cells/mL, and new growth factors were added to the culture medium too. On day 7 cells were stimulated with 1 μg/mL LPS (L6143; Sigma-Aldrich). Supernatants were collected 24 hours after LPS stimulation.
Multiplex Cytokine and Chemokine ELISA
Cell culture supernatants were analyzed by multiplex to evaluate cytokine and chemokine concentrations, using the Bead-based Multiplex for the Luminex platform (LaboSpace srl, Milan, Italy).
Study Population
A total of 30 UC patients with moderate-to-severe disease starting IFX therapy were enrolled at the IBD Unit of Fondazione Policlinico Universitario “A. Gemelli” IRCCS, and the IBD Unit of IRCCS “S. De Bellis.” The general and clinical characteristics of the study population are detailed in Table 5. Patients were evaluated endoscopically at the beginning of anti-TNF treatment (T0) and the end of the induction regimen, specifically 12 weeks after the first infusion (T1). All subjects were naïve to anti-TNF therapy and had stopped other immunosuppressant drugs at least 1 week before enrollment. The exclusion criteria were participants with diagnoses of comorbidities, such as diabetes, autoimmune disease, or any associated inflammatory or infectious disease. There were no significant differences among patients in risk factors and/or clinical variables such as sex, family history of IBD, tobacco smoking, body mass index, hemoglobin, glucose, triglycerides, total cholesterol, high-density lipoprotein, low-density lipoprotein, very-low-density lipoprotein, or systolic/diastolic blood pressure.
Table 5.
General and Clinical Characteristics of Patients With UC Enrolled in the Analysis
| Patients, n | Disease | Male/female | Age, y | Montreal classification |
|---|---|---|---|---|
| 30 | UC | 17/13 | 43.5 ± 6.8 | 7 E2S2, 4 E2S3, 19 E3S2 |
Serum samples were obtained from each UC patient at T0 and T1, and directly frozen at -80°C. The UC endoscopic classification was established using the endoscopic Mayo score of severity.7,48 At T0 all subjects had an endoscopic Mayo score ≥2. UC patients then were divided into 2 groups based on the endoscopic score at T1: endoscopic Mayo score of 0–1 (responders) and endoscopic Mayo score of ≥2 (nonresponders).
All enrolled subjects provided written informed consent.
ELISA
Sera from UC patients treated with IFX were analyzed for IL1β release in triplicate, using an ELISA kit (R&D Systems, Minneapolis, MN) following the manufacturer’s instructions. Supernatants from mice colonic tissue culture were analyzed for IL1β, IFNγ, IL6, and IL18 release in triplicate, using an ELISA kit (R&D Systems) following the manufacturer’s instructions.
Supernatants from mice stool were analyzed for N-GAL (lipocalin-2) release in triplicate, using an ELISA kit (R&D Systems) following the manufacturer’s instructions.
Multiplex Cytokine Analysis With Fluorescence-Activated Cell Sorting
Sera obtained from UC patients treated with IFX were analyzed using the MACSPlex Cytokine 12 Kit human (Miltenyi Biotec), following the manufacturer’s instructions. Flow cytometer acquisition was performed using NAVIOS (Beckman Coulter). Flow cytometer analysis was performed using Kaluza Software 1.5 (Beckman Coulter).
Multiple Regression and Correlation Analysis
The goodness-of-fit test and multiple regression were used to examine IL1β associations with other cytokines and chemokines in sera and to evaluate the correlation between the percentage of IL1β+ or caspase 8+ cells and inflammatory score in biopsy specimens. Correlation analysis was conducted using the Pearson test (r, regression). Results were considered statistically significant at P < .05.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism (San Diego, CA) statistical software release 8.0. All data were expressed as means ± SEM obtained from at least 3 independent experiments. We evaluated statistical significance with a 2-tailed Student t test, 1-way analysis of variance followed by Tukey multiple comparison as post-test, and the 2-way analysis of variance test using the Bonferroni as a post-test for the grouped analysis.
Results were considered statistically significant at P < .05.
Access to Data
All authors had access to the study data and reviewed and approved the final manuscript.
Acknowledgments
The authors are grateful to Dr Sergio Coletta, Dr Nicolò Schena, Dr Giusy Bianco, and Vito Spilotro for their precious contribution.
