Abstract
Background
Post-traumatic stress disorder (PTSD) is a psychiatric disease that develops following exposure to a traumatic event and is a stress-associated mental disorder characterized by an imbalance of neuroinflammation. Korean Red Ginseng (KRG) is the herbal supplement that is known to be involved in a variety of pharmacological activities. We aimed to investigate the effects of KRG on neuroinflammation as a potential mechanism involved in single prolonged stress (SPS) that negatively influences memory formation and consolidation and leads to cognitive and spatial impairment by regulating BDNF signaling, synaptic proteins, and the activation of NF-kB.
Methods
We analyzed the cognitive and spatial memory, and inflammatory cytokine levels during the SPS procedure. SPS model rats were injected intraperitoneally with 20, 50, or 100 mg/kg/day KRG for 14 days.
Results
KRG administration significantly attenuated the cognitive and spatial memory deficits, as well as the inflammatory reaction in the hippocampus associated with activation of NF-κB in the hippocampus induced by SPS. Moreover, the effects of KRG were equivalent to those exerted by paroxetine. In addition, KRG improved the expression of BDNF mRNA and the synaptic protein PSD-95 in the hippocampus. Taken together, these findings demonstrate that KRG exerts memory-improving actions by regulating anti-inflammatory activities and the NF-κB and neurotrophic pathway.
Conclusion
Our findings suggest that KRG is a potential functional ingredient for protecting against memory deficits in mental diseases, such as PTSD.
Keywords: Korean Red Ginseng, Post-traumatic stress disorder, Memory, Single prolonged stress, Inflammation
Graphical abstract
Korean red ginseng administration (KRG) significantly reversed the memory impairments in the Morris water maze test. KRG exerts memory-improving actions by regulating anti-inflammatory activities and the NF-κB and neurotrophic pathway.
1. Introduction
Post-traumatic stress disorder (PTSD) is the appearance of intrusive re-experiences, avoidance of reminders, negative cognition and mood after exposure to a severe traumatic event [1]. About 50% of the population is exposed to one or more severe traumatic events during their lifetime, and about 7% develop PTSD [2]. PTSD is diagnosed when the main symptoms of PTSD persist for more than 1 month, or severe psychological disease or disability occurs, which interferes with normal social and professional activities [2]. Due to the large-scale natural disasters caused by climate change, the global refugee crisis, high-intensity workplace stress, traffic accidents, terrorist attacks, sexual violence, and child abuse, the incidence rate of PTSD is increasing annually [3]. In particular, symptoms of PTSD are mainly accompanied by pathological symptoms, such as severe anxiety, depression, and impaired cognitive memory function, which causes neurological changes, including cognitive-behavioral and brain functional changes [4].
Serotonin selective reuptake inhibitors including antidepressant, antianxiety, and anticonvulsant drugs have been prescribed to alleviate symptoms, such as anxiety or cognitive impairment, caused by PTSD, but these drugs are cautionary due to serious adverse effects and long-term drug dependence [5,6]. In addition, these drugs are only intended to relieve symptoms and are not a fundamental cure.
Fear- and anxiety-based traumatic stress, such as PTSD, causes neuroinflammation due to the chronic stress-induced neurobiological response and is mainly mediated by proinflammatory cytokines [7,8]. Indeed, high concentrations of inflammatory signals, including proinflammatory cytokines accumulate in the hippocampus of animals with PTSD [9]. The pleiotropic and multi-functional nature of cytokines mediate crucial inflammatory processes in rodents and humans, and are critically involved in the regulation of PTSD. Neuroinflammation evokes adverse effects on cognitive and memory functions [10]. Therefore, resolving neuroinflammation slows the progression of memory impairment and protects against changes in brain function in patients with PTSD [11,12]. Therefore, recent studies have identified neuroinflammation as an important factor underlying PTSD, making neuroinflammation an important therapeutic target for treating PTSD [13]. Therefore, a decrease in hippocampal volume and densely located cytokine receptors are commonly observed in PTSD patients [14,15].
Additionally, some studies have shown that PTSD induces synaptic loss, and alters synaptic plasticity [16]. Also, PTSD may further impair neurotrophin-mediated signaling [17]. Brain-derived neurotrophic factor (BDNF) supports cell survival and increases synaptic plasticity by regulating inflammatory substances such as proinflammatory cytokines, and by activating nuclear factor kappa B (NF-κB) cascade signaling [18]. Therefore, BDNF signaling in the hippocampus plays an important role in PTSD neuropathology [17].
Korean Red Ginseng (KRG, Ginseng, Radix rubra) is one of the most widely used herbal health-promoting medicines with various effects, such as myocardial protection, vascular relief, and anti-stress [19]. In particular, KRG has been recognized for its variety of physiological and pharmacological activities, including recovery from fatigue, enhanced immunity, and improved blood flow, as well as anti-inflammatory, anti-oxidative, and improved memory activities [[20], [21], [22]]. The saponin fraction of KRG is effective for improving scopolamine-induced memory deficits in rats and prevents neurodegenerative disorders, such as Alzheimer's disease (AD) [23].
