Graphical abstract
Keywords: Cardiac arrest, Mitral valve prolapse, Mitral annular disjunction, Tricuspid annular disjunction
Highlights
-
•
Valvular heart disease is an uncommon but important cause of cardiac arrests.
-
•
TAD is also often seen with MAD.
-
•
High-risk features on echo include bileaflet MVP, MAD, and systolic curling.
Introduction
Out-of-hospital cardiac arrests (OHCAs) remain a pressing global health issue with poor outcomes, despite advances in resuscitation techniques. Coronary artery disease remains a top cause of OHCA worldwide.1 However, structural abnormalities in the form of bileaflet mitral valve prolapse (MVP), papillary muscle fibrosis, and mitral annular disjunction (MAD) have been found to increase arrhythmic risk.2,3 There is increasing evidence that MAD itself carries a similar significant risk as well.4 Tricuspid valve prolapse (TVP) and tricuspid annular disjunction (TAD) have been found to be associated with MVP and MAD, respectively, but appear not to be associated with a greater incidence of ventricular arrhythmias (VAs).5
In this case report, we describe a patient with OHCA secondary to ventricular fibrillation that was found to have the classical features of MVP, MAD, TVP, and TAD on multimodality cardiac imaging.
Case Presentation
A 65-year-old man with no cardiac history or family history of cardiac disease and sudden cardiac death (SCD) was admitted after a witnessed cardiac arrest while at work. Upon the arrival of the emergency medical services, the patient was in ventricular fibrillation. He was given three shocks in total before return of spontaneous circulation. He had no immediate neurological recovery and was transferred to the emergency department of our hospital, where he was intubated and started on intravenous norepinephrine and amiodarone infusions. He was then placed under targeted temperature management for 3 days before rewarming.