CRediT Authorship Contributions
Marina Liso, PhD (Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Validation: Lead; Writing – original draft: Equal)
Giulio Verna, BD (Data curation: Supporting; Formal analysis: Supporting; Investigation: Supporting; Methodology: Supporting; Validation: Supporting)
Elisabetta Cavalcanti, PhD (Formal analysis: Supporting; Investigation: Supporting; Methodology: Supporting)
Stefania De Santis, PhD (Conceptualization: Supporting; Data curation: Supporting; Formal analysis: Supporting)
Raffaele Armentano, MD (Conceptualization: Supporting; Supervision: Supporting)
Angela Tafaro, PhD (Formal analysis: Supporting; Methodology: Supporting)
Antonio Lippolis, BD (Conceptualization: Supporting; Funding acquisition: Supporting; Project administration: Supporting)
Pietro Campiglia, PhD (Data curation: Supporting; Funding acquisition: Supporting; Methodology: Supporting; Validation: Supporting)
Antonio Gasbarrini, MD (Investigation: Supporting; Supervision: Supporting)
Mauro Mastronardi, MD (Formal analysis: Supporting; Resources: Supporting)
Theresa Torres Pizarro, PhD (Conceptualization: Supporting; Investigation: Supporting; Validation: Supporting; Writing – original draft: Equal)
Fabio Cominelli, MD (Conceptualization: Supporting; Investigation: Supporting; Supervision: Supporting)
Loris Riccardo Lopetuso, MD (Data curation: Equal; Formal analysis: Equal; Writing – review & editing: Equal)
Marcello Chieppa, PhD (Conceptualization: Lead; Resources: Lead; Supervision: Lead; Writing – original draft: Equal)
Data transparency
The data presented in this study are available upon request to the corresponding author.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported by the Italian Ministry of Health grant GR-2011-02347991; Innovative solutions for patient management and therapeutic follow up in ulcerative colitis; Programma Operativo Fondo Europeo di Sviluppo Regionale (PO FESR) 2014-2020 Technological and collaborative research platforms for the fight against oncologic diseases; and Ricerca Corrente 2019 Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) “S. de Bellis.” Also supported by M.I.Cro, Inflammatory chronical intestinal disease association (M.L.); and by Programma Operativo Nazionale (PON)-Research and Innovation 2014-2020: Project AIM1801289-attività 3-linea 1 (S.D.S.).
References
- 1.Murch S.H., Lamkin V.A., Savage M.O., Walker-Smith J.A., MacDonald T.T. Serum concentrations of tumour necrosis factor alpha in childhood chronic inflammatory bowel disease. Gut. 1991;32:913. doi: 10.1136/gut.32.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsukada Y., Nakamura T., Iimura M., Iizuka B.E., Hayashi N. Cytokine profile in colonic mucosa of ulcerative colitis correlates with disease activity and response to granulocytapheresis. Am J Gastroenterol. 2002;97:2820–2828. doi: 10.1111/j.1572-0241.2002.07029.x. [DOI] [PubMed] [Google Scholar]
- 3.Braegger C.P., Nicholls S., Murch S.H., Stephens S., MacDonald T.T. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992;339:89–91. doi: 10.1016/0140-6736(92)90999-j. [DOI] [PubMed] [Google Scholar]
- 4.Sherman M., Tsynman D.N., Kim A., Arora J., Pietras T., Messing S., St Hilaire L., Yoon S., Decross A., Shah A., Saubermann L. Sustained improvement in health-related quality of life measures in patients with inflammatory bowel disease receiving prolonged anti-tumor necrosis factor therapy. J Dig Dis. 2014;15:174–179. doi: 10.1111/1751-2980.12125. [DOI] [PubMed] [Google Scholar]
