Abstract
Background and Aims
Prothrombin induced by vitamin K absence‐II (PIVKA‐II) is a serum biomarker linked to hepatocellular carcinoma (HCC), showing superiority to alpha‐fetoprotein (AFP) for early disease detection. We aimed to assess the clinical and analytical performance of the Elecsys® PIVKA‐II immunoassay in diagnosing HCC and evaluate PIVKA‐II's technical performance.
Methods
Serum samples from adult cases (i.e. patients with a first‐time HCC diagnosis; n = 168) and disease controls (i.e. patients without HCC with an at‐risk condition; n = 208) were assessed. An AFP cut‐off of 20 ng/mL was used to differentiate between HCC cases and disease controls. Clinical performance of the Elecsys PIVKA‐II assay was compared with that of comparator assays (Lumipulse G PIVKA‐II, μTASWako DCP, ARCHITECT PIVKA‐II) using receiver operating characteristic curve analysis to determine the area under the curve (AUC) values.
Results
The Elecsys PIVKA‐II assay compared favorably with comparator assays. Using a 28.4 ng/mL cut‐off, the Elecsys PIVKA‐II assay detected HCC with 86.9% sensitivity and 83.7% specificity. Clinical performance of the Elecsys PIVKA‐II assay (AUC: 90.8%) was equivalent to that of comparator assays (AUC: 88.3–89.6%). Relatively high PIVKA‐II concentrations were observed for cholangiocarcinoma and pancreatic cancer with the Elecsys assay in specificity panel analyses, indicating that high PIVKA‐II concentrations should not be used alone in the absence of other clinical data.
Conclusions
The Elecsys PIVKA‐II assay showed good analytical performance under routine laboratory conditions, comparing favorably with comparator assays. These findings support the suitability of the Elecsys PIVKA‐II assay as an aid in HCC diagnosis.
Keywords: acarboxyprothrombin, alpha‐fetoprotein, diagnosis, hepatocellular carcinoma, prothrombin induced by vitamin K absence‐II
This multicenter study assessed the clinical and analytical performance of the Elecsys® PIVKA‐II immunoassay (Roche Diagnostics International Ltd) in diagnosing hepatocellular carcinoma (HCC), versus commercially available comparators. Under routine laboratory conditions, the Elecsys PIVKA‐II immunoassay detected HCC with 86.9% sensitivity and 83.7% specificity and the clinical performance (area under the curve [AUC], 90.8%) was equivalent to comparator assays (AUC, 88.3–89.6%). These findings support the suitability of the Elecsys PIVKA‐II immunoassay as an aid in HCC diagnosis.
Introduction
In 2020, approximately 905 677 new cases of liver cancer were diagnosed worldwide, and mortality due to liver cancer was >830 000. 1 Hepatocellular carcinoma (HCC) is the most common form of liver cancer, estimated to account for >80% of the primary disease. 2
HCC often occurs in patients with chronic liver disease or cirrhosis. 2 More recently, non‐alcoholic steatohepatitis (NASH) and non‐alcoholic fatty liver disease are increasingly recognized as risk factors for HCC. 3 Patients under surveillance for such conditions, patients with suspected cirrhosis undergoing initial assessment, and patients who present with signs and symptoms such as ascites, jaundice, and hepatomegaly are often evaluated for HCC using the serum marker alpha‐fetoprotein (AFP) in combination with ultrasonography. 4 However, controversies exist regarding the value of AFP, with some studies suggesting it is the best single biomarker for HCC 5 and complements the use of ultrasound, 6 while others have questioned the sensitivity, specificity, and predictive value of AFP testing. 7 Accordingly, guidelines also differ regarding the use of serum biomarkers; ultrasound alone is proposed by the European Association for the Study of Liver, 8 while the American Association for the Study of Liver Diseases guideline includes use of an AFP cut‐off of 20 ng/mL in select circumstances. 9 The Asian Pacific Association for the Study of Liver Disease (APASL) guideline also includes the use of an AFP cut‐off of 20 ng/mL for HCC surveillance when used in combination with ultrasound. 10
Prothrombin induced by vitamin K absence‐II (PIVKA‐II; also known as des‐γ‐carboxyprothrombin [DCP]) has been identified as a serum biomarker linked to HCC. 11 , 12 PIVKA‐II has been shown to be an independent predictor of microvascular invasion in HCC 13 , 14 and superior to AFP for the early detection of HCC, being highly sensitive and specific. 11 , 15 Serologic statistical models including serum AFP and PIVKA‐II measurements have previously been developed to aid in the prognosis and diagnosis of HCC. A statistical model involving sex, age, AFP isoform L3 (AFP‐L3), AFP, and DCP (GALAD) was developed to determine the risk of HCC in patients with chronic liver disease. 16 An international case–control study showed that the GALAD score can detect early‐stage HCC and may aid in monitoring patients with NASH. 17 The GALAD score has the potential to be used as a screening tool for the detection of HCC in patients with NASH; however, further validation in a large, prospective study is warranted. A second model combining bilirubin, albumin, AFP‐L3, AFP, and DCP (BALAD) was developed to aid prognostication in HCC, and was further refined through the use of continuous variables (BALAD‐2). 18 , 19 BALAD‐2 has since been validated in an international setting and across different disease stages, supporting its use in staging and prognostication for patients with HCC. 20 , 21
A new immunoassay for the quantitative measurement of PIVKA‐II in human serum and plasma, to be used as a diagnostic aid in HCC, has been developed to complement the tumor marker portfolio on the Elecsys® automated immunoassay platform. The aim of this study was to assess the clinical performance of the Elecsys PIVKA‐II and AFP immunoassays in aiding the diagnosis of HCC and to evaluate the technical performance of PIVKA‐II, including the determination of reference range(s) for healthy adults.
Methods
Participants
This was a multicenter, prospective study designed to assess the clinical and analytical performance of the new Elecsys PIVKA‐II immunoassay (Roche Diagnostics International Ltd., Rotkreuz, Switzerland) as an aid in the diagnosis of HCC. Patients with HCC and disease controls were prospectively enrolled at seven clinics in China, Germany, Japan, and Thailand. All participants were aged 18 years and older, and provided written, informed consent prior to enrolment. One additional site provided banked HCC samples. Additional study details are provided in Methods section, Supporting information.
