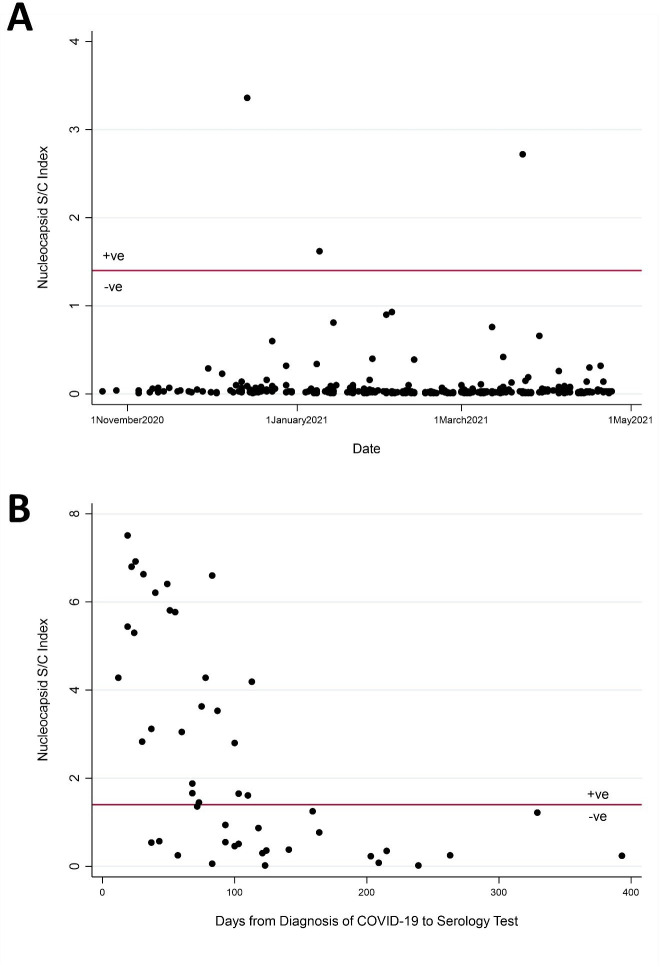
We read with interest the highly impactful CLARITY IBD study that demonstrated a diminished serological response to SARS-CoV-2 in individuals with IBD receiving infliximab as compared with those receiving vedolizumab.1 We assessed the prevalence of antibodies to SARS-CoV-2 in patients with IBD and evaluated whether serological response to SARS-CoV-2 wanes over time following infection.
Individuals with IBD from Calgary, Canada, were recruited from October 2020 to April 2021 and stratified into two cohorts: (1) serosurveillance (n=279) defined as those who were not previously diagnosed with COVID-19; and (2) COVID-19 recovered (n=45), defined as those with a molecular-confirmed diagnosis of SARS-CoV-2 infection via PCR.
Serum was analysed using the Abbott Architect SARS-CoV-2 IgG antibody to the nucleocapsid protein. A positive antibody test was defined as an index signal/cut-off ≥1.4. The assay has been validated with 95% sensitivity and >99% specificity 12 days following symptom onset and correlates to neutralising antibodies.2
We assessed the proportion, with 95% CIs, of individuals with IBD who mounted an antibody response among those undergoing serosurveillance and following infection with SARS-CoV-2. In those diagnosed with COVID-19, we calculated the days from diagnosis to serological testing and developed a logistic regression model that assessed the effect of time from COVID-19 diagnosis to serology testing (before vs after 90 days) on nucleocapsid positive versus negative antibody; adjusted for age, sex and anti-tumour necrosis factor (TNF) usage.
