Abstract
Objectives
Although hepatitis B virus (HBV) vaccination for high-risk groups including gay, bisexual and other men who have sex with men (MSM) is recommended in the UK, data on HBV immunisation coverage are limited. This study aimed to understand the prevalence of HBV infection, susceptibility and immunity due to immunisation among a high-risk population of MSM and heterosexuals who are less likely to attend sexual health services.
Methods
Residual HIV-negative serology samples archived from a national HIV self-sampling service in 2016 were tested for HBV markers using an unlinked anonymous approach. Prevalence of HBV infection, evidence of immunisation and susceptibility were calculated and stratified by individuals’ characteristics. Multinomial logistic regression was used to estimate relative risk ratios (RRRs) associated with covariates.
Results
Of 2172 samples tested, 1497 (68.9%) were from MSM and 657 (30.2%) were from heterosexuals. Susceptibility to HBV infection was 66.1% among MSM and 77.0% among heterosexuals. Only 29.9% of MSM and 17.4% of heterosexuals had serological evidence of immunisation. Current infection was 1.1% in heterosexuals and 0.2% in MSM. Adjusted analysis showed evidence of immunisation was lower among heterosexuals (RRR 0.66, 95% CI 0.50 to 0.86) and those with no previous HIV test (RRR 0.41, 95% CI 0.31 to 0.54), and higher in those of other white or other ethnicity.
Conclusions
Among MSM and heterosexual users of a self-sampling HIV service, evidence of immunisation to HBV infection was low and susceptibility to infection was comparatively high, suggesting suboptimal delivery of HBV immunisation in sexual health services.
Keywords: communicable diseases, hepatitis B, sexual health, epidemiology, serology
Introduction
Infection with the hepatitis B virus (HBV) can lead to both acute illness and chronic infection, the latter being associated with increased risk of cirrhosis and liver cancer, and can be fatal.1 The prevalence of HBV in the UK is low, with an estimated 180 000–414 000 (~0.3%) people chronically infected2 3 and with infections predominantly in migrant populations from high-prevalence countries, who likely acquired their infection in infancy in their country of birth.4 Acute HBV annual incidence is also low at 0.68 per 100 000 population in England,5 with incident infections concentrated among high-risk groups, including gay, bisexual and other men who have sex with men (MSM), particularly those with multiple sexual partners.
A safe and effective vaccine for HBV is available, and recommendations are to test and immunise higher-risk individuals, including MSM, sex workers, people who inject drugs (PWIDs), people from HBV endemic countries and sexual partners of infected or high-risk patients.6–8 In 2016, the UK signed up to the WHO goal of eliminating viral hepatitis as a public health threat by 2030.9 Improved monitoring of HBV vaccination programmes is needed to track progress against elimination targets. However, data on vaccine coverage among risk groups in the UK are currently limited; for example, HBV vaccination is recorded in Genitourinary Medicine Clinic Activity Dataset (GUMCAD), the mandatory surveillance system for STIs in England, but audits show that these data do not adequately monitor vaccine coverage,10 11 and unlinked anonymous monitoring of behavioural risk groups has only focused on reported HBV vaccination and not serological levels of immunity.12
Recent events in the UK have highlighted gaps in vaccine uptake by those at risk and in the ability to monitor access and uptake: regional clusters of A2 strain (prisoner variant) among MSM who identify as heterosexual—a high-risk group who may not attend sexual health services as they do not perceive themselves at risk but represent a bridging group with other MSM, increasing the risk of onward transmission,13 and in 2017–2018, the UK experienced a shortage of HBV vaccine due to global manufacturing issues which led to prioritisation of scarce vaccine stock and interference with MSM immunisation programmes in sexual health services.14 Although vaccine supplies have now improved, the limited information on vaccine coverage among MSM has made prioritisation of the HBV vaccine during this time more complicated; more up-to-date information is needed to inform decisions in the event of future vaccine shortages.
While work is ongoing to improve GUMCAD reporting standards for HBV vaccination in sexual health services, in the interim, serological surveys in MSM and other high-risk groups may allow us to estimate susceptibility and immunity from immunisation or natural infection. Therefore, we conducted HBV serological testing of an archive of residual samples from HIV-negative users of a national self-sampling HIV service15 to investigate the prevalence of HBV infection, immunity due to vaccination and susceptibility among a high-risk population who are less likely to attend sexual health services.16 This archive also provides an opportunity to benchmark population susceptibility prior to the start of the COVID-19 pandemic and associated social distancing measures, which are likely to impact on behaviour as well as provision of immunisation and testing services.
METHODS
Sampling strategy
In 2015, Public Health England and local authorities in England launched a national HIV self-sampling service aimed at higher-risk populations: MSM and black African men and women.15 The free home test kit is accessed online (https://freetesting.hiv/), where individuals complete an online questionnaire. Finger-prick blood samples are sent to Preventx Integrated Diagnostics for HIV testing using a Roche diagnostics fourth-generation assay. Residual sera from all HIV antigen-negative and antibody-negative specimens from the service received between 1 April 2016 and 18 November 2016 were archived. The first 2193 samples retrieved from a total of 9179 archived specimens were selected for this study. Where there was limited specimen available for testing, another sample was selected.
