Abstract
Amyotrophic lateral sclerosis is a relentlessly progressive multi-system condition. The clinical picture is dominated by upper and lower motor neuron degeneration, but extra-motor pathology is increasingly recognized, including cerebellar pathology. Post-mortem and neuroimaging studies primarily focus on the characterization of supratentorial disease, despite emerging evidence of cerebellar degeneration in amyotrophic lateral sclerosis. Cardinal clinical features of amyotrophic lateral sclerosis, such as dysarthria, dysphagia, cognitive and behavioral deficits, saccade abnormalities, gait impairment, respiratory weakness and pseudobulbar affect are likely to be exacerbated by co-existing cerebellar pathology. This review summarizes in vivo and post mortem evidence for cerebellar degeneration in amyotrophic lateral sclerosis. Structural imaging studies consistently capture cerebellar grey matter volume reductions, diffusivity studies readily detect both intra-cerebellar and cerebellar peduncle white matter alterations and functional imaging studies commonly report increased functional connectivity with supratentorial regions. Increased functional connectivity is commonly interpreted as evidence of neuroplasticity representing compensatory processes despite the lack of post-mortem validation. There is a scarcity of post-mortem studies focusing on cerebellar alterations, but these detect pTDP-43 in cerebellar nuclei. Cerebellar pathology is an overlooked facet of neurodegeneration in amyotrophic lateral sclerosis despite its contribution to a multitude of clinical symptoms, widespread connectivity to spinal and supratentorial regions and putative role in compensating for the degeneration of primary motor regions.
Key Words: amyotrophic lateral sclerosis, ataxia, cerebellum, magnetic resonance imaging, motor neuron disease, neuroimaging, neuroplasticity, pathology, primary lateral sclerosis, pseudobulbar affect
Introduction
Amyotrophic lateral sclerosis (ALS) is a relentlessly progressive neurodegenerative condition. While it is primarily characterized by the degeneration of upper and lower motor neurons, ALS is now recognized as a multi-system disorder. Cerebellar pathology in ALS has been demonstrated by a multitude of neuropathology and neuroimaging studies (Prell and Grosskreutz, 2013), but its role in the disease process is poorly characterized. Clinical reports of overt cerebellar signs are sparse, frank dysmetria, dysdiadochokinesia and ataxia are rarely reported in ALS (Machida et al., 1999; Schimke et al., 2002; Yasser et al., 2010; De Marco et al., 2015). The clinical assessment of the cerebellum in ALS is notoriously challenging due to concomitant upper and lower motor neuron degeneration. Eye-movement abnormalities, such as nystagmus (Kushner et al., 1984), pursuit (Jacobs et al., 1981; Gizzi et al., 1992; Donaghy et al., 2010) and saccadic impairment (Gizzi et al., 1992; Averbuch-Heller et al., 1998; Donaghy et al., 2010) have been consistently described in ALS. Bulbar dysfunction is a pathognomonic feature of ALS (Yunusova et al., 2019) which is often exclusively linked to corticobulbar tract and brainstem nuclei degeneration despite the likely contribution of cerebellar pathology to bulbar symptoms (Sasegbon and Hamdy, 2021). Primary lateral sclerosis (PLS) is a progressive, low-incidence upper motor neuron disorder which carries a better prognosis than ALS. A common symptom of both ALS and PLS is pseudobulbar affect, which is traditionally linked to corticobulbar disconnection, but cerebellar pathology is increasingly recognized as an important aetiological factor (Parvizi et al., 2007; Floeter et al., 2014; Christidi et al., 2018c).
Functional Anatomy
The cerebellum comprises two hemispheres with deep fissures and folia connected by the vermis. It is located in the posterior cranial fossa, inferior to the occipital and temporal lobes and posterior to the pons. Each hemisphere has three lobes, the flocculonodular lobe, anterior lobe, and posterior lobe divided by two fissures – the posterolateral fissure and the primary fissure. (Figure 1) The white matter surrounds four cerebellar nuclei, the fastigial, emboliform, globose and dentate nuclei. Functionally, the cerebellum is classically divided into three zones: (1) the vestibulocerebellum which is the flocculonodular lobe. It is primarily involved in balance and ocular reflexes. Inputs arise from vestibular nuclei and semicircular canals as well as visual inputs from superior colliculi and visual cortex and output synaps onto the vestibular nuclei; (2) the spinocerebellum which is made up of the vermis and the paravermis or intermediate zone on either side of the vermis. It is involved in the adjustment of body movements, error correction, and proprioception. It receives proprioceptive inputs from the dorsal columns of the spinal cord and trigeminal nerve and also visual and auditory inputs and has projections onto the deep cerebellar nuclei and brainstem; (3) the cerebrocerebellum which comprises the lateral hemispheres, lateral to the intermediate zone. It is involved in planning movements, motor learning as well as cognitive functions. It receives inputs from the cerebral cortex and pons and sends outputs to the thalamus and red nuclei (Fitzgerald et al., 2012). Phylogenetically, the cerebellum is divided as follows: (1) the archicerebellum which is the vestibulocerebellum, and is evolutionarily the oldest structure; (2) the paleocerebellum which is the spinocerebellum; (3) the neocerebellum which is the cerebrocerebellum, and is evolutionarily the newest structure (Fitzgerald et al., 2012). There are three major fiber bundles connecting the cerebellum to the rest of the brain known as the cerebellar peduncles. The superior cerebellar peduncle or brachium conjunctivum is the primary output pathway of the cerebellum. It is mostly made up of efferent fibers originating from the dentate nucleus projecting onto the midbrain, thalamus and medulla. The dentato-rubro-thalamo-cortical and cerebello-thalamo-cortical pathways pass through this peduncle. This peduncle is primarily involved in motor planning. The middle cerebellar peduncle or brachium pontis is primarily made up of afferent fibers originating from the pontine nuclei as part of the cortico-ponto-cerebellar tract. The inferior cerebellar peduncle (restiform and juxtarestiform bodies) is made up of both afferent and efferent fibres involved in proprioception and vestibular functions. The dorsal spinocerebellar tract passes through the inferior peduncle. This peduncle is involved in the maintenance of posture and balance (Fitzgerald et al., 2012). (Figure 1) Neurulation is the process by which the central portion of the ectoderm forms the neural plate which is the origin of the entire nervous system. At week 3 of gestation, the neural plate folds to form the neural tube, which in week 4 undergoes flexion at the region of the mesencephalon, which is the future midbrain. Above the mesencephalon is the prosencephalon, which is the future forebrain and beneath it is the rhombencephalon, which is the future hindbrain. The caudal rhombencephalon develops into the medulla oblongata and the rostral part becomes the pons and cerebellum (Fitzgerald et al., 2012).
Figure 1.
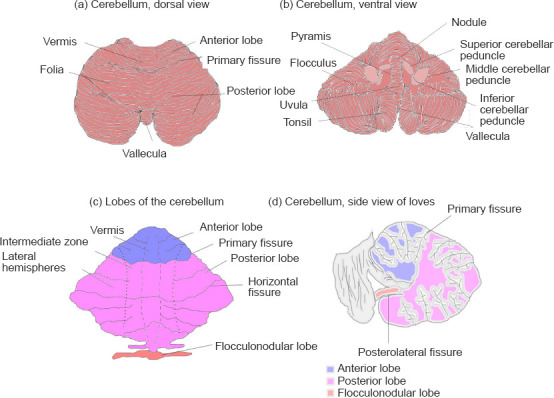
Gross anatomy of the cerebellum.
(a) Dorsal view of the cerebellum, (b) ventral view of the cerebellum, (c) lobes of the cerebellum, and (d) sagittal view of the cerebellum.
The cerebellar function has been traditionally associated with motor control and motor learning, however, physiological role in mediating cognitive and behavioral functions such as executive functions, language, spatial cognition, and mood regulation is also increasingly recognized (Buckner, 2013). The ‘Dysmetria of thought’ theory was coined to denote cognitive and emotional disorders arising from cerebellar pathology (Schmahmann, 1998). Cerebellar lobules are not only activated during motor and sensorimotor task, but during executive, working memory, language, and emotional processing paradigms (Stoodley and Schmahmann, 2009). Cerebellar pathology has been consistently implicated in impaired social cognition (Van Overwalle et al., 2015), pathological crying and laughing (Bede and Finegan, 2018; Finegan et al., 2019a), and language deficits (Runnqvist et al., 2016). Neuropsychological domains that show focal cerebellar activity on cerebellar imaging studies include attention (Allen and Courchesne, 2003), working memory (Desmond et al., 1997), and envisioning future events (Szpunar et al., 2007). Attention and visuospatial skills are noted to be particularly impaired in subjects with cerebellar insults (Malm et al., 1998). Emotional dysregulation including irritability, disinhibition, and impulsivity has been linked to lesions of the vermis (Levisohn et al., 2000). While lesions in the anterior lobe have been primarily linked to motor deficits, pathology of the posterior lobe is associated with cerebellar cognitive affective syndrome, encompassing problems with language, speech, executive and visuospatial functions and emotional affect (Tedesco et al., 2011; Argyropoulos et al., 2020). Evidence is slowly mounting that specific lobules have specific roles in mediating cognitive processes (Stoodley and Schmahmann, 2009; Argyropoulos et al., 2020). Based on the expanding spectrum of physiological roles of the cerebellum and the high number of recently published imaging studies in ALS, the main aim of this review is to summarise cerebellar neuroimaging and post-mortem findings in ALS and other motor neuron disease phenotypes. An additional objective of this manuscript is to examine the evidence for cerebellar neuroplasticity in ALS and link radiological observations to post mortem findings.
Search Strategy and Selection Criteria
A systematic literature search was performed on PubMed using the core search terms “amyotrophic lateral sclerosis”, “primary lateral sclerosis” and “motor neuron disease” combined individually with each of the following keywords as search word pairs: “cerebellum”, “cerebellar”, “magnetic resonance imaging”, “positron emission tomography”, and “post mortem”. Only original research papers were systematically reviewed. Conference abstracts, review papers, opinion pieces, and editorials were excluded. No exclusion criteria were set based on the year of publication. Based on the above criteria, a total of 69 original research papers were identified, selected, and reviewed.
Results
Imaging
Only two cerebellar studies included pre-symptomatic patients (Menke et al., 2016; Papma et al., 2017). About half of the identified studies collected genetic information, but only 11 studies stratified their ALS cohort by genotype (Tanaka et al., 1993; Canosa et al., 2016; Agosta et al., 2017; Schonecker et al., 2018; Calvo et al., 2019; Abidi et al., 2020, 2021; Hu et al., 2020). The vast majority of identified studies were cross-sectional with only 4 longitudinal imaging studies describing cerebellar changes (Keil et al., 2012; Menke et al., 2018; Calvo et al., 2019). Sample size limitations are evident, with only a minority of studies including over 100 participants. Four studies investigated over 200 participants and most of these were PET studies (Pagani et al., 2014, 2016; Canosa et al., 2020). The majority of studies were case-control studies, with only a few exceptions. While most studies reported complementary clinical or neuropsychological assessments, only a handful of studies performed clinic-radiological correlations (Verstraete et al., 2015; Consonni et al., 2019; Sala et al., 2019; Abidi et al., 2020; Canosa et al., 2021b). A minority of studies acquired data on 1.5 Tesla (T) scanners, 3T scanners were most commonly used and only one study collected data on a 7T platform (Barry et al., 2021). Over half the studies assessed multiple imaging parameters.
Grey matter alterations
While the cerebellar imaging data are characteristically challenging to quantitatively interpret, novel pipelines have been developed to evaluate infratentorial structures. Cerebellar changes are often observed on whole-brain analyses (Prell and Grosskreutz, 2013). Structural imaging readily captures cerebellar grey matter atrophy in ALS and ALS-Plus patients (Kim et al., 2017; Christidi et al., 2018d, e) and progressive cerebellar degeneration has been detected longitudinally. Cerebellar degeneration has been described in C9orf72 positive ALS patients (Agosta et al., 2017), as well as in pre-symptomatic GGGGCC hexanucleotide carriers above 40 years of age (Papma et al., 2017). A study specifically investigating genotype-associated cerebellar profiles in ALS patients revealed symmetrical patterns of cerebellar atrophy in C9orf72 ALS preferentially affecting lobules I–IV, V, VIIIA/B, IX, and the vermis (Bede et al., 2021a). In C9orf72 negative sporadic ALS patients, more focal changes were observed in lobules I–IV and V on morphometric analyses (Bede et al., 2021a). Structural cerebellar changes in the anterior lobule have been correlated to disease severity, and changes in the posterior lobule have been linked to cognitive impairment (Consonni et al., 2019). Interestingly, one study reported increased grey matter volume in the cerebellum in ALS (Qiu et al., 2019), and another detected no cerebellar atrophy (Feron et al., 2018; Schonecker et al., 2018; Additional Table 1).
Additionl table 1.