- 5.Pisani L.F., Moriggi M., Gelfi C., Vecchi M., Pastorelli L. Proteomic insights on the metabolism in inflammatory bowel disease. World J Gastroenterol. 2020;26:696–705. doi: 10.3748/wjg.v26.i7.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Horin S., Kopylov U., Chowers Y. Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun Rev. 2014;13:24–30. doi: 10.1016/j.autrev.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Lopetuso L.R., Gerardi V., Papa V., Scaldaferri F., Rapaccini G.L., Gasbarrini A., Papa A. Can we predict the efficacy of anti-TNF-alpha agents? Int J Mol Sci. 2017;18:1973. doi: 10.3390/ijms18091973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopetuso L.R., Gasbarrini A. Fighting the hype for predictors of efficacy in inflammatory bowel disease. Inflamm Bowel Dis. 2020;26:764–765. doi: 10.1093/ibd/izz274. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy N.A., Heap G.A., Green H.D., Hamilton B., Bewshea C., Walker G.J., Thomas A., Nice R., Perry M.H., Bouri S., Chanchlani N., Heerasing N.M., Hendy P., Lin S., Gaya D.R., Cummings J.R.F., Selinger C.P., Lees C.W., Hart A.L., Parkes M., Sebastian S., Mansfield J.C., Irving P.M., Lindsay J., Russell R.K., McDonald T.J., McGovern D., Goodhand J.R., Ahmad T. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4:341–353. doi: 10.1016/S2468-1253(19)30012-3. [DOI] [PubMed] [Google Scholar]
- 10.Sprakes M.B., Ford A.C., Warren L., Greer D., Hamlin J. Efficacy, tolerability, and predictors of response to infliximab therapy for Crohn's disease: a large single centre experience. J Crohns Colitis. 2012;6:143–153. doi: 10.1016/j.crohns.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Wong U., Cross R.K. Primary and secondary nonresponse to infliximab: mechanisms and countermeasures. Expert Opin Drug Metab Toxicol. 2017;13:1039–1046. doi: 10.1080/17425255.2017.1377180. [DOI] [PubMed] [Google Scholar]
- 12.West N.R., Hegazy A.N., Owens B.M.J., Bullers S.J., Linggi B., Buonocore S., Coccia M., Görtz D., This S., Stockenhuber K., Pott J., Friedrich M., Ryzhakov G., Baribaud F., Brodmerkel C., Cieluch C., Rahman N., Müller-Newen G., Owens R.J., Kühl A.A., Maloy K.J., Plevy S.E., Keshav S., Travis S.P.L., Powrie F. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med. 2017;23:579–589. doi: 10.1038/nm.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinarello C.A., Simon A., van der Meer J.W. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinarello C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cominelli F., Pizarro T.T. Interleukin-1 and interleukin-1 receptor antagonist in inflammatory bowel disease. Aliment Pharmacol Ther. 1996;10(Suppl 2):49–53. doi: 10.1046/j.1365-2036.1996.22164020.x. discussion 4. [DOI] [PubMed] [Google Scholar]
- 16.Dinarello C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281:8–27. doi: 10.1111/imr.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinarello C.A., van der Meer J.W. Treating inflammation by blocking interleukin-1 in humans. Semin Immunol. 2013;25:469–484. doi: 10.1016/j.smim.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen S., Hurd E., Cush J., Schiff M., Weinblatt M.E., Moreland L.W., Kremer J., Bear M.B., Rich W.J., McCabe D. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:614–624. doi: 10.1002/art.10141. [DOI] [PubMed] [Google Scholar]
- 19.Cohen S.B., Moreland L.W., Cush J.J., Greenwald M.W., Block S., Shergy W.J., Hanrahan P.S., Kraishi M.M., Patel A., Sun G., Bear M.B. A multicentre, double blind, randomised, placebo controlled trial of anakinra (Kineret), a recombinant interleukin 1 receptor antagonist, in patients with rheumatoid arthritis treated with background methotrexate. Ann Rheum Dis. 2004;63:1062–1068. doi: 10.1136/ard.2003.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bresnihan B., Alvaro-Gracia J.M., Cobby M., Doherty M., Domljan Z., Emery P., Nuki G., Pavelka K., Rau R., Rozman B., Watt I., Williams B., Aitchison R., McCabe D., Musikic P. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis Rheum. 1998;41:2196–2204. doi: 10.1002/1529-0131(199812)41:12<2196::AID-ART15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Opal S.M., Fisher C.J., Jr., Dhainaut J.F., Vincent J.L., Brase R., Lowry S.F., Sadoff J.C., Slotman G.J., Levy H., Balk R.A., Shelly M.P., Pribble J.P., LaBrecque J.F., Lookabaugh J., Donovan H., Dubin H., Baughman R., Norman J., DeMaria E., Matzel K., Abraham E., Seneff M. