Abstract
The purpose of this study was to investigate the effect of ultrasound-assisted enzymolysis on modified solubilization of yeast β-glucan and its related mechanism. The depolymerization effects of this system on the physicochemical properties and structural features of the degraded fragments were studied systematically. The structure and physicochemical properties of the samples showed that the solubility of yeast β-glucan achieved 75.35 % after modification; and ultrasonic enzymatic enhanced the degradation efficiency. The yeast β-glucan obtained after solubilization and modification owned better antioxidant activities. The yeast β-glucan particles become obviously smaller, sparsely dispersed in the aqueous solution and the stability was improved. In addition, the hydrogen bonds in yeast β-glucan native triple helix structure were partially broken. Moreover, the disruption of yeast β-glucan's original structure made it decreased thermostability and easier to dissolve in water. The atomic force microscope (AFM) imaging directly verified the branched-chain morphology of yeast β-glucan and the small-strand degradation fragments. Therefore, this research can provide a feasible and effective approach for improving solubility of water-insoluble yeast β-glucan to enlarge its food and biomedical applications.
Keywords: Yeast β-glucan, Ultrasound, Enzymolysis, Modification, Structural features, Depolymerization
1. Introduction
As the most abundant natural biopolymers in the world, yeast β-glucan has been widely studied for its unique physicochemical properties and biological activities, such as wound healing [1], antitumor [2], and immune enhancement [3]. Yeast β-glucan belongs to the class of β-glucan, and its structure consists of two distinct macromolecular components consisting of consecutively (1 → 3)-linked β-d-glucopyranosyl residues, with small numbers of (1 → 6)-linked branches and a minor component with consecutive (1 → 6)-linkages and (1 → 3)-branches [4]. It has received great attention in the fields of food and biomedicine because of its non-toxicity and biodegradability [5]. In addition, yeast β-d-glucan is widely used in the food industry as a thickener [6], emulsifying stabilizer [7] and fat substitute [8], because of its good water-holding, heat preservation, film-forming, and non-irritating properties. However, the intramolecular polyhydroxy groups interact to form a dense triple helix structure resulting in insolubility in water and organic solvents, such as ethanol, thereby seriously limiting its biomedical applications [9]. Consequently, it is essential to adopt modification measures to improve yeast β-glucan's water-solubility.
Nowadays, many physical and chemical approaches have been applied to increase solubility of β-glucan. However, these methods have a few drawbacks to some extent: physical methods have poor modification effect; chemical methods may cause difficult recovery and environmental pollution due to use of “non-green”acids and organic solvents. To solve this problem, extensive research has been performed over the past few years on developing effective technologies, especially simple, green and cost-effective strategies, to increase the water-solubility of yeast β-glucan.
Recently, there has been growing evidence that the simultaneous function of ultrasound and enzymes is an ideal method for polymer degradation. The complex polymeric structures of polymer transformed into smaller units with simpler branches and shorter chains after sonoenzymolysis reactions [10]. Effectiveness of this combination method is attributed to the improved the reaction rate and enzyme-substrate affinity. According to the thermodynamic results, sonoenzymolysis reactions require less energy than enzymolysis reactions [11]. Also, ultrasound depolymerizes the polymer substrate, which causes the resultant dispersed structures to be more conducive to enzyme action. Ultrasound induced cavitation disrupted YG aggregates to a coarse appearance, exposed internal structure enlarged the YG surface area [12]. Ultrasound assisted reactions are more attractive than the conventional reactions because of the improved mass transfer and reaction efficiency [11]. The facilitation of mass transfer mainly results from the ultrasonic cavitation. The mechanical effect of cavitation induced by the collage of microbubbles would uniform the mixture, showing notable enhancement of mass transfer. Under mild ultrasound conditions, oscillation and rupture of small-amplitude cavitation bubbles lead to the generation of shear forces and free radicals, which could favorably alter enzyme structures and cause the exposure of more active domains [13]. The advantages of ultrasound assisted enzymatic reactions may be summarized as follows: 1) it doesn’t introduce extra chemicals or additives; 2) it is simple and efficient; 3) it minimizes the reaction time; 4) the process does not dramatically alter the chemical structure, the properties of starch in particular [14].
Although some researches have reported that ultrasound could accelerate enzymatic reactions when combining with pectinase [10], cellulase [15], lipase [16], alpha amylase [17], and alcalase [18], there are only few reports available on ultrasound assisted β-glucanase catalyzed hydrolysis of yeast β-glucan extracted from Candida utilis. In the present work, ultrasound was introduced to act on yeast β-glucan enzymatic hydrolysis by β-glucanase. Some structural changes and physicochemical properties before and after modification of β-glucan were further investigated, helping to explore the mechanism of ultrasound assisted enzymatic process. Overall, this work explored a green and effective process for solubilizing modified β-glucan from Candida utilis.
2. Materials and methods
2.1. Materials
The β-glucan from Candida utilis with a glucan content of over 87.22% was provided by our research laboratory (Shanghai, China). β-glucanase (50 U/mg) was provided by Yuanye Bio-Technology Co., Ltd (Shanghai, China). All chemical reagents used were of analytical grade.
2.2. Sample pretreatment
β-glucanase and yeast β-glucan were dissolved in pH 5.0, 1 M citric acid-disodium hydrogen phosphate buffer respectively. β-glucanase was prepared to ultimate concentration of 10 mg/mL and yeast β-glucan solution was 5 mg/mL. Stored at 4 °C in a refrigerator for later use.
2.3. Degradation of β-glucan from Candida utilis catalyzed by β-glucanase
1 mL of β-glucanase samples was added to19 mL of β-glucan solution in conical flasks, which were placed in an electric heating constant temperature shaking water tank. In this study, a single-factor experimental design method was carried out to explore the effects of different reaction times and different reaction temperatures on the solubility of Candida utilis β-glucan. After incubation, the samples were immediately put in a boiling water bath at 100 °C for 3 min to inactivate the enzyme, and then taken out and placed in an ice water bath to cool.
2.4. Ultrasound synergistic enzymatic method of β-glucan from Candida utilis
The effects of ultrasonic intensity (10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%), ultrasonic time and temperature on the solubility of yeast β-glucan were studied with single factor experimental design method. 19 mL yeast β-glucan and 1 mL β-glucanase solution were mixed and added to the cylindrical glass reactor which was preheated to the required temperature. The ultrasonic probe was placed below the surface of the solution. Ultrasonic treatment was performed at the center of about 1 cm. The ultrasonic cell crusher JY92-IIDN (Ningbo, Ningbo Xinzhi Biological Technology Co., Ltd., China) applied in this research has a total power of 1800 W, a frequency of 22 kHz, a duty ratio of 1:1 (pulse time 1 s; intermittent time 1 s), and a probe diameter of 20 mm. During processing, the temperature probe was throughout submerged in the sample solution. Finally, the glass reactor was immediately immersed in a water bath of 100 °C boiling water for 5 min to inactivate the β-glucanase, then taken out and placed in an ice water bath for cooling.