A selection of grey and white matter studies investigating cerebellar pathology in motor neuron disease
| Study | Study participants (n, patients/HCs) | Methodology | Clinical assessments | Main findings |
|---|---|---|---|---|
| Bede et al., 2021d | 161/110 | Structural (volumetry, cortical thickness), diffusion (DTI) | ALSFRS-r, cerebellar assessment | -Cerebellar pathology confined to lobules I-V of anterior lobe in patients with sporadic ALS -Considerable posterior lobe and vermis disease burden identified in C9orf72 mutation carriers -Patients with intermediate ATXN2 expansions did not exhibit significant cerebellar pathology |
| Baek et al., 2020 | 96/47 | Diffusion (DTI) | ALSFRS-r | -Significant differences between ALS and healthy controls in cerebellar peduncle |
| De Marchi et al., 2020 | 41/0 | Diffusion (DTI) | ALSFRS-r, FVC, MMSE, RCPM, Cognitive Estimates Test, FAB, Clock Drawing Test, Digit Span test, Short Story Test, Trail Making A-B Test, Attentive Matrices, verbal fluency and comprehension, NPI | -Significant FA reduction and ADC increase in all selected regions including cerebellar peduncle |
| Consonni et al., 2019 | 66/28 | Structural (cortical thickness, cortical volume) | ALSFRS-R, phonemic fluency index, object naming, SET, stroop, RAVLT, FBI | -Disease severity correlated with cerebellar cortical volume reduction in anterior lobules -Decreased cerebellar cortical volume in posterior lobules related to cognitive |
| Qiu et al., 2019 | 60/60 | Structural (VBM), diffusion (DTI) | ALSFRS-r, MOCA | -ALS patients have increased GMV in bilateral cerebellum -Decreased FC in cerebellum anterior lobe |
| Tu et al., 2019 | 19/17 | Diffusion (DTI) | ALSFRS-r | -Significant alterations across diffusion metrics in the DRTC proximal to the motor cortex were found in both ALS and PLS patient groups -PLS patients have independent diffusion abnormality in cerebellar region of DRTC and SC tracts |
| Bede and Hardiman , 2018 | 32/69 | structural (VBM, cortical thickness), diffusion (DTI) | ALSFRS-r | -Gradually progressive cerebellar grey matter degeneration throughout three time-points |
| Christidi et al., 2018c | 56/25 | structural (VBM), diffusion (DTI) | ALSFRS-r, CNS-LS, ADI-12, neuropsychological assessment | -WM abnormalities detected in WM associative and ponto-cerebellar tracts |
| Christidi et al., 2018e | 50/25 | structural (VBM), diffusion (DTI) | ALSFRS-r, MMSE, TMT, SNST-CWIS, WCST, RAVLT, BSRT, RCFT, WAIS, Age-Scale Score | -Reduced GMV in cerebellar areas in ALS -Reduced GMV in cerebellum in ALS-Plus |
| Christidi et al., 2018d | 17/22 | structural (VBM), TMS | ALSFRS-r | -Decreased GM density in cerebellar regions in ALS |
| Feron et al., 2018 | 31/14 | Structural (VBM, volumetry) | ALSFRS-R, MRC, Ashworth scale, Berg Balance scale, CVLT, Stroop, verbal fluency test, WCST, forward and backward digit span, computational gait analysis | -No cortical changes in the cerebellum |
| Menke et al., 2018 | 16/0 | Structural (VBM), diffusion (DTI), functional (rsfMRI) | ALSFRS-r, UMN score, ACE-R | -Progressive DTI changes -Increased RD, AD, MD in cerebellum |
| Schoenecker et al., 2018 | 58/19 | Structural (volumetry) | MMSE, FTD-CDR-SOB | -No significant atrophy of cerebellar regions |
| Agosta et al., 2017 | 86/22 | Structural (cortical thickness, volume), diffusion (DTI), functional (rsfMRI) | ALSFRS-r, UMN score, MMSE, RPCM, Phonemic fluency, Semantic fluency, Digit span backward, Cognitive estimation task, WCST, Weigl test, Digit span forward, Rey’s list immediate recall, Rey’s list delay recall, Oral noun confrontation naming subtest of BADA, FBI, ALS-FTD questionnaire | -C9orf72 patients showed cerebellar and thalamic atrophy |
| Kim et al., 2017 | 47/28 | structural (VBM) | ALSFRS-R, contrasting program go, no-go test, category verbal fluency test (animal item), phonemic fluency test, Stroop test color reading and backward digit span test, forward digit span test, K-BNT, auditory comprehension test, calculation, RCFT, SVLT, Korean HVLT, K-MMSE, CDR, Caregiver-Administered Neuropsychiatric Inventory | -ALSci group show decreased volume in the left cerebellum compared to healthy -ALSci show decreased brain volume in the bilateral cerebellum compared to pure ALS |
| Papma et al., 2017 | 18/15 | structural (VBM), diffusion (DTI) | Neuropsychological tests | -C9orf72RE carriers above 40 years of age, have grey matter volume loss in cerebellum |
| Bae et al., 2016 | 42/37 | Structural (VBM), diffusion (DTI), TMS | ALSFRS-r, ACE-R, CBI-R | -More grey-matter changes in the cerebellum in ALS compared to controls -In bvFTD, severe degenerative changes observed in the cerebellum |
| Trojsi et al., 2015 | 54/18 | Structural (VBM), diffusion (DTI) | ALSFRS-R, ACE-R, FrSBe, UMN score, FVC | -ALS patients had decreased FA and increased MD and RD in left cerebellar hemisphere and brainstem precerebellar nuclei |
| Bede et al., 2014 | 27/42 | Structural (cortical thickness), diffusion | ALSFRS-r (DTI) | -Higher FA in association with male gender in cerebellum |
| Bede et al., 2015 | 36/42 | Diffusion (DTI) | 0 | -FA and RD also captured diffusivity differences in the cerebellum -Bilateral symmetrical cerebellar white matter pathology detected in C9orf72 negative cohort -C9orf72 positive patients have reduced AD, MD and RD in comparison to C9orf72 negative patients |
| Floeter et al., 2014 | 47/0 | Diffusion (DTI) | ALSFRS-r, MDRS-2, MMSE, BDI-2, FrsBe, University of California Los Angeles (UCLA) Neuropsychiatric Index | -Patients with PBA had increased MD of WM tracts underlying middle cerebellar peduncle -Disruption of corticopontocerebellar pathways supports hypothesis that PBA can be viewed as a "dysmetria" of emotional expression resulting from cerebellar dysmodulation |
| Hartung et al., 2014 | 30/37 | Diffusion (VBI) | ALSFRS-r | -Widespread WM intensity increases in cerebellum |
| McCluskey et al., 2014 | 30/0 | Structural (VBM), diffusion (DTI) | clinical assessment, ALSFRS-r | -Greater cerebellar disease in ALS-plus patients |
| Bede et al., 2013 | 39/44 | Structural (cortical thickness, VBM), diffusion (DTI) | Executive function, letter fluency, category fluency, attention, memory, language, visuospatial skills, and behavioral domains | -WM changes in C9orf72 negative group confined to corticospinal and cerebellar pathways |
| Keil et al., 2012 | 24/24 | Diffusion (DTI) | ALSFRS-r, MMSE, FAB, SF36 | -Reduced FA in cerebellum in ALS |
| Prudlo et al., 2012 | 22/21 | Diffusion (DTI) | ALSFRS-r | -Widespread white matter changes in all fibre groups of the brain including cerebellum |
| Minnerop et al., 2009 | 12/12 | Structural (VBM), relaxometry (VBR) | 0 | -Reduced WM in right middle cerebellar peduncle |
| Kassubek et al., 2005 | 22/22 | Statistical parametric mapping (SPM) and VBM | NA | -White matter alterations in cerebellum |
ACE-R: Addenbrooke’s cognitive examination score; AD: axial diffusivity; ADC: apparent diffusion coefficient; ALS: amyotrophic lateral sclerosis; ADI-12: ALS-depression inventory; ALS-FTD: amyotrophic lateral sclerosis and frontotemporal dementia; ALSci: amyotrophic lateral sclerosis with cognitive impairment; ALSFRS-r: revised amyotrophic lateral sclerosis functional rating scale; ATXN2: Ataxin 2 gene; BADA: batteria per l’analisi del deficit afasico; BDI-2: Beck depression inventory-II; BSRT: Babcock story recall test; bvFTD: Behavioral variant frontotemporal dementia; C9orf72: chromosome 9 open reading frame 72 gene; CBI-R: Cambridge behavioural inventory revised; CDR: clinical dementia rating; CNS-LS: center of neurologic study lability scale; CVLT-II: California verbal learning test II; DRTC: dentato-rubro-thalamo-cortical; DTI: diffusion tensor imaging; FA: fractional anisotropy; FAB: frontal assessment battery; FBI: frontal behavioral inventory; FC: functional connectivity; FrSBe: frontal systems behavioral evaluation; FTD-CDR-SOB: FTD modified clinical dementia rating scale sum of boxes; FVC: forced vital capacity; GM: grey matter; GMV: grey matter volume; HC: healthy control; HVLT: Hopkins verbal learning test; K-BNT: Korean version of the Boston naming test; MD: mean diffusivity; MDRS-2: Mattis dementia rating Scale-2; MMSE: mini mental state exam; MOCA: Montreal cognitive assessment; MRC: the medical research council score; NPI: neuropsychiatric inventory; PBA: Pseudobulbar affect; PLS:primary lateral sclerosis; RAVLT: Rey auditory verbal learning test; RCFT: Rey’s complex figure test-immediate recall; RD: radial diffusivity; RPCM: Raven’s progressive colored matrices; rsfMRI: resting state functional magnetic resonance imaging; SC: spino-cerebellar; SET: story-based empathy task; SF36: 36-item short form health survey; SNST-CWIS: stroop neuropsychological screening test-color word interference score; SPM: statistical parametric mapping; SVLT: Seoul verbal learning test; TMS: transcranial magnetic stimulation; TMT: trail making test; UMN: upper motor neuron; VBM: voxel based morphometry; VBR: voxel-based relaxometry; WAIS: Wechsler adult intelligence scale; WCST: Wisconsin card sorting sest; WM: white matter.
White matter changes
Cerebellar white matter alterations have also been specifically studied (Keil et al., 2012; Hartung et al., 2014; Trojsi et al., 2015) and genotype-associated patterns have recently been proposed (Bede et al., 2021a). Decreased fractional anisotropy and increased axial diffusivity and radial diffusivity in lobules I–IV, V have been observed in C9orf72 ALS, while in sporadic ALS, decreased fractional anisotropy in lobules I–IV, V, IX and crura I and II and increased radial diffusivity in lobules I–IV, V, and VI was detected (Bede et al., 2021a). Cerebro-cerebellar tractography in ALS reveals preferential white matter degeneration in the cerebellar peduncles and ponto-cerebellar tracts (Minnerop et al., 2009; Prudlo et al., 2012; Christidi et al., 2018c; Baek et al., 2020; Bharti et al., 2020; De Marchi et al., 2020; Trojsi et al., 2020; Bede et al., 2021a). Significant alterations were also observed in the dentato-rubro-thalamo-cortical tract proximal to the motor cortex in both ALS and PLS (Tu et al., 2019). Progressive diffusion tensor imaging changes were noted in the cerebellum in a longitudinal study conducted over 2 years (Menke et al., 2018; Additional Table 1).
Functional and metabolic studies
Functional magnetic resonance imaging findings are relatively inconsistent(Proudfoot et al., 2018). Both decreased (Fekete et al., 2013; Trojsi et al., 2020; Barry et al., 2021) and increased cerebro-cerebellar connectivity have been reported (Agosta et al., 2011; Zhou et al., 2013; Menke et al., 2016; Abidi et al., 2021), the latter postulated to represent a compensatory process (Abidi et al., 2021). An increase in functional connectivity has been observed between the cerebellum and a network comprising the precuneus, cingulate, and middle frontal lobe in both presymptomatic and symptomatic ALS (Menke et al., 2016). Increased functional connectivity between bilateral superior parietal lobule and right anterior inferior cerebellum is thought to correlate with disease severity (Zhou et al., 2013), but direct clinico-radiological correlations are widely seen as contentious (Verstraete et al., 2015). In the paradigm-based studies increased cerebellar activation is often observed during various motor tasks (Han and Ma, 2006; Konrad et al., 2006), and particularly in patients with upper motor neuron (UMN) predominant symptoms (Abidi et al., 2020, 2021). In ALS with cognitive impairment, alterations of regional homogeneity in the right inferior cerebellar area have been noted (Hu et al., 2020). Increased cerebellar ‘degree centrality’ was observed in the posterior lobes and bilateral cerebellum crura (Zhou et al., 2016). The involvement of the cerebellar nuclei has also been demonstrated. While no volume reductions were noted in the dentate nucleus, the resting-state functional connectivity of the dentate nucleus is thought to correlate with WM changes at the superior cerebellar peduncle (Bharti et al., 2020; Additional Table 2). Significantly reduced mean regional cerebral blood flow has been also observed in the cerebellar hemispheres (Tanaka et al., 1993). The cerebellum is rarely evaluated by spectroscopy, but one study found that the cerebellar N-acetylaspartate concentrations are unaltered in patients with motor neuron disease (Gredal et al., 1997; Additional Table 2). While some PET studies detect no changes in the cerebellum (Ludolph et al., 1992), some describe hypometabolism (Calvo et al., 2019), the majority of PET studies identify cerebellar hypermetabolism in ALS (Cistaro et al., 2012; Canosa et al., 2016; Matías-Guiu et al., 2016; Buhour et al., 2017), both in spinal-onset disease (Pagani et al., 2014; Sala et al., 2019), bulbar-onset (Sala et al., 2019), as well as in ALS-FTD (Canosa et al., 2020). The reports are conflicting, with another study reporting (Additional Table 3).
Additionl table 2.
A selection of functional magnetic resonance studies evaluating cerebellar pathology in amyotrophic lateral sclerosis: functional magnetic resonance imaging & spectroscopy
| Study | Study participants (n, patients/HCs) | Methodology | Clinical assessments | Main findings |
|---|---|---|---|---|
| Abidi et al., | 31/14 | Structural, functional (fMRI) | ALSFRS-r, CVLTII, Stroop, Verbal fluency test, WCST, digit span, MIQ-rs | -UMN predominantALS patients show increased cerebellar signal during imagined locomotion -Increased effective connectivity of striato-cerebellar and parieto-cerebellar circuits may represent compensatory process |
| Barry et al., | 12/9 | Functional (fMRI) | ALSFRS-R, SVC, quantitative muscle strength test using hand-held dynamometry | -Disruption in long range functional connectivity between superior sensorimotor cortex and bilateral cerebellar lobule VI |
| Abidi et al., | 31/14 | Structural, functional (fMRI) | ALSFRS-r, CVLTII, Stroop, Verbal fluency test, WCST, digit span | -Increased cerebellar activation in UMN predominantALS patients in comparison to healthy controls and LMN predominant ALS -Increased effective connectivity between cerebellum and caudate -Decreased connectivity between SMA and cerebellum when performing self-initiated movement -UMNp patients, a positive correlation detected between clinical variables and striato-cerebellar connectivity |
| Bharti et al., | 71/56 | Structural, functional (rsfMRI), diffusion tensor imaging (DTI) | ALSFRS-r, ECAS, finger tapping, foot tapping, UMN burden | -No dentate nucleus (DN) volumetric changes -DN rsFC correlated with WM abnormalities at superior cerebellar peduncle -Altered cerebellar rsFC connectivity with motor and extra-motor regions in ALS & impaired rsFC likely due to observed cerebellar peduncular WM damage |
| Hu et al., 2020 | 42/21 | Structural (VBM), functional (rsfMRI) | ALSFRS-r,ACER-R | -Alterations of ReHo in the right inferior cerebellar area inALS with cognitive impairment |
| Trojsi et al., 2020 | 32/21 | Structural (VBM), functional (rs-fMRI), diffusion (DTI) | ALSFRS-r, MMSE, ECAS, digit span, Stroop, fluency, RAVLT-immediate and delayed recall, RCPM, HADS, ALS-FTD-Q | -Reduced functional connectivity between bilateral hippocampus, bilateral |
| Menke et al., 2016 | 24/12 | Structural (VBM), diffusion (DTI), functional (rs-fMRI) | ALSFRS-R | -FC between cerebellum and a network comprising precuneus, cingulate & middle frontal lobe significantly higher in presymptomaticALS and symptomaticALS in comparison to controls |
| Zhou et al., 2016 | 43/44 | Functional (rsfMRI) | ALSFRS-r | -ALS patients showed significant increase of DC in the left cerebellum posterior lobes & bilateral cerebellum crus in comparison to controls |
| Fekete et al., 2013 | 40/30 | Functional (rsfMRI) | ALSFRS-r | -Widespread alterations in motor functional connectivity including in cerebellum |
| Zhou et al., 2013 | 12/12 | Functional (rsfMRI) | ALSFRS-r | -Increased FC between bilateral superior parietal lobule and right anterior inferior cerebellum found to be correlated with disease severity |
| Agosta et al., 2011 | 26/15 | Functional (rsfMRI) | ALSFRS-r, MRC score | -Significantly increased functional connectivity between left SMC and right cingulate cortex, parahippocampal gyrus, and cerebellum-crus II |
| Han and Ma, 2006 | 15/15 | Functional (fMRI) | NA | -Activation areas in ipsilateral cerebellum significantly larger inALS in comparison to HCs |
| Konrad et al., 2006 | 10/10 | Functional (fMRI) | 0 | -Activation increase observed in the cerebellum |
| Schoenfeld et al., 2005 | 6/6 | structural, functional (fMRI) | ALSFRS-R, motor task | -Difficulty-related activity in the left cerebellum observed in patients |
| Gredal et al., 1997 | 10/8 | spectroscopy (MRS) | NA | -Concentration of NAAin the cerebellum unaltered in MND patients |
| Tanaka et al., 1993 | 13/13 | regional cerebral blood flow (rCBF) and oxygen metabolism (rCMRO2) | NA | -Significant reduction in the mean rCBF was also found in the cerebellar hemispheres in progressive dementia withALS |
ACE-R: Addenbrooke’s cognitive examination score; ALS: amyotrophic lateral sclerosis; ALS-FTD-Q: amyotrophic lateral sclerosis-frontotemporal dementia-questionnaire; ALSFRS-r: revised amyotrophic lateral sclerosis functional rating scale; CVLT-II: California verbal learning test II; DC: degree centrality; DN: dentate nucleus; DTI: diffusion tensor imaging; ECAS: Edinburgh cognitive and behavioural ALS screen; FC: functional connectivity; fMRI: functional magnetic resonance imaging; HADS: familton depression rating scale; HC: healthy control; LMN: lower motor neuron; MIQ-rs: movement imagery questionnaire revised 2nd version; MMSE: mini mental state exam; MRC: the medical research council score; NAA: N-acetylaspartate; RAVLT: rey auditory verbal learning test; rCBF: regional cerebral blood flow; ReHo: regional homogeneity; RPCM: Raven’s progressive colored matrices; rsFC: resting-state functional connectivity; rsfMRI: resting state functional magnetic resonance imaging; SMA: supplementary motor area; SVC: slow vital capacity; UMN: upper motor neuron; VBM: voxel based morphometry; WCST: Wisconsin card sorting test; WM: white matter.
