Abstract
Diffuse esophageal intramural pseudo‐diverticulosis (DEIPD) is a chronic fibrosing inflammation of the esophagus of unknown origin. Its name derives from the characteristic pseudo‐diverticula formed by dilated ducts of submucosal glands. With an assumed prevalence of approximately 5–50/100 000, DEIPD is more frequent than previously estimated. It preferentially affects men between 50 and 70 years of age with a history of alcohol and tobacco abuse. Key symptoms are chronic dysphagia and food impactions. Typical endoscopic findings are multiple small, longitudinally aligned pseudo‐diverticle openings and trachealization of the esophagus. Additionally, the usually gray mucosa may show a fine‐grained pattern of very small red dots that merge into a pink tint, called “faux uni pattern.” Once established, clinical symptoms and endoscopic changes persist throughout life. Although there is no known causal therapy, complications like bolus impactions, candida infections, or reflux can and should be treated.
Keywords: bolus impaction, candidiasis, diffuse intramural pseudo‐diverticulosis, dysphagia, endoscopy, esophageal inflammation, esophagus, rare diseases

Diffuse esophageal intramural pseudo‐diverticulosis is more frequent than estimated. Gastroenterologists should know risk factors (alcohol, tobacco), symptoms (dysphagia, bolus impactions), endoscopic signs (diverticula, frosted glass look, faux‐uni pattern, trachealization).
Introduction
In 1960, Mendl and colleagues described a chronic inflammatory disease of the esophagus causing dysphagia and recurrent food impactions. Radiologically, it was characterized by multiple tiny outpouchings in the esophageal wall. 1 In the following decades, a few small series and phenomenologic reports on similar cases were published, and the term “diffuse intramural esophageal diverticulosis” or “pseudo‐diverticulosis” (DEIPD) was coined. 2 , 3 , 4 , 5 , 6 , 7 , 8 Still, most of these early works regarded it a biological oddity rather than a medical entity. Only in the 21st century did it become clear that DEIPD is a diagnosis in its own right, that it is highly symptomatic, and that it is not as exotic as previously thought. 9 , 10
Review criteria and professional perception
In January 2022, a PubMed search was conducted for the term “esophagus AND pseudodiverticulosis.” It returned 131 results. Additionally, a search for “diverticle OR diverticulosis AND esophagus” was conducted, which returned 1892 results. Narrowing the criteria to human species, English language articles, and articles containing original data left 451 results. Abstracts of these articles were then viewed manually to identify DEIPD cases and exclude other entities such as Zenker's, Kilian–Jameson, or traction diverticula. This left exactly the 131 articles found in the original search. For comparison, the search term “Eosinophilic AND esophagitis” returned 3871 results, “achalasia” 9160, and “lymphocytic AND esophagitis” 2281.
All procedures performed in our studies are in accordance with the standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
History and epidemiology
In the 60 years since its discovery, only about 320 cases of DEIPD have been reported worldwide. 11 Therefore, it was thought to be an exceptionally rare condition. 2 , 3 , 4 , 7 , 12 That changed only recently with the advent of high‐resolution endoscopy and searchable clinical databases. Within the last 6 years, three studies from three tertiary centers were published, showing very similar numbers of about 20 cases in 10 years each. 13 , 14 , 15 A fourth center, with a narrower search strategy, found 16 in 15 years. 16 Against the background of esophageal pseudo‐diverticulosis being a life‐long disease (see below), this would hint to a real prevalence in the order of between 5 and 50 in 100 000, or a total of 400 000–4 million patients worldwide. 17 Like in all orphan diseases, these numbers have to be interpreted with caution, especially since they are based on extrapolation. 17 What can be said, though, is that DEIPD qualifies as a rare disease but one that might be as common as eosinophilic esophagitis (EoE) and more common than achalasia or lymphocytic esophagitis. 18 , 19 , 20 , 21 , 22 So, many gastroenterologists will see it more than once during their career—if they do not miss it. 23
