Abstract
Purpose:
To quantify cancer risk in patients with Fuchs endothelial corneal dystrophy (FECD).
Methods:
Using the 2014–2016 Medicare Limited 5% Data Sets – Carrier Line File, U.S. Medicare fee-for-service beneficiaries (≥65 years old) with FECD and cancer were identified via International Classification of Diseases, 9th and 10th Revision (ICD-9 and ICD-10) diagnostic codes from January 1, 2014 to December 31, 2016. The main outcome measures were odds ratios (OR) of cancer at various anatomic locations in patients with versus without FECD.
Results:
Of the 1,462,740 Medicare beneficiaries, 15,534 (1.1%) patients had an ICD code for FECD. Compared to U.S. Medicare beneficiaries without FECD, FECD patients were at increased risk for the following malignancies: breast (OR: 1.32; 95% confidence interval [CI]: 1.22–1.43; p<0.001), cutaneous basal cell (OR: 1.42; 95% CI: 1.35–1.49; p<0.001), cutaneous melanoma (OR: 1.20; 95% CI: 1.03–1.40; p=0.02), cutaneous squamous cell (OR: 1.45; 95% CI: 1.38–1.53; p<0.001), ovarian (OR: 1.84; 95% CI: 1.48–2.30; p<0.001), and thyroid (OR: 1.32; 95% CI: 1.04–1.68; p=0.02). In contrast, FECD cases were at lower odds of having lung (OR: 0.81; 95% CI: 0.71–0.93; p=0.003) and prostate cancer diagnoses (OR: 0.88; 95% CI: 0.81–0.96; p=0.002).
Conclusion:
Patients with FECD ≥65 years old may be at increased risk for cancer at several anatomic locations. Further studies are needed to further explore the association of FECD and malignancy, elucidate potential disease mechanisms, and identify genetic and/or environmental risk factors.
Keywords: Fuchs endothelial corneal dystrophy, cancer, odds ratio
Introduction
Fuchs endothelial corneal dystrophy (FECD) is a highly prevalent, bilateral, late-onset heritable disorder affecting the corneal endothelium. Although only a small proportion of cases requires transplantation, FECD is the most common indication for corneal grafting worldwide, accounting for 39% of all cases.1 In the United States (U.S.), FECD was the indication for approximately 17,000 primary corneal transplants in 2018, and an unknown proportion of the more than 13,000 repeat grafts or grafts without a reported indication.2 In nearly 80% of U.S. cases, FECD involves an intronic cytosine-thymine-guanine (CTG) trinucleotide repeat (TNR) expansion in the widely expressed transcription factor 4 (TCF4) gene, also known as CTG18.1 expansion.3, 4 CTG18.1 expansion-associated FECD is inherited in an autosomal dominant pattern with incomplete penetrance and variable expressivity, with a predisposition towards females and individuals of northern European ancestry.5 The prevalence of FECD is approximately 5% in the U.S. population, thus FECD is the most common TNR expansion disorder.3, 6
Despite recent advancements in our understanding of FECD pathogenesis, it is unknown whether patients with FECD are at variable risk of systemic disease such as malignancy. Several TNR expansion disorders such as myotonic dystrophy type 1 (DM1), X-linked spinal muscular atrophy, and bulbar muscular atrophy are associated with increased cancer risk, while Huntington’s disease demonstrates decreased malignancy rates and fragile X syndrome has no cancer associations.7–10 Using the Medicare Claims 5% Limited Data Set – Carrier Line File from 2014 to 2016, we sought to quantify the risk of cancer in patients ≥65 years old with FECD in a broad insurance-based cohort.
Materials and Methods
This was a retrospective cross-sectional cohort study utilizing the Medicare Limited 5% Data Set – Carrier Line File from January 1, 2014 to December 31, 2016. Since this dataset was comprised of de-identified patient information, approval by the Mayo Clinic Institutional Review Board was not required for this study. This research complied with the Health Insurance Portability and Accountability Act and adhered to the tenets of the Declaration of Helsinki. The Medicare Limited 5% Data Set – Carrier Line File includes a 5% random sample of all individuals covered under the Medicare fee-for-service program. Individuals remained in the dataset over the 3-year period unless death or disenrollment occurred, in which case another individual was added to the dataset. Only patients ≥65 years of age were included in the study.