Although many studies have attempted to elucidate the neural mechanisms that support the memory-enhancing effect of KRG, the effect of KRG on the behavioral and neuroinflammatory responses caused by single prolonged stress (SPS)-induced memory impairment remains poorly understood. In particular, whether the anti-inflammatory effect of KRG is related to NF-κB-mediated inflammatory cytokines, the regulation of BDNF, or synaptic plasticity, has not been confirmed. Therefore, in this study, we investigated the protective effects of KRG on cognitive and spatial impairment in a PTSD-like psychiatric model by exposing rats to SPS.
2. Materials and methods
2.1. Animals and KRG administration
Male Sprague-Dawley (6–8 weeks old; 220–250 g) rats were purchased from Samtako (Seoul, Korea). The animal experiments were conducted the management of laboratory animals by the Animal Care and Use Committee of Kyung Hee University [KHUASP(SE)-21-268].
The rats were divided into six groups: a control group, an SPS only group, the KRG treatment groups, and a positive control group. The KRG treatment groups were administered KRG at concentrations of 20, 50, or 100 mg/kg for 14 days by intraperitoneal injection (i.p.), and the positive control drug paroxetine hydrochloride (15 mg/kg, PAX, Sigma-Aldrich Chemical Co., St. Louis, MO, USA) was administered. KRG was supplied by the Korean Ginseng Corp. (KT&G, Daejeon, Korea). In this study, we chose intraperitoneal administration, which can directly and rapidly confirm the pharmacological effect, instead of oral administration in which incomplete absorption occurred in the intestine similar to the previous study [19]. The experimental schedule for all drug administration, behavioral testing and sampling is presented in Fig. 1.
Fig. 1.
Experimental protocols for single prolonged stress (SPS)-induced memory impaired behaviors and Korean Red Ginseng (KRG) treatment in rats. Different groups of rats (n = 6–7/group) were used for each experimental condition.
2.2. Single prolonged stress
As already known, SPS is one of the most reliable models of PTSD, and is known to reproduce the main symptoms of PTSD [24]. The rats were subjected to restraint stress for 2 h and forced to swim in a water tank for 20 min. A rest period was provided to allow the rats to recover for 15 min, and then the rats were exposed to isoflurane until they lost consciousness. These procedures were called SPS. The rats were left alone for 7 days to further reinforce a PTSD-like condition.
2.3. Morris water maze (MWM) test
The MWM test consists of a circular water tank, an escape platform, and a computerized video-tracking system (ver. 2.5; PanLab Co., Barcelona, Spain). The tank was made of cylindrical plastic with a diameter of 200 cm and a height of 35 cm, and the walls and floor were painted white. No marks were placed on the inner wall of the tank. The tank was filled with water to a height of 25 cm and the temperature of the water was maintained at 23–25 °C. The water was made opaque with white aqueous paint so that the escape platform was not visible to the naked eye. The escape platform was a transparent acrylic cylinder with a diameter of 15 cm and height of 20 cm, and the upper surface was 1.5 cm below the water surface. The video tracker consisted of a CCD camera installed 2.5 m above the water tank and a computer, and the SMART program (ver. 2.5; PanLab) used to track and analyze the behavior of the experimental animals. Visual cues of various models were installed around the tank, and the laboratory environment and the location of the experimenter were kept constant during the experimental period, such as the experimental table, computer, and chair. During the experiment, the experimenter remained in one place to keep the spatial cues outside the maze constant. The maze was divided into quadrants of northeast, northwest, southeast, and southwest, and an escape platform was installed in the center of the southwest quadrant. The starting position of the animal was fixed, and this point was marked on the outside surface of the tank.
The rats were allowed to swim freely in the tank during training and climb up in search of hidden refuge by themselves. The rats who found the escape platform were allowed to freely observe their surroundings while staying on the shelter for 10 s and the time to reach the escape platform was recorded. Rats that could not find the escape platform within 180 s were guided to the escape platform by the experimenter, and then stayed on the escape platform for 10 s to freely observe the surroundings, and the time to reach the escape platform was recorded as 180 s. A spatial memory maintenance test was conducted on day 6 of the MWM experiment. The rats in each group were allowed to swim freely for 60 s once in the same tank from which the escape platform was removed, to find the escape platform, and the swimming path was tracked with the video tracker and stored in the computer analysis system.
2.4. Passive avoidance test (PAT)
The avoidance box is a dark black acrylic box (30 × 30 × 30 cm) with a grid floor and stainless steel rods (1 cm diameter) laid out at regular intervals on the floor, through which an electric shock can be applied to the soles of the feet. A railing (5 × 15 cm) that can barely hold a rat was installed on the outer wall of the front of the box, and a small slide door (5 × 5 cm) was installed between the railing and the avoidance box. A 50 W light illuminated the rat and was installed 45 cm above the railing. The rats were acclimated for 1 min in the avoidance box before the experiment (adaptation). Then, the rat was taken out, placed on the railing, and the 50 W light illuminated the rat. The adaptation trial ended when the rat entered the avoidance box (acquisition test). The rat was placed on the railing again, and the rat entered the avoidance box. An electric shock of 0.5 mA for 3 s was applied to the soles of the feet at the same time (learning). The rat was immediately placed back on the railing and the time until the rat entered the evasion box was measured (pre-test). The rat remembers the electric shock and tries not to enter the dark compartment box. After 24 h without an electric shock, the rat was placed on the railing and the time was measured until it entered the avoidance box (retention test). In both the acqusition and retention tests, the residence time on the railing from the rat's high-risk railing to entering the dark compartment box was recorded.
