Abstract
Parkinson’s disease, the most common movement disorder, has a strong neuroinflammatory aspect. This is evident by increased pro-inflammatory cytokines in the serum, and the presence of activated microglial cells, and inflammatory cytokines in the substantia nigra of post-mortem brains as well as cerebrospinal fluid of Parkinson’s disease patients. The central and peripheral neuroinflammatory aspects of Parkinson’s disease can be investigated in vivo via administration of the inflammagen lipopolysaccharide, a component of the cell wall of gram-negative bacteria. In this mini-review, we will critically evaluate different routes of lipopolysaccharide administration (including intranasal systemic and stereotasic), their relevance to clinical Parkinson’s disease as well as the recent findings in lipopolysaccharide mouse models. We will also share our own experiences with systemic and intrastriatal lipopolysaccharide models in C57BL/6 mice and will discuss the usefulness of lipopolysaccharide mouse models for future research in the field.
Key Words: C57BL/6 mice, intranasal models, lipopolysaccharide models, neuroinflammation, Parkinson’s disease, stereotaxic models, substantia nigra, systemic models
Introduction
Parkinson’s disease (PD) is the most common movement disorder, characterized by the degeneration of dopaminergic neurons in the substantia nigra (SN) pars compacta and their neuronal projections to the striatum. Another neuropathological hallmark of PD is the presence of Lewy bodies/Lewy neurites in the surviving dopaminergic neurons of the SN, and these are cytoplasmic inclusions made up predominantly of α-synuclein protein (Obeso et al., 2010). Although PD was originally described as a movement disorder, it is now well recognized that PD affects many other systems in the body and is associated with a plethora of non-motor symptoms such as olfactory dysfunction, anxiety, depression, and gut dysfunction. These complications can manifest years before the motor onset in PD and have a major impact on patients’ quality of life. Thus, investigation of the prodromal stage of PD is critical for identifying persons with an increased risk of PD to allow early interventions (Obeso et al., 2010).
Animal models of PD have been instrumental for elucidating PD pathogenesis and testing novel drugs. The most commonly used models in PD research include neurotoxin and genetic models. Neurotoxin models are based on exposure of experimental animals to neurotoxins such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, 6-hydroxyldopamine, rotenone and paraquat (Duty and Jenner, 2011). In contrast, genetic models are based on the disruption of genes associated with familial PD forms such as α-synuclein, leucine-rich repeat kinase 2 (LRRK2), parkin RBR E3 ubiquitin protein ligase (Parkin), PTEN-induced kinase 1 (PINK1), deglycase DJ-1 (DJ-1) and ubiquitin C-terminal hydrolase L1 (UCHL-1) as well as genes encoding for transcription factors such as nuclear receptor-related 1 protein (NURR1) and mitochondrial function (e.g., mitochondrial transcription factor A (TFAM) and mitofusin 2 (MFN2)) (Konnova and Swanberg, 2018). While these animal models have provided much insight into PD pathogenetic mechanisms such as oxidative stress, mitochondrial dysfunction, defective protein clearance and genetics involvement; generally, these models have a mild inflammatory response. In our research, we have focused on using the inflammagen lipopolysaccharide (LPS) to model PD in mice, and to better understand the neuroinflammatory aspects of PD and their impact on PD pathology and motor and non-motor symptoms. We chose to use mice in our experiments due to i) the larger genetic toolbox available for mice models; thus, opening future opportunities for developing new PD models combining genetic and environmental factors, and ii) lower costs for model development and screening of pharmaceutical agents due to the smaller animal size.
In this mini-review, we will critically evaluate the recent findings in LPS mouse models and share our own experiences with systemic and intrastriatal models in C57BL/6 mice, which may be helpful for new and experienced investigators who are interested in the neuroinflammatory aspects of PD. Furthermore, we will discuss the usefulness of these models in elucidating the pathogenesis of PD, and the future directions in this field.
Search Strategy
Relevant articles published from 2000 to 2021 were searched with Google Scholar, Scopus and PubMed using the following keywords: lipopolysaccharide; Parkinson’s disease; intranasal models; systemic models; stereotaxic models; neuroinflammation; microglial activation and neurodegeneration. Relevant articles were selected based on the title, abstract and the content of the articles.
The Relevance of Lipopolysaccharide to Clinical Parkinson’s Disease
It has been reported by Niehaus and Lange, 2003 that a laboratory worker developed cardinal motor features of PD after being accidentally exposed to LPS through an opened wound. It was confirmed years after the incident using positron emission tomography that the person had sustained an injury to the SN and cortex. Such findings indicate the deleterious potential of LPS and possible participation in clinical PD. Indeed, increased gut permeability and decreased serum lipopolysaccharide-binding protein (LBP) have been identified in PD patients (Forsyth et al., 2011). Increased gut permeability may allow entry of toxic products associated with gram-negative bacteria such as LPS into the bloodstream (Niehaus and Lange, 2003; Forsyth et al., 2011; Adams et al., 2019). LPS interacts with LBP in the bloodstream which facilitates its recognition by the circulating immune cells. Therefore, decreased serum LBP in PD patients has been associated with chronic invasion of gram-negative bacteria (Forsyth et al., 2011; Hasegawa et al., 2015). According to Matheoud et al. (2019), oral gavage of a gram-negative Citrobacter rodentium, a mouse intestinal pathogen, to PINK1–/– mice induced a loss of dopaminergic neuronal terminals in the striatum and motor impairment. These findings suggest that bacterial infections in genetically susceptible individuals could drive pathophysiological processes, resulting in the degeneration of the nigrostriatal pathway. Cumulatively, these findings imply that LPS is not just an inflammatory agent to mimic PD but could also be involved in the pathogenesis. Therefore, LPS models are highly relevant for understanding the features and pathogenesis of clinical PD.