Patients within the Medicare Limited 5% Data Set – Carrier Line File were identified as having a diagnosis if they had at least one clinical encounter during the study period in which an International Classification of Diseases, 9th and 10th Revision (ICD-9 and ICD-10) code was selected as the primary diagnosis by the billing provider. FECD cases were identified using the ICD-9 code 371.57 (endothelial corneal dystrophy) and the ICD-10 code H18.51 (endothelial corneal dystrophy). The Medicare Limited 5% Data Set – Carrier Line File lists a single diagnosis for each office visit, test, or procedure performed. Therefore, secondary diagnoses were not available. The number of FECD cases who received a corneal transplantation procedure was determined using Current Procedural Terminology (CPT) codes 65730 (keratoplasty penetrating), 65750 (keratoplasty penetrating in aphakia), and 65756 (keratoplasty procedures on the cornea). The ICD-9 and ICD-10 codes used to identify diagnoses of cancer, tobacco use, and obesity are listed in the Supplemental Table. Seventeen specific cancer diagnoses queried in this study included the following: brain, breast, choroidal melanoma, colon, cutaneous basal cell, cutaneous melanoma, cutaneous squamous cell, kidney, leukemia, lung, lymphoma, malignant carcinoid of colon, ovarian, prostate, testicular, thyroid, and uterine. All cancer associations in this study were tested a priori. Since the study population was Medicare beneficiaries (e.g., individuals ≥65 years old), we investigated associations with cancers that were more common in the elderly, assuming rare malignancies or those of childhood would not generate meaningful data. However, choroidal melanoma was included.
Overall variables were reported as frequencies and percentages. Age was reported by age category (65 to 69, 70 to 74, 75 to 79, 80 to 84, 85+ years old) since the Medicare Limited 5% Data Set – Carrier Line File only provides patient age by age category (5-year groups), and not specific age for individual patients to protect patient privacy. For ovarian and uterine cancer, percentages were reported among females only. For prostate and testicular cancer, percentages were reported among males only. Logistic regression models were used to determine if patients with FECD were more likely to have a particular cancer diagnosis compared to patients without FECD. Logistic models were also used to determine if the difference between FECD cases versus patients without FECD for a particular cancer were attributable to demographic factors. Adjusting variables in these logistic models included age, sex, race, tobacco use, and Charlson comorbidity index. The Charlson comorbidity index is a composite score of pre-existing diagnoses such as history of myocardial infarction, congestive heart failure, and peripheral vascular disease that predicts mortality rates, which we used to control for illness severity in FECD versus non-FECD subjects.11 Data were aggregated using Microsoft Excel 2010, PowerPivot, and DaxStudio 2.10.2 (Microsoft Corporation; Redmond, WA, USA). Statistical analysis was performed using RStudio version 1.2.5042 (RStudio, Inc.; Boston, MA).
Results
From January 1, 2014 to December 31, 2016, there were 1,462,740 patients ≥65 years old who were enrolled in Medicare fee-for-service plans, of which 15,534 (1.1%) patients were designated with a FECD ICD-9 or ICD-10 code during the study period (Table 1). There were 3,625 (23.3%) patients with FECD who were 70 to 74 years old, representing the highest frequency of cases by age category. Females accounted for 10,517 (67.7%) FECD cases, which was greater than that of non-FECD Medicare patients (57.7% female). FECD cases were comprised of 13,684 (88.1%) patients who were White, mirroring the racial background of the non-FECD Medicare population (84.8% White). Among FECD cases, 310 (2.0%) patients had an ICD code of tobacco abuse compared to 1.9% in those without FECD (p=0.18), and 994 (6.4%) FECD cases were assigned a diagnostic code for obesity compared to 5.7% in non-FECD cases (p=0.07). Of the 15,534 FECD cases, 885 (5.7%) subjects received a CPT code for corneal transplantation during the study period.
Table 1:
Demographic characteristics of Medicare patients with Fuchs endothelial corneal dystrophy (FECD) versus those without FECD.
