Abstract
Fibrosis is the life-threatening, excessive accumulation of extracellular matrix (ECM) and is sometimes associated with a loss of lipid-filled cells in skin and other organs. Understanding the mechanisms of fibrosis and associated lipodystrophy and their reversal may reveal new targets for therapeutic intervention. In vivo genetic models are needed to identify key targets that induce recovery from established fibrosis. Wnt signaling is activated in animal and human fibrotic diseases across organs. Here, we developed a genetically inducible and reversible Wnt activation model and show it is sufficient to cause fibrotic dermal remodeling, including ECM expansion and shrinking of dermal adipocytes. Upon withdrawal from Wnt activation, Wnt-induced fibrotic remodeling was reversed in mouse skin -- fully restoring skin architecture. Next, we demonstrated CD26/Dipeptidyl peptidase 4 (DPP4) is a Wnt/β-catenin-responsive gene and a functional mediator of fibrotic transformation. We provide genetic evidence that the Wnt/DPP4 axis is required to drive fibrotic dermal remodeling and associated with human skin fibrosis severity. Remarkably, DPP4 inhibitors can be repurposed to accelerate recovery from established Wnt-induced fibrosis. Collectively, this study identifies Wnt/DPP4 axis as a key driver of ECM homeostasis and dermal fat loss, providing therapeutic avenues to manipulate the onset and reversal of tissue-fibrosis.
Keywords: Cell Biology, Matrix Biology, Scleroderma, Wnt Signaling, Adipocytes
Introduction:
Excessive deposition of ECM proteins leads to scarring and fibrosis, inducing tissue stiffening and loss of function in virtually all organ systems, including the skin, adipose tissue, heart, intestine, and lung (Distler et al., 2019). Despite its devastating impact on nearly 5% of people worldwide annually (Zhao et al.,, 2020), no effective treatment for fibrosis exists. Interestingly, fibrosis occurs concomitantly with a loss of lipid-filled cells in several organs including adipocytes in adipose tissue and skin, and lipo-fibroblasts in the lung and liver (Hernandez-Gea and Friedman, 2011; Schmidt and Horsley, 2013; Rehan and Torday, 2014; Agha et al., 2017; DeBari and Abbott, 2020).
The skin is an excellent system to study fibrosis because it is easily accessible and has distinct ECM and dermal white adipose tissue (DWAT) layers. Fibroblasts and adipocytes arise from common progenitor populations in the skin, enabling both cell types to be targeted when designing genetic models of skin fibrosis (Atit et al.,, 2006; Rinkevich et al., 2015; Jiang et al., 2018; Shook et al., 2018). Dermal fibroblasts regulate ECM homeostasis including proteoglycans, providing structural integrity to the skin (Hunzelmann et al., 1996) Dermal adipocytes are dynamic in the skin and are associated with the hair cycle, as they undergo hypertrophy during hair growth (Festa et al., 2011) and shrink in association with hair follicle regression (Festa et al., 2011; Nicu et al., 2019; Zhang et al., 2019b). Additionally, adipocytes impact thermoregulation (Kasza et al., 2014; Alexander et al.,, 2015) can produce anti-microbial peptides in skin infection models, and can regulate inflammation after injury (Driskell et al., 2014; Zhang et al., 2015; Shook et al., 2018). Both adipocytes and fibroblasts are essential to the mechanical properties and function of skin (Ezure and Amano, 2010; 2015; Butzelaar et al., 2017; Wollina et al., 2017). Skin fibrosis with expansion of dermal ECM and reduction of DWAT likely has profound impacts on these functions. To advance our understanding of tissue-fibrosis, we need to identify signals and specific mediators that can affect multiple cell types in fibrosis, so we can harness their therapeutic potential to reverse fibrotic remodeling.
Several signaling pathways have been implicated in fibrosis development (Piersma et al., 2015; Distler et al., 2019) including TGFβ and PDGFRα signaling (Olson and Soriano, 2009; Marcelin et al., 2017). While less well studied, canonical Wnt signaling through its transducer, β-catenin, is a conserved stimulus of tissue fibrosis in many organs, including skin (Akhmetshina et al., 2012; Beyer et al., 2013; Hamburg-Shields et al., 2015; Hu et al., 2020). While activation of Wnt signaling in skin fibroblasts has been shown in human fibrotic tissues, chemically induced fibrosis in mice, and constitutive activation of β-catenin in dermal fibroblasts in genetic mouse models can induce fibrosis (Akhmetshina et al., 2012; Wei et al., 2012; Hamburg-Shields et al., 2015; Hu et al., 2020), the cellular and molecular mechanisms by which Wnt signaling promotes fibrotic phenotypes are not well understood.
Recent studies have demonstrated that Engrailed1+ (En1+) embryonic mesenchymal progenitors can produce dermal fibroblasts and dermal adipocytes and are responsible for ECM production in the skin’s dermis during development, homeostasis, and in response to fibrotic stimuli (Atit et al., 2006; Rinkevich et al., 2015; Jiang et al., 2018; Shook et al., 2018; Mascharak et al., 2021). To determine if activation of Wnt signaling in En1 embryonic mesenchymal progenitors is sufficient to induce skin fibrosis and required to maintain fibrotic phenotypes, we generated an inducible and reversible genetic mouse model to express stabilized β-catenin in En1 lineage-derived mesenchymal cells.
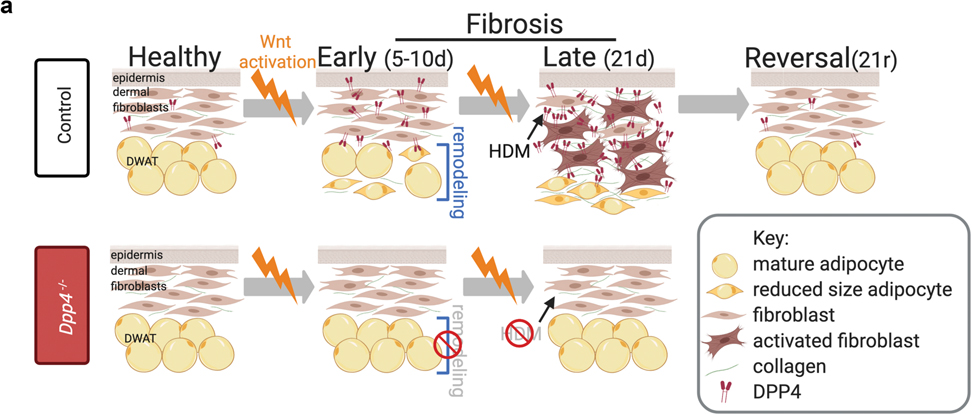
We found that several aspects of fibrotic remodeling, including dermal ECM remodeling and lipodystrophy are induced and dependent on sustained Wnt signaling activation in mesenchymal cells. To identify mechanisms by which Wnt signaling activates fibrotic phenotypes, we screened for functional mediators with roles in adipocyte and fibroblast biology. We identify DPP4 as a Wnt-responsive protein required for fibrotic remodeling of the dermis and dermal adipocyte lipodystrophy during fibrosis. We further show that pharmacological inhibition of DPP4 accelerates recovery from fibrotic ECM and DWAT remodeling in mouse skin. Collectively, these data show that the Wnt-DPP4 axis is a key regulator of fibrotic dermal transformation and recovery from established Wnt-induced fibrosis.
Results:
Fibrotic remodeling in skin is dependent on Wnt signaling activation.