Overview of Lipopolysaccharide Mouse Models
Mouse strains
The common mouse strains used in modelling PD include but are not limited to C57BL/6, 129SVJ, BALB/c and CD1 mice, and these strains have significant differences in their behaviour and response to stimulatory agents such as LPS. It is evident that acute systemic administration of LPS reduces locomotion and body weight and increases the levels of systemic and central pro-inflammatory cytokines; however, the levels of these parameters differ with the mouse strain (Rocha-Ferreira et al., 2015; Meneses et al., 2018; Piirsalu et al., 2020). To our knowledge, the specific effects of background strain have not been examined in LPS models of PD, but there is likely an effect as evident with acute response to systemic LPS. Therefore, it is important to consider the background strain for the LPS models. C57BL/6 is the most common general-purpose strain, and a background strain for genetic models. Moreover, it is the most common strain used in PD models and as a result, it was selected for our experiments.
Lipopolysaccharide from different species and strains of bacteria
There are structural differences in LPS obtained from various species and strains (the genetic variants) of bacteria that dictate pathogenicity and virulence. As a result, there tend to be varying effects when LPS is obtained from different bacterial species and strains and used in vivo, and this could be a problem for reproducibility of animal experiments (Pulendran et al., 2001). The effects of LPS from different bacterial species/strains have not been examined explicitly in LPS mouse models of PD; however, we noticed some differences based on our and other investigators’ work. For example, systemic administration of 1 mg/kg per day of LPS from Salmonella abortus equi induced degeneration of dopaminergic neurons in the SN of mice after 14–19 days (Bodea et al., 2014; Beier et al., 2017; Milde et al., 2021). However, using the same dose of LPS but from Escherichia Coli (unpublished data), we had to euthanize the mice after two injections with 1 mg/kg of LPS due to welfare concerns, suggesting varying effects. As a result, we had to systemically administer 0.3 mg/kg of LPS (Deng et al., 2021b), but this regimen did not cause dopaminergic degeneration in the midbrain, similar to another study showing no effect on the SN neurons when using LPS from the same bacterial species (Escherichia Coli, 0.25 mg/kg). Collectively, these studies (Bodea et al., 2014; Beier et al., 2017; Deneyer et al., 2019; Deng et al., 2021b; Milde et al., 2021) indicate that the bacterial species/strain and the dose of LPS should be carefully considered before animal experiments are undertaken. Unfortunately, many studies do not specify these details making it difficult to compare the treatment outcomes in different studies. Therefore, we urge the researchers to describe in their publications the bacterial species/strain source of LPS used to model PD to facilitate future research using LPS as an inflammatory agent.
Routes of administration
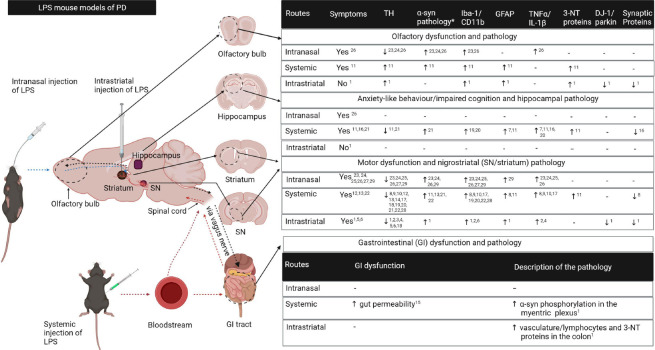
There are three common routes of administration of LPS that are used to model PD, and these are: intranasal, intraperitoneal, and stereotaxic (localized injection into the SN or striatum) and we covered these routes previously in our review paper (Deng et al., 2020). Most intranasal, intraperitoneal and intrastriatal mouse models of LPS replicate major hallmarks of PD such as degeneration of the nigrostriatal pathway, increased α-synuclein protein expression and motor deficits, providing new knowledge on the deleterious effects of neuroinflammation in PD. This review builds on our previous review (Deng et al., 2020) and will highlight the new developments in modelling PD using these three routes of LPS of administration as well as the novel route of LPS administration via drinking water recently described by (Gorecki et al., 2019) (Figure 1).
Figure 1.
A summary of the pathological changes in LPS models of PD.
Intranasal, systemic and intrastriatal administration of LPS induce pathological changes in the olfactory bulb, hippocampus, striatum, substantia nigra (SN) and the gastrointestinal tract which are associated with functional changes similar to clinical PD. Blue dotted arrows indicate propagation of LPS associated signals from the olfactory bulb to the nigrostriatal pathway; red dotted arrows – from periphery to the nigrostriatal pathway; and the blue dotted arrows – from the striatum to the olfactory bulb and gastrointestinal (GI) system. The parameters in the first row of the table apply to the olfactory bulb, hippocampus and striatum/SN but each parameter must be read vertically; α-syn pathology* refers to the increased α-synuclein protein expression, phosphorylation and/or aggregation; “-” means “not examined”. 3-NT: 3-nitrotyrosine.; α-syn: α-synuclein; DJ-1: deglycase DJ-1; GFAP: glial fibrillary acidic protein; Iba-1: Ionised calcium-binding adaptor molecule 1; IL-1β: interleukin-1β; PD: Parkinson’s disease; SN: substantia nigra; TH: tyrosine hydroxylase; TNF-α: Tumour necrosis factor-α. References: 1 (Deng et al., 2021b); 2 (Garcia et al., 2018); 3 (Gao et al., 2008); 4 (Gómez-Gálvez et al., 2016); 5 (Hunter et al., 2009); 6 (Zhang et al., 2012); 7 (Badshah et al., 2016); 8 (Beier et al., 2017); 9 (Bodea et al., 2014); 10 (Deneyer et al., 2019); 11 (Deng et al., 2021a); 12 (Jiang et al., 2017); 13 (Liu et al., 2008); 14 ( Milde et al., 2021); 15 (Kelly et al., 2014); 16 (Khan et al., 2019); 17 (Qin et al., 2013); 18 (Qin et al., 2004; 19 (Qin et al., 2007); 20 (Song et al., 2019); 21 (Zhang et al., 2019); 22 (Zheng et al., 2013); 23 (He et al., 2013); 24 (He et al., 2016); 25 (Li et al., 2015); 26 (Niu et al., 2020); 27 (Zhao et al., 2018); 28 (Liao et al., 2021); 29 (Song et al., 2020). The figure was created with BioRender.com.