2.5. Open field test (OFT)
The emotional behavioral patterns of the experimental animals were observed in the OFT. This test was conducted the day before the MWM test, and the following devices were manufactured for the OFT. The OFT used a black wooden box measuring 60 × 60 × 30 cm. An incandescent light bulb (60 W) was installed about 2.5 m above the center of the field, and the center was brighter than the surroundings, thereby alerting the rats. All tests were performed within 3 h during the night cycle, which is the activity cycle of rats, and the exposure time was 5 min. The variables measured during the 5 min of exposure are as follows. The distance that passed through the field for 5 min was measured.
2.6. Contextual fear conditioning (CFC)
The CFC was performed using a separate group of rats that did not undergo the MWM test (n = 4 for each group). The CFC test were conducted as a method to evaluate the fear response in rats. Rats were acclimatized to a conditioned chamber (CS, audible alarm) for 5 min, then re-exposed to the same chamber, and subjected to a single electric foot shock (US, 0.5 mA). After 24 h, rats were briefly re-exposed (5 min) to CS. Rats were re-exposed to tone activation (CS) in the same chamber without electric foot shock to stimulate fear memory. When rats are re-exposed to the same chamber, rats remembered, retrieved and consolidated fear memories, resulting in a freezing response.
2.7. Enzyme-linked immunoassay analysis of inflammatory mediator and NF-κB protein levels
The rats were euthanized immediately after the behavioral test, and inflammatory cytokine levels were measured in the hippocampus using enzyme-linked immunosorbent assays (ELISAs). To prevent the decomposition of unstable inflammatory markers, the hippocampus was separated and stored at −80 °C until analysis. The hippocampal brain tissue was homogenized on ice using a Polytron homogenizer in 1 × PBS, pH 7.4 containing a protease inhibitor cocktail. The lysate was collected and centrifuged at 10,000 rpm for 10–15 min, and the supernatant was collected and quantified for cytokines. The inflammatory cytokines (IL-1β, IL-4, IL-6, IL-8, IL-12, and TNF-α) and NF-κB levels in the hippocampus were quantified using commercially available ELISA kits according to the manufacturer's instructions (Abcam, Cambridge, MA, USA and Cell Signaling Technology, Danvers, MA, USA). All samples were evaluated in triplicate. A 50 μl aliquot of the substrate solution was added to each well for 20 min to induce color development, and then the quantities of proinflammatory cytokines and the NF-κB protein were measured at 450 nm using an ELISA reader.
2.8. Total RNA isolation and RT-PCR analysis
The reverse transcription-polymerase chain reaction (RT-PCR) assay was performed to confirm changes in BDNF and tropomyosin-related kinase B (TrkB) mRNA. Trizol (Sigma-Aldrich) was used to isolate total brain RNA. Total RNA was synthesized into cDNA using the reverse transcriptase (Takara Bio, Otsu, Japan) reaction. To analyze the expression levels of BDNF and TrkB in the cDNA, PCR experiments were performed using each primer. The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primer was used in the same manner as an internal standard.
2.9. Western blot
Total protein was extracted from the brain to examine the p-PSD95 protein present in the hippocampus. The hippocampus was homogenized in a lysis buffer containing a phosphatase inhibitor and a protease inhibitor (CyQUANT; Invitrogen, Carlsbad, CA, USA). The protein concentration was measured using a Bio-Rad colorimetric protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). After reacting with mouse p-PSD95 antibody (1:500, Cell Signaling Technology) a secondary antibody conjugated with peroxidase (HRP-conjugated goat anti-mouse IgG; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was added. A chemiluminescent kit (Super Signal West Pico; Pierce, Rockford, IL, USA) was used to confirm the protein treated on the membrane. Protein content was analyzed using an enhanced chemiluminescence detection system (Santa Cruz Biotechnology) and a density measurement method.
2.10. Statistical analysis
Data are expressed as mean and ± standard error. The data analysis was performed with SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). The Student's t-test was applied to evaluate the change in body weight, and individual comparisons were made using one-way analysis of variance (ANOVA) and Tukey's post-hoc test to evaluate the changes in the behavioral measurements and the cytokine profile. A p-value < 0.05 was considered significant.
3. Results
3.1. The reduction of body weight in single prolonged stress-induced rats
We measured the body weights of all rats for 14 days, and the normal rats gained body weight over time (Fig. 2). However, body weight decreased over time in the SPS-induced traumatic stress group (SPS group; p < 0.05, p < 0.01 and p < 0.001). However, in the KRG-administered group, there was no significant difference between body weight compared to the SPS group for 14 days. However, the differences in the body weights were not related to SPS-induced memory impairment. Nevertheless, as body weight is an important indicator of physiological health, and body weight deceased in the SPS-induced rats, the rats were judged to have received sufficient traumatic stress.
Fig. 2.
Results of the body weight of rats submitted to 2-week SPS. Body weights were significantly lower in SPS-exposed rats than in saline-treated (SAL) rats (significant main effect of SPS exposure versus saline-treated rats). Data are represented as the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 vs. SAL group.