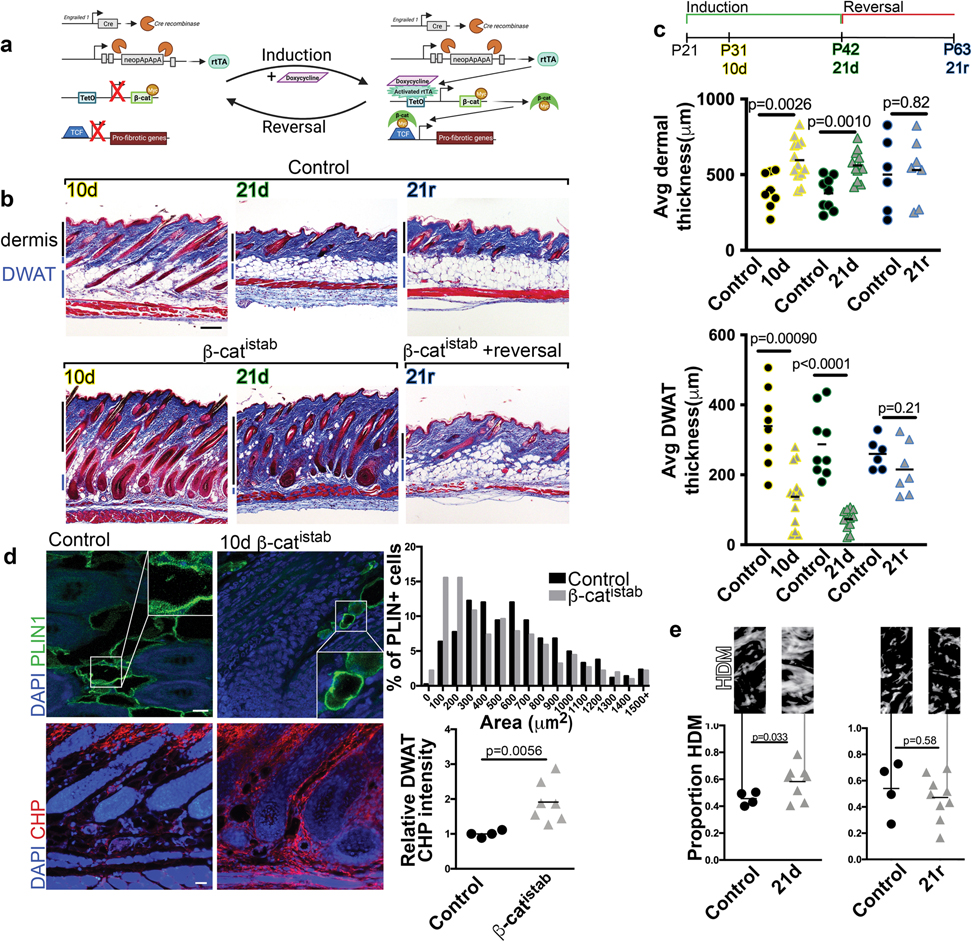
In order to determine whether Wnt activation in mesenchymal lineages is sufficient to induce and maintain fibrotic ECM and DWAT remodeling, we generated a mouse model with inducible and reversible β-catenin stabilization in the skin. To this end, we induced Wnt signaling activation in the previously-characterized embryonic En1 lineage-derived mesenchymal cells, which produce all forms of dermal fibroblasts and adipocyte stem cells and are profibrotic during skin injury and melanoma-induced fibrosis (Atit et al., 2006; Rinkevich et al., 2015; Jiang et al., 2018; Shook et al., 2018; Mascharak et al., 2021). We used En1-Cre line to recombine the Rosa26 reverse tetracycline regulator transactivator (R26rtTA), including newly En1 positive and En1 lineage-derived cells. In the presence of dietary doxycycline, R26rTA transactivates the TetO myc-tagged stabilized β-catenin (β-catistab), the signal transducer of activated canonical Wnt signaling (Figure 1a). We confirmed myc-tag and nuclear β-catenin expression in postnatal dermal fibroblasts and in the dermal adipocyte layer in mouse skin (Supplementary Figure S1). β-catistab caused significant dermal ECM expansion concomitantly with a significant decrease in DWAT thickness within 10 days of doxycycline feeding (Figure 1b and c). Quantitative histomorphometrics revealed early DWAT remodeling in fibrotic mouse skin, but we further investigated whether Wnt activation also induced changes in dermal adipocyte size. In early Wnt-induced fibrosis, mature adipocytes in β-catistab mice displayed reduced size of PLIN1+ lipid droplets, despite the increase in adipocyte lipid content that is associated with hair follicle growth (Festa et al., 2011) stimulated by sustained Wnt activation (Figure 1b and d). Hair follicle number is comparable between controls and β-catistab mouse dorsal skin (Supplementary Figure S4). These data indicated that mature adipocytes are dynamically decreasing in size early in response to Wnt activation. During early stages of Wnt induction (10d), we also found active collagen remodeling in the DWAT layer, though not in the rest of the dermis, as seen by significantly elevated Collagen Hybridizing Peptide (CHP) staining in the DWAT region (Figure 1d, 4c, and Supplementary Figure S3). With sustained Wnt activation for 21 days, β-catistab mice had progressive dermal expansion and DWAT lipodystrophy, an increase in percent area of high density collagen matrix and proteoglycans, and increased collagen fiber thickness (Figure 1b, c and e and Supplementary Figure S4 a and b). Elastin protein distribution is comparable in control and Wnt activated mice (Supplementary Figure S4d). Notably, DWAT remains diminished and shrunken adipocytes persist even after 21 days of Wnt activation (Figure 1b and d, and Supplementary Figure S2).
Figure 1: β-catistab leads to inducible and reversible dermal remodeling in mice.
a, Transgenes in doxycycline-inducible/reversible β-catenin (β-catistab) dermal fibrosis model. b, Fibrosis progression in Masson’s trichrome stained control (top) and β-catistab (bottom) dermis; black and blue bars indicate dermal and DWAT thickness; scale bar=200μm. c, Quantification of average dorsal dermal and DWAT thickness/mouse (n=6–11). d, Indirect immunofluorescence of PLIN1 (green) and CHP stain (red) in control and 10d β-catistab DWAT; quantification of area of PLIN1+ vesicles, 50/mouse (n=9) and relative corrected fluorescence CHP intensity in DWAT; scale bar=25μm. e, Quantification of avg collagen high density matrix (HDM)/40x field/mouse with HDM mask (n=4–9).
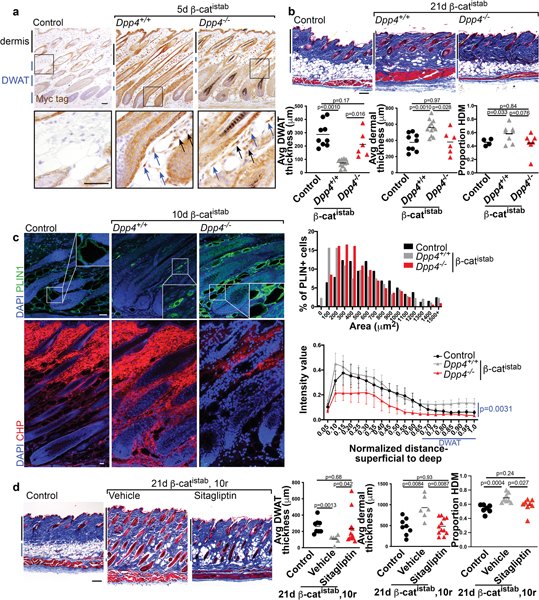
Figure 4: DPP4 mediates dermal remodeling in β-catistab dermal fibrosis model.
a, Myc-tag IHC in control, Dpp4+/+ 5d β-catistab, and Dpp4−/− 5d β-catistab dorsal dermis; blue arrows indicate adipocytes with high signal, black arrows indicate fibroblasts with high signal; scale bar=100μm. b, Masson’s trichrome stained dorsal skin of control, Dpp4+/+ 21d β-catistab, and Dpp4−/− 21d β-catistab; quantification of dermal and DWAT thickness/mouse (n=6–10) and proportion of collagen high density matrix (n=4–8); scale bar=200μm. c, Indirect immunofluorescence staining for PLIN1 (green) and CHP staining (red) in mouse skin from control, Dpp4+/+ 10d β-catistab, and Dpp4−/− 10d β-catistab mice with histogram of PLIN1+ vesicle size (n=6–9) and DWAT CHP intensity heatmap by skin depth with standard error bars; significance between DWAT region intensity values only; scale bar=25μm. d, Masson’s trichrome stained mouse skin from control, 21d β-catistab and 10d reversal with vehicle or sitagliptin treatment; scale bar=200μm; quantification of dermal, DWAT thickness, and HDM/mouse(n=6–11).
Since Wnt signaling activation in En1-lineage-derived mesenchymal cells led to progressive fibrotic remodeling, we next investigated which aspects of the fibrotic phenotype were dependent on Wnt signaling. To this end, we examined ECM and DWAT remodeling during reversal from β-catistab that is initiated by removal of doxycycline (Figure 1b and c). β-catistab mice were fed doxycycline for 21 days and subsequently given normal chow for an additional 21 days (reversal) (Figure 1b and c). In the reversal phase, the expression of myc-tagged, stabilized β-catenin protein was absent within 10 days (Supplementary Figure S1). Reversal of Wnt activation in β-catistab mice remarkably restored DWAT layer thickness, dermal thickness, percent area high density collagen matrix, proteoglycan area, and collagen fiber thickness to comparable levels to those measured in control mice (Figure 1b and c and Supplementary Figure S4). Thus, those aspects of fibrotic remodeling of the dermis and DWAT are dependent on sustained Wnt signaling activation. Taken together, these data indicate that fibrotic remodeling is dependent on sustained Wnt signaling activation in En1-lineage derived mesenchymal cells.
DPP4/CD26 is a Wnt-responsive gene during skin fibrosis.