2.5. Sonication of β-glucan from Candida utilis
The ultrasonic treatment system consisted of 19 mL yeast β-glucan solution and 1 mL citric acid-disodium hydrogen phosphate buffer (pH = 5.0). The degradation reactions were carried out at the temperature and time determined by the single factor experiment in 2.4.
2.6. Solubility of β-glucan from Candida utilis
The solubility of yeast β-glucan was measured by the aluminum box constant weight method. The pure aluminum box with lid was dried in oven at 105 °C to constant weight. It was heated for 60 min, taken it out and placed it in a desiccator to cool for 30 min, then repeatly weighed until the difference in mass between the previous does not exceed 2 mg. Yeast β-glucan was dissolved into deionized water with keeping stirring at 40 °C for 30 min. After centrifuging at 4000 rpm for 20 min, the obtained supernatant was taken in a dry and constant weight aluminum box and dried to constant weight at 105 °C, and the last constant weight value was retained. The solubility was calculated according to the following formula.
| Solubility (%) = W/(C × V) × 100 | (1) |
W (g): weight of the supernatant dried product.
V (mL): volume of the supernatant.
C (g/mL): initial solution concentration.
The expression of ultrasound intensity:
| I = P/V | (2) |
I (W/mL): intensity of ultrasound.
P (W): Ultrasonic output power.
V (mL): volume of the sample solution.
2.7. Characterization section of β-glucan from Candida utilis
2.7.1. Fourier transform infrared (FTIR) spectroscopy analysis
The FT-IR spectra of pectin samples were characterized on a spectrometer (Bruker, Germany). Samples were mixed with KBr at a ratio of 1:50 (w/w); and then ground and pressed into pellets for IR scanning in the range of 400–4000 cm−1 with 32 scans and the 2 cm−1 resolution. The obtained data were analyzed by OMNIC.
2.7.2. X-ray diffraction (XRD) analysis
Powder diffraction experiments were performed using an X-ray diffractometer (D/MAX 2200PC, Rigaku, Ltd, Japan). The powder pattern of pectin and prepared MP fractions were collected at 2θ diffraction angle from 5° to 80° (step size 0.02° 2θ, time per step: 5 s) in reflection mode. Spectra were evaluated with the MDI-Jade version 5.0.
2.7.3. Atomic-force microscopy (AFM) analysis
β-Glucan and prepared degradation fragments were dissolved in deionized water to concentrations of 1 mg/mL. After continuous stirring and incubating at 50 °C for 2 h, solutions were diluted to 10 μg/ mL; and then dropped onto a freshly cleaved mica substrate to be airdried under ambient pressure, temperature and humidity. AFM images of available samples were collected by an nGauge portable atomic force microscope (icspi, Canadian) and operated in a tapping mode in the air at room temperature and 50–60% humidity.
2.7.4. Scanning electron microscope analysis
The microstructure of β-glucan was observed on scanning electron microscope Hitachi SEM-25009(Hitachi, Japan. β-glucans were coated on the gold plate. It was analyzed under high vacuum conditions with 5 kV acceleration voltage and high vacuum conditions with a scanning electron microscope system, the image magnification is 2000 times.
2.8. Thermal stability analysis
2.8.1. Thermal gravimetric analysis (TGA)
The thermal decomposition profifiles of YG before and after modification were determined by a thermogravimetric analyzer (Pyris Diamond, USA). Solid samples were placed into aluminum crucibles with a cap with nitrogen as the purge gas. During all experiments, the temperature was increased from 30 °C to 550 °C at a constant heating rate of 15 °C/min, and a nitrogen flflow rate of 50 mL/min.
2.8.2. Differential scanning calorimetry (DSC)
Thermogravimetric Analyzer Q5000(TA, USA) was used for thermal performance of β-glucan samples. A amount of β-glucan sample was sealed in a standard aluminum crucible. During the test, the temperature was setted from 20 °C to 200 °C, the heating rate was 15 °C/min, the flow rate of nitrogen (N2) was 50 mL/min, and the thermal characteristic curve was observed.
2.9. Zeta potential measurement
The zeta potential (z, mV) of the Candida utilis β-glucan solution (0.5 mg/mL) was measured. Attractive and repulsive forces between colloidal particles play a signifificant role in the stability and physicochemical properties of the colloidal solution. The zeta potentials of solution higher than +30 mV or lower than − 30 mV is considered stable since it maintained the repulsive forces between particles during dispersion.
2.10. Antioxidant activity
2.10.1. Scavenging activity of 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radicals
The DPPH free radical scavenging activity of the samples was carried out according to a previous report with modifications [19]. To summarize, YGS samples were dissolved to obtain several solutions at different concentrations of 50, 100,150, 200, and 300 μg/mL. Ascorbic acid (VC) was used as a positive control. Then, 2 mL of the sample solutions were mixed with a DPPH ethanol solution (2 mL, 0.16 mmol/L). The mixture solution was placed under dark conditions for 40 min at room temperature. The absorbance of the reaction solution was determined at 517 nm using a 722S visible spectrophotometer (Shanghai Jinghua Technology Instrument Co., Ltd., China). The scavenging activity on DPPH radicals was calculated using the following equation.
| (3) |
where Ac is the absorbance of the control solution without sample;
Ai is the absorbance of the sample solution with DPPH radical solution;
Aj is the absorbance of the background solution without DPPH.
2.10.2. Scavenging activity of 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid ammonium salt (ABTS) radicals
The ABTS radical scavenging activity was measured using the method reported by Luo et al. [20] with slight modifications. In short, the ABTS radical mother liquor was diluted with PBS (10 mmol/L, pH 7.4) to an absorbance of 0.70 0 ± 0.020 at 734 nm. Next, several 1.0 mL samples with different concentrations of 0.1, 0.2, 0.5, 1.0, 2.0, and 5.0 mg/mL were added to ABTS radical solutions (3.0 mL). The mixture was shaken and placed for 10 min at room temperature. The absorbance was measured at 734 nm. Phosphate-buffered saline (PBS) was used as a blank control. The scavenging activity was estimated using the following formula.
| (4) |
where Ac is the absorbance of the control solution without sample;
Ai is the absorbance of the sample solution with ABTS;
Aj indicates the absorbance of the PBS solution with the sample solution.
2.10.3. Scavenging capacity of hydroxyl radicals
The hydroxyl radical scavenging activity was studied by a method modified from Li et al. [21]. Briefly, in 0.40 mL of ferrous sulfate solution (6 mmol/), 1 mL of hydrogen peroxide solution (8.8 mmol/L), 1 mL of salicylic acid solution (9 mmol/L) and 1.60 mL of distilled water were mixed and heated in a water bath at 37 °C for 15 min. Distilled water was used as a blank control. The absorbance value Ac was measured at a wavelength of 510 nm. Ai was the absorbance value measured by replacing 1.60 mL of distilled water with a certain concentration of polysaccharide solution. One milliliter of distilled water instead of hydrogen peroxide solution was used to determine the absorbance value Aj. VC solution as a positive control. Three parallel samples for each sample were used to calculate the scavenging rate of polysaccharides on hydroxyl radicals:
| (5) |
2.11. Statistical analyses
All data were recorded at least in triplicate. Differences were considered at the 95% confidence level (P ≤ 0.05). Data are presented as the mean ± SD (n = 3). One-way analysis of variance (ANOVA) was applied. Statistical analysis was implemented using IBM SPSS version 22.0 software.