3.2. Korean Red Ginseng ameliorates the impairments of cognitive and spatial memory in single prolonged stress-induced rats
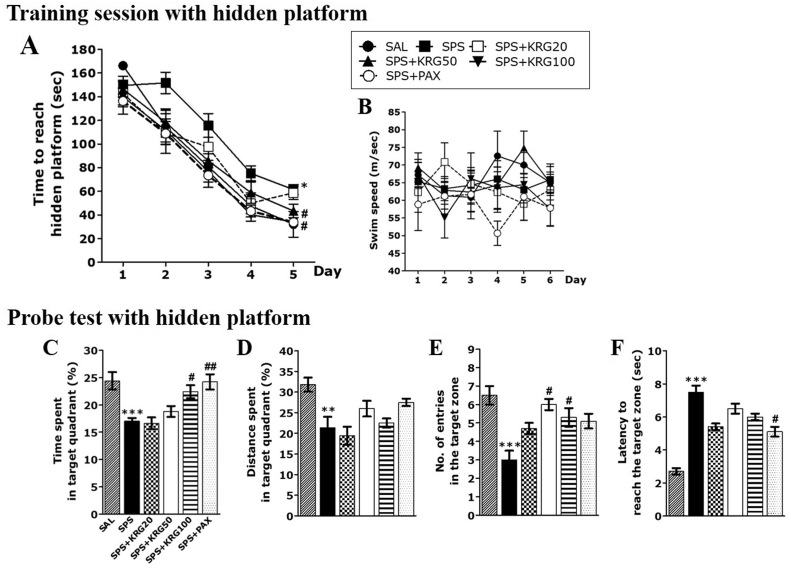
Fig. 3 shows the results of the MWM conducted to determine how KRG affected cognitive function and learning and memory abilities in the SPS animal model. The difference between the groups was significant on day 5 of training [F (5,33) = 4.124, p < 0.01](Fig. 3A). The ability of the normal rats to find the hidden platform improved daily during the 5-day training phase (acquisition phase). However, the SPS group spent more time looking for the hidden platform during the training phase than the saline-treated normal (SAL) group (p < 0.05 on day 5). As a result of analyzing the main effect of this difference by Tukey's post hoc test, the escape latency of the 100 mg/kg KRG-administered dose group was significantly shorter than that of the SPS group (p < 0.05 on day 5). The MWM results showed that the SPS group delayed learning early in the training phase, particularly from the first day of the learning session, and that the learning delay caused by this SPS procedure was suppressed by KRG. The average swimming speed was not different between the groups (Fig. 3B).
Fig. 3.
The Morris water maze (MWM) test was used to assess the effects of KRG on spatial learning and memory. Time to escape (latency) from the water onto a submerged platform during acquisition trials (A), swimming speed (B), percentages of time spent in the target quadrant (C), percentages of distance traversed in the target quadrant (D), number of entries to the target zone (E) and efficiency to reach the target zone (F) were used as outcome measures. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 vs. SAL group; #p < 0.05, ##p < 0.01 vs. SPS group.
The retention test indicated significant changes in the performance of all rats. In particular, normal rats were highly efficient in all aspects of the spatial memory test. However, rats exposed to SPS exhibited impaired spatial memory (Fig. 3C–F). However, the group administered 100 mg/kg KRG spent a significantly increased amount of time in the target area compared to the SPS group (p < 0.05). In addition, the number of entries into the target area increased significantly (p < 0.05). Impairments in learning and memory were observed in the SPS group during the MWM experiment, and these learning and memory impairments were prevented by administrating KRG for 2 weeks.
To confirm whether KRG recovers memory impairment, KRG was administered to rats with memory deficits induced by SPS, and their memory function was investigated by the PAT (Fig. 4A and B). As a result, memory performance was not different between the groups. In other words, none of the rats were cognitively impaired during the acquisition trials without electrical stimulation. However, after 24 h, the retention test showed that the SAL group had better passive avoidance learning than the SPS group (p < 0.05). The rats in the SPS + KRG100 group showed an increased latency to enter the dark compartment for retention compared to the SPS group, although this result was only marginally significant (p = 0.061).
Fig. 4.
Effects of KRG administration on the latency to enter the dark compartment during the acquisition trial (A) and retention test (B) in the passive avoidance test, and the travel distance of locomotor activity in the open-field test (C), and the percentages of time spent freezing on days 7 (D) and 14 (E) in the contextual fear conditioning test. ∗p < 0.05, ∗∗p < 0.01 vs. SAL group; #p < 0.05 vs. SPS group.
As the effect of the SPS procedure or drug administration may be delayed by changing the locomotor activity of the animal, we examined the locomotor activity of animals in an environment similar to the breeding conditions as several behavior types. As a result of behavioral analysis, all other observed behaviors were not affected by traumatic stress or KRG administration (Fig. 4C).
3.3. Korean Red Ginseng ameliorates the impaired fear memory in single prolonged stress-induced rats
Freezing behavior through conditioning training increases in rats with PTSD due to SPS. The freezing behavior of rats was measured by re-exposing them to the context when CFC was induced to evaluate the effect of KRG. The freezing behavior was measured by exposure for 5 min during CFC (Fig. 4D and E). The post-hoc test showed that freezing time was significantly longer in the SPS group than the SAL group (p < 0.01 on days 7 and 14, respectively). However, administering 100 mg/kg of KRG on day 14 significantly shortened the freezing time compared to the SPS group (p < 0.05).
