Abstract
Background
Benign prostatic hyperplasia (BPH) is a disease characterized by abnormal proliferation of the prostate, which occurs frequently in middle-aged men. In this study, we report the effect of red ginseng oil (KGC11o) on BPH.
Methods
The BPH-induced Sprague-Dawley rats were divided into seven groups: control, BPH, KGC11o 25, 50, 100, 200, and finasteride groups. KGC11o and finasteride were administered for 8 weeks. The BPH biomarkers, DHT, 5AR1, and 5AR2, androgen receptor, prostate-specific antigen (PSA), Bax, Bcl-2, and TGF-β were determined in the serum and prostate tissue. The cell viability after KGC11o treatment was determined using BPH-1 cells, and, androgen receptor, Bax, Bcl-2, and TGF-β were confirmed by western blotting.
Results
In the in vivo study, administration of KGC11o reduced prostate weight by 18%, suppressed DHT (up to 22%) and 5AR2 (up to 12%) levels from administration of 100 mg/kg KGC11o (P < 0.05). PSA was significantly downregulated dose-dependently from at the concentration of 50 mg/kg KGC11o (P < 0.05). BPH-1 cell viability significantly reduced through the treatment with KGC11o. In vitro and vivo, AR, Bcl-2 TGF-β levels reduced significantly but Bax was increased (P < 0.05).
Conclusion
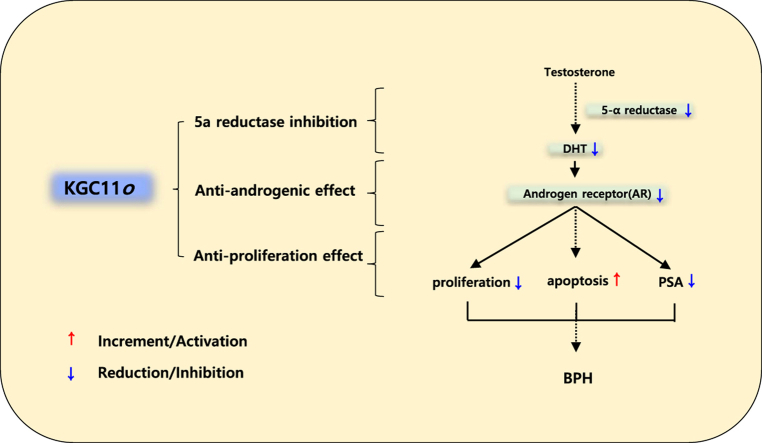
These results suggest that KGC11o may inhibit the development of BPH by significantly reducing the levels of BPH biomarkers via 5ARI, anti-androgenic effect, and anti-proliferation effect, serving as a potential functional food for treating BPH.
Keywords: Androgen receptor, 5alpha-reductase, BPH, Red ginseng oil, KGC11o
Graphical abstract
1. Introduction
In middle-aged men, the pathologic development of the prostate causes various clinical symptoms associated with the urinary system. The function of the prostate is to secrete a liquid that contributes to the volume of the semen and prolong the lifespan of sperm [1]. However, the prostate continues to develop without contraction even after the individual becomes an adult. This could lead to aggravated benign prostatic hyperplasia (BPH) [2].
The incidence of BPH increases with age. BPH occurs in approximately 60% of men over the age of 40 [3] and up to 90% at age 80 [4]. Three quarters of men with BPH complain storage and voiding symptoms of urination disorders called lower urinary tract symptoms (LUTS) [5]. BPH is accompanied with storage symptoms, such as urgency and urge incontinence, and obstructive symptoms, such as hesitancy and dysuria [6].
Although the exact mechanism of BPH has not been established, many studies have reported that BPH is based on androgen-dependent prostate development. Androgen changes during aging are suggested to be a cause of BPH [7]. Approximately 90% of the prostate androgen is DHT [8], which is converted from testosterone (T) by the enzyme 5α-reductase. Once DHT binds to AR with an affinity approximately 2–5 times higher than that of T, various growth factors, such as the epidermal growth factor (EGF) and keratinocyte growth factor (KGF) [9], are regulated, and the activity of the transforming growth factor-β (TGF-β) modulating apoptosis is also regulated [10]. Because aging men commonly have lower serum T concentrations [11] and stable DHT levels in the prostate, predominant DHT in the prostate may cause an imbalance between cell proliferation and death, leading to BPH development [9].
Finasteride and dutasteride are widely used as 5α-reductase inhibitors (5ARIs) to improve BPH. 5-ARIs prevent the conversion of T to DHT, reducing the enlarged prostate size [12]. α-adrenergic antagonists, such as tamsulosin and terazosin, are also commonly used for bladder control improvement in BPH. Although it is suggested to properly combine these two types of treatment for BPH, many studies have reported limited long-term effects or genitourinary side effects such as erectile dysfunction [13,14]. Therefore, BPH treatments with fewer side effects are of interest.
Red ginseng oil KGC11o is obtained through the extraction of supercritical fluid from processed red ginseng (Panax ginseng Meyer). Unlike red ginseng aqueous extracts containing a large amount of ginsenosides, red ginseng oil mainly has a non-saponin-based active compound and is rich in fatty acids, such as linoleic acid, and phytosterols, such as β-sitosterol [15]. Some studies have reported that red ginseng oil has antioxidant and hepatoprotective effects [16], but fewer studies have compared the oil to other extracts. Plant-derived essential oils and oil-soluble bioactive compounds have completely different bioavailability compared to that of water-soluble bioactive compounds. More attention has to be paid to red ginseng oil to establish phytotherapy with no side effects. In this study, we report the effect of red ginseng oil, KGC11o, on BPH and its mechanism of action. The study also suggests that KGC11o may be used as a novel therapeutic candidate for BPH.
