Abstract
Objective
We evaluated real-world treatment persistence and effectiveness at 1 year following initiation of IL-12/23 inhibitor ustekinumab or a tumour necrosis factor inhibitor (TNFi) for psoriatic arthritis (PsA).
Methods
PsABio (NCT02627768), a prospective, observational study, followed patients with PsA prescribed first-line to third-line ustekinumab or TNFi. Drug persistence, effectiveness (achievement of clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA) low disease activity (LDA)/remission and minimal disease activity/very low disease activity (MDA/VLDA)), and safety were assessed every 6 months. In addition to descriptive statistics, propensity score (PS)-adjusted comparisons across cohorts were performed.
Results
At 1 year, overall persistence was similar in the ustekinumab (n=317/438, 72.4%) and TNFi (n=321/455, 70.5%) groups. PS-adjusted HR (95% CI) for stopping/switching ustekinumab versus TNFi was 0.82 (0.60; 1.13). cDAPSA LDA (including remission)/remission was achieved in 55.9%/22.1% of ustekinumab-treated and 67.1%/31.7% of TNFi-treated patients; PS-adjusted ORs (95% CI) were 0.80 (0.57; 1.10) for cDAPSA LDA and 0.73 (0.49; 1.07) for remission. MDA/VLDA was achieved in 34.2%/11.9% of ustekinumab-treated and 43.1%/12.6% of TNFi-treated patients; PS-adjusted ORs (95% CI) were 0.89 (0.63; 1.26) for MDA and 0.90 (0.54; 1.49) for VLDA. The safety profiles were similar in both groups.
Conclusion
In the real-world PsABio Study, after 1 year of treatment, although unadjusted persistence was numerically slightly higher for ustekinumab versus TNFi and unadjusted effectiveness was numerically slightly higher for TNFi versus ustekinumab, the PS-adjusted comparisons demonstrated comparable overall persistence, effectiveness and safety for both modes of action in PsA.
Keywords: arthritis, psoriatic, biological therapy, tumor necrosis factor inhibitors
Key messages.
What is already known about this subject?
Although many randomised controlled trials have demonstrated efficacy and safety of biologics in psoriatic arthritis (PsA), real-world data comparing them, particularly over the long term, are lacking.
The PsABio real-world observational study provided comparative data on ustekinumab and tumour necrosis factor inhibitors (TNFi) in PsA treatment over 6 months and indicated similar efficacy.
What does this study add?
We provide 1-year analyses from the PsABio Study.
Drug persistence was similar at 1 year following treatment initiation (72.4% with ustekinumab and 70.5% with TNFi).
Drug effectiveness and safety were also similar for ustekinumab and TNFi at 1 year.
How might this impact on clinical practice or future developments?
Efficacy, safety and persistence are important considerations when making treatment decisions in PsA.
These 1-year results from the PsABio Study provide real-world evidence on factors which may impact treatment selection and help inform treatment decisions in clinical practice.
Video abstract.
Disclaimer: this video summarises a scientific article published by BMJ Publishing Group Limited (BMJ). The content of this video has not been peer-reviewed and does not constitute medical advice. Any opinions expressed are solely those of the contributors. Viewers should be aware that professionals in the field may have different opinions. BMJ does not endorse any opinions expressed or recommendations discussed. Viewers should not use the content of the video as the basis for any medical treatment. BMJ disclaims all liability and responsibility arising from any reliance placed on the content.
Introduction
Psoriatic arthritis (PsA) is a chronic immune-mediated disease, affecting approximately 20%–30% of patients with psoriasis.1 2 Patients may present with various musculoskeletal and other manifestations such as arthritis, enthesitis, dactylitis, spondyloarthritis, and skin and nail disease.1
Treatment options for PsA include non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids and disease-modifying antirheumatic drugs (DMARDs): conventional synthetic DMARDs; targeted synthetic DMARDs and biological DMARDs (bDMARDs).3 As the interleukin (IL)-12, IL-23 and IL-17 axes are critical pathways in the pathogenesis of PsA,4–6 bDMARDs directed against IL-12/IL-23 (p40), IL-23 (p19) and IL-17A, as well as tumour necrosis factor inhibitors (TNFi), have been shown to be effective.6–8 Ustekinumab, a fully human IgG1 monoclonal antibody that inhibits IL-12/IL-23,9 was the first licensed non-TNFi bDMARD therapy in psoriasis and PsA and combines efficacy against disease activity in joints and skin with a favourable safety profile.7 10 11
Owing to the significant disease heterogeneity, number of available drugs and limited head-to-head clinical trials in PsA,12 13 treatment selection is challenging. Treatment persistence is important when managing patients who require long-term treatment, in whom poor adherence (the degree of conformity to treatment recommendations relating to dose and frequency) and poor persistence can lead to suboptimal outcomes.14 15 Research has shown that the main reasons for switching to a different biologic are lack of effectiveness and adverse events (AEs),16–19 with patients who switched subsequently recording lower response rates and drug persistence than with their initial bDMARD.16 Female sex, smoking,15 17 20 presence of comorbidities18 21 and higher number of prior therapies are factors associated with poor persistence.17 Adherence, an influencing factor for persistence,22 was found to be higher in patients with longer PsA duration (>9 years).23 24 One study reported that 1-year continuation and low disease activity were predictive of 12-year persistence, indicating that better initial treatment adherence may lead to long-term persistence.25
Data on comparisons of different treatment modes of action are lacking in PsA.19 A retrospective Swedish registry study with a maximum follow-up of 10.6 years demonstrated favourable persistence with ustekinumab versus adalimumab across treatment lines.26
Six-month data from the prospective, observational PsABio cohort study of ustekinumab and TNFi treatment in patients with PsA indicated that later line of treatment, female sex and comorbidities as well as baseline disease impact, high clinical disease activity, and chronic widespread pain were shown to negatively influence treatment response.27
Here we present data on persistence, the primary outcome of PsABio, as well as clinical effectiveness, disease impact and safety after 1 year of follow-up.
