Abstract
Although there is ample evidence that central nervous system progenitor pools respond to traumatic brain injury, the reported effects are variable and likely contribute to both recovery as well as pathophysiology. Through a better understanding of the diverse progenitor populations in the adult brain and their niche-specific reactions to traumatic insult, treatments can be tailored to enhance the benefits and dampen the deleterious effects of this response. This review provides an overview of endogenous precursors, the associated effects on cognitive recovery, and the potential of exogenous cell therapeutics to modulate these endogenous repair mechanisms. Beyond the hippocampal dentate gyrus and subventricular zone of the lateral ventricles, more recently identified sites of adult neurogenesis, the meninges, as well as circumventricular organs, are also discussed as targets for endogenous repair. Importantly, this review highlights that progenitor proliferation alone is no longer a meaningful outcome and studies must strive to better characterize precursor spatial localization, transcriptional profile, morphology, and functional synaptic integration. With improved insight and a more targeted approach, the stimulation of endogenous neurogenesis remains a promising strategy for recovery following traumatic brain injury.
Key Words: cell therapy, endogenous repair, neurogenic niche, progenitors, traumatic brain injury
Traumatic Brain Injury
Traumatic brain injury (TBI) remains one of the primary causes of death and disability around the world, with estimates of more than 50 million people experiencing a TBI each year (Maas et al., 2017). Defined as brain dysfunction and pathology caused by an external force (Menon et al., 2010), TBI can result in long-term cognitive deficits (van Gils et al., 2020) as well as an increased risk of dementia (Mendez, 2017). For this reason, TBI presents a considerable health as well as economic burden, warranting a significant need for novel therapeutics.
In terms of pathophysiology, TBI is typically separated into two phases; referred to as primary and secondary injury. The primary injury corresponds to the damage inflicted at the time of trauma and is considered largely irreversible. Yet, this impact is followed by hours, days, weeks and even months of secondary damage that exacerbates the initial insult, triggering a majority of tissue loss. The secondary injury, which remains the target of most treatment strategies, is mediated by several factors, including excitotoxicity, neuroinflammation, mitochondrial dysfunction, oxidative stress, axonal degeneration, and apoptosis. While the mechanisms of secondary injury are predominantly detrimental to the injury milieu, there is also evidence for the associated activation of various central nervous system progenitor pools with potential regenerative capabilities. A better understanding of this progenitor response may be instrumental in the development of improved treatment paradigms. Further, an exogenous stem cell therapy need not result in new transplanted cells integrating with the host tissue, but rather the exogenous cells may modulate endogenous progenitor responses. This article aims to provide an overview of the niche-specific progenitor response to TBI, the associated effects of aberrant regeneration on cognitive function, and the potential application of cell therapeutics to target these events.
Search Strategy and Selection Criteria
The studies cited in the current review, published between 1993 to 2021, were searched on PubMed and Web of Science databases using the following keywords/terms: traumatic brain injury; endogenous progenitors; neural stem cells; oligodendrocyte progenitor cells; neurogenic niches; neurogenesis. Diverse variations of the search terms were applied to reach the greatest amount of literature.
Activation of Adult Neural Stem Cells in the Hippocampal Dentate Gyrus Following Traumatic Brain Injury
The hippocampal dentate gyrus subgranular zone (SGZ) is a well characterized reservoir of adult neural stem/progenitor cells, where neurogenesis is a tightly controlled process in healthy tissue (Ngwenya and Danzer, 2018) and occurs throughout an individual’s lifespan, albeit at a diminished rate over time (Kase et al., 2020). In the context of trauma, there is substantial evidence in support of injury-induced activation of SGZ progenitors and an accompanying increase neurogenesis (Dash et al., 2001; Urrea et al., 2007; Yu et al., 2008) that correlates with injury severity (Wang et al., 2016a) and may be mediated by the mammalian target of rapamycin pathway (Tee et al., 2016; Wang et al., 2016b) as well as insulin-like growth factor-1 (Carlson et al., 2014; Littlejohn et al., 2021). This has also been shown in humans following a TBI (Zheng et al., 2013).
Despite consensus on the injury-induced proliferative response, the role of endogenous progenitors in repair and injury pathogenesis remains controversial. Specifically, ablation of injury-mediated neurogenesis has been reported to limit cognitive recovery (Blaiss et al., 2011), highlighting an innate mechanism that may be responsible for some spontaneous repair. Similarly, treatments aimed at increasing SGZ neurogenesis have also been implicated in improved neurological outcome following TBI (Lu et al., 2003a). In contrast, others have reported that TBI-mediated progenitor proliferation depletes the regenerative pool (Encinas and Sierra, 2012; Neuberger et al., 2017) and the proliferative cells exhibit various morphological as well as physiological abnormalities that impair cognition (Ibrahim et al., 2016; Robinson et al., 2016; Littlejohn et al., 2020). For example, post-TBI newborn SGZ neurons have been reported to migrate ectopically towards the hilus (Ibrahim et al., 2016; Littlejohn et al., 2020), display reduced dendritic complexity, and altered directionality of processes extending through the granule cell layer (Robinson et al., 2016; Badner et al., 2021). We have reported that exogenous transplantation of human neural stem cells 30 days after TBI improves cognitive function (Haus et al., 2016; Beretta et al., 2017) while also improving the integration and directionality of endogenous newly born neurons (Badner et al., 2021). There is also recent evidence that ablation of these proliferative progenitors can limit deficits in learning and memory following repeat mild TBI (Greer et al., 2020), which correspond to other studies highlighting a role of hippocampal neurogenesis in forgetting (Frankland et al., 2013) and ectopic migration and integration of hilar granular cells in a model of epilepsy (Cameron et al., 2011). Taken together, while hippocampal neurogenesis is clearly increased by TBI, the mechanism and role in neuropathogenesis of newly born neurons on functional recovery remains uncertain. Greater neurogenesis, or prolonged neurogenesis post-TBI might not be a positive response. A better understanding of how modulation of this endogenous progenitor population and how/whether their associated response to injury can contribute to recovery is necessary for the development of effective treatment strategies aimed at endogenous repair.