Eligible HCC cases had a first‐time HCC diagnosis, confirmed radiologically according to national guidelines, or by liver biopsy. Key exclusion criteria were the presence of any other cancer (except non‐melanoma skin cancer), recurrent HCC, or current or previous treatment for HCC.
Eligible disease controls had absence of HCC confirmed by imaging within 12 months before the study and presence of one of the following: cirrhosis; non‐cirrhotic chronic hepatitis B virus (HBV); non‐cirrhotic chronic hepatitis C virus (HCV); or NASH. The key exclusion criterion was the presence of any cancer except non‐melanoma skin cancer.
Etiology groups for HCC cases and controls were classified as cirrhotic, non‐cirrhotic HBV, non‐cirrhotic HCV, non‐cirrhotic NASH, non‐cirrhotic alcoholic liver disease (ALD), or other. HCC cases were also grouped according to the Barcelona Clinic Liver Cancer (BCLC) staging (early, stages 0/A; late, stages B/C/D).
Sample handling and analysis
Serum samples were collected by venous blood draw ≥1 day before general anesthesia/surgery and frozen before shipping (see Methods section, Supporting information).
Repeatability (within‐run), intermediate precision (within‐laboratory), and reproducibility were calculated and compared against prespecified acceptance criteria. For samples with concentrations from the limit of detection to 30 ng/mL, the acceptance criteria were standard deviation (SD) ≤1.5 ng/mL (repeatability), ≤2.25 ng/mL (intermediate precision), and ≤4.5 ng/mL (reproducibility). For samples with concentrations >30 ng/mL, the acceptance criteria were coefficient of variation (CV) ≤5% (repeatability), ≤7.5% (intermediate precision), and ≤15% (reproducibility).
Samples were analyzed at three laboratories in Germany with three cobas e 411 analyzers (the master instruments) and two cobas e 601 analyzers, with one run per day for 5 days using one reagent lot (five replicates for each of the seven samples [five human serum pools plus two PreciControl samples covering the measuring range of 3.5–12 000 ng/mL]; Clinical & Laboratory Standards Institute [CLSI] EP05‐A3 criteria). Reference ranges were determined in serum samples collected from healthy individuals 20–79 years of age in Munich (n = 399) and Nuremberg (n = 412) on one cobas e 411 analyzer and two cobas e 601 analyzers.
Method comparison experiments were conducted with samples from HCC cases and disease controls on the Elecsys PIVKA‐II assay using the cobas e 601 analyzer at Microcoat GmbH (Bernried, Germany); assays using comparator platforms (Lumipulse G PIVKA‐II, μTASWako DCP, and ARCHITECT PIVKA‐II) were performed at the Life Science Research Institute (Yokohama, Japan).
Data handling
Details on sample size determination are included in the Methods section, Supporting information.
The clinical performance of the Elecsys PIVKA‐II and AFP assays was determined by co‐testing the same aliquot of HCC cases and disease control samples on the cobas e 601 analyzer. Specificity panel serum samples from patients with other benign/malignant diseases were also measured at two sites with the Elecsys PIVKA‐II and AFP assays simultaneously and the μTASWako DCP platform.
Analytical comparison of methods was performed using weighted Deming regression (CLSI EP09‐A3 criteria). Clinical performance of the different methods was assessed using receiver operating characteristic (ROC) curve analysis. Area under the curve (AUC) values were calculated. An AFP cut‐off of 20 ng/mL was prespecified to assess clinical performance of AFP to accurately differentiate between HCC cases and disease controls of 376 participants in total. For Elecsys PIVKA‐II, the clinical performance at specified specificity and sensitivity values (between 70 and 95%) was calculated, with the 95th percentile used to define the cut‐off.
Results
Study participants
In total, 473 patients were screened. Of these, 168 HCC cases and 208 disease controls were enrolled in the study; 97 screened patients were excluded (56 HCC cases and 41 disease controls) because of either inclusion/exclusion criteria not being met, incomplete sample processing, or physician or sponsor decision.
In the HCC cohort, the mean patient age was 62.86 years; 141 (83.9%) patients were men and 139 (82.7%) had cirrhotic etiology (Table S1, Supporting information). Seventy‐seven (45.8%) patients had early‐stage HCC (BCLC stages 0/A) and 91 (54.2%) had late‐stage HCC (BCLC stages B/C/D). In total, 122 (72.6%) patients in the HCC cohort had a Child–Pugh score of A, and 98 (58.3%) had an albumin‐bilirubin (ALBI) grade of 2.
In the control cohort, the mean age was 52.18 years; 126 (60.6%) participants were men and 79 (38.0%) had cirrhotic etiology. Child–Pugh scores were not available for the control cohort; however, 153 (73.6%) patients had an ALBI grade of 1.
Analytical performance
The Elecsys PIVKA‐II assay demonstrated high repeatability, intermediate precision, and reproducibility when compared against prespecified acceptance criteria. For low‐concentration samples (mean: 7.52–26.55 ng/mL), SDs ranged from 0.278 to 1.32 ng/mL for repeatability (within the ≤1.5 ng/mL criterion), from 0.334 to 1.44 ng/mL for intermediate precision (within the ≤2.25 ng/mL criterion), and from 0.619 to 1.79 ng/mL for reproducibility (within the ≤4.5 ng/mL criterion). For high‐concentration samples (mean: 359.1–10 294 ng/mL), the CV ranged from 2.05 to 2.99% for repeatability (within the ≤5% criterion), from 3.28 to 3.74% for intermediate precision (within the ≤7.5% criterion), and from 5.28 to 5.82% for reproducibility (within the ≤15% criterion).
The reference range population comprised 811 individuals: 431 (53.1%) were men and the mean age was 47.1 years. Mean PIVKA‐II concentration was 19.7 ng/mL, with values ranging from 19.1 to 20.7 ng/mL across age groups. The 95th percentile was 28.4 ng/mL. Therefore, 28.4 ng/mL was used as a cut‐off for PIVKA‐II in the clinical performance analyses.