3. Results
3.1. Effects of ultrasound on modification of Candida utilis β-glucan
3.1.1. Effects of ultrasonic intensity on the solubility of Candida utilis β-glucan
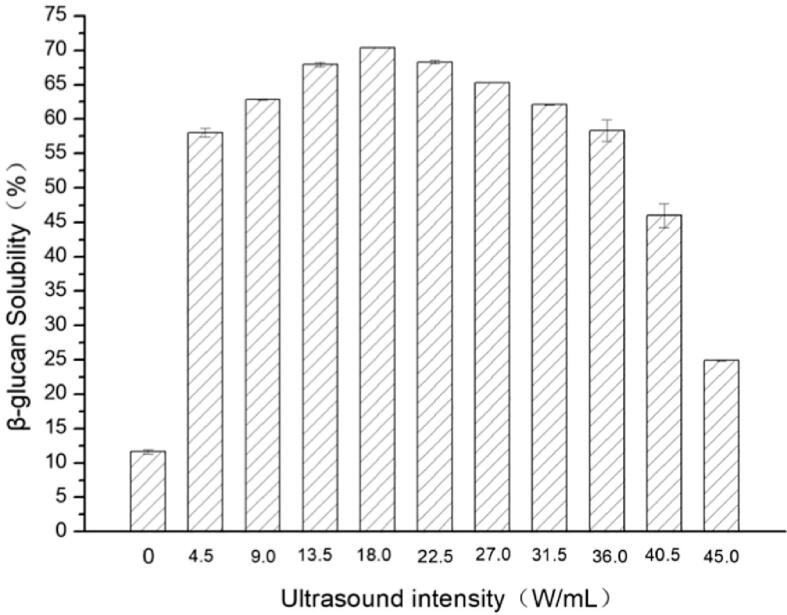
Fig. 1. showed the effect of 0–45 W/mL on the solubility of yeast β-glucan under different ultrasonic intensities. When the temperature was 30 °C and treated by the ultrasound for 10 min, it can be seen from the figure that the solubility of yeast β-glucan first increased and then decreased with the increase of ultrasonic intensity. The results were consistent with the Ultrasound-assisted pectinase process [22]. The maximum solubility of β-glucan was obtained when the ultrasonic intensity was 18.0 W/mL, and the solubility increased from 11.65 % to 70.42 % using a single enzymatic hydrolysis reaction for 10 min. When the ultrasonic intensity exceeded 18.0 W/mL, the solubility of the sonolysis reaction decreased. The enhanced solubility of the polymer enzymatic reaction in the ultrasonic field can be mainly attributed to three aspects. Firstly, the rupture of the ultrasonic cavitation bubble generated a certain intensity of shear force, which directly modify the molecular structure of the enzyme and improve the catalytic activity of the enzyme [23]. Secondly, ultrasound break macromolecular substances into smaller molecular structures, which is conducive to the formation of the reaction system [24]. Thirdly, the microjets generated by the ultrasonic cavitation effect have a certain homogenization effect on the reaction system, which promote the mass transfer process of the enzymatic reaction, enhance the collision frequency between the enzyme and the substrate, and improve the efficiency of the enzymatic reaction [25], [26]. However, from Fig. 1, the solubility of yeast β-glucan was decreased due to the excessively strong ultrasonication conditions. It was demonstrated that the increase of ultrasonic intensity promoted the decomposition process of yeast β-glucan substrate, whereas in this study, changes in β-glucanase in the ultrasonic field might affect yeast β-glucan.
Fig. 1.
The effect of ultrasonic intensity on the solubility of yeast β-glucan.
3.1.2. Effect of ultrasonic time on the solubility of Candida utilis β-glucan
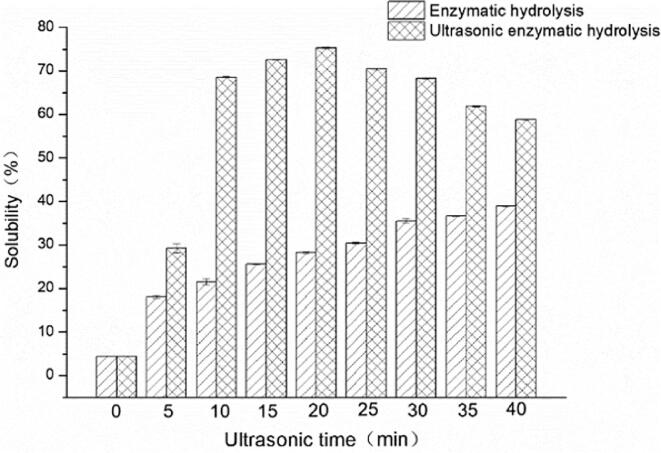
The solubility of yeast β-glucan under different ultrasonic time (0–40 min) was shown in Fig. 2, when the temperature at 30 °C and the ultrasonic intensity was 18 W/mL. The solubility of yeast β-glucan combined with ultrasonic treatment was significantly higher than that of enzymatic hydrolysis alone was illustrated. Ultrasound promoted the dissolution of yeast β-glucan within 20 min, and the solubility of yeast β-glucan reached a maximum of 75.35 % at 20 min. The solubility was increased by about 3 times was 28.28%, compared with the traditional enzymatic hydrolysis alone. However, the solubility of yeast β-glucan began to decrease, when the ultrasonic time exceeds 25 min. It indicated that long-term ultrasonic treatment might cause the inactivation of glucanase. This is consistent with the result that Zhang Qi [27] in 2015 used ultrasound to modify Schizophyllan alone and found that the zero-shear viscosity value did not change regularly with the increase of ultrasound time.
Fig. 2.
The effect of ultrasonic time on the solubility of yeast β-glucan.
3.1.3. Effect of temperature on solubility of Candida utilis β-glucan
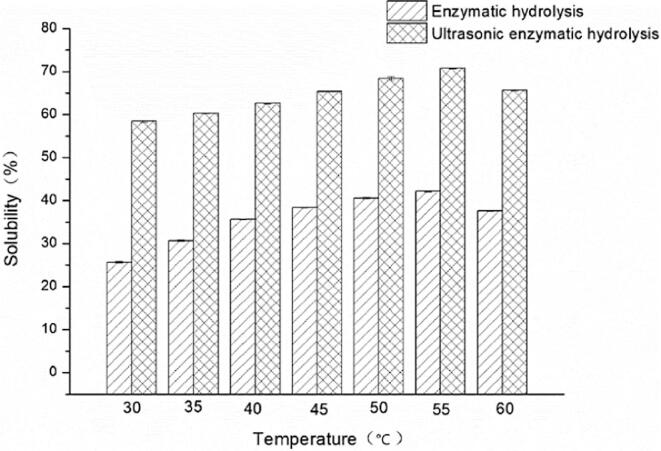
The solubility of yeast β-glucan at different temperatures of 30–60 °C was shown in Fig. 3, when the ultrasonic intensity was 18 W/mL and the treatment time was 10 min. With the increase of temperature, the solubility of yeast β-glucan firstly increased and then decreased. The solubility of yeast β-glucan reached the maximum value was as high as 70.77% at the optimum temperature of glucanase was 55 °C when enzymatic hydrolysis treatment alone and ultrasonic-assisted enzymatic solubilization modification were carried out. However, the solubility of yeast β-glucan started to decrease at 60 °C, possibly because of the thermal inactivation of glucanase and the weakening of ultrasonic cavitation. While the increased temperature led to the weakening of the ultrasonic cavitation effect, whereas the thermal degradation might be greater than the acoustic degradation [28]. The solubility of the modified yeast β-glucan by ultrasonic-assisted enzymatic solubilization was always dramatically greater than that of the traditional enzymatic treatment. It was proved that the ultrasonic had a certain promoting effect on the enzymatic hydrolysis process.