Activation of Subventricular Zone Progenitors Following Traumatic Brain Injury
The subventricular zone (SVZ) of the lateral ventricles is another well-established neurogenic niche implicated in TBI. While the literature varies based on the injury model, severity, and species of study, there is consistent data in support of a proliferative response among SVZ progenitors post-injury (Chang et al., 2016). However, as SVZ precursors are largely responsible for the replacement of olfactory bulb interneurons through the rostral migratory stream in rodents, they have potent migration capabilities that complicate analysis of their behavior following trauma paradigms. Briefly, studies have documented SVZ progenitor cell migration along the corpus callosum (Costine et al., 2015), the rostral migratory stream (Goings et al., 2004; Urrea et al., 2007), and even into the injured cerebral cortex (Sundholm-Peters et al., 2005; Radomski et al., 2013; Saha et al., 2013; Dixon et al., 2015) in response to various TBI models. Further, in contrast to the SGZ, the SVZ niche is incredibly heterogenous (Xie et al., 2020), where progenitors also display tripotential in vivo. For this reason, cell fate analysis post-injury has been susceptible to differences stemming from study design. In the context of a cortical lesion, retroviral labelling of SVZ-derived progenitors in adult mice has demonstrated that they can migrate into the corpus callosum and differentiate into oligodendrocytes, while SVZ-derived progenitors that reach the lesioned cortex become astrocytes (Goings et al., 2004). This SVZ-derived astrogenic response is reportedly mediated by Thbs4 via direct Notch1 receptor binding and endocytosis to activate downstream transcription factor expression for glia production (Benner et al., 2013). In terms of function, depletion of SVZ progenitors following TBI has been found to hinder spontaneous motor recovery and increase glial hypertrophy at the injury site (Dixon et al., 2015). Although this suggests a supportive role in cortex repair, there is evidence that progenitors in the juvenile brain, widely used in the presented studies, have a more substantial response than those in the adult (Goodus et al., 2015). Much work is needed to explore the SVZ post-TBI as a potential therapeutic target, as it is significantly less understood than the hippocampal SGZ.
Progenitor Response Among Novel Neurogenic Niches Following Traumatic Brain Injury
Beyond the aforementioned “classical” progenitor niches, there have been several more recently described sites of possible adult neurogenesis that are less understood. In particular, the circumventricular organs (CVOs) (Bennett et al., 2009, 2010), along the third and fourth ventricles, as well as the meninges (Nakagomi et al., 2015; Bifari et al., 2017; Nakagomi and Matsuyama, 2017; Decimo et al., 2021) have been characterized as novel stem cell niches in the adult brain. In vitro, cells from the CVOs form neurospheres and express neural and glial markers (Bennett et al., 2009). Akin to the other progenitor pools discussed above, the CVOs, consisting of the area postrema, median eminence, and subfornical organ, all displayed an increased proliferative response to TBI (Falnikar et al., 2018). These CVO-derived Sox2+ proliferating progenitors, especially in the area postrema, displayed doublecortin expression at 4 days post-injury, suggesting neuronal potential, although their impact on cognitive recovery remains to be assessed (Falnikar et al., 2018). Largely studied in the context of ischemia, the CVO progenitor response may be more robust in models of stroke (Sanin et al., 2013; Lin et al., 2015), or simply less studied in TBI models, thus limiting our understanding of the therapeutic implications of CVO progenitors in TBI.
Also commonly overlooked, meningeal progenitors have been primarily assessed in response to ischemia (Nakagomi et al., 2011, 2012), spinal cord injury (Decimo et al., 2011) and progressive ataxia (Kumar et al., 2014). While inferring from these studies to TBI has limitations, the data shows that meningeal progenitors can generate neurons and may play a role in recovery. Our recent wok has also demonstrated engraftment of exogenous human neural stem cells along the brain meninges post-TBI, highlight a possible interaction between exogenous and endogenous progenitors within this niche (Badner et al., 2021). Overall, much more work is needed to understand this cell population and their relevance in models of trauma.
Oligodendrocyte Progenitor Cell Response Following Traumatic Brain Injury
Also known as NG2-glia, oligodendrocyte progenitor cells (OPCs) are precursors, unrestricted to a specific niche, that differentiate into oligodendrocytes in both, the developing as well as adult brain (Dimou et al., 2008). Although their primary role is believed to be oligodendrogenesis during developmental myelination and myelin plasticity (Gibson et al., 2014), OPCs maintain strict homeostatic density as well as even distribution in the brain throughout life. Best characterized in models of demyelination, OPCs become reactive (Hampton et al., 2004), proliferate and produce oligodendrocytes (Zawadzka et al., 2010). Although the OPC population remains unchanged following small laser-induced injury, with tightly controlled cell-renewal (Hughes et al., 2013), a more severe injury also results in OPC morphological changes, proliferation and migration to the site of injury (von Streitberg et al., 2021). Further, ablation of proliferating OPCs has been reported to impair and delay wound closure (von Streitberg et al., 2021), highlighting a potential role of OPCs in the endogenous repair response. While there is still much to understand about OPCs in healthy and diseased states, especially as there is growing evidence of their synaptic integration (Bergles et al., 2000; Hughes and Appel, 2019), this cell population may also be a suitable target for enhanced recovery post-TBI.