The baseline characteristics of the two cohorts are summarised in table 1. Among the serosurveillance cohort, 1.08% (95% CI 0.22 to 3.11) tested positive for nucleocapsid antibody (table 1, figure 1A). Among those who recovered from COVID-19, 53.3% (95% CI 37.9 to 68.3) had a positive nucleocapsid antibody (table 1). The proportion of patients who were nucleocapsid antibody positive was 80.8% (95% CI 60.6 to 93.4) at 0–90 days from diagnosis and 21.7% (95% CI 7.5 to 43.7) after 90 days. Tests performed within 90 days of their diagnosis were associated with nucleocapsid seropositivity (OR: 14.1; 95% CI 3.46 to 57.6) as compared with testing beyond 90 days of COVID-19 diagnosis. An interactive dashboard displaying these data can be found here: https://kaplan-gi.shinyapps.io/COVID_Serology/.
Table 1.
Demographic and clinical characteristics of the serosurveillance and COVID-19 recovered individuals with IBD
| Baseline characteristics | Serosurveillance cohort (n=279)* | COVID-19 recovered (n=45) |
||
| Total COVID-19 recovered (n=45)* | Nucleocapsid positive (n=24)† | Nucleocapsid negative (n=21)† | ||
| Age, median (IQR) | 48.1 (36.4–59.1)‡ | 43.1 (32.2–49.8)‡ | 44.2 (33.6–55.6) | 37.9 (30.5–48.8) |
| Sex, n (%) | ||||
| Female | 156 (55.9) | 27 (60.0) | 17 (70.8) | 10 (47.6) |
| Male | 123 (44.1) | 18 (40.0) | 7 (29.2) | 11 (52.4) |
| Disease, n (%) | ||||
| Crohn’s Disease | 226 (81.0) | 29 (64.4) | 17 (70.8) | 12 (57.1) |
| Ulcerative Colitis | 53 (19.0) | 16 (35.6) | 7 (29.2) | 9 (42.9) |
| Anti-TNF§, n (%) | 138 (49.5) | 27 (60) | 15 (62.5) | 12 (57.1) |
| Ustekinumab, n (%) | 89 (32.0)‡ | 4 (8.9)‡ | 3 (12.5) | 1 (4.8) |
| Vedolizumab, n (%) | 44 (16.1) | 6 (13.3) | 2 (8.3) | 4 (19.1) |
| Tofacitinib, n (%) | 2 (0.72) | 1 (2.2) | 1 (4.2) | 0 |
| Combination therapy¶, n (%) | 54 (19.4) | 7 (15.6) | 3 (12.5) | 4 (19.1) |
| Oral prednisone, n (%) | 9 (3.2) | 0 | 0 | 0 |
| 5-ASA alone or no IBD medications, n (%) | 5 (1.8)‡ | 5 (11.1)‡ | 2 (8.3) | 3 (14.3) |
| Nucleocapsid antibody, n (%) | ||||
| Positive (≥1.4 S/C index) | 3 (1.1)‡ | 23 (51.1)‡ | NA | NA |
| Days from COVID-19 diagnosis to serology test, median (IQR)* | NA | 85 (41.5–122) | 51 (25–71)** | 123 (93–209)** |
| COVID-19 severity, n (%) | ||||
| Ambulatory | NA | 44 (97.8) | 23 (95.8) | 21 (100) |
| Hospitalised | 1 (2.2) | 1 (4.2) | 0 | |
Those previously diagnosed with COVID-19 are further stratified by positive versus negative nucleocapsid antibody test based on an S/C index of ≥1.4.
*Two individuals in the serosurveillance cohort were diagnosed with COVID-19 after their serosurveillance serology testing.
†Nucleocapsid positive or negative were based on their first serology test after COVID-19 diagnosis.
‡Significant p value comparing serosurveillance cohort to COVID-19 recovered cohort. Statistically significant at p<0.05; either defined by two-sample test of proportion (categorical variable) or Wilcoxon rank-sum test (continuous variable).
§Anti-TNF is one of golimumab, adalimumab or infliximab (originator or biosimilar).