Methods
Study design
PsABio (NCT02627768) is an observational, multinational study of patients with PsA treated with first-line to third-line ustekinumab or a TNFi by their rheumatologist, reflecting real-world practice. The study duration per participant was up to 3 years, with follow-up twice yearly. This 1-year analysis reports the first PsABio comparative drug persistence data, extended effectiveness outcomes regarding achievement of LDA or remission using clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA) definitions and minimal disease activity/very low disease activity (MDA/VLDA) as well as the patient-reported 12-item Psoriatic Arthritis Impact of Disease (PsAID-12) measure, and safety data.
Patients
Adults with PsA, who required ustekinumab or any approved TNFi (including biosimilars; online supplemental table S1) as first-line, second-line or third-line treatment, were included.
annrheumdis-2021-221640supp002.pdf (1.2MB, pdf)
Assessments
Persistence
Treatment persistence was defined as the time between initiation of bDMARD until last dose plus one dispensing interval or stop/switch to another bDMARD, or study withdrawal. For calculation of average persistence, data cut-off date for patients remaining on initial treatment was included.
cDAPSA and MDA/VLDA
cDAPSA were calculated based on the sum of four components: tender joint count for 68 joints (TJC68,), swollen joint count for 66 joints (SJC66) patient global assessment and patient pain, with scores ≤14 and ≤4 denoting cDAPSA LDA and remission, respectively.28 29 MDA and VLDA were based on attaining five and seven, respectively, out of the following seven domain cut-offs: TJC68≤1; SJC66≤1; Leeds Enthesitis Index ≤1; skin involvement assessed as body surface area (BSA) ≤3%; Health Assessment Questionnaire Disability Index (HAQ-DI) score ≤0.5; patient global assessment ≤20 (Visual Analogue Scale (VAS) in mm); and patient pain VAS ≤15.30
Patient-reported disease impact measure PsAID-12
The PsAID-12 is a validated, self-administered, weighted questionnaire that assesses the impact of PsA on patients’ lives.31 Each question is answered using a numerical rating scale, from 0 (none/no difficulty/very well) to 10 (extreme/extreme difficulty/very poorly).
Safety
Details of AEs, serious AEs and AEs of special interest (for ustekinumab defined as malignancies, serious and opportunistic infections and serious neurological disorders) were collected from the first use of ustekinumab or a TNFi in the study. All AEs that started during initial and subsequent treatments in the risk window (defined as the time between treatment initiation and 91 days after treatment stop) were reported.
Statistical analyses
The sponsor (Janssen Pharmaceuticals NV, Beerse, Belgium) oversaw the development of the statistical plan, data validation and all statistical analyses.
Populations
The safety set included all patients with baseline and any available follow-up data. Analysis of persistence and effectiveness was based on the effectiveness set, comprising all patients with baseline data and any postbaseline effectiveness data up to the upper limit of the month 12 visit window, which is up to 15 months' follow-up (including patients who switched/stopped treatment due to AEs, lack of efficacy or other reasons). For patients whose last available assessment was earlier than the lower limit of the 12-month visit window, the end-point analysis used the last observation carried forward (LOCF).
Analyses
The analysis was exploratory. No predefined hypotheses were tested and no adjustment for multiplicity was applied. Observed values and changes from baseline of effectiveness outcomes (MDA/VLDA and cDAPSA LDA/remission) were summarised at each assessment time point. cDAPSA LDA always included remission and MDA always included VLDA. Between-group differences and changes over time were described using 95% CIs. Persistence for ustekinumab and TNFi was described by Kaplan-Meier statistics and log-rank test for the effectiveness set, as well as by relevant baseline subgroups.
In addition to the descriptive statistics, comparative analyses were performed to investigate the differences between treatment cohorts in terms of persistence and effectiveness, including propensity score (PS) adjustment for imbalanced baseline demographic and disease-related covariates. In these analyses, for patients who switched/stopped their initial treatment during the 12-month observation period, the LOCF effectiveness end points were imputed as non-responders for binary end points, or as showing no improvement from baseline for continuous end points.
Results
Patients
A total of 991 participants were enrolled between December 2015 and June 2018 at 92 sites in Belgium, France, Greece, Italy, the Netherlands, the Russian Federation, Spain and the UK. For this 1-year analysis, 893 patients were included in the effectiveness analysis set (ustekinumab n=438; TNFi n=455) and 927 patients in the safety set (ustekinumab n=457; TNFi n=470; online supplemental figure S1). Of the 438 patients receiving ustekinumab, 341 (77.9%) were on a 45 mg dose, 96 (21.9%) were on a 90 mg dose and 1 (0.2%) patient was on another dose.
annrheumdis-2021-221640supp001.pdf (125.4KB, pdf)
Demographics, baseline/clinical characteristics
Patients in the ustekinumab group were older, had more comorbidities and were more likely to have had previous bDMARD exposure, but fewer patients were on concurrent methotrexate (MTX) and NSAIDs than those in the TNFi group. Ustekinumab was given as first-line treatment in 45.0%, second-line in 34.5% and third-line in 20.5% of patients versus 55.2%, 32.7% and 12.1% on TNFi, respectively (table 1). More patients in the ustekinumab versus TNFi group had severe skin involvement as assessed by BSA at baseline (table 2). Details regarding the types of previous bDMARD treatments are provided in online supplemental table S2.
Table 1.