Exogenous Cell Transplantation As a Strategy to Enhance the Endogenous Progenitor Response
Mesenchymal stem/stromal cells
Mesenchymal stem/stromal cells (MSCs) are trilineage progenitors, identified by their ability to adhere to plastic and differentiate into adipocytes, chondroblasts as well as osteoblasts (Dominici et al., 2006). Widely recognized to have potent anti-inflammatory effects (Badner et al., 2017), chiefly through trophic support, MSCs have also been implicated in driving endogenous neurogenesis. Specifically, an increased endogenous progenitor response has been reported in both, the SVZ as well as the SGZ, following MSC transplant in rat (Yoo et al., 2008; Bao et al., 2011) and mouse (Kan et al., 2011) models of cerebral ischemia. Several neurotrophic factors, either directly derived from transplanted MSCs or via their interactions with the inflammatory microenvironment, are inferred in this response. In the context of TBI, the MSC secretome alone has been found to enhance endogenous neurogenesis (Liu et al., 2020), further validating the significance of trophic support in exogenous application of these cells.
Neural stem cells
Neural stem cells (NSCs) are self-renewing precursor cells with trilineage potential, able to differentiate into neurons, oligodendrocytes, and astrocytes. Like MSCs, NSCs have been reported to secrete various neurotrophic factors (Lu et al., 2003b) that can drive endogenous neurogenesis post-ischemia (Jin et al., 2011; Mine et al., 2013) as well as Alzheimer disease (Blurton-Jones et al., 2009). However, consistent with previous studies, the work has been limited to endogenous progenitor cell counts among NSC-treated animals versus vehicle controls, highlighting a greater proliferative cell response with exogenous NSC transplantation. Nevertheless, as emphasized above, proliferation alone provides an incomplete view of the complex endogenous repair mechanism, linked to recovery as well as pathogenesis. Addressing this limitation, exogenous NSC transplantation was recently associated with an altered endogenous progenitor morphology following TBI, where NSC treatment was linked to reorganization of endogenous neural progenitor processes projection through the granule cell layer of the hippocampal SGZ (Badner et al., 2021). While this suggests that NSC transplantation may help promote an environment for healthy versus aberrant neurogenesis (Figure 1), there is a significant need to better understand the endogenous cell transcriptional profile, spatial distribution and morphology following TBI.
Figure 1.
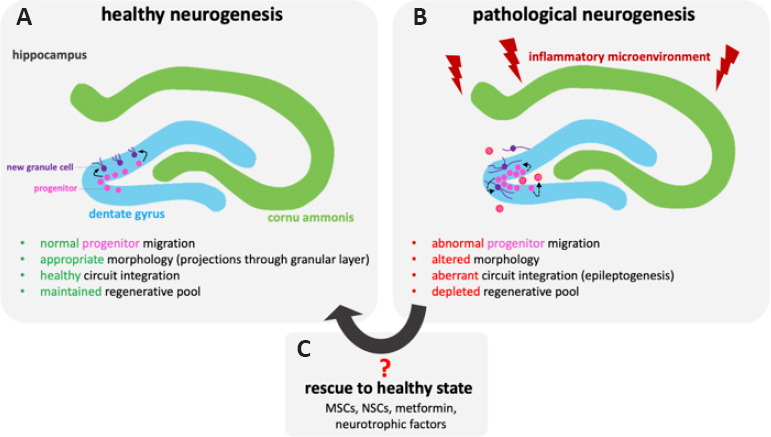
Comparison between healthy and pathological hippocampal neurogenesis following traumatic brain injury.
Overview of hippocampal neurogenesis in the dentate gyrus of healthy (A) versus injured (B) tissue. Pathology is associated with increased proliferation that may deplete the regenerative pool, aberrant circuit integration (linked to epileptogenesis) and abnormal progenitor migration as well as altered morphology. Some pathological features of neurogenesis could be potentially restored (C) through exogenous stem cell transplantation (mesenchymal stem/stromal cells or neural stem cells), metformin administration or the use of neurotrophic factors.
Non-Cellular Strategies for An Enhanced Endogenous Progenitor Response Following Traumatic Brain Injury
Metformin
The re-purposing of metformin (Potts and Lim, 2012), a drug previously applied to manage type II diabetes, has been repeatedly shown to mobilize endogenous progenitors in the hippocampus following neonatal ischemia (Dadwal et al., 2015), irradiation (Derkach et al., 2021), chemotherapy-related neurocognitive impairment (Sritawan et al., 2020) as well as TBI (DiBona et al., 2021). Most importantly, there have also been encouraging preliminary results of metformin use in survivors of pediatric brain tumors after having undergone cranial radiation (Ayoub et al., 2020). In the context of diabetes, metformin mimics a segment of the CREP binding protein, bypassing the impaired communication between the liver and pancreas to regulate glucose production. CREP binding protein has also been implicated in the atypical protein kinase C pathway responsible for recruitment of endogenous neural progenitors in the SGZ as well as the SVZ. Therefore, through mimicking the structure of CREP binding protein, metformin has been found to activate the atypical protein kinase C-pathway and stimulate endogenous neurogenesis (Wang et al., 2012). Future work should explore the spatial distribution and synaptic integration of metformin-mediated endogenous progenitors relative to injury controls, as this would give greater insight to differences in healthy versus aberrant neurogenesis.