¶Any combination of two or more of the following therapies: anti-TNF therapy, vedolizumab, ustekinumab, tofacitinib, azathioprine, 6-mercaptopurine or methotrexate.
**Significant p value comparing COVID-19 recovered nucleocapsid positive to nucleocapsid negative. Statistically significant at p<0.05; either defined by two-sample test of proportion (categorical variable) or Wilcoxon rank-sum test (continuous variable).
Anti-TNF, anti-tumour necrosis factor; NA, not applicable; S/C, signal/cut-off.
Figure 1.
The proportion of individuals with IBD who mounted an antibody response (A) Among those undergoing serosurveillance and (B) following infection with SARS-CoV-2. All clinical and serological data from this study are available open access on an online interactive dashboard: https://sc-epi.shinyapps.io/COVID_Serology/.
Seropositivity for SARS-CoV-2 nucleocapsid antibodies was found in 1% of individuals with IBD. In contrast, seropositivity in the general population in Calgary during the same time period was 1.8%, suggesting that patients with IBD were less exposed to SARS-CoV-2 or that serological response postinfection was not as robust as the general population.3
Our seropositivity was lower than prior IBD studies in the USA (3%)4 and the UK (4.3%).1 The difference in seropositivity may be explained by the lower prevalence of COVID-19 in the general population in Canada; for example, 5.4% of patients with IBD had antibodies to SARS-CoV-2 in Milan as compared with only 0.4% in regions in Europe with lower rates of infection in their general populations.5
In our study, the primary factor associated with seropositivity was the interval from diagnosis to serology testing. Three-quarters were seropositive if tested within 2 months of diagnosis, whereas no one tested after 4 months was seropositive. Limitations include sample size required to compare across therapies and not testing for neutralising antibodies or immunological memory.6
Nonetheless, these data have important clinical implications for individuals with IBD. Serosurveillance studies in IBD may only be reliable for a 3-month window of testing. Moreover, 4 months following COVID-19 diagnosis, patients with IBD may continue to be susceptible to SARS-CoV-2. Our data highlight the importance of studying sustained antibody response over time in order to develop strategies to ensure durable protection and inform public health policy.
Footnotes
Twitter: @gilkaplan, @ericbenchimol
GGK and CM contributed equally.
Collaborators: Serological Testing to Outline Protocols for COVID19 in Inflammatory Bowel Disease (STOP COVID-19 in IBD) Research Group: LeeAnn Turnbull, Maxime Delisle, Joseph W Windsor, Joshua Quan, Lisa Barrett, Charles N Bernstein, Melissa Chan, Usha Chauhan, Jose Ferraz, Sharyle Fowler, Anne Griffiths, Joan Heatherington, Jennifer Jones, Reena Khanna, M Ellen Kuenzig, Peter L Lakatos, Kate Lee, Cathy Lu, David Mack, Kerri Novak, Cynthia H Seow, Tushar Shukla, Laura Targownik, Kumanan Wilson and Sandra Zelinsky.
Contributors: GGK has full access to all data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. GGK, CM, CC, JNK, GT and RP conceived and designed the study. GGK, CM, RP, NS, MH and RJMI were responsible for clinical data. CC, JNK, GT, NS and MH were responsible for serological data. GGK, SC and LH analysed the data. GK drafted the manuscript. All authors interpreted the data and provided critical revisions of the manuscript for important intellectual content. All authors have approved the final draft of the manuscript.