Intranasal Lipopolysaccharide Mouse Models for Parkinson’s Disease Research
The dual-hit hypothesis states that PD could be initiated by airborne pathogens (such as viruses) via the invasion of olfactory and gastrointestinal systems, resulting in α-synuclein mediated neurodegenerative processes in the brain (Halliday and McCann, 2010). Congruent with this hypothesis, olfactory dysfunction is evident in 90% of PD patients and it precedes the motor onset. Additionally, PD patients exhibit Lewy body pathology in the components of the olfactory system such as the olfactory bulb, anterior olfactory nucleus, and other secondary structures before the SN (Doty, 2012). It is believed that this pathology could be initiated in the olfactory bulb; thus, it is relevant to understand the effects of external pathogens on this region (Doty, 2012). Unilateral intranasal administration of LPS (10 µg) in C57BL/6 mice is commonly carried out every second day for 3–20 weeks. A few studies using this regimen consistently showed that unilateral intranasal LPS can induce motor deficits in C57BL/6 mice although the duration of protocol varied significantly between the studies. This phenotype could have been a consequence of LPS induced inflammation, driving the degeneration of dopaminergic neurons in the SN and their neuronal projections to the striatum (He et al., 2013, 2016; Li et al., 2015; Zhao et al., 2018). Unilateral intranasal LPS also increased the expression of the α-synuclein protein in the olfactory bulb, SN, and striatum (He et al., 2013, 2016; Niu et al., 2020). Moreover, a recent study by Niu et al. (2020) showed that bilateral intranasal administration of LPS (10 µg) daily for 6 weeks increased microglial activation, interleukin 1 β (IL-1β) protein and α-synuclein pathology (e.g., monomeric, phosphorylated, and aggregated forms) as well as reduced tyrosine hydroxylase (TH) in the olfactory bulb, striatum, and SN in a manner dependent on IL-1β/IL-1 receptor signaling. These pathological changes were accompanied by motor impairment and non-motor complications such as olfactory deficits and anxiety-like behaviour. Niu and colleagues were the first to investigate α-synuclein phosphorylation and aggregation in the olfactory bulb, striatum and SN in the intranasal LPS model of PD as well as non-motor symptoms, adding to the recent developments in the field (Niu et al., 2020). Additionally, a study by Song et al. (2020) showed that bilateral intranasal LPS (3 mg/kg once every 3-days for 7 weeks) induced motor deficits, and nigral pathologies such as reduced dopaminergic neurons, increased α-synuclein protein, glial activation, and increased tumor necrosis factor α (TNF-α). The advantage of intranasal administration of LPS is that it mimics the exposure to environmental agents via the nasal cavity, making it suitable to elucidate the relationship between the olfactory system and the aetiology of PD. However, bilateral intranasal LPS administrations in mice on a daily basis for extended periods of time can be challenging for investigators and for the mice.
Systemic Lipopolysaccharide Mouse Models for Parkinson’s Disease Research
A multitude of studies have shown that there is an increased risk of PD in people with chronic systemic inflammatory diseases such as autoimmune rheumatoid arthritis, Sjögren syndrome, periodontal inflammatory disease, Crohn’s disease, and ulcerative colitis (Chen et al., 2017; Chang et al., 2018; Brudek, 2019; Ju et al., 2019). Also, elevated levels of pro-inflammatory cytokines and activated CD4+ lymphocytes have been identified in the serum and cerebrospinal fluid of PD patients, supporting the role of peripheral inflammation in PD pathogenesis (Park et al., 2019; Pajares et al., 2020). Interestingly, a significant increase in PD cases was observed among the survivors of Spanish Flu following its outbreak in 1918, emphasizing the importance of systemic inflammation in PD (Henry et al., 2010). Although the mechanisms are not clearly understood, systemic inflammatory cytokines could promote neuroinflammation via the activation of the vagal nerve, leakage of systemic cytokines to the brain parenchyma due to defects of the blood-brain barrier or activation of circumventricular organs which lack the blood-brain barrier (Konsman et al., 2002; Block et al., 2007; Dantzer et al., 2008; Perry, 2010). Therefore, the systemic LPS models may be suitable to understand the effects of systemic inflammation on dopaminergic and non-dopaminergic neurons, and the associated motor and non-motor behaviour. Most systemic mouse models of LPS have used a single injection (5 mg/kg) or 4–7 daily injections (250–300 µg or 1 mg/kg per day) to investigate PD pathology at different time points after LPS injections, and these will be highlighted below.
A single injection of LPS in mice was successful in inducing progressive dopaminergic neurodegeneration but required a high dose of LPS (5 mg/kg) and long-time (7–10 months) for the SN degeneration, increased α-synuclein protein expression and motor impairment to develop (Qin et al., 2007, 2013; Liu et al., 2008; Zheng et al., 2013; Jiang et al., 2017; Song et al., 2019; Deng et al., 2020). Importantly, some of these studies have also reported extranigral pathologies such as degeneration in the motor cortex and hippocampus as well as an increase in the gut permeability and phosphorylation of α-synuclein protein at serine 129 in the enteric nervous system (Kelly et al., 2014; Song et al., 2019). These findings suggest the utility of such models to investigate slowly progressing pathological changes and the associated motor and non-motor symptoms, resembling the progression of clinical PD. However, such models are likely to be associated with high mortality rates after the initial inflammatory insult caused by LPS injections (which is often not reported by researchers), and also, can be expensive and not very practical for many laboratories due to the long duration of the experimental protocol.