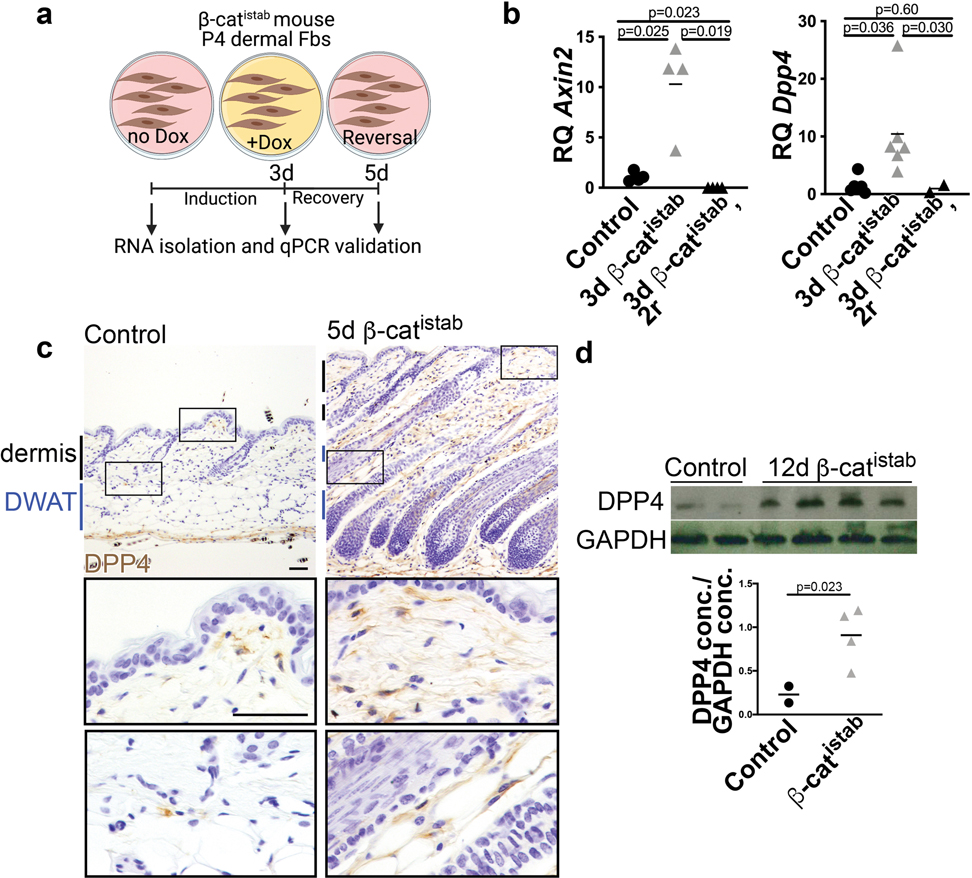
While Wnt signaling has been implicated in fibrosis of various tissues, its functional effectors are unknown. To define the mechanisms by which Wnt/β-cat signaling promotes fibrotic remodeling, we analyzed transcriptional changes associated with induction of stabilized β-catenin from primary dermal fibroblasts (Adeno-Cre; β-cateninflox3/+) (GSE 103870) (Mullin et al., 2017). One of the 20 most differentially expressed genes that were highly upregulated (47x, p<0.05) in an unbiased screen in β-catflox3/+ dermal fibroblasts was Dipeptidyl peptidase 4 (Dpp4), a multifunctional integral membrane glycoprotein and secreted serine protease which is expressed by many cell types (Gorrell, 2005; Rohrborn et al., 2016) (Figure 2a and b). In other, non-fibrotic contexts, DPP4 has been shown both to promote lipid accumulation and cause adipocyte de-differentiation in vitro (Rosmaninho-Salgado et al., 2012; Lessard et al., 2015). Dpp4 was of particular interest because it is also expressed in mouse fibrotic fibroblasts, where it has been linked to fibrotic ECM expansion (Rinkevich et al., 2015; Shook et al., 2018; Mascharak et al., 2021). Though DPP4 has been shown to play a role in skin repair and chemical models of fibrosis, previous studies have not identified the upstream regulators of its increased expression, nor have they examined its role in fibrosis-associated lipodystrophy (Rinkevich et al., 2015; Mascharak et al., 2021).
Figure 2: DPP4 expression is Wnt responsive and elevated in β-catistab dermal fibrosis model.
a, Schematic of in vitro validation. b, Dpp4 and Wnt target gene, Axin2, expression by qRT-PCR relative to housekeeping gene, Hprt, in control, β-catistab, and reversal following β-catistab dermal cells. c, DPP4 immunohistochemical staining on control and 5d β-catistab mouse skin with high magnification panels; scale bar=100μm. d, DPP4 protein quantity relative to GAPDH protein quantity in whole skin by western blot.
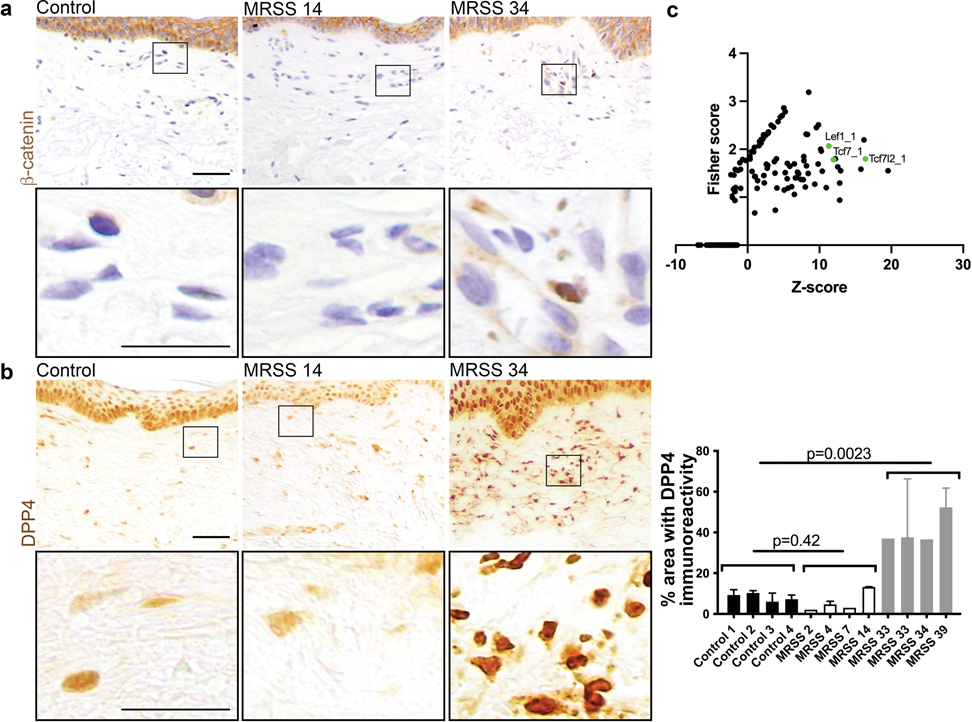
First, we validated that Dpp4 mRNA was Wnt responsive in β-catistab dermal fibroblasts in vitro and in mouse skin (Figure 2b, c, and d). DPP4 protein expression was elevated within 5 days of β-catistab, before dermal remodeling and remained elevated at 21 days of β-catistab in mouse skin (Figure 2c and Supplementary Figure S5). Elevated DPP4 immunoreactivity was present throughout the dermis including in the DWAT layer (Figure 2c, and Supplementary Figure S5). Additionally, it was increased in Bleomycin subcutaneous-injected skin within 5 days. (Supplementary Figure S5). Wnt signaling activation with a Wnt agonist CHIR99021 also induced Dpp4 mRNA expression in mature dermal adipocytes in vitro in a cell-autonomous manner (Supplementary Figure S7). Together, these data show that Dpp4 expression is Wnt-responsive in dermal mesenchymal cells. We also found that DPP4 immunoreactivity is elevated in human systemic sclerosis (SSc) corresponding to elevated dermal β-catenin (Figure 3a and b). Interestingly, DPP4 immunoreactivity corresponds with SSc disease severity compared to control human skin (Figure 3b). Analysis of human DPP4 promoter sequence (within 5KB) reveals predicted TCF/LEF family transcription factor binding sites (Figure 3c). This indicates that DPP4 is a relevant target in mouse fibrosis models, as well as in severe human SSc.
Figure 3: DPP4 and β-catenin expression are elevated in high MRSS-scored human skin.
a, IHC with α-β-catenin antibody from left to right: Control, low MRSS scored (14) human forearm biopsy sample, high MRSS scored (34) human forearm biopsy sample; scale bar= 100μm in low mag, 50μm in high mag. b, IHC with α-DPP4 antibody on same samples as in a; accompanying quantification of 1–3 fields per control and scored SSc human forearm skin sample. c, Transcription factor binding site overrepresentation analyisis using oPPOSUM showing that promoter region of DPP4 is enriched for predicted binding sites for Wnt signaling TFs, including TCF7l2 and LEF1.
DPP4/CD26 is required to mediate Wnt induced fibrotic remodeling and recovery.
Next, we tested the hypothesis that DPP4 is required to mediate Wnt induced fibrotic remodeling of both dermal ECM and DWAT. To test this hypothesis, we examined whether genetic deletion of Dpp4 rescues Wnt-induced fibrotic remodeling. Despite comparable Wnt activation, indicated by nuclear β-catenin and myc tag protein expression, in both Dpp4+/+ and Dpp4−/−; β-catistab mice, Dpp4−/−; β-catistab mice displayed increased PLIN1+ lipid vesicle size, and ultimately DWAT preservation (Figure 4a, b, and c and Supplementary Figure S6a). This was accompanied by significantly reduced collagen remodeling in the DWAT and diminished collagen remodeling throughout the dermis in Dpp4−/−; β-catistab mice in early Wnt-induced fibrosis, resulting in attenuated dermal thickening, and a comparable proportion of high density collagen matrix and similar collagen fiber thickness to controls (Figure 4b and c and Supplementary Figure S6). These results indicate that deletion of Dpp4 protects against Wnt-induced fibrosis and particularly against fibrosis-associated lipodystrophy of DWAT.