Unlinking, anonymising and data linkage
All samples were unlinked and anonymised before testing. Variables of interest were obtained from Preventx. A new study ID was created, and original identifiers were stripped from samples and self-reported demographic and risk data before testing.
Laboratory testing
The presence of antibody to hepatitis B surface antigen (anti-HBs) and antibody to hepatitis B core (anti-HBc) were determined using the BioRad Monolisa ELISA (Watford, Hertfordshire, UK) and the Murex DiaSorin (Dartford, Kent, UK) ELISA, respectively. Samples identified as anti-HBc reactive were tested for hepatitis B surface antigen (HBsAg) using the GE34/36 assay Murex DiaSorin. All ELISAs were carried out in accordance to the manufacturer’s instructions.
Data fields and definitions
The self-sampling form collected information on demographics: age, sex, ethnicity and sexuality; self-reported sexual behaviours: gender of sexual partners (men, women or both), unprotected sex in the past 12 months, number of new partners in the past 12 months, frequency of sex under the influence of alcohol or drugs; and previous HIV testing. Service users who were neither MSM nor black African were in addition asked about other risks which would be a reason for them to use the service (online supplemental table 1). All questions were multiple choice apart from age (table 1). Ethnicity was grouped based on census categories, with lower-level categories preserved if numbers are sufficient (or not shown if there are no people in that category). Sexual behaviour was defined as heterosexual, MSM or other (bisexual women and women who have sex with women only), depending on who the service user had sex with (men, women or both). Pre-exposure prophylaxis (PrEP) use was not incorporated into the measure of unprotected sex, but PrEP use was low in England in 2016 (~252 individuals), so its impact is likely to be low.17 HBV status was defined using serological markers: susceptible (HBsAg−, anti-HBc− and anti-HBs−); immune due to immunisation (HBsAg−, anti-HBc− and anti-HBs+); past infection (HBsAg−, anti-HBc+ and anti-HBs+) or (HBsAg−, anti-HBc+ and anti-HBs−); and current infection (HBsAg+, anti-HBc+ and anti-HBs−).
Table 1.
Characteristics and behaviours reported on self-sampling form and groupings for multivariable analysis
| Characteristic | Response options | Grouping for initial analysis | Grouping for multivariable analysis |
| Age | Age in years | 16–25 | 16–25 |
| 26–35 | 26–35 | ||
| 36–45 | 36–45 | ||
| 46–55 | 46–75 | ||
| 56–65 | |||
| 66–75 | |||
| Ethnicity | White British | White British/Irish | White British/Irish |
| White Irish | |||
| Black African | Black African | Black African | |
| Black Caribbean | Black other | Other | |
| Other black background | |||
| Latin American | Other or mixed | ||
| Other ethnic group | |||
| Other mixed background | |||
| White and Asian | |||
| White and black African | |||
| White and black Caribbean | |||
| Indian | South Asian | ||
| Pakistani | |||
| Bangladeshi | |||
| Chinese | Other Asian background | ||
| Other Asian background | |||
| Other white background | Other white background | Other white background | |
| New partners in last 12 months | No new partners | No new partners | No new partners |
| Just one partner | Just one partner | Just one partner | |
| 2–5 partners | 2–5 partners | 2–5 partners | |
| 6–12 partners | 6–12 partners | 6+partners | |
| More than 12 partners | More than 12 partners | ||
| Unprotected sex in last 12 months | No | No | No new partners |
| Yes, with one partner | Yes, with one partner | Just one partner | |
| Yes, with 2–5 partners | Yes, with 2–5 partners | 2–5 partners | |
| Yes, with 6–12 partners | Yes, with 6–12 partners | 6+ partners | |
| Yes, with more than 12 partners | Yes, with more than 12 partners | ||
| Last HIV test | Never tested | Never tested | Never tested |
| Within the last year | Within the last year | Previously tested | |
| Over 1 year ago | Over 1 year ago | ||
| Sex under the influence of alcohol or drugs | Never | Never | Never |
| Sometimes | Sometimes | Sometimes | |
| Usually | Usually | Usually | |
| Always | Always | Always |
sextrans-2021-055071supp001.pdf (87.4KB, pdf)
Data analysis
Results were analysed in Excel 2016 and Stata V.15. Prevalence of HBV infection (past or current), serological evidence of immunisation and susceptibility to HBV were calculated for the overall sample and by sexual orientation (heterosexual or MSM), age group, ethnicity, and behavioural risk factors. χ2 tests were used to test for differences between groups. Multinomial logistic regression was used to estimate measures of association as relative risk ratios (RRRs) and 95% CIs for current or past infection or evidence of immunisation, with those who were susceptible set as the reference group. For the multivariable analysis, groups were combined where necessary to avoid zero events and small group sizes. Black other, South Asian, other Asian and other/mixed ethnicities were grouped together due to small numbers in these groups, with black African ethnicity retained as a separate group due to being a target group for the service, and due to small sample size, those with ‘other’ sexual behaviours (18 records) were dropped from the analysis.