In contrast, repeated systemic LPS models induce earlier degeneration of dopaminergic neurons in the SN and their neuronal fibres in the striatum at 1–6 weeks post-treatment. This degeneration in the nigrostriatal pathway is accompanied by reduced dopamine levels and increased α-synuclein protein in these regions when measured at the same time point (Bodea et al., 2014; Beier et al., 2017; Zhang and Xu, 2018; Liao et al., 2021; Milde et al., 2021), but the corresponding motor phenotype is not well examined. Also, repeated systemic models of LPS could induce hippocampal inflammation (evidenced by activation of glial cells and increased proinflammatory cytokines), increased α-synuclein protein, and impaired cognitive function, but apart from these findings, the extranigral pathology and non-motor symptoms have not been explored extensively in these models (Badshah et al., 2016; Zhang and Xu, 2018; Khan et al., 2019; Song et al., 2019). In our studies, we used repeated administrations of LPS (0.3 mg/kg) for 4 days to investigate motor and non-motor pathology and behaviors in C57BL/6 mice at 19 days post-treatment (Deng et al., 2021b. We did not find dopaminergic degeneration in the midbrain after repeated systemic LPS injections however, there was a significant increase in glial markers, 3-nitrotyrosine (3-NT) proteins (indicators of oxidative stress) and α-synuclein protein. As stated earlier, olfactory and cognitive deficits are critical features of clinical PD, and these complications could be associated with pathological changes in the olfactory bulb, and hippocampus respectively. Therefore, we investigated the pathological effects of systemically induced inflammation on the olfactory bulb, and hippocampus, and the possible underlying functional and pathological changes. According to our study, systemic LPS increased glial markers and 3-NT proteins, and altered the expression of α-synuclein, TH and mature brain-derived neurotrophic factor proteins in the olfactory bulb and hippocampus. The pathological changes in the olfactory bulb and hippocampus were associated with impaired olfaction and anxiety-like behaviour observed in C57BL/6 mice (Deng et al., 2021b). Our findings for the hippocampus were consistent with the other studies (Badshah et al., 2016; Zhang and Xu, 2018). Although our study failed to induce degeneration of dopaminergic neurons and motor deficits, it could potentially be useful to understand the early non-motor aspects of PD and associated pathology.
To our knowledge, the longest study for repeated systemic injections observed dopaminergic degeneration in the SN at 42 days post-injection; thus, it could be speculated that these models may recapitulate progressive degeneration if the length of the study is extended to 7-months as in the case of single systemic injections (Beier et al., 2017). However, according to (Beier et al., 2017), repeated LPS injections did not cause progressive dopaminergic degeneration after 42 days compared to earlier time points, and there was a shift in cytokine production marked by reduced pro-inflammatory profile and increased anti-inflammatory profile. This state of reduced pro-inflammatory response is commonly referred to as tolerance, and it is classically viewed as a protective mechanism to mitigate the deleterious effects of inflammation (Pardon, 2015; Beier et al., 2017). Therefore, the reduced inflammatory response implies that the model may not be progressive but further evidence is needed. Interestingly, there is controversy around the deleterious or beneficial effects of tolerance (Pardon, 2015). While tolerance leads to reduced pro-inflammatory cytokines, which could reduce the risk of inflammation, there is an increase in anti-inflammatory cytokines. It has been proposed that elevated levels of anti-inflammatory cytokines could have deleterious effects on the brain by inhibiting the protective phenotype of microglia cells, thus may have detrimental effects on the brain (Pardon, 2015).
In summary, a single systemic injection of LPS induces a delayed progressive degeneration of the nigrostriatal pathway. In contrast, repeated systemic injections may not induce progressive degeneration, implying that other susceptibility factors such as aging, genetics and co-morbidities could be essential for a systemic inflammation to induce a robust progressive PD phenotype, which is the likely scenario in clinical PD. In our opinion, the following factors should be considered by researchers before using systemic LPS models to investigate PD: 1) The bacterial species/strain and the dose of LPS can produce variable behavioral and biochemical outcomes; 2) The systemic LPS toxicity (especially when used in high concentrations) can be a major problem in many laboratories due to animal welfare issues; 3) Repeated systemic LPS injections can cause tolerance, reducing the response to subsequent injections and thus, could result in the lack of desirable changes.
Stereotaxic Lipopolysaccharide Mouse Models for Parkinson’s Disease Research
Stereotaxic models of LPS are useful to scrutinize the specific effects of inflammation on the nigrostriatal pathway because SN has a high density of microglial cells which are the prominent mediators of neuroinflammation (Kim et al., 2000; Saijo et al., 2009). It is evident that microglial cells are activated in the SN of post-mortem brains of PD patients. These cells can produce pro-inflammatory cytokines, free radicals and chemokines that are detrimental to dopaminergic neurons (Tansey and Goldberg, 2010). The most common stereotaxic protocols used in PD research include unilateral or bilateral injections of LPS into the striatum (10–20 µg) or SN (5 µg) followed by an investigation of PD symptoms and pathology at 1–12 weeks post-treatment (Hunter et al., 2007, 2009, 2017; Zhang et al., 2012; Gómez-Gálvez et al., 2016; García et al., 2018).
The intranigral mouse models were successful in inducing SN degeneration but the other pathological markers of PD and motor/non-motor symptoms have not been well characterized in these models (Qin et al., 2004; Gao et al., 2008). One major drawback of the intranigral models is that they do not resemble the progression of clinical PD. It is believed that the degeneration of the nigrostriatal pathway begins at the dopaminergic neuronal terminals in the striatum, which then progresses to the neuronal cell bodies in the SN in clinical PD (Kordower et al., 2013); thus, the intrastriatal models are a better choice for modelling clinical PD. Also, the striatum is larger relative to the SN, and it is less challenging for investigators to perform microinjections. It has been shown that intrastriatal injections of 20 µg of LPS (bilaterally) induce inflammation in the striatum and the SN, characterized by increased glial activation and proinflammatory cytokines (Hunter et al., 2009). These pathological mechanisms could drive the progressive degeneration of neuronal fibres in the striatum and their neuronal cell bodies in the SN (between 1–12 weeks), and motor dysfunction as observed in this model (Hunter et al., 2009). To our knowledge, these intrastriatal LPS models have not examined the non-motor symptoms or extranigral pathology of PD.