2. Material and methods
2.1. Preparation of KGC11o
KGC11o is red ginseng oil produced through a supercritical fluid (CO2) extraction system (Ilshin Autoclave Co., LTD, Daejeon, Korea). Extraction methods of Red ginseng and other materials are described under the Supplementary Material.
2.2. GC-FID sample preparation (β-sitosterol analysis)
KGC11o analysis method is based on the phytosterol analysis method of KFDA (Health Functional Food Code, Ministry of Food and Drug Safety Notice No. 2021–25). The detailed methods used are described under the Supplementary Material.
2.3. Animals
Seven-week-old male Sprague-Dawley (SD) rats were purchased from Central Lab Animal Inc. (Seoul, Korea) and castrated to exclude intrinsic testosterone and received a subcutaneous injection of 3 mg/kg testosterone propionate (TP) for BPH induction. Detailed castration procedures and rat caring condition are described under Supplementary Material. All experiments were approved by the Institutional Animal Care and Use Committee of the Suwon University (Approval number: SW-IACUC-2018-004).
2.4. Assessment of serum ALT and AST
The blood from the experimental animals was collected and centrifuged, and then the serum was separated and used for test. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) serum levels were measured using a GOT/GPT kit (Asan Pharmaceutical, Seoul, Korea). Results are expressed in Karmen units using a standard curve. Result data are shown in Supplementary Material Table 1.
2.5. Measurements of DHT
Blood sera were separated by centrifuging the blood at 3000 g for 20 min at 4 °C for analysis. Prostate tissues were homogenized and went through the two freeze-thaw cycles to get supernatant for the measurement. The concentrations of DHT were determined using an enzyme-linked immunosorbent assay (ELISA) kit (BioVendor, Brno, Czech Republic) according to the manufacturer's instructions.
2.6. 5α-reductase 1 (5AR1) and 5α-reductase 2 (5AR2) determination
The collected serum and prostate tissue were used to determine 5AR1 and 5AR2 levels using the SRD5A1 and SRD5A2 ELISA kit (Cusabio Biotech, Wuhan, China).
2.7. Determination of prostate-specific antigen (PSA) serum levels
Serum PSA levels were determined using an ELISA kit (Cusabio Biotech). The serum was diluted 200 times and the OD determined at 450 nm. PSA levels were calculated using a standard curve.
2.8. Histopathological examination
After dewaxing and dehydration, the fixed prostate tissue embedded in paraffin was cut into 4-μm-thick sections and stained with hematoxylin and eosin. The sections were mounted and cover-slipped using mounting solution and then examined with a microscope under 200× magnification. The prostate epithelial thickness was measured.
2.9. Western blotting
Total protein from prostate tissue and cells was lysed using radioimmunoprecipitation assay (RIPA) buffer (Cell Signaling Technology, Danvers, USA). The Antibodies used were as follows: Bcl-2 (Santa Cruz Biotechnology, Dallas, USA), Bax (Cell Signaling Technology, Danvers, USA), TGF-β1 (Santa Cruz Biotechnology), and androgen receptor (Sigma-Aldrich, St. Louis, USA). Protein quantification and detailed western blot methods described under the Supplementary Material.
2.10. Real-time PCR
Total RNA in cells was isolated using an RNA extraction reagent (TaKaRa Bio Inc. Otsu, Shiga, Japan) and real-time PCR was performed using the Roche Lightcycler 96 Real-time PCR System (Roche, Basel, Switzerland). The primer sequences and real-time PCR temperatures and times were detailed in Supplementary Material.
2.11. Cell culture and cell viability assay
The BPH epithelial cell line, BPH-1, was provided by the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). Solution of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich) was used for cell viability analysis. Condition of cell culture and detailed cell viability assay described under the Supplementary Material.
2.12. Statistical analysis
All values are expressed as the mean ± standard deviation. Significant differences between groups were statistically analyzed using one-way analysis of variance (ANOVA) followed by Duncan's multiple range test. Significance was set at P < 0.05. All statistical analyses were performed using SPSS Statistics (Statistical Package for Social Science, version 22.0, SPSS Inc., Chicago, IL, USA).
3. Results
3.1. KGC11o active compounds
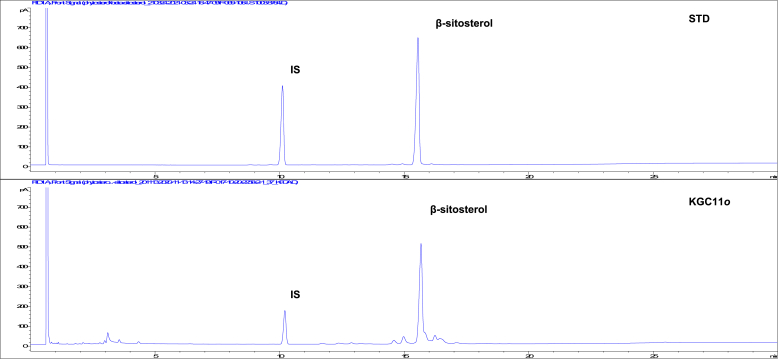
The presence of β-sitosterol in KGC11o was analyzed using a GC-FID system (Fig. 1). β-sitosterol was identified by comparing the mass spectra of the peaks with those in the mass spectrum library of NIST (Fig. 1). The β-sitosterol peak of KGC11o was observed at a retention time of 15.7 in the GC chromatogram.