Baseline demographics (effectiveness set; n=893)
| UST (n=438) | TNFi (n=455) | |
| Age years | 51.0 (12.5) (49.9; 52.2) | 48.5 (12.5) (47.3; 49.7) |
| Female, n (%) | 246 (56.2) (51.4; 60.9) | 248 (54.5) (49.8; 59.1) |
| BMI, kg/m2 | 28.6 (6.2) (27.9; 29.2) | 27.7 (5.3) (27.2; 28.2) |
| Disease duration since initial diagnosis, years | 7.5 (8.1) (6.7; 8.3) | 6.2 (6.6) (5.6; 6.9) |
| Line of bDMARD treatment, n (%) | ||
| First-line | 197 (45.0) (40.3; 49.8) | 251 (55.2) (50.5; 59.8) |
| Second-line | 151 (34.5) (30.0; 39.1) | 149 (32.7) (28.4; 37.3) |
| Third-line | 90 (20.5) (16.9; 24.6) | 55 (12.1) (9.2; 15.4) |
| csDMARD exposure, n (%) | ||
| Previous exposure | 384 (87.7) (84.2; 90.6) | 421 (92.5) (89.7; 94.8) |
| Ongoing exposure at baseline | 173 (39.5) (34.9; 44.2) | 251 (55.2) (50.5; 59.8) |
| MTX exposure ongoing at baseline | 131 (29.9) (25.7; 34.4) | 191 (42.0) (37.4; 46.7) |
| Weekly MTX dose, mg | 15.3 (5.5) (14.3; 16.3) | 15.0 (4.6) (14.3; 15.7) |
| Other treatments exposure ongoing at baseline, n (%) | ||
| NSAIDs | 240 (54.8) (50.0; 59.5) | 313 (68.8) (64.3; 73.0) |
| Glucocorticosteroids | 143 (32.6) (28.3; 37.3) | 156 (34.3) (29.9; 38.8) |
| Comorbidities present, n (%) | 301 (68.7) (64.1; 73.0) | 277 (60.9) (56.2; 65.4) |
| Cardiovascular disease/ metabolic syndrome* |
184 (42.0) (37.3; 46.8) | 162 (35.6) (31.2; 40.2) |
| Anxiety or panic disorders | 18 (4.1) (2.5; 6.4) | 18 (4.0) (2.4; 6.2) |
| Depression | 40 (9.1) (6.6; 12.2) | 29 (6.4) (4.3; 9.0) |
| GI disease or medical history of IBD | 55 (12.6) (9.6; 16.0) | 49 (10.8) (8.1; 14.0) |
| FiRST score suggestive of chronic widespread pain (scores ≥5) | 163 (39.0) (34.3; 43.9) | 126 (29.4) (25.2; 34.0) |
Data are mean (SD) (95% CI of the mean) unless otherwise stated; % is that of available data. Variables in bold indicate non-overlapping 95% CI.
*Hypertension, myocardial infarction, congestive heart failure, stroke or transient ischaemic attack, peripheral vascular disease, hyperlipidaemia, type 1 or type 2 diabetes or angina pectoris.
bDMARD, biologic disease-modifying antirheumatic drug; BMI, body mass index; csDMARD, conventional synthetic disease-modifying antirheumatic drug; FiRST, Fibromyalgia Rapid Screening Tool; GI, gastrointestinal; IBD, inflammatory bowel disease; MTX, methotrexate; NSAID, non-steroidal anti-inflammatory drug; TNFi, tumour necrosis factor inhibitor; UST, ustekinumab.
Table 2.
PsA clinical characteristics at baseline (effectiveness set)
| PsA characteristics | UST (n=438) | TNFi (n=455) |
| Psoriasis BSA, n (%) | ||
| Clear/almost clear skin | 102 (28.7) (24.1; 33.7) | 116 (33.0) (28.1; 38.1) |
| <3% but not clear/almost clear skin | 34 (9.6) (6.7; 13.1) | 53 (15.0) (11.5; 19.2) |
| 3‒10% | 124 (34.9) (30.0; 40.1) | 131 (37.2) (32.2; 42.5) |
| >10% | 95 (26.8) (22.2; 31.7) | 52 (14.8) (11.2; 18.9) |
| Axial involvement* – pure or combined with peripheral, n (%) | 153 (35.8) (31.3; 40.6) | 166 (37.4) (32.9; 42.1) |
| Oligoarticular†, n (%) | 96 (22.5) (18.6; 26.7) | 129 (29.1) (24.9; 33.5) |
| Polyarticular‡, n (%) | 286 (67.0) (62.3; 71.4) | 283 (63.7) (59.1; 68.2) |
| SJC66 | 5.9 (8.2) (5.1; 6.8) | 5.8 (7.5) (5.1; 6.6) |
| TJC68 | 12.5 (12.7) (11.2; 13.8) | 11.0 (10.5) (9.9; 12.0) |
| cDAPSA, n (%) | 30.6 (20.2) (28.5; 32.7) | 29.3 (18.6) (27.3; 31.2) |
| Remission | 10 (2.8) (1.3; 5.1) | 7 (2.0) (0.8; 4.0) |
| Low | 36 (10.1) (7.1; 13.6) | 39 (11.0) (7.9; 14.7) |
| Moderate | 141 (39.4) (34.3; 44.7) | 149 (41.9) (36.7; 47.2) |
| High | 171 (47.8) (42.5; 53.1) | 161 (45.2) (40.0; 50.6) |
| MDA§, n (%) | 16 (4.3) (2.5; 7.0) | 18 (5.1) (3.0; 7.9) |
| VLDA, n (%) | 1 (0.3)(0.0; 1.4) | 2 (0.5) (0.1; 2.0) |
| Enthesitis¶, n (%) | 192 (47.8) (42.8; 52.8) | 204 (51.3) (46.2; 56.3) |
| Dactylitis**, n (%) | 74 (17.7) (14.1; 21.7) | 90 (21.8) (17.9; 26.1) |
| PsAID-12 total score | 5.8 (2.1) (5.5; 6.0) | 5.5 (2.1) (5.3; 5.7) |
| HAQ-DI | 1.1 (0.7) (1.1; 1.2) | 1.2 (0.7) (1.1; 1.2) |
Data are mean (SD) (95% CI of the mean) unless otherwise stated; % is that of available data. Variables in bold indicate non-overlapping 95% CI.
*Pure axial PsA is defined as having only axial involvement (presence of axial disease declared by the treating rheumatologist without requirement for imaging), while combined axial PsA includes axial involvement and at least one of the following: distal interphalangeal joint involvement, monoarticular or oligoarticular PsA, polyarticular PsA, and arthritis mutilans. 2.1% of patients in the UST group and 3.2% in the TNFi group had pure axial PsA with inflammatory back pain.
†Either TJC68 and SJC66 are both non-missing and patient has <5 swollen or <5 tender joint counts, or in case TJC68 and/or SJC66 are missing monoarticular or oligoarticular PsA is indicated by the investigator.