| Characteristic | Medicare patients with FECD diagnosis | Medicare patients without FECD diagnosis population |
|---|---|---|
| Total number | 15,534 | 1,447,206 |
| Age category (years) | ||
| 65–69 | 3,614 (23.3%) | 458351 (31.7%) |
| 70–74 | 3,625 (23.3%) | 329,210 (22.7%) |
| 75–79 | 3,318 (21.4%) | 252,322 (17.4%) |
| 80–84 | 2,584 (16.6%) | 190,441 (13.2%) |
| >84 | 2,393 (15.4%) | 216,882 (15.0%) |
| Sex | ||
| Male | 5,017 (32.3%) | 613,350 (42.4%) |
| Female | 10,517 (67.7%) | 833,856 (57.6%) |
| Race/ethnicity | ||
| White | 13,684 (88.1%) | 1,227,213 (84.8%) |
| Hispanic | 205 (1.3%) | 24,732 (1.7%) |
| Asian | 233 (1.5%) | 28,576 (2.0%) |
| Black | 982 (6.3%) | 116,308 (8.0%) |
| Native American | 16 (0.1%) | 6,024 (0.4%) |
| Other | 250 (1.6%) | 27,199 (1.9%) |
| Unknown | 164 (1.1%) | 17,154 (1.2%) |
| Clinical characteristics | ||
| Tobacco use | 310 (2.0%) | 26,787 (1.9%) |
| Obesity | 994 (6.4%) | 82,983 (5.7%) |
In a multivariate logistic regression adjusted for age, race, sex (for non-sex-specific malignancies), tobacco use, and Charlson comorbidity index, patients with FECD had increased odds of the following malignancies compared to those without FECD: breast (OR: 1.32; 95% CI: 1.22 to 1.43; p<0.001), cutaneous basal cell (OR: 1.42; 95% CI: 1.35 to 1.49; p<0.001), cutaneous melanoma (OR: 1.20; 95% CI: 1.03 to 1.40; p=0.02), cutaneous squamous cell (OR: 1.45; 95% CI: 1.38 to 1.53; p<0.001), ovarian (OR: 1.84; 95% CI: 1.48 to 2.30; p<0.001), and thyroid (OR: 1.32; 95% CI: 1.04 to 1.68; p=0.02) (Table 2). In contrast, FECD cases were at lower odds of having a lung (OR: 0.81; 95% CI: 0.71 to 0.93; p=0.003) and prostate cancer diagnosis (OR: 0.88; 95% CI: 0.81 to 0.96; p=0.002). No statistically significant association was identified for brain, choroidal melanoma, colon, kidney, leukemia, lymphoma, malignant carcinoid of the colon, testicular, and uterine malignancies (Figure).
Table 2:
Odds ratio of cancer by anatomic site of Medicare patients with Fuchs endothelial corneal dystrophy (FECD) versus those without FECD.
| Cancer type | Number diagnosed in cases with FECD diagnosis (N = 15,534) |
Number diagnosed in cases without FECD diagnosis (N = 1,447,206) |
Odds ratio* (95% confidence interval) |
P-value |
|---|---|---|---|---|
| Brain | 26 (0.2%) | 3,104 (0.2%) | 0.76 (0.52–1.13) | 0.18 |
| Breast | 784 (5.0%) | 47,142 (3.3%) | 1.32 (1.22–1.43) | <0.001 |
| Choroidal melanoma | 7 (0.0%) | 477 (0.0%) | 1.29 (0.70–2.72) | 0.51 |
| Colon | 181 (1.2%) | 17,210 (1.2%) | 0.92 (0.79–1.07) | 0.30 |
| Cutaneous basal cell | 1,871 (12.0%) | 126,716 (8.8%) | 1.42 (1.35–1.49) | <0.001 |
| Cutaneous melanoma | 166 (1.1%) | 12,917 (0.9%) | 1.20 (1.03–1.40) | 0.02 |
| Cutaneous squamous cell | 1,636 (10.5%) | 106,451 (7.4%) | 1.45 (1.38–1.53) | <0.001 |
| Kidney | 87 (0.6%) | 8,893 (0.6%) | 0.93 (0.75–1.15) | 0.50 |
| Leukemia | 179 (1.2%) | 14,547 (1.0%) | 1.11 (0.96–1.29) | 0.17 |
| Lung | 247 (1.6%) | 26,297 (1.8%) | 0.81 (0.71–0.93) | 0.003 |
| Lymphoma | 159 (1.0%) | 12,235 (0.8%) | 1.17 (1.00–1.37) | 0.06 |
| Malignant carcinoid of colon | 11 (0.1%) | 634 (0.0%) | 1.61 (0.89–2.93) | 0.12 |
| Ovarian (Females only) |
83 (0.8%) | 4,220 (0.5%) | 1.84 (1.48–2.30) | <0.001 |
| Prostate (Males only) |
635 (12.7%) | 62,898(10.3%) | 0.88 (0.81–0.96) | 0.002 |
| Testicular (Males only) |
2 (0.0%) | 332 (0.1%) | 0.70 (0.18–2.83) | 0.62 |
| Thyroid | 68 (0.4%) | 4,523 (0.3%) | 1.32 (1.04–1.68) | 0.02 |
| Uterine (Females only) |
68 (0.6%) | 5,299 (0.6%) | 1.01 (0.79–1.28) | 0.96 |
N = Number of patients at risk.