Fig. 1.
Two dimensional GC-FID chromatogram of β-sitosterol standard and KGC11o. Analysis of KGC11o was performed using an DB-5 capillary column (30 × 0.32 mm, 0.25 μm; Agilent Technologies, Santa Clara, CA, USA) in a GC-FID (6890 system, Agilent Technology, USA). Samples were injected into the column and run in split mode (Split ratio = 10 : 1). The nitrogen carrier gas was programmed to maintain a constant flow rate of 2.0 mL/min. Oven temperature was initially 200 °C for 1min and then finally raised to 300 °C at 5 °C/min and maintained at 300 °C for 14 min.
3.2. Effect of KGC11o on prostate weight
TP-injected animals were administered KGC11o for 8 weeks. Blood was collected after sacrificing the animals, and the prostate was carefully dissected and weighed. The prostate weight is one of the important BPH biomarkers. The prostate weight was converted to prostate ratio and expressed as mg of prostate per 100 g of body weight. KGC11o intake significantly reduced prostate weight in rats with BPH. Table 1 shows that the prostate weight in the BPH group markedly raised compared to that of the control group; the prostate weight of rats in the KGC11o groups was significantly decreased compared to that in the BPH group. The prostate ratios of the KGC11o 100 and 200 groups were significantly lower by 18%, compared to those of the BPH group.
Table 1.
Prostate weight of rats administered with KGC11o.
| Group | Prostate weight (g) | Prostate ratioa (mg/100 g of BW) |
|---|---|---|
| Control | 0.52 ± 0.03d | 0.11 ± 0.01d |
| BPH | 0.96 ± 0.05a | 0.22 ± 0.01a |
| 25 | 0.94 ± 0.07a | 0.21 ± 0.02a |
| 50 | 0.90 ± 0.04ab | 0.21 ± 0.01a |
| 100 | 0.79 ± 0.03bc | 0.18 ± 0.01b |
| 200 | 0.76 ± 0.04c | 0.18 ± 0.01b |
| Finasteride | 0.59 ± 0.03d | 0.14 ± 0.01c |
Control: corn oil oral intake + corn oil injection; BPH: corn oil oral intake + testosterone injection; 25: TP injection + KGC11o (25 mg/kg/day); 50: 50 mg/kg KGC11o + testosterone injection; 100: 100 mg/kg KGC11o + testosterone injection; 200: 200 mg/kg KGC11o + testosterone injection; finasteride: 10 mg/kg finasteride + testosterone injection. For all the groups, testosterone was used at 3 mg/kg body weight/day. Different letters show a significant difference at p < 0.05, as determined by Duncan's multiple range test.
Prostate ratio: prostate weight (mg)/body weight (mg) x 100 (g).
3.3. Immunohistochemical analysis of prostate tissues in KGC11o-administered rats
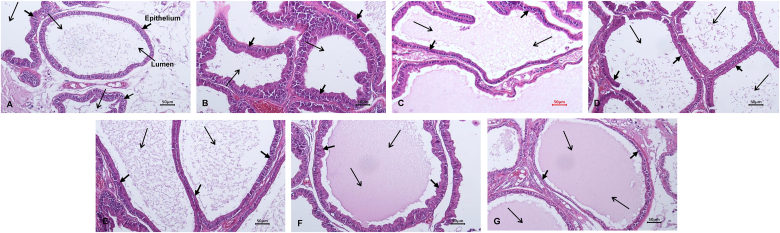
Hematoxylin-eosin staining was performed to confirm the effect of BPH induction and BPH improvement in the KGC11o groups. As shown in Fig. 2, the epithelium of the prostate tissue in the BPH group (Fig. 2B) was thicker and the lumen was narrower compared to that of the control group (Fig. 2A). In samples from the KGC11o 25 (Fig. 2C), KGC11o 50 (Fig. 2D), KGC11o 100 groups (Fig. 2E), and KGC11o 200 groups (Fig. 2F). the epithelial cell thickness decreased compared to that in samples from the BPH group (Fig. 2B), and the lumen was similar to that of the control group (Fig. 2A). The samples from the finasteride group (Fig. 2G), showed that the thickness of the epithelial cells was reduced compared to that of the BPH group and similar to that of the control group (Fig. 2A).
Fig. 2.
The effects of KGC11o on prostate tissue H&E histology of rats. Representative photomicrograhps of H&E-stained prostate tissues are presented (magnification, 100×). A: Control group, corn oil oral intake + corn oil injection, B: BPH group, corn oil oral intake + testosterone 3 mg/kg injection, C: 25 group, KGC11o 25 mg/kg + testosterone 3 mg/kg injection, D: 50 group, KGC11o 50 mg/kg + testosterone 3 mg/kg injection, E: 100 group, KGC11o 100 mg/kg + testosterone 3 mg/kg injection, F: 200 group, KGC11o 200 mg/kg + testosterone 3 mg/kg injection, G: Fina group, finasteride 10 mg/kg + testosterone 3 mg/kg injection.