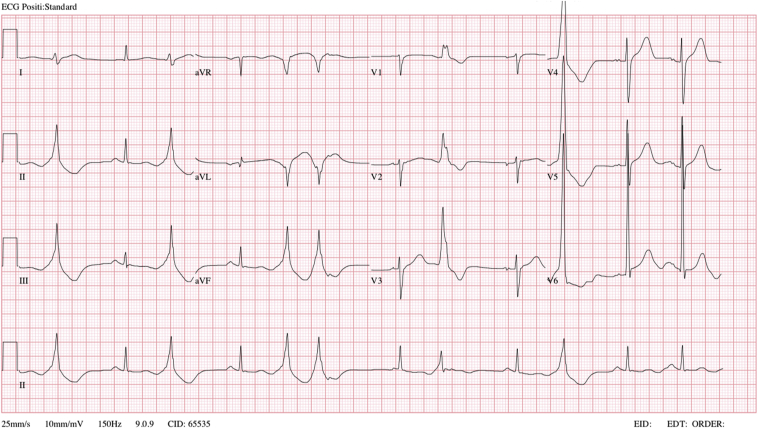
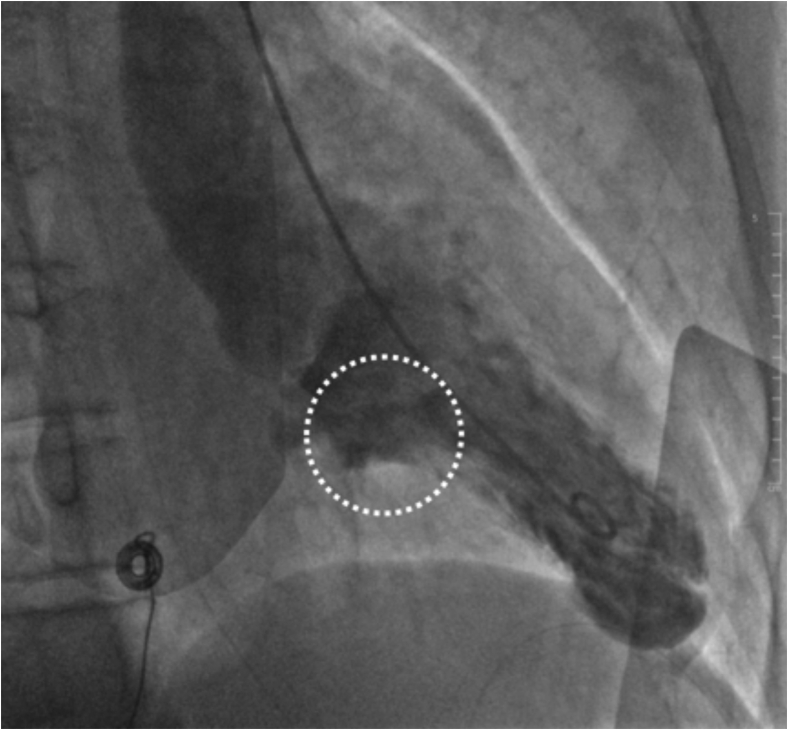
Computed tomography scan of his brain showed findings suggestive of early hypoxic ischemic encephalopathy. There were diffuse bilateral pulmonary opacities and no cardiomegaly. Blood tests revealed a normal full blood count. There were no electrolyte abnormalities, and procalcitonin was normal. An electrocardiogram (ECG) performed on arrival showed sinus rhythm with no significant ST segment abnormalities and frequent premature ventricular complexes (PVCs) of left ventricular (LV) outflow tract origin. The QT interval was borderline prolonged on the first ECG (Figure 1), but subsequent ECGs had a normal QT interval. High sensitive troponin I was mildly elevated at 23 ng/L and increased to 2,577 ng/L 4 hours later. An urgent invasive coronary angiogram showed normal and unobstructed coronary arteries. Left ventriculogram showed bulging of the basal inferior segment during systole (Figure 2, Video 1).
Figure 1.
Standard 12-lead ECG of the patient demonstrating normal sinus rhythm with frequent PVCs of LV outflow tract origin. Leftward axis with no ST segment depressions or elevations and borderline prolonged QTC interval of 508 msec.
Figure 2.
Invasive contrast-enhanced left ventriculography, left anterior oblique view, end systolic frame, demonstrating bulging of the basal inferior LV segment (white dotted circle), sometimes referred to as “ballerina-foot” deformity.
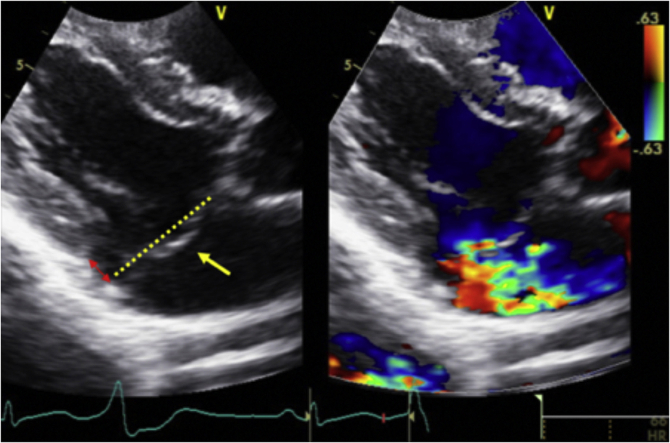
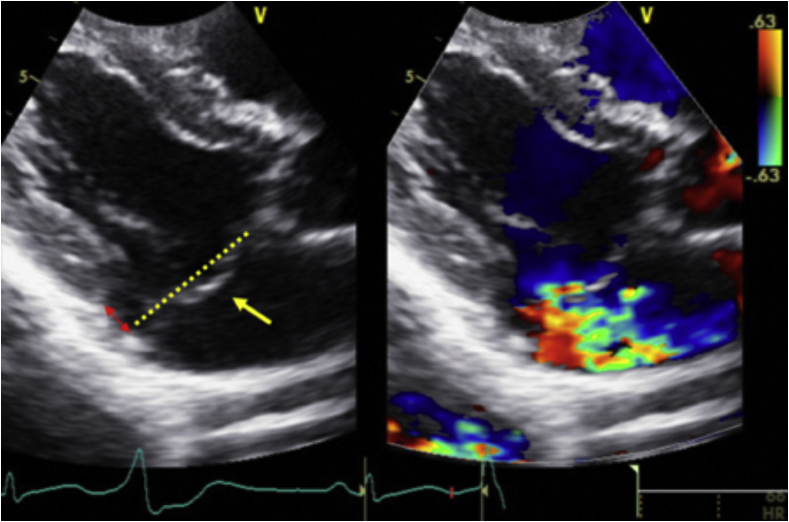
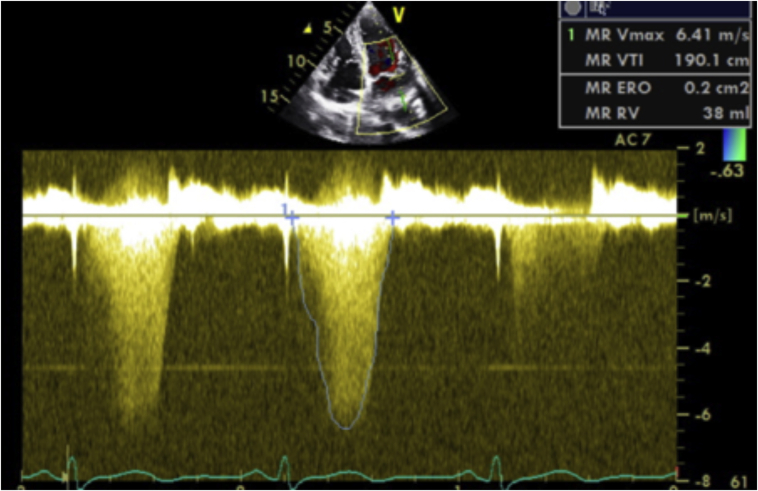
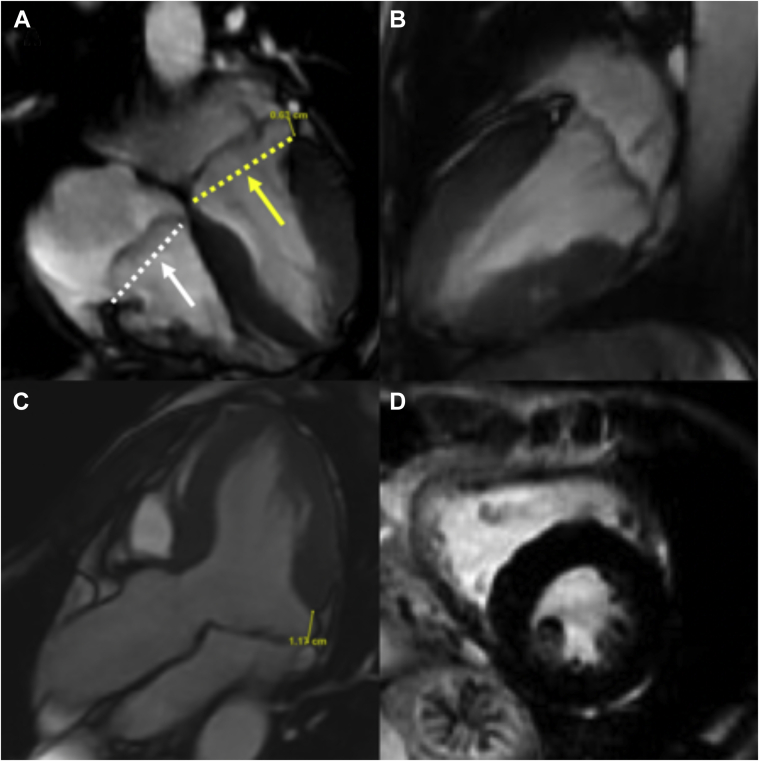