‡Either TJC68 and SJC66 are both non-missing and patient has ≥5 swollen and ≥5 tender joint counts, or in case TJC68 and/or SJC66 are missing polyarticular PsA is indicated by the investigator.
§MDA includes VLDA.
¶Enthesitis presence defined as Leeds Enthesitis Index ≥0.
**Dactylitis presence on assessment of hands and feet.
BSA, body surface area; cDAPSA, clinical Disease Activity Index for Psoriatic Arthritis; HAQ-DI, Health Assessment Questionnaire Disability Index; MDA, minimal disease activity; PsA, psoriatic arthritis; PsAID-12, 12-item Psoriatic Arthritis Impact of Disease; SJC66, swollen joint count for 66 joints; TJC68, tender joint count for 68 joints; TNFi, tumour necrosis factor inhibitor; UST, ustekinumab; VLDA, very low disease activity.
Persistence
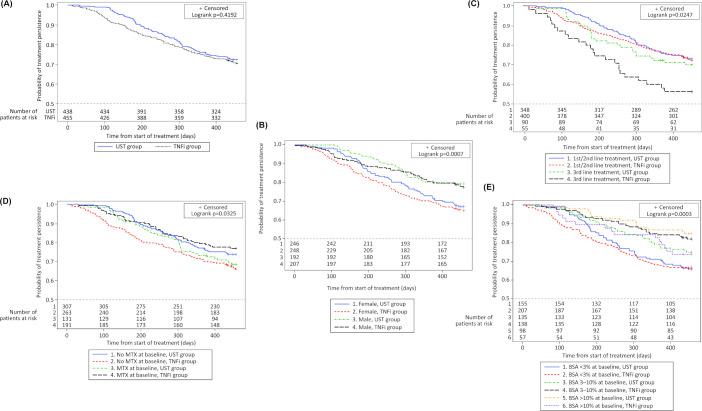
Persistence on ustekinumab and TNFi was similar at 1 year (±3 months) (figure 1A), with 72.4% of ustekinumab-treated and 70.5% of TNFi-treated patients remaining on their initial treatment. Patients stopped/switched treatment predominantly due to lack of effectiveness (ustekinumab 76.9%; TNFi 69.4%) or safety/AEs (ustekinumab 12.4%; TNFi 28.4%); others switched due to patient’s/physician’s preference, access to the drug or for guideline reasons. The PS-adjusted Cox persistence analysis confirmed the observed finding: ustekinumab versus TNFi HR (95% CI) for stopping/switching bDMARD was 0.82 (0.60; 1.13). The overall observed mean time on drug was 13.1 months (SD 3.5) for patients receiving ustekinumab versus 12.7 months (SD 4.2) for patients receiving a TNFi (a breakdown of treatment durations for individual TNFi is provided in online supplemental table S3).
Figure 1.
Kaplan-Meier plots of treatment persistence with ustekinumab versus TNFi (A) Overall, (B) By sex, (C) By treatment line, (D) By presence/absence of methotrexate and (E) By extent of skin involvement at baseline. BSA, body surface area; MTX, methotrexate; TNFi, tumour necrosis factor inhibitor; UST, ustekinumab.
Gender
Overall, as well as within both treatment cohorts, shorter drug persistence was observed in women than men (figure 1B). Comparing the treatment cohorts by means of a PS-adjusted Cox persistence analysis, no interaction was observed of the factor sex and the treatment cohort.
Axial involvement
PS-adjusted Cox analysis showed no difference in persistence between ustekinumab versus TNFi (HR: 0.83 (95% CI 0.50; 1.38)) for patients with axial involvement (defined as presence of axial disease declared by the treating rheumatologist without requirement for imaging) at baseline.
bDMARD line
Although the PS-adjusted Cox proportional hazard model did not show an overall significant interaction between the treatment lines and the treatment cohorts, the Kaplan-Meier graphs clearly showed better drug persistence in patients with first-line/second-line treatment than in patients with third-line treatment, with TNFi third-line treatment being associated with numerically shorter persistence than all other lines including ustekinumab third-line treatment (figure 1C).
Monotherapy
The observed better persistence on ustekinumab monotherapy versus TNFi monotherapy (figure 1D) was confirmed in the PS-adjusted Cox persistence analysis that showed a ustekinumab versus TNFi HR (95% CI) of 0.61 (0.42; 0.90). In patients co-treated with MTX, the observed ustekinumab and TNFi difference in persistence was not confirmed in the PS-adjusted Cox model (HR: 1.37; 95% CI 0.83; 2.26). There was no notable difference in the mean weekly MTX dose between ustekinumab and TNFi treatment groups (15.3 mg (SD 5.5) and 15.0 mg (SD 4.6), respectively).
Skin involvement
In the observed analysis, patients with more skin involvement at baseline persisted longer on their biologic than those with less skin involvement, in particular on ustekinumab (figure 1E). This was partly confirmed in the PS-adjusted Cox persistence analysis that showed a trend (p=0.0632) towards an interaction between the factor skin involvement and the treatment cohort, with longer persistence on ustekinumab in patients with baseline BSA >10% (HR: 0.41; 95% CI 0.19; 0.89).
Effectiveness
The observed proportion of patients achieving cDAPSA LDA/remission at 1 year was 55.9%/22.1% for the ustekinumab group and 67.1%/31.7% for the TNFi group; PS-adjusted ORs (95% CI) for ustekinumab versus TNFi were 0.80 (0.57; 1.10) for cDAPSA LDA and 0.73 (0.49; 1.07) for cDAPSA remission. Across all lines of treatment, the observed proportion of patients achieving MDA/VLDA was 34.2%/11.9% in the ustekinumab group and 43.1%/12.6% in the TNFi group (figure 2); PS-adjusted ORs (95% CI) for ustekinumab versus TNFi treatment were 0.89 (0.63; 1.26) for MDA and 0.90 (0.54; 1.49) for VLDA. The proportion of patients on ustekinumab or TNFi who achieved MDA at 6 months and 12 months is shown in figure 3.
Figure 2.