DPP4 functions as a soluble and cell surface protein with enzymatic and non-enzymatic functions (Gorrell, 2005; Rohrborn et al., 2016). To determine if inhibition of DPP4 enzymatic activity accelerates recovery from Wnt signaling activation induced fibrosis, we blocked DPP4’s enzymatic activity with the pharmacological inhibitor, sitagliptin (DPP4i) in vitro and in vivo (Dobrian et al., 2011; Mulvihill and Drucker, 2014). In cultured dermal adipocytes, sitagliptin treatment led to modest improvement in lipid retention (Supplementary Figure S7). We treated β-catistab mice with a high dose of sitagliptin (30mg/kg) during β-catistab reversal, after removal of doxycycline (Figure 4d) (Wang et al., 2017). β-catistab skin remained fibrotic with lipodystrophic adipocytes in the DWAT for the first 10 days of reversal. Remarkably, systemic sitagliptin treatment of β-catistab mice accelerated re-emergence lipid filled dermal adipocytes within 10 days. Dermal thickness, proportion of HDM, and fiber thickness are also decreased within 10 days in β-catistab mice treated with systemic sitagliptin (Figure 4d). Collectively, these data indicate that DPP4 is required for Wnt-induced fibrotic remodeling of DWAT and ECM and DPP4i can accelerate recovery from Wnt-induced fibrosis (Figure 5).
Figure 5: Summary schematic.
a, By 10d of β-catistab, adipocytes undergo DWAT remodeling ultimately leading to DWAT loss and collagen accumulation by 21d. DPP4 is Wnt-responsive and increased within 5d of β-catistab, prior to DWAT remodeling. Dpp4−/− mice have preservation of DWAT and reduced DWAT remodeling despite 10d β-catistab resulting in reduced dermal thickening, less high density matrix (HDM), and DWAT preservation after 21d of β-catistab.
Discussion
By developing an inducible and reversible model of Wnt activation in mesenchymal cells, we demonstrate that two hallmarks of fibrosis, ECM expansion and lipodystrophy are dependent on Wnt signaling activation. Critically, both aspects of fibrotic dermal remodeling can recover upon turning off Wnt activation. Wnt signaling is a well-known anti-adipogenic signal, but here we show that Wnt activation in mouse skin can also cause profound morphological changes and lipid loss in mature dermal adipocytes as well. We use this model also to identify a Wnt-responsive gene, Dpp4. Using genetic and pharmacological methods to target DPP4, we demonstrate that the Wnt-DPP4 axis has dual roles in fibroblasts and adipocytes and its genetic ablation and enzymatic inhibition prevent fibrogenesis and promote recovery, respectively (Figure 5).
While previous studies established that Wnt activation causes ECM expansion in the dermis with changes in expression levels of genes encoding ECM proteins independent of activation of inflammation (Akhmetshina et al., 2012; Hamburg and Atit 2012; Hamburg-Shields et al., 2015; Mastrogiannaki et al., 2016), our work further demonstrates that abrogation of Wnt signaling activation also reverses dermal ECM remodeling and leads to re-emergence of DWAT; thereby, restoring dermal architecture. We hypothesize that rescue of DWAT lipodystrophy occurs from hypertrophy of existing differentiated adipocytes, since small adipocytes persist throughout Wnt activation. However, de novo adipogenesis may also occur to restore DWAT during fibrosis recovery, as has been shown in association with the hair follicle growth cycle (Rivera-Gonzalez et al., 2016). Our data are consistent with short-term clinical trials of topical Wnt/β-catenin signaling inhibitor in human SSc skin that leads to emergence of an adipocyte cell type signature (Lafyatis et al., 2017). Our findings regarding Wnt-induced lipodystrophy will be applicable to other tissues that contain resident lipid-filled cells such as heart, lung, and liver (Hernandez-Gea and Friedman, 2011; Schmidt and Horsley, 2013; Rehan and Torday, 2014; Piersma et al., 2015; Agha et al., 2017). Our future work will explore the precise mechanism by which Wnt controls dermal architecture including a potential role in adipocyte biology. Ultimately, these data demonstrate that controlling Wnt signaling during fibrosis can promote fibrotic recovery by both reducing ECM expansion and promoting DWAT recovery.
Our data indicate that DPP4 acts downstream of Wnt signaling to profoundly impact both fibroblasts and adipocytes in the skin. Emerging studies suggest DPP4 contributes to a common pathway leading to organ fibrosis, but the mechanism of its profibrotic action has been previously unknown (Hu and Longaker, 2016). Previous studies have shown that DPP4 inhibition reduces ECM accumulation in rodent lung, liver, kidney, and skin fibrosis (Beyer et al., 2012; Shi et al., 2016; Wang et al., 2017; Zhang et al., 2019a; Chen et al., 2020; Huang et al., 2020; Lv et al., 2020). Wnt signaling is elevated in all of these models and in the human diseases they model, though a connection between Wnt signaling activation and DPP4 in fibrosis has not been previously discussed (Surendran et al., 2005; Lam et al., 2011; Beyer et al., 2012; Piersma et al., 2015). The ENCODE Consortium study using the human colon cancer cell line, HCT116, which is known to have elevated Wnt signaling, shows that the DPP4 gene at its transcriptional start site, is active (by H3K27Ac) and enriched for TCF7L2 binding (ENCODE Project Consortium 2012; Morin et al., 1997). These data demonstrate that Wnt signaling activation has the potential to regulate DPP4 transcriptionally. Our study places DPP4 expression and function downstream of Wnt activation and upstream of lipodystrophy and ECM expansion. These findings are consistent with recent work linking DPP4 with obesity, metabolic syndrome, adipocyte dedifferentiation in vitro, inhibition of adipocyte differentiation in vivo (Bouchard et al., 2009; Lamers et al., 2011; Lessard et al., 2015), scar formation (Rinkevich et al., 2015; Shook et al., 2018), and fibrosis (Hu and Longaker, 2016; Wang et al., 2017; Soare et al., 2020). Our findings also resonate with recent reports that heterogeneous fibroblast populations express DPP4 during skin homeostasis and repair (Rinkevich et al., 2015; Tabib et al., 2018; Merrick et al., 2019). The presence of putative TCF/LEF binding sites suggest direct activation of DPP4 by Wnt effectors, but further research is needed to confirm direct binding. DPP4’s peptidase activity also plays a crucial role for inflammation, wound repair, and tumorigenesis (Driskell et al., 2013; Baticic Pucar et al., 2017; Shook et al., 2018). Interestingly, DPP4 inhibition has also been linked recently to a modest decrease in hypertrophic and keloid scaring after surgery in humans (Suwanai et al., 2020). Though DPP4 has been investigated in some fibrotic tissues and has known effects on adipocyte biology, our data shows for the first time, to our knowledge, that DPP4 is a key player in fibrotic lipodystrophy.
Together, our data further demonstrate that DPP4 regulates both homeostasis of adipocyte lipid content and fibroblast ECM production to impact tissue fibrosis and promote recovery. While our data do not reveal which mesenchymal cell type expressing DPP4 mediates its impact on fibrosis, the ability of adipocytes to form myofibroblasts (Marangoni et al., 2015; Rinkevich et al., 2015; Shook et al., 2018) may increase the number of DPP4+ fibroblasts through trans differentiation as well as altered gene expression in fibroblast populations. Additionally, our in vitro findings indicate that Wnt activation can induce Dpp4 expression in differentiated dermal adipocytes (Supplementary Figure S4.2). While FDA-approved DPP4 inhibitors may be useful to accelerate clinical treatments for fibrosis prevention and/or recovery, future work identifying DPP4’s substrates in tissue fibrosis, including chemokines and metabolic regulators, may reveal additional therapeutic strategies to treat tissue fibrosis.
Our work provides several therapeutic targets to promote recovery from fibrosis including the Wnt-DPP4 axis and dermal adipocyte lipid handling. Consistent with studies on mediators of double-strand break repair in lung fibrosis reversal model (Kumar et al., 2017), our data also show that fibrosis recovery is possible in the skin. Future work defining the mechanisms by which fibrosis recovery occurs may shed light on how to promote recovery in human patients.