For the multivariable model, age group and ethnicity were included as a priori confounders, and other variables were tested for statistical significance using the likelihood ratio test and were included if the p values were <0.05. We hypothesised that the following interactions may be present for associations with evidence of immunity: (1) orientation and previous HIV testing; immunisation may be more likely to be associated with previous HIV testing for MSM than for heterosexuals due to the delivery of MSM vaccination programmes in sexual health; and (2) orientation and ethnicity; with ethnicity considered as a proxy for country of birth, those born outside the UK may be more likely to have received vaccination under universal programmes, compared with the MSM programme for those in the UK. Therefore, we first conducted a stratified analysis to compare the direction of association of other covariates when stratified by orientation, HIV testing and ethnicity (white British/Irish vs all other ethnicities) to identify any significant interactions that should be included in the model, and whether separate models should be considered for MSM and heterosexuals. As no significant differences were found under stratified analysis, a combined model without interaction terms was created.
Results
Cohort characteristics
A total of 2193 residual samples were available for HBV testing, of which 21 were excluded from subsequent analysis because of incomplete risk behaviour information. Of the final 2172 samples, 1497 (68.9%) individuals reported being MSM; 657 (30.2%) reported being heterosexual; and 18 (0.8%) reported other sexual orientation. Four-fifths (79.8%) were individuals aged <35 years, with 44.2% being <25 years; this age distribution was similar by sexual orientation (table 2). MSM were predominantly white British/Irish (76.8%), followed by other white backgrounds (12.5%) and other ethnicities (4.3%), whereas 47.9% of heterosexuals were white British/Irish, followed by black African (21.3%) and other white (11.1%) ethnicities (online supplemental table 2). Half of service users (51.7%) reported 2–5 new partners in the past 12 months, with more MSM (28.3%) than heterosexuals (9.4%) reporting 6+ partners. Unprotected sex in the past 12 months was higher among heterosexuals (90.1%) than among MSM (83.4%). Regardless of sexual orientation, almost two-thirds (62.6%) reported sex under the influence of alcohol or drugs. Previous HIV testing was higher among MSM (81.7%) than among heterosexuals (55.7%). Among the 78.7% of heterosexuals who were not of black African ethnicity, 90.3% responded to the additional risk questions, of which all reported at least one risk.
Table 2.
Demographics and HBV infection status by sexual orientation
| Characteristic | Demographics | Infection | |||||||||||
| MSM | Heterosexual | ||||||||||||
| Overall, N (%, 95% CI) | MSM, n (%, 95% CI) | Heterosexual, n (%, 95% CI) | Past infection, n (%, 95% CI) | Current infection, n (%, 95% CI) | Immunised, n (%, 95% CI) | Susceptible, n (%, 95% CI) | Total | Past infection, n (%, 95% CI) | Current infection, n (%, 95% CI) | Immunised, n (%, 95% CI) | Susceptible, n (%, 95% CI) | Total | |