Neurotrophins
While various neurotrophic factors have been linked to adult neurogenesis, brain-derived neurotrophic factor (BDNF) has been best studied (Henry et al., 2007; Choi et al., 2009; Vilar and Mira, 2016). Expressed in the SGZ, SVZ as well as the rostral migratory stream, the precise role of BDNF in neurogenesis remains muddled (Galvão et al., 2008; Choi et al., 2009). Specifically, heterozygous BDNF knockout has been shown to limit basal levels of hippocampal neurogenesis (Lee et al., 2002) and osmotic pump-mediated exogenous BDNF infusion reportedly increased hippocampal neurogenesis (Scharfman et al., 2005). In contrast, within the SVZ, neurogenesis was unaffected by knockdown of the BDNF receptor, tropomyosin receptor kinase B, in mice (Galvão et al., 2008). To further complicate interpretation, in the context of TBI, mRNA expression of BDNF and its receptors (tropomyosin receptor kinase B as well as p75) was decreased ipsilateral to the injury and increased on the contralateral side (Rostami et al., 2014). As the p75 BDNF receptor is a member of the tumor necrosis factor receptor superfamily (Baker and Reddy, 1996), it can either can either enhance or reduce neurotrophic function as well as independently induce apoptosis (Ip et al., 1993; MacPhee and Barker, 1997). For this reason, use of BDNF as a strategy for endogenous repair is likely dependant on a balance of its receptors, which would require further spatiotemporal analysis following TBI. Most importantly, BDNF is additionally hindered by limited blood-brain barrier permeability and rapid degradation (Cacialli, 2021), obstacles that would need to overcome for successful therapeutic application. There are additional review articles that provide a comprehensive summary of other neurotrophic factors and their application in TBI (Johanson et al., 2011; Houlton et al., 2019; Cacialli, 2021).
Limitations in Tracking the Endogenous Progenitor Response
Accurately tracking endogenous progenitors, especially following neurotrauma, continues to be a major challenge in the field. Therefore, some of the reported discrepancy, described throughout this review, may stem from use of unique species, strains, and tools to assess progenitor populations. Viral as well as genetic lineage tracing and, more recently, single cell sequencing, spatial transcriptomics and two-photon in vivo imaging all present distinct strengths and weaknesses in analysis. Further, as each presents a different perspective in the progenitor response, interpretation may vary widely based on the tools applied. For example, while genetic lineage tracing provides a large-scale overview of the cell population of interest as well as allowing for temporal assessment, the identified population is likely heterogenous, limiting understanding of interactions among subpopulations. In opposition, single cell sequencing highlights detailed transcriptional differences among specific subpopulations with limited spatiotemporal information. Future work will need to apply multiple approaches for a more complete picture.
Concluding Perspective
Overall, as highlighted throughout this review, there is significant evidence that TBI alone increases the activation and proliferation of various progenitor pools. Yet, as there are conflicting results, what remains unclear is the role of these progenitor responses in repair and injury pathogenesis. For this reason, there needs to be a significant shift in the tools used to study endogenous repair, especially when examining treatment strategies. As progenitor proliferation alone is no longer a meaningful outcome, studies must strive to better understand the precursor spatial localization, transcriptional profile, morphology, and circuit integration. Through greater insights into how progenitors contribute to recovery, we can identify better targets for an enhanced response. Therefore, with at least some repair potential among each reservoir of adult brain progenitors, stimulation of endogenous neurogenesis remains a promising strategy for recovery following neurotrauma.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
C-Editors: Zhao M, Liu WJ, Li JY; T-Editor: Jia Y.
References
- 1.Ayoub R, Ruddy RM, Cox E, Oyefiade A, Derkach D, Laughlin S, Ades-Aron B, Shirzadi Z, Fieremans E, MacIntosh BJ, de Medeiros CB, Skocic J, Bouffet E, Miller FD, Morshead CM, Mabbott DJ. Assessment of cognitive and neural recovery in survivors of pediatric brain tumors in a pilot clinical trial using metformin. Nat Med. 2020;26:1285–1294. doi: 10.1038/s41591-020-0985-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badner A, Reinhardt EK, Nguyen TV, Midani N, Marshall AT, Lepe CA, Echeverria K, Lepe JJ, Torrecampo V, Bertan SH, Tran S, Anderson A, Cummings BJ. Freshly thawed cryobanked human neural stem cells engraft within endogenous neurogenic niches and restore cognitive function following chronic traumatic brain injury. J Neurotrauma. 2021 doi: 10.1089/neu.2021.0045. doi: 10.1089/neu.2021.0045. [DOI] [PubMed] [Google Scholar]
- 3.Badner A, Siddiqui AM, Fehlings MG. Spinal cord injuries: how could cell therapy help? Expert Opin Biol Ther. 2017;17:529–541. doi: 10.1080/14712598.2017.1308481. [DOI] [PubMed] [Google Scholar]
- 4.Baker SJ, Reddy EP. Transducers of life and death: TNF receptor superfamily and associated proteins. Oncogene. 1996;12:1–9. [PubMed] [Google Scholar]