Method comparison was performed using 391 samples, although 10 nonclinical samples prepared from leftovers were excluded because of outlying behaviors. Weighted Deming regression analyses showed moderate agreement between the Elecsys PIVKA‐II assay and Lumipulse G PIVKA‐II (y = 5.81x + 0.57; Pearson's r = 0.883; P < 0.001); μTASWako DCP (y = 8.11x + 0.60; Pearson's r = 0.866; P < 0.001); and ARCHITECT PIVKA‐II (y = 4.68x + 0.59; Pearson's r = 0.875; P < 0.001) assays (Fig. 1).
Figure 1.
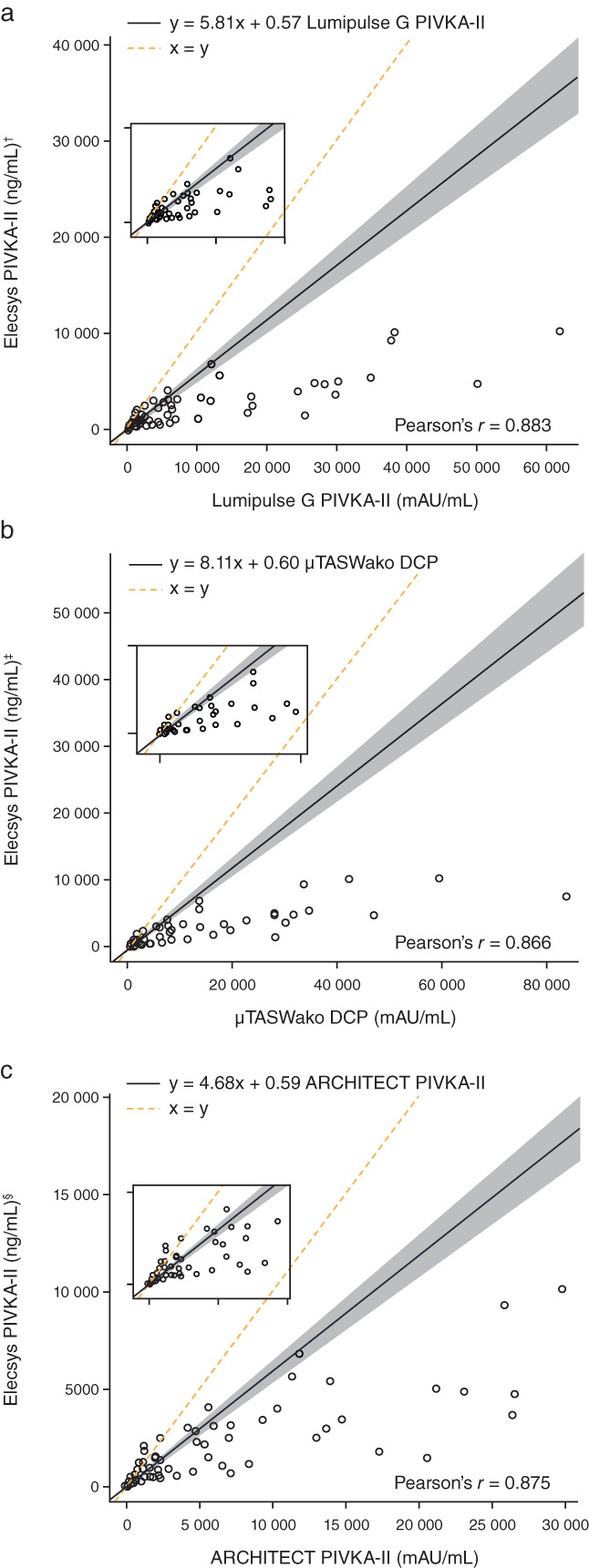
Weighted Deming regression fit of agreement between Elecsys PIVKA‐II and (a) Lumipulse G PIVKA‐II, (b) μTASWako DCP, and (c) ARCHITECT PIVKA‐II. † n = 357; ‡ n = 358; § n = 355. DCP, des‐γ‐carboxyprothrombin; PIVKA‐II, prothrombin induced by vitamin K absence‐II.
Clinical performance
Clinical performance of the Elecsys PIVKA‐II assay (AUC: 90.8%) was equivalent to that of comparator assays (AUC: 88.3–89.6%; Fig. 2a). PIVKA‐II and AFP concentrations were clearly elevated in HCC cases compared with disease controls (Fig. 3a,b). The median PIVKA‐II concentration was 301.19 ng/mL in HCC cases compared with 19.39 ng/mL in disease controls. The median AFP concentration was 24.55 ng/mL in HCC cases compared with 2.92 ng/mL in disease controls.
Figure 2.
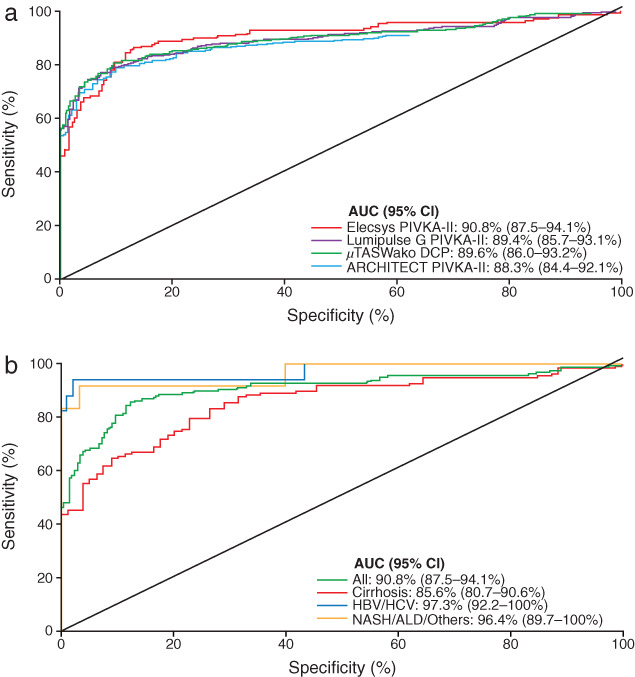
ROC analyses. (a) Elecsys PIVKA‐II, Lumipulse G PIVKA‐II, μTASWako DCP, and ARCHITECT PIVKA‐II assays for discriminating HCC cases and disease controls. (b) Elecsys PIVKA‐II for discriminating between patients with HCC and disease controls, overall and per etiology groups: cirrhosis, non‐cirrhotic HBV or HCV, non‐cirrhotic NASH or ALD, and others. ALD, alcoholic liver disease; AUC, area under the curve; CI, confidence interval; DCP, des‐γ‐carboxyprothrombin; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; NASH, non‐alcoholic steatohepatitis; PIVKA‐II, prothrombin induced by vitamin K absence‐II; ROC, receiver operating characteristic.