In our laboratory, we used unilateral injections of LPS (10 µg) into the striatum to avoid animal welfare concerns and to ensure a slow enough progression of the disease that would allow us to investigate non-motor symptoms and pathology. We found that unilateral intrastriatal administration of LPS induced degeneration of neuronal terminals in the striatum but not their cell bodies in the SN at 2-months post-surgery, resulting in mild motor deficits (Deng et al., 2021a). Our results for the SN were not consistent with previous studies which showed degeneration of the SN neurons at 2 to 4 weeks following unilateral intrastriatal injection of 10 µg of LPS (Zhang et al., 2012; Gómez-Gálvez et al., 2016; García et al., 2018). As mentioned earlier, LPS from different bacterial species/strain can produce variable effects. Therefore, the difference in the SN pathology could be because we used LPS from different bacterial species/strain compared to the studies mentioned.
The above studies by (Zhang et al., 2012; Gómez-Gálvez et al., 2016; García et al., 2018) indicated that intrastriatal inflammation propagated to the SN inducing neuroinflammation, which was signified by microglial activation and increased levels in pro-inflammatory cytokines such as tumour necrosis factor α and IL-1β. As a consequence, there was degeneration of dopaminergic neurons in the SN, accompanied by motor impairment in a manner consistent with clinical PD. Nonetheless, the authors did not investigate pathological changes in the striatum (injection site of LPS) such as the degeneration of dopaminergic neuronal terminals, oxidative stress, alterations in α-synuclein, or synaptic proteins. A thorough characterization of intrastriatal pathology would expand our understanding of the pathological changes of the nigrostriatal pathway and could facilitate identification of novel pharmaceutical targets for PD.
Of note, we found in our intrastriatal model that LPS reduced the expression of DJ-1 protein in the striatum, a protein encoded by the PARK7 gene that is mutated in clinical PD, and to our knowledge, this was a novel finding. Therefore, a reduction in DJ-1 protein in our model suggests that external inflammatory factors could modulate the expression of proteins associated with familial forms of PD to increase the susceptibility of dopaminergic neurons (Deng et al., 2021a). Additionally, we showed that the intrastriatal pathology propagated to the olfactory bulb and the colon, indicated by region-specific changes in glial markers, 3-NT proteins, synaptic proteins, and parkin, a protein also involved in familial PD. We did not find significant impairments in olfaction according to the buried food-seeking test, suggesting that the pathological changes in the olfactory bulb did not reach the threshold to induce functional changes based on this particular test. To our knowledge, our study is the first to examine the effects of intrastriatal LPS on the olfactory bulb and colon in C57BL/6 mice, and further studies are required to consolidate our findings (Deng et al., 2021a). It is not well established how intrastriatal injection of LPS may cause olfactory bulb and colonic changes. A likely pathway for the propagation of intrastriatal pathology to the olfactory bulb could be via the rostral migratory stream. During neurogenesis, this pathway facilitates the migration of stem cells from the subventricular zone of the third ventricle which is adjacent to the striatum, to the olfactory bulb. Therefore, inflammatory mediators associated with intrastriatal LPS may propagate to the olfactory bulb via this pathway. In contrast, it has been suggested that the nigrostriatal pathway connects with the gut system via the vagus nerve (Garrido-Gil et al., 2018) and this pathway could propagate intrastriatal pathology to the colon. Interestingly, it has been recently hypothesized that PD could be divided into two subtypes, a “brain-first” subtype where the pathology begins in the brain and then spreads to the peripheral nervous system; and the “gut-first” subtype where the pathology begins in the gut then spreads to the brain (Borghammer and Van Den Berge, 2019). While this hypothesis requires further confirmation, the intrastriatal as well as intranasal LPS models could be useful to elucidate the “brain-first” subtype and the spread of nigral pathology to the periphery.
In summary, 1) intrastriatal injections are technically challenging and require a good quality stereotaxic apparatus which is costly; however, it is worthy to invest in a motorized stereotaxic apparatus for greater precision and reproducibility; 2) bilateral injections induce greater and quicker degeneration compared to unilateral injection but may be associated with higher mortality; 3) intrastriatal injections are very localized and thus likely to produce specific changes; 4) intrastriatal injections appear to have the capacity to induce non-motor symptoms and pathology; thus, further characterization of these models is warranted.
Administration of Lipopolysaccharide in Drinking Water
The gastrointestinal system is a possible initiation site of PD according to the dual-hit hypothesis; thus, a model targeting this system could enhance our understanding of PD. Indeed, a recent study by Gorecki et al. (2019) indicated that administration of LPS (10 µg/mL for 12 nights) in drinking water to mice overexpressing human α-synuclein protein, induced early motor symptoms and altered microbial diversity after 12 days which did not occur in untreated mice. Interestingly, the genetic models rarely induce motor impairment on their own, making this model invaluable for investigating the combined effects of genetic and environmental factors. In addition, altered microbial diversity is associated with increased intestinal inflammation (Gorecki et al., 2019) and it would be informative to elucidate if altered microbial diversity augments neuropathological features of PD and the associated phenotype. This model is advantageous for understanding the interplay between gut dysfunction and PD pathology in the central nervous system, and it should be further characterized by future studies.