3.4. Effect of KGC11o on dihydrotestosterone (DHT) serum and prostate levels
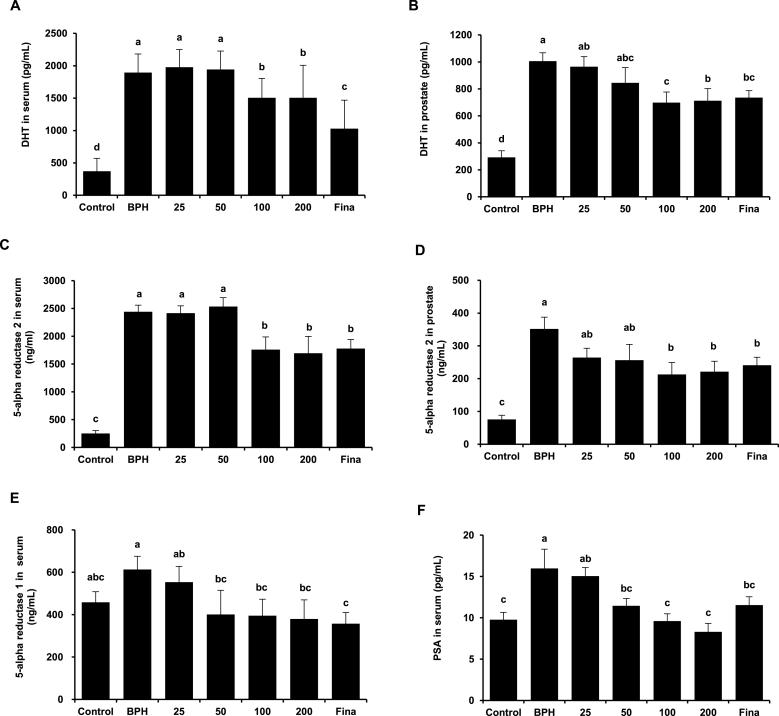
DHT are the most common androgens that regulate prostate growth [17]. The concentrations of DHT in the serum and prostate were measured to determine whether KGC11o affects androgen levels. Serum DHT levels were higher in the BPH group than those in the control group, but were lower than those in the KGC11o 100, 200, and finasteride groups (Fig. 3A). Similarly, prostate DHT concentrations in the BPH group were significantly higher than those in the control group (Fig. 3B), and decrease in concentration-dependent manner with treatment of KGC11o (p < 0.05).
Fig. 3.
The effects of KGC11o on dihydrotestosterone (DHT), 5ARI and prostate specific antigen (PSA) level. (A, B) DHT level in serum and prostate tissue showing a significant decrease with KGC11o. (C–E) Effects of KGC11o on the level of 5AR2 in the serum and prostate and that of 5AR1 in the serum. (F) Prostate specific antigen (PSA) level reduced by KGC11o dose-dependently in serum. Alphabet a, b, c and d indicate significant differences at p < 0.05 by Duncan's multiple range test (DMRT).
3.5. Effect of KGC11o on the level of 5AR2 in serum and prostate tissue, the level of 5AR1 and PSA in serum
The conversion of T to DHT in the prostate, skin, and liver [18] is catalyzed by the enzyme 5AR; and 5AR1 and 5AR2 are isoenzymes found in normal, BPH, and cancerous tissues [18]. The 5AR2 level in serum decreased by 12.0% in the KGC11o 100 group compared to that in the BPH group, similarly to that in the finasteride group (Fig. 3C). The 5AR2 level in prostate tissue was decreased concentration-dependently in the KGC11o groups compared to that in the BPH group and was significantly similar to that in the finasteride group (Fig. 3D). The concentration of 5AR1 in the BPH group increased by 33.77% compared to that in the control group. The 5AR1 concentrations in the KGC11o 50, 100, and 200 groups were reduced significantly compared to those in the BPH group (Fig. 3E).
Serum PSA levels is mainly used as an indicator of the status of prostate cancer [19] and is correlated with prostate size [20]. The concentration of PSA in the serum is shown in Fig. 3F. The PSA concentration was significantly higher in the BPH group than that in the control group. The PSA concentration in all the KGC11o groups (50, 100, and 200) except 25 group was significantly lower than that in the BPH group and were not significantly different from that in the control group.
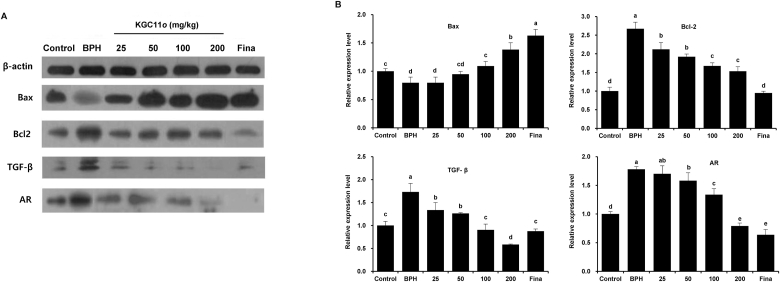
3.6. KGC11o reduces expression of Bax, Bcl-2, and TGF-β, AR 1in rat prostate
BPH development is related to uncontrolled proliferation and decreased apoptosis of prostatic cells [21]. In Fig. 4 (A and B), KGC11o groups which were administrated 100 and 200 showed a significant increase in proapoptotic protein Bax as compare to BPH group, but decreased expression of anti-apoptotic protein Bcl-2 in all KGC11o treated groups (P < 0.05). The protein expression of TGF-β 1 in KGC11o treated groups showed a significant decrease compared to BPH group (P < 0.05). AR plays an important role in prostate cell proliferation and survival, thus implicating in BPH development [22]. Also, AR expression in BPH group significantly increased, but those after KGC11o administration were significantly decreased in dose dependent manner.