Figure 3.
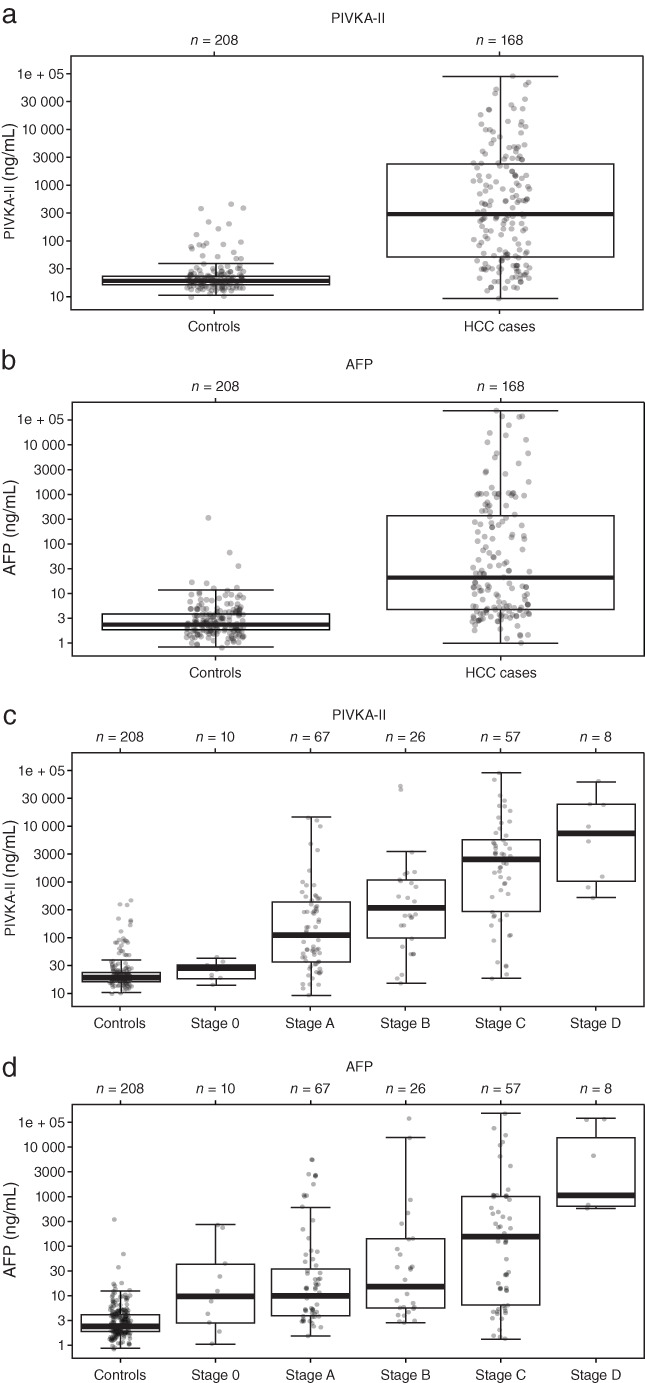
Overall distribution of (a) PIVKA‐II and (b) AFP in HCC cases and disease controls, and detailed distribution of (c) PIVKA‐II and (d) AFP according to BCLC HCC disease stage. AFP, alpha‐fetoprotein; BCLC, Barcelona Clinic Liver Cancer; HCC, hepatocellular carcinoma; PIVKA‐II, prothrombin induced by vitamin K absence‐II.
Serum PIVKA‐II and AFP concentrations correlated with HCC disease stage (Fig. 3c,d). Median PIVKA‐II and AFP concentrations were 63 and 11.7 ng/mL for early‐stage HCC, increasing to 1486 and 144 ng/mL for late‐stage HCC, respectively.
Both assays demonstrated good clinical performance for the detection of HCC (Table 1). The Elecsys PIVKA‐II assay showed high sensitivity and good specificity; sensitivity was higher for late stage versus early stage HCC (94.5 vs 77.9%). The Elecsys AFP assay showed excellent specificity and moderate sensitivity; sensitivity was higher for late stage versus early stage HCC (64.8 vs 36.4%). Cumulative data analysis resulted in an AUC value of 0.908 for Elecsys PIVKA‐II and 0.88 for Elecsys AFP, confirming their clinical performance. Clinical performance of both Elecsys PIVKA‐II and Elecsys AFP for specificity, ranging between 70 and 95%, and sensitivity, ranging between 70 and 95%, was determined (summarized in Table 2). At 95% specificity, cut‐offs for Elecsys PIVKA‐II and Elecsys AFP were 86.7 and 11.52 ng/mL, respectively, and sensitivity was 67.9 and 61.9%. Cut‐offs for Elecsys PIVKA‐II and Elecsys AFP at 90% specificity were 35.9 and 8.22 ng/mL, and sensitivity was 81 and 64.9%. Specificity at 95 and 90% sensitivity was similar between Elecsys PIVKA‐II and Elecsys AFP.
Table 1.
Clinical performance of Elecsys PIVKA‐II and Elecsys AFP assays by HCC stage
| Assay (cut‐off) | Metric, % (95% CI) | HCC stage | ||
|---|---|---|---|---|
| Early (n = 77) | Late (n = 91) | Overall (N = 168) | ||
| PIVKA‐II (28.4 ng/mL) | Sensitivity | 77.9 (67.0–86.6) | 94.5 (87.6–98.2) | 86.9 (80.8–91.6) |
| Specificity | 83.7 (77.9–88.4) | 83.7 (77.9–88.4) | 83.7 (77.9–88.4) | |
| AFP (20 ng/mL) | Sensitivity | 36.4 (25.7–48.1) | 64.8 (54.1–74.6) | 51.8 (44.0–59.5) |
| Specificity | 98.1 (95.1–99.5) | 98.1 (95.1–99.5) | 98.1 (95.1–99.5) | |
AFP, alpha‐fetoprotein; CI, confidence interval; HCC, hepatocellular carcinoma; PIVKA‐II, prothrombin induced by vitamin K absence‐II.