| Total | 2172 | 1497 | 657 | 56 (3.7, 2.9 to 4.8) |
3 (0.2, 0.1 to 0.6) |
448 (29.9, 27.7 to 32.3) |
990 (66.1, 63.7 to 68.5) |
1497 | 30; (4.6%, 3.2 to 6.4) |
7; (1.1%, 0.5 to 2.2) |
114; (17.4%, 14.6 to 20.4) |
506; (77.0%, 73.6 to 80.1) |
657 |
| Age | |||||||||||||
| 16–25 | 961 (44.2%, 42.2 to 46.3) |
636 (42.5%, 40.0 to 45.0) |
315 (47.9%, 44.1 to 51.8) |
6 (0.9%, 0.4 to 2.0) |
1; (0.2%, 0.0 to 0.9) |
168 (26.4%, 23.1 to 30.0) |
461 (72.5%, 68.9 to 75.8) |
636 | 5 (1.6%, 0.7 to 3.7) |
2 (0.6%, 0.2 to 2.3) |
43 (13.7%, 10.3 to 17.9) |
265 (84.1%, 79.7 to 87.7) |
315 |
| 26–35 | 773 (35.6%, 33.6 to 37.6) |
561 (37.5%, 35.1 to 40.0) |
206 (31.4%, 27.9 to 35.0) |
19 (3.4%, 2.2 to 5.2) |
2 (0.4%, 0.1 to 1.3) |
186 (33.2%, 29.4 to 37.2) |
354 (63.1%, 59.0 to 67.0) |
561 | 11 (5.3%, 3.0 to 9.3) |
1 (0.5%, 0.1 to 2.7) |
50 (24.3%, 18.9 to 30.6) |
144 (69.9%, 63.3 to 75.8) |
206 |
| 36–45 | 292 (13.4%, 12.1 to 14.9) |
198 (13.2%, 11.6 to 15.0) |
92 (14.0%, 11.6 to 16.9) |
16 (8.1%, 5.0 to 12.7) |
0 (0.0%, 0.0 to 1.9) |
65 (32.8%, 26.7 to 39.6) |
117 (59.1%, 52.1 to 65.7) |
198 | 12 (13.0%, 7.6 to 21.4) |
4 (4.3%, 1.7 to 10.7) |
12 (13.0%, 7.6 to 21.4) |
64 (69.6%, 59.5 to 78.0) |
92 |
| 46–75 | 146 (6.7%, 5.7 to 7.9) |
102 (6.8%, 5.6 to 8.2) |
44 (6.7%, 5.0 to 8.9) |
15 (14.7%, 9.1 to 22.9) |
0 (0.0%, 0.0 to 3.6) |
29 (28.4%, 20.6 to 37.8) |
40 (60.6%, 48.5 to 71.5) |
102 | 2 (4.5%, 1.3 to 15.1) |
0 (0.0%, 0.0 to 8.0) |
9 (20.5%, 11.2 to 34.5) |
28 (73.7%, 58.0 to 85.0) |
44 |
| Ethnicity | |||||||||||||
| White British/Irish | 1478 (68.0%, 66.1 to 70.0) |
1149 (76.8%, 74.5 to 78.8) |
315 (47.9%, 44.1 to 51.8) |
37 (3.2%, 2.3 to 4.4) |
1 (0.1%, 0.0 to 0.5) |
326 (28.4%, 25.8 to 31.0) |
785 (68.3%, 65.6 to 70.9) |
1149 | 5 (1.6%, 0.7 to 3.7) |
1 (0.3%, 0.1 to 1.8) |
44 (14.0%, 10.6 to 18.2) |
265 (84.1%, 79.7 to 87.7) |
315 |
| Black African | 153 (7.0%, 6.0 to 8.2) |
12 (0.8%, 0.5 to 1.4) |
140 (21.3%, 18.3 to 24.6) |
3 (25.0%, 8.9 to 53.2) |
0 (0.0%, 0.0 to 24.2) |
4 (33.3%, 13.8 to 60.9) |
5 (41.7%, 19.3 to 68.0) |
12 | 19 (13.6%, 8.9 to 20.2) |
5 (3.6%, 1.5 to 8.1) |
20 (14.3%, 9.4 to 21.0) |
96 (68.6%, 60.5 to 75.7) |
140 |
| Other white background | 260 (12.0%, 10.7 to 13.4) |
187 (12.5%, 10.9 to 14.3) |
73 (11.1%, 8.9 to 13.7) |
10 (5.3%, 2.9 to 9.6) |
1 (0.5%, 0.1 to 3.0) |
63 (33.7%, 27.3 to 40.7) |
113 (60.4%, 53.3 to 67.2) |
187 | 2 (2.7%, 0.8 to 9.5) |
1 (1.4%, 0.2 to 7.4) |
26 (35.6%, 25.6 to 47.1) |
44 (60.3%, 48.8 to 70.7) |
73 |
| Other | 281 (12.9%, 11.6 to 14.4) |
149 (10.0%, 8.5 to 11.6) |
129 (19.6%, 16.8 to 22.8) |
6 (4.0%, 1.9 to 8.5) |
1 (0.7%, 0.1 to 3.7) |
55 (36.9%, 29.6 to 44.9) |
87 (58.4%, 50.4 to 66.0) |
149 | 4 (3.1%, 1.2 to 7.7) |
0 (0.0%, 0.0 to 2.9) |
24 (18.6%, 12.8 to 26.2) |
101 (78.3%, 70.4 to 84.5) |
129 |
| New partners in last 12 months | |||||||||||||
| No new partners | 79 (3.6%, 2.9 to 4.5) |
35 (2.3%, 1.7 to 3.2) |
41 (6.2%, 4.6 to 8.4) |
1 (2.9%, 0.5 to 14.5) |
0 (0.0%, 0.0 to 9.9) |
9 (25.7%, 14.2 to 42.1) |
25 (71.4%, 54.9 to 83.7) |
35 | 1 (2.4%, 0.4 to 12.6) |
0 (0.0%, 0.0 to 8.6) |
13 (31.7%, 19.6 to 47.0) |
27 (65.9%, 50.5 to 78.4) |
41 |
| Just one partner | 481 (22.1%, 20.4 to 23.9) |