Fig. 4.
KGC11o on protein expressions of B-cell lymphoma 2 (Bcl-2), Bcl-2 X-associated (Bax), Transforming growth factor-β (TGF-β) and Androgen receptor (AR) in prostate tissues of TP-induced BPH rats. (A, B) Western blots revealing that KGC11o treatment increases protein levels of Bax and decreases protein levels of Bcl-2, TGF-β and AR in TP injected rats. Alphabet a, b, c and d indicate significant differences at p < 0.05 by Duncan's multiple range test (DMRT).
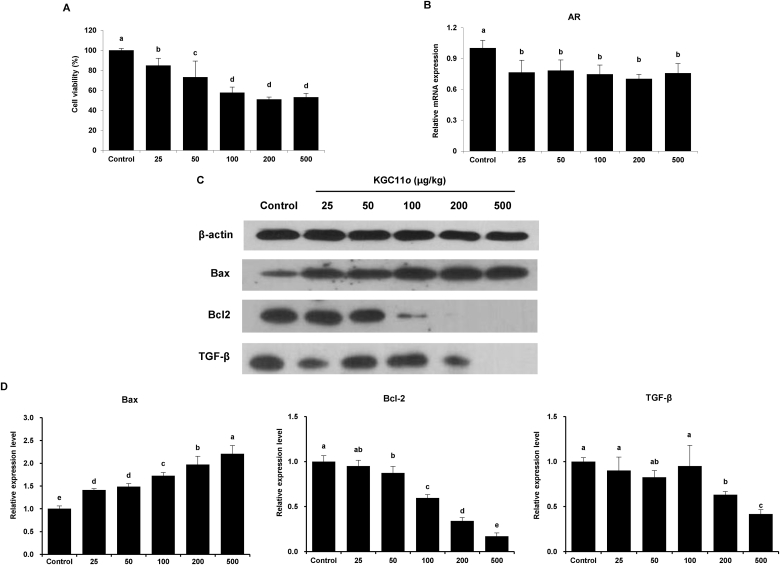
3.7. Effects of KGC11o on inhibition of proliferation in BPH-1 cells
BPH is characterized by uncontrolled prostatic growth due to cell proliferation in epithelium and stroma [23]. To investigate the effect of KGC11o on the mechanism of BPH, we used the BPH-1 cell line derived from human prostate tissue as an in vitro model. The BPH-1 cells were treated with various concentrations of KGC11o for 24 h, after which cell viability was significantly reduced in a dose-dependent manner. The differences among the KGC11o groups were significant concentrations-dependently (Fig. 5A). To investigate whether the KGC11o treatment might suppress the AR-associated pathway, we determined the AR expression level in BPH-1 cells. As shown in Fig. 5B, the KGC11o treatment significantly reduced AR levels in the KGC11o groups.
Fig. 5.
Effects of KGC11o on cell viability, AR and apoptosis related-protein expression in BPH-1 cells. BPH-1 cells were treated with various concentrations (0, 25, 50, 100, 200 and 500 μg/mL) of KGC11o. (A) Effect of KGC11o on BPH-1 cell viability. (B) The mRNA expressions of AR in BPH-1. (C, D) Protein expression of Bcl-2, Bax and TGF-β were analyzed by the western blot analysis. Alphabet a, b, c and d indicate significant differences at p < 0.05 by Duncan's multiple range test (DMRT).
To investigate whether KGC11o treatment might inhibit proliferation, Bax and Bcl-2 were determined through western blotting. As shown in Fig. 5C, Bax increased with the KGC11o treatment in a dose-dependent manner, while Bcl-2 decreased with the same treatment. In Fig. 5D, TGF-β 1 is also significantly decreased with high concentration of KGC11o treatment (200 and 500 μg/mL) (P < 0.05).
4. Discussion
The most noticeable characteristics of BPH are the increased cell proliferation and reduced apoptosis; therefore, a therapeutic strategy that enhances apoptosis or cell death in BPH tissue is desirable [24]. Recent studies suggested that red ginseng may enhance apoptosis and inhibit cell proliferation in T-induced BPH tissue to improve BPH [25]. In this study, we showed that BPH improved in vivo with KGC11o (red ginseng oil) administration, and attempted to clarify the mechanism in vitro and in vivo.
Red ginseng oil is obtained by supercritical fluid extraction from red ginseng, and unlike non-aqueous solvent extracts containing a large amount of saponins, it mainly contains non-saponin based active ingredients. According to a recent report, KGC11o has anti-inflammatory, antioxidant, and hepatoprotective effects [26]. Among the active compounds, β-sitosterol did not show a direct prostate size reduction effect, but improved symptoms of urological disease and overall urinary functions including urine flow, residual volume, and nocturia [27].