Table 2.
Cut‐offs of Elecsys PIVKA‐II and Elecsys AFP at specified specificity and sensitivity values
| PIVKA‐II | AFP | |||
|---|---|---|---|---|
| Specificity, % | Cut‐off, ng/mL | Sensitivity, % (95% CI) | Cut‐off, ng/mL | Sensitivity, % (95% CI) |
| 95 | 86.7 | 67.9 (60.2–74.8) | 11.52 | 61.9 (54.1–69.3) |
| 90 | 35.9 | 81 (74.2–86.6) | 8.22 | 64.9 (57.2–72.1) |
| 85 | 28.5 | 86.9 (80.8–91.6) | 6.00 | 73.2 (65.8–79.7) |
| 80 | 25.3 | 88.7 (82.9–93.1) | 5.28 | 77.4 (70.3–83.5) |
| 75 | 23.6 | 89.9 (84.3–94.0) | 4.71 | 81.5 (74.8–87.1) |
| 70 | 22.7 | 90.5 (85.0–94.5) | 4.27 | 86.3 (80.2–91.1) |
| Sensitivity, % | Cut‐off, ng/mL | Specificity, % (95% CI) | Cut‐off, ng/mL | Specificity, % (95% CI) |
|---|---|---|---|---|
| 95 | 18.7 | 43.3 (36.4–50.3) | 2.85 | 45.2 (38.3–52.2) |
| 90 | 23.1 | 72.1 (65.5–78.1) | 3.65 | 64.4 (57.5–70.9) |
| 85 | 31.7 | 87.5 (82.2–91.7) | 4.45 | 73.1 (66.5–79.0) |
| 80 | 36.5 | 90.4 (85.5–94.0) | 5.04 | 77.9 (71.6–83.3) |
| 75 | 51.5 | 91.8 (87.2–95.2) | 5.87 | 83.2 (77.4–88.0) |
| 70 | 63.9 | 93.3 (89.0–96.3) | 6.45 | 87.0 (81.7–91.4) |
AFP, alpha‐fetoprotein; CI, confidence interval; PIVKA‐II, prothrombin induced by vitamin K absence‐II.
Specificity and sensitivity for discrimination of HCC in early‐, late‐, and all‐stage patients at rounded cut‐offs from 20 to 1000 mAU/mL are summarized in Tables S2–S4, Supporting information for the Elecsys PIVKA‐II, Lumipulse G PIVKA‐II, μTASWako DCP, and ARCHITECT PIVKA‐II assays. At a cut‐off of 40 mAU/mL, specificity and sensitivity were comparable between the Elecsys PIVKA‐II assay (specificity: 90.9%, sensitivity: 78%) and the Lumipulse G PIVKA‐II, μTASWako DCP, and ARCHITECT PIVKA‐II assays (specificity: 84.6–89.9%, sensitivity: 81.0–82.7%) for discriminating all‐stage HCC cases.
The Elecsys PIVKA‐II assay also demonstrated high specificity in other analyses. For instance, high specificity was seen across non‐cirrhotic etiologies, ranging from 90.3% in HBV+ samples to 93.3% in samples with NASH and to 100% in HCV+ samples; specificity was lower (68.4%) for cirrhotic cases. Specificity was not determined for ALD and other factors due to small sample sizes.
The AUC for all samples was 90.8%. The AUC was highest for HBV/HCV samples (97.3%) and lowest for cirrhotic cases (85.6%; Fig. 2b). Additionally, specificity panel analyses showed that PIVKA‐II was found in lower concentrations for most other conditions tested compared with HCC, with the exception of cholangiocarcinoma and pancreatic cancer, on both Elecsys PIVKA‐II and μTASWako DCP assays (Fig. 4). In samples from patients with cholangiocarcinoma, a median PIVKA‐II concentration of 143 ng/mL (range: 14.5–22 463 ng/mL) was observed using the Elecsys PIVKA‐II assay. In samples from patients with pancreatic cancer, the median PIVKA‐II concentration was 211 ng/mL (range: 17.6–3034 ng/mL) using the Elecsys PIVKA‐II assay.
Figure 4.
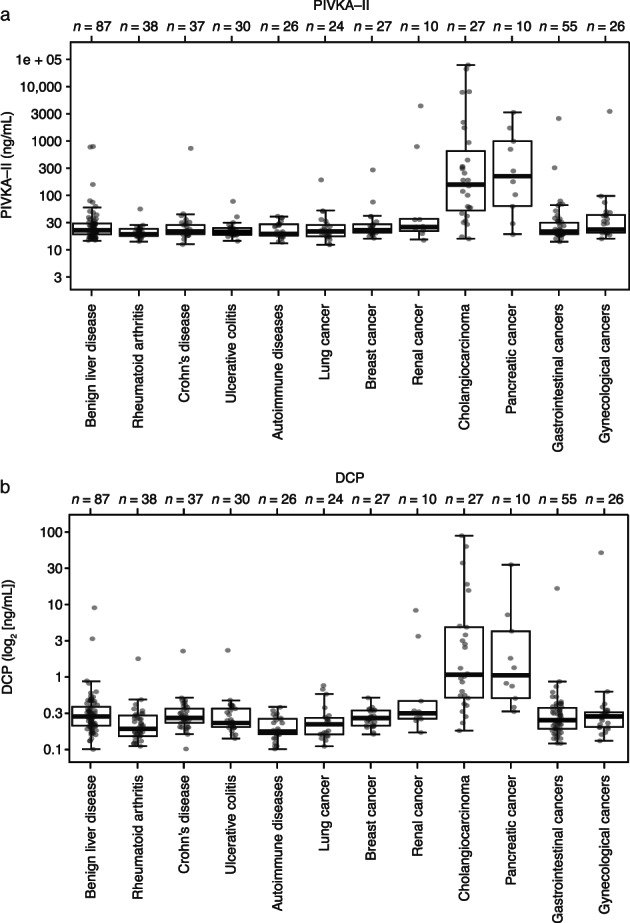
Distribution of PIVKA‐II in the subgroups of the specificity panel cohort on (a) the Elecsys PIVKA‐II assay and (b) the μTASWako DCP platform. DCP, des‐γ‐carboxyprothrombin; PIVKA‐II, prothrombin induced by vitamin K absence‐II.