Disease outcomes at month 12 for patients with PsA receiving ustekinumab or TNFi. *Main (solid) bar represents cDAPSA LDA (including remission; cDAPSA ≤13) and inset (hashed) bar represents cDAPSA remission ≤4. †Main (solid) bar represents MDA (including VLDA) and inset (hashed) bar represents VLDA. cDAPSA, clinical disease activity in psoriatic arthritis; LDA, low disease activity; MDA, minimal disease activity; PsA, psoriatic arthritis; TNFi, tumour necrosis factor inhibitor; VLDA, very low disease activity.
Figure 3.
Proportion of patients achieving MDA at month 6 (observed) and month 12 (LOCF) and PS-adjusted ORs. *The 6-month PS-adjusted OR 95% CI are from the 6-month analysis. LOCF, last observation carried forward; MDA, minimal disease activity; mo, month; obs, observed; PS, propensity score.
PsAID-12
From baseline to 1 year, both treatments improved disease impact measured by PsAID-12 (total and individual domain scores) (figure 4), with the majority of the improvement occurring by month six in both cohorts. PS-adjusted treatment comparison between the ustekinumab and TNFi groups showed similar improvement in total PsAID-12 (regression coefficient (0.14, 95% CI −0.22; 0.51), and in individual domains, except skin problems, where more improvement was observed with ustekinumab than TNFi (−0.55, 95% CI −1.04; −0.06). Within both groups, improvements in PsAID-12 and HAQ-DI showed moderate/strong positive correlation (ustekinumab: r=0.63, TNFi: r=0.70). Non-clinical aspects of PsAID-12, for example, difficulties participating in social activities and overall coping, improved with both treatments (online supplemental table S4).
Figure 4.
Mean PsAID-12 overall and domain scores at baseline and 1 year with ustekinumab (n=438) and TNFi (n=455). UST: mean (95% CI) total score improved from 5.8 (5.5; 6.0) at baseline to 3.9 (3.6; 4.1) at 6 months and 3.7 (3.4; 3.9) at 1 year. TNFi: mean (95% CI) total score improved from 5.5 (5.3; 5.7) at baseline to 3.4 (3.2; 3.7) at 6 months and 3.1 (2.9; 3.4) at 1 year. LOCF, last observation carried forward; PsA, psoriatic arthritis; PsAID-12, 12-item Psoriatic Arthritis Impact of Disease; TNFi, tumour necrosis factor inhibitor; UST, ustekinumab.
Safety
At least one AE was reported for 24.4% of all patients receiving ustekinumab and 28.7% of patients receiving a TNFi, with 4.5% and 3.4%, respectively, reporting at least one serious AE. Three patients reported at least one serious infection in both treatment groups; there were three cases of pneumonia in patients receiving a TNFi and one case each of cellulitis, skin infection and staphylococcal bacteraemia in the ustekinumab group. A similar proportion of patients reported malignancies (excluding non-melanoma skin cancer; ustekinumab: n=4; TNFi: n=3, all single events) within the first year. Non-melanoma skin cancer was reported in two ustekinumab-treated and two TNFi-treated patients. Cardiovascular AEs were reported by two ustekinumab-treated and six TNFi-treated patients over 1 year but none were major and all were arrhythmias. Of note, all but two patients experiencing cardiovascular AEs had a medical history of cardiovascular disease/metabolic syndrome. During the first year of the study, an unexplained sudden death occurred in one patient in the ustekinumab group, and one patient in the TNFi group died due to pneumonia (online supplemental table S5).
Discussion
The prospective PsABio study aims to provide comparative real-world data on treatment persistence of biologic therapy in patients with PsA. After 1 year of follow-up, drug persistence was similar for ustekinumab or a TNFi in the PS-adjusted analysis, although observed data showed slightly better persistence for ustekinumab versus TNFi. These results are in contrast to the results from recent retrospective database studies showing that patients with PsA who initiated IL-12/23 inhibitor treatment had significantly longer treatment persistence and lower discontinuation rates compared with those initiating a TNFi during 1 year follow-up32 and those initiating adalimumab during 10 years follow-up.26 Likewise, the subgroup of patients with PsA in the PSOLAR Study, a registry study of 12 095 patients with psoriasis, showed better drug persistence with ustekinumab versus TNFi.19 This difference in results of adjusted analyses between the PsABio Study and the other studies could be due to various reasons: prospective non-interventional study setting, as done here, is different from retrospective claims database or registry analysis; the ustekinumab population in the current study was heavily affected by comorbidities, chronic widespread pain, late lines of bDMARD treatment, which may have impacted drug persistence with ustekinumab in this prospective patient cohort versus the other studies, and these or additional non-assessed imbalances may not have been fully adjusted for. Also, in this study in PsA, active psoriasis was not required and many patients had clear or almost clear skin, potentially reducing the advantage of ustekinumab treatment compared with TNFi.
The current study also showed lower drug persistence in women versus men with both treatments. Third-line TNFi treatment was associated with more reduced persistence than all other lines including third-line ustekinumab treatment. This observation supports previous reports, and the strategy of changing the biologic treatment mode of action, instead of cycling through treatments with the same pathophysiological target.11 17 19
Minimal or no skin involvement was strongly associated with low persistence in both cohorts. Patients with the greatest skin involvement at baseline showed longer persistence in both treatment groups, although persistence with TNFi was shorter than with ustekinumab in patients with BSA >10%, which may indicate the importance of skin improvement for patients. This effect is also seen with a greater improvement in PsAID-12 score in patients with higher baseline BSA. These observations are consistent with other studies showing a relationship between skin involvement and treatment persistence in PsA. This is expected, as the burden of psoriasis can significantly impact morbidity, and patients’ health-related quality of life depends on successful treatment of skin symptoms.33
The differential importance of MTX co-therapy on persistence with ustekinumab versus TNFi demonstrated in this real-world study supports results from the long-term SPIRIT-H2H extension randomised controlled trial data.12 While ustekinumab persistence is independent of co-therapy with MTX, TNFi persistence without MTX is shorter than with MTX and shorter than ustekinumab with/without MTX. This may be interpreted as a function of several mechanisms: patients receiving a TNFi may develop neutralising antidrug antibodies when MTX is not given; with ustekinumab, the risk of such antidrug antibodies is described as minimal.34 Other reasons may include MTX co-therapy with TNFi being more effective for skin involvement and likely selection bias in this real-world study as more patients on TNFi versus ustekinumab were on MTX at baseline.