Materials and Methods:
Engrailed1Cre (En1Cre)(Kimmel et al., 2000); Rosa26rTA-EGFP (Belteki et al., 2005) (Jax Stock 005572); TetO-deltaN89 β-catenin (Mukherjee et al., 2010); mice were used to generate a dermal Wnt activation model of skin fibrosis. Case Western Reserve Institutional Animal Care and Use Committee approved all animal procedures in accordance with AVMA guidelines Protocol 2013–0156, approved 21 November 2014, Animal Welfare Assurance No. A3145–01 at Case Western. Dorsal dermis was harvested at the indicated timepoints (5d, 10d, 21d, 21d+ 10d reversal, 21d+ 21d reversal), drop fixed (with 10% neutral buffered formalin) or flash frozen, and used for immunohistochemical staining with β-Catenin (BD Biosciences, 1:250), myc-tag (Abcam ab9106, 1:500), Perilipin 1 (Abcam ab3526, 1:500), and CD26/DPP4 (R&D: AF954, 1:150) antibodies, western blot CD26/DPP4 (R&D: AF954, 0.25 μg/mL), other staining (Masson’s trichrome stand; RGB Trichrome: Electron Microscopy Sciences, 26357–02; Fisher Scientific, F99–10; Sigma-Aldrich, A3157 (Gaytan et al., 2020); CHP: 3Helix, bio60, 2.5 μM, (Hwang et al., 2017)). Brightfield images were captured with an Olympus BX60 microscope and Cell Sens entry software and Zeiss AX10 scope and Zen 2.6 pro software. Dermal thickness and DWAT thickness were quantified with measurement tool in Fiji/Image J software. Immunofluorescence images were imaged on an inverted confocal Leica TCS SP8 gated STED 3x microscope (DMI6000, Leica), using a 40x oil immersion objective (HC PL APO 40x/1.30 NA CS2, Oil, FWD=0.24 mm) detection by PMT detector and/or hybrid detectors and Leica LAS X software. All analysis was performed using Fiji or Cell Profiler. Dpp4−/−(Marguet et al., 2000); (Jax stock 007676) mice were crossed into the Wnt activation model and all measurements were repeated. Human samples were obtained from dorsal mid-forearm of healthy control and systemic sclerosis (SSc) human subjects after written informed consent under a protocol approved by the University of Pittsburgh Institutional Review Board. Archived de-identified patient SSc skin samples with Modified Rodnan Skin score (MRSS) were obtained from Scleroderma Center of Research Translation. They were stained using β-Catenin (Abcam ab223075, 0.25 μg/mL) or DPP4/CD26 antibodies (Abcam ab28340, 1:400). Cells isolated from postnatal day 4 mouse skin were harvested from Wnt activated and control (En1-Cre-WT) mice and some were differentiated into mature dermal adipocytes using adipocyte induction media (AIM) (DMEM (Thermofisher, 11995065) containing glucose, pyruvate, 10% fetal bovine serum (FBS), 100μM indomethacin, 1μM dexamethasone, 500μM 3-isobutyl-1-methylxanathine (IBMX), and 10μM insulin) for 8–12 days, after which they were kept in maintenance media (without dexamethasone, indomethacin, or IBMX) and treated with 7μM CHIR (Cayman, 13122) and/or 20μM sitagliptin (Cayman Chemical Company: 13252). RNA was collected after 2 or 4 days of treatment, Oil Red O stain was performed at d10 of treatment and quantified using Cell Profiler.
Data availability statement:
The data sets related to this article can be found in the GEO repository (GSE 103870, GSE 31477, GSM 945853).
Methods:
Mouse handling and lines
Engrailed1Cre (En1Cre)(Kimmel et al. 2000); Rosa26rtTA-EGFP (Belteki et al. 2005) (Jax Stock 005572); TetO-deltaN89 β-catenin (Mukherjee et al. 2010); Dpp4−/− (Marguet et al. 2000); (Jax stock 007676) lines were genotyped as previously described. The contribution of the En1Cre lineage cells in the skin has been previously described (Atit et al. 2006; Rinkevich et al. 2015; Jiang et al. 2018; Shook et al. 2018). For induction of TetO-deltaN89 β-catenin-myc tagged transgene expression in the En1Cre; R26rtTA recombined cells, 21-day old (P21) triple transgenic mice were given 6g/kg of dietary doxycycline in rodent chow (Envigo-Harlan) and 2mg/ml doxycycline in water (Sigma) for 21 days. For induction-reversal experiments, P21 mice were first treated with dietary doxycycline for 21 days (until P42) and then switched to regular chow and water. At desired time points, mice were euthanized and dorsal skin was processed for frozen or paraffin sections as previously described (Atit et al. 2006). For each experiment, mutants with litter-matched controls were studied. At least two to four litters were used for phenotypic analysis. All experiments include male and female mice.
For bleomycin-induced dermal fibrosis, (Fig. S2.1) fibrosis was induced between 6 and 8 weeks of age in wild-type or Dpp4−/− C57Bl/6 genetic background mice. Mice are given daily subcutaneous injection on their upper dorsal region with 10mg/kg of Bleomycin Sulfate (Enzo pharmaceuticals, BML-AP302–0010) in PBS for 5 days (Yamamoto et al. 1999; Wu et al. 2009). Vials of bleomycin sulfate were checked for efficacy prior to experimental treatments. To ensure injections were located to a 0.5in × 0.5in square, mice were placed under light isoflurane anesthesia prior to injections. 24 hours after the final injection, mice are euthanized and the dorsal skin was collected.
Case Western Reserve Institutional Animal Care and Use Committee approved all animal procedures in accordance with AVMA guidelines Protocol 2013–0156, approved 21 November 2014, Animal Welfare Assurance No. A3145–01 at Case Western.
Patient skin samples
Samples were obtained by performing 3 mm punch biopsies from the dorsal mid-forearm of healthy control and systemic sclerosis (SSc) human subjects after informed consent under a protocol approved by the University of Pittsburgh Institutional Review Board. Archived de-identified patient SSc skin samples with Modified Rodnan Skin score (MRSS) were obtained from Scleroderma Center of Research Translation. They were stained using β-Catenin (Abcam ab223075, 0.25μg/mL) or DPP4/CD26 antibodies (Abcam ab28340, 1:400).
Adipocyte cell culture
All data were obtained from primary dermal adipocyte progenitors isolated from wild-type CD1 background P4 dorsal skin. Approximately 1cm2 dorsal skin was removed, rinsed in sterile 1x phosphate buffered saline (PBS), minced, and placed in 2 mg/mL collagenase (Worthington, LS004196) with 2% bovine serum albumin (BSA) (Fisher, BP1600) and incubated in a 37°C rotating incubator for 45 minutes. Digested skin was filtered through a 70μM cell strainer (cat number) and plated in 60 mm tissue culture plastic plates. Cells were passaged 2–3 times when they achieved approximately 80% confluence. Adipocyte differentiation was stimulated with adipocyte induction media (AIM) (DMEM (Thermofisher, 11995065) containing glucose, pyruvate, 10% fetal bovine serum (FBS), 100μM indomethacin, 1μM dexamethasone, 500μM 3-isobutyl-1-methylxanathine (IBMX), and 10μM insulin) for 8–12 days. Duplicate cultures were kept in media containing only glucose and pyruvate. Differentiated adipocytes were enriched by trypsinizing (0.25% Trypsin EDTA (Thermofisher, 25200056)) and re-plating on a 12-well plate for treatment. Some differentiated adipocytes were kept in maintenance media only (DMEM with glucose, pyruvate, FBS, and insulin), or with additives such as 7μM CHIR (Cayman, 13122) and 20μM sitagliptin (Cayman Chemical Company, 13252). RNA was collected after 2 or 4 days of treatment. Oil Red O stain was performed at 10 days of treatment and quantified using Cell Profiler. Brightfield images of Oil Red O stained cells were imaged on an inverted widefield Leica Dmi8 microscope with a digital camera and a 10x objective (HC PL FLUOTAR 40x/0.60, Dry, FWD=3.3–1.9mm). All media was prepared fresh and changed every second day. Experiments were repeated on 3–4 biological replicates.
Histological staining and morphometrics
Dorsal mouse skin from mice of various ages (p26, p32, p42, p52, p68) was isolated, drop-fixed in 10% neutral buffered formalin for 1 hour at 4 degrees and then processed for paraffin sectioning at 7μm. Sections were stained with Masson’s trichrome (mature collagen), hematoxylin and eosin (matrix), or Verhoeff-Van Gieson (elastin) according to standard protocols. RGB Trichrome staining was performed as described (Gaytan et al. 2020) (Electron Microscopy Sciences, 26357–02; Fisher Scientific, F99–10; Sigma-Aldrich, A3157). Brightfield images were captured with an Olympus BX60 microscope and Cell Sens entry software and Zeiss AX10 scope and Zen 2.6 pro software. Dermal thickness, DWAT thickness, and adipocyte number were quantified with measurement tool in Fiji/Image J software. Data represent the average thickness in three different regions in 5–10 non-overlapping fields/mouse (Hamburg-Shields et al. 2015; Marangoni et al. 2015).