269 (18.0%, 16.1 to 20.0) |
208 (31.7%, 28.2 to 35.3) |
7 (2.6%, 1.3 to 5.3) |
1 (0.4%, 0.1 to 2.1) |
65 (24.2%, 19.4 to 29.6) |
196 (72.9%, 67.3 to 77.8) |
269 | 14 (6.7%, 4.1 to 11.0) |
3 (1.4%, 0.5 to 4.2) |
30 (14.4%, 10.3 to 19.8) |
161 (77.4%, 71.3 to 82.6) |
208 |
| 2–5 partners | 1122 (51.7%, 49.6 to 53.8) |
769 (51.4%, 48.8 to 53.9) |
346 (52.7%, 48.8 to 56.5) |
25 (3.3%, 2.2 to 4.8) |
1 (0.1%, 0.0 to 0.7) |
209 (27.2%, 24.2 to 30.4) |
534 (69.4%, 66.1 to 72.6) |
769 | 13 (3.8%, 2.2 to 6.3) |
3 (0.9%, 0.3 to 2.5) |
60 (17.3%, 13.7 to 21.7) |
270 (78.0%, 73.4 to 82.1) |
346 |
| 6+ partners | 490 (22.6%, 20.9 to 24.4) |
424 (28.3%, 26.1 to 30.7) |
62 (9.4%, 7.4 to 11.9) |
23 (5.4%, 3.6 to 8.0) |
1 (0.4%, 0.1 to 2.0) |
95 (34.8%, 29.4 to 40.6) |
165 (60.4%, 54.5 to 66.1) |
424 | 2 (3.2%, 0.9 to 11.0) |
1 (2.3%, 0.4 to 12.1) |
6 (14.0%, 6.6 to 27.3) |
35 (81.4%, 67.4 to 90.3) |
62 |
sextrans-2021-055071supp002.pdf (195.3KB, pdf)
HBV infection
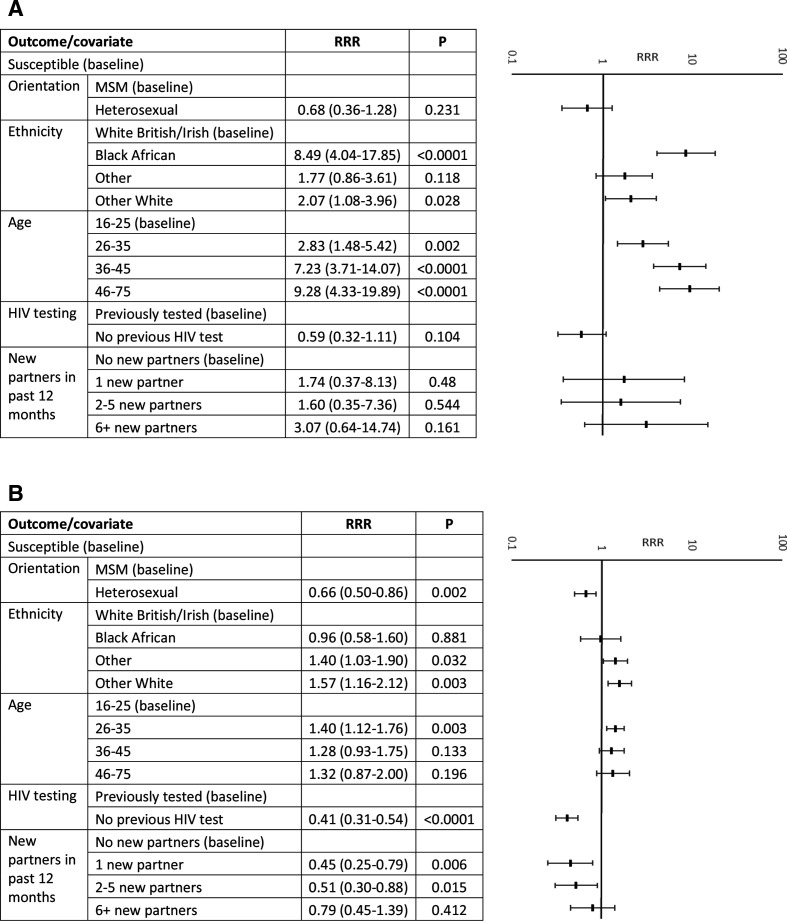
Overall, 96 (4.4%) samples were anti-HBc reactive, indicating exposure to HBV infection. Eighty-six (4.0%) had evidence of past infection, and 10 (0.5%) were reactive for HBsAg, indicating a current infection. Current HBV infection was higher among heterosexuals (1.1%, 95% CI 0.5% to 2.2%) than among MSM (0.2%, 95% CI 0.1% to 0.3%), whereas past infection was similar between the two groups (4.6%, 95% CI 3.2% to 6.4%, and 3.7%, 95% CI 2.9% to 4.8%, respectively; table 2). In the multivariable analysis, evidence of past or current HBV infection was significantly higher in all age groups compared with the age group of 16–25 years (RRR 2.84, 95% CI 1.48 to 5.42 for age 26–35 years; RRR 7.23, 95% CI 3.71 to 14.07 for age 36–45 years; and RRR 9.28, 95% CI 4.33 to 19.89 for age 46+ years), and higher among those of black African ethnicity (RRR 8.49, 95% CI 4.04 to 17.85) and other white ethnicity (RRR 2.07, 95% CI 1.08 to 3.96) compared with those of white British/Irish ethnicity (table 2 and figure 1A).
Figure 1.
Adjusted multinomial logistic regression results: RRRs and 95% CIs of (A) current or past infection with hepatitis B and (B) evidence of immunity to hepatitis B, by demographic and risk factors. MSM, men who have sex with men; RRR, relative risk ratio.
HBV immunisation
Overall, 566 (26.1%) samples had serological evidence of immunity due to immunisation (HBsAg−, anti-HBc− and anti-HBs+). Evidence of immunisation was higher among MSM than among heterosexuals (29.9%, 95% CI 27.7% to 32.3%, vs 17.4%, 95% CI 14.6% to 20.4%). In the multivariable analysis, evidence of immunisation was lower among heterosexuals than among MSM (RRR 0.66, 95% CI 0.50 to 0.86), higher among those of other (RRR 1.40, 95% CI 1.03 to 1.90) or other white (RRR 1.57, 95% CI 1.16 to 2.12) ethnicity compared with white British/Irish ethnicity, higher in the 26–35 year age group compared with the 16–25 year age group (RRR 1.40, 95% CI 1.12 to 1.76) and lower among those with one or two to five new partners in the past 12 months compared with those with no new partners (RRR 0.45, 95% CI 0.25 to 0.79, and RRR 0.51, 95% CI 0.30 to 0.88, respectively; table 2 and figure 1B).