3.4. Korean Red Ginseng reduces the pro-inflammatory cytokines and increases anti-inflammatory cytokines in the hippocampus of rats treated with single prolonged stress
Fig. 5ÃF shows the results of administering KRG on the expression of inflammatory cytokines in hippocampal tissue by ELISA. Pro-inflammatory cytokines (IL-1β, IL-6, and IL-8) increased in the SPS group (p < 0.05 and p < 0.001), whereas and anti-inflammatory cytokines (IL-12) decreased (p < 0.01). However, administrating KRG (100 mg/kg) significantly attenuated the increase in the levels of the pro-inflammatory markers IL-6 and IL-8 in the hippocampus induced by SPS (p < 0.05, respectively). In addition, administrating KRG (100 mg/kg) significantly attenuated the decrease the anti-inflammatory marker IL-12 in the hippocampus by SPS (p < 0.05).
Fig. 5.
Effects of KRG on TNF-α (A), IL-1β (B), IL-6 (C), IL-8 (D), IL-4 (E), IL-12 (F) and NF-κB (E) concentrations in the hippocampus of rats exposed to SPS using ELISA analysis. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 vs. SAL group; #p < 0.05 vs. SPS group.
3.5. Korean Red Ginseng reduces activation of NF-κB in the hippocampus of rats treated with single prolonged stress
Fig. 5G shows the results of administering KRG on the NF-κB level in hippocampal tissue by ELISA. A significant increase in NF-κB level was observed in the hippocampus of the SPS group compared to the SAL group (p < 0.01). However, KRG (100 mg/kg) significantly attenuated the increase in NF-κB levels in the hippocampus induced by SPS (p < 0.05).
3.6. Korean Red Ginseng increases the expression of BDNF mRNAs in the hippocampus of rats treated with single prolonged stress
BDNF and TrkB mRNA levels were investigated after SPS and KRG administration using PCR analysis (Fig. 6A). BDNF mRNA decreased significantly in the SPS group compared to the SAL group (p < 0.05). However, in the group subjected to the repeated KRG pretreatment, BDNF mRNA increased significantly compared to the SPS group (252.51%, p < 0.05). Also, TrkB mRNA level in the SPS group decreased compared to the SAL group, although this result was only marginally significant (p = 0.062). Unfortunately, there was no difference in TrkB mRNA in all groups.
Fig. 6.
Effects of KRG on the expression of BDNF and TrkB mRNAs in rats with SPS-induced hippocampal impairment (A). PCR bands on agarose gels and relative intensities are shown. The expression levels of BDNF and TrkB mRNAs were normalized to GAPDH mRNA as an internal control. Activation of synaptic protein, PSD-95 in the hippocampus after administration of KRG (B). Western blot analysis of protein expression levels of PSD-95. ∗p < 0.05 vs. SAL group; #p < 0.05 vs. SPS group.
3.7. Korean Red Ginseng promotes the synthesis of the PSD-95 synaptic protein in the hippocampus of rats treated with single prolonged stress
We measured the post-synaptic vesicle marker PSD-95, which is involved in improving spatial and cognitive memory by improving synaptic plasticity. As results, the PSD-95 protein level decreased in the SPS group compared to the SAL group, although this result was only marginally significant (p = 0.482). Also, the KRG treatment increased the PSD-95 protein level compared to the SPS group, although this result was only marginally significant (p = 0.094).
4. Discussion
Our study showed that SPS affects cognitive function, including learning and memory, and that SPS increased NF-κB-mediated inflammatory cytokines and decreased regulation of BDNF and the synaptic plasticity protein, PSD-95 in the hippocampus. However, administrating KRG reduced conditional fear memory, improved learning and memory impairments, inhibited the production of pro-inflammatory cytokines, and increased the production of anti-inflammatory cytokines in SPS-induced rats. In addition, the KRG treatment promoted BDNF levels and restored PSD-95 expression, a synaptic protein localized in the hippocampus. These results indicate that administering KRG significantly alleviated the cognitive dysfunction caused by SPS through regulation of the NF-kB and BDNF systems, and could serve as an effective anti-inflammatory and potential neuroprotective agent.
We investigated the dose-dependent effect of KRG to confirm optimal efficacy. We chose a dose of 100 mg/kg. This dose has already been proven effective in many studies [21]. Several studies have demonstrated that KRG enhances memory in patients with neurodegenerative memory disorders, such as AD [25], d-galactose-induced aging [26], and the LPS model [27]. In addition, KRG reduces the increase in serum corticosterone levels caused by stress [19]. Therefore, our results confirm that KRG prevented behavioral changes following PTSD-induced memory impairment and cognitive dysfunction, similar to a previous study [19].