Immunohistochemistry and Immunofluorescence
Paraffin tissue was embedded and sectioned at 7μM. Paraffin sections were deparaffinized and washed with 1xPBS. They underwent antigen retrieval in citrate buffer (10mM Tri-Sodium Citrate dyhydrate, 0.05% Tween-20, pH 6.0) for 15 minutes at 93°C in a water bath. Following 10% normal goat serum block, with 0.05% Tween-20 or 0.3% Triton for 1 hr at room-temperature, tissue was incubated with appropriate primary antibody overnight at 4°C. Primary antibodies for β-Catenin (BD Biosciences, 1:250), myc-tag (Abcam ab9106, 1:500), perilipin 1 (Abcam ab3526, 1:500), and CD26/DPP4 (R&D: AF954, 1:150) were used for brightfield immunohistochemistry or immunofluorescence as previously described (Mukherjee et al. 2010; Myung et al. 2012; Hamburg-Shields et al. 2015). After three washes in PBS- or TBS-T (PBS or TBS+ 0.3% Triton or 0.05% Tween), species-appropriate secondary antibodies conjugated to biotin (Vector) or Alexa-fluor (Thermo-Fisher) were used. Nuclei were counterstained with hematoxylin or DAPI (1:2000) before mounting in Fluoroshield (Sigma). Negative controls were used to confirm antibody specificity.
Imaging:
Brightfield Images were taken at room temperature. Brightfield images of Masson’s Trichrome staining were taken using an Olympus BX60 microscope with a digital camera (DP70, Olympus) with Cell Sens Entry software (Ver. 1.5, © Olympus Corporation 2011) with a 4x objective (Olympus UPIanFI 4x/0.13). Exposure was held constant between controls and experimental group. Immunofluorescence images were captured on an inverted confocal Leica TCS SP8 gated STED 3x microscope (DMI6000, Leica), using a 40x oil immersion objective (HC PL APO 40x/1.30 NA CS2, Oil, FWD=0.24 mm) detection by PMT detector and/or hybrid detectors and Leica LAS X software. CHP images were taken at 40x magnification on an inverted widefield Leica Dmi8 microscope (3Helix, BIO60, 2.5 μM). Max projections were generated and assembled using Fiji/ImageJ and analyzed in Cell Profiler. Images were processed and merged using Adobe Photoshop and laid out Adobe InDesign or Illustrator.
Image treatment and analysis
Adipocyte perilipin 1+ cross-sectional area:
40X confocal images were analyzed for PLIN1+ cell area in FIJI ImageJ. With FIJI’s polygon selection tool, approximately 50 adipocytes were counted per mouse from non-overlapping fields. The areas of these identified adipocytes were binned in a histogram generated in GraphPad Prism.
DPP4 Pipeline (SSc):
Prior to feeding a batch of 4x images through the Cell Profiler™ pipeline, a human forearm-skin DPP4 control image was first white-balanced using Photoshop’s image processor. The configurations of this white-balancing action were input into a white-balancing script to be used on the entire batch. These white-balanced images were loaded into Cell Profiler™. A Crop module was used to manually select an ROI of fixed size in each image. Color deconvolution was completed using an Unmix Colors module to separate the images into Hematoxylin and DAB channels. A Reduce Noise module (Size: 7, Distance: 11, Cut-off distance: 0.045) was used on the DAB channel. Unwanted objects including blood vessels and hair follicles were manually identified through an Identify Objects Manually module. These identified objects were transformed into a binary image via a Convert Objects to Image module. The area of these unwanted objects was determined and recorded by a Measure Image Area Occupied module. Using a Mask Image module (invert the mask: yes), a mask of the unwanted objects was applied into the original ROI. The resulting ROI, with unwanted objects masked out, had a threshold applied by a Threshold module via an adaptive, 3-class Otsu thresholding method in which the intermediate intensity objects were classified as foreground objects (Threshold smoothing scale:0.0, Threshold correction factor: 1.15, Lower and upper bounds on threshold: 0.15 & 1.0, Size of adaptive window: 50). The area of these threshold identified DPP4+ objects as well as the total ROI area recorded by a Measure Image Area Occupied module. These recorded area data were output by an Export To Spreadsheet module. The relevant total ROI area was determined by subtracting the unwanted area from the total ROI area. The area covered by DPP4+ objects was divided by the relevant total ROI area to give the percent coverage of DPP4 signal per image.
CHP:
Analysis pipeline was adapted from Zhang et al., (Zhang et al. 2018) 40X B-CHP images were loaded into Cell Profiler. Each original image was first split into grayscale versions of its RGB channels using a Color To Gray module (Conversion method: Split). An Identify Objects Manually module was used to trace and select an ROI composed of the entire skin under the epidermis and above the panniculus carnosus. A Convert Objects To Image module was used to convert the ROI into a binary image. A Closing module (Structuring element shape, size: disk, 50) was applied to this binary ROI to fill in any gaps left by the tracing performed in Identify Objects Manually. Using the shape of this corrected binary ROI object, a sequence of Morph (performed operation: distance) and Image Math modules are used to generate an intensity-based distance map of the ROI based on distance from the epidermis. Rescale Intensity (Rescaling method: Divide each image by the same value) modules then established how far down subsequent layering modules would extend. Divisor values for Rescale Intensity modules were changed for each image to the maximum thickness (pixel length) from the epidermis to the panniculus carnosus. Using the epidermis distance map, Threshold modules (Threshold strategy: global, thresholding method: manual, Threshold smoothing scale: 1, Manual Threshold: increased from 0 to 1 in 0.05 increments), established binary regions of increasing distance from the epidermis. Image Math modules (Operation: subtract) were then used to subtract each binary region, after threshold application, from the preceding region to establish preliminary layers. This generated 20 preliminary layers spanning the ROI. Erode Image modules (Structuring element shape, size: square, 10) were used to slightly shrink each layer and ensure no overlap. An Image Math module (Operation: add) was used to add eroded layers to produce a full map of the layers. A sequence of identification and conversion modules was used to produce a binary image containing the area occupied by the layers. Using an Identify Objects Manually layer, hair follicles and all area including and under the panniculus carnosus were selected from the original B-CHP image. Using a sequence of Mask Image and Mask Objects modules, these unwanted objects were masked out of the binary image containing the area occupied by the layers. This new masked binary image was composed of the area defined by the layers minus the area of the unwanted objects. The new binary image was converted back into objects using a Convert Image To Objects module. These objects were masked over the original layers objects to generate the final map of the layers, excluding unwanted areas. The red intensity from the original red channel was then calculated per final layer using a Measure Object Intensity module. A Measure Object Size Shape module was used to calculate the area of each final layer. The final layers were then overlaid on the original image to ensure correct functioning of the pipeline. The results were output using an Export To Spreadsheet module.
TWOMBLI analysis of matrix:
To analyze collagen matrix characteristics, 40x images of RGB Trichrome-stained mouse skin were taken (2–3 images per mouse) and non-overlapping ROIs of fixed size were drawn to exclude follicles. The fields were then deconvoluted using the RGB preset in FIJI which extracts colors using the following values: Color 1 [0 0 1], Color 2 [0 1 0], Color 3 [1 0 0]. The red-isolated fields were then fed into TWOMBLI (Wershof et al. 2021), a matrix quantification pipeline macro in FIJI/ImageJ, developed by the Sahai Lab using the parameters as follows: Contrast Saturation: 0.25 to increase contrast between fibers. Line Width: ranged from 5–30, Curvature Window: 10–100, and Minimum Branch Length: 10. Maximum Display HDM was set at an intensity level of 110/225, and Gap Analysis was not performed. Measurements from TWOMBLI were used to graph average collagen High Density Matrix and Fiber Thickness per mouse (Wershof et al. 2021).
Proteoglycan area measurements:
To analyze coverage of proteoglycans, 10x images of RGB Trichrome stained mouse skin were taken (2 images per mouse) and 3–6 non-overlapping ROIs of fixed size were drawn over the collagen-rich dermis. The area covered in blue was manually measured using the polygon tool in FIJI. The matrix area was measured by thresholding the ROI and using the Measure tool in FIJI. The area of proteoglycans was divided by the area of the matrix to give the % coverage of proteoglycans. Average proteoglycan area per mouse in collagen-rich dermal regions was graphed.
RNA extraction and qRT-PCR analysis
Total RNA was extracted from cultured cells using Trizol reagent (Thermo Fisher: 15596026) and processed for qRT-PCR analysis with 4ng of cDNA as previously described (Hamburg-Shields et al. 2015; Mullin et al. 2017). Axin2 and Dpp4 mRNA quantities were measured relative to Hprt using Taqman master mix (Thermofisher, 4304437) and probes (Thermofisher, Mm00443610_m1, Mm00494549_m1, Mm03024075_m1). Relative mRNA quantities were determined using an Applied Quantstudios Biosystems 3 PCR System. All samples were normalized to Hprt gene expression, and results are expressed as the fold change of Ct values relative to controls, using the 2−ΔΔCt formula. Complete qRT-PCR data was depicted in univariate scatter plots as previously described (Weissgerber et al. 2015). Statistical significance was determined by two-tailed, unpaired Student t-test with Welch’s correction in GraphPad Prism software.
Statistical analysis
Sample size was determined based on published studies and no statistical method was employed. The experiments were not randomized. Due to the nature of the genetic manipulations, the authors were not blinded to allocation of animals for the experiments. The authors were blinded during analyses. Individual data points on graphs represent the average value per mouse of 5–18 replicates (depending on measurement). Normality in the spread of data for each experiment was tested using Shapiro-Wilk test in GraphPad Prism software. Significance values for data sets displaying normal distributions were calculated by unpaired Student t-test (two-tailed, unequal variances) with Welch’s correction in Prism software. Paired t-tests are performed where appropriate (in vitro only). Significance for non-normal distributed data were calculated using the Mann-Whitney U-Test in Prism software. Error bars represent standard error. All p-values were included on the graphs and p-values less than 0.05 are considered statistically significant.