A formal transthoracic echocardiogram (TTE) was performed 2 days later that reported a normal-sized left ventricle (LV) with an LV ejection fraction of 54% (biplane Simpson’s method). The left atrium was normal in size (left atrium volume index = 21.8 mL/m2), and right cardiac chambers were normal in size and function. There was bileaflet MVP and systolic curling of the basal inferolateral LV segment that were suggestive of MAD (Figure 3, Video 2). Systolic curling is defined as a downward systolic motion of the posterior mitral ring, resulting in a curled appearance of the adjacent myocardium on cardiac imaging. The lateral tissue Doppler had an elevated and spiked S’ at 17 cm/sec (Figure 3). There was central mitral regurgitation (MR) of moderate severity (Figure 4). The MR occurred predominantly mid to late systole (Figure 5). The proximal isovelocity surface area radius was 0.8 cm, the effective regurgitant orifice area was 0.2 cm2, and MR regurgitant volume was 40 mL. Pulsed-wave Doppler of the pulmonary veins did not reveal systolic flow reversals. The tricuspid valve (TV) was myxomatous and prolapsed (Figure 6). On the apical four-chamber view, TAD was seen (Figure 6, Video 2). There was also mild tricuspid regurgitation seen on color flow Doppler imaging. Cardiovascular magnetic resonance imaging (CMR) showed an LV ejection fraction of 51% with no myocardial edema, which was suggestive of acute myocarditis. There was no myocardial late gadolinium enhancement (LGE). The mitral and tricuspid valves were myxomatous and prolapsed with mild mitral and tricuspid regurgitation (visual estimation). Mitral annular disjunction was seen, but TAD was not well visualized as the right heart was not studied as per the myocarditis study protocol (Figure 7, Video 3).