PS-adjusted treatment effectiveness (cDAPSA LDA/remission or MDA/VLDA) was not different for TNFi and ustekinumab at 6 months and 1 year although the observed proportions were higher with TNFi versus ustekinumab. Also, PsAID-12 scores improved in all domains between baseline and 1 year with both treatments.
Both ustekinumab and TNFi treatment have a favourable safety profile in this real-world study of patients with PsA presenting with several comorbidities. Although reported AEs and serious AE rates were similar for both groups, more patients in the TNFi group stopped/switched treatment due to AEs than in the ustekinumab group; at the same time more patients in the ustekinumab versus TNFi group stopped/switched due to lack of efficacy.
We did not evaluate outcomes in the individual dose groups of ustekinumab versus the TNFi group, as some patients received doses that were too high or too low relative to their body weight (in particular, obese patients weighing just over 100 kg). Moreover, some rheumatologists may have used a lower dose when the patient’s disease was better controlled or escalated the dose when disease activity was less well controlled; therefore, analysis of different dose groups may introduce bias. Similar complexities of dosing also apply to TNFi.
PsABio is the only prospective real-world study comparing biologics with different modes of action in patients with PsA. The prospective open design allows the analysis and publication of data as they accumulate, permitting early detection of differences. The study captures data from a real-world population across eight different countries, each with their own local guidelines and treatment preferences; data which will apply to routine patient care and management. The limitation is that the comparison between treatment cohorts had to be based on PS adjustment and not on randomisation, due to a probable selection bias in treatment choice.
This study has confirmed the strong impact of treatment line, gender and baseline extent of skin disease on persistence and demonstrated the effectiveness of ustekinumab or TNFi-based treatments in PsA, not only on physician-derived but also patient-reported outcomes, such as disease impact. The final 3-year data from the PsABio study may provide further insights, such as information about factors that may predict long-term persistence at an early stage of treatment.
Conclusion
Real-world results from the PsABio Study have demonstrated generally comparable drug persistence, efficacy and safety following 1 year of treatment with ustekinumab or a TNFi, after PS adjustment for counteracting imbalanced baseline characteristics caused by channelling bias. Patients in this study were more likely to remain on ustekinumab than TNFi when extensive skin disease was present and when MTX was not used as concomitant treatment. On unadjusted analysis, women had lower treatment persistence with both treatments versus men, indicating they may require more comprehensive multidimensional therapy.
Acknowledgments
The authors thank the investigators of all study sites—the primary investigators by study countries were: Kurt de Vlam, Marc Vanden Berghe, Marie-Joëlle Kaiser, Jan Lenaerts, Jiangang Qu, Silvana Di Romana, Johan Vanhoof (Belgium); Laure Gossec, René-Marc Flipo, Caroline Guillibert, Roland Chapurlat, Pascal Claudepierre, Bernard Combe, Arnaud Constantin, Fabienne Coury-Lucas, Philippe Goupille, Pascal Hilliquin, Frédéric Lioté, Christophe Richez, Jeremie Sellam, Eric Toussirot (France); Petros Sfikakis, Panagiotis Athanassiou, Dimitrios Boumpas; Alexandros Garyfallos, Panagiotis Georgiou, Athanasios Georgountzos, Dimitrios Kasimos, Gkikas Katsifis, Lazaros Sakkas, Prodromos Sidiropoulos, Panagiotis Vlachogiannopoulos, Dimitrios Vasilopoulos (Greece); Elisa Gremese, Marco Matucci Cerinic, Francesco Ciccia, Fabrizio Conti, Giovanna Cuomo, Rosario Foti, Enrico Fusaro, Giuliana Guggino, Florenzo Iannone, Luca Idolazzi, Giovanni Lapadula, Marta Mosca, Paolo Moscato, Roberto Perricone, Piercarlo Sarzi-Puttini, Carlo Francesco Selmi, Gabriele Valentini, Guido Valesini, (Italy); Michael Nurmohamed, Marc Bijl, Mihaela Gamala, Eduard Griep, Marc Kok, E.F.A. Leijten, Timothy Radstake, (Netherlands); Tatiana Korotaeva, Larisa Balykova, Elena Gubar, Elena Ilivanova, Irina Kushnir, Elena Loginova, Galina Lukina, Karine Lytkina, Elvira Otteva, Ruzana Samigullina, Natalia Sanina, Olga Uhanova (Russian Federation); Beatríz Joven-Ibáñez, Jaime Calvo Alén, Enrique Raya Álvarez, Eugenio Chamizo Carmona, Juan Cañete Crespillo, José Rodríguez Heredia, Ana Laiz, Julio Medina Luezas, Joaquin Maria Belzunegui Otano, Maria Consuelo Diaz-Miguel Perez, Jesús Rodríguez, Maria García Vivar (Spain); Stefan Siebert, Antoni T Y Chan, Easwaradhas Gladston Chelliah, Hector Chinoy, Lisa Dunkley, Deepak Jadon, Pauline Ho, Stephen Kelly, Ellie Korendowych, Jonathan Marks, Jonathan Packham, Tom Sheeran, Eleri Thomas (the UK). The authors also thank Cello Health MedErgy for drafting the initial version of the manuscript and providing medical writing support throughout its development.
Footnotes
Handling editor: David S Pisetsky
Twitter: @StefanSiebert1
Contributors: LG, SS, PB, JSS, TVK, EG, BJI, WN, PPS, KdV, ET, FL contributed to conceptualisation of the study; LG, SS, PB, TVK, EG, WN, PPS, KdV, MTN, ET, FL contributed to the development of study design, data curation and methodology; PB, ET, FL, MTN, KdV contributed to formal data analysis and validation of results; FL, WN provided funding acquisition and financial support for the project; LG, SS, TVK, EG, BJI, MTN, PPS, KdV, ET conducted the research; SS, EG, MTN, PPS, ET, FL planned, directed and coordinated research activity; BJI, MTN, PPS, KdV, EG provided resources and analysis tools; PB contributed to programming and implementation of computer programs; SS, ET, FL, MTN, PPS, WN, KdV, EG provided supervision of the research including mentorship; LG, SS, PB, BJI, ET, MTN, TVK, KdV, EG contributed to data visualisation and presentation; LG, PB, EG, WN, MTN, ET contributed to preparing and writing the manuscript. All authors reviewed, provided critical review at each stage and approved the final version of the manuscript. LG is the guarantor.