Odds ratios adjusted for age, race, sex (for non sex-specific malignancies), tobacco use, and Charlson comorbidity index.
Figure:
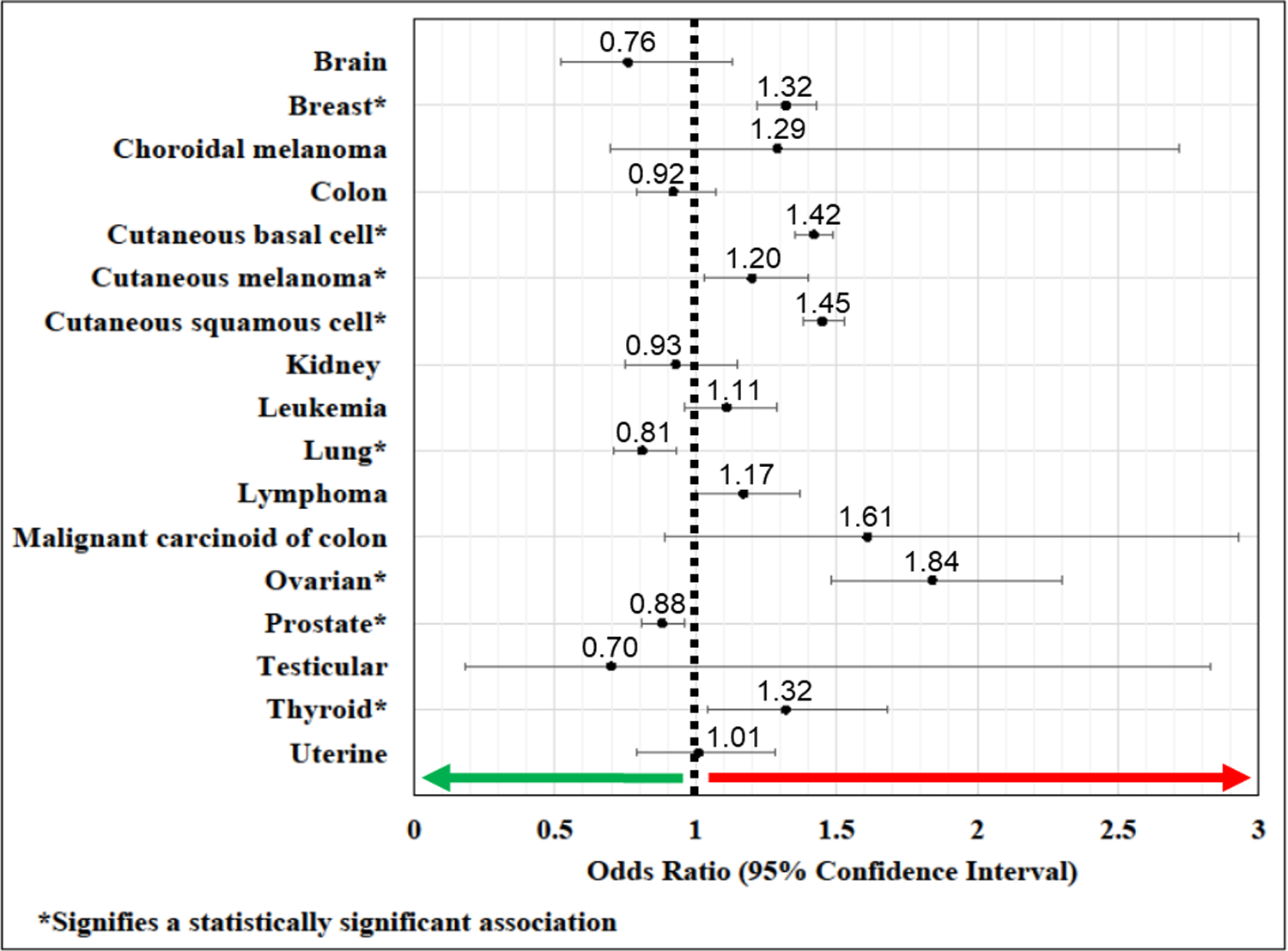
Cancer Odds Ratios in FECD versus non-FECD Subjects.