Competing interests: GGK has received honoraria for speaking or consultancy from Abbvie, Janssen, Pfizer, Amgen and Takeda. He has received research support from Ferring, Janssen, Abbvie, GlaxoSmith Kline, Merck and Shire. He has been a consultant for Gilead. He shares ownership of a patent: treatment of inflammatory disorders, autoimmune disease, and PBC. UTI Limited Partnership, assignee. Patent WO2019046959A1. PCT/CA2018/051098. 7 September 2018. CM has received consulting fees from AbbVie, Amgen, AVIR Pharma Inc, Ferring, Fresenius Kabi, Janssen, McKesson, Mylan, Takeda, Pfizer, Roche and Alimentiv (formerly Robarts Clinical Trials Inc); speaker's fees from AbbVie, AVIR Pharma Inc, Janssen, Takeda and Pfizer; research support from Pfizer. RP has received consulting fees from: AbbVie, Abbott, Alimentiv (formerly Robarts), Amgen, Arena Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim Celgene, Celltrion, Cosmos Pharmaceuticals, Eisai, Elan, Eli Lilly, Ferring, Galapagos, Genentech, Gilead Sciences, GlaxoSmith Kline, Janssen, Merck, Mylan, Oppilan Pandion, Pharma, Pandion Pharma, Pfizer, Progenity, Protagonist Therapeutics, Roche, Satisfai Health, Sandoz, Schering-Plough, Shire, Sublimity Therapeutics, Theravance Biopharma, UCB and Takeda Pharmaceuticals; speaker fees from: AbbVie, Arena Pharmaceuticals, Celgene, Eli Lilly, Ferring, Gilead Sciences, Janssen, Merck, Pfizer, Roche, Sandoz, Shire and Takeda Pharmaceuticals; advisory board: AbbVie, Amgen, Arena Pharmaceuticals, Bristol-Myers Squibb, Celgene, Celltrion, Eli Lilly, Ferring, Galapagos, Genentech, Gilead Sciences, Glaxo-Smith Kline, Janssen, Merck, Mylan, Oppilan Pharma, Pandion Pharma, Pfizer, Sandoz, Shire, Sublimity Therapeutics, Theravance Biopharma and Takeda Pharmaceuticals; research/educational support: AbbVie, Ferring, Janssen, Pfizer and Takeda. EIB has acted as a legal consultant for Hoffman La-Roche Limited and Peabody & Arnold LLP for matters unrelated to a medication used to treat IBD. CC, JNK, GT, NS, Ms Van Huyssteen, SC, Dr Hracs and RJMI: none.
Patient and public involvement: Serological Testing to Outline Protocols for COVID19 in Inflammatory Bowel Disease (STOP COVID-19 in IBD) Research Group includes the members of Crohn's and Colitis Canada's COVID-19 and IBD Taskforce, which has patient representation. Patient representative were included in several aspects of the study including collaborators on the grant, study design, interpretation, knowledge translation and authorship on the letter.
Provenance and peer review: Not commissioned; internally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
All participants provided informed consent, and the study was approved by the University of Calgary’s Conjoint Health Research Ethics Board.
References
- 1. Kennedy NA, Goodhand JR, Bewshea C, et al. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut 2021;70:865–75. 10.1136/gutjnl-2021-324388 [DOI] [PubMed] [Google Scholar]
- 2. Padoan A, Bonfante F, Pagliari M, et al. Analytical and clinical performances of five immunoassays for the detection of SARS-CoV-2 antibodies in comparison with neutralization activity. EBioMedicine 2020;62:103101. 10.1016/j.ebiom.2020.103101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Charlton CL, Nguyen L, Bailey A. Pre-Vaccine positivity of SARS-CoV-2 antibodies in Alberta, Canada during the first two waves of the COVID-19 pandemic. Submitted for Publication. mBio 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gubatan J, Levitte S, Balabanis T, et al. SARS-CoV-2 testing, prevalence, and predictors of COVID-19 in patients with inflammatory bowel disease in northern California. Gastroenterology 2020;159:1141–4. 10.1053/j.gastro.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berte’ R, Mazza S, Stefanucci MR, et al. Seroprevalence of SARS-CoV2 in IBD patients treated with biologic therapy. J Crohns Colitis 2021;15:864–8. 10.1093/ecco-jcc/jjaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021;371. 10.1126/science.abf4063. [Epub ahead of print: 05 02 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]