Supplementary Material
Acknowledgements:
We would like to thank all the past and present members of the Atit and Horsley labs that contributed intellectually to this work. We thank the CWRU Imaging Core and Yale electron microscopy core. Special thanks to Rachel Wyetzner, Qiannan Ma, Gregg DiNuoscio, David Buchner, Rodrigo Somoza-Palacios, Jixin Zhong, Meagan Kitt, Emilie Legue, Karl Liem, Suneel Apte, and Timothy Mead, Claire Reynolds for technical support and advice. We are grateful to Paresh M Atit (1942–2014) for inspiring this work.
Funding:
Global Fibrosis Fund (R.P.A), N.I.H- NIAMS R01 AR076938 (V.H. and R.P.A), NIH-NIAMS R01 AR0695505 (V.H.), R01 AR075412, R01 (V.H.), NIH-NIDCR R01 DE18470 (R.P.A), NIH-NICHD R01 HD042311 (J.P.L.), National Institutes of Arthritis Musculoskeletal and Skin Disease grants Scleroderma Center of Research Translation 1P50AR060780 (R.L.), NIH T32 Musculoskeletal Predoctoral Training Grant T32 AR 7505-31 (A.J.), NIH T32 Dermatology Predoctoral Training Grant T32 AR 7569-25 (A.J.), NIH T32 Human Genetics and Genomics Training Grant (5T32HD007149-42) (E.C.), CWRU-SOURCE fellowship (B.Z.), and Arnold and Mabel Beckman Fellowship (SK). Schematics were made with biorender.com.
Abbreviations:
- ECM
Extracellular matrix
- DPP4
Dipeptidyl peptidase 4
- DWAT
Dermal white adipose tissue
- En1
Engrailed 1
- β-catistab
β-catenin inducibly stabilized
- PLIN1
Perilipin 1
- CHP
Collagen hybridizing peptide
- HDM
High density matrix
Footnotes
Conflict of interest: RL has received consulting fees from Bristol Myers Squibb, Boehringer Ingelheim, Formation, Sanofi, Boehringer-Mannheim, Merck, Genentech/Roche, Certa, Pfizer, Magenta, Biogen and Formation, and grant support from Biogen, Corbus, Formation, Moderna, Regeneron, Pfizer, Kiniksa, Astra Zeneca, Kyowa, Kirin, and Genentech/Roche. The other authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agha El, E, Moiseenko A, Kheirollahi V, De Langhe S, Crnkovic S, Kwapiszewska G, et al. , (2017). Two-Way Conversion between Lipogenic and Myogenic Fibroblastic Phenotypes Marks the Progression and Resolution of Lung Fibrosis. Cell Stem Cell 20: 261–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, et al. , (2012). Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nature Communications 3: 735–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander CM, Kasza I, Yen E, Reeder S, Hernando D, Gallo R, et al. , (2015). Dermal white adipose tissue: a new component of the thermogenic response. J Lipid Res 56: 2061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atit R, Sgaier SK, Mohamed OA, Taketo M, Dufort D, Joyner A, et al. , (2006). Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Developmental Biology 296: 164–76. [DOI] [PubMed] [Google Scholar]
- Baticic Pucar L, Pernjak Pugel E, Detel D, Varlejen J (2017). Involvement of DPP IV/CD26 in cutaneous wound healing process in mice. Wound Repair Regen 25: 25–40. [DOI] [PubMed] [Google Scholar]
- Belteki G, Haigh J, Kabacs N, Haigh K, Sison K, Costantini F, et al. , (2005). Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Research 33: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer C, Reichert H, Akan H, Mallano T, Schramm A, Dees C, et al. , (2013). Blockade of canonical Wnt signalling ameliorates experimental dermal fibrosis. Ann Rheum Dis 72: 1255–8. [DOI] [PubMed] [Google Scholar]
- Beyer C, Schramm A, Akhmetshina A, Dees C, Kireva T, Gelse K, et al. , (2012). β-catenin is a central mediator of pro-fibrotic Wnt signaling in systemic sclerosis. Ann Rheum Dis 71: 761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard L, Faucher G, Tchernof A, Deshaies Y, Lebel S, Hould F-S, et al. , (2009). Comprehensive genetic analysis of the dipeptidyl peptidase-4 gene and cardiovascular disease risk factors in obese individuals. Acta Diabetol 46: 13–21. [DOI] [PubMed] [Google Scholar]
- Butzelaar L, Niessen FB, Talhout W, Schooneman D, Ulrich MM, Beelen R, et al. , (2017). Different properties of skin of different body sites: The root of keloid formation? Wound Repair Regen 25: 758–66. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen X, Ji Y-R, Zhu S, Bu F-T, Du X-S, et al. , (2020). PLK1 regulates hepatic stellate cell activation and liver fibrosis through Wnt/β-catenin signalling pathway. J Cell Mol Med 24: 7405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler JHW, Györfi A-H, Ramanujam M, Whitfield M, Königshoff M, Lafyatis R (2019). Shared and distinct mechanisms of fibrosis. Nature Reviews Rheumatology 15: 705–30. [DOI] [PubMed] [Google Scholar]
- Dobrian AD, Ma Q, Lindsay JW, Leone K, Ma K, Coben J, et al. , (2011). Dipeptidyl peptidase IV inhibitor sitagliptin reduces local inflammation in adipose tissue and in pancreatic islets of obese mice. Am J Physiol Endocrinol Metab 300: E410–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Jahoda CAB, Chuong C-M, Watt FM, Horsley V, (2014). Defining dermal adipose tissue. Exp Dermatol 23: 629–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, et al. , (2013). Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504: 277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCODE Project Consortium. (2012). An integrated encyclopedia of DNA elements in the human genome. Nature 489: 57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezure T, Amano S (2010). Influence of subcutaneous adipose tissue mass on dermal elasticity and sagging severity in lower cheek. Skin Res Technol 16: 332–8. [DOI] [PubMed] [Google Scholar]
- Ezure T, Amano S (2015). Increment of subcutaneous adipose tissue is associated with decrease of elastic fibres in the dermal layer. Exp Dermatol 24: 924–9. [DOI] [PubMed] [Google Scholar]
- Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, et al. , (2011). Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 146: 761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaytan F, Morales C, Reymundo C, Tena-Sempere M (2020). A novel RGB-trichrome staining method for routine histological analysis of musculoskeletal tissues. Sci Rep 10: 16659–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrell MD (2005). Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clin Sci 108: 277–92. [DOI] [PubMed] [Google Scholar]
- Hamburg EJ, Atit RP (2012). Sustained β-catenin activity in dermal fibroblasts is sufficient for skin fibrosis. J Invest Dermatol 132: 2469–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburg-Shields E, DiNuoscio GJ, Mullin NK, Lafyatis R, Atit RP, et al. , (2015). Sustained β-catenin activity in dermal fibroblasts promotes fibrosis by up-regulating expression of extracellular matrix protein-coding genes. J Pathol 235: 686–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Gea V, Friedman SL (2011). Pathogenesis of liver fibrosis. Annu Rev Pathol 6: 425–56. [DOI] [PubMed] [Google Scholar]
- Hu H-H, Cao G, Wu X-Q, Vaziri ND, Zhao Y-Y (2020). Wnt signaling pathway in aging-related tissue fibrosis and therapies. Ageing Res Rev 60: 101063. [DOI] [PubMed] [Google Scholar]
- Hu MS, Longaker MT (2016). Dipeptidyl Peptidase-4, Wound Healing, Scarring, and Fibrosis. Plastic and Reconstructive Surgery 138: 1026–31. [DOI] [PubMed] [Google Scholar]
- Huang L, Lin T, Shi M, Chen X, Wu P (2020). Liraglutide suppresses production of extracellular matrix proteins and ameliorates renal injury of diabetic nephropathy by enhancing Wnt/β-catenin signaling. AJP: Renal Physiology 319: F458–68. [DOI] [PubMed] [Google Scholar]
- Hunzelmann N, Anders S, Sollberg S, Schönherr E, Krieg T (1996). Co-ordinate induction of collagen type I and biglycan expression in keloids. Br J Dermatol 135: 394–9. [PubMed] [Google Scholar]
- Hwang J, Huang Y, Burwell TJ, Peterson NC, Connor J, Weiss SJ, et al. , (2017). In Situ Imaging of Tissue Remodeling with Collagen Hybridizing Peptides. ACS Nano 11: 9825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Correa-Gallegos D, Christ S, Stefanska A, Liu J, Ramesh P, et al. , (2018). Two succeeding fibroblastic lineages drive dermal development and the transition from regeneration to scarring. Nat Cell Biol 20: 422–31. [DOI] [PubMed] [Google Scholar]