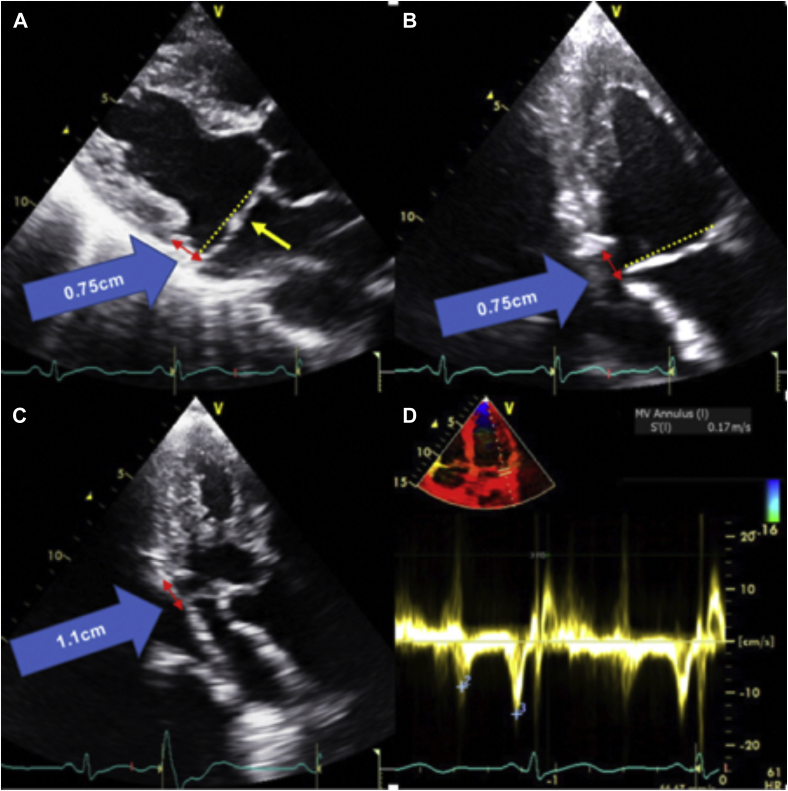
Figure 3.
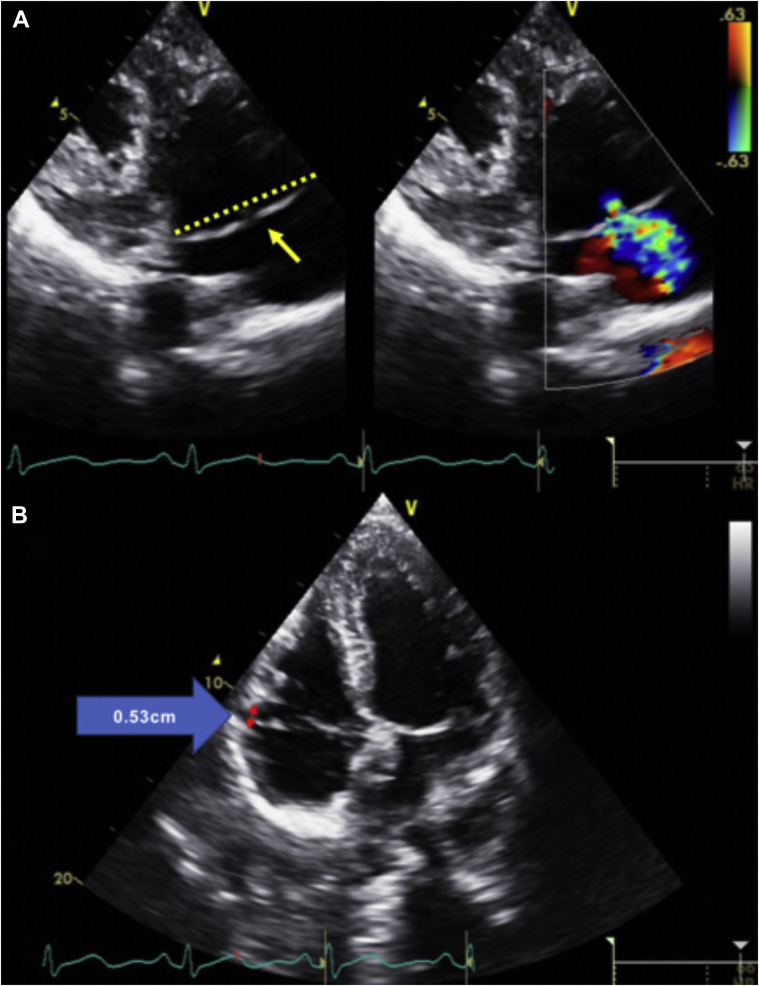
Transthoracic echocardiography images showing (A) parasternal long-axis view, end-systolic frame, demonstrating bileaflet MVP (yellow single-headed arrow) and MAD (red double-headed arrow) with a mitral annular displacement distance of 0.75 cm (blue arrow); (B) apical two-chamber view, end-systolic frame, demonstrating bileaflet MVP and MAD (red double-headed arrow) with a mitral annular displacement distance of 0.75 cm (blue arrow); (C) apical three-chamber view, end-systolic frame, also demonstrating MAD (red double-headed arrow) with a mitral annular displacement distance of 1.1 cm (blue arrow); (D) tissue Doppler imaging of the lateral mitral annulus showing an elevated lateral mitral annular S’ at 17 cm/sec.
Figure 4.
Transthoracic echocardiography image showing a parasternal long-axis view, end-systolic frame, demonstrating bileaflet MVP (yellow single-headed arrow) and the mitral annular plane (yellow dotted line), MAD (red double-headed arrow), and moderate MR on color flow Doppler imaging.
Figure 6.
Transthoracic echocardiography images show (A) right ventricular inflow view, end-systolic frame, demonstrating TVP (yellow single-headed arrow). Tricuspid annular plane is demonstrated by the yellow dotted line. There is also mild tricuspid regurgitation seen on color flow Doppler imaging. Another TTE image shows (B) apical four-chamber view, end-systolic frame, demonstrating TAD (red double-headed arrow) with a tricuspid annular displacement of 0.53 cm on the blue arrow.
Figure 5.