Clinical presentation
The most common symptom of DEIPD is chronic dysphagia or odynophagia of varying or slowly increasing duration and severity. Weight loss and malnutrition can develop, although it is not always clear whether this is due to dysphagia or other conditions (see below). Still, a remarkably high proportion of patients present with acute food impaction as first symptom and report dysphagia only when specifically asked. 24 , 25 , 26 In a recent series, almost half of all patients had at least one food bolus impaction; many had more, and one had five within 4 years. 15 , 27 These cases often require emergency endoscopy and must not be misinterpreted as EoE. 11 , 28 Risk factors for developing esophageal pseudo‐diverticulosis are fairly clear. The first patient ever reported by Mendl was a 56‐year‐old mine worker who drank 8–12 pints (approx. 4600–6800 mL) of beer and smoked 20 cigarettes per day. 1 Since then, many authors have confirmed that esophageal pseudo‐diverticulosis affects predominantly males between 50 and 70 years of age with a history of alcohol and tobacco abuse. 4 , 13 Unsurprisingly, secondary conditions such as chronic obstructive pulmanory disease (COPD), atherosclerosis, or liver cirrhosis are common in that group. 15 Other concomitant diagnoses, especially malignancies, have been reported, but because they share the same risk profile, their causality for or from DEIPD is unclear. 12 , 27
Radiology and endoscopy
From the 1960s to about the 1990s, single‐ or double‐contrast esophagography was the diagnostic method of choice. 6 , 8 Typical signs were multiple small, flask‐like outpouchings of the esophageal wall. They are about 2 mm deep and appear throughout the whole length of the organ. 29 “Intramural tracking,” that is, small contrast‐filled intramural canals connecting these diverticula, was considered pathognomonic. 30
As with many other diseases of the esophagus, radiological examinations for DEIPD have since mainly been replaced by high‐definition endoscopy. 31 Most obvious finding here—apart from wedged food—will be multiple small apertures in the esophageal wall (Figure 1). They measure between 0.25 and 5 mm in diameter and are often aligned longitudinally (Figs 2, 3, 4). Alternatively, mucosa between these openings may show a dull white swelling that resembles frosted glass (Figs 4, 6). 15 , 27 , 32 In later stages, the mucosa will be smooth but densely speckled with multiple ultrafine red dots that are visible only on close examination. Viewed from further afar, this dot pattern will merge into an evenly pink tint—a phenomenon resembling a “faux‐uni” shirt fabric (Figs 1, 2, 3, 4). Finally, fibrotic remodeling will result in a characteristic combination of a rigid, narrow lumen with multiple rings and reduced peristalsis. 15 , 26 Apart from the diverticle openings, which are sometimes difficult to spot, this phenomenon is indistinguishable from the “trachealization” of the esophagus seen in later stages of EoE (Figs 4, 5, 6). 33 , 34
Figure 1.

Esophageal pseudodiverticulosis; very discrete endoscopic signs, very mild inflammatory activity. Multiple, very small red dots on the mucosa that merge into an even tint in the background (“faux uni”). Few, small diverticle openings that can be easily overlooked. Eighty‐five years old female patient; no information on alcohol and nicotine abuse given; chronic dysphagia, weight loss. Fujinon EG‐600WR.
Figure 2.

Esophageal pseudodiverticulosis; discrete endoscopic signs, mild inflammatory activity. Multiple, very small red dots on the mucosa that merge into an even tint in the background (“faux uni”). Diverticle openings more pronounced than in Figure 1 and aligned longitudinally. Fifty‐nine years old male patient; alcohol and nicotine abuse; weight loss, multiple food impactions. Fujinon EG‐600WR.
Figure 3.
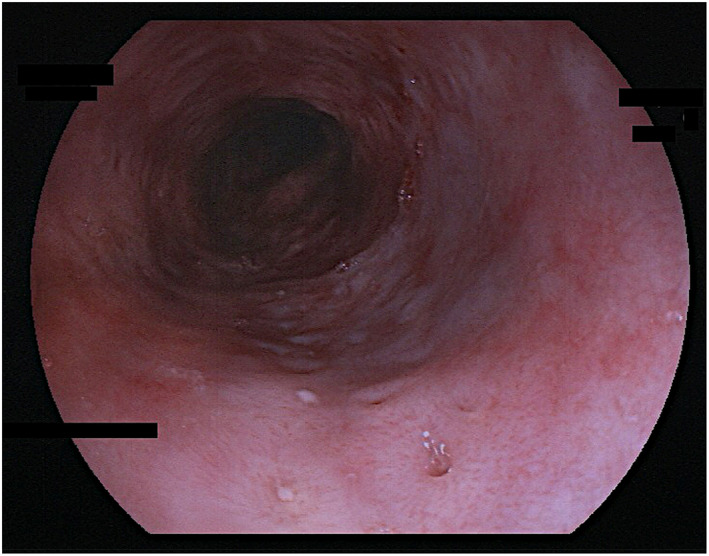
Esophageal pseudodiverticulosis; typical endoscopic signs, moderate inflammatory activity. “Faux uni” pattern and rather discrete dull‐white swelling (“frosted glass look”). Diverticle openings roughly aligned longitudinally. Sixty‐three years old male patient; alcohol and nicotine abuse; dysphagia, sore throat, multiple food impactions. Some candida hypae in mucosal biopsies. Fujinon EG‐600WR.