- 5.Bao X, Wei J, Feng M, Lu S, Li G, Dou W, Ma W, Ma S, An Y, Qin C, Zhao RC, Wang R. Transplantation of human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and endogenous neurogenesis after cerebral ischemia in rats. Brain Res. 2011;1367:103–113. doi: 10.1016/j.brainres.2010.10.063. [DOI] [PubMed] [Google Scholar]
- 6.Benner EJ, Luciano D, Jo R, Abdi K, Paez-Gonzalez P, Sheng H, Warner DS, Liu C, Eroglu C, Kuo CT. Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature. 2013;497:369–373. doi: 10.1038/nature12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett L, Yang M, Enikolopov G, Iacovitti L. Circumventricular organs: a novel site of neural stem cells in the adult brain. Mol Cell Neurosci. 2009;41:337–347. doi: 10.1016/j.mcn.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett LB, Cai J, Enikolopov G, Iacovitti L. Heterotopically transplanted CVO neural stem cells generate neurons and migrate with SVZ cells in the adult mouse brain. Neurosci Lett. 2010;475:1–6. doi: 10.1016/j.neulet.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beretta S, Cunningham KM, Haus DL, Gold EM, Perez H, López-Velázquez L, Cummings BJ. Effects of human ES-derived neural stem cell transplantation and kindling in a rat model of traumatic brain injury. Cell Transplant. 2017;26:1247–1261. doi: 10.1177/0963689717714107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- 11.Bifari F, Decimo I, Pino A, Llorens-Bobadilla E, Zhao S, Lange C, Panuccio G, Boeckx B, Thienpont B, Vinckier S, Wyns S, Bouché A, Lambrechts D, Giugliano M, Dewerchin M, Martin-Villalba A, Carmeliet P. Neurogenic radial glia-like cells in meninges migrate and differentiate into functionally integrated neurons in the neonatal cortex. Cell Stem Cell. 2017;20:360–373. doi: 10.1016/j.stem.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Blaiss CA, Yu T-S, Zhang G, Chen J, Dimchev G, Parada LF, Powell CM, Kernie SG. Temporally specified genetic ablation of neurogenesis impairs cognitive recovery after traumatic brain injury. J Neurosci Off J Soc Neurosci. 2011;31:4906–4916. doi: 10.1523/JNEUROSCI.5265-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Müller F-J, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cacialli P. Neurotrophins time point intervention after traumatic brain injury: from zebrafish to human. Int J Mol Sci. 2021;1585;22 doi: 10.3390/ijms22041585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cameron MC, Zhan RZ, Nadler JV. Morphologic integration of hilar ectopic granule cells into dentate gyrus circuitry in the pilocarpine model of temporal lobe epilepsy. J Comp Neurol. 2011;519:2175–2192. doi: 10.1002/cne.22623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson SW, Madathil SK, Sama DM, Gao X, Chen J, Saatman KE. Conditional overexpression of insulin-like growth factor-1 enhances hippocampal neurogenesis and restores immature neuron dendritic processes after traumatic brain injury. J Neuropathol Exp Neurol. 2014;73:734–746. doi: 10.1097/NEN.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang EH, Adorjan I, Mundim MV, Sun B, Dizon MLV, Szele FG. Traumatic braininjury activation of the adult subventricularzone neurogenic niche. Front Neurosci. 2016;10:332. doi: 10.3389/fnins.2016.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi SH, Li Y, Parada LF, Sisodia SS. Regulation of hippocampal progenitor cell survival, proliferation and dendritic development by BDNF. Mol Neurodegener. 2009;4:52. doi: 10.1186/1750-1326-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costine BA, Missios S, Taylor SR, McGuone D, Smith CM, Dodge CP, Harris BT, Duhaime A-C. The subventricular zone in the immature piglet brain: anatomy and exodus of neuroblasts into white matter after traumatic brain injury. Dev Neurosci. 2015;37:115–130. doi: 10.1159/000369091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dadwal P, Mahmud N, Sinai L, Azimi A, Fatt M, Wondisford FE, Miller FD, Morshead CM. Activating endogenous neural precursor cells using metformin leads to neural repair and functional recovery in a model of childhood braininjury. Stem Cell Rep. 2015;5:166–173. doi: 10.1016/j.stemcr.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dash PK, Mach SA, Moore AN. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res. 2001;63:313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Decimo I, Bifari F, Rodriguez FJ, Malpeli G, Dolci S, Lavarini V, Pretto S, Vasquez S, Sciancalepore M, Montalbano A, Berton V, Krampera M, Fumagalli G. Nestin- and doublecortin-positive cells reside in adult spinal cord meninges and participate in injury-induced parenchymal reaction. Stem Cells Dayt Ohio. 2011;29:2062–2076. doi: 10.1002/stem.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Decimo I, Dolci S, Panuccio G, Riva M, Fumagalli G, Bifari F. Meninges: a widespread niche of neural progenitors for the brain. Neuroscientist. 2021;27:506–528. doi: 10.1177/1073858420954826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derkach D, Kehtari T, Renaud M, Heidari M, Lakshman N, Morshead CM. Metformin pretreatment rescues olfactory memory associated with subependymal zone neurogenesis in a juvenile model of cranial irradiation. Cell Rep Med. 2021;2:100231. doi: 10.1016/j.xcrm.2021.