Analysis of concordance between a PIVKA‐II cut‐off of 28.4 ng/mL and AFP cut‐offs of 8.22 and 11.5 ng/mL (corresponding to 90 and 95% specificity, respectively) showed that concordance was highest for a PIVKA‐II cut‐off of >28.4 ng/mL and an AFP cut‐off of >8.22 ng/mL in patients with early‐ and late‐stage HCC (43 and 70%, respectively; Table S5, Supporting information).
Using a combination PIVKA‐II (at a cut‐off of 28.4 ng/mL) and AFP (at a cut‐off of 20 ng/mL), the overall sensitivity for HCC detection was 92% versus 87% using the Elecsys PIVKA‐II assay alone or 52% using the Elecsys AFP assay alone. The corresponding specificities were 82%, 84%, and 98%, respectively.
Discussion
This study demonstrated that the Elecsys PIVKA‐II assay compares favorably with commercially available comparator assays. Using a cut‐off of 28.4 ng/mL, the Elecsys PIVKA‐II assay detected HCC with a sensitivity of 86.9% and specificity of 83.7%. Clinical performance of the Elecsys PIVKA‐II assay (AUC: 90.8%) was equivalent to that of comparator assays (AUC: 88.3–89.6%).
Relatively high PIVKA‐II concentrations were also observed for cholangiocarcinoma and pancreatic cancer using the Elecsys PIVKA‐II assay in the specificity panel of this study, although this may be attributable to underlying conditions in these patients (e.g. cholestatic disease, cholangitis, biliary stenosis, or bile duct stone). The small number of measurable samples from patients with cholangiocarcinoma (n = 27) and pancreatic cancer (n = 10) should also be considered as a limitation. However, the elevated concentrations were confirmed using the μTASWako DCP platform; previous studies have also reported high levels of expression of PIVKA‐II in patients with intrahepatic cholangiocarcinoma and pancreatic cancer. 22 , 23 Berhane et al. found that neither PIVKA‐II nor AFP‐L3 was elevated in patients with cholangiocarcinoma or pancreatic cancer compared with healthy controls when measured using microchip capillary electrophoresis and a liquid‐phase binding assay on a μTASWako i30 analyzer. 20 Therefore, the relatively high concentrations of PIVKA‐II observed here are more likely due to differences in the patient population than differences between assays. These findings indicate that high concentrations of PIVKA‐II should not be used as a stand‐alone diagnostic tool for HCC in the absence of other clinical data, such as patient symptoms, imaging, and other laboratory tests. Currently available diagnostic biomarkers for cholangiocarcinoma are considered to be inaccurate, 24 and the only approved biomarker for pancreatic cancer, CA 19‐9, is limited by poor sensitivity and specificity. 25 Therefore, it may be beneficial to investigate the specificity and sensitivity of PIVKA‐II for these two malignancies in a future study in the setting of healthcare systems that have not incorporated PIVKA‐II in routine clinical practice.
The use of AFP and other biomarkers to detect HCC has been shown previously to be complementary to surveillance techniques and can benefit diagnostic models. 6 , 26 Our findings support the use of AFP as a biomarker for HCC. This study demonstrated the discrimination of patients with HCC from those with non‐HCC conditions using the Elecsys AFP assay. Furthermore, the results reported here for the increased overall sensitivity for HCC detection when combining PIVKA‐II and AFP assays support previously published evidence. A study of the usefulness of PIVKA‐II, AFP, and AFP‐L3 for diagnosing HCC found that the AUC was significantly (P = 0.001) higher for the combination of PIVKA‐II >40 mAU/mL and AFP >10 ng/mL versus the combination of PIVKA‐II, AFP at the same concentrations, plus AFP‐L3 >10%. 27 A real‐world study investigating the effectiveness of PIVKA‐II in detecting HCC in clinical practice demonstrated that PIVKA‐II effectively increases the detection rate of HCC and is a valid complement to AFP 12 ; these results and others 11 suggest that PIVKA‐II could therefore provide a more sensitive means of differentiating HCC from other diseases.
We used AFP 20 ng/mL as the cut‐off for HCC diagnosis, with a sensitivity of 51.8% and specificity of 98.1%. AFP 200 ng/mL, an alternative cut‐off recommended as an aid to HCC diagnosis by APASL, 10 was also analyzed but was found to be notably less sensitive (data not shown). AFP cut‐offs of 11.52 and 8.22 ng/mL, corresponding to 95 and 90% specificity, had a sensitivity of 61.9 and 64.9%, versus a sensitivity of 67.9 and 81% for PIVKA‐II at 95 and 90% specificity. For the diagnosis of early HCC, the optimal cut‐off for AFP is likely to be lower. A large multicenter phase 2 case–control study found that the optimal AFP cut‐off for the diagnosis of early HCC was 10.9 ng/mL, with a sensitivity of 66%; for very early HCC, an AFP cut‐off of 10.9 ng/mL had an AUC of 0.78 and a sensitivity of 65%. 28 Lower values of 5 and 10 ng/mL have been suggested as potential AFP cut‐offs to differentiate the risk of developing HCC in patients undergoing antiviral treatment for HBV with nucleoside/nucleotide analogs. 29 Similarly, other studies found that AFP 6 ng/mL was an appropriate cut‐off, including for patients with HBV receiving entecavir treatment 30 and patients with HCV receiving interferon treatment. 31 Accordingly, future studies investigating whether antiviral nucleoside analog treatments impact the performance of the Elecsys PIVKA‐II assay may be beneficial.
Although the Elecsys PIVKA‐II assay cannot be directly compared with other platforms because of the use of different technologies, antibodies, and detection of different carboxylated variants, this study achieves the objective of demonstrating clinical performance equivalent to that of the comparator assays. Strengths of the study include the familiarization and quality control measures, which ensure accurate performance of all study procedures. Additionally, the presented reference values may support physicians in their diagnosis of HCC.