Funding: This study was sponsored by Janssen. Medical writing and editorial support were funded by Janssen.
Disclaimer: The study sponsor was involved in the study design; the collection, analysis, and interpretation of data; report writing, and preparation, review, and approval of the manuscript; and the decision to submit the manuscript for publication under the guidance of an advisory committee consisting of the authors of this manuscript. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests: LG reports research grants from: Amgen, Galapagos, Lilly, Pfizer, Sandoz, Sanofi; consulting fees from: AbbVie, Amgen, Bristol-Myers Squibb, Galapagos, Gilead, Janssen, Lilly, Novartis, Pfizer, Samsung Bioepis, Sanofi-Aventis, UCB. SS reports non-financial support from Janssen during the conduct of the study; grants from: Boehringer-Ingelheim, Bristol-Myers Squibb; personal fees from: AbbVie, Biogen, Novartis; grants and personal fees from: Amgen (previously Celgene), GlaxoSmithKline, Janssen, UCB; all outside the submitted work. PB is a full-time employee of Janssen and owns stock at Johnson & Johnson. KdV reports personal fees from Janssen. EG reports payment or honoraria from AbbVie, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Pfizer, Sanofi. BJI has nothing to disclose. TVK reports consulting fees from MCD, Pfizer, Janssen, BiOCAD, AbbVie, Novartis, Sandoz, Lilly. FL reports non-financial support, full-time employment and restricted share units from Janssen during the conduct of the study. WN is a full-time employee of and owns stock at Johnson & Johnson. MTN reports grants and non-financial support from Janssen during the conduct of the study; grants from: Bristol-Myers Squibb, Amgen, Pfizer; grants and personal fees from: AbbVie, Eli Lilly; all outside the submitted work. PPS reports non-financial support from Janssen during the conduct of the study; grants from: UCB; personal fees from: Merck Sharpe & Dohme; grants and personal fees from: AbbVie, Lilly, Pfizer, Novartis; all outside the submitted work. ET is a full-time employee of Janssen. JSS reports grants to his institution from: AbbVie, Astro, AstraZeneca, Janssen, Lilly, Merck Sharpe & Dohme, Pfizer, and Roche; providing expert advice for or had symposia speaking engagements with: AbbVie, Amgen, AstraZeneca, Astro, Bristol-Myers Squibb, Celgene, Celltrion, Chugai, Gilead, ILTOO Pharma, Janssen, Lilly, Merck Sharp & Dohme, Novartis-Sandoz, Pfizer, R-Pharm, Roche, Samsung, Sanofi, and UCB.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. Access to anonymised individual participant-level data will not be provided for this trial as it meets one or more of the exceptions described on https://yoda.yale.edu/ under “Data Use Agreement - Janssen Pharmaceuticals DUA”.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by East of Scotland Research Ethics Service (15/ES/0166) Participants gave informed consent to participate in the study before taking part.
References
- 1. Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. The Lancet 2018;391:2273–84. 10.1016/S0140-6736(18)30830-4 [DOI] [PubMed] [Google Scholar]
- 2. Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol 2019;80:251–65. 10.1016/j.jaad.2018.06.027 [DOI] [PubMed] [Google Scholar]
- 3. Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700–12. 10.1136/annrheumdis-2020-217159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cafaro G, McInnes IB. Psoriatic arthritis: tissue-directed inflammation? Clin Rheumatol 2018;37:859–68. 10.1007/s10067-018-4012-7 [DOI] [PubMed] [Google Scholar]
- 5. Ritchlin C. Navigating the diverse immune landscapes of psoriatic arthritis. Semin Immunopathol 2021;43:279–90. 10.1007/s00281-021-00848-x [DOI] [PubMed] [Google Scholar]
- 6. Thibodaux RJ, Triche MW, Espinoza LR. Ustekinumab for the treatment of psoriasis and psoriatic arthritis: a drug evaluation and literature review. Expert Opin Biol Ther 2018;18:821–7. 10.1080/14712598.2018.1492545 [DOI] [PubMed] [Google Scholar]
- 7. Sakkas LI, Zafiriou E, Bogdanos DP. Mini review: new treatments in psoriatic arthritis. focus on the IL-23/17 axis. Front Pharmacol 2019;10:872. 10.3389/fphar.2019.00872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Queiro R, Coto-Segura P. Ustekinumab in psoriatic arthritis: need for studies from real-world evidence. Expert Opin Biol Ther 2018;18:931–5. 10.1080/14712598.2018.1504919 [DOI] [PubMed] [Google Scholar]
- 9. Davari P, Leo MS, Kamangar F, et al. Ustekinumab for the treatment of psoriatic arthritis: an update. Clin Cosmet Investig Dermatol 2014;7:243–9. 10.2147/CCID.S50003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McInnes IB, Kavanaugh A, Gottlieb AB, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 2013;382:780–9. 10.1016/S0140-6736(13)60594-2 [DOI] [PubMed] [Google Scholar]
- 11. Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis 2014;73:990–9. 10.1136/annrheumdis-2013-204655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smolen JS, Sebba A, Ruderman EM, et al. Efficacy and safety of ixekizumab with or without methotrexate in biologic-naïve patients with psoriatic arthritis: 52-week results from SPIRIT-H2H study. Rheumatol Ther 2020;7:1021–35. 10.1007/s40744-020-00250-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ruyssen-Witrand A, Perry R, Watkins C, et al. Efficacy and safety of biologics in psoriatic arthritis: a systematic literature review and network meta-analysis. RMD Open 2020;6:e001117. 10.1136/rmdopen-2019-001117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murage MJ, Tongbram V, Feldman SR, et al. Medication adherence and persistence in patients with rheumatoid arthritis, psoriasis, and psoriatic arthritis: a systematic literature review. Patient Prefer Adherence 2018;12:1483–503. 10.2147/PPA.S167508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haddad A, Gazitt T, Feldhamer I, et al. Treatment persistence of biologics among patients with psoriatic arthritis. Arthritis Res Ther 2021;23:44. 10.1186/s13075-021-02417-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glintborg B, Østergaard M, Krogh NS, et al. Clinical response, drug survival, and predictors thereof among 548 patients with psoriatic arthritis who switched tumor necrosis factor α inhibitor therapy: results from the Danish nationwide DANBIO registry. Arthritis Rheum 2013;65:1213–23. 10.1002/art.37876 [DOI] [PubMed] [Google Scholar]