Additionl table 3.
A selection of positron emission tomography studies commenting on cerebellar pathology in amyotrophic lateral sclerosis
| Study | Study participants (n, patients/HCs) | Methodology | Clinical assessments | Significant findings |
|---|---|---|---|---|
| Canosa et al., 2021b | 165/0 | F-FDG PET | FrsBe | -FrsBe apathy before-after gap positively correlated with cerebellar and pontine clusters |
| Canosa et al., 2021a | 111/40 | 18F-FDG-PET | ALSFRS-r, ECAS | -Negative correlation between medial frontal cluster and cerebellum found inALS patients may reflect cerebellar compensation |
| Canosa et al., 2020 | 274/0 | 18F-FDG-PET | ALSFRS-r, MMSE, The Letter and Category Fluency Test, FAB, Digit Span Forward and Backward, The Trail-Making Test (TMT) A and B, RAVLT, BSRT, ROCF, RPCM (CPM47) | -ALS-FTD patients showed cerebellar relative hypermetabolism |
| Calvo et al., 2019 | 101/0 | Structural, diffusion (DTI), 123I-ioflupane (123I-FP-CIT) SPECT, 18F-FDG-PET18 F-FDG-PET | ALSFRS-R, MRC score,Ashworth scale, neuropsychology battery, MDS-UPDRS | -ALS-PK patients showed a relative hypometabolism in left cerebellum |
| Sala et al., 2019 | 95/0 | 18F-FDG-PET | ALSFRS-r, cognitive tests | -Hypermetabolism in cerebellum in both spinal onset and bulbar onset ALS patients -Severity of motor symptoms correlates with cerebellar hypermetabolism in bulbar-onset ALS |
| Buhour et al., 2017 | 37/37 | Structural (VBM), FDG-PET | ALSFRS-r, MRC, Muscle Strength Scale, TMT, letter verbal fluency, episodic memory, theory of mind, Mattis | -Hypermetabolism in cerebellum |
| Canosa et al., 2016 | 170/NA | 18F-FDG-PET | NA | -Hypometabolism in frontal regions was associated to hypermetabolism in cerebellum |
| Matias-Guiu et al., 2016 | 18/24 | F-FDG PET, amyloid-PETwith (18)F-florbetaben | ALSFRS-r,ACE-III, MMSE, memory span, visuospatial span (Corsi block-tapping test), TMT, ROCF, Free and Cued Selective Reminding Test, VOSP, Stroop Color-Word Interference Test, letter verbal fluency, category fluency test, Tower of London, action fluency tests, picture-sentence matching tasks, semantic association tests, the Hayling Test, ECAS, CBI-R) Anosognosia Questionnaire for Dementia | -ALS exhibit hypometabolism in frontal area and hypermetabolism in cerebellum compared to healthy controls -Changes in metabolism in cerebellum in ALS with or without cognitive impairment |
| Pagani et al., 2016 | 259/40 | FDG-PET | ALSFRS-r | -Cerebellar/midbrain component accounted for highest accuracy in separatingALS patients from controls |
| Pagani et al., 2014 | 195/40 | (18)F-FDG-PET | 0 | -spinal patients have relative hypermetabolism in the right cerebellum |
| Cistaro et al., 2012 | 32/22 | F-FDG PET | Phonological verbal fluency test (FAS), TMT, Stroop, WCST | -Highly significant relative increases in glucose metabolism distribution in cerebellum inALS compared to HCs |
| Ludolph et al., 1992 | 18/NA | PET | Neuropsychological assessment | -No changes seen in cerebellum |
| Dalakas et al., 1987 | 12/11 | [18F]FDG-PET | 0 | -Hypometabolism did not extend to cerebellum |
ACE-III: Addenbrooke’s cognitive examination III; ALS: amyotrophic lateral sclerosis; ALS-FTD: amyotrophic lateral sclerosis and frontotemporal dementia; ALS-PK: amyotrophic lateral sclerosis with parkinsonian symptoms; ALSFRS-r: revised amyotrophic lateral sclerosis functional rating scale; BSRT: Babcock story recall test; CBI-R: Cambridge behavioural inventory revised; DTI: diffusion tensor imaging; ECAS: Edinburgh cognitive and behavioural ALS screen; F-FDG: [18]F-fluorodeoxyglucose; FAB: frontal assessment battery; FrSBe: frontal systems behavioral evaluation; HC: healthy control; MDS-UPDRS: movement disorders society unified Parkinson’s disease rating scale; MMSE: mini mental state exam; MRC: the medical research council score; PET: positron emission tomography; RAVLT: Rey auditory verbal learning test ; ROCF: Rey-Osterrieth complex figure; RPCM: Raven’s progressive colored matrices; SPECT: single-photon emission computerized tomography; TMT: trail making test; VOSP: visual object and space perception battery; WCST: Wisconsin card sorting test.
Post-mortem findings
While cerebellar changes are seldom evaluated specifically, cerebellar pathology is readily observed post-mortem (Ishihara et al., 2006; Kobayashi et al., 2011). UBQLN-positive cytoplasmic inclusions in the cerebellar granular layer (Brettschneider et al., 2012), neurofibrillary tangles (Yokota et al., 2006), and neuronal and glial TDP-43 pathology have been consistently described (Geser et al., 2008). Cerebellar degeneration has been linked to bulbar ALS (Shellikeri et al., 2017), but other studies do not identify significant cerebellar alterations (Przedborski et al., 1996; Petri et al., 2006; Additional Table 4). p62 positive, p-TDP-43 negative neuronal intranuclear inclusions (Rohrer et al., 2011) have been observed in C9orf72 and the severity of cerebellar changes are thought to depend on repeat length expansion (van Blitterswijk et al., 2013). In PLS, cerebellar involvement is clearly established. Some studies observed ballooning of dendrites in molecular and Purkinje cell layers of the cerebellum (Sugihara et al., 1999), and dentate nucleus degeneration (Kosaka et al., 2012), while others found no significant changes (Engel and Grunnet, 2000).
Additionl table 4.
A selection of post mortem studies describing cerebellar pathology in amyotrophic lateral sclerosis
| Study | Summary of findings |
|---|---|
| Jones et al., 2021 | HML6_3p21.31c consistently upregulated in ALS in cerebellum tissue |
| Yang et al., 2019 | Similar levels of nonphosphorylated TDP-43 were found in all 3 regions (motor cortex, spinal cord, cerebellar vermis) between ALS groups (ALS with repeat expansions in the ATXN2 or C9orf72 genes and sporadic disease) |
| Blitterswijk et al., 2013 | -Repeat lengths in the cerebellum were smaller than those in the frontal cortex and those in blood in C9orf72 symptomatic patients -Substantial variation in repeat sizes between samples from the cerebellum, frontal cortex, and blood -Longer repeat sizes in the cerebellum associated with survival disadvantage |
| Brettschneider et al., 2012 | UBQLN pathology showed a highly distinct pattern in ALS and FTLD-TDP cases with the C9orf72 expansion, with UBQLN-positive cytoplasmic inclusions in cerebellar granular layer |
| Kobayashi et al., 2011 | Degeneration in posterior cerebellar tract |
| Rohrer et al., 2011 | Abundant p62 positive, p-TDP-43 negative neuronal intranuclear inclusions (NIIs) observed in 6 out of 14 cases in the cerebellar granular layer in C9orf72 symptomatic patients |
| Geser et al., 2008 | Neuronal and glial TDP-43 pathology present in multiple areas of the central nervous systems of ALS patients, including cerebellum |
| Ishihara et al., 2006 | Severe and widespread degeneration in CNS including in cerebellum |
| Petri et al., 2006 | No disease-specific differences of the mRNA expression of the investigated subunits in cerebellar cortex |
| Yokota et al., 2006 | Neurofibrillary tangles (NFTs) found in cerebellum |
| Przedborski et al., 1996 | Glutathione peroxidase activity not significantly altered in the cerebellar cortex in |
| ALS compared to controls | |
| Plaitakis et al., 1988 | Glutamate levels significantly decreased in all areas investigated (frontal and cerebellar cortex and two areas of spinal cord ) |
ALS: Amyotrophic lateral sclerosis; ATXN2: Ataxin 2 gene; C9orf72: chromosome 9 open reading frame 72 gene; NFTs: neurofibrillary tangles; TDP-43: TAR DNA-binding protein 43; UBQLN: ubiquilin like.
Animal models
With few exceptions (Aguirre et al., 2005), animal models of ALS also exhibit cerebellar pathology. In SOD1(G93A) transgenic mice, PARP-immunoreactive astrocytes (Chung et al., 2004) and pERK-immunoreactive astrocytes (Chung et al., 2005) were observed in cerebellar nuclei. Reduced tau-mRNA expression was observed at the symptomatic stage of ALS (Barańczyk-Kuźma et al., 2007). Increased cerebellar TRPV4 expression (Lee et al., 2012) and enhanced LOX gene expression (Li et al., 2004) have also been shown. Cerebellar Purkinje cell degeneration and neuroinflammation were noted in MATR3 S85C knock-in mice (Kao et al., 2020), C9orf72 mice (Chew et al., 2015) as well as in SOD1 mice (Li et al., 2004).
Discussion
Neuroimaging and post-mortem infratentorial pathology in ALS
It is increasingly recognized that symptom manifestation in ALS is preceded by a long pre-symptomatic phase (Eisen et al., 2014b; Schuster et al., 2015), and to clarify the role of the cerebellum in the pathogenesis and verify proposed compensatory processes, more pre-symptomatic imaging studies are needed specifically evaluating infratentorial changes (Menke et al., 2016; Papma et al., 2017). Cerebellar findings in ALS are strikingly inconsistent; many studies report widespread cerebellar degeneration (Tu et al., 2019; Bede et al., 2021a), others detect no changes (Przedborski et al., 1996; Gredal et al., 1997; Petri et al., 2006; Schonecker et al., 2018). Both increased (Qiu et al., 2019) and decreased grey matter volumes have been reported, both increased (Agosta et al., 2011; Zhou et al., 2013; Menke et al., 2016; Abidi et al., 2021) and reduced functional cerebro-cerebellar connectivity (Trojsi et al., 2020), cerebellar hypermetabolism (Canosa et al., 2016; Matías-Guiu et al., 2016; Buhour et al., 2017) and hypometabolism (Calvo et al., 2019) have been observed. Very few imaging studies assessed disease burden in specific cerebellar lobules (Consonni et al., 2019; Barry et al., 2021; Bede et al., 2021a), cerebellar nuclei (Bharti et al., 2020) or the cerebellar peduncles (Minnerop et al., 2009; Floeter et al., 2014; Baek et al., 2020; Bharti et al., 2020; De Marchi et al., 2020). Because of the considerable clinical heterogeneity of ALS, stratification by genotype and phenotype-specific changes is essential. Delineating disease heterogeneity and objective stratification in ALS are particularly critical for drug development (Chipika et al., 2019). Only a minority of cerebellar imaging studies investigated different subtypes of ALS contrasting LMN versus UMN predominant (Abidi et al., 2020, 2021), ALS with and without cognitive impairment (Tanaka et al., 1993; Canosa et al., 2016; Schonecker et al., 2018; Hu et al., 2020), ALS-motor and ALS-plus, and C9orf72 positive and C9orf72 negative ALS (Agosta et al., 2017; Schonecker et al., 2018). Only a handful of studies included PLS (Floeter et al., 2014; Menke et al., 2018; Tu et al., 2019), PMA (Gredal et al., 1997), FTD, and ALS-FTD (Bae et al., 2016; Schonecker et al., 2018) cohorts. The strikingly different stratification strategies illustrate just one of the many methodological differences across cerebellar imaging studies that may explain the inconsistencies in the literature. It is also noteworthy that the vast majority of ALS imaging studies are cross-sectional encompassing patients at various stages of their disease trajectories adding to sample heterogeneity (Chipika et al., 2019). Sample heterogeneity (genetic, phenotypic, symptom duration) coupled with sample size limitations are likely to be key contributors to the contradictory findings. It is also widely recognized that imaging studies in ALS have an inherent inclusion bias to patients with less severe disability, limited bulbar impairment, less severe sialorrhoea, and relatively good respiratory function. Patients with frank orthopnoea, patients reliant on continuous non-invasive ventilation, patients with very poor mobility are much less likely to participate in imaging studies, therefore our insights gained from lengthy imaging protocols may not be fully representative of disease burden across the entire clinical spectrum of ALS.
There is often an expectation in imaging studies to explore clinico-radiological correlations even though cerebellar signs, gait abnormalities, eye-movement abnormalities, and cognitive deficits are confounded by coexisting UMN, LMN and frontotemporal degeneration in ALS (Verstraete et al., 2015; Christidi et al., 2018a, 2019). Nevertheless, cerebellar changes have been linked to disease severity (Consonni et al., 2019), motor symptoms (Sala et al., 2019), cognitive impairment (Consonni et al., 2019), and apathy (Canosa et al., 2021b). One study using computational gait analysis reveals the contribution of extrapyramidal deficits rather than cerebellar pathology to gait impairment highlighting the need for objective measures of clinical findings (Feron et al., 2018). The majority of cerebellar imaging studies evaluate multiple parameters which is helpful to determine which biomarkers are most sensitive to detect and track progressive cerebellar changes. Despite the overwhelming focus on spinal and supratentorial changes, cerebellar pathology has also been evaluated post-mortem (Ishihara et al., 2006). UBQLN-positive cytoplasmic inclusions (Brettschneider et al., 2012), neurofibrillary tangles (Yokota et al., 2006), and TDP-43 pathology (Geser et al., 2008) have been described in the cerebellum. The predilection to specific cerebellar lobules, vermis or deep cerebellar nuclei however has not been systematically characterized post mortem in specific ALS phenotypes and genotypes. There is a notion that neuronal networks underpinning phylogenetically “recent” skills, such as fine motor skills, vocalization, ambulation, etc. are particularly vulnerable to ALS (Eisen et al., 2014a; Eisen and Bede, 2021), which may explain the preferential atrophy of certain cerebellar regions, but these associations remain to be elucidated. It is also unfortunate that large imaging studies of well-characterized patients do not typically offer complementary post-mortem analyses to interpret their ante-mortem imaging findings.