Androgen signaling occurs through cognate receptors. It has a pivotal effect on promoting cell proliferation in BPH [20]. T is converted to activated DHT by 5AR in the prostate, and the activated DHT binds to the androgen receptor in prostate cells inducing the transcriptional activation of target genes resulting in BPH [28]. Both 5AR1 and 5AR2 isoenzymes are significantly overexpressed in BPH tissue compared to normal prostate tissue, with 5AR2 being predominant [18]. Inhibition of 5AR results in decreased conversion of T to DHT leading to the inhibition of prostatic proliferation [29]. In clinical studies of men with BPH, 5AR inhibitors (5ARI) reduce the prostate volume by approximately 20–30% [9]. 5ARIs have been used to ameliorate LUTS/BPH symptoms, and patients with larger prostates responds more effectively. Serum PSA levels correlate with prostate size [22] and the androgen-responsive PSA gene, synthesized via the AR signaling pathway, is specifically expressed in prostatic tissue [30] and upregulated as BPH progresses [31]. In patients with BPH, DHT binds to AR, in turn causings it to interact with androgen-response elements in the promoter region of PSA, thereby increasing the PSA transcriptional activity. Biological actions of androgens is defined to occur primarily through binding to the AR, a member of nuclear receptor superfamily that functions as ligand-dependent transcription factor [32]. Several reports have documented an upregulation of the AR in BPH tissue, unveiling a potential role for AR in BPH etiopathogenesis [33]. This suggests that inhibition of AR activity would be a potential key mechanism for alleviating BPH symptoms by 5ARIs.
In this study, prostate weight and, 5AR1 and 5AR2 levels were significantly decreased in rats administered KGC11o. The epithelial layer thickness was also reduced and the lumen area restored by KGC11o administration. In other reports showing BPH improvement using P. ginseng, the prostate weight, DHT levels, and epithelial layer thickness were similarly reduced as in this study [34,35]. Additionally, the upregulation of PSA levels was confirmed in TP-induced BPH rats, and administration of KGC11o reduced the PSA level in serum. The treatment with KGC11o also significantly reduced the AR expression in vitro and in vivo, and this suggests that a decrease in the AR level may contribute to the improvement of BPH.
Androgen/AR signaling affects initiation and progression of BPH by altering the expression of various growth factors which promote the growth of prostatic epithelial and stromal cells, accompanied by increased expression of epithelial-mesenchymal transition (EMT)-related molecules such as TGF-β [36]. TGF-β controls the rate of apoptosis, which affects cell proliferation, differentiation, and apoptosis signaling [36]. TGF-β was previously reported to be up-regulated in a BPH- induced animal model [37] and might play a crucial role in BPH pathogenesis by impairing the luminal epithelial barrier in the prostate [38]. In this study, TGF-β 1 was increased in the BPH group when compared to the control group but was significantly downregulated in KGC 11o treated group.
Increased cellular apoptosis indicates the improvement of BPH because it helps ameliorate the excessive hyperplasia of cells, and the Bcl-2 family of proteins plays a central role in regulating the mitochondria-mediated apoptosis pathway [15]. Bcl-2 is an anti-apoptotic protein that protects cells from apoptosis, whereas Bax is a proapoptotic protein that induces apoptosis [17]. Epithelial cells in normal prostate tissue express little or no Bcl-2, but BPH changes are associated with increased levels of Bcl-2 and decreased levels of Bax in the same tissue [23]. In this study, we confirmed that Bcl-2 levels were reduced whereas Bax levels were increased by the KGC11o treatment in the in vitro and in vivo model. These results suggest that treatment with KGC11o induced apoptosis through regulation of Bax and Bcl-2 levels, thereby suppressing the overexpression of prostate tissue and improving BPH.
In conclusion, the present study showed that similar effects are observed when comparing BPH rats administered KGC11o to those administered finasteride. The administration of KGC11o suppressed AR expression and reduced DHT levels via inhibition of 5α-reductase, thereby improving BPH partly due to the regulation of proapoptotic and antiapoptotic activity. These findings suggest that KGC11o has potential as a novel therapeutic agent for the treatment of BPH.
Acknowledgments
This work was supported by grants from the Korean Society of Ginseng.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2021.11.005.
Contributor Information
Jeongmin Lee, Email: jlee2007@khu.ac.kr.