- 17. Stober C, Ye W, Guruparan T, et al. Prevalence and predictors of tumour necrosis factor inhibitor persistence in psoriatic arthritis. Rheumatology 2018;57:158–63. 10.1093/rheumatology/kex387 [DOI] [PubMed] [Google Scholar]
- 18. Ballegaard C, Højgaard P, Dreyer L, et al. Impact of comorbidities on tumor necrosis factor inhibitor therapy in psoriatic arthritis: a population-based cohort study. Arthritis Care Res 2018;70:592–9. 10.1002/acr.23333 [DOI] [PubMed] [Google Scholar]
- 19. Menter A, Papp KA, Gooderham M, et al. Drug survival of biologic therapy in a large, disease-based registry of patients with psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Eur Acad Dermatol Venereol 2016;30:1148–58. 10.1111/jdv.13611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fagerli KM, Kearsley-Fleet L, Watson KD, et al. Long-term persistence of TNF-inhibitor treatment in patients with psoriatic arthritis. data from the British Society for Rheumatology biologics register. RMD Open 2018;4:e000596. 10.1136/rmdopen-2017-000596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iannone F, Salaffi F, Fornaro M, et al. Influence of baseline modified rheumatic disease comorbidity index (mRDCI) on drug survival and effectiveness of biological treatment in patients affected with rheumatoid arthritis, spondyloarthritis and psoriatic arthritis in real-world settings. Eur J Clin Invest 2018;48:e13013. 10.1111/eci.13013 [DOI] [PubMed] [Google Scholar]
- 22. Burudpakdee C, Khan ZM, Gala S, et al. Impact of patient programs on adherence and persistence in inflammatory and immunologic diseases: a meta-analysis. Patient Prefer Adherence 2015;9:435–48. 10.2147/PPA.S77053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smolen JS, Gladman D, McNeil HP, et al. Predicting adherence to therapy in rheumatoid arthritis, psoriatic arthritis or ankylosing spondylitis: a large cross-sectional study. RMD Open 2019;5:e000585. 10.1136/rmdopen-2017-000585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Theander E, Husmark T, Alenius G-M, et al. Early psoriatic arthritis: short symptom duration, male gender and preserved physical functioning at presentation predict favourable outcome at 5-year follow-up. Results from the Swedish early psoriatic arthritis register (SwePsA). Ann Rheum Dis 2014;73:407–13. 10.1136/annrheumdis-2012-201972 [DOI] [PubMed] [Google Scholar]
- 25. Murray K, Turk M, Alammari Y, et al. Long-term remission and biologic persistence rates: 12-year real-world data. Arthritis Res Ther 2021;23:25. 10.1186/s13075-020-02380-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geale K, Lindberg I, Paulsson EC, et al. Persistence of biologic treatments in psoriatic arthritis: a population-based study in Sweden. Rheumatol Adv Pract 2020;4:rkaa070. 10.1093/rap/rkaa070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smolen JS, Siebert S, Korotaeva TV, et al. Effectiveness of IL-12/23 inhibition (ustekinumab) versus tumour necrosis factor inhibition in psoriatic arthritis: observational PsABio study results. Ann Rheum Dis 2021;80:1419–28. 10.1136/annrheumdis-2021-220263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schoels MM, Aletaha D, Alasti F, et al. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis 2016;75:811–8. 10.1136/annrheumdis-2015-207507 [DOI] [PubMed] [Google Scholar]
- 29. Aletaha D, Alasti F, Smolen JS. Disease activity states of the DAPSA, a psoriatic arthritis specific instrument, are valid against functional status and structural progression. Ann Rheum Dis 2017;76:418–21. 10.1136/annrheumdis-2016-209511 [DOI] [PubMed] [Google Scholar]
- 30. Coates LC, Helliwell PS. Defining low disease activity states in psoriatic arthritis using novel composite disease instruments. J Rheumatol 2016;43:371–5. 10.3899/jrheum.150826 [DOI] [PubMed] [Google Scholar]
- 31. Gossec L, de Wit M, Kiltz U, et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis 2014;73:1012–9. 10.1136/annrheumdis-2014-205207 [DOI] [PubMed] [Google Scholar]
- 32. Walsh JA, Cai Q, Lin I, et al. Treatment persistence and adherence among patients with psoriatic arthritis who initiated targeted immune modulators in the US: a retrospective cohort study. Adv Ther 2021;38:2353–64. 10.1007/s12325-021-01687-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kavanaugh A, Gottlieb A, Morita A, et al. The contribution of joint and skin improvements to the health-related quality of life of patients with psoriatic arthritis: a post hoc analysis of two randomised controlled studies. Ann Rheum Dis 2019;78:1215–9. 10.1136/annrheumdis-2018-215003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jullien D, Prinz JC, Nestle FO. Immunogenicity of biotherapy used in psoriasis: the science behind the scenes. J Invest Dermatol 2015;135:31–8. 10.1038/jid.2014.295 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2021-221640supp002.pdf (1.2MB, pdf)
annrheumdis-2021-221640supp001.pdf (125.4KB, pdf)
Data Availability Statement
No data are available. Access to anonymised individual participant-level data will not be provided for this trial as it meets one or more of the exceptions described on https://yoda.yale.edu/ under “Data Use Agreement - Janssen Pharmaceuticals DUA”.