As this was a cross‐sectional study, it was not possible to assess the timing and amplitude of PIVKA‐II rise in relation to early HCC development; such an assessment would require a longitudinal study with serial samples needed for validation. Another limitation of this study is that it aimed to study the performance of HCC biomarkers alone, without considering the role of ultrasound. The performance of PIVKA‐II with or without AFP versus ultrasound was not compared, nor was the performance of serum biomarkers with ultrasound on HCC surveillance. Future studies are needed to better inform the application of these biomarkers in clinical practice.
In conclusion, the Elecsys PIVKA‐II assay demonstrated good analytical performance under routine laboratory conditions and compared favorably with commercially available comparator assays, demonstrating its suitability as an aid in HCC diagnosis. This translated as good clinical performance, which may aid in the diagnosis of HCC across all disease stages and etiologies. Combining PIVKA‐II with AFP may further increase the diagnostic performance.
Ethics approval
This study was conducted in accordance with the principles of the Declaration of Helsinki. The study protocol received approval from the joint CUHK‐NTEC Clinical Research Ethics Committee of the Prince of Wales Hospital, Hong Kong; the ethics committee of the Kinki University School of Medicine, Osaka, Japan; the Human Research Ethics Committee Faculty of Medicine, Prince of Songkla University, Hat Yai, Thailand; the ethics committee of the Medizinische Hochschule Hannover, Germany; the ethics committee of the Medical Faculty of the University of Leipzig, Germany; the ethics committee of the Medical Faculty of Goethe University Theodor‐Stern‐Kai, Frankfurt, Germany; the ethics committee of the Medical Faculty of LMU Munich, Germany; and the ethics committee of the Rhineland‐Palatinate Medical Association, Mainz, Germany. Use of prospectively collected specificity panel samples for the purpose of this study was approved by the following institutional review boards: Kiev City Clinical Oncology Center, Kiev; National Cancer Institute MOH Ukraine, Kiev; Shalimov National Institute of Surgery and Transplantation, Kiev; and Kiev City Hospital Nu, Kiev.
Supporting information
Appendix S1. Supporting information.
Acknowledgments
The authors would like to thank David Morgenstern (Roche Diagnostics) for his help with the study design, and Arndt Weinmann (University Medical Center of the Johannes Gutenberg University Mainz) for his help with data acquisition. The authors acknowledge the following individuals from the Chinese University of Hong Kong: Grace L.H. Wong, Carmen K.M. Chan, Vincent W.S. Wong, Stephen L. Chan, Siew C. Ng, Charing C.N. Chong, Ronald C.W. Ma, Simon S.M. Ng, Angel M.L. Chim, Chi‐Hang Tse, and Katherine Y.Y. Kwan. The authors also thank Naoshi Nishida from Kindai University and Katharina Ruhnke, Michaela Fink, and Karl Slusarczyk from Roche Diagnostics for their contributions. Third‐party medical writing assistance, under the guidance of the authors, was provided by Chloe Fletcher, MSc, of Ashfield MedComms (Macclesfield, UK), an Ashfield Health company, and was funded by Roche Diagnostics International Ltd (Rotkreuz, Switzerland). COBAS, COBAS E, and ELECSYS are trademarks of Roche. All other product names and trademarks are the property of their respective owners.
Declaration of conflict of interest: HLYC is on the advisory committee or review panel for GRAIL and Roche, and speaks and teaches for Roche. AV is a consultant for Roche, and speaks and teaches for Roche. TB is a consultant for Bayer, Eisai, Ipsen, Merck Sharp & Dome/Merck, and Roche. ENDeT is a consultant for AstraZeneca, Bayer, BMS, Eisai, Eli Lilly & Co., Pfizer, IPSEN, and Roche, and receives grant/research support from ArQule, AstraZeneca, BMS, Bayer, Celsion, Eli Lilly & Co., and Roche. He also speaks and teaches for BMS and Falk. MK speaks and teaches for Eisai, Bayer, Merck Sharp & Dome, Bristol Myers Squibb, Eli Lilly & Co., and EA Pharma; receives grant/research support from Gilead Sciences, Taiho, Sumitomo Dainippon Pharma, Takeda, Otsuka, EA Pharma, AbbVie, Eisai, and Ono; and is on the advisory committee or review panel for Eisai, Ono, MSD, Bristol Myers Squibb, and Roche. JT is a consultant for Amgen, Bayer Healthcare, Bristol Myers Squibb, Eisai, Ipsen, Merck Serono, Merck Sharp & Dome, Lilly ImClone, and Roche. AE is an independent contractor for Roche Diagnostics. H‐GK and JKH report no conflicts of interest. AS, KM, VR, and M‐RL are employees of Roche Diagnostics. TP receives grants/research support from Roche Diagnostics, Janssen, FibroGen, and VIR, and speaks and teaches for Bristol Myers Squibb, Gilead Sciences, Bayer, Abbott, Eisai, and Mylan Ferring.
Author contribution: Henry L Y Chan: Conceptualization, methodology, investigation, resources, writing ‐ original draft, writing ‐ review & editing, visualization, supervision. Arndt Vogel, Thomas Berg, Enrico N De Toni, Jörg Trojan, Anja Eiblmaier, Johannes Kolja Hegel, Marcus‐Rene Lisy: Investigation, resources, writing ‐ review and editing. Masatoshi Kudo: Conceptualization, investigation, resources, writing ‐ review & editing. Hanns‐Georg Klein: Validation, supervision, project administration, writing ‐ review & editing. Ashish Sharma and Teerha Piratvisuth: Methodology, Investigation, Resources, Formal analysis, Writing ‐ Review & Editing. Kairat Madin: Methodology, investigation, resources, formal analysis, writing ‐ review & editing, visualization, supervision. Vinzent Rolny: Methodology, formal analysis, data curation, writing ‐ review & editing.
Financial support: This study was funded by Roche Diagnostics GmbH (Penzberg, Germany).
Data availability statement
This study was conducted in accordance with applicable regulations. Qualified researchers may contact the following Leading Ethics Committee to request access: Ethik‐Kommission der MHH (Ethikkommission@mh‐hannover.de; Study Protocol RD002542).