Susceptibility
Overall, 1510 (69.5%) individuals tested negative for all HBV markers and were therefore classed as susceptible to HBV. Susceptibility was lower among MSM than among heterosexuals (66.1%, 95% CI 63.7% to 68.5%, vs 77.0%, 95% CI 73.6% to 80.1%; table 2).
Discussion
Our study found that 66.1% of MSM and 77.0% of heterosexuals were susceptible to HBV infection, and only 29.9% of MSM and 17.4% of heterosexuals had serological evidence of immunisation. Current infection was 1.1% in heterosexuals and 0.2% in MSM, and highest among people of black African ethnicity of any orientation (3.3%). Evidence of past infection was low at 4.0% and highest in those of black African ethnicity (14.4%). Evidence of immunisation and past infection varied by age, sexual orientation, ethnicity and HIV testing history.
Despite low levels of vaccination, prevalence of HBV current infection in MSM was low at 0.2%, similar to previous UK studies which included both HIV infected and HIV uninfected men (0%–2%)18 19 and lower than the 0.5% WHO target for vaccinated cohorts.9 Among heterosexuals, however, prevalence of current infection was 1.1%, higher than reported in previous studies of sexual health attendees (0.4%–0.9%)19–21 and prevalence surveys among PWIDs,12 22 suggesting missed opportunities in global and UK vaccination programmes.
Most current infected cases among heterosexuals were of black African ethnicity, and current infection was within the range of recently reported prevalence for this population (2.5%–6.6%).23 24 Past infection was highest among those of black African ethnicity in both crude and adjusted analysis. Population-based HBV vaccination programmes were first implemented in the early 1980s in areas of high endemicity, and by 2009 had been implemented by 177 countries as part of their national infant immunisation programmes, with estimated HBsAg prevalence in the WHO Africa region falling to 6.1%.25–28 Some individuals in our study may have acquired their infection before the introduction of universal vaccination in countries of origin, whereas infections in younger age groups may be due to programme coverage issues in countries of origin.28 Overall, past infection increased with increasing age group, which is likely associated with past exposure during time periods when the background prevalence of HBV was higher, both in the UK and in other countries of birth.
Among heterosexuals 17.4% had evidence of immunisation, compared with 29.9% among MSM. As there is no nationally commissioned programme of HBV vaccination for higher-risk heterosexuals in the UK, this figure likely represents a mix of infant and adolescent vaccination of those born overseas, and targeted immunisation in the UK for those identified as high risk.
In the multivariable analysis, evidence of immunisation was higher among those who had previously tested for HIV and higher among MSM, both of which are as expected due to the UK HBV vaccination programme in sexual health services targeting MSM and those at higher sexual exposure risk. Age was also associated with vaccination, with 26–35 year olds having higher chance of vaccination than 16–25 year olds, which would be expected as older individuals have had longer to access services, although there was no clear age gradient. There were no clear associations between behavioural risk factors and evidence of immunisation on multivariable analysis, which is surprising as behavioural risk is an indication for immunisation. However, this may be a function of our study population, which comprises higher-risk individuals who do not routinely access sexual health services or perceive themselves to be at risk, both of which are required for immunisation to occur.
Evidence of immunisation was also higher in those of other and other white ethnicities, whereas those of black African ethnicity had levels of evidence of immunisation similar to those of white British/Irish ethnicity. These findings may reflect variation in the coverage and implementation of universal vaccination programmes in the countries of origin of those born overseas.
The high level of HBV susceptibility in MSM (66.1%) and the low levels of serological evidence of immunisation in MSM (29.9%) is concerning as there are national guidelines recommending testing and vaccination of all MSM attending sexual health services.6–8 This suggests suboptimal delivery of the vaccination programme and missed opportunities for vaccination, particularly as over 80% of the MSM study population had previously tested for HIV and so were considered at risk of blood borne viruses and had contact with services. However, another recent seroepidemiological analysis of MSM attending sexual health clinics found a much higher prevalence of anti-HBs at 77% (unpublished data), supporting the hypothesis that our HIV self-sampling population may be a distinct population of MSM who are less likely to access sexual health services. Home sampling started in 2016, so our study population may have previously tested for HIV using self-sampling services rather than attending sexual health services.
Compared with traditional services, those using online self-sampling options tend to be younger, as shown by the age distribution of our samples.16 Online self-sampling also provides an alternative for those unable or reluctant to use clinic-based services due to stigma, inconvenience or perceived lack of confidentiality.29 Our findings identify a need to improve vaccine uptake among this population who may not be aware of their risk of HBV infection or eligibility for vaccination. This is more prominent during the COVID-19 pandemic, when a shift in access to online sexual health services, a sharp decline in hepatitis immunisations and potential for exacerbating existing inequalities has been observed.30 Mitigation requires tailored messaging and communications for patients, and a rethink by providers of how online sexual health services are delivered to ensure higher-risk and vulnerable individuals are identified and signposted for immunisation.