In this study, we conducted the PAT and MWM tests, which are two different types of memory tests. Our study showed that SPS severely impaired memory retention by significantly shortening step-through latency on the retention trial of the PAT. However, administrating KRG significantly restored memory retention and memory retrieval ability by prolonging step-through latency. In addition, the SPS-induced rats showed considerably longer escape latencies to reach the platform, and exhibited poor spatial learning performance [28]. SPS led to impaired memory consolidation and retrieval on the retention test [28]. However, administering KRG shortened escape latency, which resulted in short escape distances on the spatial learning test. In addition, administering KRG improved learning speed and memory consolidation ability by improving the crossing times and target distances around the target quadrant on the spatial probe trial. Our results suggest that KRG significantly improved the severe memory recall and retrieval difficulties caused by the SPS procedure. As no difference was observed between the groups on the OFT, which measures the amount of locomotor activity, KRG did not affect motor function abnormalities or motor performance improvement. The shortening of the time taken to reach the platform after administering KRG resulted from improved memory impairment, not an improvement in locomotor activity of the rats. Also, administering KRG relieved the fear memory caused by SPS.
Inflammatory storms are an important factor leading to cognitive and spatial memory impairment [29]. Many studies have demonstrated that a rapid and persistent inflammatory response destroys the memory network, increases the production of inflammatory cytokines, and changes protein expression, leading to neurodegenerative diseases, such as AD and Parkinson's disease [13,30]. Therefore, the inflammatory storm caused by the SPS animal model impaired cognitive and memory functions, and suppressing the inflammatory storm suppresses the impairment of memory function [31]. In our study, the secretion of pro-inflammatory cytokines (IL-1β, IL-6, and IL-8) increased, and secretion of the anti-inflammatory cytokines (IL-12) decreased in the hippocampus in an animal model induced by SPS, resulting in severe cognitive deficits. However, administering KRG reduced the overproduction of pro-inflammatory cytokines and suppressed the decrease of anti-inflammatory cytokines in the hippocampus induced by SPS. These anti-inflammatory reactions improve neuroinflammation by inhibiting activation of the NF-κB pathway in the hippocampus. KRG protected against memory damage and neuroinflammation caused by SPS, by activating the NF-κB pathway. These results suggest that KRG significantly weakened the behavioral changes from the SPS-induced cognitive disturbances by inhibiting neuroinflammation and activating pro- and anti-inflammatory cytokines and the NF-kB pathway.
BDNF is a widely distributed signaling protein in the central nervous system [32]. BDNF promotes the survival, growth, differentiation, and development of neurons, and plays an important role in neuronal structure and synaptic plasticity [32]. BDNF activates the NF-kB signaling cascade to regulate inflammatory substances, such as inflammatory cytokines, to induce cell survival, and to increase synaptic plasticity by activating the mTOR pathway through regulation of the Akt and ERK signals [33]. Therefore, BDNF signaling in the hippocampus plays an important role in the neuropathological mechanism of PTSD [34]. BDNF is an important mediator between cognitive impairment and neuroinflammation, and modulating BDNF signaling may be an important way to regulate neuroinflammation and cognitive function in stress-damaged rats [35]. Many studies have shown that neuroinflammation decreases the BDNF level in the hippocampus [36]. Neuroinflammation caused by SPS changes synaptic plasticity by downregulating the BDNF-TrkB pathway [37]. Our results show that SPS reduced the expression of BDNF mRNA related to memory impairment and downregulated the expression of PSD-95 in the rats. However, KRG inhibited the decrease in BDNF mRNA expression and upregulated the expression level of PSD-95 in the hippocampus induced by SPS.
Therefore, administering KRG improved cognitive functions and abilities, such as learning acquisition, memory consolidation, and the disappearance of fear memory in the SPS-induced memory impairment animal model by regulating BDNF signaling, synaptic proteins, and NF-kB activation. Our findings suggest that KRG is a useful alternative treatment for traumatic stress-related cognitive dysfunctions, such as that occurring during PTSD. In addition, there is a need to explore further the preventive effects of KRG on improving memory and its basic mechanisms in the future.
Declaration of competing interest
The authors declare no potential conflicts of interests.
Acknowledgement
This research was supported by a grant from the Korean Society of Ginseng and the Korean Ginseng Cooperation (2020).
Contributor Information
Bombi Lee, Email: bombi@khu.ac.kr.
Seikwan Oh, Email: skoh@ewha.ac.kr.