Discussion
In this investigation of U.S. Medicare-insured patients, increased risk of six distinct cancer diagnoses was observed in subjects with FECD compared to those without FECD. The strongest association was ovarian cancer, with FECD cases at a 1.8-fold increased risk. Increased malignancy risk was also observed in breast, cutaneous basal cell, cutaneous melanoma, squamous cell, and thyroid cancer. Conversely, FECD patients were at decreased risk for lung and prostate cancer. Although the observed increased risk of several malignancies was modest, the potential cancer case burden may be significant considering FECD affects approximately 5% of the U.S. population and is the most common TNR expansion disorder.4,7
The potential association between FECD and cancer is biologically plausible considering cancer risk in other TNR disorders has been previously described and 80% of FECD cases are associated with the CTG18.1 TNR expansion.3, 4 In a registry study of over 6,000 European patients, McNulty et al. found a decreased incidence rate for all cancers in patients with Huntington’s disease, potentially via TNR-mediated RNA interference of biochemical cancer pathways.9, 12 Conversely, increased malignancy risk has been reported in DM1, a TNR disease with a CTG repeat expansion in the 3’ untranslated region of the DM1 protein kinase gene.7, 8, 13 Gadalla and colleagues reported increased risk of endometrial, brain, ovarian, and colon cancer in patients with DM1 from Swedish and Danish cohorts, while Win et al. reported increased risk of thyroid cancer and choroidal melanoma in DM1 patients in a U.S. cohort.7, 8
Understanding of cancer pathogenesis in other TNR disorders may provide insight into the potential association of FECD and malignancy. Like FECD, RNA toxicity in DM1 has been proposed as a key pathogenic mechanism.14–16 In both FECD and DM1, RNA toxicity results from intra-nuclear accumulation of transcribed (CUG)n RNA, which sequesters RNA splicing factors, disrupts gene splicing regulation, and alters gene isoform expression in the affected tissues, with significant overlap in the patterns of mis-spliced genes affected by the two diseases.15, 17–19 In DM1, RNA toxicity may contribute to oncogenesis by impacting expression of tumor suppressor genes, proto-oncogenes, and mismatch repair genes,20 and upregulating β-catenin in the Wnt signaling pathway.21–24 Similar pathways may be affected in FECD, considering that the mechanism of RNA toxicity may be independent of TCF4 function. However, other studies have identified TCF4 expression changes in FECD patients containing CTG18.1 expansions.25, 26 This is important as TCF4 is a transcription factor. If changes to TCF4 expression are present, expression of downstream genes would be affected, influencing cellular pathways and mechanisms. One mechanism associated with TCF4 expression is the epithelial-mesenchymal transition, which has been linked to the pathophysiology underlying FECD.27, 28 Considering the prevalence of CTG18.1 expansion in 80% of FECD cases, the expression of the TCF4 gene in many tissues, the implicated role of TCF4 in oncogenic pathways, and shared pathogenic mechanisms with DM1, it is plausible that the CTG18.1 expansion may influence cancer pathogenesis in patients with FECD.
CTG18.1 expansion is present in 80% of FECD patients, but it is possible that other FECD-causing genetic variants may influence cancer risk. Rare genetic variants in AGBL1, COL8A2, KANK4, LAMC1, LINC00970/ATP1BP1, LOXHD1, SLC4A11, and ZEB1 have been identified in FECD.29–31 These genes typically are not considered oncogenic, with the exception of ZEB1, which is a transcription factor interacting with TCF4 in epithelial-to-mesenchymal transition (EMT) pathways.32 Other undisclosed genetic variants that cause FECD are likely.
Few systemic or environmental factors have been associated with FECD. 33,34Both smoking and body mass index may contribute to FECD severity, with smoking conferring an increased risk.33 Increased body mass index has recently been described as a risk factor for FECD severity in women,34 but obesity has also been reported as a protective factor.35 In the present study, neither tobacco use or obesity was significantly associated with FECD status, decreasing the likelihood that these factors had any influence in the association of FECD and malignancy. Furthermore, increased risk of lung cancer, which is strongly associated with tobacco abuse, was not observed in this cohort; rather, FECD patients were at decreased odds of having a lung cancer diagnosis. We acknowledge our significant under-representation of obesity given only 6% of patients had an obesity ICD code. Nevertheless, we do not anticipate FECD status to be influenced by the low frequency of obesity that was found in both FECD and non-FECD groups.