Figure 4.
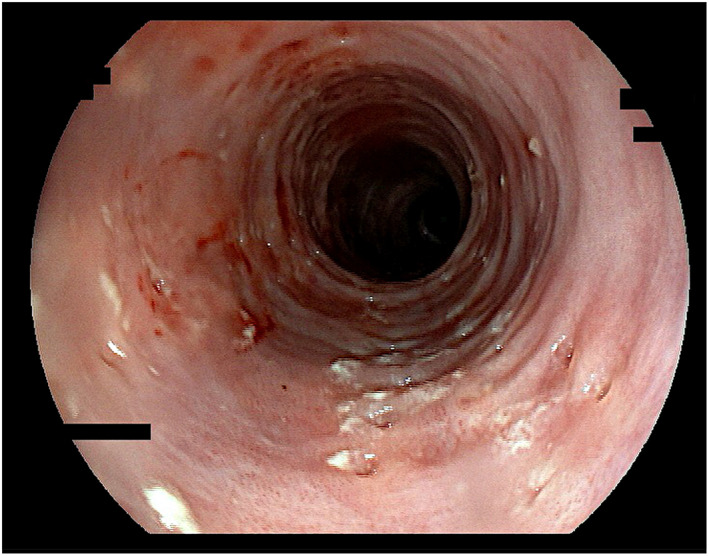
Esophageal pseudodiverticulosis; pronounced endoscopic signs, severe inflammatory activity. “Faux uni” pattern and also discrete dull‐white swelling (“frosted glass look”). Diverticle openings roughly aligned longitudinally. Multiple rings and reduced lumen (“trachealization”). Fifty‐seven years old male patient; alcohol and nicotine abuse; dysphagia. Candida hyphae in mucosal biopsies. Fujinon EG‐600WR.
Figure 6.

Esophageal pseudodiverticulosis; pronounced endoscopic signs but low inflammatory activity. Distinct white swelling of the mucosa (“frosted glass look”), more pronounced than in Figs 3, 4, 5. Diverticle openings within the swelling. Multiple rings and reduced lumen (“trachealization”). Thirty‐one years old male patient; alcohol and nicotine abuse; chronic dysphagia, weight loss. Fujinon EG‐600WR.
Figure 5.

Esophageal pseudodiverticulosis; very pronounced endoscopic signs, severe inflammatory activity. Multiple diverticle openings, some spontaneously bleeding, some oozing gray liquid. Edematous swelling of mucosa. Multiple rings and reduced lumen (“trachealization”). Massive candidiasis in mucosal biopsies. Sixty‐five years old male patient; no information on alcohol and nicotine abuse given; chronic dysphagia, presented with hematemesis. Fujinon EG‐600WR.
Histology
Since it can be difficult to differentiate between DEIPD and other types of esophagitis based on clinical or endoscopic findings alone, several biopsies should be taken.Unlike in EoE or lymphocytic esophagitis, histology in DEIPD will show a rather nonspecific mixed‐cell‐type infiltrate of the mucosa. 32 Candida hyphae can often be seen, even though the endoscopic‐macroscopic aspect might not suggest fungal infection. 15 The pseudo‐diverticula themselves are formed by inflamed and dilated ducts of submucosal esophageal glands. 35 Therefore, they are usually not seen in routine mucosal biopsies. Submucosal inflammation and fibrosis around these glands have been described in autopsy or esophagectomy specimens, but they may be also missed in mucosal biopsies. 5 , 8 In later stages, squamous cell hyperplasia or epidermoid metaplasia may develop. 15 , 36 Although symptoms vary during the course of the disease, the underlying chronic inflammation of the submucosa and its glands will continue for months or years, the end stage being submucosal fibrosis and glandular dysfunction. These would result in a narrow lumen, reduced peristalsis, and poor lubrication, which together may explain the high incidence of food impactions. 37 , 38 In this context, the pseudo‐diverticula, which gave the disease its name, would rather be a secondary phenomenon and not crucial for the development of symptoms.