Conclusions and Future Directions
In conclusion, each route of administration of LPS can be useful to elucidate specific aspects of PD pathology and has its advantages/disadvantages which need to be taken into consideration. In the future, LPS models of PD require further characterization of the non-motor symptoms (including olfactory and gut function) and the pathogenetic mechanisms such as aggregation/phosphorylation of the α-synuclein protein, mitochondrial dysfunction, and defective protein clearance to identify their ability to reproduce clinical PD and their usefulness as PD models for future research. Moreover, genetic models have limited effects on dopaminergic neurons and motor function on their own. Therefore, combining them with LPS models to generate a mixed model can be advantageous for understanding the combined effects of genetic and environmental factors in PD. Additionally, robust LPS models could be further generated by combining genetic/LPS models with LPS in drinking water which will be invaluable for investigating the concomitant effects of altered gut microbiota on the pathogenesis of PD.
Additional file: Open peer review report 1 (85.9KB, pdf) .
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open peer reviewer: Lies De Groef, University of São Paulo, Brazil.
References
- 1.Adams B, Nunes JM, Page MJ, Roberts T, Carr J, Nell TA, Kell DB, Pretorius E. Parkinson's disease: a systemic inflammatory disease accompanied by bacterial inflammagens. Front Aging Neurosci. 2019;11:210. doi: 10.3389/fnagi.2019.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badshah H, Ali T, Shafiq-ur Rehman, Faiz-ul Amin, Ullah F, Kim TH, Kim MO. Protective effect of lupeol against lipopolysaccharide-induced neuroinflammation via the p38/c-Jun N-terminal kinase pathway in the adult mouse brain. J Neuroimmune Pharmacol. 2016;11:48–60. doi: 10.1007/s11481-015-9623-z. [DOI] [PubMed] [Google Scholar]
- 3.Beier EE, Neal M, Alam G, Edler M, Wu LJ, Richardson JR. Alternative microglial activation is associated with cessation of progressive dopamine neuron loss in mice systemically administered lipopolysaccharide. Neurobiol Dis. 2017;108:115–127. doi: 10.1016/j.nbd.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 5.Bodea L-G, Wang Y, Linnartz-Gerlach B, Kopatz J, Sinkkonen L, Musgrove R, Kaoma T, Muller A, Vallar L, Di Monte DA, Balling R, Neumann H. Neurodegeneration by activation of the microglial complement–phagosome pathway. J Neurosci. 2014;34:8546–8556. doi: 10.1523/JNEUROSCI.5002-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghammer P, Van Den Berge N. Brain-first versus gut-first Parkinson's disease: a hypothesis. J Parkinsons Dis. 2019;9(2):S281–295. doi: 10.3233/JPD-191721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brudek T. Inflammatory bowel diseases and Parkinson's disease. J Parkinsons Dis. 2019;9(2):S331–344. doi: 10.3233/JPD-191729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang CC, Lin TM, Chang YS, Chen WS, Sheu JJ, Chen YH, Chen JH. Autoimmune rheumatic diseases and the risk of Parkinson disease: a nationwide population-based cohort study in Taiwan. Ann Med. 2018;50:83–90. doi: 10.1080/07853890.2017.1412088. [DOI] [PubMed] [Google Scholar]
- 9.Chen CK, Wu YT, Chang YC. Periodontal inflammatory disease is associated with the risk of Parkinson's disease: a population-based retrospective matched-cohort study. PeerJ. 2017;5:e3647. doi: 10.7717/peerj.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dantzer R, O'connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deneyer L, Albertini G, Bentea E, Massie A. Systemic LPS-induced neuroinflammation increases the susceptibility for proteasome inhibition-induced degeneration of the nigrostriatal pathway. Parkinsonism Relat Disord. 2019;68:26–32. doi: 10.1016/j.parkreldis.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Deng I, Corrigan F, Zhai G, Zhou XF, Bobrovskaya L. Lipopolysaccharide animal models of Parkinson's disease: Recent progress and relevance to clinical disease. Brain Behav Immun Health. 2020;4:100060. doi: 10.1016/j.bbih.2020.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng I, Corrigan F, Garg S, Zhou X-F, Bobrovskaya L. Further characterization of intrastriatal lipopolysaccharide model of Parkinson's disease in C57BL/6 mice. Int J Mol Sci. 2021a;22:7380. doi: 10.3390/ijms22147380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng I, Wiese MD, Zhou XF, Bobrovskaya L. The efficacy of systemic administration of lipopolysaccharide in modelling pre-motor Parkinson's disease in C57BL/6 mice. Neurotoxicology. 2021b;85:254–264. doi: 10.1016/j.neuro.2021.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Doty RL. Olfactory dysfunction in Parkinson disease. Nat Rev Neurol. 2012;8:329–339. doi: 10.1038/nrneurol.2012.80. [DOI] [PubMed] [Google Scholar]
- 16.Duty S, Jenner P. Animal models of Parkinson's disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol. 2011;164:1357–1391. doi: 10.1111/j.1476-5381.2011.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, Estes JD, Dodiya HB, Keshavarzian A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson's disease. PLoS One. 2011;6:e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM. Neuroinflammation and oxidation/nitration of α-synuclein linked to dopaminergic neurodegeneration. J Neurosci. 2008;28:7687–7698. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García C, Gómez-Cañas M, Burgaz S, Palomares B, Gómez-Gálvez Y, Palomo-Garo C, Campo S, Ferrer-Hernández J, Pavicic C, Navarrete C, Luz Bellido M, García-Arencibia M, Ruth Pazos M, Muñoz E, Fernández-Ruiz J. Benefits of VCE-003.2, a cannabigerol quinone derivative, against inflammation-driven neuronal deterioration in experimental Parkinson's disease: possible involvement of different binding sites at the PPARγreceptor. J Neuroinflammation. 2018;15:19. doi: 10.1186/s12974-018-1060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garrido-Gil P, Rodriguez-Perez AI, Dominguez-Meijide A, Guerra MJ, Labandeira-Garcia JL. Bidirectional neural interaction between central dopaminergic and gut lesions in Parkinson's disease models. Mol Neurobiol. 2018;55:7297–7316. doi: 10.1007/s12035-018-0937-8. [DOI] [PubMed] [Google Scholar]