Ultraviolet (UV) light exposure is an environmental factor implicated in FECD pathogenesis via reactive oxygen species-induced loss of corneal endothelial cells.36 In mouse models, UV-A induced the FECD phenotype through DNA damage. Increased UV light exposure in patients who develop FECD could also explain the increased risk of cutaneous malignancy observed in this study since UV light is a well-known risk factor for skin cancer.37 This association may also be in part due to race and ancestry as confounding factors. FECD is highly prevalent among predominately White U.S. and European patients, specifically those of northern European and Scandinavian decent, who are at increased risk for skin cancer.38, 39 Race adjustment was performed in our multivariate analysis, but we were unable to account for more granular ancestry-related factors such as northern versus southern European descent.
Several limitations to the findings of this study should be considered. The accuracy of claims data in database research is limited by inter-provider coding variation, incomplete data, and misclassified or less specific diagnoses (e.g., a diagnosis of corneal edema rather than FECD).40 The Medicare Limited 5% Data Sets – Carrier Line File lists only one diagnosis per visit, test or procedure, so the diagnosis of FECD as a secondary diagnosis was not discoverable in patients with more pressing ocular co-morbidities. Moreover, because the database queried was restricted to a 3-year period, only individuals seen by providers and assigned a FECD or cancer diagnosis during that period were identified. These limitations likely contributed to our under-representation of FECD diagnoses, which was 1.1% of patients in the current study compared to approximately 5% in the general population.5, 6 However, this finding was not surprising; we did not expect to identify the majority of FECD cases since many FECD cases are mild, asymptomatic, under-diagnosed, and do not require frequent follow-up visits. Rather, this study was designed to evaluate an association between diagnoses and not incidence or prevalence rates of disease. Furthermore, only Medicare beneficiaries were investigated, thus the generalizability of our findings was limited to patients ≥65 years old. As age-related diseases, FECD and some cancers are more likely to be diagnosed in older individuals, while other malignancies are more common earlier in life. Lastly, factors influencing patterns of medical care delivery of FECD and cancer may have confounded our observations. Patients with cancer may be under better medical surveillance in general and more likely to seek eye examinations. However, we did not observe a trend associating FECD with commonly screened cancers. FECD patients in the current study cohort were at increased risk for breast cancer, lower risk for prostate cancer, and no significant differential risk for colon cancer, thereby not supporting a hypothesis that our observations were dictated by surveillance bias.
In summary, these findings suggest a potential association between FECD and malignancy that has not been previously described. The strongest association identified was ovarian cancer, with FECD patients exhibiting a 1.8-fold increased risk. Although the elevated risk was moderate, the potential contribution to cancer cases may be significant considering the high prevalence of FECD in the general U.S. population. Follow-up studies in other cohorts will be essential to further investigate the novel associations observed in this study and explore whether genetic factors such as CTG18.1 expansion and/or environmental risk factors contribute to oncogenesis in patients with FECD.
Supplementary Material
Supplemental Table: International Classification of Diseases, 9th and 10th Revision diagnostic codes used to identify patients with cancer at various anatomic locations, tobacco use, and obesity.
Acknowledgements
The authors thank Dr. Eric Wieben, PhD, Mayo Clinic Department of Biochemistry and Molecular Biology, Rochester, Minnesota, for his insights and review of the manuscript.
Funding support:
This study was supported by the National Eye Institute of the National Institutes of Health under Award Numbers EY26490 and EY21727, and the Mayo Foundation under the Robert R. Waller Career Development Award. The funding sources had no role in conduct of the research and preparation of the article.
Footnotes
Conflict of Interest Statement: No conflicting relationship exists for any author.
The contents in this manuscript were presented in part as a poster presentation at the Association for Research in Vision and Ophthalmology Virtual Annual Meeting in May 2021.