Limitations
This study used samples from archived HIV-negative sera from a self-sampling HIV test service. Service users were self-selecting and are likely to represent a higher-risk population who do not attend sexual health services and perceive themselves to be at risk of HIV infection. There are limited data available to characterise the population, and no country of birth or migration status data which would help to interpret findings regarding differences by ethnicity. All risk and demographic information was self-reported and may be affected by reporting bias. Anti-HBs reactivity in the absence of anti-HBc reactivity was interpreted as evidence of immunity due to immunisation, but this does not always indicate that an individual has been immunised; without quantitative data on anti-HBs levels and the number of vaccine doses received, reactivity alone is insufficient to establish whether an individual has protective levels of anti-HBs.
Conclusions
Among MSM users of a self-sampling HIV service, evidence of immunity by immunisation to HBV infection was low, indicating suboptimal offer and uptake of immunisation in this MSM population, despite national guidelines. Current HBV infection among heterosexuals was higher than that in MSM, suggesting missed opportunities for vaccination globally and in the UK. These findings highlight the importance of monitoring vaccine uptake in high-risk populations to inform vaccine programme improvements, and need for vaccine programme delivery and monitoring to take the expansion of remote sexual health services including home sampling services, accelerated by COVID-19 into consideration. Further work is needed to understand how to facilitate access to, and information about, HBV immunisation to those who do not routinely attend in-person sexual health services to sustain population immunity against HBV and to prevent outbreaks, and to reduce health inequalities for these at-risk groups. Questions regarding HBV immunisation should be considered when setting up behavioural surveillance studies within high-risk communities.
Key messages.
Among HIV-negative MSM and heterosexual users of a self-sampling HIV service, evidence of immunisation to hepatitis B virus (HBV) infection was low and susceptibility to infection was comparatively high.
This suggests suboptimal delivery of HBV immunisation in sexual health services.
Consideration of how to facilitate access to HBV immunisation for those who do not routinely attend in-person sexual health services is required.
Acknowledgments
We acknowledge members of the National Institute for Health Research Health Protection Research Unit in BBSTI Steering Committee: Caroline Sabin, John Saunders, Catherine H Mercer, Gwenda Hughes, Greta Rait, Jackie Cassell, William Rosenberg, Tim Rhodes, Kholoud Porter, Samreen Ijaz and Sema Mandal.
Footnotes
Handling editor: Apostolos Beloukas
Contributors: SM came up with the initial concept. RS, RR and LL implemented and managed the study. SI and JL conducted the laboratory analysis. RR undertook the data analysis and had access to the complete dataset. RR drafted the initial manuscript. SM, SI, CS, RS and LL provided critical input into the manuscript. All authors reviewed and approved the final manuscript.
Funding: The research was funded by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Blood Borne and Sexually Transmitted Infections at UCL in partnership with PHE and in collaboration with the London School of Hygiene and Tropical Medicine. The views expressed are those of the authors and not necessarily those of the NIHR, the Department of Health and Social Care or Public Health England. The funders had no role in study design, data collection and analysis, or preparation of the manuscript. The final draft was circulated with the NIHR HPRU steering group prior to submission.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The datasets used and analysed during this study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This project was subject to an internal review by the Research Support and Governance Office in PHE to ensure that it was fully compliant the NHS Research Governance Framework for Health and Social Care (April 2005), and with all other current regulatory requirements. The review also covered all ethical considerations. The study was categorized as surveillance, and as no ethical issues had been identified it was decided that review by an ethics committee would not be necessary. This is in accordance with the revised guidance in the Governance Arrangements for Research Ethics Committees (GAfREC) that was released in September 2011.
References
- 1. Busch K, Thimme R. Natural history of chronic hepatitis B virus infection. Med Microbiol Immunol 2015;204:5–10. 10.1007/s00430-014-0369-7 [DOI] [PubMed] [Google Scholar]
- 2. Department of Health . Getting ahead of the curve: a strategy for combating infectious diseases (including other aspects of health protection). London: Department of Health; 2002. [Google Scholar]
- 3. Razavi-Shearer D, Gamkrelidze I, Nguyen MH, et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018;3:383–403. 10.1016/S2468-1253(18)30056-6 [DOI] [PubMed] [Google Scholar]
- 4. Hahné S, Ramsay M, Balogun K, et al. Incidence and routes of transmission of hepatitis B virus in England and Wales, 1995-2000: implications for immunisation policy. J Clin Virol 2004;29:211–20. 10.1016/j.jcv.2003.09.016 [DOI] [PubMed] [Google Scholar]
- 5. Public Health England . Acute hepatitis B (England): annual report for 2018, 2019. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/736145/hpr3118_hepB.pdf
- 6. NICE . Hepatitis B and C testing: people at risk of infection: Public health guideline [PH43], 2012. Available: https://www.nice.org.uk/guidance/ph43
- 7. Public Health England . Immunisation against infectious disease. London, UK; 2020: 143–4. [Google Scholar]
- 8. BASHH . BASHH national guidelines for the management of the viral Hepatitides; 2015.