- 21.Gómez-Gálvez Y, Palomo-Garo C, Fernández-Ruiz J, García C. Potential of the cannabinoid CB2 receptor as a pharmacological target against inflammation in Parkinson's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:200–208. doi: 10.1016/j.pnpbp.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Gorecki AM, Preskey L, Bakeberg MC, Kenna JE, Gildenhuys C, MacDougall G, Dunlop SA, Mastaglia FL, Akkari PA, Koengten F, Anderton RS. Altered gut microbiome in Parkinson's disease and the influence of lipopolysaccharide in a human α-synuclein over-expressing mouse model. Front Neurosci. 2019;13:839. doi: 10.3389/fnins.2019.00839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halliday GM, McCann H. The progression of pathology in Parkinson's disease. Ann N Y Acad Sci. 2010;1184:188–195. doi: 10.1111/j.1749-6632.2009.05118.x. [DOI] [PubMed] [Google Scholar]
- 24.Hasegawa S, Goto S, Tsuji H, Okuno T, Asahara T, Nomoto K, Shibata A, Fujisawa Y, Minato T, Okamoto A. Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson's disease. PLoS One. 2015;10:e0142164. doi: 10.1371/journal.pone.0142164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He Q, Yu W, Wu J, Chen C, Lou Z, Zhang Q, Zhao J, Wang J, Xiao B. Intranasal LPS-mediated Parkinson's model challenges the pathogenesis of nasal cavity and environmental toxins. PLoS One. 2013;8:e78418. doi: 10.1371/journal.pone.0078418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He Q, Li Yh, Guo Ss, Wang Y, Lin W, Zhang Q, Wang J, Ma Cg, Xiao BG. Inhibition of Rho-kinase by Fasudil protects dopamine neurons and attenuates inflammatory response in an intranasal lipopolysaccharide-mediated Parkinson's model. Eur J Neurosci. 2016;43:41–52. doi: 10.1111/ejn.13132. [DOI] [PubMed] [Google Scholar]
- 27.Henry J, Smeyne RJ, Jang H, Miller B, Okun MS. Parkinsonism and neurological manifestations of influenza throughout the 20th and 21st centuries. Parkinsonism Relat Disord. 2010;16:566–571. doi: 10.1016/j.parkreldis.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunter R, Ojha U, Bhurtel S, Bing G, Choi DY. Lipopolysaccharide-induced functional and structural injury of the mitochondria in the nigrostriatal pathway. Neurosci Res. 2017;114:62–69. doi: 10.1016/j.neures.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Hunter RL, Dragicevic N, Seifert K, Choi DY, Liu M, Kim HC, Cass WA, Sullivan PG, Bing G. Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J Neurochem. 2007;100:1375–1386. doi: 10.1111/j.1471-4159.2006.04327.x. [DOI] [PubMed] [Google Scholar]
- 30.Hunter RL, Cheng B, Choi DY, Liu M, Liu S, Cass WA, Bing G. Intrastriatal lipopolysaccharide injection induces parkinsonism in C57/B6 mice. J Neurosci Res. 2009;87:1913–1921. doi: 10.1002/jnr.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang MJ, Chen YH, Li L, Xu L, Liu H, Qu XL, Xu JJ, Ge BB, Qu HD. Protective effects of DL-3-n-butylphthalide in the lipopolysaccharide-induced mouse model of Parkinson's disease. Mol Med Rep. 2017;16:6184–6189. doi: 10.3892/mmr.2017.7352. [DOI] [PubMed] [Google Scholar]
- 32.Ju UH, Liu FC, Lin CS, Huang WY, Lin TY, Shen CH, Chou YC, Lin CL, Lin KT, Kao CH, Chen CH, Yang TY. Risk of Parkinson disease in Sjögren syndrome administered ineffective immunosuppressant therapies: A nationwide population-based study. Medicine. 2019;98:e14984. doi: 10.1097/MD.0000000000014984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly LP, Carvey PM, Keshavarzian A, Shannon KM, Shaikh M, Bakay RA, Kordower JH. Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson's disease. Mov Disord. 2014;29:999–1009. doi: 10.1002/mds.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan MS, Ali T, Kim MW, Jo MH, Chung JI, Kim MO. Anthocyanins improve hippocampus-dependent memory function and prevent neurodegeneration via JNK/Akt/GSK3βsignaling in LPS-treated adult mice. Mol Neurobiol. 2019;56:671–687. doi: 10.1007/s12035-018-1101-1. [DOI] [PubMed] [Google Scholar]
- 35.Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci. 2000;20:6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konnova EA, Swanberg M. Animal models of Parkinson's disease. In: Stoker TB, Greenland JC, editors. Parkinson's Disease: Pathogenesis and Clinical Aspects [Internet] Brisbane (AU): Codon Publications; 2018. [Google Scholar]
- 37.Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- 38.Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain. 2013;136:2419–2431. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li YH, He Q, Yu JZ, Liu CY, Feng L, Chai Z, Wang Q, Zhang HZ, Zhang GX, Xiao BG, Ma CG. Lipoic acid protects dopaminergic neurons in LPS-induced Parkinson's disease model. Metab Brain Dis. 2015;30:1217–1226. doi: 10.1007/s11011-015-9698-5. [DOI] [PubMed] [Google Scholar]