Pathogenesis
As esophageal pseudo‐diverticulosis is a chronic, fibrosing inflammatory disease, the question arises: what causes the inflammation?—We do not know. Alcohol and tobacco abuse are established risk factors, but since these are common and the disease is rare, additional causes must exist. 4 , 11 , 13 , 15 In principle, these could be infectious, allergic, or autoimmune. One infectious agent that has been discussed since the 1970s is candida, but no consensus on its causality in esophageal pseudo‐diverticulosis has been reached. 39 , 40 What can be deducted from case reports and small series is that the incidence and prevalence of candidiasis are comparatively high in DEIPD patients and that individuals who are negative for candida in one presentation can be positive in another. 7 , 13 , 41 , 42 On the other hand, asymptomatic candida colonization will also be found in a relevant portion of otherwise healthy individuals if only one looks close enough, and the variance between different populations is high. 43 , 44 , 45 As of today, the significance of candida infection in DEIPD remains unclear. Another conceivable mechanism of inflammation in DEIPD would be a kind of food intolerance or allergy. And, indeed, there is data suggesting the existence of an overlap syndrome between esophageal pseudo‐diverticulosis and EoE. 16 , 46 However, as this affects only a minority of patients, it may be more of a coincidence than a causality. An autoimmune component, finally, may be theoretically possible, but presently there is no data supporting it.
Therapy
Food impactions constitute an emergency and should be removed endoscopically. 15 , 26 , 27 , 47 , 48 Candida infections should always be searched for and, when found, treated according to existing guidelines or standards. 7 , 13 , 40 , 49 , 50 Since the diverticula probably cause a degree of stasis that may well predispose to chronic infection, it can be hypothesized that this infection causes fibrosis with scarring (see “Histology” above). Therefore, our own group recommends aggressive and prolonged local and systemic treatment of every candida occurrence. Additionally, proton pump inhibitors may have a beneficial effect. 25 , 51 , 52 Even when treated, the disease takes a chronic course. If discrete strictures appear, they may lead to food impactions and should be widened by balloon dilatation or bougienage. The more frequent cause of bolus impactions is trachealization though, which affetcs the whole length of the organ and is not treatable with dilatation. 13 , 51 , 53 , 54 , 55 Even in this late stage, consequent treatment of fungal infections can not only ameliorate symptoms but may also prevent stricture re‐formation after balloon dilatation. 41 At any stage of the disease, a recommendation to abstain from alcohol and nicotine should be given, even though it may not always be followed.
Conclusion and outlook
DEIPD is a chronic disease with a significant impact on the quality of life. Although rare by definition, it may have been underdiagnosed in the past. This may be partly due to its unspecific clinical signs. Additionally, because of their alcohol abuse and the slow progress of the disease, many patients may deny or downplay symptoms and would not seek medical help for a long time. For some of our own patients, presenting to the emergency department with a bolus impaction was the first contact with the healthcare system for years. Therefore, it is one goal of this article to raise awareness for DEIPD among practitioners and gastroenterologists so that it would be detected more frequently and earlier. Still, even if DEIPD becomes a widely recognized disease that is diagnosed timely and correctly, problems would remain, the two most obvious being the unknown pathomechanism and, related to it, the lack of a causal therapy. It is therefore another goal of this article to advocate further research on this disease. Possible investigations would be manometric studies to quantify the restriction in peristalsis and to determine possible pathognomonic patterns, histologic studies to further characterize the type of inflammation, and systematic microbiological investigations to determine the still enigmatic role of candida in the pathogenesis of DEIPD.
Declaration of conflict of interest: None.
Data availability statement
All relevant data are available in the article itself.