Fig. 3.
The effect of treatment time on the solubility of yeast β-glucan.
3.2. In vitro antioxidant activities of Candida utilis β-glucan
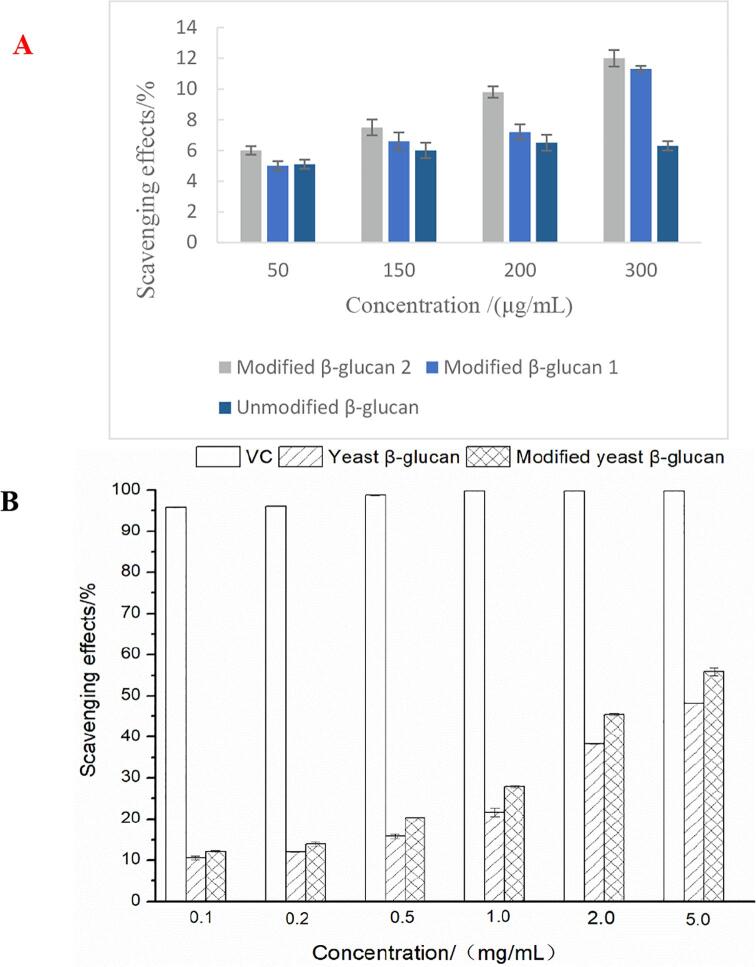
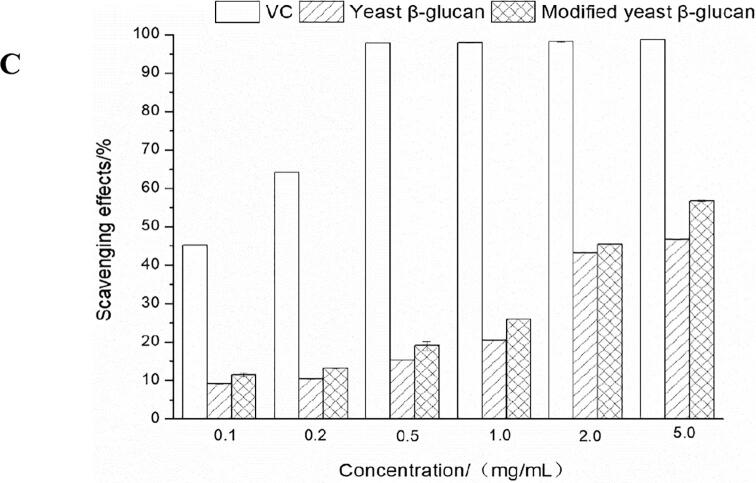
To explore the effect of ultrasonic synergistic enzymatic treatment on the scavenging ability of yeast β-glucan to scavenge DPPH free radicals. Ultrasonic co-enzymatic method 1 treatment meaned that the method of enzymatic hydrolysis and solubilization was carried out by using ultrasonic treatment alone and then using enzyme alone, and ultrasonic co-enzymatic method 2 treatment meaned the solubilization modification method of this study. The result is shown in Fig. 4-A. In the same concentration range, the trendency of the DPPH clearance rate of the polysaccharides obtained by ultrasonic synergistic enzymatic modification was similar with that of the sample. It gradually improved with the increase of the extract concentration. The polysaccharide modified by method 2 had the best scavenging effect. The scavenging rate was 12.00 % at the concentration of 300 μg/mL, followed by the polysaccharide prepared by method 1. The effect of yeast β-glucan on the scavenging ability of ABTS + and OH radicals before and after solubilization and modification was explored. In the range of 0–5.0 mg/mL, VC and yeast β-glucan before and after solubilization modification had ABTS+ scavenging ability with dose-dependent. At 1.0 mg/mL, the clearance rate of VC to ABTS+ could reach 99.84 ± 0.05 %, and then there was no significant change. When the mass concentration of yeast β-glucan and solubilized modified yeast β-glucan was 2.0 mg/mL, the clearance rates of ABTS+ were 38.32 % and 45.38 %, respectively. The scavenging ability of yeast β-glucan after modification was significantly better than that of untreated yeast β-glucan. There was no significant difference in the range of 2.0–5.0 mg/mL. And when the concentration was 5.0 mg/mL, the scavenging ability of ABTS+ was always greater than that of yeast β-glucan after solubilization and modification, but the scavenging effect of both on ABTS+ was inferior to that of VC.
Fig. 4.
Antioxidant activity in vitro before and after modified yeast β-glucan by ultrasonic enzymatic method. A: DPPH+ scavenging rate; B: ABTS+ free radical scavenging rate C: ·OH scavenging rate.