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med. 1997;25:1115–1124. doi: 10.1097/00003246-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Aschenbrenner D., Quaranta M., Banerjee S., Ilott N., Jansen J., Steere B., Chen Y.H., Ho S., Cox K., Arancibia-Cárcamo C.V., Coles M., Gaffney E., Travis S.P., Denson L., Kugathasan S., Schmitz J., Powrie F., Sansom S.N., Uhlig H.H. Deconvolution of monocyte responses in inflammatory bowel disease reveals an IL-1 cytokine network that regulates IL-23 in genetic and acquired IL-10 resistance. Gut. 2021;70:1023–1036. doi: 10.1136/gutjnl-2020-321731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGovern D., Powrie F. The IL23 axis plays a key role in the pathogenesis of IBD. Gut. 2007;56:1333–1336. doi: 10.1136/gut.2006.115402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Santis S., Kunde D., Galleggiante V., Liso M., Scandiffio L., Serino G., Pinto A., Campiglia P., Sorrentino R., Cavalcanti E., Santino A., Caruso M.L., Eri R., Chieppa M. TNFα deficiency results in increased IL-1β in an early onset of spontaneous murine colitis. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eri R.D., Adams R.J., Tran T.V., Tong H., Das I., Roche D.K., Oancea I., Png C.W., Jeffery P.L., Radford-Smith G.L., Cook M.C., Florin T.H., McGuckin M.A. An intestinal epithelial defect conferring ER stress results in inflammation involving both innate and adaptive immunity. Mucosal Immunol. 2011;4:354–364. doi: 10.1038/mi.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liso M., De Santis S., Verna G., Dicarlo M., Calasso M., Santino A., Gigante I., Eri R., Raveenthiraraj S., Sobolewski A., Palmitessa V., Lippolis A., Mastronardi M., Armentano R., Serino G., De Angelis M., Chieppa M. A specific mutation in Muc2 determines early dysbiosis in colitis-prone Winnie mice. Inflamm Bowel Dis. 2020;26:546–556. doi: 10.1093/ibd/izz279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L., Zhou Z., Yang Y., Chen N., Xiang H. Therapeutic effect of imiquimod on dextran sulfate sodium-induced ulcerative colitis in mice. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stokkers P.C., van Aken B.E., Basoski N., Reitsma P.H., Tytgat G.N., van Deventer S.J. Five genetic markers in the interleukin 1 family in relation to inflammatory bowel disease. Gut. 1998;43:33–39. doi: 10.1136/gut.43.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heresbach D., Alizadeh M., Dabadie A., Le Berre N., Colombel J.F., Yaouanq J., Bretagne J.F., Semana G. Significance of interleukin-1beta and interleukin-1 receptor antagonist genetic polymorphism in inflammatory bowel diseases. Am J Gastroenterol. 1997;92:1164–1169. [PubMed] [Google Scholar]
- 30.Thomas M.G., Bayliss C., Bond S., Dowling F., Galea J., Jairath V., Lamb C., Probert C., Timperley-Preece E., Watson A., Whitehead L., Williams J.G., Parkes M., Kaser A., Raine T. Trial summary and protocol for a phase II randomised placebo-controlled double-blinded trial of Interleukin 1 blockade in Acute Severe Colitis: the IASO trial. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-023765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruidering M., Evan G.I. Caspase-8 in apoptosis: the beginning of "the end"? IUBMB Life. 2000;50:85–90. doi: 10.1080/713803693. [DOI] [PubMed] [Google Scholar]
- 32.Schwarzer R., Jiao H., Wachsmuth L., Tresch A., Pasparakis M. FADD and caspase-8 regulate gut homeostasis and inflammation by controlling MLKL- and GSDMD-mediated death of intestinal epithelial cells. Immunity. 2020;52:978–993.e6. doi: 10.1016/j.immuni.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Prokhorova E.A., Kopeina G.S., Lavrik I.N., Zhivotovsky B. Apoptosis regulation by subcellular relocation of caspases. Sci Rep. 2018;8:12199. doi: 10.1038/s41598-018-30652-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benchoua A., Couriaud C., Guégan C., Tartier L., Couvert P., Friocourt G., Chelly J., Ménissier-de Murcia J., Onténiente B. Active caspase-8 translocates into the nucleus of apoptotic cells to inactivate poly(ADP-ribose) polymerase-2. J Biol Chem. 2002;277:34217–34222. doi: 10.1074/jbc.M203941200. [DOI] [PubMed] [Google Scholar]