References
- 1. Mendl K, McKay JM, Tanner CH. Intramural diverticulosis of the oesophagus and Rokitansky‐Aschoff sinuses in the gall‐bladder. Br. J. Radiol. 1960; 33: 496–501. [Google Scholar]
- 2. Fee BE, Dvorak AD. Intramural pseudodiverticulosis of the esophagus. Nebr. Med. J. 1976; 61: 9–13. [PubMed] [Google Scholar]
- 3. Brühlmann WF, Zollikofer CL, Maranta E et al. Intramural pseudodiverticulosis of the esophagus: report of seven cases and literature review. Gastrointest. Radiol. 1981; 6: 199–208. [DOI] [PubMed] [Google Scholar]
- 4. Sabanathan S, Salama FD, Morgan WE. Oesophageal intramural pseudodiverticulosis. Thorax. 1985; 40: 849–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Medeiros LJ, Doos WG, Balogh K. Esophageal intramural pseudodiverticulosis: a report of two cases with analysis of similar, less extensive changes in “normal” autopsy esophagi. Hum. Pathol. 1988; 19: 928–31. [DOI] [PubMed] [Google Scholar]
- 6. Farack UM, Kinnear DG, Jabbari M. Intramural pseudodiverticulosis of the esophagus‐‐a primarily radiologic diagnosis. Rofo. 1979; 130: 508–9. [DOI] [PubMed] [Google Scholar]
- 7. Levine MS, Moolten DN, Herlinger H, Laufer I. Esophageal intramural pseudodiverticulosis: a reevaluation. AJR Am. J. Roentgenol. 1986; 147: 1165–70. [DOI] [PubMed] [Google Scholar]
- 8. Wightman AJ, Wright EA. Intramural oesophageal diverticulosis: a correlation of radiological and pathological findings. Br. J. Radiol. 1974; 47: 496–8. [DOI] [PubMed] [Google Scholar]
- 9. Hahne M, Schilling D, Arnold JC, Riemann JF. Esophageal intramural pseudodiverticulosis: review of symptoms including upper gastrointestinal bleeding. J. Clin. Gastroenterol. 2001; 33: 378–82. [DOI] [PubMed] [Google Scholar]
- 10. Koyama S, Watanabe M, Iijima T. Esophageal intramural pseudodiverticulosis (diffuse type). J. Gastroenterol. 2002; 37: 644–8. [DOI] [PubMed] [Google Scholar]
- 11. Frieling T, Kreysel C, Blank M, Mülle D, Euler P, Melchior I. Not always eosinophilic esophagitis – intramural pseudodiverticulosis of the esophagus – a case report and literature review. Z. Gastroenterol. 2020; 58: 1201–7. [DOI] [PubMed] [Google Scholar]
- 12. Naoi Y, Katayama H, Tomiyoshi H. Esophageal intramural pseudodiverticulosis with esophageal cancer improved by target rotation irradiation: case report. Nihon Igaku Hoshasen Gakkai Zasshi. 1997; 57: 526–7. [PubMed] [Google Scholar]
- 13. Halm U, Lamberts R, Knigge I, Mössner J, Zachäus M. Esophageal intramural pseudodiverticulosis: endoscopic diagnosis and therapy. Dis. Esophagus. 2014; 27: 230–4. [DOI] [PubMed] [Google Scholar]
- 14. Bechtler M, Vollmer H, Vetter S, Fuchs ES, Weickert U, Jakobs R. Long‐term follow‐up after dilation in symptomatic esophageal intramural pseudodiverticulosis: an observational study in 22 cases. Endoscopy. 2014; 46: 795–7. [DOI] [PubMed] [Google Scholar]
- 15. Hentschel F, Lüth S. Clinical and endoscopic characteristics of diffuse esophageal intramural pseudo‐diverticulosis. Esophagus. 2020; 17: 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scaffidi MA, Garg A, Ro B et al. Esophageal intramural pseudodiverticulosis and concomitant eosinophilic esophagitis: a case series. Can. J. Gastroenterol. Hepatol. 2016; 2016: 1761874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Auvin S, Irwin J, Abi‐Aad P, Battersby A. The problem of rarity: estimation of prevalence in rare disease. Value Health. 2018; 21: 501–7. [DOI] [PubMed] [Google Scholar]
- 18. Richter T, Nestler‐Parr S, Babela R et al. Rare disease terminology and definitions‐a systematic global review: report of the ISPOR Rare Disease Special Interest Group. Value Health. 2015; 18: 906–14. [DOI] [PubMed] [Google Scholar]