References
- 1.Ceremuga T.E., Shellabarger P., Persson T., Fanning M., Galey P., Robinson D., Bertsch S., Ceremuga G.A., Bentley M. Effects of tetrahydropalmatine on post-traumatic stress disorder-induced changes in rat brain gene expression. J Integr Neurosci. 2013;12:513–528. doi: 10.1142/S0219635213500313. [DOI] [PubMed] [Google Scholar]
- 2.Kessler R.C. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatr. 2000;61:4–12. [PubMed] [Google Scholar]
- 3.Hopper J.W., Frewen P.A., van der Kolk B.A., Lanius R.A. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J Trauma Stress. 2007;20:713–725. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- 4.Leskin LP, White PM. Attentional networks reveal executive function deficits in posttraumatic stress disorder. Neuropsychology 007;21:275-284. [DOI] [PubMed]
- 5.Han F., Xiao B., Wen L., Shi Y. Effects of fluoxetine on the amygdala and the hippocampus after administration of a single prolonged stress to male Wistar rates: in vivo proton magnetic resonance spectroscopy findings. Psychiatr Res. 2015;232:154–161. doi: 10.1016/j.pscychresns.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi T., Morinobu S., Iwamoto Y., Yamawaki S. Effect of paroxetine on enhanced contextual fear induced by single prolonged stress in rats. Psychopharmacology. 2006;189:165–173. doi: 10.1007/s00213-006-0545-6. [DOI] [PubMed] [Google Scholar]
- 7.Ebenezer P.J., Wilson C.B., Wilson L.D., Nair A.R., Francis J. The Anti-Inflammatory Effects of Blueberries in an Animal Model of Post-Traumatic Stress Disorder (PTSD) PLoS One. 2016;11 doi: 10.1371/journal.pone.0160923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S.C., Lin C.C., Chen C.C., Tzeng N.S., Liu Y.P. Effects of Oxytocin on Fear Memory and Neuroinflammation in a Rodent Model of Posttraumatic Stress Disorder. Int J Mol Sci. 2018;19:3848. doi: 10.3390/ijms19123848. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebenezer P.J., Wilson C.B., Wilson L.D., Nair A.R. The anti-inflammatory effects of blueberries in an animal model of post-traumatic stress disorder (PTSD) PloS One. 2016;11 doi: 10.1371/journal.pone.0160923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S.C., Lin C.C., Chen C.C., Tzeng N.S., Liu Y.P. Effects of oxytocin on fear memory and neuroinflammation in a rodent model of posttraumatic stress disorder. Int J Mol Sci. 2018;19:3848–3858. doi: 10.3390/ijms19123848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai S., Wu G., Jiang Z. Glycyrrhizin treatment facilitates extinction of conditioned fear responses after a single prolonged stress exposure in rats. Cell Physiol Biochem. 2018;45:2529–2539. doi: 10.1159/000488271. [DOI] [PubMed] [Google Scholar]
- 12.Liu M., Xie J., Sun Y. TLR4/MyD88/NF-kappaB-Mediated inflammation contributes to cardiac dysfunction in rats of PTSD. Cell Mol Neurobiol. 2020;40:1029–1035. doi: 10.1007/s10571-020-00791-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunningham C., Hennessy E. Co-morbidity and systemic inflammation as drivers of cognitive decline: new experimental models adopting a broader paradigm in dementia research. Alzheimer's Res Ther. 2015;7:33–40. doi: 10.1186/s13195-015-0117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X.M., Han F., Liu D.J., Shi Y.X. Single-prolonged stress induced mitochondrial-dependent apoptosis in hippocampus in the rat model of post-traumatic stress disorder. J Chem Neuroanat. 2010;40:248–255. doi: 10.1016/j.jchemneu.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Wang W., Wang R., Xu J., Qin X., Jiang H., Khalid A., Liu D., Pan F., Ho C.S.H., Ho R.C.M. Minocycline attenuates stress-induced behavioral changes via its anti-inflammatory effects in an animal model of post-traumatic stress disorder. Front Psychiatr. 2018;9:558–568. doi: 10.3389/fpsyt.2018.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh J.Y., Kim Y.K., Kim S.N., Lee B., Jang J.H., Kwon S., Park H.J. Acupuncture modulates stress response by the mTOR signaling pathway in a rat post-traumatic stress disorder model. Sci Rep. 2018;8:11864–11874. doi: 10.1038/s41598-018-30337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M., Xie Y., Niu K., Li K. Electroacupuncture ameliorates post-traumatic stress disorder in rats via a mechanism involving the BDNF-TrkB signaling pathway. Cell Mol Biol. 2020;66:165–170. [PubMed] [Google Scholar]
- 18.Dias L., Lopes C.R., Gonçalves F.Q., Nunes A., Pochmann D., Machado N.J., Tomé A.R., Agostinho P., Cunha R.A. Crosstalk between ATP-P 2X7 and adenosine A 2A receptors controlling neuroinflammation in rats subject to repeated restraint stress. Front Cell Neurosci. 2021;1:639322–663932. doi: 10.3389/fncel.2021.639322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee B., Sur B., Lee H., Oh S. Korean Red Ginseng prevents posttraumatic stress disorder-triggered depression-like behaviors in rats via activation of the serotonergic system. J Ginseng Res. 2020;44:644–654. doi: 10.1016/j.jgr.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S., Lee Y., Cho J. Korean red ginseng extract exhibits neuroprotective effects through inhibition of apoptotic cell death. Biol Pharm Bull. 2014;37:938–946. doi: 10.1248/bpb.b13-00880. [DOI] [PubMed] [Google Scholar]