It can be demonstrated from Fig. 4-C that in the range of 0 to 5.0 mg/mL, VC and yeast β-glucan before and after solubilization modification had the ability to scavenge OH. And with the increase of the mass concentration of VC and yeast β-glucan, the scavenging ability of OH gradually increased. VC had the strongest scavenging ability to OH. At 0.5 mg/mL, the scavenging rate of VC to OH has reached 97.80 ± 0.02 %, and then with the increase of mass concentration, the scavenging rate of OH had no significance difference. The scavenging rate of hydroxyl radicals of yeast β-glucan after solubilization and modification by ultrasonic enzymatic method was significantly higher than that of untreated yeast β-glucan. The scavenging rate of hydroxyl radical of 5.0 mg/mL solubilized yeast β-glucan was 56.76 %, which was significantly higher than that of untreated yeast β-glucan, 46.65 %. In addition, as shown in Table 1, the molecular weight of modified β-glucan was significantly reduced. It was indicated that the smaller molecular weight of the polysaccharide contributed to the improvement of solubility and antioxidant activities.
Table 1.
The effect of different modification methods on the physicochemical properties of yeast β-glucan.
| Index | Modification method |
|||
|---|---|---|---|---|
| Unmodified | Enzymatic modification | Ultrasonic modification | Ultrasonic enzymatic modification | |
| Electric potential /mV | −7.34 ± 1.93a | −14.83 ± 0.56b | 16.18 ± 0.61c | −27.84 ± 0.52d |
| Particle size /μm | 43.43 ± 1.66d | 21.63 ± 0.86c | 11.37 ± 0.06b | 1.72 ± 0.05a |
| Solubility /% | 4.49 ± 0.03a | 19.89 ± 0.53b | 38.74 ± 1.20c | 75.35 ± 0.17d |
| Mw(D) | 1.99 × 106 | 1.66 × 105 | ||
3.3. Physicochemical properties of yeast β-glucan
Zeta potential measures the strength of mutual repulsion or attraction between particles, and the underlying properties reflect the stability of solutions or colloids. As the absolute value of the zeta potential increases, the system becomes more stable [29], [30]. The zeta potential of yeast β-glucan is shown in Table 1. The zeta potentials of yeast β-glucan after modification by ultrasonic synergistic enzymatic method were: −27.84 mV, respectively. After ultrasonic modification or enzymatic modification alone, the zeta sites were 16.18 mV and −14.83 mV, respectively. The molecule or dispersed particle is smaller, the Zeta potential (positive or negative) become higher, and the system is more stable. The particle size of yeast β-glucan after ultrasonic synergistic enzymatic solubilization modifier was 1.72 μm resulting in dissolution or dispersion resist aggregation. Conversely, the lower the Zeta potential (positive or negative), the more prone to condensation or condensation. In conclusion, the attractive force exceeded the repulsive force, and the Candida utilis β-glucan alone used by ultrasound or enzymatic hydrolysis was far greater than the effect of the combined action, and the dispersion was destroyed and coagulation or agglomeration occured. It demonstrated that the solubilization and modification of the yeast β-glucan solution greatly improved the stability of the yeast β-glucan aqueous solution. While the absolute value of zeta potential also showed that the yeast β-glucan sugar solution presented a relatively constant state, and the potential characteristics of polysaccharides caused changes in the electrostatic repulsion of polyelectrolytes, which may cause its network structure due to random coils. It might cause its network structure to change due to random coil winding [31].
3.4. Microstructure observation of the yeast β-glucan
3.4.1. Fourier transform infrared spectroscopy
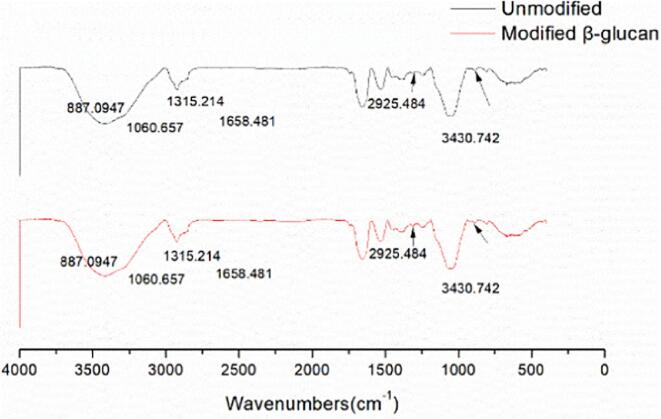
Infrared spectroscopy was carried out to identify the primary structure of Candida utilis β-glucan before and after solubilization and modification. The results are shown in Fig. 5. The infrared spectra of yeast β-glucan before and after ultrasonic enzymatic hydrolysis were basically the same. The spectrum showed a characteristic peak at 887.09 cm−1, indicating that its glycosidic bond was in β configuration. The characteristic peak at 2935 cm−1 indicated that the sample has β-1,3 bonds. The absorption band of polysaccharides was around 1454–1060 cm−1. The absorption peak at 3430 cm−1 was considered as the stretching vibration peak of O–H.
Fig. 5.
Infrared spectra of Candida utilis β-glucan before and after treatment.
3.4.2. Electron microscope observation
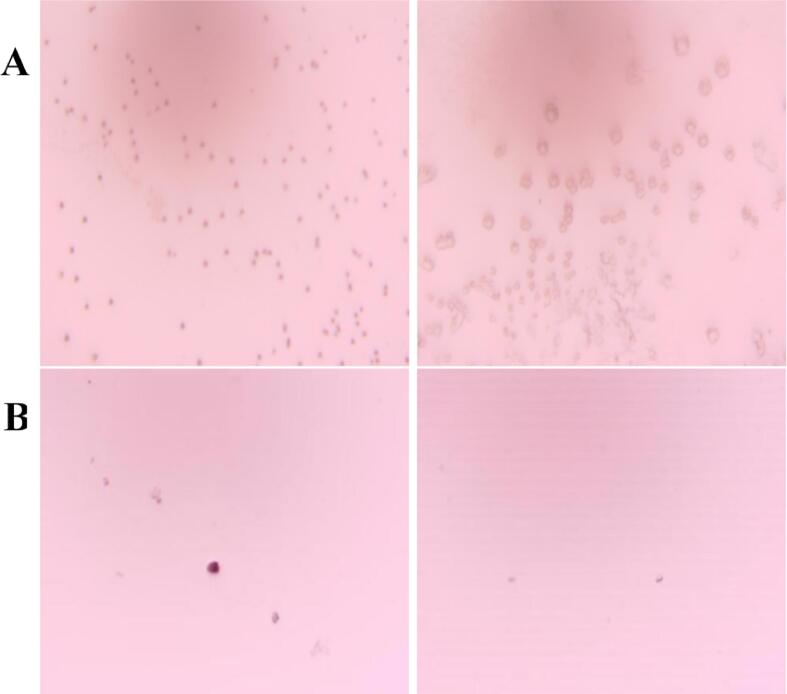
From Fig. 6 that the untreated yeast β-glucan and the solubilized and modified yeast β-glucan were prepared into an aqueous suspension of 0.1 μg/mL, and the dispersion was observed in different multiples. The original yeast β-glucan was dispersed in the aqueous solution in the form of obvious particles, and the dispersion was dense. While, yeast β-glucan after solubilization and modification by ultrasonic synergistic enzymatic method had significantly smaller particles and sparse dispersion, indicating that its solubility in aqueous solution had improved, which might be related to the destruction of its hydrogen bonds.
Fig. 6.