References
- 1.Gain P, Jullienne R, He Z, et al. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol 2016;134(2):167–73. [DOI] [PubMed] [Google Scholar]
- 2.Eye Bank Association of America. 2018 Eye Banking Statistical Report. 2019. Washington D.C. [Google Scholar]
- 3.Wieben ED, Aleff RA, Tosakulwong N, et al. A common trinucleotide repeat expansion within the transcription factor 4 (TCF4, E2–2) gene predicts Fuchs corneal dystrophy. PLoS One 2012;7(11):e49083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mootha VV, Gong X, Ku HC, Xing C. Association and familial segregation of CTG18.1 trinucleotide repeat expansion of TCF4 gene in Fuchs’ endothelial corneal dystrophy. Invest Ophthalmol Vis Sci 2014;55(1):33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu TT, Li YJ, Afshari NA, et al. Disease Expression and Familial Transmission of Fuchs Endothelial Corneal Dystrophy With and Without CTG18.1 Expansion. Invest Ophthalmol Vis Sci 2021;62(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krachmer JH, Purcell JJ Jr., Young CW, Bucher KD. Corneal endothelial dystrophy. A study of 64 families. Arch Ophthalmol 1978;96(11):2036–9. [DOI] [PubMed] [Google Scholar]
- 7.Gadalla SM, Lund M, Pfeiffer RM, et al. Cancer Risk Among Patients With Myotonic Muscular Dystrophy. JAMA 2011;306(22):2480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Win AK, Perattur PG, Pulido JS, et al. Increased Cancer Risks in Myotonic Dystrophy. Mayo Clin Proc 2012;87(2):130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNulty P, Pilcher R, Ramesh R, et al. Reduced Cancer Incidence in Huntington’s Disease: Analysis in the Registry Study. J Huntingtons Dis 2018;7(3):209–22. [DOI] [PubMed] [Google Scholar]
- 10.Sund R, Pukkala E, Patja K. Cancer incidence among persons with fragile X syndrome in Finland: a population-based study. J Intellect Disabil Res 2009;53(1):85–90. [DOI] [PubMed] [Google Scholar]
- 11.Quan H, Li B, Couris CM, et al. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data From 6 Countries. Am J Epidemiol 2011;173(6):676–82. [DOI] [PubMed] [Google Scholar]
- 12.Murmann AE, Yu J, Opal P, Peter ME. Trinucleotide Repeat Expansion Diseases, RNAi, and Cancer. Trends Cancer 2018;4(10):684–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho TH, Savkur RS, Poulos MG, et al. Colocalization of muscleblind with RNA foci is separable from mis-regulation of alternative splicing in myotonic dystrophy. J Cell Sci 2005;118(Pt 13):2923–33. [DOI] [PubMed] [Google Scholar]
- 14.Miller JW, Urbinati CR, Teng-Umnuay P, et al. Recruitment of human muscleblind proteins to (CUG)(n) expansions associated with myotonic dystrophy. EMBO J 2000;19(17):4439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du J, Aleff RA, Soragni E, et al. RNA toxicity and missplicing in the common eye disease fuchs endothelial corneal dystrophy. J Biol Chem 2015;290(10):5979–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mootha VV, Hussain I, Cunnusamy K, et al. TCF4 Triplet Repeat Expansion and Nuclear RNA Foci in Fuchs’ Endothelial Corneal Dystrophy. Invest Ophthalmol Vis Sci 2015;56(3):2003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machuca‐Tzili L, Brook D, Hilton‐Jones D. Clinical and molecular aspects of the myotonic dystrophies: a review. Muscle Nerve 2005;32(1):1–18. [DOI] [PubMed] [Google Scholar]
- 18.Sznajder ŁJ, Swanson MS. Short Tandem Repeat Expansions and RNA-Mediated Pathogenesis in Myotonic Dystrophy. Int J Mol Sci 2019;20(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin X, Miller JW, Mankodi A, et al. Failure of MBNL1-dependent post-natal splicing transitions in myotonic dystrophy. Hum Mol Genet 2006;15(13):2087–97. [DOI] [PubMed] [Google Scholar]
- 20.Thornton CA, Wymer JP, Simmons Z, et al. Expansion of the myotonic dystrophy CTG repeat reduces expression of the flanking DMAHP gene. Nat Genet 1997;16(4):407–9. [DOI] [PubMed] [Google Scholar]
- 21.Panzer S, Kuhl DP, Caskey CT. Unstable triplet repeat sequences: a source of cancer mutations? Stem Cells 1995;13(2):146–57. [DOI] [PubMed] [Google Scholar]