Transthoracic echocardiography image of apical four-chamber view, continuous-wave Doppler interrogation of the MR, demonstrating MR that occurred largely from mid to late systole.
Figure 7.
Cardiac magnetic resonance images of (A) steady state free precession (SSFP) sequence, four-chamber view, end-systolic frame, demonstrating myxomatous and prolapsed bileaflet mitral valve (yellow single-headed arrow) and TVs (white single-headed arrow). The mitral and tricuspid annular plane are shown by the yellow and white dotted lines, respectively. Mitral annular disjunction is also shown (double-headed yellow arrow) with a mitral annular displacement distance of 0.63 cm. (B) SSFP sequence, two-chamber view, end-systolic frame, demonstrating myxomatous and prolapsed bileaflet mitral valve. (C) SSFP sequence, three-chamber view, end-systolic frame, demonstrating a longitudinal MAD distance of 1.17 cm on the basal inferolateral LV wall. (D) LGE sequence, mid-LV short-axis view; no significant LGE was demonstrated in the LV.
The patient was deemed to have idiopathic ventricular fibrillation, likely secondary to MVP and MAD. He was started on daily bisoprolol and went on to have an implantable cardioverter-defibrillator (ICD). He was found to have mild cognitive impairment and was referred for neurocognitive rehabilitation and remained stable upon discharge.
Discussion
The poor outcomes associated with OHCA make it a global and local medical challenge despite large improvements in care, especially with preservation of neurological function. Improvements in community intervention and postresuscitation care remain key to improved survival, along with recognition and management of the underlying etiology.