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiBona VL, Shah MK, Krause KJ, Zhu W, Voglewede MM, Smith DM, Crockett DP, Zhang H. Metformin reduces neuroinflammation and improves cognitive functions after traumatic brain injury. Neurosci Res. 2021;172:99–109. doi: 10.1016/j.neures.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dimou L, Simon C, Kirchhoff F, Takebayashi H, Götz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci Off J Soc Neurosci. 2008;28:10434–10442. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dixon KJ, Theus MH, Nelersa CM, Mier J, Travieso LG, Yu TS, Kernie SG, Liebl DJ. Endogenous neural stem/progenitor cells stabilize the cortical microenvironment after traumatic brain injury. J Neurotrauma. 2015;32:753–764. doi: 10.1089/neu.2014.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 29.Encinas JM, Sierra A. Neural stem cell deforestation as the main force driving the age-related decline in adult hippocampal neurogenesis. Behav Brain Res. 2012;227:433–439. doi: 10.1016/j.bbr.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Falnikar A, Stratton J, Lin R, Andrews CE, Tyburski A, Trovillion VA, Gottschalk C, Ghosh B, Iacovitti L, Elliott MB, Lepore AC. Differential response in novel stem cell niches of the brain after cervical spinal cord injury and traumatic brain injury. J Neurotrauma. 2018;35:2195–2207. doi: 10.1089/neu.2017.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frankland PW, Köhler S, Josselyn SA. Hippocampal neurogenesis and forgetting. Trends Neurosci. 2013;36:497–503. doi: 10.1016/j.tins.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Galvão RP, Garcia-Verdugo JM, Alvarez-Buylla A. Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in adult mice and rats. J Neurosci Off J Soc Neurosci. 2008;28:13368–13383. doi: 10.1523/JNEUROSCI.2918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goings GE, Sahni V, Szele FG. Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 2004;996:213–226. doi: 10.1016/j.brainres.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 35.Goodus MT, Guzman AM, Calderon F, Jiang Y, Levison SW. Neural stem cells in the immature, but not the mature, subventricular zone respond robustly to traumatic brain injury. Dev Neurosci. 2015;37:29–42. doi: 10.1159/000367784. [DOI] [PubMed] [Google Scholar]
- 36.Greer K, Basso EKG, Kelly C, Cash A, Kowalski E, Cerna S, Ocampo CT, Wang X, Theus MH. Abrogation of atypical neurogenesis and vascular-derived EphA4 prevents repeated mild TBI-induced learning and memory impairments. Sci Rep. 2020;10:15374. doi: 10.1038/s41598-020-72380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hampton DW, Rhodes KE, Zhao C, Franklin RJM, Fawcett JW. The responses of oligodendrocyte precursor cells, astrocytes and microglia to a cortical stab injury, in the brain. Neuroscience. 2004;127:813–820. doi: 10.1016/j.neuroscience.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 38.Haus DL, López-Velázquez L, Gold EM, Cunningham KM, Perez H, Anderson AJ, Cummings BJ. Transplantation of human neural stem cells restores cognition in an immunodeficient rodent model of traumatic brain injury. Exp Neurol. 2016;281:1–16. doi: 10.1016/j.expneurol.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Henry RA, Hughes SM, Connor B. AAV-mediated delivery of BDNF augments neurogenesis in the normal and quinolinic acid-lesioned adult rat brain. Eur J Neurosci. 2007;25:3513–3525. doi: 10.1111/j.1460-9568.2007.05625.x. [DOI] [PubMed] [Google Scholar]
- 40.Houlton J, Abumaria N, Hinkley SFR, Clarkson AN. Therapeutic potential of neurotrophins for repair after brain injury: a helping hand from biomaterials. Front Neurosci. 2019;13:790. doi: 10.3389/fnins.2019.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hughes AN, Appel B. Oligodendrocytes express synaptic proteins that modulate myelin sheath formation. Nat Commun. 2019;4125;10 doi: 10.1038/s41467-019-12059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16:668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ibrahim S, Hu W, Wang X, Gao X, He C, Chen J. Traumatic brain injury causes aberrant migration of adult-born neurons in the hippocampus. Sci Rep. 2016;6:21793. doi: 10.1038/srep21793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ip NY, Stitt TN, Tapley P, Klein R, Glass DJ, Fandl J, Greene LA, Barbacid M, Yancopoulos GD. Similarities and differences in the way neurotrophins interact with the Trk receptors in neuronal and nonneuronal cells. Neuron. 1993;10:137–149. doi: 10.1016/0896-6273(93)90306-c. [DOI] [PubMed] [Google Scholar]
- 45.Jin K, Xie L, Mao X, Greenberg MB, Moore A, Peng B, Greenberg RB, Greenberg DA. Effect of human neural precursor cell transplantation on endogenous neurogenesis after focal cerebral ischemia in the rat. Brain Res. 2011;1374:56–62. doi: 10.1016/j.brainres.2010.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johanson C, Stopa E, Baird A, Sharma H. Traumatic brain injury and recovery mechanisms: peptide modulation of periventricular neurogenic regions by the choroid plexus-CSF nexus. J Neural Transm Vienna Austria 1996. 2011;118:115–133. doi: 10.1007/s00702-010-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kan I, Barhum Y, Melamed E, Offen D. Mesenchymal stem cells stimulate endogenous neurogenesis in the subventricular zone of adult mice. Stem Cell Rev Rep. 2011;7:404–412. doi: 10.1007/s12015-010-9190-x. [DOI] [PubMed] [Google Scholar]