- 19. Moawad FJ. Eosinophilic esophagitis: incidence and prevalence. Gastrointest. Endosc. Clin. N. Am. 2018; 28: 15–25. [DOI] [PubMed] [Google Scholar]
- 20. Sadowski DC, Ackah F, Jiang B, Svenson LW. Achalasia: incidence, prevalence and survival. A population‐based study. Neurogastroenterol. Motil. 2010; 22: e256–61. [DOI] [PubMed] [Google Scholar]
- 21. Putra J, Muller KE, Hussain ZH et al. Lymphocytic esophagitis in nonachalasia primary esophageal motility disorders: improved criteria, prevalence, strength of association, and natural history. Am. J. Surg. Pathol. 2016; 40: 1679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lucendo AJ, Santander C, Savarino E et al. Environmental, and genetic determinants in eosinophilic esophagitis: rationale, design, and study protocol of a large‐scale epidemiological study in Europe. Therap. Adv. Gastroenterol. 2022; 15: 17562848221074204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hentschel F, Lüth S. Rare inflammatory diseases of the esophagus. Internist (Berl.). 2019; 60: 533–9. [DOI] [PubMed] [Google Scholar]
- 24. Schmutz G, Zeller C, Doffoel M, Kempf F. Une cause rare de blocage alimentaire. La pseudo‐diverticulose intra‐murale de l'oesophage. Presse Med. 1983; 12: 641–2. [PubMed] [Google Scholar]
- 25. Eliakim R, Libson E, Rachmilewitz D. Diffuse intramural esophageal pseudodiverticulosis. J. Natl. Med. Assoc. 1989; 81: 93–8. [PMC free article] [PubMed] [Google Scholar]
- 26. Attila T, Marcon NE. Esophageal intramural pseudodiverticulosis with food impaction. Can. J. Gastroenterol. 2006; 20: 37–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Plavsic BM, Chen MY, Gelfand DW et al. Intramural pseudodiverticulosis of the esophagus detected on barium esophagograms: increased prevalence in patients with esophageal carcinoma. AJR Am. J. Roentgenol. 1995; 165: 1381–5. [DOI] [PubMed] [Google Scholar]
- 28. Birk M, Bauerfeind P, Deprez PH et al. Removal of foreign bodies in the upper gastrointestinal tract in adults: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2016; 48: 489–96. [DOI] [PubMed] [Google Scholar]
- 29. Hentschel F, Schreyer AG, Lüth S. Recurrent food impactions. Gut. 2021; 70: 1631–90. [DOI] [PubMed] [Google Scholar]
- 30. Canon CL, Levine MS, Cherukuri R, Johnson LF, Smith JK, Koehler RE. Intramural tracking: a feature of esophageal intramural pseudodiverticulosis. AJR Am. J. Roentgenol. 2000; 175: 371–4. [DOI] [PubMed] [Google Scholar]
- 31. Sivak MV. Gastrointestinal endoscopy: past and future. Gut. 2006; 55: 1061–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsuboi J, Tajika M, Nakamura T et al. Endoscopic features of short‐term progression of esophageal intramural pseudodiverticulosis. Endoscopy. 2010; 42(Suppl. 2): E92–3. [DOI] [PubMed] [Google Scholar]
- 33. Al‐Hussaini AA, Semaan T, El Hag IA. Esophageal trachealization: a feature of eosinophilic esophagitis. Saudi J. Gastroenterol. 2009; 15: 193–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nandy N, Rustagi T. “Trachealization” of the Esophagus. N. Engl. J. Med. 2019; 380: 177. [DOI] [PubMed] [Google Scholar]
- 35. Umlas J, Sakhuja R. The pathology of esophageal intramural pseudodiverticulosis. Am. J. Clin. Pathol. 1976; 65: 314–20. [DOI] [PubMed] [Google Scholar]
- 36. Akkari M, Shintaku M, Torii I. Development of epidermoid metaplasia of the mucosa in association with esophageal intramural pseudodiverticulosis and candidiasis. Case Rep. Gastroenterol. 2021; 15: 709–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sarosiek J. Does the healing of the esophageal mucosa improve the function of the esophageal submucosal and salivary glands? Ann. N. Y. Acad. Sci. 2016; 1380: 155–61. [DOI] [PubMed] [Google Scholar]
- 38. Sarosiek J, McCallum RW. What is the secretory potential of submucosal mucous glands within the human gullet in health and disease? Digestion. 1995; 56(Suppl. 1): 15–23. [DOI] [PubMed] [Google Scholar]