While coronary artery disease remains an important cause of OHCA, structural heart and primary arrhythmic causes need to be considered, especially in the young. Valvular disease such as MVP is an uncommon but important cause. The estimated occurrence of SCD in patients with MVP is low at 0.2%-0.4% per year.6 However, there is an increasing body of literature that suggests a strong association between mitral valve abnormalities and arrhythmic sudden death.2,7
Arrhythmic MVP
Mitral valve prolapse was first described by Barlow et al.8 in 1968 as a syndrome with a constellation of auscultatory, electrocardiographic, and mitral valve structural abnormalities. It is a common valvular condition that affects 1%-3% of the population9 and can occur in a sporadic or familial fashion. Sudden cardiac death is uncommon in the patient with MVP, and there is variable prevalence of VAs.10 Significant valvular regurgitation in MVP resulting in an overloaded LV has been associated with a high recurrence rate of VAs.11
However, uncomplicated MVPs are also seen in patients with arrhythmic SCD, and a significant majority of the patients had experienced cardiac arrest in the absence of severe MR.2,7 Echocardiography is important to identify high-risk features for SCD and VA. It allows for quick bedside assessment and is also cost effective. The following features can be seen on echocardiography: (1) Bileaflet MVP was found to be present in 70% of cases with SCD or cardiac arrest.7 (2) A high-velocity systolic spike configuration in the TTE’s tissue Doppler interrogation of the lateral mitral annulus of more than 16 cm/sec, also known as the Pickelhaube sign, has also been reported to be a risk marker for malignant arrhythmias in patients with bileaflet MVP.12 Other risk factors include leaflet redundancy and chordal abnormalities on cardiac imaging and frequent PVCs on transient cardiac event monitor.7 It has been postulated that myocardial fibrosis on CMR at the level of the papillary muscles and basal inferior LV segment provides the myocardial substrate for electrical instability.2 Among these patients, MAD was a consistent feature of arrhythmic MVP and LV fibrosis of papillary muscle and the basal inferior LV segment.3
Mitral Annular Disjunction
Mitral annular disjunction is defined as a displacement in the point of insertion of the posterior mitral valve leaflet, resulting in a separation between the left atrial wall to mitral valve junction and the basal LV attachment13 or abnormal systolic expansion of the mitral annular diameter. This is associated with an adjacent myocardial defect at the point of insertion of the posterior leaflet into the annulus. It is demonstrated on TTE, CMR, and left ventriculogram, where abnormal contractility of the basal inferior wall results in bulging and “ballerina-foot” appearance.12 It is strongly associated with floppy mitral valves, where 92% of them were associated with MAD.13 It has been postulated that morphological and functional abnormalities of the mitral annulus in the form of MAD and the systolic curling motion of the LV lead to the development of myxomatous degeneration of the leaflets, systolic increase in annulus diameter, and subsequent myocardial stretch in the basal inferior segment of the LV and papillary muscles.2
These factors contribute to the formation of PVCs and malignant arrhythmias.3 In one study, 34% of patients with MAD had VAs.4 Longitudinal MAD distance in the inferolateral wall measured on CMR correlated with PVCs per 24 hours. Also, patients with VAs were found to have a significantly larger longitudinal MAD distance on the inferolateral wall on CMR compared with those without VAs (5.0 mm vs 1.5 mm, P = .04).4 Together with papillary muscle fibrosis seen on CMR, these were found to be markers of VA in a multivariate analysis.4 Interestingly, the same study had also found that MVP was not associated with arrhythmic events, leading the authors to conclude that MAD itself was a cause of VA.4
Tricuspid Valve Prolapse and Tricuspid Annular Disjunction
Tricuspid annular disjunction is defined as a visible separation seen at end systole between the TV leaflet and RV basal myocardium. A recent study has found that TAD is a common finding among patients with MAD,5 leading to further postulation that disease of the annulus fibrosus may be the common factor that results in both left- and right-sided valvular prolapse. In addition, patients with TAD were older and had TVP. However, there was no significant association with VA, despite its association with greater MAD distance measured in multiple imaging planes.5 The authors of the same study postulated that selection bias, reduced resistance by prolapsing TV leaflets, and thinner right cardiac chamber walls could have contributed to the lower arrhythmic risk.