- 48.Kase Y, Shimazaki T, Okano H. Current understanding of adult neurogenesis in the mammalian brain: how does adult neurogenesis decrease with age? Inflamm Regen. 2020;40:10. doi: 10.1186/s41232-020-00122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar M, Csaba Z, Peineau S, Srivastava R, Rasika S, Mani S, Gressens P, El Ghouzzi V. Endogenous cerebellar neurogenesis in adult mice with progressive ataxia. Ann Clin Transl Neurol. 2014;1:968–981. doi: 10.1002/acn3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 51.Lin R, Cai J, Nathan C, Wei X, Schleidt S, Rosenwasser R, Iacovitti L. Neurogenesis is enhanced by stroke in multiple new stem cell niches along the ventricular system at sites of high BBB permeability. Neurobiol Dis. 2015;74:229–239. doi: 10.1016/j.nbd.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 52.Littlejohn EL, DeSana AJ, Williams HC, Chapman RT, Joseph B, Juras JA, Saatman KE. IGF1-stimulated posttraumatic hippocampal remodeling is not dependent on mTOR. Front Cell Dev Biol. 2021;9:663456. doi: 10.3389/fcell.2021.663456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Littlejohn EL, Scott D, Saatman KE. Insulin-like growth factor-1 overexpression increases long-term survival of posttrauma-born hippocampal neurons while inhibiting ectopic migration following traumatic brain injury. Acta Neuropathol Commun. 2020;8:46. doi: 10.1186/s40478-020-00925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu XY, Wei MG, Liang J, Xu HH, Wang JJ, Wang J, Yang XP, Lv FF, Wang KQ, Duan JH, Tu Y, Zhang S, Chen C, Li XH. Injury-preconditioning secretome of umbilical cord mesenchymal stem cells amplified the neurogenesis and cognitive recovery after severe traumatic brain injury in rats. J Neurochem. 2020;153:230–251. doi: 10.1111/jnc.14859. [DOI] [PubMed] [Google Scholar]
- 55.Lu D, Mahmood A, Zhang R, Copp M. Upregulation of neurogenesis and reduction in functional deficits following administration of DEtA/NONOate, a nitric oxide donor, after traumatic brain injury in rats. J Neurosurg. 2003a;99:351–361. doi: 10.3171/jns.2003.99.2.0351. [DOI] [PubMed] [Google Scholar]
- 56.Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003b;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 57.Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, Bragge P, Brazinova A, Büki A, Chesnut RM, Citerio G, Coburn M, Cooper DJ, Crowder AT, Czeiter E, Czosnyka M, Diaz-Arrastia R, Dreier JP, Duhaime AC, Ercole A, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- 58.MacPhee IJ, Barker PA. Brain-derived neurotrophic factor binding to the p75 neurotrophin receptor reduces TrkA signaling while increasing serine phosphorylation in the TrkA intracellular domain. J Biol Chem. 1997;272:23547–23551. doi: 10.1074/jbc.272.38.23547. [DOI] [PubMed] [Google Scholar]
- 59.Mendez MF. What is the relationship of traumatic brain injury to dementia? J Alzheimers Dis JAD. 2017;57:667–681. doi: 10.3233/JAD-161002. [DOI] [PubMed] [Google Scholar]
- 60.Menon DK, Schwab K, Wright DW, Maas AI. Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health (2010) Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. 91:1637–1640. [Google Scholar]
- 61.Mine Y, Tatarishvili J, Oki K, Monni E, Kokaia Z, Lindvall O. Grafted human neural stem cells enhance several steps of endogenous neurogenesis and improve behavioral recovery after middle cerebral artery occlusion in rats. Neurobiol Dis. 2013;52:191–203. doi: 10.1016/j.nbd.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 62.Nakagomi T, Matsuyama T. Leptomeninges: a novel stem cell niche with neurogenic potential. Stem Cell Investig. 2017;4:22. doi: 10.21037/sci.2017.03.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakagomi T, Molnár Z, Nakano-Doi A, Taguchi A, Saino O, Kubo S, Clausen M, Yoshikawa H, Nakagomi N, Matsuyama T. Ischemia-induced neural stem/progenitor cells in the pia mater following cortical infarction. Stem Cells Dev. 2011;20:2037–2051. doi: 10.1089/scd.2011.0279. [DOI] [PubMed] [Google Scholar]
- 64.Nakagomi T, Molnár Z, Taguchi A, Nakano-Doi A, Lu S, Kasahara Y, Nakagomi N, Matsuyama T. Leptomeningeal-derived doublecortin-expressing cells in poststroke brain. Stem Cells Dev. 2012;21:2350–2354. doi: 10.1089/scd.2011.0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakagomi T, Nakano-Doi A, Matsuyama T. Leptomeninges: a novel stem cell niche harboring ischemia-induced neural progenitors. Histol Histopathol. 2015;30:391–399. doi: 10.14670/HH-30.391. [DOI] [PubMed] [Google Scholar]
- 66.Neuberger EJ, Swietek B, Corrubia L, Prasanna A, Santhakumar V. Enhanced dentate neurogenesis after brain injury undermines long-term neurogenic potential and promotes seizure susceptibility. Stem Cell Rep. 2017;9:972–984. doi: 10.1016/j.stemcr.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ngwenya LB, Danzer SC. Impact of traumatic brain injury on neurogenesis. Front Neurosci. 2018;12:1014. doi: 10.3389/fnins.2018.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Potts MB, Lim DA. An old drug for new ideas: metformin promotes adult neurogenesis and spatial memory formation. Cell Stem Cell. 2012;11:5–6. doi: 10.1016/j.stem.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Radomski KL, Zhou Q, Yi KJ, Doughty ML. Cortical contusion injury disrupts olfactory bulb neurogenesis in adult mice. BMC Neurosci. 2013;14:142. doi: 10.1186/1471-2202-14-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robinson C, Apgar C, Shapiro LA. Astrocyte hypertrophy contributes to aberrant neurogenesis after traumatic brain injury. Neural Plast. 20162016:1347987. doi: 10.1155/2016/1347987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rostami E, Krueger F, Plantman S, Davidsson J, Agoston D, Grafman J, Risling M. Alteration in BDNF and its receptors, full-length and truncated TrkB and p75(NTR) following penetrating traumatic brain injury. Brain Res. 2014;1542:195–205. doi: 10.1016/j.brainres.2013.10.047. [DOI] [PubMed] [Google Scholar]