A multitude of functional magnetic resonance imaging studies showed increased cerebellar activation when performing motor tasks (Konrad et al., 2006; Prell and Grosskreutz, 2013; Proudfoot et al., 2018; Bede et al., 2021a), and resting-state functional magnetic resonance imaging studies often reveal increased cerebro-cerebellar connectivity (Agosta et al., 2011; Zhou et al., 2013; Menke et al., 2016; Abidi et al., 2021). These two observations are often interpreted as a compensatory, adaptive change to motor cortex degeneration despite the lack of supporting structural findings and compelling post mortem evidence. Neuroplasticity is an intrinsic property of the nervous system, allowing some degree of reorganization at a molecular, cellular, or anatomical level (Pascual-Leone et al., 2005) which enables the nervous system to adapt to insult and compensate for injury (Villamar et al., 2012). Effective neuroplasticity has been demonstrated in a multitude of neurological conditions such as traumatic brain injury (Villamar et al., 2012), stroke (Dimyan and Cohen, 2011), multiple sclerosis (Straudi and Basaglia, 2017), Alzheimer’s disease (Herholz et al., 2013) and spinal cord injury (Hutson and Di Giovanni, 2019), and similar adaptive mechanisms have also been proposed in ALS (Mohammadi et al., 2011). Based largely on abnormal activation patterns during motor tasks, it has been proposed that additional brain regions compensate for primary motor cortex degeneration such as the ipsilateral motor cortex, supplementary motor and premotor areas (Konrad et al., 2002), as well as in the basal ganglia (Tessitore et al., 2006) and cerebellum (Schoenfeld et al., 2005; Han and Ma, 2006; Prell and Grosskreutz, 2013; Proudfoot et al., 2018). The cerebellum in particular is thought to exhibit remarkable neuroplasticity, withstand considerable cerebellar insults (Konczak et al., 2010) and adapt to extra-cerebellar pathologies (Mitoma et al., 2020). The activation shift from primary motor areas to the cerebellum during movement execution is often interpreted as evidence of an adaptive or compensatory process, but very few structural studies (Qiu et al., 2019) and no post mortem studies have actually captured frank hypertrophy. An alternative explanation for increased functional connectivity center on the loss of cortical inhibition (Prell and Grosskreutz, 2013; Proudfoot et al., 2018).
Cerebellar findings in related neurodegenerative conditions
The link between ALS and FTD is increasingly accepted based on shared clinical, genetic, and imaging features (Omer et al., 2017; Christidi et al., 2018a, b). Frontotemporal dysfunction is a key dimension of clinical heterogeneity in ALS and the concept of an ALS-FTD spectrum or continuum is now widely accepted. Cerebellar changes have been consistently described in GGGGCC hexanucleotide repeat carriers in C9orf72 (Mahoney et al., 2012; Rohrer et al., 2015; Bede et al., 2021a; McKenna et al., 2021). While comorbid FTD was once primarily associated with hexanucleotide repeats, recent data have shown that frontotemporal degeneration is not exclusively caused by C9orf72 repeat expansions in ALS (Westeneng et al., 2016). Cerebellar changes have also been reported in FTD cohorts without ALS and GGGGCC repeat expansions (Bocchetta et al., 2016) For example, preferential vermis atrophy was described in MAPT carriers (Bocchetta et al., 2016). Other cerebellar studies of FTD stratified their patients by the clinical phenotype (Gellersen et al., 2017; Chen et al., 2018, 2020; McKenna et al., 2021) and associations were identified between cerebellar pathology and performance in specific cognitive domains such as attention, working memory, visuospatial skills, and language (Chen et al., 2018, 2020). Lobule VI, VIIb, VIIIb atrophy was noted in bvFTD and crus I and lobule VI volume loss was seen in semantic variant primary progressive aphasia (svPPA) (Chen et al., 2019). Cerebellar grey matter atrophy was captured pre-symptomatic C9orf72 carriers (Cash et al., 2018) and ALS-FTD (Tan et al., 2014). PLS is a low incidence disorder of the upper motor neurons with relatively limited imaging literature (Pioro et al., 2020) and overlapping clinical, radiological, and genetic features with ALS (Bede et al., 2019; Finegan et al., 2019b, c). Very few imaging studies have specifically commented on cerebellar changes in PLS. Intra-cerebellar white matter alterations (Finegan et al., 2021), dentato-rubro-thalamo-cortical and spino-cerebellar tract diffusivity changes (Tu et al., 2019) and increased functional connectivity were reported between the cerebellum and cortical motor, frontal and temporal areas (Meoded et al., 2015). PLS patients with cognitive deficits are thought to exhibit fractional anisotropy reductions and increased radial diffusivity in cerebellar white matter (Canu et al., 2013). In spinal muscular atrophy preferential lobule VIIIB, IX and X atrophy has been observed without notable correlations with clinical metrics (de Borba et al., 2020). Hereditary spastic paraplegia, which can be mistaken for UMN-predominant ALS or PLS, is also associated with considerable cerebellar grey and white matter degeneration (Orlacchio et al., 2004; Seidel et al., 2009; Lindig et al., 2015; Thal et al., 2015; Olivieri et al., 2019; Servelhere et al., 2021). Cerebellar changes are also commonly observed in spinal and bulbar muscular atrophy or Kennedy’s disease which is an X-linked recessive, slowly progressive, LMN-predominant motor neuron disease (Pieper et al., 2013; Pradat et al., 2020). Interestingly, cerebellar integrity metrics are typically superior in poliomyelitis survivors compared to demographically-matched healthy controls (Li Hi Shing et al., 2021b), which is often regarded as evidence of neuroplasticity and compensation for longstanding spinal anterior horn insult (Li Hi Shing et al., 2021a, c).
Clinical relevance
Though woefully understudied in ALS, cerebellar pathology is thought to contribute to pseudobulbar affect (Floeter et al., 2014; Finegan et al., 2019a), cognitive deficits (Consonni et al., 2019; Bede et al., 2021a), behavioral dysfunction (Bae et al., 2016; Canosa et al., 2021b), bulbar symptoms (Sala et al., 2019; Yunusova et al., 2019), respiratory problems (Xu and Frazier, 2002), gait impairment (Schimke et al., 2002; Yasser et al., 2010) and eye movement abnormalities (Jensen et al., 2019). The role of the cerebellum in mediating cognitive, and behavioral processes is increasingly well established (Van Overwalle et al., 2015; Guo et al., 2016; Schmahmann, 2019; Argyropoulos et al., 2021). Multi-time point longitudinal studies have shown that the corticospinal tracts and the corpus callosum are involved very early in the disease process (Schuster et al., 2016b; Bertrand et al., 2018; Querin et al., 2019a; Wen et al., 2019), whereas cerebellar changes exhibit a progressive change in the symptomatic phase of the disease (Menke et al., 2018). Regions that are affected early in the disease process may help diagnostic or phenotypic classification (Schuster et al., 2016a; Querin et al., 2018; Grollemund et al., 2019), but regions which exhibit progressive change in the later disease phases are better suited as tracking biomarkers (Schuster et al., 2015; Chipika et al., 2019). It is noteworthy that commonly used image analysis suites which evaluate cortical thickness profiles, such as FreeSurfer, only provide supratentorial segmentation, therefore many longitudinal ALS studies using this software do not evaluate cerebellar changes at all (Tahedl et al., 2021a). The prevailing clinical understanding of ALS-associated disability is overwhelmingly centered in UMN/LMN degeneration and the wider recognition of cerebellar pathology would also inform and refine multidisciplinary rehabilitation strategies, fall prevention, occupational therapy, speech and language therapy, management of pseudobulbar affect and synchronization with non-invasive ventilation. ALS is now universally regarded as part of the ALS-FTD spectrum, but mounting clinical and genetic evidence suggests that an ataxia-ALS continuum also exists. Ataxia-ALS overlap syndromes have been described in association with spinocerebellar ataxia type 1 and type 2 (Van Damme et al., 2011; Tazelaar et al., 2020). While 33 or more CAG repeats in the ATXN1 and ATXN2 genes are linked to spinocerebellar ataxia, an intermediate number of repeats in these genes is a risk factor for ALS (Neuenschwander et al., 2014; Tazelaar et al., 2020). ALS patients with intermediate ATXN1 and ATXN2 mutations often have a positive family history of spinocerebellar ataxia (Morello et al., 2020; Tazelaar et al., 2020) and spinocerebellar ataxia type 1 and type 2 patients can present with or eventually develop an ALS-like phenotype (Nanetti et al., 2009; Morello et al., 2020). Despite the emerging recognition of an ataxia-ALS continuum, ALS patients are not routinely screened for trinucleotide repeat expansions in ATXN1 and ATXN2 and a targeted cerebellar examination is seldom performed in ALS clinics (Bede et al., 2021a). Subtle cerebellar manifestations in ALS are notoriously difficult to ascertain due to confounding UMN and LMN signs which dominate the clinical picture. Spinocerebellar ataxias also share some clinical features with ALS such as dysphagia, and even muscle wasting and dementia, particularly in advanced stages (Sun et al., 2016; Antenora et al., 2017). A key lesson of the emerging ATXN1 and ATXN2 literature is that patients presenting to ataxia clinics should be carefully evaluated for UMN and LMN signs, and patients attending ALS clinics should be assessed for cerebellar signs.
Future directions
The contribution of cerebellar pathology to the clinical manifestations of ALS are poorly characterized and robust dedicated cerebellar studies are needed to dissect the role of infratentorial and supratentorial changes to motor disability, cognitive deficits, behavioral disturbances, pseudobulbar affect, dysphagia, and respiratory regulation. While putative compensatory processes were inferred from functional imaging studies, these have not been demonstrated by compelling structural and post mortem data to date. Emerging genetic data reveal an ataxia-ALS spectrum, the anatomical correlates of which have not been duly explored by dedicated imaging and post mortem studies. Cerebello-spinal connectivity is particularly poorly explored in ALS, despite emerging quantitative spinal imaging techniques (El Mendili et al., 2019; Querin et al., 2019b). From an academic perspective, the role of the cerebellum in the pathogenesis of ALS and disease propagation is not established, despite its ample projections to key ALS-associated foci; the spinal cord and motor cortex. At present, cerebellar disease burden remains an overlooked facet of ALS despite its wide-ranging clinical and academic relevance.
The field of motor neuron disease imaging has seen unprecedented technological innovations in recent years, including the emergence of ultra-high-field platforms, a gradual transition to cloud-based applications, the increasing implementation of machine-learning frameworks, emphasis on longitudinal modeling, and a shift to non-Gaussian diffusion imaging (Barritt et al., 2018; Broad et al., 2019; Bede et al., 2021b; Tahedl et al., 2021b). Methods for evaluating the cerebellum have also improved considerably; robust cerebellar analysis pipelines have been published, high-resolution cerebellar atlases have been made available and novel cerebellar normalization templates have been developed (Diedrichsen, 2006; Diedrichsen et al., 2009; Diedrichsen and Zotow, 2015; Romero et al., 2017). Thanks to momentous methodological advances in neuroimaging, animal model technologies and immunohistochemistry, the armamentarium of clinical, radiological and biological tools are finally at our disposal to conduct authoritative integrative cerebellar studies.
Conclusions
While the cerebellum is recognized to be involved in the disease process in ALS, the specific contribution of cerebellar pathology to cardinal clinical manifestations is poorly characterized. There is currently a disproportionate emphasis on supratentorial changes both in imaging and post mortem studies. Recent advances in genetics, imaging technology, and data analysis pipelines offer unprecedented opportunities to clarify the role of the cerebellum in mediating disease propagation in ALS and its contribution to clinical symptoms.
Additional files:
Additional Table 1: A selection of grey and white matter studies investigating cerebellar pathology in motor neuron disease.
Additional Table 2: A selection of functional magnetic resonance studies evaluating cerebellar pathology in amyotrophic lateral sclerosis: functional magnetic resonance imaging & spectroscopy.
Additional Table 3: A selection of positron emission tomography studies commenting on cerebellar pathology in amyotrophic lateral sclerosis.
Additional Table 4: A selection of post mortem studies describing cerebellar pathology in amyotrophic lateral sclerosis.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declared.
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Funding: This work was supported by the Spastic Paraplegia Foundation (SPF). Professor Peter Bede and the Computational Neuroimaging Group are also supported by the Health Research Board (HRB EIA-2017-019), the Irish Institute of Clinical Neuroscience (IICN), the EU Joint Programme-Neurodegenerative Disease Research (JPND), the Andrew Lydon scholarship, and the Iris O’Brien Foundation.
C-Editors: Zhao M, Liu WJ, Wang Lu; T-Editor: Jia Y.