Cardiac imaging with echocardiography is an essential first-line investigation in the diagnosis of arrhythmic MVP, MAD, and TAD. It can be performed quickly at bedside, allowing for evaluation of underlying cardiac function and structural abnormalities, specifically, in this case, valvular heart disease. Further evaluation with CMR allows for exclusion of other causes of cardiac arrest, such as dilated, hypertrophic, or arrhythmogenic right ventricular cardiomyopathies, infiltrative disease such as sarcoidosis, and inflammatory conditions resulting in myocarditis. Cardiac magnetic resonance imaging is able to assess for valvopathies, quantify the severity, and define the presence of annular disjunction clearly. Late gadolinium enhancement in CMR allows detection of fibrosis in the myocardium. In patients with MVP and annular disjunction, LGE detected in the mitral papillary muscles and basal inferior LV wall is suggestive of fibrosis and correlates with the presence of VAs.2
Early identification of patients at high risk of developing VA and SCD within a large population of asymptomatic MVP remains a major challenge. Red flags on echocardiography include MVP with bileaflet involvement, MAD, and systolic curling. These patients would benefit from further evaluation with CMR to evaluate for myocardial and papillary muscle hypertrophy and replacement fibrosis and arrhythmia surveillance. However, the utility of electrophysiologic studies in these patients is uncertain as the prognostic significance is unknown.2
Implantation of ICD as secondary prevention is indicated in OHCA survivors with MVP or MAD.14 There are no studies so far that assess the role of primary prevention ICD in MVP or MAD patients at high arrhythmic risk. A recent study has demonstrated that electrophysiologic studies and ablation can be performed to reduce PVC frequency in high-risk and symptomatic patients with drug-refractory VA.15 Surgical mitral valve repair or replacement has also been shown to reduce the burden of malignant arrhythmia in MVP patients in small studies.2 However, reduction of PVCs and VA cannot be equated to SCD risk elimination. Further studies are required to evaluate the role of these therapies for the sole purpose of primary prevention in patients with MVP or MAD at high risk for malignant arrhythmias.
Review of the literature thus far suggests that this is likely the first case report of OHCA in a patient with bilateral annular disjunction and MVP. High-risk features for VAs such as bileaflet MVP, high-velocity systolic spike configuration of the lateral mitral annulus on tissue Doppler imaging, and MAD were present in this patient. The presence of frequent PVCs of LV outflow tract origin on the initial ECG and lack of LGE in the mitral papillary muscles and basal inferior LV segment suggest that MAD, rather than MVP, is the likely underlying cause of the arrhythmic cardiac arrest. Tricuspid annular disjunction was likely to be an incidental finding associated with MAD and not the primary cause of the OHCA. As there were no hemodynamically significant valvular regurgitations, mitral or tricuspid valve repair and replacement were not indicated. Medical therapy for VAs was essential, and beta-blocker was successful in controlling the PVCs. Secondary prevention in the form of ICD remains important in the prevention of future cardiac arrests.
This case demonstrates that cardiac arrests can be caused by valvular heart disease and structural abnormalities. However, it remains a diagnosis of exclusion, and multimodality imaging is essential in ruling out other causes and confirming the diagnosis. Primary prevention therapies remain controversial, while secondary prevention with ICD is key in preventing future episodes of OHCA.
Conclusion
Cardiac structural abnormalities and valvular heart disease are an uncommon but important cause of cardiac arrests. Mitral valve prolapse, MAD, and TAD have been associated with development of VAs. This case report demonstrates that malignant VAs leading to cardiac arrest can occur in patients with MVP, MAD, and TAD. Therefore, it is important to identify high-risk features such that secondary preventive measures can be taken. Further research is required into the role of primary prevention in patients with MVP, MAD, and TAD with a high risk for malignant arrhythmias.
Acknowledgments
We thank the Cardiology Department at Tan Tock Seng Hospital for their support and clinical care of the patient.
Footnotes
Conflicts of Interest: None.
There was no external funding provided.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.case.2021.11.006.
Supplementary Data
Invasive contrast-enhanced left ventriculography, left anterior oblique view, demonstrating bulging of the basal inferior LV segment, sometimes referred to as “ballerina-foot” deformity. Note that PVCs can be seen.
Apical four-chamber view on TTE demonstrating TAD and MAD during systole.
Cardiac magnetic resonance cine loop, steady state free precession sequence, showing MAD, MVP, and TVP. Tricuspid annular disjunction is not well visualized here.