- 72.Saha B, Peron S, Murray K, Jaber M, Gaillard A. Cortical lesion stimulates adult subventricular zone neural progenitor cell proliferation and migration to the site of injury. Stem Cell Res. 2013;11:965–977. doi: 10.1016/j.scr.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 73.Sanin V, Heeß C, Kretzschmar HA, Schüller U. Recruitment of neural precursor cells from circumventricular organs of patients with cerebral ischaemia. Neuropathol Appl Neurobiol. 2013;39:510–518. doi: 10.1111/j.1365-2990.2012.01301.x. [DOI] [PubMed] [Google Scholar]
- 74.Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 75.Sritawan N, Prajit R, Chaisawang P, Sirichoat A, Pannangrong W, Wigmore P, Welbat JU. Metformin alleviates memory and hippocampal neurogenesis decline induced by methotrexate chemotherapy in a rat model. Biomed Pharmacother Biomedecine Pharmacother. 2020;131:110651. doi: 10.1016/j.biopha.2020.110651. [DOI] [PubMed] [Google Scholar]
- 76.Sundholm-Peters NL, Yang HKC, Goings GE, Walker AS, Szele FG. Subventricular zone neuroblasts emigrate toward cortical lesions. J Neuropathol Exp Neurol. 2005;64:1089–1100. doi: 10.1097/01.jnen.0000190066.13312.8f. [DOI] [PubMed] [Google Scholar]
- 77.Tee AR, Sampson JR, Pal DK, Bateman JM. The role of mTOR signalling in neurogenesis, insights from tuberous sclerosis complex. Semin Cell Dev Biol. 2016;52:12–20. doi: 10.1016/j.semcdb.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Urrea C, Castellanos DA, Sagen J, Tsoulfas P, Bramlett HM, Dietrich WD. Widespread cellular proliferation and focal neurogenesis after traumatic brain injury in the rat. Restor Neurol Neurosci. 2007;25:65–76. [PubMed] [Google Scholar]
- 79.van Gils A, Stone J, Welch K, Davidson LR, Kerslake D, Caesar D, McWhirter L, Carson A. Management of mild traumatic brain injury. Pract Neurol. 2020;20:213–221. doi: 10.1136/practneurol-2018-002087. [DOI] [PubMed] [Google Scholar]
- 80.Vilar M, Mira H. Regulation of neurogenesis by neurotrophins during adulthood: expected and unexpected roles. Front Neurosci. 2016;10:26. doi: 10.3389/fnins.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.von Streitberg A, Jäkel S, Eugenin von Bernhardi J, Straube C, Buggenthin F, Marr C, Dimou L. NG2-glia transiently overcome their homeostatic network and contribute to wound closure after brain injury. Front Cell Dev Biol. 2021;9:662056. doi: 10.3389/fcell.2021.662056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J, Gallagher D, DeVito LM, Cancino GI, Tsui D, He L, Keller GM, Frankland PW, Kaplan DR, Miller FD. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11:23–35. doi: 10.1016/j.stem.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 83.Wang X, Gao X, Michalski S, Zhao S, Chen J. Traumatic brain injury severity affects neurogenesis in adult mouse hippocampus. J Neurotrauma. 2016a;33:721–733. doi: 10.1089/neu.2015.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang X, Seekaew P, Gao X, Chen J. Traumatic brain injury stimulates neural stemcell proliferation via mammalian target of rapamycin signaling pathway activation. eNeuro. 2016b;3 doi: 10.1523/ENEURO.0162-16.2016. eneuro 0162-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie XP, Laks DR, Sun D, Poran A, Laughney AM, Wang Z, Sam J, Belenguer G, Fariñas I, Elemento O, Zhou X, Parada LF. High-resolution mouse subventricular zone stem-cell niche transcriptome reveals features of lineage, anatomy, and aging. Proc Natl Acad Sci U S A. 2020;117:31448–31458. doi: 10.1073/pnas.2014389117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yoo SW, Kim SS, Lee SY, Lee HS, Kim HS, Lee YD, Suh-Kim H. Mesenchymal stem cells promote proliferation of endogenous neural stem cells and survival of newborn cells in a rat stroke model. Exp Mol Med. 2008;40:387–397. doi: 10.3858/emm.2008.40.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu TS, Zhang G, Liebl DJ, Kernie SG. Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. J Neurosci Off J Soc Neurosci. 2008;28:12901–12912. doi: 10.1523/JNEUROSCI.4629-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zawadzka M, Rivers LE, Fancy SPJ, Zhao C, Tripathi R, Jamen F, Young K, Goncharevich A, Pohl H, Rizzi M, Rowitch DH, Kessaris N, Suter U, Richardson WD, Franklin RJM. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6:578–590. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng W, ZhuGe Q, Zhong M, Chen G, Shao B, Wang H, Mao X, Xie L, Jin K. Neurogenesis in adult human brain after traumatic brain injury. J Neurotrauma. 2013;30:1872–1880. doi: 10.1089/neu.2010.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]