References
- 1.Abidi M, de Marco G, Couillandre A, Feron M, Mseddi E, Termoz N, Querin G, Pradat PF, Bede P. Adaptive functional reorganization in amyotrophic lateral sclerosis: coexisting degenerative and compensatory changes. Eur J Neurol. 2020;27:121–128. doi: 10.1111/ene.14042. [DOI] [PubMed] [Google Scholar]
- 2.Abidi M, de Marco G, Grami F, Termoz N, Couillandre A, Querin G, Bede P, Pradat PF. Neural correlates of motor imagery of gait in amyotrophic lateral sclerosis. J Magn Reson Imaging. 2021;53:223–233. doi: 10.1002/jmri.27335. [DOI] [PubMed] [Google Scholar]
- 3.Agosta F, Valsasina P, Absinta M, Riva N, Sala S, Prelle A, Copetti M, Comola M, Comi G, Filippi M. Sensorimotor functional connectivity changes in amyotrophic lateral sclerosis. Cereb Cortex. 2011;21:2291–2298. doi: 10.1093/cercor/bhr002. [DOI] [PubMed] [Google Scholar]
- 4.Agosta F, Ferraro PM, Riva N, Spinelli EG, Domi T, Carrera P, Copetti M, Falzone Y, Ferrari M, Lunetta C, Comi G, Falini A, Quattrini A, Filippi M. Structural and functional brain signatures of C9orf72 in motor neuron disease. Neurobiol Aging. 2017;57:206–219. doi: 10.1016/j.neurobiolaging.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Aguirre N, Beal MF, Matson WR, Bogdanov MB. Increased oxidative damage to DNA in an animal model of amyotrophic lateral sclerosis. Free Radic Res. 2005;39:383–388. doi: 10.1080/10715760400027979. [DOI] [PubMed] [Google Scholar]
- 6.Allen G, Courchesne E. Differential effects of developmental cerebellar abnormality on cognitive and motor functions in the cerebellum: an fMRI study of autism. Am J Psychiatry. 2003;160:262–273. doi: 10.1176/appi.ajp.160.2.262. [DOI] [PubMed] [Google Scholar]
- 7.Antenora A, Rinaldi C, Roca A, Pane C, Lieto M, Saccà F, Peluso S, De Michele G, Filla A. The multiple faces of spinocerebellar ataxia type 2. Ann Clin Transl Neurol. 2017;4:687–695. doi: 10.1002/acn3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Argyropoulos GD, Christidi F, Karavasilis E, Velonakis G, Antoniou A, Bede P, Seimenis I, Kelekis N, Douzenis A, Papakonstantinou O, Efstathopoulos E, Ferentinos P. Cerebro-cerebellar white matter connectivity in bipolar disorder and associated polarity subphenotypes. Prog Neuropsychopharmacol Biol Psychiatry. 2021;104:110034. doi: 10.1016/j.pnpbp.2020.110034. [DOI] [PubMed] [Google Scholar]
- 9.Argyropoulos GPD, van Dun K, Adamaszek M, Leggio M, Manto M, Masciullo M, Molinari M, Stoodley CJ, Van Overwalle F, Ivry RB, Schmahmann JD. The cerebellar cognitive affective/schmahmann syndrome: a task force paper. Cerebellum. 2020;19:102–125. doi: 10.1007/s12311-019-01068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Averbuch-Heller L, Helmchen C, Horn AK, Leigh RJ, Büttner-Ennerver JA. Slow vertical saccades in motor neuron disease: correlation of structure and function. Ann Neurol. 1998;44:641–648. doi: 10.1002/ana.410440410. [DOI] [PubMed] [Google Scholar]
- 11.Bae JS, Ferguson M, Tan R, Mioshi E, Simon N, Burrell J, Vucic S, Hodges JR, Kiernan MC, Hornberger M. Dissociation of structural and functional integrities of the motor system in amyotrophic lateral sclerosis and behavioral-variant frontotemporal dementia. J Clin Neurol. 2016;12:209–217. doi: 10.3988/jcn.2016.12.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baek SH, Park J, Kim YH, Seok HY, Oh KW, Kim HJ, Kwon YJ, Sim Y, Tae WS, Kim SH, Kim BJ. Usefulness of diffusion tensor imaging findings as biomarkers for amyotrophic lateral sclerosis. Sci Rep. 2020;10:5199. doi: 10.1038/s41598-020-62049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barańczyk-Kuźma A, Usarek E, Kuźma-Kozakiewcz M, Kaźmierczak B, Gajewska B, Schwalenstocker B, Ludolph AC. Age-related changes in tau expression in transgenic mouse model of amyotrophic lateral sclerosis. Neurochem Res. 2007;32:415–421. doi: 10.1007/s11064-006-9242-4. [DOI] [PubMed] [Google Scholar]
- 14.Barritt AW, Gabel MC, Cercignani M, Leigh PN. Emerging magnetic resonance imaging techniques and analysis methods in amyotrophic lateral sclerosis. Front Neurol. 2018;9:1065. doi: 10.3389/fneur.2018.01065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barry RL, Babu S, Anteraper SA, Triantafyllou C, Keil B, Rowe OE, Rangaprakash D, Paganoni S, Lawson R, Dheel C, Cernasov PM, Rosen BR, Ratai EM, Atassi N. Ultra-high field (7T) functional magnetic resonance imaging in amyotrophic lateral sclerosis: a pilot study. Neuroimage Clin. 2021;30:102648. doi: 10.1016/j.nicl.2021.102648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bede P, Elamin M, Byrne S, Hardiman O. Sexual dimorphism in ALS: exploring gender-specific neuroimaging signatures. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:235–243. doi: 10.3109/21678421.2013.865749. [DOI] [PubMed] [Google Scholar]
- 17.Bede P, Finegan E. Revisiting the pathoanatomy of pseudobulbar affect: mechanisms beyond corticobulbar dysfunction. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19:4–6. doi: 10.1080/21678421.2017.1392578. [DOI] [PubMed] [Google Scholar]
- 18.Bede P, Chipika RH, Finegan E, Li Hi, Shing S, Doherty MA, Hengeveld JC, Vajda A, Hutchinson S, Donaghy C, McLaughlin RL, Hardiman O. Brainstem pathology in amyotrophic lateral sclerosis and primary lateral sclerosis: A longitudinal neuroimaging study. Neuroimage Clin. 2019;24:102054. doi: 10.1016/j.nicl.2019.102054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bede P, Chipika RH, Christidi F, Hengeveld JC, Karavasilis E, Argyropoulos GD, Lope J, Li Hi, Shing S, Velonakis G, Dupuis L, Doherty MA, Vajda A, McLaughlin RL, Hardiman O. Genotype-associated cerebellar profiles in ALS: focal cerebellar pathology and cerebro-cerebellar connectivity alterations. J Neurol Neurosurg Psychiatry. 2021a;92:1197–1205. doi: 10.1136/jnnp-2021-326854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bede P, Murad A, Hardiman O. Pathological neural networks and artificial neural networks in ALS: diagnostic classification based on pathognomonic neuroimaging features. J Neurol. 2021b doi: 10.1007/s00415-021-10801-5. doi: 10.1007/s00415-021-10801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertrand A, Wen J, Rinaldi D, Houot M, Sayah S, Camuzat A, Fournier C, Fontanella S, Routier A, Couratier P, Pasquier F, Habert MO, Hannequin D, Martinaud O, Caroppo P, Levy R, Dubois B, Brice A, Durrleman S, Colliot O, et al. Early cognitive,structural, and microstructural changes in presymptomatic C9orf72 carriers younger than 40 years. JAMA Neurol. 2018;75:236–245. doi: 10.1001/jamaneurol.2017.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bharti K, Khan M, Beaulieu C, Graham SJ, Briemberg H, Frayne R, Genge A, Korngut L, Zinman L, Kalra S. Involvement of the dentate nucleus in the pathophysiology of amyotrophic lateral sclerosis: A multi-center and multi-modal neuroimaging study. Neuroimage Clin. 2020;28:102385. doi: 10.1016/j.nicl.2020.102385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bocchetta M, Cardoso MJ, Cash DM, Ourselin S, Warren JD, Rohrer JD. Patterns of regional cerebellar atrophy in genetic frontotemporal dementia. Neuroimage Clin. 2016;11:287–290. doi: 10.1016/j.nicl.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brettschneider J, Van Deerlin VM, Robinson JL, Kwong L, Lee EB, Ali YO, Safren N, Monteiro MJ, Toledo JB, Elman L, McCluskey L, Irwin DJ, Grossman M, Molina-Porcel L, Lee VM, Trojanowski JQ. Pattern of ubiquilin pathology in ALS and FTLD indicates presence of C9ORF72 hexanucleotide expansion. Acta Neuropathol. 2012;123:825–839. doi: 10.1007/s00401-012-0970-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broad RJ, Gabel MC, Dowell NG, Schwartzman DJ, Seth AK, Zhang H, Alexander DC, Cercignani M, Leigh PN. Neurite orientation and dispersion density imaging (NODDI) detects cortical and corticospinal tract degeneration in ALS. J Neurol Neurosurg Psychiatry. 2019;90:404–411. doi: 10.1136/jnnp-2018-318830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buckner RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80:807–815. doi: 10.1016/j.neuron.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 27.Buhour MS, Doidy F, Mondou A, Pélerin A, Carluer L, Eustache F, Viader F, Desgranges B. Voxel-based mapping of grey matter volume and glucose metabolism profiles in amyotrophic lateral sclerosis. EJNMMI Res. 2017;7:21. doi: 10.1186/s13550-017-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calvo A, Chiò A, Pagani M, Cammarosano S, Dematteis F, Moglia C, Solero L, Manera U, Martone T, Brunetti M, Balma M, Castellano G, Barberis M, Cistaro A, Artusi CA, Vasta R, Montanaro E, Romagnolo A, Iazzolino B, Canosa A, et al. Parkinsonian traits in amyotrophic lateral sclerosis (ALS): a prospective population-based study. J Neurol. 2019;266:1633–1642. doi: 10.1007/s00415-019-09305-0. [DOI] [PubMed] [Google Scholar]
- 29.Canosa A, Pagani M, Cistaro A, Montuschi A, Iazzolino B, Fania P, Cammarosano S, Ilardi A, Moglia C, Calvo A, Chiò A. 18F-FDG-PET correlates of cognitive impairment in ALS. Neurology. 2016;86:44–49. doi: 10.1212/WNL.0000000000002242. [DOI] [PubMed] [Google Scholar]
- 30.Canosa A, Moglia C, Manera U, Vasta R, Torrieri MC, Arena V, D'Ovidio F, Palumbo F, Zucchetti JP, Iazzolino B, Peotta L, Calvo A, Pagani M, Chiò A. Metabolic brain changes across different levels of cognitive impairment in ALS: a (18)F-FDG-PET study. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323876. jnnp-2020-323876. [DOI] [PubMed] [Google Scholar]
- 31.Canosa A, Palumbo F, Iazzolino B, Peotta L, Di Pede F, Manera U, Vasta R, Grassano M, Solero L, Arena V, Moglia C, Calvo A, Chiò A, Pagani M. The interplay among education, brain metabolism, and cognitive impairment suggests a role of cognitive reserve in amyotrophic lateral sclerosis. Neurobiol Aging. 2021a;98:205–213. doi: 10.1016/j.neurobiolaging.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Canosa A, Vacchiano V, D'Ovidio F, Calvo A, Moglia C, Manera U, Vasta R, Liguori R, Arena V, Grassano M, Palumbo F, Peotta L, Iazzolino B, Pagani M, Chiò A. Brain metabolic correlates of apathy in amyotrophic lateral sclerosis: an 18F-FDG-positron emission tomography study. Eur J Neurol. 2021b;28:745–753. doi: 10.1111/ene.14637. [DOI] [PubMed] [Google Scholar]
- 33.Canu E, Agosta F, Galantucci S, Chiò A, Riva N, Silani V, Falini A, Comi G, Filippi M. Extramotor damage is associated with cognition in primary lateral sclerosis patients. PLoS One. 2013;8:e82017. doi: 10.1371/journal.pone.0082017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cash DM, Bocchetta M, Thomas DL, Dick KM, van Swieten JC, Borroni B, Galimberti D, Masellis M, Tartaglia MC, Rowe JB, Graff C, Tagliavini F, Frisoni GB, Laforce R, Jr, Finger E, de Mendonça A, Sorbi S, Rossor MN, Ourselin S, Rohrer JD, et al. Patterns of gray matter atrophy in genetic frontotemporal dementia: results from the GENFI study. Neurobiol Aging. 2018;62:191–196. doi: 10.1016/j.neurobiolaging.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Kumfor F, Landin-Romero R, Irish M, Piguet O. The cerebellum in frontotemporal dementia: a meta-analysis of neuroimaging studies. Neuropsychol Rev. 2019;29:450–464. doi: 10.1007/s11065-019-09414-7. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Landin-Romero R, Kumfor F, Irish M, Hodges JR, Piguet O. Cerebellar structural connectivity and contributions to cognition in frontotemporal dementias. Cortex. 2020;129:57–67. doi: 10.1016/j.cortex.2020.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Kumfor F, Landin-Romero R, Irish M, Hodges JR, Piguet O. Cerebellar atrophy and its contribution to cognition in frontotemporal dementias. Ann Neurol. 2018;84:98–109. doi: 10.1002/ana.25271. [DOI] [PubMed] [Google Scholar]
- 38.Chew J, Gendron TF, Prudencio M, Sasaguri H, Zhang YJ, Castanedes-Casey M, Lee CW, Jansen-West K, Kurti A, Murray ME, Bieniek KF, Bauer PO, Whitelaw EC, Rousseau L, Stankowski JN, Stetler C, Daughrity LM, Perkerson EA, Desaro P, Johnston A, et al. Neurodegeneration. C9ORF72 repeat expansions in mice cause TDP-43 pathology, neuronal loss, and behavioral deficits. Science. 2015;348:1151–1154. doi: 10.1126/science.aaa9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chipika RH, Finegan E, Li Hi, Shing S, Hardiman O, Bede P. Tracking a fast-moving disease: longitudinal markers, monitoring and clinical trial endpoints in ALS. Front Neurol. 2019;10:229. doi: 10.3389/fneur.2019.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christidi F, Karavasilis E, Rentzos M, Kelekis N, Evdokimidis I, Bede P. Clinical and radiological markers of extra-motor deficits in amyotrophic lateral sclerosis. Front Neurol. 2018a;9:1005. doi: 10.3389/fneur.2018.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christidi F, Karavasilis E, Velonakis G, Ferentinos P, Rentzos M, Kelekis N, Evdokimidis I, Bede P. The clinical and radiological spectrum of hippocampal pathology in amyotrophic lateral sclerosis. Front Neurol. 2018b;9:523. doi: 10.3389/fneur.2018.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christidi F, Karavasilis E, Ferentinos P, Xirou S, Velonakis G, Rentzos M, Zouvelou V, Zalonis I, Efstathopoulos E, Kelekis N, Evdokimidis I. Investigating the neuroanatomical substrate of pathological laughing and crying in amyotrophic lateral sclerosis with multimodal neuroimaging techniques. Amyotroph Lateral Scler Frontotemporal Degener. 2018c;19:12–20. doi: 10.1080/21678421.2017.1386689. [DOI] [PubMed] [Google Scholar]