Yoo-Hyun Lee, Email: creamut@suwon.ac.kr.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Verze P., Cai T., Lorenzetti S. The role of the prostate in male fertility, health and disease. Nat Rev Urol [Internet] 2016;13(7):379–386. doi: 10.1038/nrurol.2016.89. Available from: [DOI] [PubMed] [Google Scholar]
- 2.McNeal J. Pathology of benign prostatic hyperplasia. Insight into etiology. Urol Clin North Am [Internet. 1990;17(3):477—486. http://europepmc.org/abstract/MED/1695776 Available from: [PubMed] [Google Scholar]
- 3.Litwin M.S., Saigal C.S., Yano E.M., Avila C., Geschwind S.A., Hanley J.M., et al. Urologic diseases in America project: analytical methods and principal findings. J Urol. 2005;173(3):933–937. doi: 10.1097/01.ju.0000152365.43125.3b. [DOI] [PubMed] [Google Scholar]
- 4.McVary K.T. BPH: epidemiology and comorbidities. Am J Manag Care. 2006;12(SUPPL. 5):122–128. [PubMed] [Google Scholar]
- 5.Calais da Silva F., Marquis P., Deschaseaux P., Gineste J.L., Cauquil J., Patrick D.L. Relative importance of sexuality and quality of life in patients with prostatic symptoms. Results of an international study. Eur Urol [Internet. 1997;31(3):272–280. doi: 10.1159/000474467. https://www.karger.com/DOI/10.1159/000474467 Available from: [DOI] [PubMed] [Google Scholar]
- 6.Chen J.L., Jiang Y.H., Lee C.L., Kuo H.C. vol. 32. Tzu Chi Medical Journal; 2020. pp. 5–13. (Precision medicine in the diagnosis and treatment of male lower urinary tract symptoms suggestive of benign prostatic hyperplasia). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vignozzi L., Rastrelli G., Corona G., Gacci M., Forti G., Maggi M. Benign prostatic hyperplasia: a new metabolic disease? J Endocrinol Invest [Internet] 2014;37(4):313–322. doi: 10.1007/s40618-014-0051-3. Available from: [DOI] [PubMed] [Google Scholar]
- 8.La Vignera S., Condorelli R.A., Russo G.I., Morgia G., Calogero A.E. Endocrine control of benign prostatic hyperplasia. Andrology. 2016;4(3):404–411. doi: 10.1111/andr.12186. [DOI] [PubMed] [Google Scholar]
- 9.Carson C., Rittmaster R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology. 2003;61(4 SUPPL. 1):2–7. doi: 10.1016/s0090-4295(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 10.Kim I.Y., Zelner D.J., Sensibar J.A., Ahn H.J., Park L., Kim J.H., et al. Modulation of sensitivity to transforming growth factor-β1 (TGF-β1) and the level of type II TGF-β receptor in LNCaP cells by dihydrotestosterone. Exp Cell Res. 1996;222(1):103–110. doi: 10.1006/excr.1996.0013. [DOI] [PubMed] [Google Scholar]
- 11.Harman S.M., Metter E.J., Tobin J.D., Pearson J., Blackman M.R. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86(2):724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 12.Webber R. Therapeutic treatment for benign prostatic hyperplasia. BJU Int. 2007;99(3):697. 697. [Google Scholar]
- 13.Mysore V. Finasteride and sexual side effects. Indian Dermatol Online J. 2012 Jan;3(1):62. doi: 10.4103/2229-5178.93496. https://pubmed.ncbi.nlm.nih.gov/23130269 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirby R.S. Terazosin in benign prostatic hyperplasia: effects on blood pressure in normotensive and hypertensive men. Br J Urol [Internet] 1998;82(3):373–379. doi: 10.1046/j.1464-410x.1998.00747.x. Available from: [DOI] [PubMed] [Google Scholar]
- 15.Reyes A.W.B., Hop H.T., Arayan L.T., Huy T.X.N., Park S.J., Kim K.D., et al. The host immune enhancing agent Korean red ginseng oil successfully attenuates Brucella abortus infection in a murine model. J Ethnopharmacol [Internet] 2017;198(May 2016):5–14. doi: 10.1016/j.jep.2016.12.026. Available from: [DOI] [PubMed] [Google Scholar]
- 16.Bak M.J., Jun M., Jeong W.S. Antioxidant and hepatoprotective effects of the red ginseng essential oil in H 2O 2-treated HepG2 cells and CCL 4-treated mice. Int J Mol Sci. 2012;13(2):2314–2330. doi: 10.3390/ijms13022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rho J., Seo C.S., Park H.S., Wijerathne C.U., Jeong H.Y., Moon O.S., et al. Ulmus macrocarpa Hance improves benign prostatic hyperplasia by regulating prostatic cell apoptosis. J Ethnopharmacol [Internet] 2019;233(November 2018):115–122. doi: 10.1016/j.jep.2018.11.042. Available from: [DOI] [PubMed] [Google Scholar]
- 18.Schmidt L.J., Tindall D.J. Steroid 5 α-reductase inhibitors targeting BPH and prostate cancer. J Steroid Biochem Mol Biol [Internet] 2011;125(1–2):32–38. doi: 10.1016/j.jsbmb.2010.09.003. Available from: [DOI] [PubMed] [Google Scholar]