The microscopic morphology of yeast β-glucan before and after solubilization modification (Oil mirror × 100). A: Original yeast β-glucan; B: Yeast β-glucan after treatment.
3.4.3. Scanning electron microscope observation
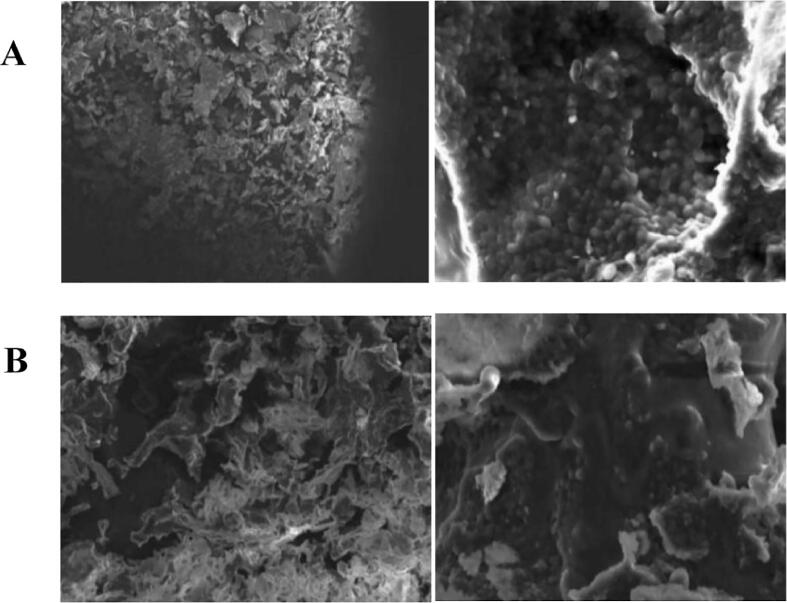
Fig. 7. showed that the morphology and structure of yeast β-glucan before and after solubilization and modification are significantly different. Yeast β-glucan existed in the form of compact aggregated clusters before treatment, and spherical aggregates (form honeycomb structure) exisedt on the rough surface of the material. Yeast β-glucan modified by ultrasonic synergistic enzymatic method, showed a loose aggregate structure, the surface of the material was reduced in spherical shape, and the structure was loose. In conclusion, the change of yeast β-glucan improved its water solubility.
Fig. 7.
Scanning electron microscopic morphology of Candida utilis β-glucan before and after solubilization and modification (×2000) A: Original yeast β-glucan; B: Yeast β-glucan after treatment.
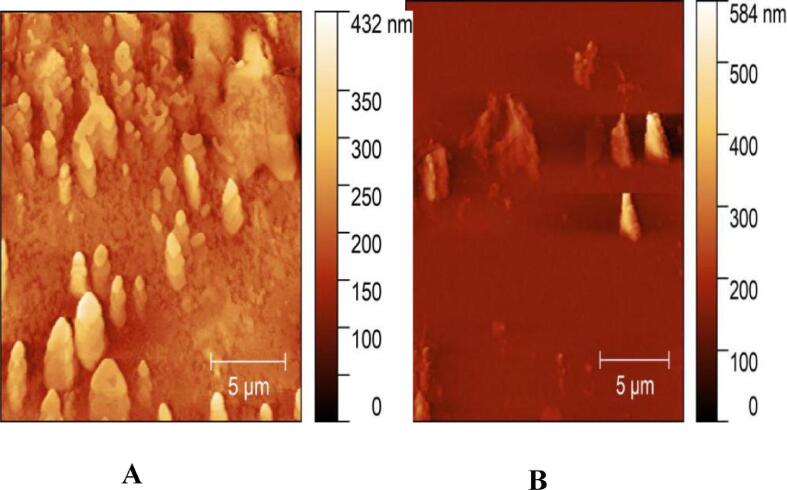
3.4.4. Atomic force microscope (AFM) observation
Fig. 8 showed that the original yeast β-glucan had obvious pile-like aggregation and dense block structure. β-glucan agglomerates of Candida utilis became smaller and relatively sparse through solubilization and modification. It proved that its solubility in aqueous solution becomes better, which might be related to the destruction of its hydrogen bonds. Besides, it indicated that ultrasonic co-enzymatic treatment destroyed the compact triple helix conformation of β-glucan, changed its structure and improved its solubility.
Fig. 8.
Atomic particle microscope observation of Candida utilis β-glucan before and after solubilization modification A: original yeast β-glucan; B: yeast β-glucan after treatment.
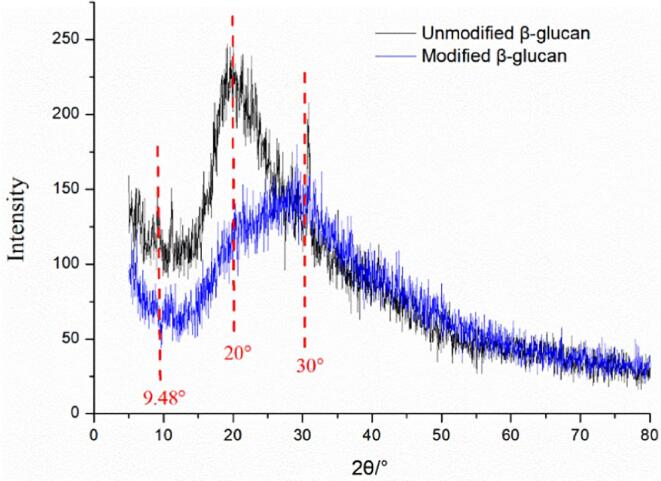
3.4.5. X-ray diffraction (XRD) observation
X-ray diffraction technology plays a role in determining the crystal structure of polysaccharide macromolecules, and other physical properties such as swelling, flexibility, tensile strength, and solubility could be predicted by analyzing the crystal structure of polysaccharides [32]. Generally, beta-glucan exhibits broad peaks due to its polymeric structure. In Fig. 9, the XRD patterns of β-glucan before and after ultrasonic synergistic enzymatic modification, in the range of diffraction angle (2θ) of 5 ∼ 80°, β-glucan before and after solubilization and modification had significant different diffraction absorption peaks. The original yeast β-glucan had the strongest absorption at 20°, while the modified yeast β-glucan had a significantly weakened absorption peak at 20°, and the strongest absorption appeared at 30°. While, the absorption peak at 5°-20° disappeared after solubilization modification. It stated clearly that the crystal structure of yeast β-glucan was damaged after modification, and the natural triple helix conformation of yeast β-glucan might be damaged.
Fig. 9.
Crystal structure changes of Candida utilis β-glucan before and after solubilization modification A: Original yeast β-glucan; B: Yeast β-glucan after treatment.