- 43.Christidi F, Karavasilis E, Velonakis G, Rentzos M, Zambelis T, Zouvelou V, Xirou S, Ferentinos P, Efstathopoulos E, Kelekis N, Evdokimidis I, Karandreas N. Motor and extra-motor gray matter integrity may underlie neurophysiologic parameters of motor function in amyotrophic lateral sclerosis: a combined voxel-based morphometry and transcranial stimulation study. Brain Imaging Behav. 2018d;12:1730–1741. doi: 10.1007/s11682-018-9841-0. [DOI] [PubMed] [Google Scholar]
- 44.Christidi F, Karavasilis E, Rentzos M, Velonakis G, Zouvelou V, Xirou S, Argyropoulos G, Papatriantafyllou I, Pantolewn V, Ferentinos P, Kelekis N, Seimenis I, Evdokimidis I, Bede P. Hippocampal pathology in amyotrophic lateral sclerosis: selective vulnerability of subfields and their associated projections. Neurobiol Aging. 2019;84:178–188. doi: 10.1016/j.neurobiolaging.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 45.Chung YH, Joo KM, Lee YJ, Shin DH, Cha CI. Reactive astrocytes express PARP in the central nervous system of SOD(G93A) transgenic mice. Brain Res. 2004;1003:199–204. doi: 10.1016/j.brainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 46.Chung YH, Joo KM, Lim HC, Cho MH, Kim D, Lee WB, Cha CI. Immunohistochemical study on the distribution of phosphorylated extracellular signal-regulated kinase (ERK) in the central nervous system of SOD1G93A transgenic mice. Brain Res. 2005;1050:203–209. doi: 10.1016/j.brainres.2005.05.060. [DOI] [PubMed] [Google Scholar]
- 47.Cistaro A, Valentini MC, Chio A, Nobili F, Calvo A, Moglia C, Montuschi A, Morbelli S, Salmaso D, Fania P, Carrara G, Pagani M. Brain hypermetabolism in amyotrophic lateral sclerosis: a FDG PET study in ALS of spinal and bulbar onset. Eur J Nucl Med Mol Imaging. 2012;39:251–259. doi: 10.1007/s00259-011-1979-6. [DOI] [PubMed] [Google Scholar]
- 48.Consonni M, Dalla Bella E, Nigri A, Pinardi C, Demichelis G, Porcu L, Gellera C, Pensato V, Cappa SF, Bruzzone MG, Lauria G, Ferraro S. Cognitive syndromes and C9orf72 mutation are not related to cerebellar degeneration in amyotrophic lateral sclerosis. Front Neurosci. 2019;13:440. doi: 10.3389/fnins.2019.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalakas MC, Hatazawa J, Brooks RA, Di Chiro G. Lowered cerebral glucose utilization in amyotrophic lateral sclerosis. Ann Neurol. 1987;22:580–586. doi: 10.1002/ana.410220504. [DOI] [PubMed] [Google Scholar]
- 50.de Borba FC, Querin G, França MC, Jr, Pradat PF. Cerebellar degeneration in adult spinal muscular atrophy patients. J Neurol. 2020;267:2625–2631. doi: 10.1007/s00415-020-09875-4. [DOI] [PubMed] [Google Scholar]
- 51.De Marchi F, Stecco A, Falaschi Z, Filippone F, Pasché A, Bebeti A, Leigheb M, Cantello R, Mazzini L. Detection of white matter ultrastructural changes for amyotrophic lateral sclerosis characterization: a diagnostic study from dti-derived data. Brain Sci. 2020;10:996. doi: 10.3390/brainsci10120996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Marco M, Merico A, Berta G, Segato N, Citton V, Baglione A, Venneri A. Morphometric correlates of dysarthric deficit in amyotrophic lateral sclerosis. Amyotroph Lateral Dcler Frontotemporal Degener. 2015;16:464–472. doi: 10.3109/21678421.2015.1056191. [DOI] [PubMed] [Google Scholar]
- 53.Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. J Neurosci. 1997;17:9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage. 2006;33:127–138. doi: 10.1016/j.neuroimage.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 55.Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 56.Diedrichsen J, Zotow E. Surface-based display of volume-averaged cerebellar imaging data. PLoS One. 2015;10:e0133402. doi: 10.1371/journal.pone.0133402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol. 2011;7:76–85. doi: 10.1038/nrneurol.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Donaghy C, Pinnock R, Abrahams S, Cardwell C, Hardiman O, Patterson V, McGivern RC, Gibson JM. Slow saccades in bulbar-onset motor neurone disease. J Neurol. 2010;257:1134–1140. doi: 10.1007/s00415-010-5478-7. [DOI] [PubMed] [Google Scholar]
- 59.Eisen A, Turner MR, Lemon R. Tools and talk: an evolutionary perspective on the functional deficits associated with amyotrophic lateral sclerosis. Muscle Nerve. 2014a;49:469–477. doi: 10.1002/mus.24132. [DOI] [PubMed] [Google Scholar]
- 60.Eisen A, Kiernan M, Mitsumoto H, Swash M. Amyotrophic lateral sclerosis: a long preclinical period? J Neurol Neurosurg Psychiatry. 2014b;85:1232–1238. doi: 10.1136/jnnp-2013-307135. [DOI] [PubMed] [Google Scholar]
- 61.Eisen A, Bede P. The strength of corticomotoneuronal drive underlies ALS split phenotypes and reflects early upper motor neuron dysfunction. Brain Behav. 2021;11:e2403. doi: 10.1002/brb3.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El Mendili MM, Querin G, Bede P, Pradat PF. Spinal cord imaging in amyotrophic lateral sclerosis: historical concepts-novel techniques. Front Neurology. 2019;10:350. doi: 10.3389/fneur.2019.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Engel PA, Grunnet M. Atypical dementia and spastic paraplegia in a patient with primary lateral sclerosis and numerous necortical beta amyloid plaques: new disorder or Alzheimer's disease variant? J Geriatr Psychiatry Neurol. 2000;13:60–64. doi: 10.1177/089198870001300203. [DOI] [PubMed] [Google Scholar]
- 64.Fekete T, Zach N, Mujica-Parodi LR, Turner MR. Multiple kernel learning captures a systems-level functional connectivity biomarker signature in amyotrophic lateral sclerosis. PLoS One. 2013;8:e85190. doi: 10.1371/journal.pone.0085190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feron M, Couillandre A, Mseddi E, Termoz N, Abidi M, Bardinet E, Delgadillo D, Lenglet T, Querin G, Welter ML, Forestier NL, Salachas F, Bruneteau G, Amador MDM, Debs R, Lacomblez L, Meininger V, Pélégrini-Issac M, Bede P, Pradat PF, et al. Extrapyramidal deficits in ALS: a combined biomechanical and neuroimaging study. J Neurol. 2018;265:2125–2136. doi: 10.1007/s00415-018-8964-y. [DOI] [PubMed] [Google Scholar]
- 66.Finegan E, Chipika RH, Li Hi, Shing S, Hardiman O, Bede P. Pathological crying and laughing in motor neuron disease: pathobiology, screening, intervention. Front Neurol. 2019a;10:260. doi: 10.3389/fneur.2019.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Finegan E, Chipika RH, Shing SLH, Hardiman O, Bede P. Primary lateral sclerosis: a distinct entity or part of the ALS spectrum? Amyotroph Lateral Scler Frontotemporal Degener. 2019b;20:133–145. doi: 10.1080/21678421.2018.1550518. [DOI] [PubMed] [Google Scholar]
- 68.Finegan E, Li Hi, Shing S, Chipika RH, Doherty MA, Hengeveld JC, Vajda A, Donaghy C, Pender N, McLaughlin RL, Hardiman O, Bede P. Widespread subcortical grey matter degeneration in primary lateral sclerosis: a multimodal imaging study with genetic profiling. Neuroimage Clin. 2019c;24:102089. doi: 10.1016/j.nicl.2019.102089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Finegan E, Shing SLH, Chipika RH, Chang KM, McKenna MC, Doherty MA, Hengeveld JC, Vajda A, Pender N, Donaghy C, Hutchinson S, McLaughlin RL, Hardiman O, Bede P. Extra-motor cerebral changes and manifestations in primary lateral sclerosis. Brain Imaging Behav. 2021;15:2283–2296. doi: 10.1007/s11682-020-00421-4. [DOI] [PubMed] [Google Scholar]
- 70.Fitzgerald MJT, Gruener G, Mtui E. Saunders. Netherlands: Elsevier; 2012. Clinical neuroanatomy and neuroscience. [Google Scholar]
- 71.Floeter MK, Katipally R, Kim MP, Schanz O, Stephen M, Danielian L, Wu T, Huey ED, Meoded A. Impaired corticopontocerebellar tracts underlie pseudobulbar affect in motor neuron disorders. Neurology. 2014;83:620–627. doi: 10.1212/WNL.0000000000000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gellersen HM, Guo CC, O'Callaghan C, Tan RH, Sami S, Hornberger M. Cerebellar atrophy in neurodegeneration—a meta-analysis. J Neurol Neurosurg Psychiatry. 2017;88:780–788. doi: 10.1136/jnnp-2017-315607. [DOI] [PubMed] [Google Scholar]
- 73.Geser F, Brandmeir NJ, Kwong LK, Martinez-Lage M, Elman L, McCluskey L, Xie SX, Lee VM, Trojanowski JQ. Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Arch Neurol. 2008;65:636–641. doi: 10.1001/archneur.65.5.636. [DOI] [PubMed] [Google Scholar]
- 74.Gizzi M, DiRocco A, Sivak M, Cohen B. Ocular motor function in motor neuron disease. Neurology. 1992;42:1037–1046. doi: 10.1212/wnl.42.5.1037. [DOI] [PubMed] [Google Scholar]
- 75.Gredal O, Rosenbaum S, Topp S, Karlsborg M, Strange P, Werdelin L. Quantification of brain metabolites in amyotrophic lateral sclerosis by localized proton magnetic resonance spectroscopy. Neurology. 1997;48:878–881. doi: 10.1212/wnl.48.4.878. [DOI] [PubMed] [Google Scholar]
- 76.Grollemund V, Pradat PF, Querin G, Delbot F, Le Chat G, Pradat-Peyre JF, Bede P. Machine learning in amyotrophic lateral sclerosis: achievements, pitfalls, and future directions. Front Neurosci. 2019;13:135. doi: 10.3389/fnins.2019.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo CC, Tan R, Hodges JR, Hu X, Sami S, Hornberger M. Network-selective vulnerability of the human cerebellum to Alzheimer's disease and frontotemporal dementia. Brain. 2016;139:1527–1538. doi: 10.1093/brain/aww003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Han J, Ma L. Functional magnetic resonance imaging study of the brain in patients with amyotrophic lateral sclerosis. Chin Med Sci J. 2006;21:228–233. [PubMed] [Google Scholar]
- 79.Hartung V, Prell T, Gaser C, Turner MR, Tietz F, Ilse B, Bokemeyer M, Witte OW, Grosskreutz J. Voxel-based MRI intensitometry reveals extent of cerebral white matter pathology in amyotrophic lateral sclerosis. PLoS One. 2014;9:e104894. doi: 10.1371/journal.pone.0104894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herholz SC, Herholz RS, Herholz K. Non-pharmacological interventions and neuroplasticity in early stage Alzheimer's disease. Expert Rev Neurother. 2013;13:1235–1245. doi: 10.1586/14737175.2013.845086. [DOI] [PubMed] [Google Scholar]
- 81.Hu T, Hou Y, Wei Q, Yang J, Luo C, Chen Y, Gong Q, Shang H. Patterns of brain regional functional coherence in cognitive impaired ALS. Int J Neurosci. 2020;130:751–758. doi: 10.1080/00207454.2019.1705806. [DOI] [PubMed] [Google Scholar]
- 82.Hutson TH, Di Giovanni S. The translational landscape in spinal cord injury: focus on neuroplasticity and regeneration. Nat Rev Neurol. 2019;15:732–745. doi: 10.1038/s41582-019-0280-3. [DOI] [PubMed] [Google Scholar]
- 83.Ishihara K, Araki S, Ihori N, Shiota J, Kawamura M, Nakano I. An autopsy case of frontotemporal dementia with severe dysarthria and motor neuron disease showing numerous basophilic inclusions. Neuropathology. 2006;26:447–454. doi: 10.1111/j.1440-1789.2006.00717.x. [DOI] [PubMed] [Google Scholar]
- 84.Jacobs L, Bozian D, Heffner RR, Jr, Barron SA. An eye movement disorder in amyotrophic lateral sclerosis. Neurology. 1981;31:1282–1287. doi: 10.1212/wnl.31.10.1282. [DOI] [PubMed] [Google Scholar]
- 85.Jensen K, Beylergil SB, Shaikh AG. Slow saccades in cerebellar disease. Cerebellum Ataxias. 2019;6:1. doi: 10.1186/s40673-018-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jones AR, Iacoangeli A, Adey BN, Bowles H, Shatunov A, Troakes C, Garson JA, McCormick AL, Al-Chalabi A. A HML6 endogenous retrovirus on chromosome 3 is upregulated in amyotrophic lateral sclerosis motor cortex. Sci Rep. 2021;11:14283. doi: 10.1038/s41598-021-93742-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kao CS, van Bruggen R, Kim JR, Chen XXL, Chan C, Lee J, Cho WI, Zhao M, Arndt C, Maksimovic K, Khan M, Tan Q, Wilson MD, Park J. Selective neuronal degeneration in MATR3 S85C knock-in mouse model of early-stage ALS. Nat Commun. 2020;5304;11 doi: 10.1038/s41467-020-18949-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kassubek J, Unrath A, Huppertz HJ, Lulé D, Ethofer T, Sperfeld AD, Ludolph AC. Global brain atrophy and corticospinal tract alterations in ALS, as investigated by voxel-based morphometry of 3-D MRI. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:213–220. doi: 10.1080/14660820510038538. [DOI] [PubMed] [Google Scholar]
- 89.Keil C, Prell T, Peschel T, Hartung V, Dengler R, Grosskreutz J. Longitudinal diffusion tensor imaging in amyotrophic lateral sclerosis. BMC Neurosci. 2012;13:141. doi: 10.1186/1471-2202-13-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim HJ, Oh SI, de Leon M, Wang X, Oh KW, Park JS, Deshpande A, Buj M, Kim SH. Structural explanation of poor prognosis of amyotrophic lateral sclerosis in the non-demented state. Eur J Neurol. 2017;24:122–129. doi: 10.1111/ene.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kobayashi Z, Tsuchiya K, Kubodera T, Shibata N, Arai T, Miura H, Ishikawa C, Kondo H, Ishizu H, Akiyama H, Mizusawa H. FALS with Gly72Ser mutation in SOD1 gene: report of a family including the first autopsy case. J Neurol Sci. 2011;300:9–13. doi: 10.1016/j.jns.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 92.Konczak J, Pierscianek D, Hirsiger S, Bultmann U, Schoch B, Gizewski ER, Timmann D, Maschke M, Frings M. Recovery of upper limb function after cerebellar stroke: lesion symptom mapping and arm kinematics. Stroke. 2010;41:2191–2200. doi: 10.1161/STROKEAHA.110.583641. [DOI] [PubMed] [Google Scholar]
- 93.Konrad C, Henningsen H, Bremer J, Mock B, Deppe M, Buchinger C, Turski P, Knecht S, Brooks B. Pattern of cortical reorganization in amyotrophic lateral sclerosis: a functional magnetic resonance imaging study. Exp Brain Res. 2002;143:51–56. doi: 10.1007/s00221-001-0981-9. [DOI] [PubMed] [Google Scholar]
- 94.Konrad C, Jansen A, Henningsen H, Sommer J, Turski PA, Brooks BR, Knecht S. Subcortical reorganization in amyotrophic lateral sclerosis. Exp Brain Res. 2006;172:361–369. doi: 10.1007/s00221-006-0352-7. [DOI] [PubMed] [Google Scholar]