- 40.Liao H, Winkler J, Wißfeld J, Shahraz A, Klaus C, Neumann H. Low molecular weight polysialic acid prevents lipopolysaccharide-induced inflammatory dopaminergic neurodegeneration in humanized SIGLEC11 transgenic mice. Glia. 2021;69:2845–2862. doi: 10.1002/glia.24073. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Qin L, Wilson B, Wu X, Qian L, Granholm AC, Crews FT, Hong JS. Endotoxin induces a delayed loss of TH-IR neurons in substantia nigra and motor behavioral deficits. Neurotoxicology. 2008;29:864–870. doi: 10.1016/j.neuro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matheoud D, Cannon T, Voisin A, Penttinen A-M, Ramet L, Fahmy AM, Ducrot C, Laplante A, Bourque M-J, Zhu L. Intestinal infection triggers Parkinson's disease-like symptoms in Pink1-/- mice. Nature. 2019;571:565–569. doi: 10.1038/s41586-019-1405-y. [DOI] [PubMed] [Google Scholar]
- 43.Meneses G, Rosetti M, Espinosa A, Florentino A, Bautista M, Díaz G, Olvera G, Bárcena B, Fleury A, Adalid-Peralta L, Lamoyi E, Fragoso G, Sciutto E. Recovery from an acute systemic and central LPS-inflammation challenge is affected by mouse sex and genetic background. PLoS One. 2018;13:e0201375. doi: 10.1371/journal.pone.0201375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milde S, van Tartwijk FW, Vilalta A, Hornik TC, Dundee JM, Puigdellívol M, Brown GC. Inflammatory neuronal loss in the substantia nigra induced by systemic lipopolysaccharide is prevented by knockout of the P2Y6 receptor in mice. J Neuroinflammation. 2021;18:1–9. doi: 10.1186/s12974-021-02280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niehaus I, Lange J. Endotoxin: is it an environmental factor in the cause of Parkinson's disease? Occup Environ Med. 2003;60:378–378. doi: 10.1136/oem.60.5.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niu H, Wang Q, Zhao W, Liu J, Wang D, Muhammad B, Liu X, Quan N, Zhang H, Zhang F. IL-1β/IL-1R1 signaling induced by intranasal lipopolysaccharide infusion regulates alpha-Synuclein pathology in the olfactory bulb, substantia nigra and striatum. Brain Pathol. 2020;30:1102–1118. doi: 10.1111/bpa.12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Obeso JA, Rodriguez-Oroz MC, Goetz CG, Marin C, Kordower JH, Rodriguez M, Hirsch EC, Farrer M, Schapira AH, Halliday G. Missing pieces in the Parkinson's disease puzzle. Nat Med. 2010;16:653–661. doi: 10.1038/nm.2165. [DOI] [PubMed] [Google Scholar]
- 48.Pajares M, I Rojo A, Manda G, Boscá L, Cuadrado A. Inflammation in Parkinson's disease: mechanisms and therapeutic implications. Cells. 2020;9:1687. doi: 10.3390/cells9071687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pardon MC. Lipopolysaccharide hyporesponsiveness: protective or damaging response to the brain? Rom J Morphol Embryol. 2015;56:903–913. [PubMed] [Google Scholar]
- 50.Park S, Kim J, Chun J, Han K, Soh H, Kang EA, Lee HJ, Im JP, Kim JS. Patients with inflammatory bowel disease are at an increased risk of Parkinson's disease: a south korean nationwide population-based study. Clin Med. 2019;8:1191. doi: 10.3390/jcm8081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perry VH. Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathol. 2010;120:277–286. doi: 10.1007/s00401-010-0722-x. [DOI] [PubMed] [Google Scholar]
- 52.Piirsalu M, Taalberg E, Lilleväli K, Tian L, Zilmer M, Vasar E. Treatment with lipopolysaccharide induces distinct changes in metabolite profile and body weight in 129Sv and Bl6 mouse strains. Front Pharmacol. 2020;11:371. doi: 10.3389/fphar.2020.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol. 2001;167:5067–5076. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qin L, Liu Y, Hong JS, Crews FT. NADPH oxidase and aging drive microglial activation, oxidative stress, and dopaminergic neurodegeneration following systemic LPS administration. Glia. 2013;61:855–868. doi: 10.1002/glia.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- 56.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rocha-Ferreira E, Phillips E, Francesch-Domenech E, Thei L, Peebles DM, Raivich G, Hristova M. The role of different strain backgrounds in bacterial endotoxin-mediated sensitization to neonatal hypoxic-ischemic brain damage. Neuroscience. 2015;311:292–307. doi: 10.1016/j.neuroscience.2015.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song G, Xi G, Li Y, Zhao Y, Qi C, Song L, Xiao B, Ma C. Double triggers, nasal induction of a Parkinson's disease mouse model. Neuroscience Letters. 2020;724:134869. doi: 10.1016/j.neulet.2020.134869. [DOI] [PubMed] [Google Scholar]
- 60.Song S, Jiang L, Oyarzabal EA, Wilson B, Li Z, Shih YI, Wang Q, Hong JS. Loss of brain norepinephrine elicits neuroinflammation-mediated oxidative injury and selective caudo-rostral neurodegeneration. Mol Neurobiol. 2019;56:2653–2669. doi: 10.1007/s12035-018-1235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tansey MG, Goldberg MS. Neuroinflammation in Parkinson's disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010;37:510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang FX, Xu RS. Juglanin ameliorates LPS-induced neuroinflammation in animal models of Parkinson's disease and cell culture via inactivating TLR4/NF-κB pathway. Biomed Pharmacother. 2018;97:1011–1019. doi: 10.1016/j.biopha.2017.08.132. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Z, Zhang K, Du X, Li Y. Neuroprotection of desferrioxamine in lipopolysaccharide-induced nigrostriatal dopamine neuron degeneration. Mol Med Rep. 2012;5:562–566. doi: 10.3892/mmr.2011.671. [DOI] [PubMed] [Google Scholar]
- 64.Zhao YF, Zhang Q, Zhang JF, Lou ZY, Zu HB, Wang ZG, Zeng WC, Yao K, Xiao BG. The synergy of aging and LPS exposure in a mouse model of Parkinson's disease. Aging Dis. 2018;9:785–797. doi: 10.14336/AD.2017.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng HF, Yang YP, Hu LF, Wang MX, Wang F, Cao LD, Li D, Mao CJ, Xiong KP, Wang JD, Liu CF. Autophagic impairment contributes to systemic inflammation-induced dopaminergic neuron loss in the midbrain. PLoS One. 2013;8:e70472. doi: 10.1371/journal.pone.0070472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.