- 35.Feltham R., Vince J.E., Lawlor K.E. Caspase-8: not so silently deadly. Clin Transl Immunol. 2017;6:e124. doi: 10.1038/cti.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gurung P., Kanneganti T.D. Novel roles for caspase-8 in IL-1β and inflammasome regulation. Am J Pathol. 2015;185:17–25. doi: 10.1016/j.ajpath.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monie T.P., Bryant C.E. Caspase-8 functions as a key mediator of inflammation and pro-IL-1β processing via both canonical and non-canonical pathways. Immunol Rev. 2015;265:181–193. doi: 10.1111/imr.12284. [DOI] [PubMed] [Google Scholar]
- 38.Shohan M., Sabzevary-Ghahfarokhi M., Bagheri N., Shirzad H., Rahimian G., Soltani A., Ghatreh-Samani M., Deris F., Tahmasbi K., Shahverdi E., Fathollahi F. Intensified Th9 response is associated with the immunopathogenesis of active ulcerative colitis. Immunol Invest. 2018;47:700–711. doi: 10.1080/08820139.2018.1486411. [DOI] [PubMed] [Google Scholar]
- 39.Corridoni D., Antanaviciute A., Gupta T., Fawkner-Corbett D., Aulicino A., Jagielowicz M., Parikh K., Repapi E., Taylor S., Ishikawa D., Hatano R., Yamada T., Xin W., Slawinski H., Bowden R., Napolitani G., Brain O., Morimoto C., Koohy H., Simmons A. Single-cell atlas of colonic CD8+ T cells in ulcerative colitis. Nat Med. 2020;26:1480–1490. doi: 10.1038/s41591-020-1003-4. [DOI] [PubMed] [Google Scholar]
- 40.Larochette V., Miot C., Poli C., Beaumont E., Roingeard P., Fickenscher H., Jeannin P., Delneste Y. IL-26, a cytokine with roles in extracellular DNA-induced inflammation and microbial defense. Front Immunol. 2019;10:204. doi: 10.3389/fimmu.2019.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X.P., Albrecht U. Dual function of interleukin-1beta for the regulation of interleukin-6-induced suppressor of cytokine signaling 3 expression. J Biol Chem. 2004 Oct 22;279(43):45279–45289. doi: 10.1074/jbc.M313072200. [DOI] [PubMed] [Google Scholar]
- 42.Obici L., Meini A., Cattalini M., Chicca S., Galliani M., Donadei S., Plebani A., Merlini G. Favourable and sustained response to anakinra in tumour necrosis factor receptor-associated periodic syndrome (TRAPS) with or without AA amyloidosis. Ann Rheum Dis. 2011;70:1511–1512. doi: 10.1136/ard.2010.143438. [DOI] [PubMed] [Google Scholar]
- 43.Sacré K., Brihaye B., Lidove O., Papo T., Pocidalo M.A., Cuisset L., Dodé C. Dramatic improvement following interleukin 1beta blockade in tumor necrosis factor receptor-1-associated syndrome (TRAPS) resistant to anti-TNF-alpha therapy. J Rheumatol. 2008;35:357–358. [PubMed] [Google Scholar]
- 44.Lobito A.A., Kimberley F.C., Muppidi J.R., Komarow H., Jackson A.J., Hull K.M., Kastner D.L., Screaton G.R., Siegel R.M. Abnormal disulfide-linked oligomerization results in ER retention and altered signaling by TNFR1 mutants in TNFR1-associated periodic fever syndrome (TRAPS) Blood. 2006;108:1320–1327. doi: 10.1182/blood-2005-11-006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simon A., Park H., Maddipati R., Lobito A.A., Bulua A.C., Jackson A.J., Chae J.J., Ettinger R., de Koning H.D., Cruz A.C., Kastner D.L., Komarow H., Siegel R.M. Concerted action of wild-type and mutant TNF receptors enhances inflammation in TNF receptor 1-associated periodic fever syndrome. Proc Natl Acad Sci U S A. 2010;107:9801. doi: 10.1073/pnas.0914118107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim W.-K., Fujimoto C., Ursea R., Mahesh S.P., Silver P., Chan C.-C., Gery I., Nussenblatt R.B. Suppression of immune-mediated ocular inflammation in mice by interleukin 1 receptor antagonist administration. Arch Ophthalmol. 2005;123:957–963. doi: 10.1001/archopht.123.7.957. [DOI] [PubMed] [Google Scholar]
- 47.Abbate A., Salloum F.N., Vecile E., Das A., Hoke N.N., Straino S., Biondi-Zoccai G.G.L., Houser J.-E., Qureshi I.Z., Ownby E.D., Gustini E., Biasucci L.M., Severino A., Capogrossi M.C., Vetrovec G.W., Crea F., Baldi A., Kukreja R.C., Dobrina A. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117:2670–2683. doi: 10.1161/CIRCULATIONAHA.107.740233. [DOI] [PubMed] [Google Scholar]
- 48.D'Haens G., Sandborn W.J., Feagan B.G., Geboes K., Hanauer S.B., Irvine E.J., Lémann M., Marteau P., Rutgeerts P., Schölmerich J., Sutherland L.R. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–786. doi: 10.1053/j.gastro.2006.12.038. [DOI] [PubMed] [Google Scholar]