- 95.Kosaka T, Fu YJ, Shiga A, Ishidaira H, Tan CF, Tani T, Koike R, Onodera O, Nishizawa M, Kakita A, Takahashi H. Primary lateral sclerosis: upper-motor-predominant amyotrophic lateral sclerosis with frontotemporal lobar degeneration--immunohistochemical and biochemical analyses of TDP-43. Neuropathology. 2012;32:373–384. doi: 10.1111/j.1440-1789.2011.01271.x. [DOI] [PubMed] [Google Scholar]
- 96.Kushner MJ, Parrish M, Burke A, Behrens M, Hays AP, Frame B, Rowland LP. Nystagmus in motor neuron disease: clinicopathological study of two cases. Ann Neurol. 1984;16:71–77. doi: 10.1002/ana.410160114. [DOI] [PubMed] [Google Scholar]
- 97.Lee JC, Joo KM, Choe SY, Cha CI. Region-specific changes in the immunoreactivity of TRPV4 expression in the central nervous system of SOD1(G93A) transgenic mice as an in vivo model of amyotrophic lateral sclerosis. J Mol Histol. 2012;43:625–631. doi: 10.1007/s10735-012-9432-0. [DOI] [PubMed] [Google Scholar]
- 98.Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123:1041–1050. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- 99.Li Hi, Shing S, Lope J, Chipika RH, Hardiman O, Bede P. Imaging data indicate cerebral reorganisation in poliomyelitis survivors: possible compensation for longstanding lower motor neuron pathology. Data Brief. 2021a;38:107316. doi: 10.1016/j.dib.2021.107316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li Hi, Shing S, Murad A, Lope J, Hardiman O, Bede P. Cerebellar remodelling decades after spinal cord insult: neuroplasticity in poliomyelitis survivors. J Integr Neurosci. 2021b doi: 10.31083/j.jin2102065. [DOI] [PubMed] [Google Scholar]
- 101.Li Hi, Shing S, Lope J, McKenna MC, Chipika RH, Hardiman O, Bede P. Increased cerebral integrity metrics in poliomyelitis survivors: putative adaptation to longstanding lower motor neuron degeneration. J Neurol Sci. 2021c;424:117361. doi: 10.1016/j.jns.2021.117361. [DOI] [PubMed] [Google Scholar]
- 102.Li PA, He Q, Cao T, Yong G, Szauter KM, Fong KS, Karlsson J, Keep MF, Csiszar K. Up-regulation and altered distribution of lysyl oxidase in the central nervous system of mutant SOD1 transgenic mouse model of amyotrophic lateral sclerosis. Brain Res Mol Brain Res. 2004;120:115–122. doi: 10.1016/j.molbrainres.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 103.Lindig T, Bender B, Hauser TK, Mang S, Schweikardt D, Klose U, Karle KN, Schüle R, Schöls L, Rattay TW. Gray and white matter alterations in hereditary spastic paraplegia type SPG4 and clinical correlations. J Neurology. 2015;262:1961–1971. doi: 10.1007/s00415-015-7791-7. [DOI] [PubMed] [Google Scholar]
- 104.Ludolph AC, Langen KJ, Regard M, Herzog H, Kemper B, Kuwert T, Bottger IG, Feinendegen L. Frontal lobe function in amyotrophic lateral sclerosis: a neuropsychologic and positron emission tomography study. Acta Neurol Scand. 1992;85:81–89. doi: 10.1111/j.1600-0404.1992.tb04003.x. [DOI] [PubMed] [Google Scholar]
- 105.Machida Y, Tsuchiya K, Anno M, Haga C, Ito T, Shimo Y, Wakeshima T, Iritani S, Ikeda K. Sporadic amyotrophic lateral sclerosis with multiple system degeneration: a report of an autopsy case without respirator administration. Acta Neuropathol. 1999;98:512–515. doi: 10.1007/s004010051117. [DOI] [PubMed] [Google Scholar]
- 106.Mahoney CJ, Beck J, Rohrer JD, Lashley T, Mok K, Shakespeare T, Yeatman T, Warrington EK, Schott JM, Fox NC, Rossor MN, Hardy J, Collinge J, Revesz T, Mead S, Warren JD. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical neuroanatomical and neuropathological features. Brain. 2012;135:736–750. doi: 10.1093/brain/awr361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Malm J, Kristensen B, Karlsson T, Carlberg B, Fagerlund M, Olsson T. Cognitive impairment in young adults with infratentorial infarcts. Neurology. 1998;51:433–440. doi: 10.1212/wnl.51.2.433. [DOI] [PubMed] [Google Scholar]
- 108.Matías-Guiu JA, Pytel V, Cabrera-Martín MN, Galán L, Valles-Salgado M, Guerrero A, Moreno-Ramos T, Matías-Guiu J, Carreras JL. Amyloid- and FDG-PET imaging in amyotrophic lateral sclerosis. Eur J Nucl Med Mol Imaging. 2016;43:2050–2060. doi: 10.1007/s00259-016-3434-1. [DOI] [PubMed] [Google Scholar]
- 109.McCluskey L, Vandriel S, Elman L, Van Deerlin VM, Powers J, Boller A, Wood EM, Woo J, McMillan CT, Rascovsky K, Grossman M. ALS-Plus syndrome: non-pyramidal features in a large ALS cohort. J Neurol Sci. 2014;345:118–124. doi: 10.1016/j.jns.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McKenna MC, Chipika RH, Li Hi, Shing S, Christidi F, Lope J, Doherty MA, Hengeveld JC, Vajda A, McLaughlin RL, Hardiman O, Hutchinson S, Bede P. Infratentorial pathology in frontotemporal dementia: cerebellar grey and white matter alterations in FTD phenotypes. J Neurol. 2021;268:4687–4697. doi: 10.1007/s00415-021-10575-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Menke RA, Proudfoot M, Wuu J, Andersen PM, Talbot K, Benatar M, Turner MR. Increased functional connectivity common to symptomatic amyotrophic lateral sclerosis and those at genetic risk. J Neurol Neurosurg Psychiatry. 2016;87:580–588. doi: 10.1136/jnnp-2015-311945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Menke RAL, Proudfoot M, Talbot K, Turner MR. The two-year progression of structural and functional cerebral MRI in amyotrophic lateral sclerosis. Neuroimage Clin. 2018;17:953–961. doi: 10.1016/j.nicl.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Meoded A, Morrissette AE, Katipally R, Schanz O, Gotts SJ, Floeter MK. Cerebro-cerebellar connectivity is increased in primary lateral sclerosis. Neuroimage Clin. 2015;7:288–296. doi: 10.1016/j.nicl.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Minnerop M, Specht K, Ruhlmann J, Grothe C, Wüllner U, Klockgether T. In vivo voxel-based relaxometry in amyotrophic lateral sclerosis. J Neurol. 2009;256:28–34. doi: 10.1007/s00415-009-0947-6. [DOI] [PubMed] [Google Scholar]
- 115.Mitoma H, Buffo A, Gelfo F, Guell X, Fucà E, Kakei S, Lee J, Manto M, Petrosini L, Shaikh AG, Schmahmann JD. Consensus paper. Cerebellar reserve: from cerebellar physiology to cerebellar disorders. Cerebellum. 2020;19:131–153. doi: 10.1007/s12311-019-01091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mohammadi B, Kollewe K, Samii A, Dengler R, Munte TF. Functional neuroimaging at different disease stages reveals distinct phases of neuroplastic changes in amyotrophic lateral sclerosis. Hum Brain Mapp. 2011;32:750–758. doi: 10.1002/hbm.21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morello G, Gentile G, Spataro R, Spampinato AG, Guarnaccia M, Salomone S, La Bella V, Conforti FL, Cavallaro S. Genomic portrait of a sporadic amyotrophic lateral sclerosis case in a large spinocerebellar ataxia type 1 family. J Per Med. 2020;10:262. doi: 10.3390/jpm10040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nanetti L, Fancellu R, Tomasello C, Gellera C, Pareyson D, Mariotti C. Rare association of motor neuron disease and spinocerebellar ataxia type 2 (SCA2): a new case and review of the literature. J Neurol. 2009;256:1926–1928. doi: 10.1007/s00415-009-5237-9. [DOI] [PubMed] [Google Scholar]
- 119.Neuenschwander AG, Thai KK, Figueroa KP, Pulst SM. Amyotrophic lateral sclerosis risk for spinocerebellar ataxia type 2 ATXN2 CAG repeat alleles: a meta-analysis. JAMA Neurol. 2014;71:1529–1534. doi: 10.1001/jamaneurol.2014.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Olivieri G, Pro S, Diodato D, Di Capua M, Longo D, Martinelli D, Bertini E, Dionisi-Vici C. Corticospinal tract damage in HHH syndrome: a metabolic cause of hereditary spastic paraplegia. Orphanet J Rare Dis. 2019;14:208. doi: 10.1186/s13023-019-1181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Omer T, Finegan E, Hutchinson S, Doherty M, Vajda A, McLaughlin RL, Pender N, Hardiman O, Bede P. Neuroimaging patterns along the ALS-FTD spectrum: a multiparametric imaging study. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:611–623. doi: 10.1080/21678421.2017.1332077. [DOI] [PubMed] [Google Scholar]
- 122.Orlacchio A, Kawarai T, Totaro A, Errico A, St George-Hyslop PH, Rugarli EI, Bernardi G. Hereditary spastic paraplegia: clinical genetic study of 15 families. Arch Neurol. 2004;61:849–855. doi: 10.1001/archneur.61.6.849. [DOI] [PubMed] [Google Scholar]
- 123.Pagani M, Chiò A, Valentini MC, Öberg J, Nobili F, Calvo A, Moglia C, Bertuzzo D, Morbelli S, De Carli F, Fania P, Cistaro A. Functional pattern of brain FDG-PET in amyotrophic lateral sclerosis. Neurology. 2014;83:1067–1074. doi: 10.1212/WNL.0000000000000792. [DOI] [PubMed] [Google Scholar]
- 124.Pagani M, Öberg J, De Carli F, Calvo A, Moglia C, Canosa A, Nobili F, Morbelli S, Fania P, Cistaro A, Chiò A. Metabolic spatial connectivity in amyotrophic lateral sclerosis as revealed by independent component analysis. Hum Brain Mapp. 2016;37:942–953. doi: 10.1002/hbm.23078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Papma JM, Jiskoot LC, Panman JL, Dopper EG, den Heijer T, Donker Kaat L, Pijnenburg YAL, Meeter LH, van Minkelen R, Rombouts S, van Swieten JC. Cognition and gray and white matter characteristics of presymptomatic C9orf72 repeat expansion. Neurology. 2017;89:1256–1264. doi: 10.1212/WNL.0000000000004393. [DOI] [PubMed] [Google Scholar]
- 126.Parvizi J, Joseph J, Press DZ, Schmahmann JD. Pathological laughter and crying in patients with multiple system atrophy-cerebellar type. Mov Disord. 2007;22:798–803. doi: 10.1002/mds.21348. [DOI] [PubMed] [Google Scholar]
- 127.Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annu Rev Neurosci. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- 128.Petri S, Kollewe K, Grothe C, Hori A, Dengler R, Bufler J, Krampfl K. GABA(A)-receptor mRNA expression in the prefrontal and temporal cortex of ALS patients. J Neurol Sci. 2006;250:124–132. doi: 10.1016/j.jns.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 129.Pieper CC, Konrad C, Sommer J, Teismann I, Schiffbauer H. Structural changes of central white matter tracts in Kennedy's disease - a diffusion tensor imaging and voxel-based morphometry study. Acta Neurol Scand. 2013;127:323–328. doi: 10.1111/ane.12018. [DOI] [PubMed] [Google Scholar]
- 130.Pioro EP, Turner MR, Bede P. Neuroimaging in primary lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21:18–27. doi: 10.1080/21678421.2020.1837176. [DOI] [PubMed] [Google Scholar]
- 131.Plaitakis A, Constantakakis E, Smith J. The neuroexcitotoxic amino acids glutamate and aspartate are altered in the spinal cord and brain in amyotrophic lateral sclerosis. Ann Neurol. 1988;24:446–449. doi: 10.1002/ana.410240314. [DOI] [PubMed] [Google Scholar]
- 132.Pradat PF, Bernard E, Corcia P, Couratier P, Jublanc C, Querin G, Morelot Panzini C, Salachas F, Vial C, Wahbi K, Bede P, Desnuelle C. The French national protocol for Kennedy's disease (SBMA): consensus diagnostic and management recommendations. Orphanet J Rare Dis. 2020;15:90. doi: 10.1186/s13023-020-01366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Prell T, Grosskreutz J. The involvement of the cerebellum in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:507–515. doi: 10.3109/21678421.2013.812661. [DOI] [PubMed] [Google Scholar]
- 134.Proudfoot M, Bede P, Turner MR. Imaging cerebral activity in amyotrophic lateral sclerosis. Front Neurol. 2018;1148;9 doi: 10.3389/fneur.2018.01148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Prudlo J, Bißbort C, Glass A, Grossmann A, Hauenstein K, Benecke R, Teipel SJ. White matter pathology in ALS and lower motor neuron ALS variants: a diffusion tensor imaging study using tract-based spatial statistics. J Neurol. 2012;259:1848–1859. doi: 10.1007/s00415-012-6420-y. [DOI] [PubMed] [Google Scholar]
- 136.Przedborski S, Donaldson D, Jakowec M, Kish SJ, Guttman M, Rosoklija G, Hays AP. Brain superoxide dismutase, catalase, and glutathione peroxidase activities in amyotrophic lateral sclerosis. Ann Neurol. 1996;39:158–165. doi: 10.1002/ana.410390204. [DOI] [PubMed] [Google Scholar]
- 137.Qiu T, Zhang Y, Tang X, Liu X, Wang Y, Zhou C, Luo C, Zhang J. Precentral degeneration and cerebellar compensation in amyotrophic lateral sclerosis: a multimodal MRI analysis. Hum Brain Mapp. 2019;40:3464–3474. doi: 10.1002/hbm.24609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Querin G, El Mendili MM, Bede P, Delphine S, Lenglet T, Marchand-Pauvert V, Pradat PF. Multimodal spinal cord MRI offers accurate diagnostic classification in ALS. J Neurol Neurosurg Psychiatry. 2018;89:1220–1221. doi: 10.1136/jnnp-2017-317214. [DOI] [PubMed] [Google Scholar]
- 139.Querin G, Bede P, El Mendili MM, Li M, Pelegrini-Issac M, Rinaldi D, Catala M, Saracino D, Salachas F, Camuzat A, Marchand-Pauvert V, Cohen-Adad J, Colliot O, Le Ber I, Pradat PF. Presymptomatic spinal cord pathology in c9orf72 mutation carriers: a longitudinal neuroimaging study. Ann Neurol. 2019a;86:158–167. doi: 10.1002/ana.25520. [DOI] [PubMed] [Google Scholar]