3.5. Thermal properties analysisof yeast β-glucan
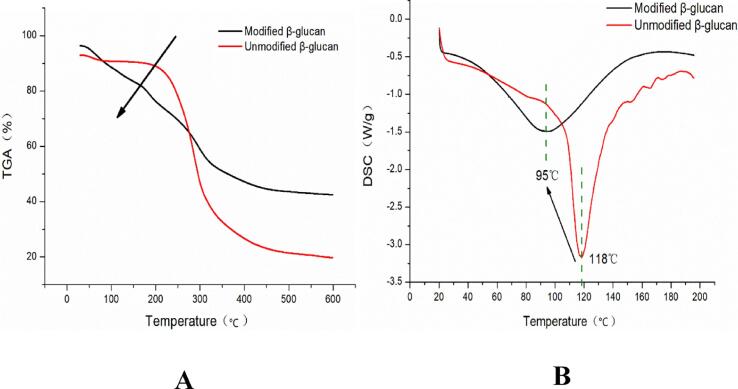
Fig. 10-A compares the TGA decomposition curve of β-glucan before and after treatment, and analyzes the thermal decomposition process of β-glucan. Modified β-glucan: 30–170 °C; original β-glucan: 30–230 °C, the weight loss rate of β-glucan before and after solubilization and modification was within 10%, which was caused by water evaporation β-glucan losed small molecule water. In the second stage, when the temperature was increased to 230 °C and 170 °C, respectively. The curve drops rapidly, the weight loss rate of modified polysaccharide increased sharply, and the mass changed greatly. The thermal decomposition temperature was dramatically reduced, indicating a decrease in its thermal stability.
Fig. 10.
TGA (A) and DSC (B) curves of Candida utilis β-glucan before and after treatment.
Fig. 10-B shows the DSC curves of β-glucan before and after treatment. The thermal transition temperatures of yeast β-glucan before and after modification were 118 °C and 95 °C, respectively. It indicated that the thermal stability decreased after the modification by ultrasonic synergistic enzymatic treatment. which was consistent with the research results of Gao Jie in 2017 [33]. It can be seen that the ultrasonic synergistic enzymatic compatibilization modified yeast β-glucan destroyed its dense intermolecular, intermolecular hydrogen bonds and its crystal structure. Resulting in its reduced thermal stability (enzymatic hydrolysis alone, ultrasonic alone). Whereas the addition of ultrasonic treatment more significantly destroyed the natural compact triple helix structure of β-glucan. This leaded to a decrease in its thermal decomposition temperature and a decrease in thermal stability. In addition, it was indicated that the molecular weight distribution of treated β-glucan was narrowed. This was in agreement with the results of scanning electron microscopy, electron microscopy and atomic force microscopy in this study.
4. Conclusion
In this research, a single-factor experiment was applied to explore a green and effective process for solubilizing and modifying Candida utilis β-glucan. The in vitro antioxidant activity, structural characteristics and physicochemical properties of yeast β-glucan before and after treatment were compared. The results showed that the solubility of Candida utilis beta-glucan was 75.35% through the ultrasonic enzymatic method, and the in vitro antioxidant capacity of the solubilized polysaccharide was significantly improved. The DPPH clearance rate of the modified polysaccharide at the concentration of 300 μg/mL was 12.00 %. The clearance rate of ABTS+ at 2.0 mg/mL was 45.38 %. The scavenging rate of the modified yeast β-glucan at 5.0 mg/mL to hydroxyl radicals reached 56.76 %. After the ultrasonic enzymatic method, the triple-helix structure of yeast β-glucan was disrupted. The dispersion was sparse, and the stability of the aqueous solution was improved. In addition, the thermal stability of β-glucan decreased after the modification with ultrasonic synergistic enzymatic treatment. Which leading the promotion of beta-glucan solubility. Due to the environmental and economic benefits, achieving environmentally sustainable food security is currently one of the biggest global challenges [34], [35]. It provided a research basis for the solubilization and modification of Candida utilis β-glucan and added value of its products, and as a theoretical reference for the structure–activity relationship of yeast β-glucan.
CRediT authorship contribution statement
Hongjie Yuan: Conceptualization, Data curation, Formal analysis, Writing – review & editing. Yan He: Investigation, Methodology, Software, Visualization, Writing – original draft. Hua Zhang: Investigation, Methodology, Software, Visualization, Writing – original draft. Xia Ma: Conceptualization, Formal analysis, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors are grateful to the Shanghai Science and Technology Commission (21142202800).
Contributor Information
Hua Zhang, Email: zhanghua@sioc.ac.cn.
Xia Ma, Email: maxia@sit.edu.cn.
References
- 1.Zhang X., Zong Y., Li Z., Yang R., Li Z., Bi Y., Prusky D. Postharvest Pichia guilliermondii treatment promotes wound healing of apple fruits. Postharvest Biol. Tec. 2020;167:111228. [Google Scholar]
- 2.Wu L., Zhao J., Zhang X., Liu S., Zhao C. Antitumor effect of soluble β-glucan as an immune stimulant. Int. J. Biol. Macromol. 2021;179:116–124. doi: 10.1016/j.ijbiomac.2021.02.207. [DOI] [PubMed] [Google Scholar]
- 3.Tang J., Zhen H., Wang N., Yan Q., Jing H., Jiang Z. Curdlan oligosaccharides having higher immunostimulatory activity than curdlan in mice treated with cyclophosphamide. Carbohyd. Polym. 2019;207:131–142. doi: 10.1016/j.carbpol.2018.10.120. [DOI] [PubMed] [Google Scholar]
- 4.Boutros J.A., Magee A.S., Cox D. Comparison of structural differences between yeast β-glucan sourced from different strains of saccharomyces cerevisiae and processed using proprietary manufacturing processes. Food Chem. 2022;367:130708. doi: 10.1016/j.foodchem.2021.130708. [DOI] [PubMed] [Google Scholar]
- 5.Chen H., Liu N., He F., Liu Q., Xu X. Specific β-glucans in chain conformations and their biological functions. Polym. J. 2022;1–27 [Google Scholar]
- 6.Nissola C., Marchioro M.L.K., de Souza Leite Mello E.V., Guidi A.C., de Medeiros D.C., da Silva C.G., de Mello J.C.P., Pereira E.A., Barbosa-Dekker A.M., Dekker R.F.H., Cunha M.A.A. Hydrogel containing (1→6)-β-D-glucan (lasiodiplodan) effectively promotes dermal wound healing. Int. J. Biol. Macromol. 2021;183:316–330. doi: 10.1016/j.ijbiomac.2021.04.169. [DOI] [PubMed] [Google Scholar]
- 7.De Iseppi A., Curioni A., Marangon M., Vincenzi S., Kantureeva G., Lomolino G. Characterization and emulsifying properties of extracts obtained by physical and enzymatic methods from an oenological yeast strain. J. Sci. Food Agr. 2019;99(13):5702–5710. doi: 10.1002/jsfa.9833. [DOI] [PubMed] [Google Scholar]
- 8.Vyrova D.V., Selezneva I.S. AIP Conference Proceedings. AIP Publishing LLC; 2019. Isolation of beta-glucan from yeast and its use as a dietary supplement for low-fat yoghurt manufacturing. Vol. 2174, No. 1, p. 020266. [Google Scholar]