- 21.Lee J., Cho J.Y., Kim W.K. Anti-inflammation effect of Exercise and Korean red ginseng in aging model rats with diet-induced atherosclerosis. Nutr Res Pract. 2014;8:284–291. doi: 10.4162/nrp.2014.8.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park J.K., Shim J.Y., Cho A.R., Cho M.R., Lee Y.J. Korean red ginseng protects against mitochondrial damage and intracellular inflammation in an animal model of type 2 diabetes mellitus. J Med Food. 2018;21:544–550. doi: 10.1089/jmf.2017.4059. [DOI] [PubMed] [Google Scholar]
- 23.Jin S.H., Park J.K., Nam K.Y., Park S.N., Jung N.P. Korean red ginseng saponins with low ratios of protopanaxadiol and protopanaxatriol saponin improve scopolamine-induced learning disability and spatial working memory in mice. J Ethnopharmacol. 1999;66:123–129. doi: 10.1016/s0378-8741(98)00190-1. [DOI] [PubMed] [Google Scholar]
- 24.Serova L.I., Laukova M., Alaluf L.G., Pucillo L., Sabban E.L. Intranasal neuropeptide Y reverses anxiety and depressive-like behavior impaired by single prolonged stress PTSD model. Eur Neuropsychopharmacol. 2014;24:142–147. doi: 10.1016/j.euroneuro.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Lee S., Youn K., Jun M. Major compounds of red ginseng oil attenuate Abeta(25-35)-induced neuronal apoptosis and inflammation by modulating MAPK/NF-kappaB pathway. Food Funct. 2018;9:4122–4134. doi: 10.1039/c8fo00795k. [DOI] [PubMed] [Google Scholar]
- 26.Park S., Kim C.S., Min J., Lee S.H., Jung Y.S. A high-fat diet increases oxidative renal injury and protein glycation in D-galactose-induced aging rats and its prevention by Korea red ginseng. J Nutr Sci Vitaminol. 2014;60:159–166. doi: 10.3177/jnsv.60.159. [DOI] [PubMed] [Google Scholar]
- 27.Sun K., Wang C.S., Guo J., Horie Y., Fang S.P., Wang F., Liu Y.Y., Liu L.Y., Yang J.Y., Fan J.Y., Han J.Y. Protective effects of ginsenoside Rb1, ginsenoside Rg1, and notoginsenoside R1 on lipopolysaccharide-induced microcirculatory disturbance in rat mesentery. Life Sci. 2007;81:509–518. doi: 10.1016/j.lfs.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Lee B., Hong R., Lim P., Cho D., Yeom M., Lee S., Kang K.S., Lee S.C., Shim I., Lee H., Hahm D.H. The ethanolic extract of Aralia continentalis ameliorates cognitive deficits via modifications of BDNF expression and anti-inflammatory effects in a rat model of post-traumatic stress disorder. BMC Compl Alternative Med. 2019;19:11–20. doi: 10.1186/s12906-018-2417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajipour S., Farbood Y., Gharib-Naseri M.K., Goudarzi G., Rashno M., Maleki H., Bakhtiari N., Nesari A., Khoshnam S.E., Dianat M., Sarkaki B., Sarkaki A. Exposure to ambient dusty particulate matter impairs spatial memory and hippocampal LTP by increasing brain inflammation and oxidative stress in rats. Life Sci. 2020;242:117210–117220. doi: 10.1016/j.lfs.2019.117210. [DOI] [PubMed] [Google Scholar]
- 30.Olsson A., Csajbok L., Ost M., Höglund K., Nylén K., Rosengren L., Nellgård B., Blennow K. Marked increase of beta-amyloid(1-42) and amyloid precursor protein in ventricular cerebrospinal fluid after severe traumatic brain injury. J Neurol. 2004;251:870–876. doi: 10.1007/s00415-004-0451-y. [DOI] [PubMed] [Google Scholar]
- 31.Gong Q.H., Pan L.L., Liu X.H., Wang Q., Huang H., Zhu Y.Z. S-propargyl-cysteine (ZYZ-802), a sulphur-containing amino acid, attenuates beta-amyloid-induced cognitive deficits and pro-inflammatory response: involvement of ERK1/2 and NF-κB pathway in rats. Amino Acids. 2011;40:601–610. doi: 10.1007/s00726-010-0685-1. [DOI] [PubMed] [Google Scholar]
- 32.Yamada K., Mizuno M., Nabeshima T. Role for brain-derived neurotrophic factor in learning and memory. Life Sci. 2002;70:735–744. doi: 10.1016/s0024-3205(01)01461-8. [DOI] [PubMed] [Google Scholar]
- 33.Mulati A., Ma S., Zhang H., Ren B., Zhao B., Wang L., Liu X., Zhao T., Kamanova S., Sair A.T., Liu Z., Liu X. Sea-buckthorn flavonoids alleviate high-fat and high-fructose diet-induced cognitive impairment by inhibiting insulin resistance and neuroinflammation. J Agric Food Chem. 2020;68:5835–5846. doi: 10.1021/acs.jafc.0c00876. [DOI] [PubMed] [Google Scholar]
- 34.Ni L., Xu Y., Dong S., Kong Y., Wang H., Lu G., Wang Y., Li Q., Li C., Du Z., Sun H., Sun L. The potential role of the HCN1 ion channel and BDNF-mTOR signaling pathways and synaptic transmission in the alleviation of PTSD. Transl Psychiatry. 2020;10:101–110. doi: 10.1038/s41398-020-0782-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrientos R.M., Sprunger D.B., Campeau S., Watkins L.R., Rudy J.W., Maier S.F. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1 beta administration. J Neuroimmunol. 2004;155:119–126. doi: 10.1016/j.jneuroim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Guan Z., Fang J. Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain Behav Immun. 2006;20:64–71. doi: 10.1016/j.bbi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Ji L.L., Peng J.B., Fu C.H., Cao D., Li D., Tong L., Wang Z.Y. Activation of Sigma-1 receptor ameliorates anxiety-like behavior and cognitive impairments in a rat model of post-traumatic stress disorder. Behav Brain Res. 2016;311:408–415. doi: 10.1016/j.bbr.2016.05.056. [DOI] [PubMed] [Google Scholar]