- 140.Querin G, El Mendili MM, Lenglet T, Behin A, Stojkovic T, Salachas F, Devos D, Le Forestier N, Del Mar Amador M, Debs R, Lacomblez L, Meininger V, Bruneteau G, Cohen-Adad J, Lehéricy S, Laforêt P, Blancho S, Benali H, Catala M, Li M, et al. The spinal and cerebral profile of adult spinal-muscular atrophy: a multimodal imaging study. Neuroimage Clin. 2019b;21:101618. doi: 10.1016/j.nicl.2018.101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rohrer JD, Lashley T, Schott JM, Warren JE, Mead S, Isaacs AM, Beck J, Hardy J, de Silva R, Warrington E, Troakes C, Al-Sarraj S, King A, Borroni B, Clarkson MJ, Ourselin S, Holton JL, Fox NC, Revesz T, Rossor MN, et al. Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain. 2011;134:2565–2581. doi: 10.1093/brain/awr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rohrer JD, Isaacs AM, Mizlienska S, Mead S, Lashley T, Wray S, Sidle K, Fratta P, Orrell RW, Hardy J, Holton J, Revesz T, Rossor MN, Warren JD. C9orf72 expansions in frontotemporal dementia and amyotrophic lateral sclerosis. Lancet Neurol. 2015;14:291–301. doi: 10.1016/S1474-4422(14)70233-9. [DOI] [PubMed] [Google Scholar]
- 143.Romero JE, Coupé P, Giraud R, Ta VT, Fonov V, Park MTM, Chakravarty MM, Voineskos AN, Manjón JV. CERES: A new cerebellum lobule segmentation method. Neuroimage. 2017;147:916–924. doi: 10.1016/j.neuroimage.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 144.Runnqvist E, Bonnard M, Gauvin HS, Attarian S, Trébuchon A, Hartsuiker RJ, Alario FX. Internal modeling of upcoming speech: A causal role of the right posterior cerebellum in non-motor aspects of language production. Cortex. 2016;81:203–214. doi: 10.1016/j.cortex.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 145.Sala A, Iaccarino L, Fania P, Vanoli EG, Fallanca F, Pagnini C, Cerami C, Calvo A, Canosa A, Pagani M, Chiò A, Cistaro A, Perani D. Testing the diagnostic accuracy of [18F]FDG-PET in discriminating spinal- and bulbar-onset amyotrophic lateral sclerosis. Eur J Nucl Med Mol Imaging. 2019;46:1117–1131. doi: 10.1007/s00259-018-4246-2. [DOI] [PubMed] [Google Scholar]
- 146.Sasegbon A, Hamdy S. The role of the cerebellum in swallowing. Dysphagia. 2021 doi: 10.1007/s00455-021-10271-x. doi: 10.1007/s00455-021-10271-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schimke N, Krampfl K, Petri S, Dengler R, Bufler J. Cerebellar symptoms in motor neuron diseases. Special form of amyotrophic lateral sclerosis plus syndrome. Nervenarzt. 2002;73:751–753. doi: 10.1007/s00115-002-1343-y. [DOI] [PubMed] [Google Scholar]
- 148.Schmahmann JD. Dysmetria of thought: clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn Sci. 1998;2:362–371. doi: 10.1016/s1364-6613(98)01218-2. [DOI] [PubMed] [Google Scholar]
- 149.Schmahmann JD. The cerebellum and cognition. Neurosci Lett. 2019;688:62–75. doi: 10.1016/j.neulet.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 150.Schoenfeld MA, Tempelmann C, Gaul C, Kuhnel GR, Duzel E, Hopf JM, Feistner H, Zierz S, Heinze HJ, Vielhaber S. Functional motor compensation in amyotrophic lateral sclerosis. J Neurol. 2005;252:944–952. doi: 10.1007/s00415-005-0787-y. [DOI] [PubMed] [Google Scholar]
- 151.Schönecker S, Neuhofer C, Otto M, Ludolph A, Kassubek J, Landwehrmeyer B, Anderl-Straub S, Semler E, Diehl-Schmid J, Prix C, Vollmar C, Fortea J, Deutsches FTLD-Konsortium, Huppertz HJ, Arzberger T, Edbauer D, Feddersen B, Dieterich M, Schroeter ML, Volk AE, et al. Atrophy in the thalamus but not cerebellum is specific for C9orf72 FTD and ALS patients - an atlas-based volumetric MRI study. Front Aging Neurosci. 2018;10:45. doi: 10.3389/fnagi.2018.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schuster C, Elamin M, Hardiman O, Bede P. Presymptomatic and longitudinal neuroimaging in neurodegeneration--from snapshots to motion picture: a systematic review. J Neurol Neurosurg Psychiatry. 2015;86:1089–1096. doi: 10.1136/jnnp-2014-309888. [DOI] [PubMed] [Google Scholar]
- 153.Schuster C, Hardiman O, Bede P. Development of an automated MRI-based diagnostic protocol for amyotrophic lateral sclerosis using disease-specific pathognomonic features: a quantitative disease-state classification study. PLoS One. 2016a;11:e0167331. doi: 10.1371/journal.pone.0167331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schuster C, Elamin M, Hardiman O, Bede P. The segmental diffusivity profile of amyotrophic lateral sclerosis associated white matter degeneration. Eur J Neurol. 2016b;23:1361–1371. doi: 10.1111/ene.13038. [DOI] [PubMed] [Google Scholar]
- 155.Seidel K, De Vos R, Derksen L, Bauer P, Riess O, den Dunnen W, Deller T, Hageman G, Rüb U. Widespread thalamic and cerebellar degeneration in a patient with a complicated hereditary spastic paraplegia (HSP) Ann Anat. 2009;191:203–211. doi: 10.1016/j.aanat.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 156.Servelhere KR, Rezende TJR, de Lima FD, de Brito MR, de França Nunes RF, Casseb RF, Pedroso JL, Barsottini OGP, Cendes F, França MC., Jr Brain damage and gene expression across hereditary spastic paraplegia subtypes. Mov Disord. 2021;36:1644–1653. doi: 10.1002/mds.28519. [DOI] [PubMed] [Google Scholar]
- 157.Shellikeri S, Karthikeyan V, Martino R, Black SE, Zinman L, Keith J, Yunusova Y. The neuropathological signature of bulbar-onset ALS: a systematic review. Neurosci Biobehav Rev. 2017;75:378–392. doi: 10.1016/j.neubiorev.2017.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 159.Straudi S, Basaglia N. Neuroplasticity-based technologies and interventions for restoring motor functions in multiple sclerosis. Adv Exp Med Biol. 2017;958:171–185. doi: 10.1007/978-3-319-47861-6_11. [DOI] [PubMed] [Google Scholar]
- 160.Sugihara H, Horiuchi M, Kamo T, Fujisawa K, Abe M, Sakiyama T, Tadokoro M. A case of primary lateral sclerosis taking a prolonged clinical course with dementia and having an unusual dendritic ballooning. Neuropathology. 1999;19:77–84. doi: 10.1046/j.1440-1789.1999.00203.x. [DOI] [PubMed] [Google Scholar]
- 161.Sun YM, Lu C, Wu ZY. Spinocerebellar ataxia: relationship between phenotype and genotype - a review. Clin Genet. 2016;90:305–314. doi: 10.1111/cge.12808. [DOI] [PubMed] [Google Scholar]
- 162.Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Proc Natl Acad Sci U S A. 2007;104:642–647. doi: 10.1073/pnas.0610082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tahedl M, Li Hi, Shing S, Finegan E, Chipika RH, Lope J, Hardiman O, Bede P. Propagation patterns in motor neuron diseases: Individual and phenotype-associated disease-burden trajectories across the UMN-LMN spectrum of MNDs. Neurobiol Aging. 2021a;109:78–87. doi: 10.1016/j.neurobiolaging.2021.04.031. [DOI] [PubMed] [Google Scholar]
- 164.Tahedl M, Li Hi, Shing S, Finegan E, Chipika RH, Lope J, Murad A, Hardiman O, Bede P. Imaging data reveal divergent longitudinal trajectories in PLS, ALS and poliomyelitis survivors: group-level and single-subject traits. Data Brief. 2021b;39:107484. doi: 10.1016/j.dib.2021.107484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tan RH, Devenney E, Dobson-Stone C, Kwok JB, Hodges JR, Kiernan MC, Halliday GM, Hornberger M. Cerebellar integrity in the amyotrophic lateral sclerosis - frontotemporal dementia continuum. PLoS ONE. 2014;9:e105632. doi: 10.1371/journal.pone.0105632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tanaka M, Kondo S, Hirai S, Sun X, Yamagishi T, Okamoto K. Cerebral blood flow and oxygen metabolism in progressive dementia associated with amyotrophic lateral sclerosis. J Neurol Sci. 1993;120:22–28. doi: 10.1016/0022-510x(93)90019-u. [DOI] [PubMed] [Google Scholar]
- 167.Tazelaar GHP, Boeynaems S, De Decker M, van Vugt JJFA, Kool L, Goedee HS, McLaughlin RL, Sproviero W, Iacoangeli A, Moisse M, Jacquemyn M, Daelemans D, Dekker AM, van der Spek RA, Westeneng HJ, Kenna KP, Assialioui A, Da Silva N Project MinE ALS Sequencing Consortium. Povedano M, et al., editors. ATXN1 repeat expansions confer risk for amyotrophic lateral sclerosis and contribute to TDP-43 mislocalization. Brain Commun. 2020;2:fcaa064. doi: 10.1093/braincomms/fcaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tedesco AM, Chiricozzi FR, Clausi S, Lupo M, Molinari M, Leggio MG. The cerebellar cognitive profile. Brain. 2011;134:3672–3686. doi: 10.1093/brain/awr266. [DOI] [PubMed] [Google Scholar]
- 169.Tessitore A, Esposito F, Monsurro MR, Graziano S, Panza D, Russo A, Migliaccio R, Conforti FL, Morrone R, Quattrone A, Di Salle F, Tedeschi G. Subcortical motor plasticity in patients with sporadic ALS: an fMRI study. Brain Res Bull. 2006;69:489–494. doi: 10.1016/j.brainresbull.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 170.Thal DR, Züchner S, Gierer S, Schulte C, Schöls L, Schüle R, Synofzik M. Abnormal paraplegin expression in swollen neurites, τ- and α-synuclein pathology in a case of hereditary spastic paraplegia SPG7 with an Ala510Val mutation. Int J Mol Sci. 2015;16:25050–25066. doi: 10.3390/ijms161025050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Trojsi F, Caiazzo G, Corbo D, Piccirillo G, Cristillo V, Femiano C, Ferrantino T, Cirillo M, Monsurro MR, Esposito F, Tedeschi G. Microstructural changes across different clinical milestones of disease in amyotrophic lateral sclerosis. PLoS One. 2015;10:e0119045. doi: 10.1371/journal.pone.0119045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Trojsi F, Di Nardo F, Caiazzo G, Siciliano M, D'Alvano G, Ferrantino T, Passaniti C, Ricciardi D, Esposito S, Lavorgna L, Russo A, Bonavita S, Cirillo M, Santangelo G, Esposito F, Tedeschi G. Hippocampal connectivity in amyotrophic lateral sclerosis (ALS): more than Papez circuit impairment. Brain Imaging Behav. 2020;15:2126–2138. doi: 10.1007/s11682-020-00408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tu S, Menke RAL, Talbot K, Kiernan MC, Turner MR. Cerebellar tract alterations in PLS and ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:281–284. doi: 10.1080/21678421.2018.1562554. [DOI] [PubMed] [Google Scholar]
- 174.van Blitterswijk M, DeJesus-Hernandez M, Niemantsverdriet E, Murray ME, Heckman MG, Diehl NN, Brown PH, Baker MC, Finch NA, Bauer PO, Serrano G, Beach TG, Josephs KA, Knopman DS, Petersen RC, Boeve BF, Graff-Radford NR, Boylan KB, Petrucelli L, Dickson DW, et al. Association between repeat sizes and clinical and pathological characteristics in carriers of C9ORF72 repeat expansions (Xpansize-72): a cross-sectional cohort study. Lancet Neurol. 2013;12:978–988. doi: 10.1016/S1474-4422(13)70210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Van Damme P, Veldink JH, van Blitterswijk M, Corveleyn A, van Vught PW, Thijs V, Dubois B, Matthijs G, van den Berg LH, Robberecht W. Expanded ATXN2 CAG repeat size in ALS identifies genetic overlap between ALS and SCA2. Neurology. 2011;76:2066–2072. doi: 10.1212/WNL.0b013e31821f445b. [DOI] [PubMed] [Google Scholar]
- 176.Van Overwalle F, D'Aes T, Marien P. Social cognition and the cerebellum: A meta-analytic connectivity analysis. Hum Brain Mapp. 2015;36:5137–5154. doi: 10.1002/hbm.23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Verstraete E, Turner MR, Grosskreutz J, Filippi M, Benatar M. Mind the gap: the mismatch between clinical and imaging metrics in ALS. Amyotroph Lateral Scler Frontotemporal Fegener. 2015;16:524–529. doi: 10.3109/21678421.2015.1051989. [DOI] [PubMed] [Google Scholar]
- 178.Villamar MF, Santos Portilla A, Fregni F, Zafonte R. Noninvasive brain stimulation to modulate neuroplasticity in traumatic brain injury. Neuromodulation. 2012;15:326–338. doi: 10.1111/j.1525-1403.2012.00474.x. [DOI] [PubMed] [Google Scholar]
- 179.Wen J, Zhang H, Alexander DC, Durrleman S, Routier A, Rinaldi D, Houot M, Couratier P, Hannequin D, Pasquier F, Zhang J, Colliot O, Le Ber I, Bertrand A. Neurite density is reduced in the presymptomatic phase of C9orf72 disease. J Neurol Neurosurg Psychiatry. 2019;90:387–394. doi: 10.1136/jnnp-2018-318994. [DOI] [PubMed] [Google Scholar]
- 180.Westeneng HJ, Walhout R, Straathof M, Schmidt R, Hendrikse J, Veldink JH, van den Heuvel MP, van den Berg LH. Widespread structural brain involvement in ALS is not limited to the C9orf72 repeat expansion. J Neurol Neurosurg Psychiatry. 2016;87:1354–1360. doi: 10.1136/jnnp-2016-313959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Xu F, Frazier DT. Role of the cerebellar deep nuclei in respiratory modulation. Cerebellum. 2002;1:35–40. doi: 10.1080/147342202753203078. [DOI] [PubMed] [Google Scholar]
- 182.Yang Y, Halliday GM, Kiernan MC, Tan RH. TDP-43 levels in the brain tissue of ALS cases with and without C9ORF72 or ATXN2 gene expansions. Neurology. 2019;93:e1748–e1755. doi: 10.1212/WNL.0000000000008439. [DOI] [PubMed] [Google Scholar]
- 183.Yasser S, Fecto F, Siddique T, Sheikh KA, Athar P. An unusual case of familial ALS and cerebellar ataxia. Amyotroph Lateral Scler. 2010;11:568–570. doi: 10.3109/17482961003636874. [DOI] [PubMed] [Google Scholar]
- 184.Yokota O, Tsuchiya K, Oda T, Ishihara T, de Silva R, Lees AJ, Arai T, Uchihara T, Ishizu H, Kuroda S, Akiyama H. Amyotrophic lateral sclerosis with dementia: an autopsy case showing many Bunina bodies, tau-positive neuronal and astrocytic plaque-like pathologies and, pallido-nigral degeneration. Acta Neuropathol. 2006;112:633–645. doi: 10.1007/s00401-006-0141-1. [DOI] [PubMed] [Google Scholar]
- 185.Yunusova Y, Plowman EK, Green JR, Barnett C, Bede P. Clinical measures of bulbar dysfunction in ALS. Front Neurol. 2019;10:106. doi: 10.3389/fneur.2019.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhou C, Hu X, Hu J, Liang M, Yin X, Chen L, Zhang J, Wang J. Altered brain network in amyotrophic lateral sclerosis: a resting graph theory-based network study at voxel-wise level. Front Neurosci. 2016;10:204. doi: 10.3389/fnins.2016.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhou F, Gong H, Li F, Zhuang Y, Zang Y, Xu R, Wang Z. Altered motor network functional connectivity in amyotrophic lateral sclerosis: a resting-state functional magnetic resonance imaging study. Neuroreport. 2013;24:657–662. doi: 10.1097/WNR.0b013e328363148c. [DOI] [PubMed] [Google Scholar]