- 9.Meng Y., Lyu F., Xu X., Zhang L. Recent advances in chain conformation and bioactivities of triple-helix polysaccharides. Biomacromolecules. 2020;21(5):1653–1677. doi: 10.1021/acs.biomac.9b01644. [DOI] [PubMed] [Google Scholar]
- 10.Ma X., Wang W., Wang D., Ding T., Ye X., Liu D. Degradation kinetics and structural characteristics of pectin under simultaneous sonochemical-enzymatic functions. Carbohyd. Polym. 2016;154:176–185. doi: 10.1016/j.carbpol.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Wang D., Ma X., Yan L., Chantapakul T., Wang W., Ding T., Liu D. Ultrasound assisted enzymatic hydrolysis of starch catalyzed by glucoamylase: Investigation on starch properties and degradation kinetics. Carbohyd. Polym. 2017;175:47–54. doi: 10.1016/j.carbpol.2017.06.093. [DOI] [PubMed] [Google Scholar]
- 12.Zheng Z., Huang Q., Luo X., Xiao Y., Cai W., Ma H. Effects and mechanisms of ultrasound-and alkali-assisted enzymolysis on production of water-soluble yeast β-glucan. Bioresour. Technol. 2019;273:394–403. doi: 10.1016/j.biortech.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y.Q., Fu E.H., Liang J.H. Effect of ultrasonic waves on the saccharification processes of lignocellulose. Chem. Eng. Technol. Indust. Chem.-Plant Equip.-Process Eng.-Biotechnol. 2008;31(10):1510–1515. [Google Scholar]
- 14.Iida Y., Tuziuti T., Yasui K., Towata A., Kozuka T. Control of viscosity in starch and polysaccharide solutions with ultrasound after gelatinization. Innov. Food. Sci. Emerg. 2008;9(2):140–146. [Google Scholar]
- 15.Szabo O.E., Csiszar E. Some factors affecting efficiency of the ultrasound-aided enzymatic hydrolysis of cotton cellulose. Carbohyd. Polym. 2017;156:357–363. doi: 10.1016/j.carbpol.2016.09.039. [DOI] [PubMed] [Google Scholar]
- 16.Cui J., Zhao Y., Liu R., Zhong C., Jia S. Surfactant-activated lipase hybrid nanoflowers with enhanced enzymatic performance. Sci. Rep-uk. 2016;6(1):1–13. doi: 10.1038/srep27928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu A., Lu J., Zheng J., Sun J., Yang L., Zhang X., Zhang Y., Lin Q. Ultrasonically aided enzymatical effects on the properties and structure of mung bean starch. Innov. Food Sci. Emerg. Technol. 2013;20:146–151. [Google Scholar]
- 18.Wang B., Meng T., Ma H., Zhang Y., Li Y., Jin J., Ye X. Mechanism study of dual-frequency ultrasound assisted enzymolysis on rapeseed protein by immobilized Alcalase. Ultrason. Sonochem. 2016;32:307–313. doi: 10.1016/j.ultsonch.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Siddhuraju P., Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agr. Food Chem. 2003;51(8):2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- 20.Luo A., He X., Zhou S., Fan Y., Luo A., Chun Z. Purification, composition analysis and antioxidant activity of the polysaccharides from Dendrobium nobile Lindl. Carbohyd. Polym. 2010;79(4):1014–1019. [Google Scholar]
- 21.Li N., Fu X., Xiao M., Wei X., Yang M., Liu Z., Mou H. Enzymatic preparation of a low-molecular-weight polysaccharide rich in uronic acid from the seaweed Laminaria japonica and evaluation of its hypolipidemic effect in mice. Food Funct. 2020;11(3):2395–2405. doi: 10.1039/c9fo02994j. [DOI] [PubMed] [Google Scholar]
- 22.Ma X.B. Zhejiang University; 2017. Reaearch on Function Routes And Mechanisms Of Ultrasound In The Enzymatic Degradation Of Pectin. [Google Scholar]
- 23.Wang D., Yan L., Ma X., Wang W., Zou M., Zhong J., Liu D. Ultrasound promotes enzymatic reactions by acting on different targets: Enzymes, substrates and enzymatic reaction systems. Int. J. Biol. Macromol. 2018;119:453–461. doi: 10.1016/j.ijbiomac.2018.07.133. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z., Bai G.e., Xu D., Cao Y. Effects of ultrasound on the kinetics and thermodynamics properties of papain entrapped in modified gelatin. Food Hydrocolloid. 2020;105:105757. [Google Scholar]
- 25.Dalagnol L.M., Silveira V.C., da Silva H.B., Manfroi V., Rodrigues R.C. Improvement of pectinase, xylanase and cellulase activities by ultrasound: Effects on enzymes and substrates, kinetics and thermodynamic parameters. Process Biochem. 2017;61:80–87. [Google Scholar]
- 26.Larsen L.R., van der Weem J., Caspers-Weiffenbach R., Schieber A., Weber F. Effects of ultrasound on the enzymatic degradation of pectin. Ultrason. Sonochem. 2021;72:105465. doi: 10.1016/j.ultsonch.2021.105465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Q. Chinese Academy of Agricultural Sciences; 2015. Immunoregulatory Activity and Preparation of Schizophyllan under Ultrasonic Treatment. [Google Scholar]
- 28.Hou F., Fan L., Ma X., Wang D., Wang W., Ding T., Liu D. Degradation of carboxymethylcellulose using ultrasound and β-glucanase: Pathways, kinetics and hydrolysates’ properties. Carbohyd. Polym. 2018;201:514–521. doi: 10.1016/j.carbpol.2018.07.092. [DOI] [PubMed] [Google Scholar]
- 29.Hubbe M.A., Rojas O.J. Colloidal stability and aggregation of lignocellulosic materials in aqueous suspension: A review. BioResources. 2008;3(4):1419–1491. [Google Scholar]
- 30.Zhong L., Fu S., Peng X., Zhan H., Sun R. Colloidal stability of negatively charged cellulose nanocrystalline in aqueous systems. Carbohyd Polym. 2012;90(1):644–649. doi: 10.1016/j.carbpol.2012.05.091. [DOI] [PubMed] [Google Scholar]
- 31.Hu H., Xu F.-J. Rational design and latest advances of polysaccharide-based hydrogels for wound healing. Biomater Sci-UK. 2020;8(8):2084–2101. doi: 10.1039/d0bm00055h. [DOI] [PubMed] [Google Scholar]
- 32.Kolsi R.B.A., Fakhfakh J., Krichen F., Jribi I., Chiarore A., Patti F.P., Blecker C., Allouche N., Belghith H., Belghith K. Structural characterization and functional properties of antihypertensive Cymodocea nodosa sulfated polysaccharide. Carbohyd. Polym. 2016;151:511–522. doi: 10.1016/j.carbpol.2016.05.098. [DOI] [PubMed] [Google Scholar]
- 33.Gao J. Chinese Academy of Agricultural Sciences; 2013. Study on the Preparation Modification and Solution Conformation of β- glucan in Saccharomyces Cerevisiae. [Google Scholar]
- 34.Lange K.W., Nakamura Y. Edible insects as future food: chances and challenges. J. Future Foods. 2021;1(1):38–46. [Google Scholar]
- 35.Barzee T.J., Cao L., Pan Z., Zhang R. Fungi for future foods. J. Future Foods. 2021;1(1):25–37. [Google Scholar]