References
- 1. Sung H, Ferlay J, Siegel RL et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021; 71: 1–41. [DOI] [PubMed] [Google Scholar]
- 2. El‐Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007; 132: 2557–76. [DOI] [PubMed] [Google Scholar]
- 3. Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2013; 10: 656–65. [DOI] [PubMed] [Google Scholar]
- 4. Bialecki ES, Di Bisceglie AM. Diagnosis of hepatocellular carcinoma. HPB (Oxford). 2005; 7: 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Song T, Wang L, Su B et al. Diagnostic value of alpha‐fetoprotein, Lens culinaris agglutinin‐reactive alpha‐fetoprotein, and des‐gamma‐carboxyprothrombin in hepatitis B virus‐related hepatocellular carcinoma. J. Int. Med. Res. 2020; 48: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahmed Mohammed HF, Roberts LR. Should AFP (or any biomarkers) be used for HCC surveillance? Curr. Hepatol. Rep. 2017; 16: 137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Llovet JM. Hepatocellular carcinoma: patients with increasing alpha‐fetoprotein but no mass on ultrasound. Clin. Gastroenterol. Hepatol. 2006; 4: 29–35. [DOI] [PubMed] [Google Scholar]
- 8. European Association for the Study of the Liver . EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 2018; 69: 182–236. [DOI] [PubMed] [Google Scholar]
- 9. Marrero JA, Kulik LM, Sirlin CB et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018; 68: 723–50. [DOI] [PubMed] [Google Scholar]
- 10. Omata M, Cheng AL, Kokudo N et al. Asia‐Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol. Int. 2017; 11: 317–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zakhary NI, Khodeer SM, Shafik HE, Abdel Malak CA. Impact of PIVKA‐II in diagnosis of hepatocellular carcinoma. J. Adv. Res. 2013; 4: 539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu R, Tan Z, Xiang X, Dan Y, Deng G. Effectiveness of PIVKA‐II in the detection of hepatocellular carcinoma based on real‐world clinical data. BMC Cancer. 2017; 17: 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pote N, Cauchy F, Albuquerque M et al. Performance of PIVKA‐II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J. Hepatol. 2015; 62: 848–54. [DOI] [PubMed] [Google Scholar]
- 14. Wu J, Xiang Z, Bai L et al. Diagnostic value of serum PIVKA‐II levels for BCLC early hepatocellular carcinoma and correlation with HBV DNA. Cancer Biomark. 2018; 23: 235–42. [DOI] [PubMed] [Google Scholar]
- 15. Svobodova S, Karlikova M, Topolcan O et al. PIVKA‐II as a potential new biomarker for hepatocellular carcinoma – a pilot study. In Vivo. 2018; 32: 1551–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson PJ, Pirrie SJ, Cox TF et al. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol. Biomarkers Prev. 2014; 23: 144–53. [DOI] [PubMed] [Google Scholar]
- 17. Best J, Bechmann LP, Sowa JP et al. GALAD score detects early hepatocellular carcinoma in an international cohort of patients with nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 2020; 18: 728–35. [DOI] [PubMed] [Google Scholar]
- 18. Toyoda H, Kumada T, Osaki Y et al. Staging hepatocellular carcinoma by a novel scoring system (BALAD score) based on serum markers. Clin. Gastroenterol. Hepatol. 2006; 4: 1528–36. [DOI] [PubMed] [Google Scholar]
- 19. Fox R, Berhane S, Teng M et al. Biomarker‐based prognosis in hepatocellular carcinoma: validation and extension of the BALAD model. Br. J. Cancer. 2014; 110: 2090–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berhane S, Toyoda H, Tada T et al. Role of the GALAD and BALAD‐2 serologic models in diagnosis of hepatocellular carcinoma and prediction of survival in patients. Clin. Gastroenterol. Hepatol. 2016; 14: 875–86. [DOI] [PubMed] [Google Scholar]
- 21. Toyoda H, Tada T, Johnson PJ et al. Validation of serological models for staging and prognostication of HCC in patients from a Japanese nationwide survey. J. Gastroenterol. 2017; 52: 1112–21. [DOI] [PubMed] [Google Scholar]
- 22. Hiraoka A, Kurose K, Kumagi T et al. Carcinoma with shared pathologic characteristics of both hepatocellular carcinoma and cholangiocarcinoma. Curr. Ther. Res. Clin. Exp. 2005; 66: 589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tartaglione S, Pecorella I, Zarrillo SR et al. Protein induced by vitamin K absence II (PIVKA‐II) as a potential serological biomarker in pancreatic cancer: a pilot study. Biochem. Med. 2019; 29: 352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Macias RIR, Kornek M, Rodrigues PM et al. Diagnostic and prognostic biomarkers in cholangiocarcinoma. Liver Int. 2019; 39: 108–22. [DOI] [PubMed] [Google Scholar]
- 25. Hasan S, Jacob R, Manne U, Paluri R. Advances in pancreatic cancer biomarkers. Oncol. Rev. 2019; 13: 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tzartzeva K, Obi J, Rich NE et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta‐analysis. Gastroenterology. 2018; 154: 1706–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park SJ, Jang JY, Jeong SW et al. Usefulness of AFP, AFP‐l3, and PIVKA‐II, and their combinations in diagnosing hepatocellular carcinoma. Medicine (Baltimore). 2017; 96: e5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marrero JA, Feng Z, Wang Y et al. Alpha‐fetoprotein, des‐gamma carboxyprothrombin, and lectin‐bound alpha‐fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009; 137: 110–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hann HW, Fu X, Myers RE et al. Predictive value of alpha‐fetoprotein in the long‐term risk of developing hepatocellular carcinoma in patients with hepatitis B virus infection – results from a clinic‐based longitudinal cohort. Eur. J. Cancer. 2012; 48: 2319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong GL, Chan HL, Tse YK et al. On‐treatment alpha‐fetoprotein is a specific tumor marker for hepatocellular carcinoma in patients with chronic hepatitis B receiving entecavir. Hepatology (Baltimore, Md.). 2014; 59: 986–95. [DOI] [PubMed] [Google Scholar]
- 31. Asahina Y, Tsuchiya K, Nishimura T et al. α‐fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology (Baltimore, Md.). 2013; 58: 1253–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information.
Data Availability Statement
This study was conducted in accordance with applicable regulations. Qualified researchers may contact the following Leading Ethics Committee to request access: Ethik‐Kommission der MHH (Ethikkommission@mh‐hannover.de; Study Protocol RD002542).