- 39. Orringer MB, Sloan H. Monilial esophagitis: an increasingly frequent cause of esophageal stenosis? Ann. Thorac. Surg. 1978; 26: 364–74. [DOI] [PubMed] [Google Scholar]
- 40. Akkari I, Ben Jazia E, Mrabet S, Jemni I. Candida albicans: a cause or a consequence of esophageal intramural pseudo‐diverticulosis? Pan Afr. Med. J. 2019; 2: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bhattacharya S, Mahmud S, McGlinchey I, Nassar AH. Intramural pseudodiverticulosis of the esophagus. Surg. Endosc. 2002. Apr; 16: 714–5. [DOI] [PubMed] [Google Scholar]
- 42. Chiba T, Iijima K, Koike T, Uno K, Asano N, Shimosegawa T. A case of severe esophageal intramural pseudodiverticulosis whose symptoms were ameliorated by oral administration of anti‐fungal medicine. Case Rep. Gastroenterol. 2012; 6: 103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Choi JH, Lee CG, Lim YJ, Kang HW, Lim CY, Choi JS. Prevalence and risk factors of esophageal candidiasis in healthy individuals: a single center experience in Korea. Yonsei Med. J. 2013; 54: 160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nassar Y, Eljabbour T, Lee H, Batool A. Possible risk factors for candida esophagitis in immunocompetent individuals. Gastroenterology Res. 2018; 11: 195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Min YW, Kim E, Son HJ, Kim JJ, Rhee PL. Antifungal treatment is not required for immunocompetent individuals with asymptomatic esophageal candidiasis. Medicine (Baltimore). 2015; 94: e1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Müller M, Will RC, Eckardt AJ. Eosinophilic esophagitis with pseudodiverticulosis: a rare coincidence. Endoscopy. 2012; 44(Suppl. 2): E71. [DOI] [PubMed] [Google Scholar]
- 47. Siba Y, Gorantla S, Gupta A, Lung E, Culpepper‐Morgan J. Esophageal intramural pseudodiverticulosis, a rare cause of food impaction: case report and review of the literature. Gastroenterol. Rep. (Oxf.). 2015; 3: 175–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Piñerúa‐Gonsálvez JF, Zambrano‐Infantino RDC, Sulbaran M. Esophageal intramural pseudodiverticulosis in a patient with food bolus impaction. Rev. Gastroenterol. Peru. 2019; 39: 362–3. [PubMed] [Google Scholar]
- 49. Ruhnke M, Rickerts V, Cornely OA et al. Diagnosis and therapy of Candida infections: joint recommendations of the German Speaking Mycological Society and the Paul‐Ehrlich‐Society for Chemotherapy. Mycoses. 2011; 54: 279–310. [DOI] [PubMed] [Google Scholar]
- 50. Rex JH, Walsh TJ, Sobel JD et al. Practice guidelines for the treatment of candidiasis. Clin. Infect. Dis. 2000; 30: 662–78. [DOI] [PubMed] [Google Scholar]
- 51. Bamidele OF, Olokoba AB, Bojuwoye MO, Barde AA. Oesophageal intramural pseudodiverticulosis: a rare endoscopic finding. Ghana Med. J. 2019; 53: 184–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Teraishi F, Fujiwara T, Jikuhara A et al. Esophageal intramural pseudodiverticulosis with esophageal strictures successfully treated with dilation therapy. Ann. Thorac. Surg. 2006; 82: 1119–21. [DOI] [PubMed] [Google Scholar]
- 53. Shapiro MJ, Sloan WC. Intramural pseudodiverticulosis of the esophagus. Ann. Otol. Rhinol. Laryngol. 1977; 86(5 Pt 1): 594–7. [DOI] [PubMed] [Google Scholar]
- 54. Chino O, Makuuchi H, Kondo Y et al. Esophageal intramural pseudodiverticulosis treated by endoscopic balloon dilatation. Tokai J. Exp. Clin. Med. 2014; 39: 137–40. [PubMed] [Google Scholar]
- 55. Yoneyama F, Kobayashi Y, Miyata K et al. Esophageal intramural pseudodiverticulosis treated by balloon dilatation: report of a case. Surg. Today. 2004; 34: 62–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are available in the article itself.