References
- 1.Pell J.P. Presentation, management, and outcome of out of hospital cardiopulmonary arrest: comparison by underlying aetiology. Heart. 2003;89:839–842. doi: 10.1136/heart.89.8.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basso C., Iliceto S., Thiene G., Perazzolo Marra M. Mitral valve prolapse, ventricular arrhythmias, and sudden death. Circulation. 2019;140:952–964. doi: 10.1161/CIRCULATIONAHA.118.034075. [DOI] [PubMed] [Google Scholar]
- 3.Perazzolo Marra M., Basso C., De Lazzari M., Rizzo S., Cipriani A., Giorgi B., et al. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ Cardiovasc Imaging. 2016;9:e005030. doi: 10.1161/CIRCIMAGING.116.005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dejgaard L.A., Skjølsvik E.T., Lie Ø.H., Ribe M., Stokke M.K., Hegbom F., et al. The mitral annulus disjunction arrhythmic syndrome. J Am Coll Cardiol. 2018;72:1600–1609. doi: 10.1016/j.jacc.2018.07.070. [DOI] [PubMed] [Google Scholar]
- 5.Aabel E.W., Chivulescu M., Dejgaard L.A., Ribe M., Gjertsen E., Hopp E., et al. Tricuspid annulus disjunction. JACC: Cardiovascular Imaging. 2021;14:1535–1543. doi: 10.1016/j.jcmg.2021.01.028. [DOI] [PubMed] [Google Scholar]
- 6.Boudoulas H., Schaal S.F., Stang J.M., Fontana M.E., Kolibash A.J., Wooley C.F. Mitral valve prolapse: cardiac arrest with long-term survival. Int J Cardiol. 1990;26:37–44. doi: 10.1016/0167-5273(90)90244-y. [DOI] [PubMed] [Google Scholar]
- 7.Han H., Ha F.J., Teh A.W., Calafiore P., Jones E.F., Johns J., et al. Mitral valve prolapse and sudden cardiac death: a systematic review. J Am Heart Assoc. 2018;7:e010584. doi: 10.1161/JAHA.118.010584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barlow J.B., Bosman C.K., Pocock W.A., Marchand P. Late systolic murmurs and non-ejection (“mid-late”) systolic clicks. An analysis of 90 patients. Heart. 1968;30:203–218. doi: 10.1136/hrt.30.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freed L.A., Levy D., Levine R.A., Larson M.G., Evans J.C., Fuller D.L., et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999;341:1–7. doi: 10.1056/NEJM199907013410101. [DOI] [PubMed] [Google Scholar]
- 10.Düren D.R., Becker A.E., Dunning A.J. Long-term follow-up of idiopathic mitral valve prolapse in 300 patients: a prospective study. J Am Coll Cardiol. 1988;11:42–47. doi: 10.1016/0735-1097(88)90164-7. [DOI] [PubMed] [Google Scholar]
- 11.Grigioni F., Enriquez-Sarano M., Ling L.H., Bailey K.R., Seward J.B., Tajik A.J., et al. Sudden death in mitral regurgitation due to flail leaflet. J Am Coll Cardiol. 1999;34:2078–2085. doi: 10.1016/s0735-1097(99)00474-x. [DOI] [PubMed] [Google Scholar]
- 12.Muthukumar L., Rahman F., Jan M.F., Shaikh A., Kalvin L., Dhala A., et al. The Pickelhaube sign. JACC: Cardiovasc Imaging. 2017;10:1078–1080. doi: 10.1016/j.jcmg.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Hutchins G.M., Moore G.W., Skoog D.K. The association of floppy mitral valve with disjunction of the mitral annulus fibrosus. N Engl J Med. 1986;314:535–540. doi: 10.1056/NEJM198602273140902. [DOI] [PubMed] [Google Scholar]
- 14.Al-Khatib S.M., Stevenson W.G., Ackerman M.J., Bryant W.J., Callans D.J., Curtis A.B., et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society. Circulation. 2018;138:e272–e391. doi: 10.1161/CIR.0000000000000549. [DOI] [PubMed] [Google Scholar]
- 15.Syed F.F., Ackerman M.J., McLeod C.J., Kapa S., Mulpuru S.K., Sriram C.S., et al. Sites of successful ventricular fibrillation ablation in bileaflet mitral valve prolapse syndrome. Circ Arrhythm Electrophysiol. 2016;9:e004005. doi: 10.1161/CIRCEP.116.004005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Invasive contrast-enhanced left ventriculography, left anterior oblique view, demonstrating bulging of the basal inferior LV segment, sometimes referred to as “ballerina-foot” deformity. Note that PVCs can be seen.
Apical four-chamber view on TTE demonstrating TAD and MAD during systole.
Cardiac magnetic resonance cine loop, steady state free precession sequence, showing MAD, MVP, and TVP. Tricuspid annular disjunction is not well visualized here.