- Kasza I, Suh Y, Wollny D, Clark RJ, Roopra A, Colman RJ, et al. , (2014). Syndecan-1 is required to maintain intradermal fat and prevent cold stress. PLoS Genet 10: e1004514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel RA, Turnbull DH, Blanquet V, Wurst W, Loomis CA, Joyner AL et al. , (2000). Two lineage boundaries coordinate vertebrate apical ectodermal ridge formation. Genes & Development 14: 1377–89. [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Fleming T, Terjung S, Gorzelanny C, Gebhardt C, Agrawal R, et al. , (2017). Homeostatic nuclear RAGE-ATM interaction is essential for efficient DNA repair. Nucleic Acids Research 45: 10595–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafyatis R, Mantero JC, Gordon J, Kishore N, Carns M, Dittrich H, et al. , (2017). Inhibition of β-Catenin Signaling in the Skin Rescues Cutaneous Adipogenesis in Systemic Sclerosis: A Randomized, Double-Blind, Placebo-Controlled Trial of C-82. Journal of Investigative Dermatology 137: 2473–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam AP, Flozak AS, Russell S, Wei J, Jain M, Mutlu GM, et al. , (2011). Nuclear β-catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation. American Journal of Respiratory Cell and Molecular Biology 45: 915–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers D, Famulla S, Wronkowitz N, Hartwig S, Lehr S, Ouwens DM, et al. , (2011). Dipeptidyl Peptidase 4 Is a Novel Adipokine Potentially Linking Obesity to the Metabolic Syndrome 60: 1917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard J, Pelletier M, Biertho L, Biron S, Marceau S, Hould F-S, et al. , (2015). Characterization of Dedifferentiating Human Mature Adipocytes from the Visceral and Subcutaneous Fat Compartments: Fibroblast-Activation Protein Alpha and Dipeptidyl Peptidase 4 as Major Components of Matrix Remodeling. PLoS ONE 10: e0122065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Q, Wang J, Xu C, Huang X, Ruan Z, Dai Y (2020). Pirfenidone alleviates pulmonary fibrosis in vitro and in vivo through regulating Wnt/GSK-3β/β-catenin and TGF-β1/Smad2/3 signaling pathways. Mol Med 26: 49–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangoni RG, Korman BD, Wei J, Wood TA, Graham LV, Whitfield ML, et al. , (2015). Myofibroblasts in Murine Cutaneous Fibrosis Originate From Adiponectin-Positive Intradermal Progenitors. Arthritis & Rheumatology 67: 1062–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelin G, Ferreira A, Liu Y, Atlan M, Aron-Wisnewsky J, Pelloux V, et al. , (2017). A PDGFRα-Mediated Switch toward CD9high Adipocyte Progenitors Controls Obesity-Induced Adipose Tissue Fibrosis. Cell Metab. [DOI] [PubMed] [Google Scholar]
- Marguet D, Baggio L, Kobayashi T, Bernard M, Pierres M, Nielsen PF, et al. , (2000). Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci USA 97: 6874–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascharak S, desJardins-Park HE, Davitt MF, Griffin M, Borrelli MR, Moore AL, et al. , (2021). Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrogiannaki M, Lichtenberger BM, Reimer A, Collins CA, Driskell RR, Watt FM. (2016). β-Catenin stabilization in skin fibroblasts causes fibrotic lesions by preventing adipocyte differentiation of the reticular dermis. J Invest Dermatol 136: 1130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick D, Sakers A, Irgebay Z, Okada C, Calvert C, Morley MP, et al. , (2019). Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science 364: eaav2501–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, et al. , (1997). Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-Catenin or APC. Science 275: 1787–1790. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Soyal SM, Li J, Ying Y, Szwarc MM, He B, et al. , (2010). A mouse transgenic approach to induce β-catenin signaling in a temporally controlled manner. Transgenic Res 20: 827–40. [DOI] [PubMed] [Google Scholar]
- Mullin NK, Mallipeddi NV, Hamburg-Shields E, Ibarra B, Khalil A, Atit RP. (2017). Wnt/β-catenin Signaling Pathway Regulates Specific lncRNAs That Impact Dermal Fibroblasts and Skin Fibrosis. Front Genet 8: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvihill EE, Drucker DJ (2014). Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev 35: 992–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicu C, Hardman JA, Pople J, Paus R (2019). Do human dermal adipocytes switch from lipogenesis in anagen to lipophagy and lipolysis during catagen in the human hair cycle? Exp Dermatol 28: 432–5. [DOI] [PubMed] [Google Scholar]
- Olson LE, Soriano P (2009). Increased PDGFRalpha activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell 16: 303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piersma B, Bank RA, Boersema M (2015). Signaling in Fibrosis: TGF-β, WNT, and YAP/TAZ Converge. Front Med (Lausanne) 2: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehan VK, Torday JS (2014). The lung alveolar lipofibroblast: an evolutionary strategy against neonatal hyperoxic lung injury. Antioxidants & redox signaling 21: 1893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, et al. , (2015). Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348: aaa2151–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Gonzalez GC, Shook BA, Andrae J, Holtrup B, Bollag K, Betsholtz C, et al. , (2016). Skin Adipocyte Stem Cell Self-Renewal Is Regulated by a PDGFA/AKT-Signaling Axis. Cell Stem Cell 19: 738–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrborn D, Brückner J, Sell H, Eckel J (2016). Reduced DPP4 activity improves insulin signaling in primary human adipocytes. Biochem Biophys Res Commun 471: 348–54. [DOI] [PubMed] [Google Scholar]
- Rosmaninho-Salgado J, Marques AP, Estrada M, Santana M, Cortez V, Grouzmann E, et al. , (2012). Dipeptidyl-peptidase-IV by cleaving neuropeptide Y induces lipid accumulation and PPAR-γ expression. Peptides 37: 49–54. [DOI] [PubMed] [Google Scholar]
- Schmidt BA, Horsley V (2013). Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development 140: 1517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Koya D, Kanasaki K (2016). Dipeptidyl peptidase-4 and kidney fibrosis in diabetes. Fibrogenesis Tissue Repair 9: 1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook BA, Wasko RR, Rivera-Gonzalez GC, Salazar-Gatzimas E, López-Giráldez F, Dash BC, et al. , (2018). Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science 362: eaar2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soare A, Györfi HA, Matei AE, Dees C, Rauber S, Wohlfahrt T, et al. , (2020). Dipeptidylpeptidase 4 as a Marker of Activated Fibroblasts and a Potential Target for the Treatment of Fibrosis in Systemic Sclerosis. Arthritis Rheumatol 72: 137–49. [DOI] [PubMed] [Google Scholar]
- Surendran K, Schiavi S, Hruska KA (2005). Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol 16: 2373–84. [DOI] [PubMed] [Google Scholar]
- Suwanai H, Watanabe R, Sato M, Odawara M, Matsumura H (2020). Dipeptidyl Peptidase-4 Inhibitor reduces the risk of developing hypertrophic scars and keloids following median sternotomy in diabetic patients: A nationwide retrospective cohort study using the national database of health insurance claims of Japan. Plast Reconstr Surg 146: 83–9. [DOI] [PubMed] [Google Scholar]
- Tabib T, Morse C, Wang T, Chen W, Lafyatis R (2018). SFRP2/DPP4 and FMO1/LSP1 Define Major Fibroblast Populations in Human Skin. J Invest Dermatol 138: 802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XM, Holz LE, Chowdhury S, Cordoba SP, Evans KA, Gall MG, et al. , (2017). The pro-fibrotic role of dipeptidyl peptidase 4 in carbon tetrachloride-induced experimental liver injury. Immunol Cell Biol 95: 443–53. [DOI] [PubMed] [Google Scholar]
- Wei J, Fang F, Lam AP, Sargent JL, Hamburg E, Hinchcliff ME, et al. , (2012). Wnt/β-catenin signaling is hyperactivated in systemic sclerosis and induces Smad-dependent fibrotic responses in mesenchymal cells. Arthritis Rheum 64: 2734–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollina U, Wetzker R, Abdel-Naser MB, Kruglikov IL. (2017). Role of adipose tissue in facial aging. Clin Interv Aging 12: 2069–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Shen Z-Y, Wang K, Li W, Shi J-M, Kombo E, et al. , (2019a). C-reactive protein exacerbates epithelial-mesenchymal transition through Wnt/β-catenin and ERK signaling in streptozocin-induced diabetic nephropathy. FASEB J 33: 6551–63. [DOI] [PubMed] [Google Scholar]
- Zhang L-J, Guerrero-Juarez CF, Hata T, Bapat SP, Ramos R, Plikus MV, et al. , (2015). Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science 347: 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Shao M, Hepler C, Zi Z, Zhao S, An YA, et al. , (2019b). Dermal adipose tissue has high plasticity and undergoes reversible dedifferentiation in mice. J Clin Invest 129: 5327–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Kwan JYY, Yip K, Liu PP, Liu F-F (2020). Targeting metabolic dysregulation for fibrosis therapy. Nat Rev Drug Discov 19: 57–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets related to this article can be found in the GEO repository (GSE 103870, GSE 31477, GSM 945853).