- 9. World Health Organisation . Global health sector strategy on viral hepatitis 2016-2021: towards ending viral hepatitis; 2016.
- 10. Hill-Tout R, Mitchell H, Hughes G. Hepatitis B vaccine coverage in MSM: a South West London audit. BASHH Annual Conference 2016 2016. [Google Scholar]
- 11. Public Health England . GUMCAD STI surveillance system. Available: https://www.gov.uk/guidance/gumcad-sti-surveillance-system
- 12. Public Health England . Unlinked anonymous monitoring (UAM) survey of HIV and viral hepatitis among PWID: 2020 report; 2020.
- 13. Shankar AG, Mandal S, Ijaz S. An outbreak of hepatitis B in men who have sex with men but identify as heterosexual. Sex Transm Infect 2016;92:227. 10.1136/sextrans-2015-052490 [DOI] [PubMed] [Google Scholar]
- 14. Public Health England . Event: Hepatitis B vaccine supplies have improved - vaccine now available for all indications. NHS England Briefing Note; 2018.
- 15. Public Health England . National HIV self-sampling service: November 2018 to October 2019: 2020.
- 16. Barnard S, Free C, Bakolis I, et al. Comparing the characteristics of users of an online service for STI self-sampling with clinic service users: a cross-sectional analysis. Sex Transm Infect 2018;94:377–83. 10.1136/sextrans-2017-053302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. aidsmap N . PrEP demand in England is rapidly accelerating – and most will want to join the trial, 2017. Available: https://www.aidsmap.com/news/aug-2017/prep-demand-england-rapidly-accelerating-and-most-will-want-join-trial [Accessed 11 May 2021].
- 18. McMillan A. The changing prevalence of hepatitis B virus infection among men who have sex with men who attended a sexually transmitted infections clinic in Edinburgh, Scotland between 1989 and 2003. Int J STD AIDS 2006;17:539–42. 10.1258/095646206778145613 [DOI] [PubMed] [Google Scholar]
- 19. Roy KM, Goldberg DJ, Wilson K, et al. Vaccination induced immunity to the hepatitis B virus among high-risk groups in Glasgow 1993-2001: evaluating the effectiveness of the United Kingdom's selective immunisation policy. Scott Med J 2008;53:13–17. 10.1258/RSMSMJ.53.4.13 [DOI] [PubMed] [Google Scholar]
- 20. Price H, Salimee S, Coelho D. Prevalence of hepatitis B and hepatitis C in a UK genitourinary medicine clinic. Int J STD AIDS 2017;28:238–41. 10.1177/0956462416641528 [DOI] [PubMed] [Google Scholar]
- 21. Kawsar M, Goh BT. Hepatitis B virus infection among Chinese residents in the United Kingdom. Sex Transm Infect 2002;78:166–8. 10.1136/sti.78.3.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Public Health England . Unlinked anonymous monitoring (UAM) survey of HIV and viral hepatitis among PWID: 2019 report; 2019.
- 23. Hopkins MJ, Todd S, Beadsworth M, et al. Consistent high prevalence of undiagnosed blood-borne virus infection in patients attending large urban emergency departments in England. J Viral Hepat 2020;27:88–91. 10.1111/jvh.13197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cochrane A, Evlampidou I, Irish C, et al. Hepatitis B infection prevalence by country of birth in migrant populations in a large UK City. J Clin Virol 2015;68:79–82. 10.1016/j.jcv.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 25. Hsu HM, Chen DS, Chuang CH, et al. Efficacy of a mass hepatitis B vaccination program in Taiwan. Studies on 3464 infants of hepatitis B surface antigen-carrier mothers. JAMA 1988;260:2231–5. [PubMed] [Google Scholar]
- 26. McMahon BJ, Bulkow LR, Singleton RJ, et al. Elimination of hepatocellular carcinoma and acute hepatitis B in children 25 years after a hepatitis B newborn and catch-up immunization program. Hepatology 2011;54:801–7. 10.1002/hep.24442 [DOI] [PubMed] [Google Scholar]
- 27. World Health Organisation . World Health Organization Weekly epidemiological record No. 40 WHO position paper on hepatitis B 2 October; 2009.
- 28. Spearman CW, Afihene M, Ally R, et al. Hepatitis B in sub-Saharan Africa: strategies to achieve the 2030 elimination targets. Lancet Gastroenterol Hepatol 2017;2:900–9. 10.1016/S2468-1253(17)30295-9 [DOI] [PubMed] [Google Scholar]
- 29. Dodds C, Mugweni E, Phillips G, et al. Acceptability of HIV self-sampling kits (TINY vial) among people of black African ethnicity in the UK: a qualitative study. BMC Public Health 2018;18:499. 10.1186/s12889-018-5256-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Public Health England . COVID-19: impact on STIs, HIV and viral hepatitis; 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
sextrans-2021-055071supp001.pdf (87.4KB, pdf)
sextrans-2021-055071supp002.pdf (195.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The datasets used and analysed during this study are available from the corresponding author on reasonable request.