- 19.Kim S.R., Ha A.W., Choi H.J., Kim S.L., Kang H.J., Kim M.H., et al. Corn silk extract improves benign prostatic hyperplasia in experimental rat model. Nutr Res Pract. 2017;11(5):373–380. doi: 10.4162/nrp.2017.11.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vickman R.E., Franco O.E., Moline D.C., Vander Griend D.J., Thumbikat P., Hayward S.W. The role of the androgen receptor in prostate development and benign prostatic hyperplasia: a review. Asian J Urol. 2020 Jul;7(3):191–202. doi: 10.1016/j.ajur.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park J., Youn D.H., Um J.Y. Aconiti Lateralis radix preparata, the dried root of Aconitum carmichaelii Debx., improves benign prostatic hyperplasia via suppressing 5-alpha reductase and inducing prostate cell apoptosis. Evid Based Complement Alternat Med. 2019 Jul 31;2019:6369132. doi: 10.1155/2019/6369132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J., Fang T., Li M., Song Y., Li J., Xue Z., et al. Pao Pereira extract attenuates testosterone-induced benign prostatic hyperplasia in rats by inhibiting 5α-reductase. Sci Rep. 2019;9(1):1–10. doi: 10.1038/s41598-019-56145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wijerathne C.U.B., Park H.S., Jeong H.Y., Song J.W., Moon O.S., Seo Y.W., et al. Quisqualis indica improves benign prostatic hyperplasia by regulating prostate cell proliferation and apoptosis. Biol Pharm Bull. 2017;40(12):2125–2133. doi: 10.1248/bpb.b17-00468. [DOI] [PubMed] [Google Scholar]
- 24.Li C., Hu W.L., Lu M.X., Xiao G.F. Resveratrol induces apoptosis of benign prostatic hyperplasia epithelial cell line (BPH-1) through p38 MAPK-FOXO3a pathway. BMC Compl Alternative Med. 2019;19(1):1–7. doi: 10.1186/s12906-019-2648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bae J.-S., Park H.-S., Park J.-W., Li S.-H., Chun Y.-S. Red ginseng and 20(S)-Rg3 control testosterone-induced prostate hyperplasia by deregulating androgen receptor signaling. J Nat Med [Internet] 2012;66(3):476–485. doi: 10.1007/s11418-011-0609-8. Available from: [DOI] [PubMed] [Google Scholar]
- 26.Bak M.J., Kim K.B., Jun M., Jeong W.S. Safety of red ginseng oil for single oral administration in Spraguee-Dawley rats. J Ginseng Res [Internet] 2014;38(1):78–81. doi: 10.1016/j.jgr.2013.11.009. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilt T.J., Ishani A., MacDonald R., Stark G., Mulrow C.D., Lau J. Cochrane Database Syst Rev [Internet; 1999. Beta-sitosterols for benign prostatic hyperplasia. (3). Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin G.G., Scott J.G. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr Relat Cancer. 2012;100(2):130–134. doi: 10.1677/ERC-08-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung Y., Park J., Kim H.L., Youn D.H., Kang J.W., Lim S., et al. Vanillic acid attenuates testosterone-induced benign prostatic hyperplasia in rats and inhibits proliferation of prostatic epithelial cells. Oncotarget. 2017;8(50):87194–87208. doi: 10.18632/oncotarget.19909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ratliff T.L. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer: Commentary. J Urol. 2006;175(3):1171. [Google Scholar]
- 31.Kim H.J., Jin B.R., An H.J. Psoralea corylifolia L. extract ameliorates benign prostatic hyperplasia by regulating prostate cell proliferation and apoptosis. J Ethnopharmacol. 2021 Jun 12;273:113844. doi: 10.1016/j.jep.2021.113844. Epub 2021 Jan 21. [DOI] [PubMed] [Google Scholar]
- 32.Heinlein C.A., Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16(10):2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- 33.Carvalho-Dias E., Miranda A., Martinho O., Mota P., Costa Â., Nogueira-Silva C., et al. Serotonin regulates prostate growth through androgen receptor modulation. Sci Rep. 2017;7(1):1–11. doi: 10.1038/s41598-017-15832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-mehi A.E., El-Sharif N.M. Modulating role of Panax Ginseng in experimentally induced benign prostatic hyperplasia in adult male albino rats. Austin J Anat [Internet. 2015;2(1):1031. https://www.researchgate.net/profile/Neveen_Shereef/publication/274249502_Austin_Journal_of_Anatomy/links/55194db40cf2d241f3562ae8.pdf (1) Available from: [Google Scholar]
- 35.Park H.K., Kim S.K., Lee S.W., Chung J.H., Lee B.C., Na S.W., et al. A herbal formula, comprising Panax ginseng and bee-pollen, inhibits development of testosterone-induced benign prostatic hyperplasia in male Wistar rats. Saudi J Biol Sci [Internet] 2017;24(7):1555–1561. doi: 10.1016/j.sjbs.2015.10.020. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Y., Lee D., Jo H., Go C., Yang J., Kang D., Kang J.S. GV1001 interacts with androgen receptor to inhibit prostate cell proliferation in benign prostatic hyperplasia by regulating expression of molecules related to epithelial-mesenchymal transition. Aging (Albany NY) 2021 Feb 4;13(3):3202–3217. doi: 10.18632/aging.202242. Epub 2021 Feb 4.PMID: 33539321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdel-Aziz A.M., Gamal El-Tahawy N.F., Salah Abdel Haleem M.A., Mohammed M.M., Ali A.I., Ibrahim Y.F. Amelioration of testosterone-induced benign prostatic hyperplasia using febuxostat in rats: the role of VEGF/TGFbeta and iNOS/COX-2. Eur J Pharmacol. 2020 Dec 15;889:173631. doi: 10.1016/j.ejphar.2020.173631. Epub 2020 Oct 5.PMID: 33031799. [DOI] [PubMed] [Google Scholar]
- 38.Li F., Pascal L.E., Wang K., Zhou Y., Balasubramani G.K., O'Malley K.J., Dhir R., He K., Stolz D., DeFranco D.B., Yoshimura N., Nelson J.B., Chong T., Guo P., He D., Wang Z. Transforming growth factor beta 1 impairs benign prostatic luminal epithelial cell monolayer barrier function. Am J Clin Exp Urol. 2020 Feb 25;8(1):9–17. eCollection 2020.PMID: 32211449. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.