- 22.Mueller CM, Hilbert JE, Martens W, et al. Hypothesis: neoplasms in myotonic dystrophy. Cancer Causes Control 2009;20(10):2009–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukong KE, Chang KW, Khandjian EW, Richard S. RNA-binding proteins in human genetic disease. Trends Genet 2008;24(8):416–25. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Torron R, Garcia-Puga M, Emparanza JI, et al. Cancer risk in DM1 is sex-related and linked to miRNA-200/141 downregulation. Neurology 2016;87(12):1250–7. [DOI] [PubMed] [Google Scholar]
- 25.Foja S, Luther M, Hoffmann K, et al. CTG18.1 repeat expansion may reduce TCF4 gene expression in corneal endothelial cells of German patients with Fuchs’ dystrophy. Graefes Arch Clin Exp Ophthalmol 2017;255(8):1621–31. [DOI] [PubMed] [Google Scholar]
- 26.Okumura N, Hayashi R, Nakano M, et al. Effect of Trinucleotide Repeat Expansion on the Expression of TCF4 mRNA in Fuchs’ Endothelial Corneal Dystrophy. Invest Ophthalmol Vis Sci 2019;60(2):779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navarrete K, Pedroso I, De Jong S, et al. TCF4 (e2–2; ITF2): a schizophrenia-associated gene with pleiotropic effects on human disease. Am J Med Genet B Neuropsychiatr Genet 2013;162b(1):1–16. [DOI] [PubMed] [Google Scholar]
- 28.Sobrado VR, Moreno-Bueno G, Cubillo E, et al. The class I bHLH factors E2–2A and E2–2B regulate EMT. J Cell Sci 2009;122(Pt 7):1014–24. [DOI] [PubMed] [Google Scholar]
- 29.Afshari NA, Igo RP Jr., Morris NJ, et al. Genome-wide association study identifies three novel loci in Fuchs endothelial corneal dystrophy. Nat Commun 2017;8:14898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biswas S, Munier FL, Yardley J, et al. Missense mutations in COL8A2, the gene encoding the alpha2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy. Hum Mol Genet 2001;10(21):2415–23. [DOI] [PubMed] [Google Scholar]
- 31.Gupta R, Kumawat BL, Paliwal P, et al. Association of ZEB1 and TCF4 rs613872 changes with late onset Fuchs endothelial corneal dystrophy in patients from northern India. Mol Vis 2015;21:1252–60. [PMC free article] [PubMed] [Google Scholar]
- 32.Kurahara H, Takao S, Maemura K, et al. Epithelial-mesenchymal transition and mesenchymal-epithelial transition via regulation of ZEB-1 and ZEB-2 expression in pancreatic cancer. J Surg Oncol 2012;105(7):655–61. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Igo RP Jr., Fondran J, et al. Association of smoking and other risk factors with Fuchs’ endothelial corneal dystrophy severity and corneal thickness. Invest Ophthalmol Vis Sci 2013;54(8):5829–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinariwala BB, Xu TT, Baratz KH, et al. Relationship of Body Mass Index With Fuchs Endothelial Corneal Dystrophy Severity and TCF4 CTG18.1 Trinucleotide Repeat Expansion. Cornea 2021. Mar 29. doi: 10.1097/ICO.0000000000002689. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 35.Zoega GM, Fujisawa A, Sasaki H, et al. Prevalence and Risk Factors for Cornea Guttata in the Reykjavik Eye Study. Ophthalmology 2006;113(4):565–9. [DOI] [PubMed] [Google Scholar]
- 36.Liu C, Miyajima T, Melangath G, et al. Ultraviolet A light induces DNA damage and estrogen-DNA adducts in Fuchs endothelial corneal dystrophy causing females to be more affected. Proc Natl Acad Sci U S A 2020;117(1):573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Gruijl FR, van Kranen HJ, Mullenders LHF. UV-induced DNA damage, repair, mutations and oncogenic pathways in skin cancer. J Photochem Photobiol B 2001;63(1):19–27. [DOI] [PubMed] [Google Scholar]
- 38.Eghrari AO, Gottsch JD. Fuchs’ corneal dystrophy. Expert Rev Ophthalmol 2010;5(2):147–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gloster HM Jr., Neal K. Skin cancer in skin of color. J Am Acad Dermatol 2006;55(5):741–60; quiz 61–4. [DOI] [PubMed] [Google Scholar]
- 40.Stein JD, Lum F, Lee PP, et al. Use of health care claims data to study patients with ophthalmologic conditions. Ophthalmology 2014;121(5):1134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table: International Classification of Diseases, 9th and 10th Revision diagnostic codes used to identify patients with cancer at various anatomic locations, tobacco use, and obesity.


